Preparation and Application of Core–Shell Nanocarbon-Based Slow-Release Foliar Fertilizer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Screening of Carbon Nanoparticles
2.3. Functionalization CN@mSiO2 Preparation and Screening of Core–Shell-Structured Nanomaterials
2.4. Urea Load Test and Preparation of Polydopamine-Coated Nano Slow-Release Fertilizer
2.5. Characterization
2.6. Urea Release Test
2.7. Seed Germination and Pot Application of Cabbage Heart
| Germination rate = Number of germinated seeds/Number of seeds for testing × 100% |
| Germination potential = Number of germination within the germination of test seeds/number of test seeds × 100% |
| Germination index = ∑ Gt/D t |
| Vitality index = Mean radicle length at day 5 of germination × germination index |
| Nitrogen utilization rate = Amount of nitrogen absorbed by the crop/Amount of nitrogen fertilizer applied × 100% |
| Nitrogen Fertilizer Residual Rate = Amount of N left in the soil/Total amount of N fertilizer applied × 100% |
| Nitrogen loss rate = 100 − Nitrogen utilization rate − Nitrogen fertilizer residual rate in soil layer |
3. Results
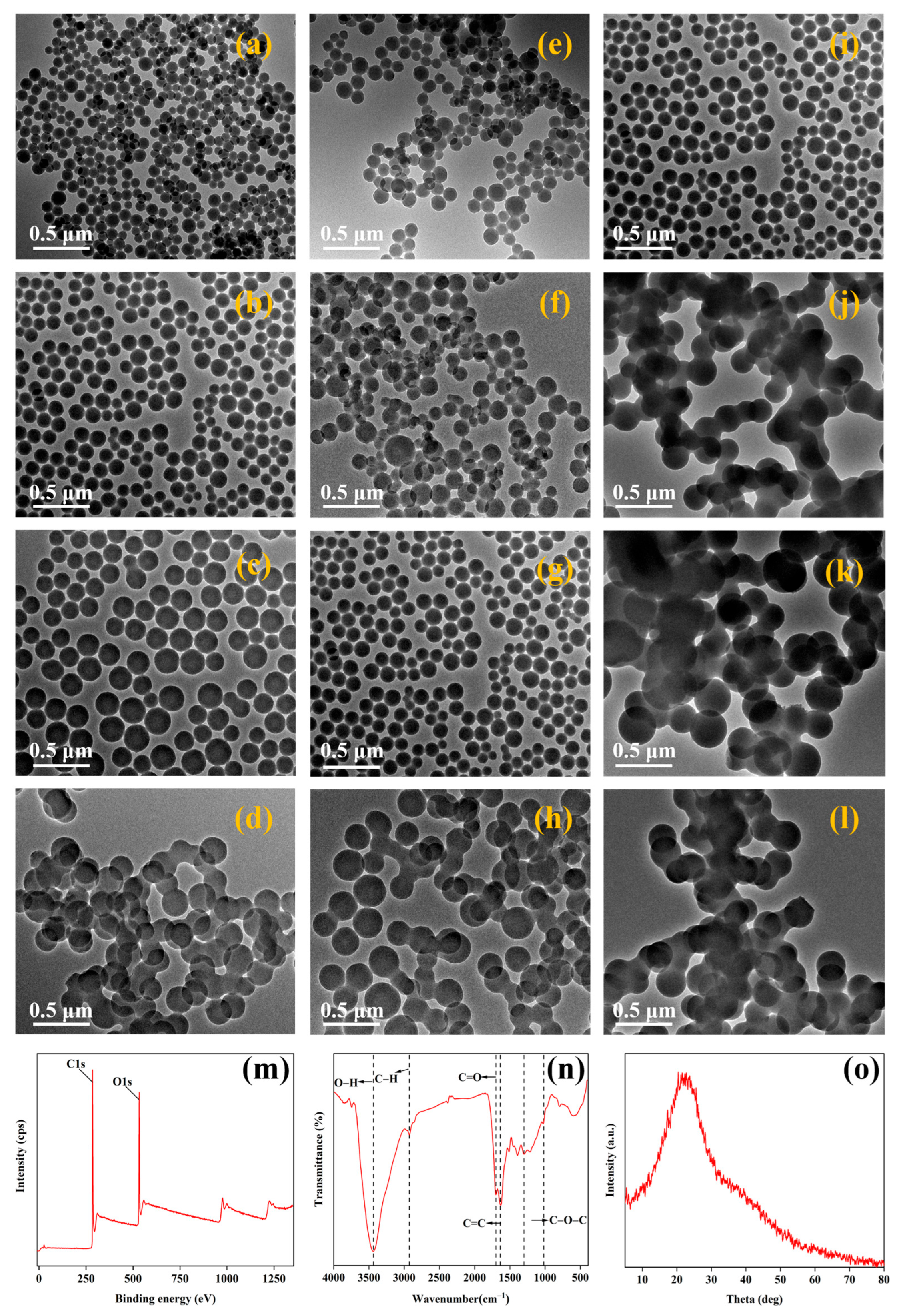
3.1. Preparation and Screening of Nanocarbon
Effect of Glucose Concentration, Reaction Time, and Temperature on Nanocarbon and Its Structural Characterization
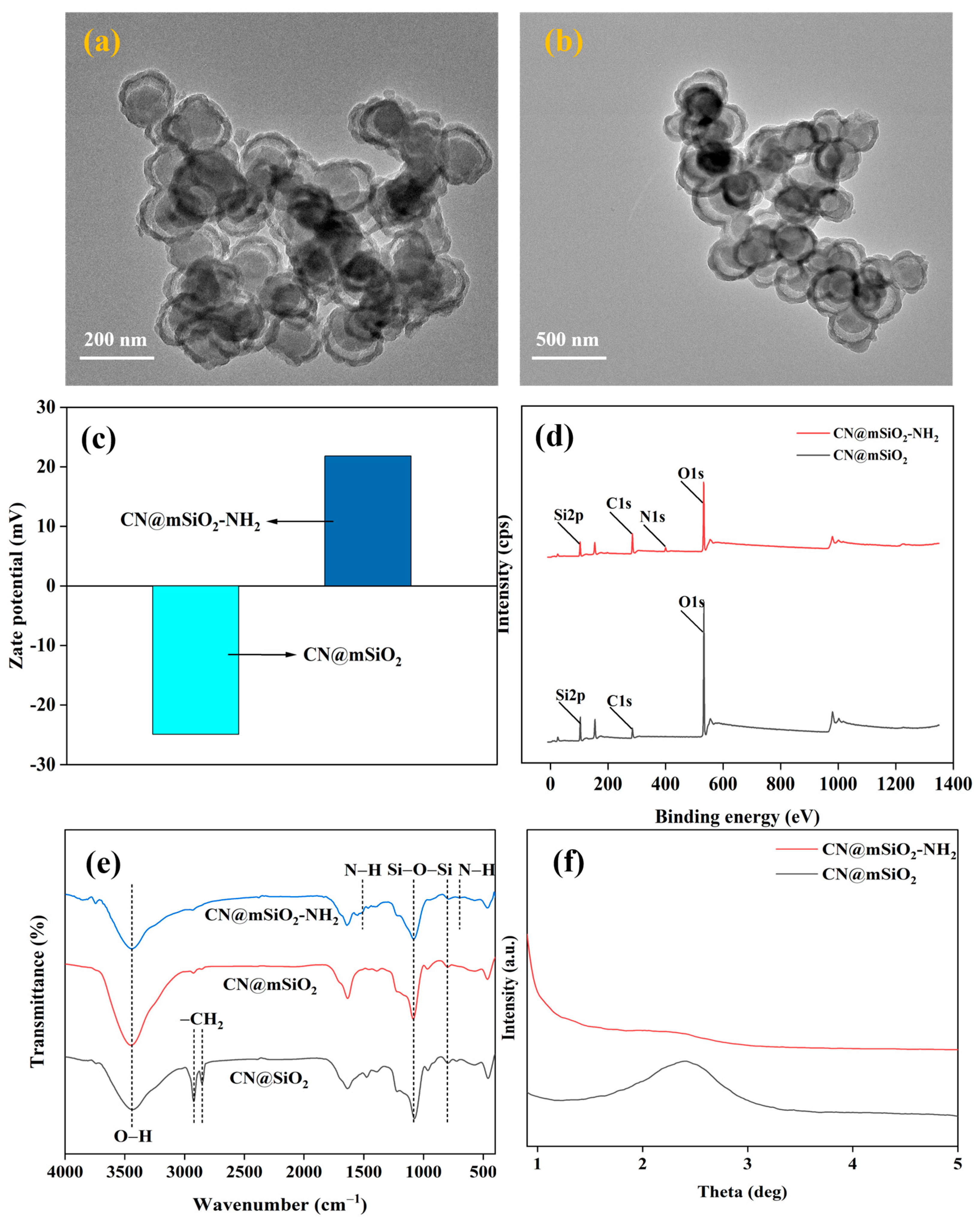
3.2. Preparation and Characterization of Functionalization CN@mSiO2 Core–Shell-Structured Nanomaterials
3.2.1. Effect of TEOS Concentration, Ammonia Concentration, and Alcohol–Water Ratio on CN@mSiO2 Core–Shell-Structured Nanomaterials
3.2.2. CN@mSiO2-NH2 Preparation and Characterization of Core–Shell-Structured Nanomaterials
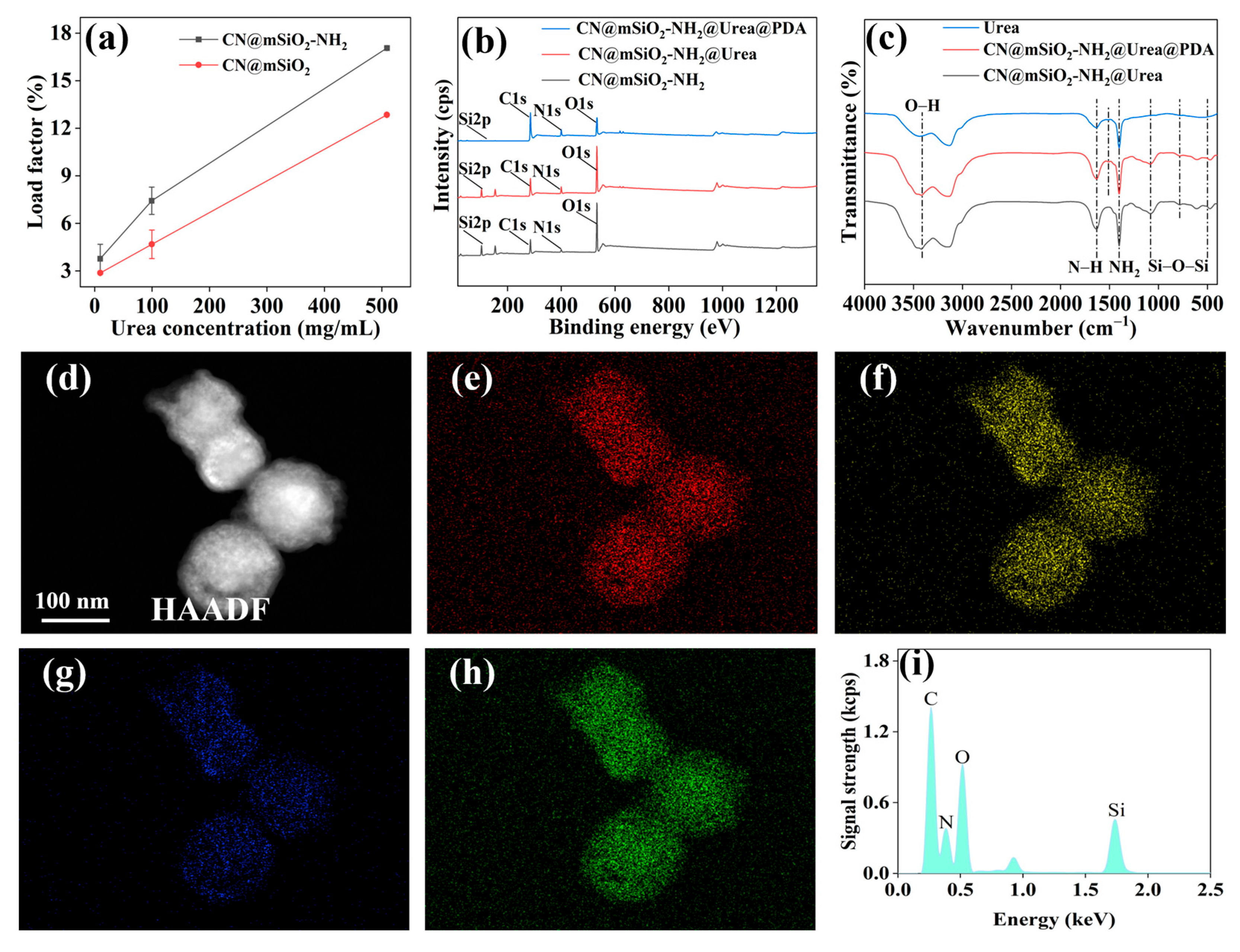
3.3. CN@mSiO2-NH2 Applications in Fertilizers
3.3.1. Loading Properties of Urea, Preparation, and Structure of CN@mSiO2-NH2@Urea@PDA Nano Slow-Release Fertilizer
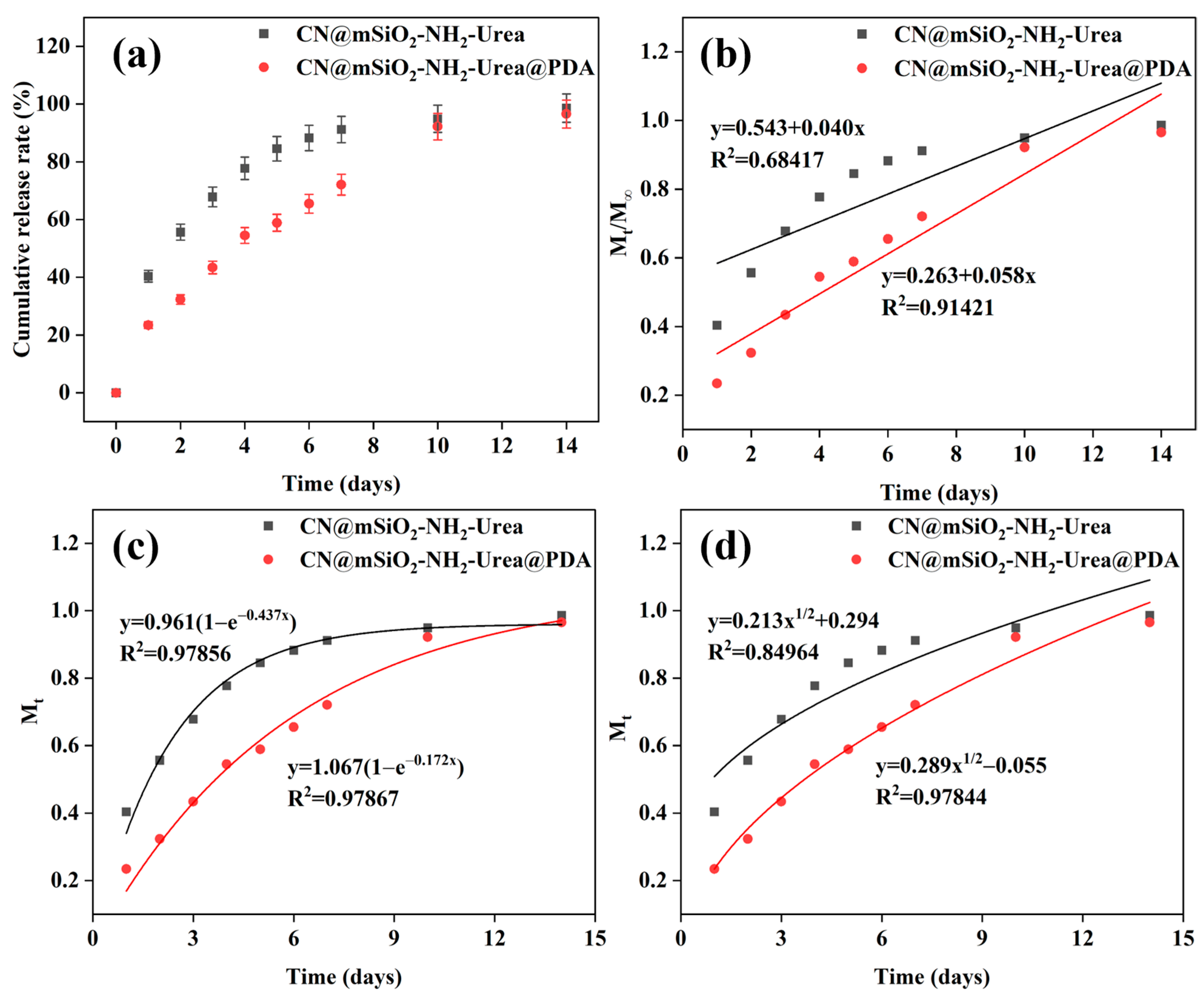
3.3.2. Release Periods of CN@mSiO2-NH2@Urea and CN@mSiO2-NH2@Urea@PDA
3.4. Evaluation of CN@mSiO2-NH2@Urea@PDA for Practical Applications
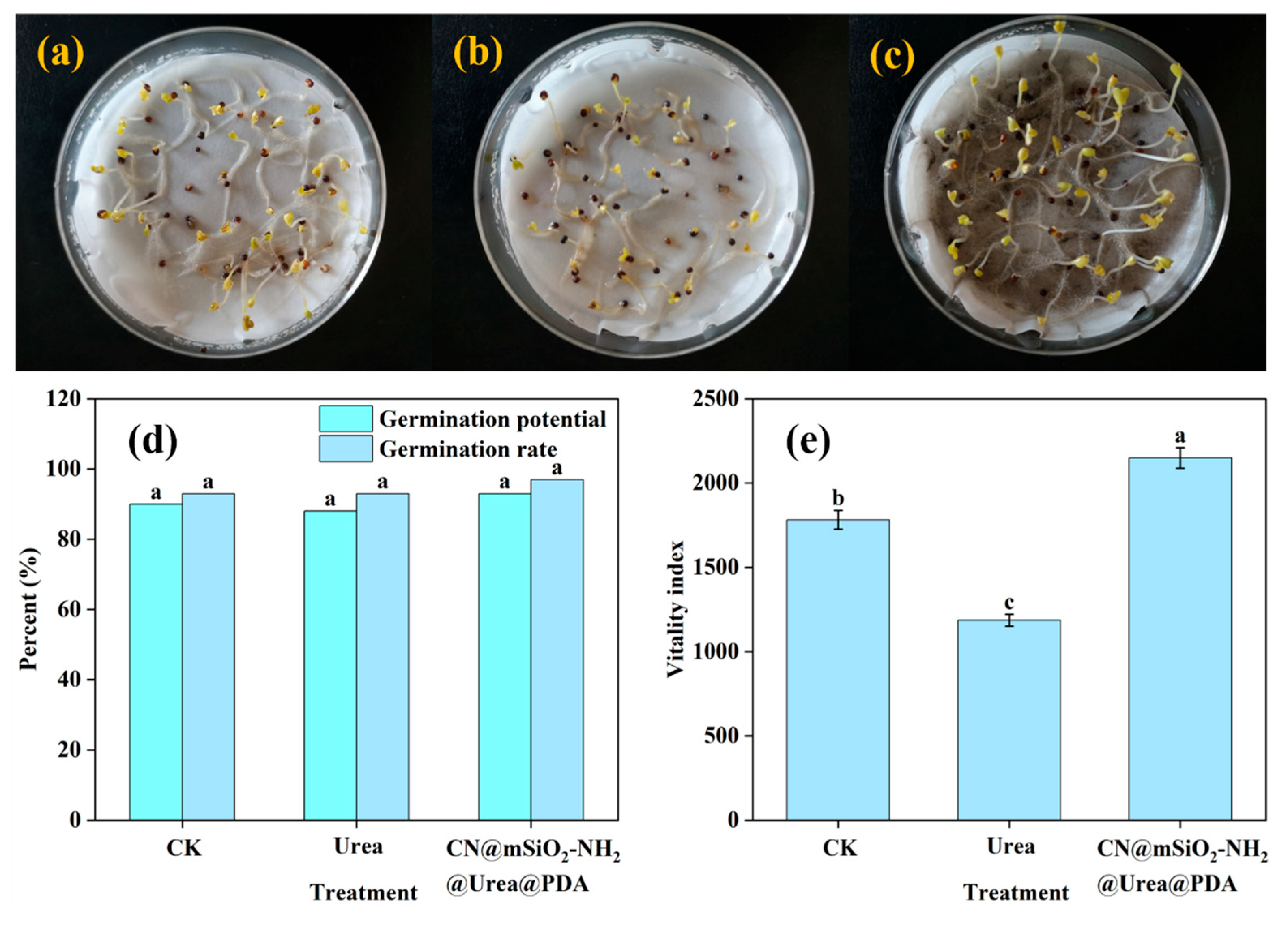
3.4.1. Seed Germination Assay
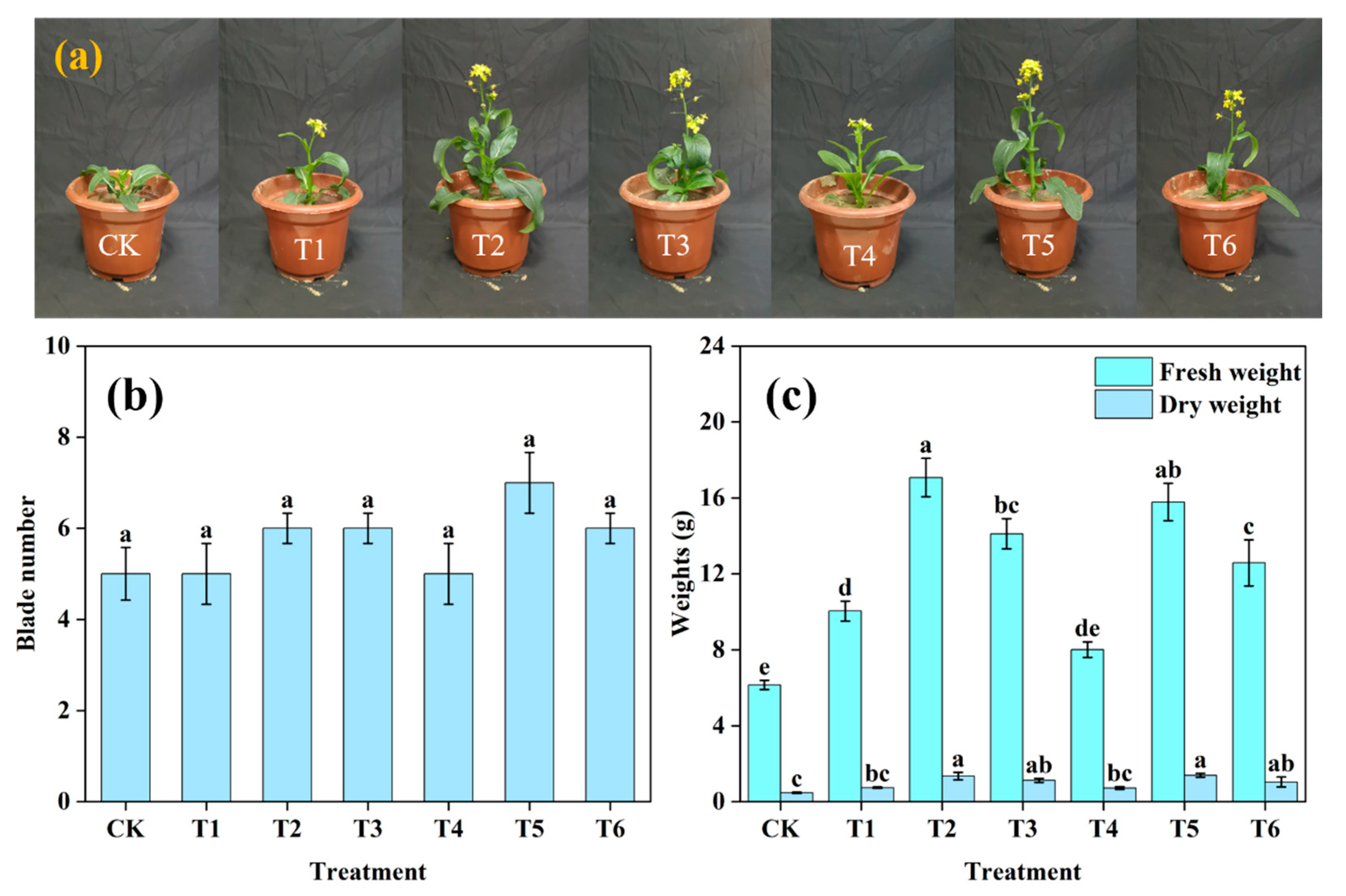
3.4.2. Pot Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CN | Carbon nanoparticles |
| mSiO2 | Mesoporous silica dioxide |
| PDA | Polydopamine |
| DA | Dopamine |
| -NH2 | Amino |
| SiO2 | Silica dioxide |
| TEOS | Tetraethyl orthosilicate |
| CTAB | Cetyltriethylammnonium bromide |
| APTES | (3-Aminopropyl) triethoxysilane |
| HCl | Hydrochloric acid |
| C | Carbon |
| N | Nitrogen |
| O | Oxygen |
| Si | Silica |
| TEM | Transmission Electron Microscope |
| SEM | Scanning Electron Microscope |
| EDS | Energy Dispersive X-Ray Spectroscopy |
| FTIR | Fourier Transform Infrared Spectroscopy |
| XRD | X-Ray Diffraction |
| XPS | X-ray Photoelectron Spectroscopy |
References
- Fukase, E.; Martin, W. Economic growth, convergence, and world food demand and supply. World Dev. 2020, 132, 12. [Google Scholar]
- Gu, B.J.; Zhang, X.M.; Lam, S.K.; Yu, Y.L.; van Grinsven, H.J.M.; Zhang, S.H.; Wang, X.X.; Bodirsky, B.L.; Wang, S.T.; Duan, J.K.; et al. Cost-effective mitigation of nitrogen pollution from global croplands. Nature 2023, 614, E19. [Google Scholar] [CrossRef]
- Li, J.Y.; Liu, Y.; Tang, Y.F.; Shao, J.F.; Xu, T.; Ma, R.H.; Jiang, Y.M.; Cheng, D.D. Optimizing fertilizer management based on controlled-release fertilizer to improve yield, quality, and reduce fertilizer application on apples. J. Soil Sci. Plant Nutr. 2022, 22, 393–405. [Google Scholar]
- Niu, Z.H.; Yi, F.; Chen, C. Agricultural insurance and agricultural fertilizer non-point source pollution: Evidence from China’s policy-based agricultural insurance pilot. Sustainability 2022, 14, 16. [Google Scholar] [CrossRef]
- Ishfaq, M.; Kiran, A.; Rehman, H.U.; Farooq, M.; Ijaz, N.H.; Nadeem, F.; Azeem, I.; Li, X.X.; Wakeel, A. Foliar nutrition: Potential and challenges under multifaceted agriculture. Environ. Exp. Bot. 2022, 200, 17. [Google Scholar]
- Li, W.C.; Fan, R.Y.; Zhou, H.J.; Zhu, Y.F.; Zheng, X.; Tang, M.Y.; Wu, X.S.; Yu, C.Z.; Wang, G.Z. Improving the utilization rate of foliar nitrogen fertilizers by surface roughness engineering of silica spheres. Environ. Sci.-Nano 2020, 7, 3526–3535. [Google Scholar]
- Xie, H.G.; Yu, G.L.; Wang, X.R.; Chu, D.Y.; Song, C.; Wang, P.; Dai, K.; Li, J.Z.; Yin, H. Sea urchin-like nanocarrier for enhancing pesticide retention and rain fastness on hydrophobic and hydrophilic crop foliage. Chem. Eng. J. 2024, 490, 12. [Google Scholar]
- Wang, M.; Sun, X.; Zhong, N.Q.; Cai, D.Q.; Wu, Z.Y. Promising approach for improving adhesion capacity of foliar nitrogen fertilizer. ACS Sustain. Chem. Eng. 2015, 3, 499–506. [Google Scholar]
- Yang, H.L.; Bai, L.F.; Geng, Z.R.; Chen, H.; Xu, L.T.; Xie, Y.C.; Wang, D.J.; Gu, H.W.; Wang, X.M. Carbon quantum dots: Preparation, optical properties, and biomedical applications. Mater. Today Adv. 2023, 18, 24. [Google Scholar] [CrossRef]
- Yang, M.; Dong, C.W.; Shi, Y. Nano fertilizer synergist effects on nitrogen utilization and related gene expression in wheat. BMC Plant Biology 2023, 23, 13. [Google Scholar]
- Adisa, I.O.; Pullagurala, V.L.R.; Peralta-Videa, J.R.; Dimkpa, C.O.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Recent advances in nano-enabled fertilizers and pesticides: A critical review of mechanisms of action. Environ. Sci.-Nano 2019, 6, 2002–2030. [Google Scholar] [CrossRef]
- Prasad, R.; Bhattacharyya, A.; Nguyen, Q.D. Nanotechnology in sustainable agriculture: Recent developments, challenges, and perspectives. Front. Microbiol. 2017, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- El-Toni, A.M.; Habila, M.A.; Labis, J.P.; Alothman, Z.A.; Alhoshan, M.; Elzatahry, A.A.; Zhang, F. Design, synthesis and applications of core-shell, hollow core, and nanorattle multifunctional nanostructures. Nanoscale 2016, 8, 2510–2531. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, P.; Luo, J.J.; Tian, L.; Tian, Z.J.; Ndayambaje, J.P. Effects of different types of nano-carbon biological fertilizers on the growth and quality of crops. Nanosci. Nanotechnol. Lett. 2019, 11, 1644–1650. [Google Scholar] [CrossRef]
- Li, D.Y.; Lu, Y.L.; Zhang, C. Superhydrophobic and electrochemical performance of CF2-modified g-C3N4 graphene composite film deposited by PECVD. Nanomaterials 2022, 12, 4387. [Google Scholar] [CrossRef]
- Liu, X.R.; Tang, X.Q.; He, Y.; Wang, F. Microemulsion nanoreactor applied for the synthesis of iron-based catalysts for carbon nanotube formation. Colloid Surf. A Physicochem. Eng. Asp. 2023, 666, 11. [Google Scholar]
- Chai, Y.R.; Feng, Y.S.; Zhang, K.; Li, J.A. Preparation of fluorescent carbon dots composites and their potential applications in biomedicine and drug delivery—A review. Pharmaceutics 2022, 14, 2482. [Google Scholar] [CrossRef] [PubMed]
- Deon, V.G.; Thesing, A.; Santana, L.R.; Costa, V.C.; Vaz, M.O.; Silva, R.M.; Mesko, M.F.; Carreno, N.L.V. Synthesis of LiNbO3 nanocrystals by microwave-assisted hydrothermal method: Formation mechanism and application to hydrogen evolution reaction. Chem. Pap. 2021, 75, 3807–3815. [Google Scholar] [CrossRef]
- Zhu, X.W.; Xu, L.J.; Cui, C.P.; Liu, Q.Z.; Wang, H.W. Preparation and characterization of zirconium carbide nano powder by hydrothermal and carbothermal reduction methods. Ceram. Int. 2024, 50, 30151–30160. [Google Scholar] [CrossRef]
- Vieira, I.R.S.; de Carvalho, A.; Conte-Junior, C.A. Recent advances in biobased and biodegradable polymer nanocomposites, nanoparticles, and natural antioxidants for antibacterial and antioxidant food packaging applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3673–3716. [Google Scholar] [CrossRef]
- Rocha, C.V.; Gonçalves, V.; da Silva, M.C.; Bañobre-López, M.; Gallo, J. PLGA-based composites for various biomedical applications. Int. J. Mol. Sci. 2022, 23, 2034. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.L.; Zhang, Y.R.; Zhu, L.L.; Miao, Y.Q.; Zhu, Y.Q.; Yue, B.B. Tailored drug delivery platforms: Stimulus-responsive core-shell structured nanocarriers. Adv. Healthc. Mater. 2024, 13, 24. [Google Scholar]
- Dulski, M.; Malarz, K.; Kuczak, M.; Dudek, K.; Matus, K.; Sulowicz, S.; Mrozek-Wilczkiewicz, A.; Nowak, A. An Organic-inorganic hybrid nanocomposite as a potential new biological agent. Nanomaterials 2024, 14, 1626. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Feng, H.H.; Liang, C.Y.; Liu, X.J.; Zeng, F.Y.; Wang, Y. Mesoporous silica as micro/nano-carrier: From passive to active cargo delivery, a mini review. J. Mater. Sci. Technol. 2017, 33, 1067–1074. [Google Scholar] [CrossRef]
- Bai, Y.X.; Zhang, H.P.; Zhu, J.S.; Shao, Y.Y.; Zhang, H. A durable superhydrophobic coating based on inherent nano/micro-integrated materials. Prog. Org. Coat. 2023, 184, 16. [Google Scholar] [CrossRef]
- Nadal, C.; Coutelier, O.; Cavalie, S.; Flaud, V.; Soulié, J.; Marty, J.D.; Destarac, M.; Tourrette, A. Polymer/silica core-shell nanoparticles with temperature-dependent stability properties. Eur. Polym. J. 2022, 168, 11. [Google Scholar] [CrossRef]
- Zhou, X.M.; Liu, Y. Preparation of core-shell Si/C/graphene composite for high-performance lithium-ion battery anodes. Synth. Met. 2024, 309, 7. [Google Scholar] [CrossRef]
- Dai, X.H.; Xu, X.M.; Yu, X.; Sun, X.; Pan, J.J.; Zhang, X.T.; Min, J. Cationic core/shell polysiloxane acrylate emulsion: Synthesis, film morphology, and performance on cotton pigment coloration. Cellulose 2022, 29, 2093–2106. [Google Scholar] [CrossRef]
- Alshahrani, A.A.; Alqarni, L.S.; Alghamdi, M.D.; Alotaibi, N.F.; Moustafa, S.M.N.; Nassar, A.M. Phytosynthesis via wasted onion peel extract of samarium oxide/ silver core/shell nanoparticles for excellent inhibition of microbes. Heliyon 2024, 10, 13. [Google Scholar] [CrossRef]
- Tang, S.Q.; Xu, X.M.; Shao, N.N.; Liang, J.M.; Xuan, W.W.; Li, O.Y.; Fei, X.C.; Jin, Y.S.; Li, Z.S. Thermodynamics-oriented crystal growth enables lignocellulose-enticed nanostructured hydroxyapatite with boosted phosphorus fertilizer utility. ACS Sustain. Chem. Eng. 2023, 11, 16680–16692. [Google Scholar] [CrossRef]
- Jakhar, A.M.; Aziz, I.; Kaleri, A.R.; Hasnain, M.; Haider, G.; Ma, J.H.; Abideen, Z. Nano-fertilizers: A sustainable technology for improving crop nutrition and food security. Nanoimpact 2022, 27, 19. [Google Scholar] [CrossRef] [PubMed]
- Gajbhiye, K.R.; Salve, R.; Narwade, M.; Sheikh, A.; Kesharwani, P.; Gajbhiye, V. Lipid polymer hybrid nanoparticles: A custom-tailored next-generation approach for cancer therapeutics. Mol. Cancer 2023, 22, 44. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Yuan, T.; Luo, X.; Liu, D.L.; Evsyukov, S.A. Effect of polydopamine surface modification on the adhesive properties of nano-injection molded metal-polymer interfaces: A molecular dynamics simulation study. Polym. Eng. Sci. 2024, 64, 1645–1657. [Google Scholar] [CrossRef]
- Ding, Z.H.; Fatollahi-Fard, F.; Kwon, I.S.; Pistorius, P.C.; Bettinger, C.J. Polydopamine nanomembranes as adhesion layers for improved corrosion resistance in low carbon steel. Adv. Eng. Mater. 2018, 20, 6. [Google Scholar] [CrossRef]
- Xiang, A.H.; Li, Y.F.; Hu, T.L.; Liu, K. Synthesis and mechanism of Mg/Al layered double oxides-silica nanocomposites for sustainable multi-nutrient delivery in agricultural applications. J. Control. Release 2024, 376, 816–828. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.Z.; Huang, M.J.; Yan, J.; Qi, T.M.; Li, T.; Liu, Y.H.; Lu, S.Y.; Liu, M.Z. Adhesive Nanocomposite for Prolonging Foliar Retention and Synergistic Weeding and Nourishing. Adv. Sustain. Syst. 2020, 4, 10. [Google Scholar] [CrossRef]
- Yu, S.J.; He, J.K.; Zhang, Z.; Sun, Z.H.; Xie, M.Y.; Xu, Y.Q.; Bie, X.; Li, Q.H.; Zhang, Y.G.; Sevilla, M.; et al. Towards negative emissions: Hydrothermal carbonization of biomass for sustainable carbon materials. Adv. Mater. 2024, 36, 44. [Google Scholar] [CrossRef]
- Cho, S.; Kim, H.; Song, D.; Jung, J.; Park, S.; Jo, H.; Seo, S.; Han, C.; Park, S.; Kwon, W.; et al. Insights into glucose-derived carbon dot synthesis via Maillard reaction: From reaction mechanism to biomedical applications. Sci. Rep. 2024, 14, 13. [Google Scholar] [CrossRef]
- Morais, R.G.; Ribeiro, L.S.; Orfao, J.J.M.; Pereira, M.F.R. Low-cost Ni-W catalysts supported on glucose/carbon nanotube hybrid carbons for sustainable ethylene glycol synthesis. Molecules 2024, 29, 3962. [Google Scholar] [CrossRef]
- Shen, W.L.; Zhu, H.H.; Cheng, X.M.; Li, X. Synthesis of mesoporous niobium phosphosilicate with high catalytic activity in the conversion of glucose to 5-hydroxymethylfurfural in water solvent. Biofuels Bioprod. Biorefining 2024, 18, 1994–2004. [Google Scholar] [CrossRef]
- Cai, W.Q.; Gu, M.M.; Jin, W.; Zhou, J.B. CTAB-functionalized C@SiO2 double-shelled hollow microspheres with enhanced and selective adsorption performance for Cr(VI). J. Alloys Compd. 2019, 777, 1304–1312. [Google Scholar]
- Wanyika, H.; Gatebe, E.; Kioni, P.; Tang, Z.Y.; Gao, Y. Mesoporous silica nanoparticles carrier for urea: Potential applications in agrochemical delivery systems. J. Nanosci. Nanotechnol. 2012, 12, 2221–2228. [Google Scholar]
- Nandeshwar, R.; Mandal, P.; Tallur, S. Portable and low-cost colorimetric sensor for detection of urea in milk samples. IEEE Sens. J. 2023, 23, 16287–16292. [Google Scholar] [CrossRef]
- Li, M.; Li, W.; Liu, S.X. Hydrothermal synthesis, characterization, and KOH activation of carbon spheres from glucose. Carbohydr. Res. 2011, 346, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Liang, F.; Wang, J.K.; Zhang, H.J.; Zhang, S.W. Preparation of mono-dispersed carbonaceous spheres via a hydrothermal process. Adv. Powder Technol. 2017, 28, 2648–2657. [Google Scholar]
- Titirici, M.M.; Antonietti, M. Chemistry and materials options of sustainable carbon materials made by hydrothermal carbonization. Chem. Soc. Rev. 2010, 39, 103–116. [Google Scholar] [CrossRef]
- Sakabe, J.; Uchida, H. Nanoparticle size control of theophylline using rapid expansion of supercritical solutions (RESS) technique. Adv. Powder Technol. 2022, 33, 7. [Google Scholar] [CrossRef]
- Rizal, S.; Mistar, E.M.; Rahman, A.A.; Khalil, H.; Oyekanmi, A.A.; Olaiya, N.G.; Abdullah, C.K.; Alfatah, T. Bionanocarbon functional material characterisation and enhancement properties in nonwoven kenaf fibre nanocomposites. Polymers 2021, 13, 2303. [Google Scholar] [CrossRef]
- Wang, Y.B.; Zhang, D.; Deng, J.; Zhou, F.; Duan, Z.Y.; Su, Q.; Pang, S.F. Mosquito’s compound eyes as inspiration for fabrication of conductive superhydrophobic nanocarbon materials from waste wheat straw. ACS Sustain. Chem. Eng. 2019, 7, 3883–3894. [Google Scholar]
- Zhang, H.J.; Li, Z.R.; Yang, X.; Zhao, Y.J.; Melhi, S.; Ge, J.P.; Cheng, T.; Tang, J. Modulator directed synthesis of size-tunable mesoporous MOFs and their derived nanocarbon-based electrocatalysts for oxygen reduction. Chem. Eng. J. 2024, 486, 7. [Google Scholar] [CrossRef]
- Zhang, C.; Gong, L.; Xiang, L.; Du, Y.; Hu, W.J.H.; Zeng, H.; Xu, Z.K. Deposition and adhesion of polydopamine on the surfaces of varying wettability. ACS Appl. Mater. Interfaces 2017, 9, 30943–30950. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.M.; Guo, Y.C.; Zeng, L.S.; Li, X.L.; Liu, X.W.; Li, J.L.; Cui, D.J. Combined urea humate and wood vinegar treatment enhances wheat-maize rotation system yields and nitrogen utilization efficiency through improving the quality of saline-alkali soils. J. Soil Sci. Plant Nutr. 2021, 21, 1759–1770. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Chen, X.; Gu, H.; Chen, H.; Huang, K.; Wang, J.; Xu, H.; Zhang, Y.; Li, W. Preparation and Application of Core–Shell Nanocarbon-Based Slow-Release Foliar Fertilizer. Nanomaterials 2025, 15, 565. https://doi.org/10.3390/nano15070565
Zhang T, Chen X, Gu H, Chen H, Huang K, Wang J, Xu H, Zhang Y, Li W. Preparation and Application of Core–Shell Nanocarbon-Based Slow-Release Foliar Fertilizer. Nanomaterials. 2025; 15(7):565. https://doi.org/10.3390/nano15070565
Chicago/Turabian StyleZhang, Ting, Xinheng Chen, Hongtao Gu, Huayi Chen, Kaichun Huang, Jinjin Wang, Huijuan Xu, Yulong Zhang, and Wenyan Li. 2025. "Preparation and Application of Core–Shell Nanocarbon-Based Slow-Release Foliar Fertilizer" Nanomaterials 15, no. 7: 565. https://doi.org/10.3390/nano15070565
APA StyleZhang, T., Chen, X., Gu, H., Chen, H., Huang, K., Wang, J., Xu, H., Zhang, Y., & Li, W. (2025). Preparation and Application of Core–Shell Nanocarbon-Based Slow-Release Foliar Fertilizer. Nanomaterials, 15(7), 565. https://doi.org/10.3390/nano15070565







