Nanocarrier-Based Eco-Friendly RNA Pesticides for Sustainable Management of Plant Pathogens and Pests
Abstract
1. Introduction
2. RNAi Technology for Gene Function Analysis and Pesticide Development
3. Advantages of RNA Pesticide Compared with Traditional Pesticides
4. Bottlenecks for Developing High-Efficiency RNA Pesticides
4.1. Instability of dsRNA
4.2. Obstacles for Delivering dsRNA
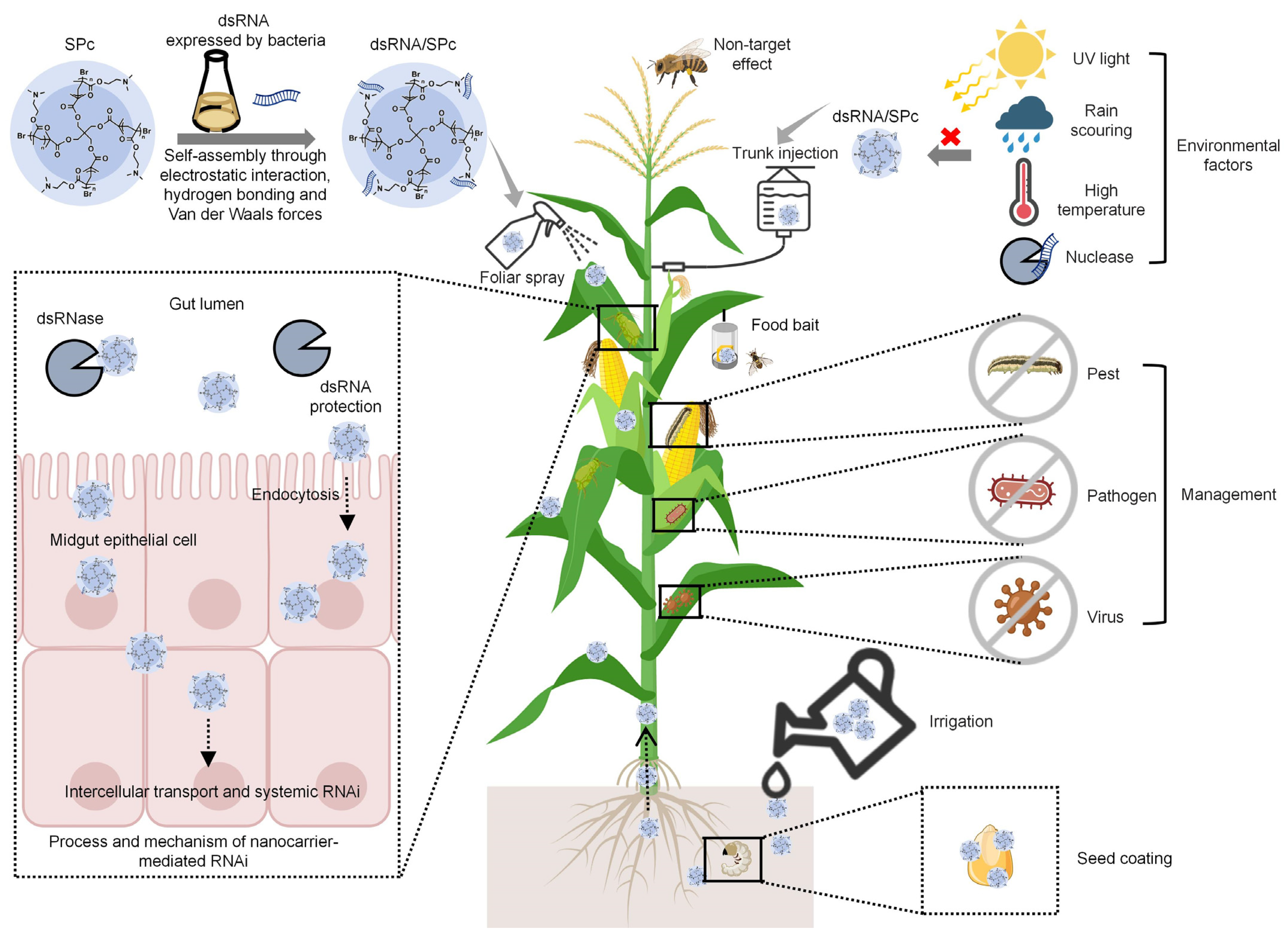
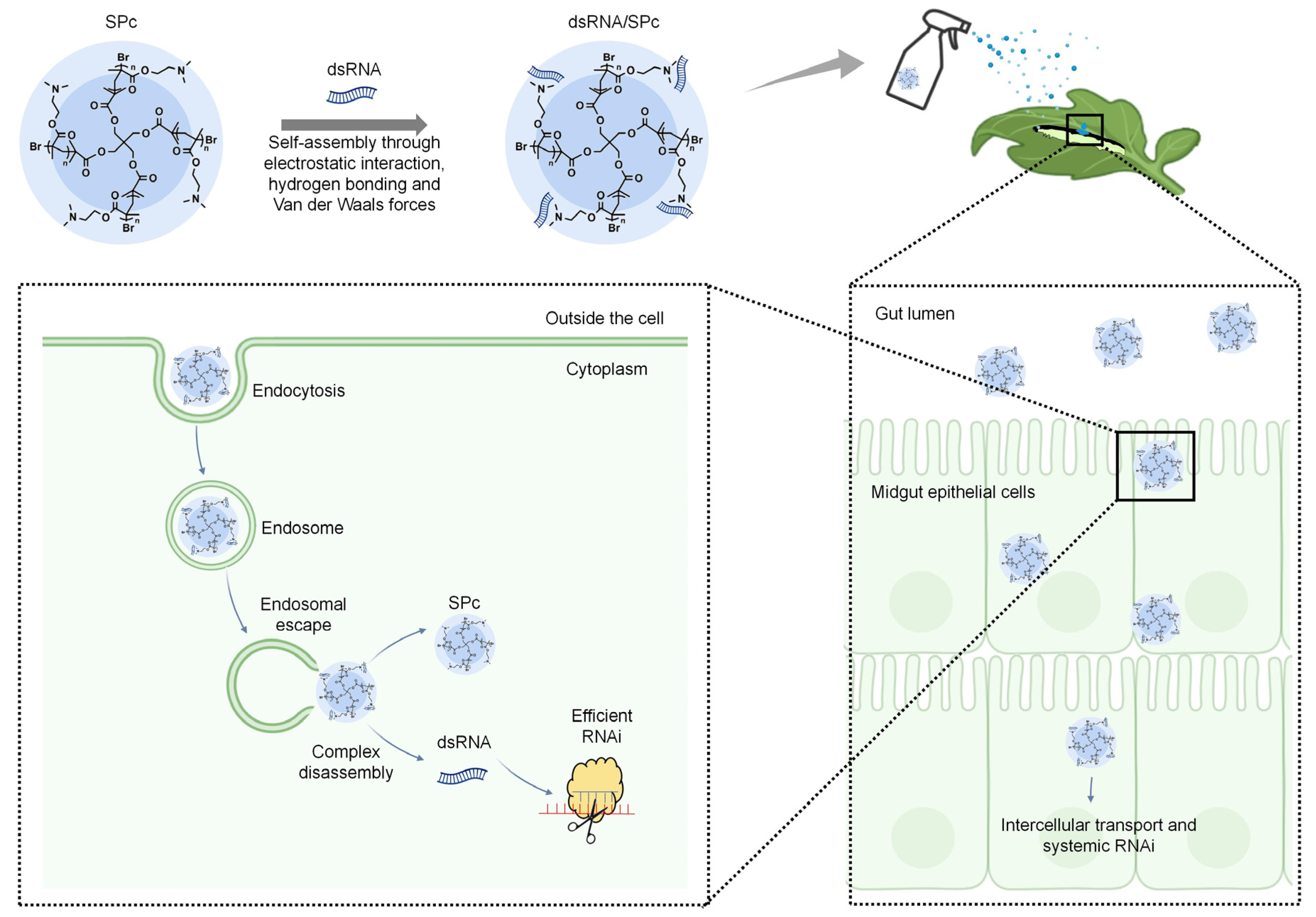
5. Process and Mechanism of Nanocarrier-Mediated RNAi
6. Potential Application of Nanocarrier-Based RNA Pesticides
6.1. Successful Cases of Nanocarrier-Mediated RNAi
6.2. Application Method of Nanocarrier-Based RNA Pesticides
7. Current Challenges and Future Perspective
7.1. Control Efficacy of RNA Pesticides
7.2. Risk Assessment of RNA Pesticides
7.3. Biosynthesis of dsRNA
Author Contributions
Funding
Conflicts of Interest
References
- Kumar, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Hassan, A.A.; Kim, K.H. Nano-based smart pesticide formulations: Emerging opportunities for agriculture. J. Control. Release 2019, 294, 131–153. [Google Scholar] [CrossRef] [PubMed]
- Lykogianni, M.; Bempelou, E.; Karamaouna, F.; Aliferis, K.A. Do pesticides promote or hinder sustainability in agriculture? The challenge of sustainable use of pesticides in modern agriculture. Sci. Total Environ. 2021, 795, 148625. [Google Scholar] [CrossRef] [PubMed]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Baum, J.A.; Bogaert, T.; Clinton, W.; Heck, G.R.; Feldmann, P.; Ilagan, O.; Johnson, S.; Plaetinck, G.; Munyikwa, T.; Pleau, M.; et al. Control of coleopteran insect pests through RNA interference. Nat. Biotechnol. 2007, 25, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Nandety, R.S.; Kuo, Y.-W.; Nouri, S.; Falk, B.W. Emerging strategies for RNA interference (RNAi) applications in insects. Bioengineered 2015, 6, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.Y.; Palli, S.R. Mechanisms, applications, and challenges of insect RNA interference. Annu. Rev. Entomol. 2020, 65, 293–311. [Google Scholar] [CrossRef]
- Price, D.R.; Gatehouse, J.A. RNAi-mediated crop protection against insects. Trends Biotechnol. 2008, 26, 393–400. [Google Scholar] [CrossRef]
- Ben-Amar, A.; Daldoul, S.; Reustle, G.M.; Krczal, G.; Mliki, A. Reverse genetics and high throughput sequencing methodologies for plant functional genomics. Cur. Genom. 2016, 17, 460–475. [Google Scholar] [CrossRef]
- Vogel, E.; Santos, D.; Mingels, L.; Verdonckt, T.-W.; Broeck, J.V. RNA interference in insects: Protecting beneficials and controlling pests. Front. Physiol. 2019, 9, 1912. [Google Scholar] [CrossRef]
- Christiaens, O.; Niu, J.; Nji Tizi Taning, C. RNAi in insects: A revolution in fundamental research and pest control applications. Insects 2020, 11, 415. [Google Scholar] [CrossRef]
- Singewar, K.; Fladung, M. Double-stranded RNA (dsRNA) technology to control forest insect pests and fungal pathogens: Challenges and opportunities. Funct. Integr. Genom. 2023, 23, 185. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yan, S.; Li, M.; Sun, L.; Dong, M.; Yin, M.; Shen, J.; Zhao, Z. NPFR regulates the synthesis and metabolism of lipids and glycogen via AMPK: Novel targets for efficient corn borer management. Int. J. Biol. Macromol. 2023, 247, 125816. [Google Scholar] [CrossRef] [PubMed]
- Hoang, B.T.L.; Fletcher, S.J.; Brosnan, C.A.; Ghodke, A.B.; Manzie, N.; Mitter, N. RNAi as a foliar spray: Efficiency and challenges to field applications. Int. J. Mol. Sci. 2022, 23, 6639. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Peng, Y.; Pu, J.; Fu, W.; Wang, J.; Han, Z. Variation in RNAi efficacy among insect species is attributable to dsRNA degradation in vivo. Insect Biochem. Mol. Biol. 2016, 77, 1–9. [Google Scholar] [CrossRef]
- Song, H.; Fan, Y.; Zhang, J.; Cooper, A.M.; Silver, K.; Li, D.; Li, T.; Ma, E.; Zhu, K.Y.; Zhang, J. Contributions of dsRNases to differential RNAi efficiencies between the injection and oral delivery of dsRNA in Locusta migratoria. Pest Manag. Sci. 2019, 75, 1707–1717. [Google Scholar] [CrossRef]
- Qiao, L.; Lan, C.; Capriotti, L.; Ah-Fong, A.; Nino Sanchez, J.; Hamby, R.; Heller, J.; Zhao, H.; Glass, N.L.; Judelson, H.S.; et al. Spray-induced gene silencing for disease control is dependent on the efficiency of pathogen RNA uptake. Plant Biotechnol. J. 2021, 19, 1756–1768. [Google Scholar] [CrossRef]
- Yoon, J.-S.; Gurusamy, D.; Palli, S.R. Accumulation of dsRNA in endosomes contributes to inefficient RNA interference in the fall armyworm, Spodoptera frugiperda. Insect Biochem. Mol. Biol. 2017, 90, 53–60. [Google Scholar] [CrossRef]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The history of nanoscience and nanotechnology: From chemical–physical applications to nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef]
- An, C.; Sun, C.; Li, N.; Huang, B.; Jiang, J.; Shen, Y.; Wang, C.; Zhao, X.; Cui, B.; Wang, C.; et al. Nanomaterials and nanotechnology for the delivery of agrochemicals: Strategies towards sustainable agriculture. J. Nanobiotechnol. 2022, 20, 11. [Google Scholar] [CrossRef]
- Wu, H.; Li, Z. Recent advances in nano-enabled agriculture for improving plant performance. Crop J. 2022, 10, 1–12. [Google Scholar] [CrossRef]
- Yan, Y.; Zhu, X.; Yu, Y.; Li, C.; Zhang, Z.; Wang, F. Nanotechnology strategies for plant genetic engineering. Adv. Mater. 2022, 34, e2106945. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Zhou, F.; Xu, Z.; He, B.; Li, M.; Shen, J.; Yin, M.; An, C. Systemically interfering with immune response by a fluorescent cationic dendrimer delivered gene suppression. J. Mater. Chem. B 2014, 2, 4653–4659. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Hu, Y.; Yan, S.; Zhou, H.; Song, D.; Yin, M.; Shen, J. A polymer/detergent formulation improves dsRNA penetration through the body wall and RNAi-induced mortality in the soybean aphid Aphis glycines. Pest Manag. Sci. 2019, 75, 1993–1999. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qian, J.; Xu, Y.; Yan, S.; Shen, J.; Yin, M. A facile-synthesized star polycation constructed as a highly efficient gene vector in pest management. ACS Sustain. Chem. Eng. 2019, 7, 6316–6322. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Zhang, B.; Gao, X.; Shi, M.; Zhang, S.; Zhong, S.; Zheng, Y.; Liu, X. Functionalized carbon dot-delivered RNA nano fungicides as superior tools to control Phytophthora pathogens through plant RdRP1 mediated spray-induced gene silencing. Adv. Funct. Mater. 2023, 33, 2213143. [Google Scholar] [CrossRef]
- Ma, Z.; Zheng, Y.; Chao, Z.; Chen, H.; Zhang, Y.; Yin, M.; Shen, J.; Yan, S. Visualization of the process of a nanocarrier-mediated gene delivery: Stabilization, endocytosis and endosomal escape of genes for intracellular spreading. J. Nanobiotechnol. 2022, 20, 124. [Google Scholar] [CrossRef]
- Zamore, P.D.; Tuschl, T.; Sharp, P.A.; Bartel, D.P. RNAi: Double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell 2000, 101, 25–33. [Google Scholar] [CrossRef]
- Wynant, N.; Santos, D.; Van Wielendaele, P.; Vanden Broeck, J. Scavenger receptor-mediated endocytosis facilitates RNA interference in the desert locust, Schistocerca gregaria. Insect Mol Biol. 2014, 23, 320–329. [Google Scholar] [CrossRef]
- Cappelle, K.; de Oliveira, C.F.R.; Van Eynde, B.; Christiaens, O.; Smagghe, G. The involvement of clathrin-mediated endocytosis and two Sid-1-like transmembrane proteins in double-stranded RNA uptake in the Colorado potato beetle midgut. Insect Mol Biol. 2016, 25, 315–323. [Google Scholar] [CrossRef]
- Hannon, G.J. RNA interference. Nature 2002, 418, 244–251. [Google Scholar] [CrossRef]
- Kennerdell, J.R.; Carthew, R.W. Use of dsRNA-mediated genetic interference to demonstrate that frizzled and frizzled 2 act in the wingless pathway. Cell 1998, 95, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Poreddy, S.; Li, J.; Baldwin, I.T. Plant-mediated RNAi silences midgut-expressed genes in congeneric lepidopteran insects in nature. BMC Plant Biol. 2017, 17, 199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Khan, S.A.; Heckel, D.G.; Bock, R. Next-generation insect-resistant plants: RNAi-mediated crop protection. Trends Biotechnol. 2017, 35, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Zotti, M.; Dos Santos, E.A.; Cagliari, D.; Christiaens, O.; Taning, C.N.T.; Smagghe, G. RNA interference technology in crop protection against arthropod pests, pathogens and nematodes. Pest Manag. Sci. 2018, 74, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Mohr, S.E.; Smith, J.A.; Shamu, C.E.; Neumüller, R.A.; Perrimon, N. RNAi screening comes of age: Improved techniques and complementary approaches. Nat. Rev. Mol. Cell Biol. 2014, 15, 591–600. [Google Scholar] [CrossRef]
- Rodrigues, T.B.; Mishra, S.K.; Sridharan, K.; Barnes, E.R.; Alyokhin, A.; Tuttle, R.; Kokulapalan, W.; Garby, D.; Skizim, N.J.; Tang, Y.; et al. First sprayable double-stranded RNA-based biopesticide product targets proteasome subunit beta type-5 in Colorado potato beetle (Leptinotarsa decemlineata). Front. Plant Sci. 2021, 12, 728652. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Wang, H.; Hu, P.; Hamby, R.; Jin, H. Small RNAs—Big players in plant-microbe interactions. Cell Host Microbe 2019, 26, 173–182. [Google Scholar] [CrossRef]
- Whyard, S.; Singh, A.D.; Wong, S. Ingested double-stranded RNAs can act as species-specific insecticides. Insect Biochem. Mol. Biol. 2009, 39, 824–832. [Google Scholar] [CrossRef]
- Wang, X.; Ji, S.; Bi, S.; Tang, Y.; Zhang, G.; Yan, S.; Wan, F.; Lü, Z.; Liu, W. A promising approach to an environmentally friendly pest management solution: Nanocarrier-delivered dsRNA towards controlling the destructive invasive pest Tuta absoluta. Environ. Sci. Nano 2023, 10, 1003–1015. [Google Scholar] [CrossRef]
- Kolge, H.; Kadam, K.; Galande, S.; Lanjekar, V.; Ghormade, V. New frontiers in pest control: Chitosan nanoparticles-shielded dsRNA as an effective topical RNAi spray for gram podborer biocontrol. ACS Appl. Bio Mater. 2021, 4, 5145–5157. [Google Scholar] [CrossRef]
- Campbell, E.M.; Budge, G.E.; Bowman, A.S. Gene-knockdown in the honey bee mite Varroa destructor by a non-invasive approach: Studies on a glutathione S-transferase. Parasit. Vectors 2010, 3, 73. [Google Scholar] [CrossRef] [PubMed]
- Garbian, Y.; Maori, E.; Kalev, H.; Shafir, S.; Sela, I. Bidirectional transfer of RNAi between honey bee and Varroa destructor: Varroa gene silencing reduces Varroa population. PLoS Pathog. 2012, 8, e1003035. [Google Scholar] [CrossRef] [PubMed]
- Paldi, N.; Glick, E.; Oliva, M.; Zilberberg, Y.; Aubin, L.; Pettis, J.; Chen, Y.; Evans, J.D. Effective gene silencing in a microsporidian parasite associated with honeybee (Apis mellifera) colony declines. Appl. Environ. Microbiol. 2010, 76, 5960–5964. [Google Scholar] [CrossRef]
- Dubelman, S.; Fischer, J.; Zapata, F.; Huizinga, K.; Jiang, C.; Uffman, J.; Levine, S.; Carson, D. Environmental fate of double-stranded RNA in agricultural soils. PLoS ONE 2014, 9, e93155. [Google Scholar] [CrossRef] [PubMed]
- Parker, K.M.; Barragán Borrero, V.; Van Leeuwen, D.M.; Lever, M.A.; Mateescu, B.; Sander, M. Environmental Fate of RNA interference pesticides: Adsorption and degradation of double-stranded RNA molecules in agricultural soils. Environ. Sci. Technol. 2019, 53, 3027–3036. [Google Scholar] [CrossRef] [PubMed]
- Petrick, J.S.; Brower-Toland, B.; Jackson, A.L.; Kier, L.D. Safety assessment of food and feed from biotechnology-derived crops employing RNA-mediated gene regulation to achieve desired traits: A scientific review. Regul. Toxicol. Pharmacol. 2013, 66, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Kogel, K.-H. New wind in the sails: Improving the agronomic value of crop plants through RNAi-mediated gene silencing. Plant Biotechnol. J. 2014, 12, 821–831. [Google Scholar] [CrossRef]
- Ivashuta, S.I.; Petrick, J.S.; Heisel, S.E.; Zhang, Y.; Guo, L.; Reynolds, T.L.; Rice, J.F.; Allen, E.; Roberts, J.K. Endogenous small RNAs in Ggrain: Semi-quantification and sequence homology to human and animal genes. Food Chem. Toxicol. 2009, 47, 353–360. [Google Scholar] [CrossRef]
- Barbier, A.J.; Jiang, A.Y.; Zhang, P.; Wooster, R.; Anderson, D.G. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 2022, 40, 840–854. [Google Scholar] [CrossRef]
- Mcdougall, P. The Cost of New Agrochemical Product Discovery, Development and Registration in 1995, 2000, 2005–8 and 2010 to 2014. R&D Expenditure in 2014 and Expectations for 2019. Available online: https://croplife.org/wp-content/uploads/2016/04/Cost-of-CP-report-FINAL.pdf (accessed on 18 November 2024).
- Palli, S.R. RNA interference in Colorado potato beetle: Steps toward development of dsRNA as a commercial insecticide. Curr. Opin. Insect Sci. 2014, 6, 1–8. [Google Scholar] [CrossRef]
- Nowara, D.; Gay, A.; Lacomme, C.; Shaw, J.; Ridout, C.; Douchkov, D.; Hensel, G.; Kumlehn, J.; Schweizer, P. HIGS: Host-induced gene silencing in the obligate biotrophic fungal pathogen Blumeria graminis. Plant Cell 2010, 22, 3130–3141. [Google Scholar] [CrossRef] [PubMed]
- Dalakouras, A.; Wassenegger, M.; Dadami, E.; Ganopoulos, I.; Pappas, M.L.; Papadopoulou, K. Genetically modified organism-free RNA interference: Exogenous application of RNA molecules in plants. Plant Physiol. 2020, 182, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Taning, C.N.; Arpaia, S.; Christiaens, O.; Dietz-Pfeilstetter, A.; Jones, H.; Mezzetti, B.; Sabbadini, S.; Sorteberg, H.-G.; Sweet, J.; Ventura, V.; et al. RNA-based biocontrol compounds: Current status and perspectives to reach the market. Pest Manag. Sci. 2020, 76, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Lü, J.; Liu, Z.; Guo, W.; Guo, M.; Chen, S.; Li, H.; Yang, C.; Zhang, Y.; Pan, H. Feeding delivery of dsHvSnf7 is a promising method for management of the pest Henosepilachna vigintioctopunctata (Coleoptera: Coccinellidae). Insects 2019, 11, 34. [Google Scholar] [CrossRef]
- Mosa, M.A.; Youssef, K. Topical delivery of host induced RNAi silencing by layered double hydroxide nanosheets: An efficient tool to decipher pathogenicity gene function of Fusarium crown and root rot in tomato. Physiol. Mol. Plant Pathol. 2021, 115, 101684. [Google Scholar] [CrossRef]
- Ray, P.; Sahu, D.; Aminedi, R.; Chandran, D. Concepts and considerations for enhancing RNAi efficiency in phytopathogenic fungi for RNAi-based crop protection using nanocarrier-mediated dsRNA delivery systems. Front. Fungal Biol. 2022, 3, 977502. [Google Scholar] [CrossRef]
- Sang, H.; Kim, J.-I. Advanced strategies to control plant pathogenic fungi by host-induced gene silencing (HIGS) and spray-induced gene silencing (SIGS). Plant Biotechnol. Rep. 2020, 14, 1–8. [Google Scholar] [CrossRef]
- Yan, S.; Yin, M.-Z.; Shen, J. Nanoparticle-based nontransformative RNA insecticides for sustainable pest control: Mechanisms, current status and challenges. Entomol. Gen. 2022, 43, 21–30. [Google Scholar] [CrossRef]
- Garbutt, J.S.; Bellés, X.; Richards, E.H.; Reynolds, S.E. Persistence of double-stranded RNA in insect hemolymph as a potential determiner of RNA interference success: Evidence from Manduca sexta and Blattella germanica. J. Insect Physiol. 2013, 59, 171–178. [Google Scholar] [CrossRef]
- Wynant, N.; Santos, D.; Verdonck, R.; Spit, J.; Van Wielendaele, P.; Broeck, J.V. Identification, functional characterization and phylogenetic analysis of double stranded RNA degrading enzymes present in the gut of the desert Locust, Schistocerca Gregaria. Insect Biochem. Mol. Biol. 2014, 46, 1–8. [Google Scholar] [CrossRef]
- Spit, J.; Philips, A.; Wynant, N.; Santos, D.; Plaetinck, G.; Broeck, J.V. Knockdown of nuclease activity in the gut enhances RNAi efficiency in the Colorado potato beetle, Leptinotarsa decemlineata, but not in the desert locust, Schistocerca gregaria. Insect Biochem. Mol. Biol. 2017, 81, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Zhao, J.; Zhu-Salzman, K.; Ji, Q.Q.; Jiang, Y.P.; Xiao, L.B.; Xu, D.J.; Xu, G.C.; Ge, L.Q.; Tan, Y.A. Gene cloning, protein expression, and enzymatic characterization of a double-stranded RNA degrading enzyme in Apolygus lucorum. Insect Sci. 2024, 31, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Zhang, J.; Li, D.; Cooper, A.M.; Silver, K.; Li, T.; Liu, X.; Ma, E.; Zhu, K.Y.; Zhang, J. A double-stranded RNA degrading enzyme reduces the efficiency of oral RNA interference in migratory locust. Insect Biochem. Mol. Biol. 2017, 86, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Adeyinka, O.S.; Riaz, S.; Toufiq, N.; Yousaf, I.; Bhatti, M.U.; Batcho, A.; Olajide, A.A.; Nasir, I.A.; Tabassum, B. Advances in exogenous RNA delivery techniques for RNAi-mediated pest control. Mol. Biol. Rep. 2020, 47, 6309–6319. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, O.; Tardajos, M.G.; Martinez Reyna, Z.L.; Dash, M.; Dubruel, P.; Smagghe, G. Increased RNAi efficacy in Spodoptera exigua via the formulation of dsRNA with guanylated polymers. Front. Physiol. 2018, 9, 316. [Google Scholar] [CrossRef]
- Shi, X.K.; Zhang, Y.W.; Zhu, K.Y.; Ma, E.B.; Zhang, J.Z.; Liu, X.J.; Wu, H.H. Comparison of the efficacy of different dsRNA delivery methods to silence antenna-rich genes in Locusta migratoria. Chin. J. Appl. Entomol. 2017, 54, 780–790. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, T.; Liu, W.; Li, D.; Gao, L.; Ma, E.; Zhu, K.Y.; Moussian, B.; Li, S.; Zhang, J. LmCht5-1 promotes pro-nymphal molting during locust embryonic development. Insect Biochem. Mol. Biol. 2018, 101, 124–130. [Google Scholar] [CrossRef]
- Yoon, J.S.; Mogilicherla, K.; Gurusamy, D.; Chen, X.; Chereddy, S.; Palli, S. Double-stranded RNA binding protein, Staufen, is required for the initiation of RNAi in coleopteran insects. Proc. Natl. Acad. Sci. USA 2018, 115, 8334–8339. [Google Scholar] [CrossRef]
- Khajuria, C.; Vélez, A.M.; Rangasamy, M.; Wang, H.; Fishilevich, E.; Frey, M.L.; Carneiro, N.P.; Gandra, P.; Narva, K.E.; Siegfried, B.D. Parental RNA interference of genes involved in embryonic development of the western corn rootworm, Diabrotica virgifera virgifera LeConte. Insect Biochem. Mol. Biol. 2015, 63, 54–62. [Google Scholar] [CrossRef]
- Yan, S.; Ren, B.Y.; Shen, J. Nanoparticle-mediated double-stranded RNA delivery system: A promising approach for sustainable pest management. Insect Sci. 2021, 28, 21–34. [Google Scholar] [CrossRef]
- Fan, Y.H.; Song, H.F.; Abbas, M.; Wang, Y.L.; Li, T.; Ma, E.B.; Cooper, A.M.; Silver, K.; Zhu, K.Y.; Zhang, J.Z. A dsRNA-degrading nuclease (dsRNase2) limits RNAi efficiency in the Asian corn borer (Ostrinia furnacalis). Insect Sci. 2021, 28, 1677–1689. [Google Scholar] [CrossRef] [PubMed]
- Winston, W.M.; Molodowitch, C.; Hunter, C.P. Systemic RNAi in C. Elegans requires the putative transmembrane protein SID-1. Science 2002, 295, 2456–2459. [Google Scholar] [CrossRef] [PubMed]
- Miyata, K.; Ramaseshadri, P.; Zhang, Y.; Segers, G.; Bolognesi, R.; Tomoyasu, Y. Establishing an in vivo assay system to identify components involved in environmental RNA interference in the western corn rootworm. PLoS ONE 2014, 9, e101661. [Google Scholar] [CrossRef] [PubMed]
- Ulvila, J.; Parikka, M.; Kleino, A.; Sormunen, R.; Ezekowitz, R.A.; Kocks, C.; Ramet, M. Double-stranded RNA is internalized by scavenger receptor-mediated endocytosis in Drosophila S2 Cells. J. Biol. Chem. 2006, 281, 14370–14375. [Google Scholar] [CrossRef]
- Xiao, D.; Gao, X.; Xu, J.; Liang, X.; Li, Q.; Yao, J.; Zhu, K.Y. Clathrin-dependent endocytosis plays a predominant role in cellular uptake of double-stranded RNA in the red flour beetle. Insect Biochem. Mol. Biol. 2015, 60, 68–77. [Google Scholar] [CrossRef]
- Ye, C.; Hu, X.S.; Wang, Z.W.; Wei, D.; Smagghe, G.; Christiaens, O.; Niu, J.; Wang, J.J. Involvement of clathrin-dependent endocytosis in cellular dsRNA uptake in aphids. I Insect Biochem. Mol. Biol. 2021, 132, 103557. [Google Scholar] [CrossRef]
- Saleh, M.C.; van Rij, R.P.; Hekele, A.; Gillis, A.; Foley, E.; O’Farrell, P.H.; Andino, R. The endocytic pathway mediates cell entry of dsRNA to induce RNAi Silencing. Nat. Cell Biol. 2006, 8, 793–802. [Google Scholar] [CrossRef]
- Avila, L.A.; Lee, S.Y.; Tomich, J.M. Synthetic in vitro delivery systems for plasmid DNA in eukaryotes. J. Nanopharm. Drug Deliv. 2014, 2, 17–35. [Google Scholar] [CrossRef][Green Version]
- Mahmoodi Chalbatani, G.; Dana, H.; Gharagouzloo, E.; Grijalvo, S.; Eritja, R.; Logsdon, C.D.; Memari, F.; Miri, S.R.; Rad, M.R.; Marmari, V. Small interfering RNAs (siRNAs) in cancer therapy: A nano-based approach. Int. J. Nanomed. 2019, 14, 3111–3128. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Zhu, K. Chitosan/double-stranded RNA nanoparticle-mediated RNA interference to silence chitin synthase genes through larval feeding in the african malaria mosquito (Anopheles gambiae). Insect Mol. Biol. 2010, 19, 683–693. [Google Scholar] [CrossRef]
- Qiao, H.; Zhao, J.; Wang, X.; Xiao, L.; Zhu-Salzman, K.; Lei, J.; Xu, D.; Xu, G.; Tan, Y.; Hao, D. An oral dsRNA delivery system based on chitosan induces G protein-coupled receptor kinase 2 Gene silencing for Apolygus lucorum Control. Pestic. Biochem. Physiol. 2023, 194, 105481. [Google Scholar] [CrossRef] [PubMed]
- Kunte, N.; McGraw, E.; Bell, S.; Held, D.; Avila, L.-A. Prospects, challenges and current status of RNAi through insect feeding. Pest Manag. Sci. 2020, 76, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Kozielski, K.L.; Tzeng, S.Y.; Green, J.J. Bioengineered nanoparticles for siRNA delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 449–468. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Shum, K.T.; Burnett, J.C.; Rossi, J.J. Nanoparticle-based delivery of RNAi therapeutics: Progress and challenges. Pharmaceuticals 2013, 6, 85–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, G.; Zheng, H.; Chen, X. Rigid nanoparticle-based delivery of anti-cancer siRNA: Challenges and opportunities. Biotechnol. Adv. 2014, 32, 831–843. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, K.; Cheng, W.; Li, J.; Liang, X.; Shen, J.; Dou, D.; Yin, M.; Yan, S. Field application of star polymer-delivered chitosan to amplify plant defense against potato late blight. Chem. Eng. J. 2021, 417, 129327. [Google Scholar] [CrossRef]
- Di Guglielmo, G.M.; Le Roy, C.; Goodfellow, A.F.; Wrana, J.L. Distinct endocytic pathways regulate TGF-β receptor signalling and turnover. Nat. Cell Biol. 2003, 5, 410–421. [Google Scholar] [CrossRef]
- Varkouhi, A.K.; Scholte, M.; Storm, G.; Haisma, H.J. Endosomal escape pathways for delivery of biologicals. J. Control. Release 2011, 151, 220–228. [Google Scholar] [CrossRef]
- Benjaminsen, R.V.; Mattebjerg, M.A.; Henriksen, J.R.; Moghimi, S.M.; Andresen, T.L. The possible “proton sponge” effect of polyethylenimine (PEI) does not include change in lysosomal pH. Mol. Ther. 2013, 21, 149–157. [Google Scholar] [CrossRef]
- Xu, Y.; Szoka, F.C. Mechanism of DNA release from cationic liposome/DNA complexes used in cell transfection. Biochemistry 1996, 35, 5616–5623. [Google Scholar] [CrossRef]
- Zelphati, O.; Szoka Jr, F.C. Mechanism of oligonucleotide release from cationic liposomes. Proc. Natl. Acad. Sci. USA 1996, 93, 11493–11498. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Niidome, T.; Aoyagi, H. Cytosolic soluble proteins induce DNA release from DNA–gene carrier complexes. J. Control. Release 2004, 98, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Moret, I.; Peris, J.E.; Guillem, V.M.; Benet, M.; Revert, F.; Dasıí, F.; Crespo, A.; Aliño, S.F. Stability of PEI–DNA and DOTAP–DNA complexes: Effect of alkaline pH, heparin and serum. J. Control. Release 2001, 76, 169–181. [Google Scholar] [CrossRef]
- Kwon, Y.J. Before and after endosomal escape: Roles of stimuli-converting siRNA/polymer interactions in determining gene silencing efficiency. Acc. Chem. Res. 2012, 45, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Lynn, D.M.; Langer, R. Degradable poly (β-Amino Esters): Synthesis, characterization, and self-assembly with plasmid DNA. J. Am. Chem. Soc. 2000, 122, 10761–10768. [Google Scholar] [CrossRef]
- Tzeng, S.Y.; Green, J.J. Subtle changes to polymer structure and degradation mechanism enable highly effective nanoparticles for siRNA and DNA delivery to human brain cancer. Adv. Healthc. Mater. 2013, 2, 468–480. [Google Scholar] [CrossRef]
- Dhandapani, R.K.; Gurusamy, D.; Howell, J.L.; Palli, S.R. Development of CS-TPP-dsRNA nanoparticles to enhance RNAi efficiency in the yellow fever mosquito, Aedes aegypti. Sci. Rep. 2019, 9, 8775. [Google Scholar] [CrossRef]
- Ragelle, H.; Riva, R.; Vandermeulen, G.; Naeye, B.; Pourcelle, V.; Le Duff, C.S.; D’Haese, C.; Nysten, B.; Braeckmans, K.; De Smedt, S.C.; et al. Chitosan nanoparticles for siRNA delivery: Optimizing formulation to increase stability and efficiency. J. Control. Release 2014, 176, 54–63. [Google Scholar] [CrossRef]
- Wang, K.; Peng, Y.; Chen, J.; Peng, Y.; Wang, X.; Shen, Z.; Han, Z. Comparison of efficacy of RNAi mediated by various nanoparticles in the rice striped stem borer (Chilo suppressalis). Pestic. Biochem. Physiol. 2020, 165, 104467. [Google Scholar] [CrossRef]
- He, B.; Chu, Y.; Yin, M.; Müllen, K.; An, C.; Shen, J. Fluorescent nanoparticle delivered dsRNA toward genetic control of insect pests. Adv. Mater. 2013, 25, 4580–4584. [Google Scholar] [CrossRef]
- Chao, Z.; Ma, Z.; Zhang, Y.; Yan, S.; Shen, J. Establishment of star polycation-based RNA interference system in all developmental stages of fall armyworm Spodoptera frugiperda. Entomol. Gen. 2023, 43, 127–137. [Google Scholar] [CrossRef]
- Yan, S.; Qian, J.; Cai, C.; Ma, Z.; Li, J.; Yin, M.; Ren, B.; Shen, J. Spray method application of transdermal dsRNA delivery system for efficient gene silencing and pest control onsoybean aphid Aphis glycines. J. Pest Sci. 2020, 93, 449–459. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, Y.; Li, M.; Chao, Z.; Du, X.; Yan, S.; Shen, J. A first greenhouse application of bacteria-expressed and nanocarrier-delivered RNA pesticide for Myzus persicae Control. J. Pest Sci. 2023, 96, 181–193. [Google Scholar] [CrossRef]
- Qiao, L.; Niño-Sánchez, J.; Hamby, R.; Capriotti, L.; Chen, A.; Mezzetti, B.; Jin, H. Artificial nanovesicles for dsRNA delivery in spray-induced gene silencing for crop protection. Plant Biotechnol. J. 2023, 21, 854–865. [Google Scholar] [CrossRef]
- Gurusamy, D.; Mogilicherla, K.; Palli, S.R. Chitosan nanoparticles help double-stranded RNA escape from endosomes and improve RNA interference in the fall armyworm, Spodoptera frugiperda. Arch. Insect Biochem. Physiol. 2020, 104, e21677. [Google Scholar] [CrossRef]
- Keppanan, R.; Karuppannasamy, A.; Nagaraja, B.C.; Thiruvengadam, V.; Kesavan, S.; Dhawane, Y.A.; Ramasamy, A. Effectiveness of chitosan nanohydrogel mediated encapsulation of EcR dsRNA against the whitefly, Bemisia tabaci Asia-I (Gennedius) (Hemiptera: Aleyordidae). Pestic. Biochem. Physiol. 2024, 198, 105712. [Google Scholar] [CrossRef]
- Zhou, H.; Wan, F.; Jian, Y.; Guo, F.; Zhang, M.; Shi, S.; Yang, L.; Li, S.; Liu, Y.; Ding, W. Chitosan/dsRNA polyplex nanoparticles advance environmental RNA interference efficiency through activating clathrin-dependent endocytosis. Int. J. Biol. Macromol. 2023, 253, 127021. [Google Scholar] [CrossRef]
- Das, S.; Debnath, N.; Cui, Y.; Unrine, J.; Palli, S.R. Chitosan, carbon quantum dot, and silica nanoparticle mediated dsRNA delivery for gene silencing in Aedes aegypti: A Comparative Analysis. ACS Appl. Mater. Interfaces 2015, 7, 19530–19535. [Google Scholar] [CrossRef]
- Zhang, X.; Mysore, K.; Flannery, E.; Michel, K.; Severson, D.W.; Zhu, K.Y.; Duman-Scheel, M. Chitosan/interfering RNA nanoparticle mediated gene silencing in disease vector mosquito larvae. J. Vis. Exp. 2015, 97, 52523. [Google Scholar] [CrossRef]
- Chen, J.; Lu, H.-R.; Zhang, L.; Liao, C.-H.; Han, Q. RNA interference-mediated knockdown of 3, 4-dihydroxyphenylacetaldehyde synthase affects larval development and adult survival in the mosquito Aedes aegypti. Parasit. Vectors 2019, 12, 1–11. [Google Scholar] [CrossRef]
- Kumar, D.R.; Kumar, P.S.; Gandhi, M.R.; Al-Dhabi, N.A.; Paulraj, M.G.; Ignacimuthu, S. Delivery of chitosan/dsRNA nanoparticles for silencing of wing development vestigial (vg) gene in Aedes aegypti Mosquitoes. Int. J. Biol. Macromol. 2016, 86, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hua, G.; Adang, M.J. Chitosan/dsiRNA nanoparticle targeting identifies AgCad1 cadherin in Anopheles gambiae Larvae as an in vivo receptor of Cry11Ba toxin of Bacillus thuringiensis Subsp. Jegathesan. Insect Biochem. Mol. Biol. 2015, 60, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.; Xiong, M.; Mao, J.; Li, W.; Jiang, G.; Zhang, W. A dsRNA delivery system based on the rosin-modified polyethylene glycol and chitosan induces gene silencing and mortality in Nilaparvata lugens. Pest Manag. Sci. 2023, 79, 1518–1527. [Google Scholar] [CrossRef] [PubMed]
- Kolge, H.; Kadam, K.; Ghormade, V. Chitosan nanocarriers mediated dsRNA delivery in gene silencing for Helicoverpa armigera biocontrol. Pestic. Biochem. Physiol. 2023, 189, 105292. [Google Scholar] [CrossRef] [PubMed]
- Taning, C.N.T.; Christiaens, O.; Berkvens, N.; Casteels, H.; Maes, M.; Smagghe, G. Oral RNAi to control Drosophila suzukii: Laboratory testing against larval and adult Stages. J. Pest Sci. 2016, 89, 803–814. [Google Scholar] [CrossRef]
- Castellanos, N.L.; Smagghe, G.; Sharma, R.; Oliveira, E.E.; Christiaens, O. Liposome encapsulation and EDTA formulation of dsRNA targeting essential genes increase oral RNAi-caused mortality in the Neotropical stink bug Euschistus heros. Pest Manag. Sci. 2019, 75, 537–548. [Google Scholar] [CrossRef]
- Gurusamy, D.; Mogilicherla, K.; Shukla, J.N.; Palli, S.R. Lipids help double-stranded RNA in endosomal escape and improve RNA interference in the fall armyworm, Spodoptera frugiperda. Arch. Insect Biochem. Physiol. 2020, 104, e21678. [Google Scholar] [CrossRef]
- Li, N.; Xu, X.; Li, J.; Hull, J.J.; Chen, L.; Liang, G. A spray-induced gene silencing strategy for Spodoptera frugiperda oviposition inhibition using nanomaterial-encapsulated dsEcR. Int. J. Biol. Macromol. 2024, 281, 136503. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Ma, Z.-Z.; Zhou, H.; Chao, Z.-J.; Yan, S.; Shen, J. Nanocarrier-delivered dsRNA suppresses wing development of green peach aphids. Insect Sci. 2022, 29, 669–682. [Google Scholar] [CrossRef]
- Wei, Z.-H.; Zhao, P.; Ning, X.-Y.; Xie, Y.Q.; Li, Z.; Liu, X.-X. Nanomaterial-encapsulated dsRNA-targeting chitin pathway—A potential efficient and eco-friendly strategy against cotton aphid, Aphis gossypii (Hemiptera: Aphididae). J. Agric. Food Chem. 2024, 72, 20905–20917. [Google Scholar] [CrossRef]
- Long, G.J.; Liu, X.Z.; Guo, H.; Zhang, M.Q.; Gong, L.L.; Ma, Y.F.; Dewer, Y.; Mo, W.J.; Ding, L.W.; Wang, Q.; et al. Oral-based nanoparticle-wrapped dsRNA delivery system: A promising approach for controlling an urban pest, Blattella germanica. J. Pest Sci. 2024, 97, 739–755. [Google Scholar] [CrossRef]
- Lu, Q.; Cui, H.; Li, W.; Liu, T.; Chen, Q.; Yang, Q. Synthetic nanoscale RNAi constructs as pesticides for the control of Locust migratoria. J. Agric. Food Chem. 2022, 70, 10762–10770. [Google Scholar] [CrossRef] [PubMed]
- Avila, L.; Chandrasekar, R.; Wilkinson, K.; Balthazor, J.; Heerman, M.; Bechard, J.; Brown, S.; Park, Y.; Dhar, S.; Reeck, G.; et al. Delivery of lethal dsRNAs in insect diets by branched amphiphilic peptide capsules. J. Control. Release 2018, 273, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Zhang, G.; Yu, C.; Qin, Y.; He, S.; Li, J.; Guo, L.; Wan, H. Characterization of CYP303A1 and its potential application based on ZIF-8 nanoparticle-wrapped dsRNA in Nilaparvata lugens (Stål). Pest Manag. Sci. 2024. [CrossRef] [PubMed]
- Xue, Q.; Li, J.; Vereecken, S.; Li, Q.; Zhi, Z.; Dubruel, P.; Taning, C.N.T.; De Schutter, K. Functionally modified graphene oxide as an alternative nanovehicle for enhanced dsRNA delivery in improving RNAi-based insect pest control. J. Agric. Food Chem. 2024, 72, 22512–22523. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, Q.; Lan, C.; Tang, T.; Wang, K.; Shen, J.; Niu, D. Nanoparticle carriers enhance RNA stability and uptake efficiency and prolong the protection against Rhizoctonia solani. Phytopathol. Res. 2023, 5, 2. [Google Scholar] [CrossRef]
- Mitter, N.; Worrall, E.A.; Robinson, K.E.; Li, P.; Jain, R.G.; Taochy, C.; Fletcher, S.J.; Carroll, B.J.; Lu, G.; Xu, Z.P. Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat. Plants 2017, 3, 16207. [Google Scholar] [CrossRef]
- Worrall, E.A.; Bravo-Cazar, A.; Nilon, A.T.; Fletcher, S.J.; Robinson, K.E.; Carr, J.P.; Mitter, N. Exogenous application of RNAi-inducing double-stranded RNA inhibits aphid-mediated transmission of a plant virus. Front Plant Sci. 2019, 10, 265. [Google Scholar] [CrossRef]
- Duanis-Assaf, D.; Shlar, I.; Galsurker, O.; Davydov, O.; Maurer, D.; Feygenberg, O.; Poverenov, E.; Fluhr, R.; Alkan, N. Nano-Clay, layered-double hydroxide (LDH), improves the efficacy of double-stranded RNA in controlling postharvest decay. Postharvest Biol. Technol. 2022, 193, 112051. [Google Scholar] [CrossRef]
- Chen, X.; Shi, T.; Tang, T.; Chen, C.; Liang, Y.; Zuo, S. Nanosheet-facilitated spray delivery of dsRNAs represents a potential tool to control Rhizoctonia solani infection. Int. J. Mol. Sci. 2022, 23, 12922. [Google Scholar] [CrossRef]
- Niño-Sánchez, J.; Sambasivam, P.T.; Sawyer, A.; Hamby, R.; Chen, A.; Czislowski, E.; Li, P.; Manzie, N.; Gardiner, D.M.; Ford, R.; et al. BioClayTM prolongs RNA interference-mediated crop protection against Botrytis Cinerea. J Integr. Plant Biol. 2022, 64, 2187–2198. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yan, S.; Xie, S.; Yin, M.; Shen, J.; Li, Z.; Zhou, Y.; Duan, L. Construction and application of star polycation nanocarrier-based microRNA delivery system in Arabidopsis and maize. J. Nanobiotechnol. 2022, 20, 219. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Arakane, Y.; Beeman, R.W.; Kramer, K.J.; Muthukrishnan, S. Functional specialization among insect chitinase family genes revealed by RNA interference. Proc. Natl. Acad. Sci. USA 2008, 105, 6650–6655. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, X.; Wang, X.; Yu, D.; Chen, B.; Kang, L. Differential responses of migratory locusts to systemic RNA interference via double-stranded RNA injection and feeding. Insect Mol. Biol. 2013, 22, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Wynant, N.; Verlinden, H.; Breugelmans, B.; Simonet, G.; Broeck, J.V. Tissue-dependence and sensitivity of the systemic RNA interference response in the desert locust, Schistocerca gregaria. Insect Biochem. Mol. Biol. 2012, 42, 911–917. [Google Scholar] [CrossRef]
- Christiaens, O.; Swevers, L.; Smagghe, G. DsRNA degradation in the pea aphid (Acyrthosiphon pisum) associated with lack of response in RNAi feeding and injection assay. Peptides 2014, 53, 307–314. [Google Scholar] [CrossRef]
- Silver, K.; Cooper, A.M.; Zhu, K.Y. Strategies for enhancing the efficiency of RNA interference in insects. Pest Manag. Sci. 2021, 77, 2645–2658. [Google Scholar] [CrossRef]
- Rodrigues, T.B.; Rieske, L.K.; Duan, J.J.; Mogilicherla, K.; Palli, S.R. Development of RNAi method for screening candidate genes to control emerald ash borer, Agrilus planipennis. Sci. Rep. 2017, 7, 7379. [Google Scholar] [CrossRef]
- Rodrigues, T.B.; Duan, J.J.; Palli, S.R.; Rieske, L.K. Identification of highly effective target genes for RNAi-mediated control of emerald ash borer, Agrilus planipennis. Sci. Rep. 2018, 8, 5020. [Google Scholar] [CrossRef]
- Li, M.; Ma, Z.; Peng, M.; Li, L.; Yin, M.; Yan, S.; Shen, J. A gene and drug co-delivery application helps to solve the short life disadvantage of RNA drug. Nano Today 2022, 43, 101452. [Google Scholar] [CrossRef]
- Yan, S.; Li, M.; Jiang, Q.; Li, M.; Hu, M.; Shi, X.; Liang, P.; Yin, M.; Gao, X.; Shen, J.; et al. Self-assembled co-delivery nanoplatform for increasing the broad-spectrum susceptibility of fall armyworm toward insecticides. J. Adv. Res. 2024. [CrossRef] [PubMed]
- Wang, Y.; Li, M.; Ying, J.; Shen, J.; Dou, D.; Yin, M.; Whisson, S.C.; Birch, P.R.; Yan, S.; Wang, X. High-efficiency green management of potato late blight by a self-assembled multicomponent nano-bioprotectant. Nat. Commun. 2023, 14, 5622. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.D.; Liu, Z.C.; Huang, S.L.; Chen, Z.Q.; Sun, Y.W.; Duan, P.F.; Ma, Y.Z.; Xia, L.Q. RNAi-mediated plant protection against aphids. Pest Manag. Sci. 2016, 72, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Khajuria, C.; Ivashuta, S.; Wiggins, E.; Flagel, L.; Moar, W.; Pleau, M.; Miller, K.; Zhang, Y.; Ramaseshadri, P.; Jiang, C.; et al. Development and characterization of the first dsRNA-resistant insect population from western corn rootworm, Diabrotica virgifera virgifera LeConte. PLoS ONE 2018, 13, e0197059. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Zhang, M.; Zhang, J. Characterization and potential mechanism of resistance to double-stranded RNA in willow leaf beetle, Plagiodera versicolora. J. Pest Sci. 2024, 97, 2217–2226. [Google Scholar] [CrossRef]
- Bachman, P.M.; Bolognesi, R.; Moar, W.J.; Mueller, G.M.; Paradise, M.S.; Ramaseshadri, P.; Tan, J.; Uffman, J.P.; Warren, J.; Wiggins, B.E.; et al. Characterization of the spectrum of insecticidal activity of a double-stranded RNA with targeted activity against Western Corn Rootworm (Diabrotica virgifera virgifera LeConte). Transgenic Res. 2013, 22, 1207–1222. [Google Scholar] [CrossRef]
- Dong, M.; Chen, D.; Che, L.; Gu, N.; Yin, M.; Du, X.; Shen, J.; Yan, S. Biotoxicity evaluation of a cationic star polymer on a predatory ladybird and cooperative pest control by polymer-delivered pesticides and ladybird. ACS Appl. Mater. Interfaces 2022, 14, 6083–6092. [Google Scholar] [CrossRef]
- De Schutter, K.; Taning, C.N.T.; Van Daele, L.; Van Damme, E.J.; Dubruel, P.; Smagghe, G. RNAi-based biocontrol products: Market status, regulatory aspects, and risk assessment. Front Insect Sci. 2022, 1, 818037. [Google Scholar] [CrossRef]
- Timmons, L.; Court, D.L.; Fire, A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 2001, 263, 103–112. [Google Scholar] [CrossRef]
- Murphy, K.A.; Tabuloc, C.A.; Cervantes, K.R.; Chiu, J.C. Ingestion of genetically modified yeast symbiont reduces fitness of an insect pest via RNA interference. Sci. Rep. 2016, 6, 22587. [Google Scholar] [CrossRef]
- Saelim, H.; Loprasert, S.; Phongdara, A. Bacillus subtilis expressing dsVP28 improved shrimp survival from WSSV challenge. ScienceAsia 2020, 46, 19–26. [Google Scholar] [CrossRef]
- Ganbaatar, O.; Cao, B.; Zhang, Y.; Bao, D.; Bao, W.; Wuriyanghan, H. Knockdown of Mythimna separata chitinase genes via bacterial expression and oral delivery of RNAi effectors. BMC Biotechnol. 2017, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Xu, J.; Palli, R.; Ferguson, J.; Palli, S.R. Ingested RNA interference for managing the populations of the Colorado potato beetle, Leptinotarsa decemlineata. Pest Manag. Sci. 2011, 67, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, M.; Zhang, H. RNA Interference of four genes in adult Bactrocera dorsalis by feeding their dsRNAs. PLoS ONE 2011, 6, e17788. [Google Scholar] [CrossRef]
- Ma, Z.Z.; Zhou, H.; Wei, Y.L.; Yan, S.; Shen, J. A novel plasmid–Escherichia coli system produces large batch dsRNAs for insect gene silencing. Pest Manag. Sci. 2020, 76, 2505–2512. [Google Scholar] [CrossRef]
- Guan, R.; Chu, D.; Han, X.; Miao, X.; Li, H. Advances in the development of microbial double-stranded RNA production systems for application of RNA interference in agricultural pest control. Front. Bioeng. Biotechnol. 2021, 9, 753790. [Google Scholar] [CrossRef]
- Maxwell, B.; Boyes, D.; Tang, J.; Rodrigues, T.; Desai, S.; Cunningham, D.; Ramachandriya, K.; Abshire, J.; Cobb, C.; Biosciences, G.; et al. Enabling the RNA Revolution; Cell-Free dsRNA Production and Control of Colorado Potato Beetle. 2018. Available online: https://www.globalengage.co.uk/pgc/docs/PosterMaxwell.pdf (accessed on 18 November 2024).
| Nanoparticle | Insect | Target Gene | Delivery Method | Reference |
|---|---|---|---|---|
| Chitosan | Chilo suppressalis | CHSA, CHSB, G3PDH | Oral feeding | [100] |
| Spodoptera frugiperda | IAP | Oral feeding | [106] | |
| Apolygus lucorum | GRK2 | Oral feeding | [82] | |
| Bemisia tabaci | ECR | Oral feeding | [107] | |
| Tetranychus cinnabarinus | TcCHIT10, Tcβ-COP, TcCHC | Oral feeding | [108] | |
| Aedes aegypti | SNF7 | Oral feeding | [109] | |
| Sema1a | Oral feeding | [110] | ||
| DOPAL synthase | Oral feeding | [111] | ||
| Vestigial gene | Oral feeding | [112] | ||
| Anopheles gambiae | Chitin synthase 1 | Oral feeding | [81] | |
| Chitin synthase 2 | Oral feeding | [110] | ||
| Cadherin1, Cadherin2 | Oral feeding | [113] | ||
| PEG–Chitosan | Nilaparvata lugens | NlCHSA | Topical application | [114] |
| Chitosan–sodium tripolyphosphate | Aedes aegypti | IAP | Oral feeding | [98] |
| Helicoverpa armigera | JHAMT, ACHE | Oral feeding | [40] | |
| Lipase, chitinase | Oral feeding | [115] | ||
| Liposom | Drosophila melanogaster | V-ATPaseE | Soaking and oral feeding | [38] |
| Drosophila suzukii | Vha26 | Oral feeding | [116] | |
| Euschistus heros | V-ATPaseA0, Muscle actin | Oral feeding | [117] | |
| Chilo suppressalis | CHSA, CHSB, G3PDH | Oral feeding | [100] | |
| Spodoptera frugiperda | IAP | Oral feeding | [118] | |
| Perylenediimide-cored cationic dendrimers | Ostrinia furnacalis | Serpin-3 | Oral feeding | [22] |
| Chitinase-like, CHT10 | Microinjection and oralfeeding | [101] | ||
| Aphis glycines | Hemocytin | Topical application | [23] | |
| Star polycation | Agrotis ypsilon | V-ATPase | Microinjection and oralfeeding | [24] |
| Spodoptera frugiperda | V-ATPaseD, chitin synthase1 | Soaking, topical application, and oral feeding | [102] | |
| ECR | Spraying | [119] | ||
| Aphis glycines | Treh, V-ATPaseD, V-ATPaseE, chitin synthase1 | Topical application and spraying | [103] | |
| Myzus persicae | vestigial, ultrabithorax | Topical application | [120] | |
| Aphis gossypii | AgCHS2, AgHK2 | Spraying | [121] | |
| Blattella germanica | BgCHS1, BgCHS2 | Oral feeding | [122] | |
| Block copolymer | Locust migratoria | LmCHS1, LmCHS2 | Oral feeding | [123] |
| Branched amphiphilic Peptide capsules | Acyrthosiphon pisum | Armet, BiP | Oral feeding | [124] |
| Tribolium castaneum | Armet, BiP | Oral feeding | [124] | |
| Carbon quantum dot | Chilo suppressalis | CHSA, CHSB, G3PDH | Oral feeding | [100] |
| Metal organic framework | Nilaparvata lugens | NlCYP303A1 | Oral feeding | [125] |
| Graphene oxide | Drosophila suzukii | vATPase | Oral feeding | [126] |
| Nanoparticle | Host | Pathogen | Target Gene | Reference |
|---|---|---|---|---|
| Star polycation | rice | Rhizoctonia solani | RsAGO1, RsAGO2 | [127] |
| Artificial nanovesicles | tomato, grape | Botrytis cinerea | Dicer-like 1, Dicer-like 2 | [105] |
| Layered double hydroxide | cowpea | Common mosaic virus | CMV2b | [128] |
| Bean common Mosaic virus | Coat protein | [129] | ||
| grape, cherry | Botrytis cinerea | erg13, erg11, erg1 | [130] | |
| maize | Rhizoctonia solani | RsCRZ1 | [131] | |
| tomato | Botrytis cinerea | BcDCL1/2, BcVDS | [132] | |
| Carbon dot | Nicotiana benthamiana, chili | Phytophthora infestans, Phytophthora sojae, Phytophthora capsici | CesA3, OSBP1 | [25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, H.; Chen, J.; Dong, M.; Shen, J.; Yan, S. Nanocarrier-Based Eco-Friendly RNA Pesticides for Sustainable Management of Plant Pathogens and Pests. Nanomaterials 2024, 14, 1874. https://doi.org/10.3390/nano14231874
Qiao H, Chen J, Dong M, Shen J, Yan S. Nanocarrier-Based Eco-Friendly RNA Pesticides for Sustainable Management of Plant Pathogens and Pests. Nanomaterials. 2024; 14(23):1874. https://doi.org/10.3390/nano14231874
Chicago/Turabian StyleQiao, Heng, Jingyi Chen, Min Dong, Jie Shen, and Shuo Yan. 2024. "Nanocarrier-Based Eco-Friendly RNA Pesticides for Sustainable Management of Plant Pathogens and Pests" Nanomaterials 14, no. 23: 1874. https://doi.org/10.3390/nano14231874
APA StyleQiao, H., Chen, J., Dong, M., Shen, J., & Yan, S. (2024). Nanocarrier-Based Eco-Friendly RNA Pesticides for Sustainable Management of Plant Pathogens and Pests. Nanomaterials, 14(23), 1874. https://doi.org/10.3390/nano14231874







