Carbon-Infiltrated Carbon Nanotube Topography Reduces the Growth of Staphylococcus aureus Biofilms
Abstract
1. Introduction
2. Materials and Methods
2.1. CICNT and Control Sample Preparation
2.2. Bacterial Strains
2.3. Bacterial Culture
2.4. Biofilm Quantification
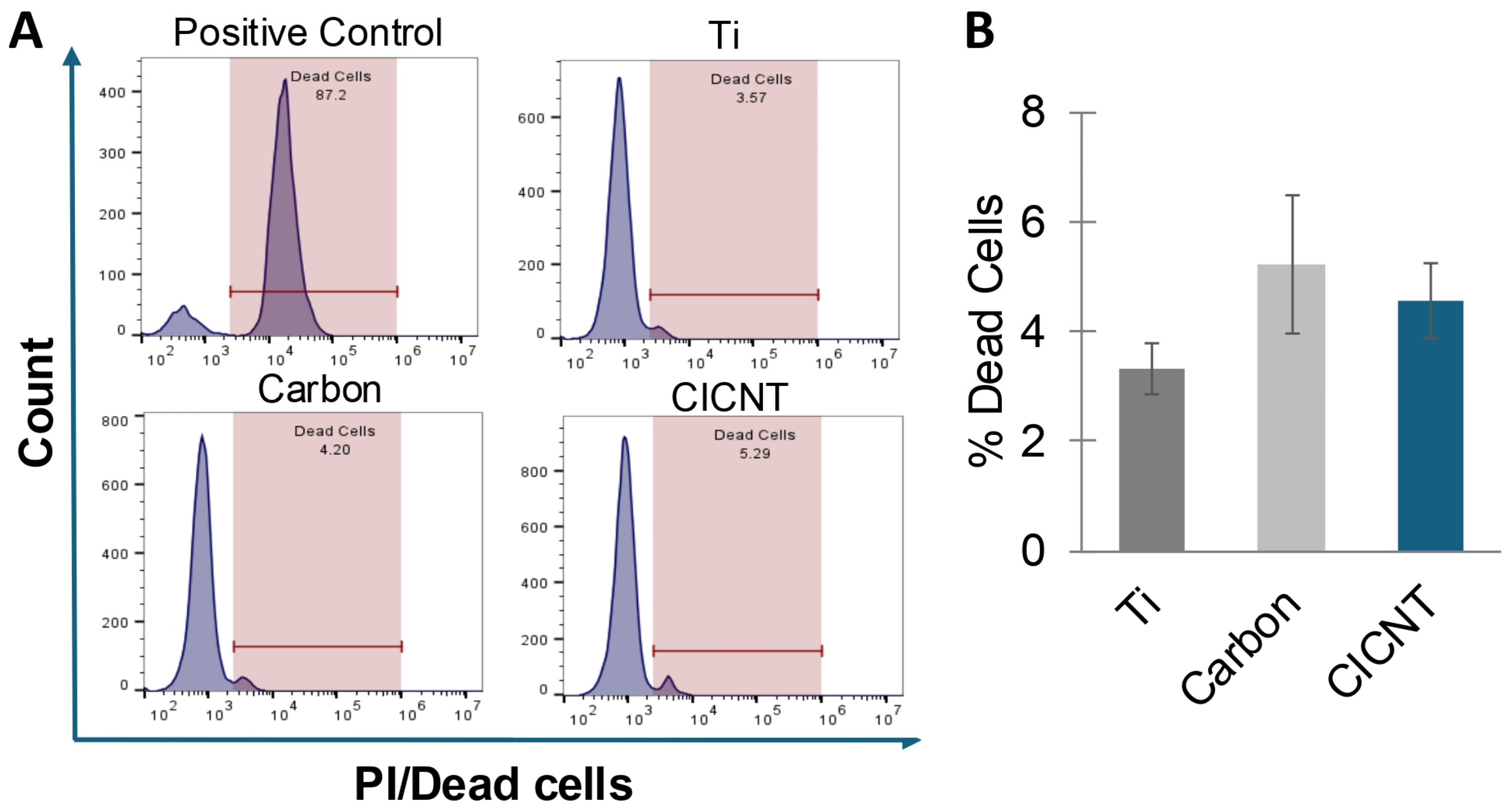
2.5. Propidium Iodide Staining and Flow Cytometry
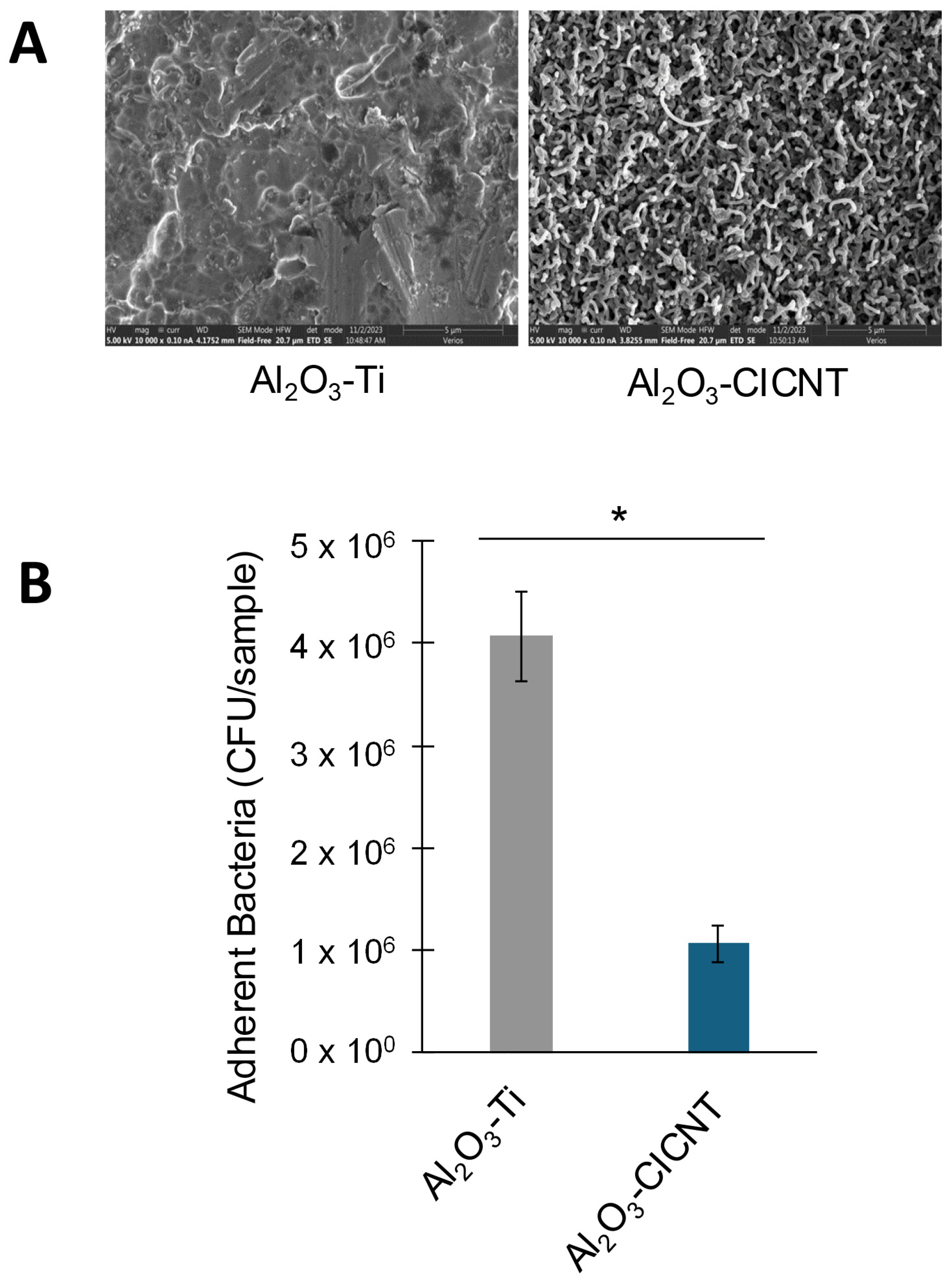
2.6. Scanning Electron Microscopy
2.7. Statistical Analysis
3. Results and Discussion
3.1. The CICNT Surface Reduces S. aureus Biofilm Growth in RPMI Media
3.2. The CICNT Topography, Rather than the Presence of Carbon, Reduces Biofilm Growth
3.3. The Reduction in Biofilm Growth on CICNT Is Not Due to Bacterial Cell Death
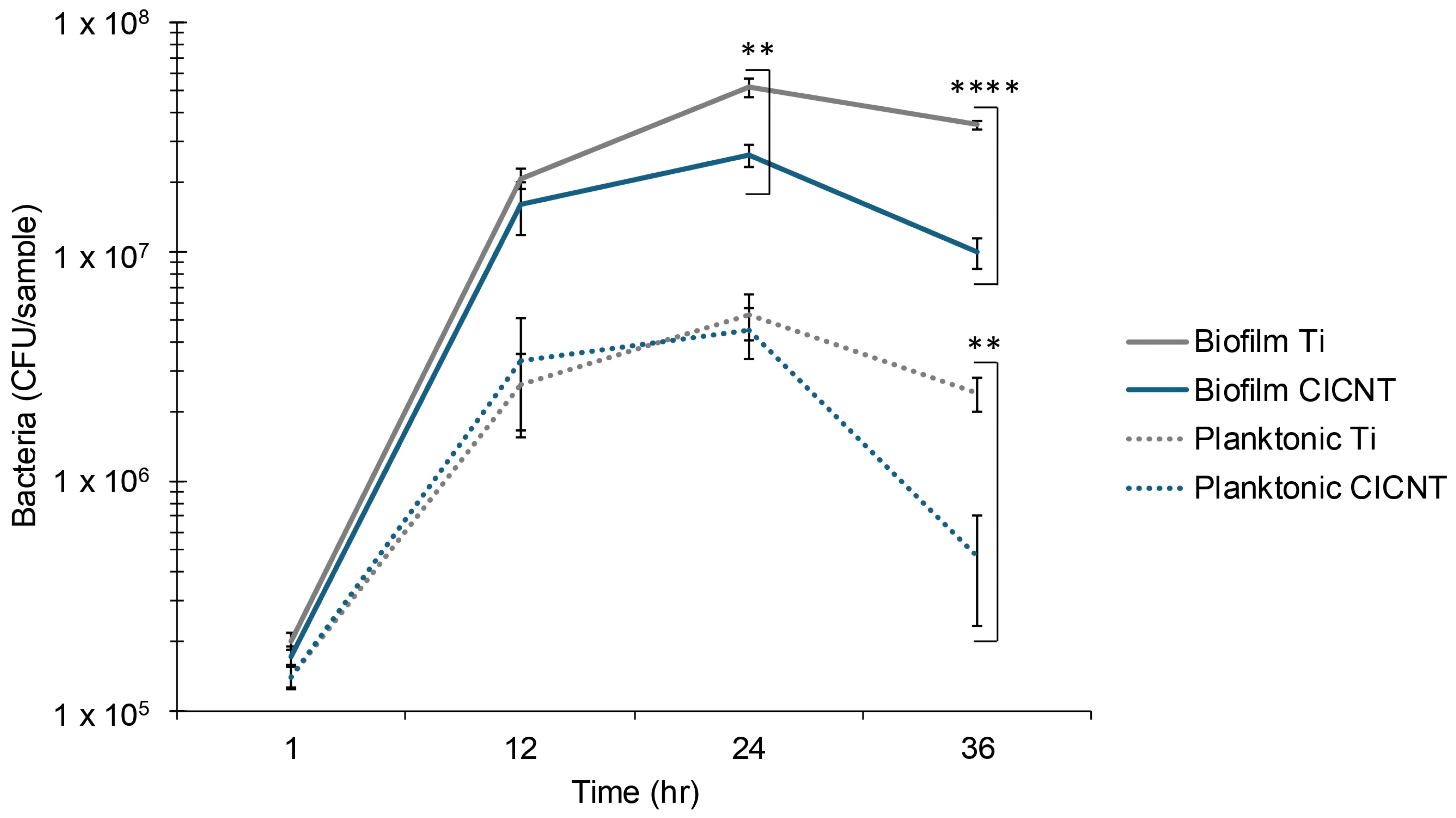
3.4. Mid to Late Biofilm Growth Is Slower on CICNT than on Ti Surfaces
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, J.A.; Yu, S.; Chen, L.; Cleveland, J.D. Rates of Total Joint Replacement in the United States: Future Projections to 2020–2040 Using the National Inpatient Sample. J. Rheumatol. 2019, 46, 1134–1140. [Google Scholar] [CrossRef]
- Shichman, I.; Roof, M.; Askew, N.; Nherera, L.; Rozell, J.C.; Seyler, T.M.; Schwarzkopf, R. Projections and Epidemiology of Primary Hip and Knee Arthroplasty in Medicare Patients to 2040-2060. JBJS Open Access 2023, 8, e22. [Google Scholar] [CrossRef]
- Ryan, S.P.; Stambough, J.B.; Huddleston, J.I., 3rd; Levine, B.R. Highlights of the 2023 American Joint Replacement Registry Annual Report. Arthroplast Today 2024, 26, 101325. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Ray, P.; Das, A.; Sharma, M. Penetration of antibiotics through Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Antimicrob. Chemother. 2010, 65, 1955–1958. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef]
- Zeller, V.; Kerroumi, Y.; Meyssonnier, V.; Heym, B.; Metten, M.A.; Desplaces, N.; Marmor, S. Analysis of postoperative and hematogenous prosthetic joint-infection microbiological patterns in a large cohort. J. Infect. 2018, 76, 328–334. [Google Scholar] [CrossRef]
- Bandara, C.D.; Singh, S.; Afara, I.O.; Wolff, A.; Tesfamichael, T.; Ostrikov, K.; Oloyede, A. Bactericidal Effects of Natural Nanotopography of Dragonfly Wing on Escherichia coli. ACS Appl. Mater. Interfaces 2017, 9, 6746–6760. [Google Scholar] [CrossRef]
- Jenkins, J.; Mantell, J.; Neal, C.; Gholinia, A.; Verkade, P.; Nobbs, A.H.; Su, B. Antibacterial effects of nanopillar surfaces are mediated by cell impedance, penetration and induction of oxidative stress. Nat. Commun. 2020, 11, 1626. [Google Scholar] [CrossRef]
- Morco, S.R.; Williams, D.L.; Jensen, B.D.; Bowden, A.E. Structural biofilm resistance of carbon-infiltrated carbon nanotube coatings. J. Orthop. Res. 2021, 40, 1953–1960. [Google Scholar] [CrossRef]
- Bowden, L.C.; Evans, J.G.W.; Miller, K.M.; Bowden, A.E.; Jensen, B.D.; Hope, S.; Berges, B.K. Carbon-infiltrated carbon nanotubes inhibit the development of Staphylococcus aureus biofilms. Sci. Rep. 2023, 13, 19398. [Google Scholar] [CrossRef]
- Kennedy, A.D.; Otto, M.; Braughton, K.R.; Whitney, A.R.; Chen, L.; Mathema, B.; Mediavilla, J.R.; Byrne, K.A.; Parkins, L.D.; Tenover, F.C.; et al. Epidemic community-associated methicillin-resistant Staphylococcus aureus: Recent clonal expansion and diversification. Proc. Natl. Acad. Sci. USA 2008, 105, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Bose, J.L.; Fey, P.D.; Bayles, K.W. Genetic tools to enhance the study of gene function and regulation in Staphylococcus aureus. Appl. Environ. Microbiol. 2013, 79, 2218–2224. [Google Scholar] [CrossRef]
- Ball, A.L.; Augenstein, E.D.; Wienclaw, T.M.; Richmond, B.C.; Freestone, C.A.; Lewis, J.M.; Thompson, J.S.; Pickett, B.E.; Berges, B.K. Characterization of Staphylococcus aureus biofilms via crystal violet binding and biochemical composition assays of isolates from hospitals, raw meat, and biofilm-associated gene mutants. Microb. Pathog. 2022, 167, 105554. [Google Scholar] [CrossRef]
- Missiakas, D.M.; Schneewind, O. Growth and laboratory maintenance of Staphylococcus aureus. Curr. Protoc. Microbiol. 2013, 28, 9C-1. [Google Scholar] [CrossRef]
- Wijesinghe, G.; Dilhari, A.; Gayani, B.; Kottegoda, N.; Samaranayake, L.; Weerasekera, M. Influence of Laboratory Culture Media on in vitro Growth, Adhesion, and Biofilm Formation of Pseudomonas aeruginosa and Staphylococcus aureus. Med. Princ. Pract. 2019, 28, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Lade, H.; Park, J.H.; Chung, S.H.; Kim, I.H.; Kim, J.M.; Joo, H.S.; Kim, J.S. Biofilm Formation by Staphylococcus aureus Clinical Isolates is Differentially Affected by Glucose and Sodium Chloride Supplemented Culture Media. J. Clin. Med. 2019, 8, 1853. [Google Scholar] [CrossRef]
- Sugimoto, S.; Sato, F.; Miyakawa, R.; Chiba, A.; Onodera, S.; Hori, S.; Mizunoe, Y. Broad impact of extracellular DNA on biofilm formation by clinically isolated Methicillin-resistant and -sensitive strains of Staphylococcus aureus. Sci. Rep. 2018, 8, 2254. [Google Scholar] [CrossRef]
- Cáceres, M.E.; Ledesma, M.M.; Lombarte Serrat, A.; Vay, C.; Sordelli, D.O.; Giacomodonato, M.N.; Buzzola, F.R. Growth conditions affect biofilms of Staphylococcus aureus producing mastitis: Contribution of MALDI-TOF-MS to strain characterization. Curr. Res. Microb. Sci. 2021, 2, 100073. [Google Scholar] [CrossRef]
- Denes, E.; Barrière, G.; Poli, E.; Lévêque, G. Alumina Biocompatibility. J. Long. Term. Eff. Med. Implants 2018, 28, 9–13. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Truong, V.K.; Watson, G.S.; Watson, J.A.; Baulin, V.A.; Pogodin, S.; Wang, J.Y.; Tobin, M.J.; et al. Natural bactericidal surfaces: Mechanical rupture of Pseudomonas aeruginosa cells by cicada wings. Small 2012, 8, 2489–2494. [Google Scholar] [CrossRef]
- Rostami, S.; Tekkesin, A.I.; Ercan, U.K.; Garipcan, B. Biomimetic sharkskin surfaces with antibacterial, cytocompatible, and drug delivery properties. Biomater. Adv. 2022, 134, 112565. [Google Scholar] [CrossRef]
- Hasan, J.; Crawford, R.J.; Ivanova, E.P. Antibacterial surfaces: The quest for a new generation of biomaterials. Trends Biotechnol. 2013, 31, 295–304. [Google Scholar] [CrossRef]
- Sorzabal-Bellido, I.; Barbieri, L.; Beckett, A.J.; Prior, I.A.; Susarrey-Arce, A.; Tiggelaar, R.M.; Fothergill, J.; Raval, R.; Diaz Fernandez, Y.A. Effect of Local Topography on Cell Division of Staphylococcus spp. Nanomaterials 2022, 12, 683. [Google Scholar] [CrossRef]
- Ellison, C.; Brun, Y.V. Mechanosensing: A regulation sensation. Curr. Biol. 2015, 25, R113–R115. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.D.; Bavi, N.; Martinac, B. Bacterial Mechanosensors. Annu. Rev. Physiol. 2018, 80, 71–93. [Google Scholar] [CrossRef] [PubMed]
- Siryaporn, A.; Kuchma, S.L.; O’Toole, G.A.; Gitai, Z. Surface attachment induces Pseudomonas aeruginosa virulence. Proc. Natl. Acad. Sci. USA 2014, 111, 16860–16865. [Google Scholar] [CrossRef]
- Gomez, S.; Bureau, L.; John, K.; Chene, E.N.; Debarre, D.; Lecuyer, S. Substrate stiffness impacts early biofilm formation by modulating Pseudomonas aeruginosa twitching motility. Elife 2023, 12, e81112. [Google Scholar] [CrossRef]
- Asp, M.E.; Ho Thanh, M.T.; Germann, D.A.; Carroll, R.J.; Franceski, A.; Welch, R.D.; Gopinath, A.; Patteson, A.E. Spreading rates of bacterial colonies depend on substrate stiffness and permeability. PNAS Nexus 2022, 1, pgac025. [Google Scholar] [CrossRef]
- de Souza Heidel, B.L.; Benson, J.; O’Keane, S.; Dodge, A.G.; Wackett, L.P.; Aksan, A. A Model for Mechanical Stress Limited Bacterial Growth and Resporulation in Confinement. ACS Appl. Mater. Interfaces 2024, 16, 41800–41809. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bowden, L.C.; Sithole, S.T.; Bowden, A.E.; Jensen, B.D.; Berges, B.K. Carbon-Infiltrated Carbon Nanotube Topography Reduces the Growth of Staphylococcus aureus Biofilms. Nanomaterials 2025, 15, 510. https://doi.org/10.3390/nano15070510
Bowden LC, Sithole ST, Bowden AE, Jensen BD, Berges BK. Carbon-Infiltrated Carbon Nanotube Topography Reduces the Growth of Staphylococcus aureus Biofilms. Nanomaterials. 2025; 15(7):510. https://doi.org/10.3390/nano15070510
Chicago/Turabian StyleBowden, Lucy C., Sidney T. Sithole, Anton E. Bowden, Brian D. Jensen, and Bradford K. Berges. 2025. "Carbon-Infiltrated Carbon Nanotube Topography Reduces the Growth of Staphylococcus aureus Biofilms" Nanomaterials 15, no. 7: 510. https://doi.org/10.3390/nano15070510
APA StyleBowden, L. C., Sithole, S. T., Bowden, A. E., Jensen, B. D., & Berges, B. K. (2025). Carbon-Infiltrated Carbon Nanotube Topography Reduces the Growth of Staphylococcus aureus Biofilms. Nanomaterials, 15(7), 510. https://doi.org/10.3390/nano15070510





