First-Principles Calculations for Glycine Adsorption Dynamics and Surface-Enhanced Raman Spectroscopy on Diamond Surfaces
Abstract
1. Introduction
2. Computational Method and Details
3. Results and Discussion
3.1. Molecular Adsorption Structure
3.2. Temperature-Dependent Adsorption Behavior
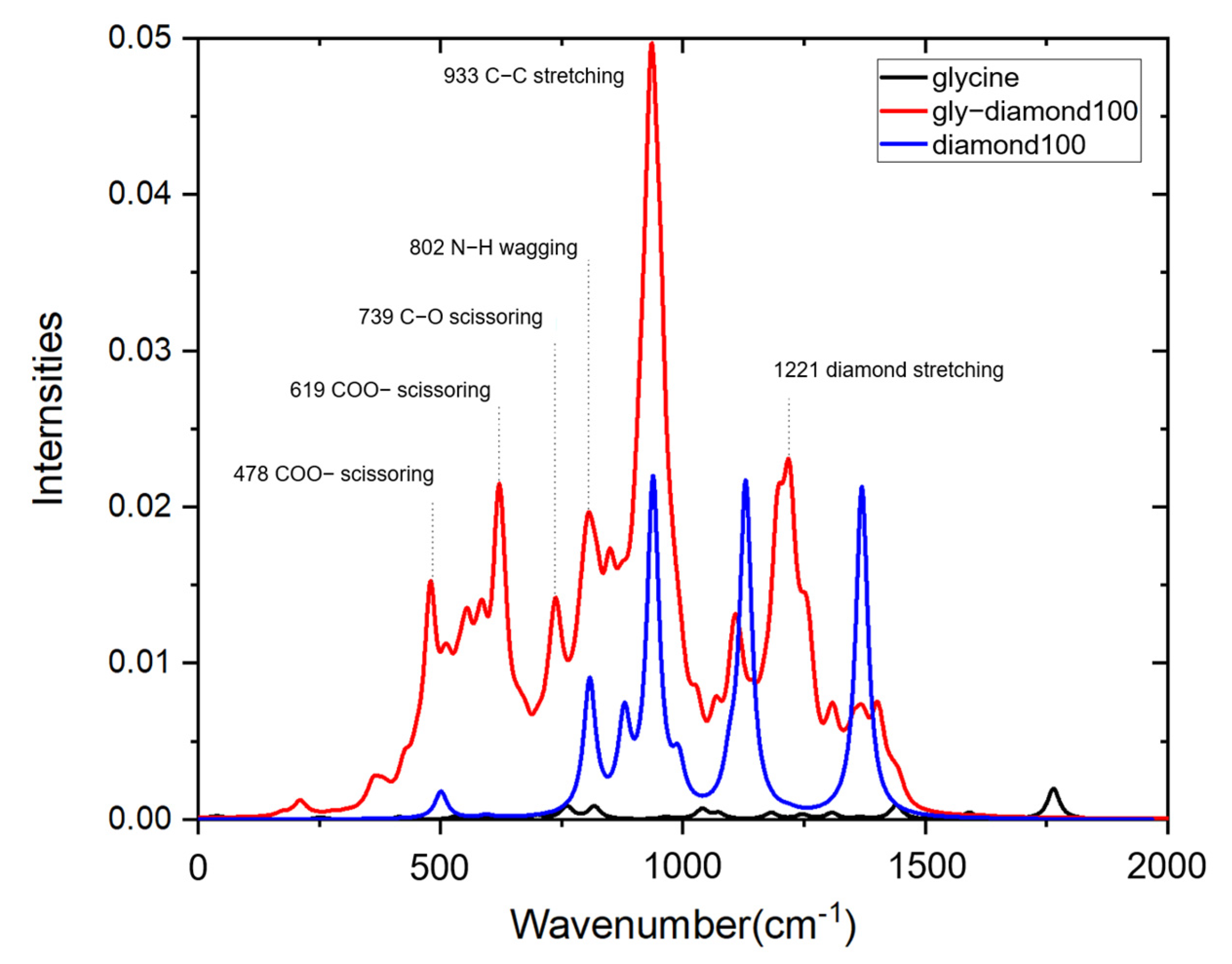
3.3. Surface-Enhanced Raman Spectroscopy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Carré, A.; Lacarrière, V. How substrate properties control cell adhesion. A physical–chemical approach. J. Adhes. Sci. Technol. 2010, 24, 815–830. [Google Scholar] [CrossRef]
- Kumar, S.; Parekh, S.H. Linking graphene-based material physicochemical properties with molecular adsorption, structure and cell fate. Commun. Chem. 2020, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Satyanarayana, U. Biochemistry; Elsevier Health Sciences: Berlin/Heidelberg, Germany, 2013; pp. 43–69. [Google Scholar]
- Lopes, I.; Piao, L.; Stievano, L.; Lambert, J.-F. Adsorption of amino acids on oxide supports: A solid-state NMR study of glycine adsorption on silica and alumina. J. Phys. Chem. C 2009, 113, 18163–18172. [Google Scholar] [CrossRef]
- Lei, G.; Xun, S.; Wei, S. Adsorption of Amino Acid Inhibitors on Iron Surface: A First-principles Investigation. Surf. Technol. 2017, 46, 228–234. [Google Scholar]
- Fang, Z.; Wang, J.; Yang, X.; Sun, Q.; Jia, Y.; Liu, H.; Xi, T.; Guan, S. Adsorption of arginine, glycine and aspartic acid on Mg and Mg-based alloy surfaces: A first-principles study. Appl. Surf. Sci. 2017, 409, 149–155. [Google Scholar] [CrossRef]
- Liu, Z.; Zhong, X.; Liu, Y.; Rao, H.; Wei, H.; Hu, W.; Nie, X.; Liu, M. Adsorption and Mechanism of Glycine on the Anatase with Exposed (001) and (101) Facets. Minerals 2022, 12, 798. [Google Scholar] [CrossRef]
- Odbadrakh, K.; Luo, X.; Lee, J.G.; Sagui, C.; Roland, C. Theoretical Investigation of the Interaction of Glycine with Diamond C (100) and C (111)(2 × 1) Surfaces. J. Phys. Chem. C 2007, 111, 12760–12767. [Google Scholar] [CrossRef]
- Li, L.; Xu, J.; Xu, L.-F.; Lian, C.-S.; Li, J.-J.; Wang, J.-T.; Gu, C.-Z. Adsorption of glycine on diamond (001): Role of bond angle of carbon atoms. Chin. Phys. B 2015, 24, 056803. [Google Scholar] [CrossRef]
- Biczysko, M.; Bloino, J.; Carnimeo, I.; Panek, P.; Barone, V. Fully ab initio IR spectra for complex molecular systems from perturbative vibrational approaches: Glycine as a test case. J. Mol. Struct. 2012, 1009, 74–82. [Google Scholar] [CrossRef]
- De La Pierre, M.; Bruno, M.; Manfredotti, C.; Nestola, F.; Prencipe, M.; Manfredotti, C. The (100), (111) and (110) surfaces of diamond: An ab initio B3LYP study. Mol. Phys. 2014, 112, 1030–1039. [Google Scholar] [CrossRef]
- Chatterjee, A.; Zhang, L.; Leung, K.T. Bidentate Surface Structures of Glycylglycine on Si (111) 7 × 7 by High-Resolution Scanning Tunneling Microscopy: Site-Specific Adsorption via N–H and O–H or Double N–H Dissociation. Langmuir 2012, 28, 12502–12508. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Hong, S. Structure of Glycine on Ge (100): Ab Initio Study of Its Scanning Tunneling Microscopy Images. J. Phys. Chem. C 2012, 116, 13890–13895. [Google Scholar] [CrossRef]
- Panikar, S.S.; Cialla-May, D.; De la Rosa, E.; Salas, P.; Popp, J. Towards translation of surface-enhanced Raman spectroscopy (SERS) to clinical practice: Progress and trends. TrAC Trends Anal. Chem. 2021, 134, 116122. [Google Scholar] [CrossRef]
- Szunerits, S.; Boukherroub, R. Different strategies for functionalization of diamond surfaces. J. Solid State Electrochem. 2008, 12, 1205–1218. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Chang, B.M.; Chang, H.C. Nanodiamond-enabled biomedical imaging. Nanomedicine 2020, 15, 1599–1616. [Google Scholar] [CrossRef]
- Zhang, A.Q.; Wang, Q.L.; Gao, Y.; Cheng, S.H.; Li, H.D. Gold-Nanoparticles/Boron-Doped-Diamond Composites as Surface-Enhanced Raman Scattering Substrates. Chin. Phys. Lett. 2020, 37, 068102. [Google Scholar] [CrossRef]
- Kumar, S.; Rai, A.K.; Singh, V.; Rai, S. Vibrational spectrum of glycine molecule. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 2741–2746. [Google Scholar] [CrossRef]
- Carnimeo, I.; Biczysko, M.; Bloino, J.; Barone, V. Reliable structural, thermodynamic, and spectroscopic properties of organic molecules adsorbed on silicon surfaces from computational modeling: The case of glycine@Si (100). Phys. Chem. Chem. Phys. 2011, 13, 16713–16727. [Google Scholar] [CrossRef]
- Whitlow, J.; Pacelli, S.; Paul, A. Multifunctional nanodiamonds in regenerative medicine: Recent advances and future directions. J. Control. Release 2017, 261, 62–86. [Google Scholar] [CrossRef]
- Yang, N. Novel Aspects of Diamond. Topics in Applied Physics; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Alfonso, D.R.; Drabold, D.A.; Ulloa, S.E. Structural, electronic, and vibrational properties of diamond (100),(111), and (110) surfaces from ab initio calculations. Phys. Rev. B 1995, 51, 14669. [Google Scholar] [CrossRef]
- Petrini, D.; Larsson, K. Origin of the reactivity on the nonterminated (100), (110), and (111) diamond surfaces: An electronic structure DFT study. J. Phys. Chem. C 2008, 112, 14367–14376. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Berendsen, H.J.; Postma, J.V.; Van Gunsteren, W.F.; DiNola, A.R.H.J.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Ge, S.P.; Zhao, X.Y.; Gai, Z.; Zhao, R.G.; Yang, W.S. Adsorption geometry of glycine on Cu (001) determined with low-energy electron diffraction and scanning tunnelling microscopy. Chin. Phys. 2002, 11, 839. [Google Scholar]
- Jensen, J.H. Glycine and the Hydrogen Bond: Toward a Model for Solvation; Iowa State University: Ames, IA, USA, 1995. [Google Scholar]
- Marquetti, I.; Desai, S. Molecular modeling the adsorption behavior of bone morphogenetic protein-2 on hydrophobic and hydrophilic substrates. Chem. Phys. Lett. 2018, 706, 285–294. [Google Scholar] [CrossRef]
- Porezag, D.; Pederson, M.R. Infrared intensities and Raman-scattering activities within density-functional theory. Phys. Rev. B 1996, 54, 7830. [Google Scholar] [CrossRef]
- Ganji, M.D.; Bakhshandeh, A. Functionalized single-walled carbon nanotubes interacting with glycine amino acid: DFT study. Phys. B Condens. Matter. 2011, 406, 4453–4459. [Google Scholar] [CrossRef]
- Pantaleone, S.; Rimola, A.; Sodupe, M. Canonical, deprotonated, or zwitterionic? II. A computational study on amino acid interaction with the TiO2 (110) rutile surface: Comparison with the anatase (101) surface. Phys. Chem. Chem. Phys. 2020, 22, 16862–16876. [Google Scholar] [CrossRef]
- Goss, J.; Wilson, T.; Morgenstern, A.; Eberhart, M. Charge density analysis attending bond torsion: A bond bundle case study. Int. J. Quantum Chem. 2018, 118, 25783. [Google Scholar] [CrossRef]
- Mishra, A.K.; Murli, C.; Verma, A.K.; Song, Y.; Kumar, M.R.S.; Sharma, S.M. Conformation and hydrogen-bond-assisted polymerization in glycine lithium sulfate at high pressures. J. Phys. Chem. A 2013, 117, 5734–5741. [Google Scholar] [CrossRef] [PubMed]
- Kadlečíková, M.; Breza, J.; Veselý, M.; Frgala, Z.; Kudrle, V.; Janča, J.; Janík, J.; Buršík, J. Raman bands in microwave plasma assisted chemical vapour deposited films. Microelectron. J. 2003, 34, 1075–1077. [Google Scholar] [CrossRef]
- Prawer, S.; Nugent, K.; Jamieson, D.; Orwa, J.; Bursill, L.; Peng, J. The Raman spectrum of nanocrystalline diamond. Chem. Phys. Lett. 2000, 332, 93–97. [Google Scholar] [CrossRef]
- Prawer, S.; Nemanich, R.J. Raman spectroscopy of diamond and doped diamond. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2004, 362, 2537–2565. [Google Scholar] [CrossRef]
- Parameswari, A.; Premkumar, S.; Premkumar, R.; Benial, A.M.F. Surface enhanced Raman spectroscopy and quantum chemical studies on glycine single crystal. J. Mol. Struct. 2016, 1116, 180–187. [Google Scholar] [CrossRef]
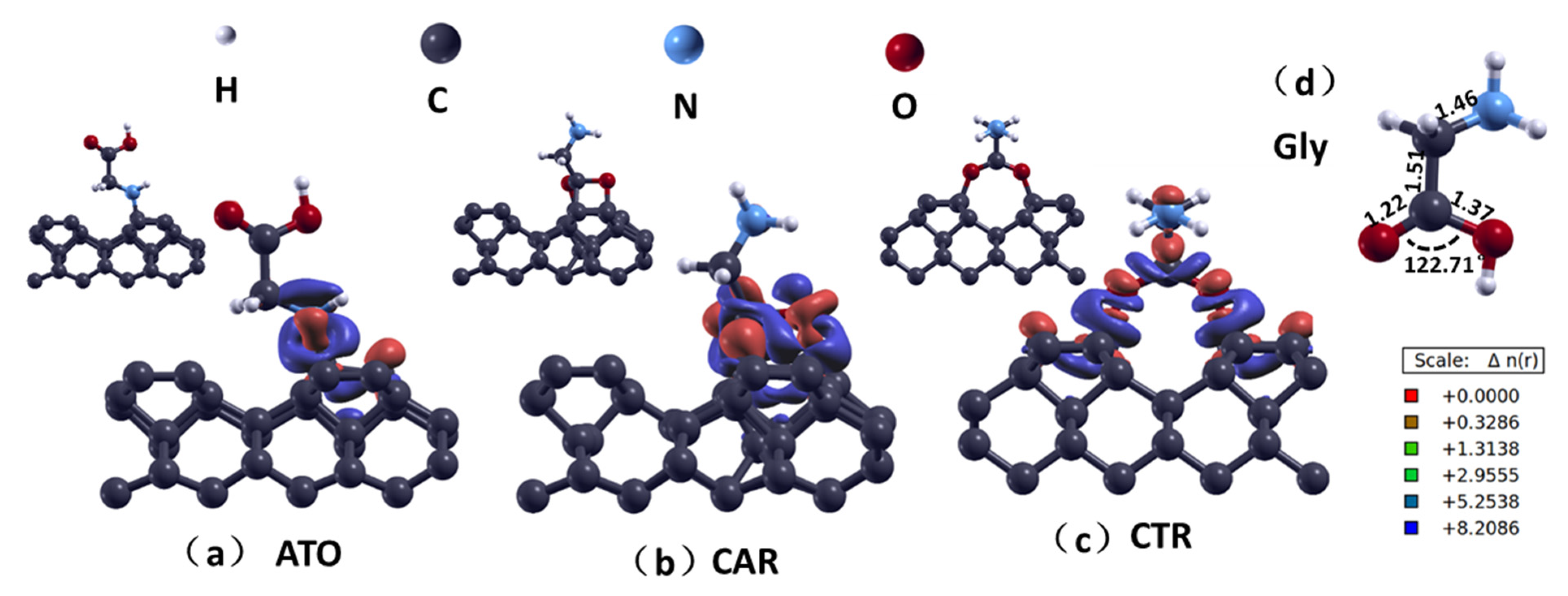
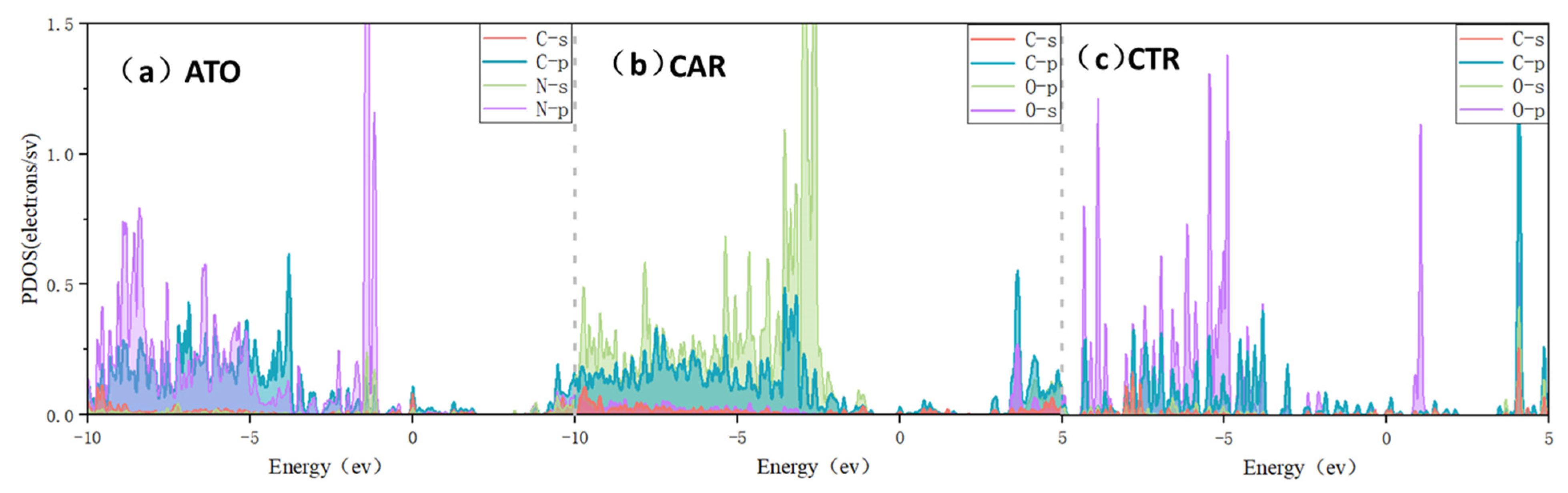
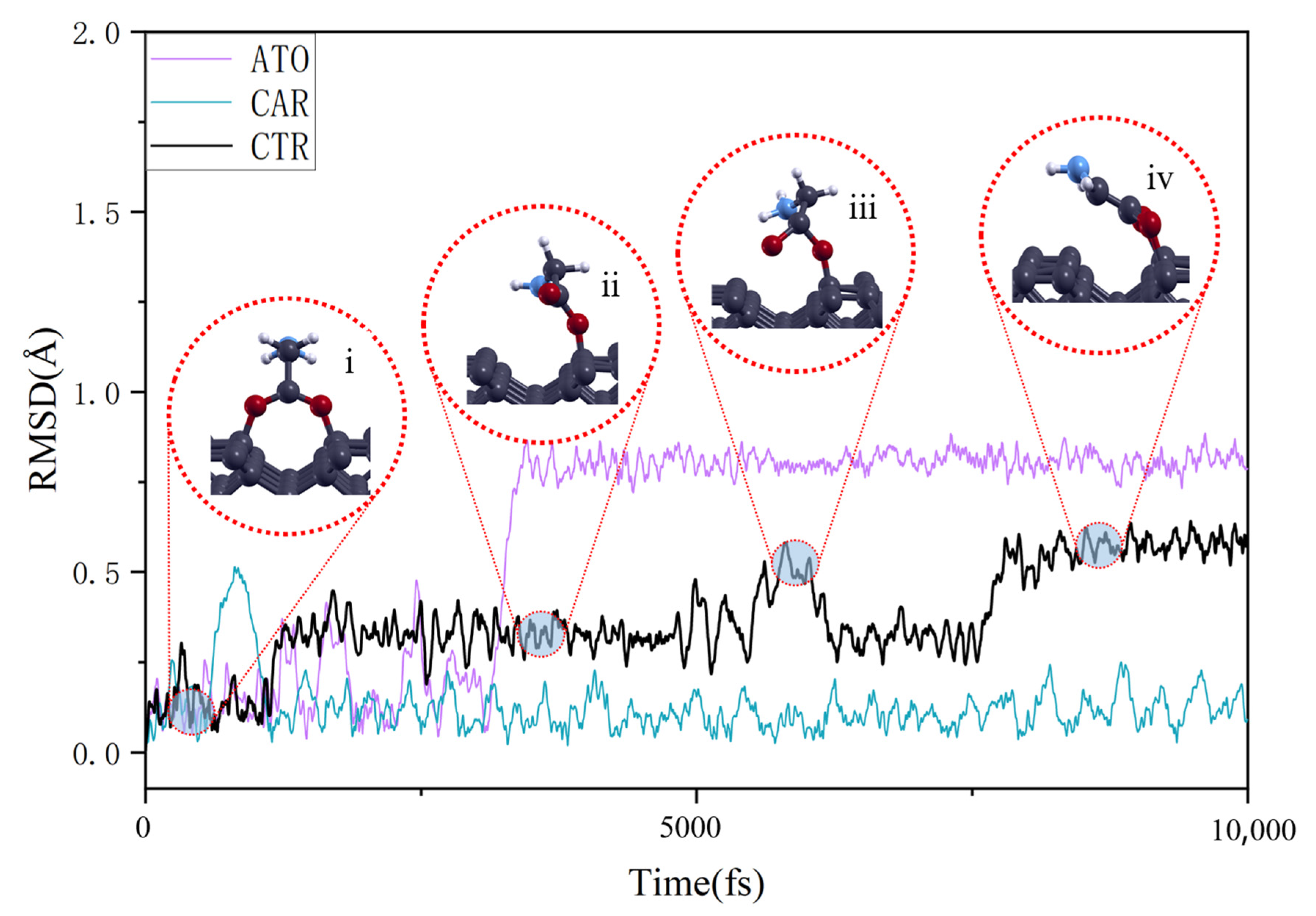
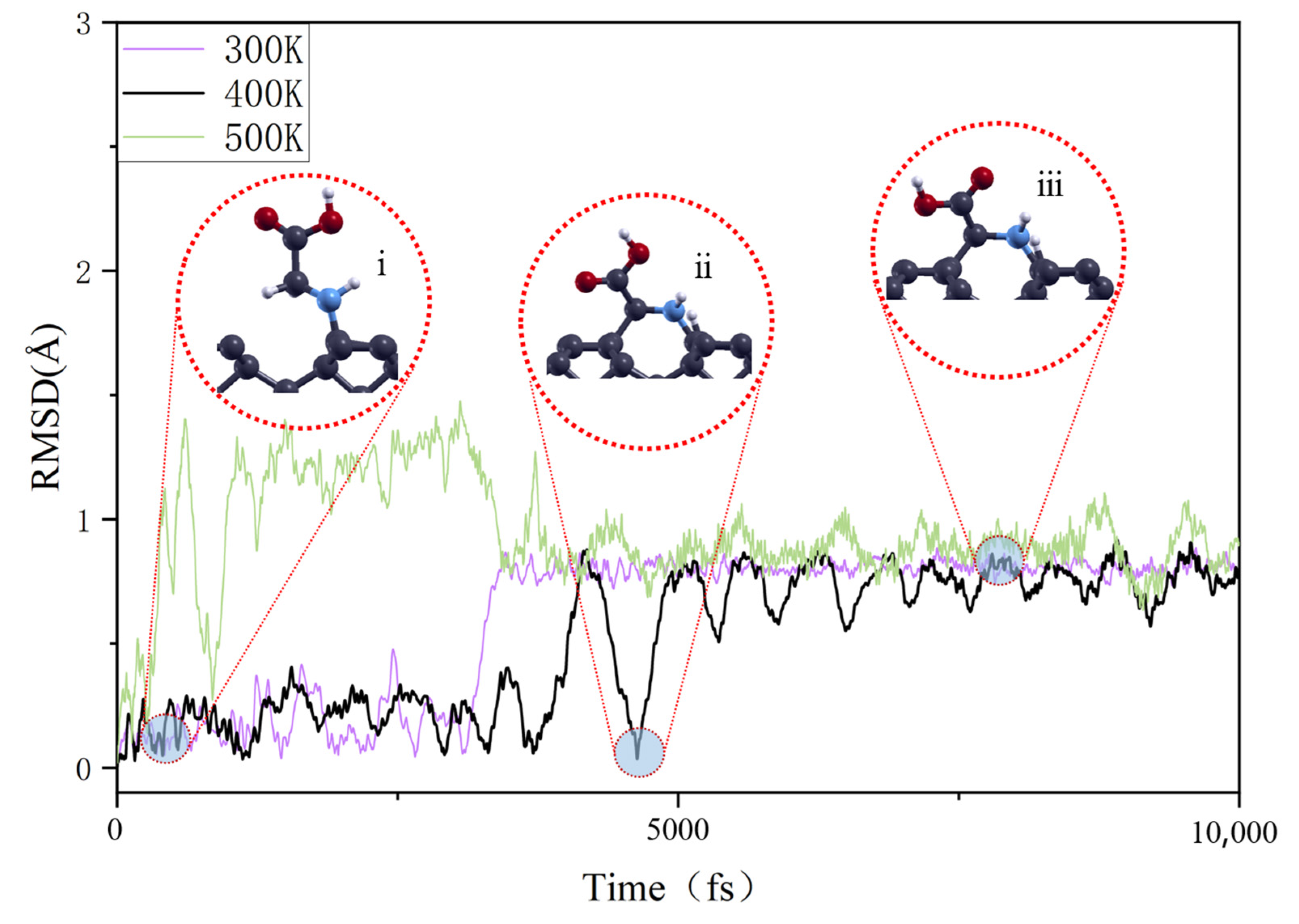
| Δdmax (Å) | Δθmax (°) | Ead (eV) | lavg (Å) | ect (e) | |
|---|---|---|---|---|---|
| ATO | 0.03 | 3.77 | 3.93 | 1.42 | 0.71 |
| CAR | 0.22 | 10.2 | 5.03 | 1.37 | 0.91 |
| CTR | 0.08 | 9.82 | 3.44 | 1.42 | 0.33 |
| Wavenumber | Intensity | Vibration Mode | ||
|---|---|---|---|---|
| Glycine | Glycine–Diamond | Glycine | Glycine–Diamond | |
| 251 | 210 | 1.51 × 10−4 | 1.2 × 10−3 | C–O bridge connection symmetric stretching |
| 411 | 368 | 1.76 × 10−4 | 2.8 × 10−3 | C–N scissor bending |
| 532 | 478 | 2.5 × 10−4 | 1.5 × 10−2 | C–O scissor bending |
| 698 | 619 | 3.2 × 10−4 | 2.1 × 10−2 | C–O scissor bending |
| 760 | 734 | 9.08 × 10−4 | 1.4 × 10−2 | C–O scissor bending |
| 815 | 806 | 8.85 × 10−4 | 0.02 | N–H out of plane wagging |
| 961 | 935 | 1.94 × 10−4 | 0.05 | C–C symmetric stretching |
| 1038 | 1023 | 7.33 × 10−4 | 8.5 × 10−3 | C–N symmetric stretching |
| 1072 | 1069 | 4.9 × 10−4 | 8 × 10−3 | C–C–N asymmetric stretching |
| 1181 | 1107 | 4.73 × 10−4 | 1.3 × 10−2 | C–H out of plane twisting |
| 1306 | 1365 | 4.64 × 10−4 | 7.4 × 10−3 | C–C scissor bending |
| 1439 | 1398 | 9.41 × 10−4 | 7.5 × 10−3 | C–H scissor bending |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Zhang, C.; An, P.; Xu, P.; Zhang, W.; Ren, Y.; Tan, X.; Yu, J. First-Principles Calculations for Glycine Adsorption Dynamics and Surface-Enhanced Raman Spectroscopy on Diamond Surfaces. Nanomaterials 2025, 15, 502. https://doi.org/10.3390/nano15070502
Sun S, Zhang C, An P, Xu P, Zhang W, Ren Y, Tan X, Yu J. First-Principles Calculations for Glycine Adsorption Dynamics and Surface-Enhanced Raman Spectroscopy on Diamond Surfaces. Nanomaterials. 2025; 15(7):502. https://doi.org/10.3390/nano15070502
Chicago/Turabian StyleSun, Shiyang, Chi Zhang, Peilun An, Pingping Xu, Wenxing Zhang, Yuan Ren, Xin Tan, and Jinlong Yu. 2025. "First-Principles Calculations for Glycine Adsorption Dynamics and Surface-Enhanced Raman Spectroscopy on Diamond Surfaces" Nanomaterials 15, no. 7: 502. https://doi.org/10.3390/nano15070502
APA StyleSun, S., Zhang, C., An, P., Xu, P., Zhang, W., Ren, Y., Tan, X., & Yu, J. (2025). First-Principles Calculations for Glycine Adsorption Dynamics and Surface-Enhanced Raman Spectroscopy on Diamond Surfaces. Nanomaterials, 15(7), 502. https://doi.org/10.3390/nano15070502





