Synthesis of ZnO Nanoparticles by Bacillus subtilis for Efficient Photocatalytic Degradation of Cyanide
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
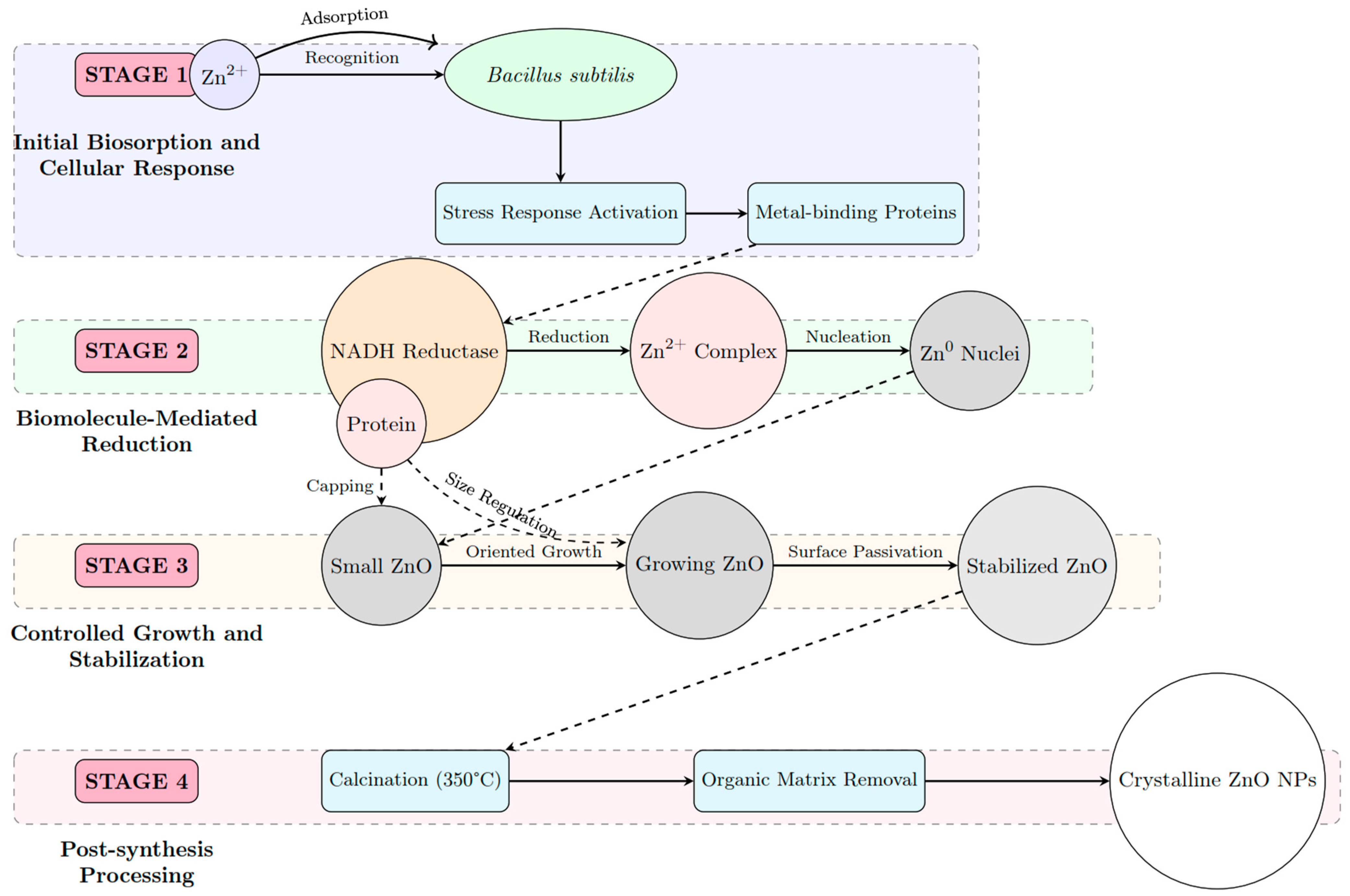
2.2. Biogenic Synthesis of ZnO Nanoparticles
2.3. Characterization of Biosynthesized ZnO Nanoparticles
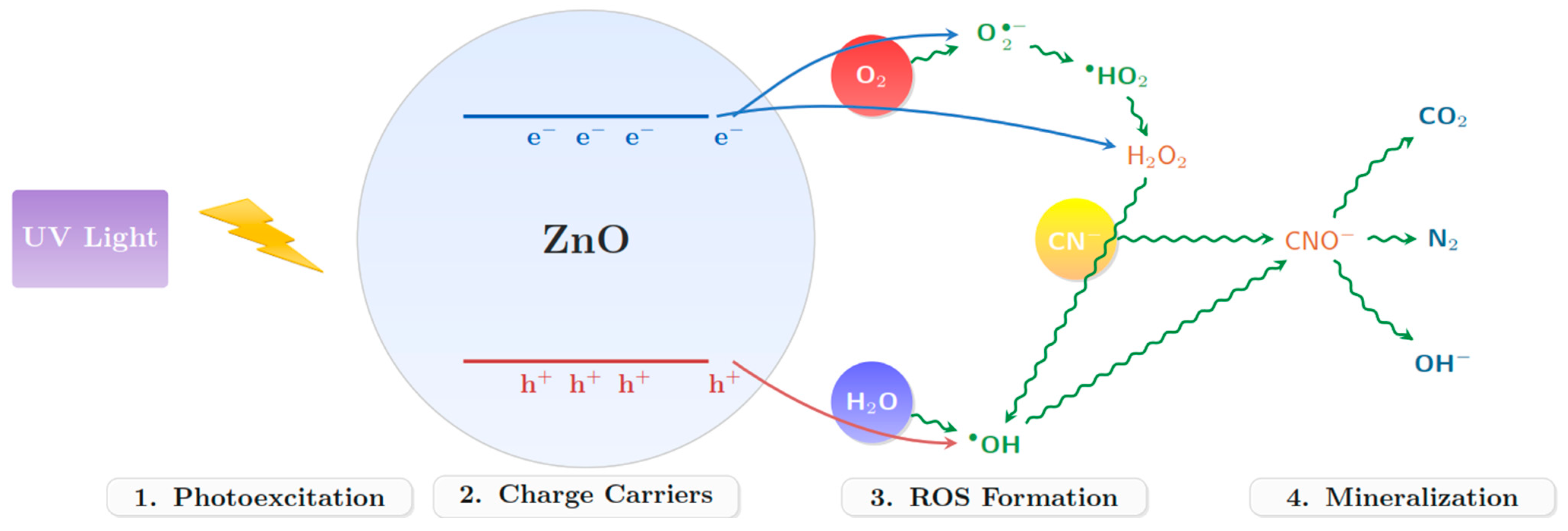
2.4. Photocatalytic Assessment of ZnO for Cyanide Degradation
3. Results
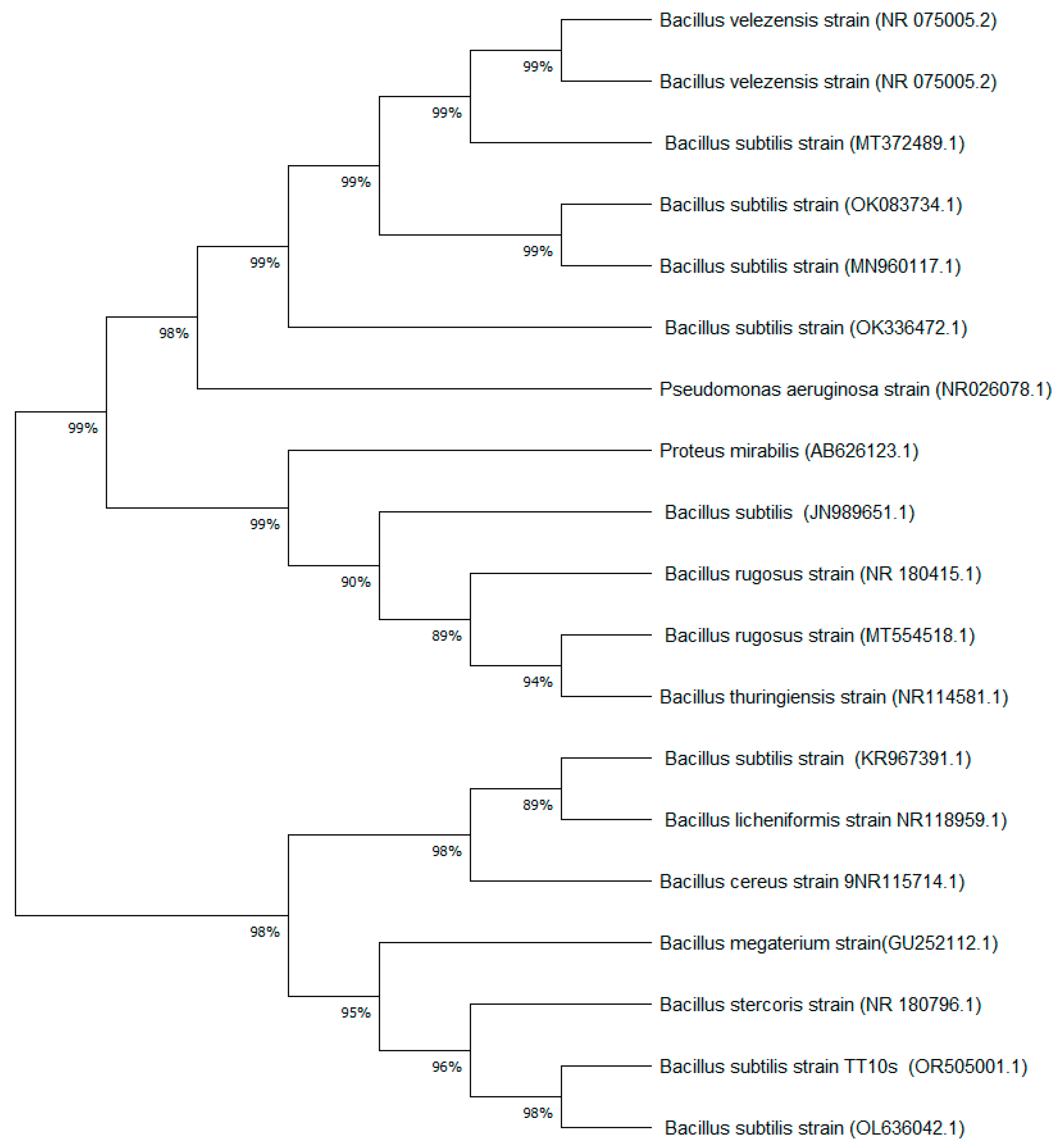
3.1. Phylogenetic Analysis of the Bacterial Strain
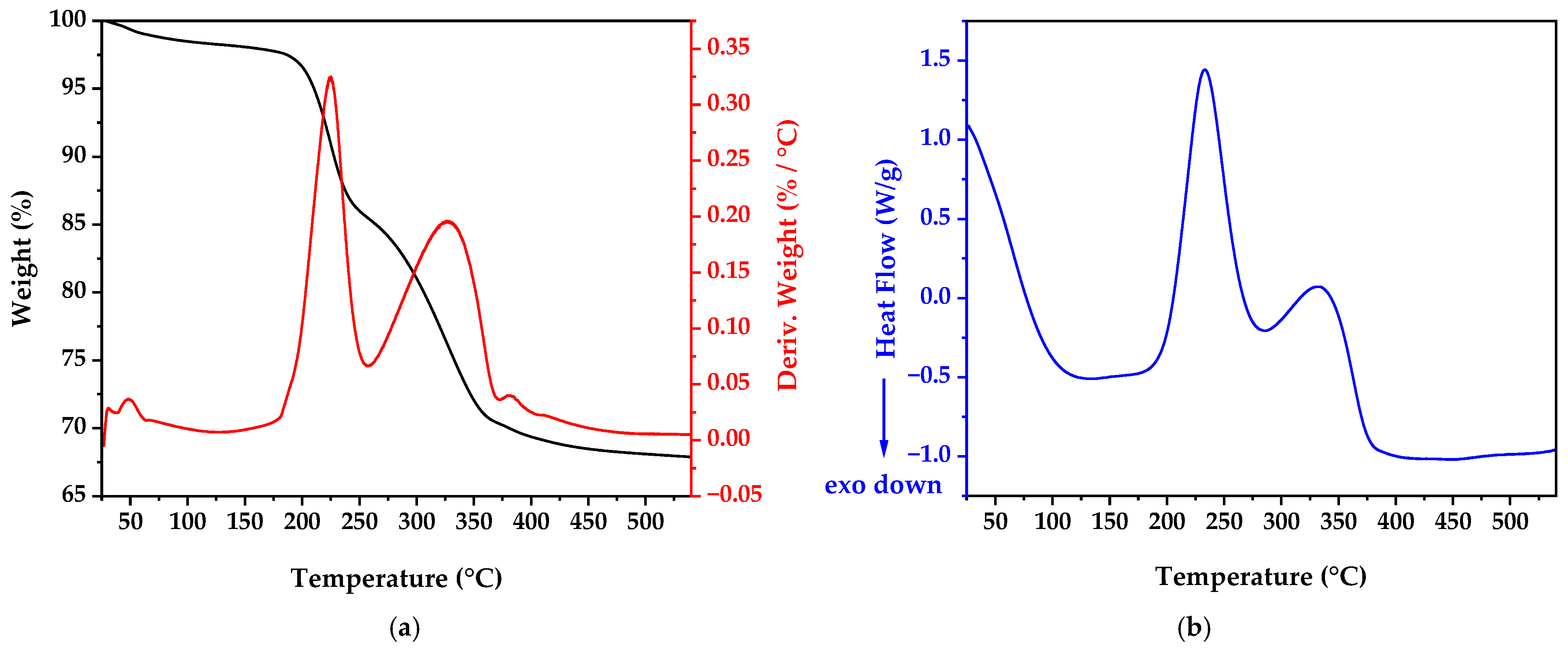
3.2. Thermal Analysis Results
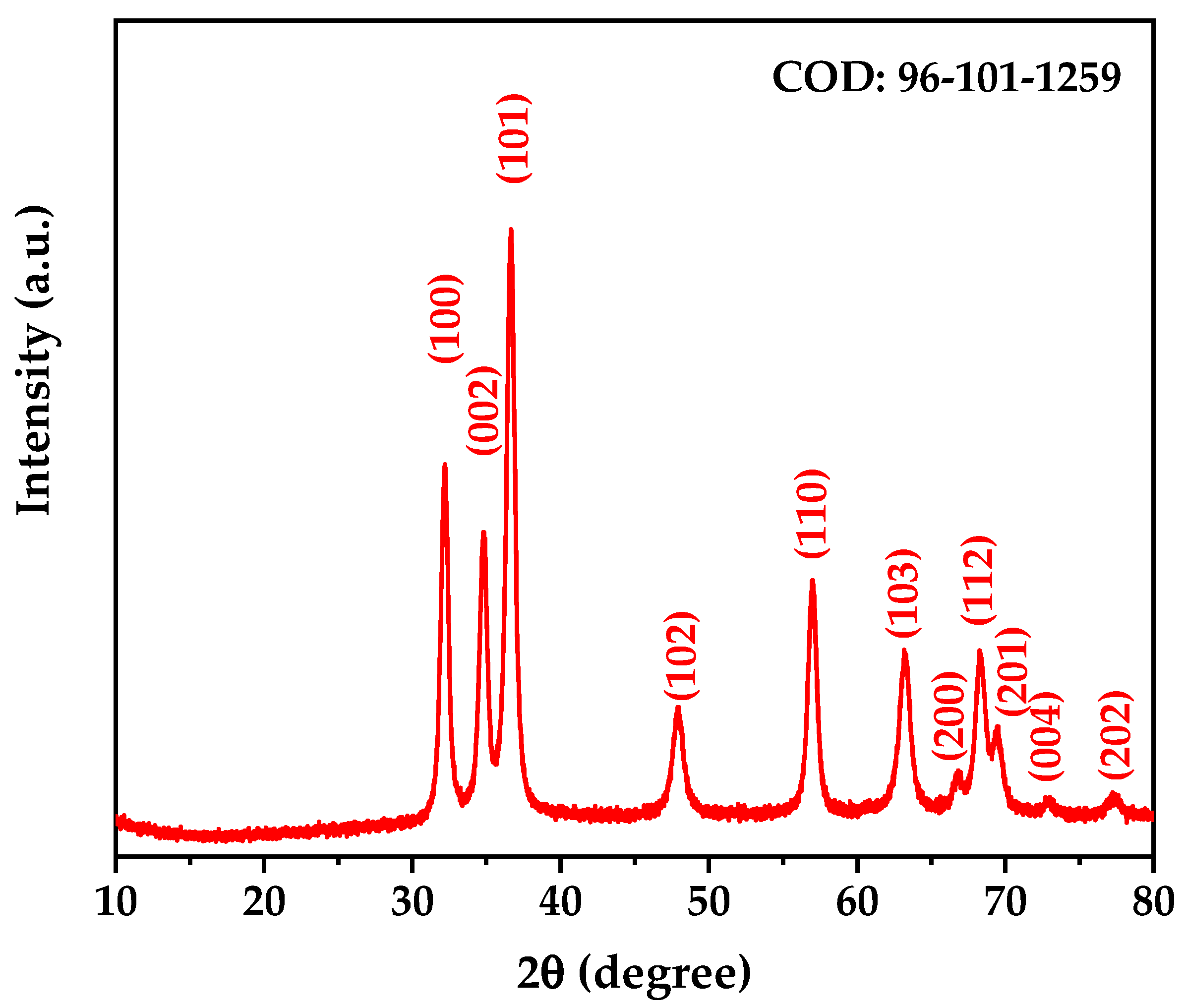
3.3. X-Ray Diffraction (XRD) Analysis After Calcination
3.4. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
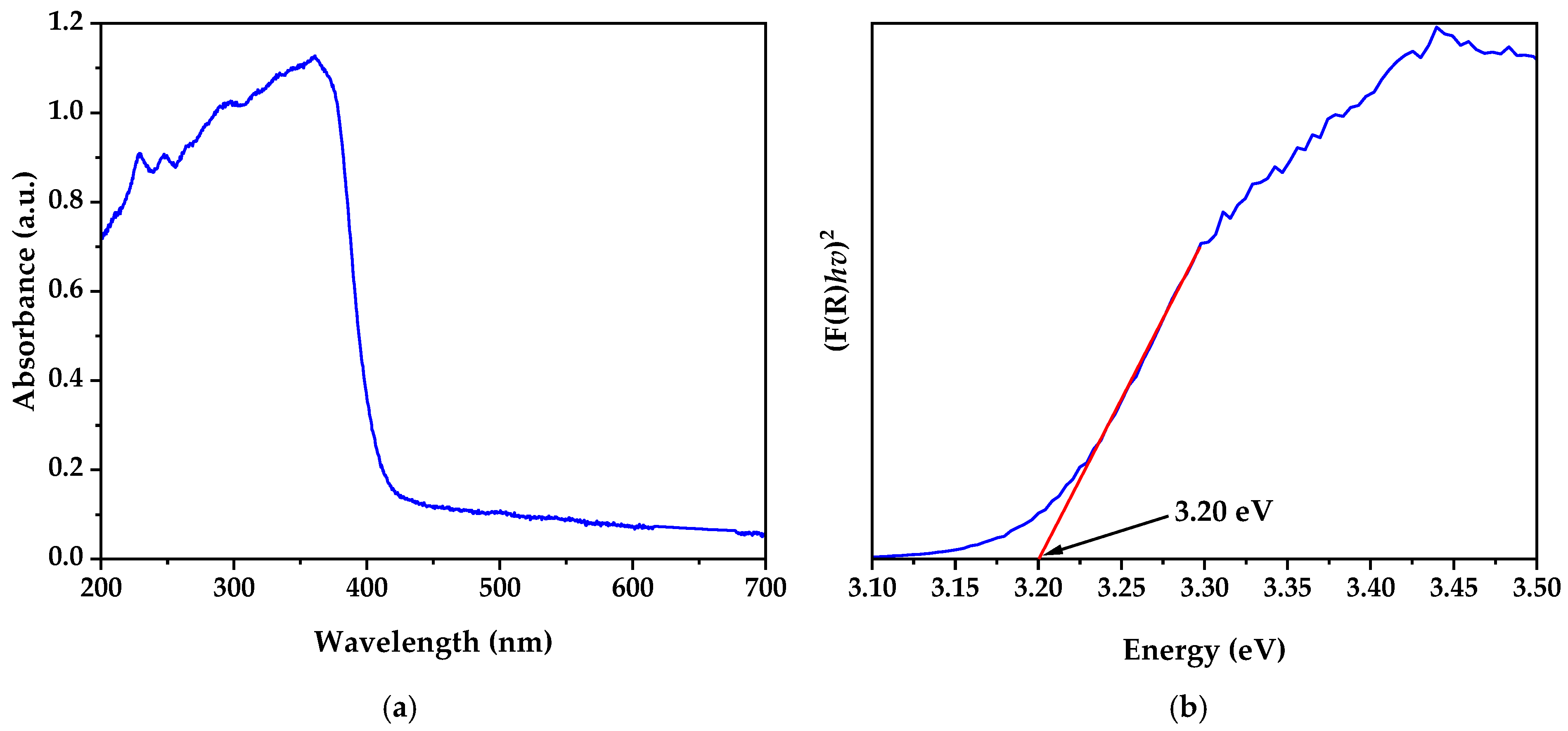
3.5. UV–Visible Spectroscopy
3.6. BET Surface Area Analysis
3.7. Scanning Electron Microscopy (SEM) Results
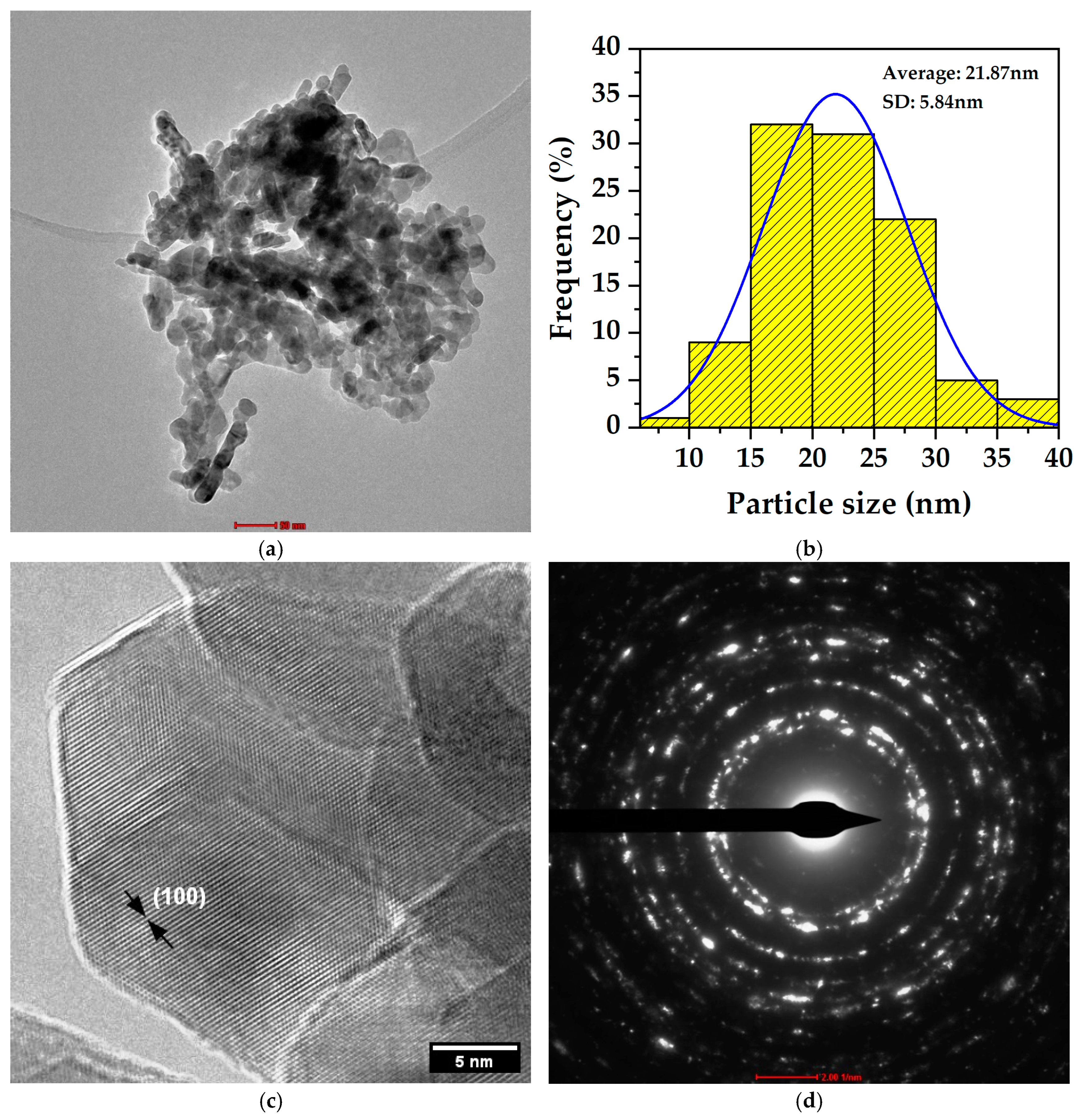
3.8. Transmission Electron Microscopy (TEM) Results
3.9. Photoluminescence Analysis
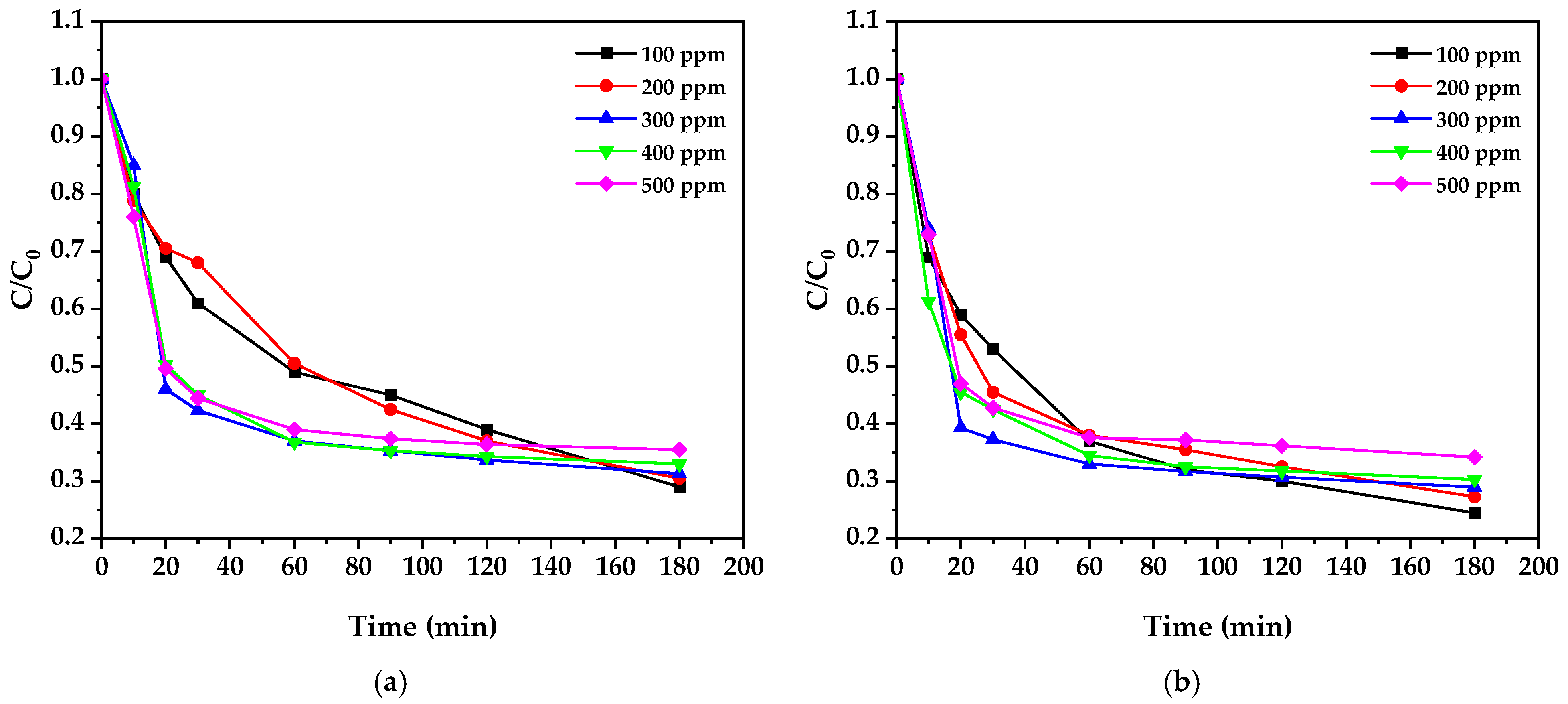
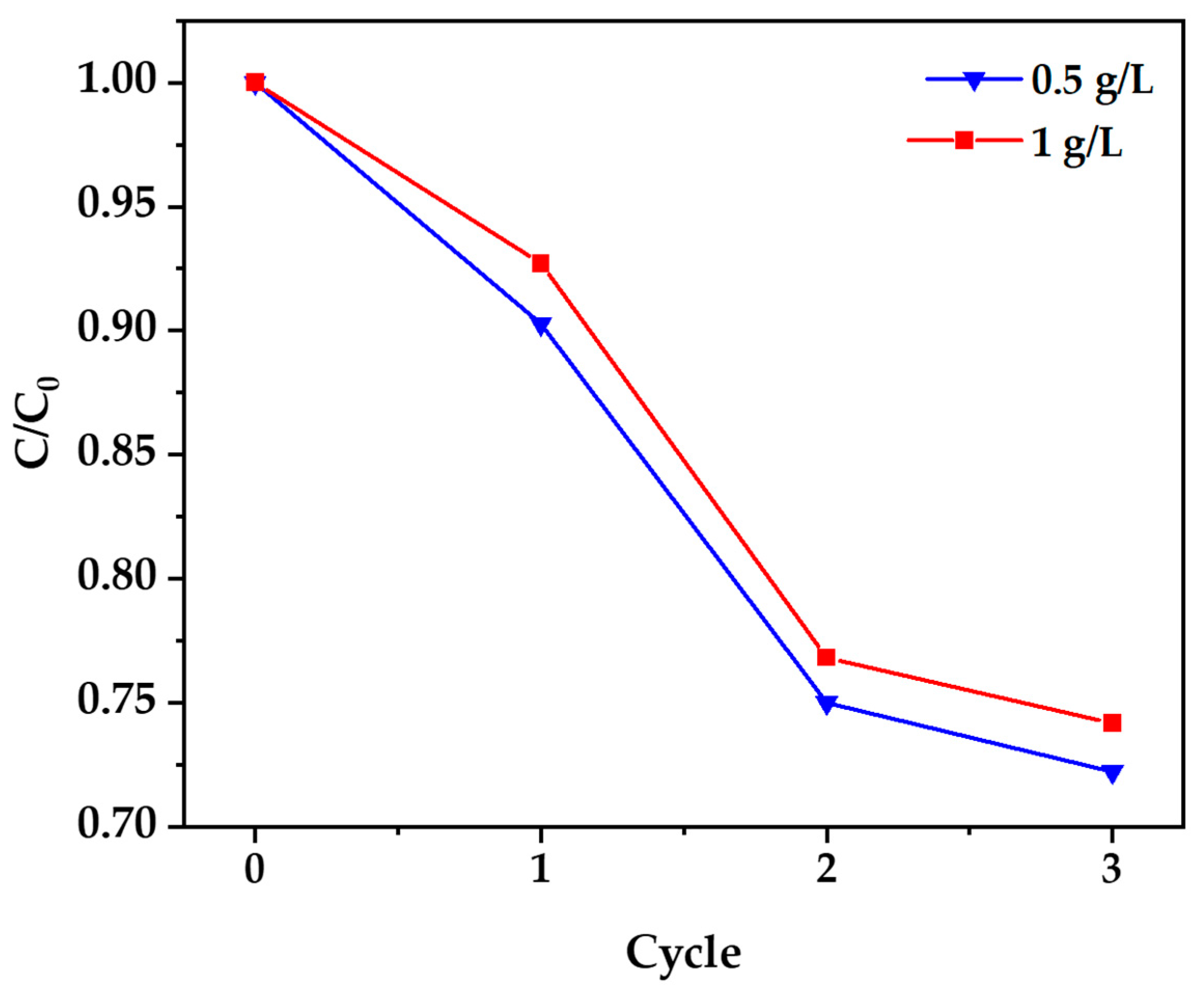
3.10. Results of Cyanide Photocatalytic Degradation
| Initial Concentration | ZnO Loading | k (min−1) | R2 | ZnO Loading | k (min−1) | R2 |
|---|---|---|---|---|---|---|
| 100 ppm | 0.5 g/L | 5.87 × 10−3 | 0.973 | 1.0 g/L | 6.64 × 10−3 | 0.979 |
| 200 ppm | 0.5 g/L | 6.23 × 10−3 | 0.968 | 1.0 g/L | 5.58 × 10−3 | 0.985 |
| 300 ppm | 0.5 g/L | 4.84 × 10−3 | 0.989 | 1.0 g/L | 4.68 × 10−3 | 0.992 |
| 400 ppm | 0.5 g/L | 4.68 × 10−3 | 0.991 | 1.0 g/L | 4.55 × 10−3 | 0.992 |
| 500 ppm | 0.5 g/L | 4.06 × 10−3 | 0.991 | 1.0 g/L | 3.98 × 10−3 | 0.992 |
| Synthesis Method | Catalyst | Bandgap (eV) | Evaluation Conditions | Degradation Efficiency (%) | Time (min) | Ref. |
|---|---|---|---|---|---|---|
| Sol-gel | ZnO sensitized with copper phthalocyanine (CuPc, 0.5% wt) | 3.2 | 30 mg/L KCN, visible light, pH 11, 0.6 g/L catalyst | 95 | 360 | [57] |
| Sol-gel | Bare ZnO | 3.2 | 10 mg/L CN−, simulated solar radiation, pH 11, 1.4 g/L catalyst | 75 | 120 | [58] |
| Room-temperature wet chemical | ZnO (prepared in water) | 3.16 | 100 mg/L KCN, UV light (365 nm), pH 8.5, 0.02 wt% catalyst | 56 | 140 | [18] |
| Precipitation | ZnO-BiOI heterojunction | 100 mg/L CN−, simulated solar light, pH 12, 15 mg catalyst in 100 mL | 97 | 35 | [53] | |
| Biogenic | ZnO | 3.2 | 100 mg/L CN−, 254nm UV light 11W, pH 10, 500 mg catalyst in 1 L | 62.4 | 180 | This work |
| Biogenic | ZnO | 3.2 | 100 mg/L CN−, 254nm UV light 11W, pH 10, 1 g catalyst in 1 L | 75.5 | 180 | This work |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suman, S.G. Challenges in Targeting Cyanide Poisoning. In Targeted Metallo-Drugs; Farkas, E., Marmion, C.J., Eds.; CRC Press: Boca Raton, FL, USA, 2023; pp. 215–238. ISBN 9781003272250. [Google Scholar]
- Halim, M.A.; Naidu, R. Cyanide contamination of soil and water: Sources, toxicity, and potential remediation strategies. In Inorganic Contaminants and Radionuclides; Elsevier: Amsterdam, The Netherlands, 2024; pp. 375–403. ISBN 9780323904001. [Google Scholar]
- Vidal-Tovar, C.; Correa-Turizo, R.; Severiche-Sierra, C.; Cabrera-Lafaurie, W. Degradation of Cyanides in Wastewater from Gold Mining: A Review of Literature. J. Eng. Appl. Sci. 2019, 14, 1475–1485. [Google Scholar] [CrossRef][Green Version]
- Alvillo-Rivera, A.; Garrido-Hoyos, S.; Buitrón, G.; Thangarasu-Sarasvathi, P.; Rosano-Ortega, G. Biological treatment for the degradation of cyanide: A review. J. Mater. Res. Technol. 2021, 12, 1418–1433. [Google Scholar] [CrossRef]
- Pérez-Cid, B.; Calvar, S.; Moldes, A.B.; Manuel Cruz, J. Effective Removal of Cyanide and Heavy Metals from an Industrial Electroplating Stream Using Calcium Alginate Hydrogels. Molecules 2020, 25, 5183. [Google Scholar] [CrossRef]
- Kuyucak, N.; Akcil, A. Cyanide and removal options from effluents in gold mining and metallurgical processes. Miner. Eng. 2013, 50, 13–29. [Google Scholar] [CrossRef]
- Parga, J.R.; Cocke, D.L. Oxidation of cyanide in a hydrocyclone reactor by chlorine dioxide. Desalination 2001, 140, 289–296. [Google Scholar] [CrossRef]
- Oleson, J.L.; Lin, H.K.; Walsh, D.E. Modeling of SO2/air cyanide destruction process. Min. Metall. Explor. 2005, 22, 199–204. [Google Scholar] [CrossRef]
- Cáceda Quiroz, C.J.; Fora Quispe, G.d.L.; Carpio Mamani, M.; Maraza Choque, G.J.; Sacari Sacari, E.J. Cyanide Bioremediation by Bacillus subtilis under Alkaline Conditions. Water 2023, 15, 3645. [Google Scholar] [CrossRef]
- Zhang, M.; Cao, Y.; Peng, B.; Tian, Y.; Barvor, J.B. Removal of copper cyanide by precipitate flotation with ammonium salts. Process Saf. Environ. Prot. 2020, 133, 82–87. [Google Scholar] [CrossRef]
- Pérez-Patiño, M.Y.; Barrera-Andrade, J.M.; Rojas-García, E.; Calzada, L.A.; Sierra-Uribe, J.H.; Falcony, C.; Valenzuela, M.A.; Albiter, E. Enhanced photocatalytic oxidation of free cyanide using hydrogen-treated TiO2: Effect of reduction temperature. Mater. Res. Express 2023, 10, 115507. [Google Scholar] [CrossRef]
- Khan, S.H.; Yadav, V.K. Advanced Oxidation Processes for Wastewater Remediation: An Overview. In Removal of Emerging Contaminants Through Microbial Processes; Shah, M.P., Ed.; Springer: Singapore, 2021; pp. 71–93. ISBN 978-981-15-5900-6. [Google Scholar]
- Sharma, D.K.; Shukla, S.; Sharma, K.K.; Kumar, V. A review on ZnO: Fundamental properties and applications. Mater. Today Proc. 2022, 49, 3028–3035. [Google Scholar] [CrossRef]
- Ramos, P.G.; Sánchez, L.A.; Rodriguez, J.M. A review on improving the efficiency of photocatalytic water decontamination using ZnO nanorods. J. Sol-Gel Sci. Technol. 2022, 102, 105–124. [Google Scholar] [CrossRef]
- Abou Zeid, S.; Leprince-Wang, Y. Advancements in ZnO-Based Photocatalysts for Water Treatment: A Comprehensive Review. Crystals 2024, 14, 611. [Google Scholar] [CrossRef]
- Saadi, H.; Khaldi, O.; Pina, J.; Costa, T.; Seixas de Melo, J.S.; Vilarinho, P.; Benzarti, Z. Effect of Co Doping on the Physical Properties and Organic Pollutant Photodegradation Efficiency of ZnO Nanoparticles for Environmental Applications. Nanomaterials 2024, 14, 122. [Google Scholar] [CrossRef] [PubMed]
- Motelica, L.; Vasile, B.-S.; Ficai, A.; Surdu, A.-V.; Ficai, D.; Oprea, O.-C.; Andronescu, E.; Jinga, D.C.; Holban, A.M. Influence of the Alcohols on the ZnO Synthesis and Its Properties: The Photocatalytic and Antimicrobial Activities. Pharmaceutics 2022, 14, 2842. [Google Scholar] [CrossRef]
- Bagabas, A.; Alshammari, A.; Aboud, M.F.; Kosslick, H. Room-temperature synthesis of zinc oxide nanoparticles in different media and their application in cyanide photodegradation. Nanoscale Res. Lett. 2013, 8, 516. [Google Scholar] [CrossRef]
- Razanamahandry, L.C.; Sackey, J.; Furqan, C.M.; Ntwampe, S.; Fosso-Kankeu, E.; Manikandan, E.; Maaza, M. Removal of Free Cyanide by a Green Photocatalyst ZnO Nanoparticle Synthesized via Eucalyptus Globulus Leaves. In Photocatalysts in Advanced Oxidation Processes for Wastewater Treatment; Fosso-Kankeu, E., Pandey, S., Ray, S.S., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 271–288. ISBN 9781119631392. [Google Scholar]
- Xu, J.; Huang, Y.; Zhu, S.; Abbes, N.; Jing, X.; Zhang, L. A review of the green synthesis of ZnO nanoparticles using plant extracts and their prospects for application in antibacterial textiles. J. Eng. Fibers Fabr. 2021, 16, 155892502110462. [Google Scholar] [CrossRef]
- Bandeira, M.; Giovanela, M.; Roesch-Ely, M.; Devine, D.M.; Da Silva Crespo, J. Green synthesis of zinc oxide nanoparticles: A review of the synthesis methodology and mechanism of formation. Sustain. Chem. Pharm. 2020, 15, 100223. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Karishma, S.; Vo, D.-V.N.; Jeevanantham, S.; Yaashikaa, P.R.; George, C.S. A review on biosynthesis of metal nanoparticles and its environmental applications. Chemosphere 2021, 264, 128580. [Google Scholar] [CrossRef]
- Hamk, M.; Akçay, F.A.; Avcı, A. Green synthesis of zinc oxide nanoparticles using Bacillus subtilis ZBP4 and their antibacterial potential against foodborne pathogens. Prep. Biochem. Biotechnol. 2023, 53, 255–264. [Google Scholar] [CrossRef]
- Vosoughian, N.; Asadbeygi, M.; Mohammadi, A.; Soudi, M.R. Green synthesis of zinc oxide nanoparticles using novel bacterium strain (Bacillus subtilis NH1-8) and their in vitro antibacterial and antibiofilm activities against Salmonellatyphimurium. Microb. Pathog. 2023, 185, 106457. [Google Scholar] [CrossRef]
- Tripathi, R.M.; Bhadwal, A.S.; Gupta, R.K.; Singh, P.; Shrivastav, A.; Shrivastav, B.R. ZnO nanoflowers: Novel biogenic synthesis and enhanced photocatalytic activity. J. Photochem. Photobiol. B Biol. 2014, 141, 288–295. [Google Scholar] [CrossRef]
- Mohd Yusof, H.; Mohamad, R.; Zaidan, U.H.; Abdul Rahman, N.A. Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: A review. J. Anim. Sci. Biotechnol. 2019, 10, 57. [Google Scholar] [CrossRef]
- Sabir, S.; Zahoor, M.A.; Waseem, M.; Siddique, M.H.; Shafique, M.; Imran, M.; Hayat, S.; Malik, I.R.; Muzammil, S. Biosynthesis of ZnO Nanoparticles Using Bacillus Subtilis: Characterization and Nutritive Significance for Promoting Plant Growth in Zea mays L. Dose Response 2020, 18, 1559325820958911. [Google Scholar] [CrossRef] [PubMed]
- Dhaka, A.; Chand Mali, S.; Sharma, S.; Trivedi, R. A review on biological synthesis of silver nanoparticles and their potential applications. Results Chem. 2023, 6, 101108. [Google Scholar] [CrossRef]
- Okaiyeto, K.; Gigliobianco, M.R.; Di Martino, P. Biogenic Zinc Oxide Nanoparticles as a Promising Antibacterial Agent: Synthesis and Characterization. Int. J. Mol. Sci. 2024, 25, 9500. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; van Nguyen, A. Synthesis, Characterization, and Photocatalytic Activity of ZnO Nanomaterials Prepared by a Green, Nonchemical Route. J. Nanomater. 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Rajabairavi, N.; Raju, C.S.; Karthikeyan, C.; Varutharaju, K.; Nethaji, S.; Hameed, A.S.H.; Shajahan, A. Biosynthesis of Novel Zinc Oxide Nanoparticles (ZnO NPs) Using Endophytic Bacteria Sphingobacterium thalpophilum. In Recent Trends in Materials Science and Applications; Ebenezar, J., Ed.; Springer: Cham, Switzerland, 2017; pp. 245–254. ISBN 978-3-319-44889-3. [Google Scholar]
- Hisana; Shahzaib, A.; Nishat, N.; Alshehri, S.M.; Ahamad, T.; Haque, Z. Biogenically fabricated Tulsi-infused ZnO–CuO nanocomposites for enhanced dye reduction and adsorption. Hybrid Adv. 2024, 5, 100145. [Google Scholar] [CrossRef]
- Falamas, A.; Marica, I.; Popa, A.; Toloman, D.; Pruneanu, S.; Pogacean, F.; Nekvapil, F.; Silipas, T.D.; Stefan, M. Size-dependent spectroscopic insight into the steady-state and time-resolved optical properties of ZnO photocatalysts. Mater. Sci. Semicond. Process. 2022, 145, 106644. [Google Scholar] [CrossRef]
- Dodd, A.C.; McKinley, A.J.; Saunders, M.; Tsuzuki, T. Effect of Particle Size on the Photocatalytic Activity of Nanoparticulate Zinc Oxide. J. Nanopart Res. 2006, 8, 43–51. [Google Scholar] [CrossRef]
- Lakshmi, N.; Anandakumar, S.; Sampathkumar, V.; Manoj, S. Synthesized ZnO nanoparticles using Sorghum Panicle for the photocatalytic degradation of pharmaceutical waste water. Desalination Water Treat. 2024, 319, 100557. [Google Scholar] [CrossRef]
- Li, G.R.; Hu, T.; Pan, G.L.; Yan, T.Y.; Gao, X.P.; Zhu, H.Y. Morphology−Function Relationship of ZnO: Polar Planes, Oxygen Vacancies, and Activity. J. Phys. Chem. C 2008, 112, 11859–11864. [Google Scholar] [CrossRef]
- Yusan, S.; Bampaiti, A.; Aytas, S.; Erenturk, S.; Aslani, M.A. Synthesis and structural properties of ZnO and diatomite-supported ZnO nanostructures. Ceram. Int. 2016, 42, 2158–2163. [Google Scholar] [CrossRef]
- Jeyachitra, R.; Senthilnathan, V.; Senthil, T.S. Studies on electrical behavior of Fe doped ZnO nanoparticles prepared via co-precipitation approach for photo-catalytic application. J. Mater. Sci. Mater. Electron. 2018, 29, 1189–1197. [Google Scholar] [CrossRef]
- Da Silva-Neto, M.L.; de Oliveira, M.C.A.; Dominguez, C.T.; Lins, R.E.M.; Rakov, N.; de Araújo, C.B.; Menezes, L.d.S.; de Oliveira, H.P.; Gomes, A.S.L. UV random laser emission from flexible ZnO-Ag-enriched electrospun cellulose acetate fiber matrix. Sci. Rep. 2019, 9, 11765. [Google Scholar] [CrossRef]
- Medina Salas, J.P.; Gamarra Gómez, F.; Sacari Sacari, E.J.; Lanchipa Ramos, W.O.; Tamayo Calderón, R.M.; Mamani Flores, E.; Yapuchura Platero, V.; Florez Ponce de León, W.D.; Sandoval, E.M.L. ZnO-CuO Nanocomposite as an Efficient Adsorbent for As(III) Removal from Water. Water 2023, 15, 4318. [Google Scholar] [CrossRef]
- Ivanova, T.; Harizanova, A.; Koutzarova, T.; Vertruyen, B. Study of ZnO sol–gel films: Effect of annealing. Mater. Lett. 2010, 64, 1147–1149. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Kong, J.; Jia, H.; Yu, M. ZnO microspheres: Controllable preparation and optical properties. Superlattices Microstruct. 2015, 86, 228–235. [Google Scholar] [CrossRef]
- Gulia, S.; Kakkar, R. ZnO Quantum Dots for Biomedical Applications. Adv. Mater. Lett. 2013, 4, 876–887. [Google Scholar] [CrossRef]
- Djurisić, A.B.; Leung, Y.H. Optical properties of ZnO nanostructures. Small 2006, 2, 944–961. [Google Scholar] [CrossRef]
- Flores, N.M.; Pal, U.; Galeazzi, R.; Sandoval, A. Effects of morphology, surface area, and defect content on the photocatalytic dye degradation performance of ZnO nanostructures. RSC Adv. 2014, 4, 41099–41110. [Google Scholar] [CrossRef]
- Can, A.; Kızılbey, K. Green Synthesis of ZnO Nanoparticles via Ganoderma Lucidum Extract: Structural and Functional Analysis in Polymer Composites. Gels 2024, 10, 576. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Pramanik, S.; Mukherjee, S.; Rajak, C.; Mukherjee, B.; Kuiri, P.K. Vibrational, optical, and photocatalytic properties of ZnO/layered carbon nanocomposite synthesized by ball milling. J. Phys. Condens. Matter 2024, 36, 395301. [Google Scholar] [CrossRef]
- Khan, A.; Mohamed, S.E.; Al-Naggar, T.I.; Albargi, H.B.; Algethami, J.S.; Abdalla, A.M. Effect of Sintering Time and Cl Doping Concentrations on Structural, Optical, and Luminescence Properties of ZnO Nanoparticles. Inorganics 2024, 12, 53. [Google Scholar] [CrossRef]
- Shen, H.; Shi, X.; Wang, Z.; Hou, Z.; Xu, C.; Duan, L.; Zhao, X.; Wu, H. Defects control and origins of blue and green emissions in sol-gel ZnO thin films. Vacuum 2022, 202, 111201. [Google Scholar] [CrossRef]
- Rana, A.U.H.S.; Shaikh, S.F.; Al-Enizi, A.M.; Agyeman, D.A.; Ghani, F.; Nah, I.W.; Shahid, A. Intrinsic Control in Defects Density for Improved ZnO Nanorod-Based UV Sensor Performance. Nanomaterials 2020, 10, 142. [Google Scholar] [CrossRef]
- Gingiguntla, B.; Akkiniraj Chandrasekar, A.; Selvan Cruz, S.A. Green Mediated Synthesis of Zinc Oxide Nanoparticles using Tamarind Bark Extract and their Applications in Textile Dye Degradation and Antimicrobial Studies. Asian J. Chem. 2023, 36, 239–246. [Google Scholar] [CrossRef]
- Farrokhi, M.; Yang, J.-K.; Lee, S.-M.; Shirzad-Siboni, M. Effect of organic matter on cyanide removal by illuminated titanium dioxide or zinc oxide nanoparticles. J. Environ. Health Sci. Eng. 2013, 11, 23. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ashiegbu, D.C.; Pilane, P.; Moma, J.; Potgieter, H. Enhanced Photocatalytic Degradation of Cyanide in Mining Wastewater Using a ZnO-BiOI Heterojunction Catalyst. Surfaces 2025, 8, 3. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Oki, A. Graphene-Coupled ZnO: A Robust NIR-Induced Catalyst for Rapid Photo-Oxidation of Cyanide. ACS Omega 2017, 2, 9095–9102. [Google Scholar] [CrossRef]
- Ahmed, S.; Annu; Chaudhry, S.A.; Ikram, S. A review on biogenic synthesis of ZnO nanoparticles using plant extracts and microbes: A prospect towards green chemistry. J. Photochem. Photobiol. B Biol. 2017, 166, 272–284. [Google Scholar] [CrossRef]
- Madhumitha, G.; Elango, G.; Roopan, S.M. Biotechnological aspects of ZnO nanoparticles: Overview on synthesis and its applications. Appl. Microbiol. Biotechnol. 2016, 100, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Maya-Treviño, M.L.; Guzmán-Mar, J.L.; Hinojosa-Reyes, L.; Hernández-Ramírez, A. Synthesis and photocatalytic activity of ZnO-CuPc for methylene blue and potassium cyanide degradation. Mater. Sci. Semicond. Process. 2018, 77, 74–82. [Google Scholar] [CrossRef]
- Núñez-Salas, R.E.; Hernández-Ramírez, A.; Hinojosa-Reyes, L.; Guzmán-Mar, J.L.; Villanueva-Rodríguez, M.; Maya-Treviño, M.d.L. Cyanide degradation in aqueous solution by heterogeneous photocatalysis using boron-doped zinc oxide. Catal. Today 2019, 328, 202–209. [Google Scholar] [CrossRef]
- Yari, K.; Seidmohammadi, A.; Khazaei, M.; Bhatnagar, A.; Leili, M. A comparative study for the removal of imidacloprid insecticide from water by chemical-less UVC, UVC/TiO2 and UVC/ZnO processes. J. Environ. Health Sci. Eng. 2019, 17, 337–351. [Google Scholar] [CrossRef]
- Shinde, S.S.; Shinde, P.S.; Bhosale, C.H.; Rajpure, K.Y. Zinc oxide mediated heterogeneous photocatalytic degradation of organic species under solar radiation. J. Photochem. Photobiol. B Biol. 2011, 104, 425–433. [Google Scholar] [CrossRef]




| Treatment Method | Operating Parameters | Removal Efficiency (%) | Environmental Impact | Ref. |
|---|---|---|---|---|
| Alkaline Chlorination | pH: 11.2, T: 25 °C | 90–99 | High chloride residuals | [7] |
| SO2/Air Process | pH: 8–9.5, T: 22–40 °C | 80–95 | SO42− generation | [8] |
| Biological Treatment | pH: 10.5, T: 30 °C, 1000 ppm | 80–100 | Minimal | [9] |
| Chemical Precipitation | pH: 5–7, T: 25 °C | 60–80 | Metal-rich sludge | [10] |
| TiO2 Photocatalysis | pH: 9–11, sunlight, 30 ppm | 70–100 | Minimal | [11] |
| Structural Parameters | Sample |
|---|---|
| ZnO | |
| Crystal structure | Hexagonal |
| Space group | P 63 m c |
| Space group number | 186 |
| a = b (Å) | 3.25168 |
| c (Å) | 5.21387 |
| α = β (°) | 90 |
| γ (°) | 120 |
| ρ (g/cm3) | 5.65 |
| D (nm) | 14.18 |
| Residual stress (%) | 0.004 |
| Rexp (%) | 5.09092 |
| Rp (%) | 2.82154 |
| Rwp (%) | 3.86143 |
| GOF | 0.75849 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quispe Cohaila, A.B.; Fora Quispe, G.d.L.; Lanchipa Ramos, W.O.; Cáceda Quiroz, C.J.; Tamayo Calderón, R.M.; Medina Salas, J.P.; Rajendran, S.; Sacari Sacari, E.J. Synthesis of ZnO Nanoparticles by Bacillus subtilis for Efficient Photocatalytic Degradation of Cyanide. Nanomaterials 2025, 15, 501. https://doi.org/10.3390/nano15070501
Quispe Cohaila AB, Fora Quispe GdL, Lanchipa Ramos WO, Cáceda Quiroz CJ, Tamayo Calderón RM, Medina Salas JP, Rajendran S, Sacari Sacari EJ. Synthesis of ZnO Nanoparticles by Bacillus subtilis for Efficient Photocatalytic Degradation of Cyanide. Nanomaterials. 2025; 15(7):501. https://doi.org/10.3390/nano15070501
Chicago/Turabian StyleQuispe Cohaila, Alberto Bacilio, Gabriela de Lourdes Fora Quispe, Wilson Orlando Lanchipa Ramos, César Julio Cáceda Quiroz, Rocío María Tamayo Calderón, Jesús Plácido Medina Salas, Saravanan Rajendran, and Elisban Juani Sacari Sacari. 2025. "Synthesis of ZnO Nanoparticles by Bacillus subtilis for Efficient Photocatalytic Degradation of Cyanide" Nanomaterials 15, no. 7: 501. https://doi.org/10.3390/nano15070501
APA StyleQuispe Cohaila, A. B., Fora Quispe, G. d. L., Lanchipa Ramos, W. O., Cáceda Quiroz, C. J., Tamayo Calderón, R. M., Medina Salas, J. P., Rajendran, S., & Sacari Sacari, E. J. (2025). Synthesis of ZnO Nanoparticles by Bacillus subtilis for Efficient Photocatalytic Degradation of Cyanide. Nanomaterials, 15(7), 501. https://doi.org/10.3390/nano15070501









