Oral Exposure to Nylon-11 and Polystyrene Nanoplastics During Early-Life in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and NPs
2.2. Fabrication of Nylon-11 NPs
2.3. Characterization of NPs
2.3.1. Dynamic Light Scattering (DLS) and Zeta Potential
2.3.2. 19F Nuclear Magnetic Resonance Spectroscopy (19F-NMR)
2.3.3. Scanning Electron Microscopy (SEM)
2.3.4. Evaluation of Endotoxin
2.3.5. Concentration of NPs
2.4. In Vivo Rat Study
2.4.1. Housing and Dose Administration
2.4.2. Cardiac Assessment
2.4.3. Quantification of Neurotransmitters and Related Metabolites in Brain Tissue
2.4.4. Metabolomics Analysis
2.4.5. Statistical and Multivariate Analysis
2.4.6. Metabolic Pathway Analysis
3. Results
3.1. Characterization of NPs
3.2. Oral Exposure of NPs to Rats
3.2.1. Ratio of Organ-to-BW
3.2.2. Cardiac Assessment
3.2.3. Concentrations of Neurotransmitters and Related Metabolites in Brain Tissue
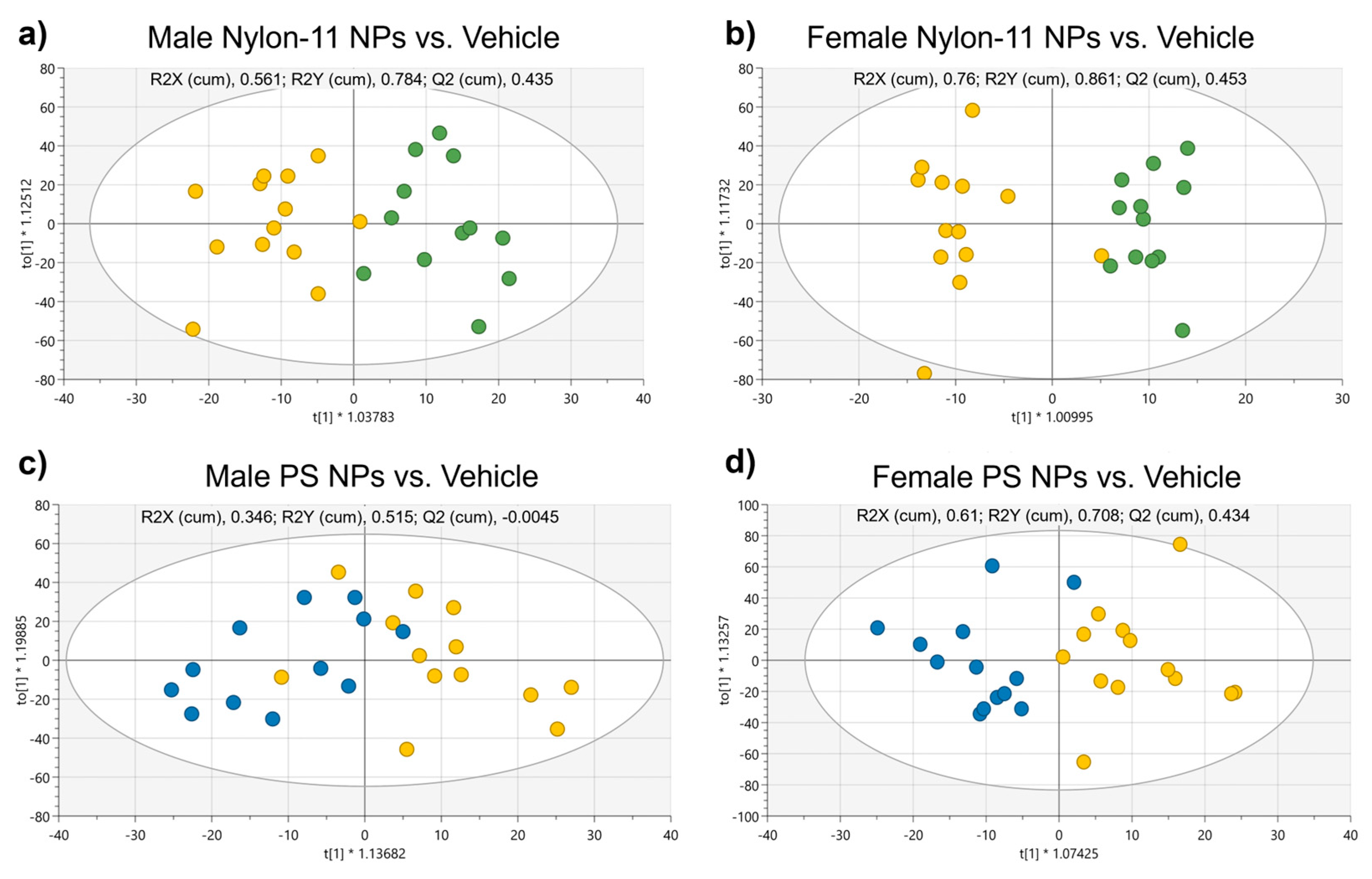
3.2.4. Metabolomics Analysis of Plasma
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiang, Y.; Jiang, L.; Zhou, Y.; Luo, Z.; Zhi, D.; Yang, J.; Lam, S.S. Microplastics and environmental pollutants: Key interaction and toxicology in aquatic and soil environments. J. Hazard. Mater. 2022, 422, 126843. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.L.; Tirelli, V.; O’connor, I.; Officer, R. Microplastics in Arctic polar waters: The first reported values of particles in surface and sub-surface samples. Sci. Rep. 2015, 5, 14947. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Z.; Chen, Y.; Yang, F.; Yao, W.; Xie, Y. Microplastics and Nanoplastics: Emerging Contaminants in Food. J. Agric. Food Chem. 2021, 69, 10450–10468. [Google Scholar] [CrossRef]
- Walkinshaw, C.; Lindeque, P.K.; Thompson, R.; Tolhurst, T.; Cole, M. Microplastics and seafood: Lower trophic organisms at highest risk of contamination. Ecotoxicol. Environ. Saf. 2020, 190, 110066. [Google Scholar] [CrossRef] [PubMed]
- De-La-Torre, G.E. Microplastics: An emerging threat to food security and human health. J. Food Sci. Technol. 2020, 57, 1601–1608. [Google Scholar] [CrossRef]
- Eerkes-Medrano, D.; Leslie, H.A.; Quinn, B. Microplastics in drinking water: A review and assessment of an emerging concern. Curr. Opin. Environ. Sci. Health 2018, 7, 69–75. [Google Scholar] [CrossRef]
- Novotna, K.; Cermakova, L.; Pivokonska, L.; Cajthaml, T.; Pivokonsky, M. Microplastics in drinking water treatment—Current knowledge and research needs. Sci. Total Environ. 2019, 667, 730–740. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Nor, N.H.M.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Wang, T.; Yi, Z.; Liu, X.; Cai, Y.; Huang, X.; Fang, J.; Shen, R.; Lu, W.; Xiao, Y.; Zhuang, W.; et al. Multimodal detection and analysis of microplastics in human thrombi from multiple anatomically distinct sites. EBioMedicine 2024, 103, 105118. [Google Scholar] [CrossRef]
- Jenner, L.C.; Rotchell, J.M.; Bennett, R.T.; Cowen, M.; Tentzeris, V.; Sadofsky, L.R. Detection of microplastics in human lung tissue using μFTIR spectroscopy. Sci. Total Environ. 2022, 831, 154907. [Google Scholar] [CrossRef] [PubMed]
- Halfar, J.; Čabanová, K.; Vávra, K.; Delongová, P.; Motyka, O.; Špaček, R.; Kukutschová, J.; Šimetka, O.; Heviánková, S. Microplastics and additives in patients with preterm birth: The first evidence of their presence in both human amniotic fluid and placenta. Chemosphere 2023, 343, 140301. [Google Scholar] [CrossRef]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Notarstefano, V.; Svelato, A.; Belloni, A.; Gioacchini, G.; Blondeel, C.; Zucchelli, E.; De Luca, C.; D’Avino, S.; Gulotta, A.; et al. Raman Microspectroscopy Detection and Characterisation of Microplastics in Human Breastmilk. Polymers 2022, 14, 2700. [Google Scholar] [CrossRef]
- Sun, J.; Sui, M.; Wang, T.; Teng, X.; Sun, J.; Chen, M. Detection and quantification of various microplastics in human endometrium based on laser direct infrared spectroscopy. Sci. Total Environ. 2023, 906, 167760. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, L.; Weng, J.; Jin, Z.; Cao, Y.; Jiang, H.; Zhang, Z. Detection and characterization of microplastics in the human testis and semen. Sci. Total Environ. 2023, 877, 162713. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Huang, X.; Bi, R.; Guo, Q.; Yu, X.; Zeng, Q.; Huang, Z.; Liu, T.; Wu, H.; Chen, Y.; et al. Detection and Analysis of Microplastics in Human Sputum. Environ. Sci. Technol. 2022, 56, 2476–2486. [Google Scholar] [CrossRef]
- Pironti, C.; Notarstefano, V.; Ricciardi, M.; Motta, O.; Giorgini, E.; Montano, L. First Evidence of Microplastics in Human Urine, a Preliminary Study of Intake in the Human Body. Toxics 2022, 11, 40. [Google Scholar] [CrossRef]
- Zhang, N.; Bin Li, Y.; He, H.R.; Zhang, J.F.; Ma, G.S. You are what you eat: Microplastics in the feces of young men living in Beijing. Sci. Total Environ. 2021, 767, 144345. [Google Scholar] [CrossRef]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, Y.; Zhang, T.; Zhang, F.; Ren, H.; Zhang, Y. Analysis of Microplastics in Human Feces Reveals a Correlation between Fecal Microplastics and Inflammatory Bowel Disease Status. Environ. Sci. Technol. 2021, 56, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Sabourian, P.; Yazdani, G.; Ashraf, S.S.; Frounchi, M.; Mashayekhan, S.; Kiani, S.; Kakkar, A. Effect of Physico-Chemical Properties of Nanoparticles on Their Intracellular Uptake. Int. J. Mol. Sci. 2020, 21, 8019. [Google Scholar] [CrossRef]
- Gatoo, M.A.; Naseem, S.; Arfat, M.Y.; Dar, A.M.; Qasim, K.; Zubair, S. Physicochemical Properties of Nanomaterials: Implication in Associated Toxic Manifestations. BioMed Res. Int. 2014, 2014, 498420. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Park, W.; Shin, H.; Choi, B.; Rhim, W.-K.; Na, K.; Han, D.K. Advanced hybrid nanomaterials for biomedical applications. Prog. Mater. Sci. 2020, 114, 100686. [Google Scholar] [CrossRef]
- Bahadar, H.; Maqbool, F.; Niaz, K.; Abdollahi, M. Toxicity of nanoparticles and an overview of current experimental models. Iran. Biomed. J. 2016, 20, 1–11. [Google Scholar] [CrossRef]
- Gupta, R.; Xie, H. Nanoparticles in Daily Life: Applications, Toxicity and Regulations. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 209–230. [Google Scholar] [CrossRef]
- Mortensen, N.P.; Pathmasiri, W.; Snyder, R.W.; Caffaro, M.M.; Watson, S.L.; Patel, P.R.; Beeravalli, L.; Prattipati, S.; Aravamudhan, S.; Sumner, S.J.; et al. Oral administration of TiO2 nanoparticles during early life impacts cardiac and neurobehavioral performance and metabolite profile in an age- and sex-related manner. Part. Fibre Toxicol. 2022, 19, 3. [Google Scholar] [CrossRef]
- Mortensen, N.P.; Caffaro, M.M.; Patel, P.R.; Snyder, R.W.; Watson, S.L.; Aravamudhan, S.; Montgomery, S.A.; Lefever, T.; Sumner, S.J.; Fennell, T.R. Biodistribution, cardiac and neurobehavioral assessments, and neurotransmitter quantification in juvenile rats following oral administration of aluminum oxide nanoparticles. J. Appl. Toxicol. 2020, 41, 1316–1329. [Google Scholar] [CrossRef] [PubMed]
- Gigault, J.; El Hadri, H.; Nguyen, B.; Grassl, B.; Rowenczyk, L.; Tufenkji, N.; Feng, S.; Wiesner, M. Nanoplastics are neither microplastics nor engineered nanoparticles. Nat. Nanotechnol. 2021, 16, 501–507. [Google Scholar] [CrossRef]
- Sly, P.; Blake, T.; Islam, Z. Impact of prenatal and early life environmental exposures on normal human development. Paediatr. Respir. Rev. 2021, 40, 10–14. [Google Scholar] [CrossRef]
- Chacko, A.; Carpenter, D.O.; Callaway, L.; Sly, P.D. Early-life risk factors for chronic nonrespiratory diseases. Eur. Respir. J. 2014, 45, 244–259. [Google Scholar] [CrossRef] [PubMed]
- Boekelheide, K.; Blumberg, B.; Chapin, R.E.; Cote, I.; Graziano, J.H.; Janesick, A.; Lane, R.; Lillycrop, K.; Myatt, L.; States, J.C.; et al. Predicting Later-Life Outcomes of Early-Life Exposures. Environ. Health Perspect. 2012, 120, 1353–1361. [Google Scholar] [CrossRef]
- Cao, J.; Xu, X.; Hylkema, M.N.; Zeng, E.Y.; Sly, P.D.; Suk, W.A.; Bergman, Å.; Huo, X. Early-life Exposure to Widespread Environmental Toxicants and Health Risk: A Focus on the Immune and Respiratory Systems. Ann. Glob. Health 2016, 82, 119–131. [Google Scholar] [CrossRef]
- Burri, P.H. Fetal and Postnatal Development of the Lung. Annu. Rev. Physiol. 1984, 46, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Georgountzou, A.; Papadopoulos, N.G. Postnatal Innate Immune Development: From Birth to Adulthood. Front. Immunol. 2017, 8, 957. [Google Scholar] [CrossRef]
- Jernigan, T.L.; Baaré, W.F.; Stiles, J.; Madsen, K.S. Postnatal brain development: Structural imaging of dynamic neurodevelopmental processes. Prog. Brain Res. 2011, 189, 77–92. [Google Scholar] [CrossRef]
- Hillery, A.; Jani, P.; Florence, A. Comparative, Quantitative Study of Lymphoid and Non-Lymphoid Uptake of 60 nm Polystyrene Particles. J. Drug Target. 1994, 2, 151–156. [Google Scholar] [CrossRef]
- Tian, F.; Razansky, D.; Estrada, G.G.; Semmler-Behnke, M.; Beyerle, A.; Kreyling, W.; Ntziachristos, V.; Stoeger, T. Surface modification and size dependence in particle translocation during early embryonic development. Inhal. Toxicol. 2009, 21, 92–96. [Google Scholar] [CrossRef]
- Walczak, A.P.; Hendriksen, P.J.; Woutersen, R.A.; van der Zande, M.; Undas, A.K.; Helsdingen, R.; van den Berg, H.H.; Rietjens, I.M.C.M.; Bouwmeester, H. Bioavailability and biodistribution of differently charged polystyrene nanoparticles upon oral exposure in rats. J. Nanopart. Res. 2015, 17, 231. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 2017, 7, 46687. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, M.; Dargahi, L.; Eslami, A.; Beirami, E.; Jahangiri-Rad, M.; Sabour, S.; Amereh, F. Neurobehavioral assessment of rats exposed to pristine polystyrene nanoplastics upon oral exposure. Chemosphere 2018, 193, 745–753. [Google Scholar] [CrossRef]
- Kik, K.; Bukowska, B.; Sicińska, P. Polystyrene nanoparticles: Sources, occurrence in the environment, distribution in tissues, accumulation and toxicity to various organisms. Environ. Pollut. 2020, 262, 114297. [Google Scholar] [CrossRef]
- Fournier, S.B.; D’errico, J.N.; Adler, D.S.; Kollontzi, S.; Goedken, M.J.; Fabris, L.; Yurkow, E.J.; Stapleton, P.A. Nanopolystyrene translocation and fetal deposition after acute lung exposure during late-stage pregnancy. Part. Fibre Toxicol. 2020, 17, 55. [Google Scholar] [CrossRef]
- Nnoruka, U.C.; Okonkwo, C.J.; Ilechukwu, I.; Okonkwo, C.J.; Belonwu, D.C. Impact of polystyrene microplastic exposure on lipid profile and oxidative stress status of male and female Wistar rats. Environ. Anal. Health Toxicol. 2022, 37, e2022024. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zhang, W.; Lin, T.; Liu, S.; Sun, Z.; Liu, F.; Yuan, Y.; Xiang, X.; Kuang, H.; Yang, B.; et al. Maternal exposure to polystyrene nanoplastics during gestation and lactation induces hepatic and testicular toxicity in male mouse offspring. Food Chem. Toxicol. 2022, 160, 112803. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.; Baek, J.Y.; Koo, J.; Park, S.; Ryu, Y.-K.; Kim, K.-S.; Zhang, S.; Chung, C.; Dogan, R.; Choi, H.-S.; et al. Maternal exposure to polystyrene nanoplastics causes brain abnormalities in progeny. J. Hazard. Mater. 2022, 426, 127815. [Google Scholar] [CrossRef]
- Harvey, N.E.; Mercer, G.V.; Stapleton, D.; Steeves, K.L.; Hanrahan, J.; Cui, M.; Aghaei, Z.; Spring, S.; Helm, P.A.; Simpson, A.J.; et al. Maternal exposure to polystyrene nanoplastics impacts developmental milestones and brain structure in mouse offspring. Environ. Sci. Adv. 2023, 2, 622–628. [Google Scholar] [CrossRef]
- Tang, J.; Bu, W.; Hu, W.; Zhao, Z.; Liu, L.; Luo, C.; Wang, R.; Fan, S.; Yu, S.; Wu, Q.; et al. Ferroptosis Is Involved in Sex-Specific Small Intestinal Toxicity in the Offspring of Adult Mice Exposed to Polystyrene Nanoplastics during Pregnancy. ACS Nano 2023, 17, 2440–2449. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Zhang, Y.; Wang, C.; Wang, X.; Zhou, J.; Shen, M.; Zhao, Y.; Fu, Z.; Jin, Y. Maternal exposure to different sizes of polystyrene microplastics during gestation causes metabolic disorders in their offspring. Environ. Pollut. 2019, 255, 113122. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.M.; Yousefi, N.; Tufenkji, N. Are There Nanoplastics in Your Personal Care Products? Environ. Sci. Technol. Lett. 2017, 4, 280–285. [Google Scholar] [CrossRef]
- Napper, I.E.; Bakir, A.; Rowland, S.J.; Thompson, R.C. Characterisation, quantity and sorptive properties of microplastics extracted from cosmetics. Mar. Pollut. Bull. 2015, 99, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Galgani, F.; Rangel-Buitrago, N. White tides: The plastic nurdles problem. J. Hazard. Mater. 2024, 470, 134250. [Google Scholar] [CrossRef]
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef]
- Statistica Research Department. Polyamide Fibers Production Globally 1975–2023. 2024. Available online: https://www.statista.com/statistics/649908/polyamide-fiber-production-worldwide/ (accessed on 20 January 2025).
- Shakiba, M.; Rezvani Ghomi, E.; Khosravi, F.; Jouybar, S.; Bigham, A.; Zare, M.; Abdouss, M.; Moaref, R.; Ramakrishna, S. Nylon—A material introduction and overview for biomedical applications. Polym. Adv. Technol. 2021, 32, 3368–3383. [Google Scholar] [CrossRef]
- Keifer, D.H. The Establishment of Modern Polymer Science by Wallace H. Carothers; American Chemical Society: Wilmington, DE, USA, 2000. [Google Scholar]
- Wypych, G. Handbook of Polymers; ChemTec Publishing: Toronto, ON, Canada, 2016. [Google Scholar]
- Kankanige, D.; Babel, S. Smaller-sized micro-plastics (MPs) contamination in single-use PET-bottled water in Thailand. Sci. Total Environ. 2020, 717, 137232. [Google Scholar] [CrossRef]
- Shruti, V.; Pérez-Guevara, F.; Elizalde-Martínez, I.; Kutralam-Muniasamy, G. First study of its kind on the microplastic contamination of soft drinks, cold tea and energy drinks—Future research and environmental considerations. Sci. Total Environ. 2020, 726, 138580. [Google Scholar] [CrossRef]
- Cho, Y.; Shim, W.J.; Jang, M.; Han, G.M.; Hong, S.H. Nationwide monitoring of microplastics in bivalves from the coastal environment of Korea. Environ. Pollut. 2020, 270, 116175. [Google Scholar] [CrossRef]
- Yaranal, N.A.; Subbiah, S.; Mohanty, K. Identification, extraction of microplastics from edible salts and its removal from contaminated seawater. Environ. Technol. Innov. 2021, 21, 101253. [Google Scholar] [CrossRef]
- Krovi, S.A.; Moreno Caffaro, M.M.; Aravamudhan, S.; Mortensen, N.P.; Johnson, L.M. Fabrication of Nylon-6 and Nylon-11 Nanoplastics and Evaluation in Mammalian Cells. Nanomaterials 2022, 12, 2699. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Liu, F.; Li, Z.; Duan, J.; Kong, Y.; Hao, M.; Ge, S.; Jiang, H.; Liu, H. Piezoelectric nylon-11 nanoparticles with ultrasound assistance for high-efficiency promotion of stem cell osteogenic differentiation. J. Mater. Chem. B 2019, 7, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
- Antonova, O.Y.; Kochetkova, O.Y.; Shlyapnikov, Y.M. ECM-Mimetic Nylon Nanofiber Scaffolds for Neurite Growth Guidance. Nanomaterials 2021, 11, 516. [Google Scholar] [CrossRef]
- Abdal-Hay, A.; Vanegas, P.; Hamdy, A.S.; Engel, F.B.; Lim, J.H. Preparation and characterization of vertically arrayed hydroxyapatite nanoplates on electrospun nanofibers for bone tissue engineering. Chem. Eng. J. 2014, 254, 612–622. [Google Scholar] [CrossRef]
- Johnson, L.M.; Mecham, J.B.; Krovi, S.A.; Moreno Caffaro, M.M.; Aravamudhan, S.; Kovach, A.L.; Fennell, T.R.; Mortensen, N.P. Fabrication of polyethylene terephthalate (PET) nanoparticles with fluorescent tracers for studies in mammalian cells. Nanoscale Adv. 2021, 3, 339–346. [Google Scholar] [CrossRef]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Desaulniers, D.; Yagminas, A.; Chu, I.; Nakai, J. Effects of Anesthetics and Terminal Procedures on Biochemical and Hormonal Measurements in Polychlorinated Biphenyl Treated Rats. Int. J. Toxicol. 2011, 30, 334–347. [Google Scholar] [CrossRef]
- Nakai, J.S.; Elwin, J.; Chu, I.; Marro, L. Effect of anaesthetics[sol ]terminal procedures on neurotransmitters from non-dosed and aroclor 1254-dosed rats. J. Appl. Toxicol. 2005, 25, 224–233. [Google Scholar] [CrossRef]
- Gelaye, B.; Sumner, S.J.; McRitchie, S.; Carlson, J.E.; Ananth, C.V.; Enquobahrie, D.A.; Qiu, C.; Sorensen, T.K.; Williams, M.A. Maternal Early Pregnancy Serum Metabolomics Profile and Abnormal Vaginal Bleeding as Predictors of Placental Abruption: A Prospective Study. PLoS ONE 2016, 11, e0156755. [Google Scholar] [CrossRef]
- Chao de la Barca, J.M.; Bakhta, O.; Kalakech, H.; Simard, G.; Tamareille, S.; Catros, V.; Callebert, J.; Gadras, C.; Tessier, L.; Reynier, P.; et al. Metabolic Signature of Remote Ischemic Preconditioning Involving a Cocktail of Amino Acids and Biogenic Amines. J. Am. Heart Assoc. 2016, 5, e003891. [Google Scholar] [CrossRef]
- Eriksson, L.; Byrne, T.; Johansson, E.; Trygg, J.; Vikström, C. Multi- and Megavariate Data Analysis Basic Principles and Applications, 3rd ed.; MKS Umetrics AB: Malmö, Sweden, 2013. [Google Scholar]
- Bylesjö, M.; Rantalainen, M.; Cloarec, O.; Nicholson, J.K.; Holmes, E.; Trygg, J. OPLS discriminant analysis: Combining the strengths of PLS-DA and SIMCA classification. J. Chemom. 2006, 20, 341–351. [Google Scholar] [CrossRef]
- Broadhurst, D.; Goodacre, R.; Reinke, S.N.; Kuligowski, J.; Wilson, I.D.; Lewis, M.R.; Dunn, W.B. Guidelines and considerations for the use of system suitability and quality control samples in mass spectrometry assays applied in untargeted clinical metabolomic studies. Metabolomics 2018, 14, 72. [Google Scholar] [CrossRef]
- United States Food and Drug Administration. Bacterial Endotoxins/Pyrogens. Available online: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/inspection-technical-guides/bacterial-endotoxinspyrogens (accessed on 21 January 2025).
- Haryadi, B.M.; Hafner, D.; Amin, I.; Schubel, R.; Jordan, R.; Winter, G.; Engert, J. Nonspherical Nanoparticle Shape Stability Is Affected by Complex Manufacturing Aspects: Its Implications for Drug Delivery and Targeting. Adv. Healthc. Mater. 2019, 8, e1900352. [Google Scholar] [CrossRef] [PubMed]
- Caputo, F.; Clogston, J.; Calzolai, L.; Rösslein, M.; Prina-Mello, A. Measuring particle size distribution of nanoparticle enabled medicinal products, the joint view of EUNCL and NCI-NCL. A step by step approach combining orthogonal measurements with increasing complexity. J. Control. Release 2019, 299, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Liu, X.; Fahr, A. Skin penetration and deposition of carboxyfluorescein and temoporfin from different lipid vesicular systems: In vitro study with finite and infinite dosage application. Int. J. Pharm. 2011, 408, 223–234. [Google Scholar] [CrossRef]
- Miao, L.; Guo, S.; Liu, Z.; Liu, S.; You, G.; Qu, H.; Hou, J. Effects of Nanoplastics on Freshwater Biofilm Microbial Metabolic Functions as Determined by BIOLOG ECO Microplates. Int. J. Environ. Res. Public Health 2019, 16, 4639. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Casabianca, L.B. Probing driving forces for binding between nanoparticles and amino acids by saturation-transfer difference NMR. Sci. Rep. 2020, 10, 12351. [Google Scholar] [CrossRef]
- Fessler, F.; Sharma, V.; Muller, P.; Stocco, A. Entry of microparticles into giant lipid vesicles by optical tweezers. Phys. Rev. E 2023, 107, L052601. [Google Scholar] [CrossRef]
- Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K.A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [Google Scholar] [CrossRef]
- Ritt, C.L.; Werber, J.R.; Wang, M.; Yang, Z.; Zhao, Y.; Kulik, H.J.; Elimelech, M. Ionization behavior of nanoporous polyamide membranes. Proc. Natl. Acad. Sci. USA 2020, 117, 30191–30200. [Google Scholar] [CrossRef]
- Michael, B.; Yano, B.; Sellers, R.S.; Perry, R.; Morton, D.; Roome, N.; Johnson, J.K.; Schafer, K. Evaluation of Organ Weights for Rodent and Non-Rodent Toxicity Studies: A Review of Regulatory Guidelines and a Survey of Current Practices. Toxicol. Pathol. 2007, 35, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Piao, Y.; Liu, Y.; Xie, X. Change Trends of Organ Weight Background Data in Sprague Dawley Rats at Different Ages. J. Toxicol. Pathol. 2013, 26, 29–34. [Google Scholar] [CrossRef]
- Yu, S.; Liu, F.; Wang, C.; Zhang, J.; Zhu, A.; Zou, L.; Han, A.; Li, J.; Chang, X.; Sun, Y. Role of oxidative stress in liver toxicity induced by nickel oxide nanoparticles in rats. Mol. Med. Rep. 2017, 17, 3133–3139. [Google Scholar] [CrossRef]
- Fan, J.; Liu, L.; Lu, Y.; Chen, Q.; Fan, S.; Yang, Y.; Long, Y.; Liu, X. Acute exposure to polystyrene nanoparticles promotes liver injury by inducing mitochondrial ROS-dependent necroptosis and augmenting macrophage-hepatocyte crosstalk. Part. Fibre Toxicol. 2024, 21, 1–19. [Google Scholar] [CrossRef]
- Kiela, P.R.; Ghishan, F.K. Physiology of Intestinal Absorption and Secretion. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar] [PubMed]
- Reynolds, H.M.; Bettini, M.L. Early-life microbiota-immune homeostasis. Front. Immunol. 2023, 14, 1266876. [Google Scholar] [CrossRef]
- Sarkar, A.; Yoo, J.Y.; Valeria Ozorio Dutra, S.; Morgan, K.H.; Groer, M. The Association between Early-Life Gut Microbiota and Long-Term Health and Diseases. J. Clin. Med. 2021, 10, 459. [Google Scholar] [CrossRef] [PubMed]
- Ibba, M.; Söll, D. Aminoacyl-tRNA Synthesis. Annu. Rev. Biochem. 2000, 69, 617–650. [Google Scholar] [CrossRef]
- Smirnova, E.V.; Lakunina, V.A.; Tarassov, I.; Krasheninnikov, I.A.; Kamenski, P.A. Noncanonical functions of aminoacyl-tRNA synthetases. Biochemistry 2012, 77, 15–25. [Google Scholar] [CrossRef]
- Farooqui, A.A.; Horrocks, L.A.; Farooqui, T. Glycerophospholipids in brain: Their metabolism, incorporation into membranes, functions, and involvement in neurological disorders. Chem. Phys. Lipids 2000, 106, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Guy, A.T.; Nagatsuka, Y.; Ooashi, N.; Inoue, M.; Nakata, A.; Greimel, P.; Inoue, A.; Nabetani, T.; Murayama, A.; Ohta, K.; et al. Glycerophospholipid regulation of modality-specific sensory axon guidance in the spinal cord. Science 2015, 349, 974–977. [Google Scholar] [CrossRef]
- Derbyshire, E.; Obeid, R. Choline, Neurological Development and Brain Function: A Systematic Review Focusing on the First 1000 Days. Nutrients 2020, 12, 1731. [Google Scholar] [CrossRef] [PubMed]
- Schverer, M.; O’Mahony, S.M.; O’riordan, K.J.; Donoso, F.; Roy, B.L.; Stanton, C.; Dinan, T.G.; Schellekens, H.; Cryan, J.F. Dietary phospholipids: Role in cognitive processes across the lifespan. Neurosci. Biobehav. Rev. 2020, 111, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Arkema. High Perfromace Polymers Medical Grades and Technical Data Sheets. Available online: https://hpp.arkema.cn/files/live/sites/shared_arkema/files/downloads/HPP/market-brochures/healthcare-medical/2020-technical-polymers-medical-grades-and-technical-data-sheets-brochure-web.pdf (accessed on 22 January 2025).
- Song, Z.; Wu, H.; Fang, X.; Feng, X.; Zhou, L. The cardiovascular toxicity of polystyrene microplastics in rats: Based on untargeted metabolomics analysis. Front. Pharmacol. 2024, 15, 1336369. [Google Scholar] [CrossRef]


| N | Hydrodynamic Diameter (nm) | PDI | Zeta Potential (mV) | Endotoxin (EU/mL) |
|---|---|---|---|---|
| Nylon-11 | 114 ± 2 | 0.18 ± 0.03 | 40 ± 1.5 | 0.054 |
| PS | 85 ± 1 | 0.08 ± 0.01 | −66 ± 2.2 | 0.048 |
| Metabolic Pathway | Nylon-11 NPs | PS NPs | ||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Aminoacyl-tRNA biosynthesis | 2.63 × 10−6 | 1.16 × 10−13 | - | 5.14 × 10−7 |
| Arginine biosynthesis | - | - | - | 3.34 × 10−4 |
| Sphingolipid metabolism | - | - | 4.61 × 10−2 | - |
| Valine, leucine, and isoleucine biosynthesis | - | 2.7 × 10−3 | - | - |
| Lipid Subclass | Nylon-11 NPs | PS NPs | ||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| 1-alkyl,2-acylglycerophosphocholines | 2.95 × 10−8 | - | 6.37 × 10−3 | 3.74 × 10−10 |
| Diacylglycerophosphocholines | 3.62 × 10−49 | 2.36 × 10−2 | 1.23 × 10−5 | 3.85 × 10−2 |
| Fatty acyl carnitines | - | - | - | 7.73 × 10−3 |
| Lysophosphatidylcholines | - | - | - | 7.73 × 10−3 |
| Phosphosphingolipids | 2.95 × 10−3 | - | 3.94 × 10−7 | - |
| Sphingomyelins | - | - | 5.56 × 10−3 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mortensen, N.P.; Caffaro, M.M.; Krovi, A.; Kim, J.; Watson, S.L.; Snyder, R.W.; Patel, P.R.; Fennell, T.R.; Johnson, L.M. Oral Exposure to Nylon-11 and Polystyrene Nanoplastics During Early-Life in Rats. Nanomaterials 2025, 15, 465. https://doi.org/10.3390/nano15060465
Mortensen NP, Caffaro MM, Krovi A, Kim J, Watson SL, Snyder RW, Patel PR, Fennell TR, Johnson LM. Oral Exposure to Nylon-11 and Polystyrene Nanoplastics During Early-Life in Rats. Nanomaterials. 2025; 15(6):465. https://doi.org/10.3390/nano15060465
Chicago/Turabian StyleMortensen, Ninell P., Maria Moreno Caffaro, Archana Krovi, Jean Kim, Scott L. Watson, Rodney W. Snyder, Purvi R. Patel, Timothy R. Fennell, and Leah M. Johnson. 2025. "Oral Exposure to Nylon-11 and Polystyrene Nanoplastics During Early-Life in Rats" Nanomaterials 15, no. 6: 465. https://doi.org/10.3390/nano15060465
APA StyleMortensen, N. P., Caffaro, M. M., Krovi, A., Kim, J., Watson, S. L., Snyder, R. W., Patel, P. R., Fennell, T. R., & Johnson, L. M. (2025). Oral Exposure to Nylon-11 and Polystyrene Nanoplastics During Early-Life in Rats. Nanomaterials, 15(6), 465. https://doi.org/10.3390/nano15060465







