Silicon/Hard Carbon Composites Synthesized from Phenolic Resin as Anode Materials for Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparations
2.2. Characterizations
2.3. Electrode Preparation and Half-Cell Fabrications
2.4. Electrochemical Measurements
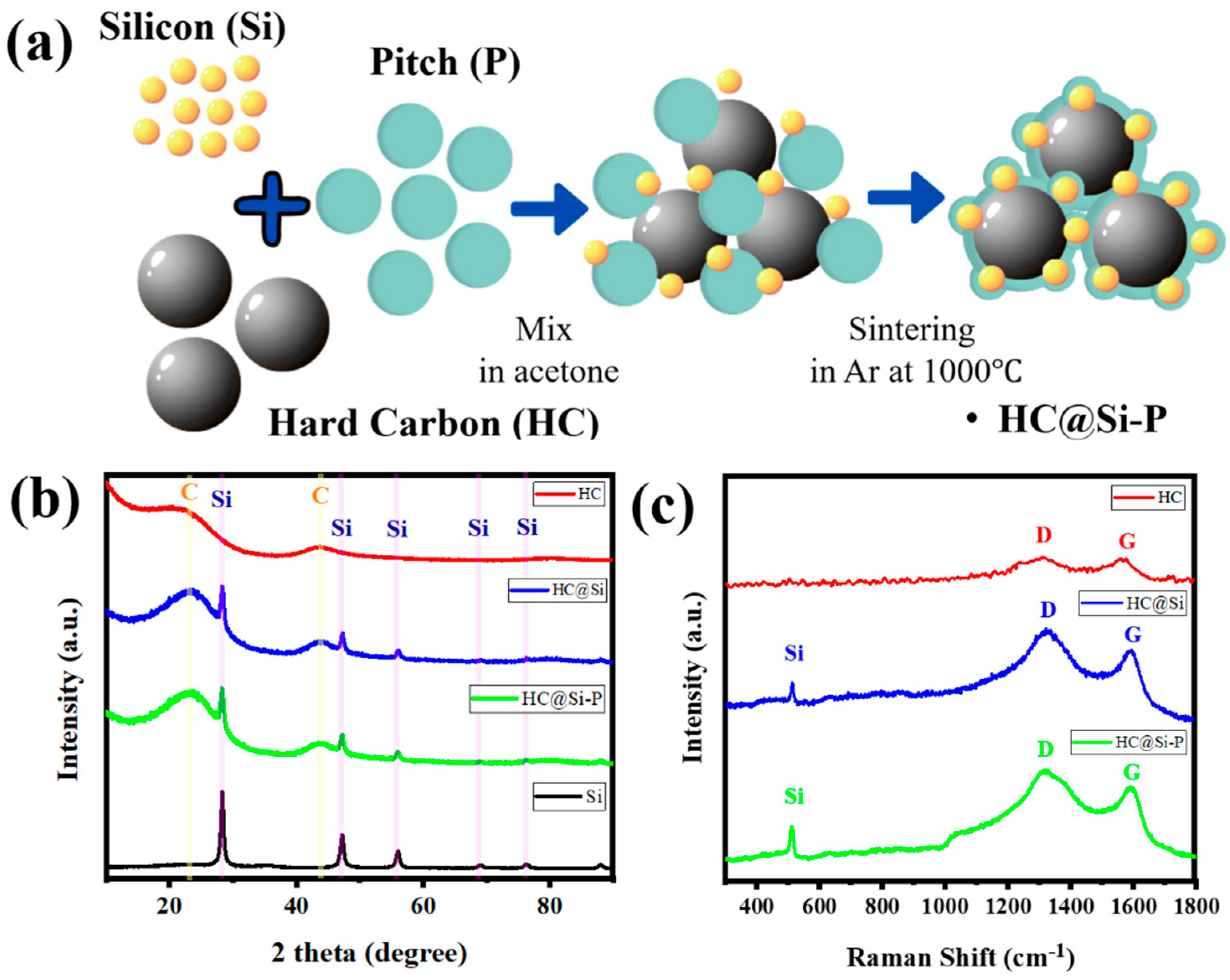
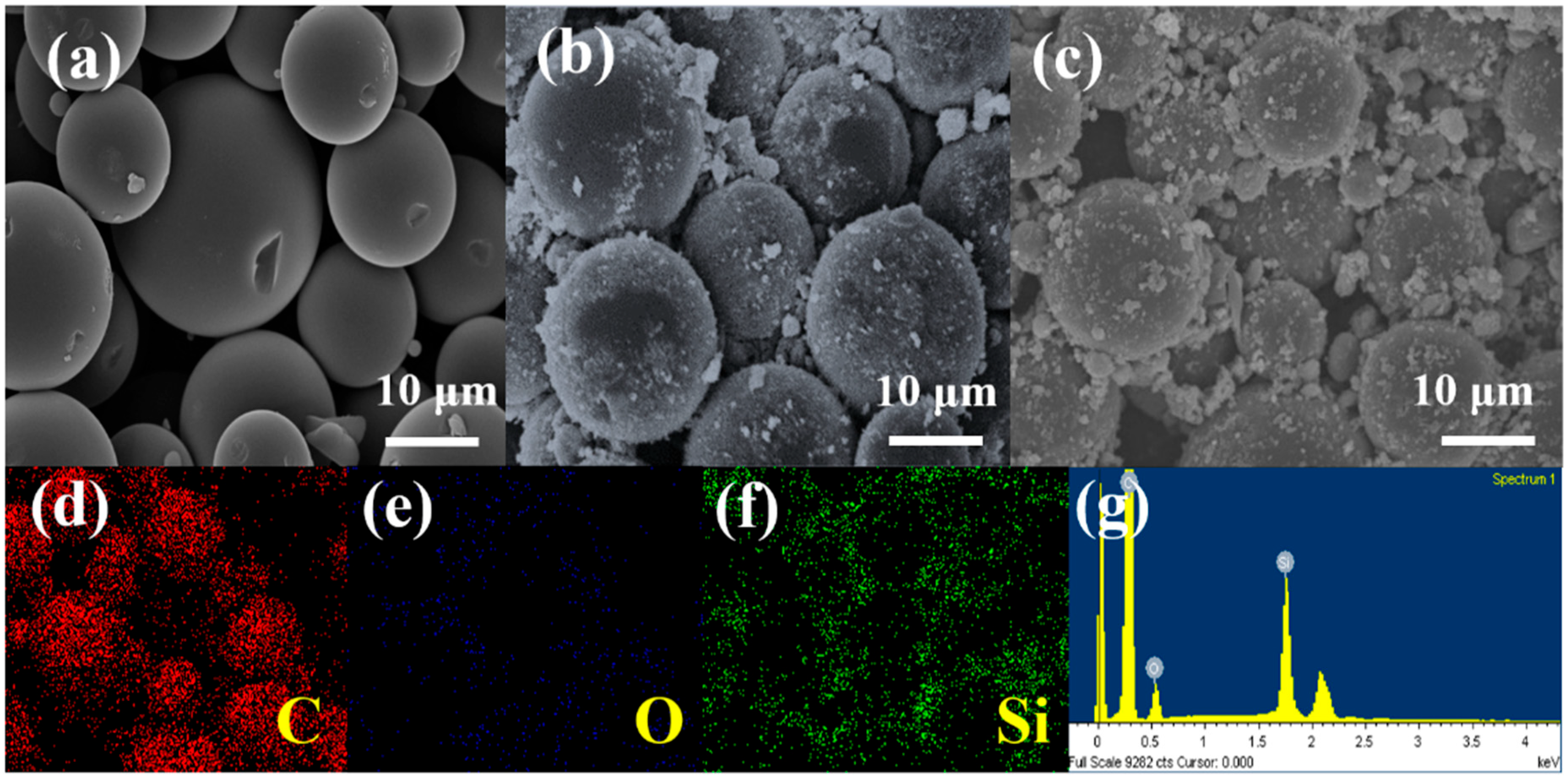
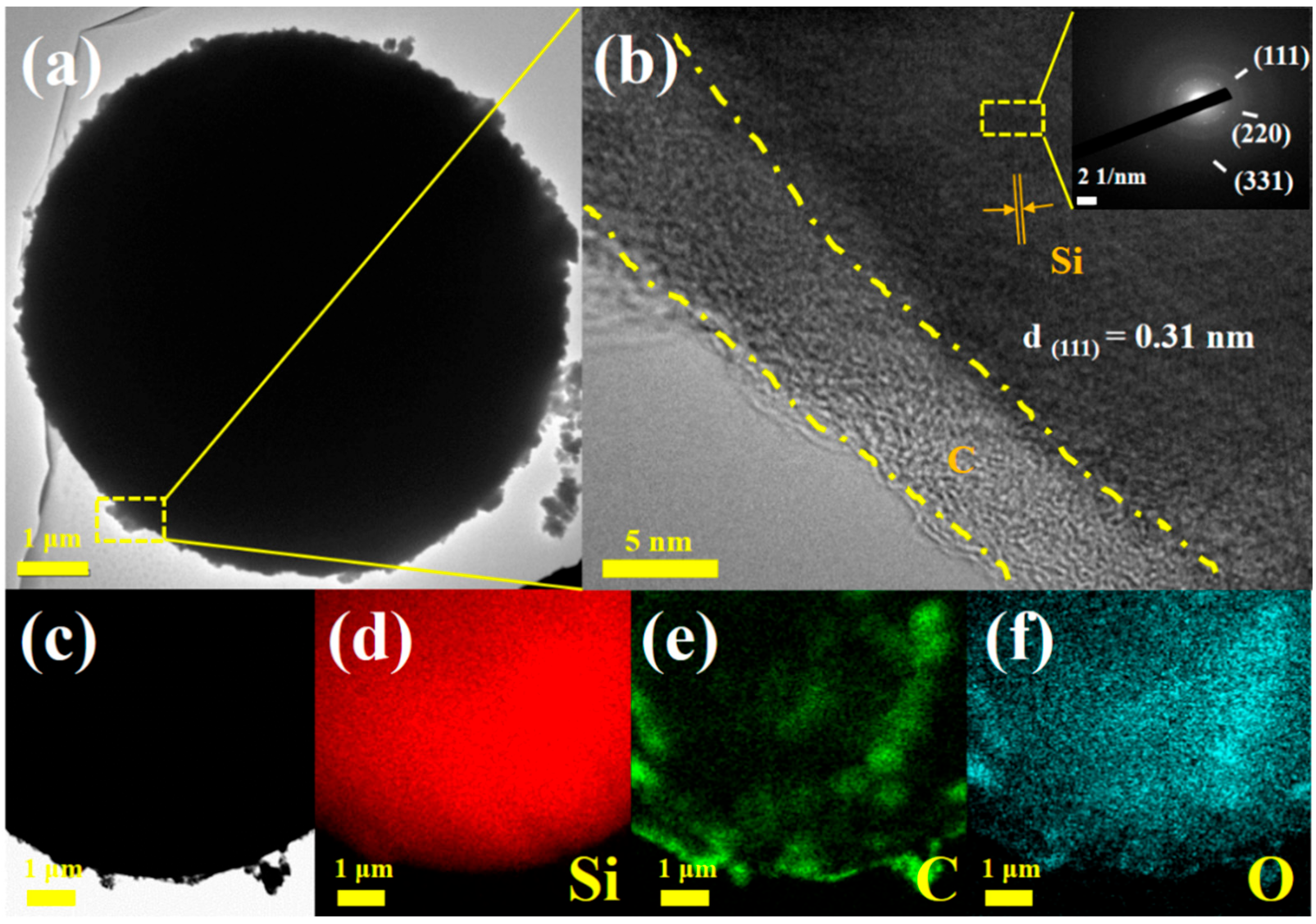
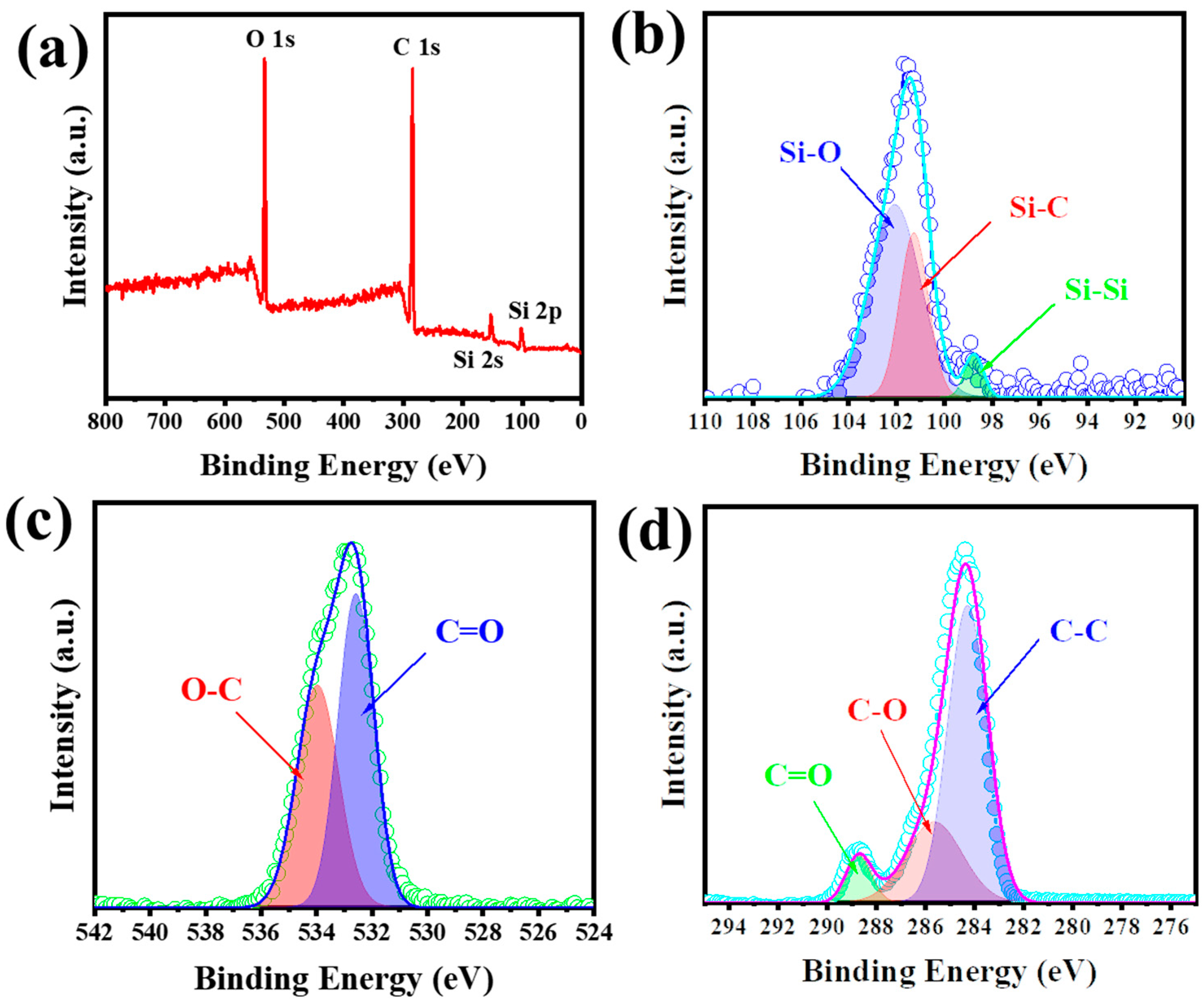
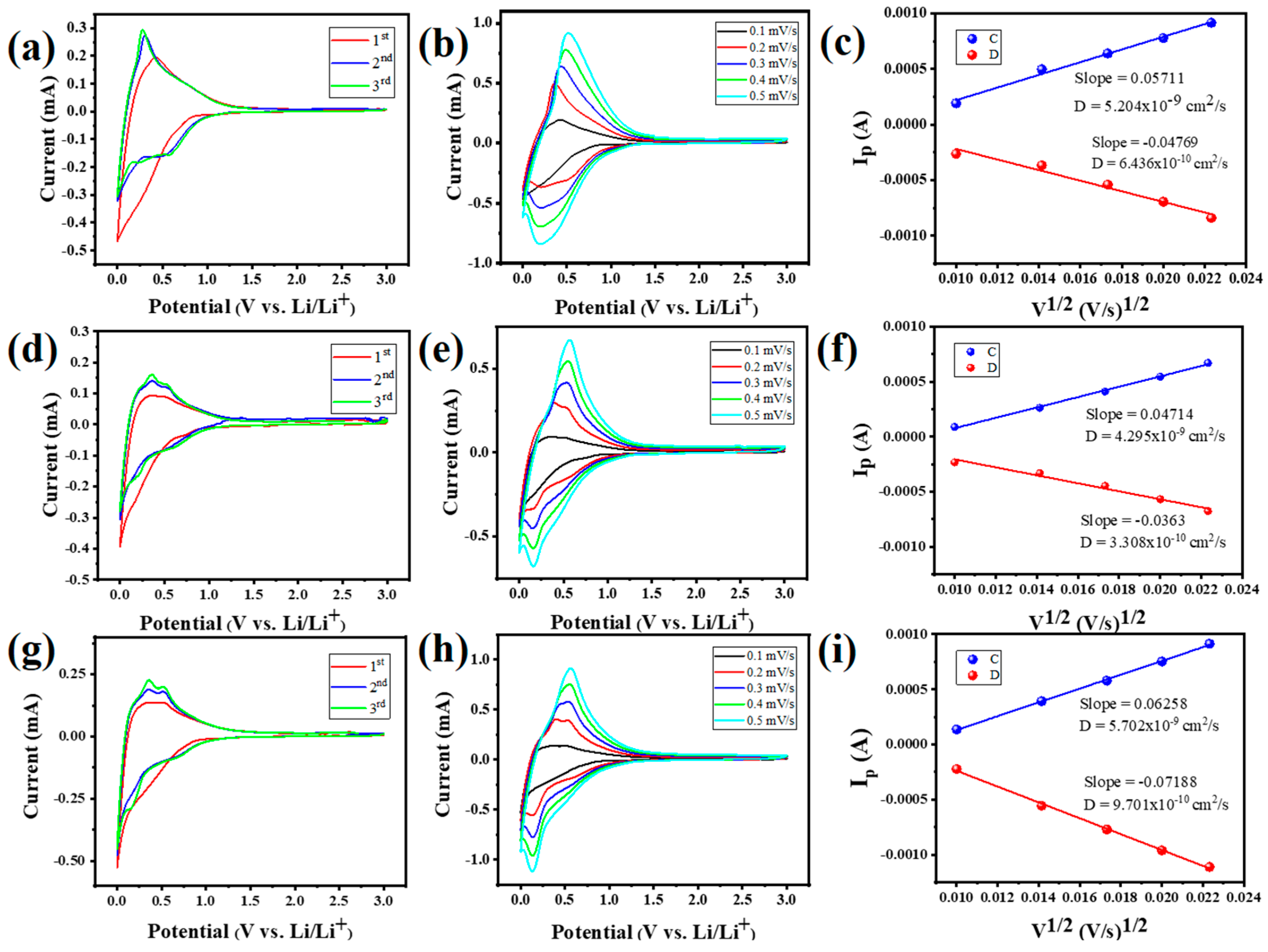
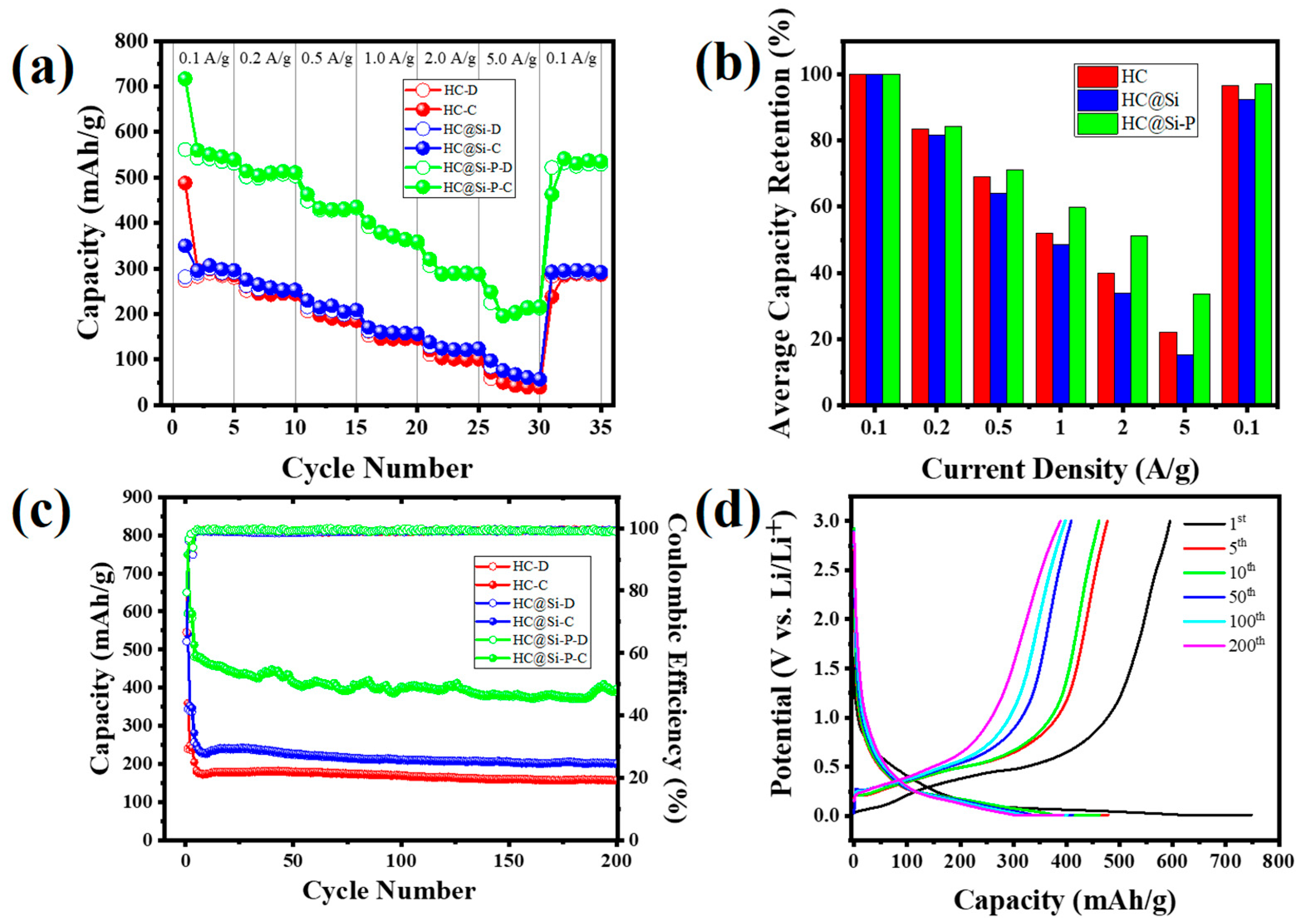
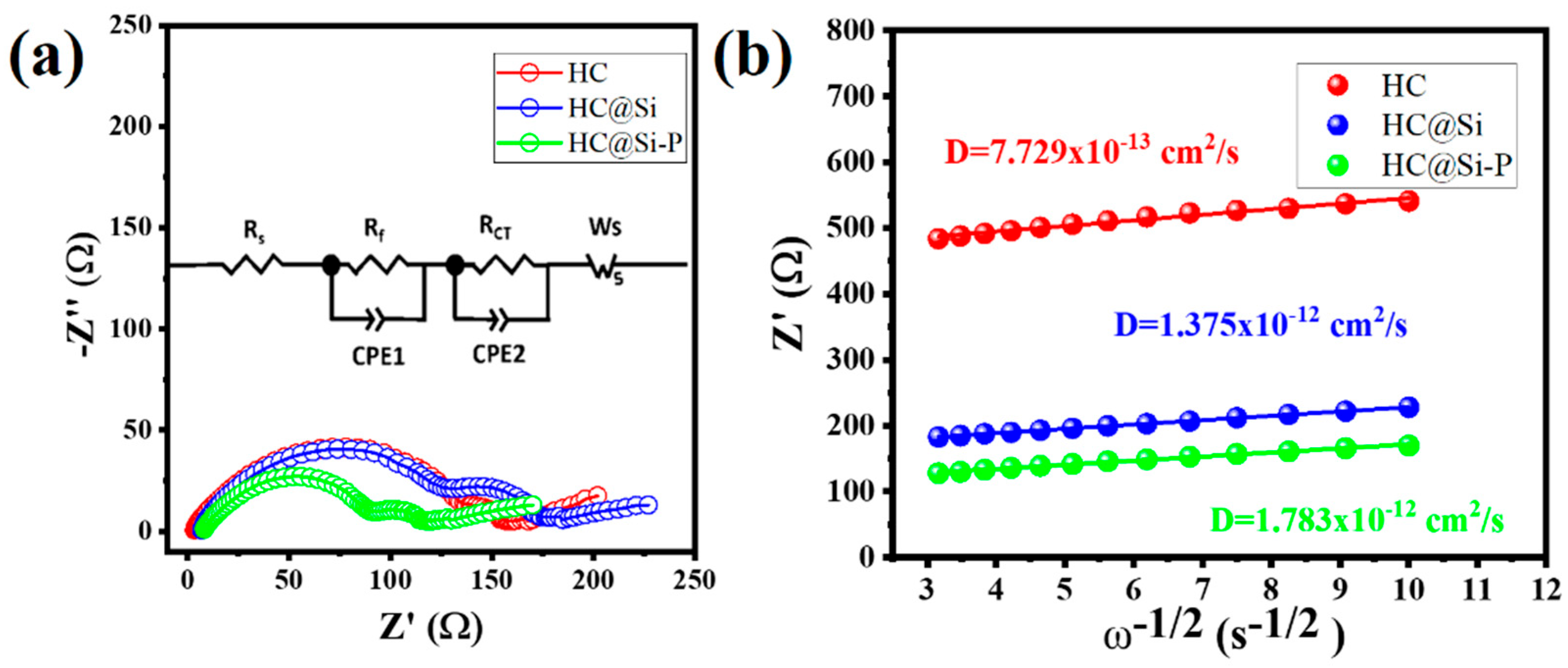
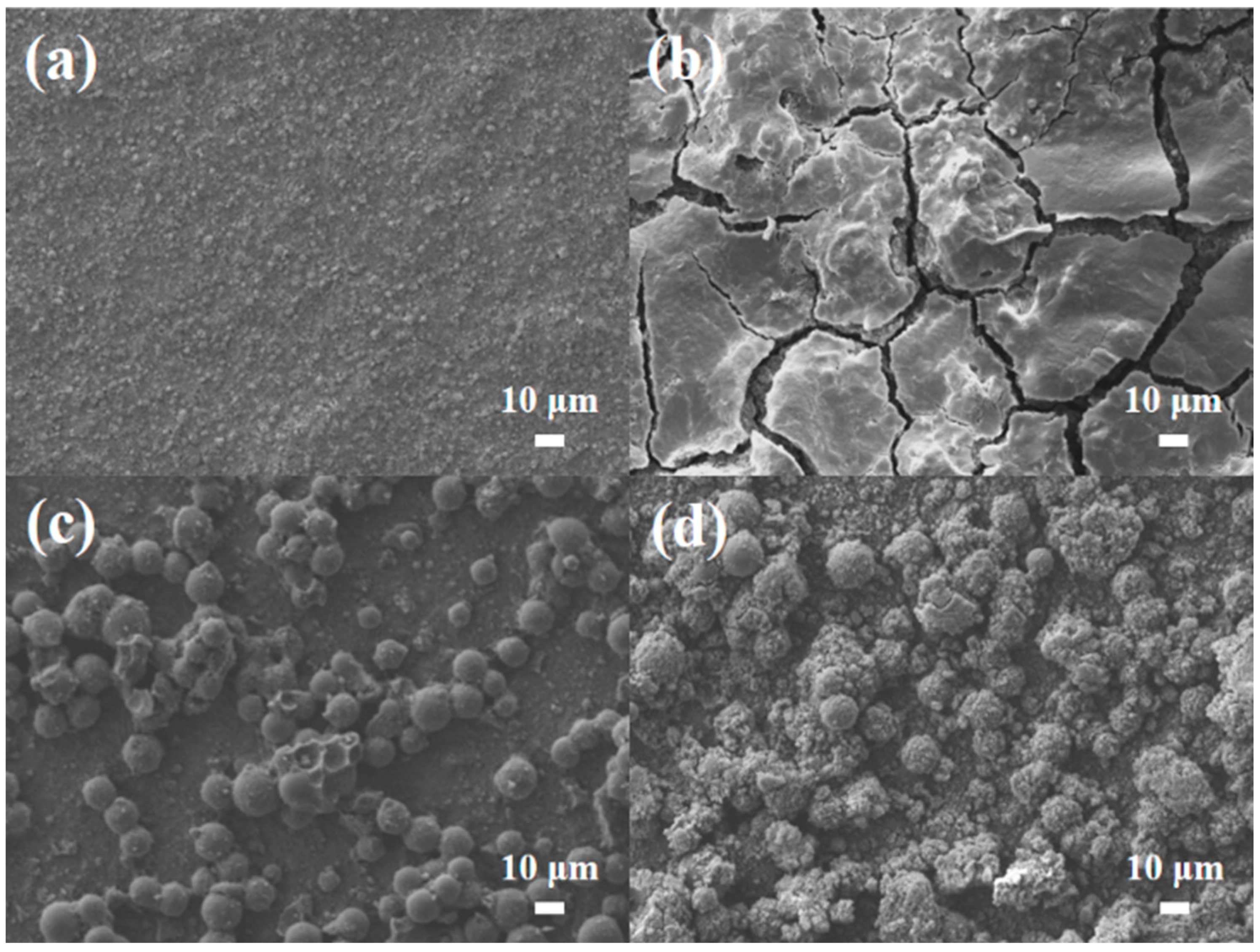
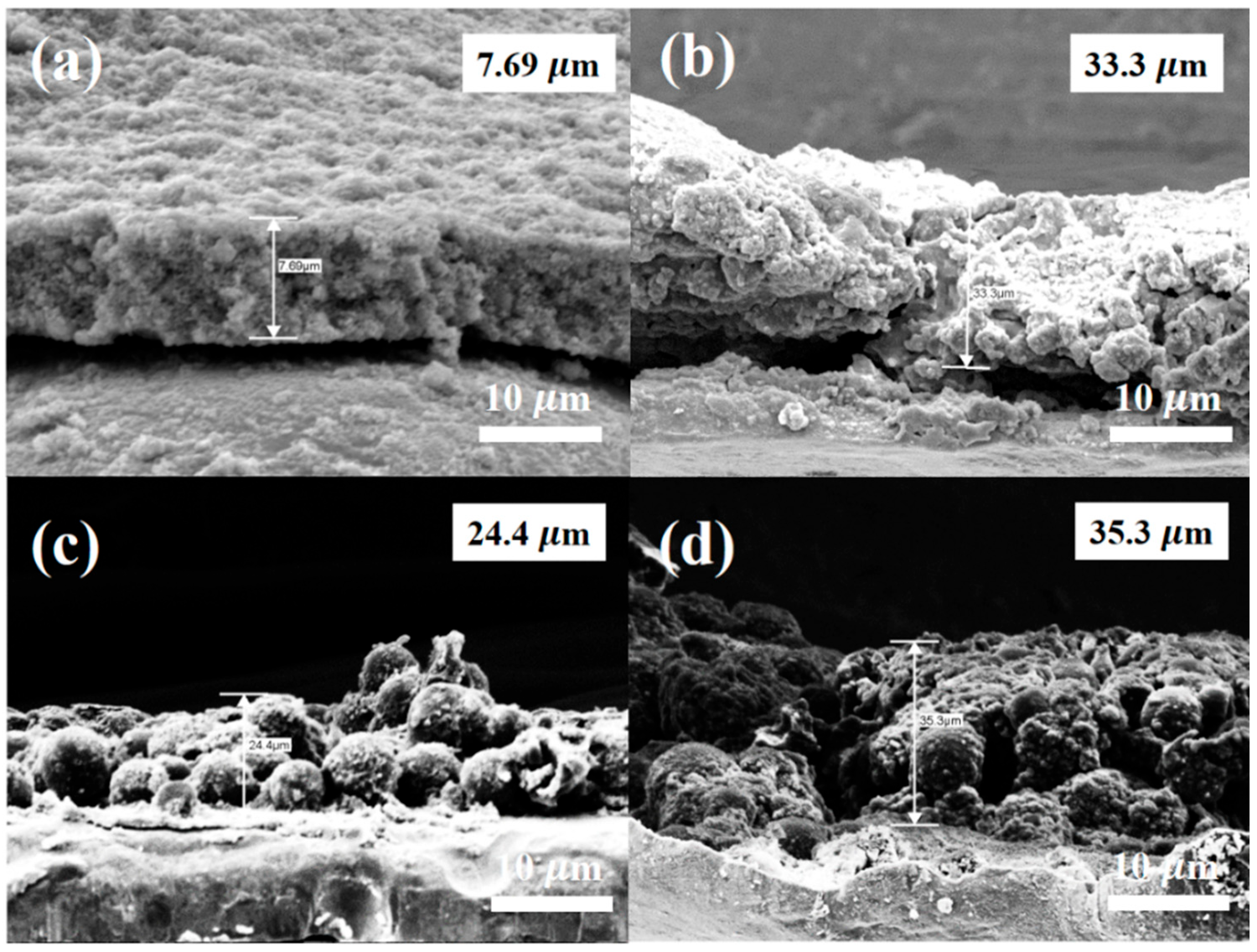
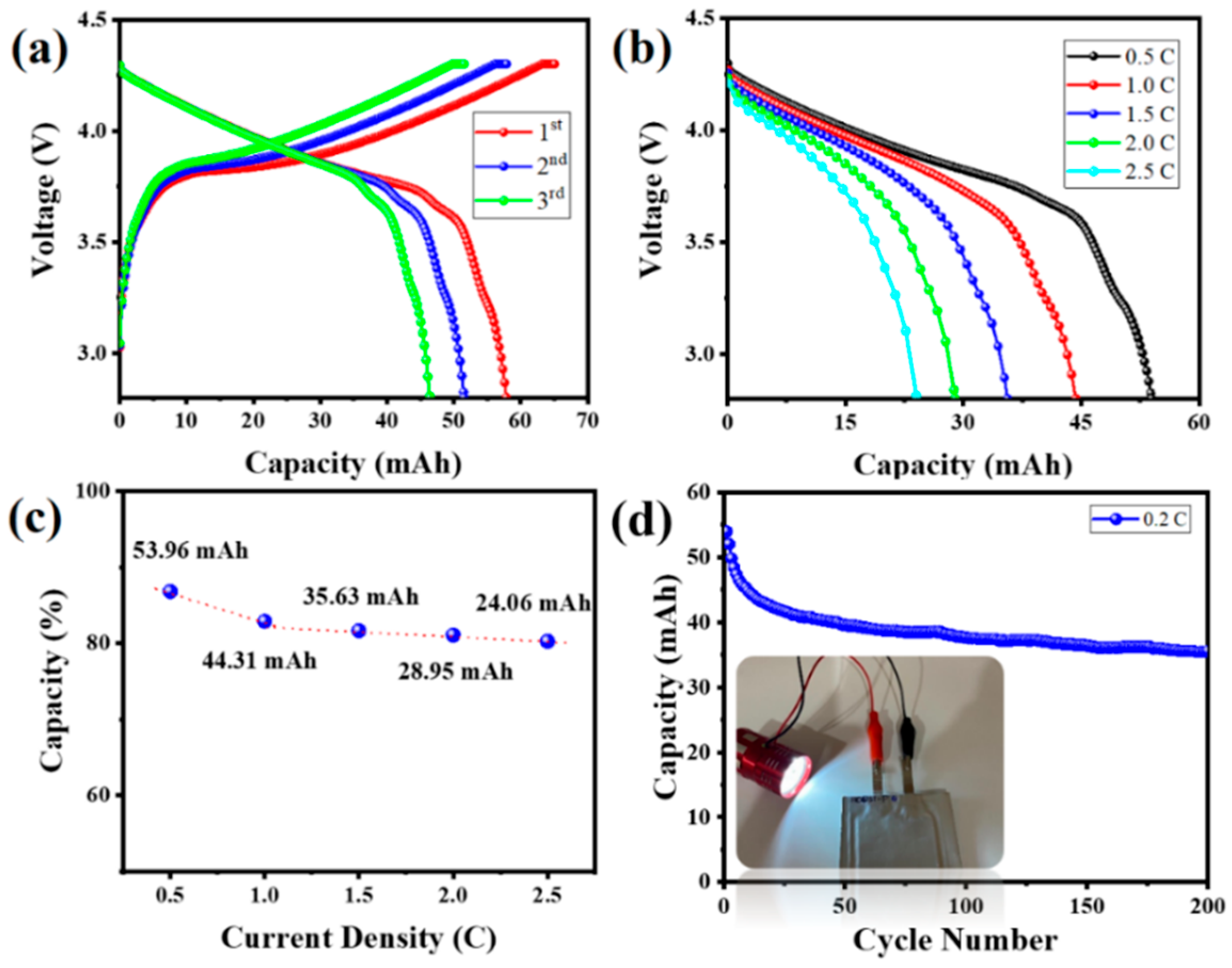
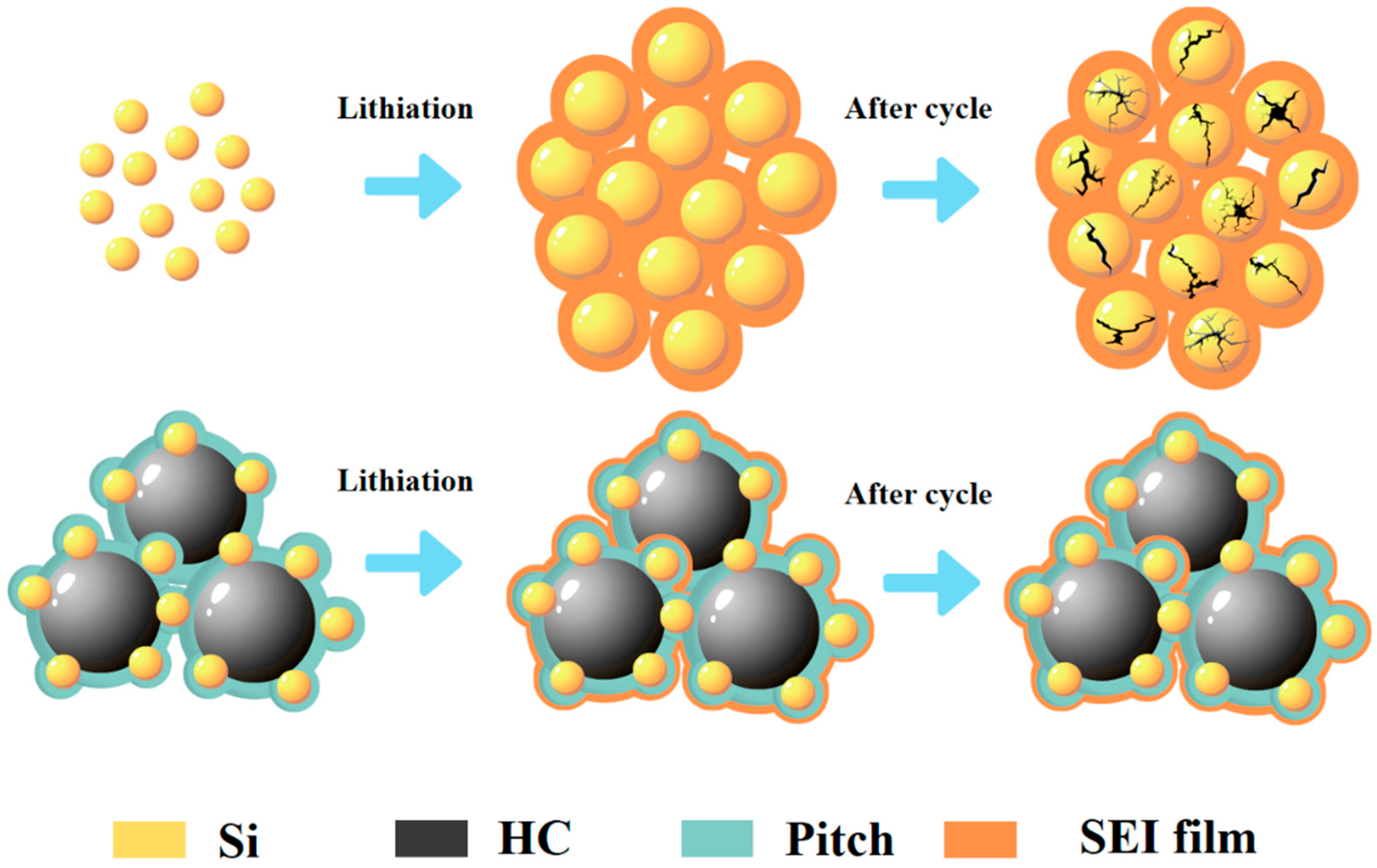
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sloop, S.; Kerr, J.; Kinoshita, K. The role of Li-ion battery electrolyte on performance decline and self-discharge. J. Power Sources 2003, 229–221, 330–337. [Google Scholar] [CrossRef]
- Yazami, R.; Reynier, Y. Mechanism of self-discharge in graphite–lithium anode. Electrochim. Acta 2002, 47, 1217–1223. [Google Scholar] [CrossRef]
- Kohler, U.; Kumpers, J.; Ullrich, M. High performance nickel-metal hydride and lithium-ion batteries. J. Power Sources 2002, 105, 139–144. [Google Scholar] [CrossRef]
- Kumar, D.R.; Kanagaraj, I.; Sukanya, R.; Karthik, R.; Hasan, M.; Thalji, M.-R.; Dhakal, G.; Milton, M.; Prakash, A.-S.; Shim, J.-J. Ti3C2Tx Filled in EMIMBF4 Semi-Solid Polymer Electrolytes for the Zinc–Metal Battery. ACS Appl. Mater. Interfaces 2024, 16, 33294–33306. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A review of lithium-ion battery safety concerns: The issues, strategies. J. Energy Chem 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Wu, F.; Maier, J.; Yu, Y. Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem. Soc. Rev. 2020, 49, 1569. [Google Scholar] [CrossRef]
- Fujimoto, H.; Tokumitsu, K.; Mabuchi, A.; Chinnasamy, N.; Kasuh, T. The anode performance of the hard carbon for the lithium-ion battery derived from the oxygen-containing aromatic precursors. J. Power Sources 2010, 195, 7452–7745. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, X.; Li, J.; Zou, Y.; Tang, J. Study of nano-porous hard carbons as anode materials for lithium-ion batteries. Mater. Chem. Phys. 2012, 135, 445–450. [Google Scholar] [CrossRef]
- Ni, J.; Huang, Y.; Gao, L. A high-performance hard carbon for Li-ion batteries and supercapacitors application. J. Power Sources 2013, 223, 306–311. [Google Scholar] [CrossRef]
- Long, W.; Fang, B.; Ignaszak, A.; Wu, Z.; Wang, Y.; Wilkinson, D. Biomass-derived nanostructured carbons and their composites as anode materials for lithium-ion batteries. Chem. Soc. Rev. 2017, 46, 7176. [Google Scholar] [CrossRef]
- Gaddam, R.; Yang, D.; Narayan, R.; Raju, K.; Kumar, N.; Zhao, X.S. Biomass derived carbon nanoparticle as anodes for high performance sodium and lithium-ion batteries. Nano Energy 2016, 23, 346–352. [Google Scholar] [CrossRef]
- Liao, L.; Ma, T.; Xiao, Y.; Wang, M.; Gao, Y.; Fang, T. Enhanced reversibility and cyclic stability of biomass-derived silicon/carbon anode material for lithium-ion battery. J. Alloys Compd. 2021, 873, 159700. [Google Scholar] [CrossRef]
- Zuo, X.; Zhu, J.; Müller-Buschbaum, P.; Cheng, Y. Silicon based lithium-ion battery anodes: A chronicle perspective review. Nano Energy 2017, 31, 113–143. [Google Scholar] [CrossRef]
- Su, X.; Wu, Q.; Li, J.; Xiao, X.; Lott, A.; Lu, W.; Sheldon, B.W.; Wu, J. Silicon-based nanomaterials for lithium-ion batteries: A Review. Adv. Energy Mater. 2014, 4, 1300882. [Google Scholar] [CrossRef]
- Feng, K.; Li, M.; Liu, W.; Kashkooli, A.; Xiao, X.; Cai, M.; Chen, Z. Silicon-based anodes for lithium-ion batteries: From fundamentals to practical applications. Small 2018, 14, 1702737. [Google Scholar] [CrossRef]
- Holzapfel, M.; Buqa, H.; Hardwick, L.J.; Hahn, M.; Würsig, A.; Scheifele, W.; Novák, P.; Kötz, R.; Veit, C.; Petrat, F. Nano silicon for lithium-ion batteries. Electrochim. Acta 2006, 52, 973–978. [Google Scholar] [CrossRef]
- Yao, Y.; McDowell, M.T.; Ryu, W.H.; Liu, N.; Hu, L.; Nix, W.D.; Cui, Y. Interconnected silicon hollow nanospheres for Lithium-ion battery anodes with long cycle life. Nano Lett. 2011, 11, 2949–2954. [Google Scholar] [CrossRef]
- Liu, N.; Hu, L.; McDowell, M.T.; Jackson, A.; Cui, Y. Prelithiated silicon nanowires as an anode for lithium-ion batteries. ACS Nano 2011, 5, 6487–6493. [Google Scholar] [CrossRef]
- Liang, B.; Liu, Y.; Xu, Y. Silicon-based materials as high-capacity anodes for next generation lithium-ion batteries. J. Power Sources 2014, 267, 469–490. [Google Scholar] [CrossRef]
- Ko, M.; Chae, S.; Ma, J.; Kim, N.; Lee, H.; Cui, Y.; Cho, J. Scalable synthesis of silicon-nanolayer-embedded graphite for high-energy lithium-ion batteries. Nat. Energy 2016, 1, 16113. [Google Scholar] [CrossRef]
- Wen, Z.; Lu, G.; Mao, S.; Kim, H.; Cui, S.; Yu, K.; Huang, X.; Hurley, P.T.; Mao, O.; Chen, J. Silicon nanotube anode for lithium-ion batteries. Electrochem. Commun. 2013, 29, 67–70. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, W.L.; Gregoire, J.; Pharr, M.; Suo, Z.; Vlassak, J.J.; Kaxiras, E. Lithium-assisted plastic deformation of silicon electrodes in lithium-ion batteries: A first-principles theoretical study. Nano Lett. 2011, 7, 2962–2967. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Yang, Y.; Hsu, C.; Cui, Y. Carbon−silicon core−shell nanowires as high-capacity electrode for lithium-ion batteries. Nano Lett. 2009, 9, 3370–3374. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Rong, J.; Fang, X.; Zhou, C. Porous doped silicon nanowires for lithium-ion battery anode with long cycle life. Nano Lett. 2012, 12, 2318–2323. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Jie, J.; Zhang, W.; Lee, S. Silicon nanowires for rechargeable lithium-ion battery anodes. Appl. Phys. Lett. 2008, 93, 033105. [Google Scholar] [CrossRef]
- Li, P.; Kim, H.; Myung, S.; Sun, Y. Diverting exploration of silicon anode into practical way: A review focused on silicon-graphite composite for lithium-ion batteries. Energy Storage Mater. 2021, 35, 550–576. [Google Scholar] [CrossRef]
- Bogart, T.D.; Oka, D.; Lu, X.; Gu, M.; Wang, C.; Korgel, B.A. Lithium-ion battery performance of silicon nanowires with carbon skin. ACS Nano 2014, 8, 915–922. [Google Scholar] [CrossRef]
- Liu, L.; Xie, F.; Lyu, J.; Zhao, T.; Li, T.; Choi, B. Tin-based anode materials with well-designed architectures for next-generation lithium-ion batteries. J. Power Sources 2016, 321, 11–35. [Google Scholar] [CrossRef]
- Kamali, A.; Fray, D.J. Tin-based materials as advanced anode materials for lithium-ion batteries: A review. Rev. Adv. Mater. Sci 2011, 27, 14–24. [Google Scholar]
- Liang, C.; Gao, M.; Pan, H.; Liu, Y.; Yan, M. Lithium alloys and metal oxides as high-capacity anode materials for lithium-ion batteries. J. Alloys Compd. 2013, 575, 246–256. [Google Scholar] [CrossRef]
- Wu, H.; Chen, J.; Hng, H.; Lou, X. Nanostructured metal oxide-based materials as advanced anodes for lithium-ion batteries. Nanoscale 2012, 4, 2526. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.; Singh, J.; Kuila, T.; Layek, R.K.; Kime, N.; Lee, J. Recent advances in graphene and its metal-oxide hybrid nanostructures for lithium-ion batteries. Nanoscale 2015, 7, 4820. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, H. Enhancing the performances of Li-ion batteries by carbon-coating: Present and future. Chem. Commun. 2012, 48, 1201–1217. [Google Scholar] [CrossRef]
- Varzi, A.; Mattarozzi, L.; Cattarin, S.; Guerriero, P.; Passerini, S. 3D Porous Cu–Zn alloys as alternative anode materials for Li-ion batteries with superior low T performance. Adv. Energy Mater. 2018, 5, 1701706. [Google Scholar] [CrossRef]
- Kwon, J.; Ryu, J.; Oh, S. Performance of electrochemically generated Li21Si5 phase for lithium-ion batteries. Electrochim. Acta 2010, 30, 8051–8055. [Google Scholar] [CrossRef]
- Wang, J.; Sun, X. Understanding and recent development of carbon coating on LiFePO4 cathode materials for lithium-ion batteries. Energy Environ. Sci. 2012, 5, 5163–5185. [Google Scholar] [CrossRef]
- Yaroslavtsev, A.; Stenina, I.A. Carbon coating of electrode materials for lithium-ion batteries. Surf. Innov. 2021, 9, 92–110. [Google Scholar] [CrossRef]
- Djian, D.; Alloin, F.; Martinet, S.; Lignier, H.; Sanchez, J.Y. Lithium-ion batteries with high charge rate capacity: Influence of the porous separator. J. Power Sources 2007, 172, 416–421. [Google Scholar] [CrossRef]
- Li, Z.H.; Zhang, H.P.; Zhang, P.; Li, G.C.; Wu, Y.P.; Zhou, X.D. Effects of the porous structure on conductivity of nanocomposite polymer electrolyte for lithium-ion batteries. J. Membr. Sci. 2008, 322, 416–422. [Google Scholar] [CrossRef]
- Hu, G.; Yu, R.; Liu, Z.; Yu, Q.; Zhang, Y.; Chen, Q.; Wu, J.; Zhou, L.; Mai, L. Study of surface oxidation layer-mediated conformal carbon coating on Si nanoparticles. ACS Appl. Mater. Interfaces 2021, 13, 3991–3998. [Google Scholar] [CrossRef]
- Lu, Z.; Li, B.; Yang, D.; Lv, H.; Xueab, M.; Zhang, C. Self-assembled silicon/phenolic resin-based carbon core–Shell nanocomposite. RSC Adv. 2018, 8, 3477–3482. [Google Scholar] [CrossRef] [PubMed]
- Huanga, Y.; Luoa, J.; Penga, J.; Shi, M.; Li, X.; Wanga, X.; Chang, B. Porous silicon–graphene–carbon composite as high-performance anode material for lithium-ion batteries. J. Energy Storage 2020, 27, 101075. [Google Scholar] [CrossRef]
- Wang, M.; Fan, L. Silicon/carbon nanocomposite pyrolyzed from phenolic resin as anode materials for lithium-ion batteries. J. Power Sources 2013, 244, 570–574. [Google Scholar] [CrossRef]
- Batool, S.; Idrees, M.; Kong, J.; Zhang, J.; Kong, S.; Dong, M.; Hou, H.; Fan, J.; Wei, H.; Guo, Z. Assessment of the electrochemical behaviour of silicon@carbon nanocomposite anode for lithium-ion batteries. J. Alloys Compd. 2020, 832, 154644. [Google Scholar] [CrossRef]
- Qian, G.; Liao, X.; Zhu, Y.; Pan, F.; Chen, X.; Yang, Y. Designing flexible lithium-ion batteries by structural engineering. ACS Energy Lett. 2019, 4, 690–701. [Google Scholar] [CrossRef]
- Luo, B.; Zhi, L. Design and construction of three-dimensional graphene-based composites for lithium-ion battery applications. Energy Environ. Sci. 2015, 8, 456–477. [Google Scholar] [CrossRef]
- Zhao, S.; Guo, Z.; Yan, K.; Wan, S.; He, F.; Suna, B.; Wanga, G. Toward high-energy-density lithium-ion batteries: Strategies for developing high-capacity lithium-rich cathode materials. Energy Storage Mater. 2021, 34, 716–734. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Y.; Li, W.; Ma, B.; Chen, X. Rational material design for ultrafast rechargeable lithium-ion batteries. Chem. Soc. Rev. 2015, 44, 5926–5940. [Google Scholar] [CrossRef]
- Ying, Q.; Wang, G.; Li, S.; Liu, T.; Qiua, J.; Li, H. Recent progress of structural designs of silicon for performance-enhanced lithium-ion batteries. Chem. Eng. J. 2020, 397, 125380. [Google Scholar]
- Isaev, I.; Salitra, G.; Soffer, A.; Cohen, Y.S.; Aurbach, D.; Fischer, J. A new approach for the preparation of anodes for Li-ion batteries based on activated hard carbon cloth with pore design. J. Power Sources 2003, 119–121, 28–33. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Deng, Z.; Kim, E.-M.; Jeong, S.-M. Facile synthesis of Si/C composites for high-performance lithium-ion battery anodes. Nanoscale 2025, 17, 6049–6057. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Shen, X.; Yao, J.; Park, J. Graphene nanosheets for enhanced lithium storage in lithium-ion batteries. Carbon 2009, 47, 2049–2053. [Google Scholar] [CrossRef]
- Fei, H.; Peng, Z.; Li, L.; Yang, Y.; Lu, W.; Samuel, E.L.G.; Fan, X.; Tour, J.M. Preparation of carbon-coated iron oxide nanoparticles dispersed on graphene sheets and applications as advanced anode materials for lithium-ion batteries. Nano Res. 2014, 7, 502–510. [Google Scholar] [CrossRef]
- Jia, H.; Gao, P.; Yang, J.; Wang, J.; Nuli, Y.; Yang, Z. Novel three-dimensional mesoporous silicon for high power lithium-ion battery anode material. Adv. Energy Mater. 2011, 1, 1036–1103. [Google Scholar] [CrossRef]
- Zuo, X.; Wang, X.; Xia, Y.; Yina, S.; Ji, Q.; Yang, Z.; Wang, M.; Zheng, X.; Qiu, B.; Liu, Z.; et al. Silicon/carbon lithium-ion battery anode with 3D hierarchical macro-/mesoporous silicon network: Self-templating synthesis via magnesiothermic reduction of silica/carbon composite. J. Power Sources 2019, 412, 93–104. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.; Qiu, X.; Ma, Y.; Kong, D.; Müllen, K.; Li, X.; Zhi, L. Stable high-capacity and high-rate silicon-based lithium battery anodes upon two-dimensional covalent encapsulation. Nature 2020, 11, 3826. [Google Scholar] [CrossRef]
- Shin, H.; Corno, J.A.; Gole, J.L.; Liu, M. Porous silicon negative electrodes for rechargeable lithium batteries. J. Power Sources 2005, 139, 314–320. [Google Scholar] [CrossRef]
- Qi, Z.; Dai, L.; Wang, Z.; Xie, L.; Chen, J.; Cheng, J.; Song, G.; Li, X.; Sun, G.; Chen, C. Optimizing the carbon coating to eliminate electrochemical interface polarization in a high-performance silicon anode for use in a lithium-ion battery. New Carbon Mater. 2022, 37, 245–258. [Google Scholar] [CrossRef]
- Guo, B.; Shu, J.; Wang, Z.; Yang, H.; Shi, L.; Liu, Y.; Chen, L. Electrochemical reduction of nano-SiO2 in hard carbon as anode material for lithium-ion batteries. Electrochem. Commun. 2008, 10, 1876–1878. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Kan, G.; Zhang, Z.; Wang, C.; Zhong, Z.; Su, F. Scalable synthesis of porous silicon/carbon microspheres as improved anode materials for Li-ion batteries. RSC Adv. 2014, 4, 43114–43120. [Google Scholar] [CrossRef]
- Sbrascini, L.; Staffolani, A.; Bottoni, L.; Darjazi, H.; Minnetti, L.; Minicucci, M.; Nobili, F. Structural and interfacial characterization of a sustainable Si/hard carbon composite anode for lithium-ion batteries. ACS Appl. Mater. Interfaces 2022, 14, 33257–33273. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Zhang, Z.; Wang, Y.; Tan, Q.; Zhong, Z.; Su, F. Preparation of porous silicon/carbon microspheres as high-performance anode materials for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 5859–5865. [Google Scholar] [CrossRef]
- Hsieh, C.; Liu, W. Effects of nitrogen doping on Si/carbon composite anode derived from Si wastes as potential active materials for Li ion batteries. J. Alloys Compd. 2019, 790, 829–836. [Google Scholar] [CrossRef]
- Luo, W.; Wang, Y.; Chou, S.; Xu, Y.; Li, W.; Kong, B.; Dou, S.; Liu, H.; Yang, J. Critical thickness of phenolic resin-based carbon interfacial layer for improving long cycling stability of silicon nanoparticle anodes. Nano Energy 2016, 27, 255–264. [Google Scholar] [CrossRef]
- Han, N.; Li, J.; Wang, X.; Zhang, C.; Liu, G.; Li, X.; Qu, J.; Peng, Z.; Zhu, X.; Zhang, L. Flexible carbon nanotubes confined yolk-shelled silicon-based anode with superior conductivity for lithium storage. Nanomaterials 2021, 11, 699. [Google Scholar] [CrossRef]
- Wei, Y.; Huang, Y.; Zeng, Y.; Zhang, Y.; Cheng, W.; Wang, W.; Jia, D.; Tang, X.; Wang, L. Designed formation of yolk−shell-Like N-doped carbon-coated Si nanoparticles by a facile method for lithium-ion battery anodes. ACS Appl. Energy Mater 2022, 5, 1471–1477. [Google Scholar] [CrossRef]
- Yang, W.; Ying, H.; Zhang, S.; Guo, R.; Wang, J.; Han, W. Electrochemical performance enhancement of porous Si lithium-ion battery anode by integrating with optimized carbonaceous materials. Electrochim. Acta 2020, 337, 135687. [Google Scholar] [CrossRef]
- Yin, L.; Parka, M.; Jeon, I.; Hwang, J.; Kim, J.; Lee, H.; Park, M.; Jeong, S.; Cho, C. Silicon nanoparticle self-incorporated in hollow nitrogen-doped carbon microspheres for lithium-ion battery anodes. Electrochim. Acta 2021, 368, 137630. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-H.; Babu, S.K.; Gregory, D.H.; Kheawhom, S.; Chang, J.-K.; Liu, W.-R. Silicon/Hard Carbon Composites Synthesized from Phenolic Resin as Anode Materials for Lithium-Ion Batteries. Nanomaterials 2025, 15, 455. https://doi.org/10.3390/nano15060455
Li Y-H, Babu SK, Gregory DH, Kheawhom S, Chang J-K, Liu W-R. Silicon/Hard Carbon Composites Synthesized from Phenolic Resin as Anode Materials for Lithium-Ion Batteries. Nanomaterials. 2025; 15(6):455. https://doi.org/10.3390/nano15060455
Chicago/Turabian StyleLi, Yu-Hsuan, Sompalli Kishore Babu, Duncan H. Gregory, Soorathep Kheawhom, Jeng-Kuei Chang, and Wei-Ren Liu. 2025. "Silicon/Hard Carbon Composites Synthesized from Phenolic Resin as Anode Materials for Lithium-Ion Batteries" Nanomaterials 15, no. 6: 455. https://doi.org/10.3390/nano15060455
APA StyleLi, Y.-H., Babu, S. K., Gregory, D. H., Kheawhom, S., Chang, J.-K., & Liu, W.-R. (2025). Silicon/Hard Carbon Composites Synthesized from Phenolic Resin as Anode Materials for Lithium-Ion Batteries. Nanomaterials, 15(6), 455. https://doi.org/10.3390/nano15060455










