Transition Metal-Mediated Preparation of Nitrogen-Doped Porous Carbon for Advanced Zinc-Ion Hybrid Capacitors
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Fe-NDPC and Other Samples
2.3. Characterization
2.4. Electrochemical Measurements
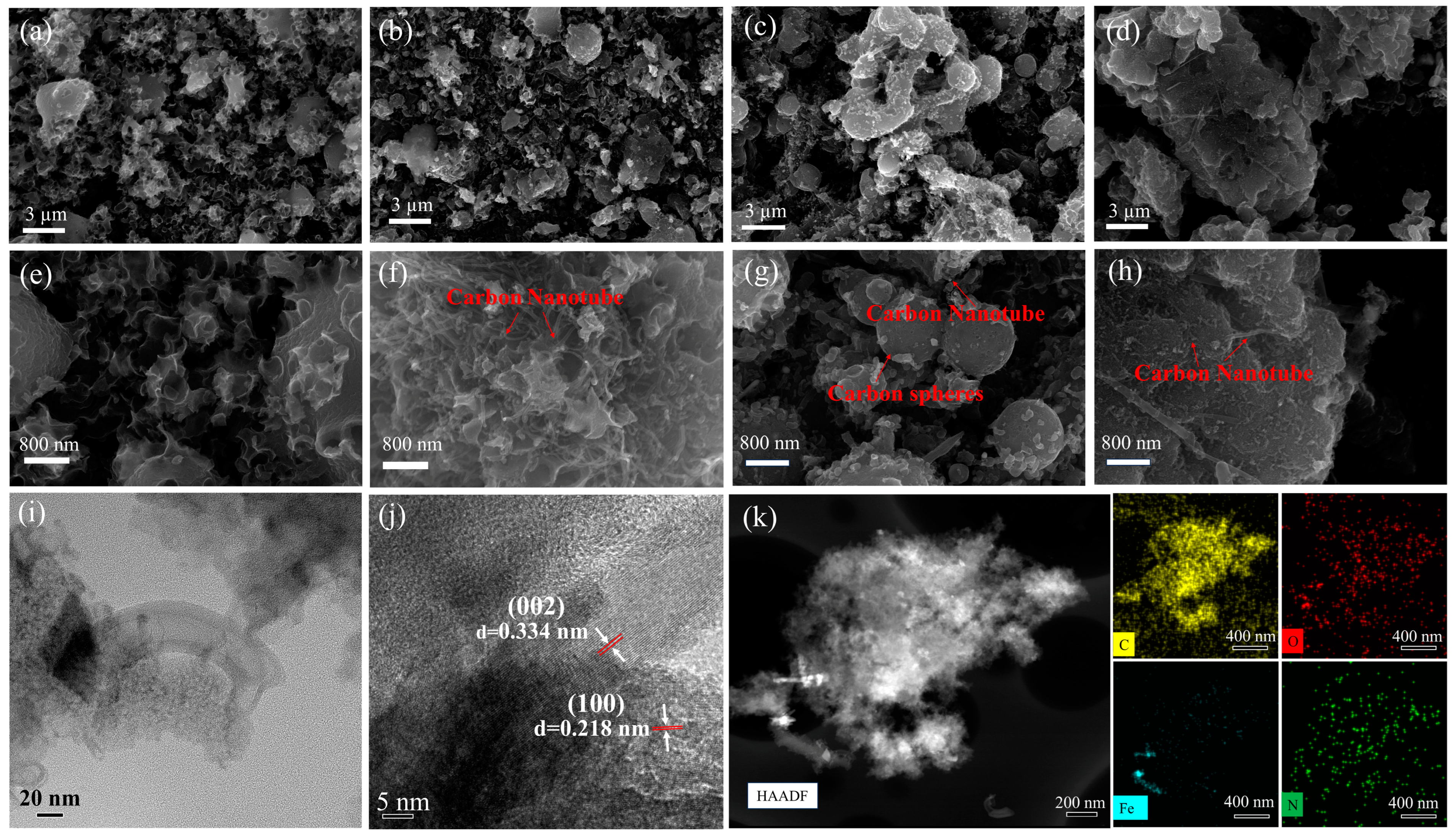
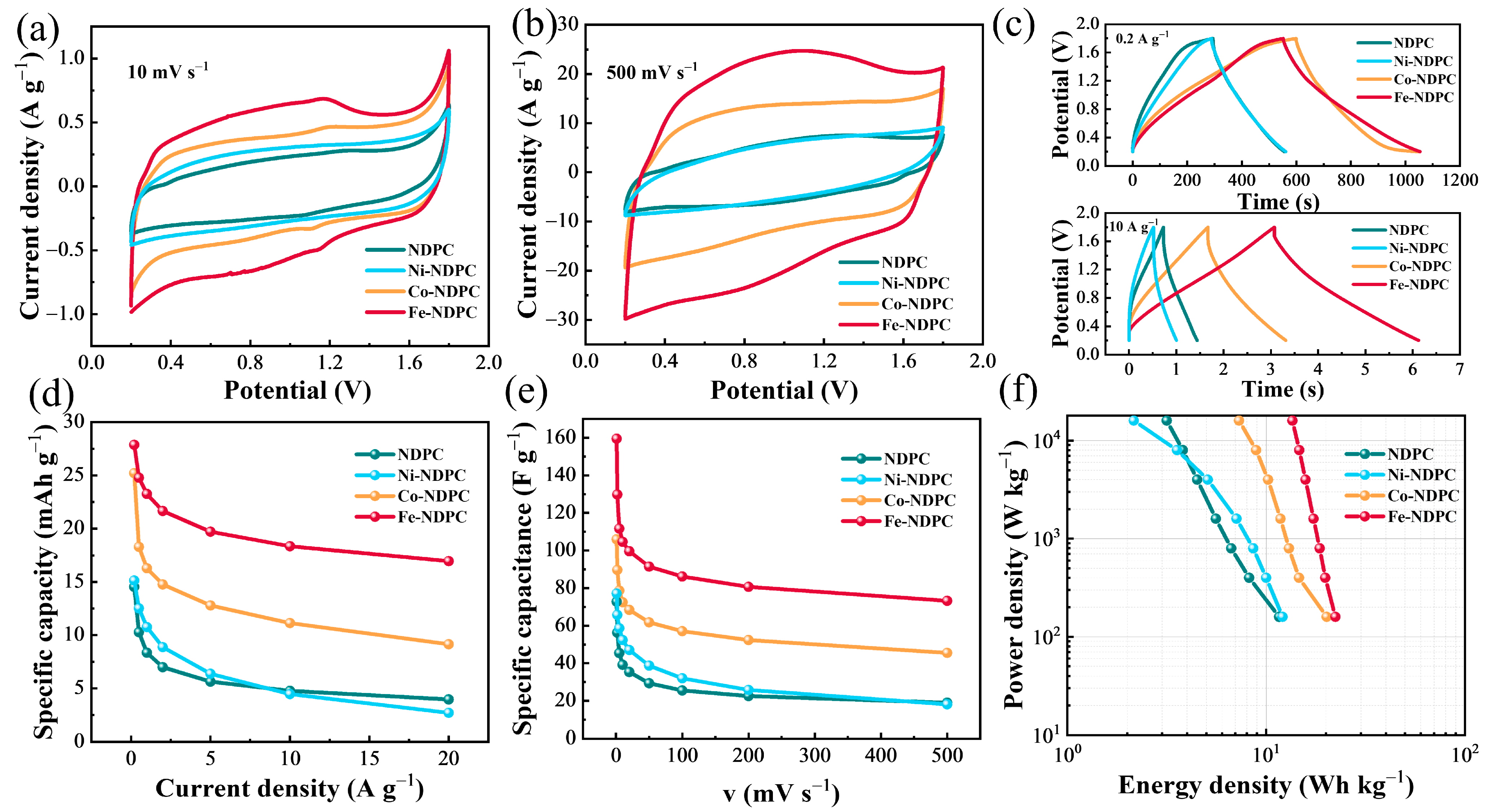
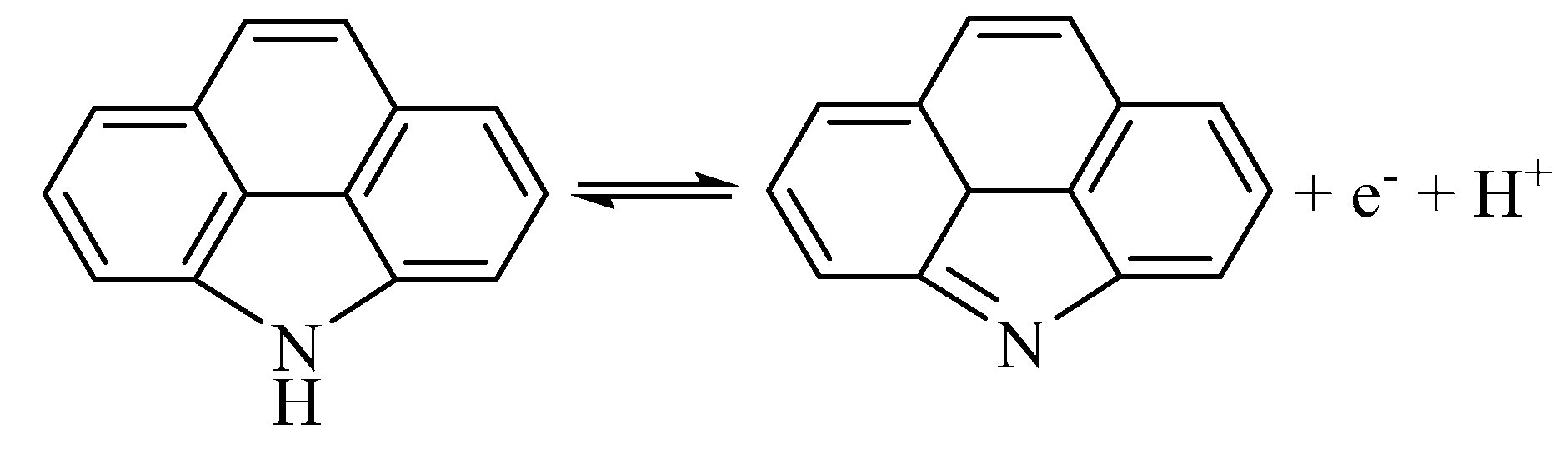
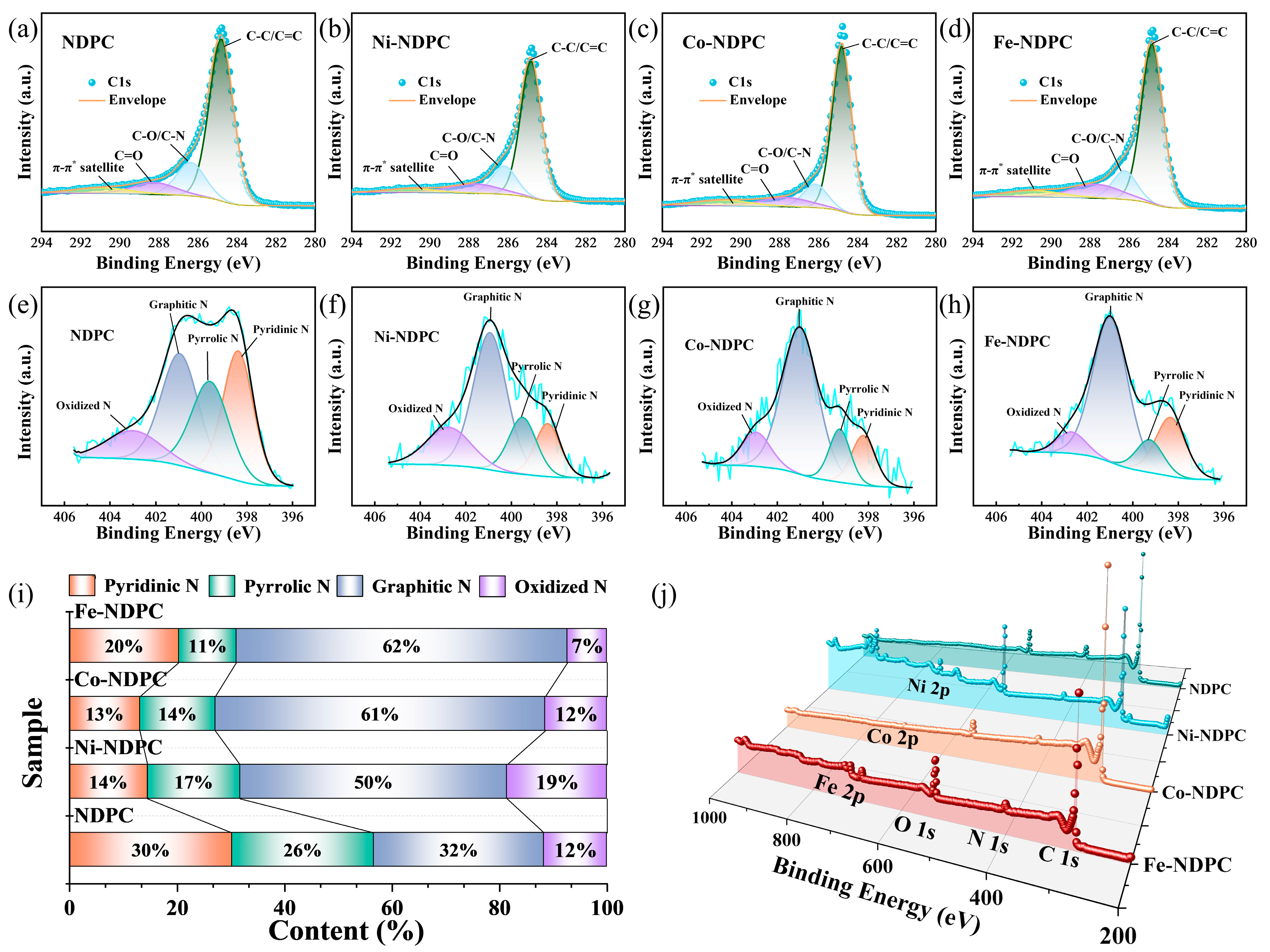
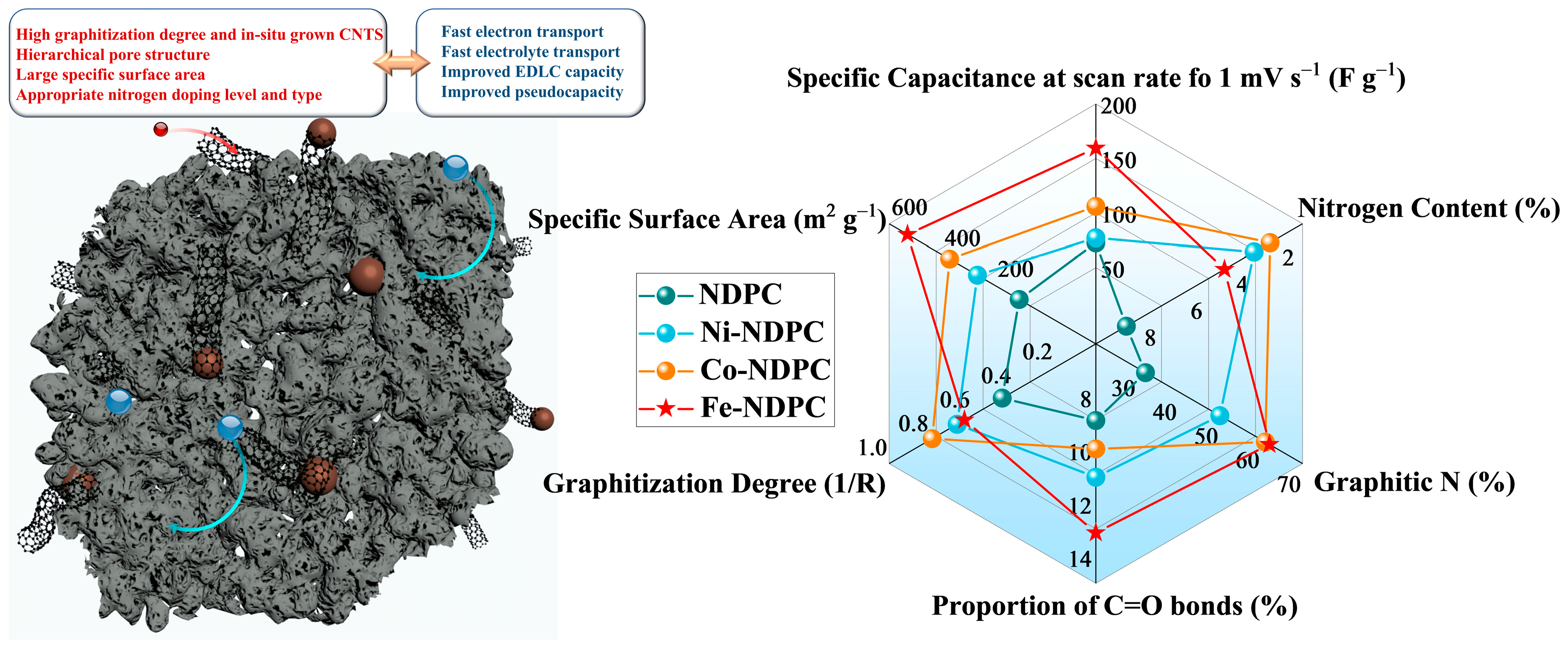
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Z.; Lv, C.; Li, W.; Wu, T.; Zhang, Q.; Tang, Y.; Shao, M.; Wang, H. Revealing the Two-Dimensional Surface Diffusion Mechanism for Zinc Dendrite Formation on Zinc Anode. Small 2021, 18, 2104148. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ge, Y.; Gao, D.; Li, Z.; Du, M.; Xing, X.; Feng, R.; Han, Y. The structural transformation of metal-organic frameworks towards 2D carbon for a desirable supercapacitor. J. Mater. Chem. C 2023, 11, 10502–10508. [Google Scholar] [CrossRef]
- Blanc, L.E.; Kundu, D.; Nazar, L.F. Scientific Challenges for the Implementation of Zn-Ion Batteries. Joule 2020, 4, 771–799. [Google Scholar] [CrossRef]
- Tang, H.; Yao, J.; Zhu, Y. Recent Developments and Future Prospects for Zinc-Ion Hybrid Capacitors: A Review. Adv. Energy Mater. 2021, 11, 2003994. [Google Scholar] [CrossRef]
- Mageto, T.; Bhoyate, S.D.; Mensah-Darkwa, K.; Kumar, A.; Gupta, R.K. Development of high-performance zinc-ion batteries: Issues, mitigation strategies, and perspectives. J. Energy Storage 2023, 70, 108081. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and Mechanisms of Asymmetric Supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mary, N.L. Review—An Overview on Supercapacitors and Its Applications. J. Electrochem. Soc. 2022, 169, 020552. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, D.; Wang, G.; Xu, Y.; Li, H.; Yan, X. An aqueous zinc-ion hybrid super-capacitor for achieving ultrahigh-volumetric energy density. Chin. Chem. Lett. 2021, 32, 926–931. [Google Scholar] [CrossRef]
- Qiu, D.; Yue, C.; Qiu, C.; Xian, L.; Li, M.; Wang, F.; Yang, R. Three-dimensional nitrogen-doped dual carbon network anode enabling high-performance sodium-ion hybrid capacitors. Electrochim. Acta 2022, 405, 139791. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, R.; Zhang, S.; Li, P.; Li, C.; Zhi, C. Recent advances and future perspectives for aqueous zinc-ion capacitors. Mater. Futures 2022, 1, 022101. [Google Scholar] [CrossRef]
- Li, H.; Ma, L.; Han, C.; Wang, Z.; Liu, Z.; Tang, Z.; Zhi, C. Advanced rechargeable zinc-based batteries: Recent progress and future perspectives. Nano Energy 2019, 62, 550–587. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, W.; Alhebshi, N.A.; Salah, N.; Alshareef, H.N. Electrochemical Zinc Ion Capacitors: Fundamentals, Materials, and Systems. Adv. Energy Mater. 2021, 11, 2100201. [Google Scholar] [CrossRef]
- Catarineu, N.R.; Lin, D.; Zhu, C.; Oyarzun, D.I.; Li, Y. High-performance aqueous zinc-ion hybrid capacitors based on 3D printed metal-organic framework cathodes. Chem. Eng. J. 2023, 465, 142544. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, T.; Song, H.; Li, X.; Liang, G.; Wang, D.; Yang, Q.; Chen, Z.; Ma, L.; Liu, Z.; et al. Effects of Anion Carriers on Capacitance and Self-Discharge Behaviors of Zinc Ion Capacitors. Angew. Chem. Int. Ed. 2020, 60, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, L. Recent advances of cathode materials for zinc-ion hybrid capacitors. Nano Energy 2023, 109, 108290. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, S.; Wu, X.; Liang, H.; Zhang, W. Status and Opportunities of Zinc Ion Hybrid Capacitors: Focus on Carbon Materials, Current Collectors, and Separators. Nano-Micro Lett. 2023, 15, 78. [Google Scholar] [CrossRef]
- Sun, Z.; Chu, S.; Jiao, X.; Li, Z.; Jiang, L. Research progress of carbon cathode materials for zinc-ion capacitors. J. Energy Storage 2024, 75, 109571. [Google Scholar] [CrossRef]
- Xue, B.; Xu, J.; Xiao, R. Ice template-assisting activation strategy to prepare biomass-derived porous carbon cages for high-performance Zn-ion hybrid supercapacitors. Chem. Eng. J. 2023, 454, 140192. [Google Scholar] [CrossRef]
- Zhang, D.; Li, L.; Gao, Y.; Wu, Y.; Deng, J. Carbon-Based Materials for a New Type of Zinc-Ion Capacitor. ChemElectroChem 2021, 8, 1541–1557. [Google Scholar] [CrossRef]
- Minakshi, M.; Samayamanthry, A.; Whale, J.; Aughterson, R.; Shinde, P.A.; Ariga, K.; Kumar Shrestha, L. Phosphorous–Containing Activated Carbon Derived From Natural Honeydew Peel Powers Aqueous Supercapacitors. Chem.—Asian J. 2024, 19, e202400622. [Google Scholar] [CrossRef]
- Minakshi, M.; Mujeeb, A.; Whale, J.; Evans, R.; Aughterson, R.; Shinde, P.A.; Ariga, K.; Shrestha, L.K. Synthesis of Porous Carbon Honeycomb Structures Derived from Hemp for Hybrid Supercapacitors with Improved Electrochemistry. ChemPlusChem 2024, 89, e202400408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cheng, R.-R.; Bi, H.-H.; Lu, Y.-H.; Ma, L.-B.; He, X.-J. A review of porous carbons produced by template methods for supercapacitor applications. New Carbon Mater. 2021, 36, 69–81. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, Z.; Shan, J.; Jin, S.; Cui, J.; Deng, Z.; Seo, M.H.; Wang, X. O, N-Codoped, Self-Activated, Holey Carbon Sheets for Low-Cost And High-Loading Zinc-Ion Supercapacitors. Adv. Funct. Mater. 2023, 34, 2302818. [Google Scholar] [CrossRef]
- Du, W.; Ang, E.H.; Yang, Y.; Zhang, Y.; Ye, M.; Li, C.C. Challenges in the material and structural design of zinc anode towards high-performance aqueous zinc-ion batteries. Energy Environ. Sci. 2020, 13, 3330–3360. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Yu, L.; Gao, J.; He, X.; Liu, H.; Guo, Y.; Zhang, G. Dual-doped carbon hollow nanospheres achieve boosted pseudocapacitive energy storage for aqueous zinc ion hybrid capacitors. Energy Storage Mater. 2021, 42, 705–714. [Google Scholar] [CrossRef]
- Liu, L.; Sun, X.; Dong, Y.; Wang, D.; Wang, Z.; Jiang, Z.; Li, A.; Chen, X.; Song, H. N-doped hierarchical porous hollow carbon spheres with multi-cavities for high performance Na-ion storage. J. Power Sources 2021, 506, 230170. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, W.; Wang, W.; Alhebshi, N.A.; Salah, N.; Alshareef, H.N. Electrochemical Zinc Ion Capacitors Enhanced by Redox Reactions of Porous Carbon Cathodes. Adv. Energy Mater. 2020, 10, 2001705. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, H.; Xie, J.; Liu, X.; Lu, X. Recent progress and challenges of carbon materials for Zn-ion hybrid supercapacitors. Carbon Energy 2020, 2, 521–539. [Google Scholar] [CrossRef]
- Zhang, W.; Yin, J.; Jian, W.; Wu, Y.; Chen, L.; Sun, M.; Schwingenschlögl, U.; Qiu, X.; Alshareef, H.N. Supermolecule-mediated defect engineering of porous carbons for zinc-ion hybrid capacitors. Nano Energy 2022, 103, 107827. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Z.; Bai, Z.; Mi, H.; Ji, C.; Pang, H.; Yu, C.; Qiu, J. High energy-power Zn-ion hybrid supercapacitors enabled by layered B/N co-doped carbon cathode. Nano Energy 2019, 66, 104132. [Google Scholar] [CrossRef]
- Liu, Z.M.; Zhong, W.S.; Li, J.X.; Hu, G.S. Preparation of nitrogen-doped porous carbons with ultra-highsurface areas for high-performance supercapacitors. Chin. J. Inorg. Chem. 2024, 40, 677–685. [Google Scholar]
- Liu, P.; Gao, Y.; Tan, Y.; Liu, W.; Huang, Y.; Yan, J.; Liu, K. Rational design of nitrogen doped hierarchical porous carbon for optimized zinc-ion hybrid supercapacitors. Nano Res. 2019, 12, 2835–2841. [Google Scholar] [CrossRef]
- Liu, Z.; Xiao, K.; Guo, H.; Ning, X.; Hu, A.; Tang, Q.; Fan, B.; Zhu, Y.; Chen, X. Nitrogen-Doped Worm-Like Graphitized Hierarchical Porous Carbon Designed for Enhancing Area-Normalized Capacitance of Electrical Double Layer Supercapacitors. Carbon 2017, 117, 163–173. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Tao, L.; Nie, Q.; Meng, F.; Zhu, S.; Cui, L.; Huang, R. Ferromagnetic carbonized polyaniline/nanodiamond hybrids for ultrabroad-band electromagnetic absorption. Carbon 2020, 164, 224–234. [Google Scholar] [CrossRef]
- Ayyanusamy, P.; Swathi, T.D.; Alphonse, R.; Minakshi, M.; Sivasubramanian, R. Synthesis of Amorphous Nickel-Cobalt Hydroxides for Ni−Zn Batteries. Chem.—A Eur. J. 2024, 30, e202402325. [Google Scholar] [CrossRef]
- Haripriya, M.; Manimekala, T.; Dharmalingam, G.; Minakshi, M.; Sivasubramanian, R. Asymmetric Supercapacitors Based on ZnCo2O4 Nanohexagons and Orange Peel Derived Activated Carbon Electrodes. Chem.—Asian J. 2024, 19, e202400202. [Google Scholar] [CrossRef]
- Wang, L.; Peng, M.; Chen, J.; Hu, T.; Yuan, K.; Chen, Y. Eliminating the Micropore Confinement Effect of Carbonaceous Electrodes for Promoting Zn-Ion Storage Capability. Adv. Mater. 2022, 34, 2203744. [Google Scholar] [CrossRef]
- Hu, W.; Chen, L.; Geng, B.; Du, M.; Shan, G.; Song, Y.; Wu, Z.; Zheng, Q. Coral-Inspired Hierarchical Structure Promotes a Win-Win on Mass Loading and Rate Capability: A Nickel-Cobalt-Zinc-Sulfide@Nickel-Cobalt Layered Double Hydroxide Electrode for a High-Performance Hybrid Supercapacitor. ACS Appl. Energy Mater. 2023, 6, 2781–2792. [Google Scholar] [CrossRef]
- Gao, T.; Luo, W.; Yang, Y.; Zhou, Y.; Xu, J.; Li, N.; Li, J.; Liu, Z. Engineering hierarchically porous carbon nanorods electrode materials for high performance zinc ion hybrid supercapacitors. Colloids Surf. A 2024, 684, 133057. [Google Scholar] [CrossRef]
- Dong, L.; Yang, W.; Yang, W.; Wang, C.; Li, Y.; Xu, C.; Wan, S.; He, F.; Kang, F.; Wang, G. High-Power and Ultralong-Life Aqueous Zinc-Ion Hybrid Capacitors Based on Pseudocapacitive Charge Storage. Nano-Micro Lett. 2019, 11, 94. [Google Scholar] [CrossRef]
- Yang, B.; Zhao, W.; Gao, Z.; Yang, J.; Shi, W.; Zhang, Y.; Su, Q.; Xu, B.; Du, G. Flexible CNT@Porous carbon sponge cathode with large mesopores for high-rate zinc-ion hybrid capacitors. Carbon 2024, 218, 118695. [Google Scholar] [CrossRef]
- Zhu, J.; Childress, A.S.; Karakaya, M.; Dandeliya, S.; Srivastava, A.; Lin, Y.; Rao, A.M.; Podila, R. Defect-Engineered Graphene for High-Energy- and High-Power-Density Supercapacitor Devices. Adv. Mater. 2016, 28, 7185–7192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Deng, S.; Wang, D.; Qing, Y.; Yan, G.; Li, L.; Wu, Y. Low-tortuosity carbon electrode derived from Wood@ZIF-67 for supercapacitor applications. Chem. Eng. J. 2023, 454, 140410. [Google Scholar] [CrossRef]
- Jiang, J.; Yao, L.; Peng, H.; Wei, G.; Tian, Y.; Sun, L.; Dai, P.; Cai, P.; Zou, Y.; Zhang, H.; et al. High-Performance Zinc-Ion Hybrid Supercapacitor from Guilin Sanhua Liquor Lees-Derived Carbon Materials. ACS Appl. Mater. Interfaces 2024, 16, 22102–22112. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.-J.; Zhu, Y.; Chen, Y.; Ren, X.-Y.; Dong, H.; Zhang, H.; Ren, Q.; Luo, H.-B.; Zou, Y.; Ren, X.-M. Acidified Nitrogen Self-Doped Porous Carbon with Superprotonic Conduction for Applications in Solid-State Proton Battery. Small 2024, 20, 2305765. [Google Scholar] [CrossRef]
- Tian, K.; Wang, J.; Cao, L.; Yang, W.; Guo, W.; Liu, S.; Li, W.; Wang, F.; Li, X.; Xu, Z.; et al. Single-site pyrrolic-nitrogen-doped sp2-hybridized carbon materials and their pseudocapacitance. Nat. Commun. 2020, 11, 3884. [Google Scholar] [CrossRef]
- Wei, J.-S.; Ding, H.; Wang, Y.-G.; Xiong, H.-M. Hierarchical Porous Carbon Materials with High Capacitance Derived from Schiff-Base Networks. ACS Appl. Mater. Interfaces 2015, 7, 5811–5819. [Google Scholar] [CrossRef]
- Wickramaarachchi, K.; Minakshi, M.; Aravindh, S.A.; Dabare, R.; Gao, X.; Jiang, Z.-T.; Wong, K.W. Repurposing N-Doped Grape Marc for the Fabrication of Supercapacitors with Theoretical and Machine Learning Models. Nanomaterials 2022, 12, 1847. [Google Scholar] [CrossRef]
- Gao, P.; Shen, B.; Zhao, P.; Shi, G.; Zhao, X. Tuning the Mn2+/Mn3+ ratio of ZnMn2O4 from spent zinc-carbon battery powder to enhance the electrochemical performance. J. Power Sources 2023, 577, 233231. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Liu, Z.; Wu, D.; Wu, H.; Xiao, K. Transition Metal-Mediated Preparation of Nitrogen-Doped Porous Carbon for Advanced Zinc-Ion Hybrid Capacitors. Nanomaterials 2025, 15, 83. https://doi.org/10.3390/nano15020083
Li M, Liu Z, Wu D, Wu H, Xiao K. Transition Metal-Mediated Preparation of Nitrogen-Doped Porous Carbon for Advanced Zinc-Ion Hybrid Capacitors. Nanomaterials. 2025; 15(2):83. https://doi.org/10.3390/nano15020083
Chicago/Turabian StyleLi, Mingcheng, Zheng Liu, Dan Wu, Huihao Wu, and Kuikui Xiao. 2025. "Transition Metal-Mediated Preparation of Nitrogen-Doped Porous Carbon for Advanced Zinc-Ion Hybrid Capacitors" Nanomaterials 15, no. 2: 83. https://doi.org/10.3390/nano15020083
APA StyleLi, M., Liu, Z., Wu, D., Wu, H., & Xiao, K. (2025). Transition Metal-Mediated Preparation of Nitrogen-Doped Porous Carbon for Advanced Zinc-Ion Hybrid Capacitors. Nanomaterials, 15(2), 83. https://doi.org/10.3390/nano15020083





