1. Introduction
Skin is the largest organ in the human body, providing the first line of defense against external damage but also serving as a wall barrier against pathogen infiltration. Moreover, it plays a key role in many vital functions, such as immunological surveillance, preventing loss of physiological fluids, synthesis of vitamin D3, and temperature regulation. After a skin injury, the immune system is activated to heal the wound quickly and prevent bacterial contamination. However, there are cases, usually seen in individuals suffering from serious illnesses such as diabetes or high levels of obesity, where the natural healing process is impaired, leading to the establishment of chronic wounds that can take a significant toll on the patient’s quality of life [
1,
2].
Recently, researchers have been considering using tissue-engineered scaffolds as wound-dressing agents that can mimic both the exterior and the mid inner layer of the skin, known as the epidermis and dermis, respectively. An ideal scaffold for wound healing, apart from supreme biocompatibility, should also possess an architectural structure and porosity that allow the exchange of nutrients but concurrently prevent the entry of harmful pathogens. In addition, the scaffold must be able to maintain its integrity but also slowly degrade in a fashion that would enable the gradual restoration of the targeted tissue [
3].
Electrospinning is a technique that creates fibrous membranes using a high-voltage source that is capable of depositing them on a conductive collector. Due to the stochastic alignment of the fibers, electrospun membranes closely resemble the morphology of the natural extracellular matrix (ECM) mesh, which plays an essential structural and functional role in the wound-healing process. In addition, they exhibit high surface area properties, making them prime scaffolds for drug encapsulation and fluid absorption [
4].
Electrospraying is based on the same fundamental principles as electrospinning. However, by manipulating particular parameters, the solvent evaporates more rapidly, leading instead to droplet sputtering. The dispersion of the liquid into droplets ensures precise delivery and deposition of substances, which plays a crucial role in their controlled release. With the progress of the electrospraying technique, new opportunities have emerged to deposit and encapsulate multiple substances such as polymers, cells, and bioactive molecules into scaffolds in a spatially controlled manner, and therefore, enhance their bioavailability. Thus, the combination of electrospinning and electrospraying opens up new possibilities for the development of scaffolds that are biomimetic, have antibacterial properties, and enable the controlled release of drugs [
5].
Poly(vinyl alcohol) (PVA) is a highly hydrophilic synthetic polymer widely used in tissue engineering due to its biocompatibility and chemical resistance. In addition, scaffolds composed of PVA provide adequate mechanical stability, and its water solubility facilitates its use in biomedical applications [
6]. However, the wide use of PVA as a scaffold for wound healing has been limited due to its low bioactivity and insufficient swelling. A method used to compensate for the drawbacks of PVA is blending it with various natural polysaccharides that enhance cell adhesion [
7]. Carrageenans are water-soluble, linear, and natural polysaccharides extracted from red seaweeds. Kappa carrageenan (KC) in particular, has one sulfate group per disaccharide unit, which endows it with a negative charge at 7.4 pH [
8]. KC has been widely used in the food industry due to its gel-forming ability, low cost, and non-toxicity. Recently, it has also gained attention in the skin tissue engineering field due to its biocompatibility, biodegradability, high fluid absorption and retention capacity, and bioactivity, as its structure resembles two major components of native extracellular matrices called endogenous glycosaminoglycans (GAGs), specifically chondroitin-4-sulfate and dermatan sulfate [
8].
Curcumin (Cur) is a natural yellow polyphenolic substance extracted from the rhizome of
Curcuma longa. Apart from its generalized use in the food industry, it has also found wide applicability in biomedical applications, as it has been reported to promote wound healing, mainly due to its anti-inflammatory properties and antibacterial activity [
9]. In addition, Cur has antioxidant properties that may aid wound healing by preventing free radicals such as reactive oxygen species, which are present at high concentrations in chronic wounds. However, its high hydrophobicity and low bioavailability are two major drawbacks that limit its utilization at higher concentrations [
10,
11].
A recent study [
12] reported on Cur-loaded PVA electrospun membranes and the Cur release from the PVA fibers as well as the antibacterial activity of the membrane against
S. aureus and
E. coli bacterial strains. They incorporated Cur into the PVA solution, which was then electrospun. The fibrous membranes showed high efficacy against both bacterial types, suggesting the potential of the electrospun membranes as wound dressings. However, the effect of the Cur-loaded membrane on cell viability and morphology was not investigated. Another report [
13] describes an ampicillin-loaded hydrogel of PVA, KC, and hyaluronic acid that indicated antibacterial activity against
S. aureus and
E. coli and no cytotoxicity in the presence of L929 fibroblasts. However, the combination of PVA and KC for the fabrication of biomimetic electrospun nanofibrous membranes for wound healing has not been investigated. Although a Cur-loaded PVA membrane has been previously reported in the literature [
14], this is the first time Cur particles are electrosprayed in a PVA/KC membrane. The main aim of this study was to fabricate nanofibrous biomimetic membranes using a natural polysaccharide (KC) and a synthetic hydrophilic polymer (PVA) and further functionalize them with Cur to endow them with antimicrobial properties. In contrast to previous reports [
15] in which Cur was loaded onto scaffolds by direct incorporation into the solution, which was then electrospun, we report on electrospraying Cur onto the electrospun membranes.
Two of the main advantages of electrospraying Cur on the nanofibrous membranes constitute the improved aqueous solubility of the poorly water-soluble Cur as well as its enhanced bioavailability. In particular, electrospraying Cur directly onto the surface of the nanofibrous membranes may improve its release kinetics compared with encapsulating it within the polymer fiber matrix. Based on other research studies reporting the release mechanisms of drugs from nanofibrous membranes [
16,
17], we assumed that in electrosprayed Cur-coated membranes, the Cur molecules are located in the outer layers of the nanofibrous membrane. Thus, there are fewer layers through which Cur diffuses, making the drug more accessible to the wound area. Moreover, the hydrophobic nature of Cur makes its mixing with water-soluble compounds rather challenging. Electrospraying overcomes this issue, as the electrospun membrane is prepared separately, and Cur is dissolved in a different solvent and electrosprayed into the membrane in a second step. Hence, we fabricated biomimetic electrospun nanofibrous membranes from PVA and KC and coated them with Cur to enhance them with antibacterial properties. Our hypothesis was to investigate the effect of two different Cur concentrations by adjusting the duration of electrospraying for the functionalization of the PVA/KC membranes with Cur on the cellular responses in fibroblasts and bacteria. To this end, we fabricated four types of electrospun nanofibrous membranes, PVA and PVA/KC via electrospinning and PVA/KC/Cur5 and PVA/KC/Cur20, in which a Cur solution was electrosprayed onto the PVA/KC membranes for 5 and 20 min, respectively. The physicochemical properties of the membranes and the release kinetics of Cur were investigated. L929 fibroblasts were cultured on fibrous scaffolds, and the cell migration along with the cell viability, cell morphology, and collagen secretion were evaluated. In addition, the antibacterial activity of the Cur-functionalized membranes against Gram-positive
S. aureus and Gram-negative
E. coli bacteria was investigated.
2. Materials and Methods
2.1. Materials
Polyvinyl alcohol (Mw 145,000 kg/mol), k-carrageenan, and curcumin (from Curcuma longa), were purchased from Sigma-Aldrich (St. Louis, MO, USA). The Presto BlueTM reagent was purchased from Invitrogen Life Technologies (Carlsbad, CA, USA). Trypsin/EDTA (0.25%) and phosphate buffer saline (PBS) were purchased from Gibco ThermoFisher Scientific (Waltham, MA, USA). Span® 80 solution, hexamethyldisilizane (HMDS), paraformaldehyde (PFA), and Sirius Red 90 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Penicillin/streptomycin (P/S) was purchased from Gibco ThermoFisher Scientific (Waltham, MA, USA). Roswell Park Memorial Institute (RPMI) medium and fetal bovine serum (FBS) were purchased from PAN-Biotech (Aidenbach, Germany). Augmentin was purchased from GlaxoSmithKline. Mueller–Hinton broth (MH-B) was purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Preparation of Electrospinning and Electrospraying Solutions
The PVA solution for electrospinning was prepared by dissolving 5% w/v PVA in distilled water. The mixture was stirred continuously with a magnetic stirrer for 3 h at 90 °C and 250 rpm to ensure complete dissolution. To produce PVA/KC membranes, the solution was obtained by preparing the PVA solution in the same manner and subsequently adding 1% w/v KC. The mixture was maintained under stirring for 4 h at 70 °C and 250 rpm. Moreover, 25 μL of Span 80 detergent was added to the mixture to lower the surface tension and facilitate the electrospinning process. To produce Cur-loaded membranes, another solution was prepared by dissolving 10% w/v Cur in 1:1 volume ratio of dimethyl sulfoxide (DMSO) and water solvent. Initially, 0.1 g of Cur was added to 0.5 mL of dimethyl sulfoxide, and after Cur was completely dissolved, 0.5 mL of water was added in a controlled manner. The solution was maintained under gentle stirring at room temperature for about 3 h.
2.3. Electrospinning and Electrospraying Process
The scaffolds used in this work were fabricated by means of a custom-made electrospinning device by CommonsLab SCE (Heraklion, Greece), similar to previous reports using a blend of PVA and a polysaccharide [
18]. The electrospinning method includes the following essential elements: a polymer solution, a horizontal syringe plunger controlled by a console, a blunt needle, a flat collector covered with aluminum foil, and a high-voltage supply with a range of 0–30 kV. A high-voltage source is applied after a polymer solution is loaded into the syringe. When the electrostatic interactions overcome the surface tension, the Taylor cone is formed, creating an accelerated jet deposited on the collector. To produce PVA and PVA/KC fibrous membranes, a total of 10 mL of polymer solution was loaded into a plastic syringe. The electrospinning parameters were set as follows: (i) flow rate at 1 mL/h, (ii) distance between the blunt needle and the grounded collector at 15 cm, (iii) applied voltage at 14 and 13 kV for the PVA and PVA/KC membranes, respectively. These parameters were selected based on previous work in the group. Various conditions were assessed to achieve stable jet formation and uniform fiber morphology, and the final conditions were selected based on the most consistent results. Although we maintained a stable processing environment, minor variations in humidity and temperature may have contributed to differences in fiber diameter and uniformity.
For the fabrication of the Cur-loaded scaffolds, the same electrospinning setup was employed, but this time the purpose was to generate Cur-loaded droplets instead of fibers via electrospraying. After 1 mL of the Cur solution was loaded into a 5 mL syringe, the distance was set at 20 cm, the flow rate at 1 mL/h, and the applied voltage at 21 kV. The Cur-loaded droplets were deposited onto the PVA/KC membranes. Two different types of PVA/KC/Cur membranes were produced; in one type, the Cur electrospraying process lasted 5 min, and in the other type, 20 min.
Table 1 summarizes the membrane types. After production, the fibrous membranes were removed from the aluminum foil and stored appropriately until use.
2.4. Physicochemical Characterization of the Electrospun Membranes
2.4.1. Fourier Transform Infrared Spectroscopy (FTIR)
The infrared spectrum of absorption for the fibrous scaffolds was measured by a Nicolet 6700 optical spectrometer within the range of 4000–400 cm−1. Measurements were performed using three samples (n = 3), and the data collected were transferred to Origin software 10.1 for graphical representation.
2.4.2. TGA
The thermal stability of the electrospun scaffolds was assessed by means of the thermogravimetric (TG) analysis using a TGA5500 thermogravimetric analyzer (TA instruments, New Castle, DE, USA). The samples were placed into a platinum pan, and their weight was recorded from 25 °C to 900 °C at a heating rate of 10 °C/min. The analysis was performed using three samples (n = 3) for each membrane composition.
2.4.3. Contact Angle Measurement
The hydrophilicity of the fibrous membranes was measured with a contact angle computing device (OCA 15 Plus, Data Physics Instruments, Filderstadt, Germany). Distilled, deionized water droplets with a volume of 4 μL were dripped onto the samples from a motorized syringe at ambient temperature and pressure. Measurements were taken at four different positions for each sample, and the average of four replicates per membrane (n = 4) was reported to ensure reproducibility.
2.4.4. Swelling and Degradation Profile
To determine the swelling capability of the scaffolds, the fibrous membranes were cut into identical shapes. After their weight was measured, they were placed into 24-well plates. They were then immersed in phosphate-buffer saline (PBS) with a pH of 7.4. At predetermined time points (1 and 24 h), the buffer was aspirated, and the excess water was carefully removed from the membrane with a paper towel. After allowing the membranes to dry for 1 h, their weight was measured again. The degree of swelling of the fibrous scaffolds was calculated using the following formula:
where W
dry is the weight of scaffolds before and W
wet is the weight after they were immersed in the buffer.
The same process as before was used to determine the degradation rate of the scaffolds, but this time the plate was placed in an incubator (Thermo Scientific™, Forma™ Steri-Cycle™ CO2 Incubator, Waltham, MA, USA) at 37 °C after immersion in phosphate-buffer saline (PBS). After 7, 14, and 21 days, their weight was measured. A high-precision scale (Analytical balance ABT 220-5DNM) with an accuracy of ±1 μg was used for the weight measurements.
2.5. Curcumin Release
The Cur release profile experiment was conducted to determine the amount of Cur released from the fiber mats. First, 0.6 × 0.6 cm Cur-loaded samples were prepared and placed in 48-well plates. The samples were then immersed in phosphate-buffer saline (PBS) mixed with 0.2% v/v tween 20. Tween 20 was added to PBS due to Cur’s insolubility in water. After the plate was incubated at 37 °C, the supernatants were collected and replaced with an equal volume of fresh PBS medium at specific time points. The amount of Cur in each collected solution was analyzed using a UV-Vis spectrophotometer at 421 nm. A calibration curve of Cur in the same buffer was used to convert the obtained absorbance values to Cur concentration. The accumulative weight of Cur over time was plotted as a function of incubation time to visualize the release kinetics.
2.6. Preparation of Scaffolds for the Fibroblasts Cell Culture
Before the cell seeding, all scaffolds were sterilized with UV light for 15 min on each side. They were then cut into squares of equal size and placed into 48-well plates. Afterward, they were immersed in 250 μL of cell culture medium for 1 h. The medium was then aspirated, and the membranes were allowed to dry for 2 h before cell seeding.
2.7. Scaffold Morphology and Cell Adhesion
To investigate cell attachment and proliferation on the different scaffolds, the membranes were visualized using scanning electron microscopy (SEM) (JEOL JSM-6390 LV, Tokyo, Japan). The cell-cultured plate was maintained in an incubator at 37 °C at 0.5% CO2. On days 3 and 7, the medium was carefully aspirated from the 48-well plate, and the samples were fixed in 4% v/v paraformaldehyde for 25 min. They were then washed with phosphate-buffer saline (PBS) and dehydrated with a series of increasing ethanol solutions (from 10 to 100% v/v). Afterward, they were immersed in hexamethyldisilazane for 30 min, and after being air-dried overnight, they were sputter-coated with gold (Baltec SCD 050, Pfäffikon, Switzerland).
2.8. Cell Viability on the Fibrous Scaffolds
Presto BlueTM Reagent, a resazurin-based solution, was used to study the viability and growth of cells on the fibrous membranes. After adding Presto BlueTM to the cells, resazurin is reduced to resorufin, a highly fluorescent compound, due to cell metabolism. The color changes are detected by the absorbance measurements. For the cell viability study, L929 fibroblast cells were seeded at a density of 7 × 104 cells/well in a 48-well plate. The number of cells per well was measured after 2, 5, and 7 days. Three independent experiments were conducted, each in triplicates per condition. The medium was aspirated, and 180 μL of fresh medium was pipetted along with 20 μL of Presto blue. The plate was then incubated for 1 h at 37 °C and 5% CO2. Subsequently, 100 μL from each well was transferred to a 96-well plate, and the absorbance of the contents of each well was measured at 570 and 600 in a spectrophotometer (Synergy HTX Multi-Mode Micro-plate Reader, BioTek, Bad Friedrichshall, Germany).
2.9. Determination of the Secreted Collagen
The Sirius Red Dye assay was used to determine collagen secretion from cells cultured on the four membrane compositions compared with the TCPS control. The negatively charged sulfonic acid groups present in the dye bind ionically to the positively charged amino groups present in the collagen species produced by the cells [
18]. After 3 × 10
4 fibroblasts were seeded into each membrane in a 48-well plate, the plate was incubated at 37 °C for 21 days. The supernatants were collected and replaced with fresh medium after 7, 14, and 21 days. At a subsequent time, when all the supernatants from all the time points were collected, 25 μL supernatants of each sample were transferred in 1.5 mL Eppendorf tubes and diluted in dH
2O to a final volume of 100 μL. To isolate the collagen precipitate, 500 μL of 0.1%
w/
v Sirius Red Dye solution in 0.5 M acetic acid was added to all tubes and centrifuged at 4 °C for 30 min at a speed of 15,000×
g. To remove the excess dye from the collagen pellet, the supernatants were then removed, and after 1 mL of 0.5 M acetic acid was added, the Eppendorf tubes were centrifuged for 10 min. This procedure was repeated several times, until the supernatants were transparent. Finally, the collagen pellet was diluted with 500 μL of 0.1 N NaOH, and 100 μL of the resulting solutions was transferred to a 96-well plate. The plate was placed in a spectrophotometer, and the absorbance values at 530 nm were recorded. The absorbance values were converted to collagen concentration in μg/mL using a standard collagen curve. Three independent experiments were conducted, each in triplicates per condition.
2.10. Cell Migration Assay
To evaluate the impact of Cur on cell migration, an in vitro wound-healing scratch assay was employed. Initially, the Cur released from pre-sterilized PVA/KC/Cur5 and PVA/KC/Cur20 scaffolds was collected. After adding 1 mL RPMI to each scaffold, the plate was incubated at 37 °C. After 48 h, the supernatants were carefully collected in Eppendorf tubes and stored appropriately until use.
At the same time, L929 cells were cultured in RPMI, and when they were 80% confluent, they were seeded in a 96-well plate at a density of 4 × 10
4 cells/well and incubated at 37 °C. When they reached confluency, the medium was aspirated, and a scratch was made mechanically in each well using a 100 μL tip. Subsequently, the wells were treated with three different media: pure RPMI medium (control) and the Cur supernatants collected earlier from the release of PVA/KC/Cur5 and PVA/KC/Cur20 scaffolds. The progress of cell migration was visualized under the microscope at 0, 24, and 48 h. The wound gap at the predetermined time points was measured using ImageJ software 1.54g with the assistance of the wound healing size tool. Three independent experiments were conducted, each with three samples (n = 3) per condition. The formula used for the calculation of the wound healing for each time point was
2.11. Antibacterial Assays
2.11.1. Preparation of the Bacterial Suspension
In this study, Gram-negative
E. coli and Gram-positive
S. aureus were used to determine the ability of Cur-loaded scaffolds to inhibit bacterial action. The strains have been described previously [
19]. The bacterial strains were inoculated into 2.5 mL Mueller–Hinton Broth (MH-B) standard media. The bacterial suspensions were then maintained in a shaking incubator at 37 °C at 250 rpm for approximately 20 h. To assess the cell number, the suspensions were diluted in MH-B, and the corresponding concentrations were determined by acquiring the optical density (OD) values using a UV-Vis spectrophotometer (Synergy HTX Multi-Mode Micro-plate Reader). The OD 0.1 at 600 nm, corresponding to 2 × 10
7 colony-forming units (CFU) per mL, was used for normalization of the bacteria cell suspension. For each experiment, after normalization of the bacteria suspension, the appropriate concentration was acquired through serial dilutions. The bacterial strains were stored as glycerol stocks at −80 °C.
2.11.2. Bacterial Cell Viability Assessment
The membranes were cut into equal shapes, and after being sterilized for 30 min on each side, they were placed on a 48-well plate. After serial dilutions, each bacterial suspension was normalized to an OD of 0.1 that corresponds to 2 × 107 CFU/mL, and bacterial suspension of 2 × 105 CFU/mL was obtained through dilution. Subsequently, 50 μL of the acquired bacterial suspension was seeded in each well of the 48-well plate, and media was supplemented at a final volume of 200 μL. The bacteria viability was determined after 3 and 24 h using the Presto BlueTM reagent in a ratio of 1:10. Subsequently, 100 μL from each well was transferred to a 96-well plate, and the OD values were recorded at 570 and 600 nm. Augmentin at a concentration of 100 μg/mL was used as positive control.
2.11.3. Morphological Observation by SEM
The membranes were cut into equal shapes and placed in a 48-well plate. After obtaining a bacterial suspension of 2 × 10
5 for both bacterial species, 50 μL of the suspensions was seeded into each sample, and an additional 150 μL of MH broth was subsequently added. The plate was then incubated at 37 °C, and after 4 and 24 h, the medium was carefully aspirated from the 48-well plate, and the samples were fixed in 4%
v/
v paraformaldehyde for 25 min. They were then washed with phosphate-buffer saline (PBS) and dehydrated with a series of increasing ethanol solutions from 10 to 100%
v/
v. They were then immersed in hexamethyldisilazane for 30 min and sputter-coated with gold (Baltec SCD 050) after overnight air-drying. The morphology of
E. coli and
S. aureus on the fibrous scaffolds was observed by a scanning electron microscope (SEM) as previously described [
20].
2.11.4. Kinetic Study
Initially, the supernatants of Cur release from the PVA/KC/Cur5 and PVA/KC/Cur20 scaffolds were obtained at specific time points. Initially, the Cur-loaded membranes were sterilized with UV radiation and were cut into identical shapes. They were then placed in 1.5 mL Eppendorf tubes, and after 500 μL of MH-B media was pipetted into each Eppendorf tube, they were maintained in a shaking incubator at 37 °C at 250 rpm. At predetermined time points (2, 6, 24 h), 150 μL of the medium was collected from each tube and was stored appropriately until use.
For the kinetic study, a 50 μL bacterial suspension of 2 × 10
5 CFU/mL was added to a 96-well plate [
20]. Subsequently, the Cur release media collected previously from the fibrous membranes was added to each well. The bacterial suspension in pure medium was used as negative control, and bacterial suspension in medium with 100 μg/mL augmentin was used as positive control. The plate was then transferred to a plate reader spectrophotometer at 37 °C, and the optical density values were measured at 600 nm every 15 min for 24 h.
2.12. Statistical Analysis
The statistical analysis of the experimental data was conducted with GraphPad Prism version 8 software. A two-way ANOVA (or mixed model) followed by Tukey’s multiple comparisons test was used to evaluate the significance between the mean values of the various scaffold compositions. A p-value < 0.05 was considered significant.
4. Discussion
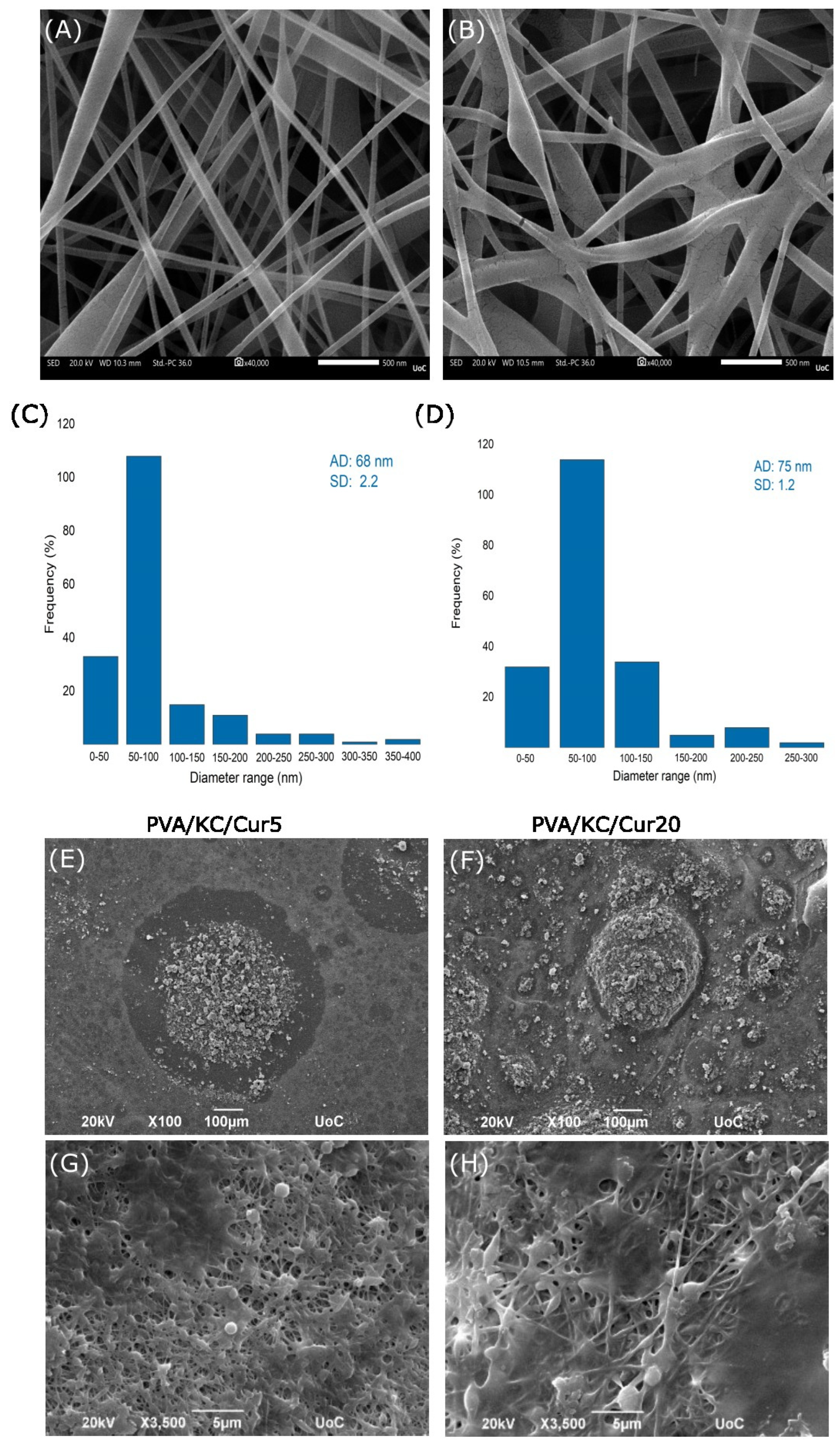
The average fiber diameter of the PVA and PVA/KC fibrous membranes was analyzed using SEM microscopy. While both membranes exhibited similar average fiber diameters, the PVA/KC membrane showed a slightly larger average diameter. This increase in fiber diameter may be attributed to the higher viscosity of the PVA/KC solution at room temperature, which affects the electrospinning process [
18]. Moreover, the slight variation in voltage during electrospinning (130 V for the PVA/KC membrane and 140 V for the PVA membrane) could also contribute to the differences in fiber morphology, as lower voltages are known to produce thicker fibers by reducing the elongation forces on the electrospinning jet [
25].
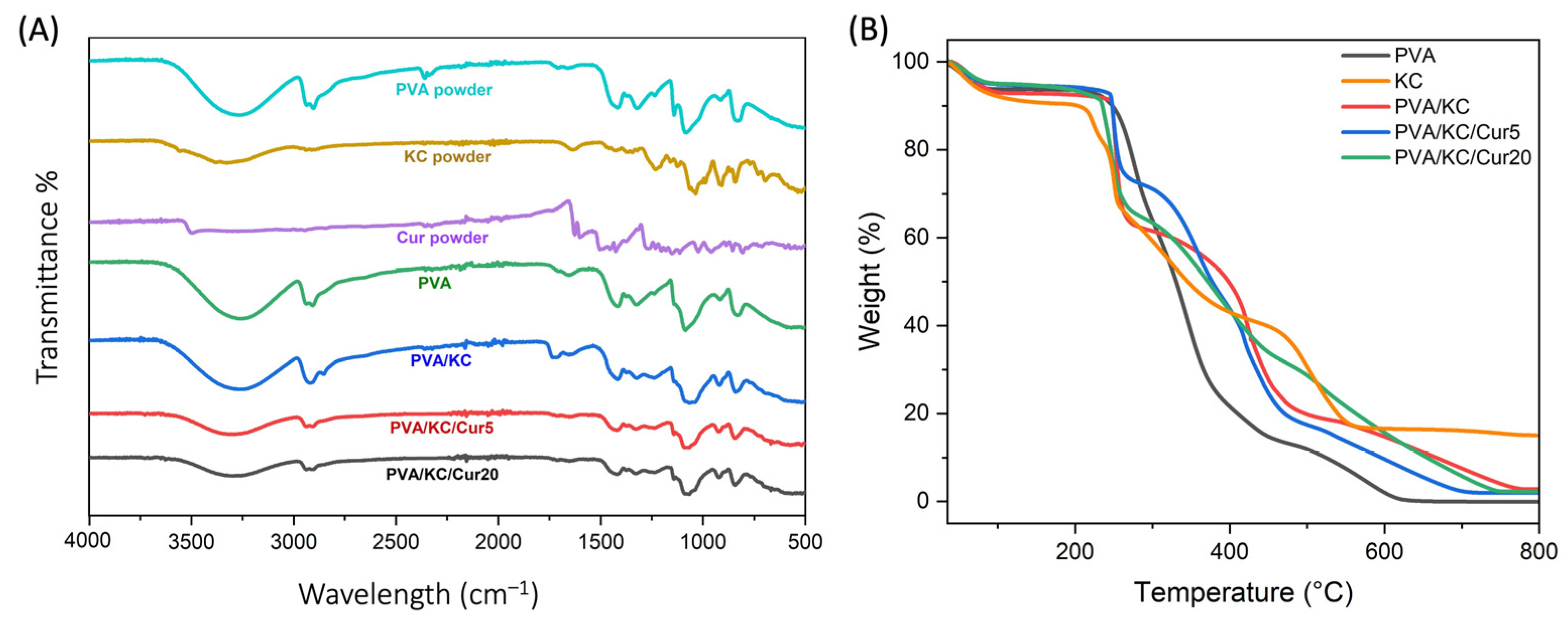
TGA analysis of the membrane compositions showed that the decomposition of KC occurs at higher temperatures, indicating that its addition enhances the thermal resistance of the membrane. This delay in weight loss compared with pure PVA can be attributed to the decomposition of the polysaccharide structure of KC, which likely involves the formation of hydrogen bonds between PVA and KC. These interactions create a more stable network, requiring more energy to break down. With the incorporation of Cur, the degradation curves shift further to higher temperatures, suggesting an additional increase in thermal stability. This enhanced stability may be due to the aromatic structure of Cur, which can form π-π interactions and additional hydrogen bonds with the PVA/KC matrix. Moreover, the PVA membrane exhibited the lowest residual mass, suggesting complete decomposition, while PVA/KC, PVA/KC/Cur5, and PVA/KC/Cur20 had higher residual masses. Among the Cur-loaded membranes, thermal resistance increased with the concentration of Cur, likely due to the increased cross-linking.
The hydrophilic properties of the membranes were evaluated using contact angle measurements. The results indicated that the PVA and PVA/KC/Cur20 membranes exhibited the lowest hydrophilicity, while the PVA/KC membrane demonstrated the highest hydrophilicity. The contact angle for the pure PVA membrane was approximately 63°, which aligns with previous studies reporting similar values for electrospun PVA membranes [
26]. The significant reduction in the contact angle observed upon the addition of KC to PVA is consistent with findings from other studies, where even a small concentration of KC in electrospun matrices drastically enhanced hydrophilicity. This can be attributed to the abundant hydroxyl and sulfate groups in the KC structure, which can form strong hydrogen bonds with water, significantly increasing the membrane’s water affinity [
27,
28]. Moreover, the hydrophilicity of the PVA/KC membrane decreased after coating it with Cur via electrospraying. The reduction in hydrophilicity became more pronounced as the Cur concentration increased. This behavior can be attributed to the hydrophobicity of Cur. This is in agreement with other studies, where Cur has been reported to increase the surface hydrophobicity when incorporated into polymer matrices [
29].
The swelling behavior of scaffolds plays a critical role in promoting faster wound healing, because scaffolds with swelling capacity aid a moist environment and prevent infections by absorbing wound exudates [
30]. Therefore, the swelling capacities of the various scaffold compositions were examined. The PVA/KC membrane exhibited the highest swelling ratio, nearly 1.5-fold higher than that of the pure PVA membrane. This significant increase in swelling can be attributed to the hydrophilic nature of KC. In contrast, the swelling capacity was reduced upon incorporation of Cur into the PVA/KC membranes, with the PVA/KC/Cur20 membrane showing a more pronounced decrease. This decrease can also be linked to the hydrophobic nature of Cur, leading to limited ability of the scaffold to absorb water.
In wound-healing applications, a scaffold must also exhibit a controlled degradation rate. It needs to slowly degrade as the new tissue grows and be gradually resorbed once the wound is healed. The degradation behavior of the membranes followed patterns observed in similar materials, with all membranes displaying a gradual increase in the degradation rate over time. The PVA/KC membrane degraded faster than the PVA membrane, which can be attributed to the enhanced hydrophilicity of KC. The increased water absorption in the PVA/KC membrane likely accelerates hydrolytic degradation. However, the addition of hydrophobic Cur into the PVA/KC membranes slowed down the degradation. This characteristic is advantageous for wound-healing scaffolds, as it allows the Cur-loaded membranes to maintain their mechanical integrity for a longer period and provide support during the stages of wound healing. This controlled degradation, combined with Cur’s inherent bioactive properties, enhances the potential of these membranes as scaffolds for skin tissue engineering.
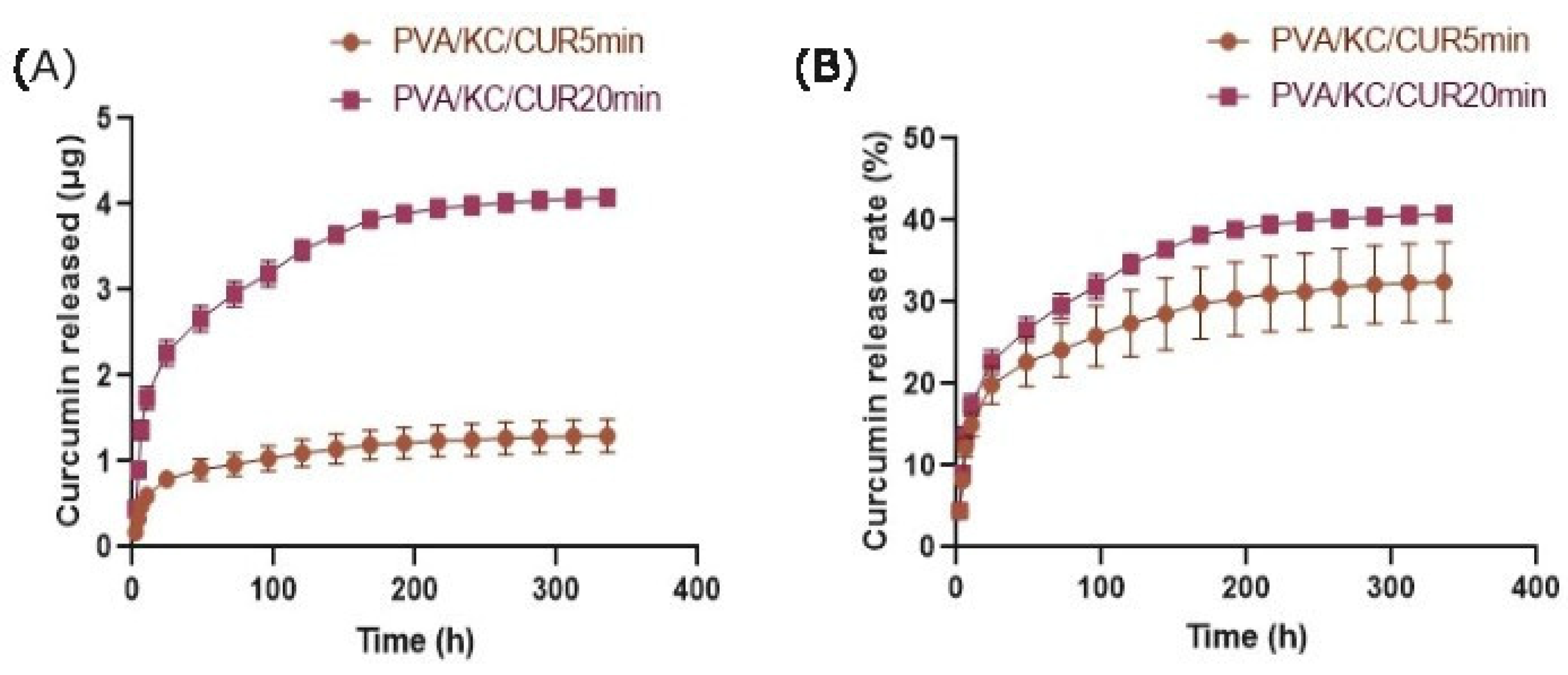
The cumulative release of Cur from the PVA/KC/Cur5 and PVA/KC/Cur20 fibrous scaffolds over 14 days was also assessed. The higher cumulative release from the PVA/KC/Cur20 membrane may be attributed to the higher initial Cur concentration, which made more Cur available for diffusion. The initial burst release of Cur from both membranes is due to the fact the Cur is attached weakly to the fibrous membrane surface [
31]. The initial burst release from both membranes could help prevent inflammation, while the sustained release ensures prolonged delivery of Cur during the critical phases of wound healing.
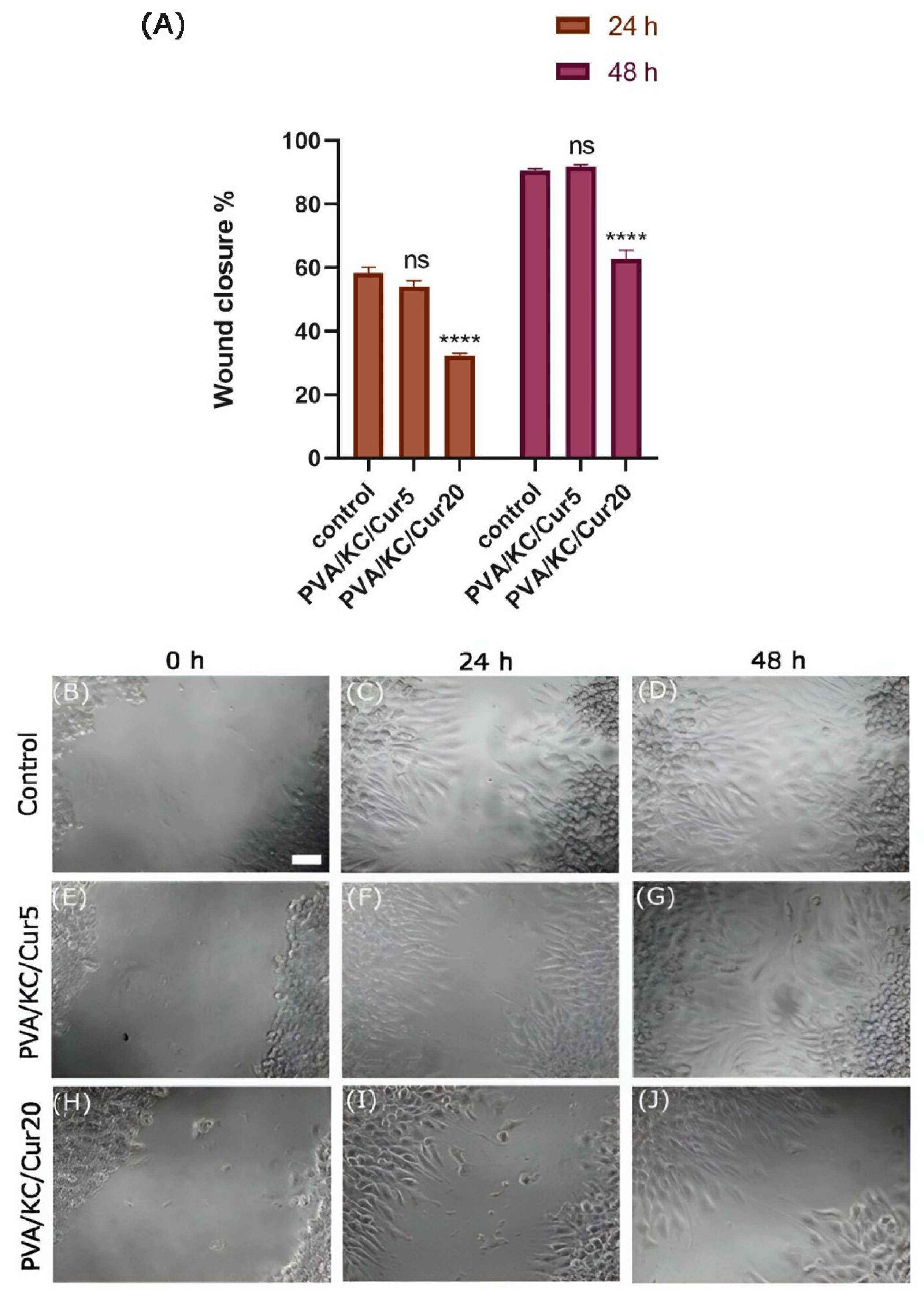
Moreover, we investigated the effect of Cur-loaded scaffolds on the migration of L929 fibroblasts. It has been reported that Cur may be beneficial for cell migration. The PVA/KC/Cur5 scaffold releasate supported wound healing at the same rate as the control group. This is consistent with other work, where Cur has been reported to enhance migration. In a recent study [
32], the effect of Cur-loaded lignin nanoparticles on the migration of L929 skin fibroblast cells was reported. The study demonstrated that the scratch surface area treated with the Cur-loaded nanoparticles resulted in higher wound healing closure compared with the control scratch surface area. In another work, Liao et al. confirmed that the released Cur could enhance the migration of HS68 cells compared with control (medium) and Cur-free PLGA nanofiber membranes [
33]. The difference in wound healing rates of the PVA/KC/Cur5 and PVA/KC/Cur20 scaffold releasates can be attributed to the toxicity of DMSO used as a solvent for the dissolution of Cur.
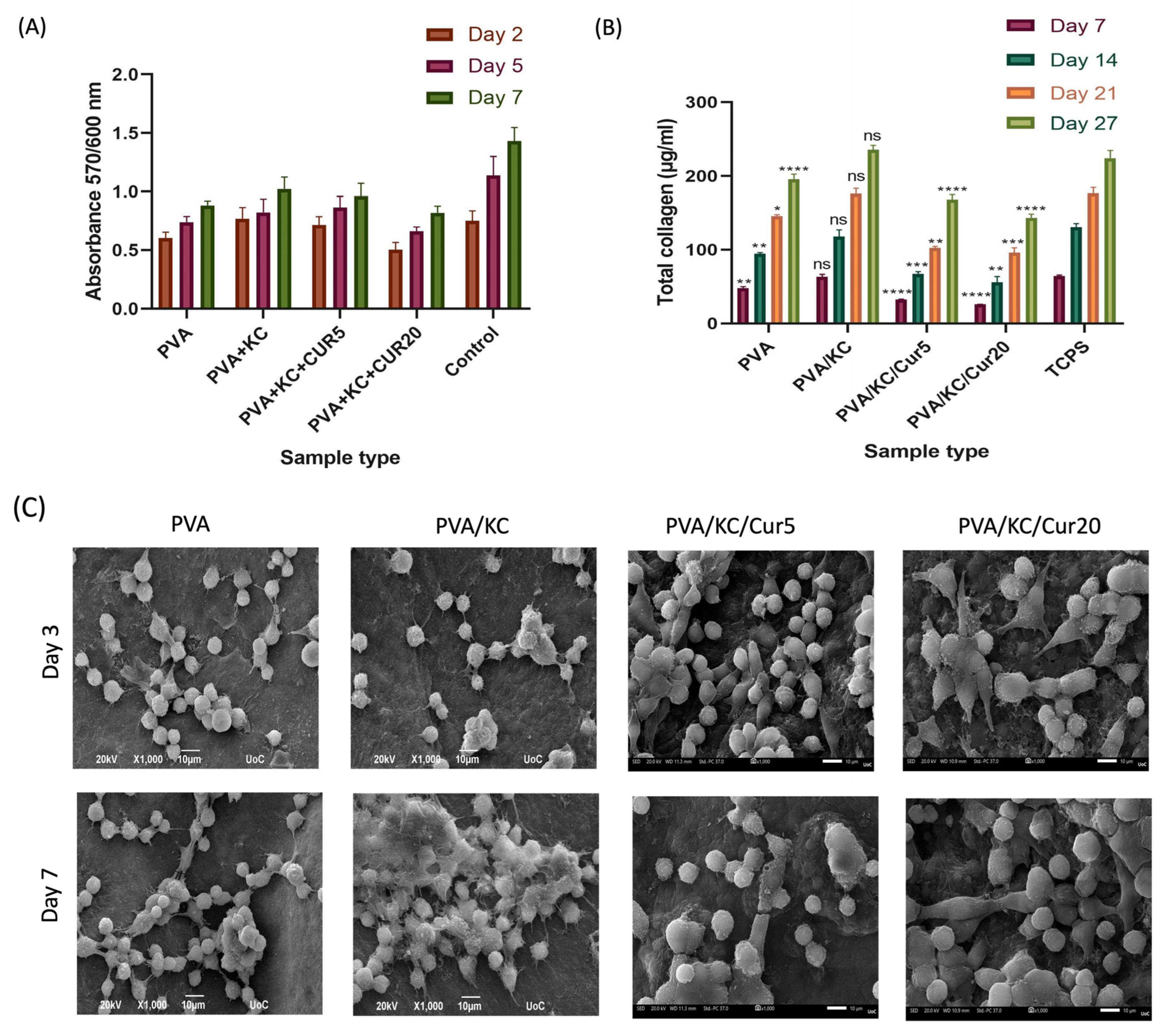
SEM images of cells seeded on the four membranes were obtained on days 3 and 7. The results demonstrated that all the membranes exhibited good biocompatibility and cell attachment. To verify the validity of the SEM findings, a quantitative cell viability assay was employed to assess the cell viability and proliferation. The results showed that the inclusion of KC in the membrane enhanced the cell viability on the PVA/KC membranes. The PVA/KC membrane displayed the highest cell viability rate among the scaffolds, followed by the PVA/KC/Cur5 membrane at all time points. This is in line with previous studies, where KC has been reported to support cell adhesion and proliferation [
34]. However, in the case of the PVA/KC/Cur20 membrane, the cell viability declined. This reduction can be attributed to the changes in the membrane’s surface roughness caused by the longer duration of electrospraying. Several research studies have reported that increased surface roughness can inhibit cell viability by hindering cell adhesion [
35].
During normal wound healing, the fibroblasts secrete collagen during the proliferation phase. After the secreted collagen forms a strong network through cross-linking, the mechanical strength of the wound is restored. Therefore, collagen secretion by fibroblast cells is crucial for the development of skin tissue and wound healing [
36]. The collagen secretion from cells seeded on all four scaffold compositions increased over time, proving the capability of the scaffolds to enable cells to produce collagen and promote wound healing. The PVA/KC membrane displayed a significant increase in collagen secretion compared with the PVA membrane. This is consistent with the cell viability experiment, wherein the PVA/KC membranes exhibited the highest cell viability. It is likely that the highest collagen production is a consequence of a better cell attachment and proliferation of cells on the PVA/KC membrane. However, the presence of Cur decreased the collagen production levels for all days.
Antibacterial experiments were conducted against
E. coli and
S. aureus strains. Consistent with previous reports, Cur showed a stronger inhibitory effect against Gram-positive
S. aureus compared with Gram-negative
E. coli [
37,
38]. This higher antibacterial efficacy against Gram-positive bacteria is thought to be due to differences in the structure and composition of the bacterial cell walls. Gram-negative bacteria such as
E. coli possess an additional outer membrane (OM) composed of lipopolysaccharides, which acts as a barrier and makes them more resistant to many drugs and antibiotics, including Cur. In contrast, Gram-positive bacteria like
S. aureus have a thicker peptidoglycan layer, but they lack the outer membrane, allowing Cur to more easily penetrate and disrupt their cellular processes [
38]. The results of the bacterial viability assessment were in line with the SEM images obtained after the bacterial strains were cultured on the membranes. In addition, the kinetic study confirmed the effect of the Cur concentration on the release rate and bacterial growth of the two strains. The PVA/KC/Cur20 membrane exhibited stronger antibacterial activity against
E. coli and
S. aureus, particularly over longer periods, compared with PVA/KC/Cur5. These results are in line with bacterial viability experiments, in which Cur-loaded membranes showed a higher efficacy against
S. aureus than
E. coli. A similar disruption in the membrane structure of
E. coli and
S. aureus was observed in relevant research reports employing antibacterial chitosan and silver ions [
19,
20]. The combination of the antibacterial and anti-inflammatory properties of Cur with the PVA/KC electrospun nanofibrous membranes highlights their potential as wound dressings.
5. Conclusions
In this study, four types of electrospun membranes, PVA, PVA/KC, PVA/KC/Cur5, and PVA/KC/Cur20, were successfully developed using the electrospinning and electrospraying techniques to identify which composition, i.e., Cur concentration, would provide optimal wound healing, in a fibroblast migration assay, and antimicrobial activity using a Gram-positive and a Gram-negative bacterial strain. Physicochemical characterization of the membranes showed that the addition of KC to the PVA scaffold significantly improved the hydrophilicity and swelling ability as well as the degradation rate of the scaffolds, making the PVA/KC membrane more suitable for tissue engineering compared with the PVA membrane. Additionally, the Cur release data revealed a sustained release profile for both Cur-loaded membranes, which is crucial for long-term therapeutic results. Evaluation of the cell migration study showed that among the two Cur-loaded membranes, PVA/KC/Cur5 facilitated faster cell migration. Moreover, studies of L929 cells on the membranes revealed that PVA/KC membrane supported better cell adhesion, proliferation, and collagen production due to its enhanced hydrophilicity and biocompatibility. However, at higher concentrations, Cur altered the surface roughness of the membranes, leading to reduced cell viability and collagen secretion. The findings from the antibacterial assays demonstrated that both Cur-loaded scaffolds exhibited antibacterial properties against both Gram-positive and Gram-negative bacteria, with PVA/KC/Cur20 exhibiting the highest antibacterial efficiency. These results emphasize the potential of Cur-loaded electrospun membranes as multifunctional wound dressings that both provide antibacterial protection and support tissue regeneration. Future studies could further focus on optimizing the Cur concentration to balance the antimicrobial effect with cell compatibility to improve the overall efficacy of the scaffold as a wound dressing for chronic wounds.