A Novel Gully-like Surface of Stainless-Steel Fiber Coated with COF-TPB-DMTP Nanoparticles for Solid-Phase Microextraction of Phthalic Acid Esters in Bottled Tea Beverages
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation
2.3. The Chromatographic Conditions
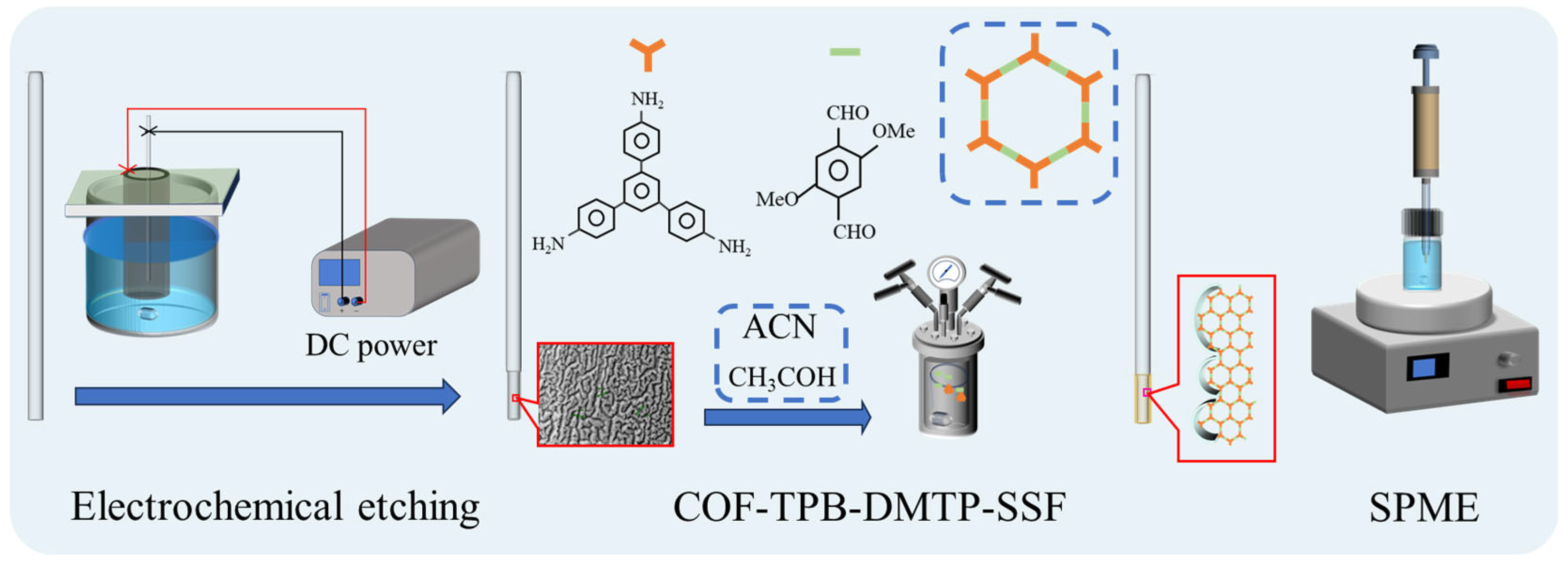
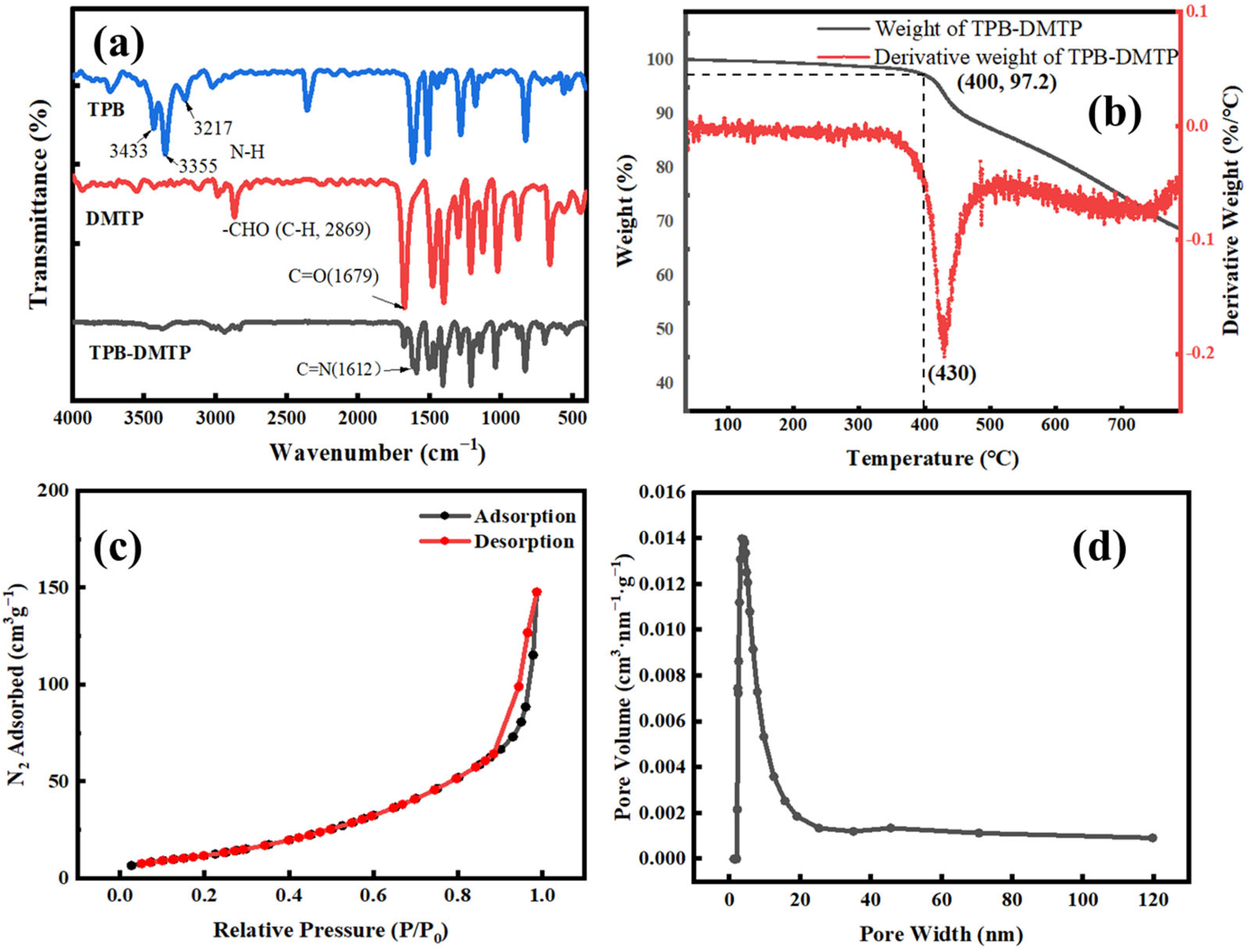
2.4. The Preparation of the COF-TPB-DMTP Used for Characterization
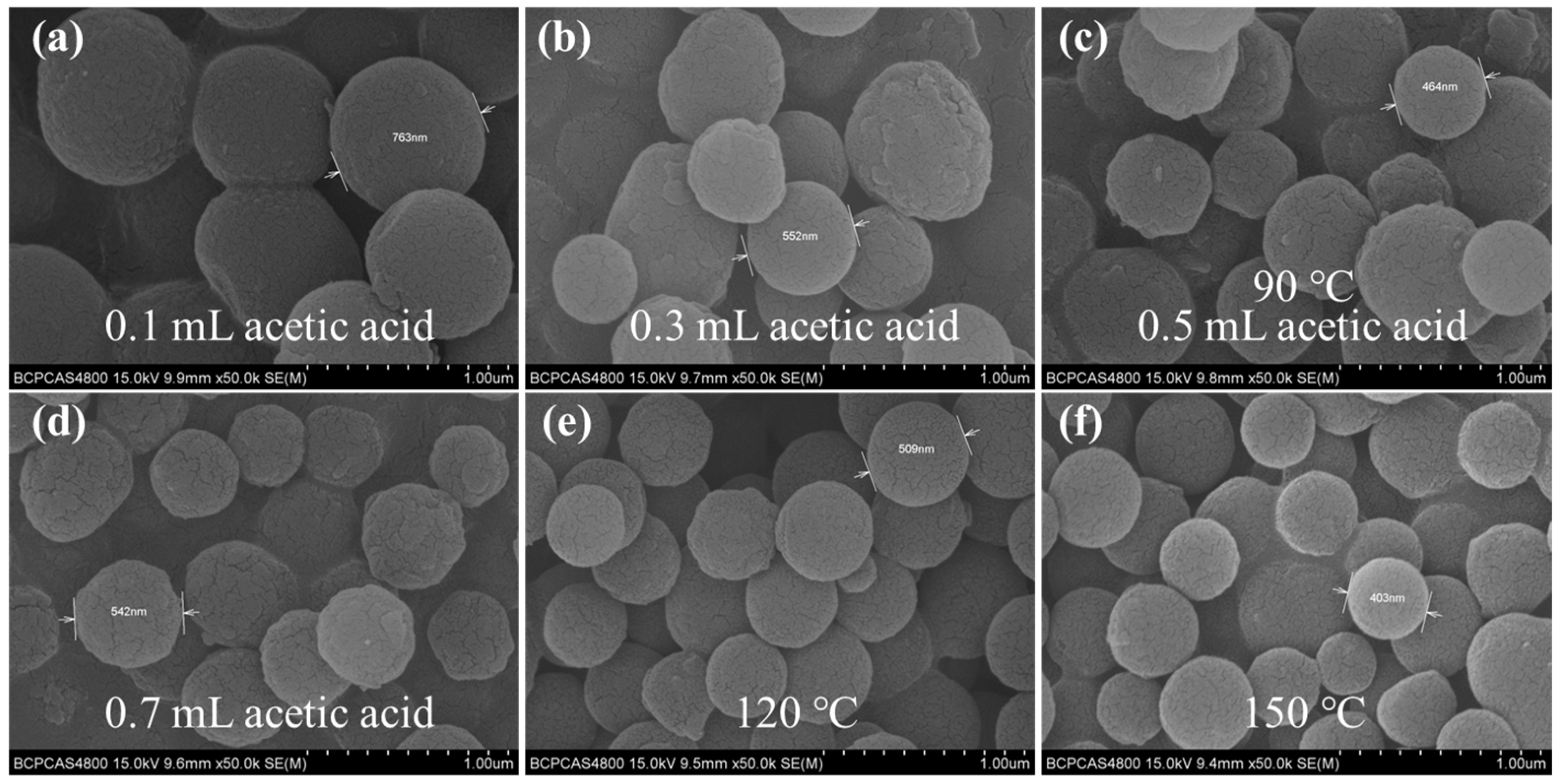
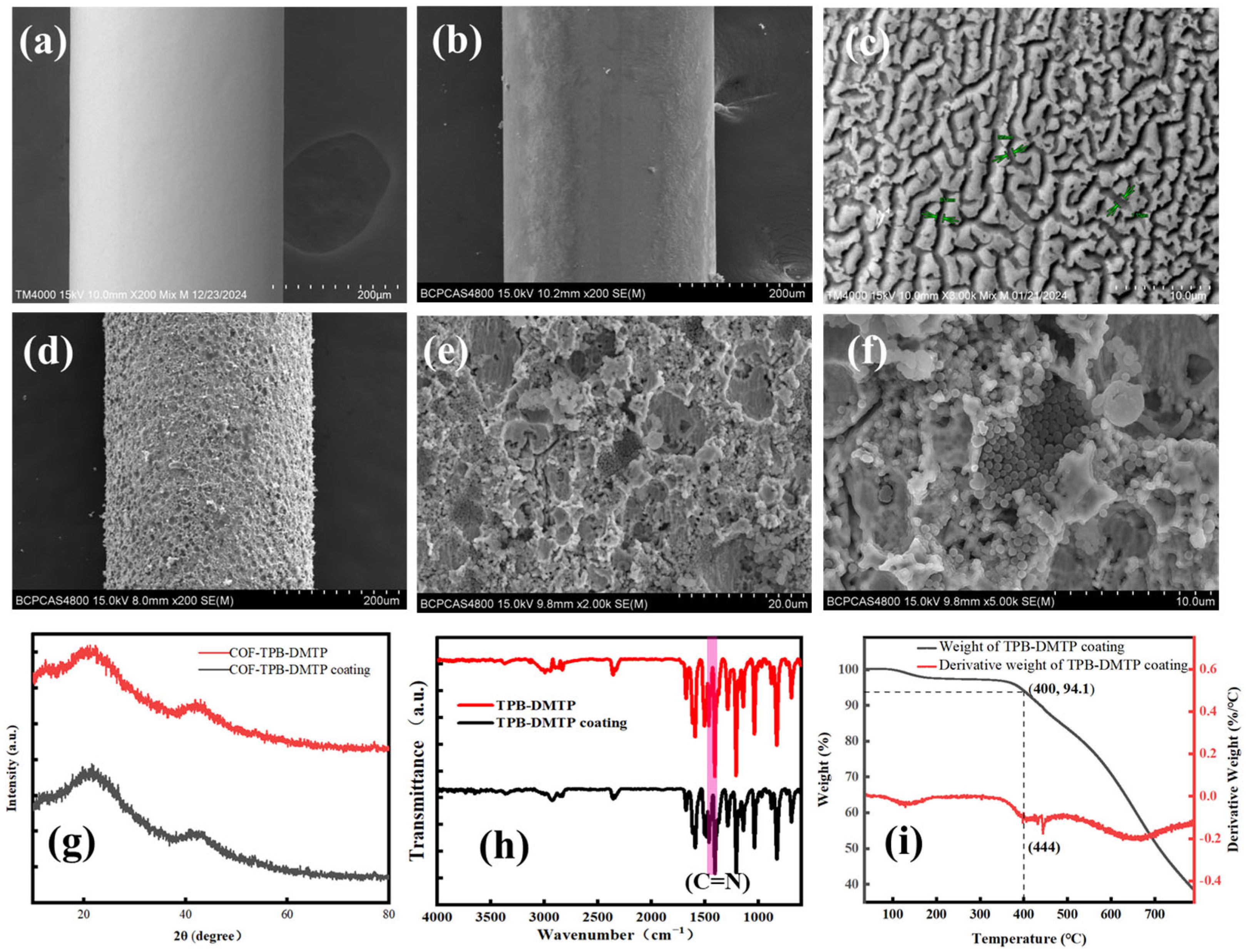
2.5. The Fabrication of the Gully-like Surface of the SSF
2.6. The Fabrication of the COF-TPB-DMTP-Coated SPME Fibers
2.7. SPME Procedure
3. Results
3.1. The Characterization of the COF-TPB-DMTP and COF-TPB-DMTP Coating
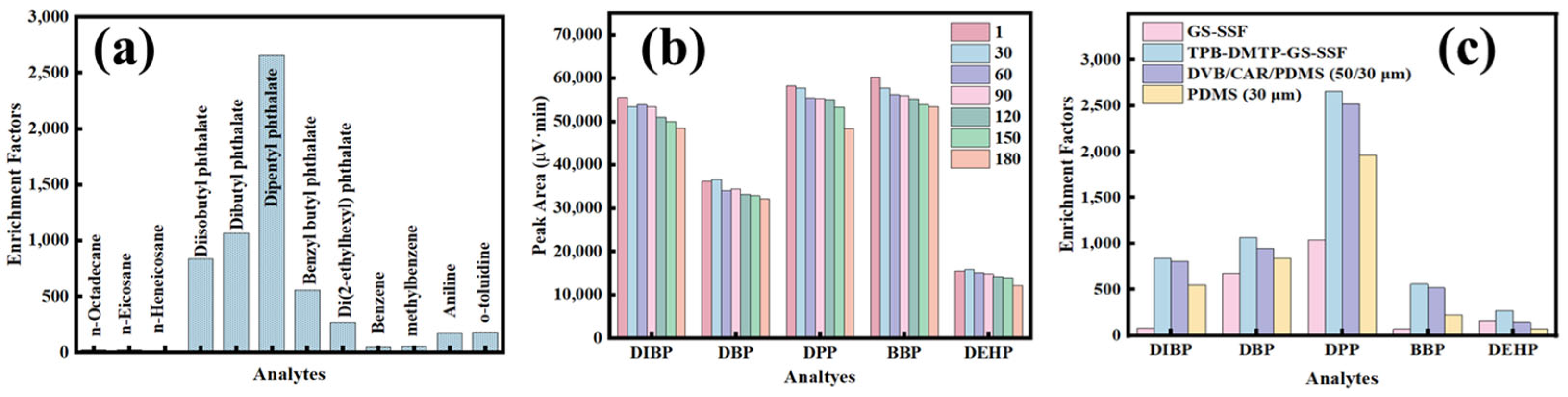
3.2. The Optimization of the Extraction Conditions
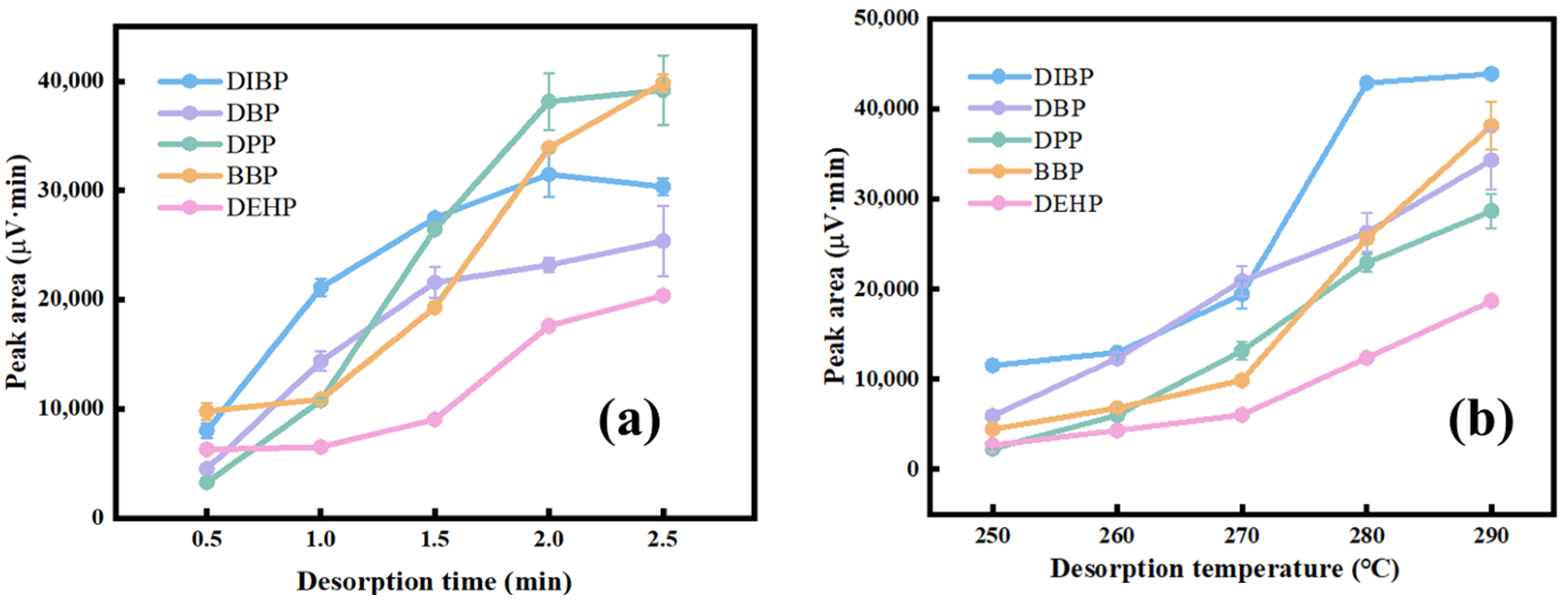
3.3. Optimization of the Desorption Conditions
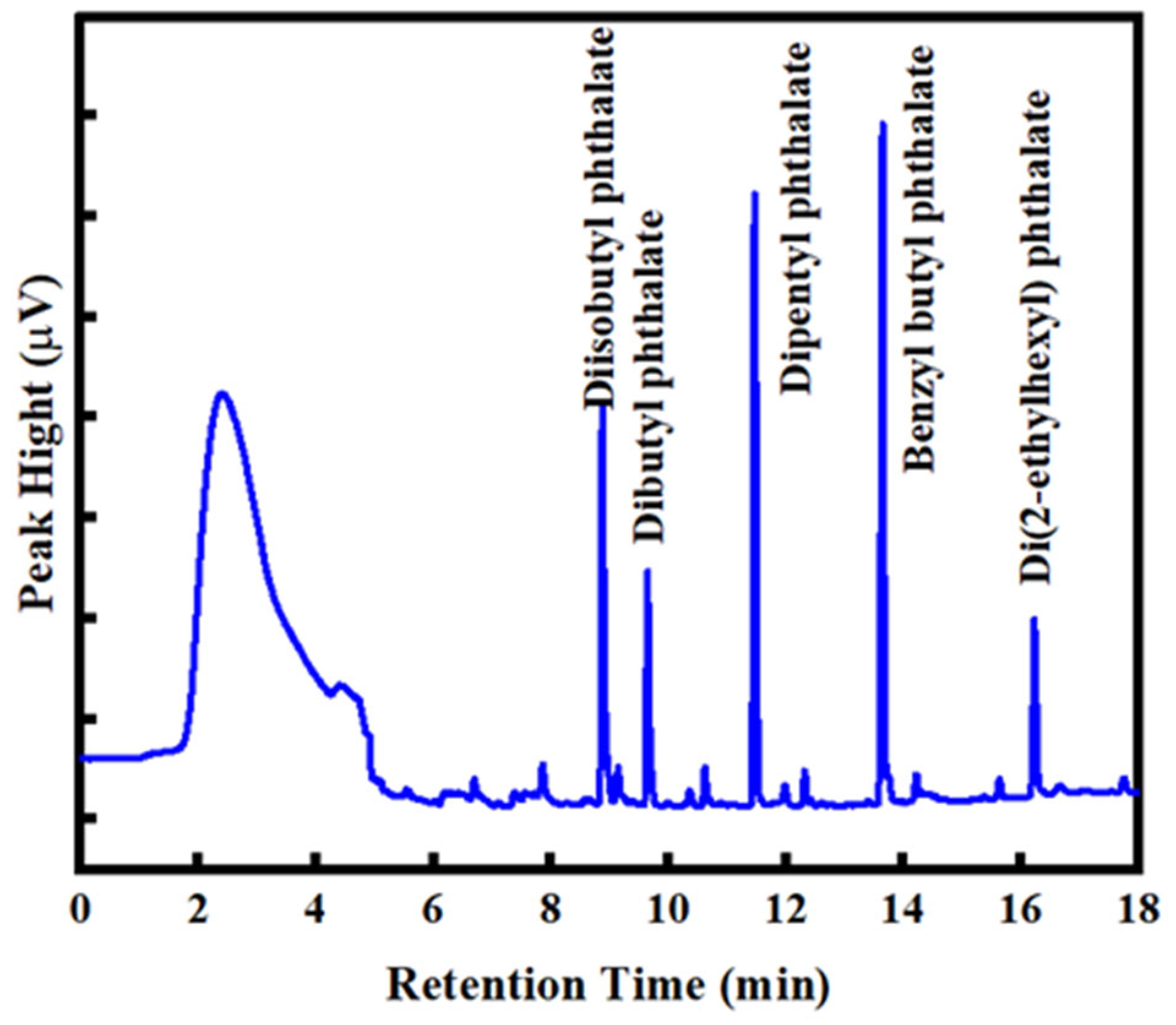
3.4. The Analytical Figures of Merit
3.5. Analysis of PAEs in Commercially Bottled Tea Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| COFs | Covalent organic frameworks |
| PAEs | Phthalic acid esters |
| SPME | Solid-phase microextraction |
| SSF | Stainless-steel fiber |
| GS-SSF | Gully-like surface of stainless-steel fiber |
| TPB | 1,3,5-tris(aminophenyl)benzene |
| DMTP | 2,5-dimethoxyterephthalaldehyde |
| DBP | Dibutyl phthalate |
| DIBP | Diisobutyl phthalate |
| BBP | Benzyl butyl phthalate |
| DPP | Dipentyl phthalate |
| DEHP | Di(2-ethylhexyl) phthalate |
| GC | Gas chromatography |
| FID | Flame ionization detector |
| FT-IR | Fourier-transform infrared |
| TGA | Thermogravimetric analysis |
| SEM | Scanning electron microscope |
| XRD | X-ray diffraction |
| LODs | Limits of detection |
| LOQs | Limits of quantification |
| RSDs | Relative standard deviations |
| EFs | Enrichment factors |
| ILs | Ionic liquids |
| MOFs | Metal–organic frameworks |
| GC-MS | Gas chromatography–mass spectrometry |
| OPs | Organophosphorus pesticides |
| PAHs | Polycyclic Aromatic Hydrocarbons |
| PCBs | Polychlorinated Biphenyls |
| OPEs | Organophosphate Esters |
References
- Sicińska, P. Di-n-Butyl Phthalate, Butylbenzyl Phthalate and Their Metabolites Induce Haemolysis and Eryptosis in Human Erythrocytes. Chemosphere 2018, 203, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Sokołowski, A.; Kończak, M.; Oleszczuk, P.; Gao, Y.; Czech, B. Environmental and Food Contamination by Phthalic Acid Esters (PAEs): Overview. Water Air Soil Pollut. 2024, 235, 313. [Google Scholar] [CrossRef]
- Pham, C.L.T.; Hoang, N.B.; Nguyen, A.V.; Le, V.; Tran, N.M.T.; Pham, K.T.; Phung, H.D.; Chu, N.C.; Hoang, A.Q.; Minh, T.B.; et al. Distribution of Phthalic Acid Esters (PAEs) in Personal Care Products and Untreated Municipal Wastewater Samples: Implications for Source Apportionment and Ecological Risk Assessment. Water Air Soil Pollut. 2025, 236, 33. [Google Scholar] [CrossRef]
- Chen, L.-B.; Gao, C.-J.; Zhang, Y.; Shen, H.-Y.; Lu, X.-Y.; Huang, C.; Dai, X.; Ye, J.; Jia, X.; Wu, K.; et al. Phthalate Acid Esters (PAEs) in Indoor Dust from Decoration Material Stores: Occurrence, Sources, and Health Risks. Toxics 2024, 12, 505. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Kannan, K. Leaching of Phthalates from Medical Supplies and Their Implications for Exposure. Environ. Sci. Technol. 2023, 57, 7675–7683. [Google Scholar] [CrossRef]
- Kumari, A.; Kaur, R. A Review on Morpho-Physiological Traits of Plants under Phthalates Stress and Insights into Their Uptake and Translocation. Plant Growth Regul. 2020, 91, 327–347. [Google Scholar] [CrossRef]
- Cai, Q.-Y.; Mo, C.-H.; Zeng, Q.-Y.; Wu, Q.-T.; Férard, J.-F.; Antizar-Ladislao, B. Potential of Ipomoea Aquatica Cultivars in Phytoremediation of Soils Contaminated with Di-n-Butyl Phthalate. Environ. Exp. Bot. Bot. 2008, 62, 205–211. [Google Scholar] [CrossRef]
- Yue, N.; Deng, C.; Li, C.; Wang, Q.; Li, M.; Wang, J.; Jin, F. Occurrence and Distribution of Phthalate Esters and Their Major Metabolites in Porcine Tissues. J. Agric. Food Chem. 2020, 68, 6910–6918. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, J.; Wang, T.; Qin, C.; Hu, X.; Mosa, A.; Ling, W. Environmental Health Risks Induced by Interaction between Phthalic Acid Esters (PAEs) and Biological Macromolecules: A Review. Chemosphere 2023, 328, 138578. [Google Scholar] [CrossRef]
- Prasad, B. Phthalate Pollution: Environmental Fate and Cumulative Human Exposure Index Using the Multivariate Analysis Approach. Environ. Sci. Process. Impacts 2021, 23, 389–399. [Google Scholar] [CrossRef]
- National Primary Drinking Water Regulations, Federal Register, Part 12, 40 CFR Part 141; U.S. Environmental Protection Agency|US EPA: Washington, DC, USA, 1991.
- GB/T5749-2022; Standards for Drinking Water Quality. National Administration of Disease Control and Prevention: Beijing, China, 2023.
- Li, X.; Wang, J.; Zhang, Q.; Li, H. Advances on the Development of Detection Methods for the Phthalate Esters in Food. J. Chromatogr. A 2015, 33, 1147. [Google Scholar] [CrossRef] [PubMed]
- Amiri, A.; Chahkandi, M.; Targhoo, A. Synthesis of Nano-Hydroxyapatite Sorbent for Microextraction in Packed Syringe of Phthalate Esters in Water Samples. Anal. Chim. Acta 2017, 950, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Arthur, C.L.; Pawliszyn, J. Solid Phase Microextraction with Thermal Desorption Using Fused Silica Optical Fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Wieczorek, M.N.; Zhou, W.; Jeleń, H.H.; Pawliszyn, J. Automated Sequential SPME Addressing the Displacement Effect in Food Samples. Food Chem. 2024, 439, 138093. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Ihara, H.; Guan, M.; Qiu, H. Preparation of Porous Carbon Nanomaterials and Their Application in Sample Preparation: A Review. Trends Anal. Chem. 2021, 143, 116421. [Google Scholar] [CrossRef]
- Xu, L.; Chen, J.; Zhang, J. Fabrication of MOF-on-MOF Composites by Surfactant-Assisted Growth Strategy for SPME of Polycyclic Aromatic Hydrocarbons. J. Hazard. Mater. 2025, 481, 136530. [Google Scholar] [CrossRef]
- Naseri, N.; Kharrazi, S.; Abdi, K.; Alizadeh, R. Fabrication of an SPME Fiber Based on ZnO@ GA Nanorods Coated onto Fused Silica as a Highly Efficient Absorbent for the Analysis of Cancer VOCs in Water and Urine. Anal. Chim. Acta 2021, 1183, 338983. [Google Scholar] [CrossRef]
- Li, Z.; Yang, M.; Shen, X.; Zhu, H.; Li, B. The Preparation of Covalent Bonding COF-TpBD Coating in Arrayed Nanopores of Stainless Steel Fiber for Solid-Phase Microextraction of Polycyclic Aromatic Hydrocarbons in Water. Int. J. Environ. Res. Public Health 2023, 20, 1393. [Google Scholar] [CrossRef]
- Pacheco-Fernández, I.; Rentero, M.; Ayala, J.H.; Pasán, J.; Pino, V. Green Solid-Phase Microextraction Fiber Coating Based on the Metal-Organic Framework CIM-80(Al): Analytical Performance Evaluation in Direct Immersion and Headspace Using Gas Chromatography and Mass Spectrometry for the Analysis of Water, Urine and Brewed Coffee. Anal. Chim. Acta 2020, 1133, 137–149. [Google Scholar] [CrossRef]
- Ghani, M.; Ghoreishi, S.M.; Masoum, S. Highly Porous Nanostructured Copper Oxide Foam Fiber as a Sorbent for Head Space Solid-Phase Microextraction of BTEX from Aqueous Solutions. Microchem. J. 2019, 145, 210–217. [Google Scholar] [CrossRef]
- Ghaemmaghami, M.; Yamini, Y.; Mousavi, K.Z. Accordion-like Ti3C2Tx MXene Nanosheets as a High-Performance Solid Phase Microextraction Adsorbent for Determination of Polycyclic Aromatic Hydrocarbons Using GC-MS. Microchim. Acta 2020, 187, 151. [Google Scholar] [CrossRef]
- Song, X.-L.; Chen, Y.; Yuan, J.-P.; Qin, Y.-J.; Zhao, R.-S.; Wang, X. Carbon Nanotube Composite Microspheres as a Highly Efficient Solid-Phase Microextraction Coating for Sensitive Determination of Phthalate Acid Esters in Water Samples. J. Chromatogr. A 2016, 1468, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Hu, W.; Luo, X.; Zhang, J. Covalent Organic Framework in Situ Grown on the Metal–Organic Framework as Fiber Coating for Solid-Phase Microextraction of Polycyclic Aromatic Hydrocarbons in Tea. Microchim Acta 2023, 190, 344. [Google Scholar] [CrossRef]
- Keshavarzi, M.; Ghorbani, M.; Mohammadi, P.; Pakseresht, M.; Ziroohi, A.; Rastegar, A. Development of a Magnetic Sorbent Based on Synthesis of MOF-on-MOF Composite for Dispersive Solid-Phase Microextraction of Five Phthalate Esters in Bottled Water and Fruit Juice Samples. Microchem. J. 2022, 182, 107934. [Google Scholar] [CrossRef]
- Jiang, X.-X.; Sun, Y.-N.; Zhang, C.; Yang, Z.; Yu, L.-Q.; Lv, Y.-K. Synthesis of SiO2@MOF-199 as a Fiber Coating for Headspace Solid-Phase Microextraction of Phthalates in Plastic Bottled Milk. Chromatographia 2022, 85, 851–863. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Yang, Q.; Zhang, S.; Chang, G.; Zang, X.; Wang, C.; Wang, Z. Covalent Triazine-Based Frameworks for Efficient Solid-Phase Microextraction of Phthalic Acid Esters from Food-Contacted Plastics. J. Chromatogr. A 2022, 1681, 463474. [Google Scholar] [CrossRef] [PubMed]
- Yue, Q.; Huang, Y.-Y.; Shen, X.-F.; Yang, C.; Pang, Y.-H. In Situ Growth of Covalent Organic Framework on Titanium Fiber for Headspace Solid-Phase Microextraction of 11 Phthalate Esters in Vegetables. Food Chem. 2020, 318, 126507. [Google Scholar] [CrossRef]
- Guo, H.; Chen, G.; Ma, J.; Jia, Q. A Triazine Based Organic Framework with Micropores and Mesopores for Use in Headspace Solid Phase Microextraction of Phthalate Esters. Microchim Acta 2019, 186, 4. [Google Scholar] [CrossRef]
- Wang, J.; Huang, S.; Wang, P.; Yang, Y. Method Development for the Analysis of Phthalate Esters in Tea Beverages by Ionic Liquid Hollow Fibre Liquid-Phase Microextraction and Liquid Chromatographic Detection. Food Control 2016, 67, 278–284. [Google Scholar] [CrossRef]
- Trujillo-Rodríguez, M.J.; Yu, H.; Cole, W.T.S.; Ho, T.D.; Pino, V.; Anderson, J.L.; Afonso, A.M. Polymeric Ionic Liquid Coatings versus Commercial Solid-Phase Microextraction Coatings for the Determination of Volatile Compounds in Cheeses. Talanta 2014, 121, 153–162. [Google Scholar] [CrossRef]
- Wang, M.; Yang, F.; Liu, L.; Cheng, C.; Yang, Y. Ionic Liquid-Based Surfactant Extraction Coupled with Magnetic Dispersive μ-Solid Phase Extraction for the Determination of Phthalate Esters in Packaging Milk Samples by HPLC. Food Anal. Methods 2017, 10, 1745–1754. [Google Scholar] [CrossRef]
- Gong, C.; Wei, Y.; Chen, M.; Liu, L.; Chow, C.; Tang, Q. Double Imprinted Photoresponsive Polymer for Simultaneous Detection of Phthalate Esters in Plastics. Eur. Polym. J. 2018, 108, 295–303. [Google Scholar] [CrossRef]
- Barciela-Alonso, M.C.; Otero-Lavandeira, N.; Bermejo-Barrera, P. Solid Phase Extraction Using Molecular Imprinted Polymers for Phthalate Determination in Water and Wine Samples by HPLC-ESI-MS. Microchem. J. 2017, 132, 233–237. [Google Scholar] [CrossRef]
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, Crystalline, Covalent Organic Frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, S.; Chen, Y.; Zhang, Z.; Ma, S. Covalent Organic Frameworks for Separation Applications. Chem. Soc. Rev. 2020, 49, 708–735. [Google Scholar] [CrossRef]
- Jin, Y.; Li, M.; Yang, Y. Covalent Organic Frameworks for Membrane Separation. Adv. Sci. 2024, 12, 2412600. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Jiang, D. Covalent Organic Frameworks for Energy Conversions: Current Status, Challenges, and Perspectives. CCS Chem. 2021, 3, 2003–2024. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Covalent Organic Frameworks (COFs) for Environmental Applications. Coord. Chem. Rev. 2019, 400, 213046. [Google Scholar] [CrossRef]
- Meng, Z.; Mirica, K.A. Covalent Organic Frameworks as Multifunctional Materials for Chemical Detection. Chem. Soc. Rev. 2021, 50, 13498–13558. [Google Scholar] [CrossRef]
- Li, M.-H.; Yang, Z.; Li, Z.; Wu, J.-R.; Yang, B.; Yang, Y.-W. Construction of Hydrazone-Linked Macrocycle-Enriched Covalent Organic Frameworks for Highly Efficient Photocatalysis. Chem. Mater. 2022, 34, 5726–5739. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Zhang, S.; Cui, P.; Wang, C.; Wang, Z. A Novel Schiff Base Network-1 Nanocomposite Coated Fiber for Solid-Phase Microextraction of Phenols from Honey Samples. Talanta 2016, 161, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, D.; Nawała, J.; Gordon, D.; Dawidziuk, B.; Popiel, S. Nanostructured Polyaniline SPME Fiber Coating for Chemical Warfare Agents Analysis. Anal. Chim. Acta 2022, 1202, 339649. [Google Scholar] [CrossRef]
- Meng, W.-K.; Liu, L.; Wang, X.; Zhao, R.-S.; Wang, M.-L.; Lin, J.-M. Polyphenylene Core-Conjugated Microporous Polymer Coating for Highly Sensitive Solid-Phase Microextraction of Polar Phenol Compounds in Water Samples. Anal. Chim. Acta 2018, 1015, 27–34. [Google Scholar] [CrossRef]
- Ma, T.-T.; Shen, X.-F.; Yang, C.; Qian, H.-L.; Pang, Y.-H.; Yan, X.-P. Covalent Immobilization of Covalent Organic Framework on Stainless Steel Wire for Solid-Phase Microextraction GC-MS/MS Determination of Sixteen Polycyclic Aromatic Hydrocarbons in Grilled Meat Samples. Talanta 2019, 201, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Zheng, Q.; He, Y.; Li, G.; Guo, W.; Lin, Z.; Zhang, L. Size-Controllable Synthesis of Uniform Spherical Covalent Organic Frameworks at Room Temperature for Highly Efficient and Selective Enrichment of Hydrophobic Peptides. J. Am. Chem. Soc. 2019, 141, 18271–18277. [Google Scholar] [CrossRef]
- Jõul, P.; Vaher, M.; Kuhtinskaja, M. Carbon Aerogel-Based Solid-Phase Microextraction Coating for the Analysis of Organophosphorus Pesticides. Anal. Methods 2021, 13, 69–76. [Google Scholar] [CrossRef]
- Cui, M.; Qiu, J.; Li, Z.; He, M.; Jin, M.; Kim, J.; Quinto, M.; Li, D. An Etched Stainless Steel Wire/Ionic Liquid-Solid Phase Microextraction Technique for the Determination of Alkylphenols in River Water. Talanta 2015, 132, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.; Chang, Q.; Zang, X.; Zhang, S.; Wang, C.; Wang, Z. Carbonized MXene-Polyvinylpyrrolidone as an Adsorbent for Solid-Phase Microextraction of Polycyclic Aromatic Hydrocarbons from Tea Beverages Prior to GC Analysis. Microchem. J. 2023, 185, 108313. [Google Scholar] [CrossRef]
- Li, J.; Wang, F.; Wu, J.-F.; Zhao, G. Stainless Steel Fiber Coated with Poly(1-Hexyl-3-Vinylimidazolium) Bromide for Solid-Phase Microextraction of Polychlorinated Biphenyls from Water Samples Prior to Their Quantitation by GC. Microchim. Acta 2017, 184, 2621–2628. [Google Scholar] [CrossRef]
- Shi, F.; Liu, J.; Liang, K.; Liu, R. Tris(Pentafluoroethyl)Trifluorophosphate-Basd Ionic Liquids as Advantageous Solid-Phase Micro-Extraction Coatings for the Extraction of Organophosphate Esters in Environmental Waters. J. Chromatogr. A 2016, 1447, 9–16. [Google Scholar] [CrossRef]
- Bagheri, H.; Javanmardi, H.; Abbasi, A.; Banihashemi, S. A Metal Organic Framework-Polyaniline Nanocomposite as a Fiber Coating for Solid Phase Microextraction. J. Chromatogr. A 2016, 1431, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Nurerk, P.; Liew, C.S.M.; Bunkoed, O.; Kanatharana, P.; Lee, H.K. Environmentally Friendly Etching of Stainless Steel Wire for Plunger-in-Needle Liquid-Phase Microextraction of Polycyclic Aromatic Hydrocarbons. Talanta 2019, 197, 465–471. [Google Scholar] [CrossRef]
- Tashakkori, P.; Tağaç, A.A.; Merdivan, M. Fabrication of Montmorillonite/Ionic Liquid Composite Coated Solid-Phase Microextraction Fibers for Determination of Phenolic Compounds in Fruit Juices by Gas Chromatography and Liquid Chromatography. J. Chromatogr. A 2021, 1635, 461741. [Google Scholar] [CrossRef]
- Li, Q.-L.; Huang, F.; Wang, X.-L.; Wang, X.; Zhao, R.-S. Multiple-Helix Cobalt(II)-Based Metal-Organic Nanotubes on Stainless Steel Fibers for Solid-Phase Microextraction of Chlorophenol and Nitrophenols from Water Samples. Microchim. Acta 2017, 184, 1817–1825. [Google Scholar] [CrossRef]
- Song, C.; Shao, Y.; Yue, Z.; Hu, Q.; Zheng, J.; Yuan, H.; Yu, A.; Zhang, W.; Zhang, S.; Ouyang, G. Sheathed In-Situ Room-Temperature Growth Covalent Organic Framework Solid-Phase Microextraction Fiber for Detecting Ultratrace Polybrominated Diphenyl Ethers from Environmental Samples. Anal. Chim. Acta 2021, 1176, 338772. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Li, J.; Zhang, S.; An, Y.; Hao, L.; Yang, X.; Wang, C.; Wang, Z.; Wu, Q. Novel N-Riched Covalent Organic Framework for Solid-Phase Microextraction of Organochlorine Pesticides in Vegetable and Fruit Samples. Food Chem. 2022, 388, 133007. [Google Scholar] [CrossRef]
- Jafari, Z.; Ghani, M.; Bakhsh Raoof, J. ZIF-8-90 @ graphene oxide reinforced porous hollow fiber coupled with deep eutectic solvent for hollow fiber solid-phase microextraction of selected phthalate esters followed by quantification through high-performance liquid chromatography-ultraviolet detection. Microchem. J. 2023, 194, 109269. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Wang, Q.; Guo, L.; Wang, C.; Wang, Z.; Zhang, S.; Wu, Q. Construction of Hypercrosslinked Polymers for High-Performance Solid Phase Microextraction of Phthalate Esters from Water Samples. J. Chromatogr. A 2021, 1641, 461972. [Google Scholar] [CrossRef]
- Baharin, S.; Sarih, N.; Mohamad, S. Novel Functionalized Polythiophene-Coated Fe3O4 Nanoparticles for Magnetic Solid-Phase Extraction of Phthalates. Polymers 2016, 117, 117. [Google Scholar] [CrossRef]
- Guo, H.; Song, N.; Wang, D.; Ma, J.; Jia, Q. A modulation approach for covalent organic frameworks: Application to solid phase microextraction of phthalate esters. Talanta 2019, 198, 277–283. [Google Scholar] [CrossRef]

| Etched Methods | Etched Solution | Time | Coating | Analytics | Ref. |
|---|---|---|---|---|---|
| Chemical etching | 1 M NaOH | 30 min | Carbon-aerogel-based | OPs | [48] |
| Chemical etching | HF (40%) | 20 min | Ionic liquids | Alkylphenols | [49] |
| Chemical etching | HF (40%) | - | Carbonized MXene-polyvinylpyrrolidone | PAHs | [50] |
| Polished | - | - | Poly(1-Hexyl-3-Vinylimidazolium) Bromide | PCBs | [51] |
| Chemical etching | 0.1 M NaOH and 0.1 M HCl | Washed | Ionic liquids | OPEs | [52] |
| Chemical etching | H2SO4 | - | MOF/PANI nanocomposites | Aromatic compounds | [53] |
| Chemical etching | FeCl3/HCl solution (20% w/v) | 15 min | - | PAHs | [54] |
| Chemical etching | Aqua regia/water (1:1) | 30 min | Montmorillonite/ionic liquid composite | Phenolic compounds | [55] |
| Chemical etching | 37% HCl | 30 min | Multiple-helix cobalt (II)-based Metal–organic nanotube | Chlorophenol and nitrophenols | [56] |
| Electrochemical etching | (CH2OH)2/HClO4 (v/v, 9:1) | 50 s | COF-TpBD | PAHs | [20] |
| Electrochemical etching | (CH2OH)2/HClO4 (v/v, 9:1); 0.1 M (CH2OH)2 of NH4F | 6 min | COF-TPB-DMTP | PAEs | This work |
| Analytes | Linear Range (µg·L−1) | R2 | LODs (µg·L−1) | LOQs (µg·L−1) | RSDs | EFs * | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Inter-Day (n = 5, %) | Intra-Day (n = 3, %) | Fiber-to-Fiber (n = 3, %) | ||||||||
| a | b | a | b | |||||||
| DIBP | 0.20–300 | 0.9932 | 0.20 | 0.67 | 4.1 | 9.3 | 4.3 | 8.7 | 8.4 | 840 |
| DBP | 0.04–100 | 0.9964 | 0.04 | 0.13 | 8.0 | 5.2 | 6.3 | 3.8 | 9.9 | 1068 |
| DPP | 0.30–100 | 0.9973 | 0.30 | 0.99 | 11.8 | 12.1 | 7.3 | 12.3 | 4.5 | 2657 |
| BBP | 0.44–500 | 0.9928 | 0.44 | 1.46 | 9.1 | 12.9 | 7.9 | 11.9 | 2.3 | 559 |
| DEHP | 0.39–500 | 0.9945 | 0.39 | 1.30 | 10.5 | 12.5 | 3.9 | 14.1 | 7.5 | 268 |
| Method | Coating | Matrix | Analytes | Linear Range (µg·L−1) | LODs (µg·L−1) | Ref. |
|---|---|---|---|---|---|---|
| HPLC-UV | ZIF-8-90@graphene oxide | Water, juice | DMP, DEP, DBP | 0.1–500 | 0.026–0.058 | [59] |
| GC-MS | MWNTs@PS | water | DBP, BBP, DEHP, DOP | 0.001–5 | 0.0012–0.018 | [24] |
| GC-MS | HCP-1 | Bottled water | DMP, DEP, DAP, DPRP, DIBP, DBP, BBP, DCHP | 0.01–10 | 0.003–0.033 | [60] |
| GC-MS-MS | TpBD-TiO2 | Vegetables | DMP, DEP, DIBP, DBP, DMPP, DPP, DHXP, BBP, DCHP, DPhP, DNOP | 0.005–50 | 0.001–0.430 | [29] |
| GC-FID | MNP@P3TArH | Bottled mineral and fresh milk | DMP, DEP, DPP, DBP, BBP, DCP, DEHP, DNOP | 0.1–50 | 0.054–0.468 | [61] |
| GC-FID | OH50%-TPB-COF | Water | DEP, DPP, DAP, DBP, BBP, DEHP | 1–100 | 0.032–0.451 | [62] |
| GC-FID | COF-TPB-DMTP | Bottled tea beverages | DIBP, DBP, BBP, DPP, DEHP | 0.04–500 | 0.04–0.44 | This work |
| Analytes | Bottled Green Tea | Jasmine Tea | Oolong Tea | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Found (µg L−1) | Recoveries (%, n = 3) | Found (µg L−1) | Recoveries (%, n = 3) | Found (µg L−1) | Recoveries (%, n = 3) | ||||
| Added a | Added b | Added a | Added b | Added a | Added b | ||||
| DIBP | ND | 106 | 87.57 | ND | 115 | 95.54 | ND | 94.3 | 91.00 |
| DBP | ND | 85.5 | 106.07 | ND | 100 | 92.86 | ND | 89.3 | 90.18 |
| DDP | ND | 89.4 | 98.37 | ND | 79.0 | 96.47 | ND | 108 | 86.53 |
| BBP | ND | 108 | 97.78 | ND | 90.0 | 102.73 | ND | 80.2 | 97.94 |
| DEHP | ND | 115 | 98.10 | ND | 114 | 96.37 | ND | 90.4 | 95.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Li, B.; Zhang, K.; Zhu, H. A Novel Gully-like Surface of Stainless-Steel Fiber Coated with COF-TPB-DMTP Nanoparticles for Solid-Phase Microextraction of Phthalic Acid Esters in Bottled Tea Beverages. Nanomaterials 2025, 15, 385. https://doi.org/10.3390/nano15050385
Yuan Y, Li B, Zhang K, Zhu H. A Novel Gully-like Surface of Stainless-Steel Fiber Coated with COF-TPB-DMTP Nanoparticles for Solid-Phase Microextraction of Phthalic Acid Esters in Bottled Tea Beverages. Nanomaterials. 2025; 15(5):385. https://doi.org/10.3390/nano15050385
Chicago/Turabian StyleYuan, Yuanyuan, Baohui Li, Keqing Zhang, and Hongtao Zhu. 2025. "A Novel Gully-like Surface of Stainless-Steel Fiber Coated with COF-TPB-DMTP Nanoparticles for Solid-Phase Microextraction of Phthalic Acid Esters in Bottled Tea Beverages" Nanomaterials 15, no. 5: 385. https://doi.org/10.3390/nano15050385
APA StyleYuan, Y., Li, B., Zhang, K., & Zhu, H. (2025). A Novel Gully-like Surface of Stainless-Steel Fiber Coated with COF-TPB-DMTP Nanoparticles for Solid-Phase Microextraction of Phthalic Acid Esters in Bottled Tea Beverages. Nanomaterials, 15(5), 385. https://doi.org/10.3390/nano15050385






