Electrochemical Synthesis of Polyaniline and Sheet-like Structure of Molybdenum Selenide (PANI@2D-MoSe2) Binary Composite for Solar Cell Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
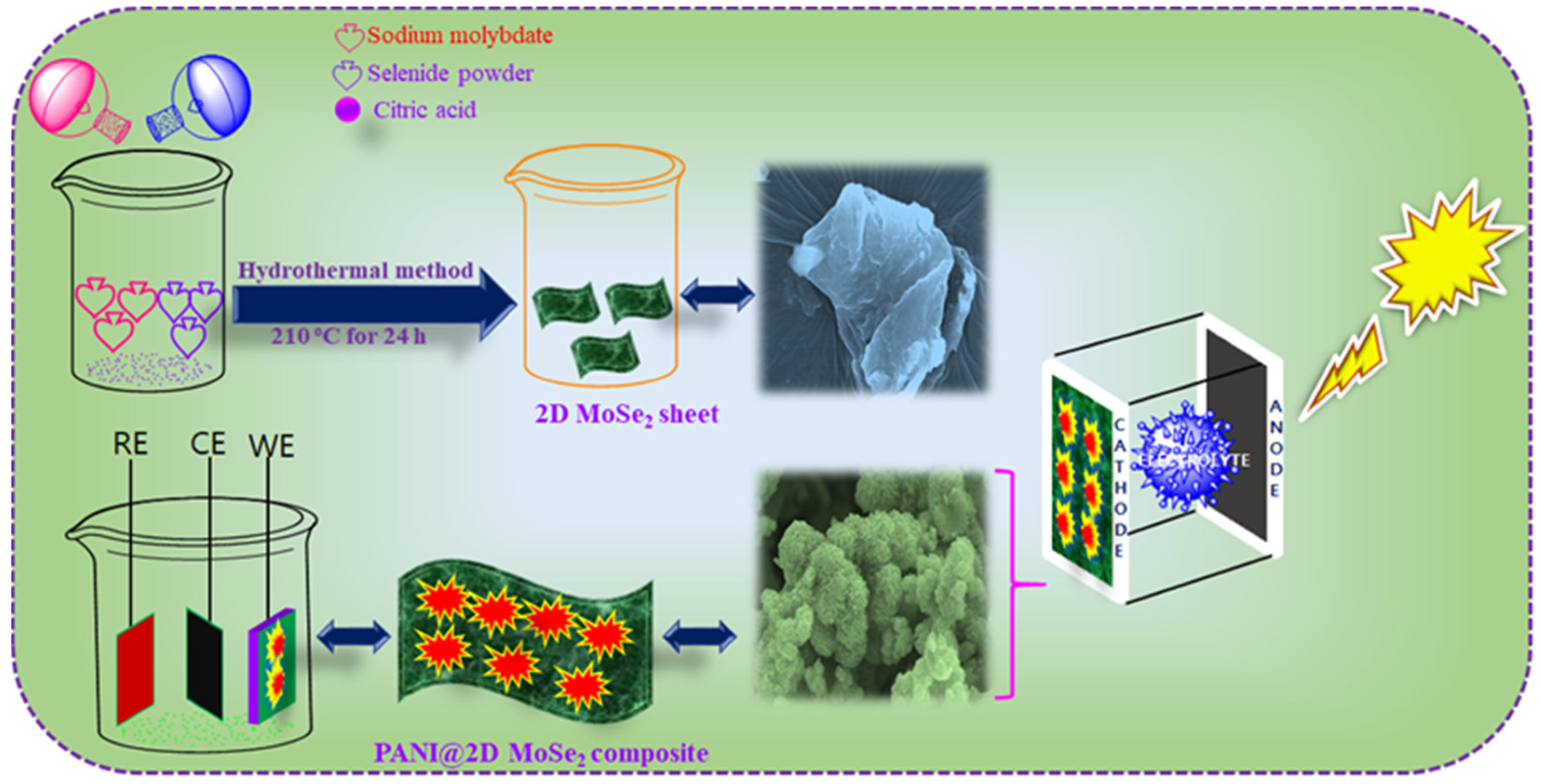
2.2. Hydrothermal Synthesis of Flower-like Two-Dimensional Molybdenum Selenide (2D MoSe2)
2.3. Electrochemical Synthesis of Polyaniline and Two-Dimensional Molybdenum Selenide (PANI@2D MoSe2) Counter Electrode
2.4. Electropolymerization Parameters
2.5. Device Fabrication
2.6. Characterization
3. Results and Discussion
3.1. Electrochemical Synthesis of PANI@2D-MoSe2 Composite
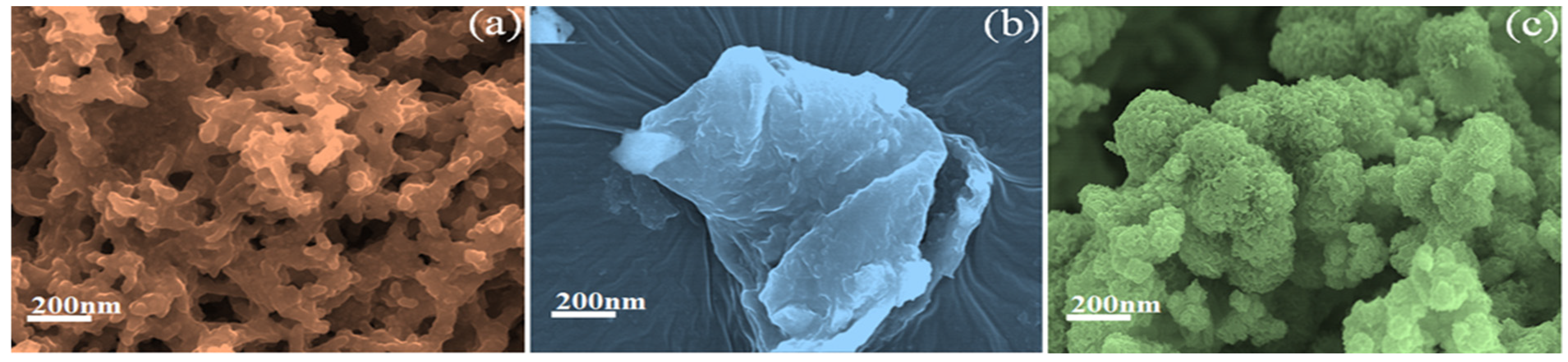
3.2. FE-SEM Image Analysis
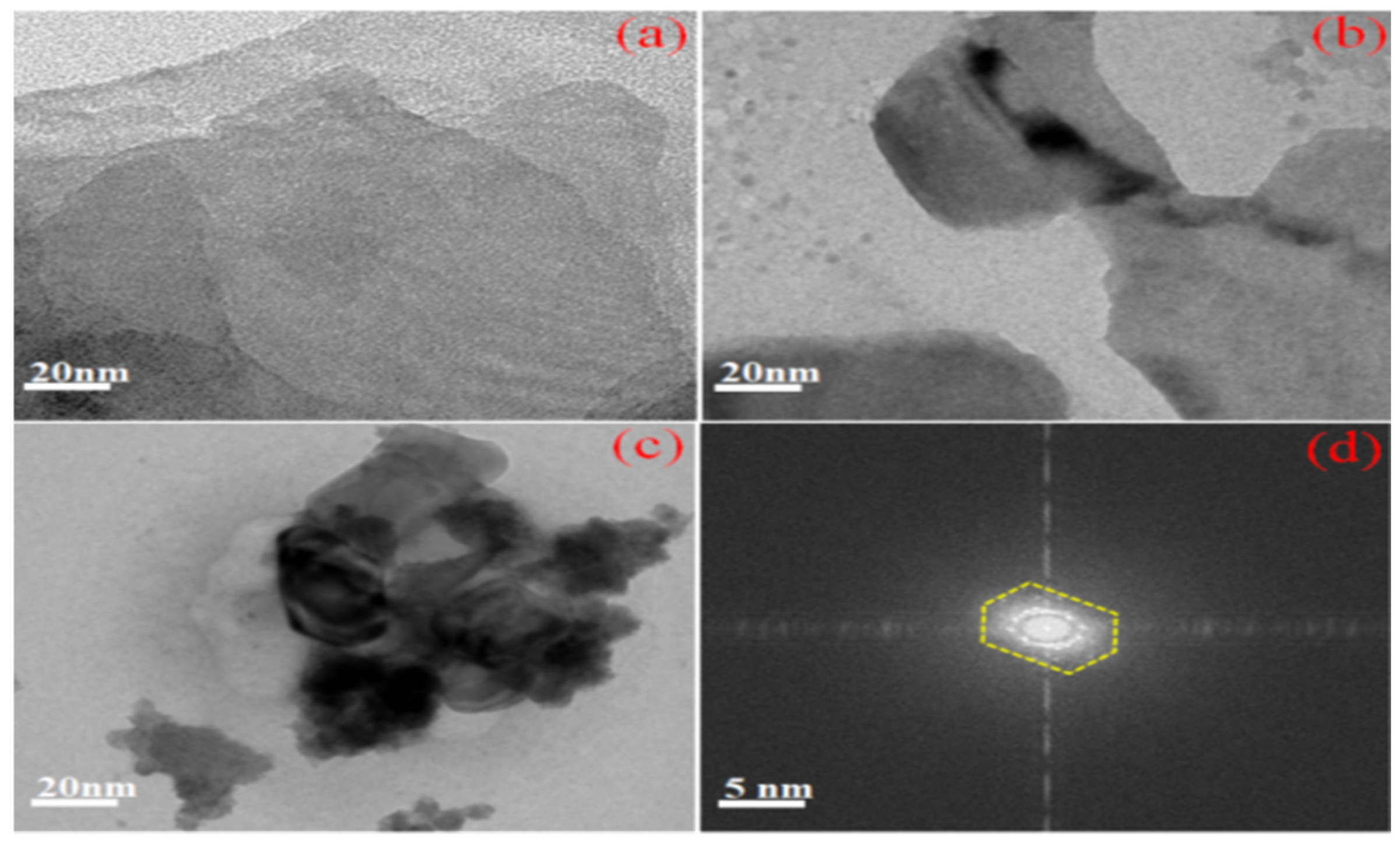
3.3. HR-TEM Image Analysis
3.4. XRD Pattern Analysis
3.5. FT-IR Spectral Analysis
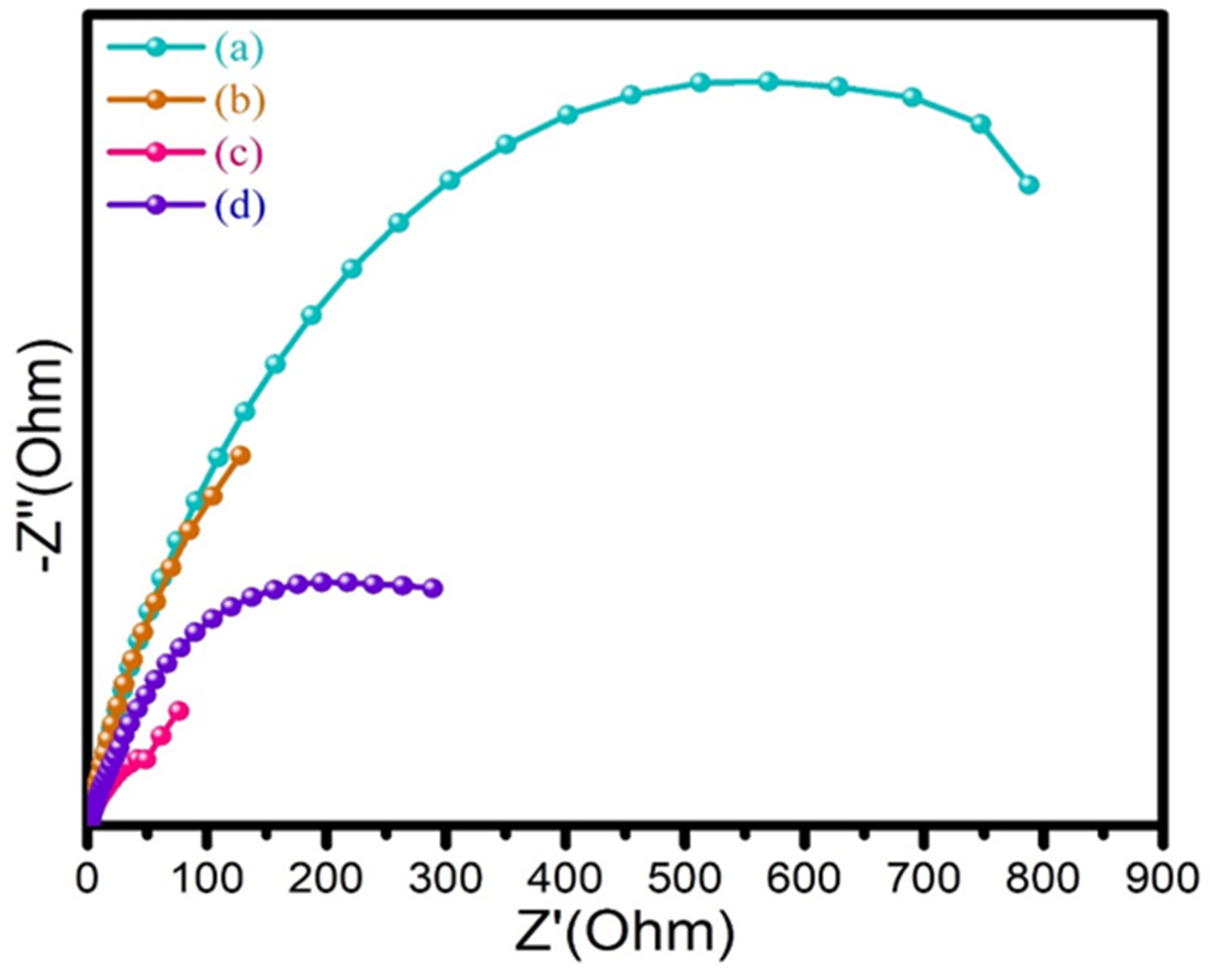
3.6. Electrochemical Impedance Spectroscopy (EIS) Studies
3.7. I–V Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alessa, H.; Wijayantha, K.G.U. A very simple flexible tandem dye-sensitized solar cell. J. Umm Al-Qura Univ. Appl. Sci. 2024, 10, 698–706. [Google Scholar] [CrossRef]
- Mozaffari, S.; Nateghi, M.R.; Zarandi, M.B. An overview of the Challenges in the commercialization of dye sensitized solar cells. Renew. Sustain. Energy Rev. 2017, 71, 675–686. [Google Scholar] [CrossRef]
- Barichello, J.; Mariani, P.; Vesce, L.; Spadaro, D.; Citro, I.; Matteocci, F.; Bartolotta, A.; Di Carlo, A.; Calogero, G. Bifacial dye-sensitized solar cells for indoor and outdoor renewable energy-based application. J. Mater. Chem. C 2023, 12, 2317–2349. [Google Scholar] [CrossRef]
- Tang, Z.; Wu, J.; Zheng, M.; Huo, J.; Lan, Z. A microporous platinum counter electrode used in dye-sensitized solar cells. Nano Energy 2013, 2, 622–627. [Google Scholar] [CrossRef]
- Wu, K.; Chen, L.; Sun, X.; Wu, M. Transition-Metal-Modified Polyaniline Nanofiber Counter Electrode for Dye-Sensitized Solar Cells. ChemElectroChem 2016, 3, 1922–1926. [Google Scholar] [CrossRef]
- Kumar, R.; Bhargava, P. Synthesis and characterization of carbon based counter electrode for dye sensitized solar cells (DSSCs) using organic precursor 2-2′Bipyridine (Bpy) as a carbon material. J. Alloys Compd. 2018, 748, 905–910. [Google Scholar] [CrossRef]
- Sarker, S.; Lee, K.S.; Seo, H.W.; Jin, Y.K.; Kim, D.M. Reduced graphene oxide for Pt-free counter electrodes of dye-sensitized solar cells. Sol. Energy 2017, 158, 42–48. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, T.; He, R.; Li, H.; Lin, H.; Li, L.; Zou, G.; Jia, Q.; Peng, H. A novel carbon nanotube/polymer composite film for counter electrodes of dye-sensitized solar cells. Polym. Chem. 2013, 4, 1680–1684. [Google Scholar] [CrossRef]
- Perminov, A.; Jurisch, M.; Bartzsch, G.; Biermann, H.; Weißgärber, T.; Volkova, O. Manufacturing Fe–TiC metal matrix composite by electron beam powder bed fusion from pre-alloyed gas atomized powder. Mater. Sci. Eng. A 2021, 813, 141130. [Google Scholar] [CrossRef]
- Ghafoor, U.; Aqeel, A.B.; Zaman, U.K.U.; Zahid, T.; Noman, M.; Ahmad, M.S. Effect of molybdenum disulfide on the performance of polyaniline based counter electrode for dye-sensitized solar cell applications. Energies 2021, 14, 3786. [Google Scholar] [CrossRef]
- Sheela, S.E.; Murugadoss, V.; Sittaramane, R.; Angaiah, S. Development of tungsten diselenide/polyaniline composite nanofibers as an efficient electrocatalytic counter electrode material for dye-sensitized solar cell. Sol. Energy 2020, 209, 538–546. [Google Scholar] [CrossRef]
- Sowbakkiyavathi, E.S.; Murugadoss, V.; Sittaramane, R.; Angaiah, S. Development of MoSe2/PANI composite nanofibers as an alternative to Pt counter electrode to boost the photoconversion efficiency of dye sensitized solar cell. J. Solid State Electrochem. 2020, 24, 2289–2300. [Google Scholar] [CrossRef]
- Singh, A.; Poddar, D.; Thakur, S.; Jha, R. Ternary composite based on MoSe2-rGO/polyaniline as an efficient counter electrode catalyst for dye sensitized solar cells. Mater. Chem. Phys. 2021, 273, 125043. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, A.; Zhang, Y.; Zhao, Z.; Ye, S.; Qin, Y. Construction of hierarchical graphene/polyaniline@polyaniline electrodes by chemical and electrochemical polymerization for high-energy supercapacitors. Electrochim. Acta 2023, 454, 142414. [Google Scholar] [CrossRef]
- Mittal, H.; Kumar, A.; Khanuja, M. MoSe2-PANI nanocomposite as Supercapacitor Electrode Material: Optimization, Mechanism and Electrochemical Performance. ChemistrySelect 2022, 7, e202201623. [Google Scholar] [CrossRef]
- Zhang, H.; He, G.; Zheng, D.; HuangFu, H.; Li, Y.; Mi, Y.; Wu, M.; Yuan, H. Higher energy density of MoSe2/polyaniline capsule nanospheres for enhanced performance supercapacitor. Nanotechnology 2023, 34, 415705. [Google Scholar] [CrossRef]
- Saha, S.; Chaudhary, N.; Mittal, H.; Gupta, G.; Khanuja, M. Inorganic–organic nanohybrid of MoS2-PANI for advanced photocatalytic application. Int. Nano Lett. 2019, 9, 127–139. [Google Scholar] [CrossRef]
- Pang, Z.; Chen, Z.; Wen, R.; Zhao, Y.; Wei, A.; Liu, J.; Tao, L.; Luo, D.; Yang, Y.; Xiao, Y.; et al. Colloidally synthesized MoSe2 nano-flowers anchored on three-dimensional porous reduced graphene oxide thin films as advanced counter electrode for dye-sensitized solar cells. J. Mater. Sci. Mater. Electron. 2017, 28, 15418–15422. [Google Scholar] [CrossRef]
- Zatirostami, A. A new electrochemically prepared composite counter electrode for dye-sensitized solar cells. Thin Solid Film. 2020, 701, 137926. [Google Scholar] [CrossRef]
- Dawo, C.; Iyer, P.K.; Chaturvedi, H. Carbon nanotubes/PANI composite as an efficient counter electrode material for dye sensitized solar cell. Mater. Sci. Eng. B 2023, 297, 116722. [Google Scholar] [CrossRef]
- Mehmood, U.; Asghar, H.; Babar, F.; Younas, M. Effect of graphene contents in polyaniline/graphene composites counter electrode material on the photovoltaic performance of dye-sensitized solar cells (DSSCSs). Sol. Energy 2020, 196, 132–136. [Google Scholar] [CrossRef]
- Velu, K.S.; Senthilkumaran, M.; Sethuraman, V.; Balaji, M.; Saravanan, C.; Ahmed, N.; Mohandoss, S.; Lee, Y.R.; Anandharaj, J.; Stalin, T. The surfactants mediated electropolymerized poly(aniline) (PANI)-reduced graphene oxide (rGO) composite counter electrode for dye-sensitized solar cell. J. Phys. Chem. Solids 2023, 173, 111121. [Google Scholar] [CrossRef]
- Al-bahrani, M.R.; Xu, X.; Ahmad, W.; Ren, X.; Su, J.; Cheng, Z.; Gao, Y. Highly efficient dye-sensitized solar cell with GNS/MWCNT/PANI as a counter electrode. Mater. Res. Bull. 2014, 59, 272–277. [Google Scholar] [CrossRef]
- Shahid, M.U.; Mohamed, N.M.; Muhsan, A.S.; Bashiri, R.; Shamsudin, A.E.; Zaine, S.N.A. Few-layer graphene supported polyaniline (PANI) film as a transparent counter electrode for dye-sensitized solar cells. Diam. Relat. Mater. 2019, 94, 242–251. [Google Scholar] [CrossRef]
- Ahmed, U.; Shahid, M.M.; Shahabuddin, S.; Abd Rahim, N.; Alizadeh, M.; Pandey, A.K.; Sagadevan, S. Pandey, Suresh Sagadevan, An efficient platform based on strontium titanate nanocubes interleaved polypyrrole nanohybrid as counter electrode for dye-sensitized solar cell. J. Alloys Compd. 2021, 860, 158228. [Google Scholar] [CrossRef]
- Chen, X.; Liu, J.; Qian, K.; Wang, J. Ternary composites of Ni–polyaniline–graphene as counter electrodes for dye-sensitized solar cells. RSC Adv. 2018, 8, 10948–10953. [Google Scholar] [CrossRef]
- Yang, P.; Duan, J.; Tang, Q. Cobalt sulfide decorated polyaniline complex counter electrodes for efficient dye-sensitized solar cells. Electrochim. Acta 2015, 184, 64–69. [Google Scholar] [CrossRef]



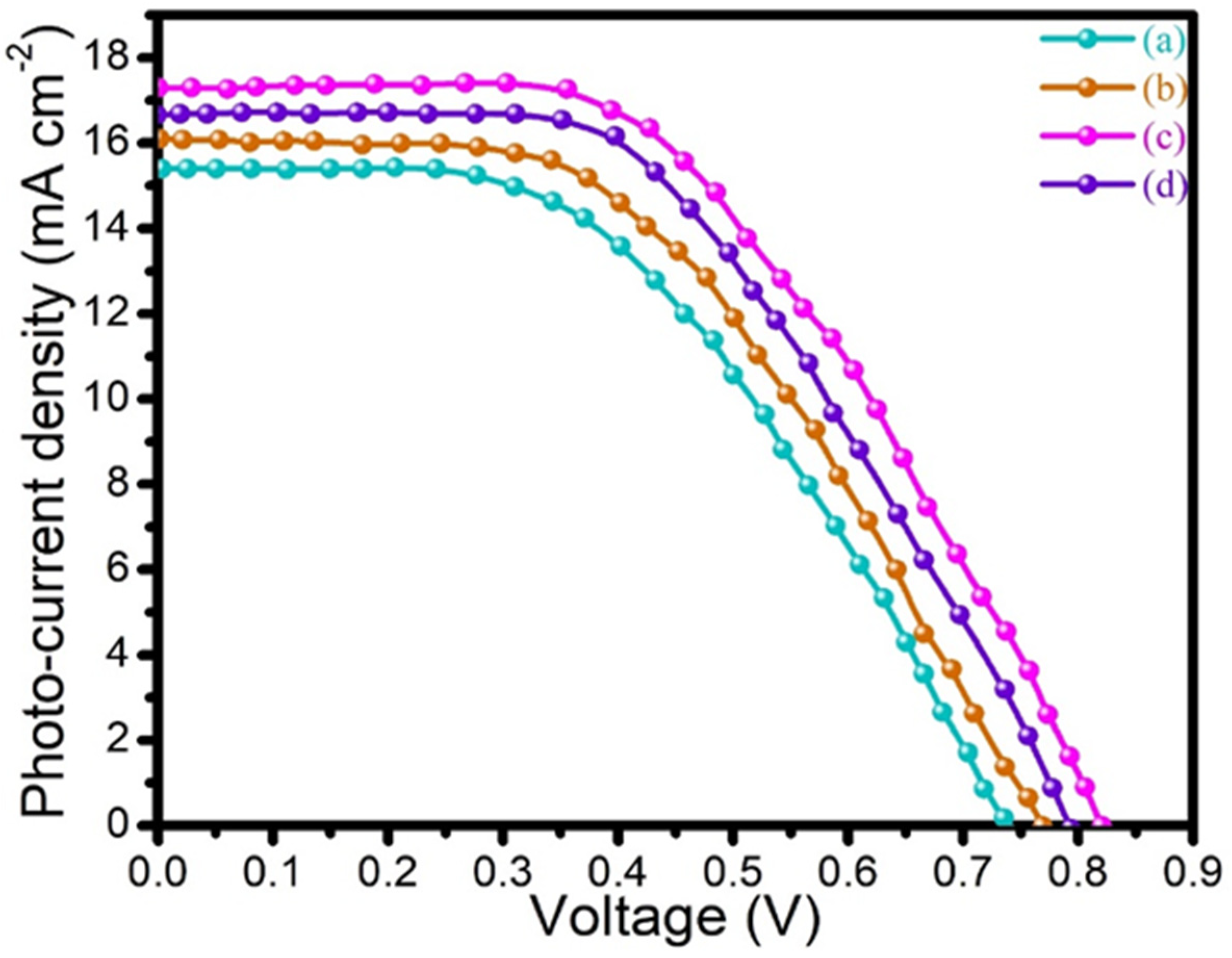
| Counter Electrode | Jsc (mA cm−2) | Voc (V) | FF (%) | Ƞ (%) |
|---|---|---|---|---|
| PANI | 15.45 | 0.73 | 0.45 | 5.07 |
| 2D-MoSe2 | 16.09 | 0.77 | 0.47 | 5.82 |
| PANI@2D-MoSe2 | 17.33 | 0.82 | 0.52 | 7.38 |
| Pt | 16.75 | 0.79 | 0.50 | 6.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manimekalai, A.; Thirumal, V.; Kim, J.; Babu, B.; Velu, K.S. Electrochemical Synthesis of Polyaniline and Sheet-like Structure of Molybdenum Selenide (PANI@2D-MoSe2) Binary Composite for Solar Cell Applications. Nanomaterials 2025, 15, 384. https://doi.org/10.3390/nano15050384
Manimekalai A, Thirumal V, Kim J, Babu B, Velu KS. Electrochemical Synthesis of Polyaniline and Sheet-like Structure of Molybdenum Selenide (PANI@2D-MoSe2) Binary Composite for Solar Cell Applications. Nanomaterials. 2025; 15(5):384. https://doi.org/10.3390/nano15050384
Chicago/Turabian StyleManimekalai, Alagumalai, Vediyappan Thirumal, Jinho Kim, Bathula Babu, and Kuppu Sakthi Velu. 2025. "Electrochemical Synthesis of Polyaniline and Sheet-like Structure of Molybdenum Selenide (PANI@2D-MoSe2) Binary Composite for Solar Cell Applications" Nanomaterials 15, no. 5: 384. https://doi.org/10.3390/nano15050384
APA StyleManimekalai, A., Thirumal, V., Kim, J., Babu, B., & Velu, K. S. (2025). Electrochemical Synthesis of Polyaniline and Sheet-like Structure of Molybdenum Selenide (PANI@2D-MoSe2) Binary Composite for Solar Cell Applications. Nanomaterials, 15(5), 384. https://doi.org/10.3390/nano15050384







