Sustainable Bacterial Cellulose Production Using Low-Cost Fruit Wastewater Feedstocks
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Fractionation Technique from Fruit Processing Wastewater
2.3. Determination of the Carbon (C) and Nitrogen (N) Content in the Fruit Processing Wastewater
2.4. Inoculum Preparation for BC Production
2.5. Preparation of Fermentation Medium and BC Production Using the Fruit Processing Wastewater Fractions
2.6. Harvesting and Purification of BC Films
2.7. Determination of BC Dry Weights
2.8. Productivity Parameters of BC Films Using Fruit Processing Wastewater Fractions
2.9. Analysis of the BC Properties
2.9.1. Thickness and Opacity Measurements of the BC Films
2.9.2. Visual Appearance of BC Films
2.9.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.9.4. Transmission Electron Microscopy (TEM)
2.9.5. X-Ray Diffractometry (XRD)
2.9.6. Differential Scanning Calorimetry (DSC) Measurements
2.9.7. Mechanical Properties
2.9.8. Water Holding Capacity (WHC)
2.10. Statistical Analysis
3. Results and Discussion
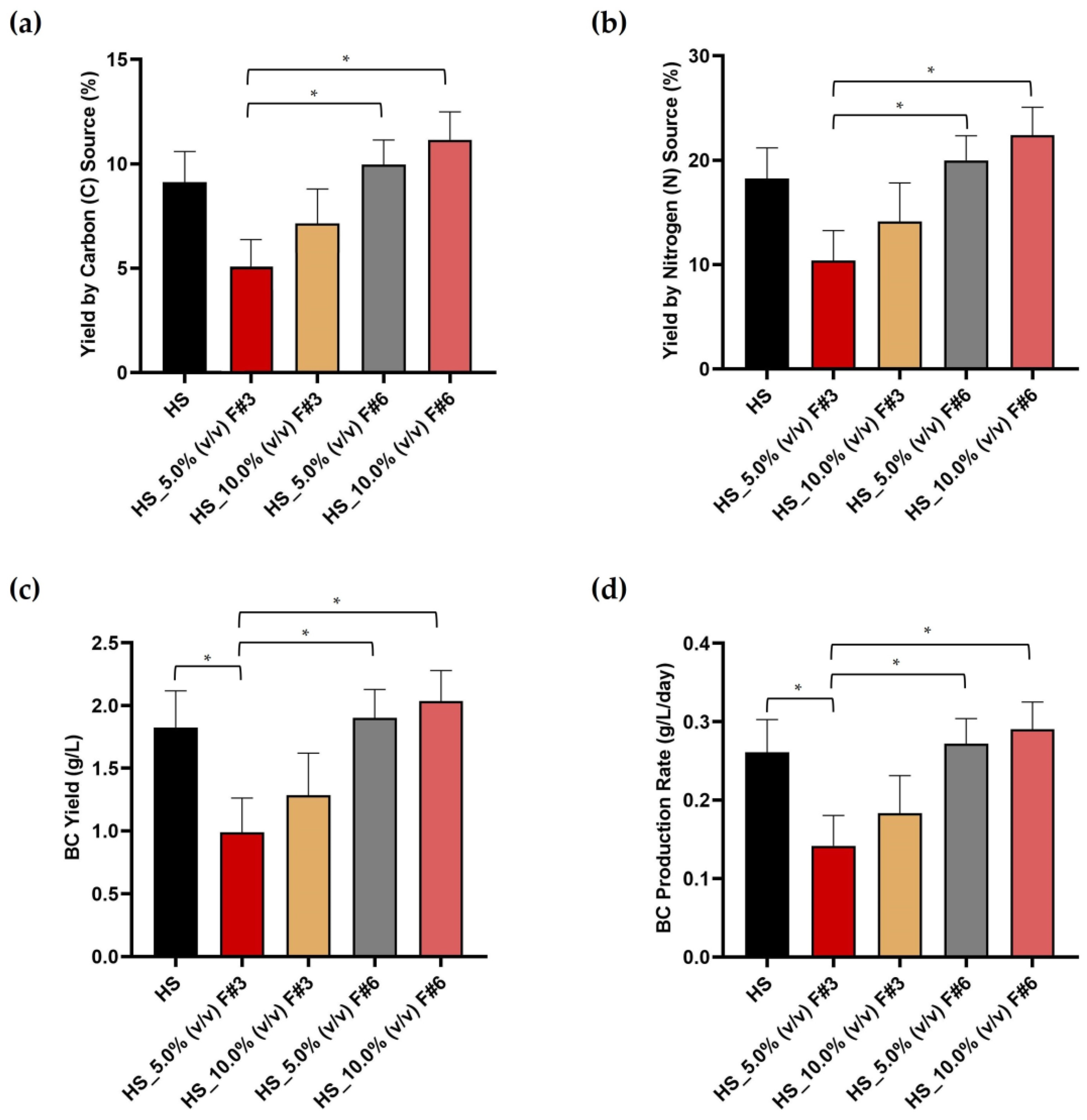
3.1. Determination of the Carbon (C) and Nitrogen (N) Content in the Fruit Processing Wastewater
3.2. Bacterial Cellulose (BC) Productivity Parameters Using Fruit Processing Wastewater Fractions
3.3. Analysis of the BC Properties
3.3.1. Visual Appearance of BC Films Produced from Fruit Processing Wastewater Fractions
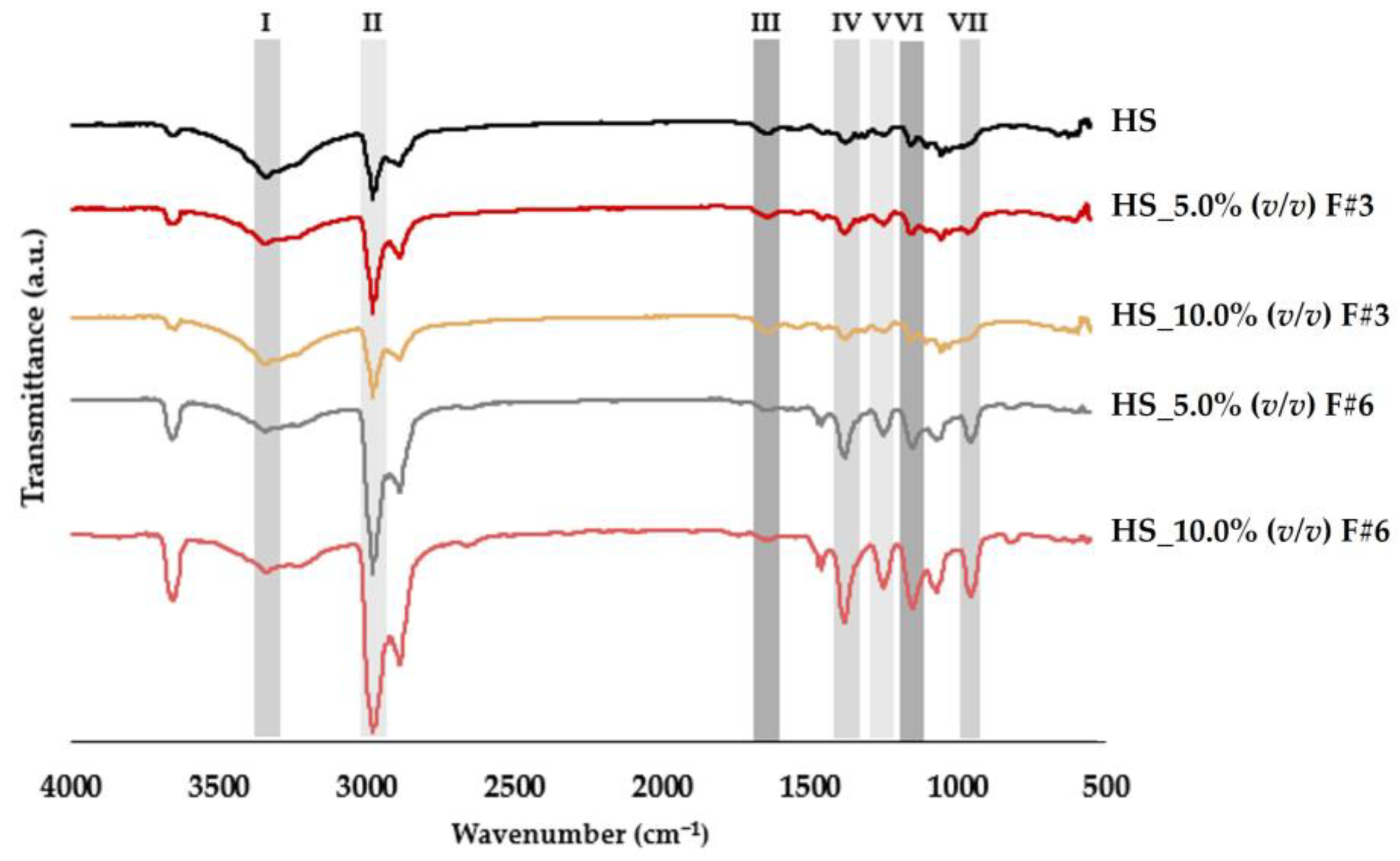
3.3.2. Fourier Transform Infrared Spectroscopy (FTIR)
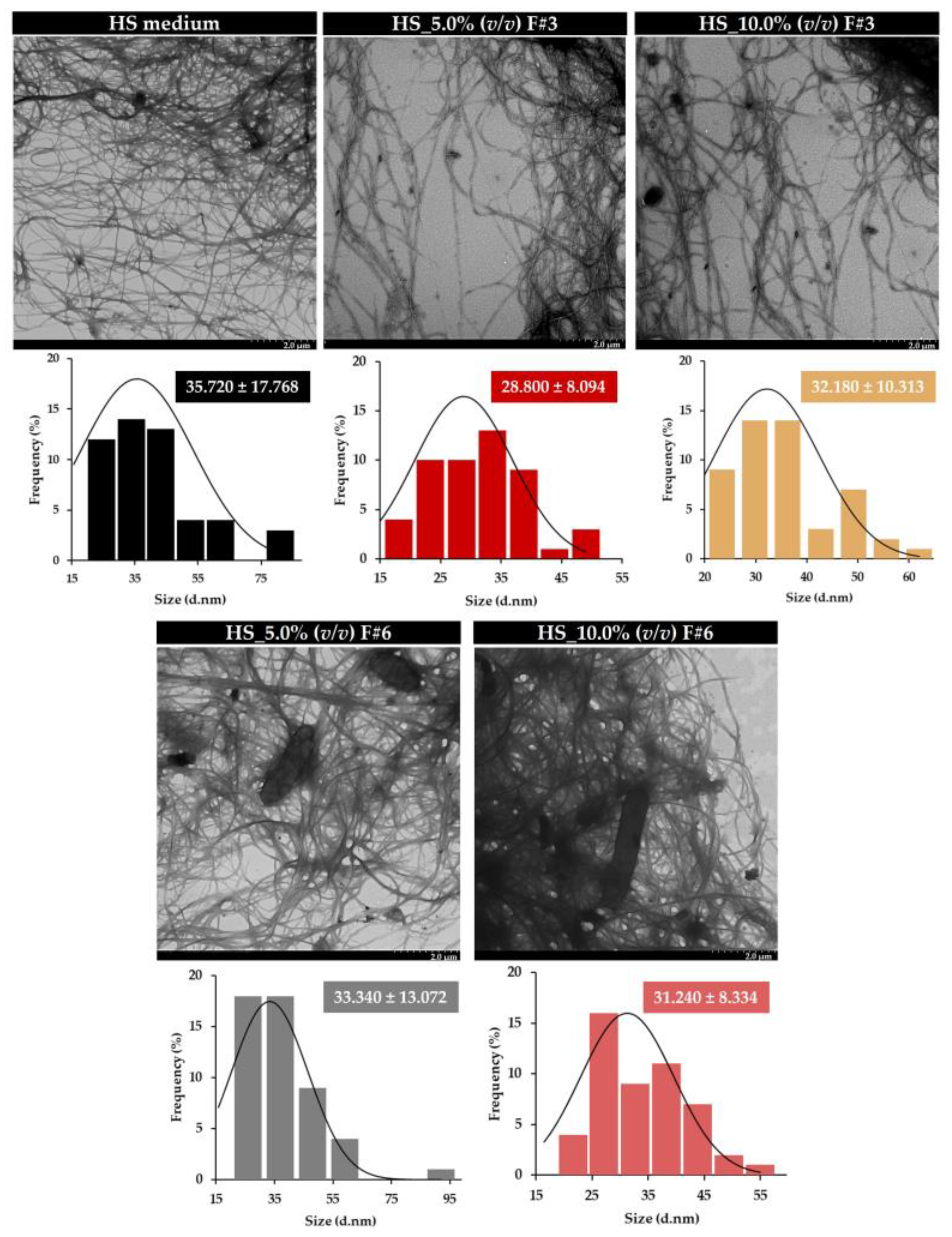
3.3.3. Transmission Electron Microscopy (TEM)
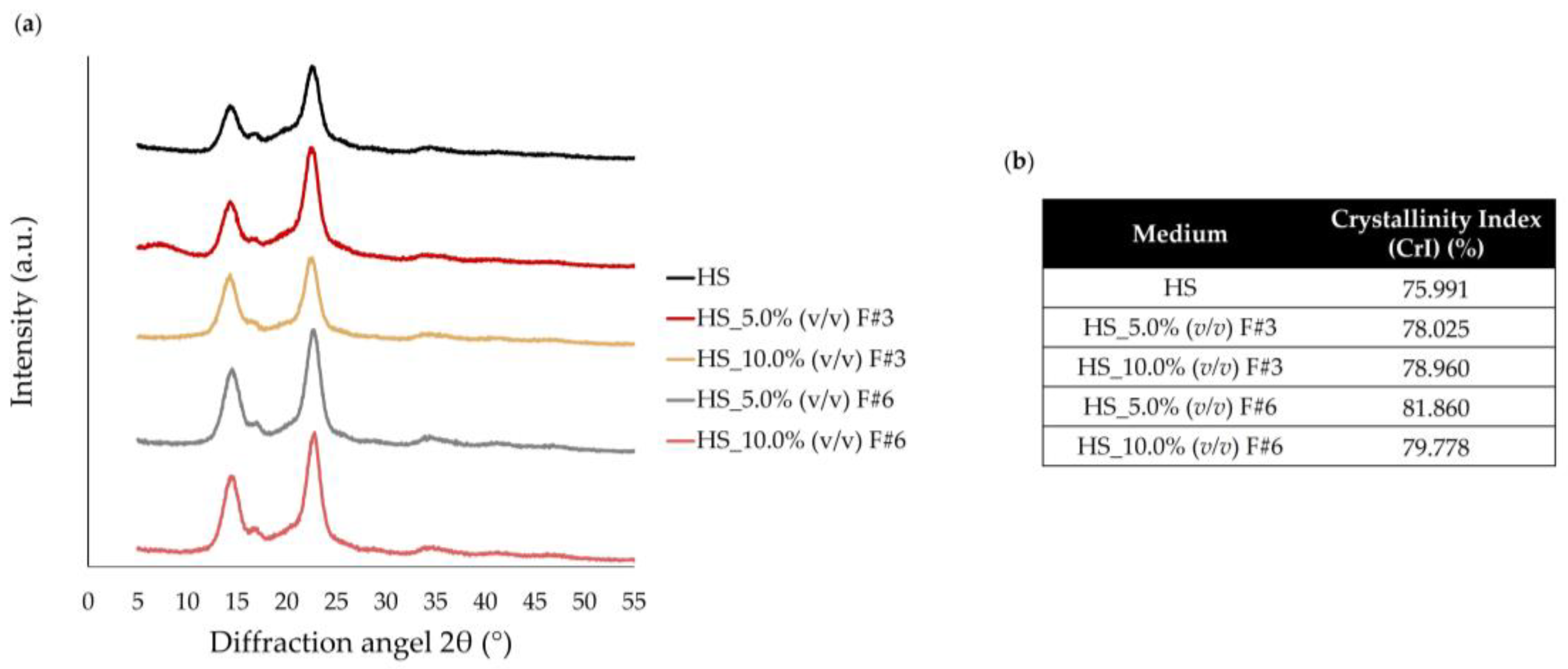
3.3.4. X-Ray Diffractometry (XRD)
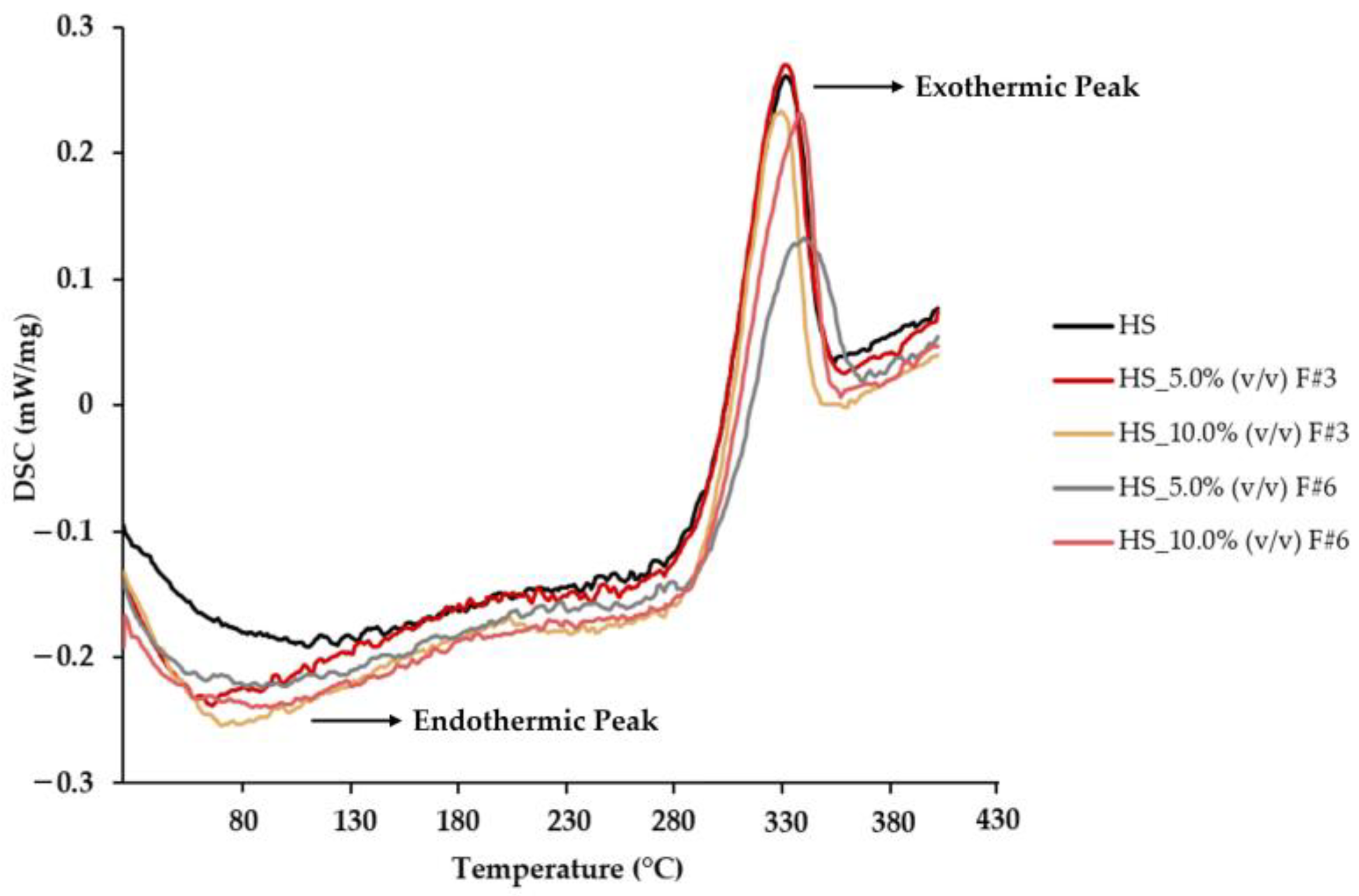
3.3.5. Differential Scanning Calorimetry (DSC) Measurements
3.3.6. Mechanical Properties
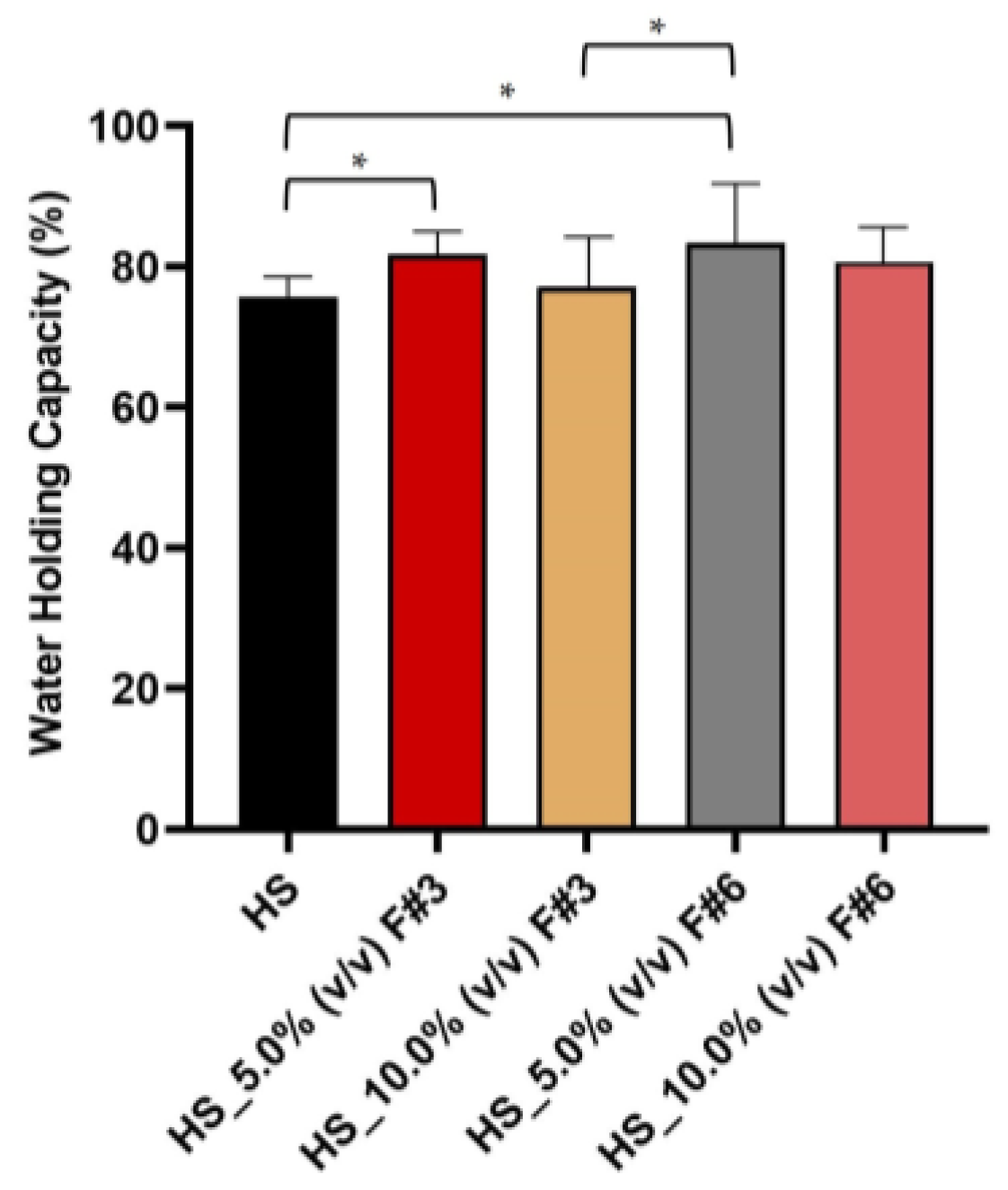
3.3.7. Water Holding Capacity (WHC)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramírez Tapias, Y.A.; Di Monte, M.V.; Peltzer, M.A.; Salvay, A.G. Bacterial Cellulose Films Production by Kombucha Symbiotic Community Cultured on Different Herbal Infusions. Food Chem. 2022, 372, 131346. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, G.B.; Blaschek, L.; Frandsen, K.E.H.; Noack, L.C.; Persson, S. Cellulose Synthesis in Land Plants. Mol. Plant 2023, 16, 206–231. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Zhu, Y.; Jiang, G.; Cao, K.; Zeng, S.; Chen, W.; Zhao, D.; Yu, H. Sustainable Cellulose and Its Derivatives for Promising Biomedical Applications. Prog. Mater. Sci. 2023, 138, 101152. [Google Scholar] [CrossRef]
- Li, Z.Y.; Azi, F.; Ge, Z.W.; Liu, Y.F.; Yin, X.T.; Dong, M.S. Bio-Conversion of Kitchen Waste into Bacterial Cellulose Using a New Multiple Carbon Utilizing Komagataeibacter Rhaeticus: Fermentation Profiles and Genome-Wide Analysis. Int. J. Biol. Macromol. 2021, 191, 211–221. [Google Scholar] [CrossRef]
- Ghozali, M.; Meliana, Y.; Chalid, M. Synthesis and Characterization of Bacterial Cellulose by Acetobacter Xylinum Using Liquid Tapioca Waste. Mater. Today Proc. 2021, 44, 2131–2134. [Google Scholar] [CrossRef]
- Greser, A.B.; Avcioglu, N.H. Optimization and Physicochemical Characterization of Bacterial Cellulose by Komagataeibacter Nataicola and Komagataeibacter Maltaceti Strains Isolated from Grape, Thorn Apple and Apple Vinegars. Arch. Microbiol. 2022, 204, 465. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.F.S.; Almeida, F.C.G.; Vinhas, G.M.; Sarubbo, L.A. Production of Bacterial Cellulose by Gluconacetobacter Hansenii Using Corn Steep Liquor as Nutrient Sources. Front. Microbiol. 2017, 8, 282716. [Google Scholar] [CrossRef]
- Martirani-VonAbercron, S.M.; Pacheco-Sánchez, D. Bacterial Cellulose: A Highly Versatile Nanomaterial. Microb. Biotechnol. 2023, 16, 1174. [Google Scholar] [CrossRef]
- Poddar, M.K.; Dikshit, P.K. Recent Development in Bacterial Cellulose Production and Synthesis of Cellulose Based Conductive Polymer Nanocomposites. Nano Select 2021, 2, 1605–1628. [Google Scholar] [CrossRef]
- Salama, A.; Saleh, A.K.; Cruz-Maya, I.; Guarino, V. Bacterial Cellulose/Cellulose Imidazolium Bio-Hybrid Membranes for In Vitro and Antimicrobial Applications. J. Funct. Biomater. 2023, 14, 60. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C. Industrial-Scale Production and Applications of Bacterial Cellulose. Front. Bioeng. Biotechnol. 2020, 8, 605374. [Google Scholar] [CrossRef] [PubMed]
- Potočnik, V.; Gorgieva, S.; Trček, J. From Nature to Lab: Sustainable Bacterial Cellulose Production and Modification with Synthetic Biology. Polymers 2023, 15, 3466. [Google Scholar] [CrossRef]
- Girard, V.D.; Chaussé, J.; Vermette, P. Bacterial Cellulose: A Comprehensive Review. J. Appl. Polym. Sci. 2024, 141, e55163. [Google Scholar] [CrossRef]
- Amorim, L.F.A.; Li, L.; Gomes, A.P.; Fangueiro, R.; Gouveia, I.C. Sustainable Bacterial Cellulose Production by Low Cost Feedstock: Evaluation of Apple and Tea by-Products as Alternative Sources of Nutrients. Cellulose 2023, 30, 5589–5606. [Google Scholar] [CrossRef]
- Revin, V.; Liyaskina, E.; Nazarkina, M.; Bogatyreva, A.; Shchankin, M. Cost-Effective Production of Bacterial Cellulose Using Acidic Food Industry by-Products. Braz. J. Microbiol. 2018, 49, 151–159. [Google Scholar] [CrossRef]
- Quijano, L.; Rodrigues, R.; Fischer, D.; Tovar-Castro, J.D.; Payne, A.; Navone, L.; Hu, Y.; Yan, H.; Pinmanee, P.; Poon, E.; et al. Bacterial Cellulose Cookbook: A Systematic Review on Sustainable and Cost-Effective Substrates. J. Bioresour. Bioprod. 2024, 9, 379–409. [Google Scholar] [CrossRef]
- Akintunde, M.O.; Adebayo-Tayo, B.C.; Ishola, M.M.; Zamani, A.; Horváth, I.S. Bacterial Cellulose Production from Agricultural Residues by Two Komagataeibacter sp. Strains. Bioengineered 2022, 13, 10010. [Google Scholar] [CrossRef]
- Öz, Y.E.; Kalender, M. A Novel Static Cultivation of Bacterial Cellulose Production from Sugar Beet Molasses: Series Static Culture (SSC) System. Int. J. Biol. Macromol. 2023, 225, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, N.; Suresh, S. Production of Cellulose from Sugarcane Molasses Using Gluconacetobacter Intermedius SNT-1: Optimization & Characterization. J. Clean. Prod. 2016, 112, 71–80. [Google Scholar] [CrossRef]
- Gomes, F.P.; Silva, N.H.C.S.; Trovatti, E.; Serafim, L.S.; Duarte, M.F.; Silvestre, A.J.D.; Neto, C.P.; Freire, C.S.R. Production of Bacterial Cellulose by Gluconacetobacter Sacchari Using Dry Olive Mill Residue. Biomass Bioenergy 2013, 55, 205–211. [Google Scholar] [CrossRef]
- Tsouko, E.; Pilafidis, S.; Dimopoulou, M.; Kourmentza, K.; Sarris, D. Bioconversion of Underutilized Brewing By-Products into Bacterial Cellulose by a Newly Isolated Komagataeibacter Rhaeticus Strain: A Preliminary Evaluation of the Bioprocess Environmental Impact. Bioresour. Technol. 2023, 387, 129667. [Google Scholar] [CrossRef] [PubMed]
- Hasanin, M.S.; Abdelraof, M.; Hashem, A.H.; El Saied, H. Sustainable Bacterial Cellulose Production by Achromobacter Using Mango Peel Waste. Microb. Cell Factories 2023, 22, 24. [Google Scholar] [CrossRef]
- Zhao, H.; Xia, J.; Wang, J.; Yan, X.; Wang, C.; Lei, T.; Xian, M.; Zhang, H. Production of Bacterial Cellulose Using Polysaccharide Fermentation Wastewater as Inexpensive Nutrient Sources. Biotechnol. Biotechnol. Equip. 2018, 32, 350–356. [Google Scholar] [CrossRef]
- Leonarski, E.; Cesca, K.; Pinto, C.C.; González, S.Y.G.; de Oliveira, D.; Poletto, P. Bacterial Cellulose Production from Acerola Industrial Waste Using Isolated Kombucha Strain. Cellulose 2022, 29, 7613–7627. [Google Scholar] [CrossRef]
- Ye, J.; Zheng, S.; Zhang, Z.; Yang, F.; Ma, K.; Feng, Y.; Zheng, J.; Mao, D.; Yang, X. Bacterial Cellulose Production by Acetobacter Xylinum ATCC 23767 Using Tobacco Waste Extract as Culture Medium. Bioresour. Technol. 2019, 274, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Guo, X.; Zhang, S.; Han, S.f.; Yang, G.; Jönsson, L.J. Bacterial Cellulose Production from Cotton-Based Waste Textiles: Enzymatic Saccharification Enhanced by Ionic Liquid Pretreatment. Bioresour. Technol. 2012, 104, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Asgharnejad, H.; Khorshidi Nazloo, E.; Madani Larijani, M.; Hajinajaf, N.; Rashidi, H. Comprehensive Review of Water Management and Wastewater Treatment in Food Processing Industries in the Framework of Water-Food-Environment Nexus. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4779–4815. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, V.; Ali, I.; Marjub, M.M.; Rene, E.R.; Soto, A.M.F. Wastewater in the Food Industry: Treatment Technologies and Reuse Potential. Chemosphere 2022, 293, 133553. [Google Scholar] [CrossRef] [PubMed]
- Blyashyna, M.; Zhukova, V.; Sabliy, L. Processes of Biological Wastewater Treatment for Nitrogen, Phosphorus Removal by Immobilized Microorganisms. East.-Eur. J. Enterp. Technol. 2018, 2, 30–37. [Google Scholar] [CrossRef][Green Version]
- Howard, I.; Espigares, E.; Lardelli, P.; Martín, J.L.; Espigares, M. Evaluation of Microbiological and Physicochemical Indicators for Wastewater Treatment. Environ. Toxicol. 2004, 19, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Nishat, A.; Yusuf, M.; Qadir, A.; Ezaier, Y.; Vambol, V.; Ijaz Khan, M.; Ben Moussa, S.; Kamyab, H.; Sehgal, S.S.; Prakash, C.; et al. Wastewater Treatment: A Short Assessment on Available Techniques. Alex. Eng. J. 2023, 76, 505–516. [Google Scholar] [CrossRef]
- Iqbal, A.; Zan, F.; Siddiqui, M.A.; Nizamuddin, S.; Chen, G. Integrated Treatment of Food Waste with Wastewater and Sewage Sludge: Energy and Carbon Footprint Analysis with Economic Implications. Sci. Total Environ. 2022, 825, 154052. [Google Scholar] [CrossRef]
- Jayaseelan, M.; Usman, M.; Somanathan, A.; Palani, S.; Muniappan, G.; Jeyakumar, R.B. Microalgal Production of Biofuels Integrated with Wastewater Treatment. Sustainability 2021, 13, 8797. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, S.; Sharma, S. Biopolymer in Wastewater Treatment; Springer: Cham, Switzerland, 2022; pp. 323–351. [Google Scholar] [CrossRef]
- Velmozhina, K.; Shinkevich, P.; Zhazhkov, V.; Politaeva, N.; Korablev, V.; Vladimirov, I.; Morales, T.C. Production of Biohydrogen from Microalgae Biomass after Wastewater Treatment and Air Purification from CO2. Processes 2023, 11, 2978. [Google Scholar] [CrossRef]
- Güven, H.M.; Ateş, H. A Holistic Approach to the Recovery of Valuable Substances from the Treatment Sludge Formed from Chemical Precipitation of Fruit Processing Industry Wastewater. Sci. Total Environ. 2024, 917, 170372. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Khanashyam, A.C.; Mundanat, A.S.; Shah, K.; Babu, K.S.; Thorakkattu, P.; Al-Asmari, F.; Pandiselvam, R. Valorization of Fruit Waste for Bioactive Compounds and Their Applications in the Food Industry. Foods 2023, 12, 556. [Google Scholar] [CrossRef]
- Al-Tayawi, A.N.; Sisay, E.J.; Beszédes, S.; Kertész, S. Wastewater Treatment in the Dairy Industry from Classical Treatment to Promising Technologies: An Overview. Processes 2023, 11, 2133. [Google Scholar] [CrossRef]
- Lee, S.Y.; Stuckey, D.C. Separation and Biosynthesis of Value-Added Compounds from Food-Processing Wastewater: Towards Sustainable Wastewater Resource Recovery. J. Clean. Prod. 2022, 357, 131975. [Google Scholar] [CrossRef]
- Keskin, B.; Ersahin, M.E.; Ozgun, H.; Koyuncu, I. Pilot and Full-Scale Applications of Membrane Processes for Textile Wastewater Treatment: A Critical Review. J. Water Process Eng. 2021, 42, 102172. [Google Scholar] [CrossRef]
- Ma, R.; Li, J.; Zeng, P.; Duan, L.; Dong, J.; Ma, Y.; Yang, L. The Application of Membrane Separation Technology in the Pharmaceutical Industry. Membranes 2024, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Birniwa, A.H.; Habibu, S.; Abdullahi, S.S.; Mohammad, R.E.A.; Hussaini, A.; Magaji, H.; Al-dhawi, B.N.S.; Noor, A.; Jagaba, A.H. Membrane Technologies for Heavy Metals Removal from Water and Wastewater: A Mini Review. Case Stud. Chem. Environ. Eng. 2024, 9, 100538. [Google Scholar] [CrossRef]
- Ezugbe, E.O.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Gomes, A.C.; Gonçalves, I.C.; De Pinho, M.N. The Role of Adsorption on Nanofiltration of Azo Dyes. J. Memb. Sci. 2005, 255, 157–165. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods for the Examination of Water & Wastewater; American Public Health Association: Washington, DC, USA, 2005; p. 1200. [Google Scholar]
- Teixeira, G.G.; Santos, P.M. Simple and Cost-Effective Approaches for Quantification of Reducing Sugar Exploiting Digital Image Analysis. J. Food Compos. Anal. 2022, 113, 104719. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Han, J.H.; Floros, J.D. Casting Antimicrobial Packaging Films and Measuring Their Physical Properties and Antimicrobial Activity. J. Plast. Film Sheeting 1997, 13, 287–298. [Google Scholar] [CrossRef]
- Salari, M.; Sowti Khiabani, M.; Rezaei Mokarram, R.; Ghanbarzadeh, B.; Samadi Kafil, H. Development and Evaluation of Chitosan Based Active Nanocomposite Films Containing Bacterial Cellulose Nanocrystals and Silver Nanoparticles. Food Hydrocoll. 2018, 84, 414–423. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Khan, T.; Park, J.K. Water Holding and Release Properties of Bacterial Cellulose Obtained by in Situ and Ex Situ Modification. Carbohydr. Polym. 2012, 88, 596–603. [Google Scholar] [CrossRef]
- Barshan, S.; Rezazadeh-Bari, M.; Almasi, H.; Amiri, S. Optimization and Characterization of Bacterial Cellulose Produced by Komagatacibacter Xylinus PTCC 1734 Using Vinasse as a Cheap Cultivation Medium. Int. J. Biol. Macromol. 2019, 136, 1188–1195. [Google Scholar] [CrossRef]
- Dulekgurgen, E.; Doǧruel, S.; Karahan, Ö.; Orhon, D. Size Distribution of Wastewater COD Fractions as an Index for Biodegradability. Water Res. 2006, 40, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, A.; Gentili, A.; Isidori, M.; Monaco, P.; Nardelli, A.; Parrella, A.; Temussi, F. Environmental Effects Caused by Olive Mill Wastewaters: Toxicity Comparison of Low-Molecular-Weight Phenol Components. J. Agric. Food Chem. 2003, 51, 1005–1009. [Google Scholar] [CrossRef]
- Wang, F.; Smith, D.W.; Gamal El-Din, M. Aged Raw Landfill Leachate: Membrane Fractionation, O3 Only and O3/H2O2 Oxidation, and Molecular Size Distribution Analysis. Water Res. 2006, 40, 463–474. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Souiad, F.; Fernandes, A.; Baía, A.; Pacheco, M.J.; Ciríaco, L.; Bendaoud-Boulahlib, Y.; Lopes, A. Treatment of Fruit Processing Wastewater by Electrochemical and Activated Persulfate Processes: Toxicological and Energetic Evaluation. Environ. Res. 2022, 209, 112868. [Google Scholar] [CrossRef] [PubMed]
- Srikandace, Y.; Apriyana, A.Y.; Zahrad, S.A.; Ramdhani, W.; Asri, P.P.P.; Andriani, D.; Abdullah, A.H.D.; Syampurwadi, A.; Satoto, R.; Karina, M. Bacterial Cellulose Production by Komagataeibacter Xylinus Using Rice-Washed Water and Tofu Processing Wastewater with the Addition of Sodium Glutamate. Fibers Polym. 2022, 23, 1190–1196. [Google Scholar] [CrossRef]
- Leonarski, E.; Cesca, K.; Zanella, E.; Stambuk, B.U.; de Oliveira, D.; Poletto, P. Production of Kombucha-like Beverage and Bacterial Cellulose by Acerola Byproduct as Raw Material. LWT 2021, 135, 110075. [Google Scholar] [CrossRef]
- Jittaut, P.; Hongsachart, P.; Audtarat, S.; Dasri, T. Production and Characterization of Bacterial Cellulose Produced by Gluconacetobacter Xylinus BNKC 19 Using Agricultural Waste Products as Nutrient Source. Arab. J. Basic. Appl. Sci. 2023, 30, 221–230. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.; Liu, S.; Tang, J. Bacterial Cellulose Nanofibril-Based Pickering Emulsions: Recent Trends and Applications in the Food Industry. Foods 2022, 11, 4064. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, H.; Jin, M.; Zheng, F. Biosynthesis of Antibacterial Bacterial Cellulose In-Situ Using Yellow Wine Wastewater through Co-Fermentation. Ind. Crops Prod. 2024, 222, 119715. [Google Scholar] [CrossRef]
- Güzel, M. Valorisation of Bread Wastes via the Bacterial Cellulose Production. Biomass Convers. Biorefinery 2024, 15, 4777–4790. [Google Scholar] [CrossRef]
- Revin, V.V.; Dolganov, A.V.; Liyaskina, E.V.; Nazarova, N.B.; Balandina, A.V.; Devyataeva, A.A.; Revin, V.D. Characterizing Bacterial Cellulose Produced ByKomagataeibacter Sucrofermentans H-110 on Molasses Medium and Obtaining a Biocomposite Based on It for the Adsorption of Fluoride. Polymers 2021, 13, 1422. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.J.; Ida, E.I.; Spinosa, W.A. Nutritional Supplementation with Amino Acids on Bacterial Cellulose Production by Komagataeibacter Intermedius: Effect Analysis and Application of Response Surface Methodology. Appl. Biochem. Biotechnol. 2022, 194, 5017–5036. [Google Scholar] [CrossRef] [PubMed]
- Uğurel, C.; Öğüt, H. Optimization of Bacterial Cellulose Production by Komagataeibacter Rhaeticus K23. Fibers 2024, 12, 29. [Google Scholar] [CrossRef]
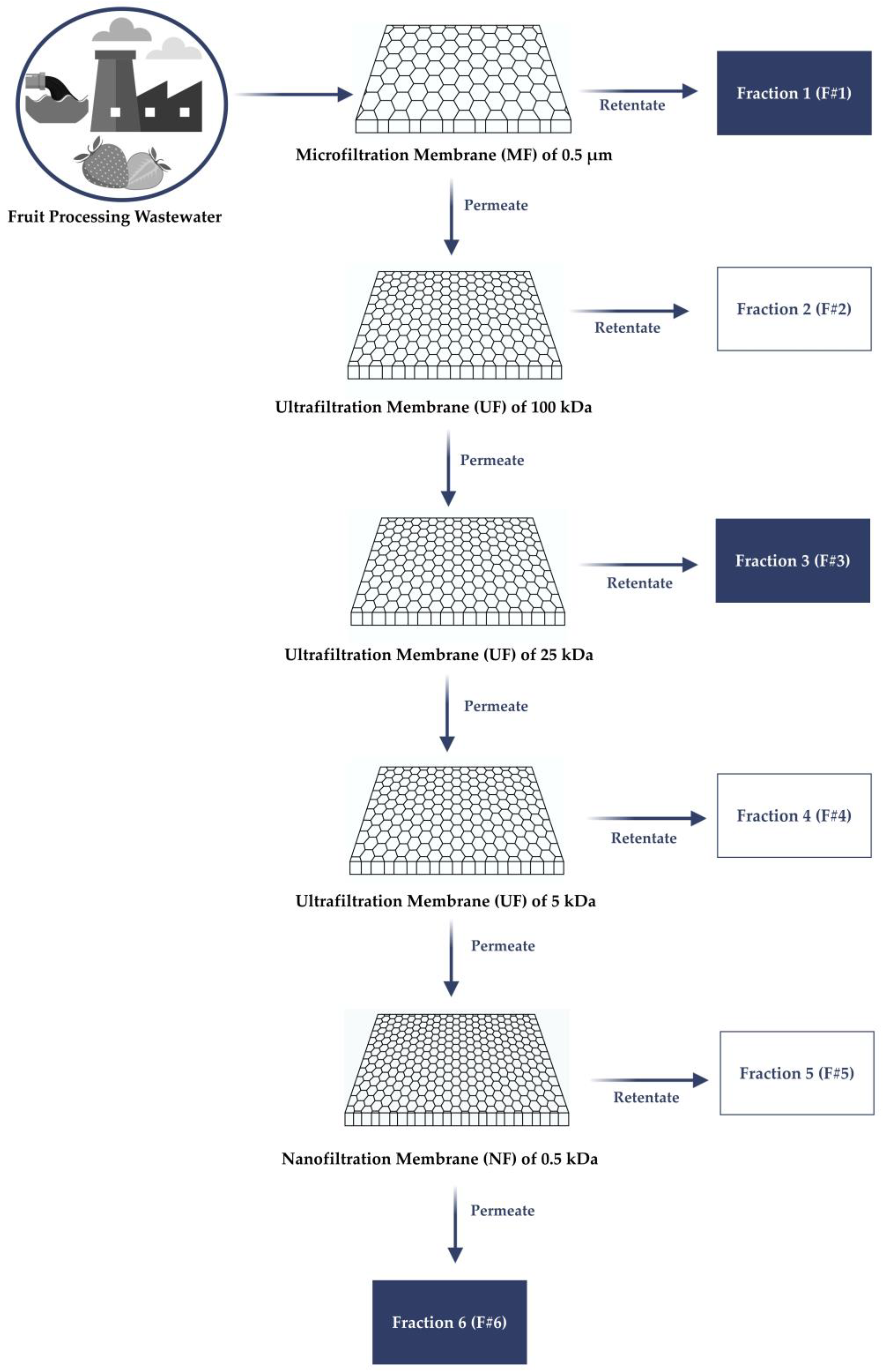
| Process | Designation | Selectivity (1) | Hydraulic Permeability (L/m2/h) | Operation Limits |
|---|---|---|---|---|
| MF | MFP5 | 0.5 µm | 264 | pH: 1–11 Pressure: 1–3 bar Temperature: 5–60 °C |
| UF | GR40PP | 100 kDa | 91 | pH: 2–12 Pressure: 1–10 bar Temperature: 5–75 °C |
| GR60PP | 25 kDa | 36 | ||
| GR90PP | 5 kDa | 29 | ||
| NF | NFT | >99% | 7 | pH: 3–9 Pressure: 15–35 bar Temperature: 5–50 °C |
| Media Designation | Carbon and Nitrogen Sources |
|---|---|
| HS | 2.0% (w/v) Glucose |
| HS_5.0% (v/v) F#1 | 2.0% (w/v) Glucose 0.5% (w/v) Peptone 0.5% (w/v) Yeast extract 5.0% (v/v) F#1 |
| HS_10.0% (v/v) F#1 | 2.0% (w/v) Glucose 0.5% (w/v) Peptone 0.5% (w/v) Yeast extract 10.0% (v/v) F#1 |
| HS_5.0% (v/v) F#3 | 2.0% (w/v) Glucose 0.5% (w/v) Peptone 0.5% (w/v) Yeast extract 5.0% (v/v) F#3 |
| HS_10.0% (v/v) F#3 | 2.0% (w/v) Glucose 0.5% (w/v) Peptone 0.5% (w/v) Yeast extract 10.0% (v/v) F#3 |
| HS_5.0% (v/v) F#6 | 2.0% (w/v) Glucose 0.5% (w/v) Peptone 0.5% (w/v) Yeast extract 5.0% (v/v) F#6 |
| HS_10.0% (v/v) F#6 | 2.0% (w/v) Glucose 0.5% (w/v) Peptone 0.5% (w/v) Yeast extract 10.0% (v/v) F#6 |
| Parameter (Units) | (Mean ± Standard Deviation) |
|---|---|
| pH | 11.5 ± 0.8 |
| Electrical conductivity (mS/cm) | 1.80 ± 0.07 |
| Total suspended solids (g/L) | 2.52 ± 0.09 |
| Chemical oxygen demand (COD) (g/L) | 12.85 ± 0.60 |
| Total dissolved nitrogen (N) (mg/L) | 6.44 ± 0.31 |
| Parameter (Units) | F#1 (>0.50 µm) | F#3 (5–25 kDa) | F#6 (<0.50 kDa) |
|---|---|---|---|
| pH | 6.08 ± 0.27 | 5.98 ± 0.16 | 6.70 ± 0.23 |
| Chemical oxygen demand (g/L) | 23.32 ± 1.64 | 15.26 ± 0.51 | 0.76 ± 0.03 |
| Reducing sugars (g/L) | 0.78 ± 0.09 | 0.85 ± 0.11 | 0.74 ± 0.06 |
| Total dissolved nitrogen (mg/L) | n.d. | 6.18 ± 0.15 | 2.70 ± 0.07 |
| Samples | Thickness (mm) | Opacity (Abs 600 nm mm−1) | Whiteness Index (WI) | Color Change (ΔE) |
|---|---|---|---|---|
| HS | 0.424 ± 0.050 | 6.892 ± 0.042 | 71.080 ± 3.622 | - |
| HS_5.0% (v/v) F#3 | 0.326 ± 0.040 | 7.862 ± 0.063 | 75.382 ± 1.475 | 4.705 ± 1.528 |
| HS_10.0% (v/v) F#3 | 0.439 ± 0.195 | 6.933 ± 0.116 | 75.859 ± 1.552 | 5.630 ± 1.051 |
| HS_5.0% (v/v) F#6 | 0.871 ± 0.234 | 3.049 ± 0.017 | 75.506 ± 1.729 | 4.505 ± 1.502 |
| HS_10.0% (v/v) F#6 | 0.908 ± 0.181 | 3.527 ± 0.094 | 73.307 ± 2.501 | 3.132 ± 1.603 |
| Tensile Strength (MPa) | Young’s Modulus (MPa) | Elongation at Break (%) | |
|---|---|---|---|
| HS | 12.771 ± 4.445 | 837.633 ± 269.340 | 1.514 ± 0.181 |
| HS_5.0% (v/v) F#3 | 20.132 ± 5.656 | 3706.835 ± 1420.495 | 0.647 ± 0.413 |
| HS_10.0% (v/v) F#3 | 19.221 ± 7.099 | 1986.560 ± 1019.253 | 1.099 ± 0.605 |
| HS_5.0% (v/v) F#6 | 23.149 ± 4.041 | 4827.031 ± 2171.323 | 0.545 ± 0.225 |
| HS_10.0% (v/v) F#6 | 22.755 ± 8.449 | 1645.328 ± 870.893 | 1.182 ± 0.185 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouro, C.; Gomes, A.; Gomes, A.P.; Gouveia, I.C. Sustainable Bacterial Cellulose Production Using Low-Cost Fruit Wastewater Feedstocks. Nanomaterials 2025, 15, 271. https://doi.org/10.3390/nano15040271
Mouro C, Gomes A, Gomes AP, Gouveia IC. Sustainable Bacterial Cellulose Production Using Low-Cost Fruit Wastewater Feedstocks. Nanomaterials. 2025; 15(4):271. https://doi.org/10.3390/nano15040271
Chicago/Turabian StyleMouro, Cláudia, Arlindo Gomes, Ana P. Gomes, and Isabel C. Gouveia. 2025. "Sustainable Bacterial Cellulose Production Using Low-Cost Fruit Wastewater Feedstocks" Nanomaterials 15, no. 4: 271. https://doi.org/10.3390/nano15040271
APA StyleMouro, C., Gomes, A., Gomes, A. P., & Gouveia, I. C. (2025). Sustainable Bacterial Cellulose Production Using Low-Cost Fruit Wastewater Feedstocks. Nanomaterials, 15(4), 271. https://doi.org/10.3390/nano15040271












