Gas-Mediated Dynamic Structure Evolution of Bimetallic Alloy Catalysts
Abstract
1. Introduction
2. Gas-Mediated Dynamic Structure Evolution of Alloy Catalysts
2.1. Gas-Mediated Surface Reconstruction
2.1.1. Oxidation-Driven Surface Reconstruction of Alloy Catalysts
2.1.2. Reduction-Driven Surface Reconstruction of Alloy Catalysts
2.1.3. Redox Driven Surface Reconstruction of Alloy Catalysts
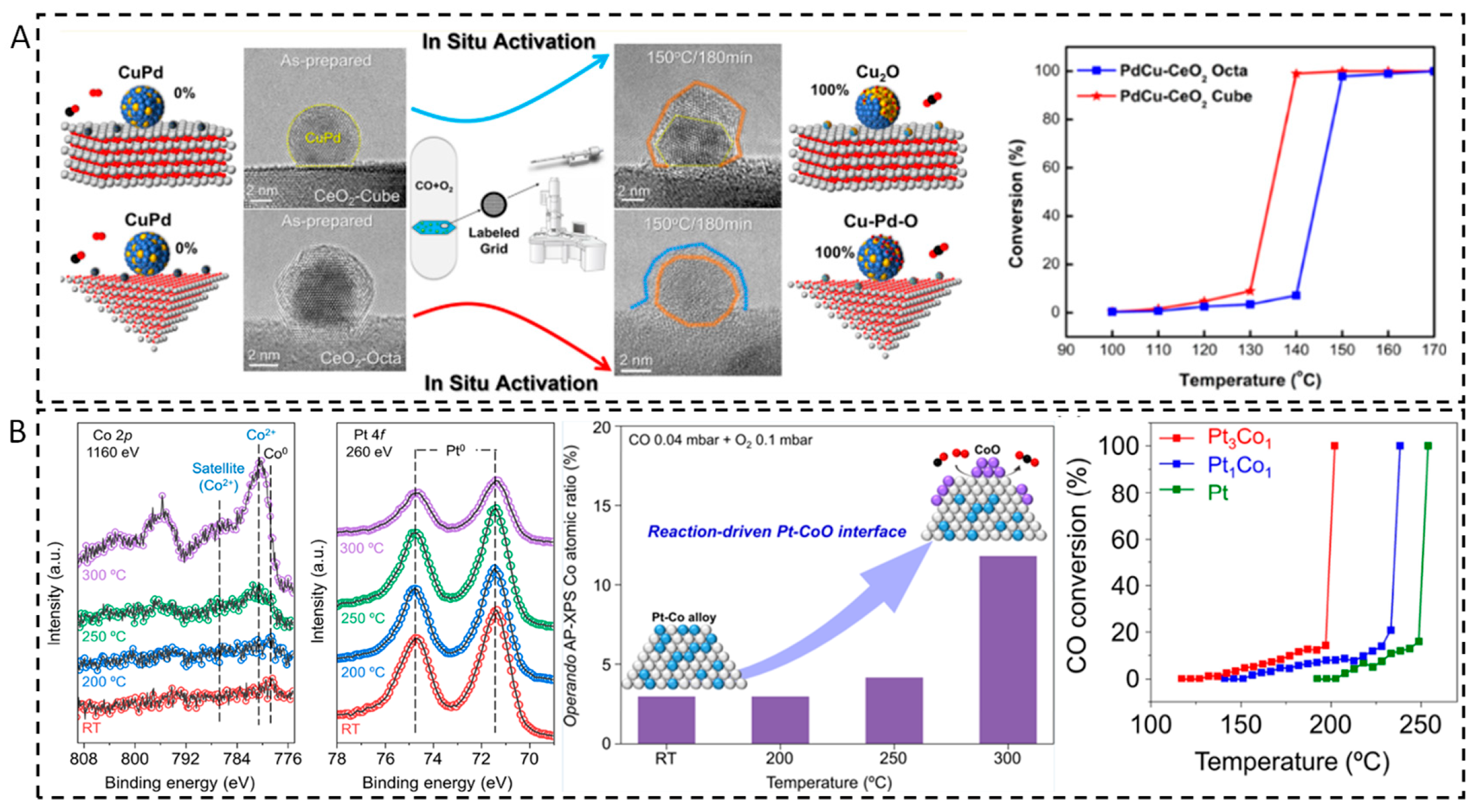
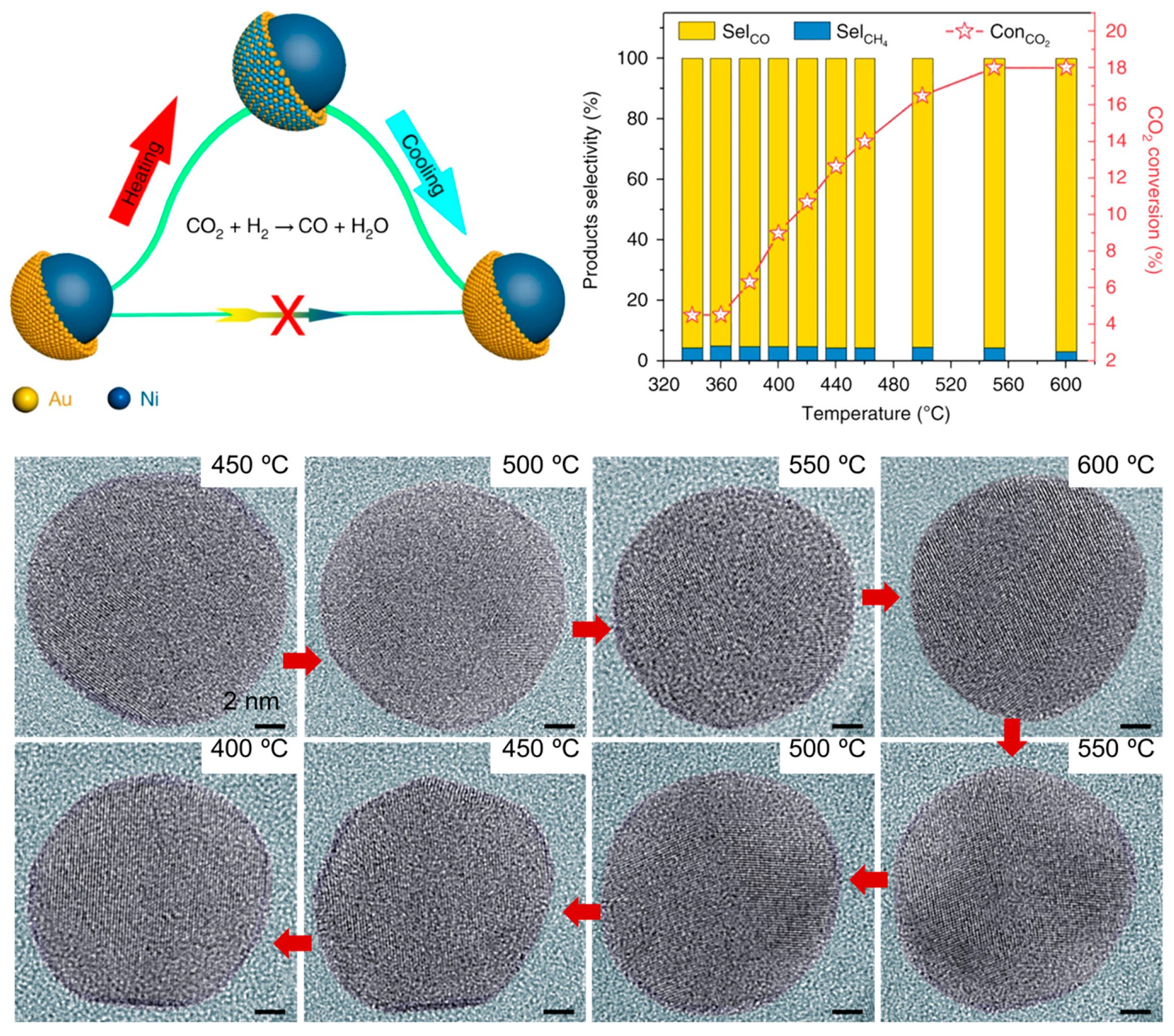
2.1.4. Dynamic Structure–Activity Relationship During Reaction
2.2. Gas-Mediated Compositional Segregation
2.2.1. Alloy Surface Segregation Under Heating Conditions
2.2.2. Oxidation-Driven Surface Segregation
2.2.3. Reduction-Driven Surface Segregation
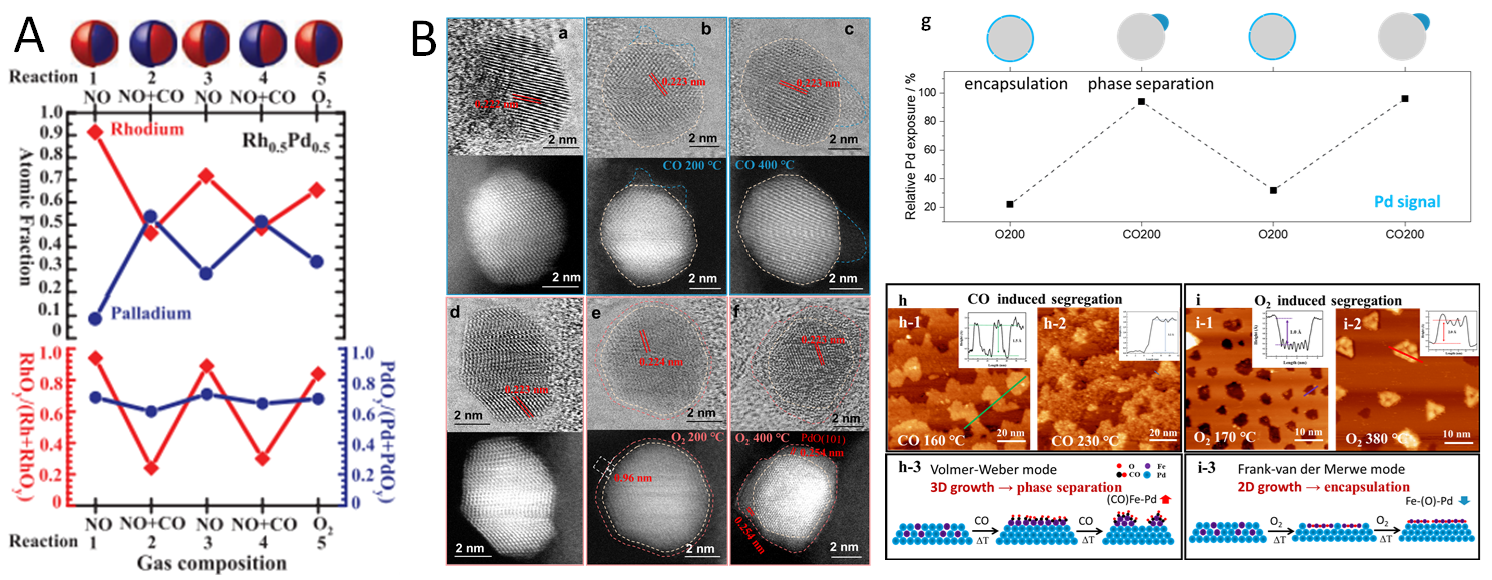
2.2.4. Redox-Driven Surface Segregation
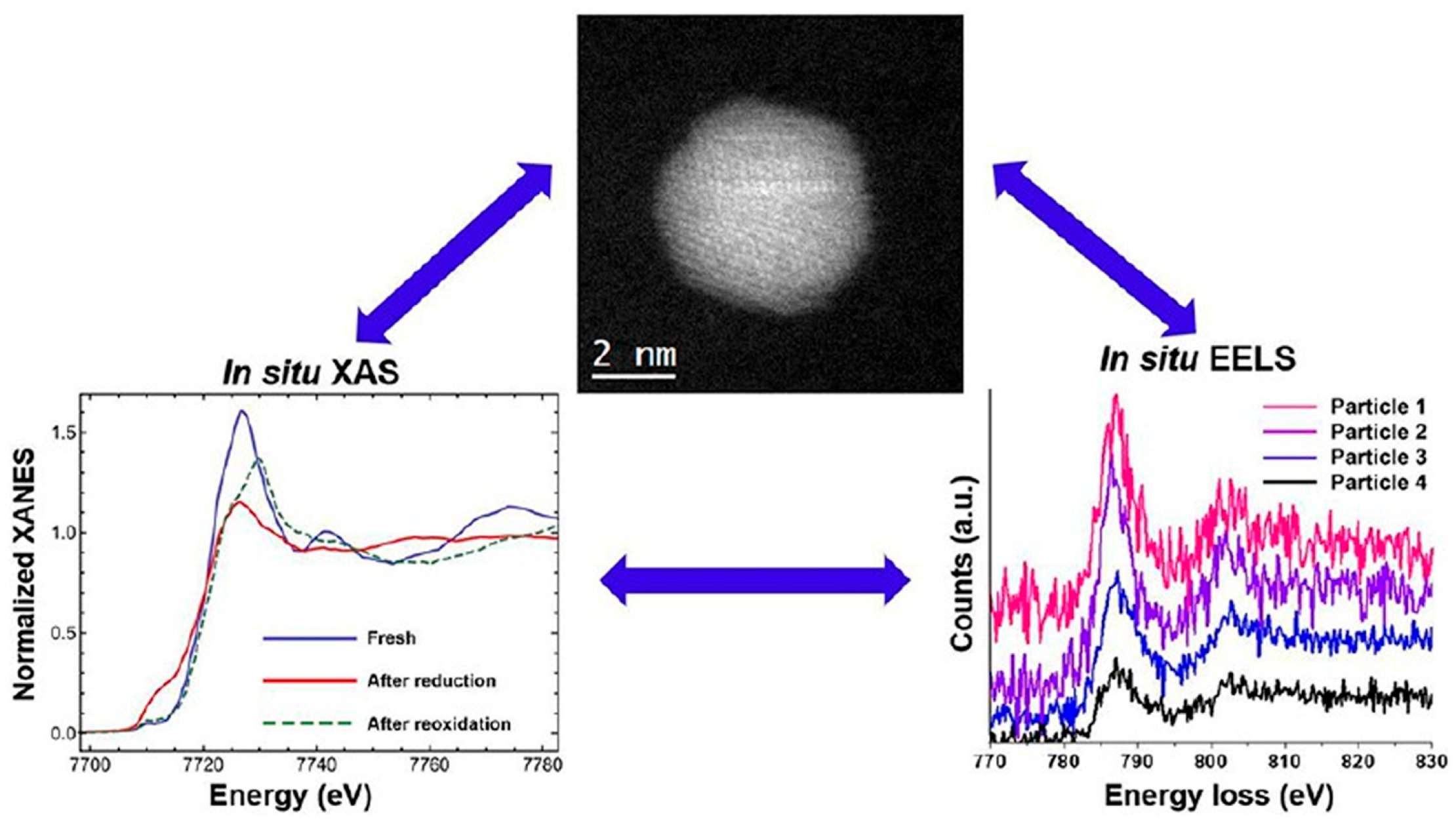
2.2.5. Dynamic Structure-Activity Relationship During Reaction
2.3. Gas-Mediated Dynamic Phase Transition
2.3.1. Alloy Phase Transition Under Vacuum or H2 Atmosphere
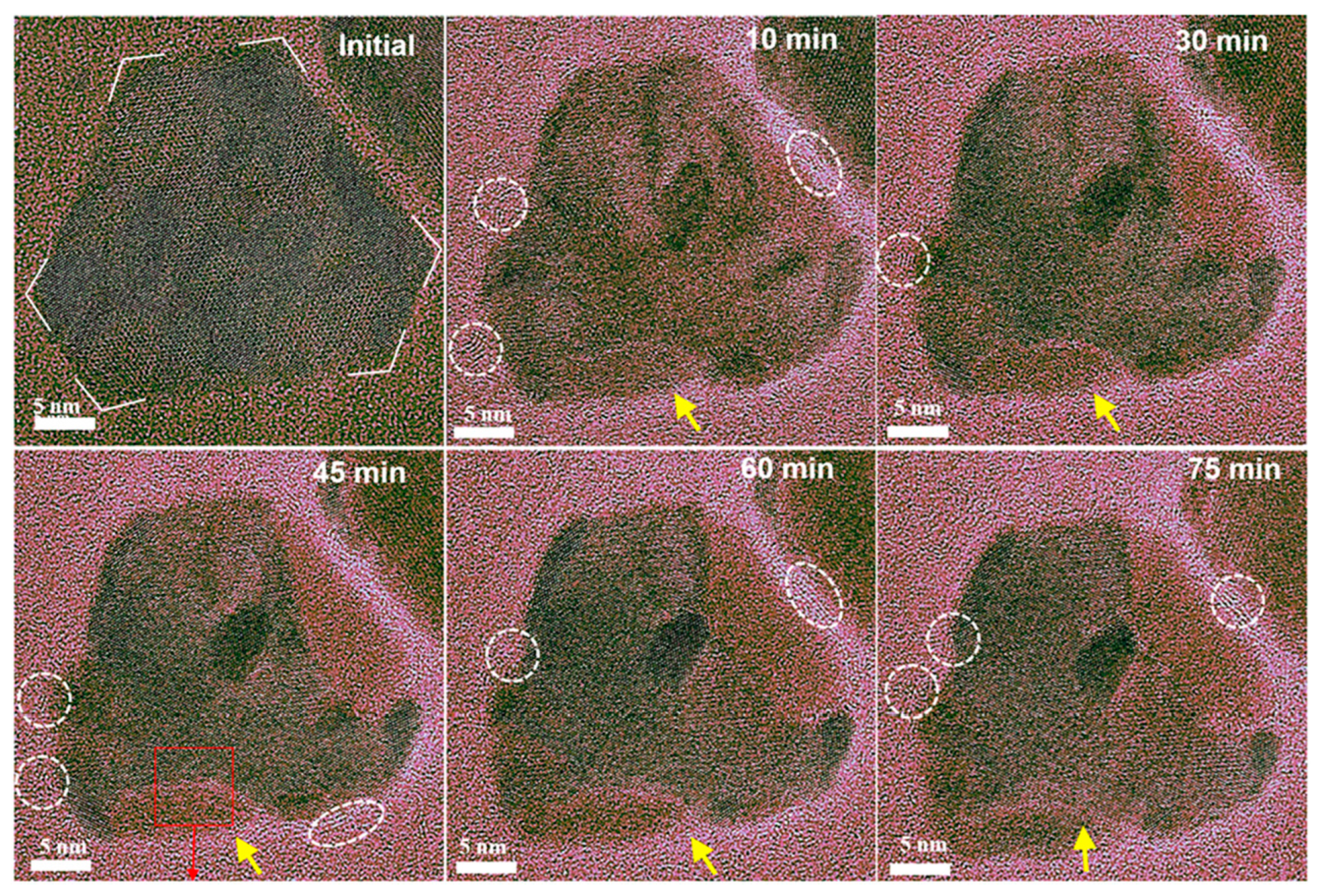
2.3.2. Dynamic Structure–Activity Relationship During Reaction
2.4. Gas-Mediated Compositional Alloying
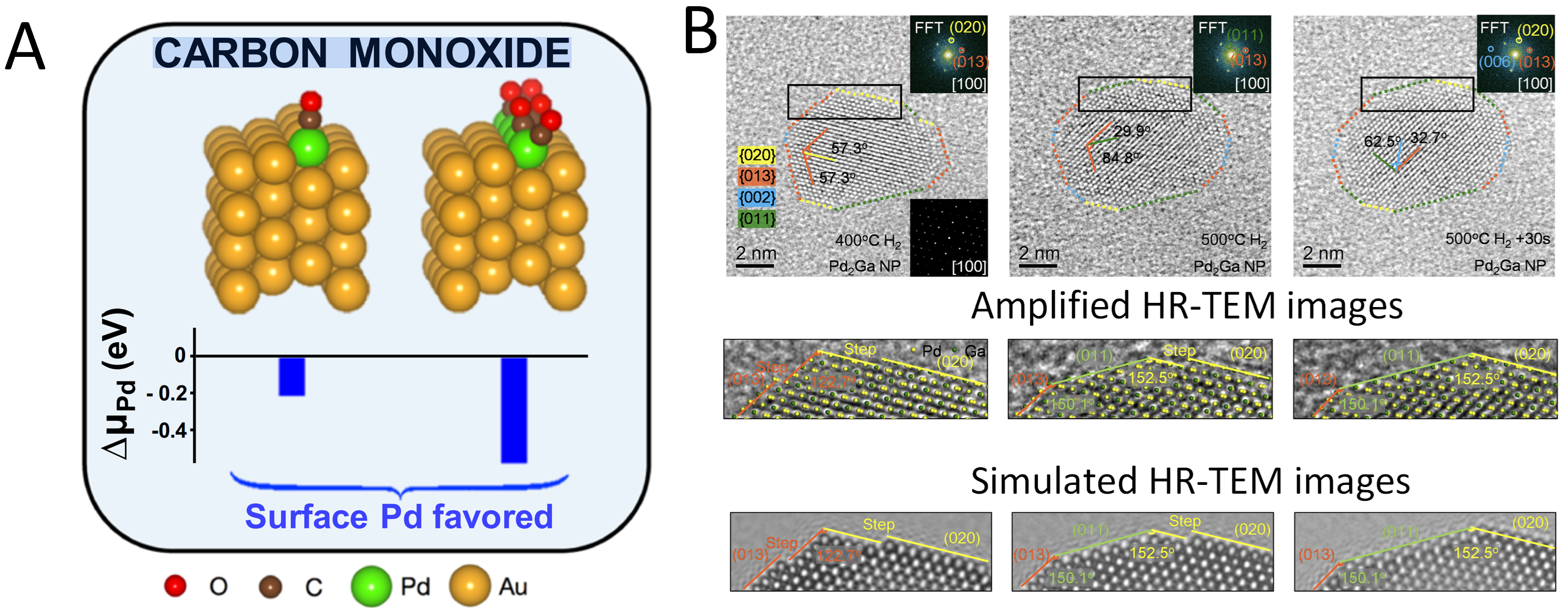
2.4.1. Reduction-Driven Alloying of Bimetallic Nanoparticles
2.4.2. Dynamic Structure–Activity Relationship During Reaction
3. In Situ Characterizations for Dynamics of Bimetallic Alloys
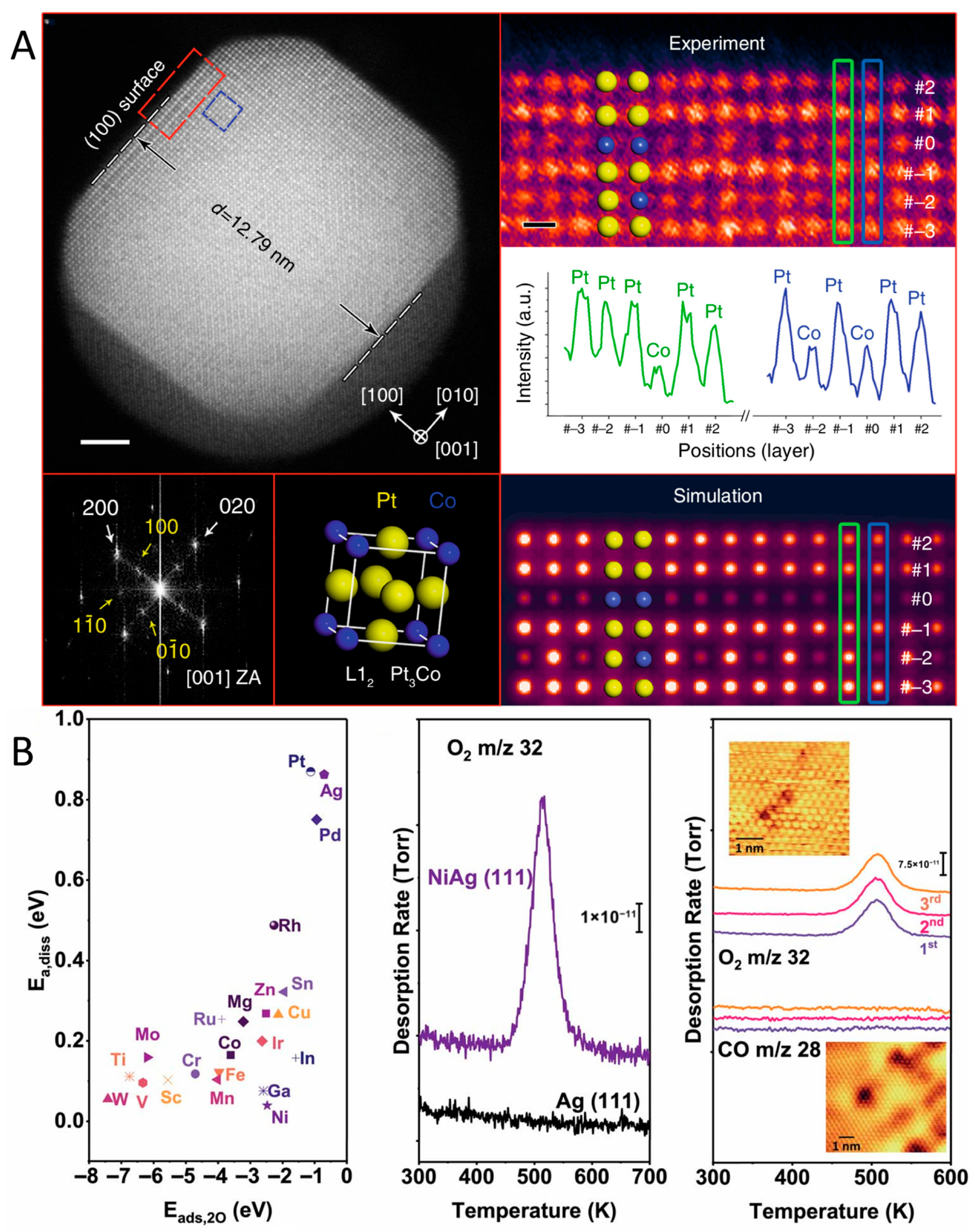
3.1. In Situ TEM Characterization
3.2. NAP-STM Characterization
4. Summary and Outlook
- (1)
- Improving the structural stability of alloy catalysts under reaction conditions. Alloy catalysts, when subjected to high temperatures or prolonged reaction conditions, are prone to structural degradations such as particle sintering, and surface coarsening, which inevitably leads to a decline in catalytic activity and selectivity. Consequently, enhancing the stability the active structure of alloy catalysts remains a critical research priority during reaction.
- (2)
- Overcoming in situ TEM resolution and sensitivity limitations caused by environments and electron beam effects. When conducting in situ imaging characterizations, the clear differences between the experimental environments and the actual working conditions of catalysts must be carefully considered. These differences may include the reactor dimensions, gas pressure, flow rate, and other parameters. In in situ TEM, gas molecule scattering and the window membrane in gas cell holders can adversely affect imaging quality. Moreover, electron beam irradiation can induce structural modifications in catalysts through the electron transfer process and localized heating effects. Taking these factors into account, in situ TEM techniques still require further advancements in both resolution and sensitivity, particularly in achieving higher temporal and spatial resolution, to more accurately capture the dynamic structural evolution of alloy catalysts under realistic operating conditions. Bridging the gap between the model in situ studies and industrial conditions now remains a key direction for the predictive design of active catalysts.
- (3)
- Developing the advanced and intelligentized methodology for automated tracking of atomic or nanoparticle trajectories. In situ TEM experiments of catalysts can generate massive volumes of image and video data, which is used to capture dynamic structural and compositional changes at high temporal and spatial resolution. The enormous quantity and diversity of these datasets often exceed the capacity of conventional manual analysis methods, which are typically time-consuming and prone to the existence of subjective bias. Therefore, developing fast, accurate, and efficient data processing methodologies are needed, such as machine-learning-assisted image recognition and advanced image recognition algorithms for the automatic tracking of atomic or nanoparticle trajectories.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cao, L.; Lu, J. Atomic-scale engineering of metal–oxide interfaces for advanced catalysis using atomic layer deposition. Catal. Sci. Technol. 2020, 10, 2695–2710. [Google Scholar] [CrossRef]
- Vesborg, P.C.K.; Jaramillo, T.F. Addressing the terawatt challenge: Scalability in the supply of chemical elements for renewable energy. RSC Adv. 2012, 2, 7933–7947. [Google Scholar] [CrossRef]
- Wang, X.; Ruditskiy, A.; Xia, Y.N. Rational design and synthesis of noble-metal nanoframes for catalytic and photonic applications. Natl. Sci. Rev. 2016, 3, 520–533. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, S.; Gilroy, K.D.; Cai, Z.; Xia, Y. Icosahedral nanocrystals of noble metals: Synthesis and applications. Nano Today 2017, 15, 121–144. [Google Scholar] [CrossRef]
- Nakaya, Y.; Furukawa, S. Catalysis of alloys: Classification, principles, and design for a variety of materials and reactions. Chem. Rev. 2023, 123, 5859–5947. [Google Scholar] [CrossRef]
- Bera, P.; Hegde, M.S. Noble metal ions in CeO2 and TiO2: Synthesis, structure and catalytic properties. RSC Adv. 2015, 5, 94949–94979. [Google Scholar] [CrossRef]
- Rood, S.; Eslava, S.; Manigrasso, A.; Bannister, C. Recent advances in gasoline three-way catalyst formulation: A review. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2019, 234, 936–949. [Google Scholar] [CrossRef]
- Hu, Z.; Wan, C.Z.; Lui, Y.K.; Dettling, J.; Steger, J.J. Design of a novel Pd three-way catalyst: Integration of catalytic functions in three dimensions. Catal. Today 1996, 30, 83–89. [Google Scholar] [CrossRef]
- Yin, X.; Li, S.; Deng, J.; Zhao, Y.; Wang, J.; Chen, Y. Efficient control of automotive emission by Pt-based and Rh-based three-way catalysts: The critical role of phase structure of ceria-zirconia support. Sep. Purif. Technol. 2025, 356, 129707. [Google Scholar] [CrossRef]
- Clauss, D.; Martin, V.; Nelayah, J.; Chattot, R.; Bordet, P.; Drnec, J.; Mirolo, M.; Dubau, L.; Maillard, F. A Model approach to uncover the role of the IrOx crystallographic structure and chemistry on OER activity and stability via annealing a sacrificial template. ACS Catal. 2025, 15, 2654–2665. [Google Scholar] [CrossRef]
- Zhang, T.; Zheng, P.; Gao, J.; Liu, X.; Ji, Y.; Tian, J.; Zou, Y.; Sun, Z.; Hu, Q.; Chen, G.; et al. Simultaneously activating molecular oxygen and surface lattice oxygen on Pt/TiO2 for low-temperature CO oxidation. Nat. Commun. 2024, 15, 6827. [Google Scholar] [CrossRef]
- Xu, D.; Jin, Y.; He, B.; Fang, X.; Chen, G.; Qu, W.; Xu, C.; Chen, J.; Ma, Z.; Chen, L.; et al. Electronic communications between active sites on individual metallic nanoparticles in catalysis. Nat. Commun. 2024, 15, 8614. [Google Scholar] [CrossRef]
- Aso, R.; Tamaoka, T.; Yoshida, H.; Hojo, H.; Sano, H.; Midoh, Y.; Einaga, H.; Tanigaki, T.; Murakami, Y. Direct visualization of surface structure and charge states of ceria-supported gold catalysts under redox conditions. Adv. Sci. 2025, 12, e08554. [Google Scholar] [CrossRef]
- Li, H.; Abdelgaid, M.; Paudel, J.R.; Holzapfel, N.P.; Augustyn, V.; McKone, J.R.; Mpourmpakis, G.; Crumlin, E.J. Operando unveiling of hydrogen spillover mechanisms on tungsten oxide surfaces. J. Am. Chem. Soc. 2025, 147, 6472–6479. [Google Scholar] [CrossRef]
- Duan, C.; Liu, J.; Li, Z.; Shi, R.; Zhao, J.; Waterhouse, G.I.N.; Wen, X.D.; Zhang, L.P.; Wu, L.Z.; Zhang, T. Efficient photocatalytic propane direct dehydrogenation to propylene over PtO2 clusters. Adv. Mater. 2025, 37, e2411648. [Google Scholar] [CrossRef]
- Wang, J.Q.; Song, L.J.; Huo, J.T.; Gao, M.; Zhang, Y. Designing advanced amorphous/nanocrystalline alloys by controlling the energy state. Adv. Mater. 2024, 36, e2311406. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Gong, Y.; Li, S.; Wu, J.; Lu, Z.; Li, Q.; Wang, L.; Wu, S.; Zhang, J. Single-atom alloys materials for CO2 and CH4 catalytic conversion. Adv. Mater. 2024, 36, e2311628. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.; Hammer, B.; Stoltze, P.; Skriver, H.L.; Nørskov, J.K. Surface electronic structure and reactivity of transition and noble metals. J. Mol. Catal. A Chem. 1997, 115, 421–429. [Google Scholar] [CrossRef]
- Rossmeisl, J.; Qu, Z.W.; Zhu, H.; Kroes, G.J.; Nørskov, J.K. Electrolysis of water on oxide surfaces. J. Electroanal. Chem. 2007, 607, 83–89. [Google Scholar] [CrossRef]
- Bligaard, T.; Nørskov, J.K. Ligand effects in heterogeneous catalysis and electrochemistry. Electrochim. Acta 2007, 52, 5512–5516. [Google Scholar] [CrossRef]
- Xin, H.; Holewinski, A.; Schweitzer, N.; Nikolla, E.; Linic, S. Electronic structure engineering in heterogeneous catalysis: Identifying novel alloy catalysts based on rapid screening for materials with desired electronic properties. Top Catal. 2012, 55, 376–390. [Google Scholar] [CrossRef]
- Wei, Z.; Yu, S.; Li, C. Research development of anti-CO poisoning in electrocatalytic methanol oxidation processes: A review. Catal. Sci. Technol. 2024, 14, 5128–5142. [Google Scholar] [CrossRef]
- Liu, Y.; Chi, M.; Mazumder, V.; More, K.L.; Soled, S.; Henao, J.D.; Sun, S. Composition-controlled synthesis of bimetallic PdPt nanoparticles and their electro-oxidation of methanol. Chem. Mater. 2011, 23, 4199–4203. [Google Scholar] [CrossRef]
- Li, B.; Higgins, D.C.; Zhu, S.; Li, H.; Wang, H.; Ma, J.; Chen, Z. Highly active Pt–Ru nanowire network catalysts for the methanol oxidation reaction. Catal. Commun. 2012, 18, 51–54. [Google Scholar] [CrossRef]
- Hwang, S.J.; Kim, S.K.; Lee, J.G.; Lee, S.C.; Jang, J.H.; Kim, P.; Lim, T.H.; Sung, Y.E.; Yoo, S.J. Role of electronic perturbation in stability and activity of Pt-based alloy nanocatalysts for oxygen reduction. J. Am. Chem. Soc. 2012, 134, 19508–19511. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.W.; Wang, H.Y.; Feng, L.; Zhu, J.Z.; Liu, J.X.; Li, W.X. Crystal-phase engineering in heterogeneous catalysis. Chem. Rev. 2024, 124, 164–209. [Google Scholar] [CrossRef]
- Zhuang, J.; Wang, D. Recent advances of single-atom alloy catalyst: Properties, synthetic methods and electrocatalytic applications. Mater. Today Catal. 2023, 2, 100009. [Google Scholar] [CrossRef]
- Lin, F.; Li, M.; Zeng, L.; Luo, M.; Guo, S. Intermetallic nanocrystals for fuel-cells-based electrocatalysis. Chem. Rev. 2023, 123, 12507–12593. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, Z.; Jin, R.; Zhu, C.; Zhao, C.; Lin, Y.; Guan, Q.; Cao, L.; Wang, H.; Li, S.; et al. Conjugated dual size effect of core-shell particles synergizes bimetallic catalysis. Nat. Commun. 2023, 14, 530. [Google Scholar] [CrossRef] [PubMed]
- Tritsaris, G.A.; Greeley, J.; Rossmeisl, J.; Nørskov, J.K. Atomic-scale modeling of particle size effects for the oxygen reduction reaction on Pt. Catal. Lett. 2011, 141, 909–913. [Google Scholar] [CrossRef]
- Yang, X.F.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T. Single-atom catalysts: A new frontier in heterogeneous catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Peles, A.; Shoemaker, K. Electrocatalysis on platinum nanoparticles: Particle size effect on oxygen reduction reaction activity. Nano Lett. 2011, 11, 3714–3719. [Google Scholar] [CrossRef]
- Sheng, T.; Tian, N.; Zhou, Z.-Y.; Lin, W.-F.; Sun, S.-G. Designing Pt-based electrocatalysts with high surface energy. ACS Energy Lett. 2017, 2, 1892–1900. [Google Scholar] [CrossRef]
- Zhang, J.; Vukmirovic, M.B.; Sasaki, K.; Nilekar, A.U.; Mavrikakis, M.; Adzic, R.R. Mixed-metal Pt monolayer electrocatalysts for enhanced oxygen reduction kinetics. J. Am. Chem. Soc. 2005, 127, 12480–12481. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, L.; Lyu, Z.; Xie, M.; Chen, R.; Jin, W.; Mavrikakis, M.; Xia, Y. Janus Nanocages of Platinum-Group Metals and Their Use as Effective Dual-Electrocatalysts. Angew. Chem. Int. Ed. 2021, 60, 10384–10392. [Google Scholar] [CrossRef] [PubMed]
- Asano, M.; Kawamura, R.; Sasakawa, R.; Todoroki, N.; Wadayama, T. Oxygen reduction reaction activity for strain-controlled Pt-based model alloy catalysts: Surface strains and direct electronic effects induced by alloying elements. ACS Catal. 2016, 6, 5285–5289. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, K.; Liu, Q.; Qin, J.; Jiang, Q.; Yang, B.; Yin, F. Ni2+-directed anisotropic growth of PtCu nested skeleton cubes boosting electroreduction of oxygen. Adv. Sci. 2022, 9, e2104927. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Qin, J.; Leng, D.Y.; Liu, Q.R.; Xu, X.Y.; Yang, B.; Yin, F. Tunable strain drives the activity enhancement for oxygen reduction reaction on Pd@Pt core-shell electrocatalysts. J. Power Sources 2021, 485, 229340. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, K.; Gu, Q.; Jiang, Q.; Qin, J.; Leng, D.; Liu, Q.; Yang, B.; Yin, F. Optimized oxygen reduction activity by tuning shell component in Pd@Pt-based core-shell electrocatalysts. J. Colloid Interface Sci. 2021, 604, 301–309. [Google Scholar] [CrossRef]
- Mahbub, M.A.A.; Junqueira, J.R.C.; Wang, X.; Zhang, J.; Dieckhöfer, S.; Seisel, S.; Das, D.; Schuhmann, W. Dynamic transformation of functionalized bismuth to catalytically active surfaces for CO2 reduction to formate at high current densities. Adv. Funct. Mater. 2023, 34, 2307752. [Google Scholar] [CrossRef]
- Tao, F.; Salmeron, M. Surface restructuring and predictive design of heterogeneous catalysts. Science 2024, 386, eadq0102. [Google Scholar] [CrossRef]
- Chao, H.Y.; Venkatraman, K.; Moniri, S.; Jiang, Y.; Tang, X.; Dai, S.; Gao, W.; Miao, J.; Chi, M. In situ and emerging transmission electron microscopy for catalysis research. Chem. Rev. 2023, 123, 8347–8394. [Google Scholar] [CrossRef]
- Xiao, M.; Sun, H.; Meng, Y.; Zhu, F. Advances of in situ transmission electron microscopy research on gas phase catalyst particles. Catal. Sci. Technol. 2024, 14, 2040–2063. [Google Scholar] [CrossRef]
- Li, S.; Wang, G.; Lv, H.; Lin, Z.; Liang, J.; Liu, X.; Wang, Y.G.; Huang, Y.; Wang, G.; Li, Q. Constructing gradient orbital coupling to induce reactive metal-support interaction in Pt-carbide electrocatalysts for efficient methanol oxidation. J. Am. Chem. Soc. 2024, 146, 17659–17668. [Google Scholar] [CrossRef]
- Jalil, A.; Happel, E.E.; Cramer, L.; Hunt, A.; Hoffman, A.S.; Waluyo, I.; Montemore, M.M.; Christopher, P.; Sykes, E.C.H. Nickel promotes selective ethylene epoxidation on silver. Science 2025, 387, 869–873. [Google Scholar] [CrossRef] [PubMed]
- Crozier, P.A.; Leibovich, M.; Haluai, P.; Tan, M.; Thomas, A.M.; Vincent, J.; Mohan, S.; Marcos Morales, A.; Kulkarni, S.A.; Matteson, D.S.; et al. Visualizing nanoparticle surface dynamics and instabilities enabled by deep denoising. Science 2025, 387, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Shi, J.; Liu, S.; Li, G.; Pei, F.; Chen, Y.; Deng, J.; Zheng, Q.; Li, J.; Zhao, C.; et al. Visualizing interfacial collective reaction behaviour of Li-S batteries. Nature 2023, 621, 75–81. [Google Scholar] [CrossRef]
- Salvato, M.; Crescenzi, M.; Scagliotti, M.; Castrucci, P.; Boninelli, S.; Caruso, G.M.; Liu, Y.; Mikkelsen, A.; Timm, R.; Nahas, S.; et al. Nanometric moire stripes on the surface of Bi2Se3 topological insulator. ACS Nano 2022, 16, 13860–13868. [Google Scholar] [CrossRef]
- Feng, Q.; Zhu, C.; Sheng, G.; Sun, T.; Li, Y.; Zhu, Y. Four-dimensional scanning transmission electron microscopy: From material microstructures to physicochemical properties. Acta Phys. Chim. Sin. 2023, 39, 2210017. [Google Scholar] [CrossRef]
- Baber, A.E.; Xu, F.; Dvorak, F.; Mudiyanselage, K.; Soldemo, M.; Weissenrieder, J.; Senanayake, S.D.; Sadowski, J.T.; Rodriguez, J.A.; Matolin, V.; et al. In situ imaging of Cu2O under reducing conditions: Formation of metallic fronts by mass transfer. J. Am. Chem. Soc. 2013, 135, 16781–16784. [Google Scholar] [CrossRef]
- Fu, Q.; Li, W.X.; Yao, Y.; Liu, H.; Su, H.Y.; Ma, D.; Gu, X.K.; Chen, L.; Wang, Z.; Zhang, H.; et al. Interface-confined ferrous centers for catalytic oxidation. Science 2010, 328, 1141–1144. [Google Scholar] [CrossRef] [PubMed]
- Tao, F.; Grass, M.E.; Zhang, Y.; Butcher, D.R.; Renzas, J.R.; Liu, Z.; Chung, J.Y.; Mun, B.S.; Salmeron, M.; Somorjai, G.A. Reaction-driven restructuring of Rh-Pd and Pt-Pd core-shell nanoparticles. Science 2008, 322, 932–934. [Google Scholar] [CrossRef] [PubMed]
- Andersson, K.J.; Calle-Vallejo, F.; Rossmeisl, J.; Chorkendorff, I. Adsorption-driven surface segregation of the less reactive alloy component. J. Am. Chem. Soc. 2009, 131, 2404–2407. [Google Scholar] [CrossRef]
- Ma, T.; Fu, Q.; Su, H.Y.; Liu, H.Y.; Cui, Y.; Wang, Z.; Mu, R.T.; Li, W.X.; Bao, X.H. Reversible structural modulation of Fe-Pt bimetallic surfaces and its effect on reactivity. ChemPhysChem 2009, 10, 1013–1016. [Google Scholar] [CrossRef]
- Kim, H.Y.; Henkelman, G. CO Adsorption-driven surface segregation of Pd on Au/Pd bimetallic surfaces: Role of defects and effect on CO oxidation. ACS Catal. 2013, 3, 2541–2546. [Google Scholar] [CrossRef]
- McCue, A.J.; Anderson, J.A. CO induced surface segregation as a means of improving surface composition and enhancing performance of CuPd bimetallic catalysts. J. Catal. 2015, 329, 538–546. [Google Scholar] [CrossRef]
- Wu, C.H.; Liu, C.; Su, D.; Xin, H.L.; Fang, H.-T.; Eren, B.; Zhang, S.; Murray, C.B.; Salmeron, M.B. Bimetallic synergy in cobalt–palladium nanocatalysts for CO oxidation. Nat. Catal. 2018, 2, 78–85. [Google Scholar] [CrossRef]
- Xu, C.; Han, S.; Zhao, Q.; Liu, S.; Liu, W.; Li, Y.; Shen, W. Structure evolution of Cu3Pd single-particles under CO2 hydrogenation. Chem. Eng. J. 2024, 494, 153208. [Google Scholar] [CrossRef]
- Sandoval-Diaz, L.; Cruz, D.; Vuijk, M.; Ducci, G.; Hävecker, M.; Jiang, W.; Plodinec, M.; Hammud, A.; Ivanov, D.; Götsch, T.; et al. Metastable nickel–oxygen species modulate rate oscillations during dry reforming of methane. Nat. Catal. 2024, 7, 161–171. [Google Scholar] [CrossRef]
- Zhang, R.P.; He, B.; Liu, X.; Lu, A.H. Hydrogen spillover-driven dynamic evolution and migration of iron oxide for structure regulation of versatile magnetic nanocatalysts. J. Am. Chem. Soc. 2023, 145, 25834–25841. [Google Scholar] [CrossRef]
- Usoltsev, O.; Stoian, D.; Skorynina, A.; Kozyr, E.; Njoroge, P.N.; Pellegrini, R.; Groppo, E.; van Bokhoven, J.A.; Bugaev, A. restructuring of palladium nanoparticles during oxidation by molecular oxygen. Small 2024, 20, e2401184. [Google Scholar] [CrossRef]
- Lee, J.; Christopher, P. Does H2 temperature-programmed reduction always probe solid-state redox chemistry? the case of Pt/CeO2. Angew. Chem. Int. Ed. 2024, 64, e202414388. [Google Scholar] [CrossRef]
- Ricchebuono, A.; Vottero, E.; Bonavia, D.; Lazzarini, P.; Pellegrini, R.; Crocellà, V.; Porcaro, N.G.; Checchia, S.; Ferri, D.; Piovano, A.; et al. CO-induced dynamic behavior of Al2O3-supported Pd nanoparticles at room temperature. ACS Catal. 2024, 14, 13736–13746. [Google Scholar] [CrossRef]
- Dai, S.; You, Y.; Zhang, S.; Cai, W.; Xu, M.; Xie, L.; Wu, R.; Graham, G.W.; Pan, X. In situ atomic-scale observation of oxygen-driven core-shell formation in Pt3Co nanoparticles. Nat. Commun. 2017, 8, 204. [Google Scholar] [CrossRef]
- Cramer, L.A.; Liu, Y.; Deshlahra, P.; Sykes, E.C.H. Dynamic restructuring induced oxygen activation on AgCu near-surface alloys. J. Phys. Chem. Lett. 2020, 11, 5844–5848. [Google Scholar] [CrossRef] [PubMed]
- van Spronsen, M.A.; Daunmu, K.; O’Connor, C.R.; Egle, T.; Kersell, H.; Oliver-Meseguer, J.; Salmeron, M.B.; Madix, R.J.; Sautet, P.; Friend, C.M. Dynamics of surface alloys: Rearrangement of Pd/Ag(111) induced by CO and O2. J. Phys. Chem. C 2018, 123, 8312–8323. [Google Scholar] [CrossRef]
- Luneau, M.; Guan, E.; Chen, W.; Foucher, A.C.; Marcella, N.; Shirman, T.; Verbart, D.M.A.; Aizenberg, J.; Aizenberg, M.; Stach, E.A.; et al. Enhancing catalytic performance of dilute metal alloy nanomaterials. Commun. Chem. 2020, 3, 46. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, Y.; Chen, J.; Li, S.; Huang, X.; Willinger, M.G.; Zhang, W.; Liu, Y.; Zhang, B. Patterning the consecutive Pd3 to Pd1 on Pd2Ga surface via temperature-promoted reactive metal-support interaction. Sci. Adv. 2022, 8, eabq5751. [Google Scholar] [CrossRef]
- Zhou, C.; Ngan, H.T.; Lim, J.S.; Darbari, Z.; Lewandowski, A.; Stacchiola, D.J.; Kozinsky, B.; Sautet, P.; Boscoboinik, J.A. Dynamical study of adsorbate-induced restructuring kinetics in bimetallic catalysts using the PdAu(111) model system. J. Am. Chem. Soc. 2022, 144, 15132–15142. [Google Scholar] [CrossRef]
- Ouyang, M.; Papanikolaou, K.G.; Boubnov, A.; Hoffman, A.S.; Giannakakis, G.; Bare, S.R.; Stamatakis, M.; Flytzani-Stephanopoulos, M.; Sykes, E.C.H. Directing reaction pathways via in situ control of active site geometries in PdAu single-atom alloy catalysts. Nat. Commun. 2021, 12, 1549. [Google Scholar] [CrossRef] [PubMed]
- Gibson, E.K.; Beale, A.M.; Catlow, C.R.A.; Chutia, A.; Gianolio, D.; Gould, A.; Kroner, A.; Mohammed, K.M.H.; Perdjon, M.; Rogers, S.M.; et al. Restructuring of AuPd nanoparticles studied by a combined XAFS/DRIFTS approach. Chem. Mater. 2015, 27, 3714–3720. [Google Scholar] [CrossRef]
- Hansen, C.; Zhou, W.; Brack, E.; Wang, Y.; Wang, C.; Paterson, J.; Southouse, J.; Copéret, C. Decoding the promotional effect of iron in bimetallic Pt–Fe-nanoparticles on the low temperature reverse water–gas shift reaction. J. Am. Chem. Soc. 2024, 146, 27555–27562. [Google Scholar] [CrossRef]
- Marcella, N.; Lim, J.S.; Plonka, A.M.; Yan, G.; Owen, C.J.; van der Hoeven, J.E.S.; Foucher, A.C.; Ngan, H.T.; Torrisi, S.B.; Marinkovic, N.S.; et al. Decoding reactive structures in dilute alloy catalysts. Nat. Commun. 2022, 13, 832. [Google Scholar] [CrossRef]
- Pielsticker, L.; Zegkinoglou, I.; Divins, N.J.; Mistry, H.; Chen, Y.T.; Kostka, A.; Boscoboinik, J.A.; Cuenya, B.R. Segregation phenomena in size-selected bimetallic CuNi nanoparticle catalysts. J. Phys. Chem. B 2018, 122, 919–926. [Google Scholar] [CrossRef]
- Tang, M.; Yuan, W.; Ou, Y.; Li, G.; You, R.; Li, S.; Yang, H.; Zhang, Z.; Wang, Y. Recent progresses on structural reconstruction of nanosized metal catalysts via controlled-atmosphere transmission electron microscopy: A review. ACS Catal. 2020, 10, 14419–14450. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Y.F.; Li, B.; Yang, B.; Li, L. Resolving the active role of isolated transition metal species in Ni-based catalysts for dry reforming of methane. ACS Catal. 2024, 14, 4164–4174. [Google Scholar] [CrossRef]
- Zhang, X.; Han, S.; Zhu, B.; Zhang, G.; Li, X.; Gao, Y.; Wu, Z.; Yang, B.; Liu, Y.; Baaziz, W.; et al. Reversible loss of core–shell structure for Ni–Au bimetallic nanoparticles during CO2 hydrogenation. Nat. Catal. 2020, 3, 411–417. [Google Scholar] [CrossRef]
- Jiang, Y.; Wong, Z.M.; Yan, H.; Tan, T.L.; Mirsaidov, U. Revealing Multistep Phase Separation in Metal Alloy Nanoparticles with In Situ Transmission Electron Microscopy. ACS Nano 2025, 19, 3886–3894. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-S.; Choi, H.; Kim, D.; Song, H.C.; Oh, Y.; Jeong, B.; Lee, J.; Kim, K.-J.; Shin, J.W.; Byon, H.R.; et al. Catalytic boosting on AuCu bimetallic nanoparticles by oxygen-induced atomic restructuring. Appl. Catal. B Environ. 2023, 331, 122704. [Google Scholar] [CrossRef]
- Foucher, A.C.; Marcella, N.; Lee, J.D.; Rosen, D.J.; Tappero, R.; Murray, C.B.; Frenkel, A.I.; Stach, E.A. Structural and valence state modification of cobalt in CoPt nanocatalysts in redox conditions. ACS Nano 2021, 15, 20619–20632. [Google Scholar] [CrossRef]
- Yao, Y.; Goodman, D.W. In situ IR spectroscopic studies of Ni surface segregation induced by CO adsorption on Cu-Ni/SiO2 bimetallic catalysts. Phys. Chem. Chem. Phys. 2014, 16, 3823–3829. [Google Scholar] [CrossRef]
- Wang, Q.; Xia, G.-J.; Zhao, Z.L.; Zhu, Y.; Shi, X.; Huang, L.; Wang, Y.-G.; Gu, M. Atomic origin of CO-interaction effect of PtPb@Pt catalyst revealed by in situ environmental transmission electron microscopy. Nano Energy 2020, 76, 105099. [Google Scholar] [CrossRef]
- Silva, T.A.G.; Teixeira-Neto, É.; Borges, L.R.; Neves-Garcia, T.; Braga, A.H.; Rossi, L.M. From AuPd nanoparticle alloys towards core-shell motifs with enhanced alcohol oxidation activity. ChemCatChem 2023, 15, e202300180. [Google Scholar] [CrossRef]
- Foucher, A.C.; Owen, C.J.; Shirman, T.; Aizenberg, J.; Kozinsky, B.; Stach, E.A. Atomic-Scale STEM analysis shows structural changes of Au–Pd nanoparticles in various gaseous environments. J. Phys. Chem. C 2022, 126, 18047–18056. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Liu, F.; Li, M.; Zhang, Y.; Cai, J.; Li, Y.; Yang, F.; Yin, F.; Lu, J.; et al. Revealing dynamics and competitive mechanism of gas-induced surface segregation of PdFe0.08 dilute alloy by multi-dimensional imaging. J. Phys. Chem. Lett. 2024, 15, 11737–11744. [Google Scholar] [CrossRef]
- Beaumont, S.K.; Alayoglu, S.; Pushkarev, V.V.; Liu, Z.; Kruse, N.; Somorjai, G.A. Exploring surface science and restructuring in reactive atmospheres of colloidally prepared bimetallic CuNi and CuCo nanoparticles on SiO2 in situ using ambient pressure X-ray photoelectron spectroscopy. Faraday Discuss 2013, 162, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Dong, Z.; Yang, S.; Cui, R.; Zhang, L.; Chen, X.; Luo, L. Phase Separation of CuPd Alloy Nanocatalysts in CO Oxidation. ACS Nano 2024, 19, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Lim, A.M.H.; Yan, H.; Zeng, H.C.; Mirsaidov, U. Phase segregation in PdCu alloy nanoparticles during CO oxidation reaction at atmospheric pressure. Adv. Sci. 2023, 10, e2302663. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, L.; Du, M.; Tian, C.; Zhao, G.; Liu, Z.; Liang, Z.; Hou, K.; Chen, J.; Liu, X.; et al. Unraveling distinct effects between CuOx and PtCu alloy sites in Pt-Cu bimetallic catalysts for CO oxidation at different temperatures. Nat. Commun. 2024, 15, 5598. [Google Scholar] [CrossRef]
- Song, Y.; Kim, D.; Hong, S.; Kim, T.-S.; Kim, K.-J.; Park, J.Y. Bimetallic synergy from a reaction-driven metal oxide–metal interface of Pt–Co bimetallic nanoparticles. ACS Catal. 2023, 13, 13777–13785. [Google Scholar] [CrossRef]
- Ghosh, T.; Liu, X.; Sun, W.; Chen, M.; Liu, Y.; Li, Y.; Mirsaidov, U. Revealing the origin of low-temperature activity of Ni-Rh nanostructures during CO oxidation reaction with operando TEM. Adv. Sci. 2022, 9, e2105599. [Google Scholar] [CrossRef]
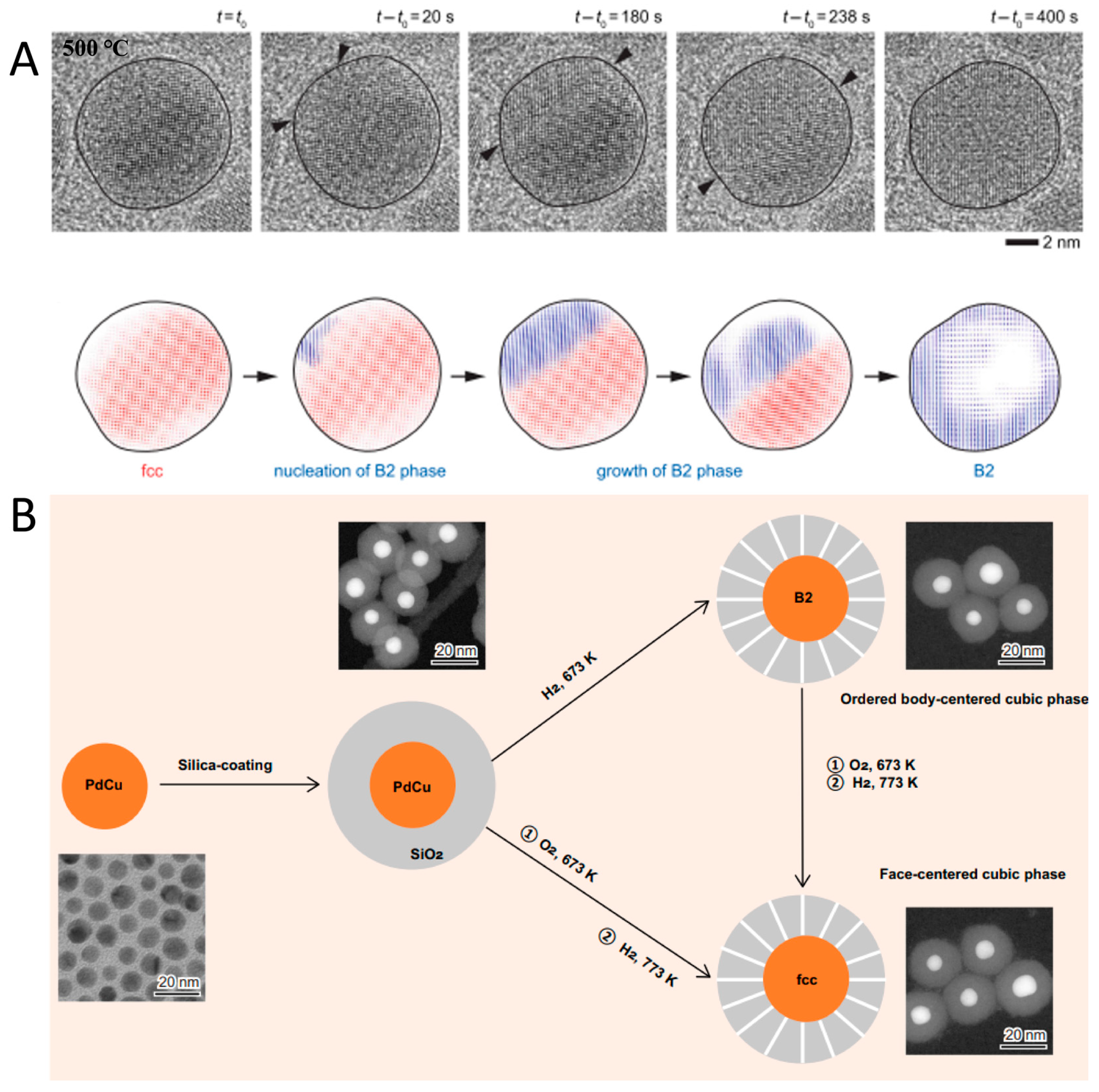
- Jiang, Y.; Duchamp, M.; Ang, S.J.; Yan, H.; Tan, T.L.; Mirsaidov, U. Dynamics of the fcc-to-bcc phase transition in single-crystalline PdCu alloy nanoparticles. Nat. Commun. 2023, 14, 104. [Google Scholar] [CrossRef]
- Liu, S.; Li, Y.; Yu, X.; Han, S.; Zhou, Y.; Yang, Y.; Zhang, H.; Jiang, Z.; Zhu, C.; Li, W.X.; et al. Tuning crystal-phase of bimetallic single-nanoparticle for catalytic hydrogenation. Nat. Commun. 2022, 13, 4559. [Google Scholar] [CrossRef]
- Qiu, Y.; Xin, L.; Li, Y.; McCrum, I.T.; Guo, F.; Ma, T.; Ren, Y.; Liu, Q.; Zhou, L.; Gu, S.; et al. BCC-phased PdCu alloy as a highly active electrocatalyst for hydrogen oxidation in alkaline electrolytes. J. Am. Chem. Soc. 2018, 140, 16580–16588. [Google Scholar] [CrossRef]
- Lim, J.; Cullen, D.A.; Stavitski, E.; Lee, S.W.; Hatzell, M.C. Atomically ordered PdCu electrocatalysts for selective and stable electrochemical nitrate reduction. ACS Energy Lett. 2023, 8, 4746–4752. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Sun, M.; Xu, J.; Zhao, X.; Zhou, R.; Pan, B.; Wang, L.; Han, N.; Huang, B.; Li, Y. Phase-dependent electrocatalytic CO2 reduction on Pd3Bi nanocrystals. Angew. Chem. Int. Ed. 2021, 60, 21741–21745. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Chen, Z.; Wei, R.; Yang, C.; Wang, Y.; Xu, J.; Zhang, H.; Guan, A.; Chen, J.; Sham, T.-K.; et al. Selective CO-to-acetate electroreduction via intermediate adsorption tuning on ordered Cu–Pd sites. Nat. Catal. 2022, 5, 251–258. [Google Scholar] [CrossRef]
- Tong, W.; Huang, B.; Wang, P.; Li, L.; Shao, Q.; Huang, X. Crystal-phase-engineered PdCu electrocatalyst for enhanced ammonia synthesis. Angew. Chem. Int. Ed. 2020, 59, 2649–2653. [Google Scholar] [CrossRef]
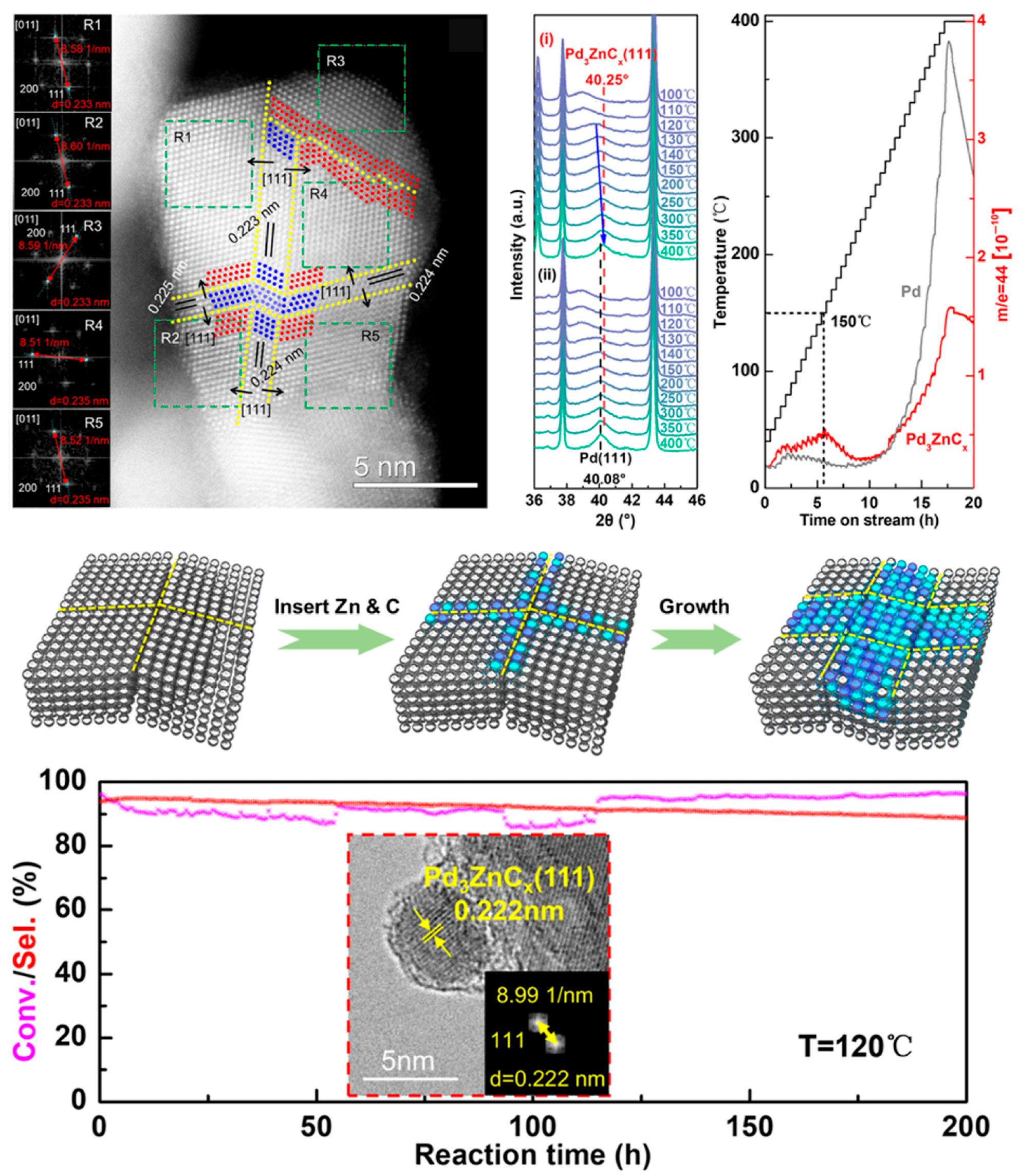
- Chen, H.; Li, L.; Zhao, Z.J.; Yang, B.; Zhang, Y.; Liu, X.; Gu, Q.; Yu, Z.; Yang, X.; Gong, J.; et al. Co-infiltration and dynamic formation of Pd3ZnCx intermetallic carbide by syngas boosting selective hydrogenation of acetylene. Nat. Commun. 2024, 15, 9850. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, X.; Gu, Q.; Sun, Z.; Wang, H.; Cao, L.; Xu, Y.; Li, S.; Yang, B.; Wei, S.; et al. Breaking the conversion-selectivity trade-off in methanol synthesis from CO2 using dual intimate oxide/metal interfaces. J. Am. Chem. Soc. 2024, 146, 28885–28894. [Google Scholar] [CrossRef]
- Liu, X.; Gu, Q.; Zhang, Y.; Xu, X.; Wang, H.; Sun, Z.; Cao, L.; Sun, Q.; Xu, L.; Wang, L.; et al. Atomically thick oxide overcoating stimulates low-temperature reactive metal-support interactions for enhanced catalysis. J. Am. Chem. Soc. 2023, 145, 6702–6709. [Google Scholar] [CrossRef] [PubMed]
- Ouvrard, A.; Alyabyeva, N.; Zakaria, A.M.; Yuan, K.; Dablemont, C.; Lazzari, R.; Charra, F.; Bourguignon, B. Change of composition and surface plasmon resonance of Pd/Au core/shell nanoparticles triggered by CO adsorption. J. Chem. Phys. 2024, 161, 124713. [Google Scholar] [CrossRef]
- Yang, F.; Wang, Y.; Cui, Y.; Yang, X.; Zhu, Y.; Weiss, C.M.; Li, M.; Chen, G.; Yan, Y.; Gu, M.D.; et al. Sub-3 nm Pt@Ru toward outstanding hydrogen oxidation reaction performance in alkaline media. J. Am. Chem. Soc. 2023, 145, 27500–27511. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Yang, J.; Zhang, Z.; Jiang, X.; Liu, W.; Qiao, B.; Mu, J.; Wang, F. Strong metal–support interaction facilitated multicomponent alloy formation on metal oxide support. J. Am. Chem. Soc. 2023, 145, 22671–22684. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, W.; Li, Y.; Zhao, W.; Han, S.; Shen, W. Pt3Ti intermetallic alloy formed by strong metal–support interaction over Pt/TiO2 for the selective hydrogenation of acetophenone. ACS Catal. 2023, 13, 4030–4041. [Google Scholar] [CrossRef]
- Niu, Z.; Chen, S.; Yu, Y.; Lei, T.; Dehestani, A.; Schierle-Arndt, K.; Yang, P. Morphology-controlled transformation of Cu@Au core-shell nanowires into thermally stable Cu3Au intermetallic nanowires. Nano Res. 2020, 13, 2564–2569. [Google Scholar] [CrossRef]
- Du, P.; Zhang, Y.; Qi, R.; Gu, Q.; Xu, X.; Wang, A.; Zhu, B.; Yang, B.; Zhang, T. Domino effect of catalysis: Coherence between reaction network and catalyst restructuring accelerating surface carburization for CO2 hydrogenation. J. Am. Chem. Soc. 2025, 147, 19622–19631. [Google Scholar] [CrossRef]
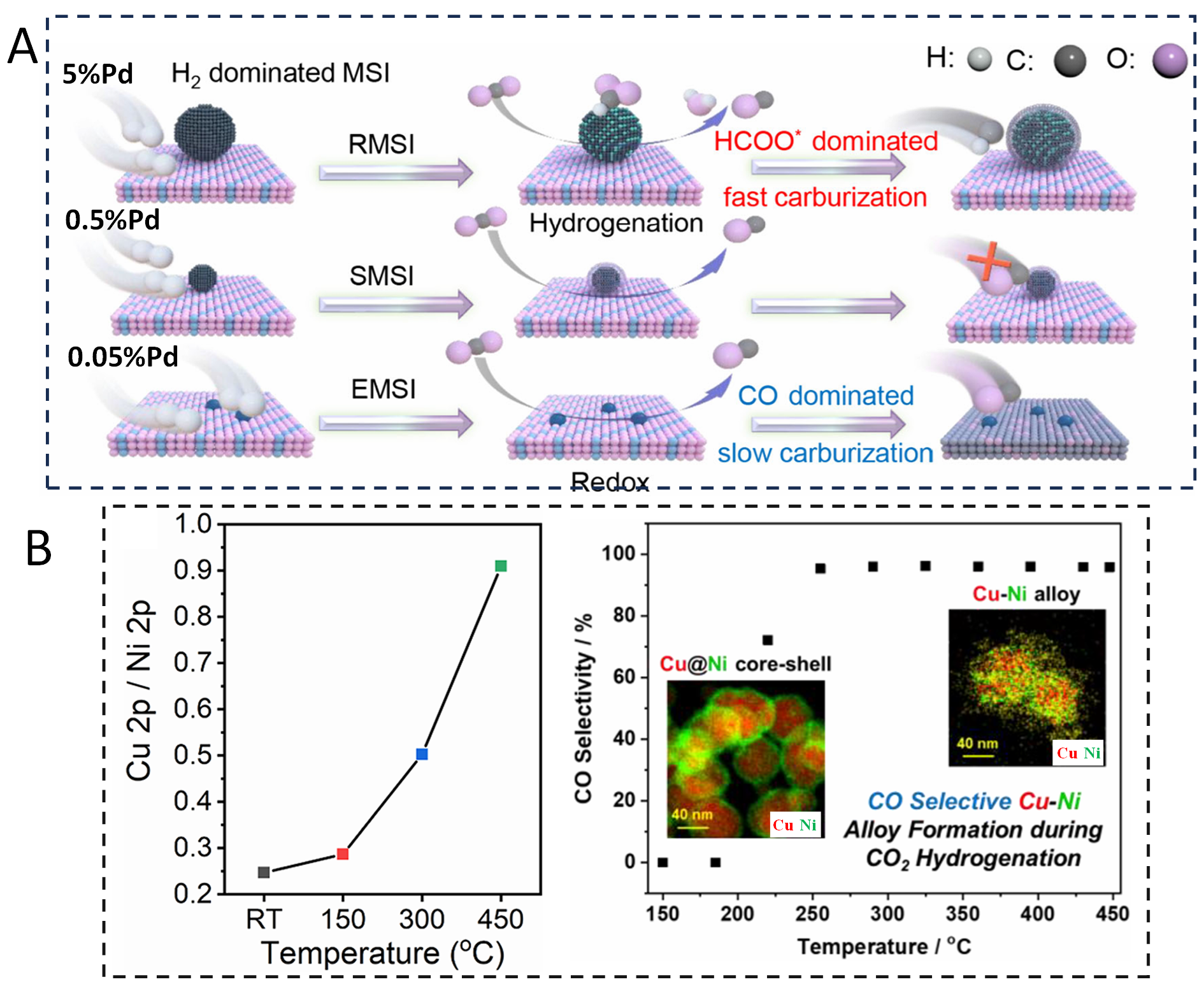
- Reddy, K.P.; Kim, D.; Hong, S.; Kim, K.J.; Ryoo, R.; Park, J.Y. Tuning CO2 hydrogenation selectivity through reaction-driven restructuring on Cu-Ni bimetal catalysts. ACS Appl. Mater. Interfaces 2023, 15, 9373–9381. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, Z.; Gao, T.; Wang, Z. Experimental and theoretical study of Pd-Pt/In2O3 bimetallic catalysts for enhancing methanol production from CO2. Chem. Eng. J. 2024, 483, 149370. [Google Scholar] [CrossRef]
- De Broglie, L. Waves and quanta. Nature 1923, 112, 540. [Google Scholar] [CrossRef]
- Knoll, M.; Ruska, E. Das elektronenmikroskop. Z. Phys. 1932, 78, 318–339. [Google Scholar] [CrossRef]
- Tang, M.; Li, S.; Chen, S.; Ou, Y.; Hiroaki, M.; Yuan, W.; Zhu, B.; Yang, H.; Gao, Y.; Zhang, Z.; et al. Facet-dependent oxidative strong metal-support interactions of palladium-TiO2 determined by in situ transmission electron microscopy. Angew. Chem. Int. Ed. 2021, 60, 22339–22344. [Google Scholar] [CrossRef]
- Yuan, W.; Zhu, B.; Fang, K.; Li, X.Y.; Hansen, T.W.; Ou, Y.; Yang, H.; Wagner, J.B.; Gao, Y.; Wang, Y.; et al. In situ manipulation of the active Au-TiO2 interface with atomic precision during CO oxidation. Science 2021, 371, 517–521. [Google Scholar] [CrossRef]
- Gu, J.Y.; Cai, Z.F.; Wang, D.; Wan, L.J. Single-molecule imaging of iron-phthalocyanine-catalyzed oxygen reduction reaction by in situ scanning tunneling microscopy. ACS Nano 2016, 10, 8746–8750. [Google Scholar] [CrossRef]
- Valls Mascaró, F.; Koper, M.T.M.; Rost, M.J. Step bunching instability and its effects in electrocatalysis on platinum surfaces. Nat. Catal. 2024, 7, 1165–1172. [Google Scholar] [CrossRef]
- Yuan, W.; Zhu, B.; Li, X.Y.; Hansen, T.W.; Ou, Y.; Fang, K.; Yang, H.; Zhang, Z.; Wagner, J.B.; Gao, Y.; et al. Visualizing H2O molecules reacting at TiO2 active sites with transmission electron microscopy. Science 2020, 367, 428–430. [Google Scholar] [CrossRef] [PubMed]
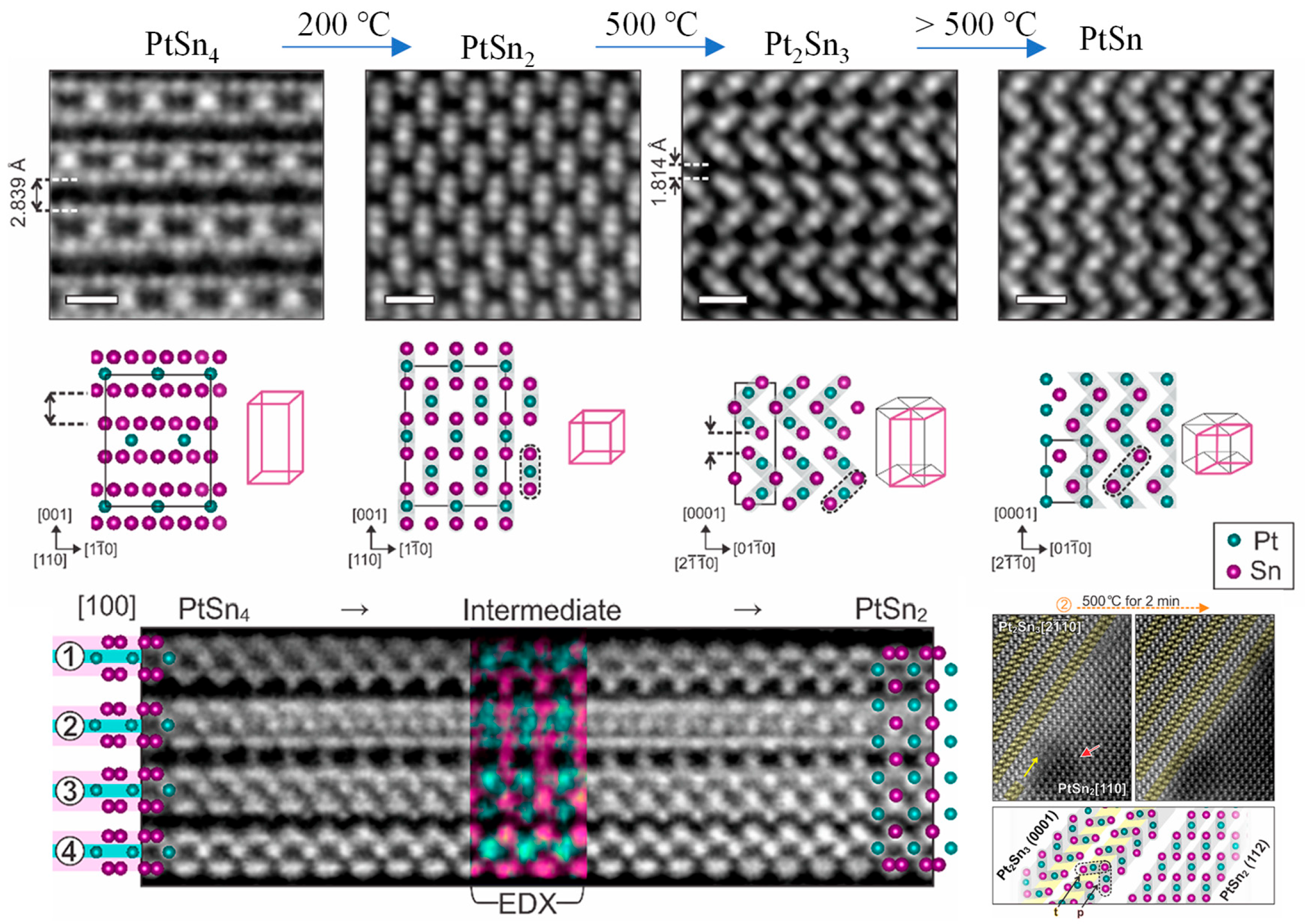
- Yun, H.; Zhang, D.; Birol, T.; Wang, J.P.; Mkhoyan, K.A. Structural anisotropy-driven atomic mechanisms of phase transformations in the Pt-Sn system. Nano Lett. 2023, 23, 7576–7583. [Google Scholar] [CrossRef]
- Binnig, G.; Rohrer, H.; Gerber, C.; Weibel, E. Surface studies by scanning tunneling microscopy. Phys. Rev. Lett. 1982, 49, 57–61. [Google Scholar] [CrossRef]
- Laegsgaard, E.; Österlund, L.; Thostrup, P.; Rasmussen, P.B.; Stensgaard, I.; Besenbacher, F. A high-pressure scanning tunneling microscope. Rev. Sci. Instrum. 2001, 72, 3537–3542. [Google Scholar] [CrossRef]
- Hulsken, B.; Van Hameren, R.; Gerritsen, J.W.; Khoury, T.; Thordarson, P.; Crossley, M.J.; Rowan, A.E.; Nolte, R.J.; Elemans, J.A.; Speller, S. Real-time single-molecule imaging of oxidation catalysis at a liquid-solid interface. Nat. Nanotechnol. 2007, 2, 285–289. [Google Scholar] [CrossRef]
- Kyriakou, G.; Boucher, M.B.; Jewell, A.D.; Lewis, E.A.; Lawton, T.J.; Baber, A.E.; Tierney, H.L.; Flytzani-Stephanopoulos, M.; Sykes, E.C. Isolated metal atom geometries as a strategy for selective heterogeneous hydrogenations. Science 2012, 335, 1209–1212. [Google Scholar] [CrossRef] [PubMed]
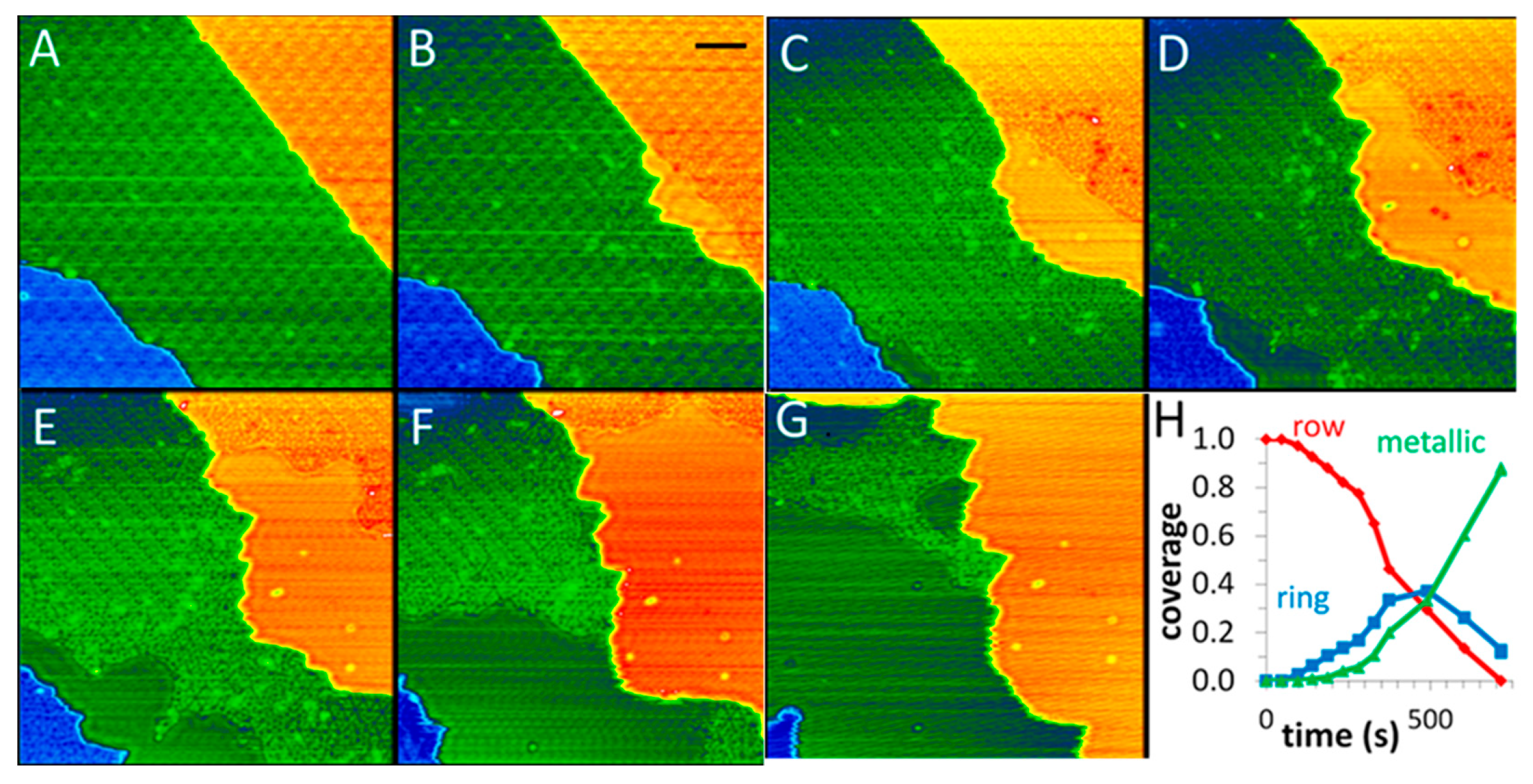
- Vestergaard, E.K.; Vang, R.T.; Knudsen, J.; Pedersen, T.M.; An, T.; Laegsgaard, E.; Stensgaard, I.; Hammer, B.; Besenbacher, F. Adsorbate-induced alloy phase separation: A direct view by high-pressure scanning tunneling microscopy. Phys. Rev. Lett. 2005, 95, 126101. [Google Scholar] [CrossRef] [PubMed]
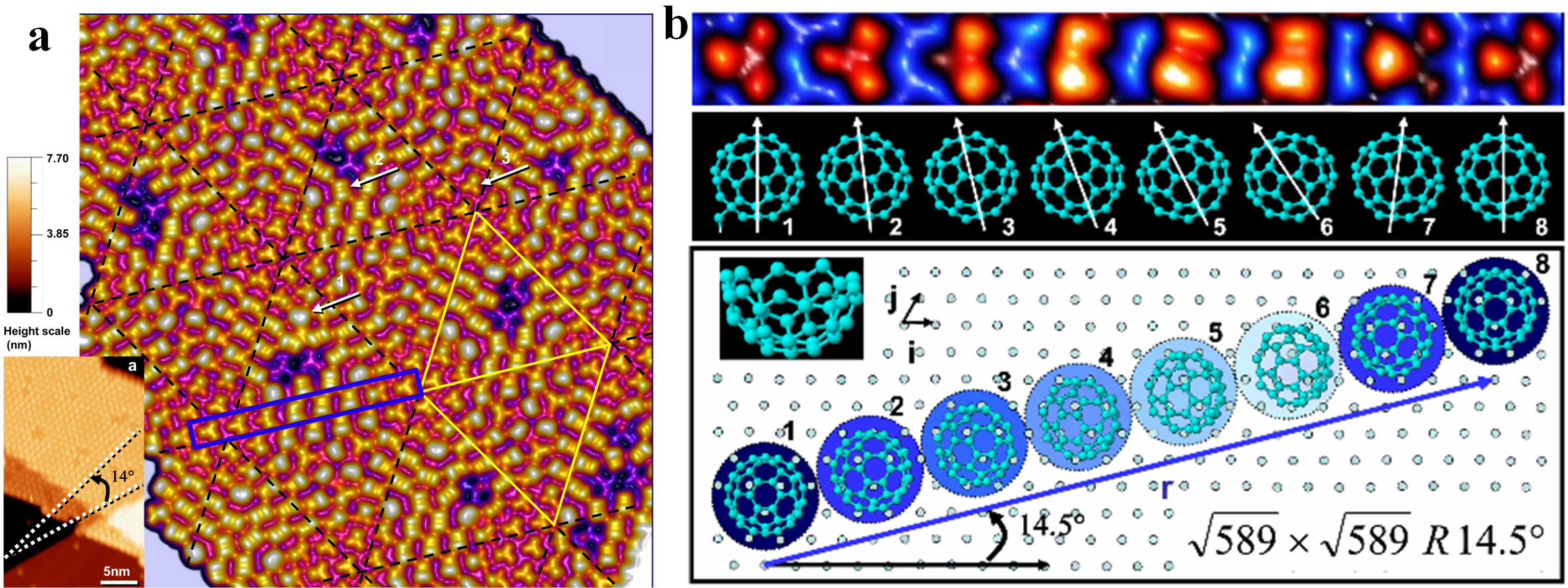
- Schull, G.; Berndt, R. Orientationally ordered (7×7) superstructure of C60 on Au(111). Phys. Rev. Lett. 2007, 99, 226105. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Du, P.; Yang, B. Gas-Mediated Dynamic Structure Evolution of Bimetallic Alloy Catalysts. Nanomaterials 2025, 15, 1828. https://doi.org/10.3390/nano15231828
Zhang Y, Du P, Yang B. Gas-Mediated Dynamic Structure Evolution of Bimetallic Alloy Catalysts. Nanomaterials. 2025; 15(23):1828. https://doi.org/10.3390/nano15231828
Chicago/Turabian StyleZhang, Yafeng, Pengfei Du, and Bing Yang. 2025. "Gas-Mediated Dynamic Structure Evolution of Bimetallic Alloy Catalysts" Nanomaterials 15, no. 23: 1828. https://doi.org/10.3390/nano15231828
APA StyleZhang, Y., Du, P., & Yang, B. (2025). Gas-Mediated Dynamic Structure Evolution of Bimetallic Alloy Catalysts. Nanomaterials, 15(23), 1828. https://doi.org/10.3390/nano15231828