Abstract
Beyond the extensively studied two-dimensional transition metal dichalcogenides, a wide range of non-stoichiometric transition metal sulfides, such as molybdenum sulfides and tungsten sulfides (Mo2S3, W2S3, Mo6S8, Mo6S6, NiMo3S4), have attracted significant attention for their promising applications in sensing, catalysis, and energy storage. It is necessary to review the current advanced progress of non-stoichiometric transition metal sulfides for various applications. Here, we systematically summarize the synthesis strategies of the non-stoichiometric transition metal sulfides, encompassing methods such as the molten salt synthesis method, high-metal-content growth strategy, and others. Particular emphasis is placed on how variations in the metal-to-sulfur ratio give rise to distinct crystal structures and electronic properties, and how these features influence their conductivity, stability, and performance. This review will deepen the understanding of the state of the art of non-stoichiometric transition metal sulfides, including the synthesis, characterization, modification, and various applications.
1. Introduction
Two-dimensional transition metal dichalcogenides (TMDs) possess tunable electronic structures, excellent catalytic properties, and strong excitonic effects, which have been widely applied in various fields [1,2,3,4,5,6,7,8]. Beyond the TMDs, a wide range of non-stoichiometric transition metal sulfides (NSTMSs) have also been applied in the field of sensing, catalysis, and energy storage due to their unique physicochemical properties. A large number of NSTMSs, such as Mo2S3, W2S3, Mo6S8, Mo6S6, NiMo3S4, have been explored [9,10,11,12,13,14,15]. Their electronic structures can be readily tailored through intrinsic defects, intercalated atoms, or elemental doping [16,17,18,19,20]. Compared to their stoichiometric counterparts, these NSTMSs also exhibit superior performance in sensing, catalysis, and energy storage applications. Despite their non-stoichiometric composition, these transition metal chalcogenides retain the layered crystal structure and weak interlayer van der Waals interactions characteristic of their stoichiometric counterparts, enabling them to be similarly processed into nanosheets through standard exfoliation or chemical methods. These unique properties have made NSTMSs a research hotspot in materials science, driving the development of novel conceptual devices [21,22,23,24,25].
Advanced progress of NSTMSs for sensing, catalysis, and energy storage has been made in recent years. Zhao et al. fabricated Mo2S3 nanowire grids via liquid–liquid interfacial self-assembly, which exhibited uniform morphology and exceptional flexibility, demonstrating outstanding performance in flexible strain sensors and high-energy-density full-cell systems [26]. Tan et al. proposed replacing Mo6S8 synthesized by traditional methods with Mo6S8 encapsulated in carbon fiber (Mo6S8@CF). This approach solves the problem of Mo6S8′s tendency to aggregate and sinter at high temperatures, which reduces catalytic efficiency, while significantly improving its performance in nitroaromatic hydrocarbon electroreduction [27]. Liu et al. constructed Mo6S6 nanostructures through electron beam lithography. Though not yet practically applied, it exhibits theoretically high catalytic potential in electrocatalytic hydrogen evolution due to its unique electronic states and surface-active site distribution [28]. The applicability of NSTMSs further extends to magnetic systems and photonic platforms [29]. Advancements in novel synthesis techniques have provided fresh insights for structural optimization and performance enhancement [30,31,32,33,34,35,36,37]. These innovative synthesis approaches not only address the critical challenge of preparing NSTMSs but also establish a material foundation for performance optimization across diverse application scenarios [38,39,40,41,42,43,44].
In this work, we systematically summarize and analyze the synthesis strategies, structural diversity, and functional applications of non-stoichiometric transition metal sulfides. First, we provide a comprehensive overview of the synthetic approaches and applications of M2S3 (M = Mo, W) and its based composites, highlighting their promising potential in sensor technologies and catalysis technologies. Second, we further explored M6S8 compounds, with a particular focus on Mo6S8 due to its high conductivity and reversible electrochemical intercalation, demonstrating significant potential for applications in lithium-ion batteries. Finally, we selected Mo6S6 as a representative of the important transition metal sulfide M6S6 family, providing a detailed discussion on its structural formation mechanisms and synthesis methods, while highlighting its promising properties. By summarizing the synthetic approaches and functional applications of these transition metal sulfides, this work contributes to a deeper understanding of their broad prospects in sensing, catalysis, and energy storage.
2. M2S3 and Their Applications
M2S3 (M = Mo, W) has shown broad application prospects in various fields such as sensing, catalysis, electrochemical energy storage, and electronic devices. The structural characteristics and functional applications of different forms of M2S3 and M2S3 matrix composites are systematically analyzed, with special attention to their advantages and future directions in sensor, catalyst, and energy storage applications [45]. Notably, nanowire-networked Mo2S3 exhibits superior sensitivity and selectivity in energy storage and biosensing applications due to its high conductivity and open framework, which facilitates efficient electron transport pathways. Layered Mo2S3 nanosheets with adjustable layer spacing have been widely used in humidity sensors. At the same time, existing studies have shown that W2S3 has been widely used as a high-efficiency catalyst in hydrogen evolution reactions. In order to further broaden the horizons of hydrogen evolution reaction catalysts, we note that Mo2S3 matrix composites have abundant surface-active sites and show great potential in hydrogen evolution reactions (HER) and oxygen evolution reactions (OER), making them promising candidates for advanced catalytic applications [46]. Looking forward, the synthesis of these functional materials could be revolutionized by emerging machine learning-assisted techniques, such as Bayesian optimization for reaction parameter screening, which may enable precise control over morphology and defect engineering to further enhance their catalytic and sensing performance.
2.1. Ultrathin Mo2S3 Nanowire Network
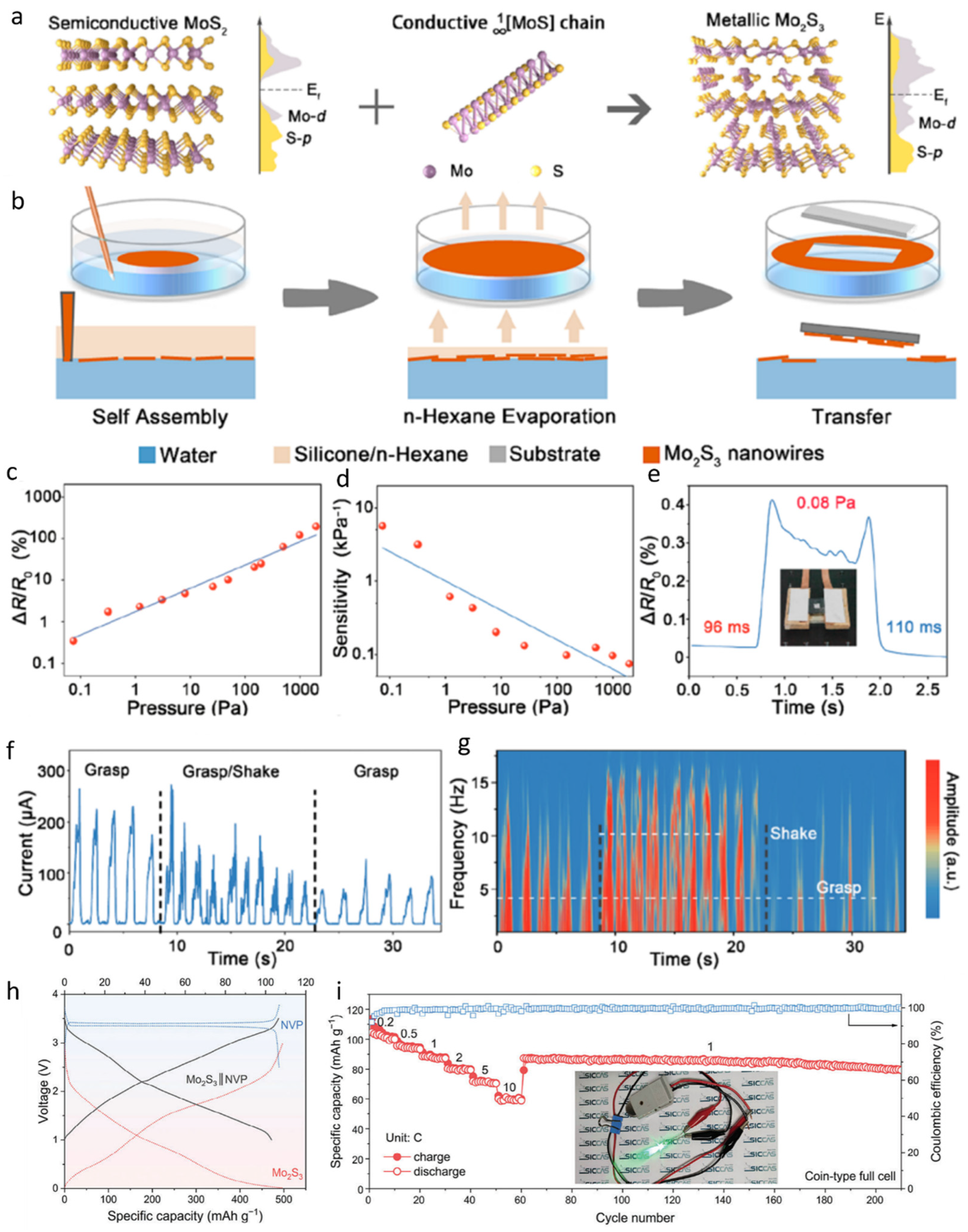
At present, Mo2S3 is being widely used in the field of sensing and energy storage [47,48,49,50,51]. In order to synthesize Mo2S3, a molten salt reaction method was employed to insert conductive MoS bonds into semiconducting MoS2 (Figure 1a). This process led to the formation of numerous MoS clusters within the interlayers of MoS2. Fermi level analysis revealed that, prior to insertion, the interstitial gaps of MoS2 exhibited Fermi levels close to zero. However, after the incorporation of MoS bonds, a pronounced peak in the Mo-d Fermi level was observed, confirming the successful intercalation and the formation of Mo2S3. For the fabrication of large-area, ultrathin Mo2S3 nanowire networks, a liquid–liquid interfacial self-assembly technique was adopted (Figure 1b). A biphasic mixture of water and n-hexane was used, into which a pre-prepared Mo2S3/ethanol solution was injected at the interface. The diffusion of ethanol into the aqueous phase generated a Marangoni force, and by controlling the injection volume, the Mo2S3 nanowires were uniformly distributed along the interface. Subsequent evaporation of n-hexane resulted in the nanowire network floating on the water surface, from which it could be transferred onto various substrates. Additionally, the incorporation of silica gel into the n-hexane phase was found to enhance the stability of the assembly. The pressure-sensing performance of the Mo2S3 nanowires was systematically evaluated. As the applied pressure was increased from 0 to 1000 Pa, the relative resistance of the nanowires increased proportionally (Figure 1c), while the sensitivity decreased linearly with pressure (Figure 1d). At the lower static response limit, a relative resistance change exceeding 0.3% enabled the detection of pressure variations as small as 0.08 Pa, with a response time of 96 ms and a recovery time of 110 ms (Figure 1e). These results indicate that the nanowires can rapidly respond to and recover from subtle external pressure changes. Owing to their high adaptability and sensitivity, Mo2S3 nanowires have been applied in monitoring applications for Parkinson’s disease. The sensing mechanism is based on detecting the electrical current fluctuations caused by high-frequency oscillations superimposed during the grasping of objects. Specifically, the current profile becomes irregular during gripping and gradually returns to a smooth baseline upon cessation of oscillatory activity (Figure 1f,g). Thus, these nanowires demonstrate significant potential for real-time monitoring of motor fluctuations in Parkinson’s patients. Mo2S3 nanowires also exhibit broad applicability in the field of flexible electronics. By fabricating Mo2S3 nanofilms for use in full-cell systems, the galvanostatic charge–discharge (GCD) curves (Figure 1h) and cycling performance (Figure 1i) were analyzed. The results demonstrate that the full cell exhibits excellent electrochemical performance, enabling stable charge–discharge cycles. Moreover, even after extensive cycling, the device maintains a high capacitance retention, highlighting its promising potential for energy storage applications.
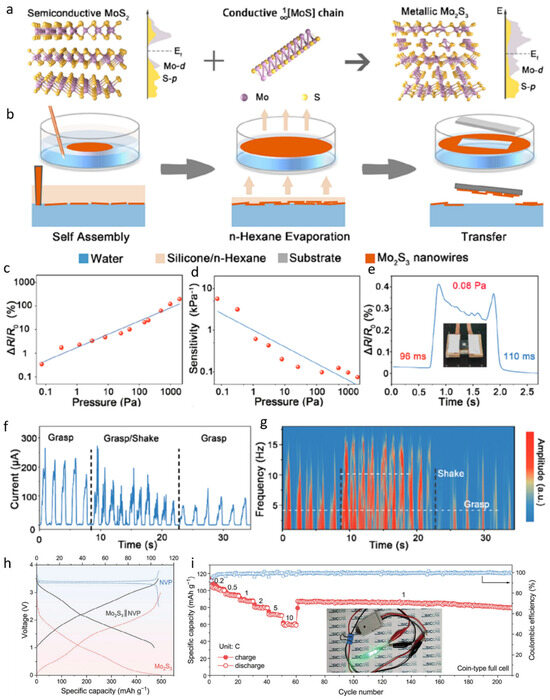
Figure 1.
Ultrathin Mo2S3 nanowire networks designed for piezoresistive electronic skin and full-cell battery systems. (a) The conceptual illustration of its preparation shows the insertion of highly conductive MoS chains containing Mo−Mo zigzag structures into MoS2 layers. (b) The nanowire network is formed via liquid–liquid interface self-assembly. (c) The relative resistance changes in the network with increasing pressure. (d) The sensitivity performance of the sensor under applied pressure. (e) The detection limit, response, and recovery time of the sensor, along with a digital photograph from the test (the inset shows the weight of a small piece of paper with an area of 1 cm2). (f,g) Real-time response to current and frequency signals during Parkinson-like grasping movements. Reproduced with permission [26]. Copyright 2023, American Chemical Society. (h) The GCD curves of a coin-type full cell with a freestanding Mo2S3 film as the anode and NVP as the cathode. (i) The rate and cycling performance of the full cell, with the inset showing an LED bulb powered by the battery. Reproduced with permission [47]. Copyright 2024, Wiley-Blackwell.
2.2. Layered Mo2S3 Nanosheets
While Mo2S3 nanowires have demonstrated exceptional electronic transport properties and catalytic activity owing to their distinctive one-dimensional architecture, recent research efforts have increasingly focused on two-dimensional nanonets to overcome inherent limitations in specific surface area and active site exposure. This paradigm shift is driven by the superior structural advantages of Mo2S3 nanonets—their highly porous, interconnected networks not only optimize charge transport pathways but also maximize electrolyte permeability, collectively elevating their catalytic efficiency and energy storage capabilities to new heights [52,53,54,55,56,57].
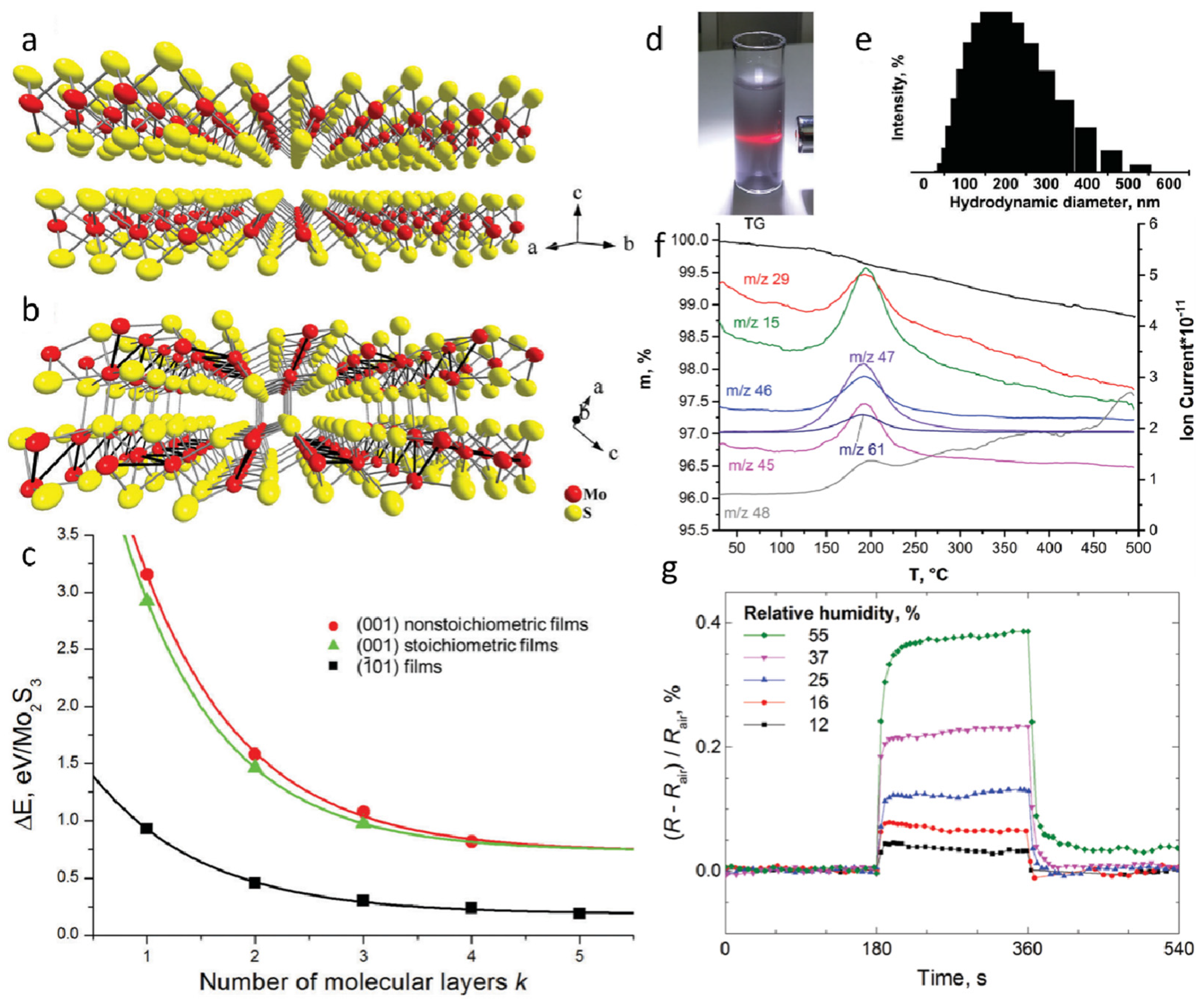
The crystal structure of MoS2 consists of S–Mo–S layers arranged in a three-atomic-layer configuration, where the interlayer spacing is relatively large, and the interlayer interactions are weak (Figure 2a). In contrast, Mo2S3 features independent metallic bonds in its crystalline structure, with Mo–Mo bonding along the b-axis, rendering it significantly more stable than MoS2 (Figure 2b). Mo2S3 can be regarded as MoS2 with inserted Mo atoms, leading to a quasi-layered structure in which adjacent layers are held together by weak van der Waals interactions. Previous studies have investigated the influence of different surface terminations and thicknesses on the relative stability of Mo2S3 nanosheets (Figure 2c). Three types of Mo2S3 films were considered: (001) non-stoichiometric film, (001) stoichiometric film, and (101) film. The results indicate that Mo2S3 nanosheets with (101) surface termination exhibit the highest stability among all configurations, with their stability remaining unaffected by stoichiometry and thickness. The relative energy of all three nanosheet types increases with thickness, rapidly approaching the value of surface formation energy. The colloidal dispersions formed by ultrasonic treatment of Mo2S3 in organic media have been explored, with a series of solvents commonly used for MoS2 liquid-phase exfoliation tested to evaluate the suitability of Mo2S3 for similar processing. The solvent-based dispersions exhibited a grayish appearance and displayed the Tyndall effect, confirming the presence of colloidal particles in the liquid phase (Figure 2d). Dynamic light scattering (DLS) measurements of the dispersions revealed a relatively broad particle size distribution, suggesting a uniform colloidal state (Figure 2e). These findings clearly demonstrate that bulk quasi-layered Mo2S3 can be successfully exfoliated via ultrasonic treatment. To further characterize the material, thermogravimetric and mass spectrometric analyses were conducted using simultaneous thermogravimetric–differential scanning calorimetry (TG-DSC) and evolved gas analysis–mass spectrometry (EGA-MS). These analyses confirmed the presence of solvent molecules in the solid phase obtained from dimethyl sulfoxide (DMSO)-based colloidal dispersions (Figure 2f). The mass loss and corresponding ion current increase indicate that DMSO molecules are the primary gas-phase products released during this stage. The observed decomposition temperature aligns well with the boiling point of DMSO (189 °C), suggesting that Mo atoms generated upon Mo–S bond cleavage may be stabilized through complexation with solvent molecules or their residual species. Finally, Mo2S3 nanosheets were tested for their electrical resistance response to humidity variations (Figure 2g). A sensing device fabricated using Mo2S3 exhibited a significant increase in resistance upon exposure to water vapor, which rapidly returned to its initial value upon removal from the testing chamber. The humidity sensing performance of Mo2S3 nanosheets surpasses that of several other nanomaterial-based sensors, including those based on two-dimensional MoS2, ZnO nanowires, and graphene oxide. While these materials demonstrate certain sensitivity to humidity changes, they often suffer from slower response times or limited stability. In contrast, Mo2S3 exhibits a unique quasi-layered structure and enhanced stability, resulting in faster response and recovery times, as well as improved repeatability. Owing to its unique quasi-layered structure, high stability, and rapid humidity response, Mo2S3 represents a promising candidate for next-generation sensing materials. Future research should focus on surface functionalization to enhance solvent exfoliation efficiency, explore heterostructural integration with other two-dimensional materials, and expand its applications in flexible electronics and environmental monitoring.
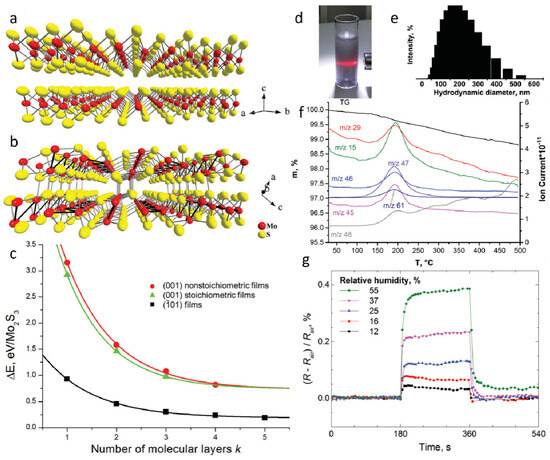
Figure 2.
A DFT study and experimental evidence reveal the cleavage behavior of Mo2S3 under sonication in the liquid phase. (a) Crystal structure of layered MoS2. (b) Crystal structure of quasi-layered Mo2S3. (c) Relative energy variations of Mo2S3 nanosheets as a function of thickness and cleavage mode. (d) Photograph of Mo2S3 dispersed in an ethanol–water mixture (volume ratio 1:1). (e) Dynamic light scattering analysis of Mo2S3 dispersion in the ethanol–water mixture (volume ratio 1:1). (f) Thermogravimetric–differential scanning calorimetry (TG–DSC) and evolved gas analysis–mass spectrometry (EGA–MS) characterization of Mo2S3 precipitated from the colloidal dispersion in DMSO. (g) Time-dependent resistance measurements of Mo2S3 thin films in an aqueous medium. Reproduced with permission [52]. Copyright 2017, Royal Society of Chemistry.
Moreover, the unique quasi-layered structure and high stability exhibited by Mo2S3 nanosheets not only contribute to their outstanding humidity sensing performance but also provide a robust foundation for energy storage applications. Compared with conventional MoS2, which often suffers from limited electrical conductivity and relatively sluggish ion diffusion in its semiconducting 2H phase, Mo2S3 offers a higher density of active sites and more efficient electron transport pathways owing to its non-stoichiometric composition and defect-rich structure [58]. In energy storage devices, critical factors such as electrical conductivity, interfacial reaction activity, and structural stability strongly influence electrochemical performance. Mo2S3 nanosheets demonstrate significant promise for high-performance applications in lithium-ion batteries, supercapacitors, and related systems, enabling both efficient charge storage and rapid charge–discharge behavior. Furthermore, the rapid response and recovery observed in humidity sensing reflect the material’s excellent structural reversibility and chemical stability in electrolyte environments, implying favorable durability and cycling stability under electrochemical conditions. These characteristics highlight the advantages of Mo2S3 over traditional disulfide-based materials and underscore the potential of integrating sensing and energy storage functionalities in next-generation Mo2S3-based nanodevices.
2.3. W2S3 Nanosheets
W2S3 has emerged as a promising candidate owing to its unique crystal structure, rich defect chemistry, and favorable electrical conductivity. These features suggest strong potential for W2S3 in catalytic and energy storage applications. The following section highlights recent developments in the synthesis and functional exploration of W2S3, aiming to provide insights into its advantages over conventional layered dichalcogenides.
WS2 is a common catalyst with decent performance in HER, but its catalytic activity and conductivity are limited. In contrast, W2S3 features a unique non-centrosymmetric structure that induces spontaneous polarization and metallic conductivity, significantly enhancing its catalytic performance. Experimental results show that W2S3 nanosheets exhibit lower overpotentials and higher catalytic activity than WS2 in both acidic and alkaline media, even outperforming commercial Pt/C catalysts under alkaline conditions. Additionally, W2S3 demonstrates excellent stability in practical electrolyzers, maintaining performance during long-term high-current operation. Theoretical calculations further confirm that W2S3 has an optimal hydrogen adsorption free energy, supporting its efficient catalytic mechanism.
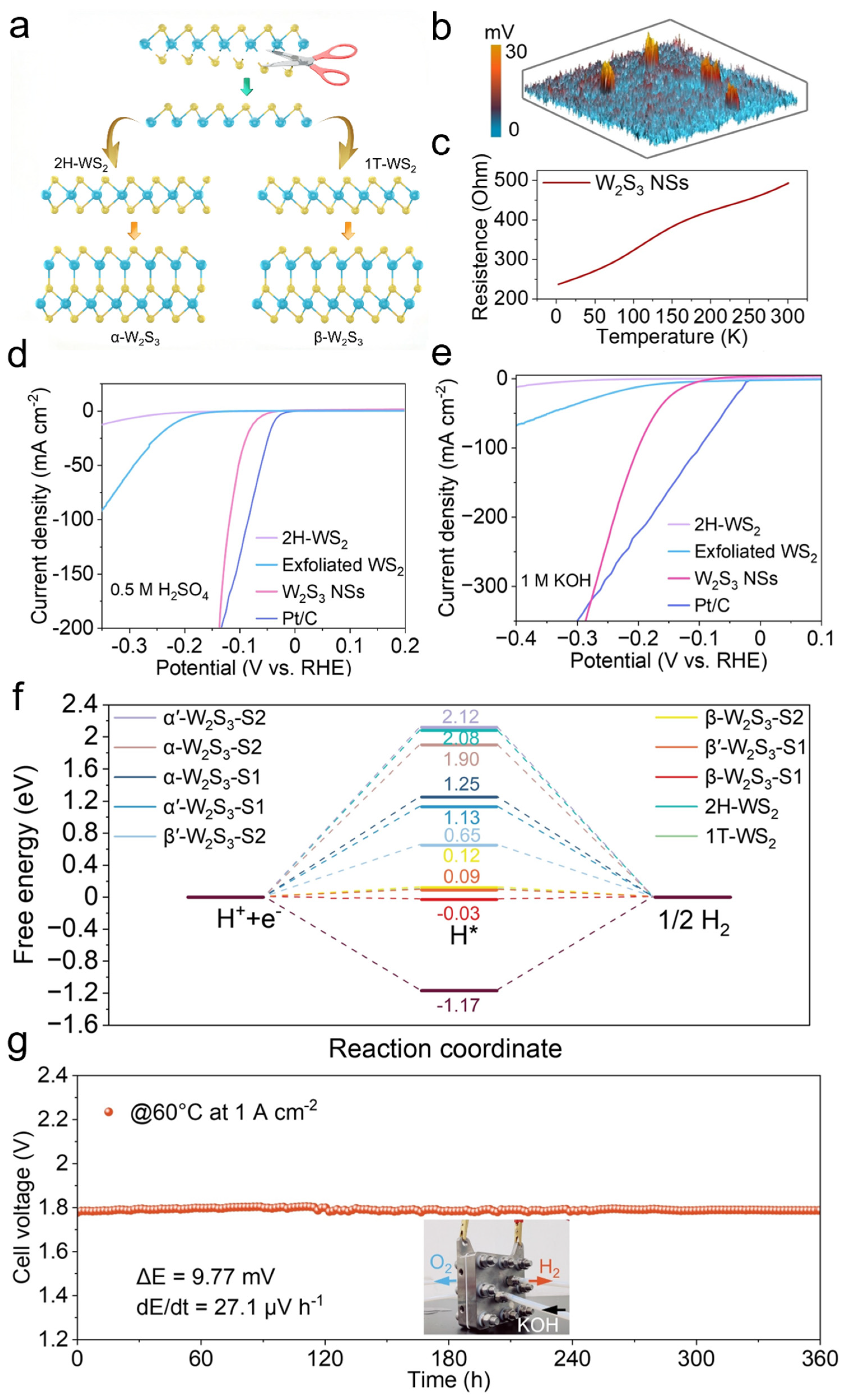
W2S3 has been extensively utilized as a catalyst in HER, significantly enhancing reaction efficiency. To synthesize W2S3, researchers initially obtained metastable 1T-phase WS2 (octahedral coordination) and stable 2H-phase WS2 (trigonal prismatic coordination) through shear exfoliation and rearrangement of WS2 layers [59,60,61,62,63,64]. Subsequently, monolayer WS fragments were covalently bonded with 2H- and 1T-WS2 layers to form α’-W2S3 and β’-W2S3 derivatives (Figure 3a). To fabricate W2S3 nanosheets (NSs), a high-metal-content growth strategy was employed, wherein the chemical ratio of tungsten to sulfur was precisely controlled to facilitate the crystallization of W2S3. The obtained W2S3 NSs were characterized by atomic force microscopy (AFM) and Kelvin probe force microscopy (KPFM) to evaluate surface potential variations. The potential mapping revealed highly distinct spherical domains with significant potential differences (Figure 3b). This surface potential disparity primarily arises from spontaneous polarization induced by the novel non-centrosymmetric structure of W2S3. Furthermore, temperature-dependent electrical measurements demonstrated that the resistance of W2S3 increased with rising temperature, indicating its metallic nature (Figure 3c). To evaluate the HER performance of W2S3 NSs in both acidic and alkaline media, a three-electrode system was utilized in 0.5 M H2SO4 and 1 M KOH solutions. The catalytic activity of W2S3 NSs was benchmarked against exfoliated WS2, 2H-WS2, and commercial Pt/C (20 wt%). In acidic solutions, W2S3 NSs exhibited significantly lower overpotentials and superior catalytic activity compared to WS2 and 2H-WS2, approaching the performance of commercial Pt/C (20 wt%) (Figure 3d). Remarkably, in alkaline solutions, W2S3 NSs outperformed commercial Pt/C (20 wt%) under high current densities (Figure 3e). To further elucidate the origin of the electrocatalytic activity of W2S3 NSs and investigate the HER catalytic mechanism, density functional theory (DFT) calculations were conducted. The Gibbs free energy of hydrogen adsorption (ΔGH*) serves as a crucial parameter for evaluating HER catalytic activity, where a ΔGH* value closer to zero indicates optimal adsorption–desorption equilibrium and enhanced HER performance. Among all examined sites, β-W2S3 exhibited the lowest ΔGH* values, suggesting its superior HER catalytic activity (Figure 3f). To assess the feasibility of large-scale HER applications, W2S3 NSs were employed as the cathode in a flow-type anion exchange membrane (AEM) electrolyzer, with commercial IrO2 as the anode. Under 30% KOH at 60 °C, the W2S3-based electrolyzer demonstrated excellent operational stability, maintaining a steady voltage at a current density of 1.0 A cm−2 for 360 h without significant degradation (Figure 3g). By leveraging a stoichiometrically controlled growth strategy coupled with theoretical structural predictions, W2S3 has been successfully developed as a highly efficient HER catalyst. Its outstanding performance in both acidic and alkaline conditions highlights its potential as a promising cathode material for large-scale hydrogen production.
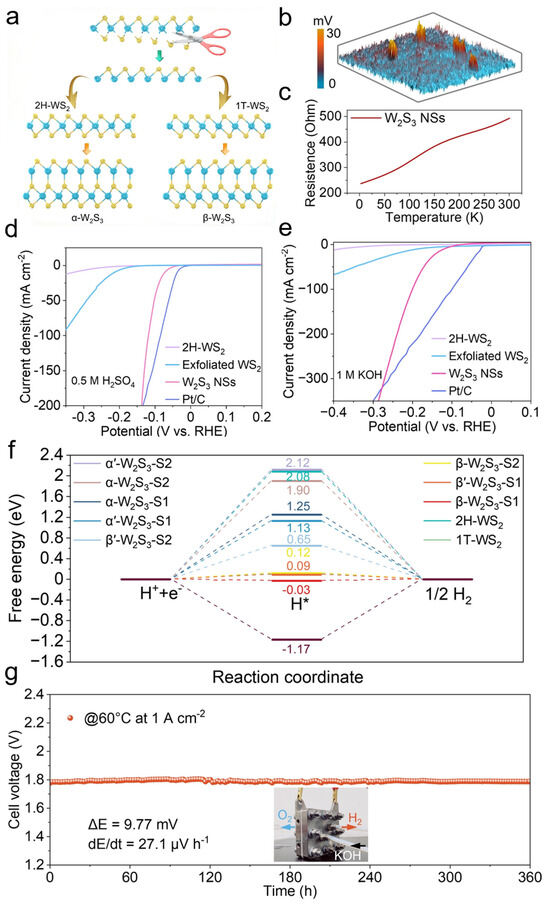
Figure 3.
W2S3 for efficient biphasic pH hydrogen evolution reaction. (a) Schematic illustration of the synthesis of W2S3. (b) Surface potential of W2S3 nanosheets measured by Kelvin probe force microscopy (KPFM). (c) Temperature-dependent resistance curve of W2S3 nanosheets. (d,e) HER performance in 0.5 M H2SO4 and 1 M KOH. (f) Gibbs free energy diagrams of W2S3 with different structures. (g) Durability test of the catalyst fabricated from W2S3 nanosheets in an anion exchange membrane (AEM) electrolyzer. (Inset: Photograph of the AEM electrolyzer setup). Reproduced with permission [59]. Copyright 2024, John Wiley and Sons Ltd.
2.4. Mo2S3 Based Composites
Through the research of the existing M2S3 in the field of catalysis, people have gradually turned their attention to the matrix composites composed of M2S3. Building upon the intrinsic properties of Mo2S3 nanostructures, recent studies have focused on the design and synthesis of Mo2S3-based composite materials to further enhance functional performance [65,66]. Among these, NiMo3S4 composites have attracted significant attention due to their synergistic effects, combining the advantageous characteristics of both Mo2S3 and nickel sulfides. This composite not only improves electrical conductivity and structural stability but also introduces additional active sites, thereby boosting catalytic and electrochemical activities.
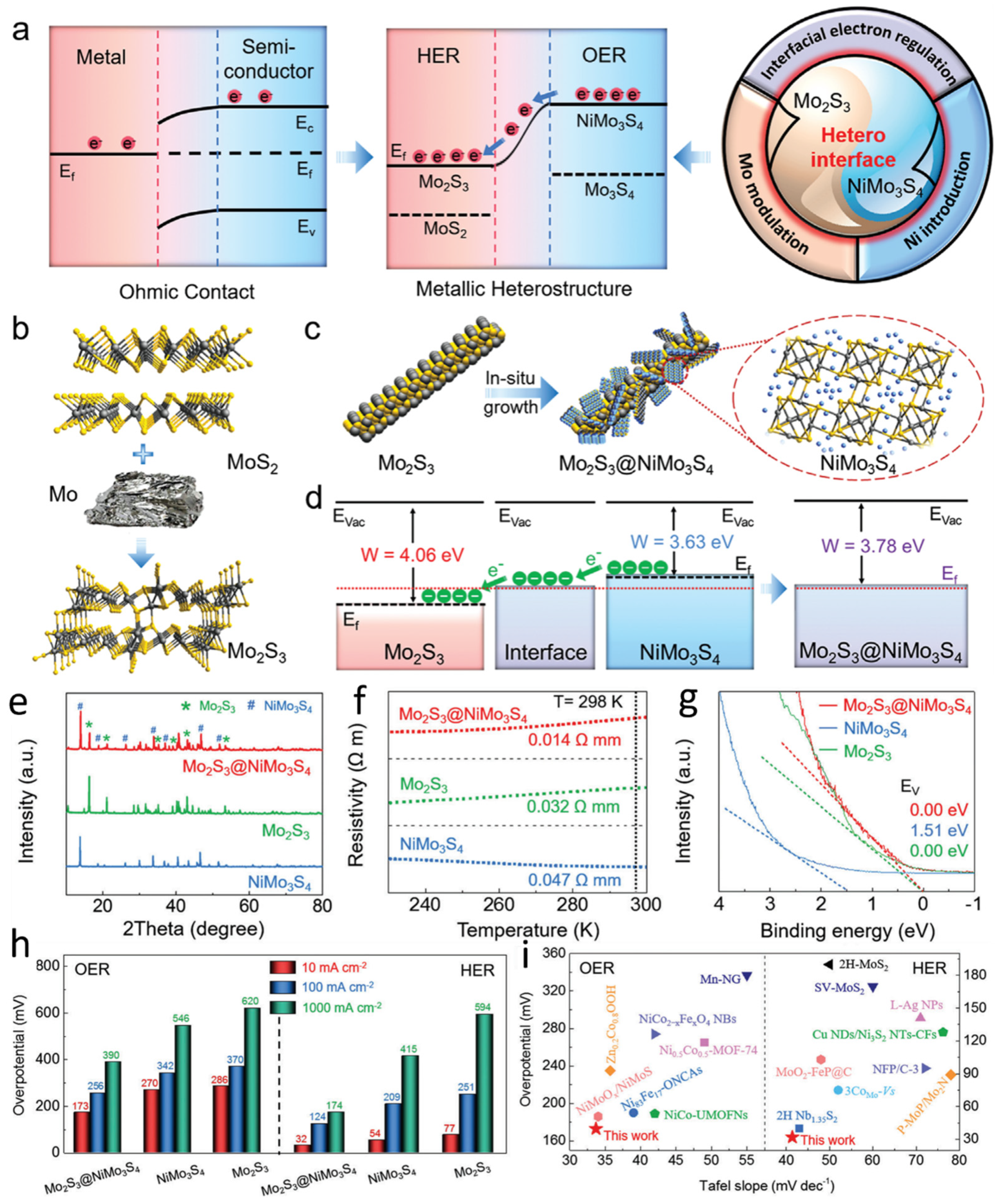
Electrochemical water splitting, encompassing the hydrogen evolution reaction and oxygen evolution reaction, represents a sustainable approach for continuous hydrogen production [67,68,69,70,71,72]. However, traditional serial fabrication methods face challenges in achieving durable and rapid semiconductor heterostructures for electrode preparation. To address this issue, Mo-modulated molybdenum disulfide (MoS2) phases can be introduced to construct metal sulfide supports, facilitating the epitaxial growth of metal heterostructures (Figure 4a). This strategy enables the formation of Mo2S3@NiMo3S4 heterostructures, where each component plays a complementary catalytic role. The Mo2S3 phase primarily enhances HER, whereas the NiMo3S4 phase mainly promotes OER. The incorporation of Ni, which possesses asymmetric 3d orbitals, can reconfigure with the vacant 4d orbitals of Mo in the sulfide framework, driving electron transfer from the OER site to the HER site and providing abundant active sites, thereby accelerating hydrogen generation. Mo2S3 is synthesized via the reaction of MoS2 with Mo, while Mo2S3@NiMo3S4 is fabricated through the in situ growth of NiMo3S4 nanosheets on Mo2S3 nanorods. To synthesize Mo2S3@NiMo3S4 nanorods, an excess of Mo was first introduced to transform the semiconductor MoS2 into metallic Mo2S3 (Figure 4b). Subsequently, NiMo3S4 nanosheets were grown in situ on the Mo2S3 nanorod surface via a hydrothermal process, forming the Mo2S3@NiMo3S4 metal heterostructure (Figure 4c). The high Fermi-level metallic Mo2S3 serves as an efficient charge transport medium at the interface. Moreover, compared to pure metallic Mo, Mo2S3 features shorter Mo–Mo bonds, which generate delocalized electrons and electronic states, thereby facilitating HER. The electronic band structures of Mo2S3, NiMo3S4, and Mo2S3@NiMo3S4 demonstrate that the formation of the heterointerface promotes favorable electron migration from NiMo3S4 to Mo2S3 (Figure 4d). The crystal structures of the synthesized samples were further characterized using X-ray diffraction (XRD) analysis. The characteristic peaks of the Mo2S3 phase confirm its successful formation, while the distinctive peaks of the NiMo3S4 phase validate the presence of NiMo3S4 (Figure 4e). These observations unequivocally confirm the successful synthesis of Mo2S3@NiMo3S4. Furthermore, the electrical conductivity of the samples was investigated via temperature-dependent resistivity measurements (Figure 4f). At 298 K, Mo2S3@NiMo3S4 exhibits significantly lower resistivity than Mo2S3 and NiMo3S4, indicating superior electrical conductivity. Additionally, the resistivity of both Mo2S3 and Mo2S3@NiMo3S4 decreases with decreasing temperature, confirming their metallic nature. Ultraviolet photoelectron spectroscopy (UPS) analysis was conducted to further validate the low-energy electronic features of Mo2S3, NiMo3S4, and Mo2S3@NiMo3S4, substantiating the metallic characteristics of Mo2S3 and Mo2S3@NiMo3S4 (Figure 4g). The catalytic performance of Mo2S3, NiMo3S4, and Mo2S3@NiMo3S4 was subsequently evaluated. The overpotentials for OER and HER at 10, 100, and 1000 mA·cm−2 were measured in a 1.0 M KOH solution saturated with N2 at room temperature, using Mo2S3, NiMo3S4, and commercial Pt/C as reference samples (Figure 4h). The synthesized Mo2S3@NiMo3S4 catalyst exhibits lower overpotentials for both OER and HER, outperforming commercial RuO2, Pt/C, and recently reported electrocatalysts (Figure 4i).
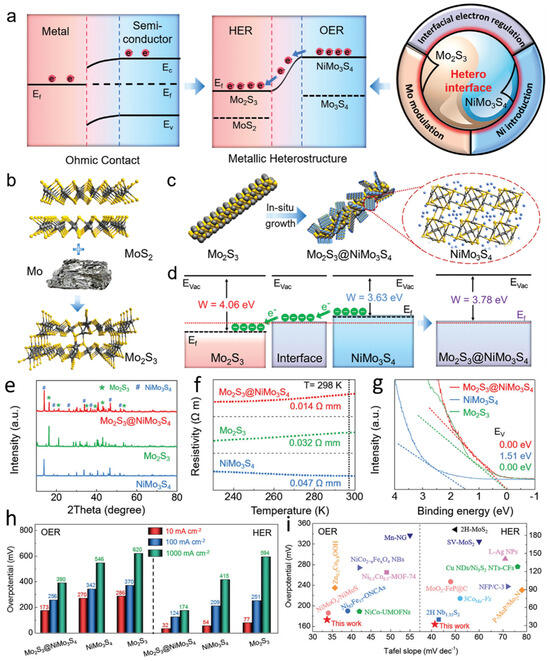
Figure 4.
Bimetallic regulation enables high-current-density, efficient overall water splitting (a) Evolution of water-splitting catalysts. (b) Schematic illustration of the transformation from MoS2 to metallic Mo2S3. (c) Schematic representation of the epitaxial construction of Mo2S3@NiMo3S4. (d) Schematic diagram of the energy band structures of Mo2S3 and NiMo3S4 (Evac = vacuum energy level, Ef = Fermi level, W = work function). (e) Temperature-dependent resistivity curves of Mo2S3, NiMo3S4, and Mo2S3@NiMo3S4. (f) Ultraviolet photoelectron spectroscopy (UPS) spectra in the low-energy region for Mo2S3, NiMo3S4, and Mo2S3@NiMo3S4. (g) UPS photoelectron spectra of Mo2S3, NiMo3S4, and Mo2S3@NiMo3S4. (h) Overpotentials of Mo2S3, NiMo3S4, and Mo2S3@NiMo3S4 for the OER and HER. (i) Kinetics and catalytic activity of OER and HER. Reproduced with permission [65]. Copyright 2023, American Chemical Society.
Mo2S3@NiMo3S4 demonstrates exceptional OER and HER electrocatalytic performance in alkaline media, primarily due to the high activity and electrical conductivity provided by interfacial electronic engineering within its metal heterostructure [73]. This study addresses the limitations of traditional catalysts in terms of conductivity and activity through interface electronic modulation and metallic heterostructure design, offering a novel strategy for developing highly efficient and stable bifunctional electrocatalysts.
3. M6S8 and Their Applications
While M2S3 and its composite materials have demonstrated promising properties in sensing, catalysis, and energy storage, M6S8 has also attracted widespread attention. In this context, Mo6S8, a representative member of the Chevrel-phase family, has emerged as a particularly intriguing candidate [74,75,76,77,78]. Its unique cluster-based structure enables efficient ion intercalation, excellent structural stability, and multi-electron redox activity, making it highly attractive for applications in advanced batteries and electrocatalysis. Recent advances in AI-driven characterization (e.g., convolutional neural network-based defect analysis) are expected to provide deeper insights into the structure-activity relationships of these Chevrel-phase materials, particularly in understanding their exceptional ion transport mechanisms at atomic scales. The following section discusses recent advances in the use of M6S8 for energy-related applications, highlighting its performance advantages and underlying mechanisms.
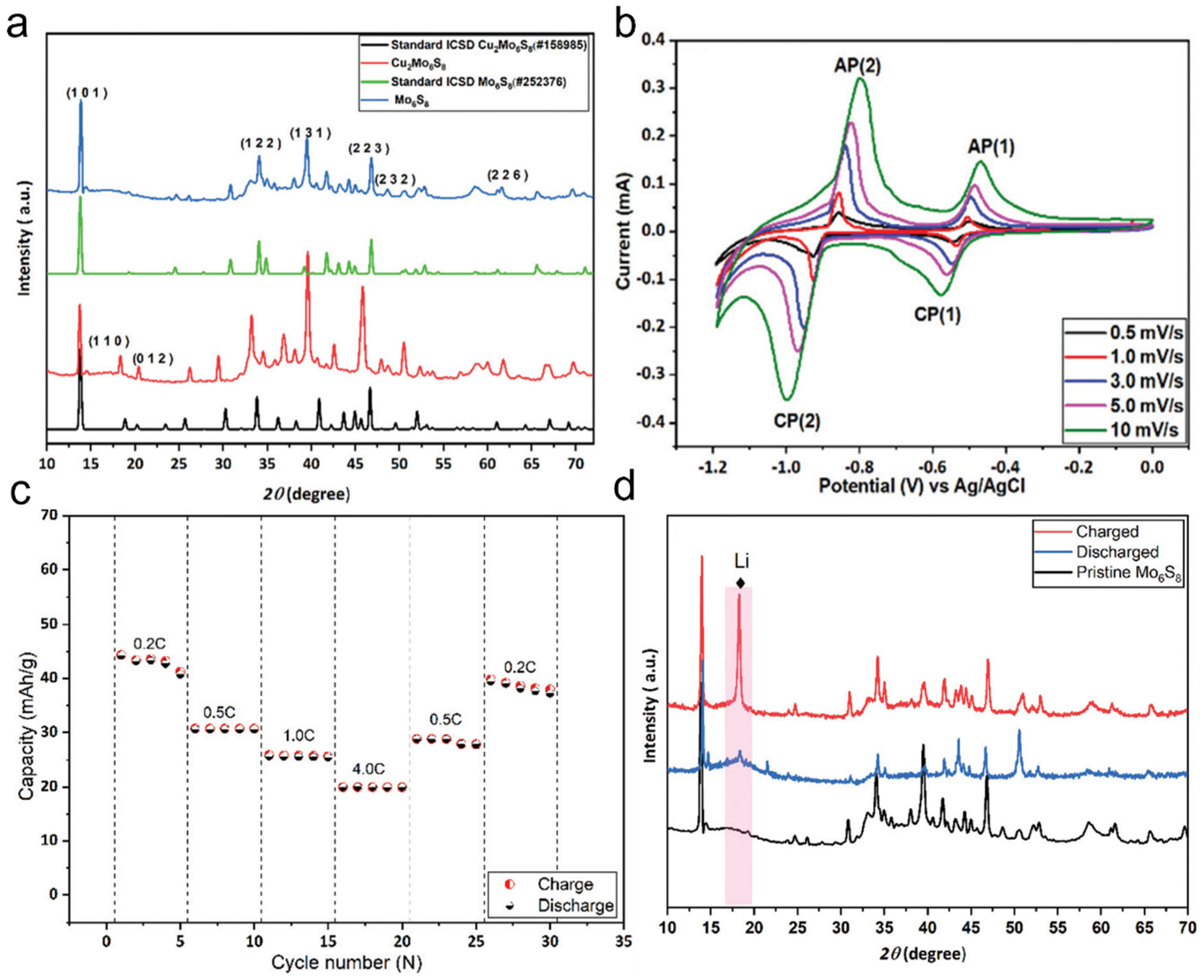
Chevrel-phase Mo6S8 has been widely studied as an anode material due to its unique crystal structure. The traditional preparation method involves first synthesizing Cu2Mo6S8 and then removing Cu to obtain Mo6S8. To obtain Cu2Mo6S8, CuS, MoS2, and elemental Mo were mixed in a stoichiometric ratio and heated at 1000 °C for 24 h, forming Cu2Mo6S8. The X-ray diffraction (XRD) patterns of Cu2Mo6S8 and Mo6S8 (Figure 5a) show that the diffraction peaks of Cu2Mo6S8 are highly consistent with the standard Cu2Mo6S8 pattern (ICSD#158985). Furthermore, the strong peak at 13.8° and the minimal peak intensity at 14.4°, corresponding to MoS2, indicate the high purity of the synthesized Cu2Mo6S8. The obtained Cu2Mo6S8 was then leached in 6 M HCl, yielding Chevrel-phase Mo6S8. When used as an anode material for lithium-ion batteries, Mo6S8 exhibits excellent electrochemical performance during the lithiation/delithiation process. Its cyclic voltammetry (CV) curves (Figure 5b) show a small peak separation between the anodic and cathodic reactions, suggesting fast lithium-ion insertion kinetics. The overpotential is significantly lower than that of conventional anode materials, such as VO2(B), V2O5, and LiTi2(PO4)3. This outstanding performance is primarily attributed to the nanoscale morphology of Mo6S8, which shortens the lithium-ion diffusion path and enhances kinetic performance. The rate capability and cycling stability of Mo6S8 were further evaluated by assembling Mo6S8-based coin cells with LiMn2O4 as the cathode and conducting charge–discharge tests at different rates (Figure 5c). The results indicate that the specific capacity decreases at higher charge–discharge rates, mainly due to the limited lithium-ion diffusion rate rather than electrode degradation. This finding suggests that Mo6S8 exhibits excellent structural stability. In the rate recovery test, when the charge–discharge rate was restored to its initial value, the battery capacity nearly returned to its first-cycle level, demonstrating outstanding high-rate stability. To gain deeper insight into the structural evolution of Mo6S8 during lithium-ion insertion/extraction, in situ XRD characterization was performed on cycled electrodes (Figure 5d). The XRD results reveal that during the charging process, Mo6S8 undergoes a phase transformation, forming LixMo6S8 (0 < x < 4). However, in the fully discharged state, the reappearance of a distinct diffraction peak at 18° indicates that the original structure of Mo6S8 is restored, confirming the reversibility of this phase transition. In summary, Chevrel-phase Mo6S8 exhibits exceptional lithium-ion insertion kinetics, superior rate performance, and high structural stability as an anode material for lithium-ion batteries. Its unique nanoscale morphology shortens the lithium-ion diffusion path, enhances kinetic response, and maintains excellent cycling stability even at high charge–discharge rates. Additionally, the reversible phase transition further ensures structural integrity during long-term cycling, making Mo6S8 a highly promising high-performance anode material.
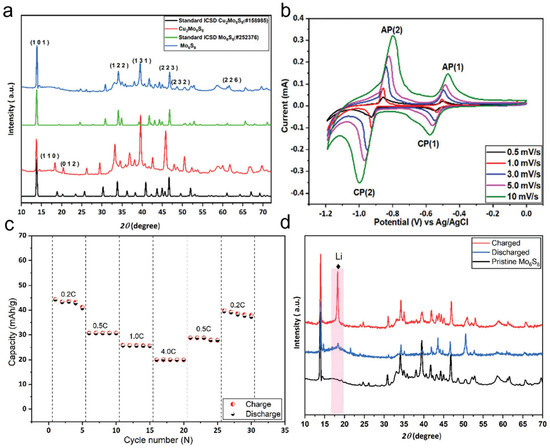
Figure 5.
Chevrel-phase Mo6S8 as an active electrode material for lithium-ion batteries (a) Powder X-ray diffraction (P-XRD) patterns of Cu2Mo6S8 and Mo6S8 along with their corresponding standard patterns. (b) Cyclic voltammetry (CV) curves of the Mo6S8 electrode at different scan rates. (c) Rate performance of the lithium-ion battery at various current densities. (d) P-XRD characterization of the Mo6S8 electrode. Reproduced with permission [75]. Copyright 2022, Royal Society of Chemistry.
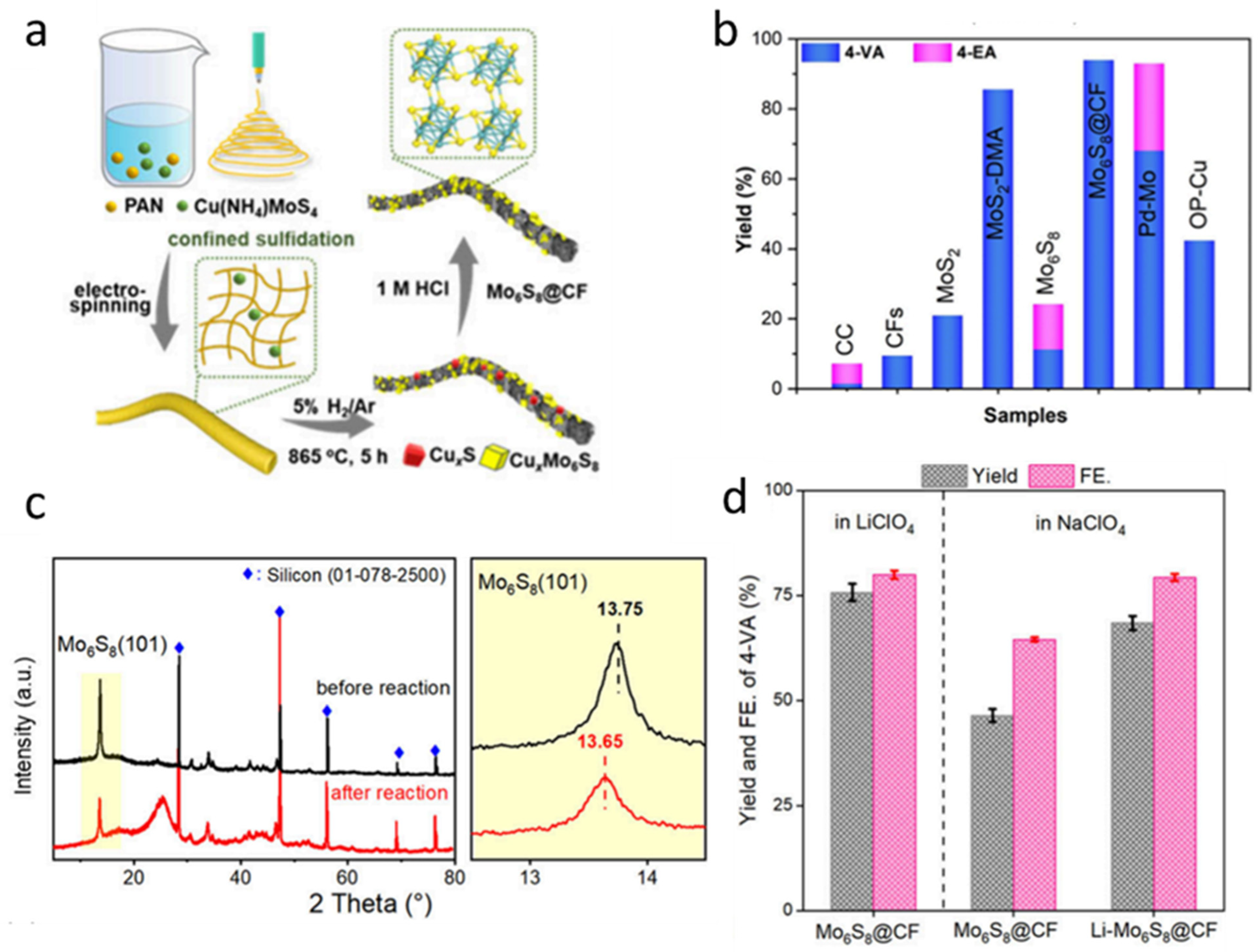
However, conventional synthesis methods often lead to severe aggregation and sintering of the active materials at high temperatures, which significantly reduces the catalytic efficiency [79,80]. Therefore, Mo6S8 nanoparticles wrapped in carbon fiber (Mo6S8@CF) were selected as an alternative to overcome these limitations. Mo6S8@CF was synthesized through a combination of electrospinning and high-temperature sulfurization. Initially, a homogeneous electrospinning precursor solution was prepared by dissolving polyacrylonitrile (PAN) and copper ammonium thiomolybdate (Cu (NH4) MoS4) (Figure 6a). The solution was then subjected to electrospinning under a high-voltage electric field to form nanofibers, effectively confining the Cu (NH4) MoS4 within the PAN matrix. Subsequently, the obtained composite fibers were annealed in a 5% H2/Ar atmosphere at 865 °C for 5 h, during which CuxS and CuxMo6S8 intermediates were formed. Finally, these intermediates were treated with 1.0 M hydrochloric acid (HCl) to selectively remove the copper-containing phases, yielding phase-pure Mo6S8@CF. To evaluate the catalytic performance of Mo6S8@CF for the electroreduction of nitroarenes, the production efficiencies of 4-vinylaniline (4-VA) and 4-ethylaniline (4-EA) at −0.45 V were compared under identical conditions (Figure 6b). Mo6S8@CF exhibits significantly higher catalytic efficiency than other tested catalysts. The Mo6S8 structure contains cavities within its nanofibers that are capable of hosting metal ions. To verify the possible intercalation of metal ions, X-ray diffraction (XRD) analysis was conducted on the samples after the electrochemical reduction process (Figure 6c). A distinct shift in the (101) diffraction peak of Mo6S8 was observed, indicating that Li+ ions were successfully inserted into the Mo6S8 lattice. This intercalation leads to a noticeable lattice expansion, as evidenced by the peak shift. The electrocatalytic reduction performance of Mo6S8@CF electrodes was compared in LiClO4 and NaClO4 electrolytes, as illustrated in Figure 6d. It is evident from the figure that the reaction rate in LiClO4 solution is significantly higher than that in NaClO4. Moreover, in NaClO4 solution, the catalytic rate of Li-Mo6S8@CF is also markedly superior to that of Mo6S8@CF. These results fully verify the possibility of using Mo6S8@CF instead of Mo6S8 as a high-performance lithium-ion battery cathode.
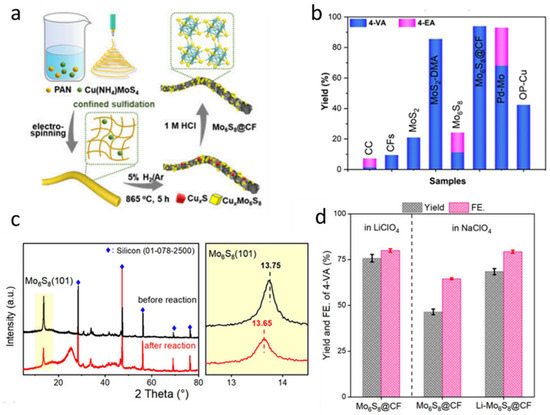
Figure 6.
Preparation and reaction performance of in situ Li+-intercalated nanosized Chevrel-phase Mo6S8 catalyst. (a) Schematic illustration of the preparation of Mo6S8@CF (b) comparison of product yields using different catalysts (c) XRD patterns of Mo6S8 before and after the electrochemical reaction (d) Electrocatalytic performance of Mo6S8@CF in 0.1 M LiClO4 and NaClO4 electrolytes after 3 h, and Li-Mo6S8@CF tested in 0.1 M NaClO4 with 10 mM 4-NS. Reproduced with permission [27]. Copyright 2025, American Chemical Society.
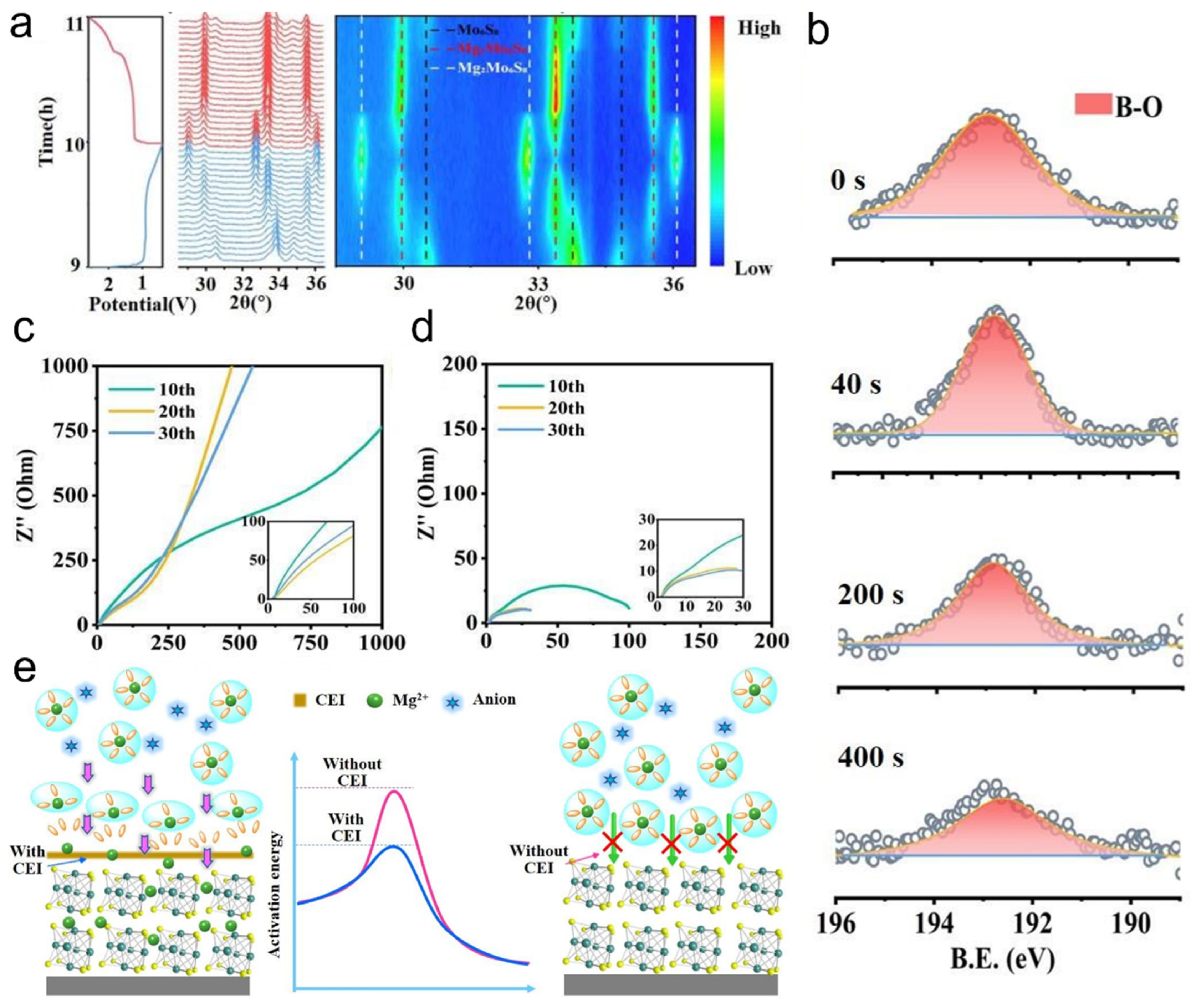
With the strong demand for increased energy density in batteries, Mg is widely used as a metal anode due to its high volumetric specific capacity, low cost, high natural abundance, and sustainability [81,82]. The use of a chlorine-containing adsorption layer on the surface of Mo6S8 on the cathode can reduce its activation energy, thereby effectively transferring Mg2+. However, chloride tends to corrode the metal on the anode surface, so the use of LiBxOy as the CEI type improves the stability of the cathode. The XRD pattern of the Mo6S8//Mg cell was observed by performing a charge and discharge reaction (Figure 7a). When discharged, MgMo6S8 is formed as an intermediate product, and finally Mg2Mo6S8 is generated. However, when charging, Mg2Mo6S8 is converted into MgMo6S8, but the Mg2+ in it cannot continue to obtain electrons to generate Mg, because at low Mg content, Mg2+ has a unique ring with low potential energy, so the diffusion in MgMo6S8 is slower, so there will be coexistence of MgMo6S8 and Mo6S8 at the cathode. X-ray photoelectron spectroscopy (XPS) was performed on the Mo6S8 surface at 2.0 eV (Figure 7b). A strong B-O peak was found at around 192.5 eV, corresponding to the MgBxOy species. At the same time, it was found that the peak of the signal continued to decrease over time. This phenomenon illustrates the formation of a homogeneous CEI on the surface of the cathode made of Mo6S8. The electrochemical resistance of Mo6S8//Mg cells after different cycles was tested at voltages of 2.0 V (Figure 7c) and 2.6 V (Figure 7d), respectively. It is found that the interfacial impedance is significantly lower than that of 2.0 V at 2.6 V. And as the number of cycles increases, the interface impedance gradually decreases. Therefore, at higher voltages, Mg2+ is easier to transfer. Based on the above experimental process, the surface CEI working mechanism of Mo6S8 was predicted. [B(hfip)4]− decompose first to form CEI and Mg2+ at high voltage, CEI reduces its activation energy and accelerates the movement of Mg2+, while Mg2+ on the surface of Mo6S8 without CEI is difficult to pass. Therefore, the storage performance of Mg2+ in the cathode can be improved (Figure 7e). Under high voltage, [B(hfip)4]− decomposes to form the CEI layer, which reduces the activation energy of Mg2+, accelerates its migration, and improves the magnesium storage performance of Mo6S8. This study demonstrates that interfacial chemistry optimization significantly enhances the magnesium storage performance of the Mo6S8 cathode, advancing the practical development of magnesium batteries.
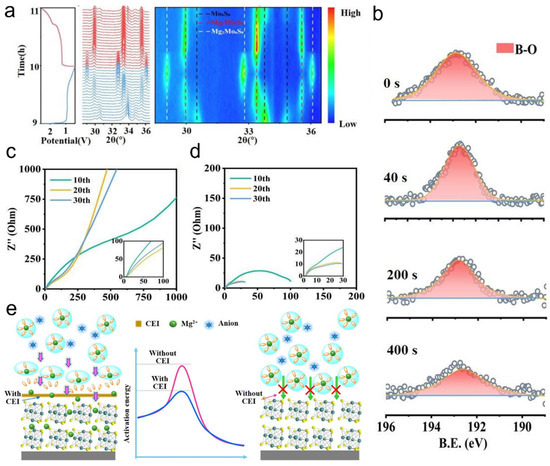
Figure 7.
Cathode electrolyte interface (CEI) facilitates fast Mg2+ transport in Mo6S8: (a) In situ XRD patterns of the Mo6S8//Mg battery at a cutoff voltage of 2.6 V during charging. (b) Depth-profiled B 1s XPS spectra of the Mo6S8 cathode after cycling at 2.6 V. (c,d) EIS of Mo6S8//Mg battery at 2.0 V and 2.6 V under different cutoff voltages. (e) Schematic illustration of the CEI-mediated interfacial mechanism on the Mo6S8 particle surface. Reproduced with permission [81]. Copyright 2023, John Wiley and Sons Ltd.
4. Other Non-Stoichiometric Metal Sulfides
Nanowires are typical 1D systems that hold potential for applications spanning from flexible nanodevices to ultrasmall functional interconnects. Among them, nanowires composed of transition metals and chalcogens, especially M6×6 nanowires (M = transition metal; X = chalcogen), have potential applications in 1D electron channels, spintronics, optoelectronics, and catalysis due to their authentic sub-nanometer width (<1 nm) and intrinsic metallicity [83]. However, there are still many challenges in the fabrication of M6×6 nanowires, especially single nanowires. Traditional methods, such as physical vapor deposition, can make the purification of individual nanowires difficult. At present, the latest research shows that the treatment of trimethyl molybdenum disulfide (TMDC) monolayer by electron beam irradiation technology is a clean and efficient preparation method [84,85,86].
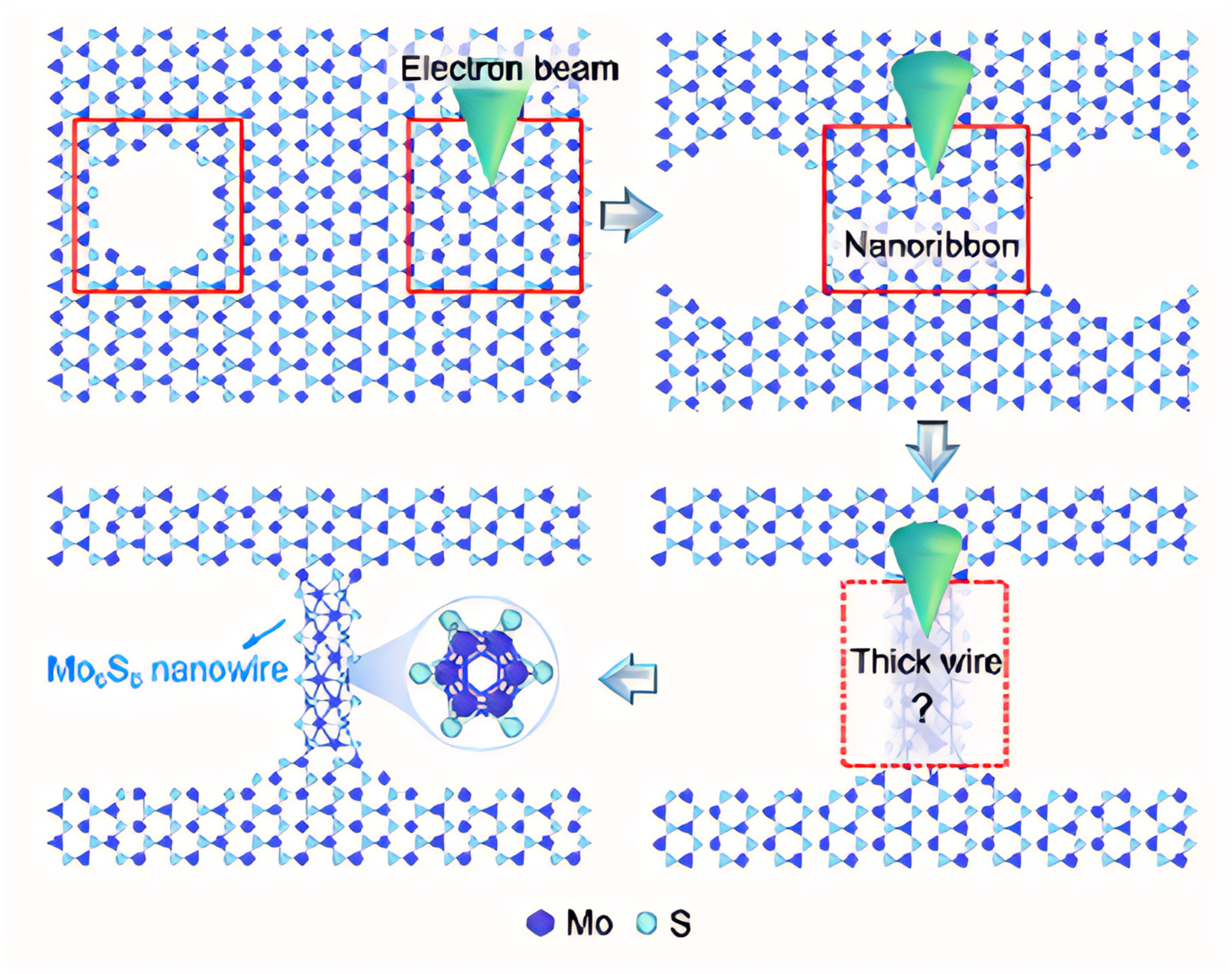
Firstly, MoS2 is a layered crystal, in which Mo atoms are sandwiched between two layers of S atoms, and when irradiated by a high-energy electron beam, they will preferentially excite and sputter S atoms with lower binding energy, resulting in the formation of S vacancies and defects in local areas. This initial etching disrupts the original lattice equilibrium. Then, due to the bond breakage and the uneven distribution of local energy in the defect region, the remaining Mo atoms and some S atoms began to diffuse and migrate, undergoing atomic rearrangement in the defect edge region. This atomic migration and self-organization effect prompts the atoms to be reconstructed in a new arrangement, transforming from a two-dimensional structure to a narrow nanoribbon. With the continuous action of the electron beam and the contribution of the local heat shock effect, the atoms inside the nanoribbon are further adjusted, and finally form a one-dimensional Mo6S6 nanowire with a new crystal structure, which is not only stable, but may also aggregate and fuse in the local region and evolve into a thicker wire-like structure (Figure 8).
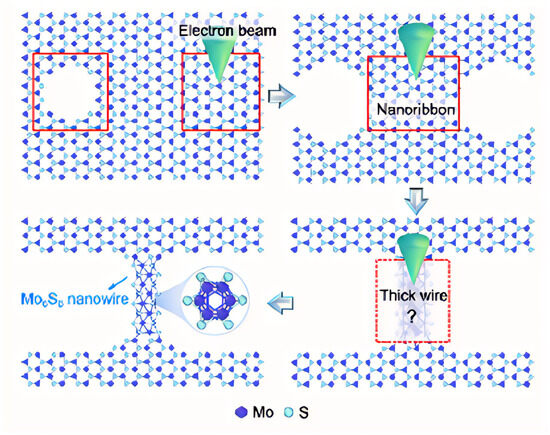
Figure 8.
The schematic diagram of a single Mo6S6 nanowire prepared from a monolayer 1H-phase MoS2 material via electron beam lithography. The red box indicates the region exposed to the electron beam. Reproduced with permission [28]. Copyright 2024, American Chemical Society.
This study reveals the unexplored mechanism of M6×6 single nanowire formation, which provides an important basis for further understanding the narrowing process of TMDC nanoribbons under electron irradiation [87]. Though not yet practically applied, it exhibits theoretically high catalytic potential in electrocatalytic hydrogen evolution due to its unique electronic states and surface-active site distribution.
5. Conclusions and Prospects
Scalable synthesis methods for non-stoichiometric transition metal sulfides have been explored to meet industrial demands in sensing, catalysis, and energy storage. These methods provide viable routes for translating laboratory-scale materials into industrial applications. Despite significant advances in their application for sensing, catalysis, and energy storage, several challenges and opportunities remain to be addressed to fully realize their potential. First, the precise correlation between crystal structure, defect engineering, and catalytic activity warrants deeper exploration. Tailoring the active sites via controlled sulfur vacancies, phase engineering, or heteroatom doping could further enhance HER performance while improving material stability under operational conditions. Studies by Denis Gentili et al. have demonstrated that defect engineering in transition metal dichalcogenides can achieve precise spatial control through advanced techniques such as electrochemical nanopatterning [88]. Second, these non-stoichiometric transition metal sulfides exhibit multifunctional characteristics. Owing to their exceptional electron transport properties and tunable surface-active sites, these materials simultaneously achieve the trifecta of high-precision sensing, efficient catalysis, and high-capacity energy storage. This unique combination opens new avenues for developing integrated or multifunctional energy devices. Future investigations should focus on elucidating the fundamental mechanisms governing this multifunctionality, thereby enabling the rational design of materials that seamlessly combine rapid charge-transfer kinetics with robust ion-intercalation capabilities. From an application perspective, scaling synthesis methods while maintaining precise structural control remains a critical hurdle. Additionally, integrating these materials into practical device architectures—such as flexible electrodes, hybrid electrolyzers, or tandem energy storage–conversion units—requires multidisciplinary approaches bridging materials science, engineering, and system design.
In summary, this work systematically reviews the synthesis strategies, structural diversity, and functional applications of NSTMS, highlighting their unique advantages over stoichiometric counterparts in terms of sensing, catalysis, and energy storage. The M2S3, M6S8, and M6S6 series deliver superior performance in flexible sensors, lithium-ion batteries, and electrocatalysis through defect engineering, intercalation, or doping of custom electronic structures. Advancements in synthesis techniques, such as liquid–liquid interface self-assembly, address key challenges in material preparation while opening up new opportunities for structural optimization. The article highlights the importance of several advanced characterization techniques, such as X-ray diffraction, scanning electron microscopy, and transmission electron microscopy, which play a crucial role in uncovering the crystal structure, surface morphology, and defect distribution of these materials. Furthermore, density functional theory calculations have become indispensable tools for predicting the electronic properties and catalytic behavior of these sulfides, providing valuable theoretical support and guidance. With these calculations, material design and optimization can be more precisely targeted to meet specific application needs. To harness the full potential of these materials, the article further explores their applications in sensors, ion batteries, and other energy storage devices, highlighting their advantages in improving device efficiency, extending lifespan, and enhancing stability. This review highlights the critical role of NSTMS in driving innovation in next-generation devices. Future research should focus on scalable synthesis, mechanistic studies of structure-property relationships, and integration into actual systems to maximize their potential. This work provides a basic perspective for the further exploration of NSTMS in functional materials science.
Author Contributions
Writing—original draft preparation, X.X.; writing—review and editing, M.Z.; supervision, Y.L. and L.W.; project administration, J.W. and Z.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
As the guest editor of “The development path of atomically dispersed catalysts”, I would like to express my sincere gratitude to all the authors for publishing their valuable research findings in this Special Issue. We also extend our thanks to all the reviewers and editorial staff for their contributions to the creation of this Special Issue.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Huang, Y.; Sun, Y.; Zheng, X.; Aoki, T.; Pattengale, B.; Huang, J.; He, X.; Bian, W.; Younan, S.; Williams, N. Atomically Engineering Activation Sites onto Metallic 1T-MoS2 Catalysts for Enhanced Electrochemical Hydrogen Evolution. Nat. Commun. 2019, 10, 982. [Google Scholar] [CrossRef] [PubMed]
- Fa, Y.; Piacentini, A.; Macco, B.; Kalisch, H.; Heuken, M.; Vescan, A.; Wang, Z.; Lemme, M.C. Contact Resistance Optimization in MoS2 Field-Effect Transistors through Reverse Sputtering-Induced Structural Modifications. ACS Appl. Mater. Interfaces 2025, 17, 24526–24534. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Yang, L.; Huang, J.; Chen, W.; Li, B.; Yang, S.; Yang, R.; Zeng, Z.; Tang, Z.; Gui, X. Controlling Sulfurization of 2D Mo2C Crystal for Mo2C/MoS2-Based Memristor and Artificial Synapse. npj Flex. Electron. 2022, 6, 93. [Google Scholar] [CrossRef]
- Rodrigues Pela, R.; Vona, C.; Lubeck, S.; Alex, B.; Gonzalez Oliva, I.; Draxl, C. Critical Assessment of G0W0 Calculations for 2D Materials: The Example of Monolayer MoS2. npj Comput. Mater. 2024, 10, 77. [Google Scholar] [CrossRef]
- Chen, X.; Park, Y.J.; Kang, M.; Kang, S.-K.; Koo, J.; Shinde, S.M.; Shin, J.; Jeon, S.; Park, G.; Yan, Y. CVD-Grown Monolayer MoS2 in Bioabsorbable Electronics and Biosensors. Nat. Commun. 2018, 9, 1690. [Google Scholar] [CrossRef]
- Chen, X.; Shinde, S.M.; Dhakal, K.P.; Lee, S.W.; Kim, H.; Lee, Z.; Ahn, J.-H. Degradation Behaviors and Mechanisms of MoS2 Crystals Relevant to Bioabsorbable Electronics. NPG Asia Mater. 2018, 10, 810–820. [Google Scholar] [CrossRef]
- Liu, J.; Goswami, A.; Jiang, K.; Khan, F.; Kim, S.; McGee, R.; Li, Z.; Hu, Z.; Lee, J.; Thundat, T. Direct-Current Triboelectricity Generation by a Sliding Schottky Nanocontact on MoS2 Multilayers. Nat. Nanotechnol. 2018, 13, 112–116. [Google Scholar] [CrossRef]
- Lin, M.; Trubyanov, M.; Lee, H.W.; Ivanov, A.S.; Zhou, X.; Zhang, P.; Zhang, Y.; Wang, Q.; Tan, G.S.X.; Novoselov, K.S. Enhanced CO2 Hydrogenation to Methanol Using out-of-Plane Grown MoS2 Flakes on Amorphous Carbon Scaffold. Small 2025, 21, 2408592. [Google Scholar] [CrossRef]
- Onga, M.; Zhang, Y.; Ideue, T.; Iwasa, Y. Exciton Hall Effect in Monolayer MoS2. Nat. Mater. 2017, 16, 1193–1197. [Google Scholar] [CrossRef]
- Fu, X.; Li, T.; Cai, B.; Miao, J.; Panin, G.N.; Ma, X.; Wang, J.; Jiang, X.; Li, Q.; Dong, Y. Graphene/MoS2−xOx/Graphene Photomemristor with Tunable Non-Volatile Responsivities for Neuromorphic Vision Processing. Light Sci. Appl. 2023, 12, 39. [Google Scholar] [CrossRef]
- Moradi, K.; Ashrafi, M.; Salimi, A.; Melander, M.M. Hierarchical MoS2@ NiFeCo-Mo (Doped)-Layered Double Hydroxide Heterostructures as Efficient Alkaline Water Splitting (Photo) Electro-catalysts. Small 2025, 21, 2409097. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Kim, K.-H.; Keneipp, R.; Jung, M.; Trainor, N.; Chen, C.; Zheng, J.; Redwing, J.M.; Kang, J.; Drndić, M. High Current and Carrier Densities in 2D MoS2/AlScN Field-Effect Transistors via Ferroelectric Gating and Ohmic Contacts. ACS Nano 2025, 19, 8985–8996. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.; Choi, M.K.; Liu, S.; Kim, M.; Park, O.K.; Im, C.; Kim, J.; Qin, X.; Lee, G.J.; Cho, K.W. Human Eye-Inspired Soft Optoelectronic Device Using High-Density MoS2-Graphene Curved Image Sensor Array. Nat. Commun. 2017, 8, 1664. [Google Scholar] [CrossRef] [PubMed]
- Bui, M.; Rost, S.; Auge, M.; Tu, J.; Zhou, L.; Aguilera, I.; Blügel, S.; Ghorbani-Asl, M.; Krasheninnikov, A.; Hashemi, A. Low-Energy Se Ion Implantation in MoS2 Monolayers. npj 2D Mater. Appl. 2022, 6, 42. [Google Scholar]
- Zhao, L.; Wang, X.; Zhang, Z.; Ji, Y.; Guo, J.; Du, Z.; Cheng, G. Realizing the Ultrafast Recovery of the Monolayer MoS2-Based NH3 Sensor by Gas-Ion-Gate. ACS Appl. Mater. Interfaces 2025, 17, 17465–17475. [Google Scholar] [CrossRef]
- Torres-Cavanillas, R.; Morant-Giner, M.; Escorcia-Ariza, G.; Dugay, J.; Canet-Ferrer, J.; Tatay, S.; Cardona-Serra, S.; Giménez-Marqués, M.; Galbiati, M.; Forment-Aliaga, A. Spin-Crossover Nanoparticles Anchored on MoS2 Layers for Heterostructures with Tunable Strain Driven by Thermal or Light-Induced Spin Switching. Nat. Chem. 2021, 13, 1101–1109. [Google Scholar] [CrossRef]
- Mondal, S.; Dilly Rajan, K.; Patra, L.; Rathinam, M.; Ganesh, V. Sulfur Vacancy-Induced Enhancement of Piezocatalytic H2 Production in MoS2. Small 2025, 21, 2411828. [Google Scholar] [CrossRef]
- Wu, X.; Yu, X.; Tian, Z.; Li, H.; Xu, J. Thermodynamically Stable Synthesis of the 1T-MoS2/g-CN Superstructure with Rapid Redox Kinetics for Robust Capacitive Energy Storage. ACS Nano 2025, 19, 9292–9303. [Google Scholar] [CrossRef]
- Chhowalla, M.; Amaratunga, G.A. Thin Films of Fullerene-like MoS2 Nanoparticles with Ultra-Low Friction and Wear. Nature 2000, 407, 164–167. [Google Scholar] [CrossRef]
- Das, S.; Wang, Y.; Dai, Y.; Li, S.; Sun, Z. Ultrafast Transient Sub-Bandgap Absorption of Monolayer MoS2. Light Sci. Appl. 2021, 10, 27. [Google Scholar] [CrossRef]
- Verma, A.K.; Rahman, M.A.; Vashishtha, P.; Guo, X.; Sehrawat, M.; Mitra, R.; Giridhar, S.P.; Waqar, M.; Bhoriya, A.; Murdoch, B.J. Oxygen-Passivated Sulfur Vacancies in Monolayer MoS2 for Enhanced Piezoelectricity. ACS Nano 2025, 19, 3478–3489. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Liao, Z.; Duan, Y.; Fu, X.; Huang, Y.; Li, G. Graphene Oxide Wrapped-Silica Microspheres Humidity Sensor with Fast Response/Recovery, High Sensitivity, and Selectivity for Pig Respiratory Rate Monitoring. Sci. China Technol. Sci. 2025, 68, 1220203. [Google Scholar] [CrossRef]
- Zang, W.; Li, P.; Fu, Y.; Xing, L.; Xue, X. Hydrothermal Synthesis of Co–ZnO Nanowire Array and Its Application as Piezo-Driven Self-Powered Humidity Sensor with High Sensitivity and Repeatability. RSC Adv. 2015, 5, 84343–84349. [Google Scholar] [CrossRef]
- Cheng, Y.; Fu, P.; Yu, Z.; Yang, X.; Zhang, Y.; Yuan, A.; Liu, H.; Du, J.; Chen, L. Modulation of the Multiphase Phosphorus/Sulfide Heterogeneous Interface via Rare Earth for Solar-enhanced Water Splitting at Industrial-level Current Densities. Carbon Neutralization 2024, 3, 873–887. [Google Scholar] [CrossRef]
- Majumder, S.; Sett, A.; Goswami, D.K.; Bhattacharyya, T.K. Pseudo Electron Injection in Amine-Modified MoS2-Based Sensor for Humidity Monitoring. IEEE Trans. Electron Devices 2021, 68, 5173–5178. [Google Scholar] [CrossRef]
- Zhao, C.; Fang, Y.; Chen, H.; Zhang, S.; Wan, Y.; Riaz, M.S.; Zhang, Z.; Dong, W.; Diao, L.; Ren, D.; et al. Ultrathin Mo2S3 Nanowire Network for High-Sensitivity Breathable Piezoresistive Electronic Skins. ACS Nano 2023, 17, 4862–4870. [Google Scholar] [CrossRef]
- Tan, J.; Feng, L.; Shao, J.; Zhang, W.; Qin, H.; Liu, H.; Shu, Y.; Yang, L.; Meng, Y.; Tang, Y. In Situ Li+ Intercalation into Nanosized Chevrel Phase Mo6S8 toward Efficient Electrochemical Nitroarene Reduction. J. Am. Chem. Soc. 2025, 147, 10118–10128. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, T.; Li, D. Why Does a Transition Metal Dichalcogenide Nanoribbon Narrow into a Nanowire under Electron Irradiation? J. Am. Chem. Soc. 2024, 146, 33874–33882. [Google Scholar] [CrossRef]
- Lin, Z.; Carvalho, B.R.; Kahn, E.; Lv, R.; Rao, R.; Terrones, H.; Pimenta, M.A.; Terrones, M. Defect Engineering of Two-Dimensional Transition Metal Dichalcogenides. 2D Mater. 2016, 3, 022002. [Google Scholar] [CrossRef]
- Qin, N.; Dai, F.; Xue, Y.; Gao, D.; Liu, Y.; Zhang, Y.; Chen, J.; Yang, Q. Acanthosphere-like Bimetallic Sulfide Cu9S5/Mo2S3/NF as Bifunctional Catalyst for Water Splitting. J. Electroanal. Chem. 2024, 964, 118338. [Google Scholar] [CrossRef]
- Pan, Q.; Liu, Y.; Zhao, L. Co9S8/Mo2S3 Nanorods on CoS2 Laminar Arrays as Advanced Electrode with Superior Rate Properties and Long Cycle Life for Asymmetric Supercapacitors. Chem. Eng. J. 2018, 351, 603–612. [Google Scholar] [CrossRef]
- Huang, X.; Sha, W.; He, S.; Zhao, L.; Li, S.; Lv, C.; Lou, C.; Xu, X.; Wang, J.; Pan, H. Defect-Rich Mo2S3 Loaded Wood-Derived Carbon Acts as a Spacer in Lithium–Sulfur Batteries: Forming a Polysulfide Capture Net and Promoting Fast Lithium Flux. Nanoscale 2023, 15, 7870–7876. [Google Scholar] [CrossRef]
- Schutte, W.; Disselborg, F.; De Boer, J. Determination of the Two-Dimensional Incommensurately Modulated Structure of Mo2S3. Struct. Sci. 1993, 49, 787–794. [Google Scholar] [CrossRef]
- Yang, B.; Jin, F.; Pan, X.; Jin, X.; Jin, Z. Directional Electron Transfer in CuInS2/Mo2S3 S-Scheme Heterojunctions for Efficient Photocatalytic Hydrogen Production. ACS Appl. Mater. Interfaces 2024, 16, 36333–36342. [Google Scholar] [CrossRef]
- Gemming, S.; Seifert, G.; Vilfan, I. Li Doped Mo6S6 Nanowires: Elastic and Electronic Properties. Phys. Status Solidi B 2006, 243, 3320–3324. [Google Scholar] [CrossRef]
- Xu, K.; Lin, Y.; Shi, Q.; Fu, Y.; Yang, Y.; Zhang, Z.; Wu, J. Mechanical Enhancement and Weakening in Mo6S6 Nanowire by Twisting. Chin. Phys. B 2023, 32, 046204. [Google Scholar] [CrossRef]
- Ren, Z.; Sun, Y.; Lei, Q.; Zhang, W.; Zhao, Y.; Yao, Z.; Si, J.; Li, Z.; Ren, X.; Sun, X. Accumulative Delocalized Mo 4d Electrons to Bound the Volume Expansion and Accelerate Kinetics in Mo6S8 Cathode for High-Performance Aqueous Cu2+ Storage. ACS Nano 2023, 17, 19144–19154. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xu, Y.; Zuo, Q.; Shi, J.; Wu, X.; Liu, L.; Sheng, J.; Jiang, P.; Ben-Gal, A. Evaluating and Improving Soil Water and Salinity Stress Response Functions for Root Water Uptake. Agric. Water Manag. 2023, 287, 108451. [Google Scholar] [CrossRef]
- Sun, D.; Lu, W.; Le, D.; Ma, Q.; Aminpour, M.; Alcántara Ortigoza, M.; Bobek, S.; Mann, J.; Wyrick, J.; Rahman, T.S. An MoSx Structure with High Affinity for Adsorbate Interaction. Angew. Chem.-Ger. Ed. 2012, 124, 10430. [Google Scholar] [CrossRef]
- Niu, S.; Zheng, J. Mo2S3@ Ni3S2 Nanowries on Nickel Foam as a Highly-Stable Supercapacitor Material. J. Alloys Compd. 2018, 737, 809–814. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, W.; Pan, J.; Fang, Y.; Wang, F.; Huang, F. Urchin-like Mo2S3 Prepared via a Molten Salt Assisted Method for Efficient Hydrogen Evolution. Chem. Commun. 2018, 54, 12714–12717. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jankowski, P.; Njel, C.; Bauer, W.; Li, Z.; Meng, Z.; Dasari, B.; Vegge, T.; Lastra, J.M.G.; Zhao-Karger, Z. Dual Role of Mo6S8 in Polysulfide Conversion and Shuttle for Mg–S Batteries. Adv. Sci. 2022, 9, 2104605. [Google Scholar] [CrossRef]
- Paskach, T.J.; Hilsenbeck, S.J.; Thompson, R.K.; McCarley, R.E.; Schrader, G.L. Synthesis and Characterization of a Novel Platinum Molybdenum Sulfide Containing the Mo6S8 Cluster. J. Alloys Compd. 2000, 311, 169–180. [Google Scholar] [CrossRef]
- Xu, K.; Deng, S.; Liang, T.; Cao, X.; Han, M.; Zeng, X.; Zhang, Z.; Yang, N.; Wu, J. Efficient Mechanical Modulation of the Phonon Thermal Conductivity of Mo6S6 Nanowires. Nanoscale 2022, 14, 3078–3086. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhu, F.; Cheng, H.; Komarneni, S.; Ma, J. In-Situ Growth of Ni3S2@ Mo2S3 Catalyst on Mo-Ni Foam for Degradation of p-Nitrophenol with a Good Synergetic Effect by Using Ozone. J. Environ. Chem. Eng. 2023, 11, 111477. [Google Scholar] [CrossRef]
- Panchu, S.; Dhani, S.; Chuturgoon, A.; Swart, H.; Moodley, M. Neodymium YAG Laser Chemical Vapor Deposition Growth of Luminescent Mo2S3 Nanocrystals Using Bulk MoS2 and Its Structural, Optical Properties and Caspase-Mediated Apoptosis in THP-1 Monocytic Cells. Mater. Today Chem. 2020, 17, 100315. [Google Scholar] [CrossRef]
- Lv, Z.; Zhao, C.; Xie, M.; Cai, M.; Peng, B.; Ren, D.; Fang, Y.; Dong, W.; Zhao, W.; Lin, T. 1D Insertion Chains Induced Small-Polaron Collapse in MoS2 2D Layers Toward Fast-Charging Sodium-Ion Batteries. Adv. Mater. 2024, 36, 2309637. [Google Scholar] [CrossRef]
- Nishanthi, S.; Yadav, K.K.; Baruah, A.; Ganguli, A.K.; Jha, M. New Sustainable and Environmental Friendly Process of Synthesis of Highly Porous Mo2S3 Nanoflowers in Cooking Oil and Their Electrochemical Properties. Electrochim. Acta 2019, 300, 177–185. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, Y.; Zhang, G.; Li, R.; Sun, X. Site-Controlled Synthesis and Mechanism of Three-Dimensional Mo2S3 Flowers. Appl. Surf. Sci. 2012, 263, 410–415. [Google Scholar] [CrossRef]
- Guo, Z.; Yu, F.; Chen, Z.; Shi, Z.; Wang, J.; Wang, X. Stabilized Mo2S3 by FeS2 Based Porous Solar Evaporation Systems for Highly Efficient Clean Freshwater Collection. Sol. Energy Mater. Sol. Cells 2020, 211, 110531. [Google Scholar] [CrossRef]
- Chen, C.; Li, S.; Liu, J.; Yu, H.; Sun, S.; Xu, Y.; Li, H. Hierarchical NiCo-LDHs/Metal Sulfides (V3S4, CuS, Mo2S3) Heterostructures with Improved Electrochemical Properties for Asymmetric Supercapacitors: A Comparative Study. J. Alloys Compd. 2024, 981, 173682. [Google Scholar] [CrossRef]
- Kozlova, M.N.; Enyashin, A.N.; Grayfer, E.D.; Kuznetsov, V.A.; Plyusnin, P.E.; Nebogatikova, N.A.; Zaikovskii, V.I.; Fedorov, V.E. A DFT Study and Experimental Evidence of the Sonication-Induced Cleavage of Molybdenum Sulfide Mo2S3 in Liquids. J. Mater. Chem. C 2017, 5, 6601–6610. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Yang, J.; Saeys, M.; Joachim, C. Surface Reconstruction of MoS2 to Mo2S3. Surf. Sci. 2008, 602, 2628–2633. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Zha, X.; Luo, Y.; Hu, Y.; Chen, G.; He, X. Interfacial Chemical Bond and Oxygen Vacancies Modulated Mo2S3/BiOBr High-Low Junctions for Enhanced Photocatalysis Gatifloxacin Degradation. Appl. Surf. Sci. 2023, 641, 158548. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, M.; Du, X.; Zhang, X. Synthesis of M-NiS/Mo2S3 (M=Co, Fe, Ce and Bi) Nanoarrays as Efficient Electrocatalytic Hydrogen Evolution Reaction Catalyst in Fresh and Seawater. Int. J. Hydrogen Energy 2024, 62, 532–540. [Google Scholar] [CrossRef]
- Wu, S.; Yang, X.; Cui, T.; Feng, Q.; Zhou, S.; Xu, X.; Zhao, H.; Wu, L.; He, Y.; Yang, Q. Tubular-like NiS/Mo2S3 Microspheres as Electrode Material for High-Energy and Long-Life Asymmetric Supercapacitors. Colloids Surf. Physicochem. Eng. Asp. 2021, 628, 127332. [Google Scholar] [CrossRef]
- Xia, H.; Qin, H.; Zhang, Y.; Yin, H.; Li, Q.; Pan, F.; Xia, D.; Li, D.; Xu, H. Modulate 1O2 by Passivate Oxygen Vacancy to Boosting the Photocatalytic Performance of Z-Scheme Mo2S3/BiOCl Heterostructure. Sep. Purif. Technol. 2021, 266, 118547. [Google Scholar] [CrossRef]
- Canadell, E.; LeBeuze, A.; El Khalifa, M.A.; Chevrel, R.; Whangbo, M.H. Origin of Metal Clustering in Transition-Metal Chalcogenide Layers MX2 (M = Nb, Ta, Mo, Re; X = S, Se). J. Am. Chem. Soc. 1989, 111, 3778–3782. [Google Scholar] [CrossRef]
- Xie, L.; Wang, L.; Liu, X.; Zhao, W.; Liu, S.; Huang, X.; Zhao, Q. Tetra-Coordinated W2S3 for Efficient Dual-pH Hydrogen Production. Angew. Chem. Int. Ed. 2024, 63, e202316306. [Google Scholar] [CrossRef]
- Occelli, M.; Rennard, R. Hydrotreating Catalysts Containing Pillared Clays. Catal. Today 1988, 2, 309–319. [Google Scholar] [CrossRef]
- Schellenberger, A.; Jaegermann, W.; Pettenkofer, C.; Kamaratos, M.; Papageorgopoulos, C. Li Insertion into 2H—WS2: Electronic Structure and Reactivity of the UHV In-situ Prepared Interface. Berichte Bunsenges. Für Phys. Chem. 1994, 98, 833–841. [Google Scholar] [CrossRef]
- Mendoza, I.; Camardo, J.; Moleiro, F.; Castellanos, A.; Medina, V.; Gomez, J.; Acquatella, H.; Casal, H.; Tortoledo, F.; Puigbo, J. Sustained Ventricular Tachycardia in Chronic Chagasic Myocarditis: Electrophysiologic and Pharmacologic Characteristics. Am. J. Cardiol. 1986, 57, 423–427. [Google Scholar] [CrossRef]
- Gong, Y.; Duan, R.; Hu, Y.; Wu, Y.; Zhu, S.; Wang, X.; Wang, Q.; Lau, S.P.; Liu, Z.; Tay, B.K. Reconfigurable and Nonvolatile Ferroelectric Bulk Photovoltaics Based on 3R-WS2 for Machine Vision. Nat. Commun. 2025, 16, 230. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, X.; Li, W.; Guo, Q.; Feng, Z.; Huang, C.; Ren, Y.; Cai, Y.; Zhou, X.; Wang, J. Remote Epitaxy of Single-Crystal Rhombohedral WS2 Bilayers. Nat. Commun. 2024, 15, 4130. [Google Scholar] [CrossRef]
- Wu, T.; Xu, S.; Zhang, Z.; Luo, M.; Wang, R.; Tang, Y.; Wang, J.; Huang, F. Bimetal Modulation Stabilizing a Metallic Heterostructure for Efficient Overall Water Splitting at Large Current Density. Adv. Sci. 2022, 9, 2202750. [Google Scholar] [CrossRef]
- Jiang, J.; Gao, M.; Sheng, W.; Yan, Y. Hollow Chevrel-phase NiMo3S4 for Hydrogen Evolution in Alkaline Electrolytes. Angew. Chem. 2016, 128, 15466–15471. [Google Scholar] [CrossRef]
- Guillevic, J.; Bars, O.; Grandjean, D. Structural Study of Molybdenum Sulfides and Selenides. 3: Crystal Structure of Nickel Molybdenum Sulfide (NiMo3S4). J. Solid State Chem. Fr. 1976, 7, 158–162. [Google Scholar] [CrossRef]
- Kong, D.; Wang, Y.; Von Lim, Y.; Huang, S.; Zhang, J.; Liu, B.; Chen, T.; Yang, H.Y. 3D Hierarchical Defect-Rich NiMo3S4 Nanosheet Arrays Grown on Carbon Textiles for High-Performance Sodium-Ion Batteries and Hydrogen Evolution Reaction. Nano Energy 2018, 49, 460–470. [Google Scholar] [CrossRef]
- Kong, D.; Wang, Y.; Huang, S.; Von Lim, Y.; Wang, M.; Xu, T.; Zang, J.; Li, X.; Yang, H.Y. Defect-Engineered 3D Hierarchical NiMo3S4 Nanoflowers as Bifunctional Electrocatalyst for Overall Water Splitting. J. Colloid Interface Sci. 2022, 607, 1876–1887. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.; Ma, T.; Hu, Z.; Liu, Q.; Zhan, C.; Li, Y.; Bowen, C.; Lu, H.; Liu, Y. Delocalization of D-Electrons Induced by Cation Coupling in Ultrathin Chevrel-Phase NiMo3S4 Nanosheets for Efficient Electrochemical Water Splitting. Appl. Catal. B Environ. 2023, 338, 123007. [Google Scholar] [CrossRef]
- Guillevic, J.; Bars, M.O.; Grandjean, D. Etude Structurale de Combinaisons Sulfurées et Séleniées Du Molybdène. III. Structure Cristalline de NiMo3S4. J. Solid State Chem. 1973, 7, 158–162. [Google Scholar] [CrossRef]
- Pallavolu, M.R.; Vallem, S.; Nallapureddy, R.R.; Adem, S.; Joo, S.W. Self-Assembled Hierarchical Silkworm-Type Bimetallic Sulfide (NiMo3S4) Nanostructures Developed on Sg-C3N4 Sheets: Promising Electrode Material for Supercapacitors. ACS Appl. Energy Mater. 2023, 6, 812–821. [Google Scholar] [CrossRef]
- Wu, T.; Xu, Z.; Wang, X.; Luo, M.; Xia, Y.; Zhang, X.; Li, J.; Liu, J.; Wang, J.; Wang, H.-L. Surface-Confined Self-Reconstruction to Sulfate-Terminated Ultrathin Layers on NiMo3S4 toward Biomass Molecule Electro-Oxidation. Appl. Catal. B Environ. 2023, 323, 122126. [Google Scholar] [CrossRef]
- Geng, L.; Lv, G.; Xing, X.; Guo, J. Reversible Electrochemical Intercalation of Aluminum in Mo6S8. Chem. Mater. 2015, 27, 4926–4929. [Google Scholar] [CrossRef]
- Elgendy, A.; Papaderakis, A.A.; Cai, R.; Polus, K.; Haigh, S.J.; Walton, A.S.; Lewis, D.J.; Dryfe, R.A. Nanocubes of Mo6S8 Chevrel Phase as Active Electrode Material for Aqueous Lithium-Ion Batteries. Nanoscale 2022, 14, 10125–10135. [Google Scholar] [CrossRef]
- Paskach, T.J.; Schrader, G.L.; McCarley, R.E. Synthesis of Methanethiol from Methanol over Reduced Molybdenum Sulfide Catalysts Based on the Mo6S8 Cluster. J. Catal. 2002, 211, 285–295. [Google Scholar] [CrossRef]
- Tong, Y.; Gao, A.; Zhang, Q.; Gao, T.; Yue, J.; Meng, F.; Gong, Y.; Xi, S.; Lin, Z.; Mao, M. Cation-Synergy Stabilizing Anion Redox of Chevrel Phase Mo6S8 in Aluminum Ion Battery. Energy Storage Mater. 2021, 37, 87–93. [Google Scholar] [CrossRef]
- Mao, M.; Lin, Z.; Tong, Y.; Yue, J.; Zhao, C.; Lu, J.; Zhang, Q.; Gu, L.; Suo, L.; Hu, Y.-S. Iodine Vapor Transport-Triggered Preferential Growth of Chevrel Mo6S8 Nanosheets for Advanced Multivalent Batteries. ACS Nano 2019, 14, 1102–1110. [Google Scholar] [CrossRef]
- Lu, K.; Liu, Y.; Chen, J.; Zhang, Z.; Cheng, Y. Redox Catalytic and Quasi-Solid Sulfur Conversion for High-Capacity Lean Lithium Sulfur Batteries. ACS Nano 2019, 13, 14540–14548. [Google Scholar] [CrossRef]
- Liu, C.; Liu, P. Mechanistic Study of Methanol Synthesis from CO2 and H2 on a Modified Model Mo6S8 Cluster. ACS Catal. 2015, 5, 1004–1012. [Google Scholar] [CrossRef]
- Wang, D.; Du, X.; Chen, G.; Song, F.; Du, J.; Zhao, J.; Ma, Y.; Wang, J.; Du, A.; Cui, Z. Cathode Electrolyte Interphase (CEI) Endows Mo6S8 with Fast Interfacial Magnesium-Ion Transfer Kinetics. Angew. Chem. Int. Ed. 2023, 62, e202217709. [Google Scholar] [CrossRef]
- Saito, T.; Yamamoto, N.; Yamagata, T.; Imoto, H. Synthesis of [Mo6S8(PEt3)6] by Reductive Dimerization of a Trinuclear Molybdenum Chloro Sulfido Cluster Complex Coordinated with Triethylphosphine and Methanol: A Molecular Model for Superconducting Chevrel Phases. J. Am. Chem. Soc. 1988, 110, 1646–1647. [Google Scholar] [CrossRef]
- Pu, M.; Peng, R.-J.; Yuan, J.-H.; Wang, J. One-Dimensional Mo6S6 Nanowire for Potential Application in Gas Sensing. Mater. Sci. Semicond. Process 2025, 188, 109239. [Google Scholar] [CrossRef]
- Yang, W.-D.; Zhao, R.-D.; Guo, F.-Y.; Xiang, J.; Loy, S.; Liu, L.; Dai, J.-Y.; Wu, F.-F. Interface Engineering of Hybrid ZnCo2O4@ Ni2.5Mo6S6.7 Structures for Flexible Energy Storage and Alkaline Water Splitting. Chem. Eng. J. 2023, 454, 140458. [Google Scholar] [CrossRef]
- Teng, J.; Cao, J.; Ouyang, T.; Yao, Y.; Chen, C.; Wei, X. Stability and Electronic Properties of α/β-Mo6S6 Nanowires Encapsulated inside Carbon Nanotubes. Phys. E Low-Dimens. Syst. Nanostruct. 2021, 134, 114891. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Tuxen, A.; Levisen, M.; Lægsgaard, E.; Gemming, S.; Seifert, G.; Lauritsen, J.V.; Besenbacher, F. Atomic-Scale Structure of Mo6S6 Nanowires. Nano Lett. 2008, 8, 3928–3931. [Google Scholar] [CrossRef]
- Popov, I.; Gemming, S.; Okano, S.; Ranjan, N.; Seifert, G. Electromechanical Switch Based on Mo6S6 Nanowires. Nano Lett. 2008, 8, 4093–4097. [Google Scholar] [CrossRef]
- Gentili, D.; Calabrese, G.; Lunedei, E.; Borgatti, F.; Mirshokraee, S.A.; Benekou, V.; Tseberlidis, G.; Mezzi, A.; Liscio, F.; Candini, A. Tuning Electronic and Functional Properties in Defected MoS2 Films by Surface Patterning of Sulphur Atomic Vacancies. Small Methods 2025, 9, 2401486. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).