Dual Synthetic Pathways for Organotin-Functionalized Mesoporous Silica Nanoparticles: Targeted Therapeutic Platforms with Folic Acid and PEI Formulation
Abstract
1. Introduction
2. Materials and Methods
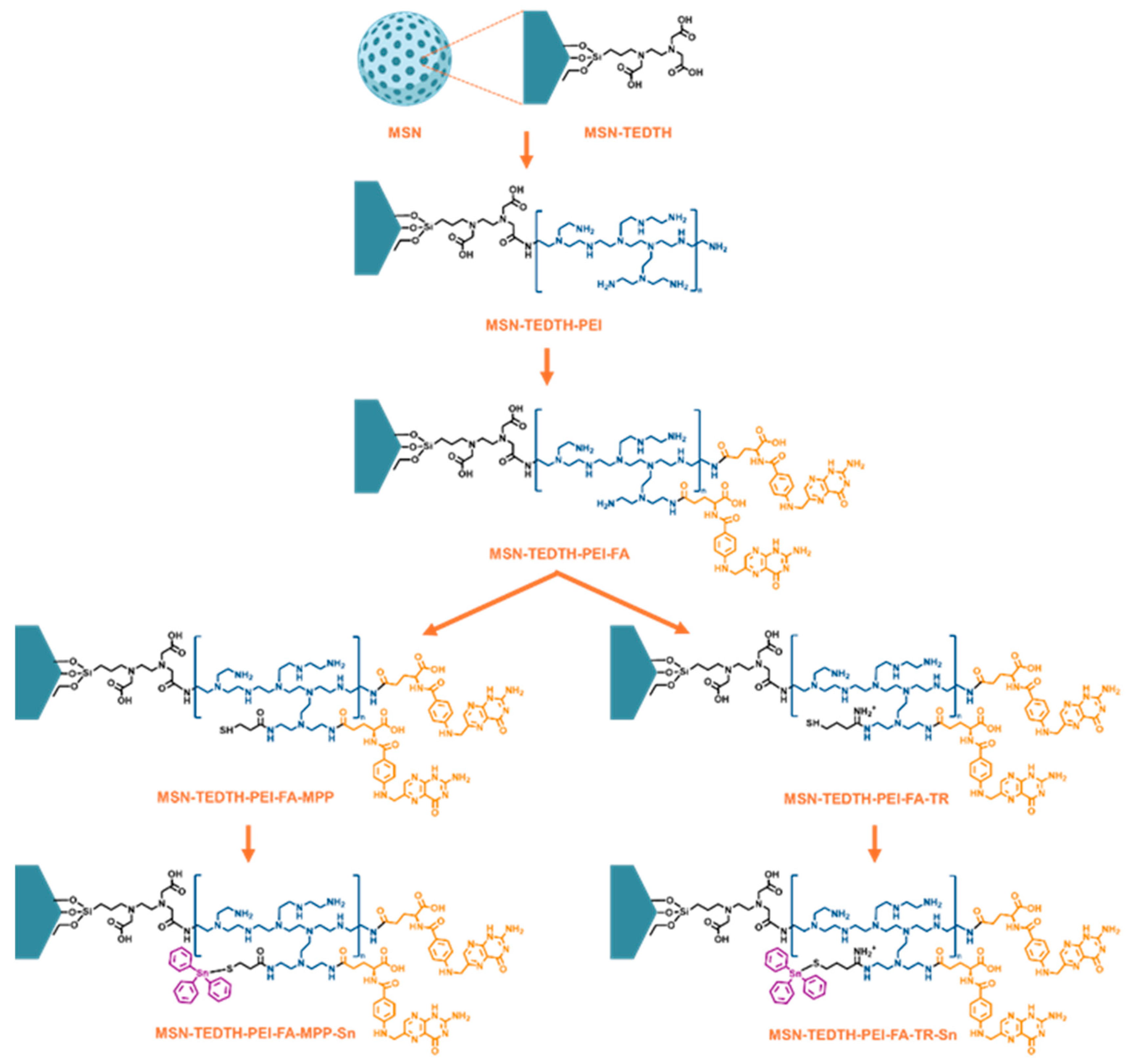
2.1. Synthesis and Characterization of Materials
2.1.1. General Condition on the Synthesis and Characterization of the Materials
2.1.2. Synthesis of MSN
2.1.3. Activation of the MSN and Functionalization with the TEDTH Ligand. Preparation of MSN-TEDTH
2.1.4. Formulation with PEI. Preparation of MSN-TEDTH-PEI
2.1.5. Functionalization with Folic Acid. Preparation of MSN-TEDTH-PEI-FA
2.1.6. Functionalization with Ligands MPP and TR. Preparation of MSN-TEDTH-PEI-FA-MPP and MSN-TEDTH-PEI-FA-TR
2.1.7. Functionalization with Triphenyltin(IV) Chloride. Preparation of MSN-TEDTH-PEI-FA-MPP-Sn and MSN-TEDTH-PEI-FA-TR-Sn
2.1.8. Metal Release Studies (ICP-AES)
2.1.9. Experimental Details on Z-Potential and Dynamic Light Scattering (DLS)
2.2. Antitumoral Activity of the Synthesized Materials
2.2.1. Cell Line and Cell Culture Conditions
2.2.2. MTT Cytotoxicity Assay
3. Results and Discussion
3.1. Synthesis, Functionalization and Characterization of the Studied Nanostructured Materials
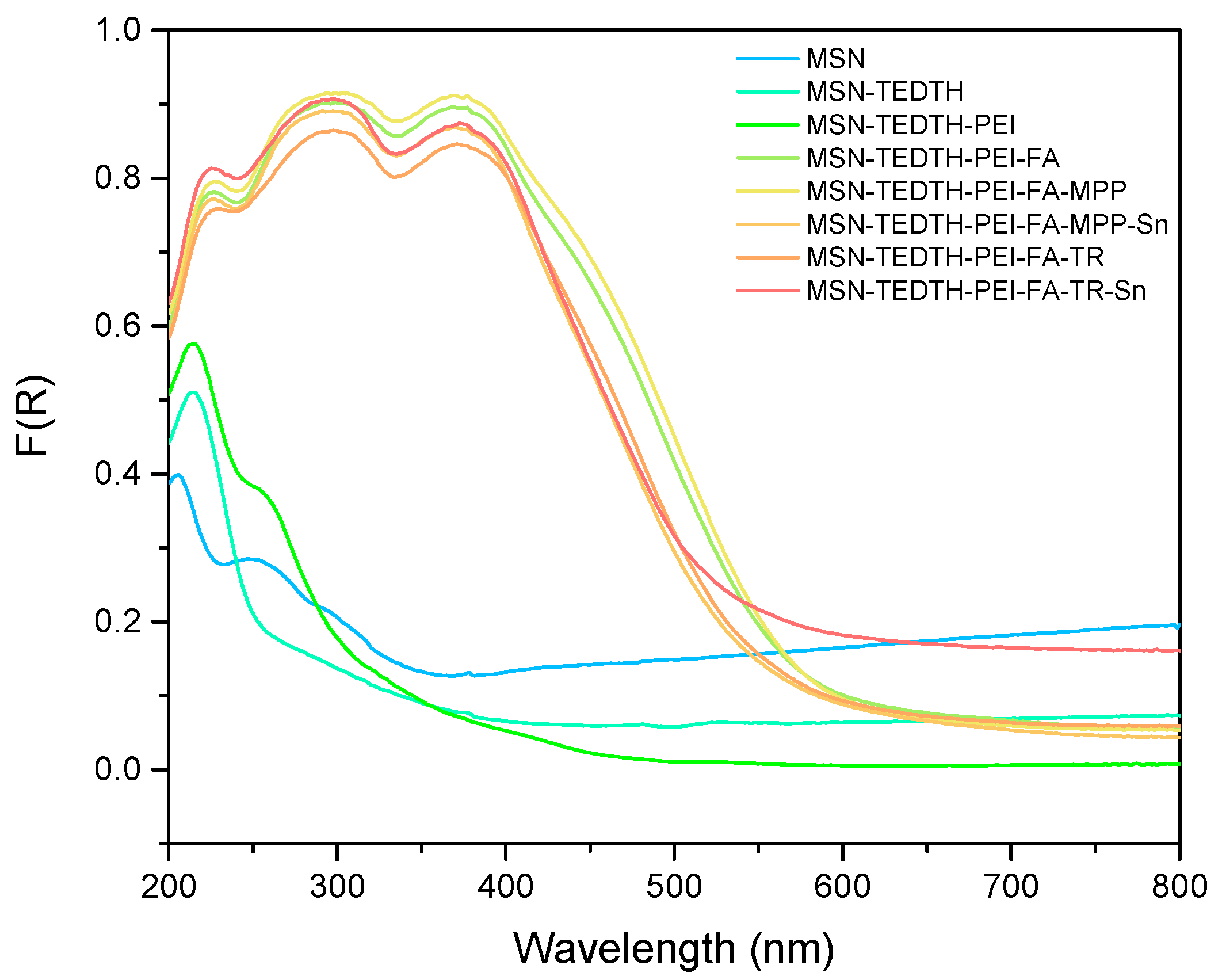
3.1.1. UV–Visible Spectroscopy Studies (DR UV-Vis)
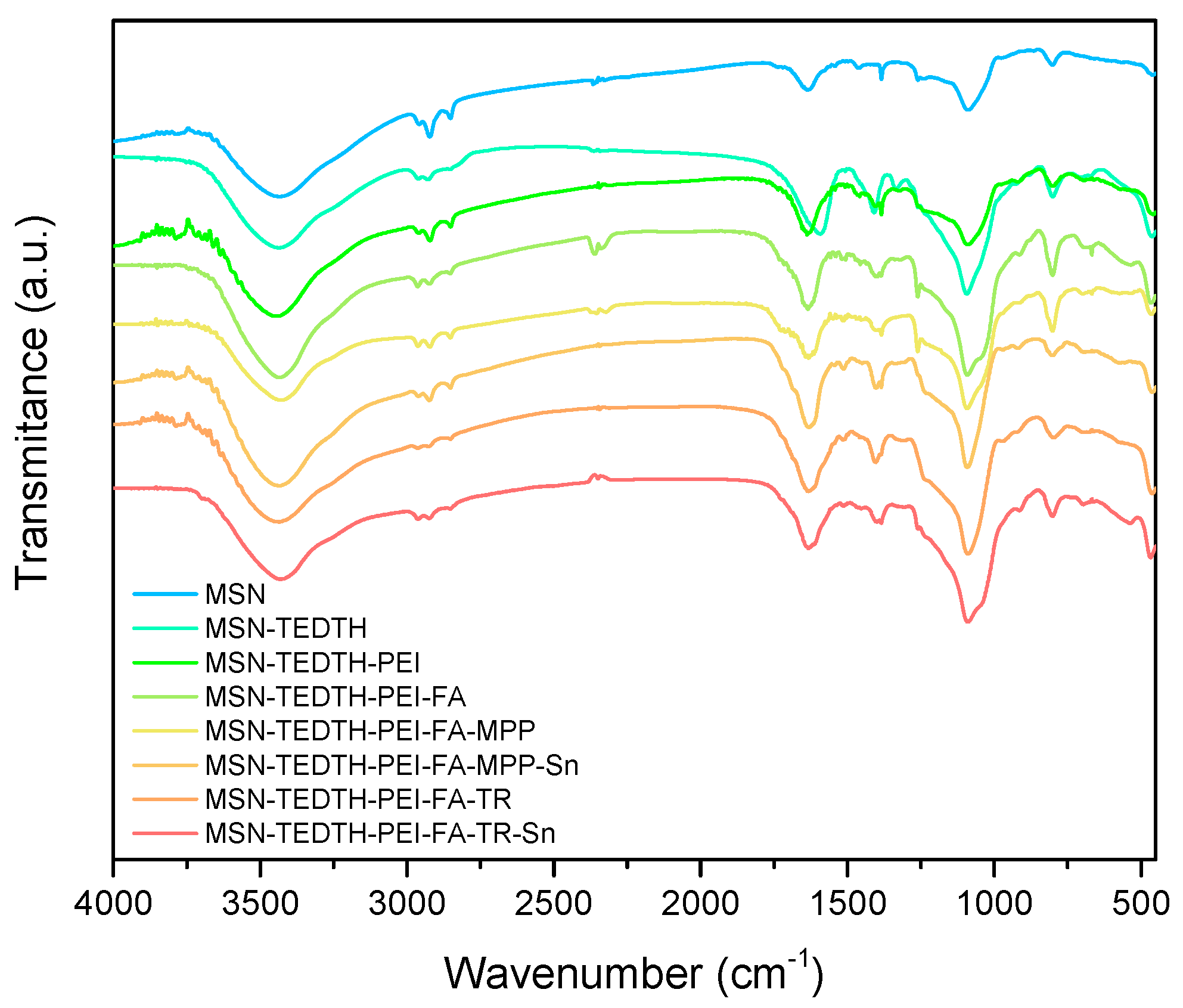
3.1.2. FT-IR Studies
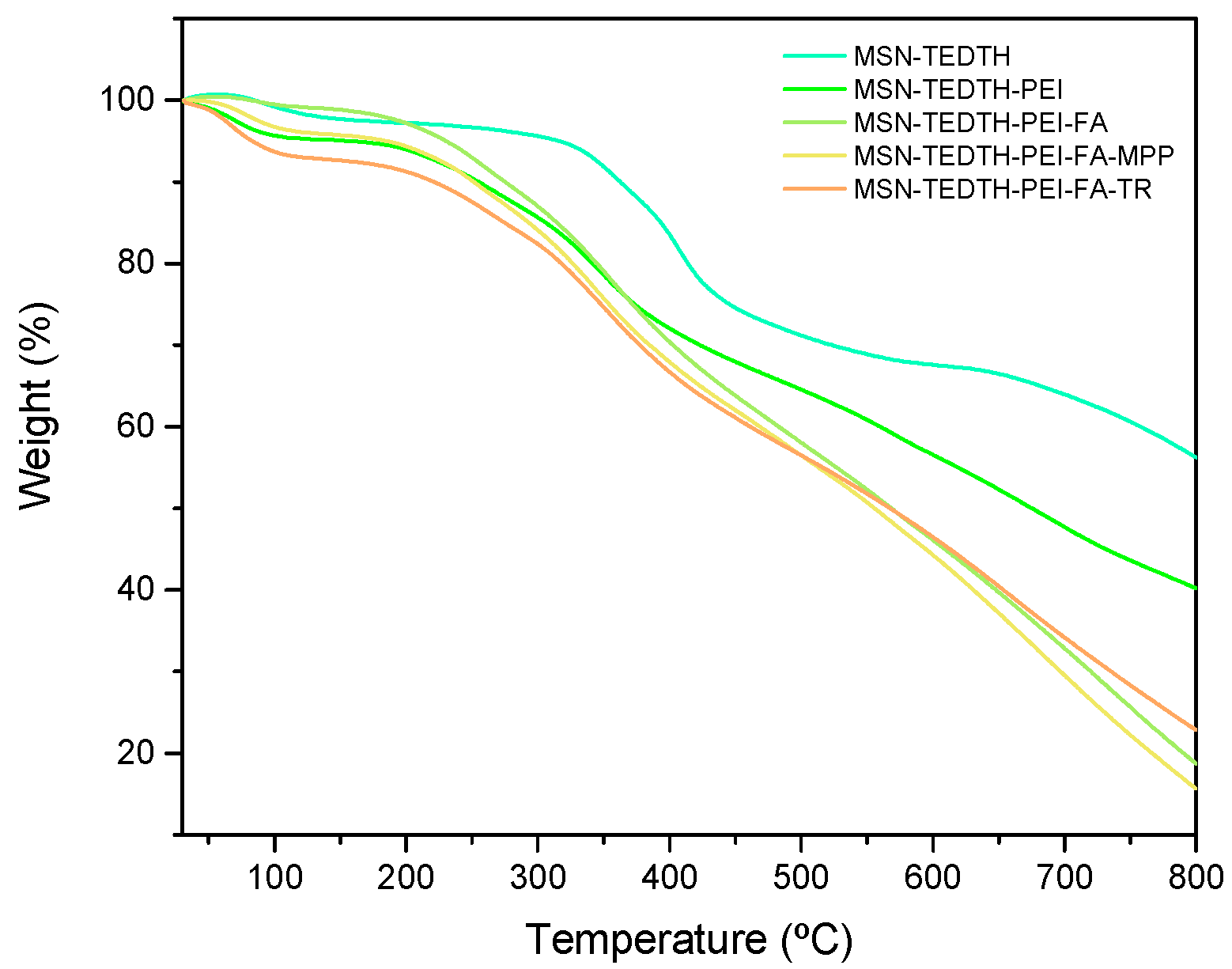
3.1.3. Quantification of Interesting Molecules by TG, ICP and XFR
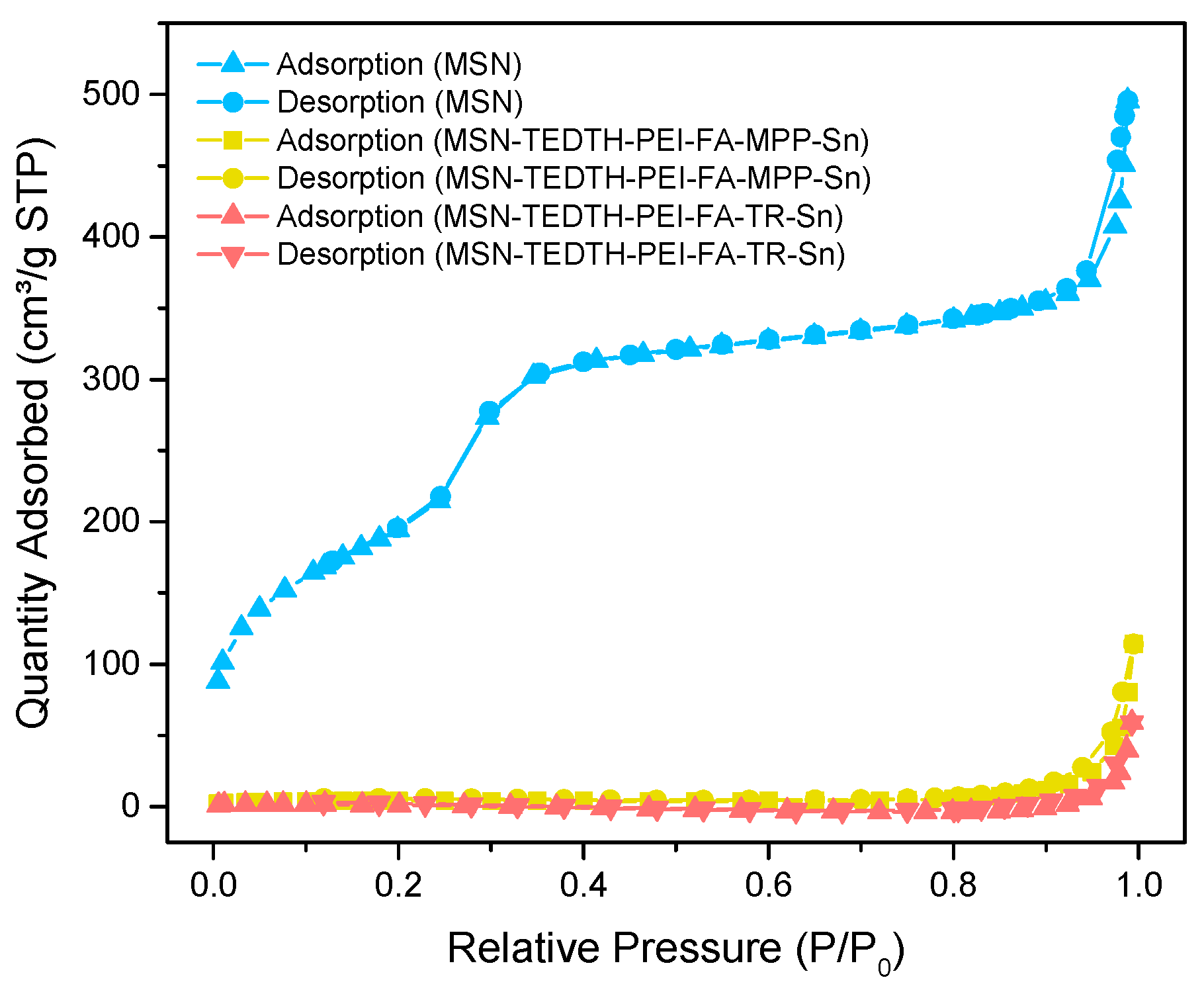
3.1.4. Nitrogen Adsorption-Desorption Isotherms (BET)
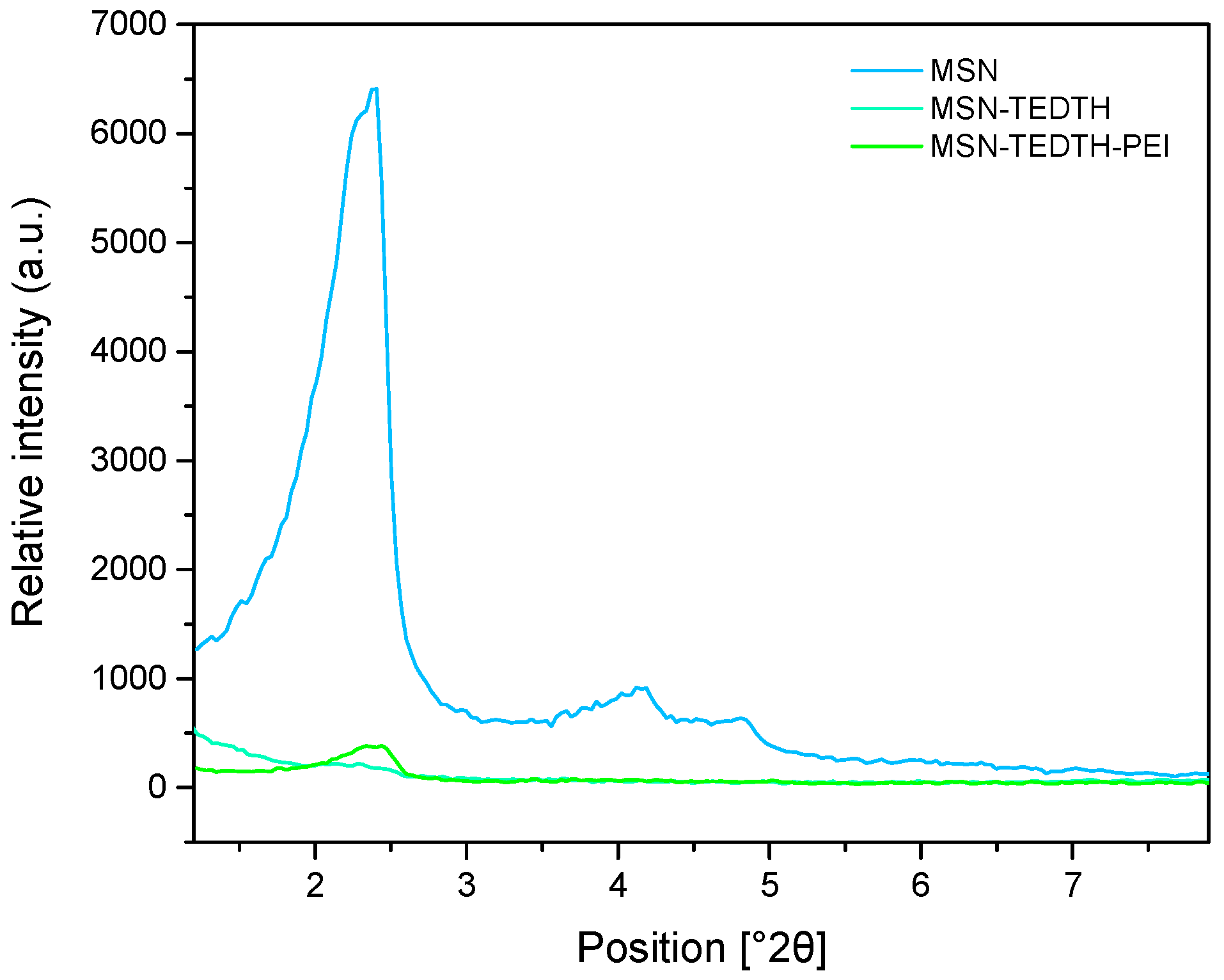
3.1.5. Powder X-Ray Diffraction Studies (XRD)
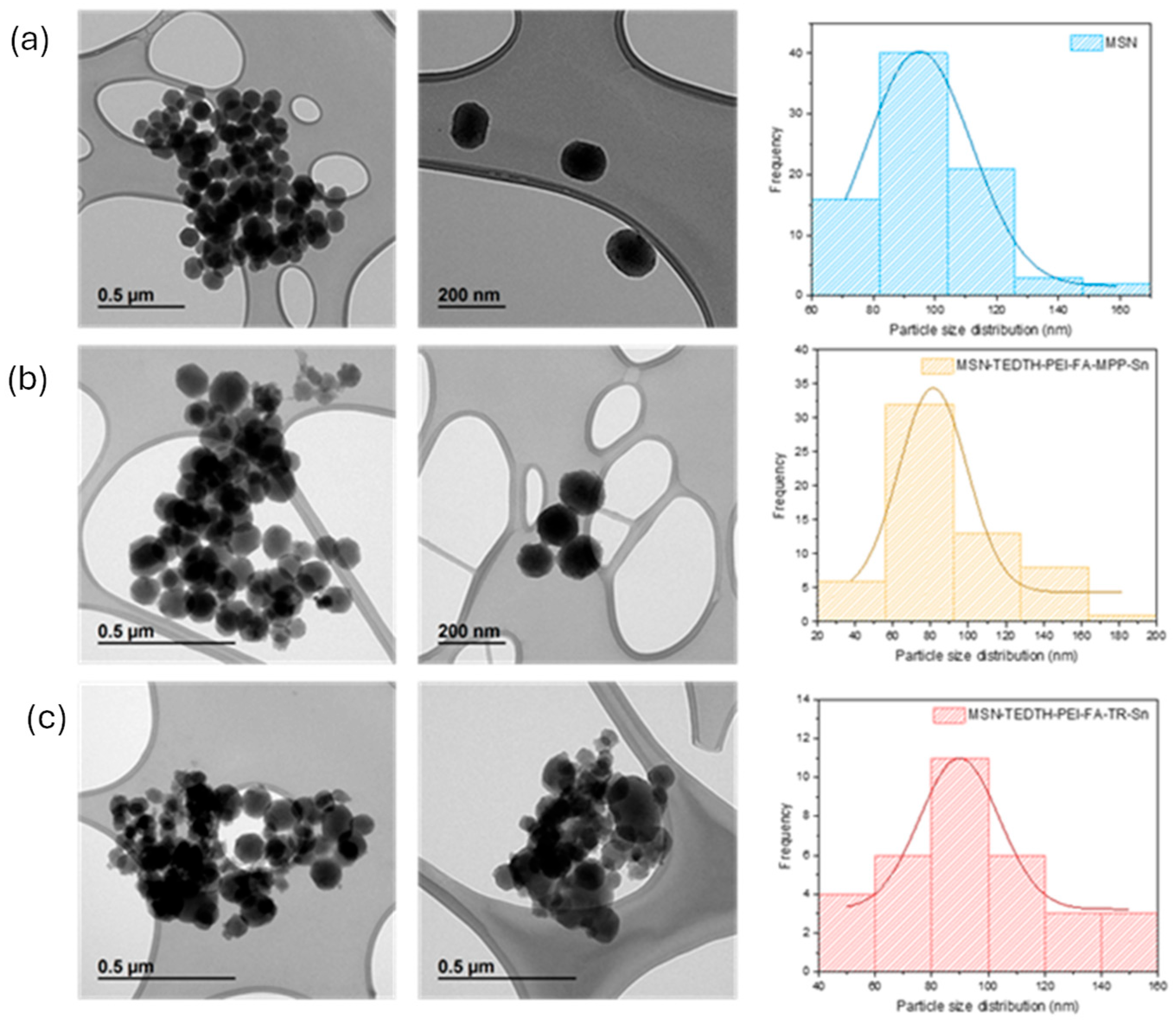
3.1.6. Electronic Microscopy Studies (TEM)
3.1.7. Field Emission Scanning Electron Microscopy (FEG-SEM)
3.2. Studies in a Simulated Biological Environment
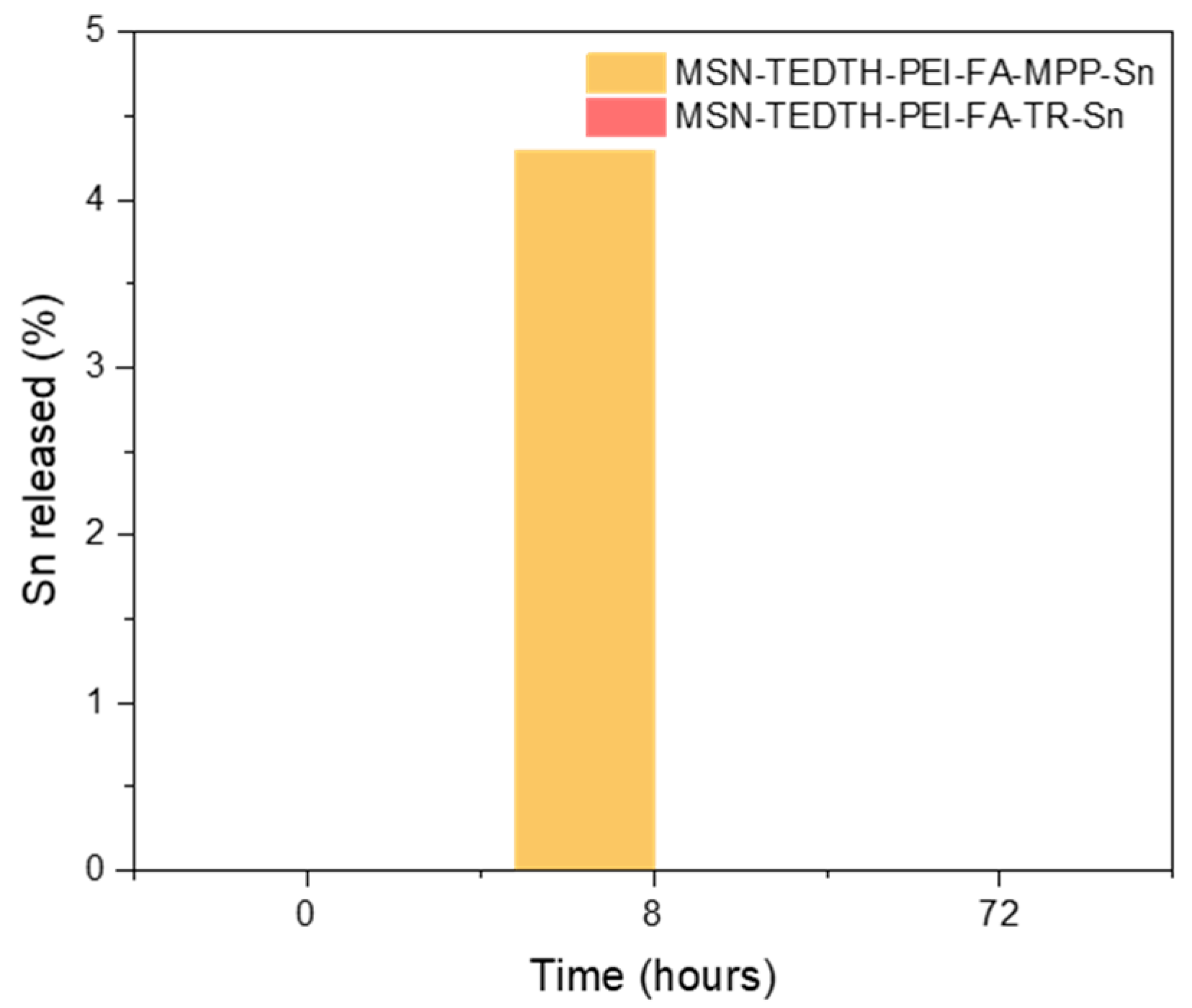
3.2.1. Release Studies
3.2.2. Z-Potential and Dynamic Light Scattering (DLS)
3.3. Biological Studies
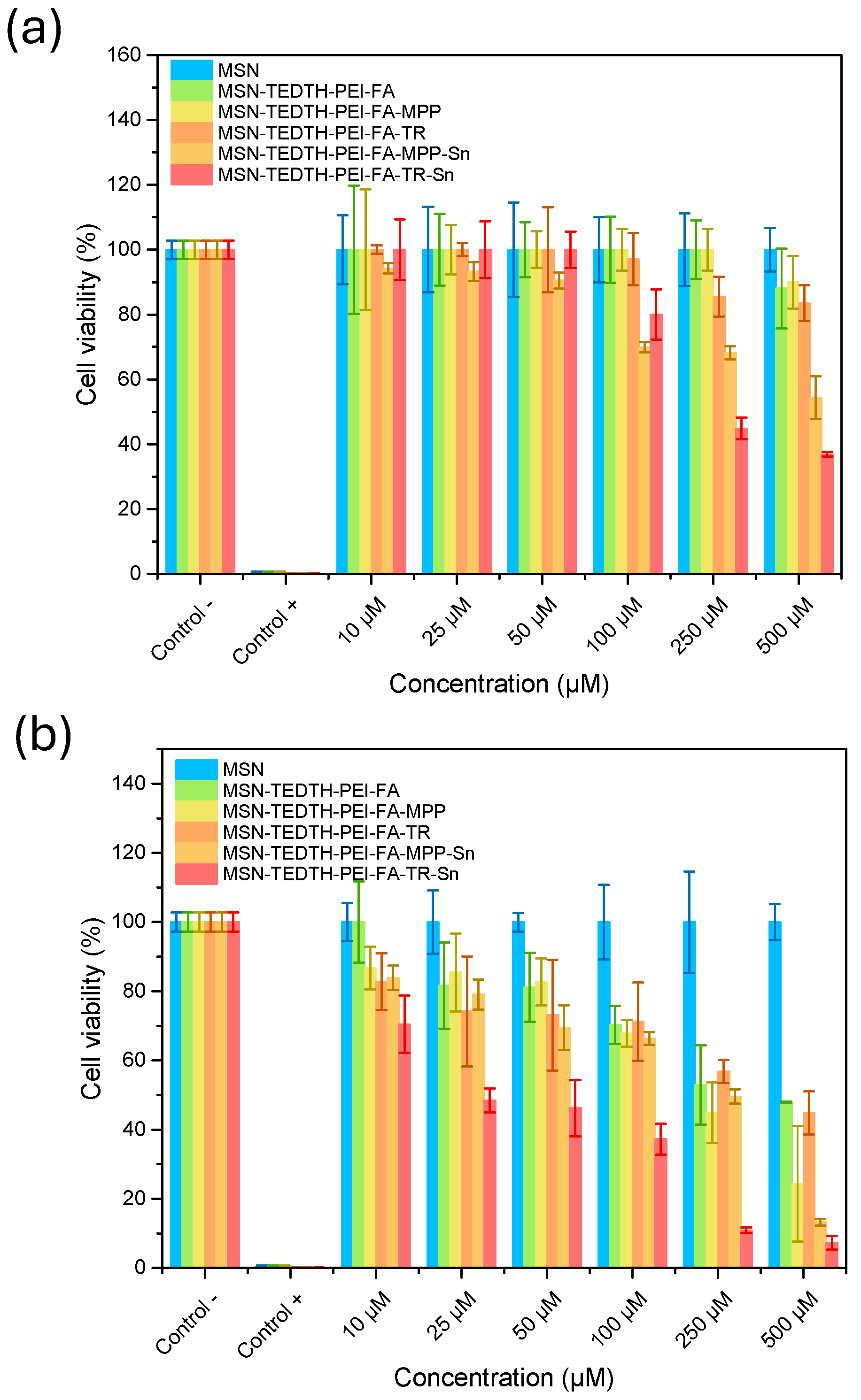
MTT Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaluđerović, G.N.; Pantelić, N.Đ. Advanced Nanomaterials in Biomedical Applications. Nanomaterials 2024, 14, 1668. [Google Scholar] [CrossRef]
- Hosny, S.; Mohamed, L.Z.; Ragab, M.S.; Alomoush, Q.K.; Abdalla, E.M.; Aly, S.A. Nanomaterials in Biomedical Applications: Opportunities and Challenges—A Review. Chem. Pap. 2025, 79, 2657. [Google Scholar] [CrossRef]
- Chowdhury, E.H.; Akaike, T. Bio-Functional Inorganic Materials: An Attractive Branch of Gene-Based Nano-Medicine Delivery for 21st Century. Curr. Gene Ther. 2005, 5, 669. [Google Scholar] [CrossRef]
- Sao, R.; Vaish, R.; Sinha, N. Multifunctional Drug Delivery Systems Using Inorganic Nanomaterials: A Review. J. Nanosci. Nanotechnol. 2015, 15, 1960. [Google Scholar] [CrossRef]
- Bhise, K.; Sau, S.; Alsaab, H.; Kashaw, S.K.; Tekade, R.K.; Iyer, A.K. Nanomedicine for Cancer Diagnosis and Therapy: Advancement, Success and Structure–Activity Relationship. Ther. Deliv. 2017, 8, 1003. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S.; Kang, D.D.; Dong, Y. Biomaterials for in Situ Cell Therapy. BMEMat 2023, 1, 12039. [Google Scholar] [CrossRef]
- Lima-De-Faria, J.; Figueiredo, M.O. Classification, Notation, and Ordering on a Table of Inorganic Structure Types. J. Solid State Chem. 1976, 16, 7. [Google Scholar] [CrossRef]
- Schubert, U.S.; Hüsing, N. Synthesis of Inorganic Materials; John Wiley & Sons: Hoboken, NJ, USA, 2019; ISBN 978-3-527-34457-4. [Google Scholar]
- Frickenstein, A.N.; Hagood, J.M.; Britten, C.N.; Abbott, B.S.; McNally, M.W.; Vopat, C.A.; Patterson, E.G.; MacCuaig, W.M.; Jain, A.; Walters, K.B.; et al. Mesoporous Silica Nanoparticles: Properties and Strategies for Enhancing Clinical Effect. Pharmaceutics 2021, 13, 570. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Colilla, M.; Izquierdo-Barba, I.; Manzano, M. Mesoporous Silica Nanoparticles for Drug Delivery: Current Insights. Molecules 2018, 23, 47. [Google Scholar] [CrossRef]
- Bakhshian Nik, A.; Zare, H.; Razavi, S.; Mohammadi, H.; Torab Ahmadi, P.; Yazdani, N.; Bayandori, M.; Rabiee, N.; Izadi Mobarakeh, J. Smart Drug Delivery: Capping Strategies for Mesoporous Silica Nanoparticles. Microporous Mesoporous Mater. 2020, 299, 110115. [Google Scholar] [CrossRef]
- Mohamed Isa, E.D.; Ahmad, H.; Abdul Rahman, M.B.; Gill, M.R. Progress in Mesoporous Silica Nanoparticles as Drug Delivery Agents for Cancer Treatment. Pharmaceutics 2021, 13, 152. [Google Scholar] [CrossRef]
- Chidambaram, M.; Manavalan, R.; Kathiresan, K. Nanotherapeutics to Overcome Conventional Cancer Chemotherapy Limitations. J. Pharm. Pharm. Sci. 2011, 14, 67. [Google Scholar] [CrossRef]
- Kaur, R.; Bhardwaj, A.; Gupta, S. Cancer Treatment Therapies: Traditional to Modern Approaches to Combat Cancers. Mol. Biol. Rep. 2023, 50, 9663. [Google Scholar] [CrossRef]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced Targeted Therapies in Cancer: Drug Nanocarriers, the Future of Chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52. [Google Scholar] [CrossRef]
- Sarma, K.; Akther, M.H.; Ahmad, I.; Afzal, O.; Altamimi, A.S.A.; Alossaimi, M.A.; Jaremko, M.; Emwas, A.-H.; Gautam, P. Adjuvant Novel Nanocarrier-Based Targeted Therapy for Lung Cancer. Molecules 2024, 29, 1076. [Google Scholar] [CrossRef]
- Hubbell, J.A.; Chilkoti, A. Nanomaterials for Drug Delivery. Science 2012, 337, 303. [Google Scholar] [CrossRef]
- Ma, J.; Jemal, A. Breast Cancer Statistics. In Breast Cancer Metastasis and Drug Resistance: Progress and Prospects; Ahmad, A., Ed.; Springer: New York, NY, USA, 2013; ISBN 978-1-4614-5647-6. [Google Scholar]
- Nikolaou, M.; Pavlopoulou, A.; Georgakilas, A.G.; Kyrodimos, E. The Challenge of Drug Resistance in Cancer Treatment: A Current Overview. Clin. Exp. Metastasis 2018, 35, 309. [Google Scholar] [CrossRef]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug Resistance in Cancer: An Overview. Cancers 2014, 6, 1769. [Google Scholar] [CrossRef]
- He, Q.; Shi, J. MSN Anti-Cancer Nanomedicines: Chemotherapy Enhancement, Overcoming of Drug Resistance, and Metastasis Inhibition. Adv. Mater. 2014, 26, 391. [Google Scholar] [CrossRef]
- Guan, Y.-F.; Li, G.-R.; Wang, R.-J.; Yi, Y.-T.; Yang, L.; Jiang, D.; Zhang, X.-P.; Peng, Y. Application of Next-Generation Sequencing in Clinical Oncology to Advance Personalized Treatment of Cancer. Chin. J. Cancer 2012, 31, 463. [Google Scholar] [CrossRef]
- Osborne, C.K.; Hobbs, K.; Trent, J.M. Biological Differences among MCF-7 Human Breast Cancer Cell Lines from Different Laboratories. Breast Cancer Res. Treat. 1987, 9, 111. [Google Scholar] [CrossRef]
- Termini, J.M.; Silver, Z.A.; Connor, B.; Antonopoulos, A.; Haslam, S.M.; Dell, A.; Desrosiers, R.C. HEK293T Cell Lines Defective for O-Linked Glycosylation. PLoS ONE 2017, 12, e0179949. [Google Scholar] [CrossRef]
- Merlin, J.-L.; Barberi-Heyob, M.; Bachmann, N. In Vitro Comparative Evaluation of Trastuzumab (Herceptin®) Combined with Paclitaxel (Taxol®) or Docetaxel (Taxotere®) in HER2-Expressing Human Breast Cancer Cell Lines. Ann. Oncol. 2002, 13, 1743. [Google Scholar] [CrossRef]
- Lammers, T.; Kiessling, F.; Hennink, W.E.; Storm, G. Drug Targeting to Tumors: Principles, Pitfalls and (Pre-) Clinical Progress. J. Control. Release 2012, 161, 175. [Google Scholar] [CrossRef]
- Tagde, P.; Kulkarni, G.T.; Mishra, D.K.; Kesharwani, P. Recent Advances in Folic Acid Engineered Nanocarriers for Treatment of Breast Cancer. J. Drug Deliv. Sci. Technol. 2020, 56, 101613. [Google Scholar] [CrossRef]
- Choudhury, H.; Pandey, M.; Wen, L.P.; Cien, L.K.; Xin, H.; Yee, A.N.J.; Lee, N.J.; Gorain, B.; Amin, M.C.I.M.; Pichika, M.R. Folic Acid Conjugated Nanocarriers for Efficient Targetability and Promising Anticancer Efficacy for Treatment of Breast Cancer: A Review of Recent Updates. Curr. Pharm. Des. 2020, 26, 5365. [Google Scholar] [CrossRef]
- Khosravian, P.; Shafiee Ardestani, M.; Khoobi, M.; Ostad, S.N.; Dorkoosh, F.A.; Akbari Javar, H.; Amanlou, M. Mesoporous Silica Nanoparticles Functionalized with Folic Acid/Methionine for Active Targeted Delivery of Docetaxel. OncoTargets Ther. 2016, 9, 7315. [Google Scholar] [CrossRef]
- Sarkar, A.; Ghosh, S.; Chowdhury, S.; Pandey, B.; Sil, P.C. Targeted Delivery of Quercetin Loaded Mesoporous Silica Nanoparticles to the Breast Cancer Cells. Biochim. Et Biophys. Acta (BBA) Gen. Subj. 2016, 1860, 2065. [Google Scholar] [CrossRef]
- Díaz-García, D.; Montalbán-Hernández, K.; Mena-Palomo, I.; Achimas-Cadariu, P.; Rodríguez-Diéguez, A.; López-Collazo, E.; Prashar, S.; Ovejero Paredes, K.; Filice, M.; Fischer-Fodor, E.; et al. Role of Folic Acid in the Therapeutic Action of Nanostructured Porous Silica Functionalized with Organotin(IV) Compounds against Different Cancer Cell Lines. Pharmaceutics 2020, 12, 512. [Google Scholar] [CrossRef]
- Arjmand, F.; Parveen, S.; Tabassum, S.; Pettinari, C. Organo-Tin Antitumor Compounds: Their Present Status in Drug Development and Future Perspectives. Inorganica Chim. Acta 2014, 423, 26. [Google Scholar] [CrossRef]
- Hadjikakou, S.K.; Hadjiliadis, N. Antiproliferative and Anti-Tumor Activity of Organotin Compounds. Coord. Chem. Rev. 2009, 253, 235. [Google Scholar] [CrossRef]
- Niu, L.; Li, Y.; Li, Q. Medicinal Properties of Organotin Compounds and Their Limitations Caused by Toxicity. Inorganica Chim. Acta 2014, 423, 2. [Google Scholar] [CrossRef]
- Ovejero Paredes, K.; Díaz-García, D.; García-Almodóvar, V.; Lozano Chamizo, L.; Marciello, M.; Díaz-Sánchez, M.; Prashar, S.; Gómez-Ruiz, S.; Filice, M. Multifunctional Silica-Based Nanoparticles with Controlled Release of Organotin Metallodrug for Targeted Theranosis of Breast Cancer. Cancers 2020, 12, 187. [Google Scholar] [CrossRef]
- Díaz-García, D.; Sommerova, L.; Martisova, A.; Skoupilova, H.; Prashar, S.; Vaculovic, T.; Kanicky, V.; del Hierro, I.; Hrstka, R.; Gómez-Ruiz, S. Mesoporous Silica Nanoparticles Functionalized with a Dialkoxide Diorganotin(IV) Compound: In Search of More Selective Systems against Cancer Cells. Microporous Mesoporous Mater. 2020, 300, 110154. [Google Scholar] [CrossRef]
- García-Almodóvar, V.; Ovejero-Paredes, K.; Díaz-García, D.; Méndez-Arriaga, J.M.; Prashar, S.; Filice, M.; Gómez-Ruiz, S. Albumin-Loaded Silica Nanomaterials Functionalized with Organotin(IV) Agents: Theranostic Materials Against Triple-Negative Breast Cancer. Adv. Ther. 2024, 7, 2400114. [Google Scholar] [CrossRef]
- Ali, S.; Shahzadi, S.; Imtiaz-ud-Din. Anticarcinogenicity and Toxicity of Organotin(IV) Complexes: A Review. Iran. J. Sci. Technol. Trans. A Sci. 2018, 42, 505. [Google Scholar] [CrossRef]
- Devi, J.; Yadav, J. Recent Advancements in Organotin(IV) Complexes as Potential Anticancer Agents. Anti-Cancer Agents Med. Chem. 2018, 18, 335. [Google Scholar] [CrossRef]
- Yamada, H.; Urata, C.; Higashitamori, S.; Aoyama, Y.; Yamauchi, Y.; Kuroda, K. Critical Roles of Cationic Surfactants in the Preparation of Colloidal Mesostructured Silica Nanoparticles: Control of Mesostructure, Particle Size, and Dispersion. ACS Appl. Mater. Interfaces 2014, 6, 3491. [Google Scholar] [CrossRef]
- Varache, M.; Bezverkhyy, I.; Bouyer, F.; Chassagnon, R.; Baras, F.; Bouyer, F. Improving Structural Stability of Water-Dispersed MCM-41 Silica Nanoparticles through Post-Synthesis pH Aging Process. J. Nanoparticle Res. 2015, 17, 356. [Google Scholar] [CrossRef]
- Sun, X.; Wang, N.; Yang, L.-Y.; Ouyang, X.-K.; Huang, F. Folic Acid and PEI Modified Mesoporous Silica for Targeted Delivery of Curcumin. Pharmaceutics 2019, 11, 430. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Wang, M.; Ma, Y.; Xia, W.; Gu, H. A Mesoporous Silica Nanoparticle—PEI—Fusogenic Peptide System for siRNA Delivery in Cancer Therapy. Biomaterials 2013, 34, 1391. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yue, M.; Guo, T.; Li, F.; Li, Z.; Yang, D.; Lin, M. PEI-PEG-Coated Mesoporous Silica Nanoparticles Enhance the Antitumor Activity of Tanshinone IIA and Serve as a Gene Transfer Vector. Evid.-Based Complement. Altern. Med. 2021, 2021, 6756763. [Google Scholar] [CrossRef]
- Hanafi-Bojd, M.Y.; Jaafari, M.R.; Ramezanian, N.; Xue, M.; Amin, M.; Shahtahmassebi, N.; Malaekeh-Nikouei, B. Surface Functionalized Mesoporous Silica Nanoparticles as an Effective Carrier for Epirubicin Delivery to Cancer Cells. Eur. J. Pharm. Biopharm. 2015, 89, 248. [Google Scholar] [CrossRef] [PubMed]
- Badihi, R.; Mahmoudi, A.; Sazegar, M.R.; Nazari, K. A Study on Co-Modification of MSNs with Some Transition Metals and Polyethyleneimine (PEI) as a Versatile Strategy for Efficient Delivery of Short Oligonucleotides. Chem. Papers. 2022, 76, 7023. [Google Scholar] [CrossRef]
- Man, K.; Brunet, M.Y.; Jones, M.-C.; Cox, S.C. Engineered Extracellular Vesicles: Tailored-Made Nanomaterials for Medical Applications. Nanomaterials 2020, 10, 1838. [Google Scholar] [CrossRef]
- Yu, H.-P.; Zhu, Y.-J. Guidelines Derived from Biomineralized Tissues for Design and Construction of High-Performance Biomimetic Materials: From Weak to Strong. Chem. Soc. Rev. 2024, 53, 4490. [Google Scholar] [CrossRef]
- Shao, W.; Paul, A.; Abbasi, S.; Chahal, P.S.; Mena, J.A.; Montes, J.; Kamen, A.; Prakash, S. A Novel Polyethyleneimine-Coated Adeno-Associated Virus-like Particle Formulation for Efficient siRNA Delivery in Breast Cancer Therapy: Preparation and In Vitro Analysis. Int. J. Nanomed. 2012, 7, 1575. [Google Scholar]
- Zhao, Y.; Trewyn, B.G.; Slowing, I.I.; Lin, V.S.-Y. Mesoporous Silica Nanoparticle-Based Double Drug Delivery System for Glucose-Responsive Controlled Release of Insulin and Cyclic AMP. J. Am. Chem. Soc. 2009, 131, 8398. [Google Scholar] [CrossRef]
- Dembélé, J.; Liao, J.-H.; Liu, T.-P.; Chen, Y.-P. Overcoming Cytosolic Delivery Barriers of Proteins Using Denatured Protein-Conjugated Mesoporous Silica Nanoparticles. ACS Appl. Mater. Interfaces 2023, 15, 432. [Google Scholar] [CrossRef]
- António, M.; Lima, T.; Vitorino, R.; Daniel-da-Silva, A.L. Label-Free Dynamic Light Scattering Assay for C-Reactive Protein Detection Using Magnetic Nanoparticles. Anal. Chim. Acta 2022, 1222, 340169. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, S.G.; Oh, E.J.; Chung, H.Y.; Han, S.I.; Kim, E.J.; Seo, S.Y.; Ghim, H.D.; Yeum, J.H.; Choi, J.H. Antimicrobial Polyethyleneimine-Silver Nanoparticles in a Stable Colloidal Dispersion. Colloids Surf. B Biointerfaces 2011, 88, 505. [Google Scholar] [CrossRef] [PubMed]
- García-Almodóvar, V.; Ardiles, P.D.R.; Prashar, S.; Páez, P.L.; Gómez-Ruiz, S. Unleashing the Antibacterial and Antibiofilm Potential of Silica-Based Nanomaterials Functionalized with an Organotin(IV) Compound. J. Mater. Chem. B 2024, 12, 9056. [Google Scholar] [CrossRef]
- Lazarević, S.S.; Janković-Častvan, I.M.; Jokić, B.M.; Janaćković, D.T.; Petrović, R.D. Sepiolite Functionalized with N-[3-(Trimethoxysilyl)Propyl]-Ethylenediamine Triacetic Acid Trisodium Salt. Part I: Preparation and Characterization. J. Serbian Chem. Soc. 2015, 80, 1193. [Google Scholar] [CrossRef]
- Hashida, T.; Tashiro, K.; Aoshima, S.; Inaki, Y. Structural Investigation on Water-Induced Phase Transitions of Poly(Ethylene Imine). 1. Time-Resolved Infrared Spectral Measurements in the Hydration Process. Macromolecules 2002, 35, 4330. [Google Scholar] [CrossRef]
- Ugalde-Arbizu, M.; Aguilera-Correa, J.J.; García-Almodóvar, V.; Ovejero-Paredes, K.; Díaz-García, D.; Esteban, J.; Páez, P.L.; Prashar, S.; San Sebastian, E.; Filice, M.; et al. Dual Anticancer and Antibacterial Properties of Silica-Based Theranostic Nanomaterials Functionalized with Coumarin343, Folic Acid and a Cytotoxic Organotin(IV) Metallodrug. Pharmaceutics 2023, 15, 560. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodríguez-Reinoso, F.; Rouquerol, S.K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution. IUPAC Tech. Rep. 2025, 87, 1051. [Google Scholar]
- Zeng, W.; Bai, H. High-Performance CO2 Capture on Amine-Functionalized Hierarchically Porous Silica Nanoparticles Prepared by a Simple Template-Free Method. Adsorption 2016, 22, 117. [Google Scholar] [CrossRef]
- Purnawira, B.; Purwaningsih, H.; Ervianto, Y.; Pratiwi, V.M.; Susanti, D.; Rochiem, R.; Purniawan, A. Synthesis and Characterization of Mesoporous Silica Nanoparticles (MSNp) MCM 41 from Natural Waste Rice Husk. IOP Conf. Ser. Mater. Sci. Eng. 2019, 541, 012018. [Google Scholar] [CrossRef]
- Hethnawi, A.; Nassar, N.N.; Vitale, G. Preparation and Characterization of Polyethylenimine-Functionalized Pyroxene Nanoparticles and Its Application in Wastewater Treatment. Colloids Surf. A Physicochem. Eng. Asp. 2017, 525, 20. [Google Scholar] [CrossRef]
- Pham, D.T.; Saelim, N.; Tiyaboonchai, W. Crosslinked fibroin nanoparticles using EDC or PEI for drug delivery: Physicochemical properties, crystallinity and structure. J. Mater. Sci. 2018, 53, 14087. [Google Scholar] [CrossRef]
- ImageJ. Available online: https://imagej.net/ij/ (accessed on 23 September 2025).
- Doane, T.L.; Chuang, C.-H.; Hill, R.J.; Burda, C. Nanoparticle ζ-Potentials. Acc. Chem. Res. 2012, 45, 317. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Li, L.; Chen, D. Mesoporous Silica Nanoparticles: Synthesis, Biocompatibility and Drug Delivery. Adv. Mater. 2012, 24, 1504. [Google Scholar] [CrossRef] [PubMed]
- Díaz-García, D.; Fischer-Fodor, E.; Vlad, C.I.; Méndez-Arriaga, J.M.; Prashar, S.; Gómez-Ruiz, S. Study of Cancer Cell Cytotoxicity, Internalization and Modulation of Growth Factors Induced by Transferrin-Conjugated Formulations of Metallodrug-Functionalized Mesoporous Silica Nanoparticles. Microporous Mesoporous Mater. 2021, 323, 111238. [Google Scholar] [CrossRef]
| Materials | Weight (%) | mmol/g | Sn (%) | Other Elements (%) |
|---|---|---|---|---|
| MSN-TEDTH | 31.89 (TEDTH) | 0.74 | - | - |
| MSN-TEDTH-PEI | 11.12 (PEI) | 2.64 | - | - |
| MSN-TEDTH-PEI-FA | 16.46 (FA) | 0.39 | - | - |
| MSN-TEDTH-PEI-FA-MPP | 0.54 (MPP) | 0.06 | ||
| MSN-TEDTH-PEI-FA-TR | 6.85 (TR) | 0.66 | ||
| MSN-TEDTH-PEI-FA-MPP-Sn | - | - | 0.314 ± 0.001 | 3.56 (S) 30.96 (Cl) |
| MSN-TEDTH-PEI-FA-TR-Sn | - | - | 0.526 ± 0.001 | 10.82 (S) 11.40 (Cl) |
| Materials | (hkl) | 2θ (°) | dhkl (Å) |
|---|---|---|---|
| MSN | 100 | 2.41 | 36.57 |
| 110 | 4.17 | 21.18 | |
| 200 | 4.84 | 18.25 | |
| MSN-TEDTH | 100 | 2.31 | - |
| MSN-TEDTH-PEI | 100 | 2.47 | 35.67 |
| Materials | Release (%) |
|---|---|
| MSN-TEDTH-PEI-FA-MPP-Sn (0 h) | <0.1 |
| MSN-TEDTH-PEI-FA-TR-Sn (0 h) | <0.1 |
| MSN-TEDTH-PEI-FA-MPP-Sn (8 h) | 4.3 |
| MSN-TEDTH-PEI-FA-TR-Sn (8 h) | <0.1 |
| MSN-TEDTH-PEI-FA-MPP-Sn (72 h) | <0.1 |
| MSN-TEDTH-PEI-FA-TR-Sn (72 h) | <0.1 |
| Materials | Z-Potential (mV) | Standard Deviation (mV) |
|---|---|---|
| MSN-TEDTH-PEI-FA-MPP-Sn | −25.821 a | 1.011 |
| MSN-TEDTH-PEI-FA-TR-Sn | −21.918 a | 1.137 |
| MSN-TEDTH-PEI-FA-MPP-Sn | −8.608 b | 3.056 |
| MSN-TEDTH-PEI-FA-TR-Sn | −8.105 b | 2.834 |
| Materials | IC50 (µM) | Metal [Sn] (µM) |
|---|---|---|
| MSN | >500 | - |
| MSN-TEDTH-PEI-FA | >500 | - |
| MSN-TEDTH-PEI-FA-MPP | >500 | - |
| MSN-TEDTH-PEI-FA-TR | >500 | - |
| MSN-TEDTH-PEI-FA-MPP-Sn | >500 | >1.60 |
| MSN-TEDTH-PEI-FA-TR-Sn | 228.10 | 1.20 |
| Materials | IC50 (µM) | Metal [Sn] (µM) |
|---|---|---|
| MSN | >500 | - |
| MSN-TEDTH-PEI-FA | 397.90 | - |
| MSN-TEDTH-PEI-FA-MPP | 216.50 | - |
| MSN-TEDTH-PEI-FA-TR | 391.70 | - |
| MSN-TEDTH-PEI-FA-MPP-Sn | 246.48 | 0.78 |
| MSN-TEDTH-PEI-FA-TR-Sn | 23.97 | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Almodóvar, V.; Prashar, S.; Gómez-Ruiz, S. Dual Synthetic Pathways for Organotin-Functionalized Mesoporous Silica Nanoparticles: Targeted Therapeutic Platforms with Folic Acid and PEI Formulation. Nanomaterials 2025, 15, 1791. https://doi.org/10.3390/nano15231791
García-Almodóvar V, Prashar S, Gómez-Ruiz S. Dual Synthetic Pathways for Organotin-Functionalized Mesoporous Silica Nanoparticles: Targeted Therapeutic Platforms with Folic Acid and PEI Formulation. Nanomaterials. 2025; 15(23):1791. https://doi.org/10.3390/nano15231791
Chicago/Turabian StyleGarcía-Almodóvar, Victoria, Sanjiv Prashar, and Santiago Gómez-Ruiz. 2025. "Dual Synthetic Pathways for Organotin-Functionalized Mesoporous Silica Nanoparticles: Targeted Therapeutic Platforms with Folic Acid and PEI Formulation" Nanomaterials 15, no. 23: 1791. https://doi.org/10.3390/nano15231791
APA StyleGarcía-Almodóvar, V., Prashar, S., & Gómez-Ruiz, S. (2025). Dual Synthetic Pathways for Organotin-Functionalized Mesoporous Silica Nanoparticles: Targeted Therapeutic Platforms with Folic Acid and PEI Formulation. Nanomaterials, 15(23), 1791. https://doi.org/10.3390/nano15231791








