Controlling the Orientation of MoS2 Films on Mo Metal Thin Film Through Sulfur Flux Regulation: A Novel Reaction-Diffusion Model
Abstract
1. Introduction
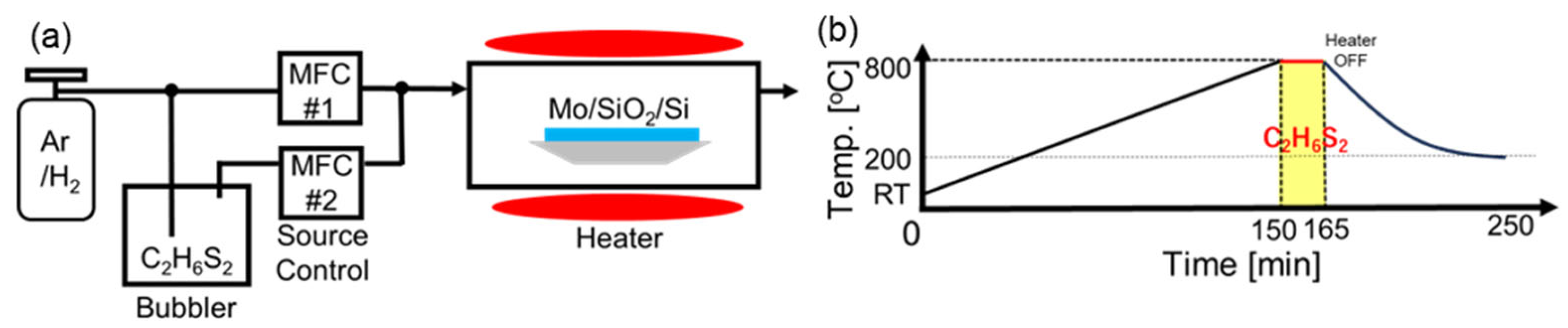
2. Materials and Methods
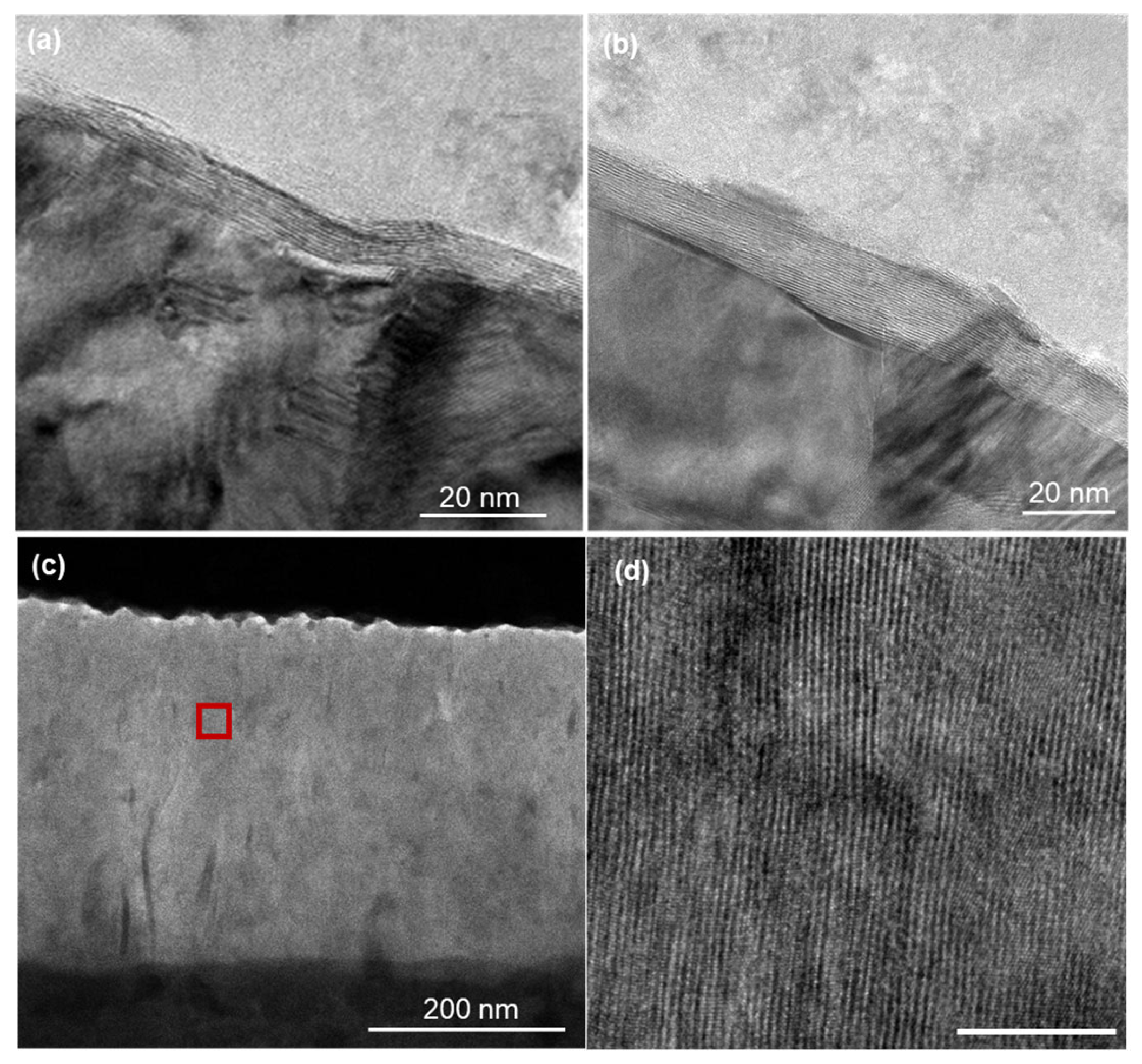
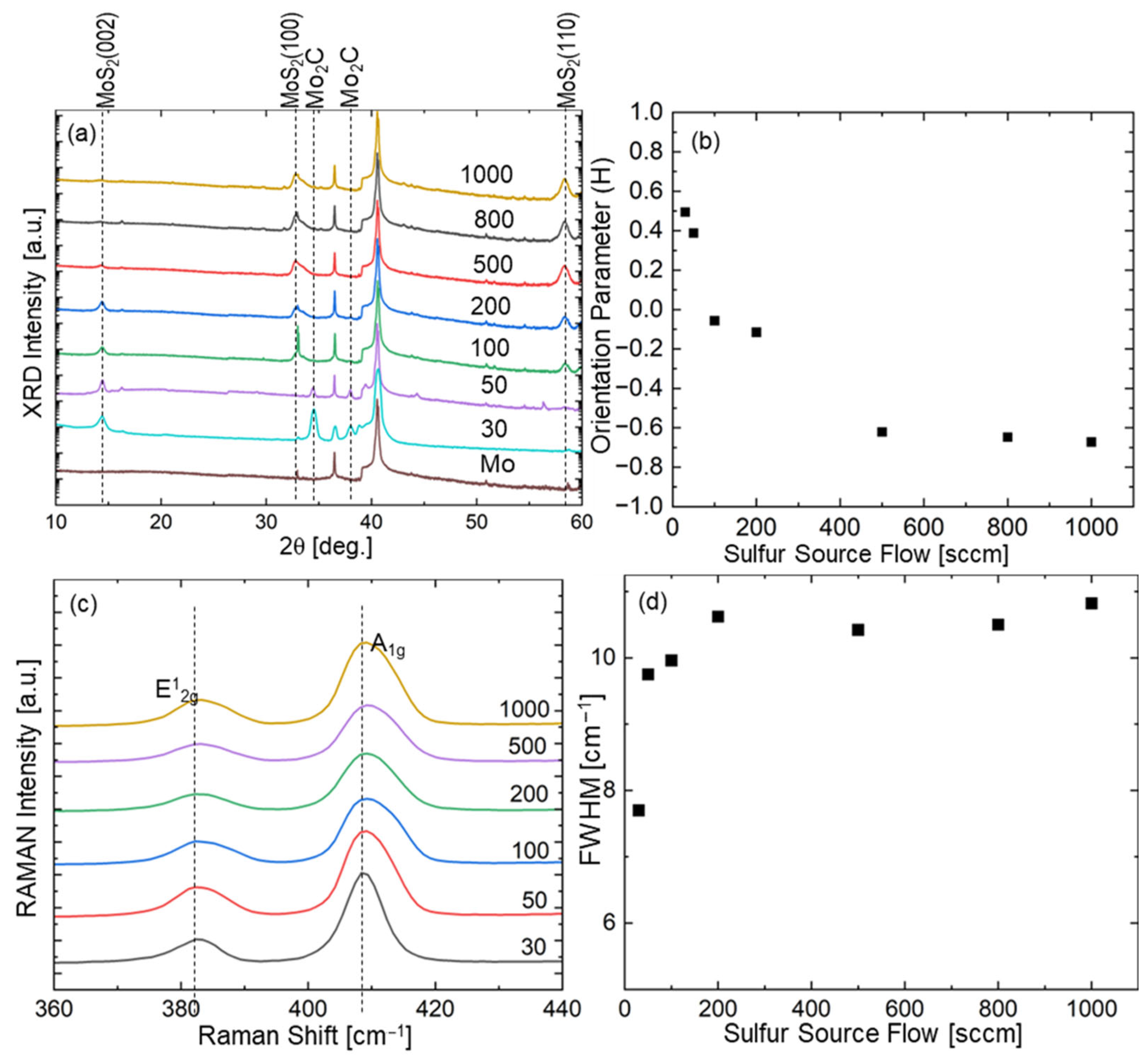
3. Results
4. Discussion
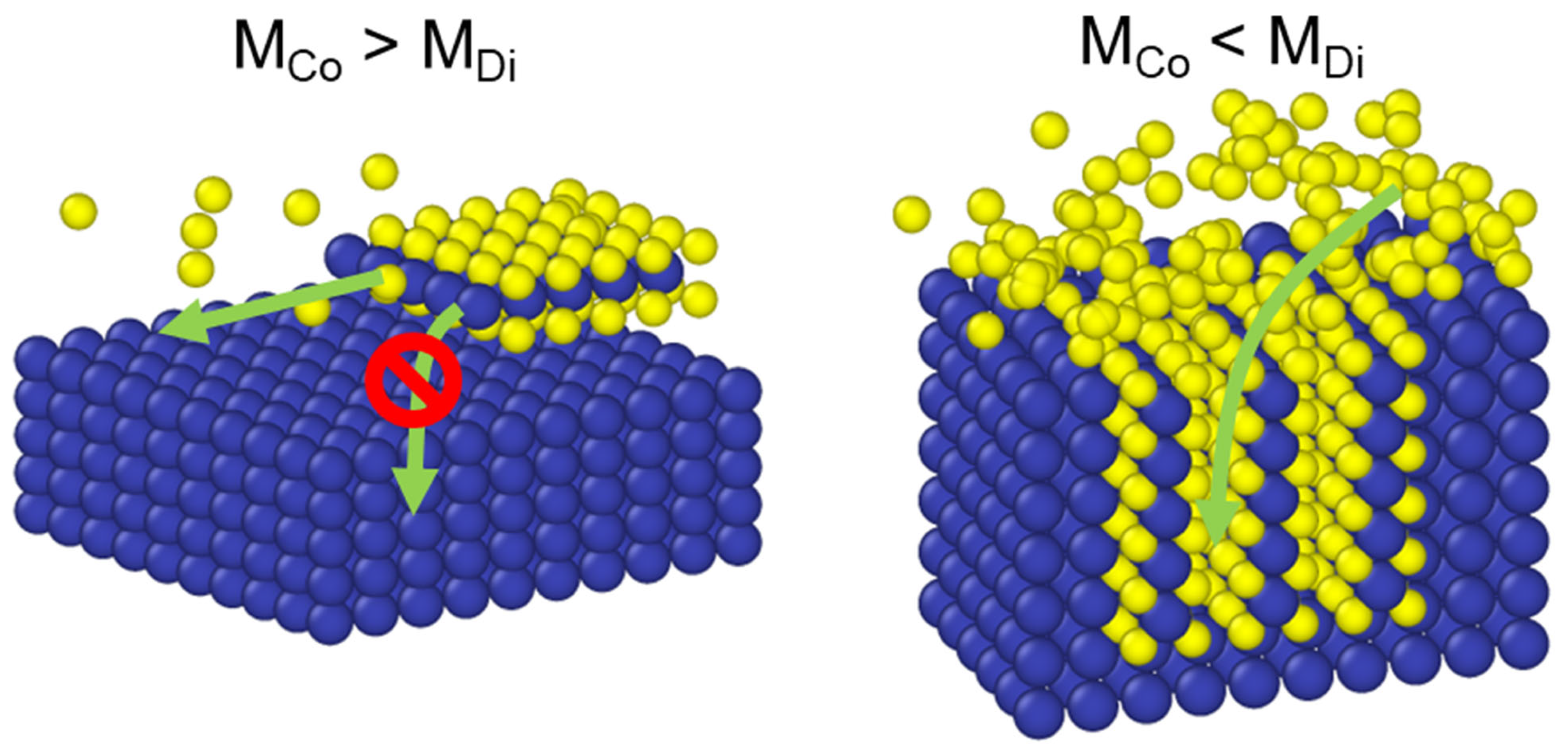
4.1. Reaction-Diffusion Model Development
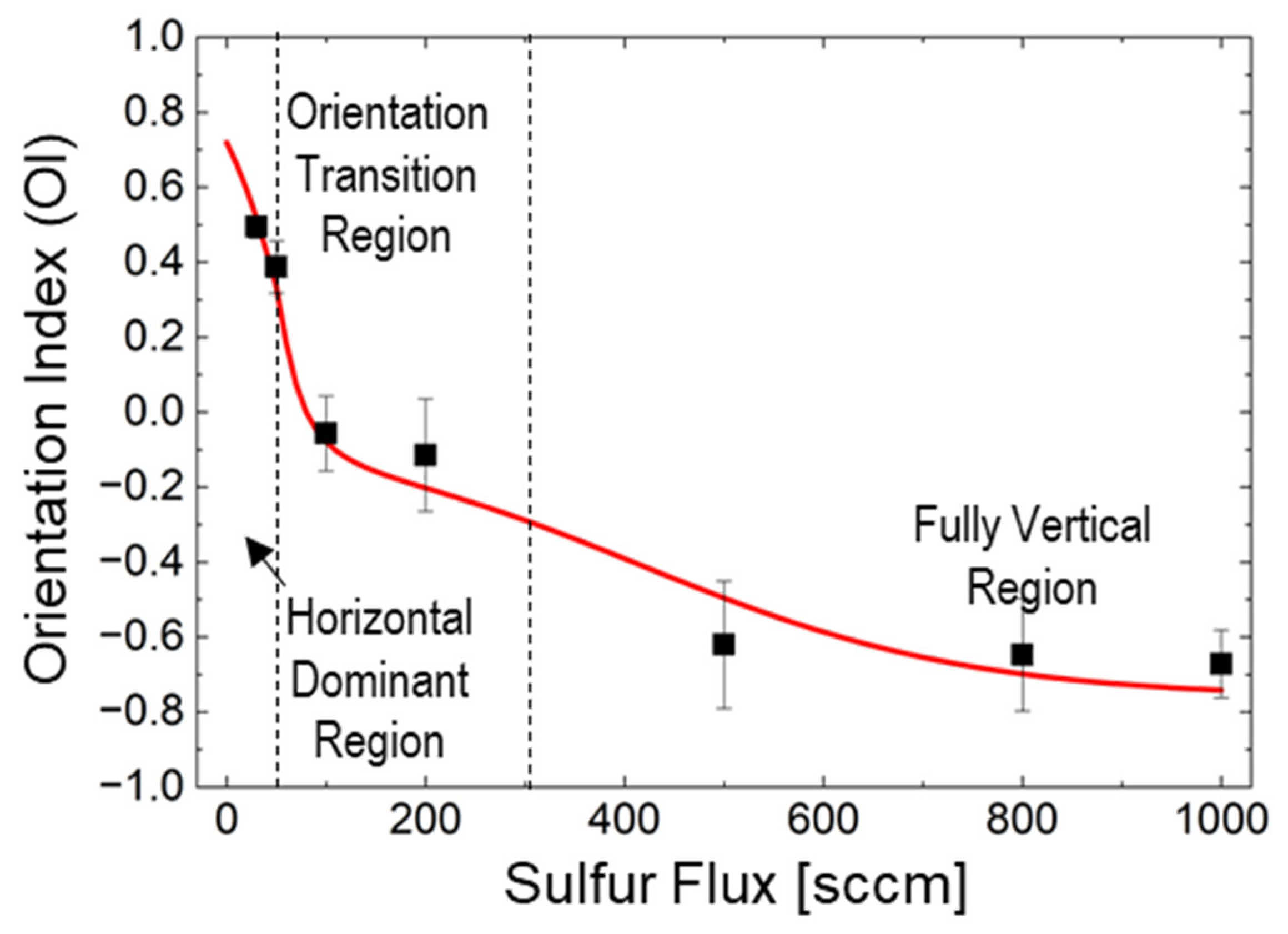
4.2. Modeling Orientation Components
- ‑
- F1 ≈ 50 sccm: This threshold marks the transition from horizontal to mixed orientation, where H(F1) = R(F1).
- ‑
- F2 ≈ 300 sccm: This threshold indicates the shift from mixed to vertical-dominated orientation, where R(F2) = V(F2).
4.2.1. Horizontal-Dominant Region (0–50 sccm)
4.2.2. Transition Region (50–300 sccm)
4.2.3. Vertical-Dominant Region (>300 sccm)
4.3. Role of Grain Boundaries in Diffusion Pathways
- (i)
- The random orientation layer shows multiple growth fronts intersecting at various angles (as indicated by arrows in Figures S1–S3), with no systematic correlation to the underlying Mo grain structure visible in Figure 3c.
- (ii)
- The stochastic distribution of these collision zones shows no preferential spacing or alignment that would correspond to the Mo grain size determined by XRD.
- (iii)
- The growth directions (arrows) converge from multiple orientations toward collision points, consistent with surface nucleation followed by vertical propagation, rather than vertical growth originating from linear features (which would be expected if Mo grain boundaries were the primary nucleation sites).
4.4. Implications for Film Transfer and Device Integration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, C.; Cao, X.; Wu, X.J.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.H.; et al. Recent advances in ultrathin two-dimensional nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Kum, H.; Bae, S.-H.; Shim, J.; Kim, H.; Kong, L.; Meng, Y.; Wang, K.; Kim, C.; Kim, J. Path towards graphene commercialization from lab to market. Nat. Nanotechnol. 2019, 14, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Peng, H.; Liu, Z. Synthesis challenges for graphene industry. Nat. Mater. 2019, 18, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Bando, M.; Kimura, N.; Shimizu, K.; Kadono, K.; Umezu, N.; Miyahara, K.; Hayazaki, S.; Nagai, S.; Mizuguchi, Y.; et al. Production of a 100-m-long high-quality graphene transparent conductive film by roll-to-roll chemical vapor deposition and transfer process. Appl. Phys. Lett. 2013, 102, 023112. [Google Scholar] [CrossRef]
- Banszerus, L.; Schmitz, M.; Engels, S.; Dauber, J.; Oellers, M.; Haupt, F.; Watanabe, K.; Taniguchi, T.; Beschoten, B.; Stampfer, C. Ultrahigh-mobility graphene devices from chemical vapor deposition on reusable copper. Sci. Adv. 2015, 1, e1500222. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Wu, S.; He, Q.; Tan, C.; Wang, Y.; Zhang, H. Graphene-based electrochemical sensors. Small 2013, 9, 1160–1172. [Google Scholar] [CrossRef]
- Polat, E.O.; Balci, O.; Kakenov, N.; Uzlu, H.B.; Kocabas, C.; Dahiya, R. Synthesis of large area graphene for high performance in flexible optoelectronic devices. Sci. Rep. 2015, 5, 16744. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Sun, Z.; Hasan, T.; Ferrari, A.C. Graphene photonics and optoelectronics. Nat. Photonics 2010, 4, 611–622. [Google Scholar] [CrossRef]
- Yang, N.; Zhai, J.; Wang, D.; Chen, Y.; Jiang, L. Two-Dimensional Graphene Bridges Enhanced Photoinduced Charge Transport in Dye-Sensitized Solar Cells. ACS Nano 2010, 4, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Hu, Y.H. Recent advances in graphene-based materials for fuel cell applications. Energy Sci. Eng. 2021, 9, 958–983. [Google Scholar] [CrossRef]
- Kim, J.; Chang, H.; Bae, G.; Choi, M.; Jeon, S. Graphene-based thermoelectric materials: Toward sustainable energy-harvesting systems. Chem. Commun. 2025, 61, 5050–5063. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, K.G.; Zhao, H.L. Graphene-based nanomaterials for lithium-ion batteries. J. Mater. Chem. 2010, 20, 5983–5992. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Shao, Y.; Kaner, R.B. Graphene for batteries, supercapacitors and beyond. Nat. Rev. Mater. 2016, 1, 16033. [Google Scholar] [CrossRef]
- Qian, Y.; Lyu, Z.; Zhang, Q.; Lee, T.H.; Kang, T.K.; Sohn, M.; Shen, L.; Kim, D.H.; Kang, D.J. High-performance flexible energy storage devices based on graphene decorated with flower-shaped MoS2 heterostructures. Micromachines 2023, 14, 297. [Google Scholar] [CrossRef]
- Jang, H.; Park, Y.J.; Chen, X.; Das, T.; Kim, M.S.; Ahn, J.H. Graphene-based flexible and stretchable electronics. Adv. Mater. 2016, 28, 4184–4202. [Google Scholar] [CrossRef]
- Li, X.; Magnuson, C.W.; Venugopal, A.; Tromp, R.M.; Hannon, J.B.; Vogel, E.M.; Colombo, L.; Ruoff, R.S. Large-area graphene single crystals grown by low-pressure chemical vapor deposition of methane on copper. J. Am. Chem. Soc. 2011, 133, 2816–2819. [Google Scholar] [CrossRef]
- Avouris, P.; Dimitrakopoulos, C. Graphene: Synthesis and applications. Mater. Today 2012, 15, 86–97. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Thayil, R.; Krishna, K.G.; Chinthamreddy, A.; Parne, S.R. 2D MoS2 for Next-Generation Electronics and Optoelectronics: From Material Properties to Manufacturing Challenges and Future Prospects. Small 2025, 21, 2412467. [Google Scholar] [CrossRef]
- Mattevi, C.; Kim, H.; Chhowalla, M. A review of chemical vapour deposition of graphene on copper. J. Mater. Chem. 2011, 21, 3324–3334. [Google Scholar] [CrossRef]
- Chen, L.; Xu, H.; Gao, J.; Warner, J.H.; Castell, M.R. Step-edge-guided nucleation and growth of aligned WSe2 on sapphire via a layer-over-layer growth mode. ACS Nano 2015, 9, 8368–8375. [Google Scholar] [CrossRef] [PubMed]
- Sharbidre, R.S.; Narute, P.; Byen, J.C.; Kim, D.; Park, J.; Park, B.C.; Hong, S.-G. In-Column Backscattered Electron Microscopy for a Rapid Identification of the Number of Layers in MoS2 Nanosheets: Implications for Electronic and Optoelectronic Devices. ACS Appl. Nano Mater. 2024, 7, 153–162. [Google Scholar] [CrossRef]
- Orofeo, C.M.; Suzuki, S.; Sekine, Y.; Hibino, H. Scalable synthesis of layer-controlled WS2 and MoS2 sheets by sulfurization of thin metal films. Appl. Phys. Lett. 2014, 105, 083112. [Google Scholar] [CrossRef]
- Ji, Q.; Kan, M.; Zhang, Y.; Guo, Y.; Ma, D.; Shi, J.; Sun, Q.; Chen, Q.; Zhang, Y.; Liu, Z. Unravelling orientation distribution and merging behavior of monolayer MoS2 domains on sapphire. Nano Lett. 2015, 15, 198–205. [Google Scholar] [CrossRef]
- Jeon, J.; Jang, S.K.; Jeon, S.M.; Yoo, G.; Lee, Y.H.; Park, J.H. Layer-controlled CVD growth of large-area two-dimensional MoS2 films. Nanoscale 2015, 7, 1688–1695. [Google Scholar] [CrossRef]
- Ho, Y.T.; Ma, C.H.; Luong, T.T.; Wei, L.L.; Yen, T.C.; Hsu, W.T.; Chang, W.H.; Chu, Y.C.; Tu, Y.Y.; Pande, K.P.; et al. Layered MoS2 grown on c-sapphire by pulsed laser deposition. Phys. Status Solidi RRL 2015, 9, 187–191. [Google Scholar] [CrossRef]
- Youn, H.Y.; Park, S.J.; Kim, M.H.; Lee, J.S.; Kim, T.W.; Park, C.G. Soft sputtering of large-area 2D MoS2 layers using isolated plasma soft deposition for humidity sensors. Adv. Mater. 2024, 37, 2414800. [Google Scholar] [CrossRef]
- Ajayeoba, Y.A.; Sobola, D.; Kaspar, P.; Papes, M.; Gablech, I.; Horak, P.; Dallaev, R. Surface structural probing and photoelectrochemical characterization of electrodeposited MoS2 nanostructured thin film. Sci. Rep. 2025, 15, 14077. [Google Scholar] [CrossRef]
- Singh, A.K. 2D Layered Transition Metal Dichalcogenides (MoS2): Synthesis, Applications and Theoretical Aspects. Appl. Mater. Today 2018, 13, 242–270. [Google Scholar] [CrossRef]
- Ikram, M.; Raza, A.; Ali, S. Two-Dimensional Transition Metal Dichalcogenides: Synthesis and Applications; Springer: Berlin/Heidelberg, Germany, 2019; ISBN 978-981-13-9045-6. [Google Scholar]
- Khan, K.; Tareen, A.K.; Aslam, M.; Wang, R.; Zhang, Y.; Mahmood, A.; Ouyang, Z.; Zhang, H.; Guo, Z. Recent developments in emerging two-dimensional materials and their applications. J. Mater. Chem. C 2020, 8, 387–440. [Google Scholar] [CrossRef]
- Kumar, V.P.; Panda, D.K. Review—Next Generation 2D Material Molybdenum Disulfide (MoS2): Properties, Applications and Challenges. ECS J. Solid State Sci. Technol. 2022, 11, 033012. [Google Scholar] [CrossRef]
- Mouloua, D.; Rajput, N.S.; Foran, E.; Cloutier, S.G.; Konstantatos, G.; Jouiad, M.; El Khakani, M.A. Tuning the Optoelectronic Properties of Pulsed Laser Deposited “3D”-MoS2 Films via the Degree of Vertical Alignment of Their Constituting Layers. Adv. Opt. Mater. 2024, 12, 2302966. [Google Scholar] [CrossRef]
- Tian, X.; Wang, S.; Li, H.; Li, M.; Chen, T.; Xiao, X.; Wang, Y. Recent advances in MoS2-based nanomaterial sensors for room-temperature gas detection: A review. Sens. Diagn. 2023, 2, 1118–1154. [Google Scholar] [CrossRef]
- Kočí, M.; Izsák, T.; Vanko, G.; Sojková, M.; Hrdá, J.; Szabó, O.; Husák, M.; Végsö, K.; Varga, M.; Kromka, A. Improved Gas Sensing Capabilities of MoS2/Diamond Heterostructures at Room Temperature. ACS Appl. Mater. Interfaces 2023, 15, 34206–34214. [Google Scholar] [CrossRef]
- Kumar, R.; Liu, X.; Zhang, J.; Kumar, M. Room-temperature gas sensors under photoactivation: From metal oxides to 2D materials. Nano-Micro Lett. 2020, 12, 164. [Google Scholar] [CrossRef]
- Kim, J.; Takahashi, K.; Takaetsu, Y.; Funatsu, A. Characterization of Vertically Aligned MoS2 Thin Film on Mo Electrode for Hydrogen Evolution Catalyst. J. Jpn. Inst. Energy 2021, 100, 283–287. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, H.; Li, S.; Wang, R.; Sun, X.; Zhou, M.; Zhou, J.; Lou, X.W.; Xie, Y. Defect-rich MoS2 ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution. Adv. Mater. 2013, 25, 5807–5813. [Google Scholar] [CrossRef]
- Zhao, X.; Rui, K.; Dou, S.X.; Sun, W. Heterostructures for electrochemical hydrogen evolution reaction: A review. Adv. Funct. Mater. 2018, 28, 1803291. [Google Scholar] [CrossRef]
- Stern, C.; Grinvald, S.; Kirshner, M.; Sinai, O.; Oksman, M.; Alon, H.; Meiron, O.E.; Bar-Sadan, M.; Houben, L.; Naveh, D. Growth mechanisms and electronic properties of vertically aligned MoS2. Sci. Rep. 2018, 8, 16480. [Google Scholar] [CrossRef] [PubMed]
- Shokhen, V.; Miroshnikov, Y.; Gershinsky, G.; Gotlib, N.; Stern, C.; Naveh, D.; Zitoun, D. On the impact of vertical alignment of MoS2 for efficient lithium storage. Sci. Rep. 2017, 7, 3280. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Shen, J.; Liu, Y.; Woods, J.M.; Sun, Y.; Cha, J.J. Metal seed layer thickness-induced transition from vertical to horizontal growth of MoS2 and WS2. Nano Lett. 2014, 14, 6842–6849. [Google Scholar] [CrossRef]
- He, M.; Kong, F.; Yin, G.; Lv, Z.; Sun, X.; Shi, H.; Gao, B. Enhanced hydrogen evolution reaction activity of hydrogen-annealed vertical MoS2 nanosheets. RSC Adv. 2018, 8, 14369–14376. [Google Scholar] [CrossRef]
- Sojková, M.; Vegso, K.; Mrkyvkova, N.; Hagara, J.; Hutár, P.; Rosová, A.; Čaplovičová, M.; Ludacka, U.; Skákalová, V.; Majková, E.; et al. Tuning the orientation of few-layer MoS2 films using one-zone sulfurization. RSC Adv. 2019, 9, 29645–29651. [Google Scholar] [CrossRef]
- Shaji, A.; Vegso, K.; Sojkova, M.; Hulman, M.; Nadazdy, P.; Hutar, P.; Pribusova Slusna, L.; Hrda, J.; Bodik, M.; Hodas, M.; et al. Orientation of Few-Layer MoS2 Films: In-Situ X-ray Scattering Study During Sulfurization. J. Phys. Chem. C 2021, 125, 9461–9471. [Google Scholar] [CrossRef]
- Zhu, H.; Nayir, N.; Choudhury, T.H.; Bansal, A.; Huet, B.; Zhang, K.; Puretzky, A.A.; Bachu, S.; York, K.; McKnight, T.V.; et al. Step engineering for nucleation and domain orientation control in WSe2 epitaxy on c-plane sapphire. Nat. Nanotechnol. 2023, 18, 1295–1302. [Google Scholar] [CrossRef]
- Gurarslan, A.; Yu, Y.; Su, L.; Yu, Y.; Suarez, F.; Yao, S.; Zhu, Y.; Ozturk, M.; Zhang, Y.; Cao, L. Surface-energy-assisted perfect transfer of centimeter-scale monolayer and few-layer MoS2 films onto arbitrary substrates. ACS Nano 2014, 8, 11522–11528. [Google Scholar] [CrossRef]
- Desai, S.B.; Madhvapathy, S.R.; Amani, M.; Kiriya, D.; Hettick, M.; Tosun, M.; Zhou, Y.; Dubey, M.; Ager III, J.W.; Chrzan, D.; et al. Gold-mediated exfoliation of ultralarge optoelectronically-perfect monolayers. Adv. Mater. 2016, 28, 4053–4058. [Google Scholar] [CrossRef]
- Meyer, B. Elemental sulfur. Chem. Rev. 1976, 76, 367–388. [Google Scholar] [CrossRef]
- Zeng, H.; Wen, Y.; Yin, L.; Cheng, R.; Wang, H.; Liu, C.; He, J. Recent developments in CVD growth and applications of 2D transition metal dichalcogenides. Front. Phys. 2023, 18, 53603. [Google Scholar] [CrossRef]
- Choi, S.H.; Stephen, B.; Park, J.H.; Lee, J.S.; Kim, S.M.; Yang, W.; Kim, K.K. Water-assisted synthesis of molybdenum disulfide film with single organic liquid precursor. Sci. Rep. 2017, 7, 1983. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Viswanath, B. Horizontally and vertically aligned growth of strained MoS2 layers with dissimilar wetting and catalytic behaviors. CrystEngComm 2017, 19, 5068–5078. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Yap, C.C.R.; Tay, B.K.; Edwin, T.H.T.; Olivier, A.; Baillargeat, D. From bulk to monolayer MoS2: Evolution of Raman scattering. Adv. Funct. Mater. 2012, 22, 1385–1390. [Google Scholar] [CrossRef]
- Gong, C.; Lin, J.; Wang, X.; Shi, G.; Lei, S.; Lin, Z.; Zou, X.; Ye, G.; Vajtai, R.; Yakobson, B.I.; et al. Metal contacts on physical vapor deposited monolayer MoS2. ACS Nano 2013, 7, 11350–11357. [Google Scholar] [CrossRef]
- Shang, S.L.; Lindwall, G.; Wang, Y.; Redwing, J.M.; Anderson, T.; Liu, Z.K. Lateral versus vertical growth of two-dimensional layered transition-metal dichalcogenides: Thermodynamic insight into MoS2. Nano Lett. 2016, 16, 5742–5750. [Google Scholar] [CrossRef]
- Kong, D.; Wang, H.; Cha, J.J.; Pasta, M.; Koski, K.J.; Yao, J.; Cui, Y. Synthesis of MoS2 and MoSe2 films with vertically aligned layers. Nano Lett. 2013, 13, 1341–1347. [Google Scholar] [CrossRef]
- Wang, H.; Tsai, C.; Kong, D.; Chan, K.; Abild-Pedersen, F.; Nørskov, J.K.; Cui, Y. Transition-metal doped edge sites in vertically aligned MoS2 catalysts for enhanced hydrogen evolution. Nano Res. 2015, 8, 566–575. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Chang, Y.-H.; Hsu, C.-L.; Wei, K.-H.; Chiang, C.-Y.; Li, L.-J. Comparative study on MoS2 and WS2 for electrocatalytic water splitting. Int. J. Hydrogen Energy 2013, 38, 12302–12309. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Chen, Z.; Reinecke, B.N.; Jaramillo, T.F. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nat. Mater. 2012, 11, 963–969. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Ike, M.; Tokuda, K. Controlling the Orientation of MoS2 Films on Mo Metal Thin Film Through Sulfur Flux Regulation: A Novel Reaction-Diffusion Model. Nanomaterials 2025, 15, 1783. https://doi.org/10.3390/nano15231783
Kim J, Ike M, Tokuda K. Controlling the Orientation of MoS2 Films on Mo Metal Thin Film Through Sulfur Flux Regulation: A Novel Reaction-Diffusion Model. Nanomaterials. 2025; 15(23):1783. https://doi.org/10.3390/nano15231783
Chicago/Turabian StyleKim, Joonam, Masakazu Ike, and Kenichi Tokuda. 2025. "Controlling the Orientation of MoS2 Films on Mo Metal Thin Film Through Sulfur Flux Regulation: A Novel Reaction-Diffusion Model" Nanomaterials 15, no. 23: 1783. https://doi.org/10.3390/nano15231783
APA StyleKim, J., Ike, M., & Tokuda, K. (2025). Controlling the Orientation of MoS2 Films on Mo Metal Thin Film Through Sulfur Flux Regulation: A Novel Reaction-Diffusion Model. Nanomaterials, 15(23), 1783. https://doi.org/10.3390/nano15231783





