Highly Efficient Cellulose Nanofiber/Halloysite Nanotube Separators for Sodium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
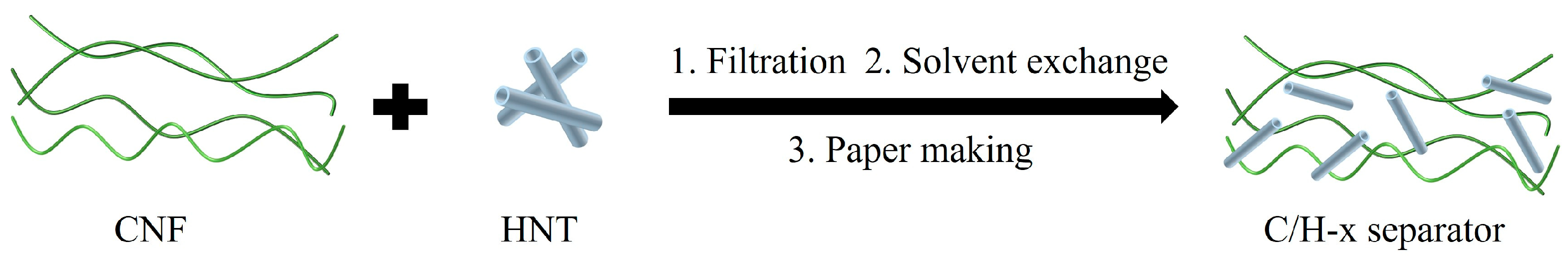
2.2. Preparation of CNF/HNT Separators
2.3. Characterization
3. Results and Discussion
3.1. Preparation of Composite Separators
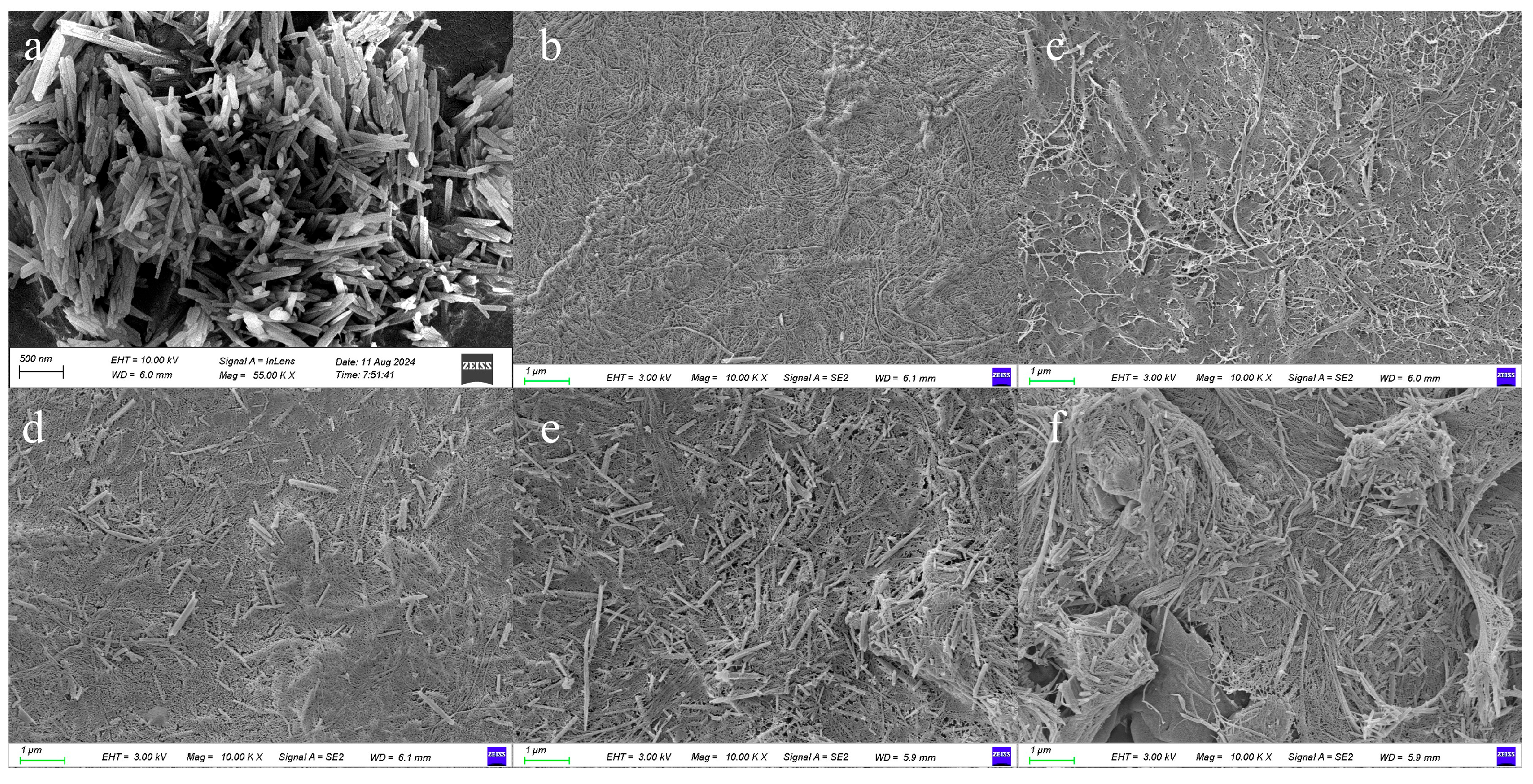
3.2. Morphology Study of Composite Separators
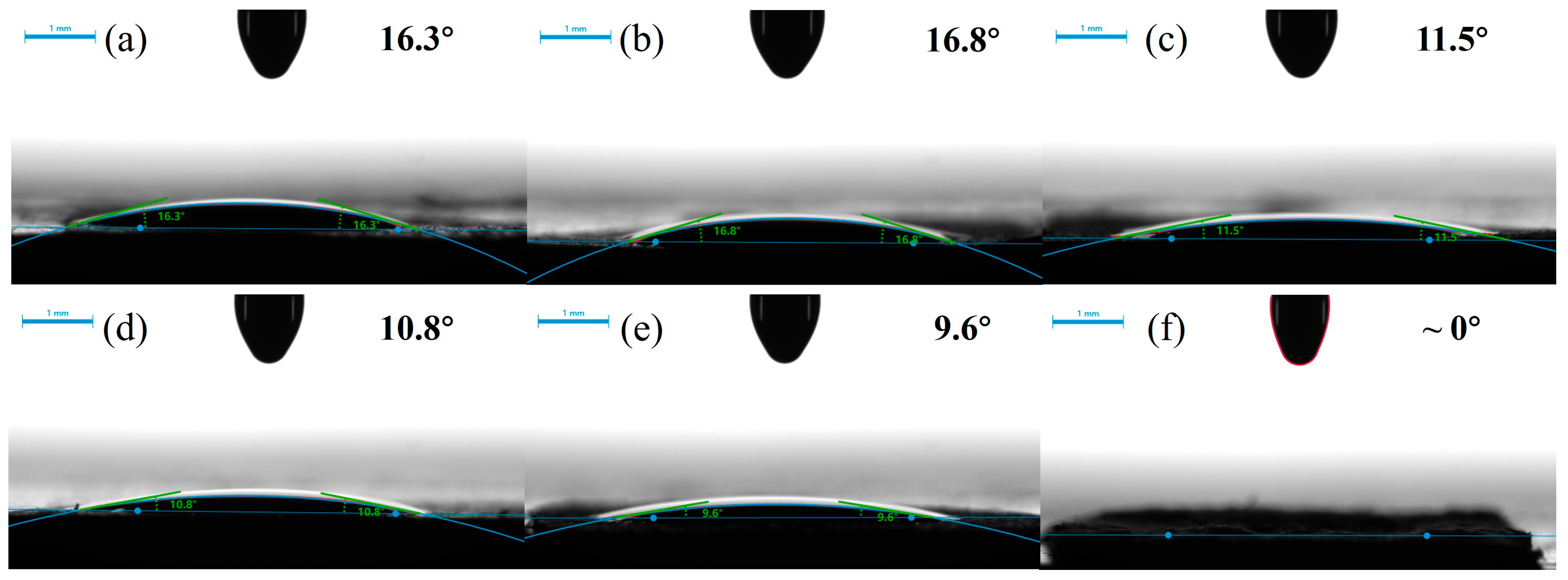
3.3. Wettability of Composite Separators
3.4. Mechanical Properties of Composite Separators
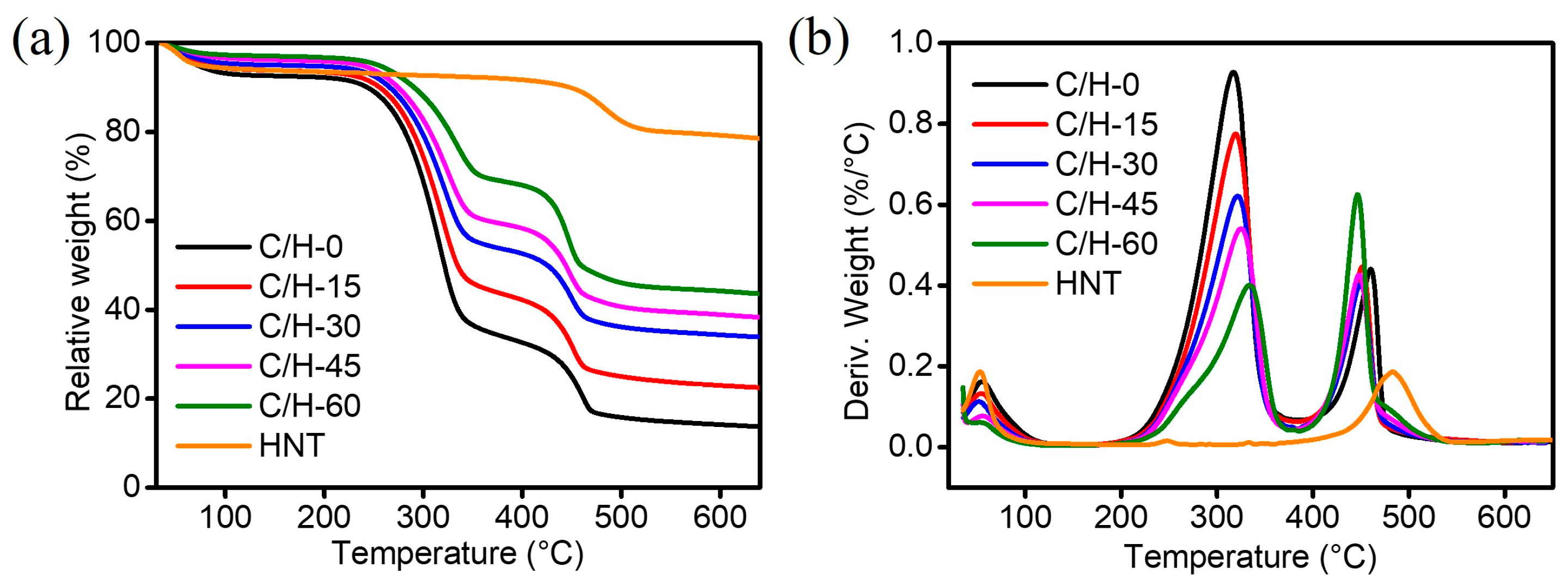
3.5. Thermal Stability of Composite Separators
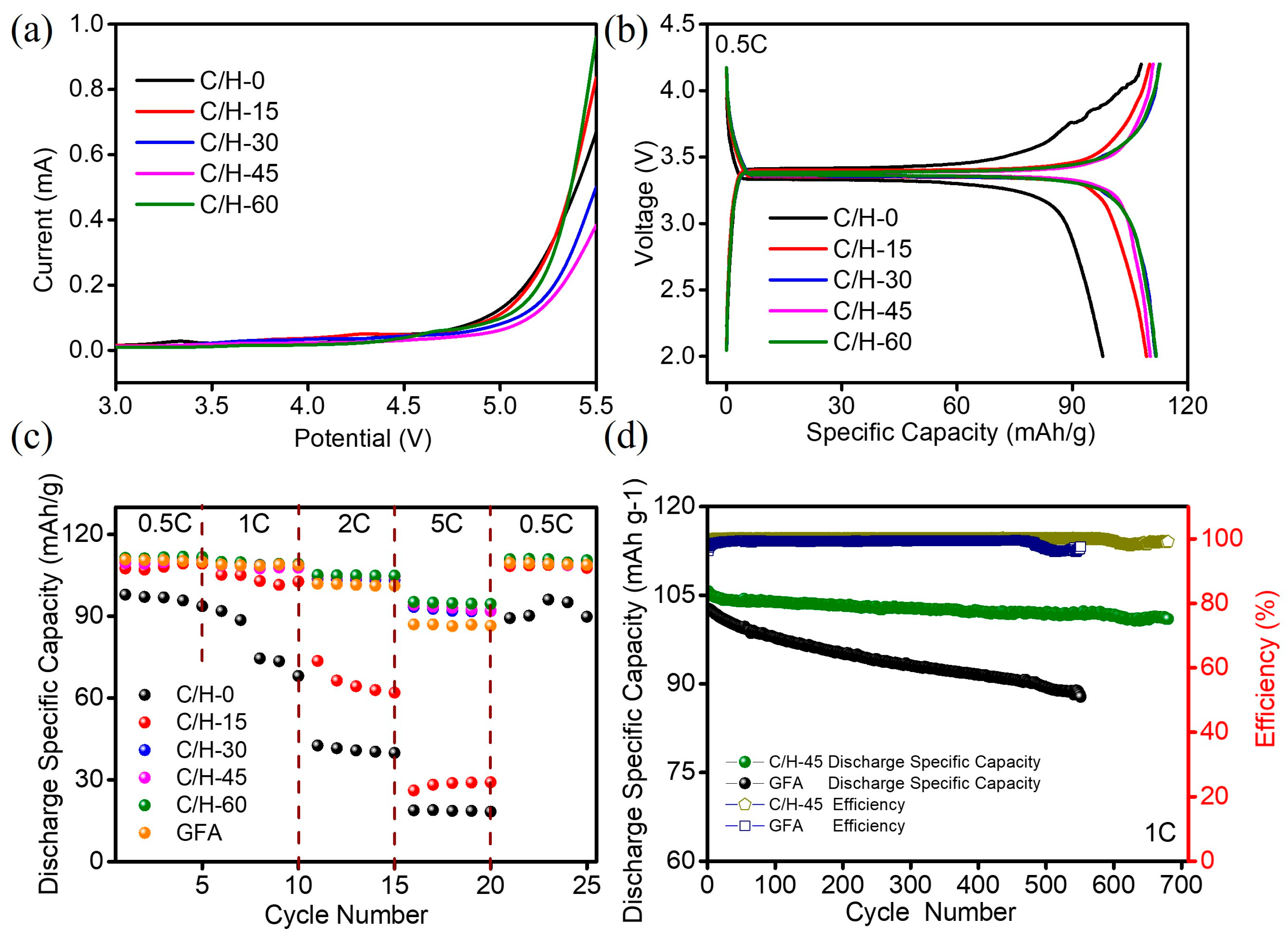
3.6. Performance of Sodium-Ion Batteries with Different Composite Separators
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xue, Z.; Zhu, D.; Shan, M.; Wang, H.; Zhang, J.; Cui, G.; Hu, Z.; Gordon, K.C.; Xu, G.; Zhu, M. Functional separator materials of sodium-ion batteries: Grand challenges and industry perspectives. Nano Today 2024, 55, 102175. [Google Scholar] [CrossRef]
- Yu, W.; Shen, L.; Lu, X.; Han, J.; Geng, N.; Lai, C.; Xu, Q.; Peng, Y.; Min, Y.; Lu, Y. Novel Composite Separators Based on Heterometallic Metal-Organic Frameworks Improve the Performance of Lithium-Ion Batteries. Adv. Energy Mater. 2023, 13, 2204055. [Google Scholar] [CrossRef]
- Gao, Z.; Wen, R.; Deng, H.; Luo, L.; Cui, X.; Yang, Z.; Zheng, Z.; Zhang, J. Composite Membrane of Poly (vinylidene fluoride) and 2D Ni(OH)2 Nanosheets for High-Performance Lithium-Ion Battery. ACS Appl. Polym. Mater. 2022, 4, 960–970. [Google Scholar] [CrossRef]
- Gao, Z.; Luo, L.; Wen, R.; Song, X.; Gao, Z.; Zheng, Z.; Zhang, J. A multifunctional composite membrane for high-safety lithium-ion batteries. J. Mater. Chem. A 2023, 11, 1774–1784. [Google Scholar] [CrossRef]
- Wu, D.; Dong, N.; Wang, R.; Qi, S.; Liu, B.; Wu, D. In situ construction of High-safety and Non-flammable polyimide “Ceramic” Lithium-ion battery separator via SiO2 Nano-Encapsulation. Chem. Eng. J. 2021, 420, 129992. [Google Scholar] [CrossRef]
- Ryu, J.; Han, D.-Y.; Hong, D.; Park, S. A polymeric separator membrane with chemoresistance and high Li-ion flux for high-energy-density lithium metal batteries. Energy Storage Mater. 2022, 45, 941–951. [Google Scholar] [CrossRef]
- Peng, B.; Wan, G.; Ahmad, N.; Yu, L.; Ma, X.; Zhang, G. Recent Progress in the Emerging Modification Strategies for Layered Oxide Cathodes toward Practicable Sodium Ion Batteries. Adv. Energy Mater. 2023, 13, 2300334. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, Y.; Sun, F.; Osenberg, M.; Hilger, A.; Manke, I.; Cao, R.; Dou, S.X.; Fan, H.J. Designing Solvated Double-Layer Polymer Electrolytes with Molecular Interactions Mediated Stable Interfaces for Sodium Ion Batteries. Angew. Chem. Int. Edit. 2023, 62, e202219000. [Google Scholar] [CrossRef] [PubMed]
- Wostoupal, O.S.; Wang, X.; Liu, Q.; Xu, T.; Zhang, Z. Electrolyte Compatible Separator Materials for Sodium-Ion Battery. J. Electrochem. Soc. 2025, 172, 060515. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, H.; Tong, X.; Zhuo, H.; Yang, W.; Chen, Y.; Shi, G.; Chen, Z.; Zhong, L.; Peng, X. Amphoteric Supramolecular Nanofiber Separator for High-Performance Sodium-Ion Batteries. Energy Environ. Mater. 2024, 7, e12735. [Google Scholar] [CrossRef]
- Yu, L.; Gu, J.; Pan, C.; Zhang, J.; Wei, Z.; Zhao, Y. Recent developments of composite separators based on high-performance fibers for lithium batteries. Compos. Part A Appl. S 2022, 162, 107132. [Google Scholar] [CrossRef]
- Zhu, T.; Liu, H.; Sun, C.; Ma, Z.; Zuo, X.; Nan, J. Paper-Based Composite Separator with Thermotolerant and Flame-Retardant Sodium Silicate Sheath for High-Performance Sodium-Ion Batteries. ACS Appl. Energy Mater. 2024, 7, 1212–1223. [Google Scholar] [CrossRef]
- Babiker, D.M.D.; Usha, Z.R.; Wan, C.; Hassaan, M.M.E.; Chen, X.; Li, L. Recent progress of composite polyethylene separators for lithium/sodium batteries. J. Power Sources 2023, 564, 232853. [Google Scholar] [CrossRef]
- Heidari, A.A.; Mahdavi, H. Recent Development of Polyolefin-Based Microporous Separators for Li−Ion Batteries: A Review. Chem. Rec. 2019, 20, 570–595. [Google Scholar] [CrossRef]
- Ma, X.; Chen, Z.; Zhang, T.; Zhang, X.; Ma, Y.; Guo, Y.; Wei, Y.; Ge, M.; Hou, Z.; Zi, Z. Efficient utilization of glass fiber separator for low-cost sodium-ion batteries. Int. J. Min. Met. Mater. 2023, 30, 1878–1886. [Google Scholar] [CrossRef]
- Li, J.; Meng, L.; Chen, J.; Chen, X.; Wang, Y.; Xiao, Z.; Wang, H.; Liang, D.; Xie, Y. Encapsulation of polyethylene glycol in cellulose-based porous capsules for latent heat storage and light-to-thermal conversion. Front. Chem. Sci. Eng. 2023, 17, 1038–1050. [Google Scholar] [CrossRef]
- Li, J.; Meng, L.; Xu, Y.; Wang, Y.; Xiao, Z.; Wang, H.; Liang, D.; Xie, Y. Hybrid nanoparticles of quaternary ammonium cellulose derivatives and citric acid for enhancing the antibacterial activity of polyvinyl alcohol composites. Cellulose 2023, 30, 3625–3638. [Google Scholar] [CrossRef]
- Meng, L.; Li, J.; Fan, X.; Wang, Y.; Xiao, Z.; Wang, H.; Liang, D.; Xie, Y. Improved mechanical and antibacterial properties of polyvinyl alcohol composite films using quaternized cellulose nanocrystals as nanofillers. Compos. Sci. Technol. 2023, 232, 109885. [Google Scholar] [CrossRef]
- Sheng, J.; Chen, T.; Wang, R.; Zhang, Z.; Hua, F.; Yang, R. Ultra-light cellulose nanofibril membrane for lithium-ion batteries. J. Membrane Sci. 2020, 595, 117550. [Google Scholar] [CrossRef]
- Liu, A.; Li, S.; Jiang, Z.; Du, J.; Tao, Y.; Lu, J.; Cheng, Y.; Wang, H. A renewable membrane with high ionic conductivity and thermal stability for Li-ion batteries. J. Power Sources 2022, 521, 230947. [Google Scholar] [CrossRef]
- Liu, J.; Yang, K.; Mo, Y.; Wang, S.; Han, D.; Xiao, M.; Meng, Y. Highly safe lithium-ion batteries: High strength separator from polyformaldehyde/cellulose nanofibers blend. J. Power Sources 2018, 400, 502–510. [Google Scholar] [CrossRef]
- Chen, P.; Lin, X.; Yang, B.; Gao, Y.; Xiao, Y.; Li, L.; Zhang, H.; Li, L.; Zheng, Z.; Wang, J.; et al. Cellulose Separators for Rechargeable Batteries with High Safety: Advantages, Strategies, and Perspectives. Adv. Funct. Mater. 2024, 34, 2409368. [Google Scholar] [CrossRef]
- Liu, H.; Tao, R.; Guo, C.; Zhang, W.; Liu, X.; Guo, P.; Zhang, T.; Liang, J. Lithiated halloysite nanotube/cross-linked network polymer composite artificial solid electrolyte interface layer for high-performance lithium metal batteries. Chem. Eng. J. 2022, 429, 132239. [Google Scholar] [CrossRef]
- Zhou, Q.; Hussain, S.; Peng, J.; Zhang, B.; Wang, L. Multi-channeled halloysite nanotube-blended polybenzimidazole separators for enhancing lithium-ion battery performance. J. Membr. Sci. 2025, 714, 123417. [Google Scholar] [CrossRef]
- Huang, C.; Ji, H.; Guo, B.; Luo, L.; Xu, W.; Li, J.; Xu, J. Composite nanofiber membranes of bacterial cellulose/halloysite nanotubes as lithium ion battery separators. Cellulose 2019, 26, 6669–6681. [Google Scholar] [CrossRef]
- Li, J.; Jia, H.; Ma, S.; Xie, L.; Wei, X.-X.; Dai, L.; Wang, H.; Su, F.; Chen, C.-M. Separator Design for High-Performance Supercapacitors: Requirements, Challenges, Strategies, and Prospects. ACS Energy Lett. 2022, 8, 56–78. [Google Scholar] [CrossRef]
- Toivonen, M.S.; Onelli, O.D.; Jacucci, G.; Lovikka, V.; Rojas, O.J.; Ikkala, O.; Vignolini, S. Anomalous-Diffusion-Assisted Brightness in White Cellulose Nanofibril Membranes. Adv. Mater. 2018, 30, 1704050. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Chien, L.-H.; Kuo, P.-L. High thermal and electrochemical stability of a SiO2 nanoparticle hybird–polyether cross-linked membrane for safety reinforced lithium-ion batteries. RSC Adv. 2016, 6, 18089–18095. [Google Scholar] [CrossRef]
- He, X.; Ni, Y.; Hou, Y.; Lu, Y.; Jin, S.; Li, H.; Yan, Z.; Zhang, K.; Chen, J. Insights into the Ionic Conduction Mechanism of Quasi-Solid Polymer Electrolytes through Multispectral Characterization. Angew. Chem. Int. Edit. 2021, 60, 22672–22677. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; An, B.; Li, X.; Zai, Z.; Wu, S.; Ma, J.; Zhang, L. Effect of different electronegative oxygen atoms of cellulose nanofibrils on the formation and photocatalytic property of ZnO/cellulose composite. Appl. Surf. Sci. 2023, 637, 157974. [Google Scholar] [CrossRef]
- Tang, Y.; Ye, L.; Zhang, Z.; Friedrich, K. Interlaminar fracture toughness and CAI strength of fibre-reinforced composites with nanoparticles—A review. Compos. Sci. Technol. 2013, 86, 26–37. [Google Scholar] [CrossRef]
- Huang, D.; Zhang, Z.; Zheng, Y.; Quan, Q.; Wang, W.; Wang, A. Synergistic effect of chitosan and halloysite nanotubes on improving agar film properties. Food Hydrocolloid. 2020, 101, 105471. [Google Scholar] [CrossRef]
- Dong, N.; Wang, J.; Chen, N.; Liu, B.; Tian, G.; Qi, S.; Sun, G.; Wu, D. In Situ Reinforcing: ZrO2-Armored Hybrid Polyimide Separators for Advanced and Safe Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2021, 9, 6250–6257. [Google Scholar] [CrossRef]
- Yanilmaz, M.; Lu, Y.; Dirican, M.; Fu, K.; Zhang, X. Nanoparticle-on-nanofiber hybrid membrane separators for lithium-ion batteries via combining electrospraying and electrospinning techniques. J. Membrane Sci. 2014, 456, 57–65. [Google Scholar] [CrossRef]


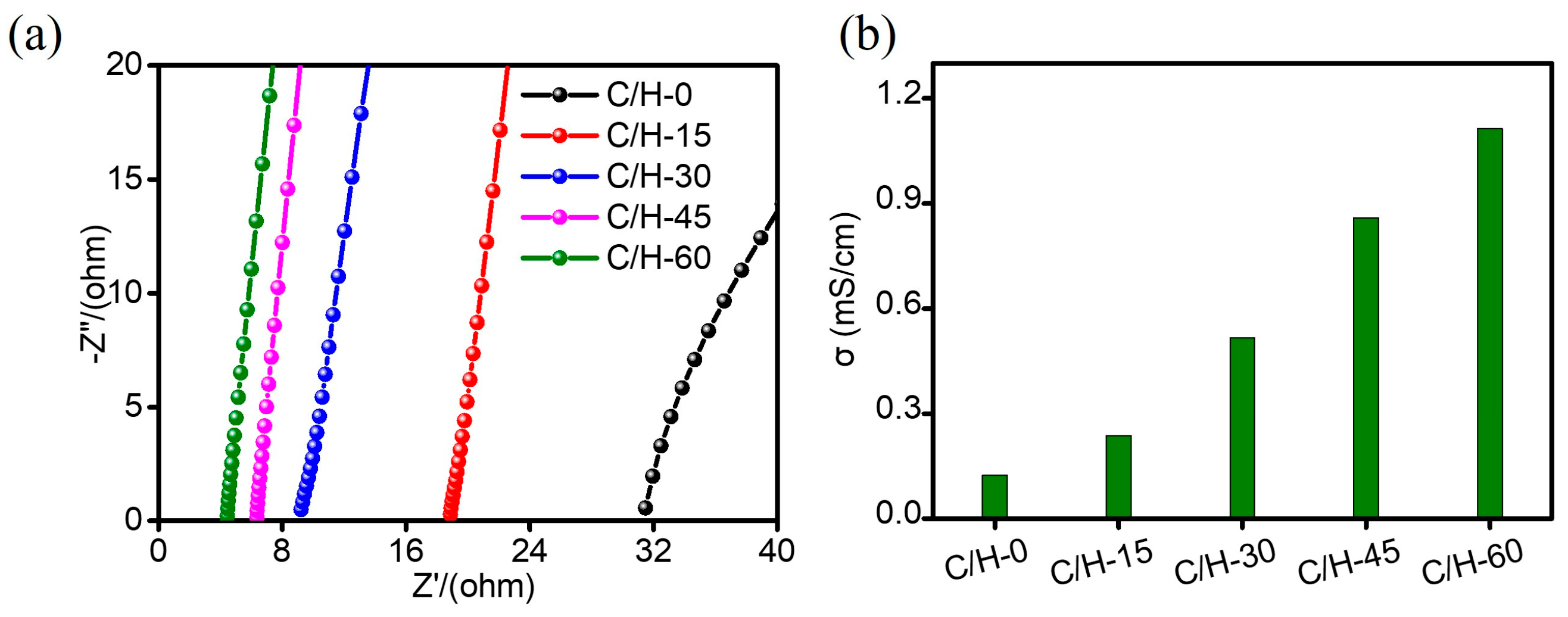
| Samples | C/H-0 | C/H-15 | C/H-30 | C/H-45 | C/H-60 |
|---|---|---|---|---|---|
| Mass ratio of HNT (%) | 0 | 15 | 30 | 45 | 60 |
| Thickness (mm) | 0.078 | 0.089 | 0.094 | 0.108 | 0.100 |
| Porosity (%) | 33.61 | 41.84 | 45.03 | 53.58 | 69.08 |
| Electrolyte uptake (%) | 42.74 | 49.14 | 63.64 | 82.67 | 91.30 |
| Bulk resistance (Rb, Ω) | 31.48 | 18.88 | 9.213 | 6.353 | 4.425 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Guan, Q.; Wei, H.; Zhang, M.; Ren, S.; Dong, L.; Li, Z.; Yang, S.; Zhang, X. Highly Efficient Cellulose Nanofiber/Halloysite Nanotube Separators for Sodium-Ion Batteries. Nanomaterials 2025, 15, 1745. https://doi.org/10.3390/nano15221745
Li J, Guan Q, Wei H, Zhang M, Ren S, Dong L, Li Z, Yang S, Zhang X. Highly Efficient Cellulose Nanofiber/Halloysite Nanotube Separators for Sodium-Ion Batteries. Nanomaterials. 2025; 15(22):1745. https://doi.org/10.3390/nano15221745
Chicago/Turabian StyleLi, Jiangwei, Qian Guan, Hualiang Wei, Mengju Zhang, Suxia Ren, Lili Dong, Zaifeng Li, Shuhua Yang, and Xiuqiang Zhang. 2025. "Highly Efficient Cellulose Nanofiber/Halloysite Nanotube Separators for Sodium-Ion Batteries" Nanomaterials 15, no. 22: 1745. https://doi.org/10.3390/nano15221745
APA StyleLi, J., Guan, Q., Wei, H., Zhang, M., Ren, S., Dong, L., Li, Z., Yang, S., & Zhang, X. (2025). Highly Efficient Cellulose Nanofiber/Halloysite Nanotube Separators for Sodium-Ion Batteries. Nanomaterials, 15(22), 1745. https://doi.org/10.3390/nano15221745







