Recent Advances in Polymer-Coated Metal and Metal Oxide Nanoparticles: From Design to Promising Applications
Abstract
1. Introduction
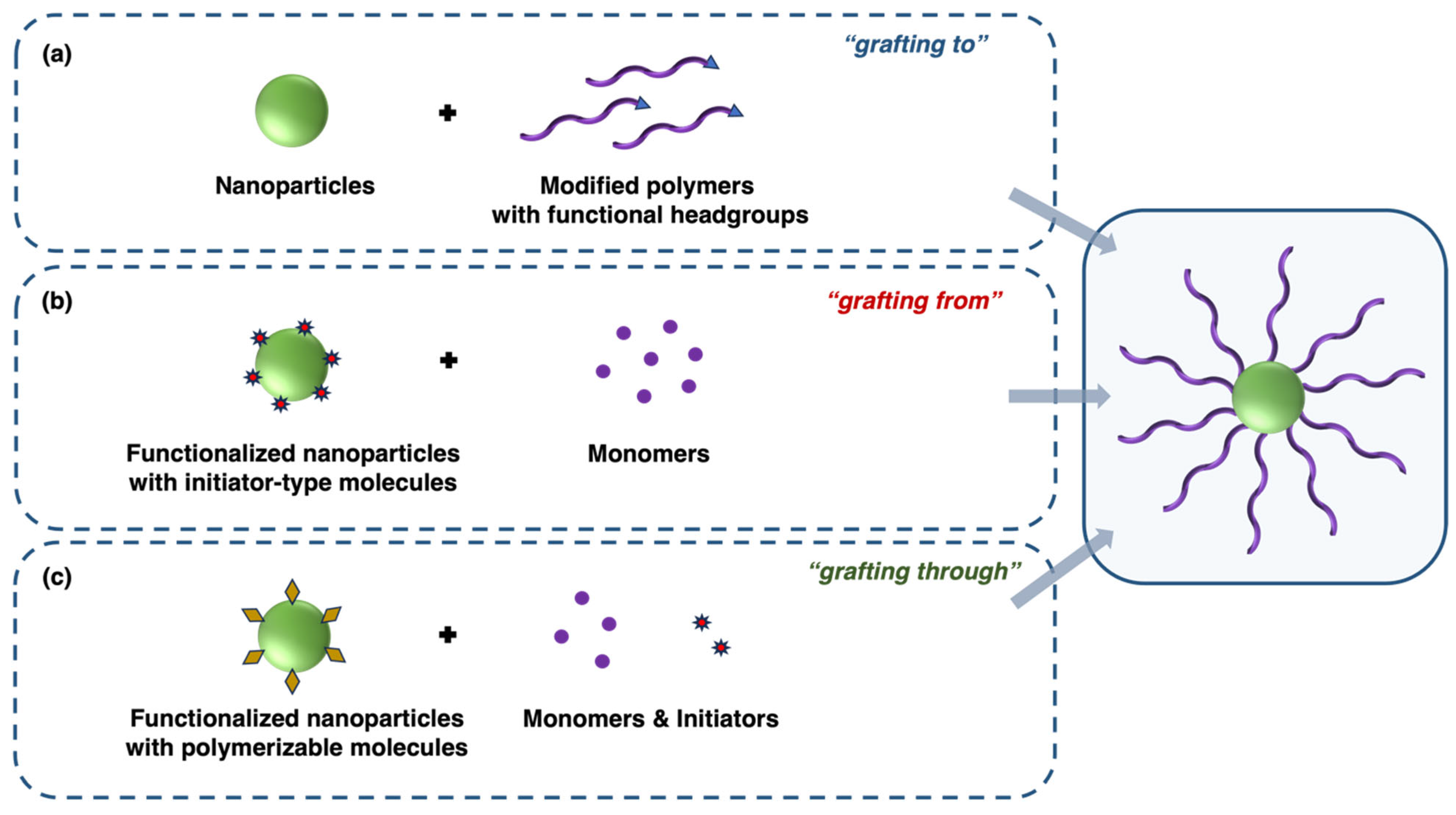
2. Modification of Metal and Metal Oxide Nanoparticles with Polymer Coatings
2.1. Grafting to Method (Post-Synthesis Polymer Coating)
2.2. Grafting from Method (Surface-Initiated Polymerization)
2.3. Grafting Through Method
2.4. In Situ Approach
2.5. Layer-by-Layer (LbL) Assembly
2.6. Specific Polymerization Methods
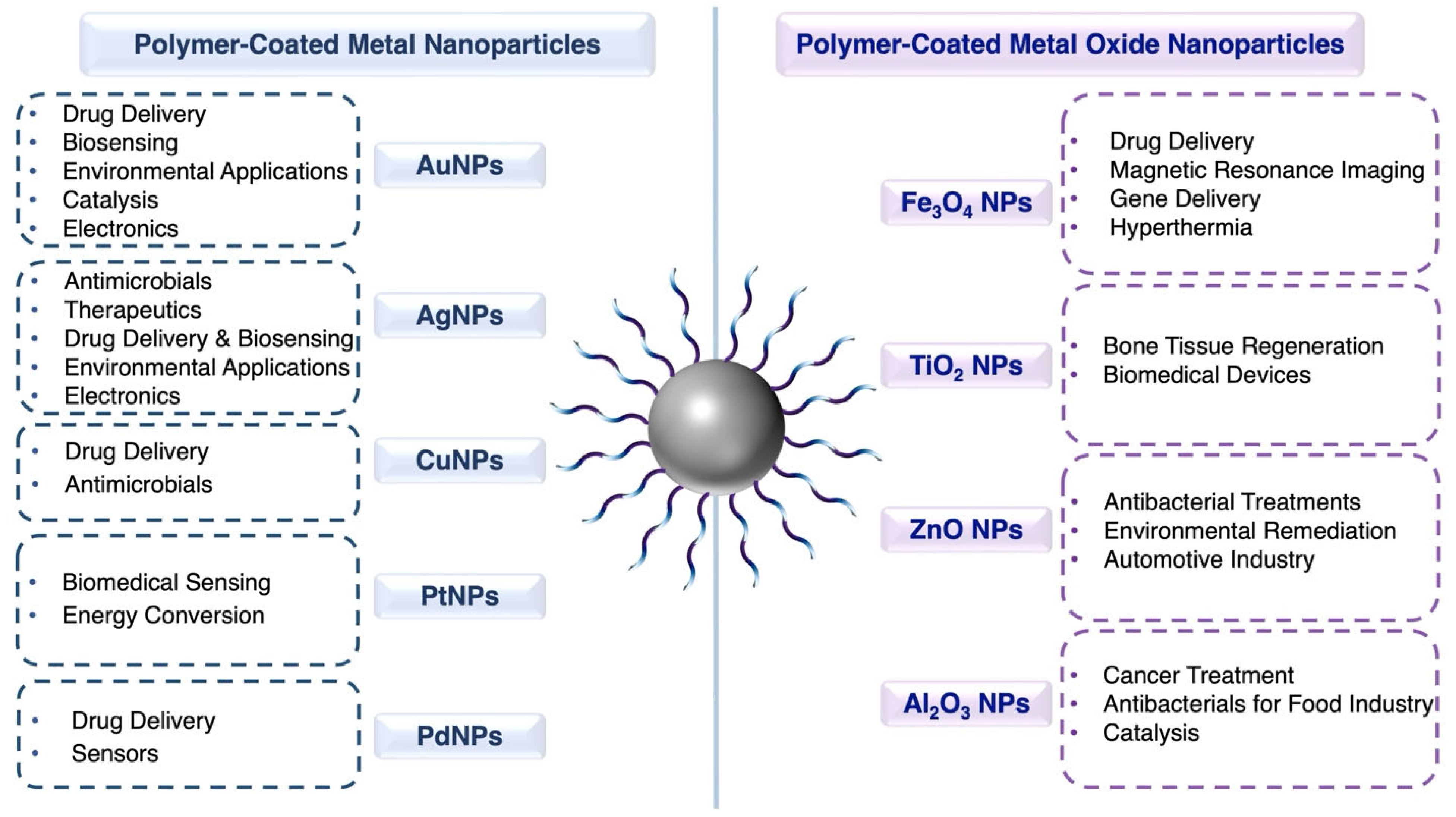
3. Structures, Properties, and Applications of Polymer-Coated Metal Nanoparticles
3.1. Gold Nanoparticles (AuNPs)
3.1.1. Polymer-Coated AuNPs in Targeted Drug Delivery
3.1.2. Polymer-Coated AuNPs in Biosensing
3.1.3. Polymer-Coated AuNPs in Environmental Applications and Catalysis
3.1.4. Polymer-Coated AuNPs in Electronics
3.2. Silver Nanoparticles (AgNPs)
3.2.1. Polymer-Coated AgNPs in Antimicrobials and Therapeutics
3.2.2. Polymer-Coated AgNPs in Targeted Drug Delivery and Biosensing
3.2.3. Polymer-Coated AgNPs in Environmental Applications
3.2.4. Polymer-Coated AgNPs in Electronics
3.3. Copper Nanoparticles (CuNPs)
3.3.1. Polymer-Coated CuNPs in Drug Delivery
3.3.2. Polymer-Coated CuNPs in Antimicrobials and Environmental Applications
3.4. Platinum Nanoparticles (PtNPs)
3.4.1. Polymer-Coated PtNPs in Biomedical Sensing
3.4.2. Polymer-Coated PtNPs in Energy Conversion
3.5. Palladium Nanoparticles (PdNPs)
3.5.1. Polymer-Coated PdNPs in Drug Delivery
3.5.2. Polymer-Coated PdNPs in Sensors
4. Structures, Properties, and Applications of Polymer-Coated Metal Oxide Nanoparticles
4.1. Iron Oxide Nanoparticles (Fe3O4 NPs)
4.1.1. Polymer-Coated Fe3O4 NPs in Drug Delivery
4.1.2. Polymer-Coated Fe3O4 NPs in Magnetic Resonance Imaging (MRI)
4.1.3. Polymer-Coated Fe3O4 NPs in MRI-Guided Drug Delivery
4.1.4. Polymer-Coated Fe3O4 NPs in Gene Delivery
4.1.5. Polymer-Coated Fe3O4 NPs in Hyperthermia
4.2. Titanium Dioxide Nanoparticles (TiO2 NPs)
4.2.1. Polymer-Coated TiO2 NPs in Bone Tissue Regeneration
4.2.2. Polymer-Coated TiO2 NPs in Biomedical Devices
4.3. Zinc Oxide Nanoparticles (ZnO NPs)
4.3.1. Polymer-Coated ZnO NPs in Antibacterial Treatments and Environmental Remediation
4.3.2. Polymer-Coated ZnO NPs in the Automotive Industry
4.4. Aluminum Oxide Nanoparticles (Al2O3 NPs)
4.4.1. Polymer-Coated Al2O3 NPs in Cancer Treatment
4.4.2. Polymer-Coated Al2O3 NPs in Antibacterial Applications in the Food Industry
4.4.3. Polymer-Coated Al2O3 NPs in Catalysis
5. Future Perspectives and Outlook
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sim, S.; Wong, N. Nanotechnology and Its Use in Imaging and Drug Delivery (Review). Biomed. Rep. 2021, 14, 42. [Google Scholar] [CrossRef]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical–Physical Applications to Nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef]
- Malik, S.; Muhammad, K.; Waheed, Y. Nanotechnology: A Revolution in Modern Industry. Molecules 2023, 28, 661. [Google Scholar] [CrossRef]
- Pokrajac, L.; Abbas, A.; Chrzanowski, W.; Dias, G.M.; Eggleton, B.J.; Maguire, S.; Maine, E.; Malloy, T.; Nathwani, J.; Nazar, L.; et al. Nanotechnology for a Sustainable Future: Addressing Global Challenges with the International Network4Sustainable Nanotechnology. ACS Nano 2021, 15, 18608–18623. [Google Scholar] [CrossRef] [PubMed]
- Mashaghi, S.; Jadidi, T.; Koenderink, G.; Mashaghi, A. Lipid Nanotechnology. Int. J. Mol. Sci. 2013, 14, 4242–4282. [Google Scholar] [CrossRef]
- Sarkar, S.; Guibal, E.; Quignard, F.; SenGupta, A.K. Polymer-Supported Metals and Metal Oxide Nanoparticles: Synthesis, Characterization, and Applications. J. Nanopart. Res. 2012, 14, 715. [Google Scholar] [CrossRef]
- Felix, L.C.; Ortega, V.A.; Ede, J.D.; Goss, G.G. Physicochemical Characteristics of Polymer-Coated Metal-Oxide Nanoparticles and Their Toxicological Effects on Zebrafish (Danio rerio) Development. Environ. Sci. Technol. 2013, 47, 6589–6596. [Google Scholar] [CrossRef]
- Shapiro, E.M. Biodegradable, Polymer Encapsulated, Metal Oxide Particles for MRI-based Cell Tracking. Magn. Reson. Med. 2015, 73, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Chavali, M.S.; Nikolova, M.P. Metal Oxide Nanoparticles and Their Applications in Nanotechnology. SN Appl. Sci. 2019, 1, 607. [Google Scholar] [CrossRef]
- Nair, G.M.; Sajini, T.; Mathew, B. Advanced Green Approaches for Metal and Metal Oxide Nanoparticles Synthesis and Their Environmental Applications. Talanta Open 2022, 5, 100080. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Sasidharan, M.; Jin, W. Earth-Abundant Transition Metal and Metal Oxide Nanomaterials: Synthesis and Electrochemical Applications. Prog. Mater. Sci. 2019, 106, 100574. [Google Scholar] [CrossRef]
- Teske, S.; Detweiler, C. The Biomechanisms of Metal and Metal-Oxide Nanoparticles’ Interactions with Cells. Int. J. Environ. Res. Public Health 2015, 12, 1112–1134. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, A.; Jackson, K.; Morgan, C.; Anderson, A.; Britt, D.W. A Review of Metal and Metal-Oxide Nanoparticle Coating Technologies to Inhibit Agglomeration and Increase Bioactivity for Agricultural Applications. Agronomy 2020, 10, 1018. [Google Scholar] [CrossRef]
- Zhang, X.-D.; Wu, D.; Shen, X.; Chen, J.; Sun, Y.-M.; Liu, P.-X.; Liang, X.-J. Size-Dependent Radiosensitization of PEG-Coated Gold Nanoparticles for Cancer Radiation Therapy. Biomaterials 2012, 33, 6408–6419. [Google Scholar] [CrossRef]
- Tejamaya, M.; Römer, I.; Merrifield, R.C.; Lead, J.R. Stability of Citrate, PVP, and PEG Coated Silver Nanoparticles in Ecotoxicology Media. Environ. Sci. Technol. 2012, 46, 7011–7017. [Google Scholar] [CrossRef]
- Cestarollo, L.; Utomo, N.; Htet, H.W.; Chen, Y.; Archer, L.A.; El-Ghazaly, A. Amplifying Magneto-Mechanical Performance of Magnetorheological Elastomers through Surface Functionalization of Iron Nanoparticles. ACS Appl. Mater. Interfaces 2025, 17, 15849–15858. [Google Scholar] [CrossRef]
- Manuja, A.; Kumar, B.; Kumar, R.; Chhabra, D.; Ghosh, M.; Manuja, M.; Brar, B.; Pal, Y.; Tripathi, B.N.; Prasad, M. Metal/Metal Oxide Nanoparticles: Toxicity Concerns Associated with Their Physical State and Remediation for Biomedical Applications. Toxicol. Rep. 2021, 8, 1970–1978. [Google Scholar] [CrossRef]
- Manuja, A.; Raguvaran, R.; Kumar, B.; Kalia, A.; Tripathi, B.N. Accelerated Healing of Full Thickness Excised Skin Wound in Rabbits Using Single Application of Alginate/Acacia Based Nanocomposites of ZnO Nanoparticles. Int. J. Biol. Macromol. 2020, 155, 823–833. [Google Scholar] [CrossRef]
- Chopra, M.; Bernela, M.; Kaur, P.; Manuja, A.; Kumar, B.; Thakur, R. Alginate/Gum Acacia Bipolymeric Nanohydrogels—Promising Carrier for Zinc Oxide Nanoparticles. Int. J. Biol. Macromol. 2015, 72, 827–833. [Google Scholar] [CrossRef]
- Rezaei, B.; Harun, A.; Wu, X.; Iyer, P.R.; Mostufa, S.; Ciannella, S.; Karampelas, I.H.; Chalmers, J.; Srivastava, I.; Gómez-Pastora, J.; et al. Effect of Polymer and Cell Membrane Coatings on Theranostic Applications of Nanoparticles: A Review. Adv. Healthc. Mater. 2024, 13, 2401213. [Google Scholar] [CrossRef]
- Abdulraheem, M.I.; Moshood, A.Y.; Gourkhede, P.H.; Xu, L.; Zang, Y.; Cadenas-Pliego, G.; Raghavan, V.; Hu, J. Advancements in Polymeric Nanoparticles Reinforced with Metallic Nanoparticles: Synthesis Techniques, Properties Enhancements, and Emerging Functionalities. Polym. Bull. 2025, 82, 5201–5239. [Google Scholar] [CrossRef]
- Chandra Mohanta, S.; Saha, A.; Sujatha Devi, P. PEGylated Iron Oxide Nanoparticles for pH Responsive Drug Delivery Application. Mater. Today Proc. 2018, 5, 9715–9725. [Google Scholar] [CrossRef]
- Walker, M.; Will, I.; Pratt, A.; Chechik, V.; Genever, P.; Ungar, D. Magnetically Triggered Release of Entrapped Bioactive Proteins from Thermally Responsive Polymer-Coated Iron Oxide Nanoparticles for Stem-Cell Proliferation. ACS Appl. Nano Mater. 2020, 3, 5008–5013. [Google Scholar] [CrossRef]
- Bédard, M.F.; De Geest, B.G.; Skirtach, A.G.; Möhwald, H.; Sukhorukov, G.B. Polymeric Microcapsules with Light Responsive Properties for Encapsulation and Release. Adv. Colloid Interface Sci. 2010, 158, 2–14. [Google Scholar] [CrossRef]
- Polívková, M.; Hubáček, T.; Staszek, M.; Švorčík, V.; Siegel, J. Antimicrobial Treatment of Polymeric Medical Devices by Silver Nanomaterials and Related Technology. Int. J. Mol. Sci. 2017, 18, 419. [Google Scholar] [CrossRef]
- Mondal, S.K.; Chakraborty, S.; Manna, S.; Mandal, S.M. Antimicrobial Nanoparticles: Current Landscape and Future Challenges. RSC Pharm. 2024, 1, 388–402. [Google Scholar] [CrossRef]
- Sahoo, J.; Sarkhel, S.; Mukherjee, N.; Jaiswal, A. Nanomaterial-Based Antimicrobial Coating for Biomedical Implants: New Age Solution for Biofilm-Associated Infections. ACS Omega 2022, 7, 45962–45980. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Guo, X.; Wu, Y.; Chen, X.; Feng, L.; Xie, N.; Shen, G. Nanotechnology’s Frontier in Combatting Infectious and Inflammatory Diseases: Prevention and Treatment. Sig. Transduct. Target Ther. 2024, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Froelich, A.; Jakubowska, E.; Wojtyłko, M.; Jadach, B.; Gackowski, M.; Gadziński, P.; Napierała, O.; Ravliv, Y.; Osmałek, T. Alginate-Based Materials Loaded with Nanoparticles in Wound Healing. Pharmaceutics 2023, 15, 1142. [Google Scholar] [CrossRef]
- Valenzuela-Amaro, H.M.; Aguayo-Acosta, A.; Meléndez-Sánchez, E.R.; De La Rosa, O.; Vázquez-Ortega, P.G.; Oyervides-Muñoz, M.A.; Sosa-Hernández, J.E.; Parra-Saldívar, R. Emerging Applications of Nanobiosensors in Pathogen Detection in Water and Food. Biosensors 2023, 13, 922. [Google Scholar] [CrossRef]
- Ahmad, F.; Salem-Bekhit, M.M.; Khan, F.; Alshehri, S.; Khan, A.; Ghoneim, M.M.; Wu, H.-F.; Taha, E.I.; Elbagory, I. Unique Properties of Surface-Functionalized Nanoparticles for Bio-Application: Functionalization Mechanisms and Importance in Application. Nanomaterials 2022, 12, 1333. [Google Scholar] [CrossRef] [PubMed]
- Mout, R.; Moyano, D.F.; Rana, S.; Rotello, V.M. Surface Functionalization of Nanoparticles for Nanomedicine. Chem. Soc. Rev. 2012, 41, 2539. [Google Scholar] [CrossRef]
- Guerra, F.D.; Attia, M.F.; Whitehead, D.C.; Alexis, F. Nanotechnology for Environmental Remediation: Materials and Applications. Molecules 2018, 23, 1760. [Google Scholar] [CrossRef]
- Losetty, V.; Lakkaboyana, S.K.; Chappidi, H.Y.; Venkateswarlu, K.; Trilaksana, H.; Koduru, J.R.; Yuzir, A.; Atanase, L.I.; Seepana, P.K.; Knani, S. Transformative Applications of Polymer-Based Metal Oxide Nanocomposites in Medicine, Industry, and Environmental Remediation: A Review. J. Inorg. Organomet. Polym. Mater. 2025, 1–33. [Google Scholar] [CrossRef]
- Naseem, T.; Durrani, T. The Role of Some Important Metal Oxide Nanoparticles for Wastewater and Antibacterial Applications: A Review. J. Environ. Chem. Ecotoxicol. 2021, 3, 59–75. [Google Scholar] [CrossRef]
- Matei, E.; Predescu, A.M.; Râpă, M.; Țurcanu, A.A.; Mateș, I.; Constantin, N.; Predescu, C. Natural Polymers and Their Nanocomposites Used for Environmental Applications. Nanomaterials 2022, 12, 1707. [Google Scholar] [CrossRef]
- Narayan, N.; Meiyazhagan, A.; Vajtai, R. Metal Nanoparticles as Green Catalysts. Materials 2019, 12, 3602. [Google Scholar] [CrossRef]
- Arya, R.K.; Thapliyal, D.; Pandit, A.; Gora, S.; Banerjee, C.; Verros, G.D.; Sen, P. Polymer Coated Functional Catalysts for Industrial Applications. Polymers 2023, 15, 2009. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.F.; Resina, L.; Ferreira, F.C.; Sanjuan-Alberte, P.; Esteves, T. Conductive Core–Shell Nanoparticles: Synthesis and Applications. J. Phys. Chem. C 2024, 128, 11083–11100. [Google Scholar] [CrossRef]
- Kishore Kumar, D.; Kříž, J.; Bennett, N.; Chen, B.; Upadhayaya, H.; Reddy, K.R.; Sadhu, V. Functionalized Metal Oxide Nanoparticles for Efficient Dye-Sensitized Solar Cells (DSSCs): A Review. Mater. Sci. Energy Technol. 2020, 3, 472–481. [Google Scholar] [CrossRef]
- Pérez Mendoza, A.E.; Andronescu, C.; Olean-Oliveira, A. Design of Conducting Polymer/Metal-Based Nanocomposites as Electrocatalysts for Electrochemical Energy Conversion. Synth. Met. 2024, 307, 117662. [Google Scholar] [CrossRef]
- Boyer, C.; Whittaker, M.R.; Bulmus, V.; Liu, J.; Davis, T.P. The Design and Utility of Polymer-Stabilized Iron-Oxide Nanoparticles for Nanomedicine Applications. NPG Asia Mater. 2010, 2, 23–30. [Google Scholar] [CrossRef]
- Navaf, M.; Sunooj, K.V.; Aaliya, B.; Akhila, P.P.; Sudheesh, C.; Mir, S.A.; George, J. Impact of Metal and Metal Oxide Nanoparticles on Functional and Antimicrobial Activity of Starch Nanocomposite Film; A Review. Meas. Food 2023, 11, 100099. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, S.; He, J.; Nie, Z. Polymers and Inorganic Nanoparticles: A Winning Combination towards Assembled Nanostructures for Cancer Imaging and Therapy. Nano Today 2021, 36, 101046. [Google Scholar] [CrossRef]
- Khdary, N.H.; Almuarqab, B.T.; El Enany, G. Nanoparticle-Embedded Polymers and Their Applications: A Review. Membranes 2023, 13, 537. [Google Scholar] [CrossRef]
- Aguilar, N.M.; Perez-Aguilar, J.M.; González-Coronel, V.J.; Soriano Moro, J.G.; Sanchez-Gaytan, B.L. Polymers as Versatile Players in the Stabilization, Capping, and Design of Inorganic Nanostructures. ACS Omega 2021, 6, 35196–35203. [Google Scholar] [CrossRef]
- Agboola, O.D.; Benson, N.U. Physisorption and Chemisorption Mechanisms Influencing Micro (Nano) Plastics-Organic Chemical Contaminants Interactions: A Review. Front. Environ. Sci. 2021, 9, 678574. [Google Scholar] [CrossRef]
- Abarca-Cabrera, L.; Fraga-García, P.; Berensmeier, S. Bio-Nano Interactions: Binding Proteins, Polysaccharides, Lipids and Nucleic Acids onto Magnetic Nanoparticles. Biomater. Res. 2021, 25, 12. [Google Scholar] [CrossRef] [PubMed]
- Knapp, T.V.; Hasan, M.R.; Niebuur, B.-J.; Widmer-Cooper, A.; Kraus, T. Stabilization of Apolar Nanoparticle Dispersions by Molecular Additives. Langmuir 2024, 40, 13527–13537. [Google Scholar] [CrossRef]
- Süer, N.C.; Acaroğlu Degitz, İ.; Sungur, P.; Bayır, A.; Eren, T. Production of Au/Phosphonium Polymer Nanoparticles. Eur. Polym. J. 2021, 156, 110599. [Google Scholar] [CrossRef]
- Purohit, P.; Bhatt, A.; Mittal, R.K.; Abdellattif, M.H.; Farghaly, T.A. Polymer Grafting and Its Chemical Reactions. Front. Bioeng. Biotechnol. 2023, 10, 1044927. [Google Scholar] [CrossRef]
- Zdyrko, B.; Luzinov, I. Polymer Brushes by the “Grafting to” Method. Macromol. Rapid Commun. 2011, 32, 859–869. [Google Scholar] [CrossRef]
- Zoppe, J.O.; Ataman, N.C.; Mocny, P.; Wang, J.; Moraes, J.; Klok, H.-A. Surface-Initiated Controlled Radical Polymerization: State-of-the-Art, Opportunities, and Challenges in Surface and Interface Engineering with Polymer Brushes. Chem. Rev. 2017, 117, 1105–1318. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Qi, H.; Han, L.; Barbash, D.; Li, C.Y. Towards Controlled Polymer Brushes via a Self-Assembly-Assisted-Grafting-to Approach. Nat. Commun. 2016, 7, 11119. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.L.M.; Castanheira, E.J.; Alves, S.P.C.; Baleizão, C.; Farinha, J.P. Grafting with RAFT—gRAFT Strategies to Prepare Hybrid Nanocarriers with Core-Shell Architecture. Polymers 2020, 12, 2175. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Jamison, A.C.; Lee, T.R. Entropy-Driven Conformational Control of α,ω-Difunctional Bidentate-Dithiol Azo-Based Adsorbates Enables the Fabrication of Thermally Stable Surface-Grafted Polymer Films. ACS Appl. Mater. Interfaces 2016, 8, 15691–15699. [Google Scholar] [CrossRef]
- Messina, M.S.; Messina, K.M.M.; Bhattacharya, A.; Montgomery, H.R.; Maynard, H.D. Preparation of Biomolecule-Polymer Conjugates by Grafting-from Using ATRP, RAFT, or ROMP. Prog. Polym. Sci. 2020, 100, 101186. [Google Scholar] [CrossRef]
- Jordan, R.; Ulman, A. Surface Initiated Living Cationic Polymerization of 2-Oxazolines. J. Am. Chem. Soc. 1998, 120, 243–247. [Google Scholar] [CrossRef]
- Jordan, R.; Ulman, A.; Kang, J.F.; Rafailovich, M.H.; Sokolov, J. Surface-Initiated Anionic Polymerization of Styrene by Means of Self-Assembled Monolayers. J. Am. Chem. Soc. 1999, 121, 1016–1022. [Google Scholar] [CrossRef]
- Rowe, M.D.; Hammer, B.A.G.; Boyes, S.G. Synthesis of Surface-Initiated Stimuli-Responsive Diblock Copolymer Brushes Utilizing a Combination of ATRP and RAFT Polymerization Techniques. Macromolecules 2008, 41, 4147–4157. [Google Scholar] [CrossRef]
- Bhat, R.R.; Tomlinson, M.R.; Wu, T.; Genzer, J. Surface-Grafted Polymer Gradients: Formation, Characterization, and Applications. In Surface-Initiated Polymerization II; Jordan, R., Ed.; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2006; Volume 198, pp. 51–124. ISBN 978-3-540-30251-3. [Google Scholar]
- Gao, C.; Yan, D. Hyperbranched Polymers: From Synthesis to Applications. Prog. Polym. Sci. 2004, 29, 183–275. [Google Scholar] [CrossRef]
- Galvin, C.J.; Genzer, J. Applications of Surface-Grafted Macromolecules Derived from Post-Polymerization Modification Reactions. Prog. Polym. Sci. 2012, 37, 871–906. [Google Scholar] [CrossRef]
- Suresh, D.; Goh, P.S.; Ismail, A.F.; Hilal, N. Surface Design of Liquid Separation Membrane through Graft Polymerization: A State of the Art Review. Membranes 2021, 11, 832. [Google Scholar] [CrossRef]
- Michalek, L.; Mundsinger, K.; Barner-Kowollik, C.; Barner, L. The Long and the Short of Polymer Grafting. Polym. Chem. 2019, 10, 54–59. [Google Scholar] [CrossRef]
- Chinthamanipeta, P.S.; Kobukata, S.; Nakata, H.; Shipp, D.A. Synthesis of Poly(Methyl Methacrylate)–Silica Nanocomposites Using Methacrylate-Functionalized Silica Nanoparticles and RAFT Polymerization. Polymer 2008, 49, 5636–5642. [Google Scholar] [CrossRef]
- Azani, M.-R.; Hassanpour, A. High-Performance Polymer Nanocomposites: Advanced Fabrication Methods and Critical Insights. J. Polym. Res. 2024, 31, 168. [Google Scholar] [CrossRef]
- Alshangiti, D.M.; El-damhougy, T.K.; Zaher, A.; Madani, M.; Mohamady Ghobashy, M. Revolutionizing Biomedicine: Advancements, Applications, and Prospects of Nanocomposite Macromolecular Carbohydrate-Based Hydrogel Biomaterials: A Review. RSC Adv. 2023, 13, 35251–35291. [Google Scholar] [CrossRef]
- Dzhardimalieva, G.I.; Uflyand, I.E. Preparation of Metal-Polymer Nanocomposites by Chemical Reduction of Metal Ions: Functions of Polymer Matrices. J. Polym. Res. 2018, 25, 255. [Google Scholar] [CrossRef]
- Manojkumar, K.; Sivaramakrishna, A.; Vijayakrishna, K. A Short Review on Stable Metal Nanoparticles Using Ionic Liquids, Supported Ionic Liquids, and Poly(Ionic Liquids). J. Nanopart. Res. 2016, 18, 103. [Google Scholar] [CrossRef]
- Kelarakis, A. In Situ Generation of Nanoparticles on and within Polymeric Materials. Polymers 2024, 16, 1611. [Google Scholar] [CrossRef]
- Darwish, M.S.A.; Mostafa, M.H.; Al-Harbi, L.M. Polymeric Nanocomposites for Environmental and Industrial Applications. Int. J. Mol. Sci. 2022, 23, 1023. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.; Rahdar, A. LbL Nano-Assemblies: A Versatile Tool for Biomedical and Healthcare Applications. Nanomaterials 2022, 12, 949. [Google Scholar] [CrossRef]
- Bose, S.; Robertson, S.F.; Bandyopadhyay, A. Surface Modification of Biomaterials and Biomedical Devices Using Additive Manufacturing. Acta Biomater. 2018, 66, 6–22. [Google Scholar] [CrossRef]
- Sahebalzamani, M.; Ziminska, M.; McCarthy, H.O.; Levingstone, T.J.; Dunne, N.J.; Hamilton, A.R. Advancing Bone Tissue Engineering One Layer at a Time: A Layer-by-Layer Assembly Approach to 3D Bone Scaffold Materials. Biomater. Sci. 2022, 10, 2734–2758. [Google Scholar] [CrossRef]
- Borges, J.; Mano, J.F. Molecular Interactions Driving the Layer-by-Layer Assembly of Multilayers. Chem. Rev. 2014, 114, 8883–8942. [Google Scholar] [CrossRef]
- Yadav, A.S.; Tran, D.T.; Teo, A.J.T.; Dai, Y.; Galogahi, F.M.; Ooi, C.H.; Nguyen, N.-T. Core–Shell Particles: From Fabrication Methods to Diverse Manipulation Techniques. Micromachines 2023, 14, 497. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiong, J.; Gao, X.; Huang, J.; Feng, Z.; Chen, Z.; Zhu, Y. Recent Advances in 3D G-C3N4 Composite Photocatalysts for Photocatalytic Water Splitting, Degradation of Pollutants and CO2 Reduction. J. Alloys Compd. 2019, 802, 196–209. [Google Scholar] [CrossRef]
- Norrish, R.G.W.; Brookman, E.F. The Mechanism of Polymerization Reactions. I. The Polymerization of Styrene and Methyl Methacrylate. Proc. R. Soc. Lond. A 1939, 171, 147–171. [Google Scholar] [CrossRef]
- Chen, M.; Zhong, M.; Johnson, J.A. Light-Controlled Radical Polymerization: Mechanisms, Methods, and Applications. Chem. Rev. 2016, 116, 10167–10211. [Google Scholar] [CrossRef]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Cowie, A.C. Recent Advances in Photo-Crosslinkable Hydrogels for Biomedical Applications. BioTechniques 2019, 66, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, A.; Clochard, M.-C.; Coqueret, X.; Dispenza, C.; Driscoll, M.S.; Ulański, P.; Al-Sheikhly, M. Polymerization Reactions and Modifications of Polymers by Ionizing Radiation. Polymers 2020, 12, 2877. [Google Scholar] [CrossRef]
- Barsbay, M.; Güven, O. Nanostructuring of Polymers by Controlling of Ionizing Radiation-Induced Free Radical Polymerization, Copolymerization, Grafting and Crosslinking by RAFT Mechanism. Radiat. Phys. Chem. 2020, 169, 107816. [Google Scholar] [CrossRef]
- Nasef, M.M.; Gupta, B.; Shameli, K.; Verma, C.; Ali, R.R.; Ting, T.M. Engineered Bioactive Polymeric Surfaces by Radiation Induced Graft Copolymerization: Strategies and Applications. Polymers 2021, 13, 3102. [Google Scholar] [CrossRef] [PubMed]
- Dispenza, C.; Giacomazza, D.; Jonsson, M. Micro- to Nanoscale Bio-Hybrid Hydrogels Engineered by Ionizing Radiation. Biomolecules 2020, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Matyjaszewski, K. Advanced Materials by Atom Transfer Radical Polymerization. Adv. Mater. 2018, 30, 1706441. [Google Scholar] [CrossRef]
- Wang, Y.; Lorandi, F.; Fantin, M.; Matyjaszewski, K. Atom Transfer Radical Polymerization in Dispersed Media with Low-Ppm Catalyst Loading. Polymer 2023, 275, 125913. [Google Scholar] [CrossRef]
- Tian, X.; Ding, J.; Zhang, B.; Qiu, F.; Zhuang, X.; Chen, Y. Recent Advances in RAFT Polymerization: Novel Initiation Mechanisms and Optoelectronic Applications. Polymers 2018, 10, 318. [Google Scholar] [CrossRef]
- Keddie, D.J. A Guide to the Synthesis of Block Copolymers Using Reversible-Addition Fragmentation Chain Transfer (RAFT) Polymerization. Chem. Soc. Rev. 2014, 43, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Fairbanks, B.D.; Gunatillake, P.A.; Meagher, L. Biomedical Applications of Polymers Derived by Reversible Addition—Fragmentation Chain-Transfer (RAFT). Adv. Drug Deliv. Rev. 2015, 91, 141–152. [Google Scholar] [CrossRef]
- Purohit, V.B.; Pięta, M.; Pietrasik, J.; Plummer, C.M. Recent Advances in the Ring-Opening Polymerization of Sulfur-Containing Monomers. Polym. Chem. 2022, 13, 4858–4878. [Google Scholar] [CrossRef]
- Harrier, D.D.; Guironnet, D. Design Rules for Performing Water-Sensitive Ring-Opening Polymerizations in an Aqueous Dispersion. Polym. Chem. 2022, 13, 2459–2468. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Pitsikalis, M.; Pispas, S.; Iatrou, H. Polymers with Complex Architecture by Living Anionic Polymerization. Chem. Rev. 2001, 101, 3747–3792. [Google Scholar] [CrossRef]
- Gleede, T.; Rieger, E.; Liu, L.; Bakkali-Hassani, C.; Wagner, M.; Carlotti, S.; Taton, D.; Andrienko, D.; Wurm, F.R. Alcohol- and Water-Tolerant Living Anionic Polymerization of Aziridines. Macromolecules 2018, 51, 5713–5719. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Jin, Y.; Lin, X.; Chen, M. Recent Advances in Living Cationic Polymerization with Emerging Initiation/Controlling Systems. Macromol. Rapid Commun. 2021, 42, 2100148. [Google Scholar] [CrossRef]
- Destephen, A.; Ballard, N. On the Limitations of Cationic Polymerization of Vinyl Monomers in Aqueous Dispersed Media. Polym. Chem. 2021, 12, 6444–6455. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, D.; Lyu, J.; A, S.; Xu, Q.; Newland, B.; Matyjaszewski, K.; Tai, H.; Wang, W. Complex Polymer Architectures through Free-Radical Polymerization of Multivinyl Monomers. Nat. Rev. Chem. 2020, 4, 194–212. [Google Scholar] [CrossRef] [PubMed]
- Moad, G.; Chiefari, J.; Chong, Y.K.; Krstina, J.; Mayadunne, R.T.A.; Postma, A.; Rizzardo, E.; Thang, S.H. Living Free Radical Polymerization with Reversible Addition—Fragmentation Chain Transfer (the Life of RAFT). Polym. Int. 2000, 49, 993–1001. [Google Scholar] [CrossRef]
- Higashimura, H.; Kobayashi, S. Oxidative Polymerization. In Encyclopedia of Polymer Science and Technology; Mark, H.F., Ed.; Wiley: Hoboken, NJ, USA, 2016; pp. 1–37. ISBN 978-1-118-63389-2. [Google Scholar]
- Ghazzy, A.; Naik, R.R.; Shakya, A.K. Metal–Polymer Nanocomposites: A Promising Approach to Antibacterial Materials. Polymers 2023, 15, 2167. [Google Scholar] [CrossRef]
- Unser, S.; Bruzas, I.; He, J.; Sagle, L. Localized Surface Plasmon Resonance Biosensing: Current Challenges and Approaches. Sensors 2015, 15, 15684–15716. [Google Scholar] [CrossRef]
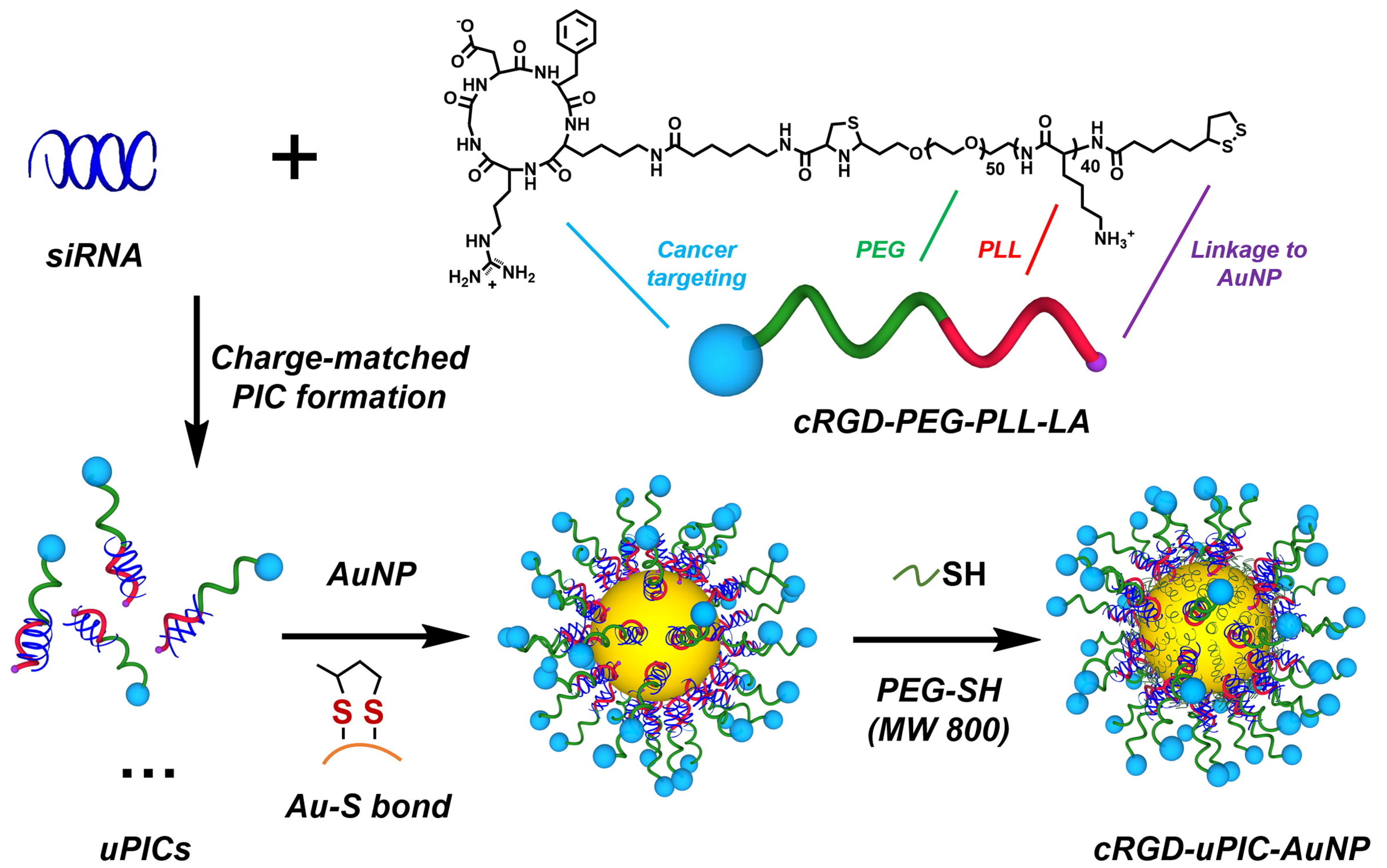
- Yi, Y.; Kim, H.J.; Mi, P.; Zheng, M.; Takemoto, H.; Toh, K.; Kim, B.S.; Hayashi, K.; Naito, M.; Matsumoto, Y.; et al. Targeted Systemic Delivery of siRNA to Cervical Cancer Model Using Cyclic RGD-Installed Unimer Polyion Complex-Assembled Gold Nanoparticles. J. Control. Release 2016, 244, 247–256. [Google Scholar] [CrossRef]
- Brazzale, C.; Mastrotto, F.; Moody, P.; Watson, P.D.; Balasso, A.; Malfanti, A.; Mantovani, G.; Caliceti, P.; Alexander, C.; Jones, A.T.; et al. Control of Targeting Ligand Display by pH-Responsive Polymers on Gold Nanoparticles Mediates Selective Entry into Cancer Cells. Nanoscale 2017, 9, 11137–11147. [Google Scholar] [CrossRef]
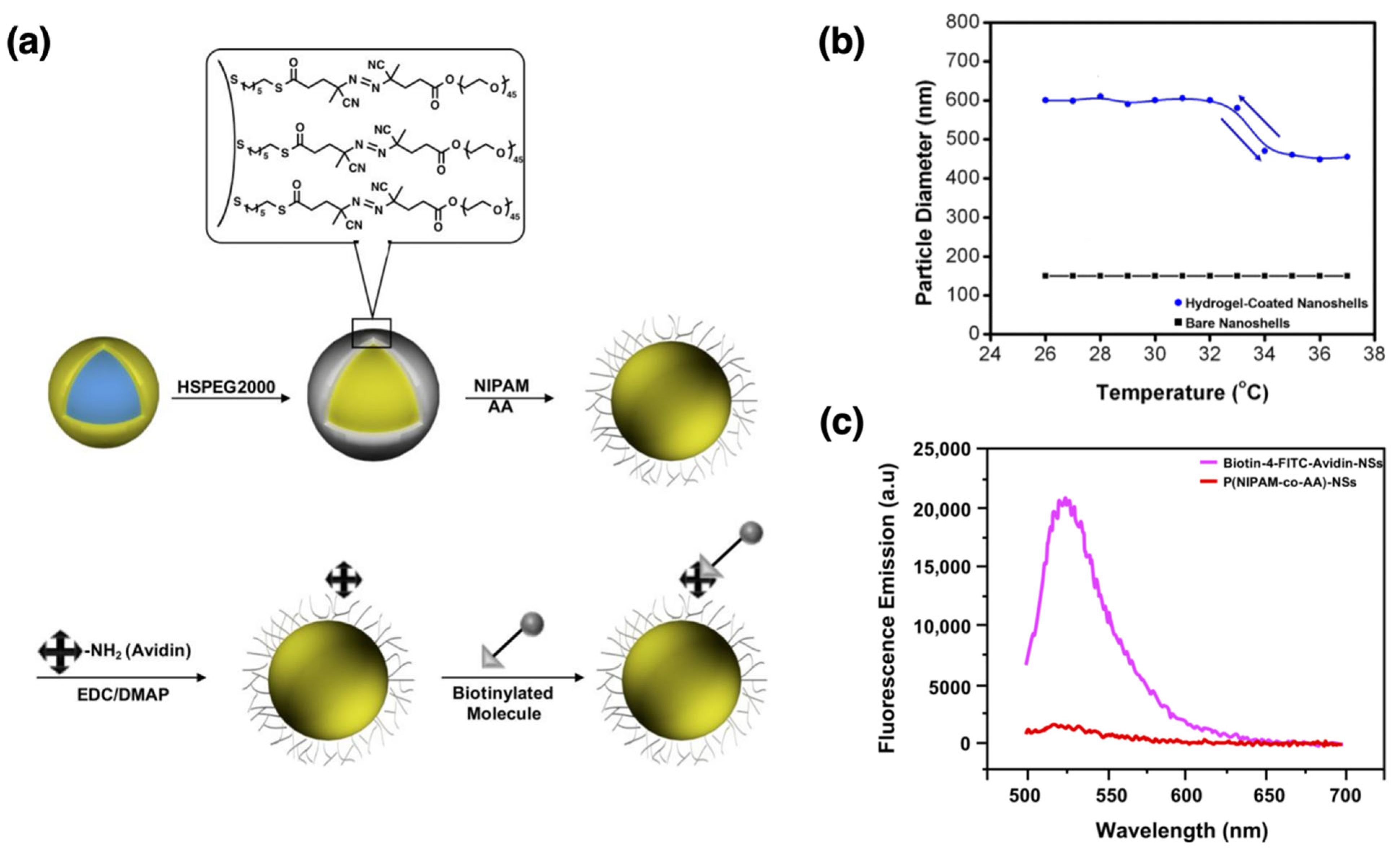
- Park, H.; Srisombat, L.; Jamison, A.; Liu, T.; Marquez, M.; Park, H.; Lee, S.; Lee, T.-C.; Lee, T. Temperature-Responsive Hydrogel-Coated Gold Nanoshells. Gels 2018, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Takara, M.; Toyoshima, M.; Seto, H.; Hoshino, Y.; Miura, Y. Polymer-Modified Gold Nanoparticles via RAFT Polymerization: A Detailed Study for a Biosensing Application. Polym. Chem. 2014, 5, 931–939. [Google Scholar] [CrossRef]
- Wang, R.; Schirmer, L.; Wieduwilt, T.; Förster, R.; Schmidt, M.A.; Freudenberg, U.; Werner, C.; Fery, A.; Rossner, C. Colorimetric Biosensors Based on Polymer/Gold Hybrid Nanoparticles: Topological Effects of the Polymer Coating. Langmuir 2022, 38, 12325–12332. [Google Scholar] [CrossRef] [PubMed]
- Subair, R.; Tripathi, B.P.; Formanek, P.; Simon, F.; Uhlmann, P.; Stamm, M. Polydopamine Modified Membranes with in Situ Synthesized Gold Nanoparticles for Catalytic and Environmental Applications. Chem. Eng. J. 2016, 295, 358–369. [Google Scholar] [CrossRef]
- Langer, N.; LeGrand, M.; Kedem, O. Cationic Polymer Coating Increases the Catalytic Activity of Gold Nanoparticles toward Anionic Substrates. ACS Appl. Mater. Interfaces 2023, 15, 29160–29169. [Google Scholar] [CrossRef]
- Reiser, B.; González-García, L.; Kanelidis, I.; Maurer, J.H.M.; Kraus, T. Gold Nanorods with Conjugated Polymer Ligands: Sintering-Free Conductive Inks for Printed Electronics. Chem. Sci. 2016, 7, 4190–4196. [Google Scholar] [CrossRef]
- Klos, M.A.H.; González-García, L.; Kraus, T. Mechanically Robust, Inkjet-Printable Polymer Nanocomposites with Hybrid Gold Nanoparticles and Metal-like Conductivity. ACS Appl. Mater. Interfaces 2024, 16, 31576–31585. [Google Scholar] [CrossRef]
- Niyonshuti, I.I.; Krishnamurthi, V.R.; Okyere, D.; Song, L.; Benamara, M.; Tong, X.; Wang, Y.; Chen, J. Polydopamine Surface Coating Synergizes the Antimicrobial Activity of Silver Nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 40067–40077. [Google Scholar] [CrossRef]
- Bera, A.; Ghosh, P.; Goswami, K.; De, P. Amino Acid-Based Polymer-Coated Silver Nanoparticles as Insulin Fibril Inhibitors. ACS Appl. Nano Mater. 2023, 6, 8705–8716. [Google Scholar] [CrossRef]
- Qiu, L.; Li, J.-W.; Hong, C.-Y.; Pan, C.-Y. Silver Nanoparticles Covered with pH-Sensitive Camptothecin-Loaded Polymer Prodrugs: Switchable Fluorescence “Off” or “On” and Drug Delivery Dynamics in Living Cells. ACS Appl. Mater. Interfaces 2017, 9, 40887–40897. [Google Scholar] [CrossRef]
- Kato, R.; Uesugi, M.; Komatsu, Y.; Okamoto, F.; Tanaka, T.; Kitawaki, F.; Yano, T. Highly Stable Polymer Coating on Silver Nanoparticles for Efficient Plasmonic Enhancement of Fluorescence. ACS Omega 2022, 7, 4286–4292. [Google Scholar] [CrossRef]
- Skiba, M.; Pivovarov, A.; Vorobyova, V. The Plasma-Induced Formation of PVP-Coated Silver Nanoparticles and Usage in Water Purification. Chem. Chem. Technol. 2020, 14, 47–54. [Google Scholar] [CrossRef]
- Khalil, A.M.; Hassan, M.L.; Ward, A.A. Novel Nanofibrillated Cellulose/Polyvinylpyrrolidone/Silver Nanoparticles Films with Electrical Conductivity Properties. Carbohydr. Polym. 2017, 157, 503–511. [Google Scholar] [CrossRef]
- Wang, X.; Hu, A.; Du, K.; Feng, F. Biomimetic Polymer-Templated Copper Nanoparticles Stabilize a Temozolomide Intermediate for Chemotherapy against Glioblastoma Multiforme. ACS Appl. Bio Mater. 2021, 4, 8004–8012. [Google Scholar] [CrossRef] [PubMed]
- Bogdanović, U.; Vodnik, V.; Mitrić, M.; Dimitrijević, S.; Škapin, S.D.; Žunič, V.; Budimir, M.; Stoiljković, M. Nanomaterial with High Antimicrobial Efficacy—Copper/Polyaniline Nanocomposite. ACS Appl. Mater. Interfaces 2015, 7, 1955–1966. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Zhao, Q.; Liu, J.; Zhang, Y. Antibacterial Behavior of Halloysite Nanotubes Decorated with Copper Nanoparticles in a Novel Mixed Matrix Membrane for Water Purification. Environ. Sci. Water Res. Technol. 2015, 1, 874–881. [Google Scholar] [CrossRef]
- Deng, H.-H.; Lin, X.-L.; Liu, Y.-H.; Li, K.-L.; Zhuang, Q.-Q.; Peng, H.-P.; Liu, A.-L.; Xia, X.-H.; Chen, W. Chitosan-Stabilized Platinum Nanoparticles as Effective Oxidase Mimics for Colorimetric Detection of Acid Phosphatase. Nanoscale 2017, 9, 10292–10300. [Google Scholar] [CrossRef]
- Fenoy, G.E.; Maza, E.; Zelaya, E.; Marmisollé, W.A.; Azzaroni, O. Layer-by-Layer Assemblies of Highly Connected Polyelectrolyte Capped-Pt Nanoparticles for Electrocatalysis of Hydrogen Evolution Reaction. Appl. Surf. Sci. 2017, 416, 24–32. [Google Scholar] [CrossRef]
- Bharathiraja, S.; Bui, N.Q.; Manivasagan, P.; Moorthy, M.S.; Mondal, S.; Seo, H.; Phuoc, N.T.; Vy Phan, T.T.; Kim, H.; Lee, K.D.; et al. Multimodal Tumor-Homing Chitosan Oligosaccharide-Coated Biocompatible Palladium Nanoparticles for Photo-Based Imaging and Therapy. Sci. Rep. 2018, 8, 500. [Google Scholar] [CrossRef]
- Baghayeri, M.; Alinezhad, H.; Tarahomi, M.; Fayazi, M.; Ghanei-Motlagh, M.; Maleki, B. A Non-Enzymatic Hydrogen Peroxide Sensor Based on Dendrimer Functionalized Magnetic Graphene Oxide Decorated with Palladium Nanoparticles. Appl. Surf. Sci. 2019, 478, 87–93. [Google Scholar] [CrossRef]
- Kumalasari, M.R.; Alfanaar, R.; Andreani, A.S. Gold Nanoparticles (AuNPs): A Versatile Material for Biosensor Application. Talanta Open 2024, 9, 100327. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Gold Nanoparticles: Optical Properties and Implementations in Cancer Diagnosis and Photothermal Therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent Biomedical Applications of Gold Nanoparticles: A Review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef]
- Ferrari, E. Gold Nanoparticle-Based Plasmonic Biosensors. Biosensors 2023, 13, 411. [Google Scholar] [CrossRef] [PubMed]
- Pellas, V.; Hu, D.; Mazouzi, Y.; Mimoun, Y.; Blanchard, J.; Guibert, C.; Salmain, M.; Boujday, S. Gold Nanorods for LSPR Biosensing: Synthesis, Coating by Silica, and Bioanalytical Applications. Biosensors 2020, 10, 146. [Google Scholar] [CrossRef]
- Brinson, B.E.; Lassiter, J.B.; Levin, C.S.; Bardhan, R.; Mirin, N.; Halas, N.J. Nanoshells Made Easy: Improving Au Layer Growth on Nanoparticle Surfaces. Langmuir 2008, 24, 14166–14171. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Rhéaume, É.; Lesage, F.; Kakkar, A. Synthetic Methodologies to Gold Nanoshells: An Overview. Molecules 2018, 23, 2851. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Hoang, S.; Winey, K.I.; Composto, R.J. Size-Dependent Electrostatic Adsorption of Polymer-Grafted Gold Nanoparticles on Polyelectrolyte Brushes. ACS Appl. Mater. Interfaces 2024, 16, 61083–61095. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.-Y.; Huang, J.; Chen, C.-Y.; Wang, Z.-X.; Xie, H. Silver Nanoparticles: Synthesis, Medical Applications and Biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef]
- Wei, L.; Lu, J.; Xu, H.; Patel, A.; Chen, Z.-S.; Chen, G. Silver Nanoparticles: Synthesis, Properties, and Therapeutic Applications. Drug Discov. Today 2015, 20, 595–601. [Google Scholar] [CrossRef]
- Ibrahim, N.; Akindoyo, J.O.; Mariatti, M. Recent Development in Silver-Based Ink for Flexible Electronics. J. Sci. Adv. Mater. Devices 2022, 7, 100395. [Google Scholar] [CrossRef]
- Gould, A.L.; Kadkhodazadeh, S.; Wagner, J.B.; Catlow, C.R.A.; Logsdail, A.J.; Di Vece, M. Understanding the Thermal Stability of Silver Nanoparticles Embedded in A-Si. J. Phys. Chem. C 2015, 119, 23767–23773. [Google Scholar] [CrossRef]
- Liu, S.; Han, G.; Zhang, M.; Xiong, B.; Xu, J.; Zhu, J. Grafting of Poly(Methyl Methacrylate) with Different Sulfur-Containing End Groups on Gold Nanoparticles and Their Interfacial Assembly into Superlattice Films. Macromolecules 2023, 56, 5567–5574. [Google Scholar] [CrossRef]
- Ajayakumar, M.R.; Alcón, I.; Bromley, S.T.; Veciana, J.; Rovira, C.; Mas-Torrent, M. Direct Covalent Grafting of an Organic Radical Core on Gold and Silver. RSC Adv. 2017, 7, 20076–20083. [Google Scholar] [CrossRef]
- Singh, N.; Khanna, P.K. In Situ Synthesis of Silver Nano-Particles in Polymethylmethacrylate. Mater. Chem. Phys. 2007, 104, 367–372. [Google Scholar] [CrossRef]
- Xiong, Y.; Luo, G.; Chen, C.; Yuan, H.; Shen, Q.; Li, M. In Situ Synthesis of Zero-Valent Silver Nanoparticles in Polymethylmethacrylate under High Temperature. Appl. Surf. Sci. 2012, 258, 5822–5826. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef]
- Ponce, A.A.; Klabunde, K.J. Chemical and Catalytic Activity of Copper Nanoparticles Prepared via Metal Vapor Synthesis. J. Mol. Catal. A Chem. 2005, 225, 1–6. [Google Scholar] [CrossRef]
- Rojas, B.; Soto, N.; Villalba, M.; Bello-Toledo, H.; Meléndrez-Castro, M.; Sánchez-Sanhueza, G. Antibacterial Activity of Copper Nanoparticles (CuNPs) against a Resistant Calcium Hydroxide Multispecies Endodontic Biofilm. Nanomaterials 2021, 11, 2254. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.-B.; Wang, J.-F.; Chuang, K.-J.; Cheng, H.-Y.; Chang, K.-C.; Ma, C.-M. Preparing Copper Nanoparticles and Flexible Copper Conductive Sheets. Nanomaterials 2022, 12, 360. [Google Scholar] [CrossRef] [PubMed]
- Tomotoshi, D.; Kawasaki, H. Surface and Interface Designs in Copper-Based Conductive Inks for Printed/Flexible Electronics. Nanomaterials 2020, 10, 1689. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Huang, M.; Liu, X.; Sun, D.; Shao, C. In Situ Generation of Fluorescent Copper Nanoclusters Embedded in Monolithic Eggshell Membrane: Properties and Applications. Materials 2018, 11, 1913. [Google Scholar] [CrossRef]
- Mallick, K.; Witcomb, M.J.; Scurrell, M.S. In Situ Synthesis of Copper Nanoparticles and Poly(o-Toluidine): A Metal–Polymer Composite Material. Eur. Polym. J. 2006, 42, 670–675. [Google Scholar] [CrossRef]
- Mohammed Safiullah, S.; Abdul Wasi, K.; Anver Basha, K. Preparation of Poly(Glycidyl Methacrylate)–Copper Nanocomposite by in-Situ Suspension Polymerization—A Novel Synthetic Method. Mater. Lett. 2014, 133, 60–63. [Google Scholar] [CrossRef]
- Khan, M.A.R.; Mamun, M.S.A.; Ara, M.H. Review on Platinum Nanoparticles: Synthesis, Characterization, and Applications. Microchem. J. 2021, 171, 106840. [Google Scholar] [CrossRef]
- Dutta, S.; Hazra, R.; Kar, A.; Ghosh, P.; Patra, P. Platinum Nanoparticles: Tiny Titans in Therapy. Discov. Mater. 2024, 4, 16. [Google Scholar] [CrossRef]
- Phan, T.T.V.; Huynh, T.-C.; Manivasagan, P.; Mondal, S.; Oh, J. An Up-To-Date Review on Biomedical Applications of Palladium Nanoparticles. Nanomaterials 2019, 10, 66. [Google Scholar] [CrossRef]
- Wang, W.; Nadagouda, M.N.; Mukhopadhyay, S.M. Advances in Matrix-Supported Palladium Nanocatalysts for Water Treatment. Nanomaterials 2022, 12, 3593. [Google Scholar] [CrossRef]
- Astruc, D. Palladium Nanoparticles as Efficient Green Homogeneous and Heterogeneous Carbon−Carbon Coupling Precatalysts: A Unifying View. Inorg. Chem. 2007, 46, 1884–1894. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Ostrom, C. Palladium-Based Nanomaterials: Synthesis and Electrochemical Applications. Chem. Rev. 2015, 115, 11999–12044. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.-W.; Fan, S.-X.; Wang, F.; Sun, L.-D.; Zheng, X.-Y.; Yan, C.-H. Porous Pd Nanoparticles with High Photothermal Conversion Efficiency for Efficient Ablation of Cancer Cells. Nanoscale 2014, 6, 4345–4351. [Google Scholar] [CrossRef]
- Molahalli, V.; Sharma, A.; Bijapur, K.; Soman, G.; Shetty, A.; Sirichandana, B.; Patel, B.G.M.; Chattham, N.; Hegde, G. Properties, Synthesis, and Characterization of Cu-Based Nanomaterials. In ACS Symposium Series; Srivastava, A., Srivastava, A., Eds.; American Chemical Society: Washington, DC, USA, 2024; Volume 1466, pp. 1–33. ISBN 978-0-8412-9681-7. [Google Scholar]
- Bi, S.; Li, K.; Chen, X.; Fu, W.; Chen, L.; Sheng, H.; Yang, Q. Preparation and Catalytic Properties of Composites with Palladium Nanoparticles and Poly(Methacrylic Acid) Microspheres. Polym. Compos. 2014, 35, 2251–2260. [Google Scholar] [CrossRef]
- Mondal, K.; Sharma, A. Recent Advances in the Synthesis and Application of Photocatalytic Metal–Metal Oxide Core–Shell Nanoparticles for Environmental Remediation and Their Recycling Process. RSC Adv. 2016, 6, 83589–83612. [Google Scholar] [CrossRef]
- Nguyen, M.D.; Tran, H.-V.; Xu, S.; Lee, T.R. Fe3O4 Nanoparticles: Structures, Synthesis, Magnetic Properties, Surface Functionalization, and Emerging Applications. Appl. Sci. 2021, 11, 11301. [Google Scholar] [CrossRef]
- Israel, L.L.; Galstyan, A.; Holler, E.; Ljubimova, J.Y. Magnetic Iron Oxide Nanoparticles for Imaging, Targeting and Treatment of Primary and Metastatic Tumors of the Brain. J. Control. Release 2020, 320, 45–62. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Visible-Light Active Titanium Dioxide Nanomaterials with Bactericidal Properties. Nanomaterials 2020, 10, 124. [Google Scholar] [CrossRef]
- Li, X.; Xiong, J.; Tang, Z.; He, W.; Wang, Y.; Wang, X.; Zhao, Z.; Wei, Y. Recent Progress in Metal Oxide-Based Photocatalysts for CO2 Reduction to Solar Fuels: A Review. Molecules 2023, 28, 1653. [Google Scholar] [CrossRef]
- Mao, Y.; Li, Y.; Zou, Y.; Shen, X.; Zhu, L.; Liao, G. Solvothermal Synthesis and Photocatalytic Properties of ZnO Micro/Nanostructures. Ceram. Int. 2019, 45, 1724–1729. [Google Scholar] [CrossRef]
- Musil, J.; Blažek, J.; Zeman, P.; Prokšová, Š.; Šašek, M.; Čerstvý, R. Thermal Stability of Alumina Thin Films Containing γ-Al2O3 Phase Prepared by Reactive Magnetron Sputtering. Appl. Surf. Sci. 2010, 257, 1058–1062. [Google Scholar] [CrossRef]
- Sundaresan, V.; Menon, J.U.; Rahimi, M.; Nguyen, K.T.; Wadajkar, A.S. Dual-Responsive Polymer-Coated Iron Oxide Nanoparticles for Drug Delivery and Imaging Applications. Int. J. Pharm. 2014, 466, 1–7. [Google Scholar] [CrossRef]
- Huang, J.; Shu, Q.; Wang, L.; Wu, H.; Wang, A.Y.; Mao, H. Layer-by-Layer Assembled Milk Protein Coated Magnetic Nanoparticle Enabled Oral Drug Delivery with High Stability in Stomach and Enzyme-Responsive Release in Small Intestine. Biomaterials 2015, 39, 105–113. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, B.; Wang, L.; Wang, J.; Li, X.; Yang, G.; Gao, F. Superparamagnetic Iron Oxide Nanoparticles Coated with Different Polymers and Their MRI Contrast Effects in the Mouse Brains. Appl. Surf. Sci. 2015, 326, 32–38. [Google Scholar] [CrossRef]
- Hou, Z.; Ren, M.; Wang, K.; Yang, Y.; Xu, J.; Zhu, J. Deformable Block Copolymer Microparticles by Controllable Localization of pH-Responsive Nanoparticles. Macromolecules 2020, 53, 473–481. [Google Scholar] [CrossRef]
- Yang, K.; Liu, Y.; Liu, Y.; Zhang, Q.; Kong, C.; Yi, C.; Zhou, Z.; Wang, Z.; Zhang, G.; Zhang, Y.; et al. Cooperative Assembly of Magneto-Nanovesicles with Tunable Wall Thickness and Permeability for MRI-Guided Drug Delivery. J. Am. Chem. Soc. 2018, 140, 4666–4677. [Google Scholar] [CrossRef]
- Park, W.; Yang, H.N.; Ling, D.; Yim, H.; Kim, K.S.; Hyeon, T.; Na, K.; Park, K.-H. Multi-Modal Transfection Agent Based on Monodisperse Magnetic Nanoparticles for Stem Cell Gene Delivery and Tracking. Biomaterials 2014, 35, 7239–7247. [Google Scholar] [CrossRef] [PubMed]
- Kakwere, H.; Leal, M.P.; Materia, M.E.; Curcio, A.; Guardia, P.; Niculaes, D.; Marotta, R.; Falqui, A.; Pellegrino, T. Functionalization of Strongly Interacting Magnetic Nanocubes with (Thermo) Responsive Coating and Their Application in Hyperthermia and Heat-Triggered Drug Delivery. ACS Appl. Mater. Interfaces 2015, 7, 10132–10145. [Google Scholar] [CrossRef] [PubMed]
- Rezk, A.I.; Bhattarai, D.P.; Park, J.; Park, C.H.; Kim, C.S. Polyaniline-Coated Titanium Oxide Nanoparticles and Simvastatin-Loaded Poly(ε-Caprolactone) Composite Nanofibers Scaffold for Bone Tissue Regeneration Application. Colloids Surf. B Biointerfaces 2020, 192, 111007. [Google Scholar] [CrossRef]
- Haque, S.U.; Duteanu, N.; Nasar, A. Inamuddin Polythiophene-Titanium Oxide (PTH-TiO2) Nanocomposite: As an Electron Transfer Enhancer for Biofuel Cell Anode Construction. J. Power Sources 2022, 520, 230867. [Google Scholar] [CrossRef]
- Bharathi, D.; Ranjithkumar, R.; Chandarshekar, B.; Bhuvaneshwari, V. Preparation of Chitosan Coated Zinc Oxide Nanocomposite for Enhanced Antibacterial and Photocatalytic Activity: As a Bionanocomposite. Int. J. Biol. Macromol. 2019, 129, 989–996. [Google Scholar] [CrossRef]
- Vyavhare, K.; Timmons, R.B.; Erdemir, A.; Edwards, B.L.; Aswath, P.B. Robust Interfacial Tribofilms by Borate- and Polymer-Coated ZnO Nanoparticles Leading to Improved Wear Protection under a Boundary Lubrication Regime. Langmuir 2021, 37, 1743–1759. [Google Scholar] [CrossRef] [PubMed]
- Rajan, Y.C.; Inbaraj, B.S.; Chen, B.H. Synthesis and Characterization of Poly(γ-Glutamic Acid)-Based Alumina Nanoparticles with Their Protein Adsorption Efficiency and Cytotoxicity towards Human Prostate Cancer Cells. RSC Adv. 2015, 5, 15126–15139. [Google Scholar] [CrossRef]
- Burmistrov, D.E.; Serov, D.A.; Simakin, A.V.; Baimler, I.V.; Uvarov, O.V.; Gudkov, S.V. A Polytetrafluoroethylene (PTFE) and Nano-Al2O3 Based Composite Coating with a Bacteriostatic Effect against E. coli and Low Cytotoxicity. Polymers 2022, 14, 4764. [Google Scholar] [CrossRef]
- Abdel-Naby, A.S.; Nabil, S.; Aldulaijan, S.; Ababutain, I.M.; Alghamdi, A.I.; Almubayedh, S.; Khalil, K.D. Synthesis, Characterization of Chitosan-Aluminum Oxide Nanocomposite for Green Synthesis of Annulated Imidazopyrazol Thione Derivatives. Polymers 2021, 13, 1160. [Google Scholar] [CrossRef]
- Wahajuddin, A. Superparamagnetic Iron Oxide Nanoparticles: Magnetic Nanoplatforms as Drug Carriers. Int. J. Nanomed. 2012, 7, 3445. [Google Scholar] [CrossRef] [PubMed]
- Guardia, P.; Di Corato, R.; Lartigue, L.; Wilhelm, C.; Espinosa, A.; Garcia-Hernandez, M.; Gazeau, F.; Manna, L.; Pellegrino, T. Water-Soluble Iron Oxide Nanocubes with High Values of Specific Absorption Rate for Cancer Cell Hyperthermia Treatment. ACS Nano 2012, 6, 3080–3091. [Google Scholar] [CrossRef]
- Shokrollahi, H. A Review of the Magnetic Properties, Synthesis Methods and Applications of Maghemite. J. Magn. Magn. Mater. 2017, 426, 74–81. [Google Scholar] [CrossRef]
- Białek, M.; Zhang, J.; Yu, H.; Ansermet, J.-P. Antiferromagnetic Resonance in α-Fe2O3 up to Its Néel Temperature. Appl. Phys. Lett. 2022, 121, 032401. [Google Scholar] [CrossRef]
- Mishra, M.; Chun, D.-M. α-Fe2O3 as a Photocatalytic Material: A Review. Appl. Catal. A 2015, 498, 126–141. [Google Scholar] [CrossRef]
- Ma, J.; Lian, J.; Duan, X.; Liu, X.; Zheng, W. α-Fe2O3: Hydrothermal Synthesis, Magnetic and Electrochemical Properties. J. Phys. Chem. C 2010, 114, 10671–10676. [Google Scholar] [CrossRef]
- Li, Q.; Kartikowati, C.W.; Horie, S.; Ogi, T.; Iwaki, T.; Okuyama, K. Correlation between Particle Size/Domain Structure and Magnetic Properties of Highly Crystalline Fe3O4 Nanoparticles. Sci. Rep. 2017, 7, 9894. [Google Scholar] [CrossRef] [PubMed]
- Issa, B.; Obaidat, I.; Albiss, B.; Haik, Y. Magnetic Nanoparticles: Surface Effects and Properties Related to Biomedicine Applications. Int. J. Mol. Sci. 2013, 14, 21266–21305. [Google Scholar] [CrossRef]
- Kolhatkar, A.; Jamison, A.; Litvinov, D.; Willson, R.; Lee, T. Tuning the Magnetic Properties of Nanoparticles. Int. J. Mol. Sci. 2013, 14, 15977–16009. [Google Scholar] [CrossRef]
- Teja, A.S.; Koh, P.-Y. Synthesis, Properties, and Applications of Magnetic Iron Oxide Nanoparticles. Prog. Cryst. Growth Charact. Mater. 2009, 55, 22–45. [Google Scholar] [CrossRef]
- Foster, L.M.; Worthen, A.J.; Foster, E.L.; Dong, J.; Roach, C.M.; Metaxas, A.E.; Hardy, C.D.; Larsen, E.S.; Bollinger, J.A.; Truskett, T.M.; et al. High Interfacial Activity of Polymers “Grafted through” Functionalized Iron Oxide Nanoparticle Clusters. Langmuir 2014, 30, 10188–10196. [Google Scholar] [CrossRef]
- Na, H.B.; Song, I.C.; Hyeon, T. Inorganic Nanoparticles for MRI Contrast Agents. Adv. Mater. 2009, 21, 2133–2148. [Google Scholar] [CrossRef]
- Rahman, M. Magnetic Resonance Imaging and Iron-Oxide Nanoparticles in the Era of Personalized Medicine. Nanotheranostics 2023, 7, 424–449. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Bai, R.; Yi, J.; Zhou, H.; Xian, J.; Chen, C. Designing Smart Iron Oxide Nanoparticles for MR Imaging of Tumors. Chem. Biomed. Imaging 2023, 1, 315–339. [Google Scholar] [CrossRef] [PubMed]
- Lapusan, R.; Borlan, R.; Focsan, M. Advancing MRI with Magnetic Nanoparticles: A Comprehensive Review of Translational Research and Clinical Trials. Nanoscale Adv. 2024, 6, 2234–2259. [Google Scholar] [CrossRef]
- Deng, J.; Wen, X.; Wang, Q. Solvothermal in Situ Synthesis of Fe3O4-Multi-Walled Carbon Nanotubes with Enhanced Heterogeneous Fenton-like Activity. Mater. Res. Bull. 2012, 47, 3369–3376. [Google Scholar] [CrossRef]
- Barrow, M.; Taylor, A.; Murray, P.; Rosseinsky, M.J.; Adams, D.J. Design Considerations for the Synthesis of Polymer Coated Iron Oxide Nanoparticles for Stem Cell Labelling and Tracking Using MRI. Chem. Soc. Rev. 2015, 44, 6733–6748. [Google Scholar] [CrossRef] [PubMed]
- Seynhaeve, A.L.B.; Amin, M.; Haemmerich, D.; Van Rhoon, G.C.; Ten Hagen, T.L.M. Hyperthermia and Smart Drug Delivery Systems for Solid Tumor Therapy. Adv. Drug Deliv. Rev. 2020, 163–164, 125–144. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.Y.; Kim, M.J.; Kim, H.I.; Park, J.; Baek, S.H. Hyperthermia Treatment as a Promising Anti-Cancer Strategy: Therapeutic Targets, Perspective Mechanisms and Synergistic Combinations in Experimental Approaches. Antioxidants 2022, 11, 625. [Google Scholar] [CrossRef]
- Behrouzkia, Z.; Joveini, Z.; Keshavarzi, B.; Eyvazzadeh, N.; Aghdam, R.Z. Hyperthermia: How Can It Be Used? Oman Med. J. 2016, 31, 89–97. [Google Scholar] [CrossRef]
- Cohen, Y.; Shoushan, S.Y. Magnetic Nanoparticles-Based Diagnostics and Theranostics. Curr. Opin. Biotechnol. 2013, 24, 672–681. [Google Scholar] [CrossRef]
- Thirunavukkarasu, G.K.; Cherukula, K.; Lee, H.; Jeong, Y.Y.; Park, I.-K.; Lee, J.Y. Magnetic Field-Inducible Drug-Eluting Nanoparticles for Image-Guided Thermo-Chemotherapy. Biomaterials 2018, 180, 240–252. [Google Scholar] [CrossRef]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef] [PubMed]
- Hajareh Haghighi, F.; Mercurio, M.; Cerra, S.; Salamone, T.A.; Bianymotlagh, R.; Palocci, C.; Romano Spica, V.; Fratoddi, I. Surface Modification of TiO2 Nanoparticles with Organic Molecules and Their Biological Applications. J. Mater. Chem. B 2023, 11, 2334–2366. [Google Scholar] [CrossRef]
- Eddy, D.R.; Permana, M.D.; Sakti, L.K.; Sheha, G.A.N.; Solihudin; Hidayat, S.; Takei, T.; Kumada, N.; Rahayu, I. Heterophase Polymorph of TiO2 (Anatase, Rutile, Brookite, TiO2 (B)) for Efficient Photocatalyst: Fabrication and Activity. Nanomaterials 2023, 13, 704. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Tu, K.-J.; Deng, J.-P.; Lo, Y.-S.; Wu, C.-H. Markedly Enhanced Surface Hydroxyl Groups of TiO2 Nanoparticles with Superior Water-Dispersibility for Photocatalysis. Materials 2017, 10, 566. [Google Scholar] [CrossRef] [PubMed]
- Rozman, N.; Nadrah, P.; Cornut, R.; Jousselme, B.; Bele, M.; Dražić, G.; Gaberšček, M.; Kunej, Š.; Škapin, A.S. TiO2 Photocatalyst with Single and Dual Noble Metal Co-Catalysts for Efficient Water Splitting and Organic Compound Removal. Int. J. Hydrogen Energy 2021, 46, 32871–32881. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 Photocatalysis: Design and Applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Khlyustova, A.; Sirotkin, N.; Kusova, T.; Kraev, A.; Titov, V.; Agafonov, A. Doped TiO2: The Effect of Doping Elements on Photocatalytic Activity. Mater. Adv. 2020, 1, 1193–1201. [Google Scholar] [CrossRef]
- Yu, W.; Zhao, L.; Chen, F.; Zhang, H.; Guo, L.-H. Surface Bridge Hydroxyl-Mediated Promotion of Reactive Oxygen Species in Different Particle Size TiO2 Suspensions. J. Phys. Chem. Lett. 2019, 10, 3024–3028. [Google Scholar] [CrossRef]
- Ashkarran, A.A.; Hamidinezhad, H.; Haddadi, H.; Mahmoudi, M. Double-Doped TiO2 Nanoparticles as an Efficient Visible-Light-Active Photocatalyst and Antibacterial Agent under Solar Simulated Light. Appl. Surf. Sci. 2014, 301, 338–345. [Google Scholar] [CrossRef]
- Abutalib, M.M.; Rajeh, A. Preparation and Characterization of Polyaniline/Sodium Alginate-Doped TiO2 Nanoparticles with Promising Mechanical and Electrical Properties and Antimicrobial Activity for Food Packaging Applications. J. Mater. Sci. Mater. Electron. 2020, 31, 9430–9442. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, G.; Meng, X.; Zhang, X.; Yu, L. Promoting the Catalytic Activities of Polyanilines for L-Lactic Acid Condensation by Calcium-Doping: A Biocompatible Strategy. Chin. Chem. Lett. 2025, 36, 111069. [Google Scholar] [CrossRef]
- Rasmussen, M.; Abdellaoui, S.; Minteer, S.D. Enzymatic Biofuel Cells: 30 Years of Critical Advancements. Biosens. Bioelectron. 2016, 76, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Balta, Z.; Bilgin Simsek, E.; Saloglu, D. Bio-Inspired Functional Photocatalyst: Lipase Enzyme Functionalized TiO2 with Excellent Photocatalytic, Enzymatic, and Antimicrobial Performance. J. Photochem. Photobiol. A Chem. 2023, 438, 114565. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, E.T.; Neoh, K.G.; Tan, K.L. Oxidative Graft Polymerization of Aniline on Modified Si(100) Surface. Macromolecules 2001, 34, 3133–3141. [Google Scholar] [CrossRef]
- Anjum, S.; Hashim, M.; Malik, S.A.; Khan, M.; Lorenzo, J.M.; Abbasi, B.H.; Hano, C. Recent Advances in Zinc Oxide Nanoparticles (ZnO NPs) for Cancer Diagnosis, Target Drug Delivery, and Treatment. Cancers 2021, 13, 4570. [Google Scholar] [CrossRef]
- Öztel, H.O.; Akçay, N.; Algün, G. Deep-Level Transient Spectroscopy Analysis of Interface Defects in Ce:ZnO/p-Si Heterostructures. J. Mater. Sci. Mater. Electron 2024, 35, 1247. [Google Scholar] [CrossRef]
- Raha, S.; Ahmaruzzaman, M. ZnO Nanostructured Materials and Their Potential Applications: Progress, Challenges and Perspectives. Nanoscale Adv. 2022, 4, 1868–1925. [Google Scholar] [CrossRef]
- Uribe-López, M.C.; Hidalgo-López, M.C.; López-González, R.; Frías-Márquez, D.M.; Núñez-Nogueira, G.; Hernández-Castillo, D.; Alvarez-Lemus, M.A. Photocatalytic Activity of ZnO Nanoparticles and the Role of the Synthesis Method on Their Physical and Chemical Properties. J. Photochem. Photobiol. A Chem. 2021, 404, 112866. [Google Scholar] [CrossRef]
- Cao, D.; Shu, X.; Zhu, D.; Liang, S.; Hasan, M.; Gong, S. Lipid-Coated ZnO Nanoparticles Synthesis, Characterization and Cytotoxicity Studies in Cancer Cell. Nano Converg. 2020, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Mohd Yusof, H.; Abdul Rahman, N.; Mohamad, R.; Zaidan, U.H.; Samsudin, A.A. Optimization of Biosynthesis Zinc Oxide Nanoparticles: Desirability-Function Based Response Surface Methodology, Physicochemical Characteristics, and Its Antioxidant Properties. OpenNano 2022, 8, 100106. [Google Scholar] [CrossRef]
- Canta, M.; Cauda, V. The Investigation of the Parameters Affecting the ZnO Nanoparticle Cytotoxicity Behaviour: A Tutorial Review. Biomater. Sci. 2020, 8, 6157–6174. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, W.; Li, Q.; Liu, H.; Wang, X. Preparations and Applications of Zinc Oxide Based Photocatalytic Materials. Adv. Sens. Energy Mater. 2023, 2, 100069. [Google Scholar] [CrossRef]
- Singh, K.; Nancy; Bhattu, M.; Singh, G.; Mubarak, N.M.; Singh, J. Light-Absorption-Driven Photocatalysis and Antimicrobial Potential of PVP-Capped Zinc Oxide Nanoparticles. Sci. Rep. 2023, 13, 13886. [Google Scholar] [CrossRef]
- Prokhorov, E.; Luna-Bárcenas, G.; Yáñez Limón, J.M.; Gómez Sánchez, A.; Kovalenko, Y. Chitosan-ZnO Nanocomposites Assessed by Dielectric, Mechanical, and Piezoelectric Properties. Polymers 2020, 12, 1991. [Google Scholar] [CrossRef]
- Ren, B.; Gao, L.; Xie, B.; Li, M.; Zhang, S.; Zu, G.; Ran, X. Tribological Properties and Anti-Wear Mechanism of ZnO@graphene Core-Shell Nanoparticles as Lubricant Additives. Tribol. Int. 2020, 144, 106114. [Google Scholar] [CrossRef]
- Hernaiz, M.; Elexpe, I.; Aranzabe, E.; Fernández, B.; Fernández, X.; Fernández, S.; Cortada-García, M.; Aguayo, A.T. Study of the Effect of ZnO Functionalization on the Performance of a Fully Formulated Engine Oil. Nanomaterials 2023, 13, 2540. [Google Scholar] [CrossRef]
- Hassanpour, P.; Panahi, Y.; Ebrahimi-Kalan, A.; Akbarzadeh, A.; Davaran, S.; Nasibova, A.N.; Khalilov, R.; Kavetskyy, T. Biomedical Applications of Aluminium Oxide Nanoparticles. Micro Nano Lett. 2018, 13, 1227–1231. [Google Scholar] [CrossRef]
- Matori, K.; Wah, L.; Hashim, M.; Ismail, I.; Zaid, M. Phase Transformations of α-Alumina Made from Waste Aluminum via a Precipitation Technique. Int. J. Mol. Sci. 2012, 13, 16812–16821. [Google Scholar] [CrossRef]
- Suchanek, W.L. Hydrothermal Synthesis of Alpha Alumina (α-Al2O3) Powders: Study of the Processing Variables and Growth Mechanisms. J. Am. Ceram. Soc. 2010, 93, 399–412. [Google Scholar] [CrossRef]
- Gholizadeh, Z.; Aliannezhadi, M.; Ghominejad, M.; Tehrani, F.S. High Specific Surface Area γ-Al2O3 Nanoparticles Synthesized by Facile and Low-Cost Co-Precipitation Method. Sci. Rep. 2023, 13, 6131. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, G.; Kim, J.; Lee, S.; Kim, J.-C.; Ryoo, R.; Lee, H. Anchoring Catalytically Active Species on Alumina via Surface Hydroxyl Group for Durable Surface Reaction. Appl. Catal. B Environ. 2023, 325, 122325. [Google Scholar] [CrossRef]
- Mallakpour, S.; Khadem, E. Studies of Surface Functional Modification of α-Al2O3 Nanoparticles Using Organic Chain Dicarboxylic Acid Containing Trimellitylimido-Amino Acid-Based Diacids Via Post Modification Method. Synth. React. Inorg. Met. -Org. Nano-Met. Chem. 2015, 45, 1773–1779. [Google Scholar] [CrossRef]






| Metal Core | Polymer Coating | Polymer Synthesis Method | Hybrid Nanomaterial Formation Mechanism | Applications | Ref. |
|---|---|---|---|---|---|
| AuNPs | cRGD-PEG-PLL-LA | Anionic | Grafting to | Drug delivery | [102] |
| AuNPs | lipoyl-[(MCH)26-b-(GMA)53] | RAFT | Grafting to | Drug delivery | [103] |
| AuNShs | P(NIPAM-co-AA) | Free radical | Grafting from | Drug delivery | [104] |
| AuNPs | poly(AcMan-r-AAm) | RAFT | Grafting to | Biosensing | [105] |
| AuNPs | Star-shaped PEG | N/A | Grafting to | Biosensing | [106] |
| AuNPs | DOPA, PEI | Self-polymerization, N/A | In situ | Environmental | [107] |
| AuNIs | PAH | N/A | Physisorption | Catalysis | [108] |
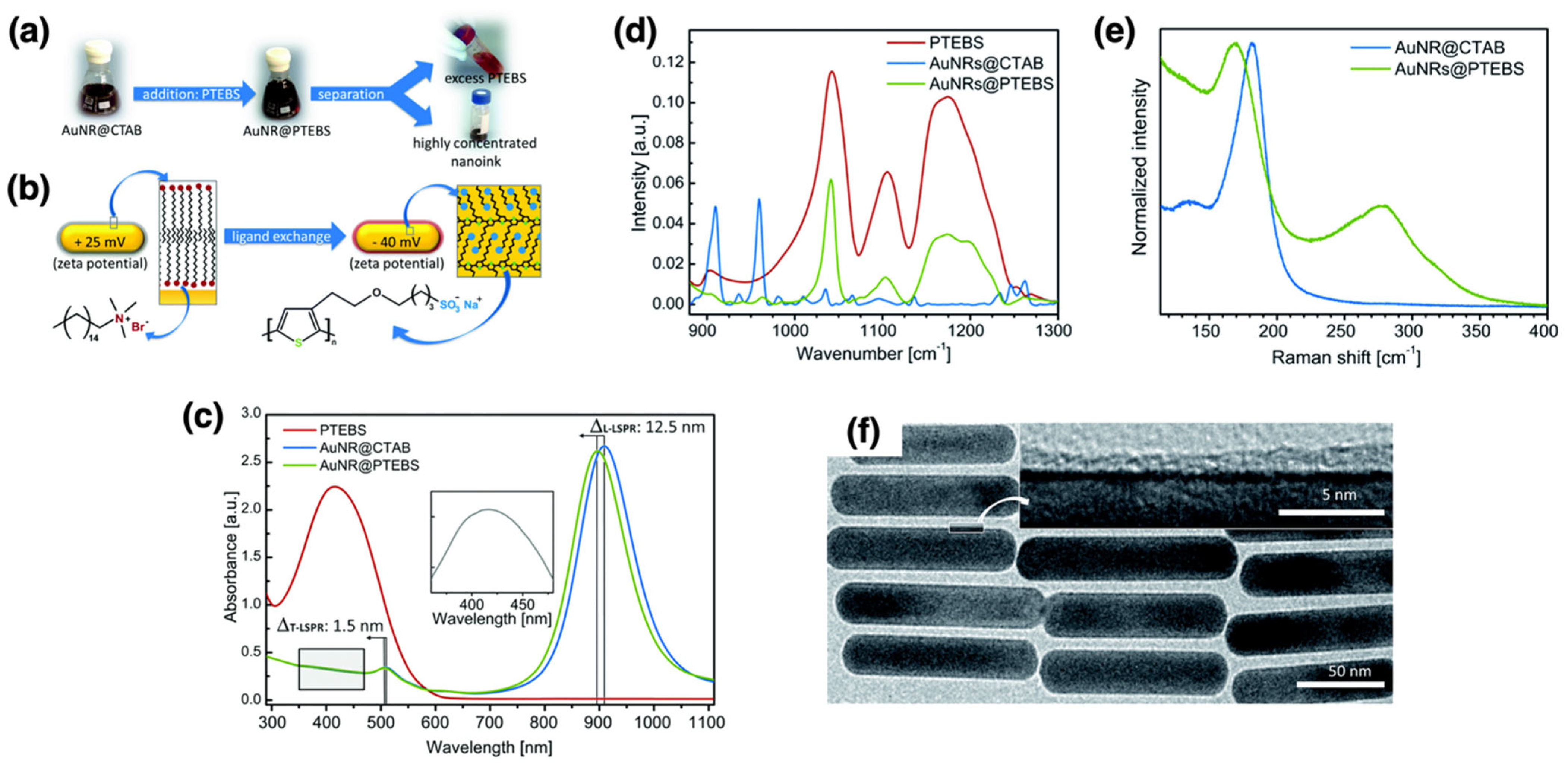
| AuNRs | PTEBS | N/A | Grafting to | Electronics | [109] |
| AuNPs | PEDOT:PSS, PVA | N/A | Grafting to | Electronics | [110] |
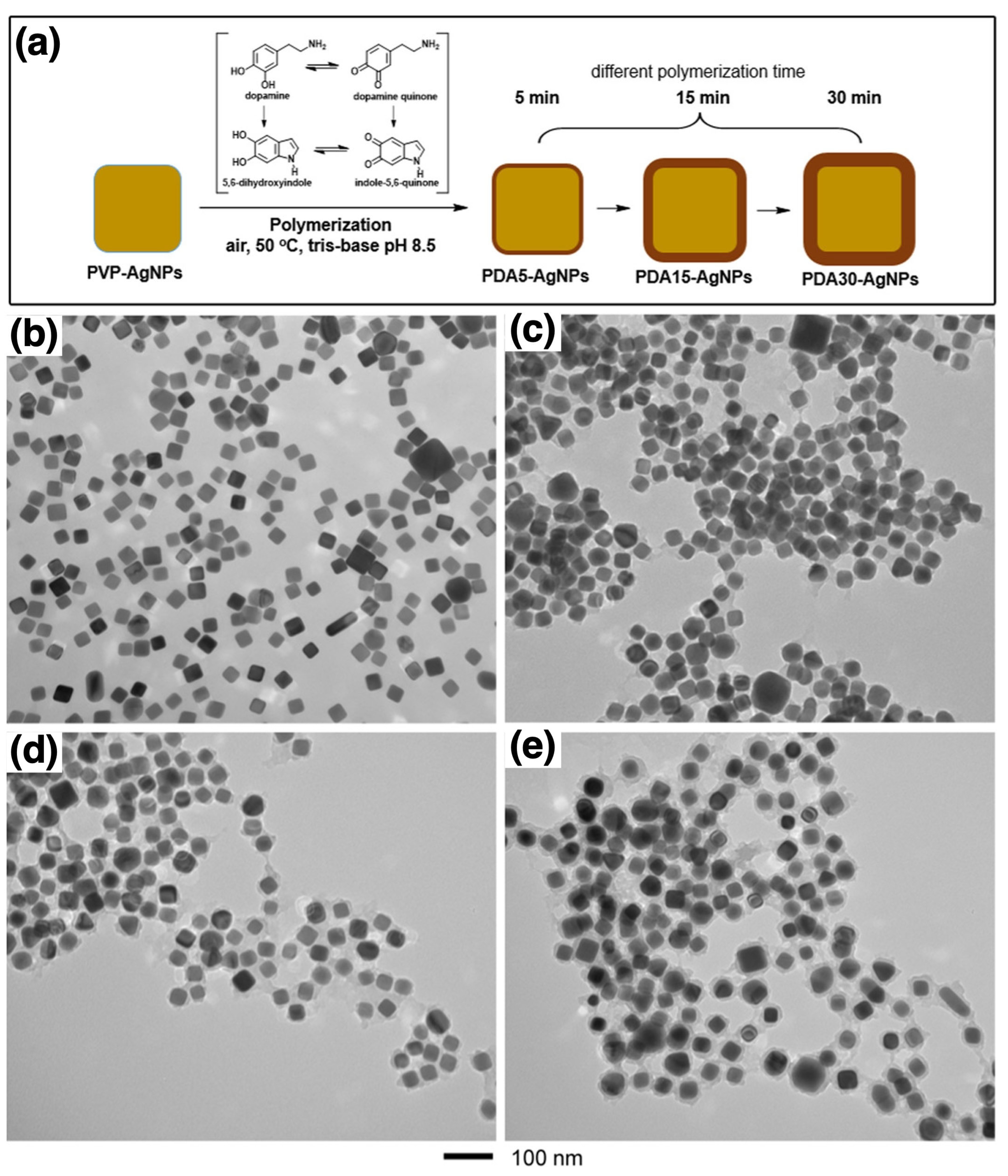
| AgNPs | PDA | Self-polymerization | Coordination | Antimicrobials | [111] |
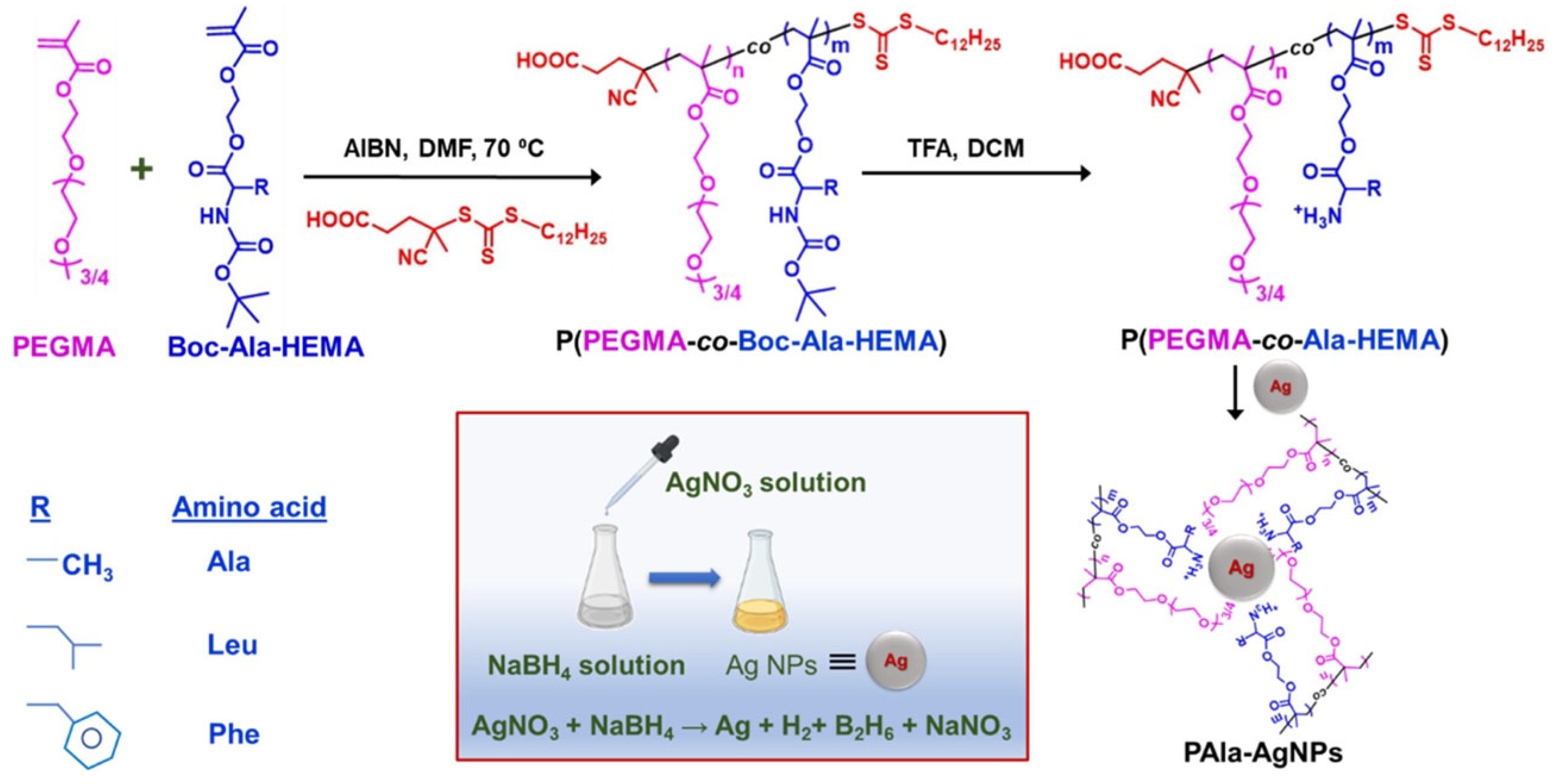
| AgNPs | P(PEGMA-co-R-HEMA) | RAFT | Physisorption | Therapeutics | [112] |
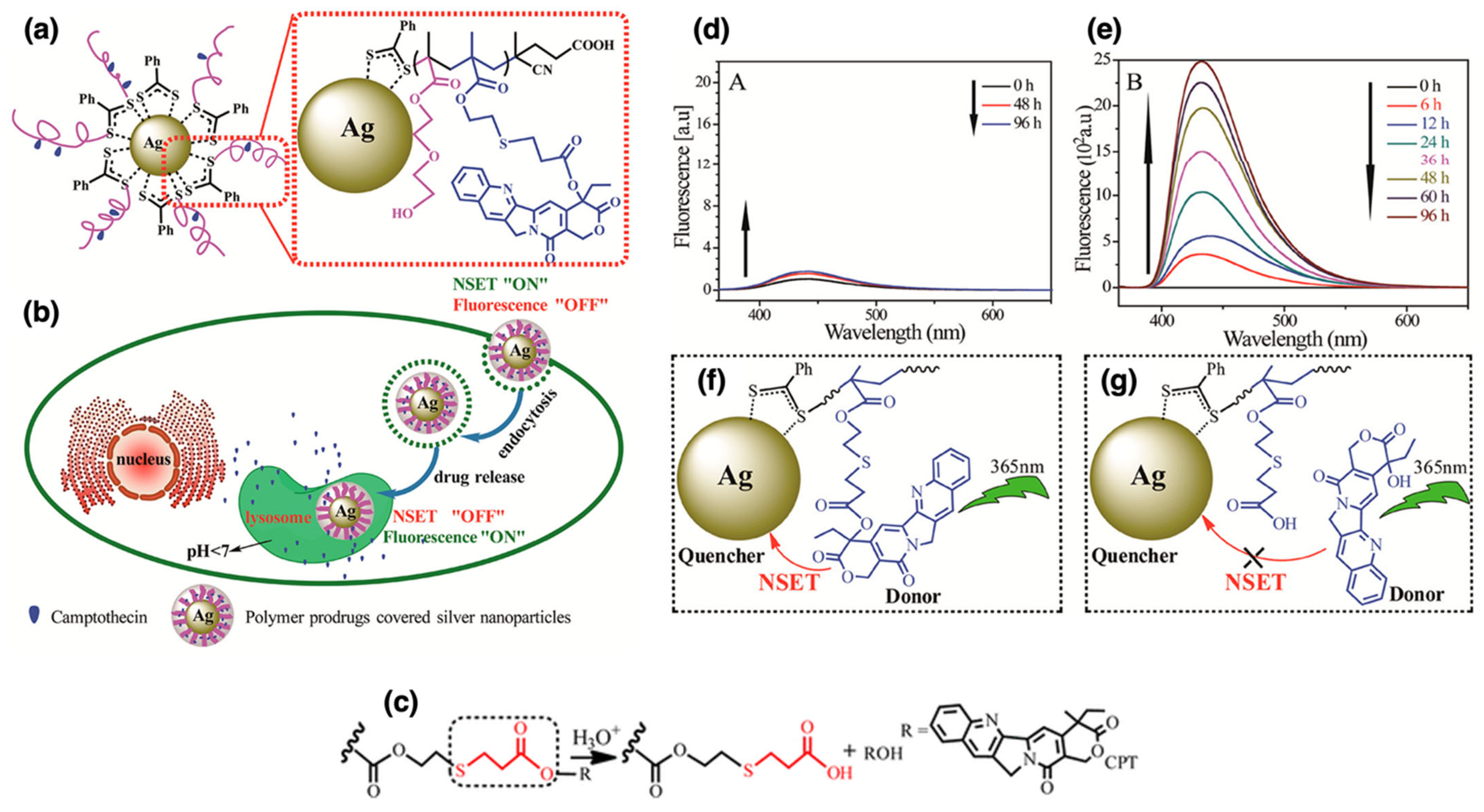
| AgNPs | P(HEO2MA-co-MACPT) | RAFT | Grafting to | Drug delivery | [113] |
| AgNPs | PLL-PEG4-SPDP | N/A | Grafting to | Biosensing | [114] |
| AgNPs | PVP | N/A | In situ | Environmental | [115] |
| AgNPs | NFC-PVP | N/A | In situ | Electronics | [116] |
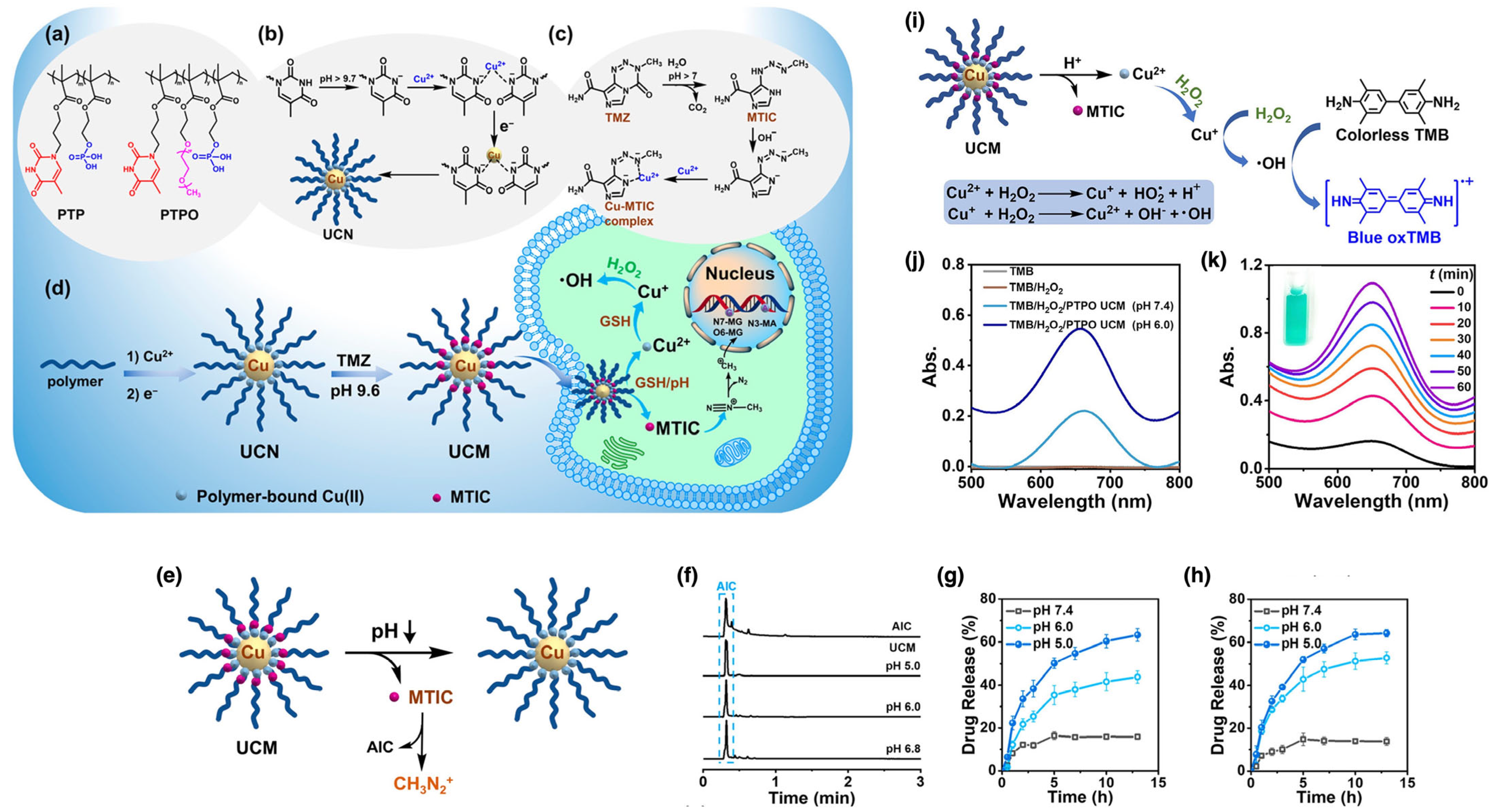
| CuNPs | PTP, PTPO | Free radical | In situ | Drug delivery | [117] |
| CuNPs | PANI | In situ | In situ | Antimicrobials | [118] |
| CuNPs | P4VP | Reversible ATRP | In situ | Environmental | [119] |
| PtNPs | Chitosan | N/A | In situ | Biosensing | [120] |
| PtNPs | PDDA, PSS | N/A | Layer-by-layer | Energy conversion | [121] |
| PdNPs | COS-RGD | N/A | Grafting to | Drug delivery | [122] |
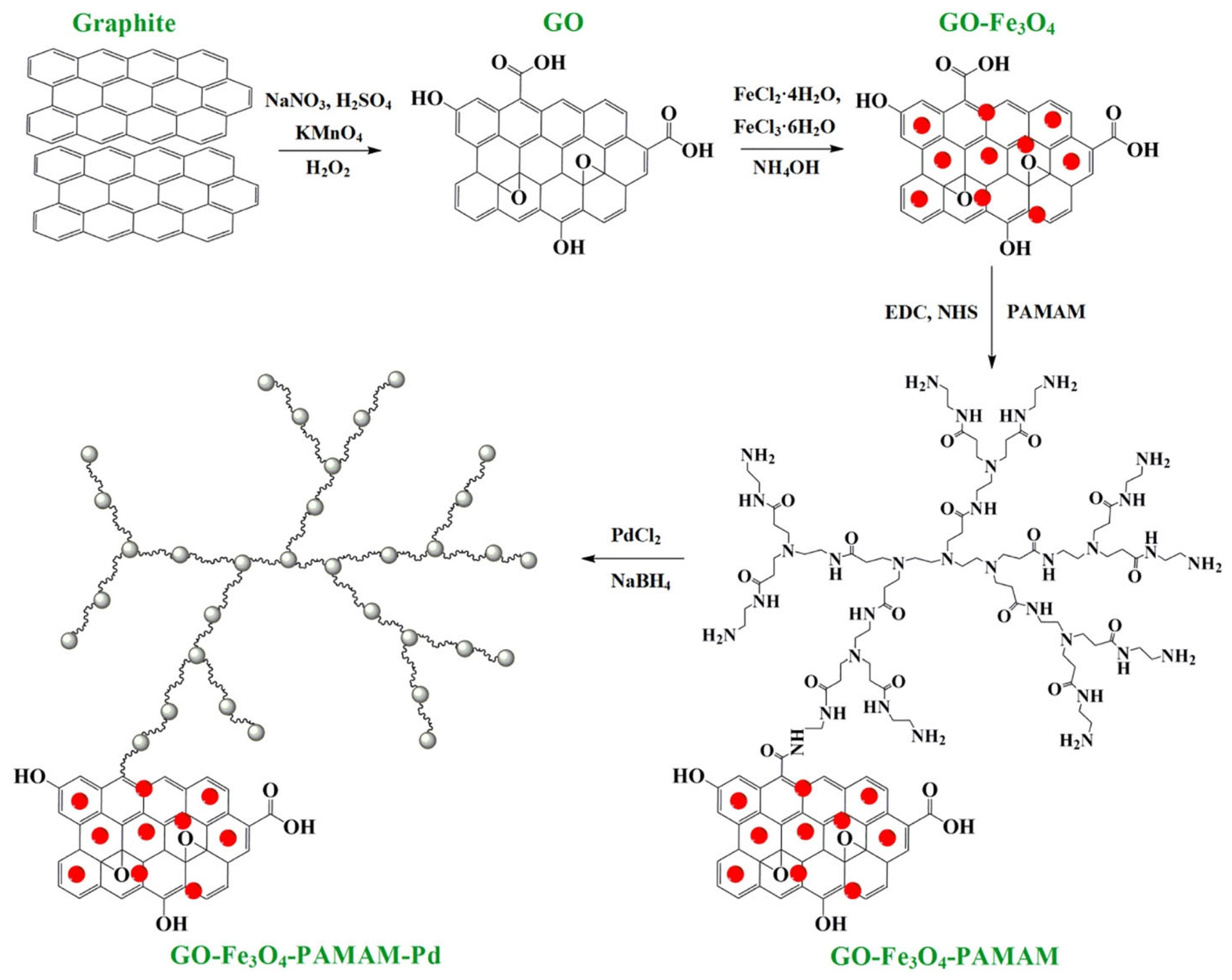
| PdNPs | PAMAM dendrimer | Divergent method | In situ | Sensors | [123] |
| Applications | Core Material | Polymer Coating | Coating Mechanism | Typical Size/Shell Thickness/Grafting Density | Stability/Cytotoxicity | Key Performance Notes | Ref. |
|---|---|---|---|---|---|---|---|
| Drug Delivery | AuNPs | cRGD-PEG-PLL-LA | Grafting to | Core: ~20 nm; polymer shell: ~8 nm | Stable in serum; no observed cytotoxicity | Targeted siRNA delivery; strong tumor inhibition | [102] |
| AuNPs | lipoyl-[(MCH)26-b-(GMA)53] | Grafting to | Core: ~15–20 nm; polymer shell ~10–12 nm at pH 7.4, shrinking to ~5–7 nm at pH 6.5 | Stable in physiological media; no significant cytotoxicity in KB and MCF-7 cells | pH-triggered “hide-and-reveal” folate targeting; significantly higher uptake at pH 6.5 compared to pH 7.4 | [103] | |
| AuNShs | P(NIPAM-co-AA) | Grafting from | Core: ~120 nm; polymer shell: ~20–30 nm | Reversible collapse at LCST (~40 °C) | NIR-responsive heating and on-demand drug release | [104] | |
| AgNPs | P(HEO2MA-co-MACPT) | Grafting to | Core: ~50 nm; polymer shell: ~10 nm | Stable at pH 7.4; release triggered in acidic environments | Monitored CPT delivery via fluorescence “off/on” signal | [113] | |
| CuNPs | PTP, PTPO | In situ | PTP- and PTPO-stabilized NPs: ~20–30 nm in TEM | pH/GSH-responsive release with reduced side effects | Dual chemo + chemodynamic therapy in GBM | [117] | |
| PdNPs | COS-RGD | Grafting to | Core: ~22 nm; polymer shell: ~2–3 nm | Stable under physiological conditions; pH-triggered drug release; low systemic toxicity in mouse models | RGD-mediated tumor targeting and strong NIR photothermal response; significant tumor suppression in vivo | [122] | |
| Biosensing | AuNPs | poly(AcMan-r-AAm) | Grafting to | Core: ~40 nm; polymer shell: ~6–14 nm | Salt-responsive colloidal stability | Multivalent mannose-driven ConA recognition | [105] |
| AuNPs | Star shaped PEG | Grafting to | Core: ~19 nm; polymer shell: ~25–30 nm | Stable in buffer due to PEG steric repulsion | 4-arm PEG yields stronger IL-8 sensing response than 8-arm PEG | [106] | |
| AgNPs | PLL-PEG4-SPDP | Grafting to | Core: ~80 nm; polymer shell: ~4 nm in TEM | Stable dispersion | 26x fluorescence enhancement; high sensitivity biosensing | [114] | |
| PtNPs | Chitosan | In situ | Ch-PtNPs: ~2 nm | Biocompatible due to chitosan coating | ACP sensing, LOD ~0.016 U·L−1 | [120] | |
| Environmental | AuNPs | DOPA, PEI | In situ | N/A (No size information) | Stable under continuous use, low NP leaching | High catalytic efficiency for PNP and dye degradation | [107] |
| AgNPs | PVP | In situ | PVP-coated AgNPs: ~40–50 nm increasing PVP % reduces size) | Stable and well-dispersed; effective against E. coli and S. aureus | Water purification; effective bacteria removal | [115] | |
| CuNPs | P4VP | In situ | Core CuNPs: ~10 nm; HNTs: 0.5–2 μm length; inner diameter 20–30 nm; shell thickness 15–20 nm | Minimal leaching; stable antibacterial action | 94.5% bacteriostasis vs. E. coli, enhanced membrane hydrophilicity and water flux; suitable for water purification | [119] | |
| Electronics | AuNRs | PTEBS | Grafting to | Core NRs: ~25 nm (short axis) × ~110 nm (long axis); ligand shell thickness: ~0.7–2.1 nm | Stable in polar media; 1+ year conductivity retention | Sinter-free inks; resistivity ~10−6–10−7 Ω·m; flexible printed films | [109] |
| AuNPs | PEDOT:PSS, PVA | Grafting to | Core NPs: 47 nm; film thickness with PVA: 0.4 to 1.0 μm | Stable 280 days; low resistivity; strong adhesion | High conductivity (2.1 × 105 S·m−1); sinter-free; flexible | [110] | |
| AgNPs | NFC-PVP | In situ | PVP-AgNPs: ~25 nm | Stable dispersion within polymer network; no aggregation reported | Improved conductivity and flexibility for wearable electronics | [116] | |
| Antimicrobials | AgNPs | PDA | Coordination | Core PVP-AgNPs: ~32 nm; PDA-capped AgNPs: ~36–54 nm | Stable; ROS-enhanced antibacterial action | Enhanced antibacterial action via PDA-mediated ROS | [108] |
| CuNPs | PANI | In situ | CuNPs ~6 nm uniformly dispersed in PANI matrix | PANI prevents CuNP aggregation; stable dispersion | Strong antimicrobial activity; synergistic membrane-disruptive effects | [118] | |
| Therapeutics | AgNPs | P(PEGMA-co-R-HEMA) | Physisorption | Ag core: ~16 nm; polymer-coated sizes: ~28–51 nm depending on amino acid | Stable colloidal dispersion | Inhibits and reverses insulin fibrils | [112] |
| Catalysis | AuNIs | PAH | Physisorption | PAH layer thickness: 0.54 nm | Stable coating; maintains AuNI morphology | Enhanced catalytic activity via electrostatic enrichment of anionic reactants | [108] |
| Energy Conversion | PtNPs | PDDA, PSS | Layer-by-layer | Pt core: ~2.6 nm; PDDA-capped NPs: ~11 nm | Uniform film formation in LbL assemblies | Efficient HER electrocatalysis | [121] |
| Sensors | PdNPs | PAMAM dendrimer | In situ | N/A (No size information) | Stable immobilization via dendrimer anchoring; magnetic recyclability | H2O2 sensing; linear range 0.05–160 μM; LOD 0.01 μM | [123] |
| Metal Oxide Core | Polymer Coating | Polymer Synthesis Method | Hybrid Nanomaterial Formation Mechanism | Applications | Ref. |
|---|---|---|---|---|---|
| Fe3O4 NPs | PAC | Free radical | Grafting through | Drug delivery | [165] |
| Fe3O4 NPs | CN | N/A | Layer-by-layer | Drug delivery | [166] |
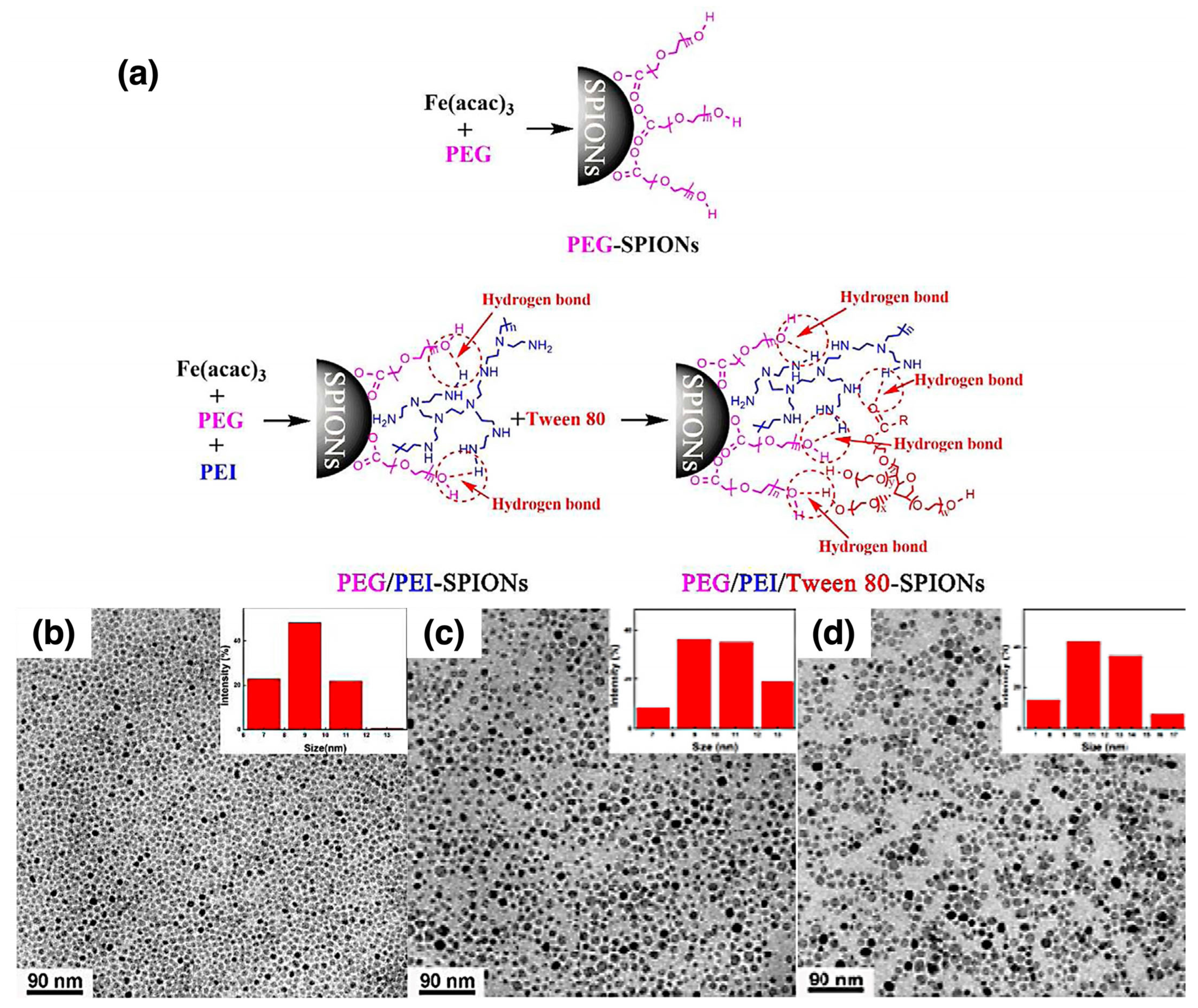
| Fe3O4 NPs | PEG/PEI/Tween 80 | N/A | In situ | MRI | [167] |
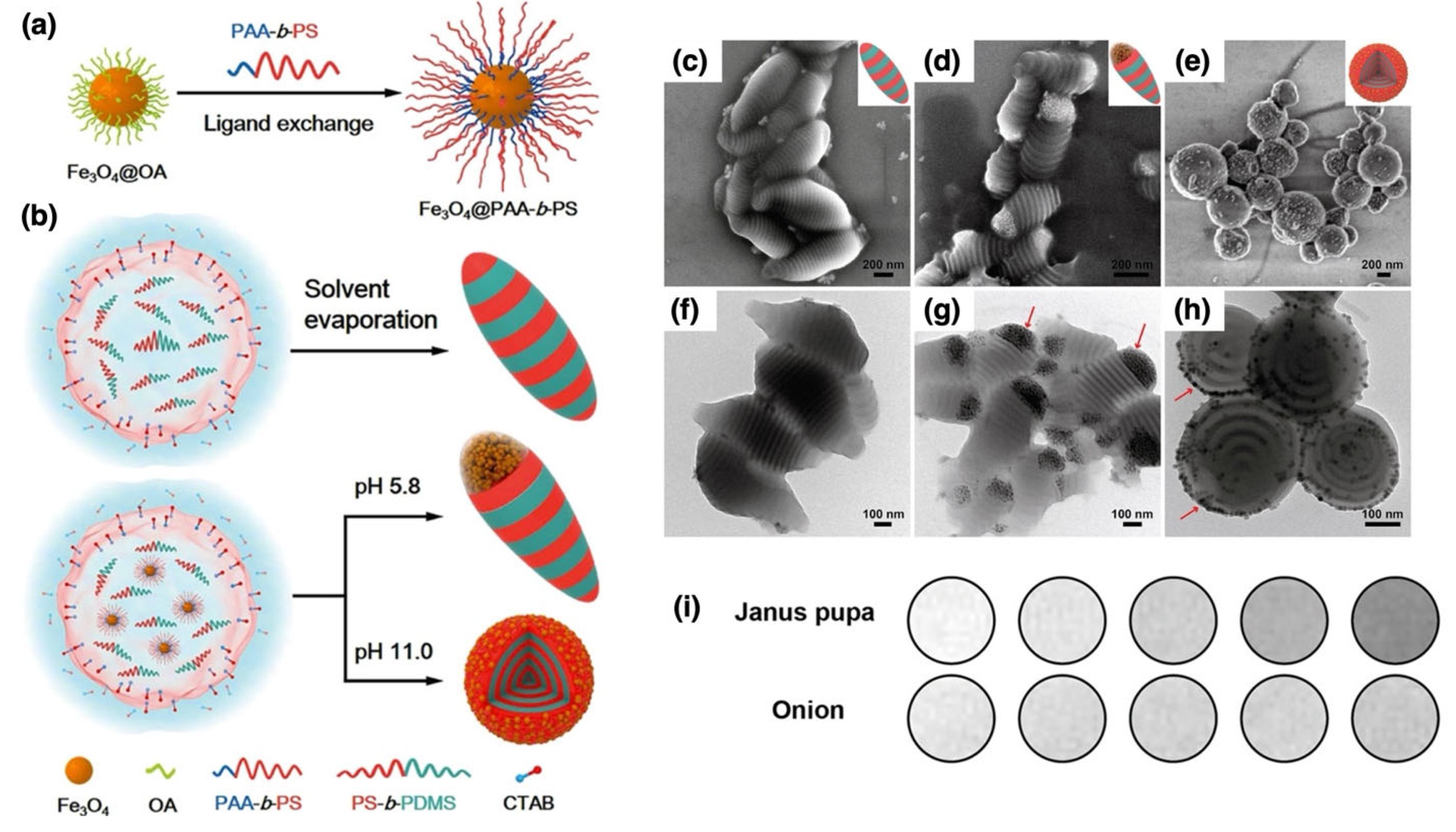
| Fe3O4 NPs | PS-b-PDMS | ATRP | Grafting to | MRI | [168] |
| Fe3O4 NPs | PS-b-PAA, PS-PEO-SH | N/A, RAFT | Grafting to | MRI-guided drug delivery | [169] |
| Fe3O4 NPs | CFP, R-PEI | ROP, N/A | Grafting to | Gene delivery | [170] |
| Fe3O4 NPs | PNIPAAM | RAFT | Grafting from | Hyperthermia | [171] |
| TiO2 NPs | PANI | Oxidative chemical polymerization | Physisorption | Bone tissue regeneration | [172] |
| TiO2 NPs | PTH | Oxidative chemical polymerization | Grafting from | Biomedical devices | [173] |
| ZnO NPs | Chitosan | N/A | Physisorption | Antibacterials | [174] |
| ZnO NPs | Borate- and methacrylate-based polymer films | Plasma polymerization | N/A | Automotive industry | [175] |
| Al2O3 NPs | γ-PGA | N/A | Physisorption | Cancer treatment | [176] |
| Al2O3 NPs | PTFE | N/A | N/A | Antibacterials | [177] |
| Al2O3 NPs | Chitosan | N/A | Physisorption | Catalysis | [178] |
| Applications | Core Material | Polymer Coating | Coating Mechanism | Typical Size/Shell Thickness/Grafting Density | Stability/Cytotoxicity | Key Performance Notes | Ref. |
|---|---|---|---|---|---|---|---|
| Drug delivery | Fe3O4 NPs | PAC | Grafting through | Core: ~10 nm; polymer shell: ~70 nm (~150 nm final) | No significant cytotoxicity up to 500 μg·mL−1 | Dual-responsive DOX release (heat and pH); R11 peptide enhances PC3 cell uptake | [165] |
| Fe3O4 NPs | CN | Layer-by-layer | Core: ~10 nm; hydrodynamic diameter ~24 nm (shell: ~7–9 nm) | Stable in gastric pH; enzyme-responsive | Enhanced oral DOX absorption | [166] | |
| MRI | Fe3O4 NPs | PEG/PEI/Tween 80 | In situ | PEG-SPIONs: ~9 nm; PEG/PEI/Tween 80-SPIONs: ~11–12 nm | Stable in water; PEG slows clearance and reduces toxicity | PEG-SPIONs provide enhanced MRI brain contrast and prolonged circulation | [167] |
| Fe3O4 NPs | PS-b-PDMS | Grafting to | Core: ~15 nm; polymer shell increases interparticle spacing to ~21 nm | pH-responsive morphology | Janus pupa-like particles provide enhanced T2 MRI contrast | [168] | |
| Antibacterials | ZnO NPs | Chitosan | Physisorption | Rod-like grains ~20–150 nm (FE-SEM) | Stable in aqueous media; strong antibacterial effect | Photocatalytically degrades congo red and methylene blue under sunlight | [174] |
| Al2O3 NPs | PTFE | N/A | Al2O3 NPs: ~50 nm | Stable dispersion (zeta potential: ~+50 mV); low cytotoxicity to fibroblasts | Generates ROS and inhibits E. coli growth; suitable for food-contact antibacterial coatings | [177] | |
| MRI-guided drug delivery | Fe3O4 NPs | PS-b-PAA, PS-PEO-SH | Grafting to | Membrane thickness tunable ~10–93 nm (monolayer-multilayer vesicles) | Stable colloidal dispersions, superparamagnetic behavior retained | Controlled release via membrane thickness; RGD functionalization enabled tumor targeting and MRI-guided therapy | [169] |
| Gene delivery | Fe3O4 NPs | CFP, R-PEI | Grafting to | Oleic acid-capped Fe3O4 NPs: ~15 nm; with polymer shell: ~40 nm | Stable, positively charged in aqueous media | Efficient gene delivery; MRI and fluorescence cell tracking | [170] |
| Hyperthermia | Fe3O4 NPs | PNIPAAM | Grafting from | RAFT agent-capped NPs: ~19 nm; polymer layer: ~1.5 nm thick | LCST tunable above 37 °C (stable at body temperature; collapses under hyperthermia) | Heat-triggered or pH-triggered DOX release; retains superparamagnetism; >90% cell viability | [171] |
| Bone tissue regeneration | TiO2 NPs | PANI | Physisorption | Fibers ~0.5–0.8 μm (diameter varies with TiO2/PANI loading) | Stable, bioactive surface | Enhances osteogenesis and controlled SIM release | [172] |
| Biomedical devices | TiO2 NPs | PTH | Grafting from | PTH-TiO2 nanocomposite: ~70–90 nm | Maintains ~88% activity (14 days) | Enhanced GOx-mediated bioanode performance; improved electron transport in BFCs | [173] |
| Automotive industry | ZnO NPs | Borate- and methacrylate-based polymer films | N/A | ZnO NPs: ~10–30 nm | Stable under high load/heat | ~95% wear reduction | [175] |
| Cancer treatment | Al2O3 NPs | γ-PGA | Physisorption | Core: ~5 nm, polymer-coated: ~7 nm | Stable, negatively charged dispersion; enhanced cancer cell cytotoxicity (PC-3) | Selective adsorption of positively charged proteins (e.g., lysozyme); ROS-mediated cancer cell killing | [176] |
| Catalysis | Al2O3 NPs | Chitosan | Physisorption | Al2O3 NPs: ~50 nm | Reusable catalyst up to ≥4 cycles with minimal activity loss | Enhanced base-catalyzed synthesis yield; heterogeneous, green catalyst enabling easy separation | [178] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atik, R.; Islam, R.; Ariza Gonzalez, M.; Chinwangso, P.; Lee, T.R. Recent Advances in Polymer-Coated Metal and Metal Oxide Nanoparticles: From Design to Promising Applications. Nanomaterials 2025, 15, 1744. https://doi.org/10.3390/nano15221744
Atik R, Islam R, Ariza Gonzalez M, Chinwangso P, Lee TR. Recent Advances in Polymer-Coated Metal and Metal Oxide Nanoparticles: From Design to Promising Applications. Nanomaterials. 2025; 15(22):1744. https://doi.org/10.3390/nano15221744
Chicago/Turabian StyleAtik, Refia, Rafiqul Islam, Melissa Ariza Gonzalez, Pailinrut Chinwangso, and T. Randall Lee. 2025. "Recent Advances in Polymer-Coated Metal and Metal Oxide Nanoparticles: From Design to Promising Applications" Nanomaterials 15, no. 22: 1744. https://doi.org/10.3390/nano15221744
APA StyleAtik, R., Islam, R., Ariza Gonzalez, M., Chinwangso, P., & Lee, T. R. (2025). Recent Advances in Polymer-Coated Metal and Metal Oxide Nanoparticles: From Design to Promising Applications. Nanomaterials, 15(22), 1744. https://doi.org/10.3390/nano15221744








