VOC Gas Sensors Based on Zinc Stannate Nanoparticles Decorated with Silver
Abstract
1. Introduction
2. Materials and Methods
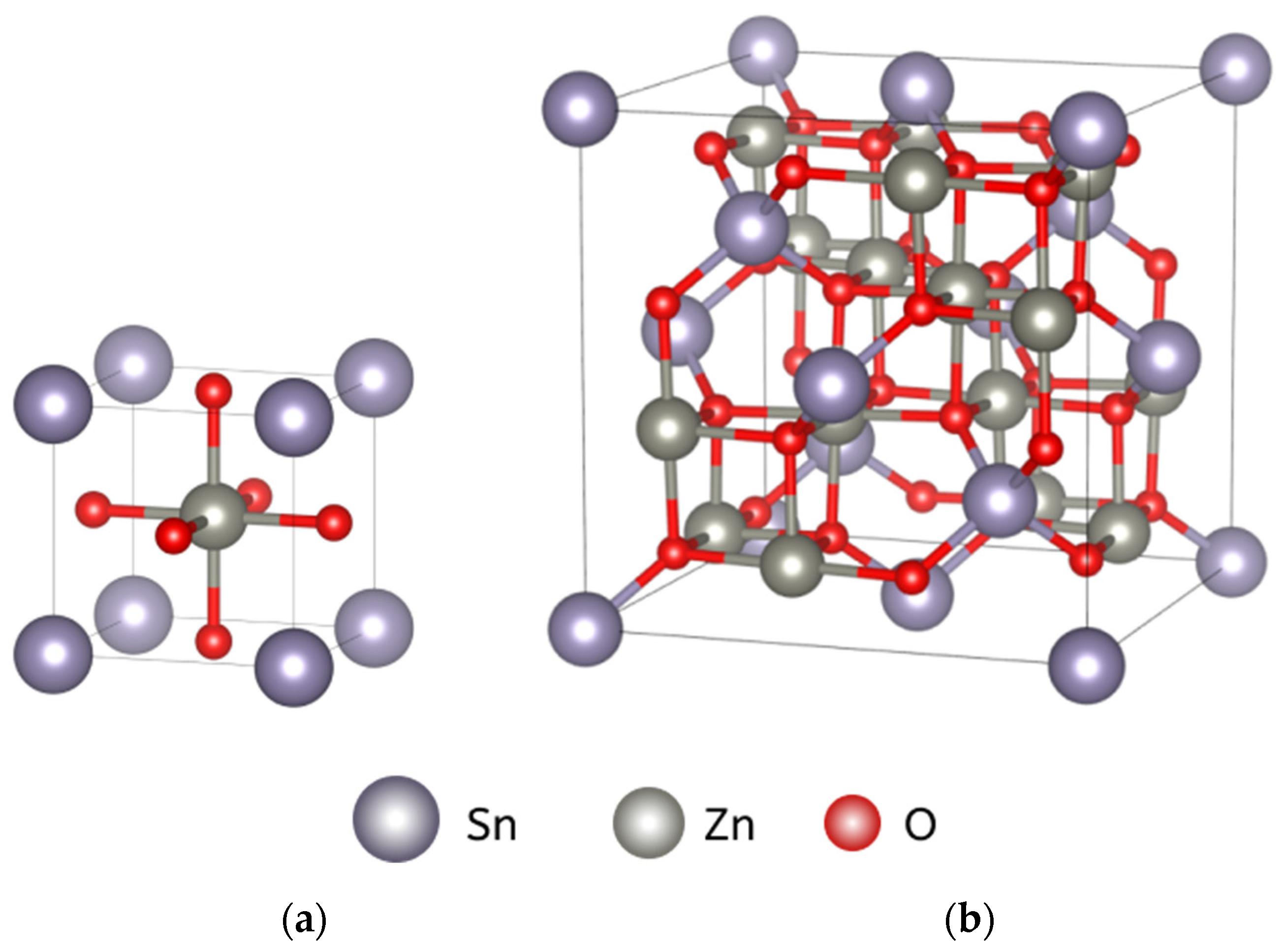
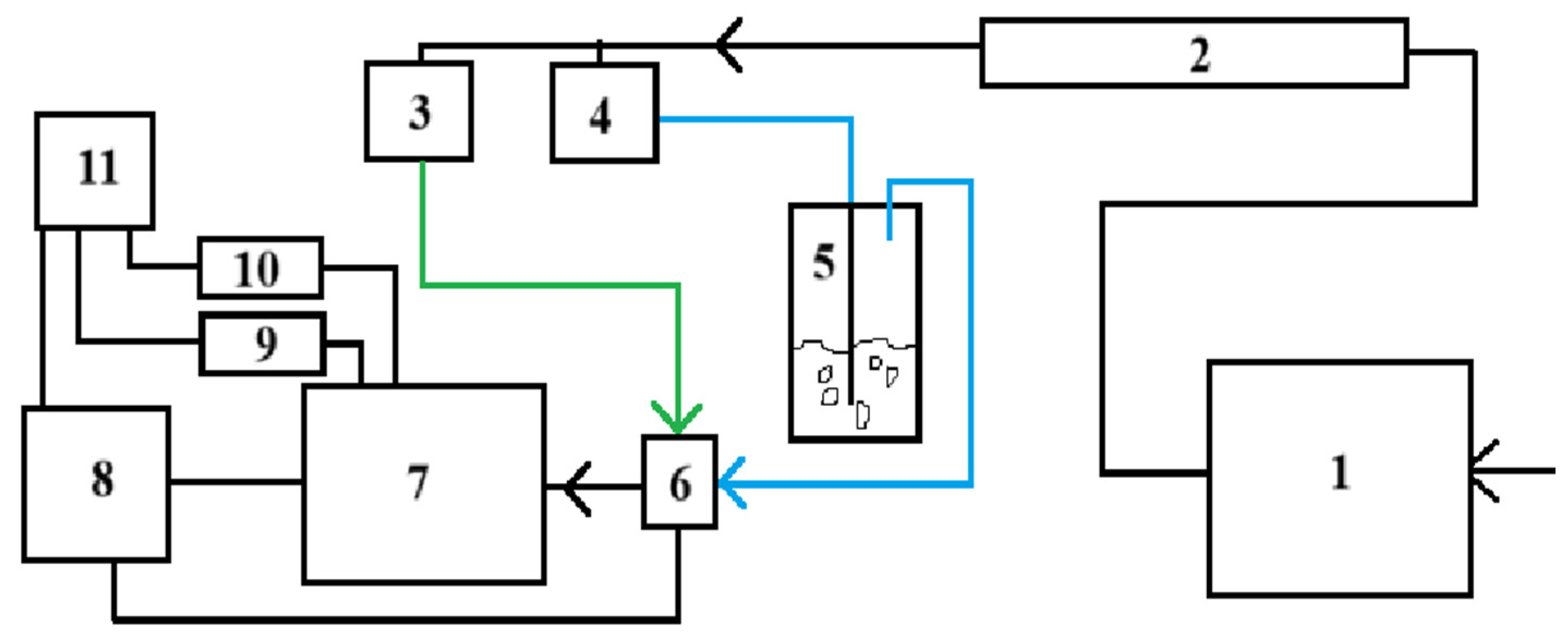
2.1. Sample Fabrication
2.2. Preparation of Ag Nanoparticles and Decoration of Zinc Stannate
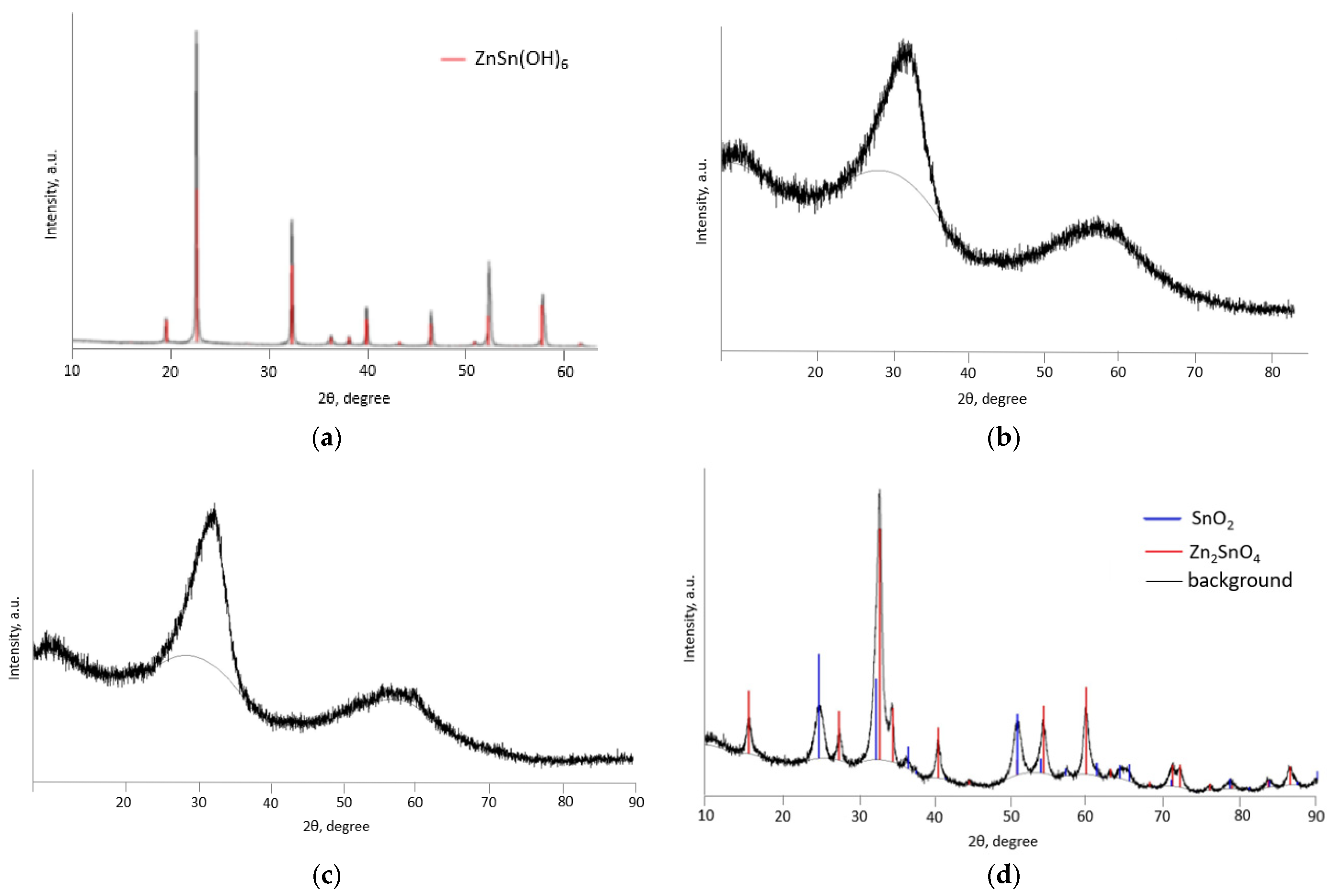
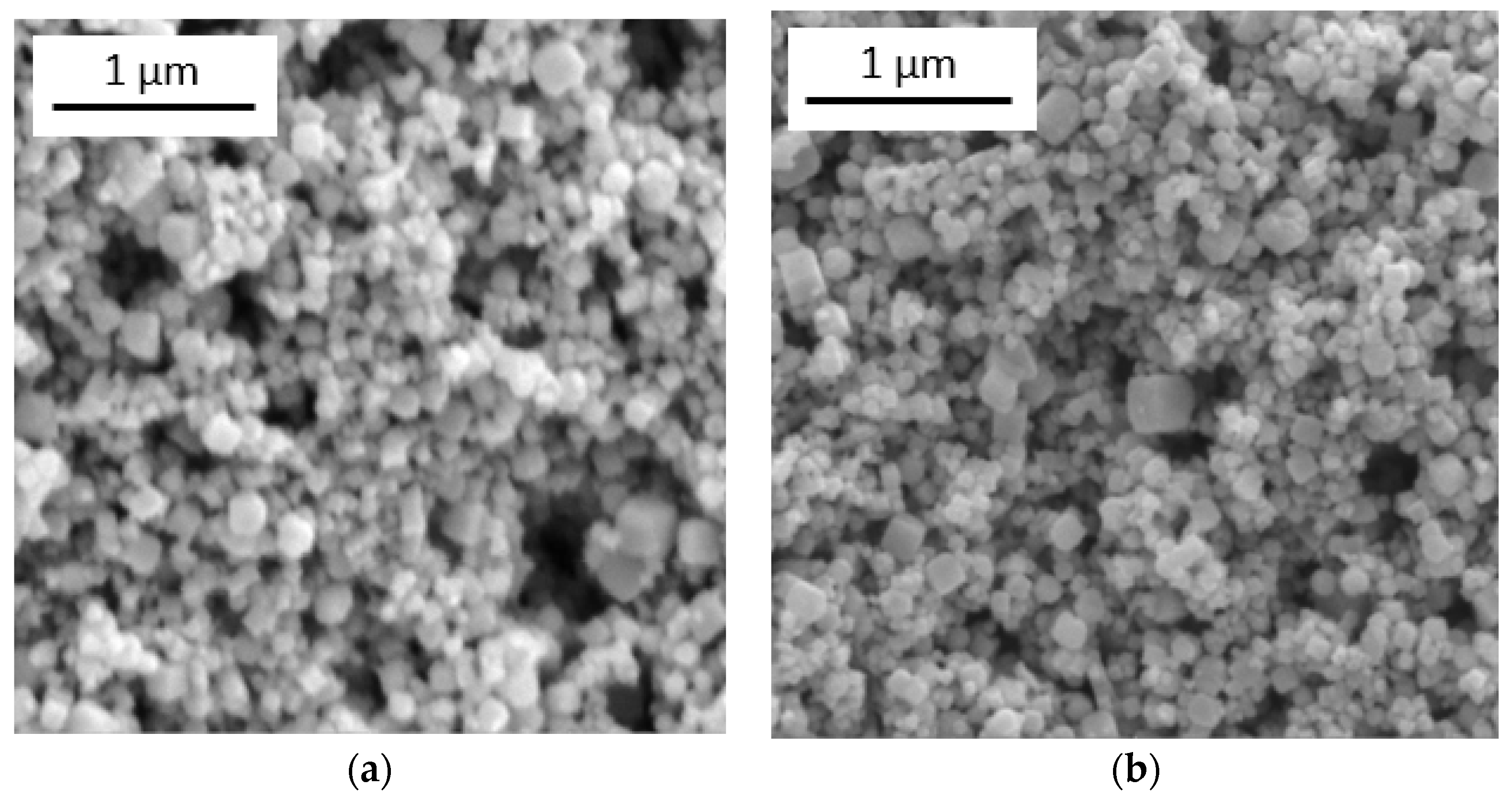
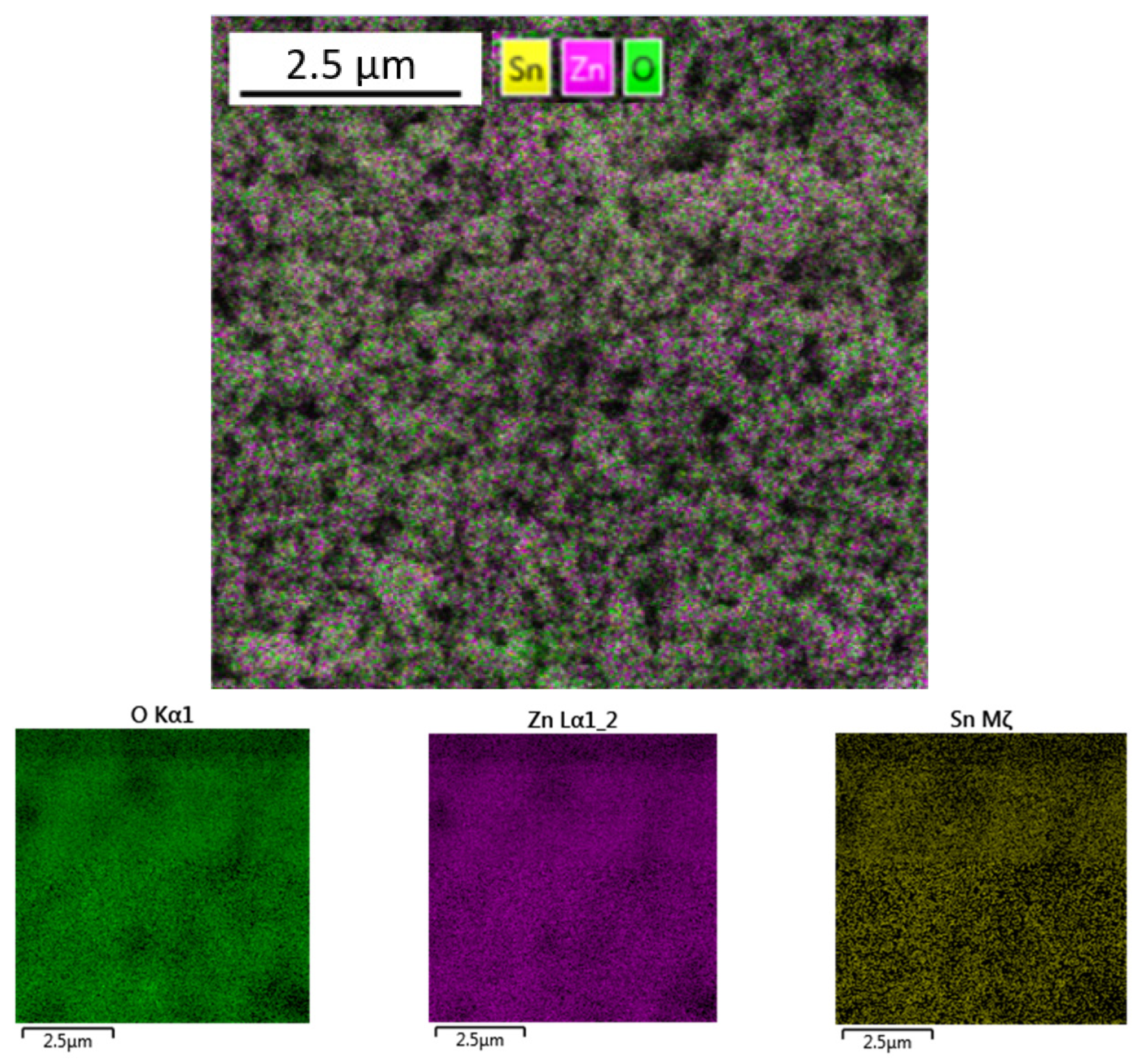
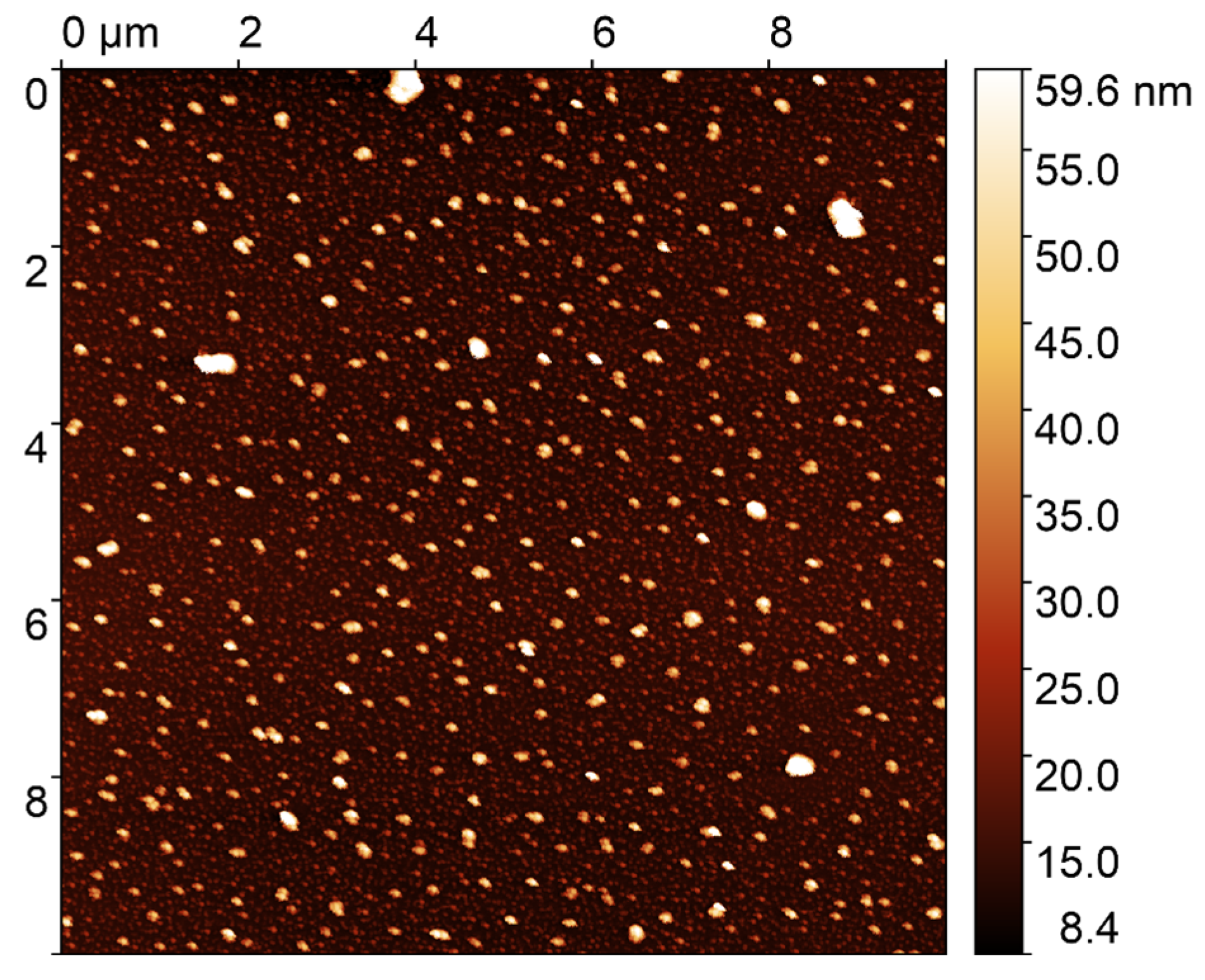
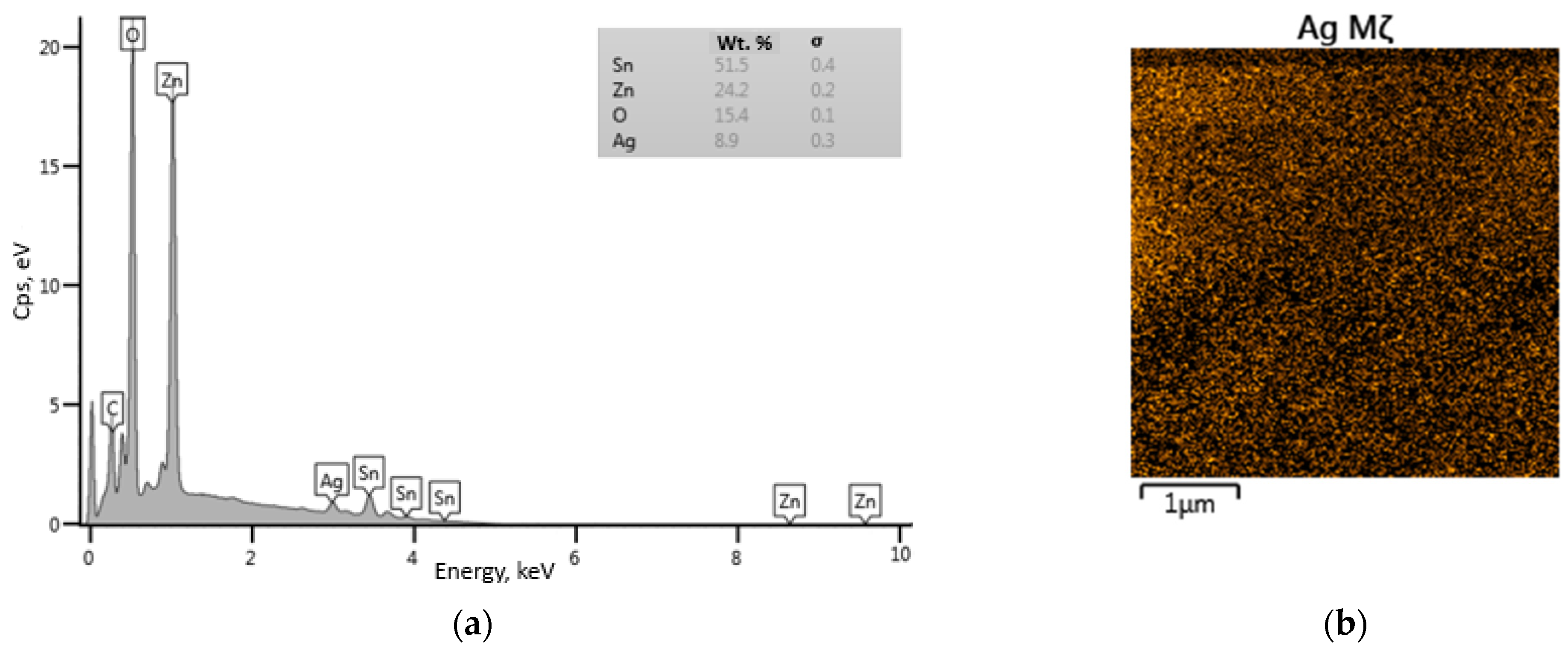
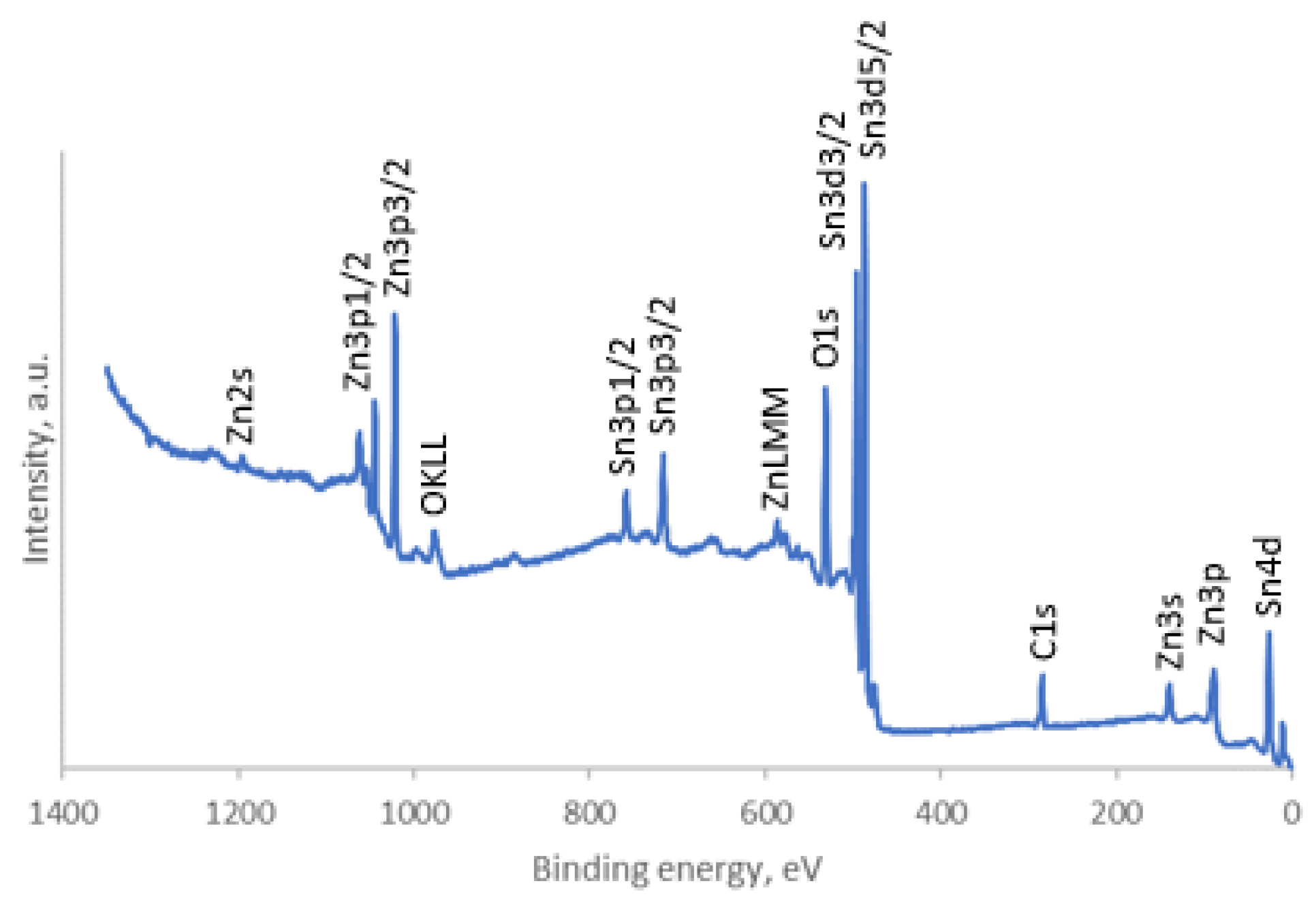
2.3. Characterization of Sample
2.4. Sensor Fabrication and Measurement
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Korotcenkov, G. Electrospun Metal Oxide Nanofibers and Their Conductometric Gas Sensor Application. Part 2: Gas Sensors and Their Advantages and Limitations. Nanomaterials 2021, 11, 1555. [Google Scholar] [CrossRef] [PubMed]
- Hashtroudi, H.; Mackinnon, I.D.R.; Shafiei, M. Emerging 2D hybrid nanomaterials: Towards enhanced sensitive and selective conductometric gas sensors at room temperature. J. Mater. Chem. C 2020, 8, 13108–13126. [Google Scholar] [CrossRef]
- Wawrzyniak, J. Advancements in Improving Selectivity of Metal Oxide Semiconductor Gas Sensors Opening New Perspectives for Their Application in Food Industry. Sensors 2023, 23, 9548. [Google Scholar] [CrossRef] [PubMed]
- Petrov, V.V.; Sysoev, V.V.; Starnikova, A.P.; Volkova, M.G.; Kalazhokov, Z.K.; Storozhenko, V.Y.; Khubezhov, S.A.; Bayan, E.M. Synthesis, Characterization and Gas Sensing Study of ZnO-SnO2 Nanocomposite Thin Films. Chemosensors 2021, 9, 124. [Google Scholar] [CrossRef]
- Nalimova, S.S.; Maksimov, A.I.; Matyushkin, L.B.; Moshnikov, V.A. Current State of Studies on Synthesis and Application of Zinc Stannate (Review). Glass Phys. Chem. 2019, 45, 251–260. [Google Scholar] [CrossRef]
- Anitha, A.; Ponnusamy, V. Optical and electrochemical studies on single-phase ZnSnO3 nanostructures—A photosensitive approach. Surf. Interfaces 2024, 51, 104747. [Google Scholar] [CrossRef]
- Hanh, N.H.; Van Duy, L.; Hung, C.M.; Van Duy, N.; Heo, Y.-W.; Van Hieu, N.; Hoa, N.D. VOC gas sensor based on hollow cubic assembled nanocrystal Zn2SnO4 for breath analysis. Sens. Actuators A 2020, 302, 111834. [Google Scholar] [CrossRef]
- Shomakhov, Z.V.; Nalimova, S.S.; Rybina, A.A.; Buzovkin, S.S.; Kalazhokov, Z.; Moshnikov, V.A. Improving the sensor characteristics of binary and ternary oxide nanosystems. Phys. Chem. Asp. Study Clust. Nanostruct. Nanomater. 2023, 15, 879–887. [Google Scholar] [CrossRef]
- Xiong, Y.; Lin, Y.; Wang, X.; Zhao, Y.; Tian, J. Defect engineering on SnO2 nanomaterials for enhanced gas sensing performances. Adv. Powder Mater. 2022, 1, 100033. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J. The Oxygen Vacancy Defect of ZnO/NiO Nanomaterials Improves Photocatalytic Performance and Ammonia Sensing Performance. Nanomaterials 2022, 12, 433. [Google Scholar] [CrossRef]
- Bora, T.; Al-Hinai, M.H.; Al-Hinai, A.T.; Dutta, J. Phase Transformation of Metastable ZnSnO3 Upon Thermal Decomposition by In-Situ Temperature-Dependent Raman Spectroscopy. J. Am. Ceram. Soc. 2015, 98, 4044–4049. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, T.; Fan, H.; Lu, G.; Kang, M. Synthesis and gas-sensing properties of ZnSnO3 cubic nanocages and nanoskeletons. Sens. Actuators B 2009, 143, 449–453. [Google Scholar] [CrossRef]
- Xu, J.; Jia, X.; Lou, X.; Shen, J. One-step hydrothermal synthesis and gas sensing property of ZnSnO3 microparticles. Solid-State Electron. 2006, 50, 504–507. [Google Scholar] [CrossRef]
- Kovacheva, D.; Petrov, K. Preparation of crystalline ZnSnO3 from Li2SnO3 by low-temperature ion exchange. Solid State Ion. 1998, 109, 327–332. [Google Scholar] [CrossRef]
- Ibrahim, D.M.; Gaber, A.A.; Reda, A.E.; Abdel Aziz, D.A.; Ajiba, N.A. Structural, optical, and dielectric properties of sol-gel derived perovskite ZnSnO3 nanomaterials. J. Sol-Gel Sci. Technol. 2024, 112, 703–714. [Google Scholar] [CrossRef]
- Jie, J.; Wang, G.; Han, X.; Fang, J.; Yu, Q.; Liao, Y.; Xu, B.; Wang, Q.; Hou, J. Growth of Ternary Oxide Nanowires by Gold-Catalyzed Vapor-Phase Evaporation. J. Phys. Chem. B 2004, 108, 8249–8253. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Liao, X.; Yang, W. A simple method to synthesize single-crystalline Zn2SnO4 (ZTO) nanowires and their photoluminescence properties. Nanotechnology 2005, 16, 2928–2931. [Google Scholar] [CrossRef]
- Ge, Q.; Liu, C.; Zhao, Y.; Wang, N.; Zhang, X.; Feng, C.; Zhang, S.; Wang, H.; Jiang, W.; Liu, S.; et al. Phase evolution in preparing ZnSnO3 powders by precipitation method. Appl. Phys. A 2021, 127, 89. [Google Scholar] [CrossRef]
- Chang, C.-J.; Lin, C.-Y.; Chen, J.-K.; Hsu, M.-H. Ce-doped ZnO nanorods based low operation temperature NO2 gas sensors. Ceram. Int. 2014, 40, 10867–10875. [Google Scholar] [CrossRef]
- Zhu, L.-Y.; Ou, L.-X.; Mao, L.-W.; Wu, X.-Y.; Liu, Y.-P.; Lu, H.-L. Advances in Noble Metal-Decorated Metal Oxide Nanomaterials for Chemiresistive Gas Sensors: Overview. Nano-Micro Lett. 2023, 15, 89. [Google Scholar] [CrossRef]
- Lai, H.-Y.; Chen, C.-H. Highly sensitive room-temperature CO gas sensors: Pt and Pd nanoparticle-decorated In2O3 flower-like nanobundles. J. Mater. Chem. 2012, 22, 13204. [Google Scholar] [CrossRef]
- Meng, X.; Bi, M.; Xiao, Q.; Gao, W. Ultra-fast response and highly selectivity hydrogen gas sensor based on Pd/SnO2 nanoparticles. Int. J. Hydrogen Energy 2022, 47, 3157–3169. [Google Scholar] [CrossRef]
- Kafil, V.; Sreenan, B.; Hadj-Nacer, M.; Wang, Y.; Yoon, J.; Greiner, M.; Chu, P.; Wang, X.; Fadali, M.S.; Zhu, X. Review of noble metal and metal-oxide-semiconductor based chemiresistive hydrogen sensors. Sens. Actuators A 2024, 373, 115440. [Google Scholar] [CrossRef]
- Nogami, K.; Kishimoto, K.; Hashimoto, Y.; Watanabe, H.; Hishii, Y.; Ma, Q.; Niki, T.; Kotani, T.; Kiwa, T.; Shoji, S.; et al. Self-growth of silver tree-like fractal structures with different geometries. Appl. Phys. A 2022, 128, 860. [Google Scholar] [CrossRef]
- He, Q.; Zi, J.; Huang, B.; Yan, L.; Fa, W.; Li, D.; Zhang, Y.; Gao, Y.; Zheng, Z. Controlled growth and thermal decomposition of well-dispersed and uniform ZnSn(OH)6 submicrocubes. J. Alloys Compd. 2014, 607, 193–197. [Google Scholar] [CrossRef]
- Fan, H.; Zeng, Y.; Xu, X.; Lv, N.; Zhang, T. Hydrothermal synthesis of hollow ZnSnO3 microspheres and sensing properties toward butane. Sens. Actuators B 2011, 153, 170–175. [Google Scholar] [CrossRef]
- Li, B.; Luo, L.; Xiao, T.; Hu, X.; Lu, L.; Wang, J.; Tang, Y. Zn2SnO4–SnO2 heterojunction nanocomposites for dye-sensitized solar cells. J. Alloys Compd. 2011, 509, 2186–2191. [Google Scholar] [CrossRef]
- Nalimova, S.S.; Shomakhov, Z.V.; Moshnikov, V.A.; Bobkov, A.A.; Ryabko, A.A.; Kalazhokov, Z.K. An X-ray Photoelectron Spectroscopy Study of Zinc Stannate Layer Formation. Tech. Phys. 2020, 65, 1087–1090. [Google Scholar] [CrossRef]
- Sá, B.S.; Zito, C.A.; Perfecto, T.M.; Volanti, D.P. Porous ZnSnO3 nanocubes as a triethylamine sensor. Sens. Actuators B 2021, 338, 129869. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Wu, J.M. Effect of Controlled Oxygen Vacancy on H2-Production through the Piezocatalysis and Piezophototronics of Ferroelectric R3C ZnSnO3 Nanowires. Adv. Funct. Mater. 2020, 30, 1907619. [Google Scholar] [CrossRef]
- Naberezhnyi, D.; Rumyantseva, M.; Filatova, D.; Batuk, M.; Hadermann, J.; Baranchikov, A.; Khmelevsky, N.; Aksenenko, A.; Konstantinova, E.; Gaskov, A. Effects of Ag Additive in Low Temperature CO Detection with In2O3 Based Gas Sensors. Nanomaterials 2018, 8, 801. [Google Scholar] [CrossRef] [PubMed]
- Barathy, T.R.; Yadav, P.V.K.; Mondal, A.; Ramakrishnan, K.; Jarugala, J.; Liu, C.; Reddy, Y.A.K. Enhanced response of WO3 thin film through Ag loading towards room temperature hydrogen gas sensor. Chemosphere 2024, 353, 141545. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Metal oxide composites in conductometric gas sensors: Achievements and challenges. Sens. Actuators B 2017, 244, 182–210. [Google Scholar] [CrossRef]
- Ma, S.; Shen, L.; Ma, S.; Wen, J.; Xu, J. Emerging zinc stannate and its application in volatile organic compounds sensing. Coord. Chem. Rev. 2023, 490, 215217. [Google Scholar] [CrossRef]
- Park, S.; Ko, H.; Kim, S.; Lee, C. Role of the interfaces in multiple networked one-dimensional core-shell nanostructured gas sensors. ACS Appl. Mater. Interfaces 2014, 6, 9595–9600. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, X.; Chang, X.; Li, J.; Pan, L.; Jiang, Y.; Gao, W.; Gao, C.; Sun, S. Metal-organic framework-derived porous SnO2 nanosheets with grain sizes comparable to Debye length for formaldehyde detection with high response and low detection limit. Sens. Actuators B 2021, 347, 130599. [Google Scholar] [CrossRef]
- Marikutsa, A.; Rumyantseva, M.; Konstantinova, E.A.; Gaskov, A. The Key Role of Active Sites in the Development of Selective Metal Oxide Sensor Materials. Sensors 2021, 21, 2554. [Google Scholar] [CrossRef]
- Zhou, T.; Sang, Y.; Sun, Y.; Wu, C.; Wang, X.; Tang, X.; Zhang, T.; Wang, H.; Xie, C.; Zeng, D. Gas Adsorption at Metal Sites for Enhancing Gas Sensing Performance of ZnO@ZIF-71 Nanorod Arrays. Langmuir 2019, 35, 3248. [Google Scholar] [CrossRef]
- Cheng, I.-K.; Wang, J.-H.; Tsai, C.-Y.; Chen, Y.-S.; Pan, F.-M. Correlation of surface processes with characteristic sensing responses of PdO thin films to ethanol. Appl. Surf. Sci. 2019, 473, 589. [Google Scholar] [CrossRef]
- Chiang, H.; Bhan, A. Catalytic consequences of hydroxyl group location on the rate and mechanism of parallel dehydration reactions of ethanol over acidic zeolites. J. Catal. 2010, 271, 251. [Google Scholar] [CrossRef]
- Barbosa, M.S.; Suman, P.H.; Kim, J.J.; Tuller, H.L.; Orlandi, M.O. Investigation of electronic and chemical sensitization effects promoted by Pt and Pd nanoparticles on single-crystalline SnO nanobelt-based gas sensors. Sens. Actuators B 2019, 301, 127055. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, Y.; Wang, C.; Ma, J.; Xu, L.; Lv, C.; Sun, Y. Investigation of doping effects of different noble metals for ethanol gas sensors based on mesoporous In2O3. Nanotechnology 2021, 32, 305503. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Kumar, S.; Agrawal, H.; Moinuddin, M.G.; Kumar, M.; Sharma, S.K.; Awasthi, K. An efficient hydrogen gas sensor based on hierarchical Ag/ZnO hollow microstructures. Sens. Actuators B 2021, 346, 130510. [Google Scholar] [CrossRef]
- Wang, S.; Jia, F.; Wang, X.; Hu, L.; Sun, Y.; Yin, G.; Zhou, T.; Feng, Z.; Kumar, P.; Liu, B. Fabrication of ZnO Nanoparticles Modified by Uniformly Dispersed Ag Nanoparticles: Enhancement of Gas Sensing Performance. ACS Omega 2020, 5, 5209. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. A high-response formaldehyde sensor based on fibrous Ag-ZnO/In2O3 with multi-level heterojunctions. J. Hazard. Mater. 2021, 413, 125352. [Google Scholar] [CrossRef]
- Zhang, C.; Huan, Y.; Li, Y.; Luo, Y.; Debliquy, M. Low concentration isopropanol gas sensing properties of Ag nanoparticles decorated In2O3 hollow spheres. J. Adv. Ceram. 2022, 11, 379. [Google Scholar] [CrossRef]
- Kim, M.Y.; Hwang, J.Y.; Mirzaei, A.; Choi, S.W.; Kim, S.I.; Kim, H.S.; Kim, S.J.; Roh, J.W.; Choi, M.S.; Lee, K.H.; et al. NO2 Gas Sensing Properties of Ag-Functionalized Porous ZnO Sheets. Adsorpt. Sci. Technol. 2023, 2023, 9021169. [Google Scholar] [CrossRef]
- Sima, Z.; Song, P.; Wang, Q. Ag nanoparticles decorated ZnSnO3 hollow cubes for enhanced formaldehyde sensing performance at low temperature. Appl. Surf. Sci. 2023, 614, 156215. [Google Scholar] [CrossRef]
- Kamble, C.; Narwade, S.; Mane, R. Detection of acetylene (C2H2) gas using Ag-modified ZnO/GO nanorods prepared by a hydrothermal synthesis. Mater. Sci. Semicond. Proc. 2023, 153, 107145. [Google Scholar] [CrossRef]
- Guo, L.; Liang, H.; An, D.; Yang, H. One-step hydrothermal synthesis of uniform Ag-doped SnO2 nanoparticles for highly sensitive ethanol sensing. Physica E 2023, 151, 115717. [Google Scholar] [CrossRef]
- Wang, Z.; Haidry, A.A.; Xie, L.; Zavabeti, A.; Li, Z.; Yin, W.; Fomekong, R.L.; Saruhan, B. Acetone sensing applications of Ag modified TiO2 porous nanoparticles synthesized via facile hydrothermal method. Appl. Surf. Sci. 2020, 533, 147383. [Google Scholar] [CrossRef]
- Anusha; Ani, A.; Poornesh, P.; Antony, A.; Bhaghyesh; Shchetinin, I.V.; Nagaraja, K.K.; Chattopadhyay, S.; Vinayakumar, K.B. Impact of Ag on the Limit of Detection towards NH3-Sensing in Spray-Coated WO3 Thin-Films. Sensors 2022, 22, 2033. [Google Scholar] [CrossRef]
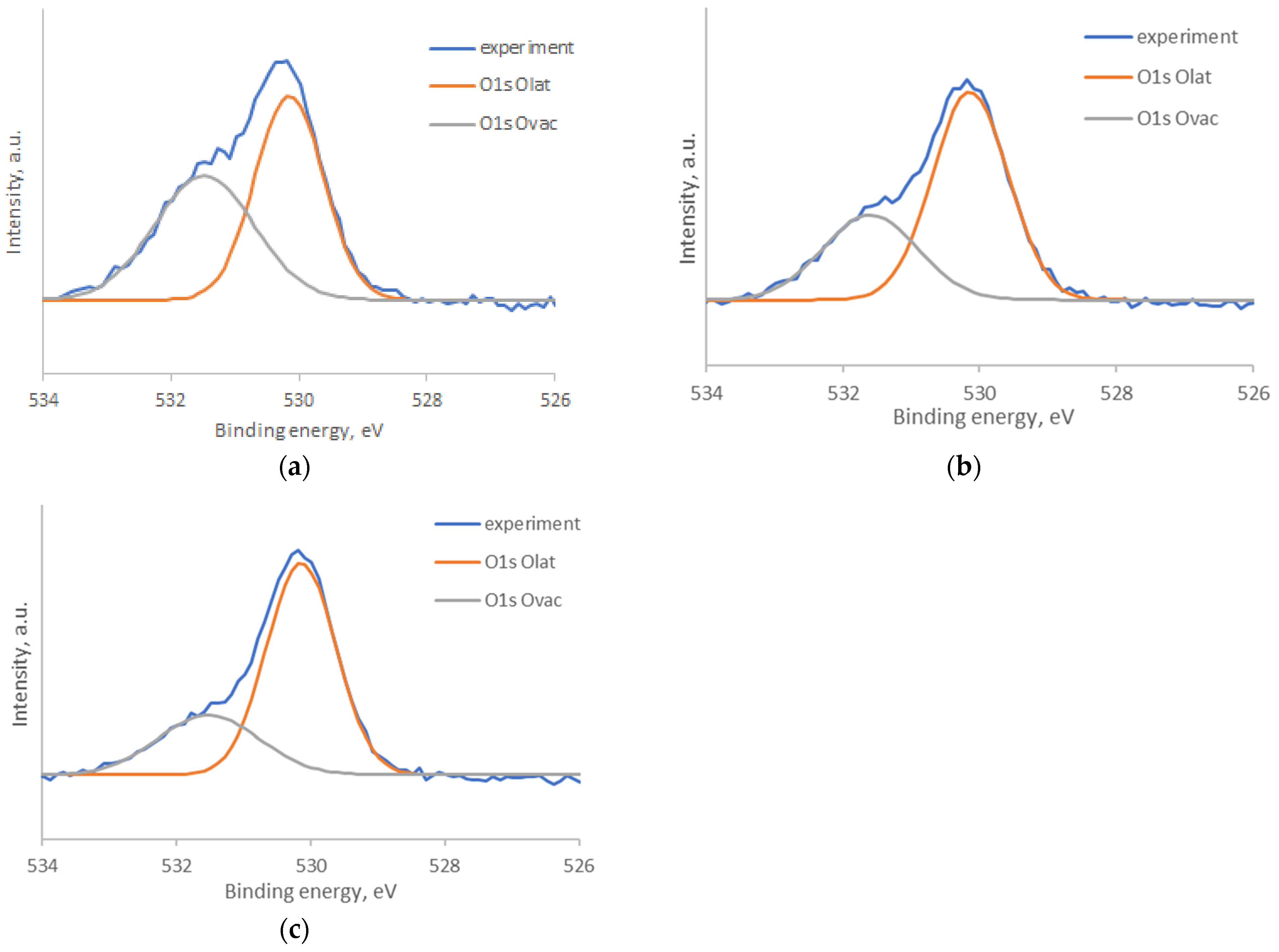
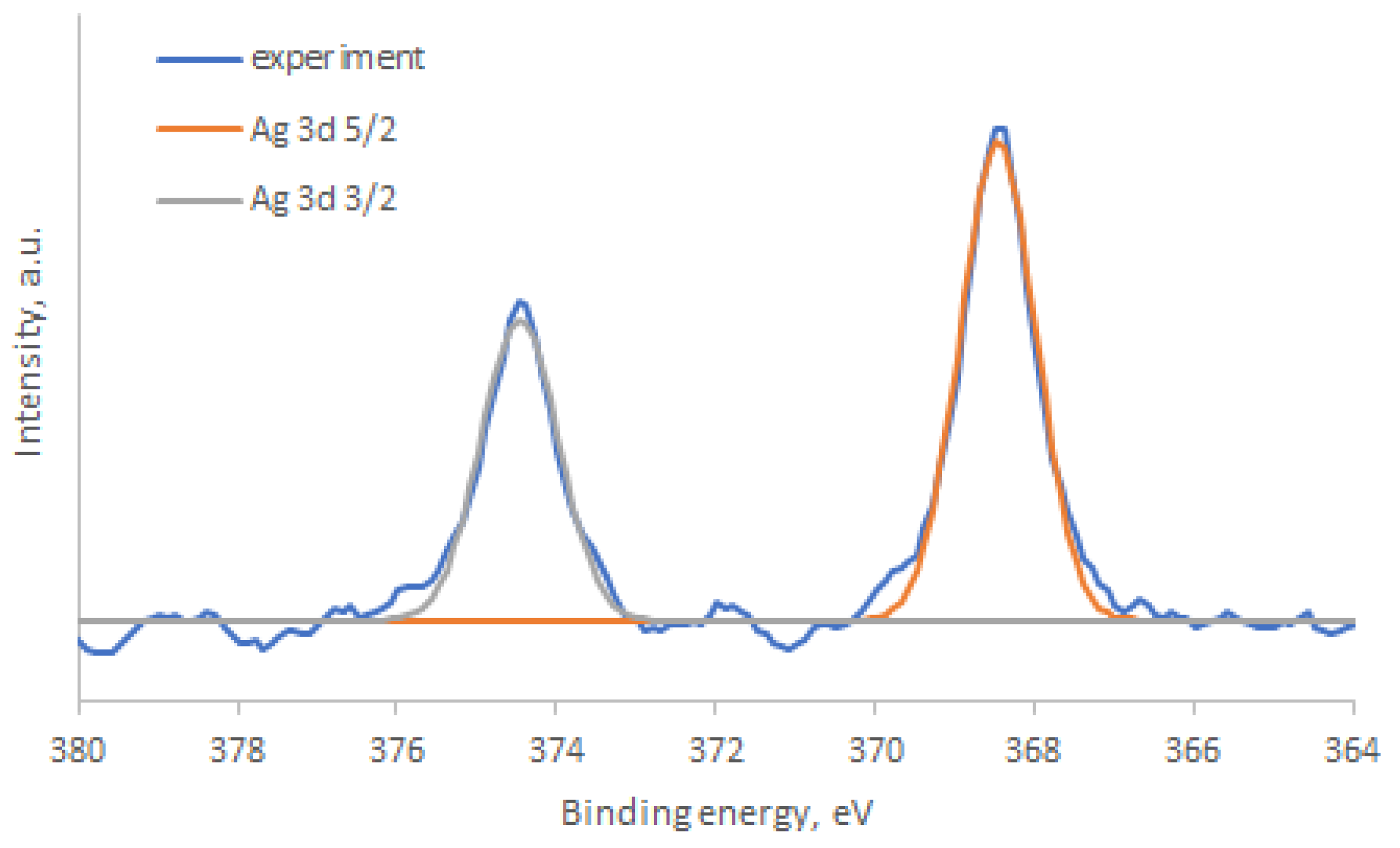
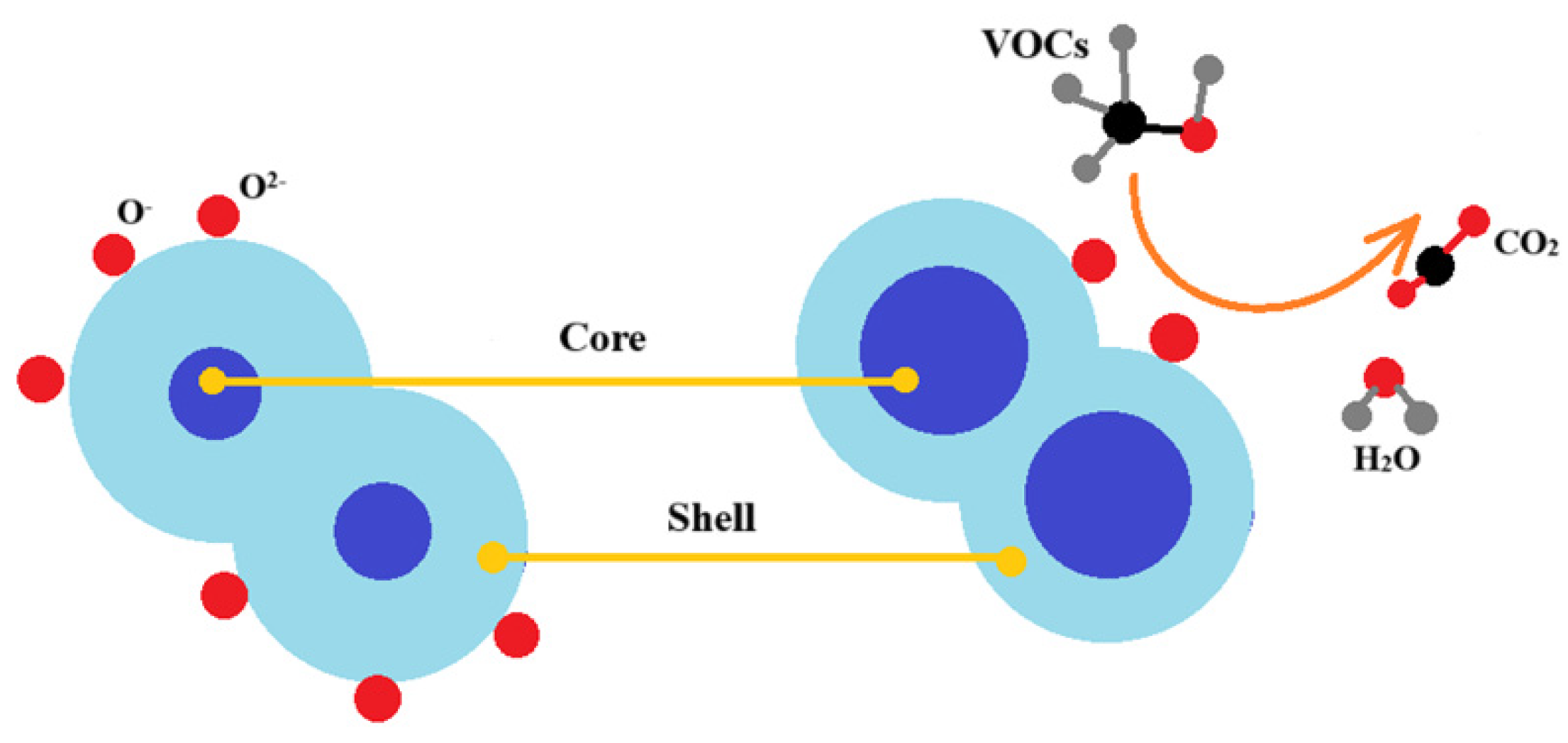
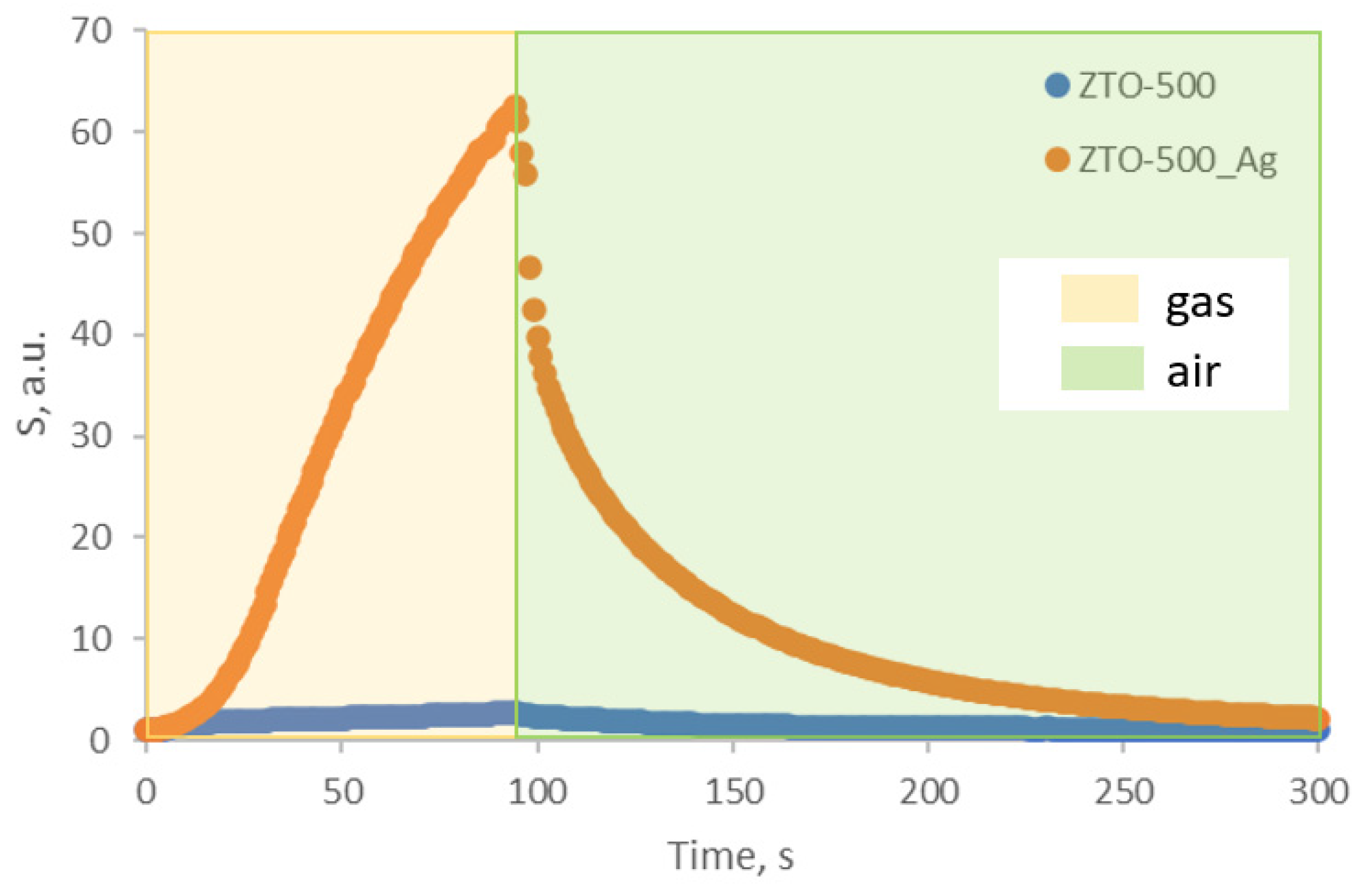
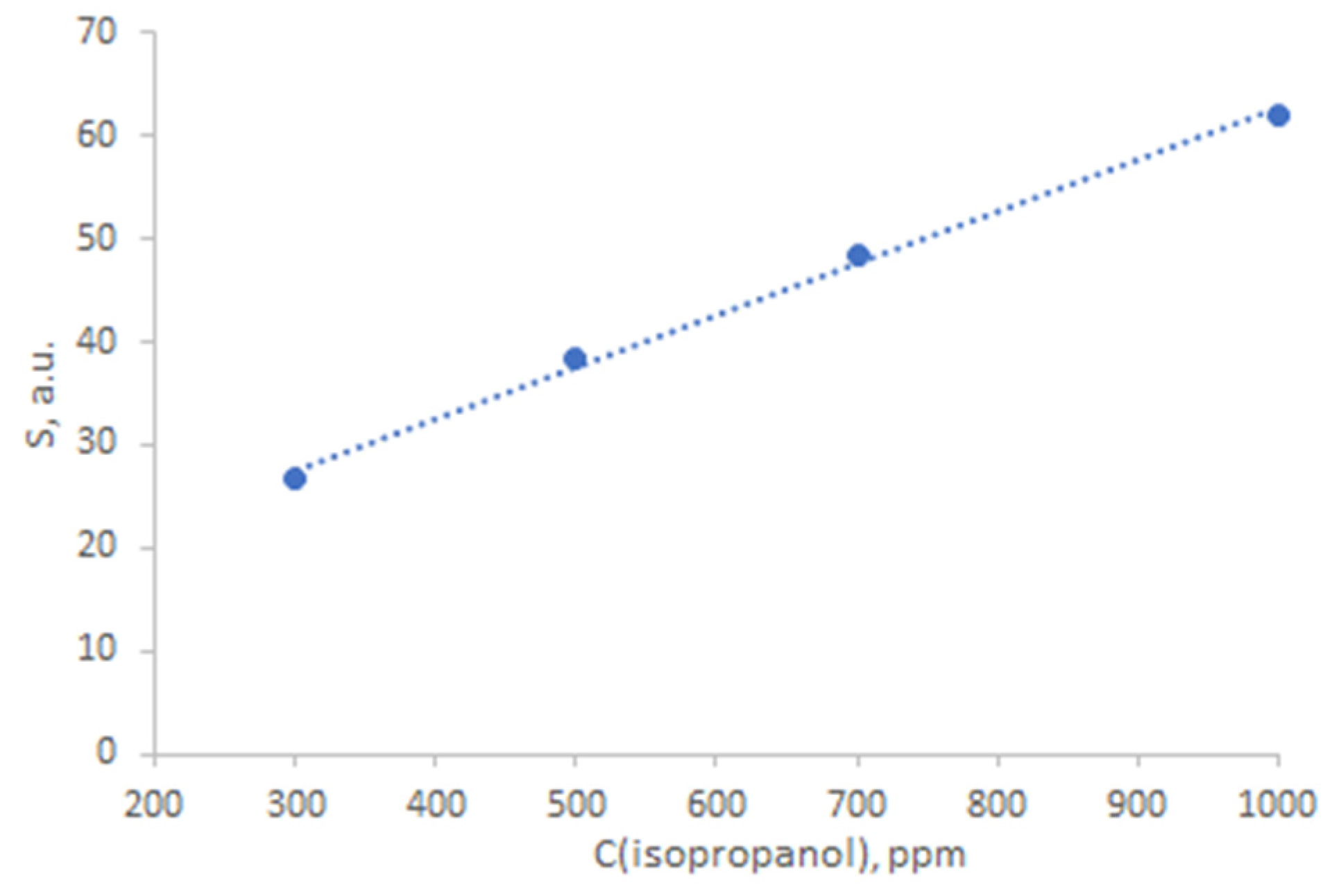
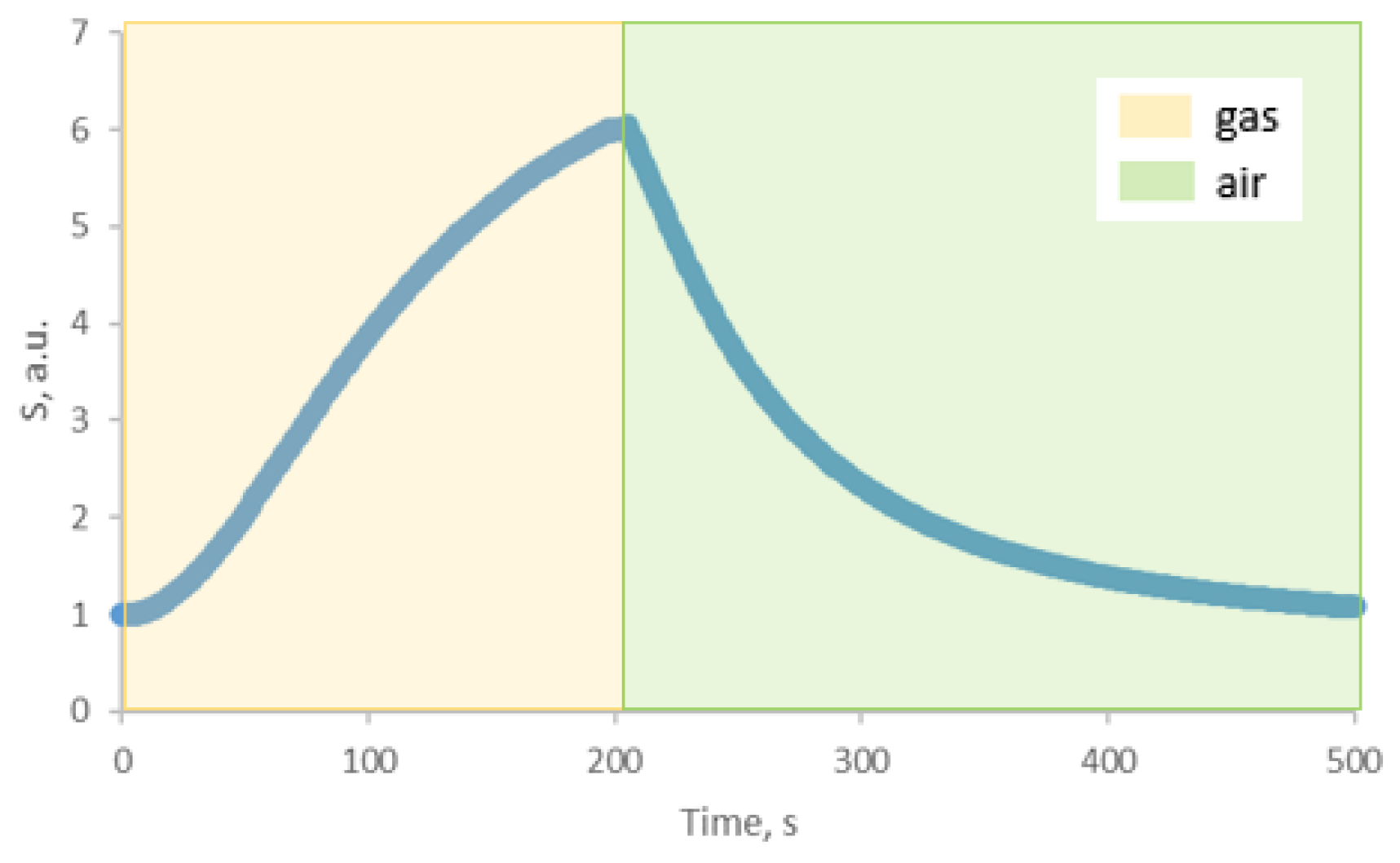
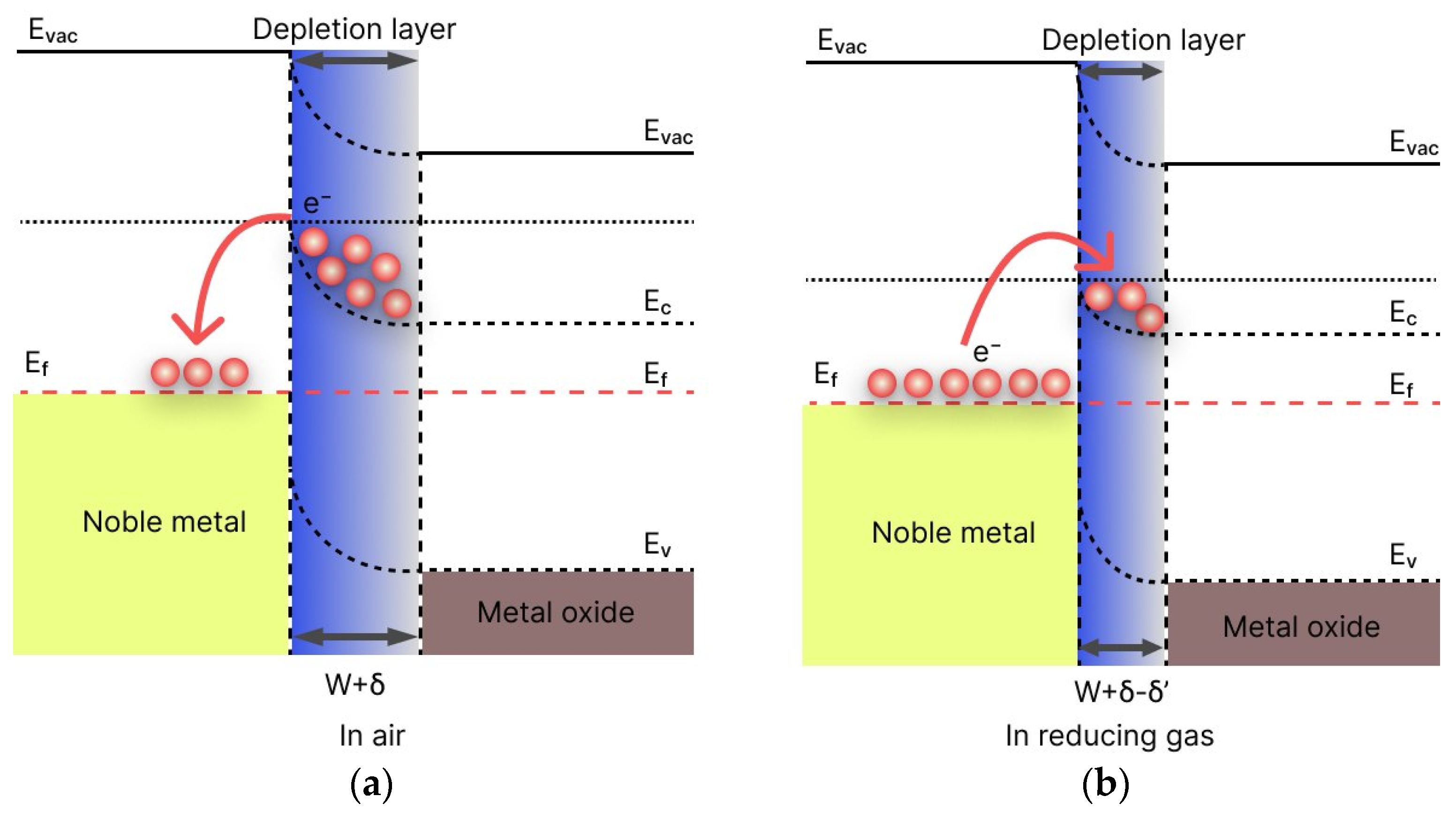
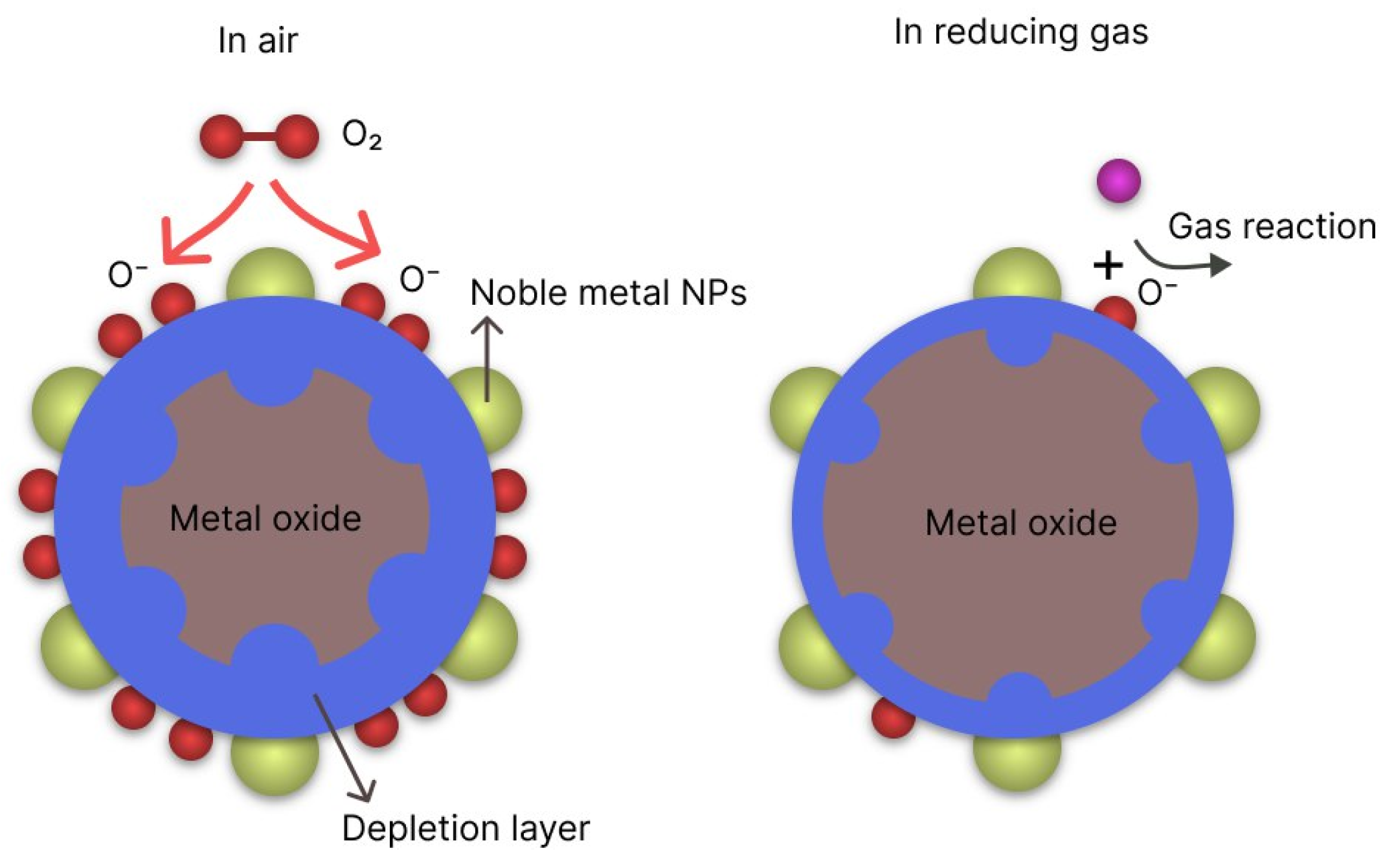
| Structure | Gas | Response | Response/Recovery Time, s |
|---|---|---|---|
| ZTO-300 | isopropyl alcohol | 6.26 | 143/313 |
| acetone | 1.56 | 143/88 | |
| ethanol | 1.59 | 61/110 | |
| ZTO-500 | isopropyl alcohol | 3 | 108/254 |
| acetone | 7.1 | 18/282 | |
| ethanol | 2.2 | 34/235 | |
| ZTO-700 | isopropyl alcohol | 1.48 | 62/60 |
| acetone | 2.37 | 106/159 | |
| ethanol | 1.75 | 179/120 |
| Isopropyl Alcohol (1000 ppm) | Acetone (1000 ppm) | Ethanol (1000 ppm) | ||||
|---|---|---|---|---|---|---|
| ZTO-500 | ZTO_Ag-500 | ZTO-500 | ZTO_Ag-500 | ZTO-500 | ZTO_Ag-500 | |
| Response | 3 | 62 | 7.1 | 4.22 | 2.2 | 35.6 |
| Response time, s | 177 | 58 | 60 | 220 | 34 | 73 |
| Recovery time, s | 254 | 457 | 282 | 238 | 235 | 442 |
| Sensor Material | Target Gas (Concentration) | Response, Ra/Rg | Temperature, °C | Reference |
|---|---|---|---|---|
| Ag-ZnO | H2, 300 ppm | 5.79 | 250 | [43] |
| Ag-ZnO | Ethanol, 100 ppm | 148 | 320 | [44] |
| Ag-ZnO/In2O3 | HCHO, 100 ppm | 186 | 260 | [45] |
| Ag-In2O3 | Isopropanol, 5 ppm | 5.2 | 300 | [46] |
| Ag-ZnO | NO2, 10 ppm | 17.18 | 200 | [47] |
| Ag-ZnSnO3 | HCHO, 50 ppm | 26.7 | 100 | [48] |
| Ag–ZnO/GO | C2H2, 100 ppm | 2.87 | 250 | [49] |
| Ag/SnO2 | Ethanol, 50 ppm | 135 | 180 | [50] |
| Ag-TiO2 | Acetone, 100 ppm | 13.9 | 275 | [51] |
| Ag-WO3 | NH3, 5 ppm | 3.29 | 200 | [52] |
| Ag-ZnSnO3 | Isopropanol, 1000 ppm | 6 | 150 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nalimova, S.S.; Shomakhov, Z.V.; Kozodaev, D.A.; Rybina, A.A.; Buzovkin, S.S.; Bui, C.D.; Novikov, I.A.; Moshnikov, V.A. VOC Gas Sensors Based on Zinc Stannate Nanoparticles Decorated with Silver. Nanomaterials 2024, 14, 1993. https://doi.org/10.3390/nano14241993
Nalimova SS, Shomakhov ZV, Kozodaev DA, Rybina AA, Buzovkin SS, Bui CD, Novikov IA, Moshnikov VA. VOC Gas Sensors Based on Zinc Stannate Nanoparticles Decorated with Silver. Nanomaterials. 2024; 14(24):1993. https://doi.org/10.3390/nano14241993
Chicago/Turabian StyleNalimova, Svetlana S., Zamir V. Shomakhov, Dmitry A. Kozodaev, Arina A. Rybina, Sergey S. Buzovkin, Cong D. Bui, Ivan A. Novikov, and Vyacheslav A. Moshnikov. 2024. "VOC Gas Sensors Based on Zinc Stannate Nanoparticles Decorated with Silver" Nanomaterials 14, no. 24: 1993. https://doi.org/10.3390/nano14241993
APA StyleNalimova, S. S., Shomakhov, Z. V., Kozodaev, D. A., Rybina, A. A., Buzovkin, S. S., Bui, C. D., Novikov, I. A., & Moshnikov, V. A. (2024). VOC Gas Sensors Based on Zinc Stannate Nanoparticles Decorated with Silver. Nanomaterials, 14(24), 1993. https://doi.org/10.3390/nano14241993






