In Vitro Antibacterial Efficacy of a New TiO2-Cu-Coated Titanium Surface for Biomedical Applications
Abstract
1. Introduction
2. Methods
2.1. Materials
2.2. Scanning Electron Microscopy (SEM) and Energy Dispersive Spectroscopy (SEM-EDS)
2.3. Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES)
2.4. Xray Photoelectron Spectroscopy (XPS)
2.5. Ultraviolet Photoelectron Spectroscopy (UPS)
2.6. Profilometry
2.7. Sessile Dynamic Contact Angle
2.8. Cu Release Profile
2.9. Optical Densitometry
2.10. Antibacterial Activity Assay Measured by CFU Count
2.11. Presto Blue Assay
2.12. Live-Dead Staining for Confocal Imaging
2.13. Statistical Methods
3. Results and Discussion
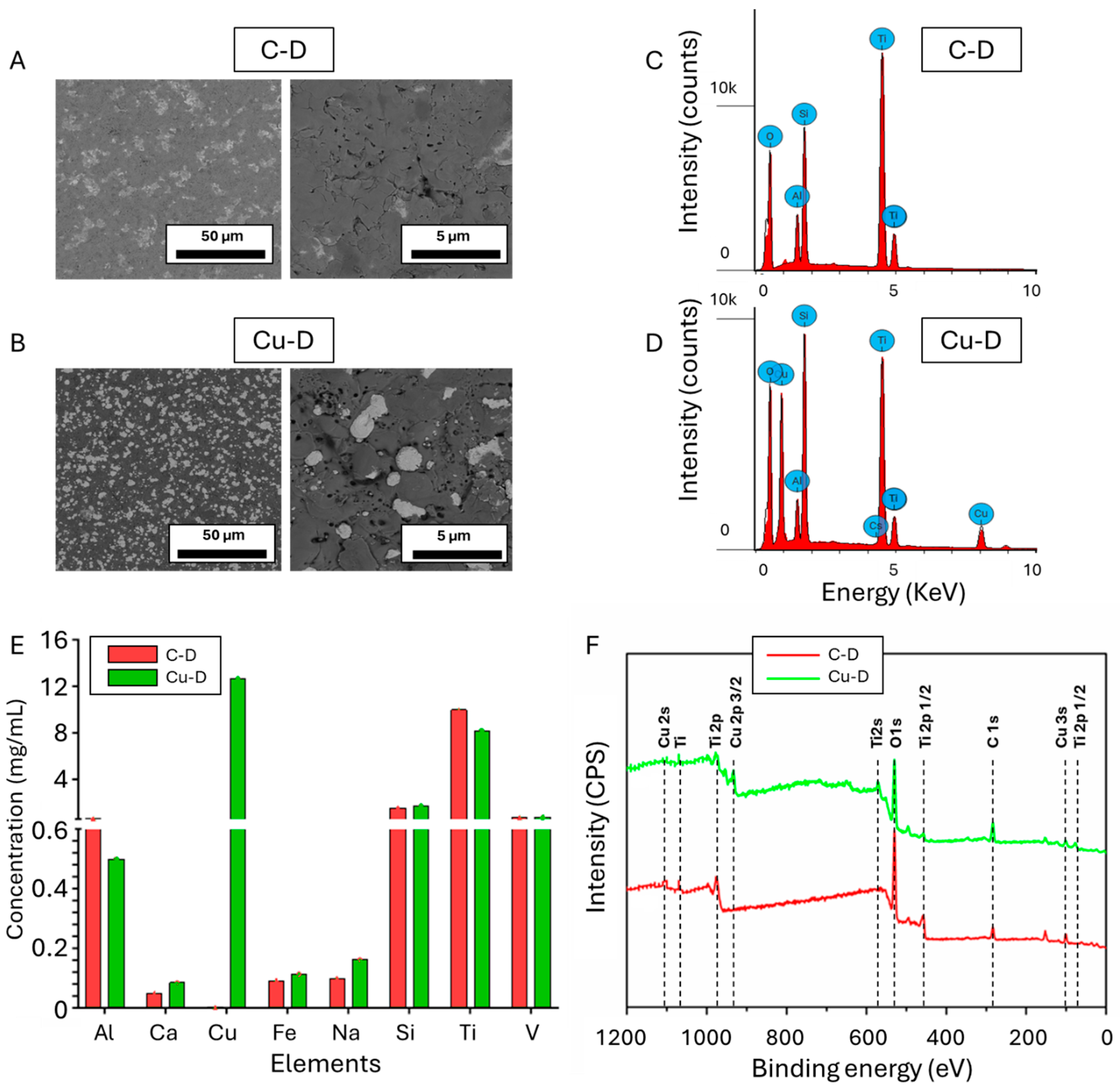
3.1. SEM Observation and EDS Elemental Analysis
3.2. Cu Surface Elemental Concentration Measured Through Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES)
3.3. X-Ray Photoelectron Spectroscopy (XPS)
3.4. Ultraviolet Photoelectron Spectroscopy (UPS)
3.5. Surface Profilometer
3.6. Contact Angle–Dynamic Mode
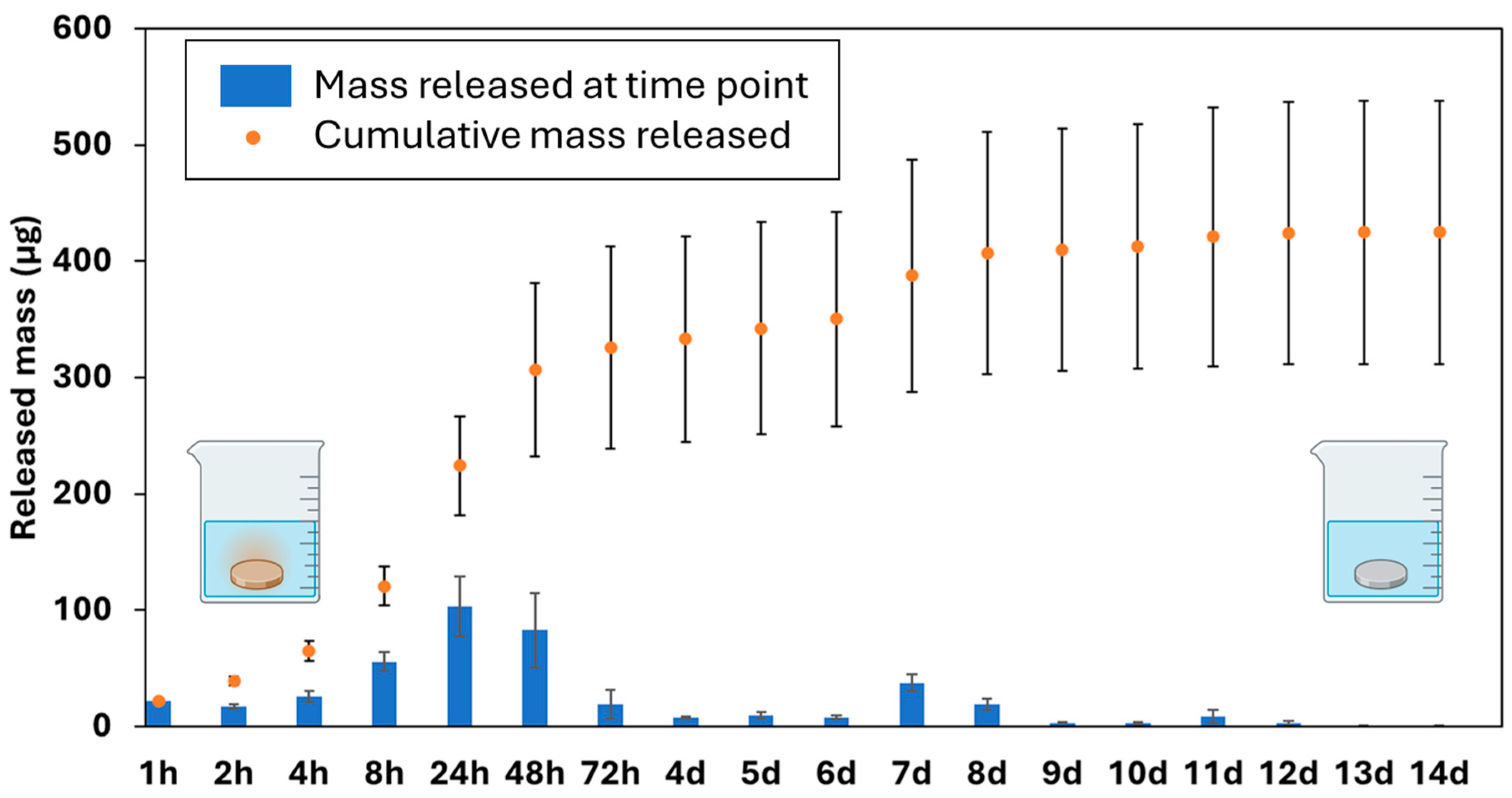
3.7. Cu Release Kinetics via ICP-OES
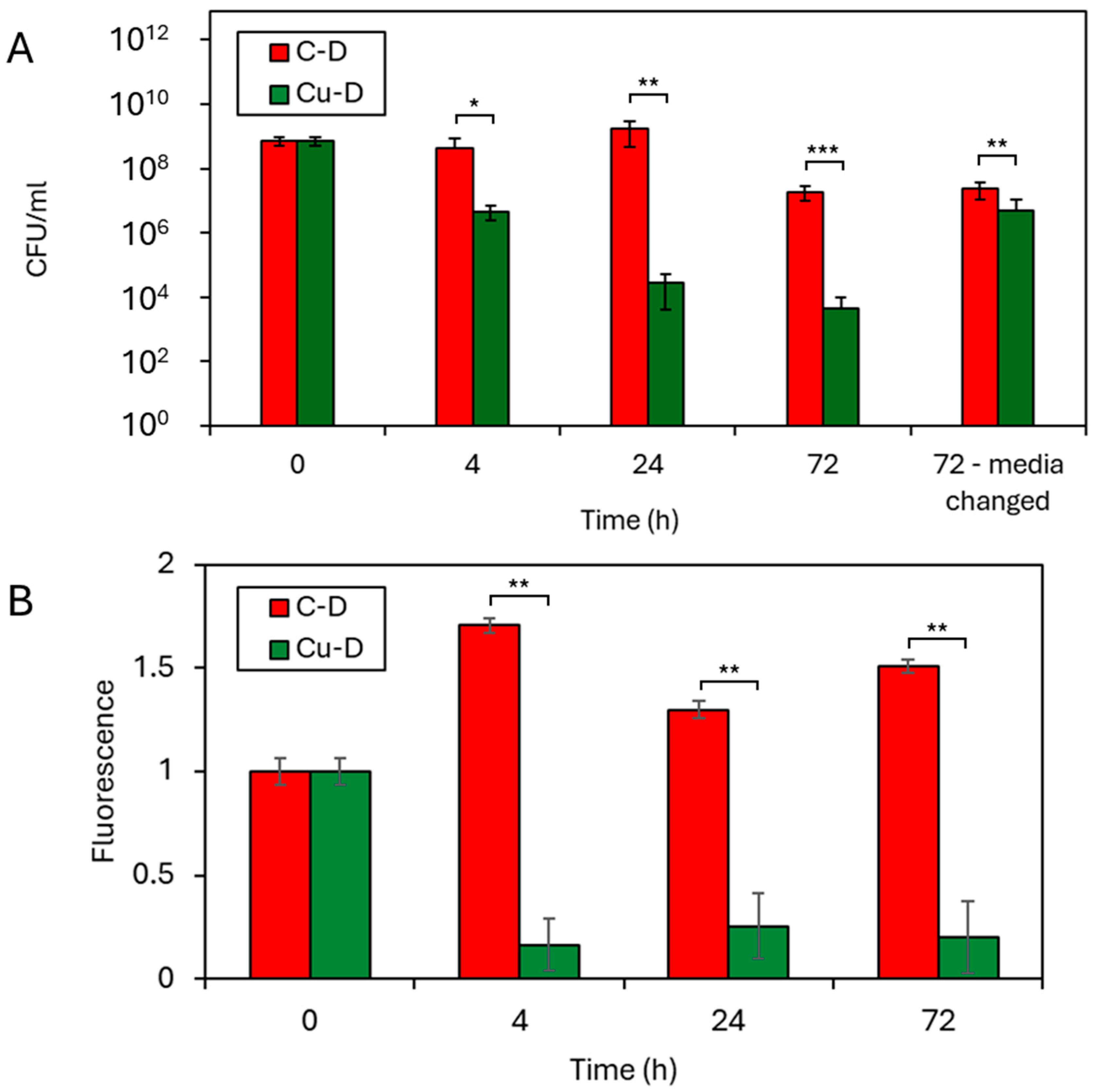
3.8. CFU Counting by Serial Dilution Technique
3.9. Presto Blue Assay
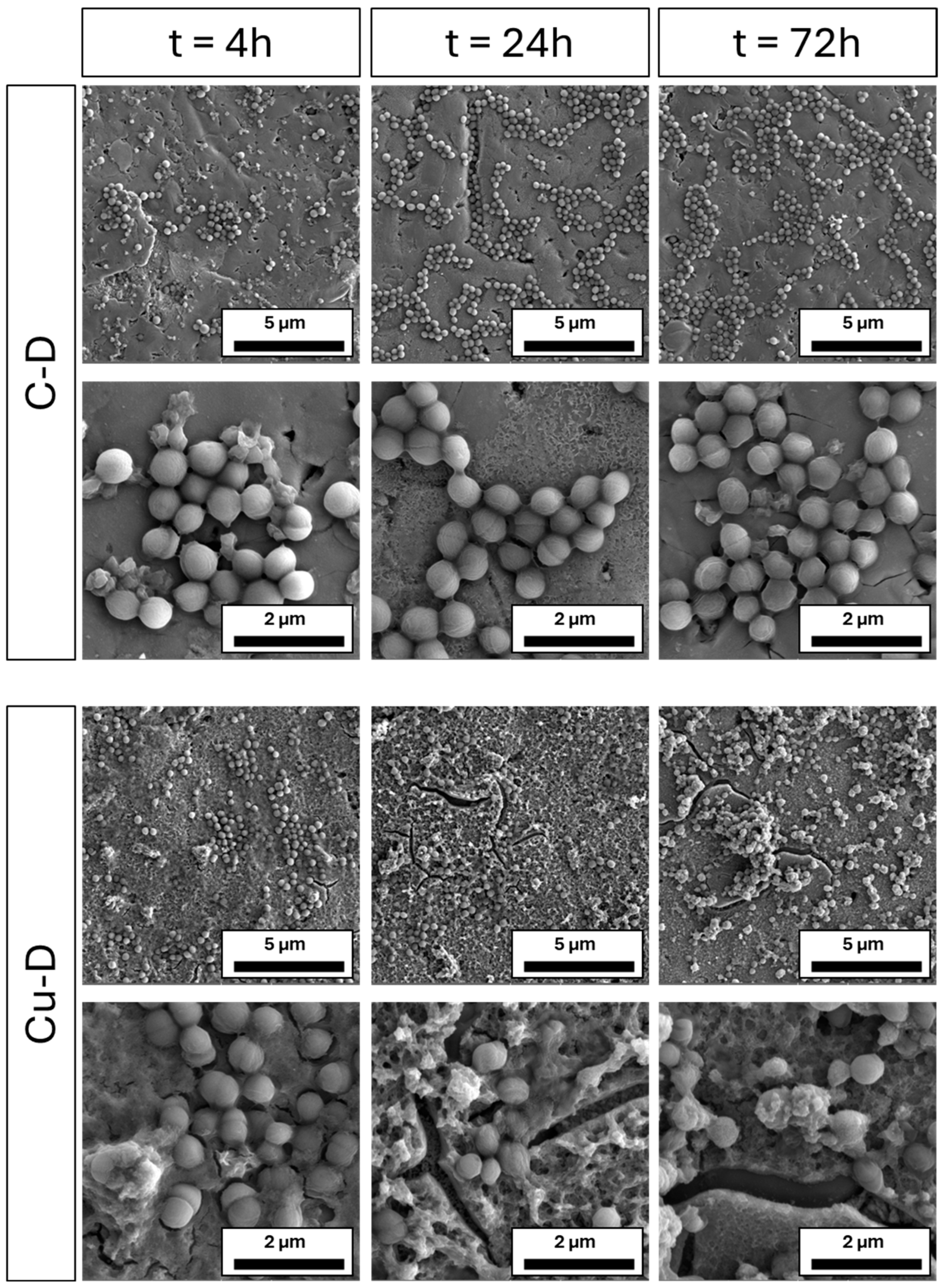
3.10. SEM Imaging of S. aureus Colonisation
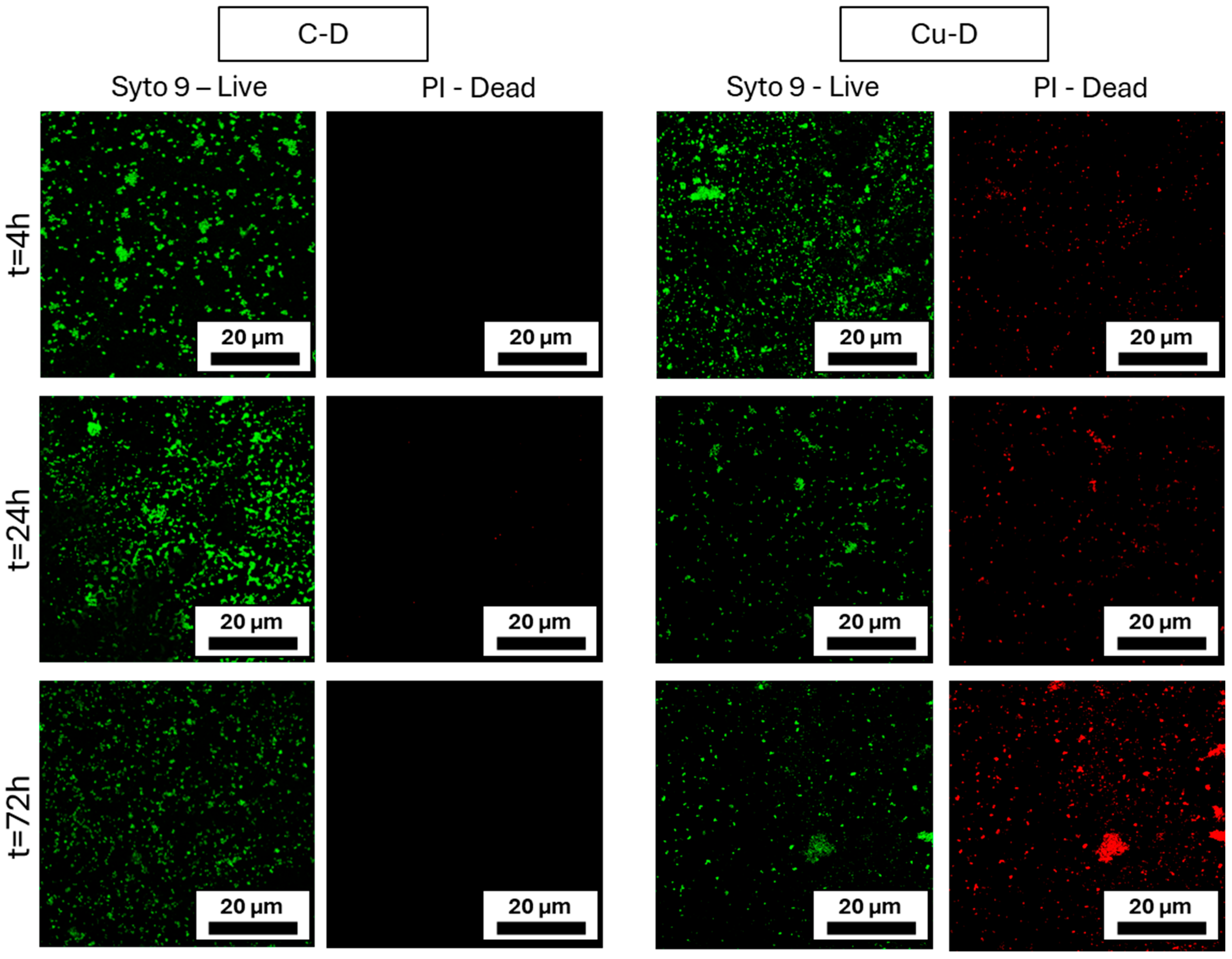
3.11. Live/Dead Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meroni, G.; Tsikopoulos, A.; Tsikopoulos, K.; Allemanno, F.; Martino, P.A.; Soares Filipe, J.F. A journey into animal models of human osteomyelitis: A review. Microorganisms 2022, 10, 1135. [Google Scholar] [CrossRef] [PubMed]
- Miclau, T. Open fracture management: Critical issues. OTA Int. 2020, 3, e074. [Google Scholar] [CrossRef]
- Hoekstra, H.; Smeets, B.; Metsemakers, W.J.; Spitz, A.C.; Nijs, S. Economics of open tibial fractures: The pivotal role of length-of-stay and infection. Health Econ. Rev. 2017, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Castillo, I.A.; Heiner, J.A.; Meremikwu, R.I.; Kellam, J.; Warner, S.J. Where are we in 2022? A summary of 11,000 open tibia fractures over 4 decades. J. Orthop. Trauma 2023, 37, e326–e334. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Lu, Y.; Feng, Q.; He, X.; Li, Z.; Zhang, K. Relationship between time to surgical debridement and the incidence of infection in patients with open tibial fractures. Orthop. Surg. 2020, 12, 524–532. [Google Scholar] [CrossRef]
- Schwarz, E.M.; Parvizi, J.; Gehrke, T.; Aiyer, A.; Battenberg, A.; Brown, S.A.; Callaghan, J.J.; Citak, M.; Egol, K.; Garrigues, G.E.; et al. 2018 international consensus meeting on musculoskeletal infection: Research priorities from the general assembly questions. J. Orthop. Res. 2019, 37, 997–1006. [Google Scholar] [CrossRef]
- Bachoura, A.; Guitton, T.G.; Smith, R.M.; Vrahas, M.S.; Zurakowski, D.; Ring, D. Infirmity and injury complexity are risk factors for surgical-site infection after operative fracture care. Clin. Orthop. Relat. Res. 2011, 469, 2621–2630. [Google Scholar] [CrossRef]
- Trampuz, A.; Zimmerli, W. Diagnosis and treatment of infections associated with fracture-fixation devices. Injury 2006, 37 (Suppl. S2), S59–S66. [Google Scholar] [CrossRef]
- Dicks, K.V.; Lewis, S.S.; Durkin, M.J.; Baker, A.W.; Moehring, R.W.; Chen, L.F.; Sexton, D.J.; Anderson, D.J. Surveying the surveillance: Surgical site infections excluded by the January 2013 updated surveillance definitions. Infect. Control Hosp. Epidemiol. 2014, 35, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Thakore, R.V.; Greenberg, S.E.; Shi, H.; Foxx, A.M.; Francois, E.L.; Prablek, M.A.; Nwosu, S.K.; Archer, K.R.; Ehrenfeld, J.M.; Obremskey, W.T.; et al. Surgical site infection in orthopedic trauma: A case-control study evaluating risk factors and cost. J. Clin. Orthop. Trauma 2015, 6, 220–226. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Lau, E.; Watson, H.; Schmier, J.K.; Parvizi, J. Economic burden of periprosthetic joint infection in the United States. J. Arthroplast. 2012, 27, 61–65.e61. [Google Scholar] [CrossRef]
- Metsemakers, W.J.; Handojo, K.; Reynders, P.; Sermon, A.; Vanderschot, P.; Nijs, S. Individual risk factors for deep infection and compromised fracture healing after intramedullary nailing of tibial shaft fractures: A single centre experience of 480 patients. Injury 2015, 46, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Galvain, T.; Chitnis, A.; Paparouni, K.; Tong, C.; Holy, C.E.; Giannoudis, P.V. The economic burden of infections following intramedullary nailing for a tibial shaft fracture in England. BMJ Open 2020, 10, e035404. [Google Scholar] [CrossRef]
- Jernigan, J.A. Is the burden of Staphylococcus aureus among patients with surgical-site infections growing? Infect. Control Hosp. Epidemiol. 2004, 25, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.J.; Sexton, D.J.; Kanafani, Z.A.; Auten, G.; Kaye, K.S. Severe surgical site infection in community hospitals: Epidemiology, key procedures, and the changing prevalence of methicillin-resistant Staphylococcus aureus. Infect. Control Hosp. Epidemiol. 2007, 28, 1047–1053. [Google Scholar] [CrossRef]
- McConoughey, S.J.; Howlin, R.; Granger, J.F.; Manring, M.M.; Calhoun, J.H.; Shirtliff, M.; Kathju, S.; Stoodley, P. Biofilms in periprosthetic orthopedic infections. Future Microbiol 2014, 9, 987–1007. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Ehrlich, G.D.; Montanaro, L. Biofilm-based implant infections in orthopaedics. Adv. Exp. Med. Biol. 2015, 830, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, W.; Sendi, P. Orthopaedic biofilm infections. APMIS J. Pathol. Microbiol. Immunol. 2017, 125, 353–364. [Google Scholar] [CrossRef]
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef]
- Raschke, M.J.; Rosslenbroich, S.B.; Fuchs, T.F. Antibiotic coated nails. In Intramedullary Nailing: A Comprehensive Guide; Rommens, P.M., Hessmann, M.H., Eds.; Springer: London, UK, 2015; pp. 555–563. [Google Scholar]
- Goodman, S.B.; Yao, Z.; Keeney, M.; Yang, F. The future of biologic coatings for orthopaedic implants. Biomaterials 2013, 34, 3174–3183. [Google Scholar] [CrossRef]
- Bohara, S.; Suthakorn, J. Surface coating of orthopedic implant to enhance the osseointegration and reduction of bacterial colonization: A review. Biomater. Res. 2022, 26, 26. [Google Scholar] [CrossRef]
- Boot, W.; Foster, A.L.; Guillaume, O.; Eglin, D.; Schmid, T.; D’Este, M.; Zeiter, S.; Richards, R.G.; Moriarty, T.F. An Antibiotic-Loaded Hydrogel Demonstrates Efficacy as Prophylaxis and Treatment in a Large Animal Model of Orthopaedic Device-Related Infection. Front. Cell. Infect. Microbiol. 2022, 12, 826392. [Google Scholar] [CrossRef]
- Foster, A.L.; Boot, W.; Stenger, V.; D’Este, M.; Jaiprakash, A.; Eglin, D.; Zeiter, S.; Richards, R.G.; Moriarty, T.F. Single-stage revision of MRSA orthopedic device-related infection in sheep with an antibiotic-loaded hydrogel. J. Orthop. Res. 2021, 39, 438–448. [Google Scholar] [CrossRef]
- Stewart, S.; Barr, S.; Engiles, J.; Hickok, N.J.; Shapiro, I.M.; Richardson, D.W.; Parvizi, J.; Schaer, T.P. Vancomycin-modified implant surface inhibits biofilm formation and supports bone-healing in an infected osteotomy model in sheep: A proof-of-concept study. J. Bone Jt. Surg. Am. 2012, 94, 1406–1415. [Google Scholar] [CrossRef]
- Gimeno, M.; Pinczowski, P.; Mendoza, G.; Asín, J.; Vázquez, F.J.; Vispe, E.; García-Álvarez, F.; Pérez, M.; Santamaría, J.; Arruebo, M.; et al. Antibiotic-eluting orthopedic device to prevent early implant associated infections: Efficacy, biocompatibility and biodistribution studies in an ovine model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Jorge-Mora, A.; Amhaz-Escanlar, S.; Fernandez-Pose, S.; García-Iglesias, A.; Mandia-Mancebo, F.; Franco-Trepat, E.; Guillán-Fresco, M.; Pino-Minguez, J. Commercially available antibiotic-laden PMMA-covered locking nails for the treatment of fracture-related infections—A retrospective case analysis of 10 cases. J. Bone Jt. Infect. 2019, 4, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Inoue, D.; Kabata, T.; Ohtani, K.; Kajino, Y.; Shirai, T.; Tsuchiya, H. Inhibition of biofilm formation on iodine-supported titanium implants. Int. Orthop. 2017, 41, 1093–1099. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Choi, H.; Kushida, Y.; Bhayana, B.; Wang, Y.; Hamblin, M.R. Broad-spectrum antimicrobial effects of photocatalysis using titanium dioxide nanoparticles are strongly potentiated by addition of potassium iodide. Antimicrob. Agents Chemother. 2016, 60, 5445–5453. [Google Scholar] [CrossRef]
- Bosch, E.H.; van Doorne, H.; de Vries, S. The lactoperoxidase system: The influence of iodide and the chemical and antimicrobial stability over the period of about 18 months. J. Appl. Microbiol. 2000, 89, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z. Nanosurface modification of Ti64 implant by anodic fluorine-doped alumina/titania for orthopedic application. Mater. Chem. Phys. 2022, 281, 125867. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Le, P.T.M.; Shintani, S.A.; Takadama, H.; Ito, M.; Ferraris, S.; Spriano, S. Iodine-loaded calcium titanate for bone repair with sustainable antibacterial activity prepared by solution and heat treatment. Nanomaterials 2021, 11, 2199. [Google Scholar] [CrossRef] [PubMed]
- Inoue, D.; Kabata, T.; Kajino, Y.; Shirai, T.; Tsuchiya, H. Iodine-supported titanium implants have good antimicrobial attachment effects. J. Orthop. Sci. 2019, 24, 548–551. [Google Scholar] [CrossRef]
- Daeschlein, G. Antimicrobial and antiseptic strategies in wound management. Int. Wound J. 2013, 10 (Suppl. S1), 9–14. [Google Scholar] [CrossRef] [PubMed]
- Lollobrigida, M.; Filardo, S.; Sessa, R.; Di Pietro, M.; Bozzuto, G.; Molinari, A.; Lamazza, L.; Vozza, I.; De Biase, A. Antibacterial activity and impact of different antiseptics on biofilm-contaminated implant surfaces. Appl. Sci. 2019, 9, 5467. [Google Scholar] [CrossRef]
- Tripathy, A.; Sen, P.; Su, B.; Briscoe, W.H. Natural and bioinspired nanostructured bactericidal surfaces. Adv. Colloid Interface Sci. 2017, 248, 85–104. [Google Scholar] [CrossRef]
- Oopath, S.V.; Baji, A.; Abtahi, M.; Luu, T.Q.; Vasilev, K.; Truong, V.K. Nature-inspired biomimetic surfaces for controlling bacterial attachment and biofilm development. Adv. Mater. Interfaces 2023, 10, 2201425. [Google Scholar] [CrossRef]
- Beddoes, C.M.; Case, C.P.; Briscoe, W.H. Understanding nanoparticle cellular entry: A physicochemical perspective. Adv. Colloid Interface Sci. 2015, 218, 48–68. [Google Scholar] [CrossRef]
- Wang, L.; Guo, X.; Zhang, H.; Liu, Y.; Wang, Y.; Liu, K.; Liang, H.; Ming, W. Recent advances in superhydrophobic and antibacterial coatings for biomedical materials. Coatings 2022, 12, 1469. [Google Scholar] [CrossRef]
- Linklater, D.P.; Ivanova, E.P. Nanostructured antibacterial surfaces—What can be achieved? Nano Today 2022, 43, 101404. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.J. Metal-based antimicrobial strategies. Microb. Biotechnol. 2017, 10, 1062–1065. [Google Scholar] [CrossRef] [PubMed]
- Birkett, M.; Dover, L.; Cherian Lukose, C.; Wasy Zia, A.; Tambuwala, M.M.; Serrano-Aroca, Á. Recent advances in metal-based antimicrobial coatings for high-touch surfaces. Int. J. Mol. Sci. 2022, 23, 1162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Zhao, X.; Hu, J.; Wang, R.; Fu, S.; Qin, G. Antibacterial metals and alloys for potential biomedical implants. Bioact. Mater. 2021, 6, 2569–2612. [Google Scholar] [CrossRef]
- Fordham, W.R.; Redmond, S.; Westerland, A.; Cortes, E.G.; Walker, C.; Gallagher, C.; Medina, C.J.; Waecther, F.; Lunk, C.; Ostrum, R.F.; et al. Silver as a bactericidal coating for biomedical implants. Surf. Coat. Technol. 2014, 253, 52–57. [Google Scholar] [CrossRef]
- Santo, C.E.; Quaranta, D.; Grass, G. Antimicrobial metallic copper surfaces kill StapShylococcus haemolyticus via membrane damage. Microbiologyopen 2012, 1, 46–52. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A mini review of antibacterial properties of ZnO nanoparticles. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- Read, S.A.; Obeid, S.; Ahlenstiel, C.; Ahlenstiel, G. The role of zinc in antiviral immunity. Adv. Nutr. 2019, 10, 696–710. [Google Scholar] [CrossRef]
- Mijnendonckx, K.; Leys, N.; Mahillon, J.; Silver, S.; Van Houdt, R. Antimicrobial silver: Uses, toxicity and potential for resistance. Biometals 2013, 26, 609–621. [Google Scholar] [CrossRef]
- Liu, Q.; Li, A.; Liu, S.; Fu, Q.; Xu, Y.; Dai, J.; Li, P.; Xu, S. Cytotoxicity of biodegradable zinc and its alloys: A systematic review. J. Funct. Biomater. 2023, 14, 206. [Google Scholar] [CrossRef]
- Kumar, K.; Gill, R.; Batra, U. Challenges and opportunities for biodegradable magnesium alloy implants. Mater. Technol. 2018, 33, 153–172. [Google Scholar] [CrossRef]
- Lin, J.; Nguyen, N.-Y.T.; Zhang, C.; Ha, A.; Liu, H.H. Antimicrobial properties of MgO nanostructures on magnesium substrates. ACS Omega 2020, 5, 24613–24627. [Google Scholar] [CrossRef]
- Vincent, M.; Hartemann, P.; Engels-Deutsch, M. Antimicrobial applications of copper. Int. J. Hyg. Environ. Health 2016, 219 Pt A, 585–591. [Google Scholar] [CrossRef]
- Vincent, M.; Duval, R.E.; Hartemann, P.; Engels-Deutsch, M. Contact killing and antimicrobial properties of copper. J. Appl. Microbiol. 2018, 124, 1032–1046. [Google Scholar] [CrossRef]
- Grass, G.; Rensing, C.; Solioz, M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.P. Effect of copper-impregnated composite bed linens and patient gowns on healthcare-associated infection rates in six hospitals. J. Hosp. Infect. 2018, 100, e130–e134. [Google Scholar] [CrossRef] [PubMed]
- Noyce, J.O.; Michels, H.; Keevil, C.W. Potential use of copper surfaces to reduce survival of epidemic meticillin-resistant Staphylococcus aureus in the healthcare environment. J. Hosp. Infect. 2006, 63, 289–297. [Google Scholar] [CrossRef]
- Bryce, E.A.; Velapatino, B.; Akbari Khorami, H.; Donnelly-Pierce, T.; Wong, T.; Dixon, R.; Asselin, E. In vitro evaluation of antimicrobial efficacy and durability of three copper surfaces used in healthcare. Biointerphases 2020, 15, 011005. [Google Scholar] [CrossRef]
- Charles, M.K.; Williams, T.C.; Nakhaie, D.; Woznow, T.; Velapatino, B.; Lorenzo-Leal, A.C.; Bach, H.; Bryce, E.A.; Asselin, E. In vitro assessment of antibacterial and antiviral activity of three copper products after 200 rounds of simulated use. BioMetals 2023, 37, 849–856. [Google Scholar] [CrossRef]
- Montero, D.A.; Arellano, C.; Pardo, M.; Vera, R.; Gálvez, R.; Cifuentes, M.; Berasain, M.A.; Gómez, M.; Ramírez, C.; Vidal, R.M. Antimicrobial properties of a novel copper-based composite coating with potential for use in healthcare facilities. Antimicrob. Resist. Infect. Control 2019, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Kalapos, C.; Nie, X.; Murphy, M.; Hussein, R.; Zhang, J. Superhydrophilicity and antibacterial property of a Cu-dotted oxide coating surface. Ann. Clin. Microbiol. Antimicrob. 2010, 9, 25. [Google Scholar] [CrossRef]
- Isa, N.N.C.; Mohd, Y.; Mohamad, S.A.S.; Zaki, M.H.M. Antibacterial activity of copper coating electrodeposited on 304 stainless steel substrate. In AIP Conference Proceedings; AIP Publishing LLC.: Melville, NY, USA, 2017; p. 020009. [Google Scholar]
- Bharadishettar, N.; Bhat, K.U.; Bhat Panemangalore, D. Coating technologies for copper based antimicrobial active surfaces: A perspective review. Metals 2021, 11, 711. [Google Scholar] [CrossRef]
- Giraldo-Osorno, P.M.; Turner, A.B.; Barros, S.M.; Büscher, R.; Guttau, S.; Asa’ad, F.; Trobos, M.; Palmquist, A. Anodized Ti6Al4V-ELI, electroplated with copper is bactericidal against Staphylococcus aureus and enhances macrophage phagocytosis. J. Mater. Sci. Mater. Med. 2025, 36, 14. [Google Scholar] [CrossRef]
- Hoene, A.; Prinz, C.; Walschus, U.; Lucke, S.; Patrzyk, M.; Wilhelm, L.; Neumann, H.G.; Schlosser, M. In vivo evaluation of copper release and acute local tissue reactions after implantation of copper-coated titanium implants in rats. Biomed. Mater. 2013, 8, 035009. [Google Scholar] [CrossRef] [PubMed]
- Burghardt, I.; Lüthen, F.; Prinz, C.; Kreikemeyer, B.; Zietz, C.; Neumann, H.G.; Rychly, J. A dual function of copper in designing regenerative implants. Biomaterials 2015, 44, 36–44. [Google Scholar] [CrossRef]
- Dawari, C.K.; Gunell, M.; Mönkkönen, K.; Suvanto, M.; Saarinen, J.J. Antibacterial activity of electrodeposited copper and zinc on metal injection molded (MIM) micropatterned WC-CO hard metals. Coatings 2022, 12, 485. [Google Scholar] [CrossRef]
- Van Hengel, I.; Tierolf, M.; Valerio, V.; Minneboo, M.; Fluit, A.; Fratila-Apachitei, L.; Apachitei, I.; Zadpoor, A. Self-defending additively manufactured bone implants bearing silver and copper nanoparticles. J. Mater. Chem. B 2020, 8, 1589–1602. [Google Scholar] [CrossRef]
- Popova, A.D.; Sheveyko, A.N.; Kuptsov, K.A.; Advakhova, D.Y.; Karyagina, A.S.; Gromov, A.V.; Krivozubov, M.S.; Orlova, P.A.; Volkov, A.V.; Slukin, P.V.; et al. Osteoconductive, osteogenic, and antipathogenic plasma electrolytic oxidation coatings on titanium implants with BMP-2. ACS Appl. Mater. Interfaces 2023, 15, 37274–37289. [Google Scholar] [CrossRef]
- Augustin, A.; Thaira, H.; Udaya Bhat, K.; Udupa, K.R. Effect of electrodeposited copper thin film on the morphology and cell death of E. coli; an electron microscopic study. In Biotechnology and Biochemical Engineering: Select Proceedings of ICACE 2015; Springer: Berlin/Heidelberg, Germany, 2016; pp. 227–232. [Google Scholar]
- Chen, J.; He, Z.; Zheng, S.; Gao, W.; Wang, Y. Ultrasonic-assisted electrodeposition of Cu-TiO2 nanocomposite coatings with long-term antibacterial activity. ACS Appl. Mater. Interfaces 2024, 16, 66695–66705. [Google Scholar] [CrossRef]
- Mamleyev, E.R.; Falk, F.; Weidler, P.G.; Heissler, S.; Wadhwa, S.; Nassar, O.; Shyam Kumar, C.; Kübel, C.; Wöll, C.; Islam, M. Polyaramid-based flexible antibacterial coatings fabricated using laser-induced carbonization and copper electroplating. ACS Appl. Mater. Interfaces 2020, 12, 53193–53205. [Google Scholar] [CrossRef] [PubMed]
- Zeiger, M.; Solioz, M.; Edongué, H.; Arzt, E.; Schneider, A.S. Surface structure influences contact killing of bacteria by copper. MicrobiologyOpen 2014, 3, 327–332. [Google Scholar] [CrossRef]
- Ciacotich, N.; Din, R.U.; Sloth, J.J.; Møller, P.; Gram, L. An electroplated copper–silver alloy as antibacterial coating on stainless steel. Surf. Coat. Technol. 2018, 345, 96–104. [Google Scholar] [CrossRef]
- Banthia, S.; Hazra, C.; Sen, R.; Das, S.; Das, K. Electrodeposited functionally graded coating inhibits Gram-positive and Gram-negative bacteria by a lipid peroxidation mediated membrane damage mechanism. Mater. Sci. Eng. C 2019, 102, 623–633. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, J.; Yan, T.; Han, Y. Fibroblast responses and antibacterial activity of Cu and Zn co-doped TiO2 for percutaneous implants. Appl. Surf. Sci. 2018, 434, 633–642. [Google Scholar] [CrossRef]
- Kharitonov, D.S.; Kasach, A.A.; Sergievich, D.S.; Wrzesińska, A.; Bobowska, I.; Darowicki, K.; Zielinski, A.; Ryl, J.; Kurilo, I.I. Ultrasonic-assisted electrodeposition of Cu-Sn-TiO2 nanocomposite coatings with enhanced antibacterial activity. Ultrason. Sonochemistry 2021, 75, 105593. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ye, X.; Zhang, L.; Shao, Y.; Zhou, X.; Lu, M.; Chu, C.; Xue, F.; Bai, J. Corrosion and antimicrobial behavior of stainless steel prepared by one-step electrodeposition of silver at the grain boundaries. Surf. Coat. Technol. 2022, 439, 128428. [Google Scholar] [CrossRef]
- El Sayed, M.A.; Elazab, N.T.; Gassoumi, M.; Ibrahim, M.A. Nanocrystalline silver coatings on steel by electrodeposition from non-polluting aqueous baths and its antibacterial activity. J. Taiwan Inst. Chem. Eng. 2022, 132, 104212. [Google Scholar] [CrossRef]
- Kirmanidou, Y.; Sidira, M.; Bakopoulou, A.; Tsouknidas, A.; Prymak, O.; Papi, R.; Choli-Papadopoulou, T.; Epple, M.; Michailidis, N.; Koidis, P.; et al. Assessment of cytotoxicity and antibacterial effects of silver nanoparticle-doped titanium alloy surfaces. Dent. Mater. 2019, 35, e220–e233. [Google Scholar] [CrossRef]
- Reyes-Vidal, Y.; Suarez-Rojas, R.; Ruiz, C.; Torres, J.; Ţălu, Ş.; Méndez, A.; Trejo, G. Electrodeposition, characterization, and antibacterial activity of zinc/silver particle composite coatings. Appl. Surf. Sci. 2015, 342, 34–41. [Google Scholar] [CrossRef]
- Zhao, Q.; Yi, L.; Jiang, L.; Ma, Y.; Lin, H.; Dong, J. Surface functionalization of titanium with zinc/strontium-doped titanium dioxide microporous coating via microarc oxidation. Nanomed. Nanotechnol. Biol. Med. 2019, 16, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Uribe, P.; Ruiz, J.; Ortiz, C.; Blanco, S.; Gutierrez, J. Antibacterial activity of ZnO nanoparticle coatings formed by electrophoretic deposition. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2020; p. 012007. [Google Scholar]
- ISO 11137; Sterilization of Health Care Products. International Organization for Standardization: Geneva, Switzerland, 2025.
- ISO 21920-2:2021; Geometrical Product Specifications (GPS)—Surface Texture: Profile. International Organization for Standardization: Geneva, Switzerland, 2022.
- ISO 10993-12:2021; Biological Evaluation of Medical Devices. International Organization for Standardization: Geneva, Switzerland, 2021.
- Heidenau, F.; Mittelmeier, W.; Detsch, R.; Haenle, M.; Stenzel, F.; Ziegler, G.; Gollwitzer, H. A novel antibacterial titania coating: Metal ion toxicity and in vitro surface colonization. J. Mater. Sci. Mater. Med. 2005, 16, 883–888. [Google Scholar] [CrossRef]
- Haenle, M.; Fritsche, A.; Zietz, C.; Bader, R.; Heidenau, F.; Mittelmeier, W.; Gollwitzer, H. An extended spectrum bactericidal titanium dioxide (TiO2) coating for metallic implants: In vitro effectiveness against MRSA and mechanical properties. J. Mater. Sci. Mater. Med. 2011, 22, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Gargioni, C.; Borzenkov, M.; D’Alfonso, L.; Sperandeo, P.; Polissi, A.; Cucca, L.; Dacarro, G.; Grisoli, P.; Pallavicini, P.; D’Agostino, A.; et al. Self-assembled monolayers of copper sulfide nanoparticles on glass as antibacterial coatings. Nanomaterials 2020, 10, 352. [Google Scholar] [CrossRef]
- Fiedler, J.; Kolitsch, A.; Kleffner, B.; Henke, D.; Stenger, S.; Brenner, R.E. Copper and silver ion implantation of aluminium oxide-blasted titanium surfaces: Proliferative response of osteoblasts and antibacterial effects. Int. J. Artif. Organs 2011, 34, 882–888. [Google Scholar] [CrossRef]
- Cherenda, N.N.; Basalai, A.V.; Shymanski, V.I.; Uglov, V.V.; Astashynski, V.M.; Kuzmitski, A.M.; Laskovnev, A.P.; Remnev, G.E. Modification of Ti-6Al-4V alloy element and phase composition by compression plasma flows impact. Surf. Coat. Technol. 2018, 355, 148–154. [Google Scholar] [CrossRef]
- Zegan, G.; Cimpoesu, N.; Agop, M.; Stirbu, I.; Chicet, D.; Istrate, B.; Alexandru, A.; Prisacariu, B. Improving the HA deposition process on ti-based advanced alloy through sandblasting. Optoelectron. Adv. Mater.—Rapid Commun. 2016, 10, 279–284. [Google Scholar]
- Yuda, A.; Supriadi, S.; Saragih, A. Surface Modification of Ti-Alloy Based Bone Implant by Sandblasting. In AIP Conference Proceedings; AIP Publishing LLC.: Melville, NY, USA, 2019; Volume 2193, p. 020015. [Google Scholar]
- Polishetty, A.; Manoharan, V.; Littlefair, G.; Sonavane, C. Machinability assessment of Ti-6Al-4V for aerospace applications. ASME Early Career Tech. J. 2013, 12, 53–58. [Google Scholar]
- Liang, T.; Wang, Y.; Zeng, L.; Liu, Y.; Qiao, L.; Zhang, S.; Zhao, R.; Li, G.; Zhang, R.; Xiang, J.; et al. Copper-doped 3D porous coating developed on Ti-6Al-4V alloys and its in vitro long-term antibacterial ability. Appl. Surf. Sci. 2020, 509, 144717. [Google Scholar] [CrossRef]
- Zhou, X.; Thompson, G.E.; Skeldon, P.; Shimizu, K.; Habazaki, H.; Wood, G.C. The valence state of copper in anodic films formed on Al-1at.% Cu alloy. Corrosion 2005, 47, 1299–1306. [Google Scholar] [CrossRef]
- Whitten, J.E. Ultraviolet photoelectron spectroscopy: Practical aspects and best practices. Appl. Surf. Sci. Adv. 2023, 13, 100384. [Google Scholar] [CrossRef]
- Mansfeldova, V.; Zlamalova, M.; Tarabkova, H.; Janda, P.; Vorokhta, M.; Piliai, L.; Kavan, L. Work function of TiO2 (anatase, rutile, and brookite) single crystals: Effects of the environment. J. Phys. Chem. C 2021, 125, 1902–1912. [Google Scholar] [CrossRef]
- Schultz, T.; Schlesinger, R.; Niederhausen, J.; Henneberger, F.; Sadofev, S.; Blumstengel, S.; Vollmer, A.; Bussolotti, F.; Yang, J.P.; Kera, S.; et al. Tuning the work function of GaN with organic molecular acceptors. Phys. Rev. B 2016, 93, 125309. [Google Scholar] [CrossRef]
- Kashiwaya, S.; Morasch, J.; Streibel, V.; Toupance, T.; Jaegermann, W.; Klein, A. The work function of TiO2. Surfaces 2018, 1, 73–89. [Google Scholar] [CrossRef]
- Luo, Y.; Niu, L.; Wang, Y.; Wen, P.; Gong, Y.; Li, C.; Xu, S. Regulating the work function of Cu2O films via crystal facet engineering with enhanced charge transfer and SERS activity. Appl. Surf. Sci. 2023, 607, 155095. [Google Scholar] [CrossRef]
- Su, M.; Liang, Z.; Liu, P.; Yue, S. Preparation of high quality Cu2O crystal and its opto-electronic properties. Mater. Lett. 2016, 170, 80–84. [Google Scholar] [CrossRef]
- Kumar, S.; Datta, S.; Dey, V.; Roy, D.N. Formation of nanocones and generation of negative potential on stainless steel surfaces by electrochemical etching synergistically reduce pseudomonas aeruginosa’s biofilm. Surf. Rev. Lett. 2025, 32, 2450109. [Google Scholar] [CrossRef]
- Rocha, S.S.d.; Adabo, G.L.; Henriques, G.E.P.; Nóbilo, M.A.d.A. Vickers hardness of cast commercially pure titanium and Ti-6Al-4V alloy submitted to heat treatments. Braz. Dent. J. 2006, 17, 126–129. [Google Scholar] [CrossRef]
- Jianchao, Y.; Wang, G.; Rong, Y. Experimental study on the surface integrity and chip formation in the micro cutting process. Procedia Manuf. 2015, 1, 655–662. [Google Scholar] [CrossRef]
- Chen, C.J.; Ding, S.J.; Chen, C.C. Effects of surface conditions of titanium dental implants on bacterial adhesion. Photomed. Laser Surg. 2016, 34, 379–388. [Google Scholar] [CrossRef]
- Gallardo-Moreno, A.M.; Pacha-Olivenza, M.A.; Saldaña, L.; Pérez-Giraldo, C.; Bruque, J.M.; Vilaboa, N.; González-Martín, M.L. In vitro biocompatibility and bacterial adhesion of physico-chemically modified Ti6Al4V surface by means of UV irradiation. Acta Biomater. 2009, 5, 181–192. [Google Scholar] [CrossRef]
- Dong, Y.; Li, X.; Tian, L.; Bell, T.; Sammons, R.L.; Dong, H. Towards long-lasting antibacterial stainless steel surfaces by combining double glow plasma silvering with active screen plasma nitriding. Acta Biomater. 2011, 7, 447–457. [Google Scholar] [CrossRef]
- Sharifahmadian, O.; Salimijazi, H.; Fathi, M.; Mostaghimi, J.; Pershin, L. Relationship between surface properties and antibacterial behavior of wire arc spray copper coatings. Surf. Coat. Technol. 2013, 233, 74–79. [Google Scholar] [CrossRef]
- Cao, B.; Zheng, Y.; Xi, T.; Zhang, C.; Song, W.; Burugapalli, K.; Yang, H.; Ma, Y. Concentration-dependent cytotoxicity of copper ions on mouse fibroblasts in vitro: Effects of copper ion release from TCu380A vs TCu220C intra-uterine devices. Biomed. Microdevices 2012, 14, 709–720. [Google Scholar] [CrossRef]
- Ruparelia, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008, 4, 707–716. [Google Scholar] [CrossRef]
- Wennergren, D.; Bergdahl, C.; Selse, A.; Ekelund, J.; Sundfeldt, M.; Möller, M. Treatment and re-operation rates in one thousand and three hundred tibial fractures from the Swedish Fracture Register. Eur. J. Orthop. Surg. Traumatol. 2021, 31, 143–154. [Google Scholar] [CrossRef]
- Benson, D.R.; Riggins, R.S.; Lawrence, R.M.; Hoeprich, P.D.; Huston, A.C.; Harrison, J.A. Treatment of open fractures: A prospective study. J. Trauma Acute Care Surg. 1983, 23, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Prinz, C.; Elhensheri, M.; Rychly, J.; Neumann, H.G. Antimicrobial and bone-forming activity of a copper coated implant in a rabbit model. J. Biomater. Appl. 2017, 32, 139–149. [Google Scholar] [CrossRef]
- Hill, P.; Clasper, J.; Parker, S.; Watkins, P. Early intramedullary nailing in an animal model of a heavily contaminated fracture of the tibia. J. Orthop. Res. 2002, 20, 648–653. [Google Scholar] [CrossRef]
- Moriarty, T.; Schmid, T.; Post, V.; Samara, E.; Kates, S.; Schwarz, E.; Zeiter, S.; Richards, R. A large animal model for a failed two-stage revision of intramedullary nail-related infection by methicillin-resistant Staphylococcus aureus. Eur. Cell. Mater. 2017, 34, 83–98. [Google Scholar] [CrossRef]
- Banstola, A.; Reynolds, J.N.J. The Sheep as a Large Animal Model for the Investigation and Treatment of Human Disorders. Biology 2022, 11, 1251. [Google Scholar] [CrossRef] [PubMed]


| Coating | Deposition Process | Substrate Characteristics | In Vitro Model Design | Outcomes | Reference |
|---|---|---|---|---|---|
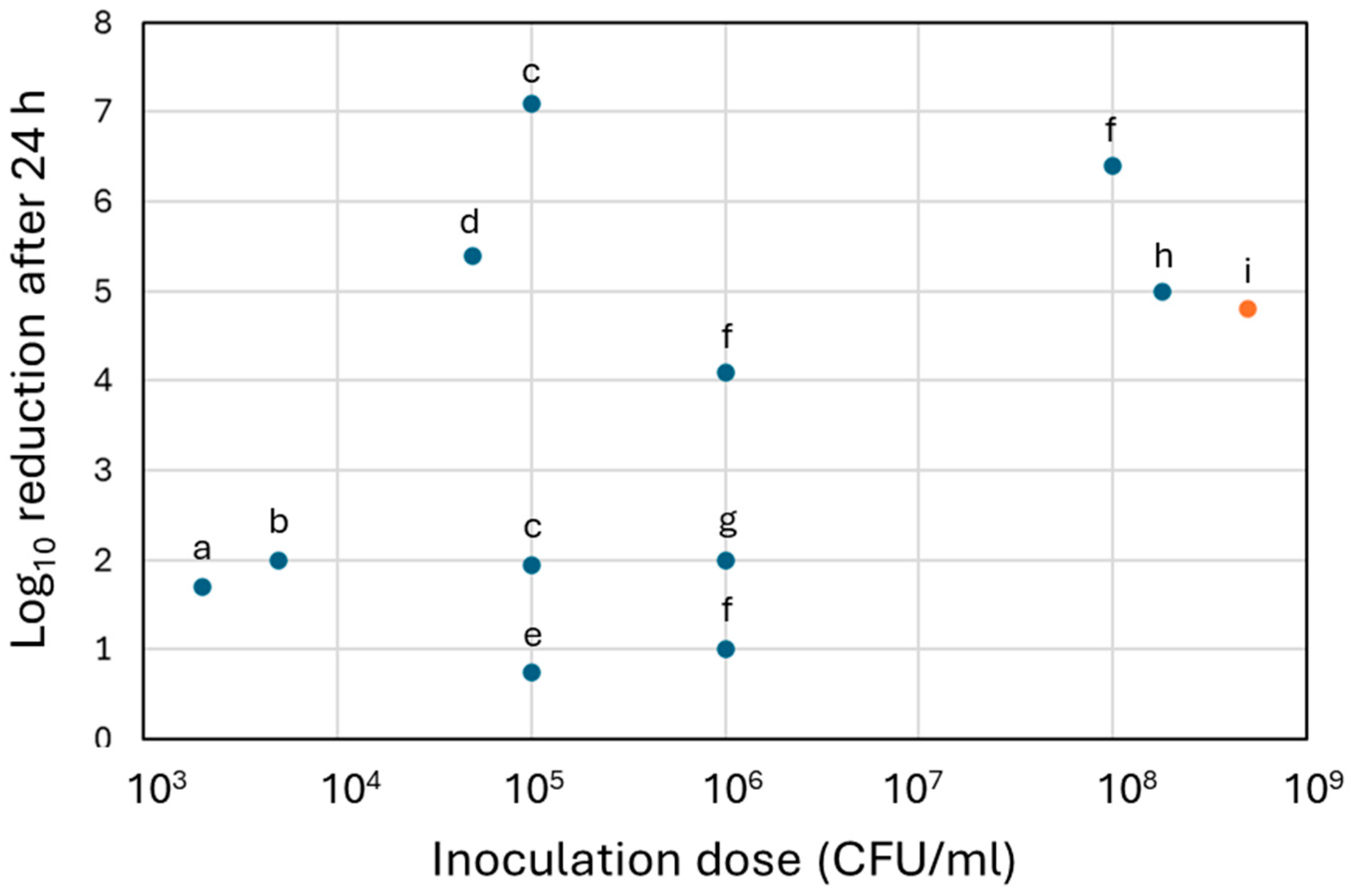
| Cu | Plasma electrolytic oxidation | Ti6Al4V Plates, 5 × 5 × 1 mm | S. aureus CFU count, 2, 4, 6, 8 and 24 h timepoints 5 × 104 CFU/mL dose | Adhered bacteria on samples decreased to undetectable levels after 2 h and further timepoints. Compared to control samples, this corresponded to 3 log10, 4 log10, 5.39 log10 reductions after 4 h, 6 h and 24 h. | Hoene et al., 2013 [65] |
| Plasma electrolytic oxidation | Ti6Al4V Plates, 5 × 5 × 1 mm Cu concentrations 0.05–0.5 mM | S. aureus CFU count, 6, 24 and 48 h timepoints 5 × 103 CFU/mL dose | Reduction in bacterial growth was more significant with higher Cu concentration. For 0.5 mM, 2 log10 reductions were observed at 24 and 48 h. | Burghardt et al., 2015 [66] | |
| Plasma electrolytic oxidation | Ti6Al4V Discs, ⌀ 12 mm, height 3 mm | S. aureus CFU count, 4 and 24 h timepoints 105 CFU/mL dose | No bactericidal effect observed after 4 h. 1.95 log10 (98.86%) to 7.10 log10 (99.99%) reductions after 24 h, depending on culture media. | Giraldo-Osorno et al., 2025 [64] | |
| Electroplating | Tungsten carbide-cobalt plates | S. aureus CFU count, 24 h timepoint 1.8 × 108 CFU/mL dose | 5.0 log10 reduction. | Dawari et al., 2022 [67] | |
| Plasma electrolytic oxidation | Ti6Al4V Rods, ⌀ 0.5 mm, height 10 mm | S. aureus CFU count, overnight 2 × 103 CFU/mL dose | 1.7 log10 reduction. | Van Hengel et al., 2020 [68] | |
| Plasma electrolytic oxidation | Grade 4 Ti Plates, 15 × 15 × 3 mm | MRSA and E. coli CFU count, 6 and 24 h timepoints 105–106 CFU/ml | 6 log10 reduction against E. coli after 6 h. 1 log10 (90%) and 2 log10 (99%) reductions against MRSA after 6 and 24 h. | Popova et al., 2023 [69] | |
| Plasma electrolytic oxidation | AA1100 Al Plates, 25 × 25 × 5 mm | MRSA, E. coli and E. faecium CFU count, 1, 2 and 3 h timepoint. 1.5 × 108 CFU/mL dose | For all strains, bacterial concentration decreased faster than control Al plates. At 3 h, 1.70 log10, 7.98 log10, and 1.86 log10 reductions were observed for E. coli, MRSA and E. faecium. | Nie et al., 2010 [61] | |
| Electrophoresis | 304 stainless steel Plates, 20 × 20 × 1 mm | S. aureus and E. coli Time-of-contact, 5, 10, 15, 20, 25 and 30 min 105 CFU dose | Complete reduction in E. coli after 5 min of exposure, and of S. aureus after 10 min (5.0 log10 reduction). | Isa et al., 2017 [62] | |
| Electroplating | Al Plates, 100 mm2 | E. coli CFU count, 6 h timepoint 107 CFU/mL dose | 1.4 log10 (96%) reduction. | Augustin et al., 2016 [70] | |
| Ultrasonic-assisted electrophoresis | Al2O3-coated Al | E. coli CFU count, 1 h timepoint 105 CFU/mL dose | 5.0 log10 reduction. | Chen et al., 2024 [71] | |
| Electroplating | Carbonised polyaramid Plates, 10 × 10 mm | E. coli and B. subtilis CFU count, 75 min timepoint | 3.0 log10 reduction for both strains. | Mamleyev et al., 2020 [72] | |
| Electrophoresis | Microstructured copper foils, 2.5 × 2.5 × 0.05 cm | E. coli. Time-of-contact, 20, 40, 60, 100 and 180 min 2 × 108 CFU/mL dose | Complete reduction in E. coli after 60 min of exposure, or 100 min for surface non-microstructured (8.3 log10 reduction). | Zeiger et al., 2014 [73] | |
| Cu-Ag | Plasma electrolytic oxidation | Ti6Al4V Rods, ⌀ 0.5 mm, height 10 mm | S. aureus CFU count, overnight 2 × 103 CFU/mL dose | 8.4 log10 reduction. | Van Hengel et al., 2020 [68] |
| Electroplating | AISI 316L steel Plates, 10 × 20 × 1 mm | S. aureus and E. coli CFU count, 0.5, 4 and 24 h timepoints 106 and 108 CFU/mL doses in PBS and BHI broth | For S. aureus, gradual bacterial growth reductions were observed across timepoints. At 24 h, 4.1 log10 reduction for small dose and 6.4 log10 reduction for high dose in PBS were observed, but only 1.0 log10 reduction (90.0%) for small dose in broth. For E. coli, 2.5–2.7 log10 reductions were observed for all timepoints for small dose in PBS. | Ciacotich et al., 2018 [74] | |
| Cu, Cu-SiC | Pulse reverse electrodeposition | Annealed Cu Plates, 2 cm × 2 cm × 100 μm | E. coli and B. subtilis CFU count, 4, 8, 12, 16, 20 and 24 h timepoints 1.5 × 108 CFU dose | Only 1.11 log10 reductions for both E. coli and B. subtilis at 24 h with the Cu coating, but 7.14 and 10 log10 reductions at 24 h with the Cu-SiC coating. | Banthia et al., 2019 [75] |
| Cu, Cu-Zn | Plasma electrolytic oxidation | Ti6Al4V Discs, ⌀ 14 mm, height 2 mm | S. aureus CFU count, 6 and 24 h timepoints 105 CFU/mL dose | 78% and 82% growth reduction at 6 and 24 h for the Cu coating. Up to 90% and 92% reduction at 6 and 24 h for the Cu coating with the highest concentration of Zn. | Zhang et al., 2018 [76] |
| Cu-Sn and Cu-Sn-TiO2 | Ultrasonic-assisted electrophoresis | Stainless steel Plates, 4 cm2 Different concentrations of Cu, Sn and TiO2 | E. coli CFU count, 20 and 30 min timepoints 9.2 × 105 CFU dose | No reduction in Cu-Sn coatings. Highest reduction after 30 min was 57%, for 82.4% Cu−10% Sn−7.6% Ti. Highest reductions rates observed with combination with UV exposure. | Kharitonov et al., 2021 [77] |
| Ag | Electroplating | 304 stainless steel Plates, 50 × 50 × 1 mm | S. aureus and E. coli CFU count, 72 h timepoint 6 × 105 CFU/mL dose | Antibacterial rate against E.coli. and S. aureus. reached 99.88% (2.9 log10) and 97.92% (1.7 log10), respectively. | Wang et al., 2022 [78] |
| Electrophoresis | Stainless steel Plates, 3.5 × 2.5 cm | S. aureus and E. coli Zone of inhibition | Inhibition zone diameters were 1.5–2 cm against S. aureus and 1.5–1.9 cm against E. coli (depending on the deposition parameters investigated in the study). | El Sayed et al., 2022 [79] | |
| Plasma electrolytic oxidation | Ti6Al4V Rods, ⌀ 0.5 mm, height 10 mm | S. aureus CFU count, overnight 2 × 103 CFU/mL dose | 8.4 log10 reduction. | Van Hengel et al., 2020 [68] | |
| Electrophoresis | Ti6Al4V Discs, ⌀ 6 mm, height 1 mm | P. gingivalis and P. intermedia CFU count, 24 h timepoint 5 and 30 nm particle size 100, 200 and 300 ppm particle concentration. | Decrease in bacterial population increased gradually with the concentration of Ag particles. Higher reduction was observed with smaller particles. Maximum reductions were 29.9% and 26%, for the 5 nm/300 ppm and 30 nm/300 ppm groups. | Kirmanidou et al., 2019 [80] | |
| Zn-Ag | Electroplating | AISI 1018 steel Plates, 10 × 15 cm | S. aureus and E. coli Time-of-contact, 1, 15 and 30 min 0–14.0 mg/cm3 Ag concentrations | Zn with 4.3 mg/cm3 Ag reduced E. coli and S. aureus growth by 94.1% and 91.0% after 1 min, and 95.4% and 98.4%, after 30 min. Results were similar for higher concentrations of Ag. | Reyes-Vidal et al., 2015 [81] |
| Zn | Electroplating | Tungsten carbide-cobalt plates | S. aureus CFU count, 24 h timepoint 1.8 × 108 CFU/mL dose | 2.0 log10 reduction. | Dawari et al., 2022 [67] |
| Electroplating | AISI 1018 steel Plates, 10 × 15 cm | S. aureus and E. coli Time-of-contact, 1, 15 and 30 min | Zn alone reduced E. coli and S. aureus growth by 87.0% and 89.5% after 1 min, and 80.5% and 83.73%, after 30 min. | Reyes-Vidal et al., 2015 [81] | |
| Zn, Sr, Zn-Sr | Microarc oxidation | TA2 Ti Discs, ⌀ 14.75 mm, height 1 mm | S. aureus CFU count, 24 h timepoint 104 CFU/mL dose | 60% and 80% reduction in bacterial growth on Sr and Zn-Sr coatings. Complete reduction of bacterial growth on Zn coatings (4.0 log10 reduction). | Zhao et al., 2019 [82] |
| ZnO | Electrophoresis | SAE 1020 carbon steel Bar, ⌀ 12 mm, height 50 mm | MRSA and E. coli. Zone of inhibition 20, 30, 40 and 50 ppm particle concentration | Bacterial reductions ranged from 71.4% (20 ppm particle concentration) to 99.97% (50 ppm particle concentration). | Uribe et al., 2020 [83] |
| MgO | Electrophoresis | Mg Plates, 10 × 10 mm | S. aureus CFU count, 24 h timepoint 6 × 106 CFU/mL dose | 6.9 log10 reduction compared to Ti control substrate, and 3.9 log10 reduction compared to Mg control substrate. | Lin et al., 2020 [52] |
| Group | Timepoints & Media Change | |
|---|---|---|
| Negative control | C-D sample No bacteria | 4 h, 24 h, 72 h No media change |
| 72 h Media change at 24 h and 48 h | ||
| Positive control | C-D sample S. aureus, 1–5 × 108 CFU/mL | 4 h, 24 h, 72 h No media change |
| 72 h Media change at 24 h and 48 h | ||
| Experimental | Cu-D sample S. aureus, 1–5 × 108 CFU/mL | 4 h, 24 h, 72 h No media change |
| 72 h Media change at 24 h and 48 h | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandian, M.; Cavelier, S.; Guttau, S.; Cometta, S.; Fernando, J.; Kobbe, P.; Hutmacher, D.W. In Vitro Antibacterial Efficacy of a New TiO2-Cu-Coated Titanium Surface for Biomedical Applications. Nanomaterials 2025, 15, 1742. https://doi.org/10.3390/nano15221742
Pandian M, Cavelier S, Guttau S, Cometta S, Fernando J, Kobbe P, Hutmacher DW. In Vitro Antibacterial Efficacy of a New TiO2-Cu-Coated Titanium Surface for Biomedical Applications. Nanomaterials. 2025; 15(22):1742. https://doi.org/10.3390/nano15221742
Chicago/Turabian StylePandian, Mahalakshmi, Sacha Cavelier, Simone Guttau, Silvia Cometta, Joseph Fernando, Philipp Kobbe, and Dietmar W. Hutmacher. 2025. "In Vitro Antibacterial Efficacy of a New TiO2-Cu-Coated Titanium Surface for Biomedical Applications" Nanomaterials 15, no. 22: 1742. https://doi.org/10.3390/nano15221742
APA StylePandian, M., Cavelier, S., Guttau, S., Cometta, S., Fernando, J., Kobbe, P., & Hutmacher, D. W. (2025). In Vitro Antibacterial Efficacy of a New TiO2-Cu-Coated Titanium Surface for Biomedical Applications. Nanomaterials, 15(22), 1742. https://doi.org/10.3390/nano15221742










