Removal of Cadmium and Lead from Tires Discarded in the Open Sea with Multicomponent Nanoparticles from Sugarcane Bagasse
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Chemicals
2.3. The Extract Black Berry (Rubus glaucus) Preparation
2.4. Preparation of Sugarcane Bagasse
2.5. Synthesis of Nanoparticles (NPs)
2.6. Metal Readings from Sugarcane Bagasse
2.7. Lead and Cadmium Analysis of Tire Samples
2.8. Materials and Equipment Used in the Characterization of NPs
2.9. Removal of Lead and Cadmium
3. Results
3.1. Antioxidant Capacity and Polyphenol Concentration
3.2. Analysis of Metals in Sugarcane Bagasse
3.3. Concentration and Removal of Lead and Cadmium in Contaminated Aquatic Systems
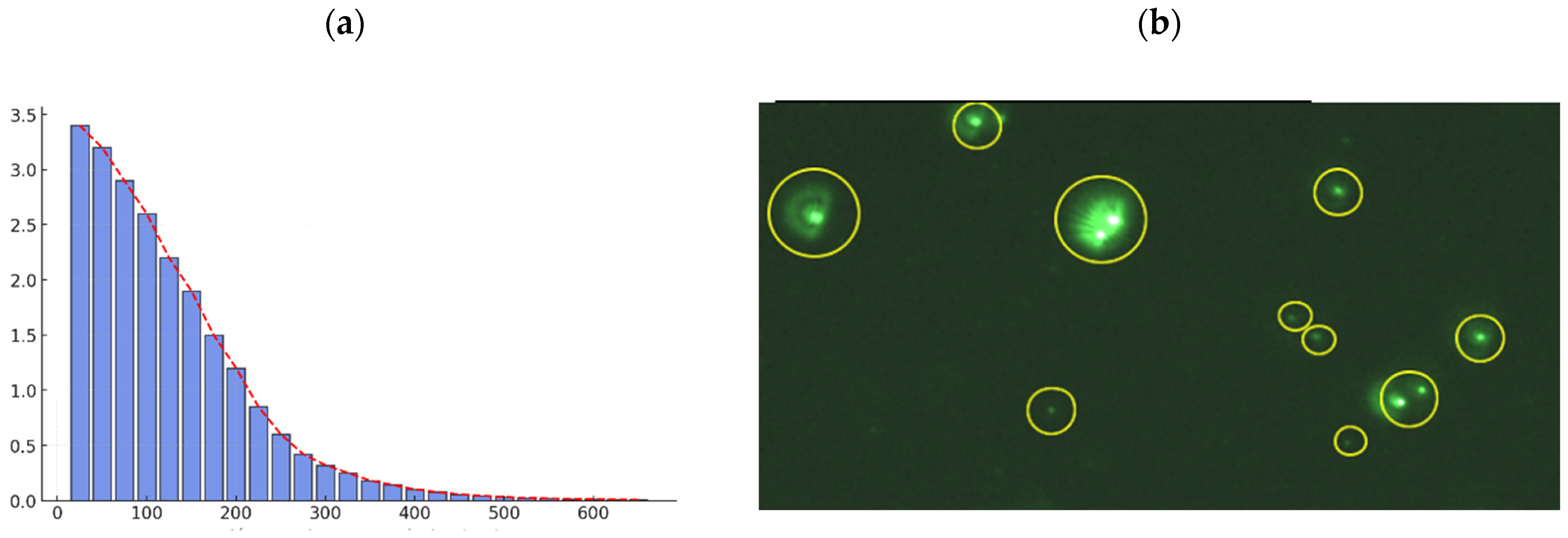
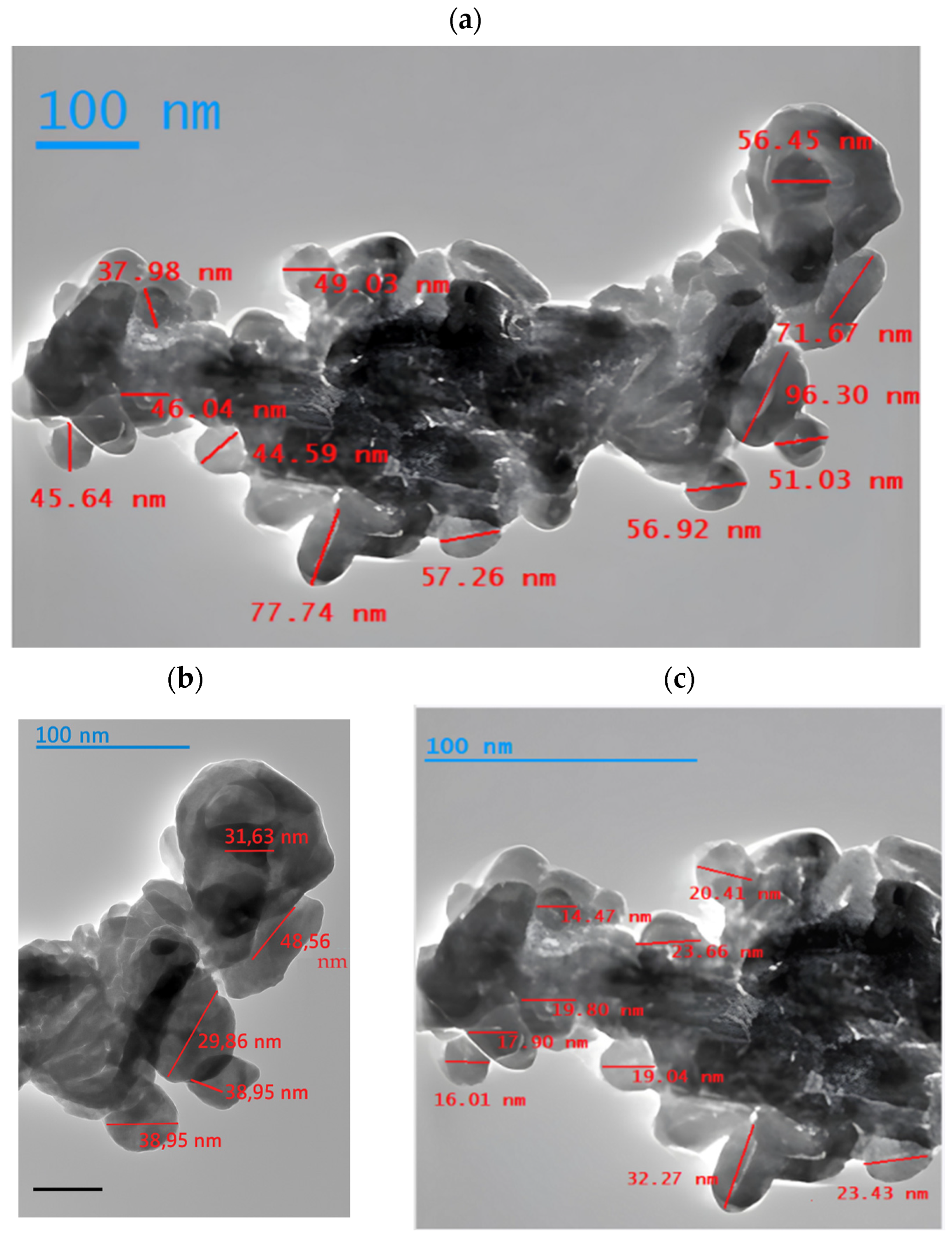
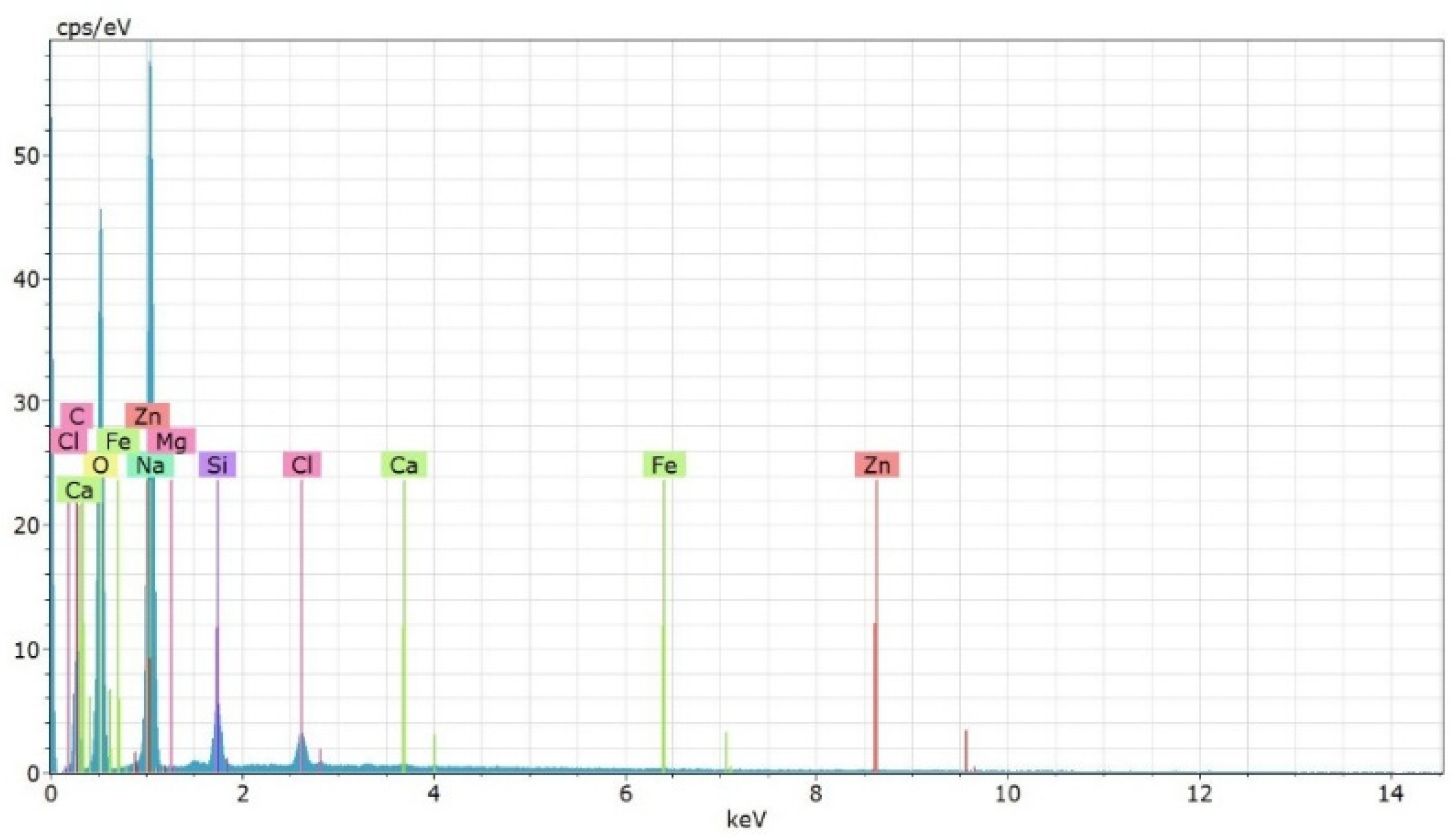
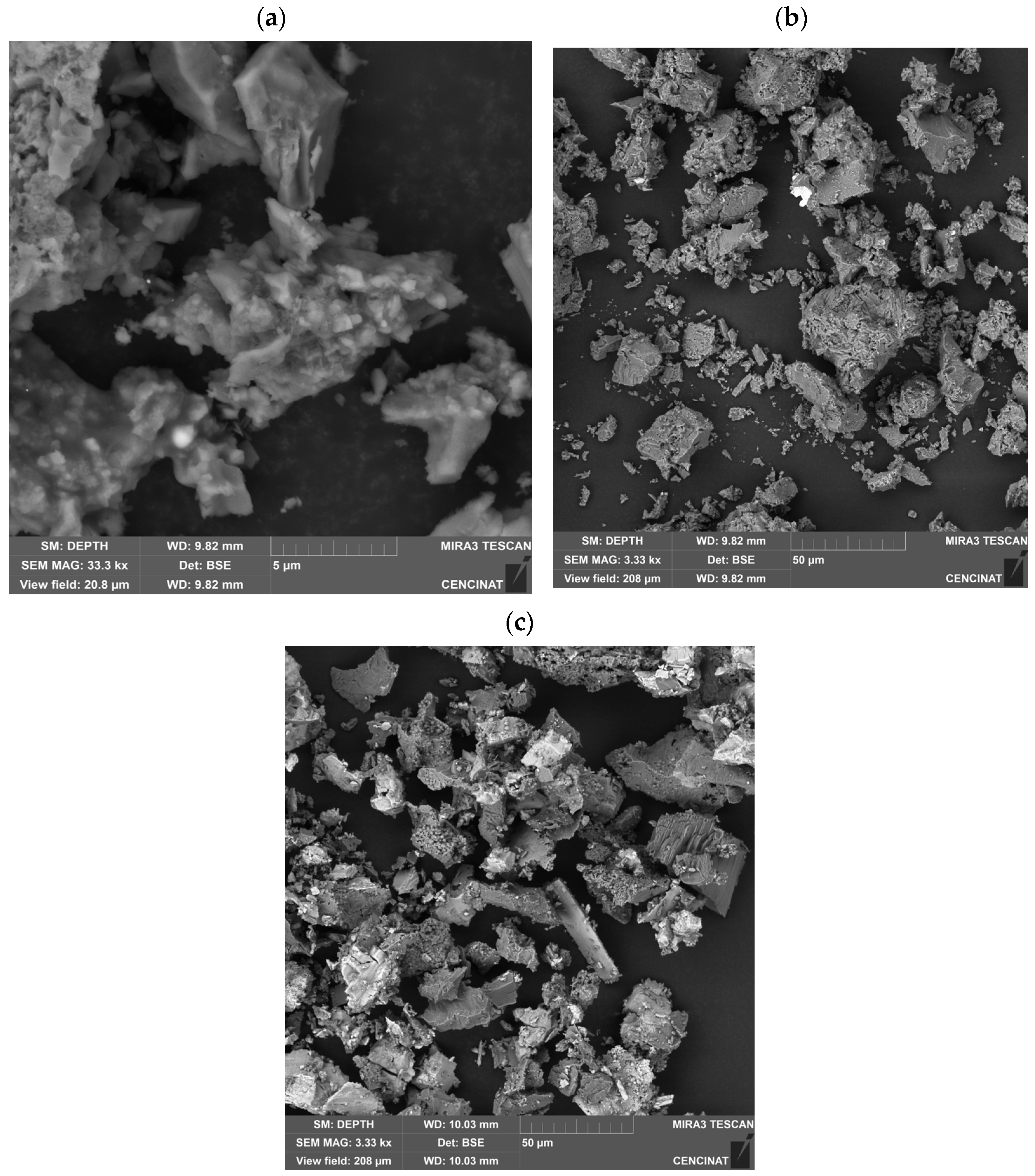
3.4. Characterization of Multicomponent Nanoparticles
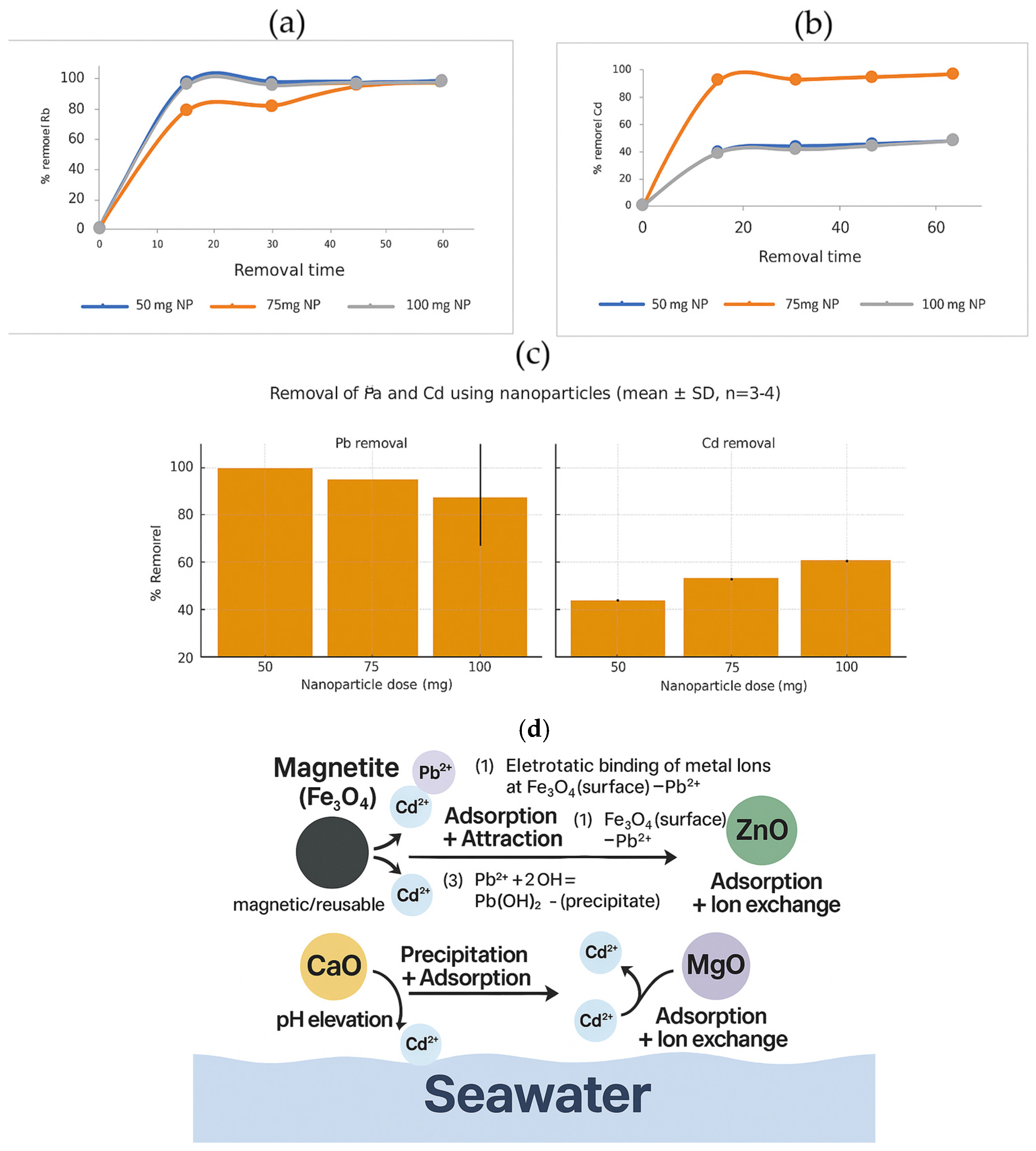
3.5. Removal of Cadmium and Lead from Contaminated Seawater Using Oxide Nanoparticles Synthesized from Sugarcane Bagasse
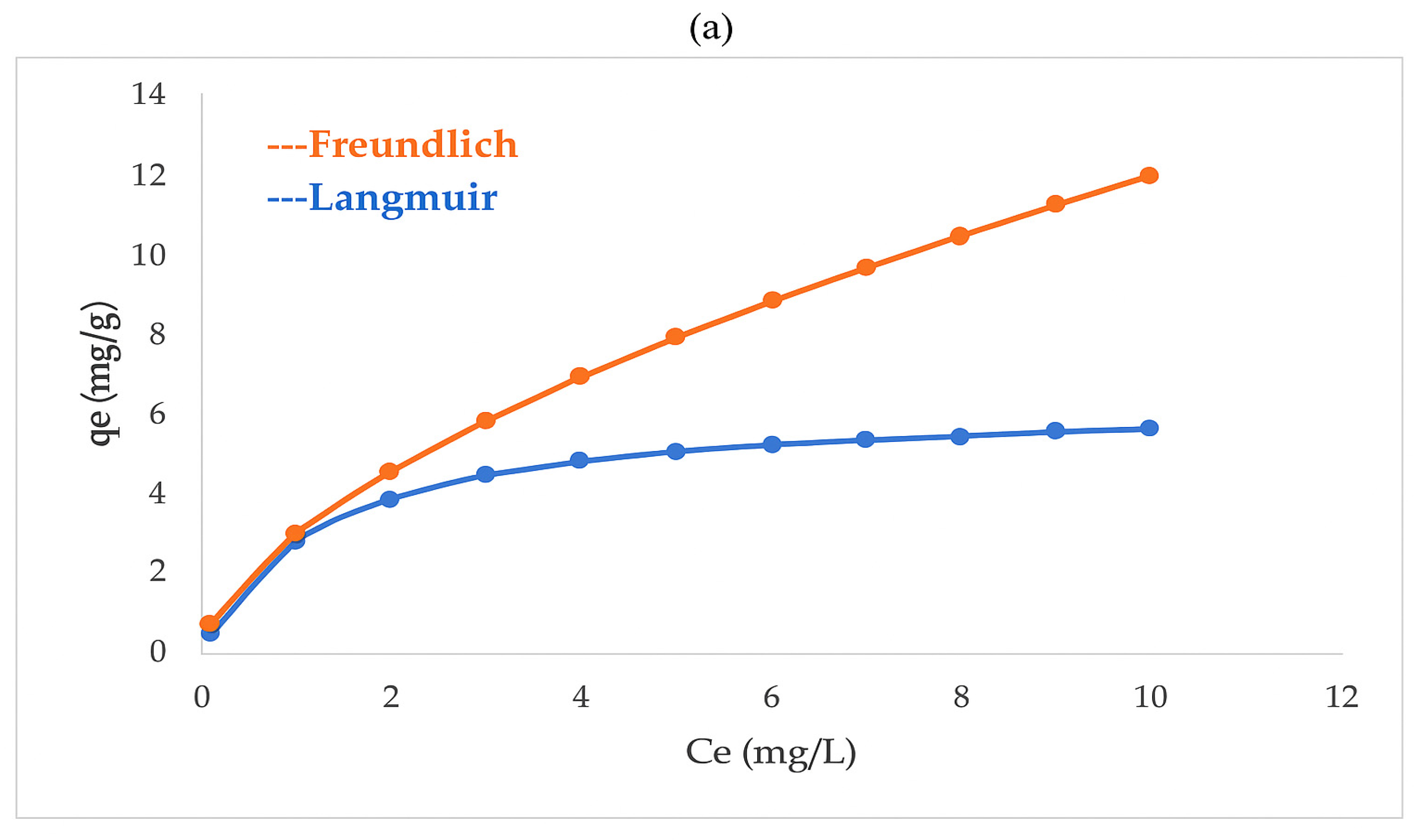
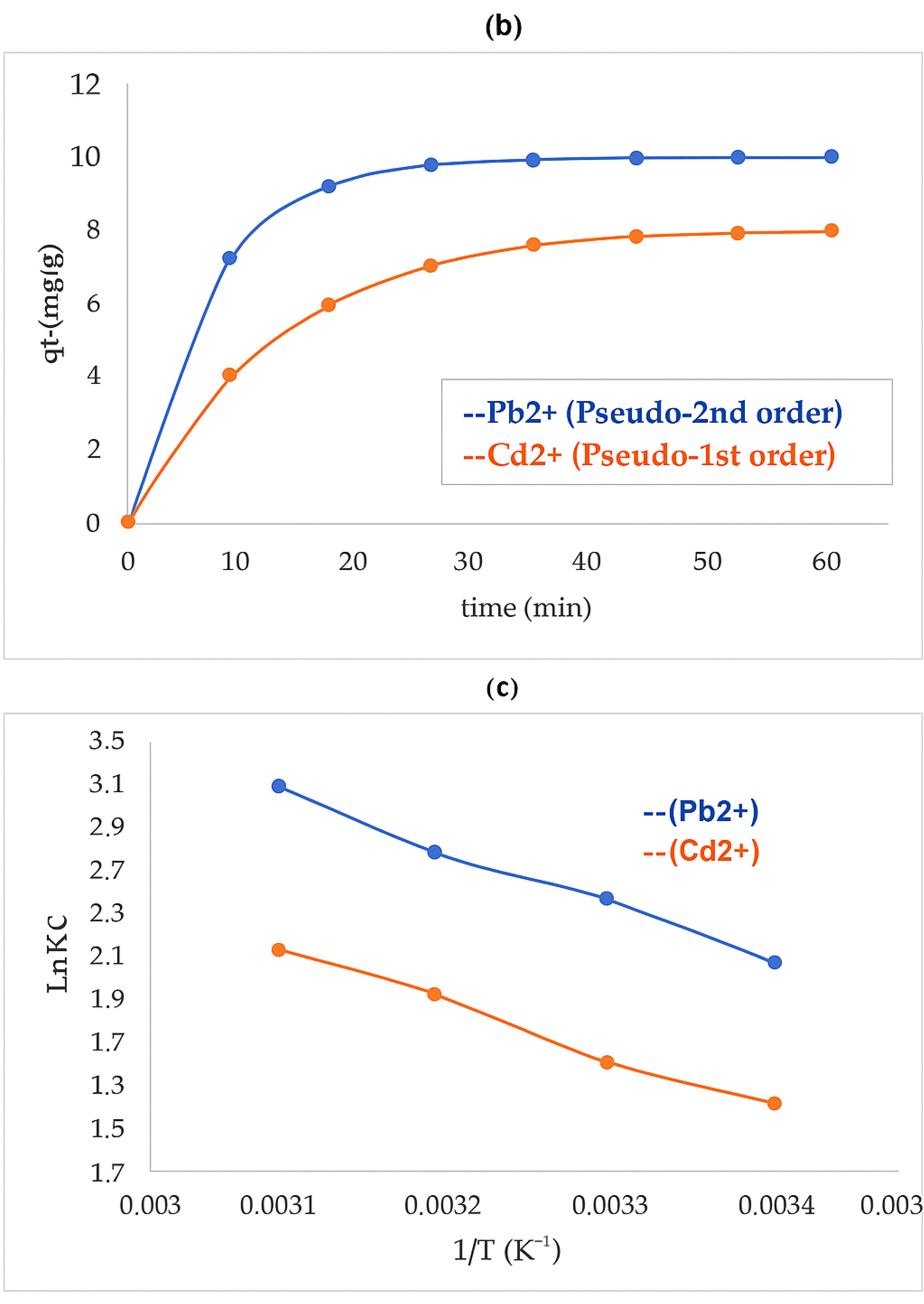
3.6. Adsorption Equilibrium, Kinetic, and Thermodynamic Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garabiza, B.; Prudente, E.; Quinde, K. La aplicación del modelo de economía circular en Ecuador: Estudio de caso. Rev. Espac. 2021, 42, 222–237. [Google Scholar] [CrossRef]
- Elnour, M.G.; Laz, H.A. Tire hazardous, disposal and recycling. J. Appl. Ind. Sci. 2014, 2, 63–74. [Google Scholar]
- Padilla, L.; Díaz, Á.; Anzules, W. Eco-management of end-of-life tires: Advances and challenges for the Ecuadorian case. Waste Manag. Res. 2024, 43, 181–191. [Google Scholar] [CrossRef]
- Karthikeyan, J.; Rupesh, K.; Arumugam, A.; Sudalai, S. Utilization of Waste Tires Toward Concrete Production and Decomposition of Tires by Pyrolysis. In Proceedings of the International Conference on Advances and Innovations in Recycling Engineering, Virtual, 6–7 August 2021; Springer Nature: Singapore, 2021; pp. 81–92. [Google Scholar]
- Martínez, J. An overview of the end-of-life tires status in some Latin American countries: Proposing pyrolysis for a circular economy. Renew. Sustain. Energy Rev. 2021, 144, 4–5. [Google Scholar] [CrossRef]
- ECUADORTIMES. EcuadorTimes.net, Galapagos Is Released from 9,600 Used Tires. Available online: https://ecuadortimes.net/galapagos-is-released-from-9600-used-tires/ (accessed on 24 March 2024).
- Espinosa-Aquino, B.; Durany, X.G.; Vargas, R.Q. The Role of Informal Waste Management in Urban Metabolism: A Review of Eight Latin American Countries. Sustainability 2023, 15, 1826. [Google Scholar] [CrossRef]
- Pabón, S.E.; Benítez, R.; Sarria, R.A.; Gallo, J.A. Contaminación del agua por metales pesados, métodos de análisis y tecnologías de remoción. Entre Cienc. Ing. 2020, 14, 9–18. [Google Scholar] [CrossRef]
- Jeong, Y.; Lee, S.; Woo, S.-H. Chemical Leaching from Tire Wear Particles with Various Treadwear Ratings. Int. J. Environ. Res. Public Health 2022, 19, 6006. [Google Scholar] [CrossRef]
- Kole, P.J.; Löhr, A.J.; Van Belleghem, F.G.A.J.; Ragas, A.M.J. Wear and Tear of Tyres: A Stealthy Source of Microplastics in the Environment. Int. J. Environ. Res. Public Health 2017, 14, 1265. [Google Scholar] [CrossRef] [PubMed]
- Jadaa, W.; Mohammed, H.K. Heavy Metals—Definition, Natural and Anthropogenic Sources of Releasing into Ecosystems, Toxicity, and Removal Methods—An Overview Study. J. Ecol. Eng. 2023, 24, 249–271. [Google Scholar] [CrossRef]
- Malpica, A.C.; De la Cruz Ríos, M. Toxicidad de Metales Pesados en Zooplancton de las Lagunas del Distrito de Chongos alto; Universidad Nacional del Centro de Perú: Huancayo, Peru, 2015. [Google Scholar]
- Torres, P.; Llopis, A.; Melo, C.; Rodrigues, A. Environmental Impact of Cadmium in a Volcanic Archipelago: Research Challenges Related to a Natural Pollution Source. J. Mar. Sci. Eng. 2023, 11, 100. [Google Scholar] [CrossRef]
- Morrow, H. Cadmium and cadmium alloys. In Kirk-Othmer, Encyclopedia of Chemical Technology; Wiley: Hoboken, NJ, USA, 2010; pp. 1–36. [Google Scholar]
- Londoño-Franco, L.F.; Londoño-Muñoz, P.T.; Muñoz-García, F.G. Los riesgos de los metales pesados en la salud humana y animal. Biotecnol. Sect. Agropecu. Agroindustrial 2016, 14, 145–153. [Google Scholar] [CrossRef]
- Wu, D.; Hu, Y.; Cheng, H.; Ye, X. Detection Techniques for Lead Ions in Water: A Review. Molecules 2023, 28, 3601. [Google Scholar] [CrossRef]
- Smail, J.R. What’s in the Ocean? Am. Biol. Teach. 1981, 43, 312–316. [Google Scholar] [CrossRef]
- Jaspal, D.; Malviya, A.; El Allaoui, B.; Zari, N.; Bouhfid, R.; Qaiss, A.K.; Bhagwat, S. Emerging advances of composite membranes for seawater pre-treatment: A review. Water Sci. Technol. 2023, 88, 408–429. [Google Scholar] [CrossRef]
- Peycheva, K.; Stancheva, M.; Georgieva, S.; Makedosnki, L. Heavy Metals in Water, Sediments and Marine Fishes from Bulgarian Black Sea; Medical University of Varna: Varna, Bulgaria, 2017. [Google Scholar]
- Manev, I.; Kirov, V.; Neshovska, H. Heavy metals accumulation in Black Sea ecosystems: Seawater, sediment, algae, benthic organisms. Tradit. Mod. Vet. Med. 2020, 5, 88–99. [Google Scholar]
- Coatu, V.; Ţigănuş, D.; Oros, A.; Lazăr, L. Analysis of hazardous substance contamination of the marine ecosystem in the Romanian Black Sea coast, part of the marine strategy framework directive (2008/56/EEC) implementation. Cercet. Mar. 2013, 43, 174–186. [Google Scholar]
- Cartaya, O.; Reynaldo, I.; Peniche, C. Cinética de adsorción de iones cobre (II) por una mezcla de oligogalacturónidos. Rev. Iberoam. Polim. 2008, 9, 473–479. [Google Scholar]
- Farooq, U.; Kozinski, J.A.; Khan, M.A.; Athar, M. Biosorption of heavy metal ions using wheat based biosorbents—A review of the recent literature. Bioresour. Technol. 2010, 101, 5043–5053. [Google Scholar] [CrossRef] [PubMed]
- Dermont, G.; Bergeron, M.; Mercier, G.; Richer-Laflèche, M. Soil washing for metal removal: A review of physical/chemical technologies and field applications. J. Hazard. Mater. 2008, 152, 1–3. [Google Scholar] [CrossRef]
- Acar, Y.B.; Alshawabkeh, A.N. Principles of electrokinetic remediation. Environ. Sci. Technol. 1993, 27, 2638–2647. [Google Scholar] [CrossRef]
- Abaidoo, R.C.; Keraita, B.; Drechsel, P.; Maxwell, P.D.A.A.S. Soil Biology and Agriculture in the Tropics; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010; Volume 21, pp. 280–281. [Google Scholar]
- Dixit, R.; Wasiullah, D.; Malaviya, D.; Pandiyan, K.; Singh, U.B.; Sahu, A.; Shukla, R.; Singh, B.P.; Rai, J.P.; Sharma, P.K.; et al. Bioremediation of Heavy Metals from Soil and Aquatic Environment: An Overview of Principles and Criteria of Fundamental Processes. Sustainability 2015, 7, 2189–2212. [Google Scholar] [CrossRef]
- Zheng, X.-J.; Li, Q.; Peng, H.; Zhang, J.-X.; Chen, W.-J.; Zhou, B.-C.; Chen, M. Remediation of Heavy Metal-Contaminated Soils with Soil Washing: A Review. Sustainability 2022, 14, 13058. [Google Scholar] [CrossRef]
- Sala, L.F.; García, S.I.; González, J.C.; Frascaroli, M.I.; Bellú, S.; Mangiameli, F.; Blanes, P.; Mogetta, M.H.; Andreu, V.; Atria, A.M.; et al. Biosorción para la eliminación de metales pesados en aguas de desecho. An. Real Soc. Española Quim. 2010, 106, 114–120. [Google Scholar]
- Das, N. Recovery of precious metals through biosorption—A review. Hydrometallurgy 2010, 103, 180–189. [Google Scholar] [CrossRef]
- Sabreena, S.; Hassan, S.; Bhat, S.A.; Kumar, V.; Ganai, B.A.; Ameen, F. Phytoremediation of Heavy Metals: An Indispensable Contrivance in Green Remediation Technology. Plants 2022, 11, 1255. [Google Scholar] [CrossRef]
- Testa, G.; Corinzia, S.A.; Cosentino, S.L.; Ciaramella, B.R. Phytoremediation of Cadmium-, Lead-, and Nickel-Polluted Soils by Industrial Hemp. Agronomy 2023, 13, 995. [Google Scholar] [CrossRef]
- Halsband, C.; Sørensen, L.; Booth, A.M.; Herzke, D. Car Tire Crumb Rubber: Does Leaching Produce a Toxic Chemical Cocktail in Coastal Marine Systems? Front. Environ. Sci. 2020, 8, 125. [Google Scholar] [CrossRef]
- Klöckner, P.; Reemtsma, T.; Eisentraut, P.; Braun, U.; Ruhl, A.S.; Wagner, S. Tire and road wear particles in road environment—Quantification and assessment of particle dynamics by Zn determination after density separation. Chemosphere 2019, 222, 714–721. [Google Scholar] [CrossRef]
- Baensch-Baltruschat, B.; Kocher, B.; Stock, F.; Reifferscheid, G. Tyre and road wear particles (TRWP)—A review of generation, properties, emissions, human health risk, ecotoxicity, and fate in the environment. Sci. Total. Environ. 2020, 733, 13782. [Google Scholar] [CrossRef] [PubMed]
- Rajkowska-Myśliwiec, M.; Protasowicki, M.; Witczak, A. The Mobility and Distribution of Lead and Cadmium in the Ecosystems of Two Lakes in Poland and Their Effect on Humans and the Environment. Water 2025, 17, 2255. [Google Scholar] [CrossRef]
- De Silva, M.; Cao, G.; Tam, M. Nanomaterials for the Removal and detection of heavy metals: A review. Environ. Sci. Nano 2025, 12, 2154–2176. [Google Scholar] [CrossRef]
- Qasem, N.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. Nature 2021, 11, 36. [Google Scholar] [CrossRef]
- Khan, S.; Naushad, M.; Al-Gheethi, A.; Iqbal, J. Engineered nanoparticles for removal of pollutants from wastewater: Current status and future prospects of nanotechnology for remediation strategies. J. Environ. Chem. Eng. 2021, 9, 106–160. [Google Scholar] [CrossRef]
- Andrade-Zavaleta, K.; Chacon-Laiza, Y.; Asmat-Campos, D.; Raquel-Checca, N. Green Synthesis of Superparamagnetic Iron Oxide Nanoparticles with Eucalyptus globulus Extract and Their Application in the Removal of Heavy Metals from Agricultural Soil. Molecules 2022, 27, 1367. [Google Scholar] [CrossRef]
- Mohamed, A.; Atta, R.R.; Kotp, A.A.; El-Ela, F.I.A.; El-Raheem, H.A.; Farghali, A.; Alkhalifah, D.H.M.; Hozzein, W.N.; Mahmoud, R. Green synthesis and characterization of iron oxide nanoparticles for the removal of heavy metals (Cd2+ and Ni2+) from aqueous solutions with Antimicrobial Investigation. Sci. Rep. 2023, 13, 7277. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef]
- Patsula, V.; Moskvin, M.; Dutz, S.; Horák, D. Size-dependent magnetic properties of iron oxide nanoparticles. J. Phys. Chem. Solids 2016, 88, 24–30. [Google Scholar] [CrossRef]
- Contreras, A.M. Nanopartículas de Sílice Obtenidas a Partir del Bagazo de Caña Para la Desinfección de Escherichia Coli y Pseudomona aeruginosa; Universidad de los Andes: Bogota, Columbia, 2021; pp. 6–8. [Google Scholar]
- Seroka, N.; Taziwa, R.; Khotseng, L. Extraction and Synthesis of Silicon Nanoparticles (SiNPs) from Sugarcane Bagasse Ash: A Mini-Review. Appl. Sci. 2022, 12, 2310. [Google Scholar] [CrossRef]
- Ajala, E.O.; Ighalo, J.O.; Ajala, M.A.; Adeniyi, A.G.; Ayanshola, A.M. Sugarcane bagasse: A biomass sufficiently applied for improving global energy, environment and economic sustainability. Bioresour. Bioprocess. 2021, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- García-Zaldívar, O.; Albuerne-Torres, S.; Álvarez-Delgado, A.; Brown-Gómez, A.; Aranda, P.; Ruiz-Hitzky, E.; González, Y. Modification of Sugarcane Bagasse Derivatives with Superparamagnetic Iron Oxide Nanoparticles for the Extraction of Hydrocarbons. Rev. Cuba. Física 2023, 40, 78–84. [Google Scholar]
- Abdelhamid, H.N.; Mathew, A.P. Cellulose-Based Materials for Water Remediation: Adsorption, Catalysis, and Antifouling. Front. Chem. Eng. 2021, 3, 790314. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef]
- Murgueitio Herrera, E.; Jacome, G.; Stael, C.; Arroyo, G.; Izquierdo, A.; Debut, A.; Delgado, P.; Montalvo, G. Green Synthesis of Metal Nanoparticles with Borojó (Borojoa patinoi) Extracts and Their Application in As Removal in Water Matrix. Nanomaterials 2024, 14, 1526. [Google Scholar] [CrossRef]
- Do, Q.; Angkawijaya, A.; Tran-Nguyen, P.; Huynh, L.; Soetaredjo, F.; Ismadji, S.; Ju, Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal Processing Enhances the Nutritional Value of Tomatoes by Increasing Total Antioxidant Activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Murgueitio, E.; Izquierdo, A.; Salgado, D.; Cumbal, L. Removal of Phenanthrene from Contaminated Soils by Applying nZVI-Rg. and Subsequently with Biostimulation. In Proceedings of the XV Multidisciplinary International Congress on Science and Technology, Quito, Equador, 19–23 October 2021; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 115–131. [Google Scholar]
- Blois, M. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- AEaton; Clesceri, L.; Grenberg, A. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1995. [Google Scholar]
- Murgueitio, E.; Cumbal, L.; Abril, M.; Izquierdo, A.; Debut, A.; Tinoco, O. Green Synthesis of Iron Nanoparticles: Application on the Removal of Petroleum Oil from Contaminated Water and Soils. J. Nanotechnol. 2018, 1, 4184769. [Google Scholar] [CrossRef]
- Lim, Y.; Lim, T.; Tee, J. Antioxidant properties of several tropical fruits: A comparative study. Food Chem. 2007, 103, 1003–1008. [Google Scholar] [CrossRef]
- Vasco, C.; Ruales, J.; Kamal-Eldin, A. Total phenolic compounds and antioxidant capacities of major fruits from Ecuador. Food Chem. 2008, 111, 816–823. [Google Scholar] [CrossRef]
- Canilha, L.; Santos, V.T.O.; Rocha, G.J.M.; e Silva, J.B.A.; Giulietti, M.; Silva, S.S.; Felipe, M.G.A.; Ferraz, A.; Milagres, A.M.F.; Carvalho, W. A study on the pretreatment of a sugarcane bagasse sample with dilute sulfuric acid. J. Ind. Microbiol. Biotechnol. 2011, 38, 1467–1475. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.; Kim, T. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 42, 199. [Google Scholar] [CrossRef]
- Li, M.; Pu, Y.; Ragauskas, A.J. Current Understanding of the Correlation of Lignin Structure with Biomass Recalcitrance. Front. Chem. 2016, 45, 4. [Google Scholar] [CrossRef] [PubMed]
- Mood, S.H.; Golfeshan, A.H.; Tabatabaei, M.; Jouzani, G.S.; Najafi, G.H.; Gholami, M.; Ardjmand, M. Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew. Sustain. Energy Rev. 2013, 27, 77–93. [Google Scholar] [CrossRef]
- Heuzé, V.; Tran, G.; Archimède, H. Sugarcane bagasse. Feedipedia, a Programme by INRAE, CIRAD, AFZ and FAO, Last updated 8 October 2015.
- Bridhikitti, A.; Kaewsuk, J.; Karaket, N.; Somchat, K.; Friend, R.; Sallach, B.; Chong, J.P.J.; Redeker, K.R. Sources and Magnitude of Heavy Metals in Sugarcane Plantation Soils with Different Agricultural Practices and Their Implications on Sustainable Waste-to-Foods Strategy in the Sugar–Ethanol Industry. Sustainability 2023, 15, 14816. [Google Scholar] [CrossRef]
- Waqar, R.; Kaleem, M.; Iqbal, J.; Minhas, L.A.; Haris, M.; Chalgham, W.; Ahmad, A.; Mumtaz, A.S. Kinetic and Equilibrium Studies on the Adsorption of Lead and Cadmium from Aqueous Solution Using Scenedesmus sp. Sustainability 2023, 15, 6024. [Google Scholar] [CrossRef]
- Bouida, L.; Rafatullah, M.; Kerrouche, A.; Qutob, M.; Alosaimi, A.M.; Alorfi, H.S.; Hussein, M.A. A Review on Cadmium and Lead Contamination: Sources, Fate, Mechanism, Health Effects and Remediation Methods. Water 2022, 14, 3432. [Google Scholar] [CrossRef]
- Page, T.; Almeda, R.; Koski, M.; Bournaka, E.; Nielsen, T. Toxicity of tyre wear particle leachates to marine phytoplankton. Aquat. Toxicol. 2022, 252, 106299. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Zhao, Y.; Pan, Y.; Zhang, Z.; Liu, S.; Chen, X.; Zhang, J.; Wu, T. Tire wear particles in the marine environment: Sources, migration, ecological risk and control strategy. Front. Mar. Sci. 2025, 12, 1668826. [Google Scholar] [CrossRef]
- Praipipat, P.; Ngamsurach, P.; Sanghuayprai, A. Modification of sugarcane bagasse with iron(III) oxide-hydroxide to improve its adsorption property for removing lead(II) ions. Sci. Rep. 2023, 13, 1467. [Google Scholar] [CrossRef]
- Lim, J.; Yeap, S.P.; Che, H.X.; Low, S.C. Characterization of magnetic nanoparticle by dynamic light scattering. Nanoscale Res. Lett. 2013, 8, 381. [Google Scholar] [CrossRef] [PubMed]
- Bagbi, Y.; Sarswat, A.; Mohan, D.; Pandey, A.; Solanki, P.R. Lead and Chromium Adsorption from Water using L-Cysteine Functionalized Magnetite (Fe3O4) Nanoparticles. Sci. Rep. 2017, 7, 7672. [Google Scholar] [CrossRef] [PubMed]
- Campaña, A.; Guillén, A.; Rivas, R.; Akle, V.; Cruz, J.; Osma, J. Functionalization and Evaluation of Inorganic Adsorbents for the Removal of Cadmium in Wastewater. Molecules 2021, 26, 4150. [Google Scholar] [CrossRef] [PubMed]
- Ehrampoush, M.; Miria, M.; Salmani, M.; Mahvi, A. Cadmium removal from aqueous solution by green synthesis iron oxide nanoparticles with tangerine peel extract. J. Environ. Health Sci. Eng. 2015, 13, 84. [Google Scholar] [CrossRef]
- Stoian, O.; Covaliu, C.I.; Paraschiv, G.; (Traistaru), G.-A.C.; Niță-Lazăr, M.; Matei, E.; Biriş, S.Ș.; Tudor, P. Magnetite Oxide Nanomaterial Used for Lead Ions Removal from Industrial Wastewater. Materials 2021, 14, 2831. [Google Scholar] [CrossRef]
- Zhang, Z.; Kong, J. Novel magnetic Fe3O4@C nanoparticles as adsorbents for removal of organic dyes from aqueous solution. J. Hazard. Mater. 2011, 193, 325–329. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-Ray Diffraction, 3rd ed.; Pearson: London, UK, 2014. [Google Scholar]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.; Zolfaghari, B. Green synthesis of metal nanoparticles using plants. Green Chem. 2014, 13, 2638–2650. [Google Scholar] [CrossRef]
- Cornell, R.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Özgür, U.; Alivov, Y.; Liu, C.; Teke, A.; Reshchikov, M.; Doğan, S.; Morkoc, A. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 41301. [Google Scholar] [CrossRef]
- Gražulis, S.; Daškevič, A.; Merkys, A.; Chateigner, D.; Lutterotti, Q.; Quiros, M.; Le Bail, A. Crystallography Open Database (COD): An open-access collection of crystal structures and platform for world-wide collaboration. Nucleic Acids Res. 2012, 40, D420–D427. [Google Scholar] [CrossRef]
- International Centre for Diffraction Data (ICDD). Powder Diffraction File (PDF-4+ Database); ICDD: Newtown Square, PA, USA, 2024. [Google Scholar]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Iwuozor, K.O.; Ogunfowora, L.A.; Oyekunle, I.P. Review on Sugarcane-Mediated Nanoparticle Synthesis: A Green Approach. Sugar Tech 2021, 24, 1186–1197. [Google Scholar] [CrossRef]
- Toledo-Jaldin, H.P.; Sánchez-Mendieta, V.; Blanco-Flores, A.; López-Téllez, G.; Vilchis-Nestor, A.R.; Martín-Hernández, O. Low-cost sugarcane bagasse and peanut shell magnetic-composites applied in the removal of carbofuran and iprodione pesticides. Environ. Sci. Pollut. Res. 2020, 27, 7872–7885. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.T.T.; Milani, P.A.; Consonni, J.L.; Labuto, G.; Carrilho, E.N.V.M. Nanomodified sugarcane bagasse biosorbent: Synthesis, characterization, and application for Cu(II) removal from aqueous medium. Environ. Sci. Pollut. Res. 2020, 28, 24744–24755. [Google Scholar] [CrossRef] [PubMed]
- Yew, Y.P.; Shameli, K.; Miyake, M.; Kuwano, N.; Khairudin, N.B.B.A.; Mohamad, S.E.B.; Lee, K.X. Green Synthesis of Magnetite (Fe3O4) Nanoparticles Using Seaweed (Kappaphycus alvarezii) Extract. Nanoscale Res. Lett. 2016, 11, 276. [Google Scholar] [CrossRef]
- Rezende, C.; de Medeiros, E.; de Oliveira, L.; de Jesus Neto, A. Chemical and morphological characterization of sugarcane bagasse submitted to diluted acid and alkaline pretreatment. Biotechnol. Biofuels 2011, 5, 36. [Google Scholar]
- Hartmann, M.; Blouin, S.; Misof, B.; Fratzl-Zelman, N.; Roschger, P.; Berzlanovich, A.; Gruber, G.M.; Brugger, P.C.; Zwerina, J.; Fratzl, P. Quantitative Backscattered Electron Imaging of Bone Using a Thermionic or a Field Emission Electron Source. Calcif. Tissue Int. 2021, 109, 190–202. [Google Scholar] [CrossRef]
- Wystalska, K.; Kowalczyk, M.; Kamizela, T.; Worwąg, M.; Zabochnicka, M. Properties and Possibilities of Using Biochar Composites Made on the Basis of Biomass and Waste Residues Ferryferrohydrosol Sorbent. Materials 2024, 17, 2646. [Google Scholar] [CrossRef] [PubMed]
- Ford, R.; Wilkin, R.; Puls, R. Monitored Natural Attenuation of Inorganic Contaminants in Ground Water; Naiontal Risk Management Research Laboratory, US Environmental Protection Agency: Washington, DC, USA, 2007; Volume 2.
- Mensah, M.; Lewis, D.; Boadi, N.; Awudza, J. Heavy metal pollution and the role of inorganic nanomaterials in environmental remediation. R. Soc. Open Sci. 2021, 8, 10. [Google Scholar] [CrossRef]
- Wang, P.; Shen, X.; Qiu, S.; Zhang, L.; Ma, Y.; Liang, J. Clay-Based Materials for Heavy Metals Adsorption: Mechanisms, Advancements, and Future Prospects in Environmental Remediation. Crystals 2024, 14, 1046. [Google Scholar] [CrossRef]
- Zheng, K.; Guang, Z.; Wang, Z.; Liu, Y.; Cheng, X.; Liu, Y. Robust Adsorption of Pb(II) and Cd(II) by GLDA-Intercalated ZnAl-LDH: Structural Engineering, Mechanistic Insights, and Environmental Applications. Coatings 2025, 15, 613. [Google Scholar] [CrossRef]
- Bazarkina, E.; Pokrovski, G.; Zotov, A.; Hazemann, J. Structure and stability of cadmium chloride complexes in hydrothermal fluids. Chem. Geol. 2010, 276, 1–17. [Google Scholar] [CrossRef]
- Byrne, R. Inorganic speciation of dissolved elements in seawater: The influence of pH on concentration ratios. Geochem. Trans. 2002, 3, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Naeem, A.; Ahmad, I.; Fozia, F.; Almutairi, M.H.; Aslam, M.; Israr, M.; Almutairi, B.O.; Ullah, Z. Synthesis of Plant-Mediated Iron Oxide Nanoparticles and Optimization of Chemically Modified Activated Carbon Adsorbents for Removal of As, Pb, and Cd Ions from Wastewater. ACS Omega 2023, 9, 317–329. [Google Scholar] [CrossRef]
- Singh, V.; Singh, N.; Rai, S.N.; Kumar, A.; Singh, A.K.; Singh, M.P.; Sahoo, A.; Shekhar, S.; Vamanu, E.; Mishra, V. Heavy Metal Contamination in the Aquatic Ecosystem: Toxicity and Its Remediation Using Eco-Friendly Approaches. Toxics 2023, 11, 147. [Google Scholar] [CrossRef]
- Alswat, A.; Ashmali, A.; Alqasmi, T.; Alhassani, H.; Alshorifi, F. Role of nanohybrid NiO–Fe3O4 in enhancing the adsorptive performance of activated carbon synthesized from Yemeni-Khat leave in removal of Pb (II) and Hg (II) from aquatic systems. Heliyon 2023, 9, e14301. [Google Scholar] [CrossRef]
- Ding, H.; Liu, J.; Li, Q.; Liu, Z.; Xia, K.; Hu, L.; Wu, X.; Yan, Q. Highly effective adsorption and passivation of Cd from wastewater and soil by MgO- and Fe3O4-loaded biochar nanocomposites. Front. Environ. Sci. 2023, 11, 1239842. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Yoon, M. Rapid removal of cadmium ions using green-synthesized Fe3O4 nanoparticles capped with diethyl-4-(4 amino-5-mercapto-4H-1,2,4-triazol-3-yl)phenyl phosphonate. RSC Adv. 2015, 5, 65444–65453. [Google Scholar] [CrossRef]
- Kataria, N.; Garg, V. Green synthesis of Fe3O4 nanoparticles loaded sawdust carbon for cadmium (II) removal from water: Regeneration and mechanism. Chemosphere 2018, 172, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Hassan, P.; Rasheed, R.; Zargoosh, K. Cadmium and Lead Removal from Aqueous Solution Using Magnetite Nanoparticles Biofabricated from Portulaca oleracea Leaf Extract. J. Nanomater. 2022, 2022, 1024554. [Google Scholar] [CrossRef]
- Safari, M.; Rezaee, R.; Soltani, R. Dual immobilization of magnetite nanoparticles and biosilica within alginate matrix for the adsorption of Cd(II) from aquatic phase. Sci. Rep. 2022, 12, 11473. [Google Scholar] [CrossRef]
- Rayaroth, M.; Oh, D.; Lee, C.; Chang, Y. Simultaneous removal of heavy metals and dyes in water using a MgO-coated Fe3O4 nanocomposite: Role of micro-mixing effect induced by bubble generation. Chemosphere 2022, 294, 133788. [Google Scholar] [CrossRef]
- Hafez, E.; Alharbi, K.; Gharib, H.; Omara, A.; Elatafi, E.; Hamada, M.M.; Rashwan, E.; Alshaal, T. Synergistic Effect of Sugarcane Bagasse and Zinc Oxide Nanoparticles on Eco-Remediation of Cadmium-Contaminated Saline Soils in Wheat Cultivation. Plants 2024, 14, 85. [Google Scholar] [CrossRef]
- Somyanonthanakun, W.; Greszta, A.; Roberts, A.J.; Thongmee, S. Sugarcane Bagasse-Derived Activated Carbon as a Potential Material for Lead Ions Removal from Aqueous Solution and Supercapacitor Energy Storage Application. Sustainability 2023, 15, 5566. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Afsari Sardari, S.; Afsharnia, M.; Qasemi, M.; Shams, M. Removal of toxic lead from aqueous solution using a low-cost adsorbent. Sci. Rep. 2023, 13, 3278. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Gafaru, I.; Cobbina, S.J.; Michael, K. Green-synthesized magnetic iron oxide nanoparticles for the adsorptive removal of CD2+ and PB2+ from aqueous solution. Discov. Water 2025, 5, 92. [Google Scholar] [CrossRef]
- Raji, Z.; Karim, A.; Karam, A.; Khalloufi, S. Adsorption of Heavy Metals: Mechanisms, Kinetics, and Applications of Various Adsorbents in Wastewater Remediation—A Review. Waste 2023, 1, 775–805. [Google Scholar] [CrossRef]
- Azizi, S.; Mahdavi Shahri, M.; Mohamad, R. Green synthesis of zinc oxide nanoparticles for enhanced adsorption of lead ions from aqueous solutions: Equilibrium, kinetic and thermodynamic studies. Molecules 2017, 22, 831. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, A.O.; Royahu, C.O.; Ahmad, A.; Dele-Afolabi, T.T.; Alshammari, M.B.; Imteaz, M. A novel oyster shell biocomposite for the efficient adsorptive removal of cadmium and lead from aqueous solution: Synthesis, process optimization, modelling and mechanism studies. PLoS ONE 2024, 19, e0294286. [Google Scholar] [CrossRef]
- Suleiman, M.; El-Sheikh, S.; Mohamed, E.; El Raey, M.; El Sherbiny, S.; Morsy, F.; El-Hout, S.; Sheta, S. Green synthesis of ZnO-NPs using sugarcane bagasse waste: Phytochemical assessment of extract and biological study of nanoparticles. Dalton Trans. 2024, 53, 18494–18505. [Google Scholar] [CrossRef]
- Alanazi, A.; Habila, M.; Al Othman, Z.; Badjah-Hadj-Ahmed, A. Synthesis and Characterization of Zinc Oxide Nanoparticle Anchored Carbon as Hybrid Adsorbent Materials for Effective Heavy Metals Uptake from Wastewater. Crystals 2024, 14, 447. [Google Scholar] [CrossRef]
- Bharti; Jangwan, J.S.; Kumar, S.; Kumar, V.; Kumar, A.; Kumar, D. A review on the capability of zinc oxide and iron oxides nanomaterials, as a water decontaminating agent: Adsorption and photocatalysis. Appl. Water Sci. 2022, 12, 56. [Google Scholar] [CrossRef]


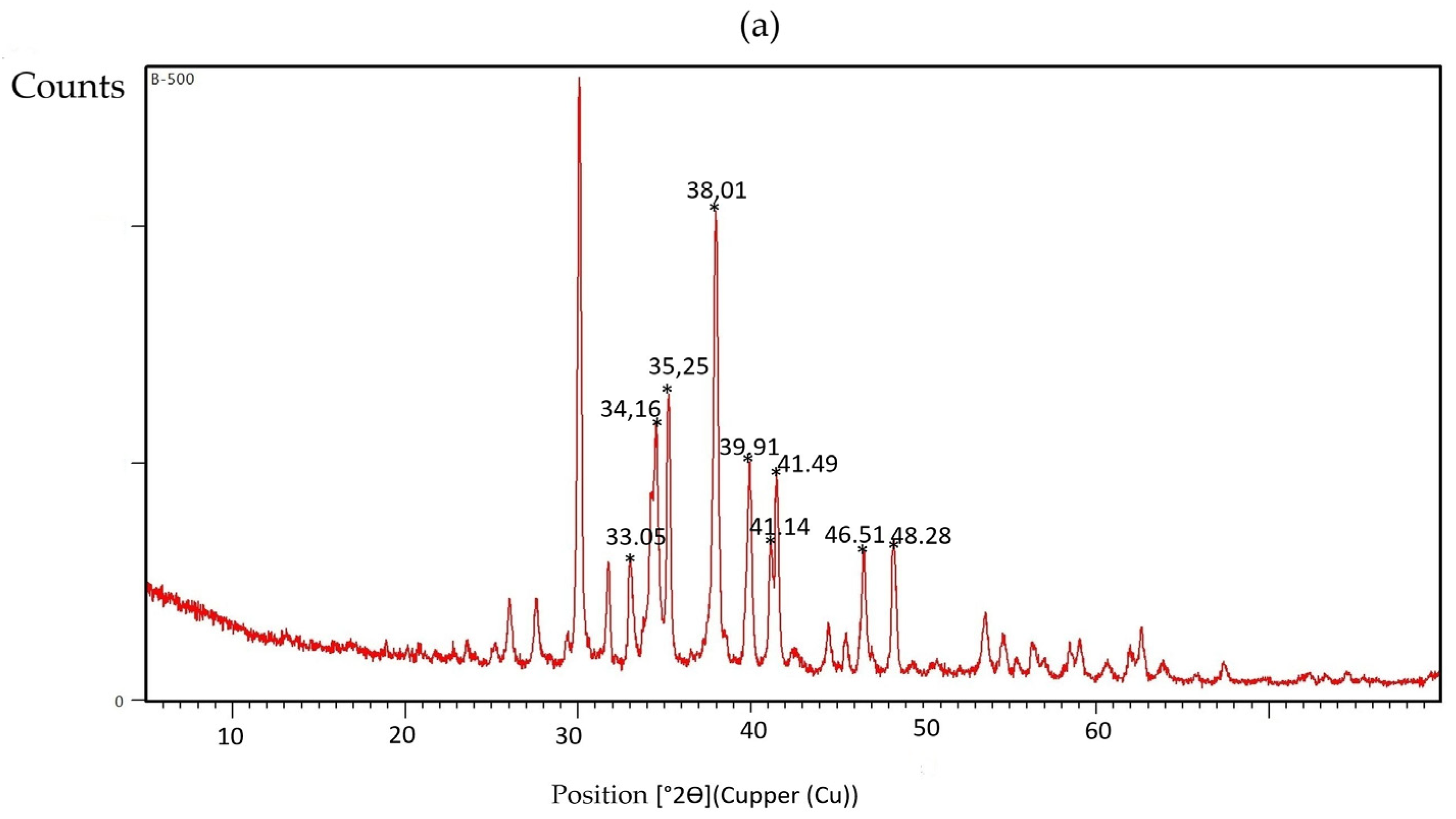
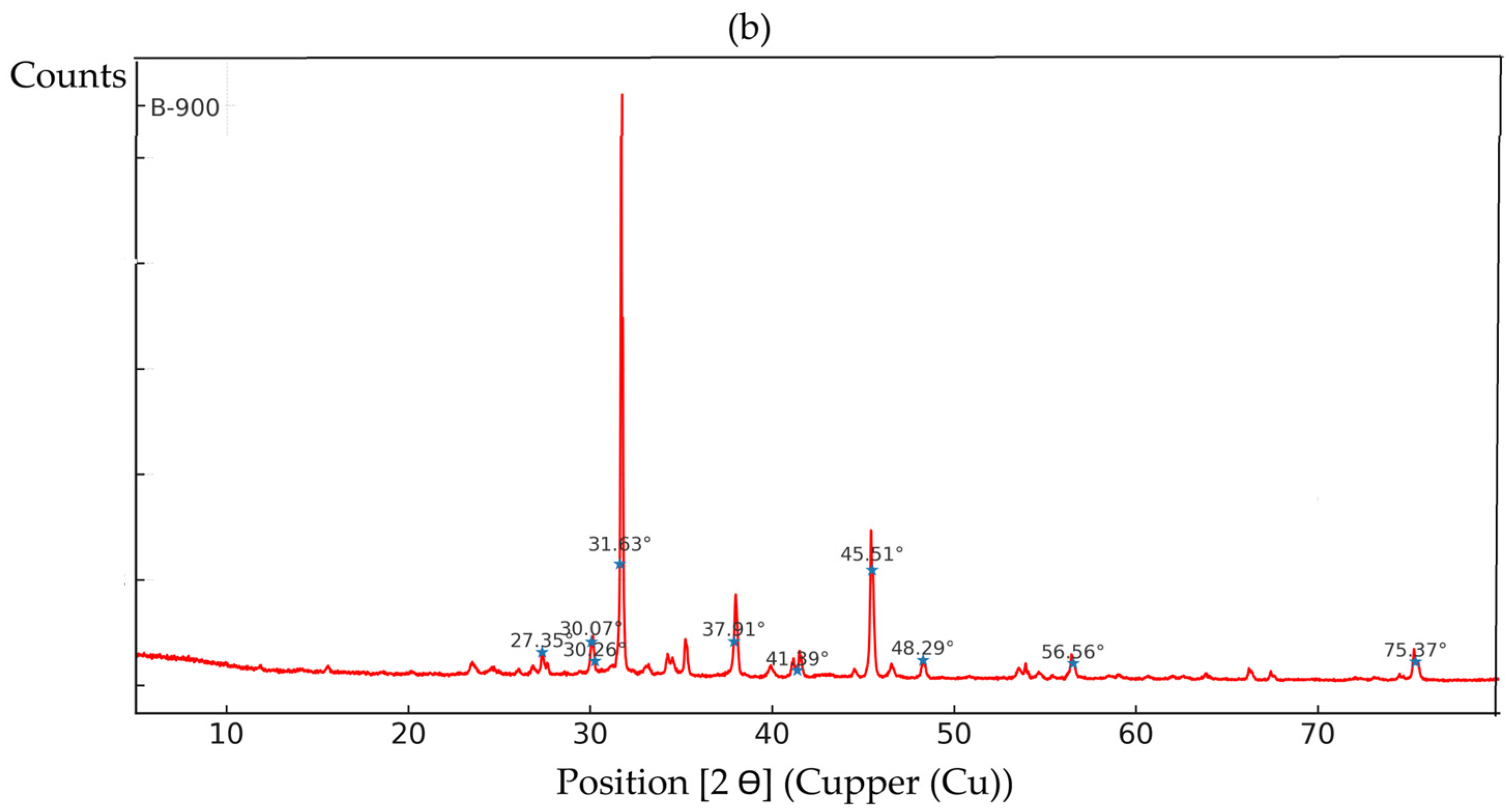
| 2θ (°) | Probable Phase | Typical (hkl) Plane | Composition |
|---|---|---|---|
| ~30.09 | CaCO3 (calcite/aragonite) or Fe3O4 | (104)/(220) | Ca/Fe |
| ~31.75 | ZnO | (100) | Zn |
| ~33.03 | Fe2O3 (hematite) | (104) | Fe |
| ~34.27/34.51 | ZnO/Ca(OH)2/FeO | (002)/(110) | Zn/Ca/Fe |
| ~35.25 | Fe3O4 (magnetite) | (311) | Fe |
| ~38.01 | ZnO/FeO | (101) | Zn/Fe |
| ~39.91 | CaO | (200) | Ca |
| ~41.14/41.49 | CaO/ZnO | — | Ca/Zn |
| ~46.51 | Fe3O4/ZnO | (400)/(102) | Fe/Zn |
| ~48.28 | ZnO | (102) | Zn |
| Element | Norm. wt.% ± 1σ |
|---|---|
| O | 63 ± 8 |
| Na | 34.1 ± 2.3 |
| Si | 1.54 ± 0.09 |
| Cl | 0.94 ± 0.05 |
| Mg | 0.12 ± 0.03 |
| Ca | 0.057 ± 0.022 |
| Fe | 0.027 ± 0.021 |
| Zn | <0.02 (trace) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murgueitio-Herrera, E.; Carpio, P.; Bungacho, P.; Tapia, L.T.; Camacho, C.; Debut, A. Removal of Cadmium and Lead from Tires Discarded in the Open Sea with Multicomponent Nanoparticles from Sugarcane Bagasse. Nanomaterials 2025, 15, 1700. https://doi.org/10.3390/nano15221700
Murgueitio-Herrera E, Carpio P, Bungacho P, Tapia LT, Camacho C, Debut A. Removal of Cadmium and Lead from Tires Discarded in the Open Sea with Multicomponent Nanoparticles from Sugarcane Bagasse. Nanomaterials. 2025; 15(22):1700. https://doi.org/10.3390/nano15221700
Chicago/Turabian StyleMurgueitio-Herrera, Erika, Pablo Carpio, Paola Bungacho, Luis Tipán Tapia, Christian Camacho, and Alexis Debut. 2025. "Removal of Cadmium and Lead from Tires Discarded in the Open Sea with Multicomponent Nanoparticles from Sugarcane Bagasse" Nanomaterials 15, no. 22: 1700. https://doi.org/10.3390/nano15221700
APA StyleMurgueitio-Herrera, E., Carpio, P., Bungacho, P., Tapia, L. T., Camacho, C., & Debut, A. (2025). Removal of Cadmium and Lead from Tires Discarded in the Open Sea with Multicomponent Nanoparticles from Sugarcane Bagasse. Nanomaterials, 15(22), 1700. https://doi.org/10.3390/nano15221700









