N–O–S Co–Doped Hierarchical Porous Carbons Prepared by Mild KOH Activation of Ammonium Lignosulfonate for High–Performance Supercapacitors
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
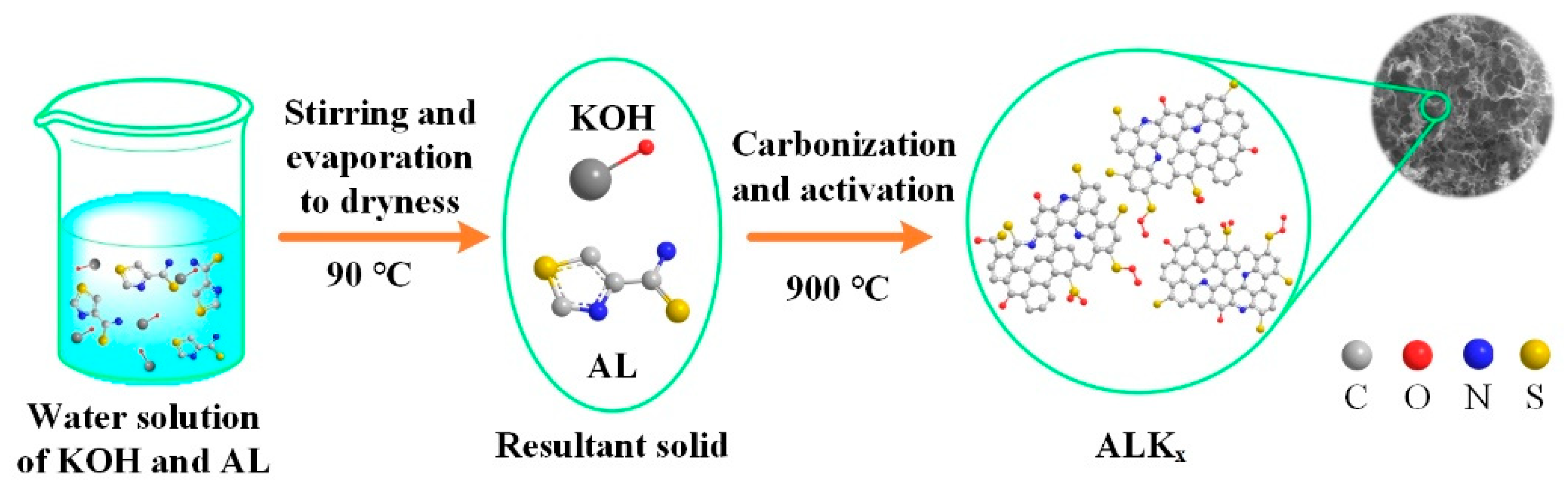
2.2. Preparation of ALKx
2.3. Characterizations
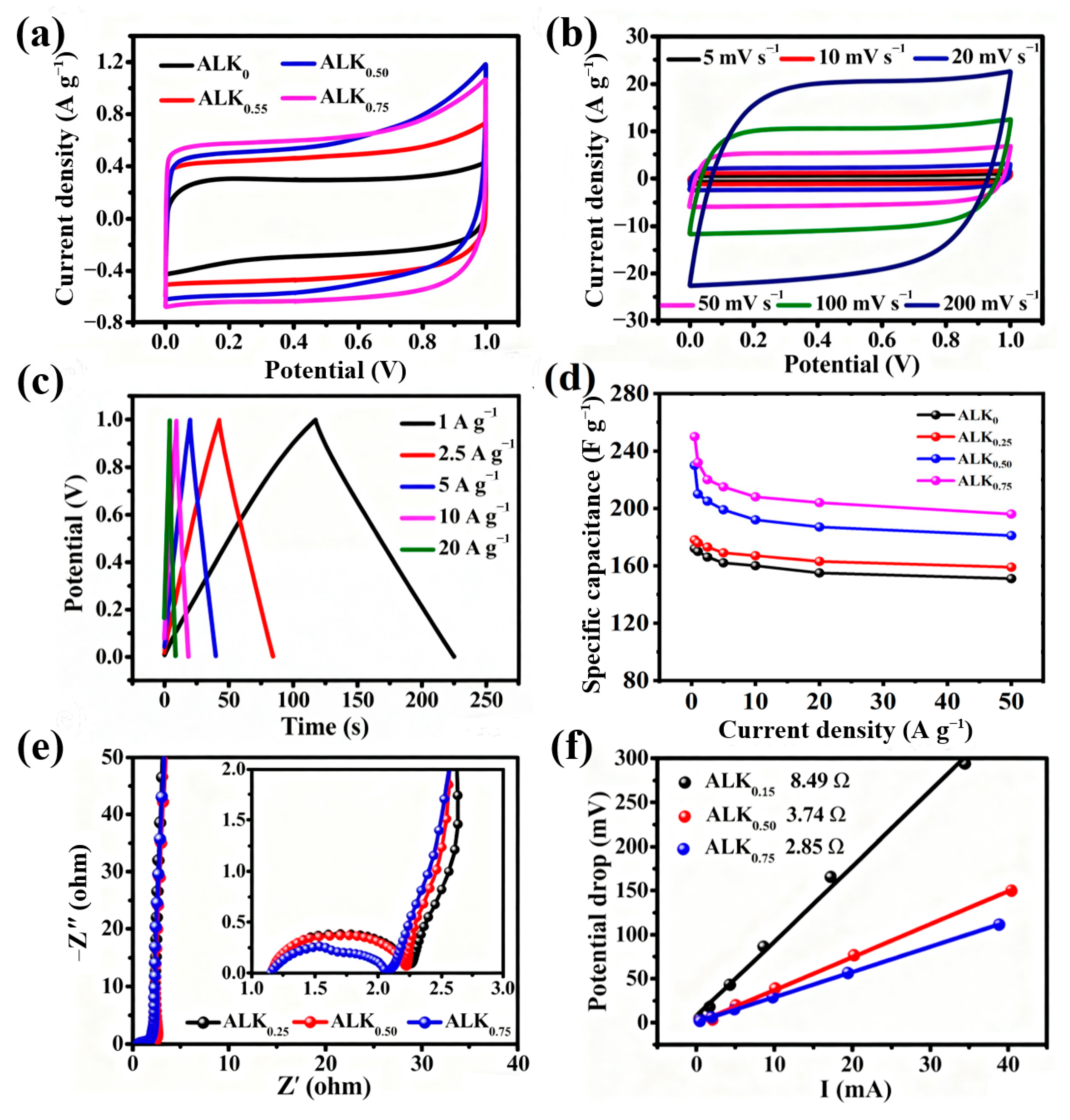
2.4. Electrochemical Measurements
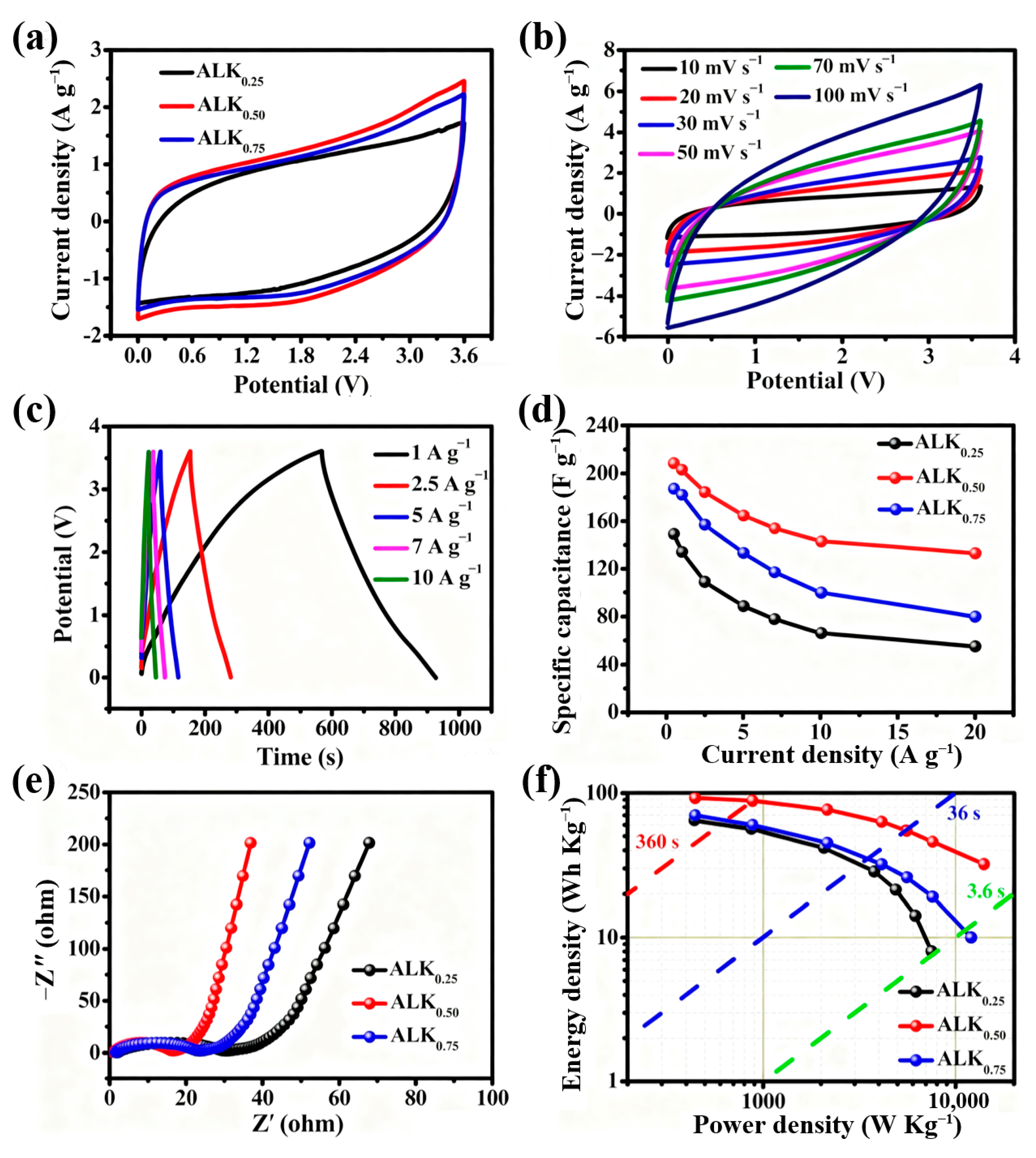
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lian, Y.; Yu, G.Y.; Lu, L.J.; Guo, H.X.; Wang, J.N.; Dai, Y.; Tang, X.Y.; Zhang, H.H. Controllable thickness carbon sheet under anion and cation co-doping for supercapacitors and capacitive deionization. Carbon 2024, 225, 119097. [Google Scholar] [CrossRef]
- You, B.; Jiang, J.H.; Fan, S.J. Three-Dimensional Hierarchically Porous All-Carbon Foams for Supercapacitor. ACS Appl. Mater. Interfaces 2014, 6, 15302–15308. [Google Scholar] [CrossRef]
- Zhai, Z.Z.; Zhang, L.H.; Du, T.M.; Ren, B.; Xu, Y.L.; Wang, S.S.; Miao, J.F.; Liu, Z.F. A review of carbon materials for supercapacitors. Mater. Des. 2022, 221, 19. [Google Scholar] [CrossRef]
- Yang, H.T.; Abdurashid, A.; Jamal, R.; Abdiryim, T.; Wang, X.G.; Song, K.; Zhou, Y.Q.; Liu, Y.J.; Fan, N.N. Boron and nitrogen co-doped carbon nano framework composites for high performance energy storage. Carbon 2025, 235, 120028. [Google Scholar] [CrossRef]
- Anjana, P.M.; Aminabhavi, T.M. Supercapattery: Energy storage devices combining functionalities of battery electrodes and supercapacitor electrodes. J. Energy Storage 2025, 134, 118265. [Google Scholar] [CrossRef]
- Li, W.Q.; Yang, X.Q.; Yuan, C.; Wang, X.; Huo, X.J.; Ye, Y.; Qian, Z.T.; Qin, Z.H. Optimizing the degree of structural disorder in coal-based porous carbon for enhancing supercapacitor capacitance performance. Fuel 2026, 404, 136378. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Jiang, H.M.; Wang, Q.S.; Zheng, J.Q.; Meng, C.G. Kelp-derived three-dimensional hierarchical porous N, O-doped carbon for flexible solid-state symmetrical supercapacitors with excellent performance. Appl. Surf. Sci. 2018, 447, 876–885. [Google Scholar] [CrossRef]
- Wang, M.R.; Liu, B.; Chen, H.B.; Yang, D.G.; Li, H.M. N/O Codoped Porous Carbons with Layered Structure for High-Rate Performance Supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 11219–11227. [Google Scholar] [CrossRef]
- Sun, Y.K.; Xu, D.; He, Z.J.; Zhang, Z.H.; Fan, L.W.; Wang, S.R. Green fabrication of pore-modulated carbon aerogels using a biological template for high-energy density supercapacitors. J. Mater. Chem. A 2023, 11, 20011–20020. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Li, B.Q.; Huang, Y.J.; Wang, Y.M.; Chen, J.C.; Wei, D.Q.; Feng, Y.J.; Jia, D.C.; Zhou, Y. Molten salt synthesis of nitrogen and oxygen enriched hierarchically porous carbons derived from biomass via rapid microwave carbonization for high voltage supercapacitors. Appl. Surf. Sci. 2018, 439, 712–723. [Google Scholar] [CrossRef]
- Zhu, C.W.; Yan, J.J. Fabricating of N/O-codoping porous carbon interpenetrating networks for high energy aqueous supercapacitor. J. Energy Storage 2022, 52, 105047. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Cui, X.; Yang, X.Y.; Jiang, W.; Yuan, Z.Q.; Wan, J.F.; Liu, Y.F.; Ma, F.W. Green synthesis of N/B co-doped layered porous carbon with high gravimetric and volumetric capacitance for supercapacitor. J. Power Sources 2025, 630, 236118. [Google Scholar] [CrossRef]
- Liu, C.F.; Liu, Y.C.; Yi, T.Y.; Hu, C.C. Carbon materials for high-voltage supercapacitors. Carbon 2019, 145, 529–548. [Google Scholar] [CrossRef]
- Singh, P.; Singh, A.; Saini, R.; Deepika; Kulriya, P.; Kumar, R. Advancements in graphene-based nanostructured conducting polymer hybrid composite electrodes for high-performance supercapacitors. J. Power Sources 2025, 630, 236176. [Google Scholar] [CrossRef]
- An, Y.F.; Yang, Y.Y.; Hu, Z.G.; Guo, B.S.; Wang, X.T.; Yang, X.; Zhang, Q.C.; Wu, H.Y. High-performance symmetric supercapacitors based on carbon nanosheets framework with graphene hydrogel architecture derived from cellulose acetate. J. Power Sources 2017, 337, 45–53. [Google Scholar] [CrossRef]
- Maeng, J.; Park, G.H.; Ji, J.; Jang, D.; Pittala, S.; Ha, J.; Bae, H.; Mangishetti, S.R.; Kim, W.B. N-doped carbon nanotube-graphene nanoarchitecture electrodes with solid-state biopolymer electrolyte for high performance flexible supercapacitors. Carbon 2025, 243, 120545. [Google Scholar] [CrossRef]
- Sun, Y.K.; He, Z.J.; Fan, H.Y.; Wang, S.R. Nitrogen configuration modulation of porous graphitic carbon nanosheets for superior capacitive energy storage. J. Power Sources 2024, 614, 235027. [Google Scholar] [CrossRef]
- Liu, Y.J.; Liu, Q.; Li, C.J.; Li, W.Y.; Zhang, B.Y.; Liu, Z.Q.; Ying, A.G. Engineering lateral and vertical heterostructures for supercapacitors with unprecedented energy density. Appl. Surf. Sci. 2023, 609, 11. [Google Scholar] [CrossRef]
- Liang, Y.N.; Guo, J.; Zhang, H.; Brett, D.J.L.; Gadipelli, S. Superior supercapacitor performance with tuneable 2D/3D morphological microporous carbons of zeolitic imidazolate frameworks synthesized by recycling mother liquors. Chem. Eng. J. 2024, 489, 151190. [Google Scholar] [CrossRef]
- Li, Z.M.; Xiao, D.W.; Li, Z.H.; Xu, Z.M.; Dou, H.; Zhang, X.G. Optimizing EMIMBF4-based electrolyte with LiBr redox medium for enhanced supercapacitors. J. Energy Storage 2024, 89, 111735. [Google Scholar] [CrossRef]
- Guan, X.; Li, X.; Zhao, X.; Wang, Z.G.; Zhang, L.L.; Ma, J.X. Recent advances in hierarchical porous carbon materials derived from lignin in black liquor toward high-performance supercapacitors: A review. J. Energy Storage 2025, 111, 115380. [Google Scholar] [CrossRef]
- Miao, X.F.; Chen, J.Y.; Zhong, C.Y.; Tong, J.; Yang, H.Y.; Yang, X.F.; Qiao, X.B.; Song, Z.X.; Zhang, L. Rational Design of Hierarchical Structure Electrodes to Suppress Shuttle Diffusion in Redox-Enhanced Supercapacitors. ACS Appl. Mater. Interfaces 2024, 16, 69303–69315. [Google Scholar] [CrossRef]
- Li, H.J.; Li, Y.Y.; Li, Y.; Shen, H.Y.; Zhu, S.M.; Zhu, Y.Y.; Lian, K. Facile synthesis of heteroatom-doped hierarchical porous carbon with small mesopores for high-performance supercapacitors. J. Energy Storage 2024, 77, 110000. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.P.; Qiu, L.L.; Xiao, J.; Wu, F.P.; Cao, J.P.; Bai, Y.H.; Liu, F.J. Insights into the KOH activation parameters in the preparation of corncob-based microporous carbon for high-performance supercapacitors. Diam. Relat. Mater. 2022, 129, 109331. [Google Scholar] [CrossRef]
- Dai, J.X.; Li, G.C.; Hu, Y.P.; Han, L. Hollow carbon spheres anchored with nitrogen-doped carbon dots for high-performance supercapacitors. J. Energy Storage 2024, 83, 110640. [Google Scholar] [CrossRef]
- Khadem, A.H.; Ul Hasan, T.; Rahman, A.; Smriti, S.A.; Alimuzzaman, S. Fabrication, properties, and performance of graphene-based textile fabrics for supercapacitor applications: A review. J. Energy Storage 2022, 56, 105988. [Google Scholar] [CrossRef]
- Smolin, Y.Y.; Van Aken, K.L.; Boota, M.; Soroush, M.; Gogotsi, Y.; Lau, K.K.S. Engineering Ultrathin Polyaniline in Micro/Mesoporous Carbon Supercapacitor Electrodes Using Oxidative Chemical Vapor Deposition. Adv. Mater. Interfaces 2017, 4, 8. [Google Scholar] [CrossRef]
- Poornima, B.H.; Vijayakumar, T. N, S co-doped and boron doped Artocarpus heterophyllus wood derived activated carbon for supercapacitor application. Electrochim. Acta 2025, 512, 145491. [Google Scholar] [CrossRef]
- Bai, Z.Y.; Yang, F.; Sun, L.J.; Jia, Y.M.; Xie, Y.L. Synthesis and Supercapacitor Performance of Boron-Doped Phenolic Resin-Based Mesoporous Carbon. Chemistryselect 2025, 10, e202404942. [Google Scholar] [CrossRef]
- Jiang, Q.; Cai, Y.Q.; Sang, X.M.; Zhang, Q.X.; Ma, J.; Chen, X.G. Nitrogen-Doped Carbon Materials As Supercapacitor Electrodes: A Mini Review. Energy Fuels 2024, 38, 10542–10559. [Google Scholar] [CrossRef]
- Tong, Q.J.; Zhang, Z.H.; Luo, Q.T.; Gu, K.; Yang, W.Q. Regulating and Unraveling Electrochemical Behavior of Hierarchically-Densifying Mesoporous Apocynum Carbon for High performance Supercapacitor. Batter. Supercaps 2025, 8, e202400450. [Google Scholar] [CrossRef]
- Kraiwattanawong, K. A review on the development of a porous carbon-based as modeling materials for electric double layer capacitors. Arab. J. Chem. 2022, 15, 103625. [Google Scholar] [CrossRef]
- Su, H.; Huang, H.C.; Zhao, S.L.; Zhou, Y.H.; Xu, S.M.; Pan, H.; Gu, B.N.; Chu, X.; Deng, W.; Zhang, H.P.; et al. Understanding the Ion-Sorption Dynamics in Functionalized Porous Carbons for Enhanced Capacitive Energy Storage. ACS Appl. Mater. Interfaces 2020, 12, 2773–2782. [Google Scholar] [CrossRef] [PubMed]
- Farma, R.; Yunita, A.; Apriyani, I.; Awitdrus, A.; Deraman, M.; Omar, F.S.; Shamsudin, S.A.; Othman, M.A.R. Coin-Shaped Carbon Prepared with Cerbera manghas Fibers via Multistage Carbonization and Activation for High-Performance Symmetrical Supercapacitors. Chem. Eng. Technol. 2024, 47, 1071–1081. [Google Scholar] [CrossRef]
- Liu, H.; Liu, R.M.; Xu, C.; Ren, Y.M.; Tang, D.X.; Zhang, C.G.; Li, F.; Wei, X.L.; Zhang, R.L. Oxygen-nitrogen-sulfur self-doping hierarchical porous carbon derived from lotus leaves for high-performance supercapacitor electrodes. J. Power Sources 2020, 479, 228799. [Google Scholar] [CrossRef]
- Zhao, G.Y.; Chen, C.; Yu, D.F.; Sun, L.; Yang, C.H.; Zhang, H.; Sun, Y.; Besenbacher, F.; Yu, M. One-step production of O-N-S co-doped three-dimensional hierarchical porous carbons for high-performance supercapacitors. Nano Energy 2018, 47, 547–555. [Google Scholar] [CrossRef]
- Yang, W.; Yang, W.; Song, A.L.; Gao, L.J.; Su, L.; Shao, G.J. Supercapacitance of nitrogen-sulfur-oxygen co-doped 3D hierarchical porous carbon in aqueous and organic electrolyte. J. Power Sources 2017, 359, 556–567. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Han, M.; Wang, B.; Li, Y.B.; Lei, L.Y.; Wang, K.J.; Wang, Y.; Zhang, L.; Feng, H.X. Superior supercapacitors based on nitrogen and sulfur co-doped hierarchical porous carbon: Excellent rate capability and cycle stability. J. Power Sources 2017, 358, 112–120. [Google Scholar] [CrossRef]
- Ilnicka, A.; Skorupska, M.; Szkoda, M.; Zarach, Z.; Lukaszewicz, J.P. N-doped carbon materials as electrodes for highly stable supercapacitors. Mater. Res. Lett. 2023, 11, 213–221. [Google Scholar] [CrossRef]
- Xiao, X.; Song, L.; Wang, Q.L.; Wang, Z.C.; Wang, H.Y.; Chu, J.C.; Liu, J.M.; Liu, X.R.; Bian, Z.T.; Zhao, X.X. Hierarchical hollow-tubular porous carbon microtubes prepared via a mild method for supercapacitor electrode materials with high volumetric capacitance. RSC Adv. 2022, 12, 16257–16266. [Google Scholar] [CrossRef]
- Padilla-Martinez, E.D.; Sánchez-Rodriguez, C.E.; Rios-González, J.A.; López-Sandoval, R. Effect of KOH impregnation conditions on the porosity and capacitance of coconut fiber-derived activated carbons. J. Phys. Chem. Solids 2026, 208, 113030. [Google Scholar] [CrossRef]
- Gabryelczyk, A.; Yadav, S.; Swiderska-Mocek, A.; Altaee, A.; Lota, G. From waste to energy storage: Calcinating and carbonizing chicken eggshells into electrode materials for supercapacitors and lithium-ion batteries. RSC Adv. 2023, 13, 24162–24173. [Google Scholar] [CrossRef] [PubMed]
- Nazhipkyzy, M.; Kurmanbayeva, G.; Seitkazinova, A.; Varol, E.A.; Li, W.L.; Dinistanova, B.; Issanbekova, A.; Mashan, T. Activated Carbon Derived from Cucumber Peel for Use as a Supercapacitor Electrode Material. Nanomaterials 2024, 14, 686. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Zhang, W.F.; Zhang, H.; Zhang, J.L.; Zhang, H.M.; Cao, G.P.; Han, M.F.; Yang, Y.S. Sustainable nitrogen-containing hierarchical porous carbon spheres derived from sodium lignosulfonate for high-performance supercapacitors. Carbon 2018, 132, 280–293. [Google Scholar] [CrossRef]
- Chen, C.; Yu, D.F.; Zhao, G.Y.; Du, B.S.; Tang, W.; Sun, L.; Sun, Y.; Besenbacher, F.; Yu, M. Three-dimensional scaffolding framework of porous carbon nanosheets derived from plant wastes for high-performance supercapacitors. Nano Energy 2016, 27, 377–389. [Google Scholar] [CrossRef]
- Wang, D.; Lee, S.H.; Kim, J.; Park, C.B. “Waste to Wealth”: Lignin as a Renewable Building Block for Energy Harvesting/Storage and Environmental Remediation. ChemSusChem 2020, 13, 2807–2827. [Google Scholar] [CrossRef]
- Wang, H.; Niu, H.T.; Wang, H.J.; Wang, W.Y.; Jin, X.; Wang, H.X.; Zhou, H.; Lin, T. Micro-meso porous structured carbon nanofibers with ultra-high surface area and large supercapacitor electrode capacitance. J. Power Sources 2021, 482, 228986. [Google Scholar] [CrossRef]
- Huang, G.X.; Liu, Y.B.; Chen, H.; Xing, B.L.; Li, Y.Y.; Yao, Y.H.; Jia, J.B.; Liu, Q.R.; Zhang, C.X. A novel grafting-template method to prepare three-dimensional hierarchical porous carbon with high surface area and electrical conductivity for superior-performance supercapacitors. J. Power Sources 2021, 482, 12. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, W.D.; Fan, X.; Hou, R.R.; Bai, X.; Zhang, X.Y.; Li, Y.; Zhao, G.M.; Liang, P. Porous carbon prepared by thermal dissolution of coal and biomass for high-performance supercapacitors. Fuel 2024, 369, 131677. [Google Scholar] [CrossRef]
- Ma, Z.D.; Wang, W.B.; Bai, Q.H.; Zhou, F.; Ma, C.D.; Shen, Y.H. Three-dimensional nitrogen-doping hierarchically porous carbon derived from polyacrylamide-lignocellulose aerogel for high energy density supercapacitors. J. Power Sources 2025, 631, 236304. [Google Scholar] [CrossRef]
- Lu, W.J.; Si, Y.X.; Zhao, C.R.; Chen, T.Q.; Li, C.; Zhang, C.; Wang, K.B. Biomass-derived carbon applications in the field of supercapacitors: Progress and prospects. Chem. Eng. J. 2024, 495, 153311. [Google Scholar] [CrossRef]
- Said, M.B.; Ahmed, Z.; Debiemme-Chouvy, C.; Cachet, H.; Louhichi, B.; Chtourou, R.; Charradi, K. New electrodes made of a cellulose hydrogel/CNT/polyaniline composite for high-performance supercapacitors. J. Energy Storage 2025, 135, 118273. [Google Scholar] [CrossRef]
- Niu, J.; Shao, R.; Liang, J.J.; Dou, M.L.; Li, Z.L.; Huang, Y.Q.; Wang, F. Biomass-derived mesopore-dominant porous carbons with large specific surface area and high defect density as high performance electrode materials for Li-ion batteries and supercapacitors. Nano Energy 2017, 36, 322–330. [Google Scholar] [CrossRef]
- Yuan, R.C.; Guo, Y.W.; Gurgan, I.; Siddique, N.; Li, Y.S.; Jang, S.; Noh, G.A.; Kim, S.H. Raman spectroscopy analysis of disordered and amorphous carbon materials: A review of empirical correlations. Carbon 2025, 238, 120214. [Google Scholar] [CrossRef]
- Liu, W.H.; Liu, M.J.; Liu, X.; Gao, C.; Xia, Y.Y.; Guo, C.; Wang, S.J.; Kong, F.G. Novel nitrogen-doped bamboo-structure carbon nanotubes as superior sulfur host for high-performance lithium-sulfur batteries. J. Alloys Compd. 2022, 923, 166376. [Google Scholar] [CrossRef]
- Liu, Y.B.; Huang, G.X.; Li, Y.Y.; Yao, Y.H.; Liu, Q.R.; Xing, B.L.; Jia, J.B.; Zhang, C.X. Structural evolution of porous graphitic carbon nanosheets based on quinonyl decomposition for supercapacitor electrodes. Appl. Surf. Sci. 2021, 537, 12. [Google Scholar] [CrossRef]
- Sun, N.; Guan, Z.R.X.; Liu, Y.W.; Cao, Y.L.; Zhu, Q.Z.; Liu, H.; Wang, Z.X.; Zhang, P.; Xu, B. Extended “Adsorption-Insertion” Model: A New Insight into the Sodium Storage Mechanism of Hard Carbons. Adv. Energy Mater. 2019, 9, 14. [Google Scholar] [CrossRef]
- Mao, Y.Q.; Dong, G.H.; Li, Y.Q.; Huang, P.; Fu, S.Y. One-step fabrication of N/O self-doped porous carbon derived from 2-MeIM for high-performance supercapacitor electrode. J. Energy Storage 2023, 74, 109263. [Google Scholar] [CrossRef]
- Huo, S.L.; Liu, M.Q.; Wu, L.L.; Liu, M.J.; Xu, M.; Ni, W.; Yan, Y.M. Methanesulfonic acid-assisted synthesis of N/S co-doped hierarchically porous carbon for high performance supercapacitors. J. Power Sources 2018, 387, 81–90. [Google Scholar] [CrossRef]
- Qian, X.; Miao, L.; Jiang, J.; Ping, G.; Xiong, W.; Lv, Y.; Liu, Y.; Gan, L.; Zhu, D.; Liu, M. Hydrangea-like N/O codoped porous carbons for high-energy supercapacitors. Chem. Eng. J. 2020, 388, 124208. [Google Scholar] [CrossRef]
- Song, Z.Y.; Miao, L.; Li, L.C.; Zhu, D.Z.; Lv, Y.K.; Xiong, W.; Duan, H.; Wang, Z.W.; Gan, L.H.; Liu, M.X. A universal strategy to obtain highly redox-active porous carbons for efficient energy storage. J. Mater. Chem. A 2020, 8, 3717–3725. [Google Scholar] [CrossRef]
- Fu, F.B.; Yang, D.J.; Zhao, B.W.; Fan, Y.K.; Liu, W.F.; Lou, H.M.; Qiu, X.Q. Boosting capacitive performance of N, S co-doped hierarchical porous lignin-derived carbon via self-assembly assisted template-coupled activation. J. Colloid Interface Sci. 2023, 640, 698–709. [Google Scholar] [CrossRef]
- Zhang, T.T.; Liu, P.; Zhong, Y.; Zheng, J.; Deng, K.; Lv, X.B.; Li, H.J.; Tian, W.; Ji, J.Y. N, S co-doped branched carbon nanotubes with hierarchical porous structure and electron/ion transfer pathways for supercapacitors and lithium-ion batteries. Carbon 2022, 198, 91–100. [Google Scholar] [CrossRef]
- Wu, C.H.; Qin, Y.Y.; Zhao, Z.; Zhang, G.H.; Bian, Z.T.; Zhao, X.X.; Fang, L.Y.; Sun, Y.Y.; Zhang, L.G.; Zhang, K.Y. Osmanthus fragrans-derived hierarchically porous carbons for high-performance supercapacitors. J. Alloys Compd. 2025, 1036, 182201. [Google Scholar] [CrossRef]
- Isaqu, J.P.; Huang, C.W.; Chih, J.K.; Huang, B.Y.; Subramani, M.; Tsao, I.Y.; Chang, B.K.; Su, C.Y. Selective Nitrogen Doping at Hole Edges of Holey Graphene: Enhancing Ionic Transport Mechanisms for High-Performance Supercapacitors. Small Methods 2025, 9, 2402038. [Google Scholar] [CrossRef] [PubMed]
- Malavekar, D.; Pawar, D.; Bagde, A.; Khot, S.; Sankapal, S.; Bachankar, S.; Patil, S.; Lokhande, C.D.; Kim, J.H. Advancements in graphene and its derivatives based composite Materials: A comprehensive review on Synthesis, Characterization, and supercapacitive charge storage. Chem. Eng. J. 2024, 501, 157533. [Google Scholar] [CrossRef]
- Cai, X.; Zhang, K.X.; Chen, W.X.; Hu, X.Q. Deciphering the capacitive behavior of heteroatom-doped carbon materials with small mesopores. Energy Storage Mater. 2025, 82, 104584. [Google Scholar] [CrossRef]
- Liu, Y.B.; Huang, G.X.; Li, Y.Y.; Yao, Y.H.; Zhang, F.M.; Xing, B.L.; Zhang, C.X. N-O-S Co-doped Hierarchical Porous Carbons Derived from Calcium Lignosulfonate for High-Performance Supercapacitors. Energy Fuels 2020, 34, 3909–3922. [Google Scholar] [CrossRef]
- Xiu, S.J.; Kim, D.K.; Kang, Y.J.; Duan, S.M.; Wang, Q.; Chen, T.Y.; Piao, Y.Z.; Suk, J.; Jin, X.Z.; Quan, B. One-step synthesis of nitrogen and sulfur co-doped hierarchical porous carbon derived from acesulfame potassium as a dual-function agent for supercapacitors and lithium-sulfur batteries. J. Energy Storage 2023, 66, 107214. [Google Scholar] [CrossRef]

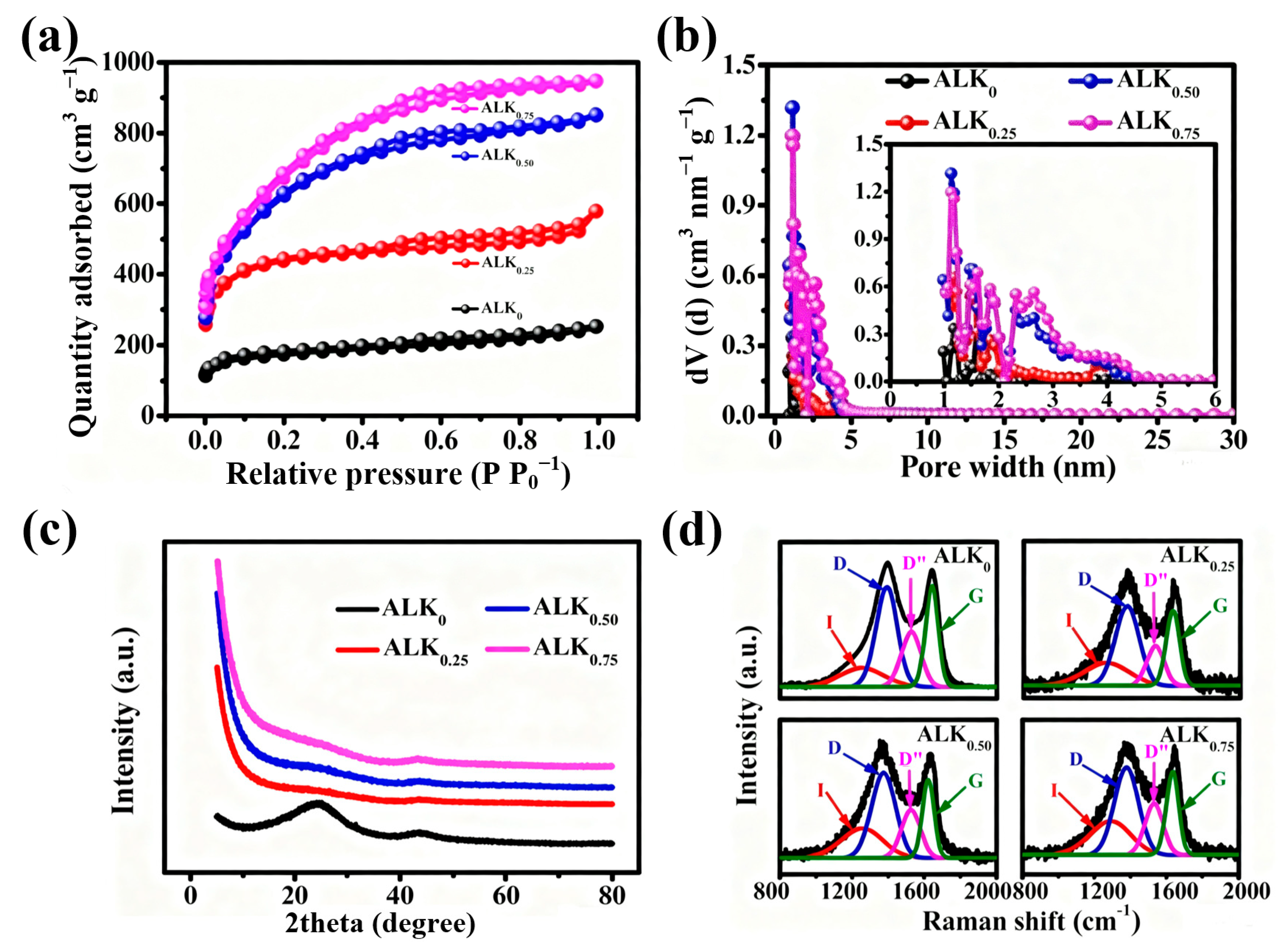

| Samples | SBET a (m2 g−1) | Vt b (cm3 g−1) | Vmic c (cm3 g−1) | Vmes d (cm3 g−1) | Vmes Vt−1 e (%) |
|---|---|---|---|---|---|
| ALK0 | 646 | 0.39 | 0.20 | 0.19 | 48.7 |
| ALK0.25 | 1625 | 0.90 | 0.61 | 0.29 | 32.2 |
| ALK0.50 | 2208 | 1.32 | 0.56 | 0.76 | 57.6 |
| ALK0.75 | 2406 | 1.47 | 0.48 | 0.99 | 67.3 |
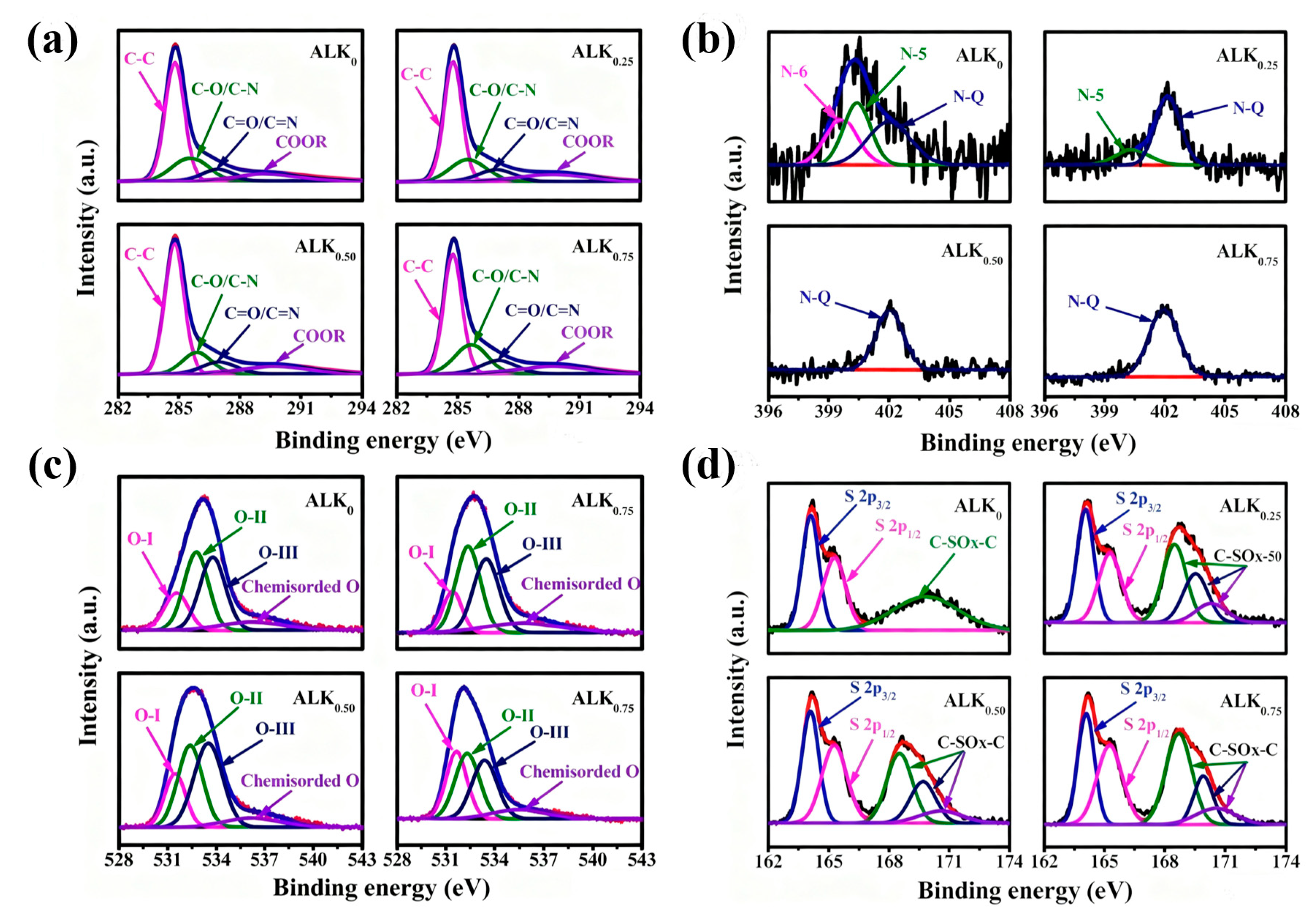
| Samples | Carbon (at.%) | Nitrogen (at.%) | Oxygen (at.%) | Sulfur (at.%) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total C | C-C | C-O/ C-N | C=O/ C=N | COOR | Total N | N-6 | N-5 | N-Q | Total O | O-I | O-II | O-III | Chemist- ordered-O | Total S | S 2p3/2 | S 2p1/2 | C-SOx-C | |
| ALK0 | 90.3 | 53.2 | 18.2 | 6.6 | 12.3 | 1.9 | 0.5 | 0.6 | 0.8 | 6.8 | 1.2 | 2.5 | 2.2 | 0.9 | 1.0 | 0.3 | 0.3 | 0.4 |
| ALK0.25 | 89.7 | 50.7 | 18.4 | 8.1 | 12.5 | 1.0 | 0 | 0.2 | 0.8 | 7.8 | 1.1 | 2.8 | 2.9 | 1.0 | 1.5 | 0.4 | 0.4 | 0.7 |
| ALK0.50 | 88.5 | 48.3 | 18.9 | 8.6 | 12.7 | 0.7 | 0 | 0 | 0.7 | 8.9 | 1.8 | 3.0 | 3.0 | 1.1 | 1.9 | 0.5 | 0.5 | 0.9 |
| ALK0.75 | 86.8 | 45.1 | 19.4 | 9.3 | 13.0 | 0.7 | 0 | 0 | 0.7 | 10.0 | 2.5 | 3.2 | 3.0 | 1.3 | 2.5 | 0.5 | 0.6 | 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Xue, X.; Zhang, Y.; Zhang, C.; Li, W.; Jia, C.; Tian, J. N–O–S Co–Doped Hierarchical Porous Carbons Prepared by Mild KOH Activation of Ammonium Lignosulfonate for High–Performance Supercapacitors. Nanomaterials 2025, 15, 1633. https://doi.org/10.3390/nano15211633
Jiang Z, Xue X, Zhang Y, Zhang C, Li W, Jia C, Tian J. N–O–S Co–Doped Hierarchical Porous Carbons Prepared by Mild KOH Activation of Ammonium Lignosulfonate for High–Performance Supercapacitors. Nanomaterials. 2025; 15(21):1633. https://doi.org/10.3390/nano15211633
Chicago/Turabian StyleJiang, Zhendong, Xiaoxiao Xue, Yaojie Zhang, Chuanxiang Zhang, Wenshu Li, Chaoyi Jia, and Junwei Tian. 2025. "N–O–S Co–Doped Hierarchical Porous Carbons Prepared by Mild KOH Activation of Ammonium Lignosulfonate for High–Performance Supercapacitors" Nanomaterials 15, no. 21: 1633. https://doi.org/10.3390/nano15211633
APA StyleJiang, Z., Xue, X., Zhang, Y., Zhang, C., Li, W., Jia, C., & Tian, J. (2025). N–O–S Co–Doped Hierarchical Porous Carbons Prepared by Mild KOH Activation of Ammonium Lignosulfonate for High–Performance Supercapacitors. Nanomaterials, 15(21), 1633. https://doi.org/10.3390/nano15211633





