Dielectric Properties of Co-Doped TiO2 with Mg and Nb for Energy Storage Applications
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
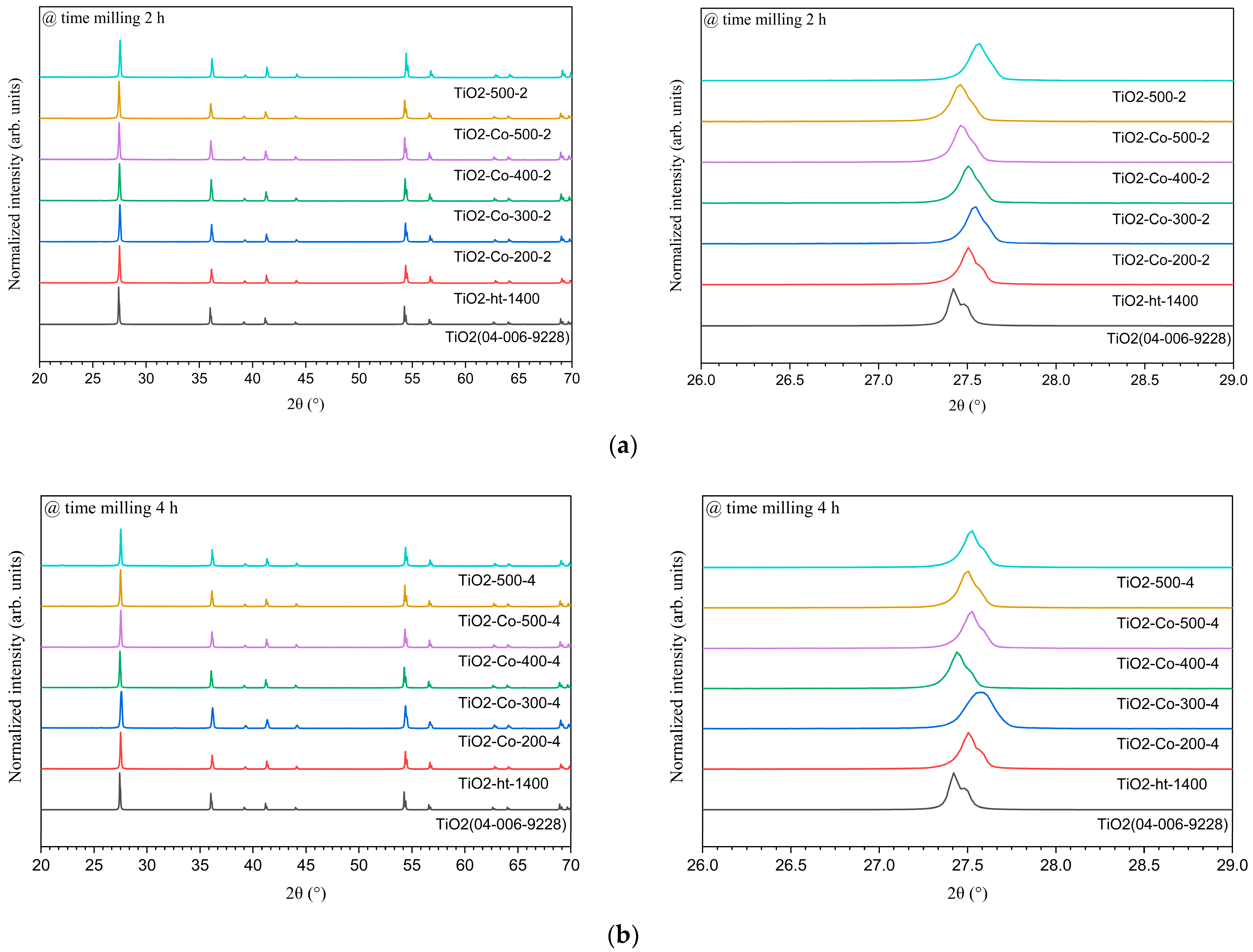
3.1. Structural Analysis
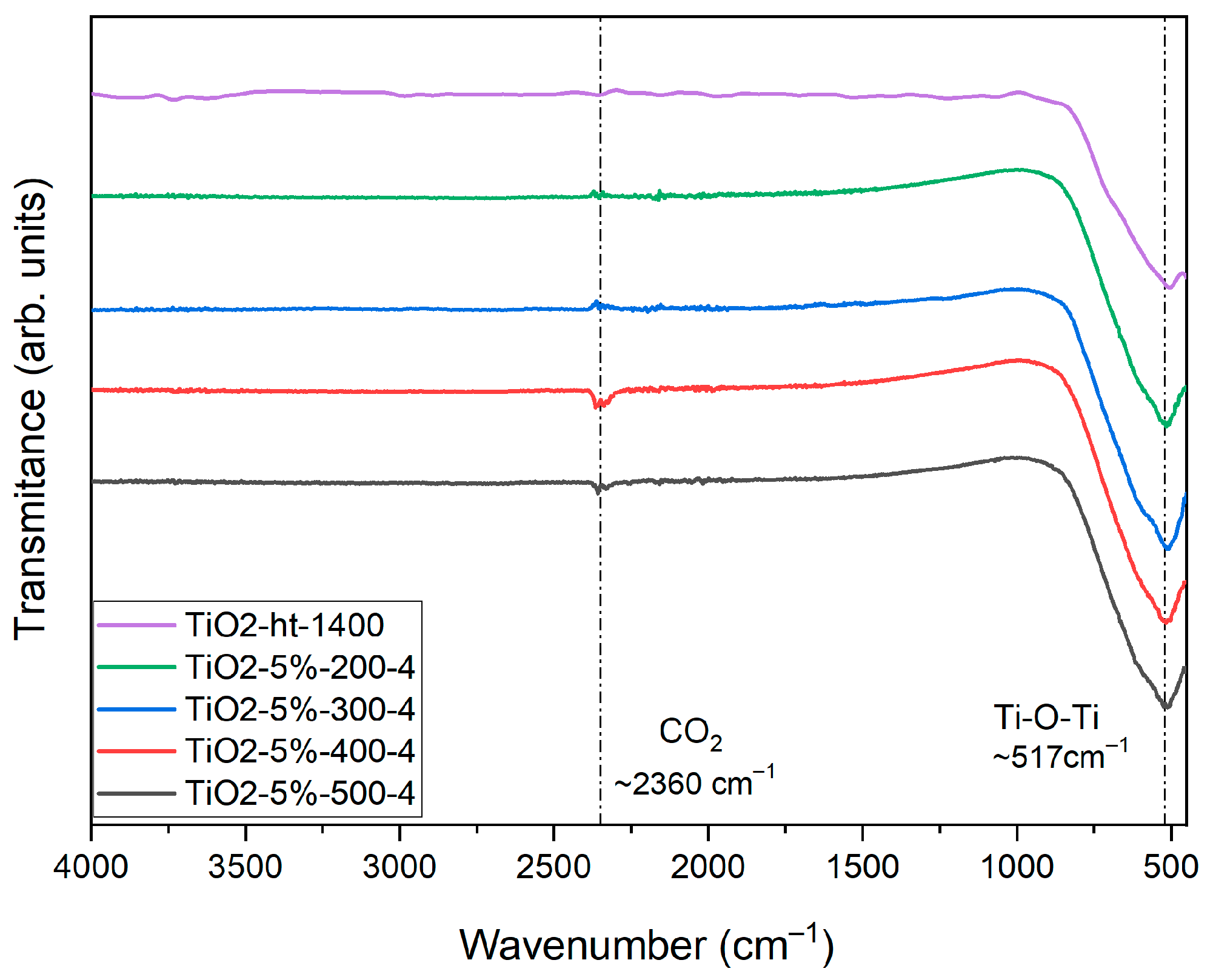
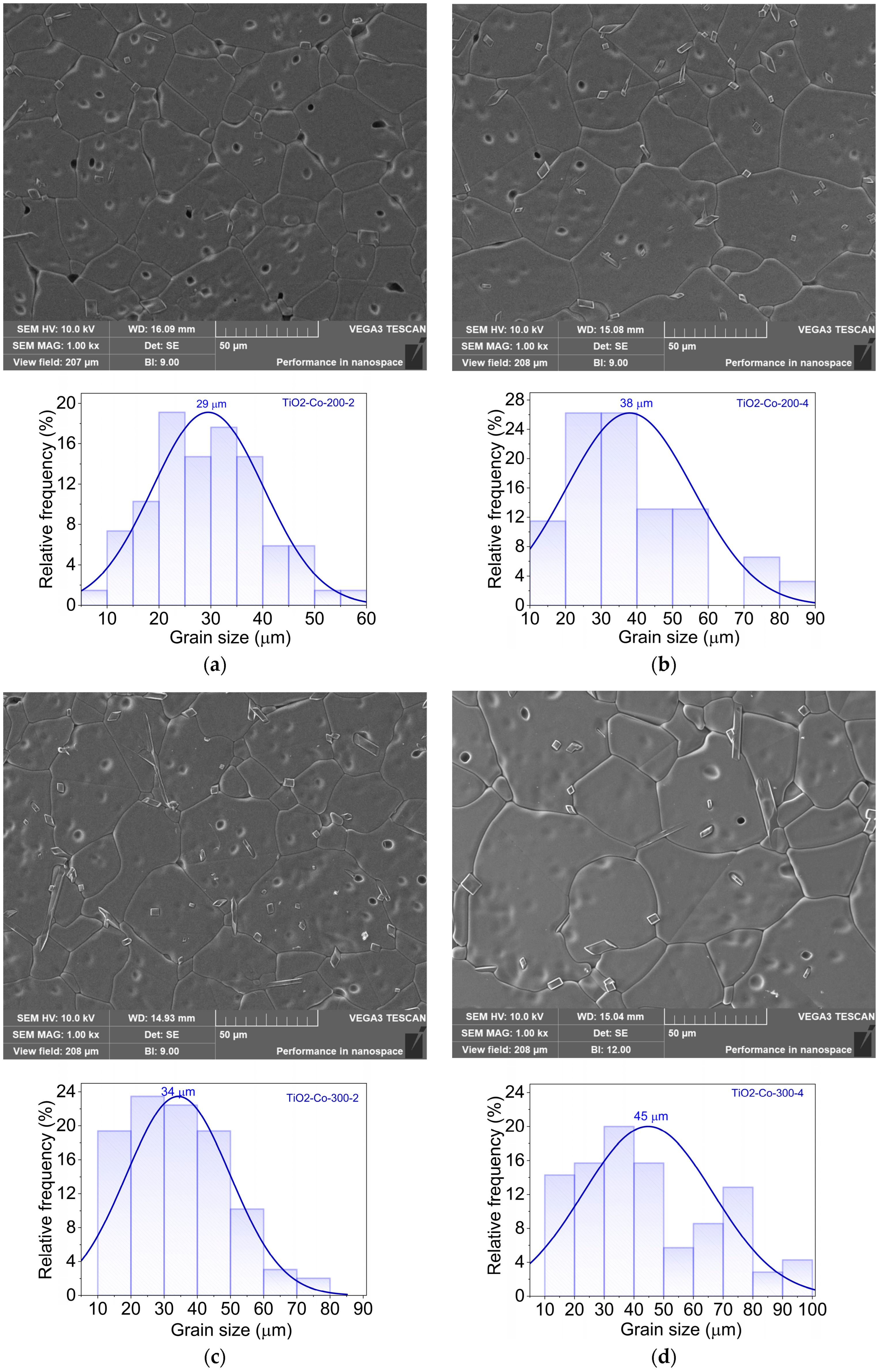
3.2. Morphological and Composition Analysis
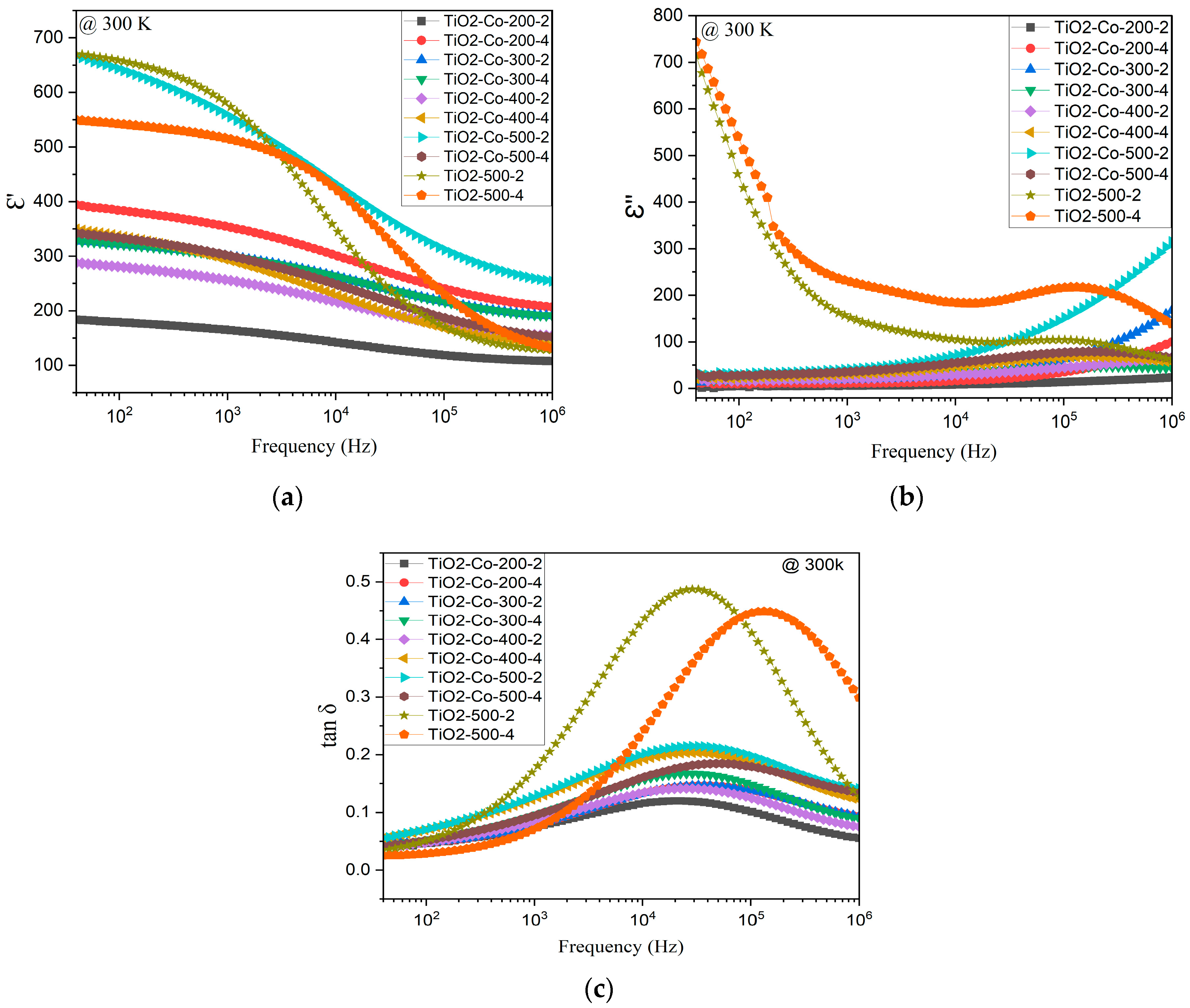
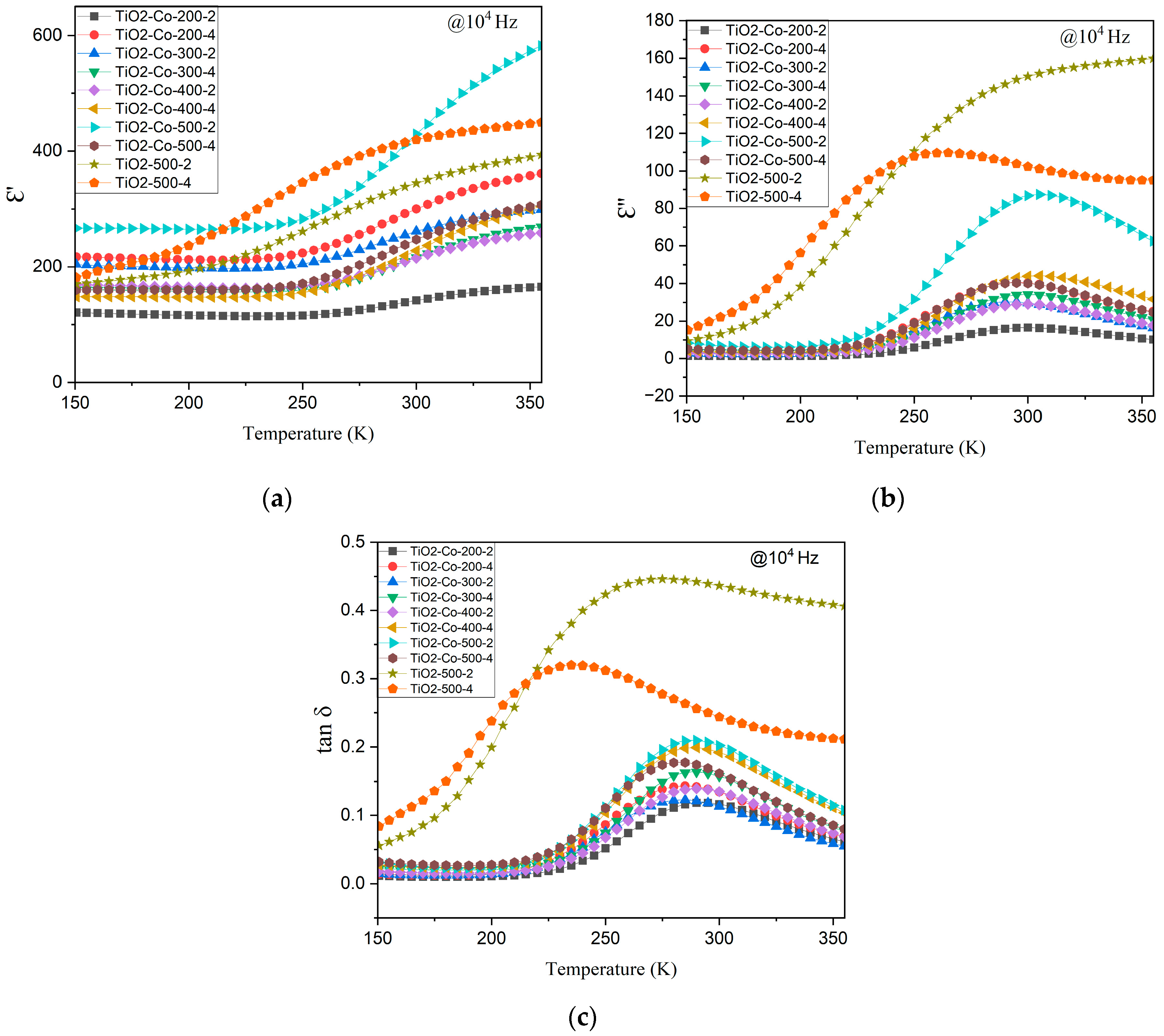
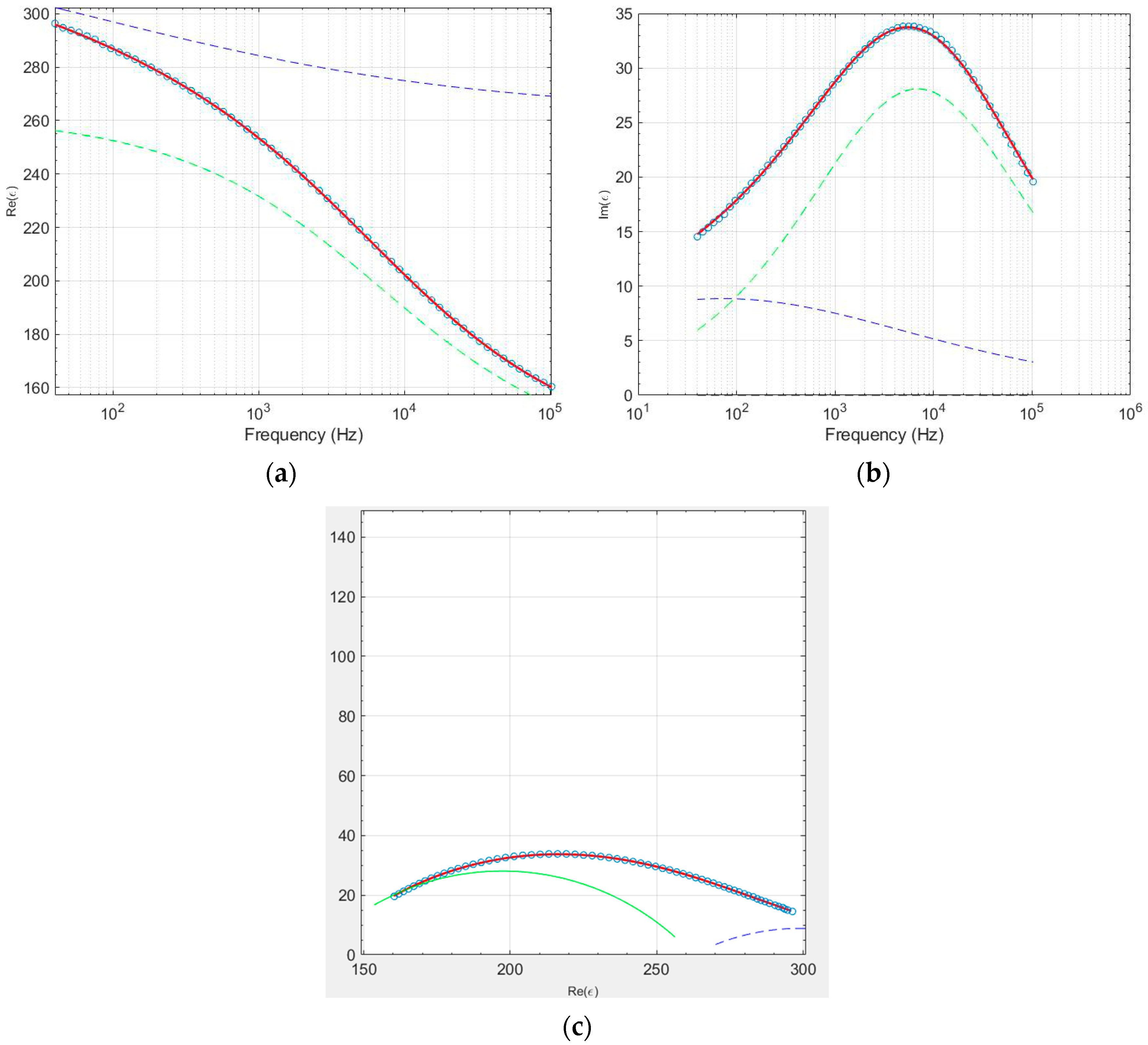
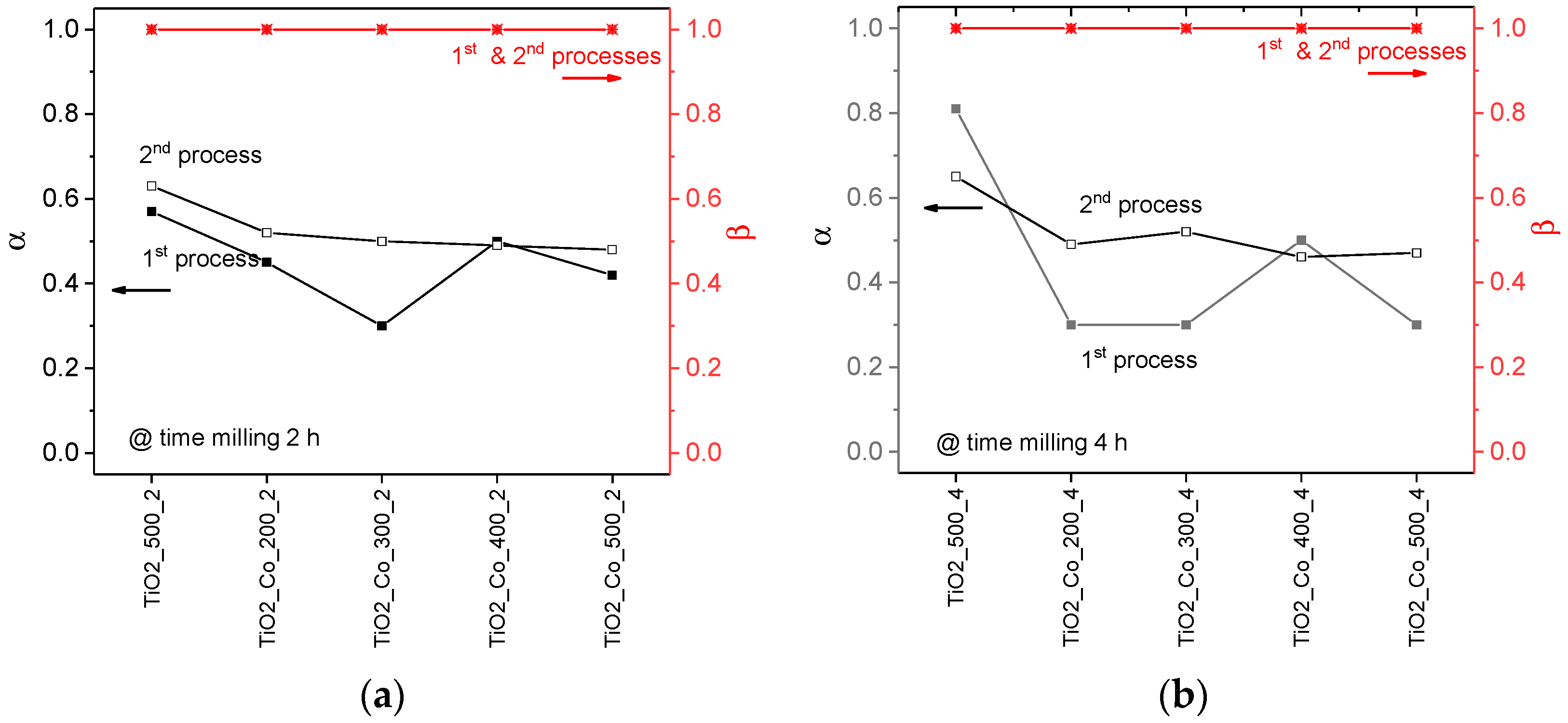
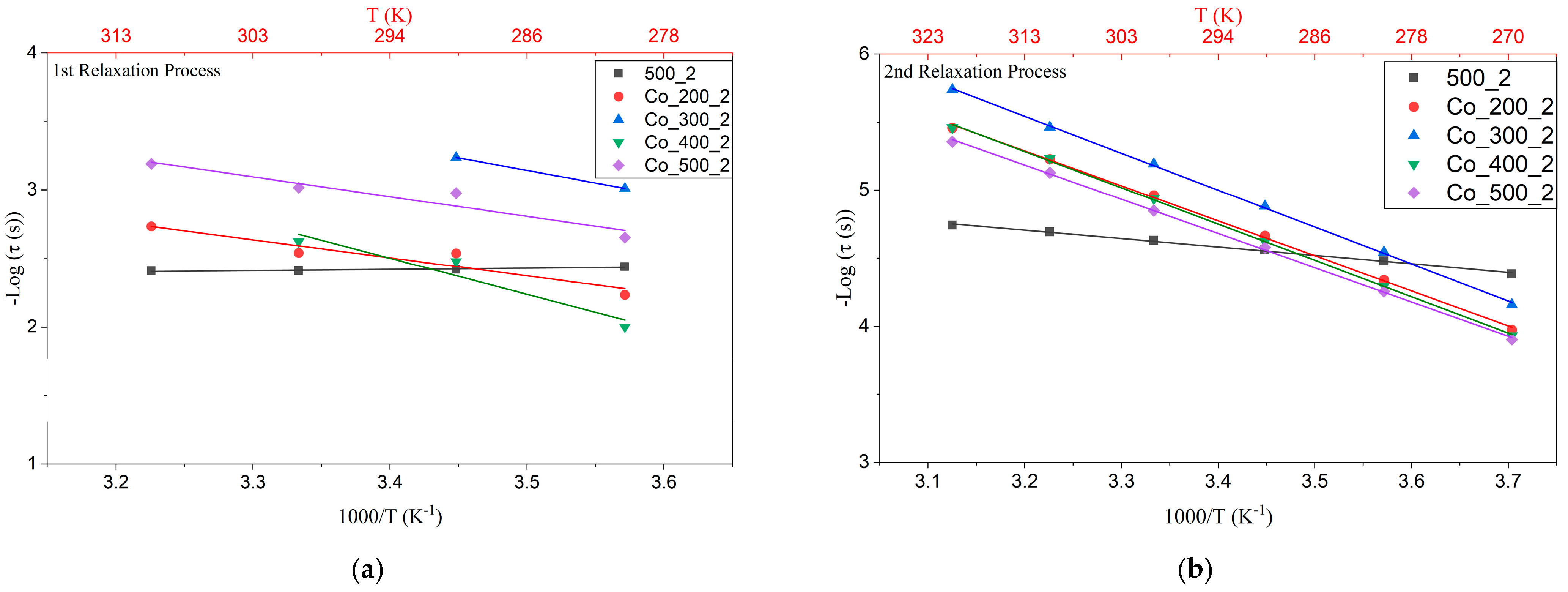
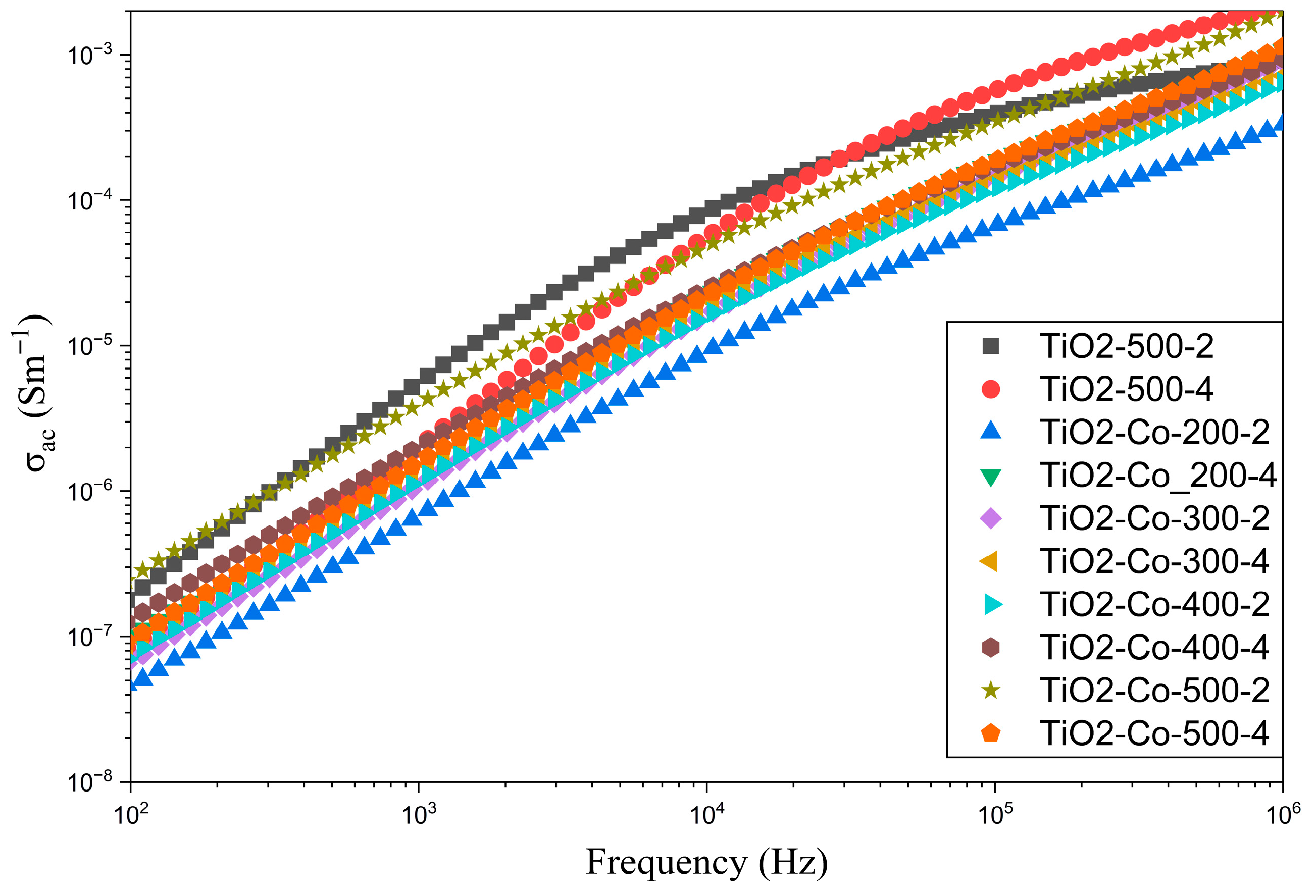
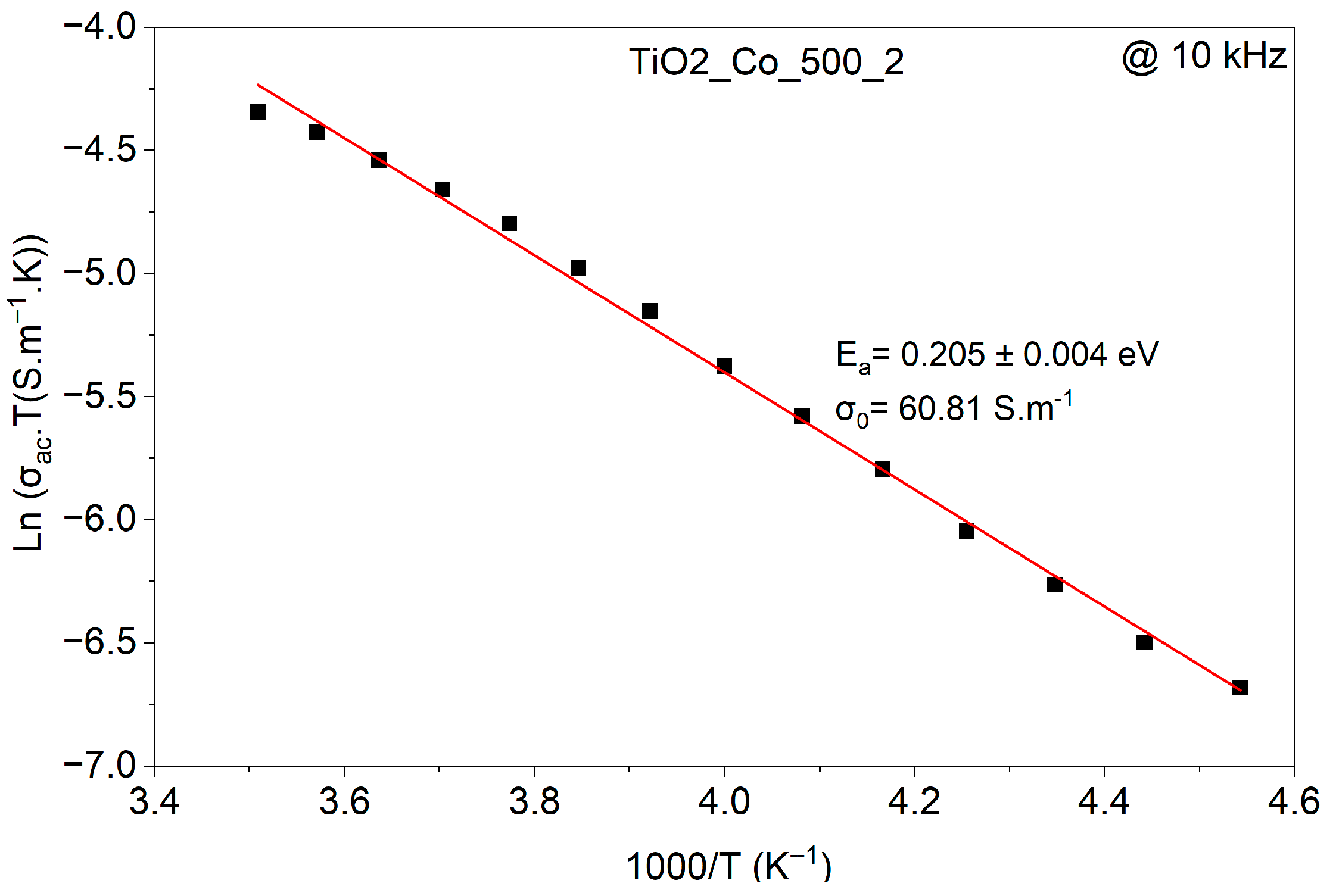
3.3. Electrical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diebold, U. The Surface Science of Titanium Dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Zhou, W.; Cao, M.; Wang, H.; Hao, H.; Yao, Z.; Liu, H. Defect Structure Design of TiO2 Ceramics with Colossal Permittivity by Doping with Ti Metal Powder. Ceram. Int. 2022, 48, 16723–16729. [Google Scholar] [CrossRef]
- Suchikova, Y.; Nazarovets, S.; Konuhova, M.; Popov, A.I. Binary Oxide Ceramics (TiO2, ZnO, Al2O3, SiO2, CeO2, Fe2O3, and WO3) for Solar Cell Applications: A Comparative and Bibliometric Analysis. Ceramics 2025, 8, 119. [Google Scholar] [CrossRef]
- Liang, X.; Yu, S.; Meng, B.; Wang, X.; Yang, C.; Shi, C.; Ding, J. Advanced TiO2-Based Photoelectrocatalysis: Material Modifications, Charge Dynamics, and Environmental–Energy Applications. Catalysts 2025, 15, 542. [Google Scholar] [CrossRef]
- Nachaithong, T.; Chanlek, N.; Moontragoon, P.; Thongbai, P. The Primary Origin of Excellent Dielectric Properties of (Co, Nb) Co-doped Tio2 Ceramics: Electron-pinned Defect Dipoles vs. Internal Barrier Layer Capacitor Effect. Molecules 2021, 26, 3230. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, J.; Gai, Z.; Li, J.; Liu, J.; Wang, J.; Wang, C.; Wang, X. Annealing Effects on the Structural and Dielectric Properties of (Nb + In) Co-Doped Rutile TiO2 Ceramics. RSC Adv. 2019, 9, 8364–8368. [Google Scholar] [CrossRef]
- Tsuji, K.; Han, H.S.; Guillemet-Fritsch, S.; Randall, C.A. Dielectric Relaxation and Localized Electron Hopping in Colossal Dielectric (Nb,In)-Doped TiO2 Rutile Nanoceramics. Phys. Chem. Chem. Phys. 2017, 19, 8568–8574. [Google Scholar] [CrossRef]
- Mingmuang, Y.; Chanlek, N.; Moontragoon, P.; Srepusharawoot, P.; Thongbai, P. Effects of Sn4+ and Ta5+ Dopant Concentration on Dielectric and Electrical Properties of TiO2: Internal Barrier Layer Capacitor Effect. Results Phys. 2022, 42, 106029. [Google Scholar] [CrossRef]
- Li, Z.; Wu, J.; Xiao, D.; Zhu, J.; Wu, W. Colossal Permittivity in Titanium Dioxide Ceramics Modified by Tantalum and Trivalent Elements. Acta Mater. 2016, 103, 243–251. [Google Scholar] [CrossRef]
- Li, W.; Qiu, S.; Chen, N.; Du, G. Enhanced Dielectric Response in Mg-Doped CaCu3Ti4O12 Ceramics. J. Mater. Sci. Technol. 2010, 26, 682–686. [Google Scholar] [CrossRef]
- Hu, W.; Liu, Y.; Withers, R.L.; Frankcombe, T.J.; Norén, L.; Snashall, A.; Kitchin, M.; Smith, P.; Gong, B.; Chen, H.; et al. Electron-Pinned Defect-Dipoles for High-Performance Colossal Permittivity Materials. Nat. Mater. 2013, 12, 821–826. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, N.; Li, Q.; Yu, Y.; Zhang, J.; Li, Y.; Wang, H. Dielectric Relaxations in Rutile TiO2. J. Am. Ceram. Soc. 2015, 98, 148–153. [Google Scholar] [CrossRef]
- Cheng, X.; Li, Z.; Wu, J. Colossal Permittivity in Ceramics of TiO2 Co-Doped with Niobium and Trivalent Cation. J. Mater. Chem. A Mater. 2015, 3, 5805–5810. [Google Scholar] [CrossRef]
- Ke, S.; Li, T.; Ye, M.; Lin, P.; Yuan, W.; Zeng, X.; Chen, L.; Huang, H. Origin of Colossal Dielectric Response in (In + Nb) Co-Doped TiO2 Rutile Ceramics: A Potential Electrothermal Material. Sci. Rep. 2017, 7, 10144. [Google Scholar] [CrossRef]
- Mckubre, M.; Macdonald, D.; Strømme, M. Impedance Spectroscopy: Theory, Experiment, and Applications, 3rd ed.; Wiley: Hoboken, NJ, USA, 2018. [Google Scholar]
- Yang, C.; Tse, M.Y.; Wei, X.; Hao, J. Colossal Permittivity of (Mg + Nb) Co-Doped TiO2 Ceramics with Low Dielectric Loss. J. Mater. Chem. C Mater. 2017, 5, 5170–5175. [Google Scholar] [CrossRef]
- El Mragui, A.; Logvina, Y.; da Silva, L.P.; Zegaoui, O.; da Silva, J.C.E. Synthesis of Fe-and Co-Doped TiO2 with Improved Photocatalytic Activity under Visible Irradiation toward Carbamazepine Degradation. Materials 2019, 12, 3874. [Google Scholar] [CrossRef]
- Khan, M.A.M.; Siwach, R.; Kumar, S.; Alhazaa, A.N. Role of Fe Doping in Tuning Photocatalytic and Photoelectrochemical Properties of TiO2 for Photodegradation of Methylene Blue. Opt. Laser Technol. 2019, 118, 170–178. [Google Scholar] [CrossRef]
- Wang, X.W.; Zheng, Y.P.; Liang, B.K.; Zhang, G.; Shi, Y.C.; Zhang, B.H.; Xue, L.L.; Shang, S.Y.; Shang, J.; Yin, S.Q.; et al. Preparation and Properties of La and Nb Co-Doped TiO2 Colossal Dielectric Ceramic Materials. J. Mater. Sci. Mater. Electron. 2020, 31, 16044–16052. [Google Scholar] [CrossRef]
- Patterson, E.A.; Kwon, S.; Huang, C.C.; Cann, D.P. Effects of ZrO2 Additions on the Dielectric Properties of CaCu3Ti4O12. Appl. Phys. Lett. 2005, 87, 182911. [Google Scholar] [CrossRef]
- Yu, M.; Sun, H.; Huang, X.; Yan, Y.; Zhang, W. In Situ-Formed and Low-Temperature-Deposited Nb:TiO2 Compact-Mesoporous Layer for Hysteresis-Less Perovskite Solar Cells with High Performance. Nanoscale Res. Lett. 2020, 15, 135. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, S.; Wan, P.; Sun, J.; Hood, Z.D. Introducing Ti3+ Defects Based on Lattice Distortion for Enhanced Visible Light Photoreactivity in TiO2 Microspheres. RSC Adv. 2017, 7, 32461–32467. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, C.; Xie, Y.; Wang, L.; Ding, J.; Zhang, J.; Wan, H.; Guan, G. Intentionally Introducing Oxygen Vacancies and Ti3+ Defects on the Surface of Bi4Ti3O12 Nanosheets for Promoting the Photoreduction of CO2 to CH3OH. ACS Appl. Nano Mater. 2024, 7, 3012–3023. [Google Scholar] [CrossRef]
- Xu, W.; Russo, P.A.; Schultz, T.; Koch, N.; Pinna, N. Niobium-Doped Titanium Dioxide with High Dopant Contents for Enhanced Lithium-Ion Storage. ChemElectroChem 2020, 7, 4016–4023. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-Ray Photoelectron Spectroscopy; Perkin-Elmer Corporation Physical Electronics Division: Norwalk, CT, USA, 1995. [Google Scholar]
- Tsyganov, A.; Morozova, N.; Vikulova, M.; Asmolova, A.; Zotov, I.; Bainyashev, A.; Gorokhovsky, A.; Gorshkov, N. Thermal Behavior of La3+, Ni2+ and Sn4+ Co-Doped CaCu3Ti4O12 Ceramics Dielectric Response. Inorg. Chem. Commun. 2024, 160, 111914. [Google Scholar] [CrossRef]
- Tsyganov, A.; Morozova, N.; Vikulova, M.; Asmolova, A.; Artyukhov, D.; Zotov, I.; Gorokhovsky, A.; Gorshkov, N. The Effect of Lithium Doping on the Dielectric Properties of Solid Solutions LixCa(1−x)Cu3Ti4O12 (x = 0.01–0.1). J. Compos. Sci. 2023, 7, 282. [Google Scholar] [CrossRef]
- Havriliak, S.; Negami, S. A Complex Plane Representation of Electronical and Mechanical Relaxation. Polymer 1967, 8, 161–210. [Google Scholar] [CrossRef]
- Melo, B.; Loureiro, F.J.; Fagg, D.P.; Costa, L.; Graça, M. DFRTtoEIS An Easy Approach to Verify the Consistency of a DFRT Generated from an Impedance Spectrum. Electrochim. Acta 2021, 366, 137429. [Google Scholar] [CrossRef]
- Maglione, M.; Vikhnin, S.; Liu Springer, G.K. Polarons, Free Charge Localisation and Effective Dielectric Permittivity in Oxides. arXiv 2010, arXiv:1006.3719. [Google Scholar] [CrossRef]
- Deskins, N.A.; Dupuis, M. Electron Transport via Polaron Hopping in Bulk TiO2: A Density Functional Theory Characterization. Phys. Rev. B Condens. Matter Mater. Phys. 2007, 75, 195212. [Google Scholar] [CrossRef]
- Yagi, E.; Hasiguti, R.R.; Aono, M. Electronic Conduction above 4 K of Slightly Reduced Oxygen-Deficient Rutile TiO2−x. Phys. Rev. B Condens. Matter 1996, 54, 7945–7956. [Google Scholar] [CrossRef]
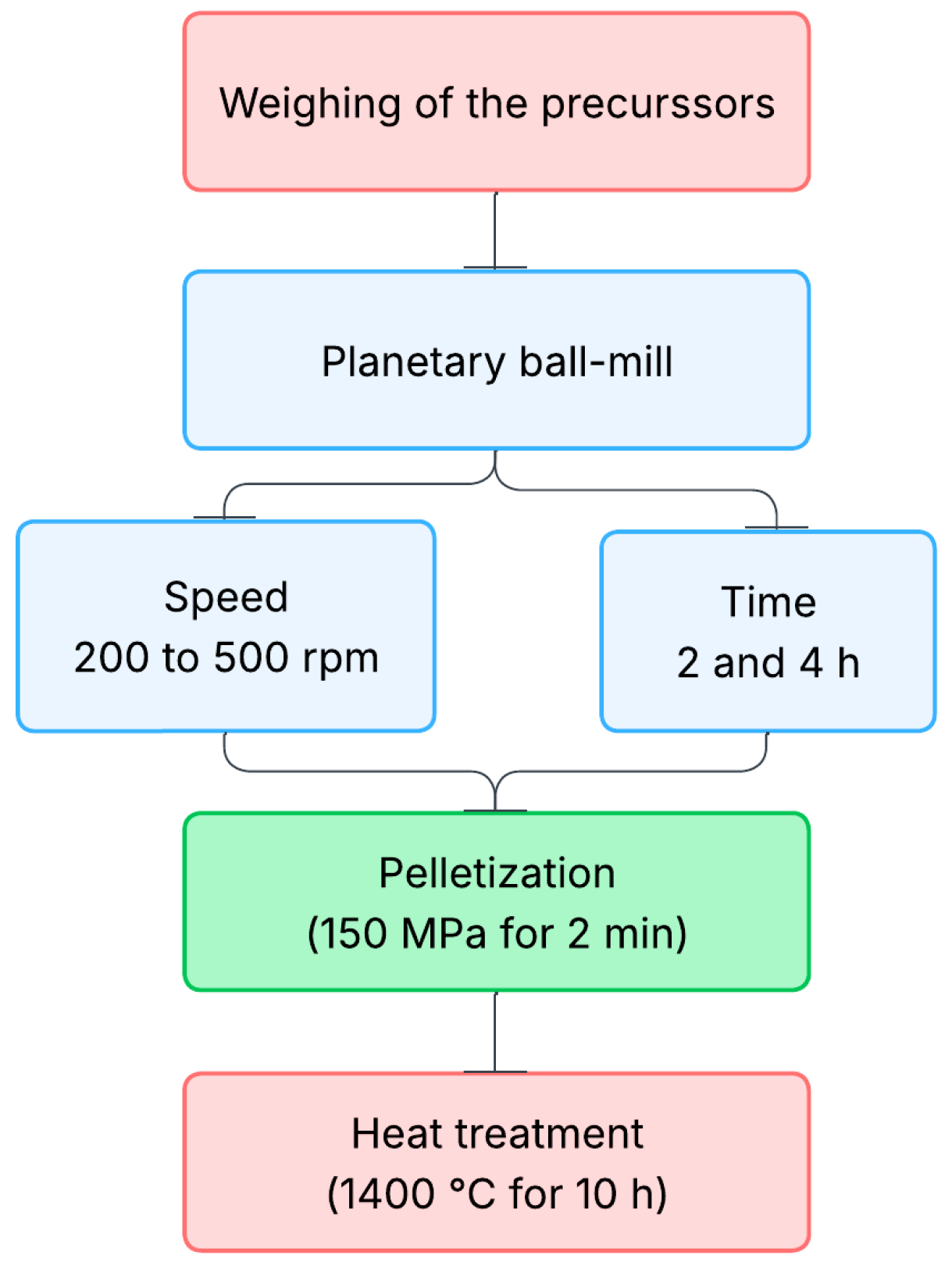
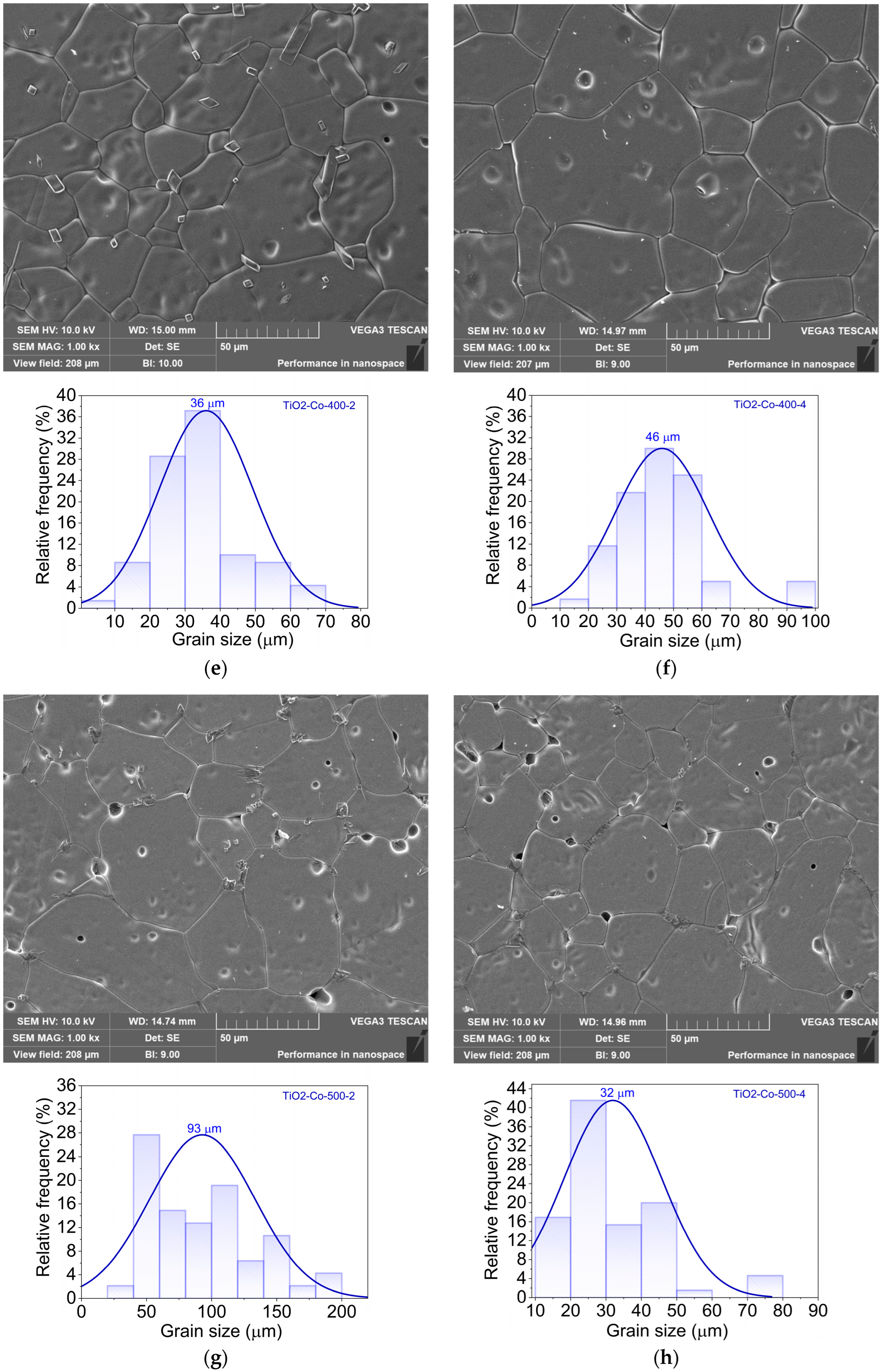
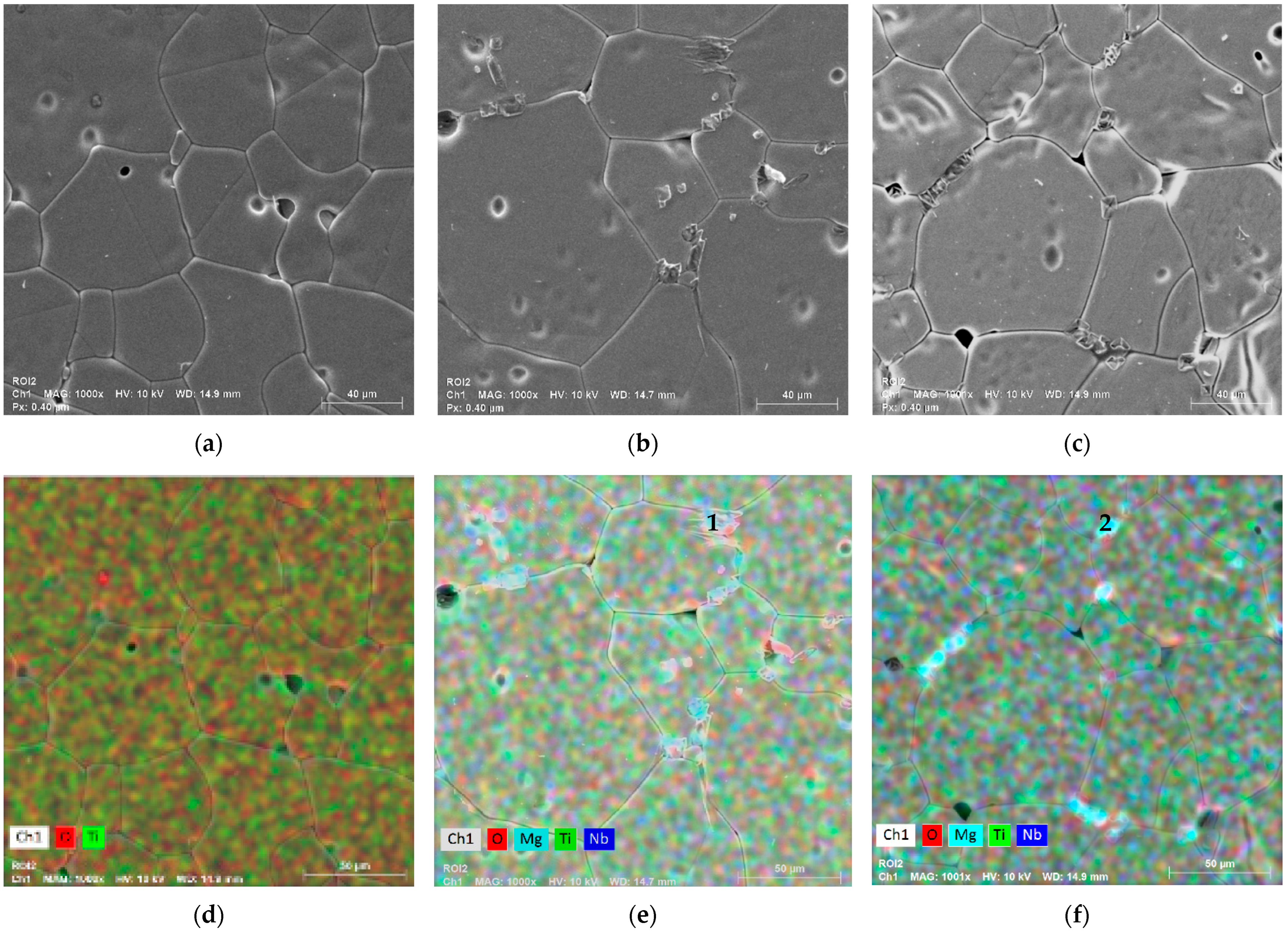
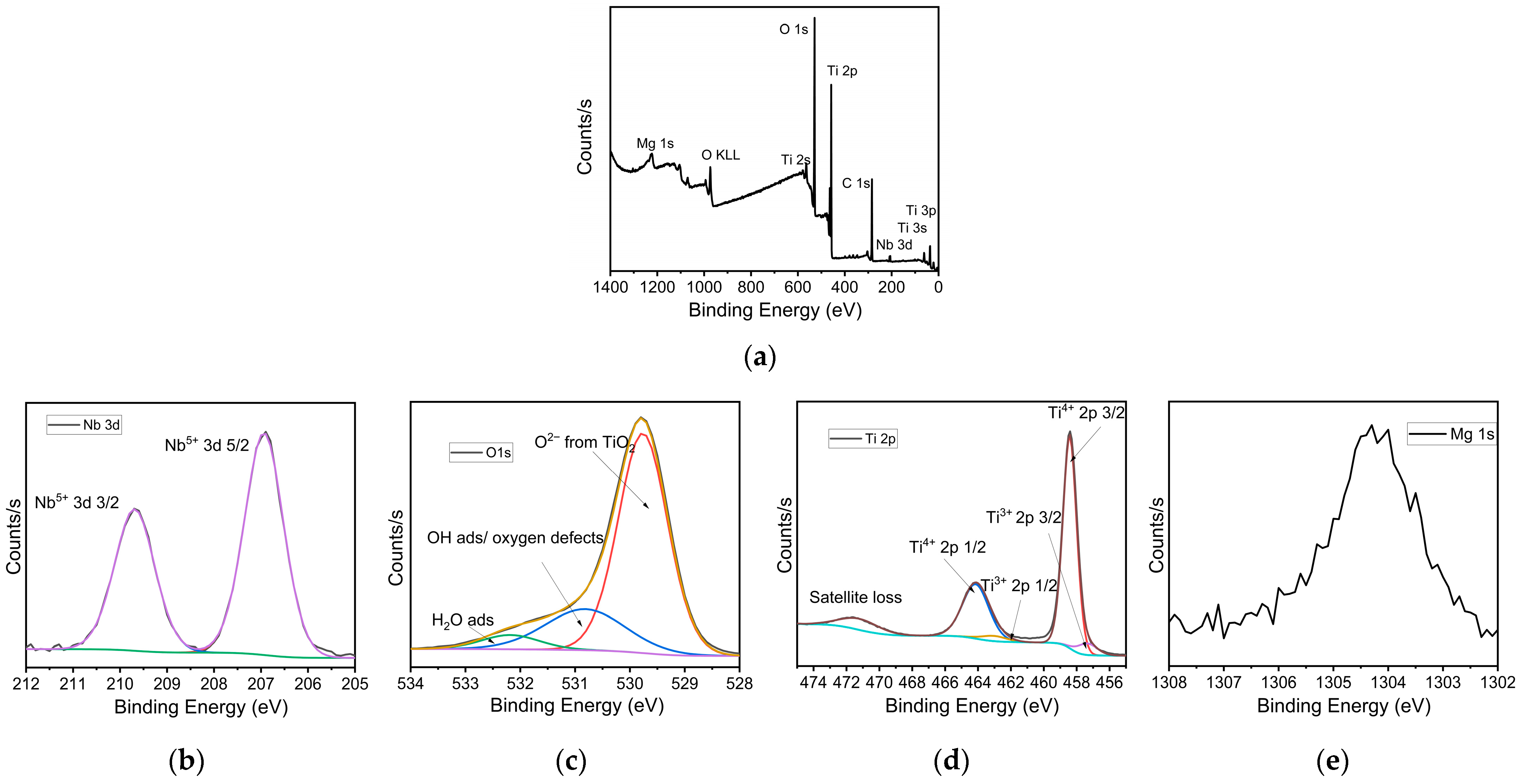
| Sample | Composition | Speed (rpm) | Time (h) |
|---|---|---|---|
| TiO2-500-2 | TiO2 | 500 | 2 |
| TiO2-500-4 | TiO2 | 500 | 4 |
| TiO2-Co-200-2 | (Nb½Mg½)0.05Ti0.95O2 | 200 | 2 |
| TiO2-Co-200-4 | (Nb½Mg½)0.05Ti0.95O2 | 200 | 4 |
| TiO2-Co-300-2 | (Nb½Mg½)0.05Ti0.95O2 | 300 | 2 |
| TiO2-Co-300-4 | (Nb½Mg½)0.05Ti0.95O2 | 300 | 4 |
| TiO2-Co-400-2 | (Nb½Mg½)0.05Ti0.95O2 | 400 | 2 |
| TiO2-Co-400-4 | (Nb½Mg½)0.05Ti0.95O2 | 400 | 4 |
| TiO2-Co-500-2 | (Nb½Mg½)0.05Ti0.95O2 | 500 | 2 |
| TiO2-Co-500-4 | (Nb½Mg½)0.05Ti0.95O2 | 500 | 4 |
| Sample | TiO2-500-4 | TiO2-Co-200-4 | TiO2-Co-300-4 | TiO2-Co-400-4 | TiO2-Co-500-4 |
|---|---|---|---|---|---|
| Crystalline phase | Rutile TiO2 | Rutile TiO2 | Rutile TiO2 | Rutile TiO2 | Rutile TiO2 |
| Crystalline system | Tetragonal | Tetragonal | Tetragonal | Tetragonal | Tetragonal |
| Space group | P42/mnm | P42/mnm | P42/mnm | P42/mnm | P42/mnm |
| Volume (Å3) | 62.41551 | 62.64447 | 62.68064 | 62.67346 | 62.65655 |
| Goodness-of-fit | 3.29971 | 2.85369 | 3.15049 | 3.06936 | 3.23643 |
| Atomic % | Ti | O | Nb | Mg |
|---|---|---|---|---|
| TiO2-500-2 | 30.22 ± 2.79 | 69.78 ± 0.89 | - | - |
| TiO2-Co-500-2 | 27.59 ± 2.42 | 70.82 ± 0.86 | 0.63 ± 0.13 | 0.96 ± 0.07 |
| TiO2-Co-500-4 | 27.87 ± 2.19 | 70.72 ± 0.81 | 0.66 ± 0.12 | 0.77 ± 0.05 |
| 1 | 28.54 ± 1.49 | 60.14 ± 4.71 | 0.13 ± 0.05 | 11.19 ± 0.56 |
| 2 | 24.63 ± 1.38 | 64.55 ± 5.20 | 0.21 ± 0.06 | 10.58 ±0.57 |
| Sample | ε′ (10 kHz, 300 K) | tan δ (10 kHz, 300 K) | Reference |
|---|---|---|---|
| TiO2-Co-500-2 | 429 | 0.18 | This study |
| TiO2-Co-200-2 | 180 | 0.11 | This study |
| (Mg,Nb) co-doped rutile TiO2 (Mg1/3Nb2/3)xTi(1−x)O2, x = 0.5–7% | ~2 × 104–4 × 104 | ~0.008–0.02 | [16] |
| (In,Nb) co-doped rutile TiO2 | ~1 × 104–1 × 105 | <0.05 | [14] |
| (Sn,Ta) co-doped rutile TiO2 (Sn1/2Ta1/2)x ti(1−x)O2, x = 1–5% | ~3.6 × 104–4 × 104 | ~0.015–0.06 | [8] |
| (La,Nb) co-doped rutile TiO2 (La1/2Nb1/2)x ti(1−x)O2, x = 2–7% | ~3 × 104–6 × 104 | ~0.3–0.35 | [19] |
| (Co,Nb) co-doped rutile TiO2 | ~7 × 103–2.5 × 104 | <0.1 | [5] |
| Samples | 1st Process Ea (eV) (τ1) | 2nd Process Ea (eV) (τ2) | Ea (eV) (σac) |
|---|---|---|---|
| TiO2-500-2 | 0.02 ± 0.01 | 0.12 ± 0.01 | 0.115 ± 0.001 |
| TiO2-500-4 | 0.04 ± 0.02 | 0.12 ± 0.01 | 0.098 ± 0.001 |
| TiO2-Co-200-2 | 0.26 ± 0.06 | 0.51 ± 0.01 | 0.221 ± 0.004 |
| TiO2-Co-200-4 | 0.02 ± 0.01 | 0.53 ± 0.01 | 0.205 ± 0.003 |
| TiO2-Co-300-2 | 0.36 ± 0.04 | 0.54 ± 0.01 | 0.205 ± 0.003 |
| TiO2-Co-300-4 | 0.92 ± 0.16 | 0.57 ± 0.01 | 0.209 ± 0.005 |
| TiO2-Co-400-2 | 0.52 ± 0.15 | 0.53 ± 0.01 | 0.211 ± 0.004 |
| TiO2-Co-400-4 | 0.66 ± 0.11 | 0.55 ± 0.01 | 0.210 ± 0.003 |
| TiO2-Co-500-2 | 0.29 ± 0.06 | 0.50 ± 0.01 | 0.219 ± 0.003 |
| TiO2-Co-500-4 | 0.89 ± 0.13 | 0.55 ± 0.01 | 0.200 ± 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferchaud, L.; Carvalho, J.P.F.; Gavinho, S.R.; Amaral, F.; Toderascu, L.I.; Socol, G.; Costa, L.C.; Benzerga, R.; Teixeira, S.S. Dielectric Properties of Co-Doped TiO2 with Mg and Nb for Energy Storage Applications. Nanomaterials 2025, 15, 1632. https://doi.org/10.3390/nano15211632
Ferchaud L, Carvalho JPF, Gavinho SR, Amaral F, Toderascu LI, Socol G, Costa LC, Benzerga R, Teixeira SS. Dielectric Properties of Co-Doped TiO2 with Mg and Nb for Energy Storage Applications. Nanomaterials. 2025; 15(21):1632. https://doi.org/10.3390/nano15211632
Chicago/Turabian StyleFerchaud, L., J. P. F. Carvalho, S. R. Gavinho, F. Amaral, L. I. Toderascu, G. Socol, L. C. Costa, R. Benzerga, and S. Soreto Teixeira. 2025. "Dielectric Properties of Co-Doped TiO2 with Mg and Nb for Energy Storage Applications" Nanomaterials 15, no. 21: 1632. https://doi.org/10.3390/nano15211632
APA StyleFerchaud, L., Carvalho, J. P. F., Gavinho, S. R., Amaral, F., Toderascu, L. I., Socol, G., Costa, L. C., Benzerga, R., & Teixeira, S. S. (2025). Dielectric Properties of Co-Doped TiO2 with Mg and Nb for Energy Storage Applications. Nanomaterials, 15(21), 1632. https://doi.org/10.3390/nano15211632












