Enhanced Thermoelectric Performance of β-Ag2Se/RGO Composites Synthesized by Cold Sintering Process for Ambient Energy Harvesting
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
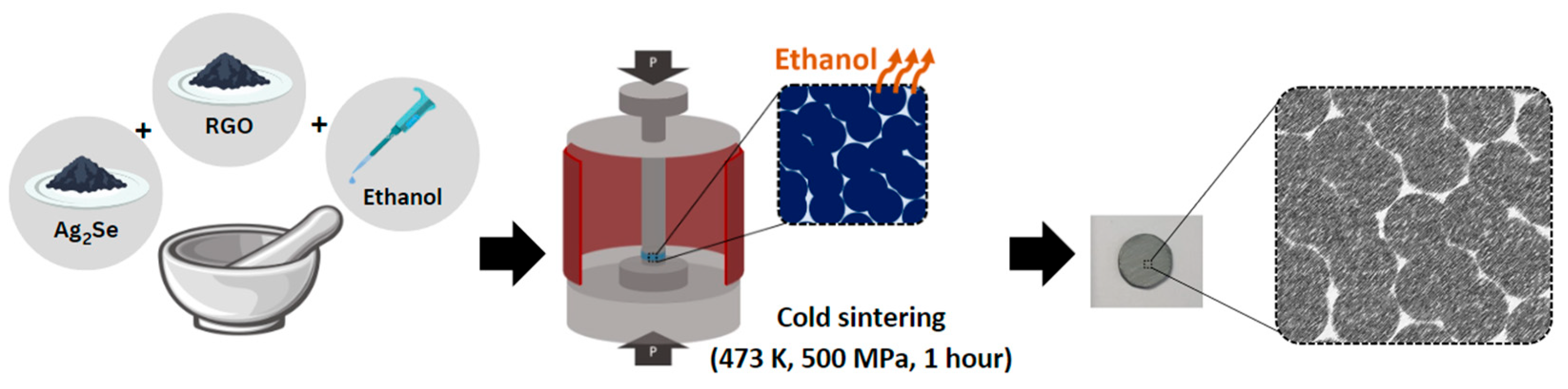
2.2. Synthesis
2.3. Characterization
3. Results and Discussion
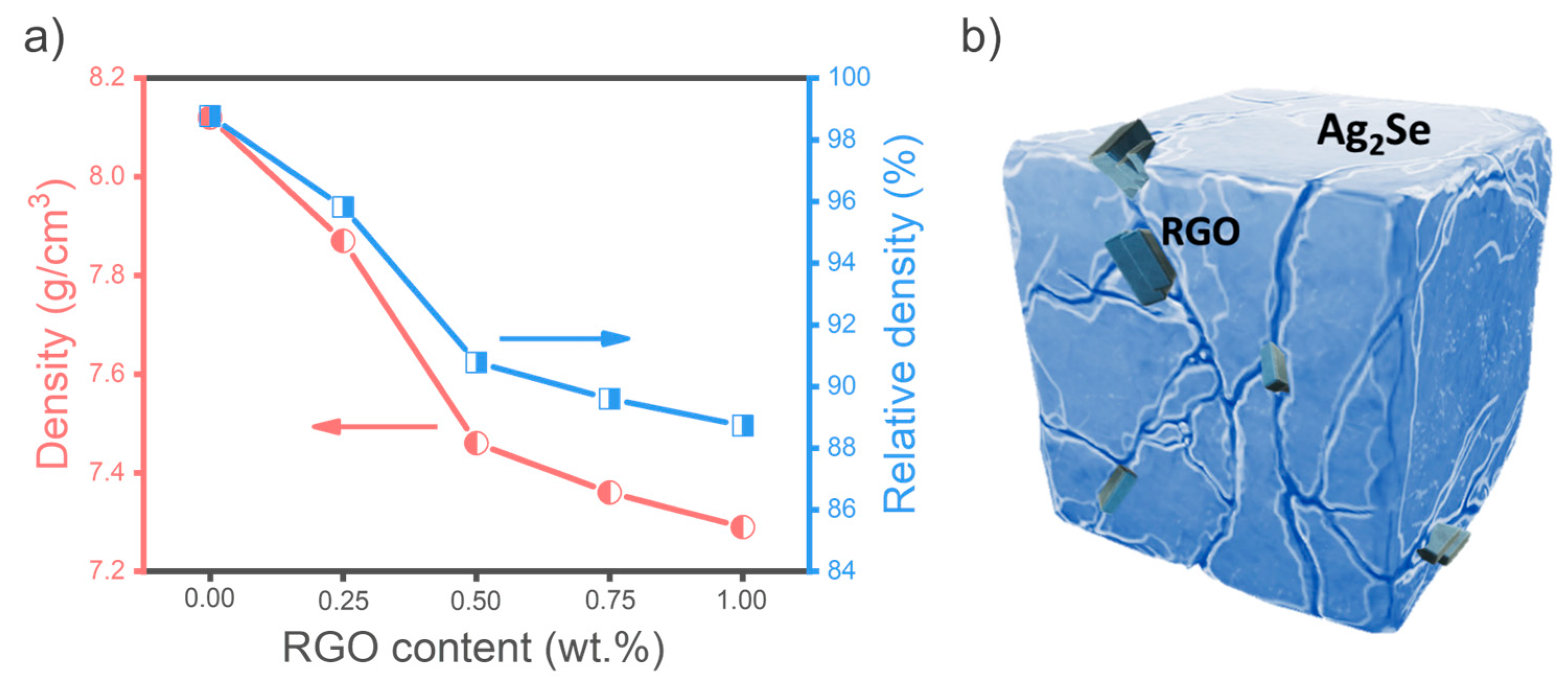
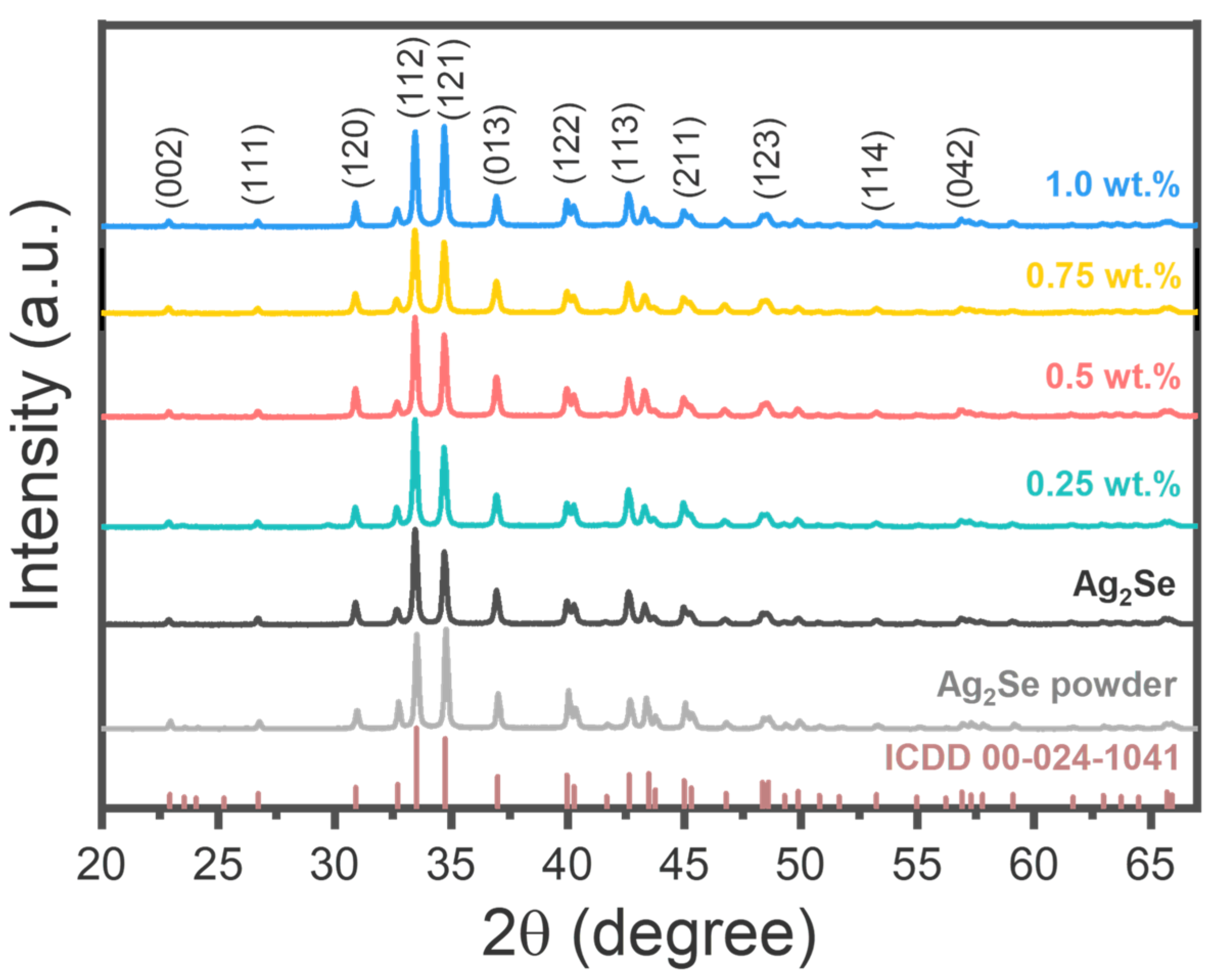
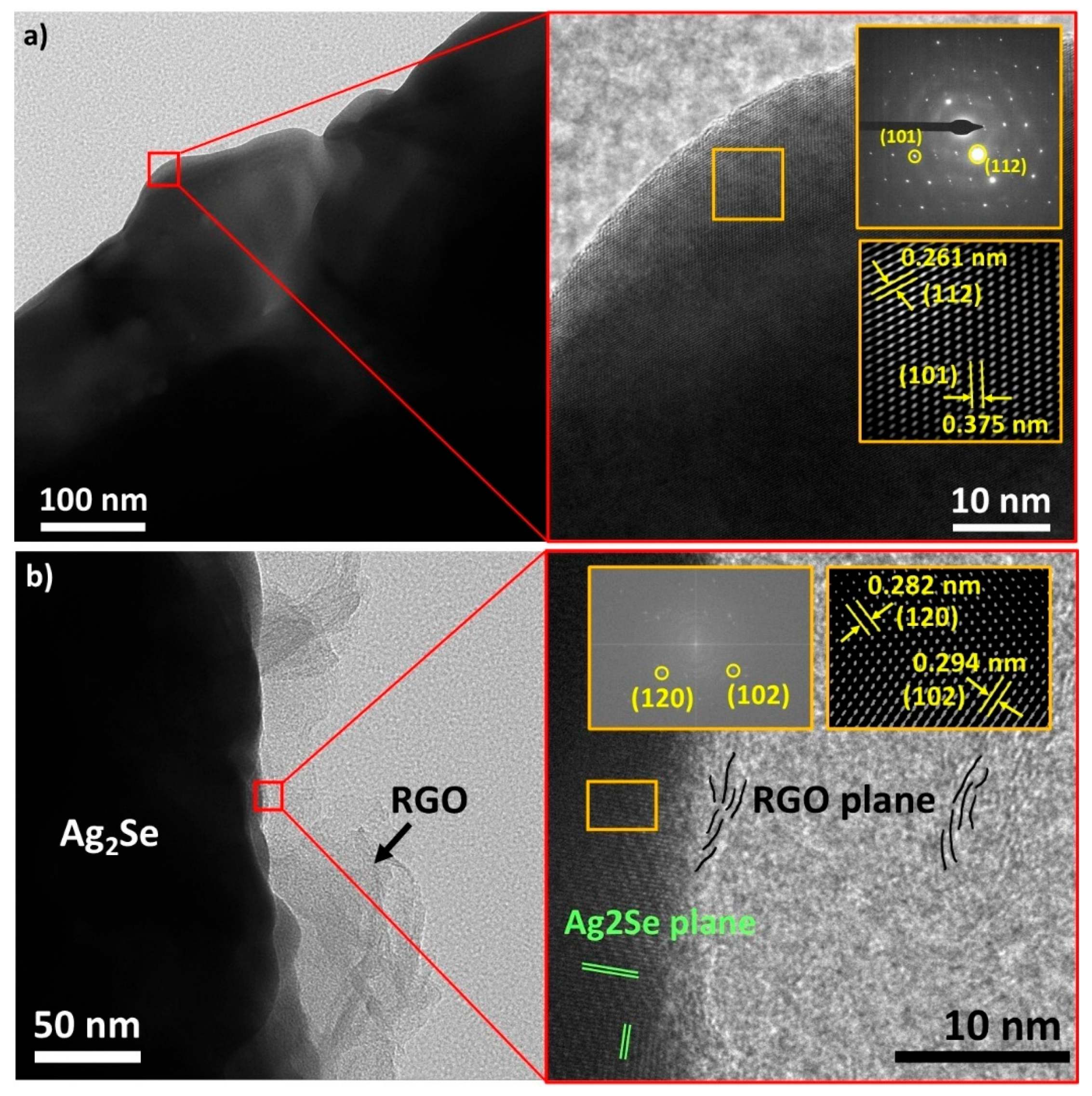
3.1. Physical and Microstructural Analysis
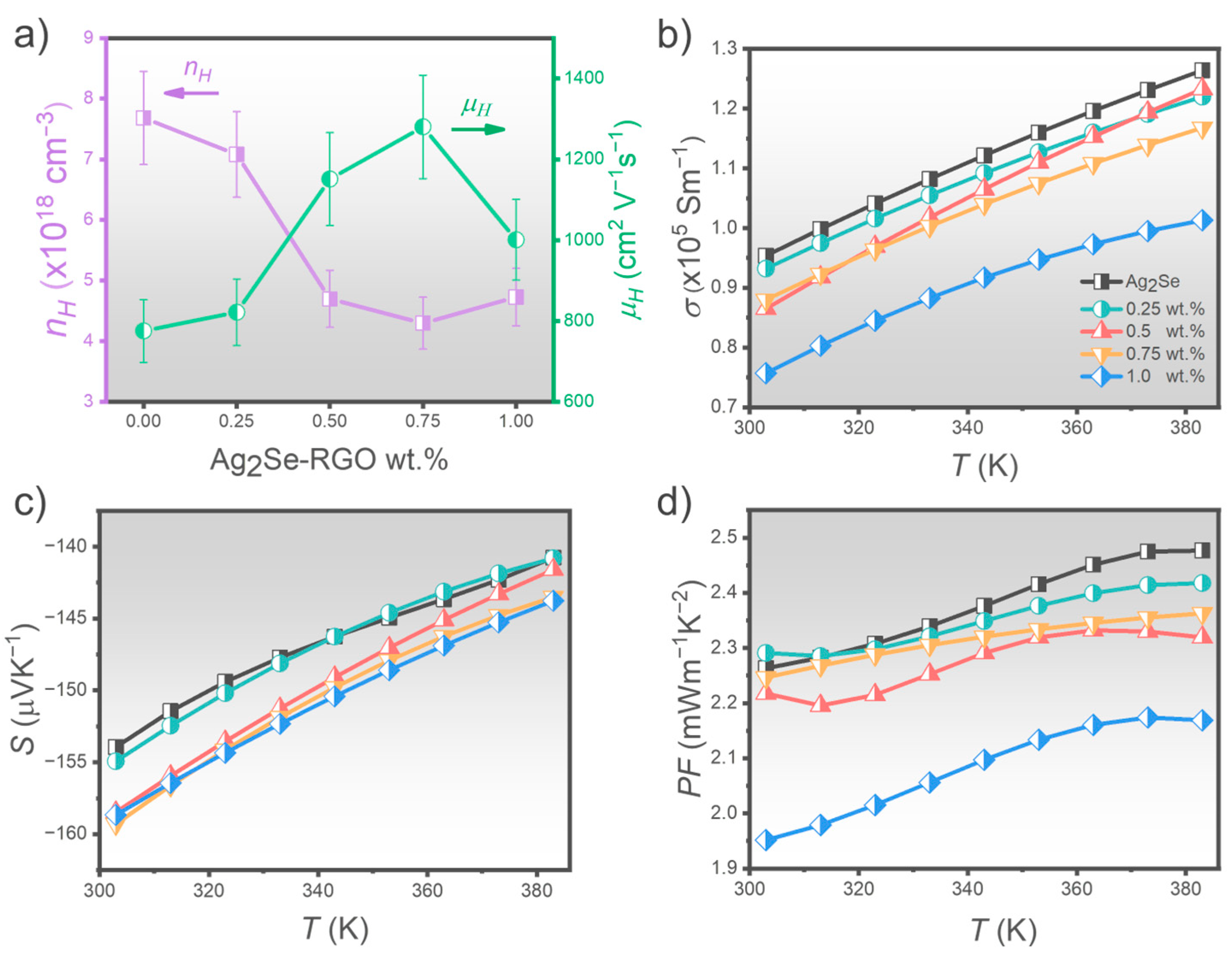
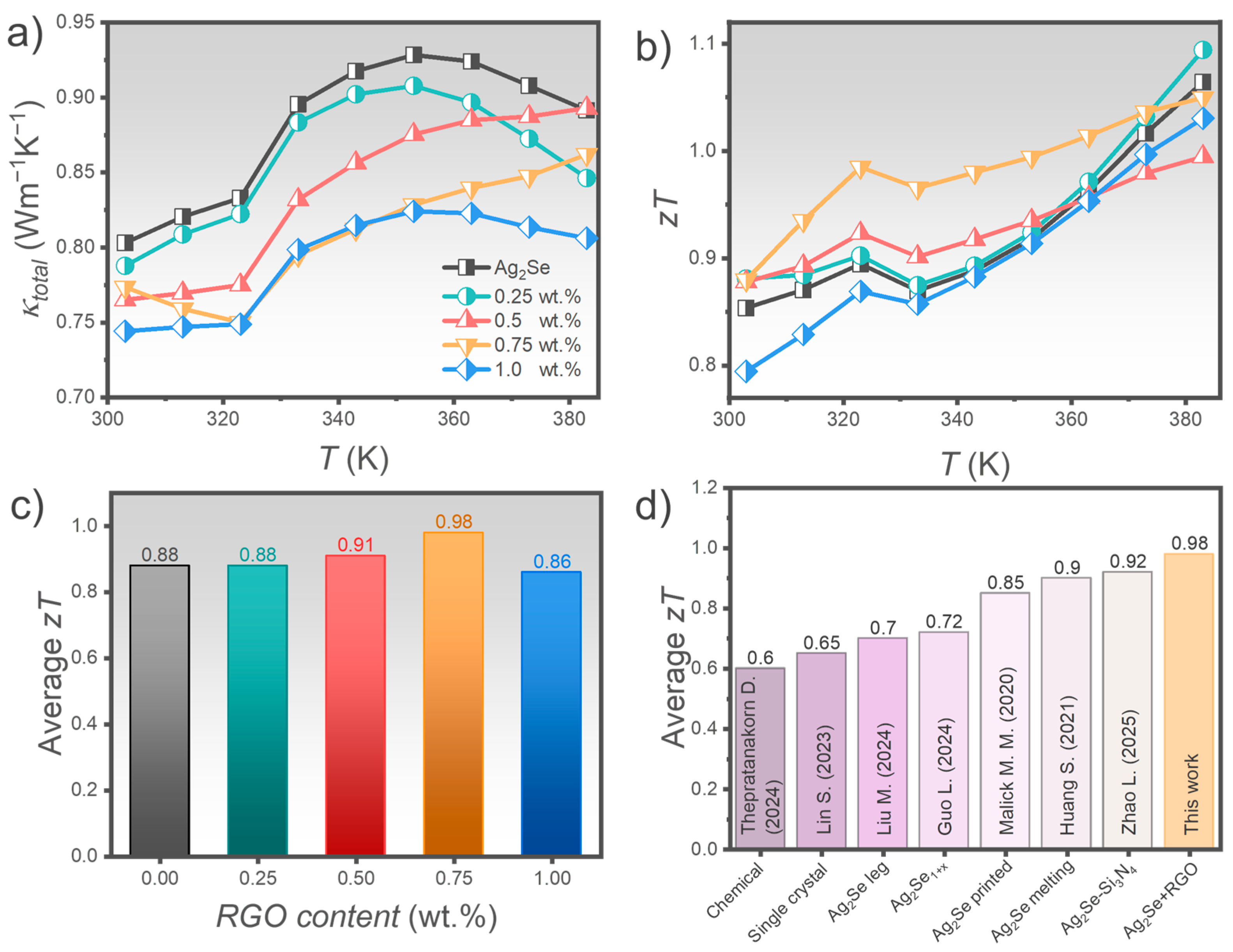
3.2. Transport and Thermoelectric Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Østergaard:, P.A.; Duic, N.; Noorollahi, Y.; Kalogirou, S. Advances in renewable energy for sustainable development. Renew. Energy 2023, 219, 119377. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, L.-D. Thermoelectric materials: Energy conversion between heat and electricity. J. Mater. 2015, 1, 92–105. [Google Scholar] [CrossRef]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef]
- Mamur, H.; Bhuiyan, M.; Korkmaz, F.; Nil, M. A review on bismuth telluride (Bi2Te3) nanostructure for thermoelectric applications. Renew. Sustain. Energy Rev. 2018, 82, 4159–4169. [Google Scholar] [CrossRef]
- Guin, S.N.; Chatterjee, A.; Negi, D.S.; Datta, R.; Biswas, K. High thermoelectric performance in tellurium free p-type AgSbSe2. Energy Environ. Sci. 2013, 6, 2603–2608. [Google Scholar] [CrossRef]
- Ferhat, M.; Nagao, J. Thermoelectric and transport properties of β-Ag2Se compounds. J. Appl. Phys. 2000, 88, 813–816. [Google Scholar] [CrossRef]
- Ding, Y.; Qiu, Y.; Cai, K.; Yao, Q.; Chen, S.; Chen, L.; He, J. High performance n-type Ag2Se film on nylon membrane for flexible thermoelectric power generator. Nat. Commun. 2019, 10, 841. [Google Scholar] [CrossRef] [PubMed]
- Palaporn, D.; Pinitsoontorn, S.; Kurosaki, K.; Snyder, G.J. Porous Ag2Se Fabricated by a Modified Cold Sintering Process with the Average zT Around Unity Near Room Temperature. Adv. Mater. Technol. 2023, 9, 2301242. [Google Scholar] [CrossRef]
- Guo, L.; Lu, X.; Hou, Y.; Zhang, X.; Li, R.; Jin, M.; Lin, S. Stoichiometric manipulation to enhance the thermoelectric and mechanical performance of Ag2Se1+x. Chem. Phys. Lett. 2024, 840, 141132. [Google Scholar] [CrossRef]
- Jood, P.; Ohta, M. Temperature-Dependent Structural Variation and Cu Substitution in Thermoelectric Silver Selenide. ACS Appl. Energy Mater. 2020, 3, 2160–2167. [Google Scholar] [CrossRef]
- Yang, D.; Su, X.; Meng, F.; Wang, S.; Yan, Y.; Yang, J.; He, J.; Zhang, Q.; Uher, C.; Kanatzidis, M.G.; et al. Facile room temperature solventless synthesis of high thermoelectric performance Ag2Se via a dissociative adsorption reaction. J. Mater. Chem. A 2017, 5, 23243–23251. [Google Scholar] [CrossRef]
- Nunna, R.; Qiu, P.; Yin, M.; Chen, H.; Hanus, R.; Song, Q.; Zhang, T.; Chou, M.-Y.; Agne, M.T.; He, J.; et al. Ultrahigh thermoelectric performance in Cu2Se-based hybrid materials with highly dispersed molecular CNTs. Energy Environ. Sci. 2017, 10, 1928–1935. [Google Scholar] [CrossRef]
- Sondors, R.; Gavars, D.; Spalva, E.; Kons, A.; Lohmus, R.; Volkova, M.; Meija, R.; Andzane, J. Synthesis and enhanced room-temperature thermoelectric properties of CuO–MWCNT hybrid nanostructured composites. Nanoscale Adv. 2023, 6, 697–704. [Google Scholar] [CrossRef]
- Palaporn, D.; Iadrat, P.; Yurata, T.; Changtong, C.; Pinitsoontorn, S. Enhanced thermoelectric properties of Ag2Se by manipulation in carrier concentration via acetylene carbon black nanocomposites. Results Phys. 2024, 65, 107967. [Google Scholar] [CrossRef]
- Santhosh, R.; Abinaya, R.; Archana, J.; Ponnusamy, S.; Harish, S.; Navaneethan, M. Controlled grain boundary interfaces of reduced graphene oxide in Ag2Se matrix for low lattice thermal conductivity and enhanced power factor for thermoelectric applications. J. Power Sources 2022, 525, 231045. [Google Scholar] [CrossRef]
- Du, Y.; Li, J.; Xu, J.; Eklund, P. Thermoelectric Properties of Reduced Graphene Oxide/Bi2Te3 Nanocomposites. Energies 2019, 12, 2430. [Google Scholar] [CrossRef]
- Li, C.; Qin, X.; Li, Y.; Li, D.; Zhang, J.; Guo, H.; Xin, H.; Song, C. Simultaneous increase in conductivity and phonon scattering in a graphene nanosheets/(Bi2Te3 )0.2(Sb2Te3)0.8 thermoelectric nanocomposite. J. Alloys Compd. 2016, 661, 389–395. [Google Scholar] [CrossRef]
- Thanh, T.T.; Van Du, N.; Bae, J.; Choi, S.Y.; Ahmed, T.; Khan, S.A.; Cho, J.Y.; Nam, W.H.; Le, D.D.; Lee, S. Combined effect of donor doping and RGO (reduced graphene oxide) coating in La/Nb-doped SrTiO3 thermoelectrics. Solid State Sci. 2021, 122, 106774. [Google Scholar] [CrossRef]
- Chueachot, R.; Nakhowong, R. Achieving thermoelectric performance of rGO/Bi0.5Sb1.5Te3/Cu2Se1–xTex composites through the scattering engineering strategy. J. Materiomics. 2023, 10, 1091–1100. [Google Scholar] [CrossRef]
- Wu, C.; Li, J.; Fan, Y.; Xing, J.; Gu, H.; Zhou, Z.; Lu, X.; Zhang, Q.; Wang, L.; Jiang, W. The effect of reduced graphene oxide on microstructure and thermoelectric properties of Nb-doped A-site-deficient SrTiO3 ceramics. J. Alloys Compd. 2019, 786, 884–893. [Google Scholar] [CrossRef]
- Okhay, O.; Tkach, A. Impact of Graphene or Reduced Graphene Oxide on Performance of Thermoelectric Composites. C 2021, 7, 37. [Google Scholar] [CrossRef]
- Amollo, T.A.; Mola, G.T.; Kirui, M.S.K.; Nyamori, V.O. Graphene for Thermoelectric Applications: Prospects and Challenges. Crit. Rev. Solid State Mater. Sci. 2017, 43, 133–157. [Google Scholar] [CrossRef]
- Bark, H.; Ko, M.; Lee, M.; Lee, W.; Hong, B.; Lee, H. Thermoelectric Properties of Thermally Reduced Graphene Oxide Observed by Tuning the Energy States. ACS Sustain. Chem. Eng. 2018, 6, 7468–7474. [Google Scholar] [CrossRef]
- Rahman, J.U.; Van Du, N.; Nam, W.H.; Shin, W.H.; Lee, K.H.; Seo, W.-S.; Kim, M.H.; Lee, S. Grain Boundary Interfaces Controlled by Reduced Graphene Oxide in Nonstoichiometric SrTiO3-δ Thermoelectrics. Sci. Rep. 2019, 9, 8624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, Y.; Wang, S. Enhancing thermoelectric properties of organic composites through hierarchical nanostructures. Sci. Rep. 2013, 3, 3448. [Google Scholar] [CrossRef] [PubMed]
- Palaporn, D.; Kurosaki, K.; Pinitsoontorn, S. Effect of Sintering Temperature on the Thermoelectric Properties of Ag2Se Fabricated by Spark Plasma Sintering with High Compression. Adv. Energy Sustain. Res. 2023, 4, 2300082. [Google Scholar] [CrossRef]
- Chiu, W.-T.; Chen, C.-L.; Chen, Y.-Y. A strategy to optimize the thermoelectric performance in a spark plasma sintering process. Sci. Rep. 2016, 6, 23143. [Google Scholar] [CrossRef] [PubMed]
- Palaporn, D.; Parse, N.; Tanusilp, S.-A.; Silpawilawan, W.; Kurosaki, K.; Pinitsoontorn, S. Synthesis of Silicon and Higher Manganese Silicide Bulk Nano-composites and Their Thermoelectric Properties. J. Electron. Mater. 2020, 49, 2920–2927. [Google Scholar] [CrossRef]
- Xu, Y.; Yan, M.; Jiang, E.; Zheng, Z.; Wang, H.; Duan, B.; Li, G.; Zhai, P. Thermoelectric properties of Ag2Te prepared by one-step hot-pressing method. Mater. Lett. 2023, 339, 134100. [Google Scholar] [CrossRef]
- Namhongsa, W.; Omoto, T.; Fujii, Y.; Seetawan, T.; Kosuga, A. Effect of the crystal structure on the electronic structure and electrical properties of thermoelectric GeSb6Te10 prepared by hot pressing. Scr. Mater. 2017, 133, 96–100. [Google Scholar] [CrossRef]
- Delaizir, G.; Bernard-Granger, G.; Monnier, J.; Grodzki, R.; Kim-Hak, O.; Szkutnik, P.-D.; Soulier, M.; Saunier, S.; Goeuriot, D.; Rouleau, O.; et al. A comparative study of Spark Plasma Sintering (SPS), Hot Isostatic Pressing (HIP) and microwaves sintering techniques on p-type Bi2Te3 thermoelectric properties. Mater. Res. Bull. 2012, 47, 1954–1960. [Google Scholar] [CrossRef]
- Galotta, A.; Sglavo, V.M. The cold sintering process: A review on processing features, densification mechanisms and perspectives. J. Eur. Ceram. Soc. 2021, 41, 1–17. [Google Scholar] [CrossRef]
- Ndayishimiye, A.; Sengul, M.Y.; Sada, T.; Dursun, S.; Bang, S.H.; Grady, Z.A.; Tsuji, K.; Funahashi, S.; van Duin, A.C.; Randall, C.A. Roadmap for densification in cold sintering: Chemical pathways. Open Ceram. 2020, 2, 100019. [Google Scholar] [CrossRef]
- Tee, S.Y.; Tan, X.Y.; Wang, X.; Lee, C.J.J.; Win, K.Y.; Ni, X.P.; Teo, S.L.; Seng, D.H.L.; Tanaka, Y.; Han, M.-Y. Aqueous Synthesis, Doping, and Processing of n-Type Ag2Se for High Thermoelectric Performance at Near-Room-Temperature. Inorg. Chem. 2022, 61, 6451–6458. [Google Scholar] [CrossRef]
- dos Santos, A.M.; Thomazini, D.; Gelfuso, M.V. Cold sintering and thermoelectric properties of Ca3Co4O9 ceramics. Ceram. Int. 2020, 46, 14064–14070. [Google Scholar] [CrossRef]
- Zhu, B.; Su, X.; Shu, S.; Luo, Y.; Tan, X.Y.; Sun, J.; Sun, D.; Zhang, H.; Zhang, Q.; Suwardi, A.; et al. Cold-Sintered Bi2Te3-Based Materials for Engineering Nanograined Thermoelectrics. ACS Appl. Energy Mater. 2022, 5, 2002–2010. [Google Scholar] [CrossRef]
- Piyasin, P.; Palaporn, D.; Kurosaki, K.; Pinitsoontorn, S. High-performance thermoelectric properties of Cu2Se fabricated via cold sintering process. Solid State Sci. 2024, 149, 107448. [Google Scholar] [CrossRef]
- Jabr, A.; Jones, H.N.; Argüelles, A.P.; Trolier-McKinstry, S.; Randall, C.; Bermejo, R. Scaling up the cold sintering process of ceramics. J. Eur. Ceram. Soc. 2023, 43, 5319–5329. [Google Scholar] [CrossRef]
- Theprattanakorn, D.; Kaewmaraya, T.; Pinitsoontorn, S. Boosting thermoelectric efficiency of Ag2Se through cold sintering process with Ag nano-precipitate formation. Int. J. Miner. Met. Mater. 2024, 31, 2760–2769. [Google Scholar] [CrossRef]
- Kongsip, N.; Kaewmaraya, T.; Kamwanna, T.; Pinitsoontorn, S. Enhancing thermoelectric properties of silver selenide through cold sintering process using aqua regia as a liquid medium. Next Mater. 2024, 3, 100136. [Google Scholar] [CrossRef]
- Synthesis, Characterization, and Electrochemical Properties of Reduced Graphene Oxide Produced from Sugarcane Bagasse for Supercapacitor Applications. Biointerface Res. Appl. Chem. 2024, 14, 109. [CrossRef]
- Zhao, D.; Wang, X.; Wu, D. Enhanced Thermoelectric Properties of Graphene/Cu2SnSe3 Composites. Crystals 2017, 7, 71. [Google Scholar] [CrossRef]
- Zaferani, S.H.; Ghomashchi, R.; Vashaee, D. Assessment of Thermoelectric, Mechanical, and Microstructural Reinforcement Properties of Graphene-Mixed Heterostructures. ACS Appl. Energy Mater. 2021, 4, 3573–3583. [Google Scholar] [CrossRef]
- Fang, C.; de Groot, R.; Wiegers, G. Ab initio band structure calculations of the low-temperature phases of Ag2Se, Ag2Te and Ag3AuSe2. J. Phys. Chem. Solids 2002, 63, 457–464. [Google Scholar] [CrossRef]
- Rameshkumar, S.; Jaiganesh, G.; Jayalakshmi, V.; Palanivel, B. Ab initio calculation of structural stability, electronic and optical properties of Ag2Se. AIP Conf. Proc. 2015, 1665, 090024. [Google Scholar] [CrossRef]
- Shin, W.H.; Ahn, K.; Jeong, M.; Yoon, J.S.; Song, J.M.; Lee, S.; Seo, W.S.; Lim, Y.S. Enhanced thermoelectric performance of reduced graphene oxide incorporated bismuth-antimony-telluride by lattice thermal conductivity reduction. J. Alloys Compd. 2017, 718, 342–348. [Google Scholar] [CrossRef]
- Huang, L.; Lu, J.; Ma, D.; Ma, C.; Zhang, B.; Wang, H.; Wang, G.; Gregory, D.H.; Zhou, X.; Han, G. Facile in situ solution synthesis of SnSe/rGO nanocomposites with enhanced thermoelectric performance. J. Mater. Chem. A 2019, 8, 1394–1402. [Google Scholar] [CrossRef]
- Bin Rahaman, A.; Sarkar, A.; Singha, T.; Chakraborty, K.; Dutta, S.; Pal, T.; Ghosh, S.; Datta, P.K.; Banerjee, D. Electrical transport properties and ultrafast optical nonlinearity of rGO–metal chalcogenide ensembles. Nanoscale Adv. 2020, 2, 1573–1582. [Google Scholar] [CrossRef]
- C, A.; Abinaya, R.; Mani, N.; Krishnan, H.S. Strain Effect in the Layered MoS2-rGO Heterostructure with Enhanced Performance for Flexible Thermoelectric Applications. ACS Appl. Energy Mater. 2025, 8, 1589–1597. [Google Scholar] [CrossRef]
- Lin, S.; Guo, L.; Wang, X.; Liu, Y.; Wu, Y.; Li, R.; Shao, H.; Jin, M. Revealing the promising near-room-temperature thermoelectric performance in Ag2Se single crystals. J. Mater. 2023, 9, 754–761. [Google Scholar] [CrossRef]
- Mallick, M.; Rösch, A.G.; Franke, L.; Gall, A.; Ahmad, S.; Gesswein, H.; Mazilkin, A.; Kuebel, C.; Lemmer, U. New frontier in printed thermoelectrics: Formation of β-Ag2Se through thermally stimulated dissociative adsorption leads to high ZT. J. Mater. Chem. A 2020, 8, 16366–16375. [Google Scholar] [CrossRef]
- Huang, S.; Wei, T.-R.; Chen, H.; Xiao, J.; Zhu, M.; Zhao, K.; Shi, X. Thermoelectric Ag2Se: Imperfection, Homogeneity, and Reproducibility. ACS Appl. Mater. Interfaces 2021, 13, 60192–60199. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, H.; Dong, J.; Zhang, G.; Cao, Q.; Ding, Z.; Wang, S.; Wang, J.; Li, Z. Engineering Ag2Se thermoelectrics via amorphous nano-Si3N4: A dual-functional strategy for enhanced zT and mechanical strength. J. Mater. Chem. C 2025, 13, 17961–17969. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, X.; Zhang, S.; Pei, Y. Ag2Se as a tougher alternative to n-type Bi2Te3 thermoelectrics. Nat. Commun. 2024, 15, 6580. [Google Scholar] [CrossRef] [PubMed]

| Sample | RGO wt.% | Phase |
|---|---|---|
| Ag2Se-powder | - | Powder |
| Ag2Se | - | Pellet |
| Ag2Se-0.25% | 0.25 | Pellet |
| Ag2Se-0.5% | 0.5 | Pellet |
| Ag2Se-0.75% | 0.75 | Pellet |
| Ag2Se-1% | 1.0 | Pellet |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palaporn, D.; Darmawan, I.; Piyasin, P.; Pinitsoontorn, S. Enhanced Thermoelectric Performance of β-Ag2Se/RGO Composites Synthesized by Cold Sintering Process for Ambient Energy Harvesting. Nanomaterials 2025, 15, 1631. https://doi.org/10.3390/nano15211631
Palaporn D, Darmawan I, Piyasin P, Pinitsoontorn S. Enhanced Thermoelectric Performance of β-Ag2Se/RGO Composites Synthesized by Cold Sintering Process for Ambient Energy Harvesting. Nanomaterials. 2025; 15(21):1631. https://doi.org/10.3390/nano15211631
Chicago/Turabian StylePalaporn, Dulyawich, Ikhwan Darmawan, Piyawat Piyasin, and Supree Pinitsoontorn. 2025. "Enhanced Thermoelectric Performance of β-Ag2Se/RGO Composites Synthesized by Cold Sintering Process for Ambient Energy Harvesting" Nanomaterials 15, no. 21: 1631. https://doi.org/10.3390/nano15211631
APA StylePalaporn, D., Darmawan, I., Piyasin, P., & Pinitsoontorn, S. (2025). Enhanced Thermoelectric Performance of β-Ag2Se/RGO Composites Synthesized by Cold Sintering Process for Ambient Energy Harvesting. Nanomaterials, 15(21), 1631. https://doi.org/10.3390/nano15211631







