Ion-Exchanged Beta-Based Cobalt Catalyst for Efficient Degradation of Aqueous Dye Acid Orange II
Abstract
1. Introduction
2. Experimental Section
2.1. Sample Preparation
2.2. Sample Characterization
2.3. Catalytic Tests
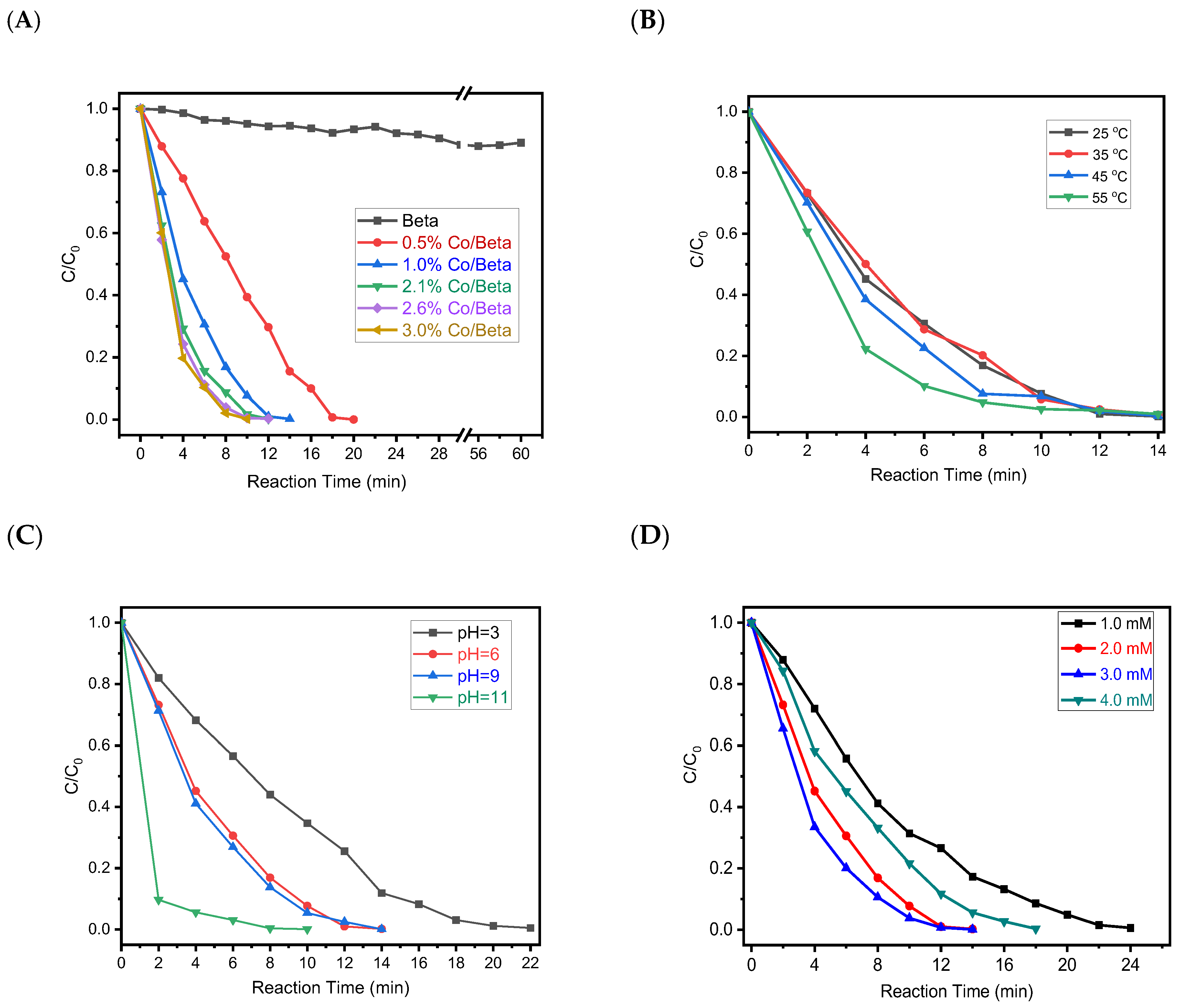
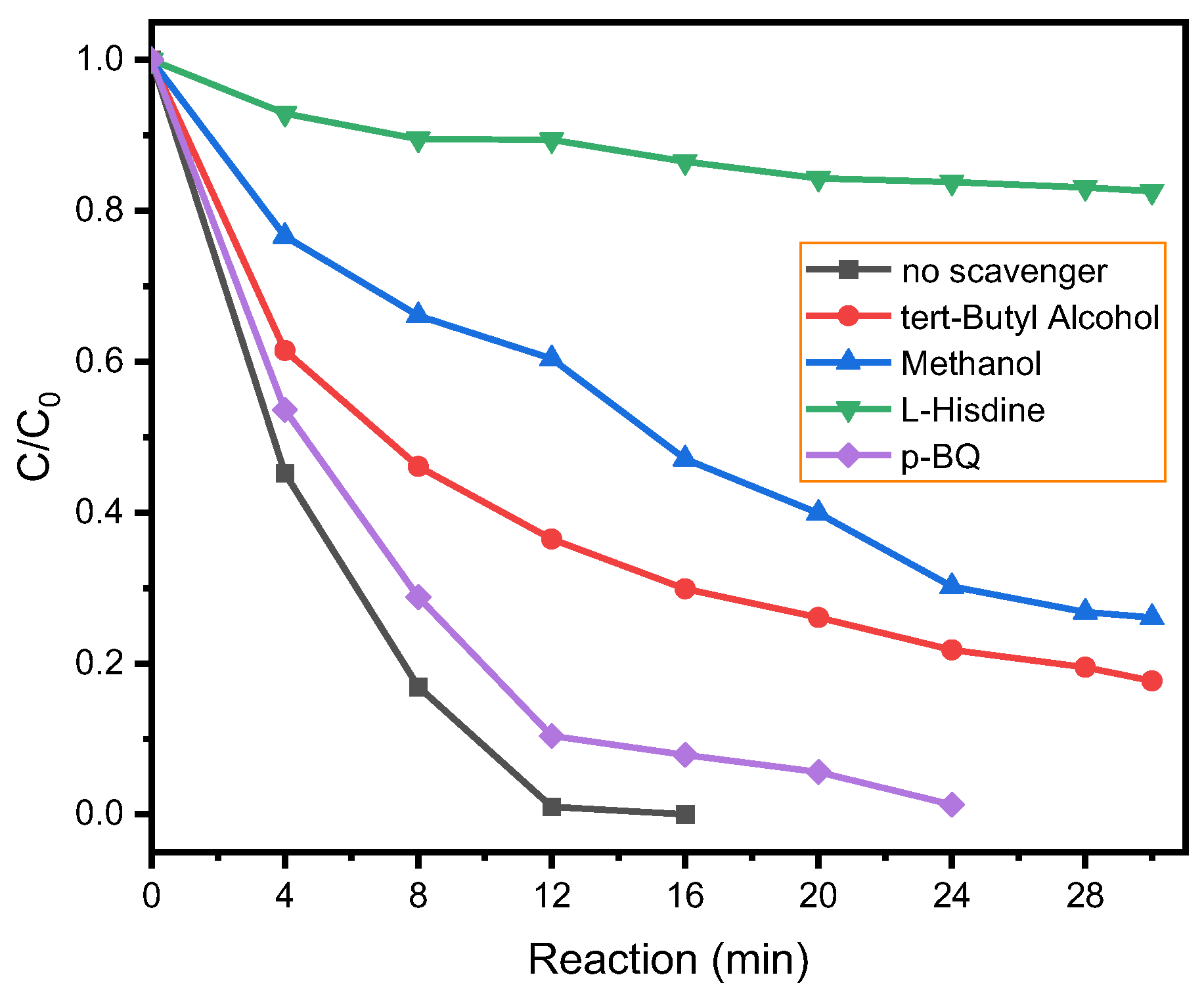
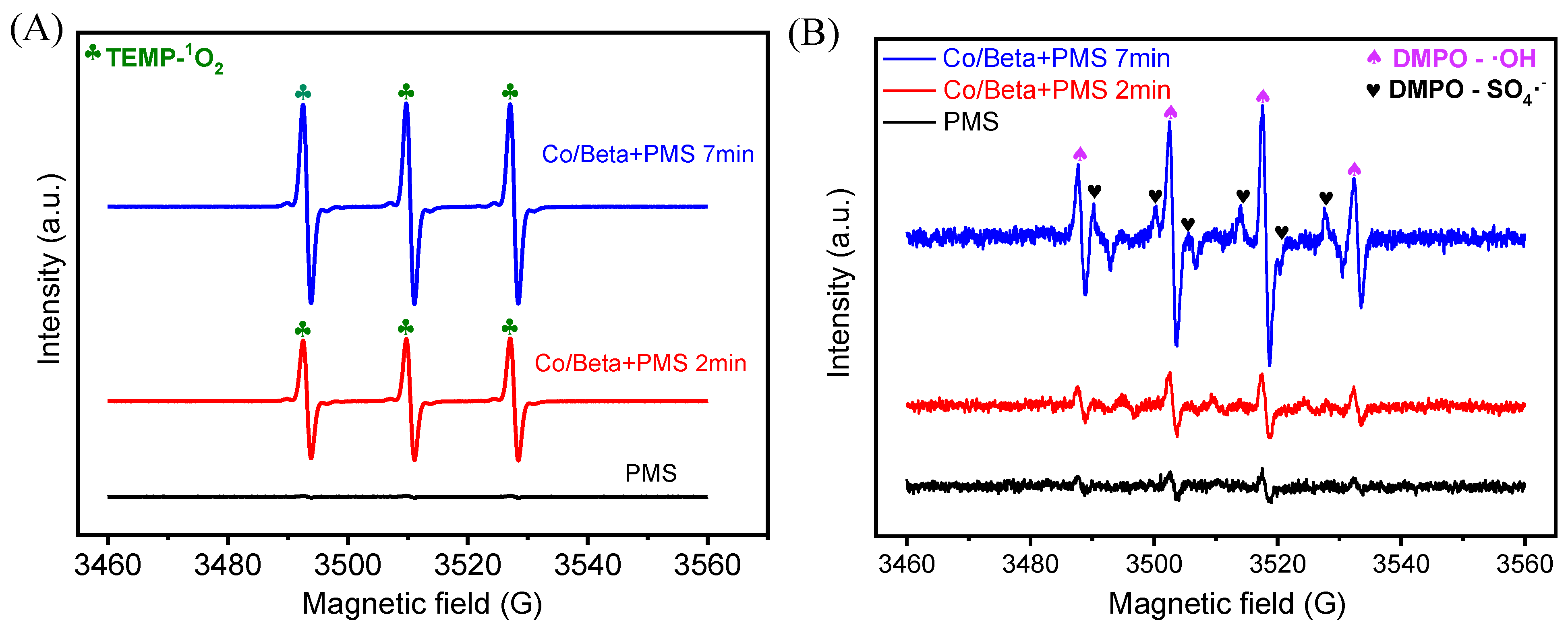
3. Results and Discussion
| Catalysts | CAO7 (mM) | CPMS (mM) | CCatalyst (g·L−1) | t (min) | Removal Efficiency (%) b | References |
|---|---|---|---|---|---|---|
| CUST-562 | 0.06 | 0.16 g/L a | 0.12 | 30 | 93.5 | [45] |
| HDCo@C-800 | 0.03 | 0.76 g/L a | 0.1 | 60 | >99.5 | [46] |
| CoCuAl-LDOs | 0.06 | 0.1 g/L a | 0.1 | 30 | >99.5 | [47] |
| Co-HPNC | 0.1 | 1.0 | 0.05 | 10 | 98.1 | [48] |
| Co-Fc-MOFs | 0.06 | 4.0 | 0.25 | 90 | 83.7 | [49] |
| Co-CoO@BC | 0.06 | 1.0 | 0.06 | 20 | 95.0 | [50] |
| Co–MIL-101(Fe) | 0.1 | 8.0 | 0.2 | 180 | 98.0 | [51] |
| Co/SBA-15 | 0.2 | 5.0 | 0.5 | 90 | >99.5 | [52] |
| Co3O4/NF | 0.1 | 0.5 | 2.0 mM a | 30 | >99.5 | [53] |
| nano-Co3O4 | 0.2 | 2.0 | 0.5 | 30 | >99.5 | [54] |
| Co/ZSM-5 | 0.2 | 2.0 | 0.3 | 16 | >99.5 | Pw c |
| Co/Y | 0.2 | 2.0 | 0.3 | 14 | >99.5 | Pw c |
| Co/Beta | 0.2 | 2.0 | 0.3 | 10 | >99.5 | Pw c |
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Samadi, A.; Xie, M.; Li, J.; Shon, H.; Zheng, C.; Zhao, S. Polyaniline-based adsorbents for aqueous pollutants removal: A review. Chem. Eng. J. 2021, 418, 129425. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Z.; Wang, Y.; Bai, Z.; Zheng, T.; Dai, X.; Liu, S.; Gui, D.; Liu, W.; Chen, M.; et al. A mesoporous cationic thorium-organic framework that rapidly traps anionic persistent organic pollutants. Nat. Commun. 2017, 8, 1354. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Repo, E.; Yin, D.; Meng, Y.; Jafari, S.; Sillanpaa, M. EDTA-Cross-Linked β-Cyclodextrin: An environmentally friendly bifunctional adsorbent for simultaneous adsorption of metals and cationic dyes. Environ. Sci. Technol. 2015, 49, 10570–10580. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, X.; Meng, Y.; Pan, G.; Ni, Z.; Xia, S. Layered double hydroxides-based photocatalysts and visible-light driven photodegradation of organic pollutants: A review. Chem. Eng. J. 2020, 392, 123684. [Google Scholar] [CrossRef]
- Ji, J.; Yan, Q.; Yin, P.; Mine, S.; Matsuoka, M.; Xing, M. Defects on CoS2-x: Tuning redox reactions for sustainable degradation of organic pollutants. Angew. Chem. Int. Ed. 2021, 60, 2903–2908. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Ye, W.; Xie, M.; Seo, D.; Luo, J.; Wan, Y.; van der Bruggen, B. Environmental impacts and remediation of dye-containing wastewater. Nat. Rev. Earth Environ. 2023, 4, 785–803. [Google Scholar] [CrossRef]
- Singh, A.; Pal, D.; Mohammad, A.; Alhazmi, A.; Haque, S.; Yoon, T.; Srivastava, N.; Gupta, V.K. Biological remediation technologies for dyes and heavy metals in wastewater treatment: New insight. Bioresour. Technol. 2022, 343, 126154. [Google Scholar] [CrossRef]
- Zhang, S.; Hedtke, T.; Zhu, Q.; Sun, M.; Weon, S.; Zhao, Y.; Stavitski, E.; Elimelech, M.; Kim, J. Membrane-confined iron oxychloride nanocatalysts for highly efficient heterogeneous fenton water treatment. Environ. Sci. Technol. 2021, 55, 9266–9275. [Google Scholar] [CrossRef]
- Alsbaiee, A.; Smith, B.; Xiao, L.; Ling, Y.; Helbling, D.; Dichtel, W.R. Rapid removal of organic micropollutants from water by a porous β-cyclodextrin polymer. Nature 2016, 529, 190–194. [Google Scholar] [CrossRef]
- Hunge, Y.; Yadav, A.; Khan, S.; Takagi, K.; Suzuki, N.; Teshima, K.; Terashima, C.; Fujishima, A. Photocatalytic degradation of bisphenol A using titanium dioxide@nanodiamond composites under UV light illumination. J. Colloid Interface Sci. 2021, 582, 1058–1066. [Google Scholar] [CrossRef]
- Yu, C.; Wu, Z.; Liu, R.; Dionysiou, D.; Yang, K.; Wang, C.; Liu, H. Novel fluorinated Bi2MoO6 nanocrystals for efficient photocatalytic removal of water organic pollutants under different light source illumination. Appl. Catal. B Environ. 2017, 209, 1–11. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.; Li, W.; Zhao, M.; Hou, T.; Zhou, J.; Yang, B. PAN/Zein/BiOCl fibrous membranes with ultrafast liquid transport channels and petal-textured energy conversion layers for oil-water separation and photocatalytic degradation. Appl. Surf. Sci. 2025, 695, 162845. [Google Scholar] [CrossRef]
- Ren, Z.; Che, X.; Wang, Q.; Zhang, F.; Han, J.; Zhou, C.; Liang, L.; Zhu, J.; Li, Z.; Li, G.; et al. RuO2/MnOOH heterojunctions promotes the formation of high-valent metal-oxo species for ultrafast removal of organic pollutants. Appl. Catal. B Environ. Energy 2025, 371, 125176. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Wu, G.; Shen, Q.; Zhang, W.; Guo, J.; Huang, L.; Feng, L.; Yuan, C.; Sun, F. CoFe2S4-modified CNTs catalyst to activate peroxymonosulfate under a wide range pH for high-efficient tetracycline degradation via radical and non-radical paths. Sep. Purif. Technol. 2025, 363, 131972. [Google Scholar] [CrossRef]
- Shang, Y.; Xu, X.; Gao, B.; Wang, S.; Duan, X. Single-atom catalysis in advanced oxidation processes for environmental remediation. Chem. Soc. Rev. 2021, 50, 5281–5322. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Xie, X.; Zhong, H.; Xiao, F.; Xie, R.; Liu, B.; Bao, H.; Hu, D.; Zhang, P.; Huang, H. Efficient catalytic elimination of toxic volatile organic compounds via advanced oxidation process wet scrubbing with bifunctional cobalt sulfide/activated carbon catalysts. Environ. Sci. Technol. 2024, 58, 8846–8856. [Google Scholar] [CrossRef]
- ElAsmar, R.; Baalbaki, A.; Abou Khalil, Z.; Naim, S.; Bejjani, A.; Ghauch, A. Iron-based metal organic framework MIL-88-A for the degradation of naproxen in water through persulfate activation. Chem. Eng. J. 2021, 405, 126701. [Google Scholar] [CrossRef]
- Lan, M.; Li, Y.; Wang, C.; Li, X.; Cao, J.; Meng, L.; Gao, S.; Ma, Y.; Ji, H.; Xing, M. Multi-channel electron transfer induced by polyvanadate in metal-organic framework for boosted peroxymonosulfate activation. Nat. Commun. 2024, 15, 7208. [Google Scholar] [CrossRef]
- Gu, C.; Wang, S.; Zhang, A.; Liu, C.; Jiang, J.; Yu, H. Tuning electronic structure of metal-free dual-site catalyst enables exclusive singlet oxygen production and in-situ utilization. Nat. Commun. 2024, 15, 5771. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, D.; Ge, X.; Yu, J.; Zhao, Y.; Bu, Y. Anchored cobalt nanoparticles on layered perovskites for rapid peroxymonosulfate activation in antibiotic degradation. Adv. Mater. 2024, 36, 2402935. [Google Scholar] [CrossRef]
- Li, J.; He, Y.; Tan, L.; Zhang, P.; Peng, X.; Oruganti, A.; Yang, G.; Abe, H.; Wang, Y.; Tsubaki, N. Integrated tuneable synthesis of liquid fuels via Fischer-Tropsch technology. Nat. Catal. 2018, 1, 787–793. [Google Scholar] [CrossRef]
- Roth, W.J.; Nachtigall, P.; Morris, R.E.; Cejka, J. Two-dimensional zeolites: Current status and perspectives. Chem. Rev. 2014, 114, 4807–4837. [Google Scholar] [CrossRef]
- Pan, X.; Jiao, F.; Miao, D.; Bao, X. Oxide-zeolite-based composite catalyst concept that enables syngas chemistry beyond Fischer-Tropsch synthesis. Chem. Rev. 2021, 121, 6588–6609. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yi, X.; Hui, Y.; Wang, L.; Chen, W.; Qin, Y.; Wang, M.; Ma, J.; Chu, X.; Wang, Y.; et al. Isolated boron in zeolite for oxidative dehydrogenation of propane. Science 2021, 372, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Babucci, M.; Zhang, Y.; Lund, A.; Liu, L.; Li, J.; Chen, Y.; Hoffman, A.; Bare, S.R.; Han, Y.; et al. Propane dehydrogenation catalyzed by isolated Pt atoms in siozn-oh nests in dealuminated zeolite beta. J. Am. Chem. Soc. 2021, 143, 21364–21378. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Qin, G.; Han, J.; Zhang, W.; Wang, N.; Zheng, Y.; Jiang, Q.; Ji, T.; Yuan, Z.; Xiao, J.; et al. Atomic insight into the local structure and microenvironment of isolated Co-motifs in mfi zeolite frameworks for propane dehydrogenation. J. Am. Chem. Soc. 2022, 144, 12127–12137. [Google Scholar] [CrossRef]
- Hu, G.; Yang, J.; Duan, X.; Farnood, R.; Yang, C.; Yang, J.; Liu, W.; Liu, Q. Recent developments and challenges in zeolite-based composite photocatalysts for environmental applications. Chem. Eng. J. 2021, 417, 129209. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Nguyen, H.; Ahmed, S.F.; Rajamohan, N.; Yusuf, M.; Sharma, A.; Arunkumar, P.; Deepanraj, B.; Tran, H.T.; Al-Gheethi, A.; et al. Emerging waste-to-wealth applications of fly ash for environmental remediation: A review. Environ. Res. 2023, 227, 115800. [Google Scholar] [CrossRef]
- Liaquat, I.; Munir, R.; Abbasi, N.A.; Sadia, B.; Muneer, A.; Younas, F.; Younas, M.F.; Zahid, M.; Noreen, S. Exploring zeolite-based composites in adsorption and photocatalysis for toxic wastewater treatment: Preparation, mechanisms, and future perspectives. Environ. Pollut. 2024, 349, 123922. [Google Scholar] [CrossRef]
- Zhang, G.; Choi, W.; Kim, S.H.; Hong, S.B. Selective photocatalytic degradation of aquatic pollutants by titania encapsulated into FAU-type zeolites. J. Hazard. Mater. 2011, 188, 198–205. [Google Scholar] [CrossRef]
- Liu, L.; Lu, J.; Yang, Y.; Ruettinger, W.; Gao, X.; Wang, M.; Lou, H.; Wang, Z.; Liu, Y.; Tao, X.; et al. Dealuminated Beta zeolite reverses Ostwald ripening for durable copper nanoparticle catalysts. Science 2024, 383, 94–101. [Google Scholar] [CrossRef]
- Verboekend, D.; Nuttens, N.; Locus, R.; Van Aelst, J.; Verolme, P.; Groen, J.C.; Perez-Ramirez, J.; Sels, B.F. Synthesis, characterisation, and catalytic evaluation of hierarchical faujasite zeolites: Milestones, challenges, and future directions. Chem. Soc. Rev. 2016, 45, 3331–3352. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, J.; Corma, A. Applications of zeolites to C1 Chemistry: Recent advances, challenges, and opportunities. Adv. Mater. 2020, 32, 2002927. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; van Bokhoven, J.A. Water structures on acidic zeolites and their roles in catalysis. Chem. Soc. Rev. 2024, 53, 3065–3095. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, W.; Gao, Y.; Lin, P.; Liu, J.; Zhang, J.; Huang, T. The catalyst derived from the sulfurized Co-doped metal-organic framework (MOF) for peroxymonosulfate (PMS) activation and its application to pollutant removal. Sep. Purif. Technol. 2022, 285, 120362. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Luo, P.; Li, C.; Zeng, F.; Sun, T.; Lei, H.; Liu, X.; Liu, C. Peroxymonosulfate activated M-MOF-74 (M = Co, Fe, Ni) visible light photocatalysts for methylene blue degradation enhancement. Phys. Chem. Chem. Phys. 2025, 27, 10387–10398. [Google Scholar] [CrossRef]
- Wang, M.; Wang, F.; Wang, P.; Chu, H.; Fu, H.; Zhao, C.; Wang, C.; Zhao, Y. Highly efficient and selective organic pollutants degradation via peroxymonosulfate activation over micron-sized Co-MOF: Nearly 100% singlet oxygen mechanism. Sep. Purif. Technol. 2023, 326, 124806. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, G.; Chen, Y.; Han, B.; Xia, K.; Zhou, C. Utilizing cobalt-doped materials as heterogeneous catalysts to activate peroxymonosulfate for organic pollutant degradation: A critical review. Environ. Sci. Wat. Res. 2021, 7, 1197–1211. [Google Scholar] [CrossRef]
- Lin, K.; Chen, B. Prussian blue analogue derived magnetic carbon/cobalt/iron nanocomposite as an efficient and recyclable catalyst for activation of peroxymonosulfate. Chemosphere 2017, 166, 146–156. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, X.; Wei, J.; Chen, M.; Bi, J.; Bao, Y. Understanding the Iron-Cobalt synergies in ZSM-5: Enhanced peroxymonosulfate activation and organic pollutant degradation. ACS Omega 2022, 7, 17811–17821. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, D.; Feng, P.; Shi, S.; Wang, C.; Zheng, A.; Lu, G.; Tian, Z. Synthesis of zeolite Beta containing ultra-small CoO particles for ethylbenzene oxidation. Chin. J. Catal. 2017, 38, 1207–1215. [Google Scholar] [CrossRef]
- Dedecek, J.; Capek, L.; Kaucky, D.; Sobalik, Z.; Wichterlova, B. Siting and distribution of the Co ions in beta zeolite: A UV-Vis-NIR and FTIR study. J. Catal. 2002, 211, 198–207. [Google Scholar] [CrossRef]
- Li, H.; Wan, J.; Ma, Y.; Wang, Y. Synthesis of novel core-shell Fe0@Fe3O4 as heterogeneous activator of persulfate for oxidation of dibutyl phthalate under neutral conditions. Chem. Eng. J. 2016, 301, 315–324. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Zhang, D.; Lan, Y.; Guo, J. CuO-Co3O4@CeO2 as a heterogeneous catalyst for efficient degradation of 2,4-dichlorophenoxyacetic acid by peroxymonosulfate. J. Hazard. Mater. 2020, 381, 121209. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Lin, Z.; Hu, X.; Su, Z. Significantly enhanced activation of peroxymonosulfate by a 3D Co-based MOF for the efficient degradation of organic dyes. J. Mol. Struct. 2024, 1312, 138564. [Google Scholar] [CrossRef]
- Hou, J.; Yu, W.; Zhang, R.; He, X.; Zhu, H.; Li, X. Confined highly dispersed trace cobalt species in shell porous carbon for activating peroxymonosulfate: Role of superoxide radical and singlet oxygen. J. Environ. Chem. Eng. 2023, 11, 111307. [Google Scholar] [CrossRef]
- Luo, L.; Wang, Y.; Zhu, M.; Cheng, X.; Zhang, X.; Meng, X.; Huang, X.; Hao, H. Co−Cu−Al layered double oxides as heterogeneous catalyst for enhanced degradation of organic pollutants in wastewater by activating peroxymonosulfate: Performance and synergistic effect. Ind. Eng. Chem. Res. 2019, 58, 8699–8711. [Google Scholar] [CrossRef]
- Long, Y.; Li, S.; Yang, P.; Chen, X.; Liu, W.; Zhan, X.; Xue, C.; Liu, D.; Huang, W. Synthesis of ZIF-67 derived honeycomb porous Co/NC catalyst for AO7 degradation via activation of peroxymonosulfate. Sep. Purif. Technol. 2022, 286, 120470. [Google Scholar] [CrossRef]
- Lv, X.; Leng, Y.; Wang, R.; Wei, Y.; Ren, X.; Guo, W. Persulfate activation by ferrocene-based metal–organic framework microspheres for efficient oxidation of orange acid 7. Environ. Sci. Pollut. Res. 2022, 29, 34464–34474. [Google Scholar] [CrossRef]
- Sun, C.; Li, M.; Shen, X.; Chen, F.; Qiao, Y.; Zhang, Z.; Liu, J.; Liu, R.; Fan, H. Sodium percarbonate (SPC) activation by magnetic cobalt-based catalysts for organic pollutants degradation: Performance and mechanism. J. Environ. Chem. Eng. 2025, 13, 116184. [Google Scholar] [CrossRef]
- Duan, M.; Guan, Z.; Ma, Y.; Wan, J.; Wang, Y.; Qu, Y. A novel catalyst of MIL-101(Fe) doped with Co and Cu as persulfate activator: Synthesis, characterization, and catalytic performance. Chem. Pap. 2018, 72, 235–250. [Google Scholar] [CrossRef]
- Cai, C.; Kang, S.; Xie, X.; Liao, C. Ultrasound-assisted heterogeneous peroxymonosulfate activation with Co/SBA-15 for the efficient degradation of organic contaminant in water. J. Hazard. Mater. 2020, 385, 121519. [Google Scholar] [CrossRef]
- Yuan, R.; Hu, L.; Yu, P.; Wang, H.; Wang, Z.; Fang, J. Nanostructured Co3O4 grown on nickel foam: An efficient and readily recyclable 3D catalyst for heterogeneous peroxymonosulfate activation. Chemosphere 2018, 198, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, J.; Qiao, X.; Wang, D.; Cai, X. Performance of nano-Co3O4/peroxymonosulfate system: Kinetics and mechanism study using Acid Orange 7 as a model compound. Appl. Catal. B Environ. 2008, 80, 116–121. [Google Scholar] [CrossRef]
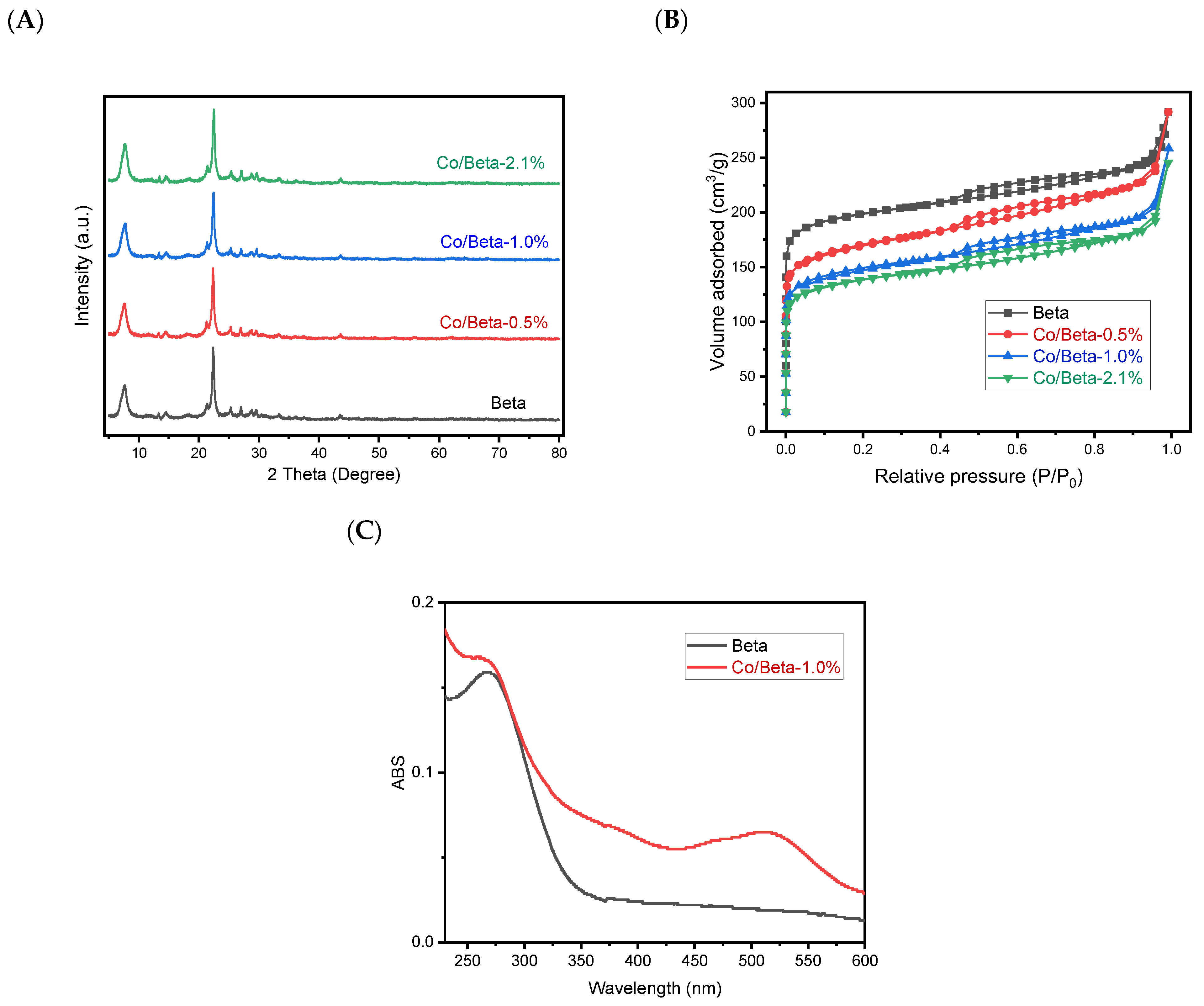
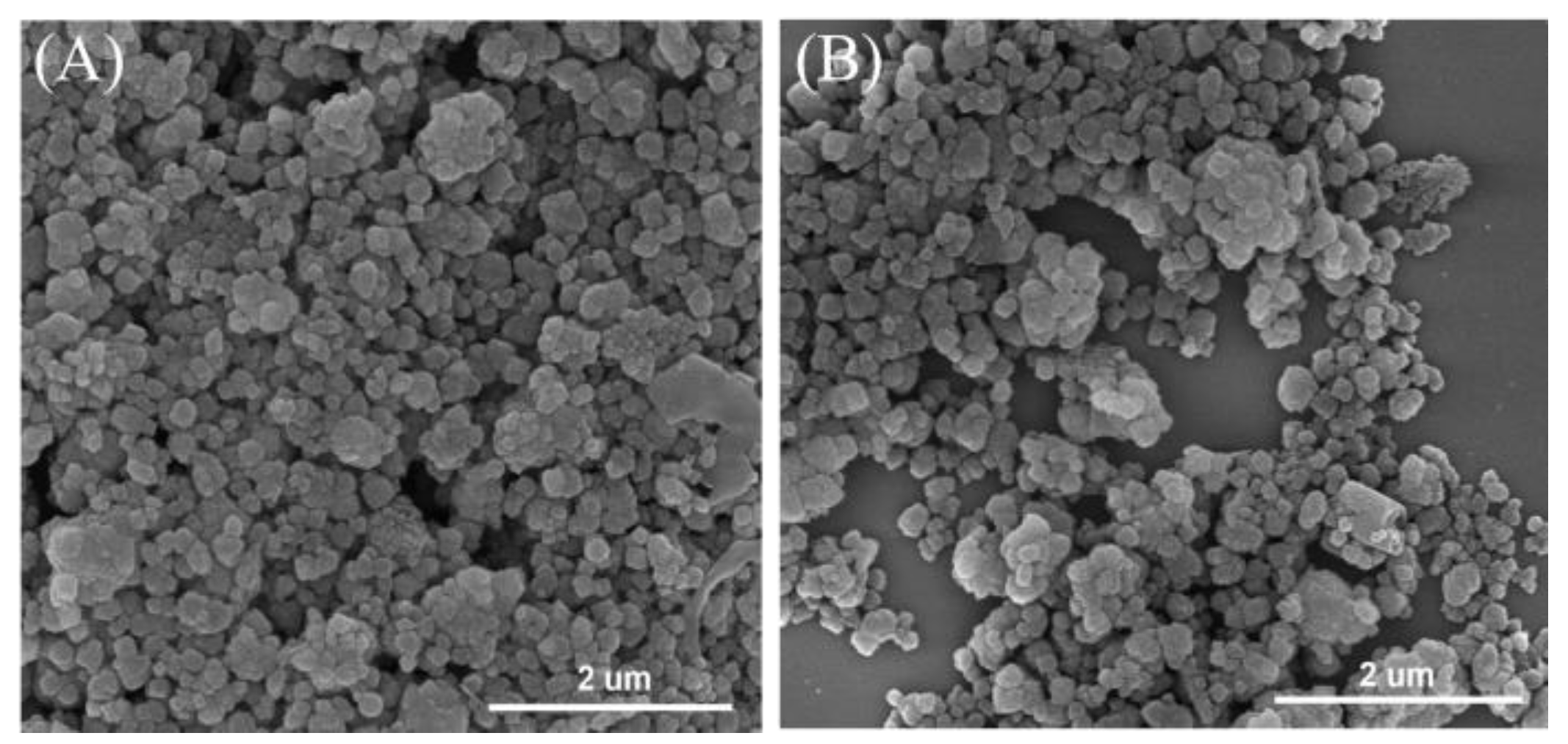
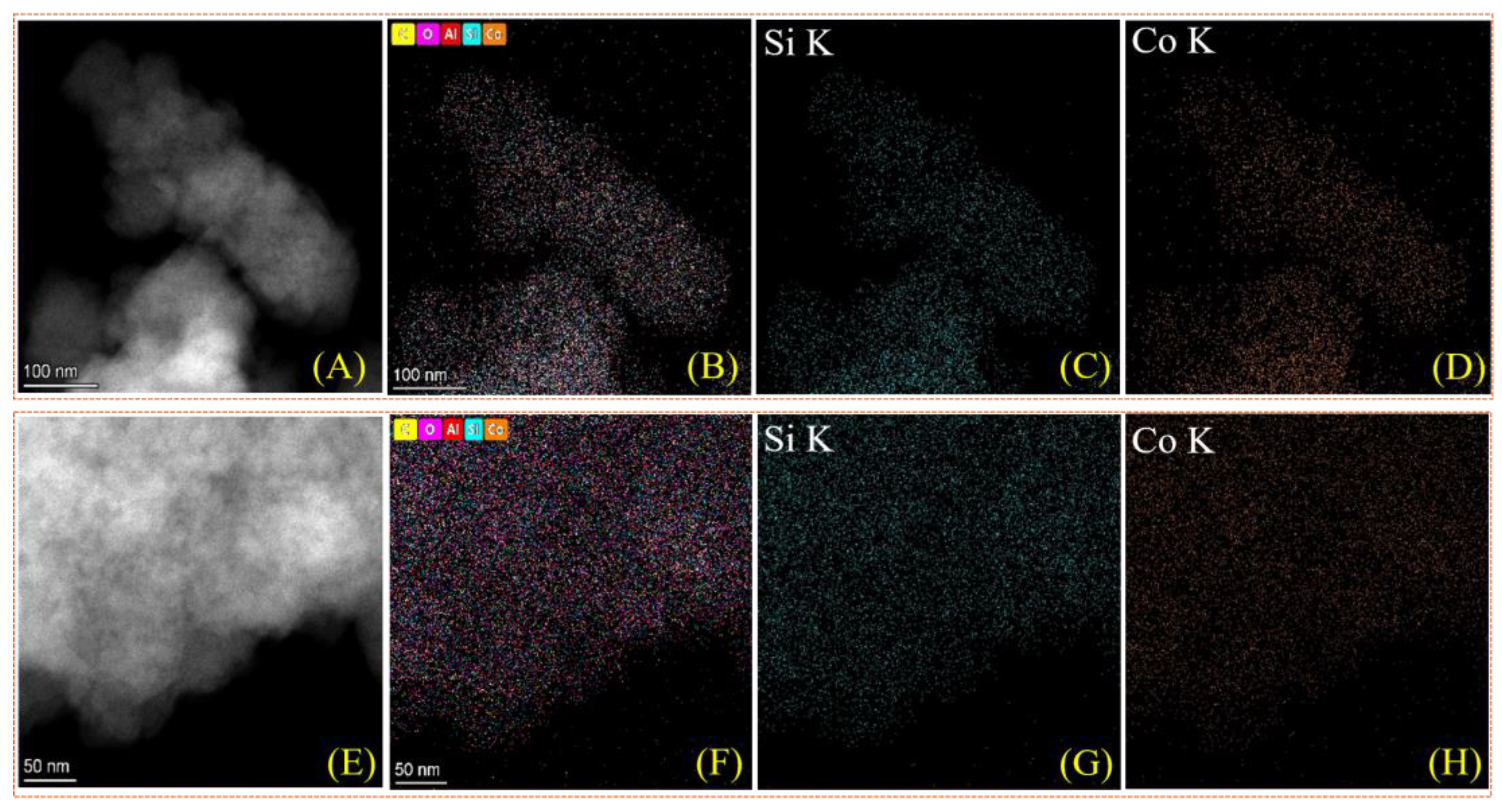
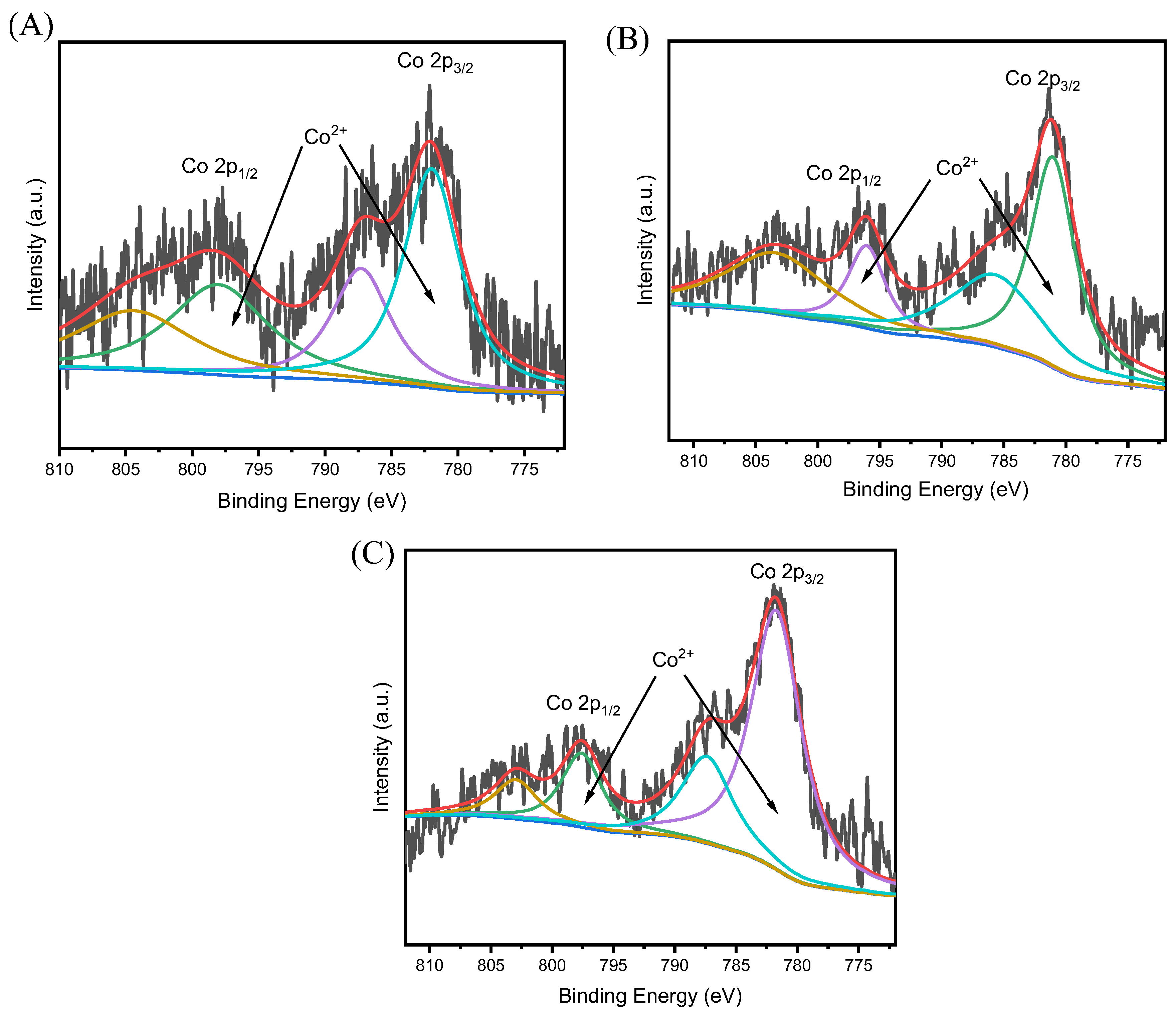

| Catalysts | Co(wt%) a | Surface Area (m2/g) b | Pore Volume (cm3/g) c |
|---|---|---|---|
| Beta | 0 | 554 | 0.23 |
| Co/Beta-0.5% | 0.5 | 545 | 0.22 |
| Co/Beta-1.0% | 1.0 | 524 | 0.22 |
| Co/Beta-2.1% | 2.1 | 475 | 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, E.; Liao, X.; He, C.; Xu, S.; Li, H. Ion-Exchanged Beta-Based Cobalt Catalyst for Efficient Degradation of Aqueous Dye Acid Orange II. Nanomaterials 2025, 15, 1630. https://doi.org/10.3390/nano15211630
Fu E, Liao X, He C, Xu S, Li H. Ion-Exchanged Beta-Based Cobalt Catalyst for Efficient Degradation of Aqueous Dye Acid Orange II. Nanomaterials. 2025; 15(21):1630. https://doi.org/10.3390/nano15211630
Chicago/Turabian StyleFu, En, Xiang Liao, Chun He, Shaodan Xu, and Huanxuan Li. 2025. "Ion-Exchanged Beta-Based Cobalt Catalyst for Efficient Degradation of Aqueous Dye Acid Orange II" Nanomaterials 15, no. 21: 1630. https://doi.org/10.3390/nano15211630
APA StyleFu, E., Liao, X., He, C., Xu, S., & Li, H. (2025). Ion-Exchanged Beta-Based Cobalt Catalyst for Efficient Degradation of Aqueous Dye Acid Orange II. Nanomaterials, 15(21), 1630. https://doi.org/10.3390/nano15211630







