Light Absorption and Scattering Properties of Ag@TiO2 Nanosphere Dimer for Photocatalytic Water Purification
Abstract
1. Introduction
2. Materials and Methods
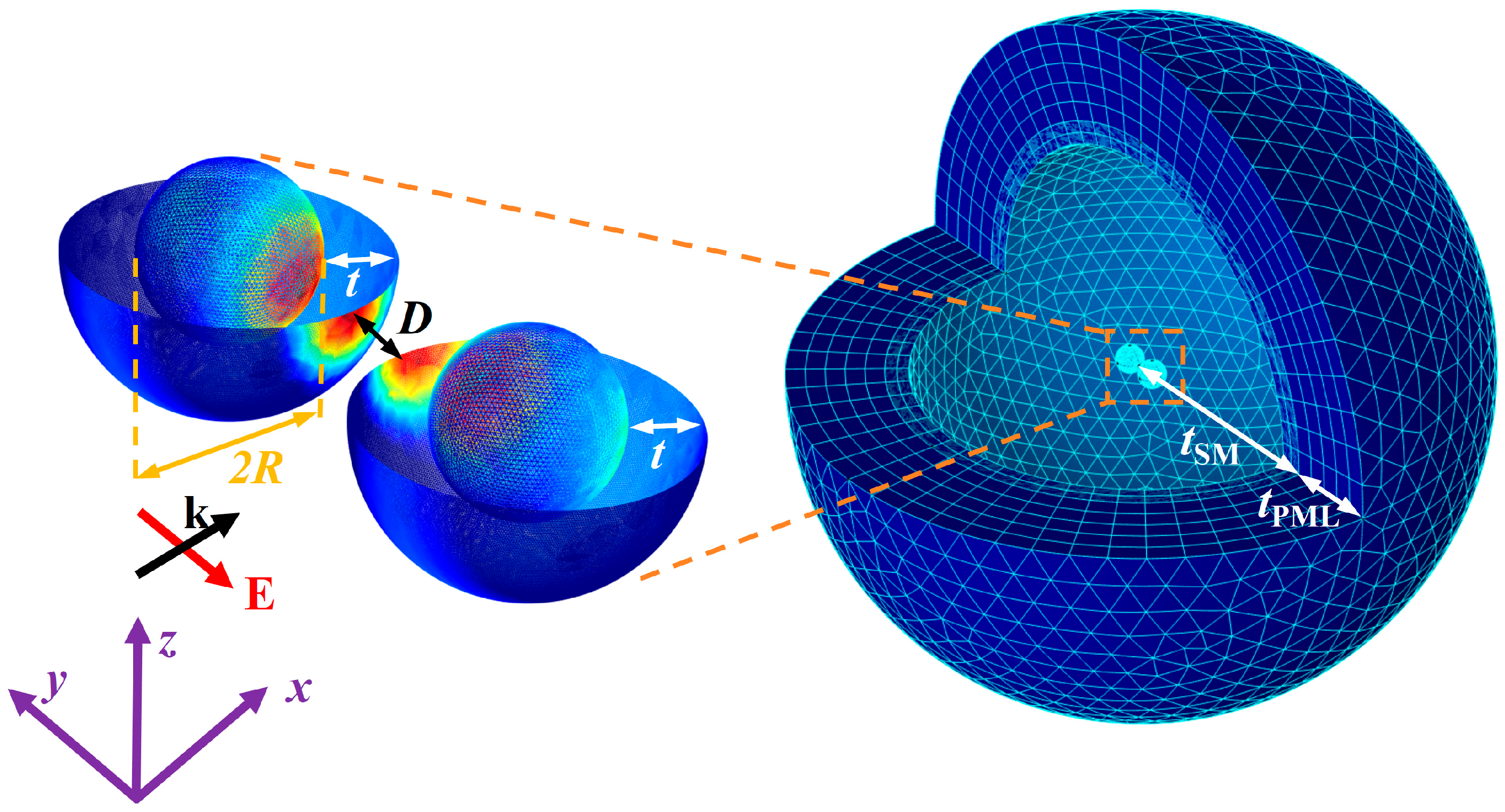
2.1. Simulation Model
2.2. Calculation of Absorption and Scattering Properties
2.3. Multipole Decomposition of Scattered Spectra
2.4. Heat Transfer Calculation
3. Results and Discussion
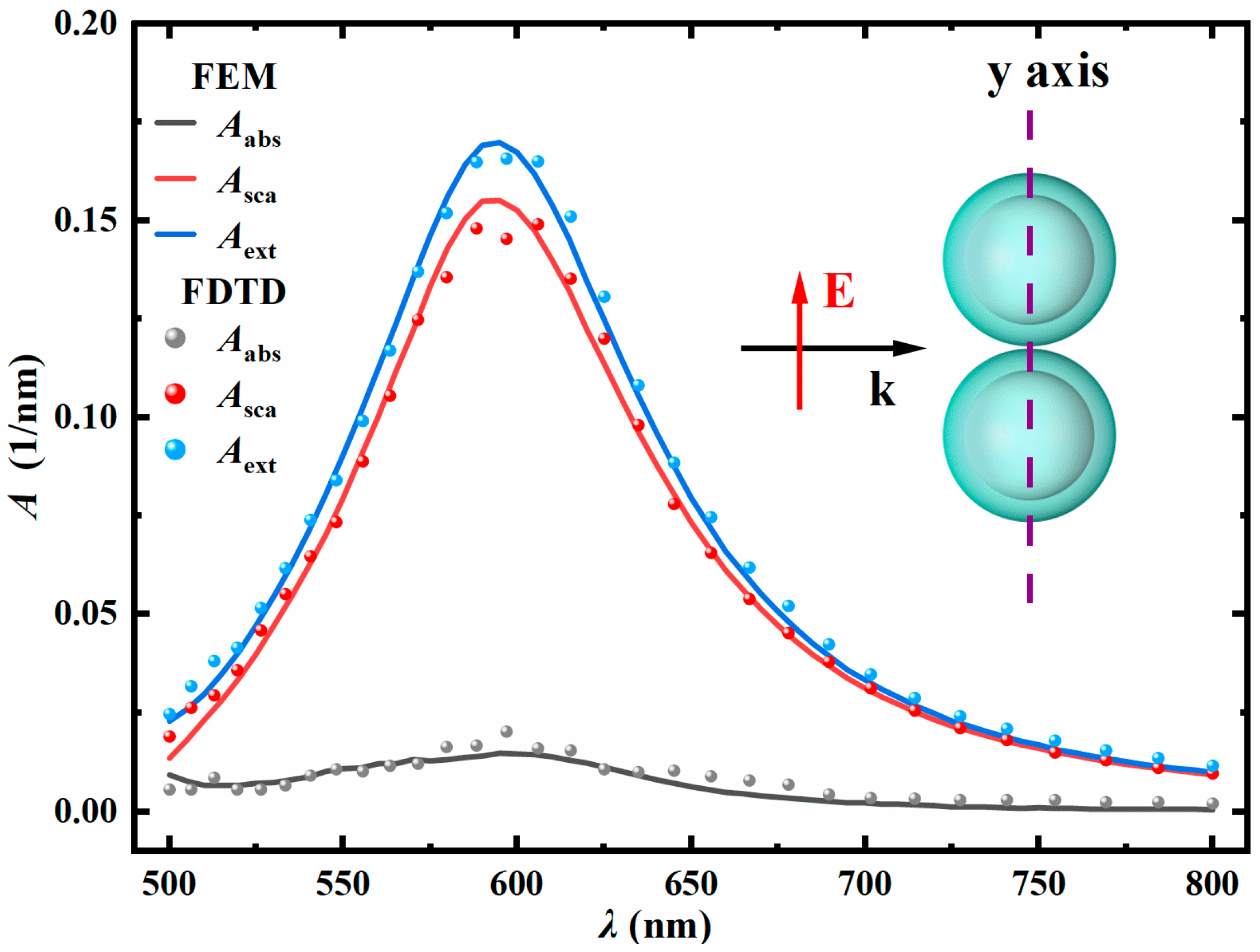
3.1. Numerical Verification of FEM
3.2. The Influence of Internal Factors Within the Dimer
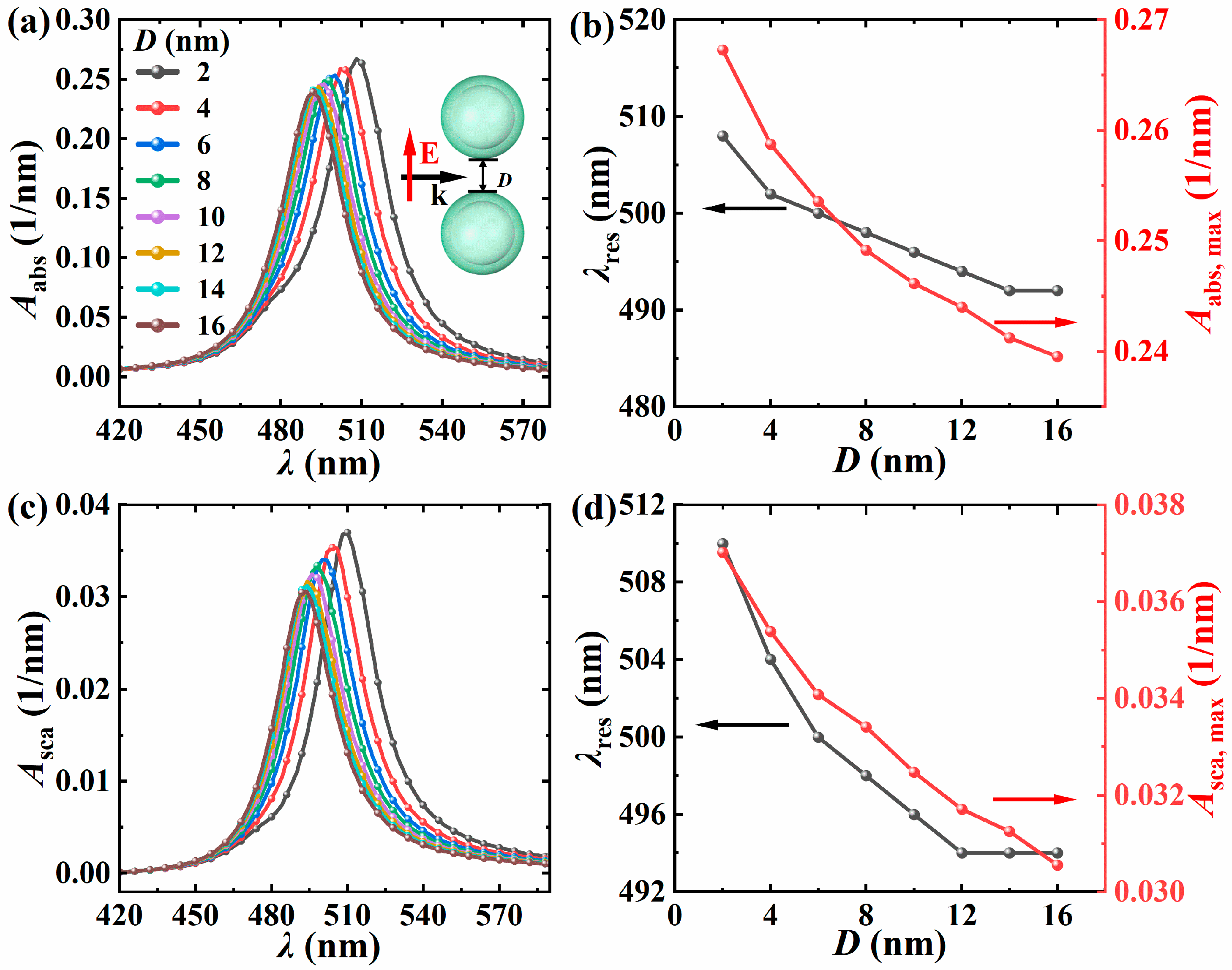
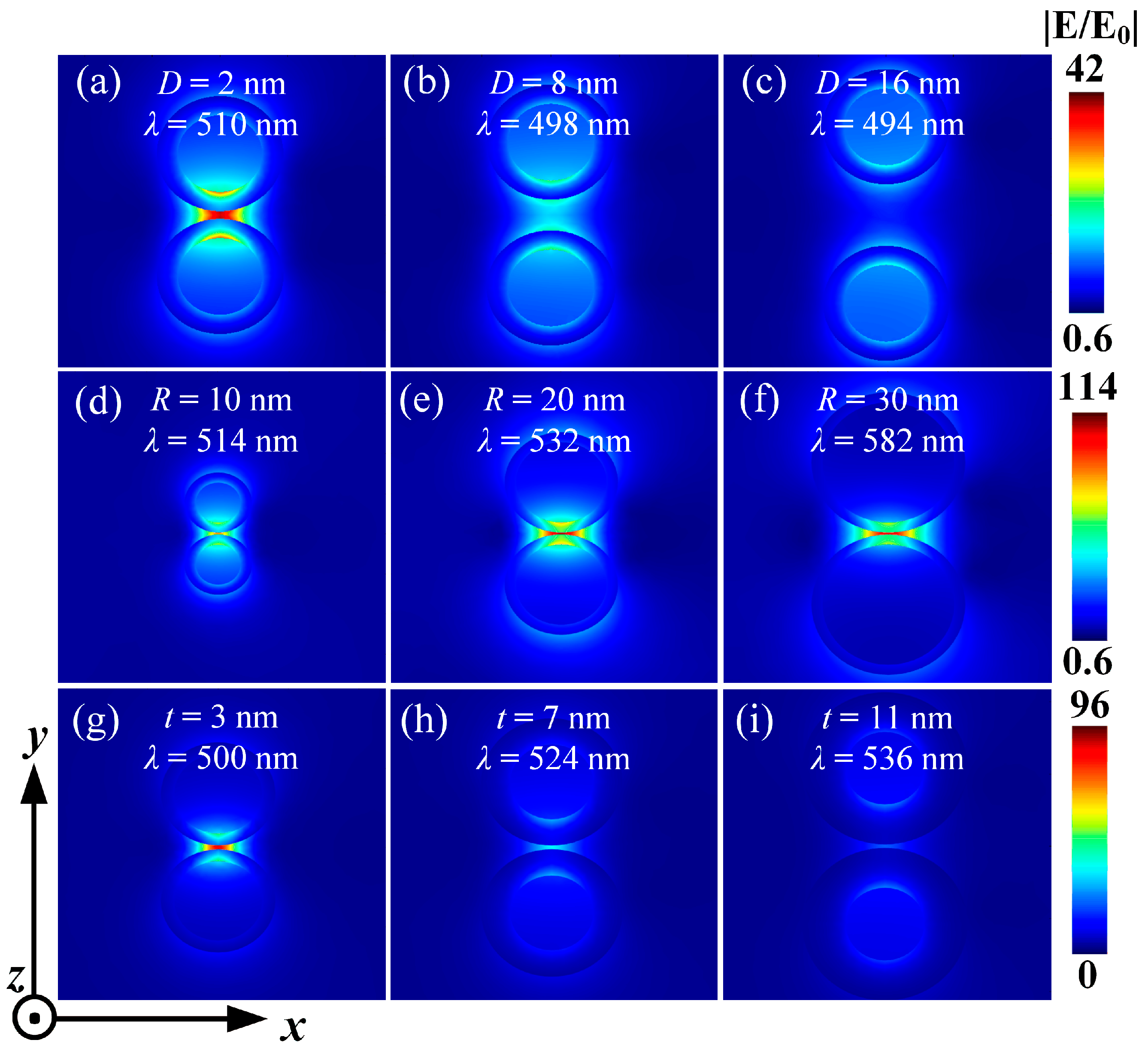
3.2.1. The Influence of Particle Spacing
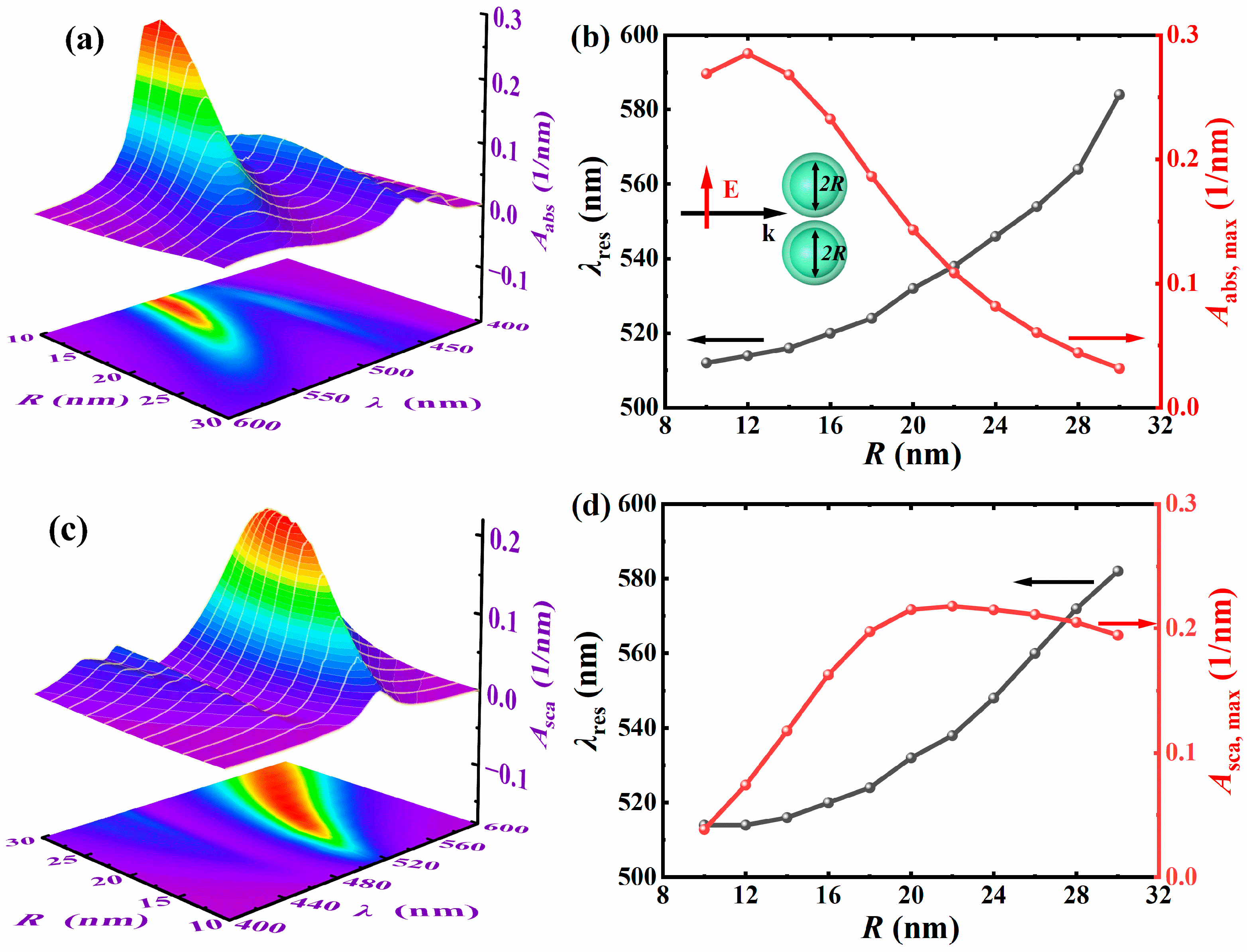
3.2.2. The Impact of Kernel Radius
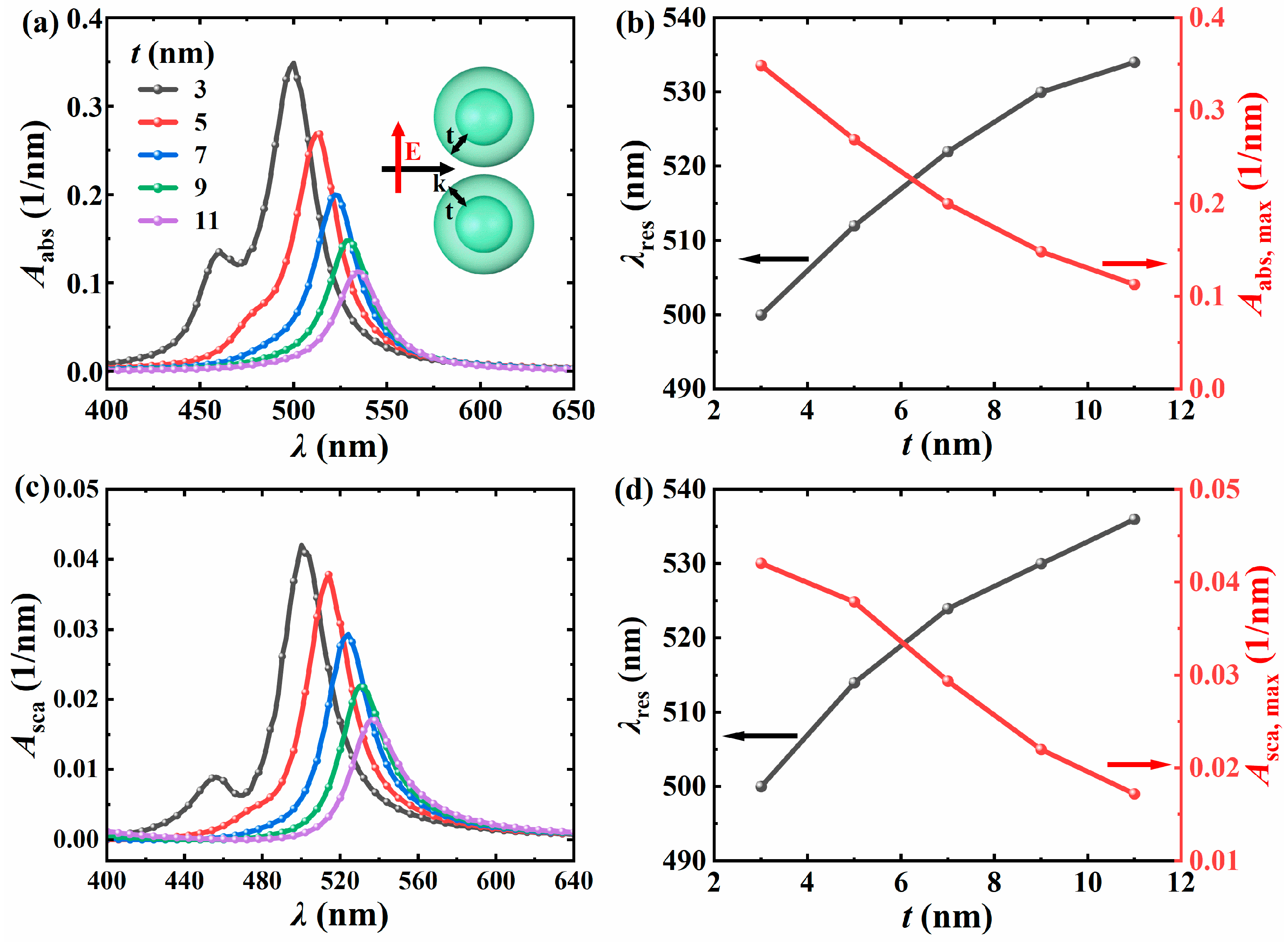
3.2.3. The Influence of Shell Thickness
3.2.4. The Influence of Size on Electric Field Enhancement
3.3. Influence of External Factors on Dimer Structure
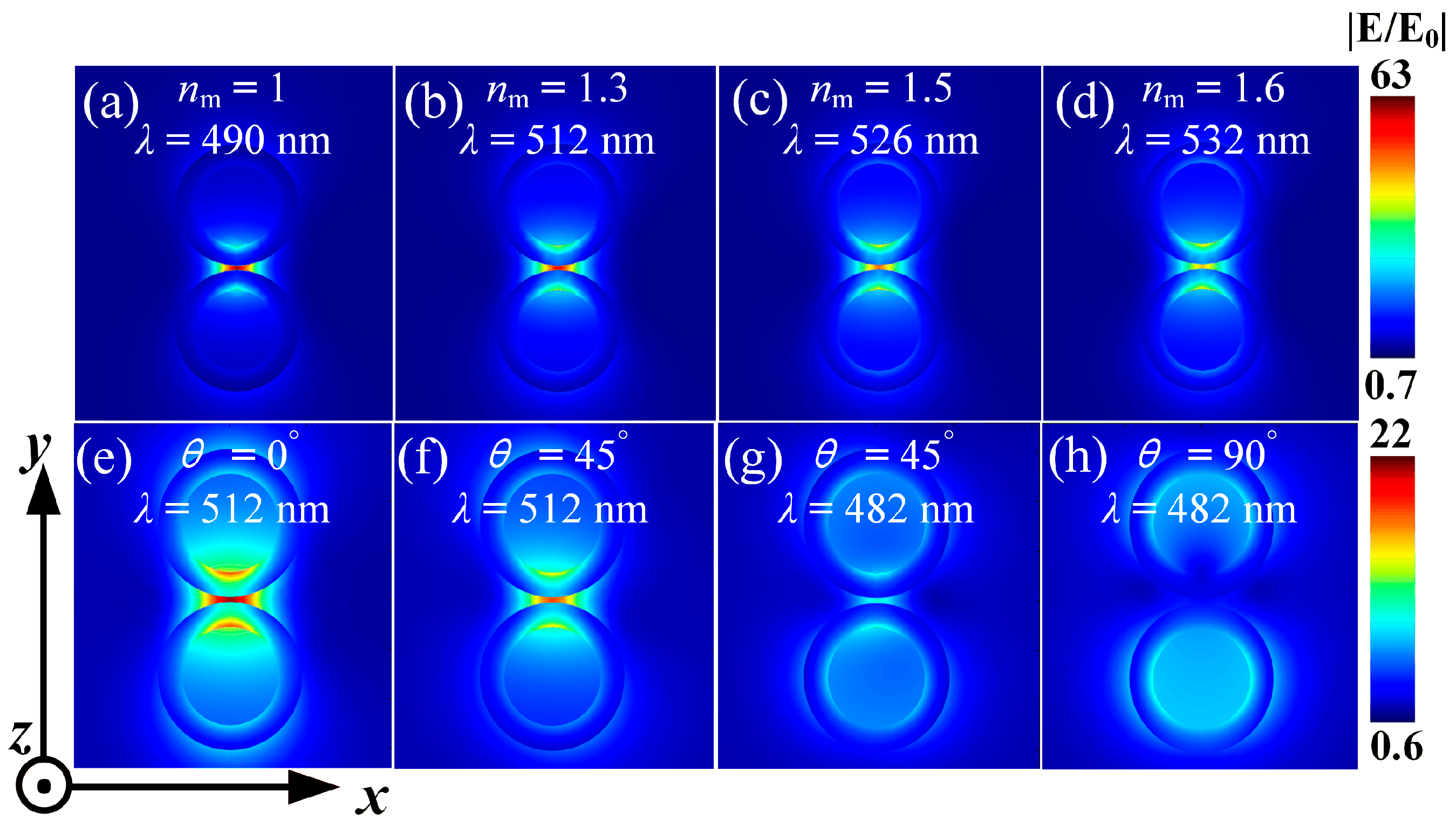
3.3.1. The Influence of the Refractive Index of the SM
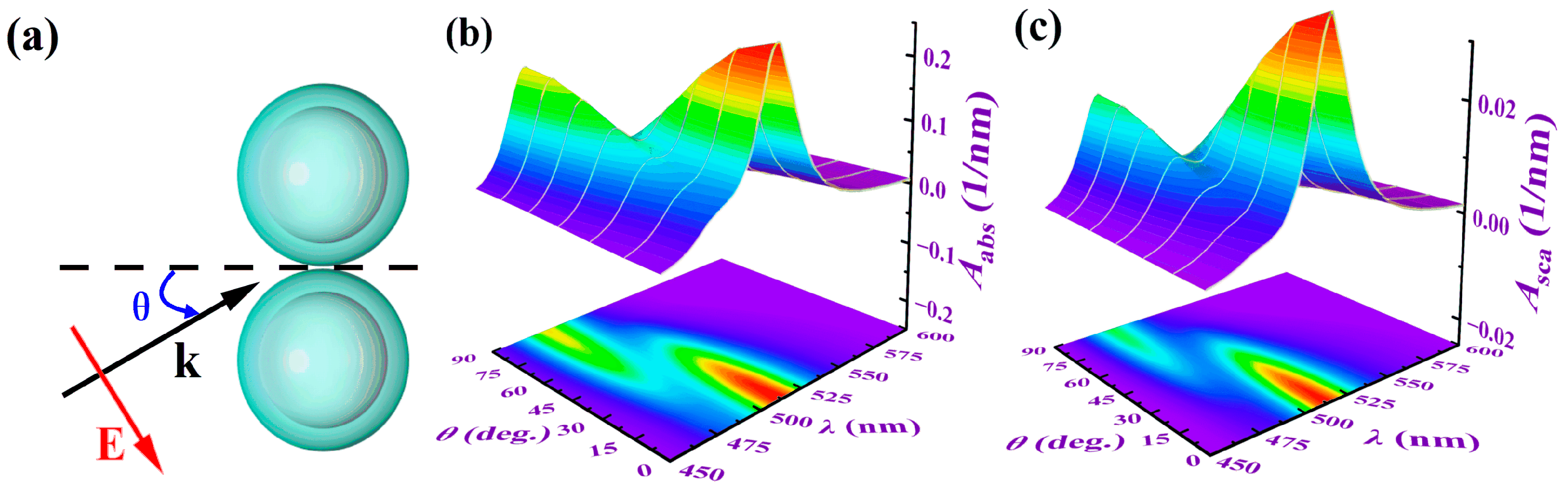
3.3.2. The Influence of Incident Light Direction Angle
3.3.3. The Influence of SM Refractive Index and Incident Direction Angle on the Enhancement of Electric Field
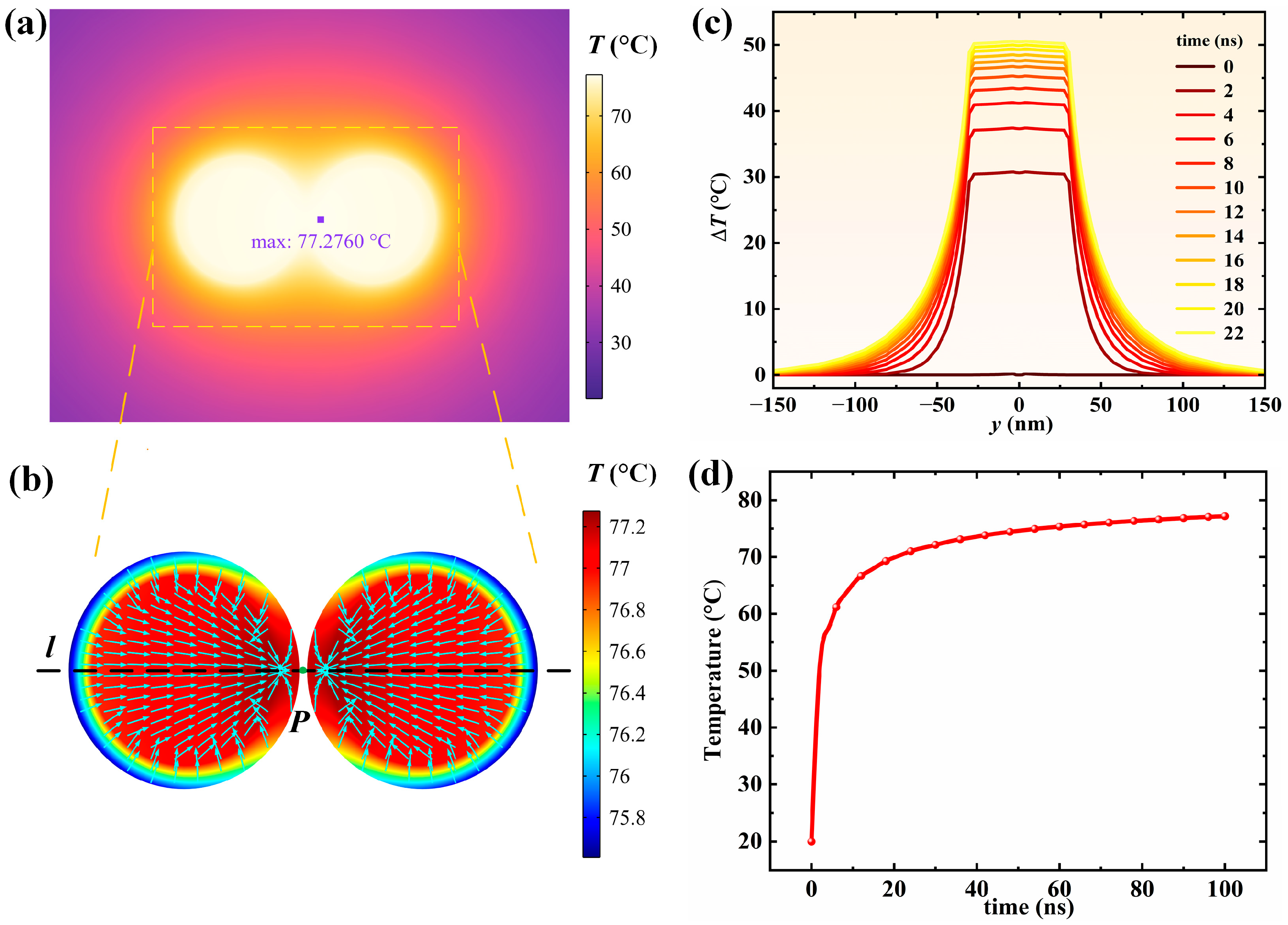
3.4. Photothermal Effect of Dimer Compounds
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Garrick, D.E.; Hall, J.W.; Dobson, A.; Damania, R.; Grafton, R.Q.; Hope, R.; Hepburn, C.; Bark, R.; Boltz, F.; De Stefano, L.; et al. Valuing Water for Sustainable Development. Science 2017, 358, 1003–1005. [Google Scholar] [CrossRef]
- Kümmerer, K.; Dionysiou, D.D.; Olsson, O.; Fatta-Kassinos, D. A Path to Clean Water. Science 2018, 361, 222–224. [Google Scholar] [CrossRef]
- Mukherjee, A.; Coomar, P.; Sarkar, S.; Johannesson, K.H.; Fryar, A.E.; Schreiber, M.E.; Ahmed, K.M.; Alam, M.A.; Bhattacharya, P.; Bundschuh, J.; et al. Arsenic and Other Geogenic Contaminants in Global Groundwater. Nat. Rev. Earth Environ. 2024, 5, 312–328. [Google Scholar] [CrossRef]
- Münzel, T.; Hahad, O.; Lelieveld, J.; Aschner, M.; Nieuwenhuijsen, M.J.; Landrigan, P.J.; Daiber, A. Soil and Water Pollution and Cardiovascular Disease. Nat. Rev. Cardiol. 2025, 22, 71–89. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S.; Guo, L.; Chen, L.; Yu, Z. Chlorination Contributes to Multi-Antibiotic Resistance in a Pilot-Scale Water Distribution System. Water Supply 2021, 21, 4369–4381. [Google Scholar] [CrossRef]
- Beattie, R.E.; Skwor, T.; Hristova, K.R. Survivor Microbial Populations in Post-Chlorinated Wastewater Are Strongly Associated with Untreated Hospital Sewage and Include Ceftazidime and Meropenem Resistant Populations. Sci. Total Environ. 2020, 740, 140186–140207. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.-B.; Guo, M.-T.; Yang, J. Fate of Antibiotic Resistant Bacteria and Genes during Wastewater Chlorination: Implication for Antibiotic Resistance Control. PLoS ONE 2015, 10, e0119403. [Google Scholar] [CrossRef]
- Putra, H.S.A.; Ma’rufi, I.; Ellyke, E. Analisis risiko kesehatan lingkungan sisa klor (Cl2) pada ZAMP perumda air minum tugu tirta kota malang. Pustaka Kesehat. 2022, 10, 107. [Google Scholar] [CrossRef]
- Bolan, N.S.; Bell, K.; Krishan, A.K.; Chung, J.-W. Irrigating Horticultural Crops with Recycled Water: An Australian Perspective. J. Hortic. Sci. 2011, 6, 1–20. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Kader, S.; Islam, M.S.; Khan, M.Z.H. Photocatalytic Activity Improvement and Application of UV-TiO2 Photocatalysis in Textile Wastewater Treatment: A Review. J. Environ. Chem. Eng. 2019, 7, 103248–103308. [Google Scholar] [CrossRef]
- Ouyang, Z.; Yang, Y.; Zhang, C.; Zhu, S.; Qin, L.; Wang, W.; He, D.; Zhou, Y.; Luo, H.; Qin, F. Recent Advances in Photocatalytic Degradation of Plastics and Plastic-Derived Chemicals. J. Mater. Chem. A 2021, 9, 13402–13441. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A Historical Overview and Future Prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8287. [Google Scholar] [CrossRef]
- Park, H.; Park, Y.; Kim, W.; Choi, W. Surface Modification of TiO2 Photocatalyst for Environmental Applications. J. Photochem. Photobiol. C 2013, 15, 1–20. [Google Scholar] [CrossRef]
- Lawless, D.; Serpone, N.; Meisel, D. Role of Hydroxyl Radicals and Trapped Holes in Photocatalysis. A Pulse Radiolysis Study. J. Phys. Chem. 1991, 95, 5166–5170. [Google Scholar] [CrossRef]
- Kesselman, J.M.; Weres, O.; Lewis, N.S.; Hoffmann, M.R. Electrochemical Production of Hydroxyl Radical at Polycrystalline Nb-Doped TiO2 Electrodes and Estimation of the Partitioning between Hydroxyl Radical and Direct Hole Oxidation Pathways. J. Phys. Chem. B 1997, 101, 2637–2643. [Google Scholar] [CrossRef]
- Tada, H.; Jin, Q.; Kobayashi, H. Prediction of the Main Route in the TiO2-photocatalyzed Degradation of Organic Compounds in Water by Density Functional Calculations. ChemPhysChem 2012, 13, 3457–3461. [Google Scholar] [CrossRef] [PubMed]
- Naya, S.; Kume, T.; Akashi, R.; Fujishima, M.; Tada, H. Red-Light-Driven Water Splitting by Au(Core)–CdS(Shell) Half-Cut Nanoegg with Heteroepitaxial Junction. J. Am. Chem. Soc. 2018, 140, 1251–1254. [Google Scholar] [CrossRef]
- Narayan, M.R. Review: Dye Sensitized Solar Cells Based on Natural Photosensitizers. Renew. Sustain. Energy Rev. 2011, 16, 208–215. [Google Scholar] [CrossRef]
- Garcia, M.A. Surface Plasmons in Metallic Nanoparticles: Fundamentals and Applications. J. Phys. D Appl. Phys. 2012, 45, 389501. [Google Scholar] [CrossRef]
- Hong, D.; Lyu, L.-M.; Koga, K.; Shimoyama, Y.; Kon, Y. Plasmonic ag@TiO2 Core–Shell Nanoparticles for Enhanced CO2 Photoconversion to CH4. ACS Sustain. Chem. Eng. 2019, 7, 18955–18964. [Google Scholar] [CrossRef]
- Wumaier, D.; Tuersun, P.; Li, S.; Li, Y.; Wang, M.; Xu, D. Light Absorption Analysis and Optimization of ag@TiO2 Core-Shell Nanospheroid and Nanorod. Nanomaterials 2024, 14, 325. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, X.; Zhang, R.; Wang, J.; Zhou, Z.; Xia, Y.; Li, N.; Chen, D.; Zhou, Z.; Fan, X. Local Polarization Piezoelectric Electric Field Promoted Water Dissociation for Hydroxyl Radical Generation under Ambient Humidity Condition. Adv. Mater. 2025, 37, 2418554–2418565. [Google Scholar] [CrossRef]
- Li, J.; Cushing, S.K.; Bright, J.; Meng, F.; Senty, T.R.; Zheng, P.; Bristow, A.D.; Wu, N. Ag@Cu2 O Core-Shell Nanoparticles as Visible-Light Plasmonic Photocatalysts. ACS Catal. 2013, 3, 47–51. [Google Scholar] [CrossRef]
- Zhao, J.; Pinchuk, A.O.; McMahon, J.M.; Li, S.; Ausman, L.K.; Atkinson, A.L.; Schatz, G.C. Methods for Describing the Electromagnetic Properties of Silver and Gold Nanoparticles. Acc. Chem. Res. 2008, 41, 1710–1720. [Google Scholar] [CrossRef] [PubMed]
- Myroshnychenko, V.; Rodríguez-Fernández, J.; Pastoriza-Santos, I.; Funston, A.M.; Novo, C.; Mulvaney, P.; Liz-Marzán, L.M.; García De Abajo, F.J. Modelling the Optical Response of Gold Nanoparticles. Chem. Soc. Rev. 2008, 37, 1792–1807. [Google Scholar] [CrossRef]
- Amirjani, A.; Sadrnezhaad, S.K. Computational Electromagnetics in Plasmonic Nanostructures. J. Mater. Chem. C 2021, 9, 9791–9819. [Google Scholar] [CrossRef]
- Hövel, H.; Fritz, S.; Hilger, A.; Kreibig, U.; Vollmer, M. Width of Cluster Plasmon Resonances: Bulk Dielectric Functions and Chemical Interface Damping. Phys. Rev. B 1993, 48, 18178–18188. [Google Scholar] [CrossRef]
- Kreibig, U.; Vollmer, M. Optical Properties of Metal Clusters; Springer Series in Materials Science; Springer: Berlin/Heidelberg, Germany, 1995; Volume 25, ISBN 978-3-642-08191-0. [Google Scholar]
- Johnson, P.B.; Christy, R.W. Optical Constants of the Noble Metals. Phys. Rev. B 1972, 6, 4370–4379. [Google Scholar] [CrossRef]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; Wiley: New York, NY, USA, 1983; ISBN 978-0-471-05772-7. [Google Scholar]
- Moroz, A. Electron Mean-Free Path in Metal-Coated Nanowires. J. Opt. Soc. Am. B 2011, 28, 1130–1138. [Google Scholar] [CrossRef]
- Sharma, R.; Sarkar, A.; Jha, R.; Kumar Sharma, A.; Sharma, D. Sol-gel–Mediated Synthesis of TiO2 Nanocrystals: Structural, Optical, and Electrochemical Properties. Int. J. Appl. Ceram. Technol. 2020, 17, 1400–1409. [Google Scholar] [CrossRef]
- Alaee, R.; Rockstuhl, C.; Fernandez-Corbaton, I. An Electromagnetic Multipole Expansion beyond the Long-Wavelength Approximation. Opt. Commun. 2018, 407, 17–21. [Google Scholar] [CrossRef]
- Schwinger, J.; DeRaad, L.L.; Milton, K.A.; Tsai, W.-Y. Classical Electrodynamics; Perseus Books: Reading, MA, USA, 1998; ISBN 978-0-7382-0056-9. [Google Scholar]
- Baffou, G.; Quidant, R.; García De Abajo, F.J. Nanoscale Control of Optical Heating in Complex Plasmonic Systems. ACS Nano 2010, 4, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 97th ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-4987-5429-3. [Google Scholar]
- Brandl, D.W.; Oubre, C.; Nordlander, P. Plasmon Hybridization in Nanoshell Dimers. J. Chem. Phys. 2005, 123, 024701. [Google Scholar] [CrossRef] [PubMed]



| Material | Density ( [kg/m3]) | Specific Heat Capacity (Cp [J/kg/K]) | Thermal Conductivity (k [W/m/K]) |
|---|---|---|---|
| Ag | 10,500 | 235 | 429 |
| TiO2 | 3800 | 686 | 6.7 |
| H2O | 997 | 4182 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, B.; Tuersun, P.; Li, S.; He, G.; Dou, F.; Lv, S. Light Absorption and Scattering Properties of Ag@TiO2 Nanosphere Dimer for Photocatalytic Water Purification. Nanomaterials 2025, 15, 1618. https://doi.org/10.3390/nano15211618
Pu B, Tuersun P, Li S, He G, Dou F, Lv S. Light Absorption and Scattering Properties of Ag@TiO2 Nanosphere Dimer for Photocatalytic Water Purification. Nanomaterials. 2025; 15(21):1618. https://doi.org/10.3390/nano15211618
Chicago/Turabian StylePu, Bojun, Paerhatijiang Tuersun, Shuyuan Li, Guoming He, Fengyi Dou, and Shuqi Lv. 2025. "Light Absorption and Scattering Properties of Ag@TiO2 Nanosphere Dimer for Photocatalytic Water Purification" Nanomaterials 15, no. 21: 1618. https://doi.org/10.3390/nano15211618
APA StylePu, B., Tuersun, P., Li, S., He, G., Dou, F., & Lv, S. (2025). Light Absorption and Scattering Properties of Ag@TiO2 Nanosphere Dimer for Photocatalytic Water Purification. Nanomaterials, 15(21), 1618. https://doi.org/10.3390/nano15211618





