Advancing Earth-Abundant CZTSSe Solar Cells: Recent Progress in Efficiency and Defect Engineering
Abstract
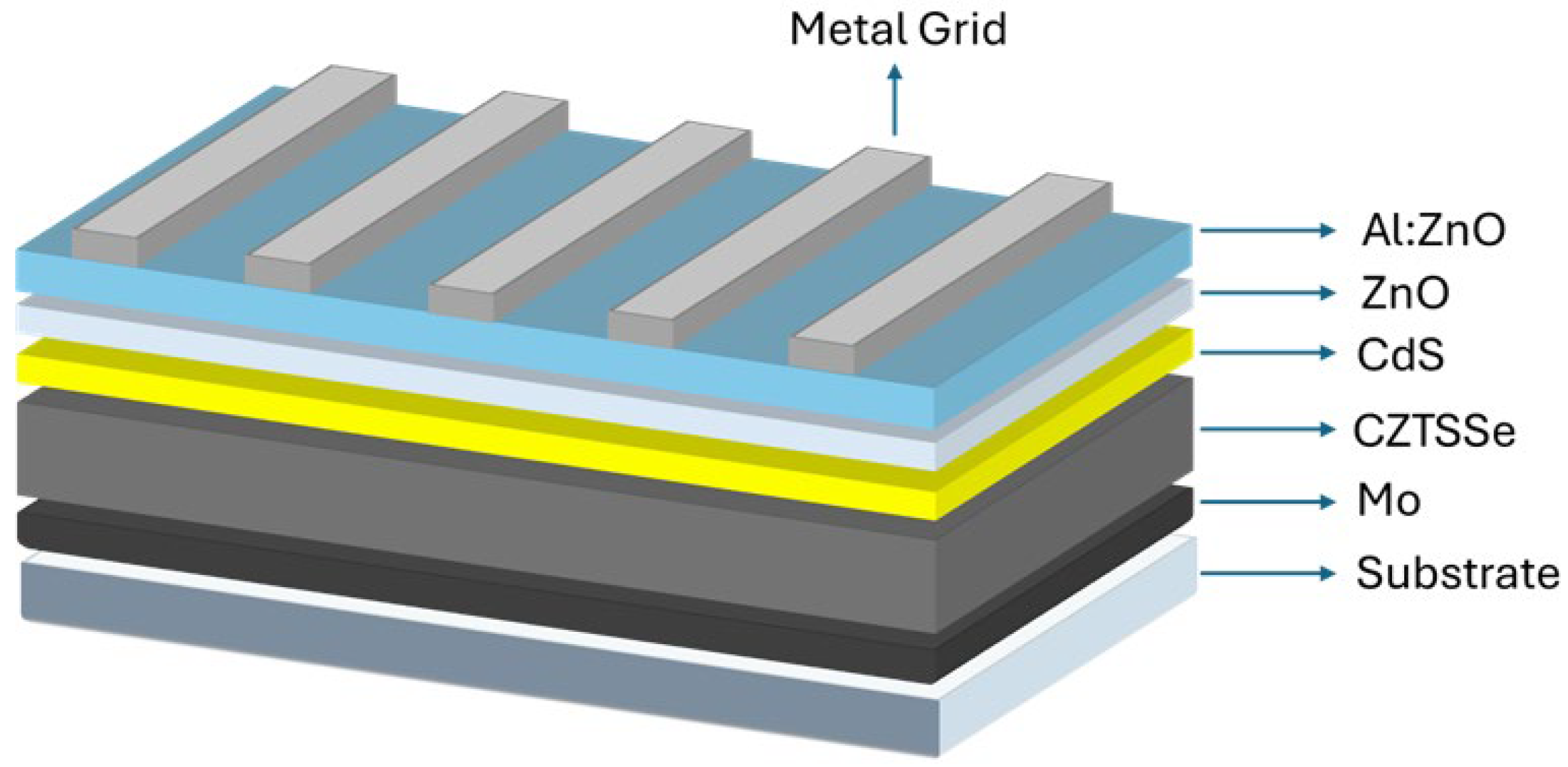
1. Introduction
- Section 2 presents the key challenges that limit CZTSSe solar cell efficiency, including Voc deficit, defect formation, and secondary phases.
- Section 3 reviews recent efficiency improvement strategies such as doping/alloying, interface engineering, and morphology control.
- Section 4 provides outlook and commercialization aspects, including cost, scalability, flexible substrates, and stability.
- Section 5 concludes with a critical summary and future research directions.
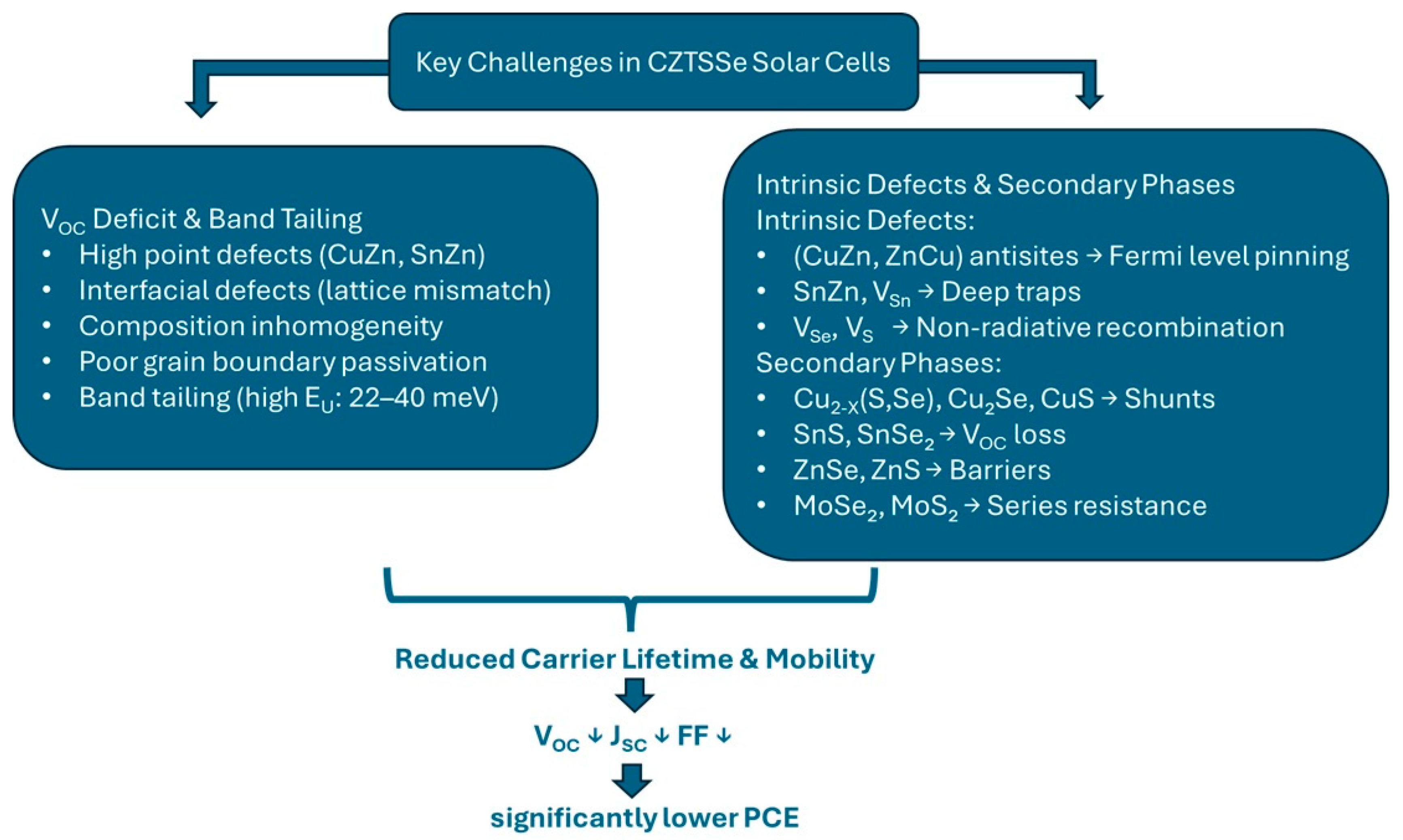
2. Key Challenges Limiting CZTSe Solar Cell Efficiency
2.1. Open-Circuit Voltage (VOC) Deficit and Band Tailing in CZTSSe Solar Cells
- Highly concentrated point defects and defect clusters in the CZTSSe absorber layer [36];
- Interfacial defects caused by lattice mismatches [60];
- Changes in electrostatic and band potential caused by local composition inhomogeneity [61];
- Poorly passivated grain boundaries that act as recombination sites [2].
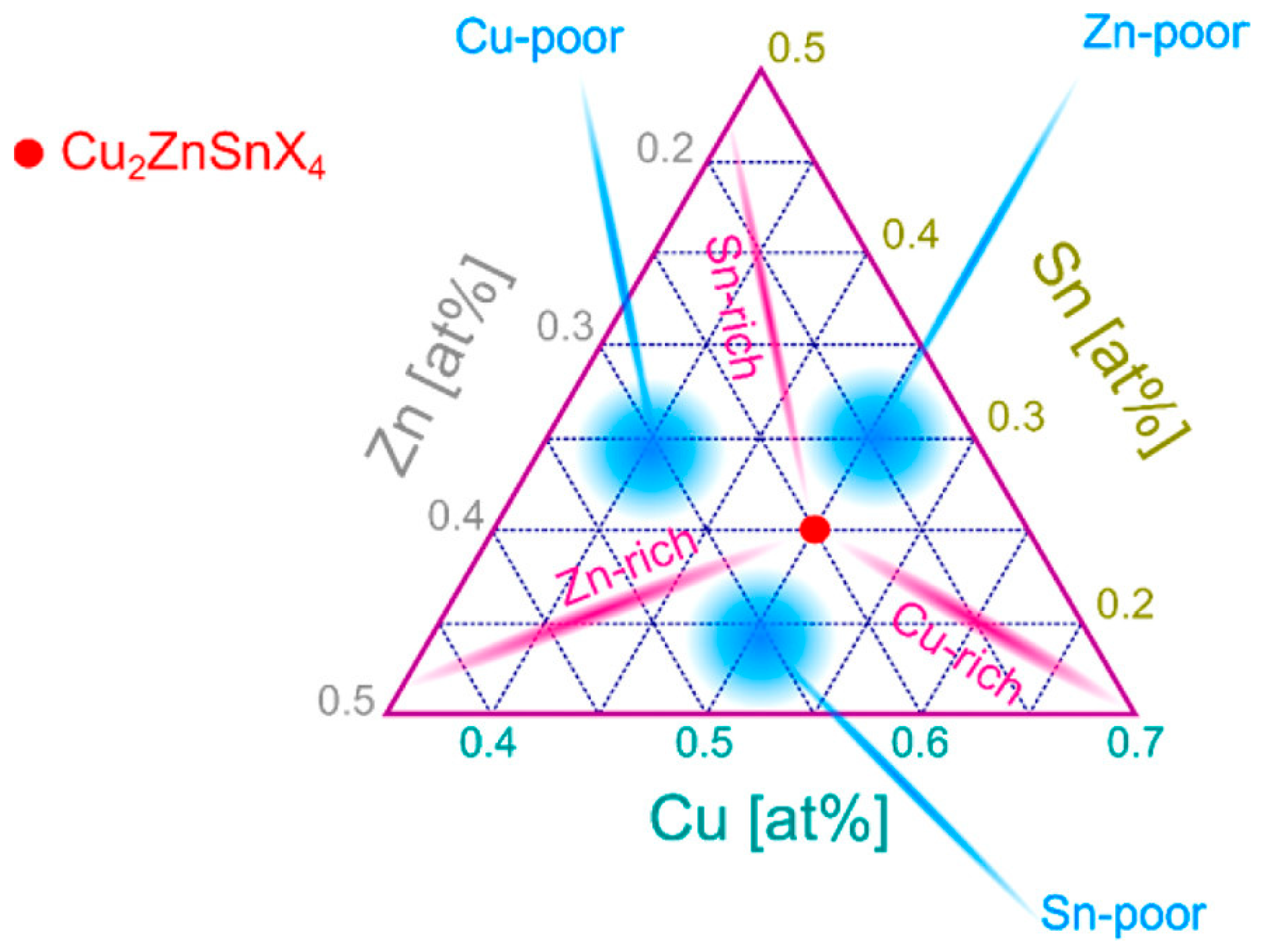
2.2. Intrinsic Defects and Secondary Phases in CZTSSe Solar Cells
2.2.1. Intrinsic Defects
- CuZn Disorder and Antisite Defects (CuZn, ZnCu):
- Sn-Related Defects (SnZn, VSn) and Complexes:
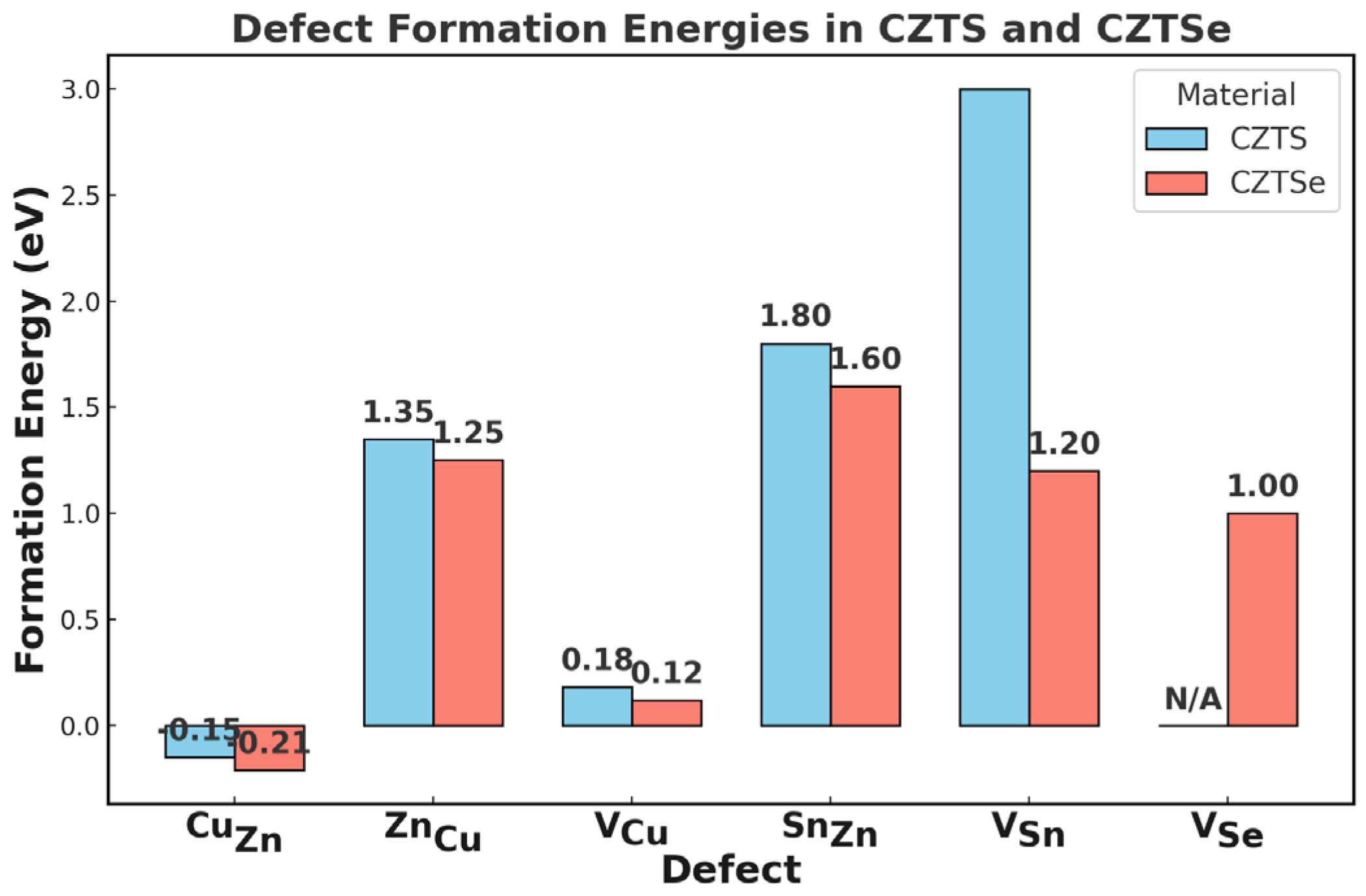
- Anion Vacancies (VSe, VS):
- Other Defects (VCu):
2.2.2. Secondary Phases
- Cu–S/Se Phases (Cu2−x(S,Se), Cu2Se, CuS):
- Sn–S/Se Phases (SnSe, SnSe2, SnS, SnS2):
- Zn–S/Se Phases (ZnSe, ZnS):
- Molybdenum Chalcogenides (MoSe2, MoS2):
- Ternary Phases (e.g., Cu2SnSe3)
2.2.3. How It Affects the Performance of the Whole Device
- Apply strict stoichiometric control;
- Optimize annealing/selenization protocols;
- Develop effective defect passivation and secondary phase suppression strategies.
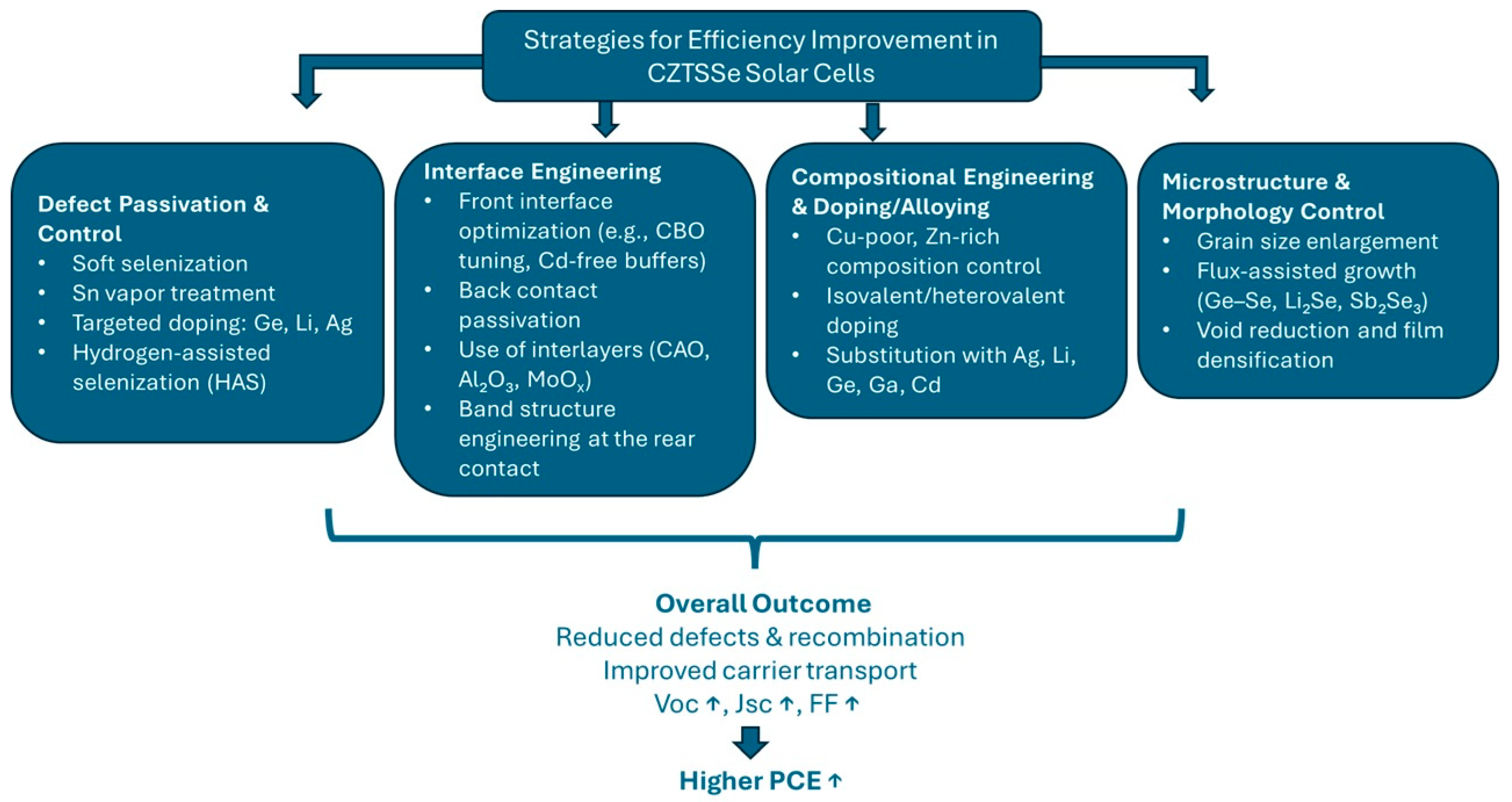
3. Strategies for Efficiency Improvement
3.1. Defect Passivation and Control
3.1.1. Soft Selenization and Tin Vapor Treatment
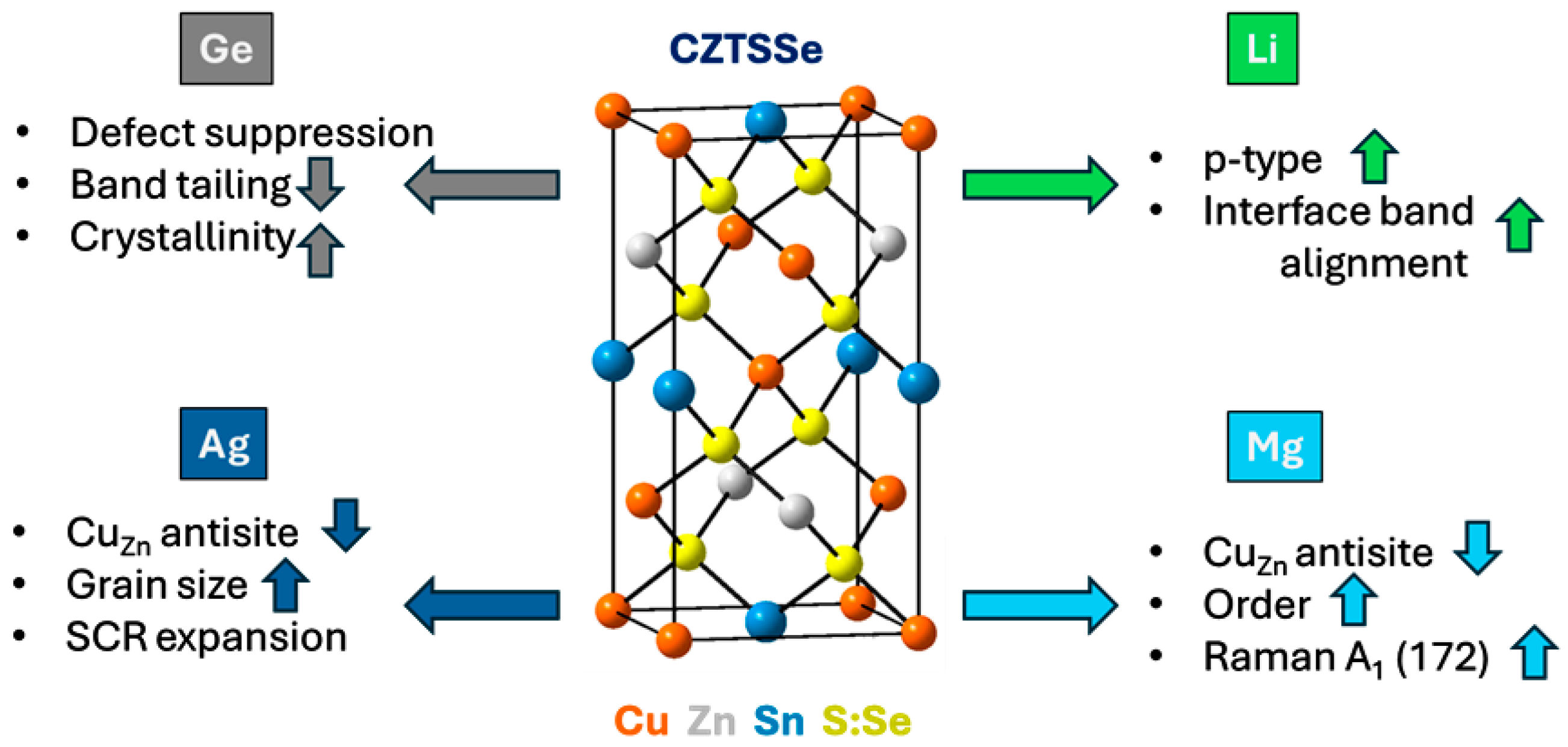
3.1.2. Isovalent and Alkali Metal Doping
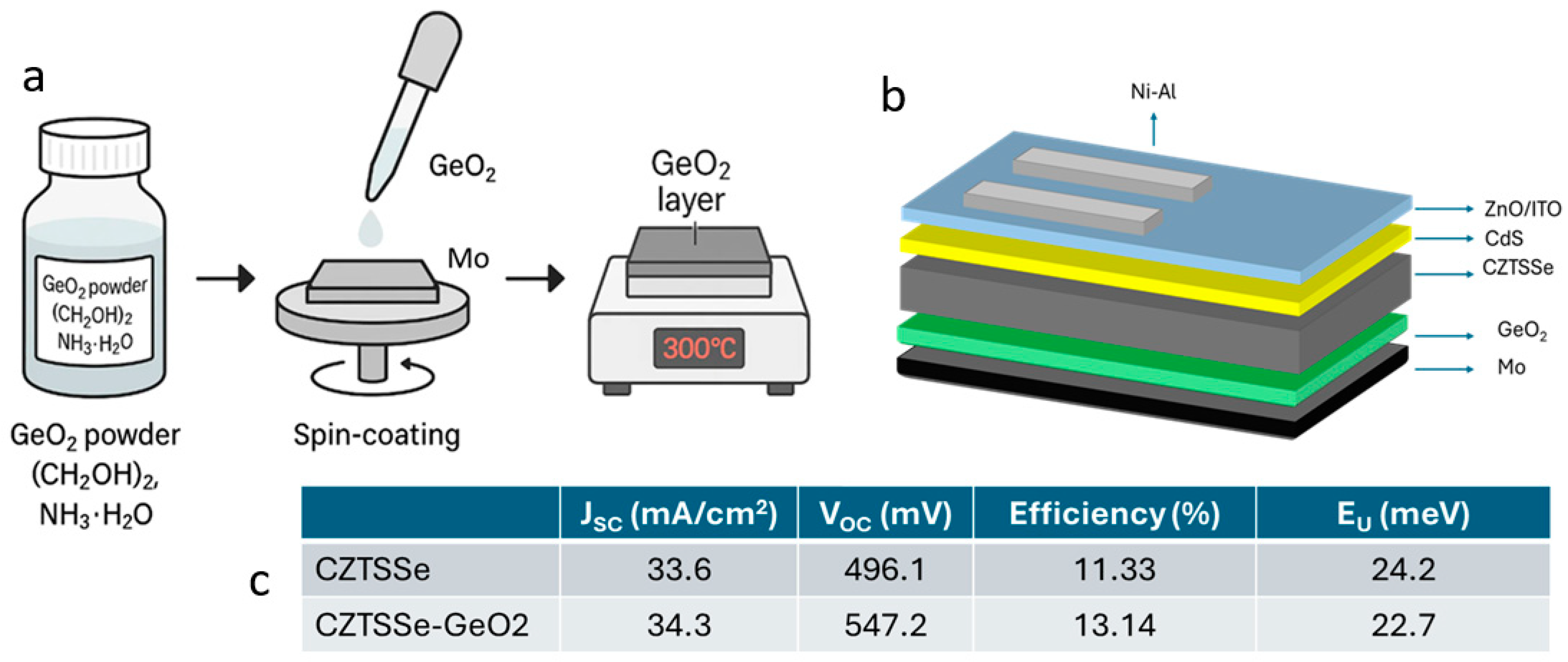
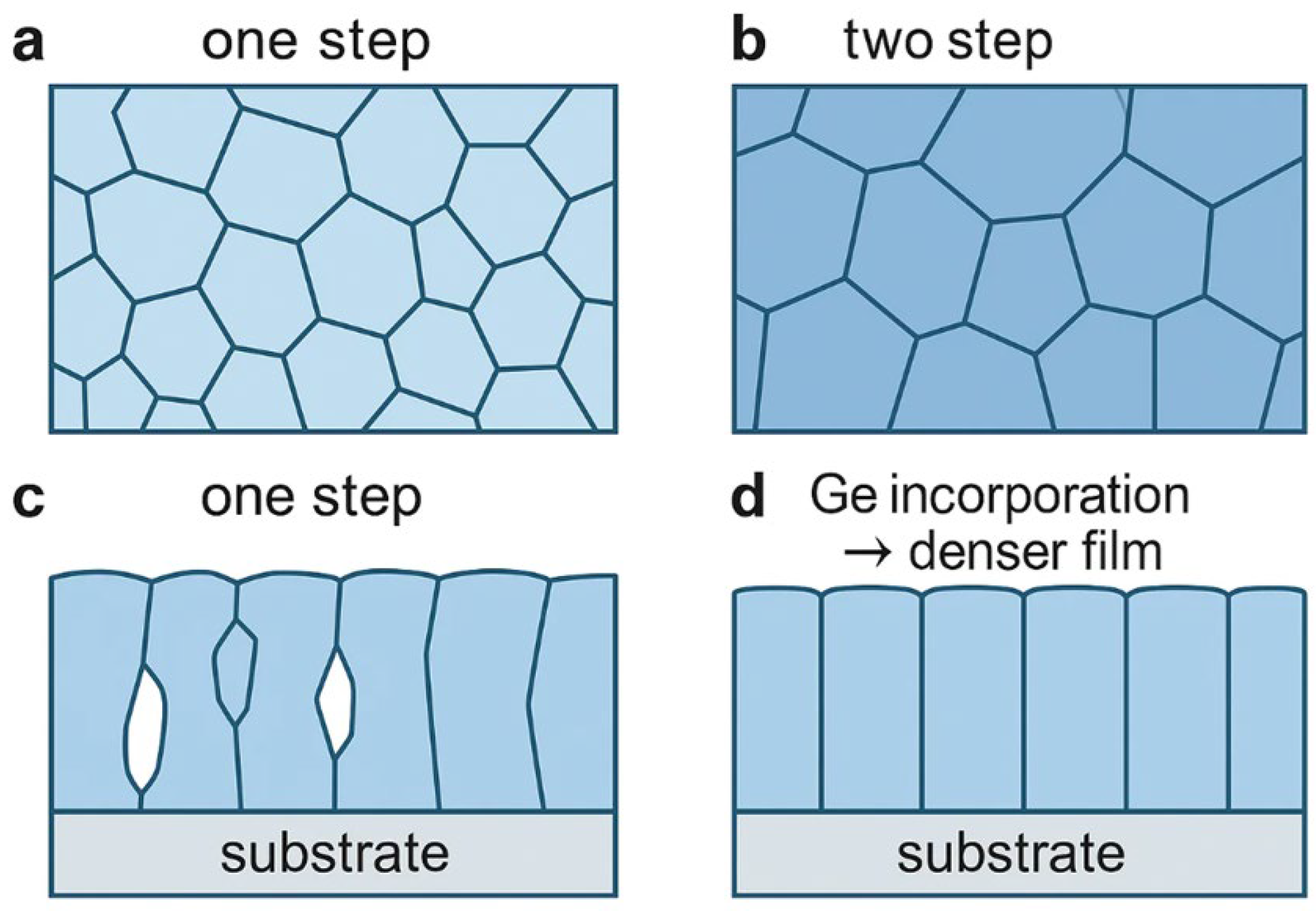
- Germanium (Ge) Doping:
- Lithium (Li) Doping:
- Silver (Ag) Alloying:
- Magnesium (Mg) Doping:
3.1.3. Hydrogen-Assisted Selenization (HAS)

3.2. Interface Engineering
3.2.1. Front Interface Optimization (Absorber/Buffer Layer)

3.2.2. Optimization of the Back Interface (Absorber/Back Contact)
3.3. Compositional Engineering and Doping/Alloying
| Dopant | Substitution Target | Key Benefits | Max. Reported PCE (%) | Reference |
|---|---|---|---|---|
| Ag | Cu | Reduces CuZn antisite defects, enlarges the depletion region width, improves grain growth, enhances band alignment, suppresses non-radiative recombination | 13.38 | [10] |
| Ge | Sn | Raises CBM, suppresses SnZn, improves morphology, back grading, reduces tailing | 14.67 | [15] |
| Li | Cu/Zn | Increases hole concentration, passivates GBs, improves alignment, synergistic with Ag | 13.11 | [10] |
| Ga | Zn/Sn | Suppresses deep defects, increases depletion width, reduces Urbach energy | 12.12 | [2] |
| Cd | Zn | Forms buried junction, increases hole density, improves lifetime | 12.6 | [144] |
| Ag + Li | Cu + Zn | Synergistic defect passivation and doping, highest FF and PCE | 14.91 | [10] |
| Ag + Ge | Cu + Sn | Improves front/back interface, grain growth, recombination suppression | 8.46 | [32] |
| Ge + Cd | Sn + Zn | Combines conductivity and band structure benefits | 11.6 | [145] |
3.4. Microstructure and Morphology Control
3.5. Commercialization Aspects
3.5.1. Cost Competitiveness
3.5.2. Scalability and Manufacturability
3.5.3. Device Stability
3.5.4. Flexible Substrate Integration
4. Advanced Characterization Techniques in CZTSSe Solar Cells
| Technique | Purpose in CZTSSe | Typical Target or Defect | Reference |
|---|---|---|---|
| XRD | Phase identification, crystallinity, alloying effects | CZTS(e), ZnSe, MoSe2, secondary phases | [178,179] |
| Raman Spectroscopy | Detecting secondary phases, Cu-Zn disorder, S/Se ratio | CTS, ZnS, SnS2, phase segregation | [11,180,181,182] |
| PL/TRPL | Evaluating band tailing and recombination activity | CuZn/SnZn-related tail states, minority carrier lifetime | [183,184,185] |
| DLCP | Bulk defect profiling | CuZn, SnZn clusters, acceptor levels | [186,187] |
| SIMS | Elemental depth profiling, dopant distribution | Ge, Ag, Li gradients; Se and MoSe2 interface regions | [188,189] |
| EBIC/XBIC | Mapping charge collection efficiency | Spatial inhomogeneities, recombination centers | [190,191] |
| KPFM | Surface potential variation, work function mapping | Grain boundary barriers | [192,193] |
| SEM/TEM (with EDS/EELS) | Film morphology, grain size, thickness, nanoscale defect analysis | Grain boundaries, voids, secondary phases, antisite clusters | [93,194,195] |
| J–V & QE (Device-level tests) | Linking absorber quality to device performance | VOC deficit, FF losses, series resistance shunt resistance, carrier collection, spectral response | [196,197] |
| In situ/Operando spectroscopy | Real-time tracking of defect dynamics during annealing or illumination | Defect evolution, phase transitions, band tailing | [117,198,199] |
| Phase | Major XRD Peaks (2θ, °) | Notes | Reference |
|---|---|---|---|
| CZTS (kesterite) | 28.5, 47.3, 56.1 | Often overlaps with ZnS and CTS; needs Raman confirmation | [209,210,211] |
| CZTSe | 27.2, 45.2, 53.6 | Slight shift due to larger Se radius | [212,213] |
| ZnS | 28.5, 47.5 | Overlaps with CZTS; cannot be unambiguously identified by XRD alone | [214,215] |
| Cu2SnS3 (CTS) | 28.4, 47.3 | Overlaps with CZTS; Raman needed to distinguish | [216,217] |
| MoSe2 | 13.4, 31.8, 38.3, 47.9, 56.1 | Back contact phase; thickness and formation condition-dependent | [218] |
| ZnSe | 27.2, 45.2, 53.6 | Can appear near back contact in Zn-rich systems | [78,219] |
| SnS | 22.0, 26.0, 27.5, 31.0, 44.8, 53.4 | Orthorhombic α-SnS; peaks shift with morphology; overlaps partly with CZTS | [220] |
| SnS2 | 15.0, 28.2, 32.1, 50.1 | Can overlap CZTS (28.5°); appears under Sn-rich conditions | [221] |
| Cu2−xSe | ~27.0°, ~44.0–44.7° | Highly conductive; forms under Cu-rich conditions; creates shunt path | [87,222,223] |
| SnSe2 | 14.4, 31.15 | Layered phase; common in Sn-rich or Se-rich films; affects VOC stability | [224] |
| Cu2SnSe3 (CTSe) | 28.44°, 32.96°, 47.31°, 56.13 | Overlaps with CZTSe; requires Raman confirmation | [181,210] |
| Phase | Characteristic Raman Peaks (cm−1) | Notes | Reference |
|---|---|---|---|
| CZTS (kesterite) | ~337, 287, 367 | Strong peak at 337 cm−1; mode shift indicates S/Se ratio | [226,227] |
| CZTSe | ~196, 174, 231 | Main peak at 196 cm−1 | [228,229] |
| ZnS | ~274 (TO), ~351 (LO), | Cubic ZnS (sphalerite); TO and LO are reliable modes | [230] |
| CTS | ~294, 354 | Distinction from CZTS benefits from complementary techniques | [231] |
| SnS | 68, 94, 162, 191, 219, 288 | Orthorhombic α-SnS | [232] |
| SnS2 | ~205 (Eg), 312–315 (A1g), ~340 (A2u, weak) | Hexagonal phase; 205 and 312–315 cm−1 are dominant Raman modes, while the ~340 cm−1 mode is weak and rarely observed | [233] |
| MoSe2 | ~240 (A1g), ~285 (E2g1) | Appears when Mo reacts with Se during annealing | [234] |
| ZnSe | 205, 250, 495 | Zinc-blended ZnSe; overlaps with CZTSe, needs careful analysis | [235] |
| Cu2SnSe3 (CTSe) | 178, 204, 231, 248 | Often confused with CZTSe; Raman confirmation required | [236] |
| Cu2−xSe | 260–270 | Cu-rich phase; metallic character, introduces shunt paths | [237,238] |
5. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, A.; He, M.; Green, M.A.; Sun, K.; Hao, X. A critical review on the progress of kesterite solar cells: Current strategies and insights. Adv. Energy Mater. 2023, 13, 2203046. [Google Scholar] [CrossRef]
- Zhao, X.; Pan, Y.; Chen, W.; Yang, W.; Zeng, Q.; Li, L.; Liao, X.; Zhang, Z.; Liu, S.; Lai, Y. Insights into the efficiency improvement for CZTSSe solar cells with over 12% efficiency via Ga incorporation. Adv. Funct. Mater. 2023, 33, 2301377. [Google Scholar] [CrossRef]
- He, M.; Yan, C.; Li, J.; Suryawanshi, M.P.; Kim, J.; Green, M.A.; Hao, X. Kesterite solar cells: Insights into current strategies and challenges. Adv. Sci. 2021, 8, 2004313. [Google Scholar] [CrossRef]
- Quadir, S.; Qorbani, M.; Lai, Y.-R.; Sabbah, A.; Thong, H.T.; Hayashi, M.; Chen, C.Y.; Chen, K.H.; Chen, L.C. Impact of cation substitution in (AgxCu1−x)2ZnSnSe4 absorber-based solar cells toward 10% efficiency: Experimental and theoretical analyses. Sol. RRL 2021, 5, 2100441. [Google Scholar] [CrossRef]
- Taskesen, T.; Neerken, J.; Schoneberg, J.; Pareek, D.; Steininger, V.; Parisi, J.; Gütay, L. Device characteristics of an 11.4% CZTSe solar cell fabricated from sputtered precursors. Adv. Energy Mater. 2018, 8, 1703295. [Google Scholar] [CrossRef]
- Li, Y.; Cui, C.; Wei, H.; Shao, Z.; Wu, Z.; Zhang, S.; Wang, X.; Pang, S.; Cui, G. Suppressing element inhomogeneity enables 14.9% efficiency CZTSSe solar cells. Adv. Mater. 2024, 36, 2400138. [Google Scholar] [CrossRef]
- Wang, D.; Wu, J.; Guo, H.; Wu, M.; Wu, L.; Zhang, S.; Ao, J.; Wang, H.; Zhang, Y. Tuning the work function of the metal back contact toward efficient Cu2ZnSnSe4 solar cells. Sol. RRL 2021, 5, 2000391. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, Q.; Guo, J.; Zhang, Y.; Han, Y.; Ao, J.; Jeng, M.-J.; Liu, F.; Liu, W.; Zhang, Y. Over 10% efficient pure CZTSe solar cell fabricated by electrodeposition with Ge doping. Sol. RRL 2020, 4, 2000059. [Google Scholar] [CrossRef]
- Wang, W.; Winkler, M.T.; Gunawan, O.; Gokmen, T.; Todorov, T.K.; Zhu, Y.; Mitzi, D.B. Device characteristics of CZTSSe thin-film solar cells with 12.6% efficiency. Adv. Energy Mater. 2014, 4, 1301465. [Google Scholar] [CrossRef]
- Zhong, X.; Wang, J.; Han, L.; Chi, J.; Liu, T.; Kou, D.; Zhou, W.; Zhou, Z.; Yuan, S.; Meng, Y. Li, Ag Co-Doping Enables Efficient Kesterite Solar Cell with a High Fill Factor of 74.30%. Adv. Funct. Mater. 2025, 35, 2418548. [Google Scholar] [CrossRef]
- Batibay, D.; Ocak, Y.S.; Genisel, M.F.; Turan, R. Co-sputtered Cu2ZnTi(S:Se)4 absorbers for thin film solar cells. Renew. Energy 2020, 145, 1672–1676. [Google Scholar] [CrossRef]
- Yi, Q.; Wu, J.; Zhao, J.; Wang, H.; Hu, J.; Dai, X.; Zou, G. Tuning bandgap of p-type Cu2Zn(Sn, Ge)(S, Se)4 semiconductor thin films via aqueous polymer-assisted deposition. ACS Appl. Mater. Interfaces 2017, 9, 1602–1608. [Google Scholar] [CrossRef]
- Gong, Y.; Jimenez-Arguijo, A.; Medaille, A.G.; Moser, S.; Basak, A.; Scaffidi, R.; Carron, R.; Flandre, D.; Vermang, B.; Giraldo, S. Li-Doping and Ag-Alloying Interplay Shows the Pathway for Kesterite Solar Cells with Efficiency Over 14%. Adv. Funct. Mater. 2024, 34, 2404669. [Google Scholar] [CrossRef]
- Wang, J.; Lou, L.; Yin, K.; Meng, F.; Xu, X.; Jiao, M.; Zhang, B.; Shi, J.; Wu, H.; Luo, Y. Vacancy enhanced cation ordering enables> 15% efficiency in Kesterite solar cells. arXiv 2024, arXiv:2404.05974. [Google Scholar] [CrossRef]
- Tao, S.; Wang, H.; Jia, M.; Han, J.; Wu, Z.; Zhou, J.; Baranova, M.; Zhu, H.; Zhao, M.; Zhuang, D. Ge-Doped CZTSe Solar Cell Efficiency Beyond 14% by Suppressing Recombination. Adv. Funct. Mater. 2025, 35, 2423251. [Google Scholar] [CrossRef]
- Wang, A.; Chang, N.L.; Sun, K.; Xue, C.; Egan, R.J.; Li, J.; Yan, C.; Huang, J.; Rong, H.; Ramsden, C. Analysis of manufacturing cost and market niches for Cu2ZnSnS4(CZTS) solar cells. Sustain. Energy Fuels 2021, 5, 1044–1058. [Google Scholar] [CrossRef]
- Pedrazzetti, L.; Marchi, C.; Khalil, M.I.; Lucotti, A.; Le Donne, A.; Binetti, S.; Magagnin, L. One Step Cu-Zn-Sn Alloy Deposition As a Precursor for CZTS Absorber in Photovoltaics. In Electrochemical Society Meeting Abstracts 231; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2017; p. 1068. [Google Scholar] [CrossRef]
- Li, J.; Sun, K.; Yuan, X.; Huang, J.; Green, M.A.; Hao, X. Emergence of flexible kesterite solar cells: Progress and perspectives. npj Flex. Electron. 2023, 7, 16. [Google Scholar] [CrossRef]
- Sivasankar, S.M.; Amorim, C.d.O.; Cunha, A.F.d. Progress in thin-film photovoltaics: A review of key strategies to enhance the efficiency of CIGS, CdTe, and CZTSSe solar cells. J. Compos. Sci. 2025, 9, 143. [Google Scholar] [CrossRef]
- Keller, J.; Kiselman, K.; Donzel-Gargand, O.; Martin, N.M.; Babucci, M.; Lundberg, O.; Wallin, E.; Stolt, L.; Edoff, M. High-concentration silver alloying and steep back-contact gallium grading enabling copper indium gallium selenide solar cell with 23.6% efficiency. Nat. Energy 2024, 9, 467–478. [Google Scholar] [CrossRef]
- Green, M.A.; Dunlop, E.D.; Yoshita, M.; Kopidakis, N.; Bothe, K.; Siefer, G.; Hinken, D.; Rauer, M.; Hohl-Ebinger, J.; Hao, X. Solar cell efficiency tables (Version 64). Prog. Photovolt. Res. Appl. 2024, 32, 425–441. [Google Scholar] [CrossRef]
- Qi, Y.; Wei, N.; Li, Y.; Kou, D.; Zhou, W.; Zhou, Z.; Meng, Y.; Yuan, S.; Han, L.; Wu, S. Passivating SnZn Defect and Optimizing Energy Level Alignment via Organic Silicon Salt Incorporation toward Efficient Solution-Processed CZTSSe Solar Cells. Adv. Funct. Mater. 2024, 34, 2308333. [Google Scholar] [CrossRef]
- Guo, R.; Li, X.; Jiang, Y.; Zhou, T.; Xia, Y.; Wang, P.; Liang, Y.; Sui, Y.; Yao, B.; Liu, Y. Regulating SnZn defects and optimizing bandgap in the Cu2ZnSn(S, Se)4 absorption layer by Ge gradient doping for efficient kesterite solar cells. Ceram. Int. 2024, 50, 18329–18336. [Google Scholar] [CrossRef]
- Siebentritt, S. Why are kesterite solar cells not 20% efficient? Thin Solid Film. 2013, 535, 1–4. [Google Scholar] [CrossRef]
- Tombak, A.; Kilicoglu, T.; Ocak, Y.S. Solar cells fabricated by spray pyrolysis deposited Cu2CdSnS4 thin films. Renew. Energy 2020, 146, 1465–1470. [Google Scholar] [CrossRef]
- Sun, Q.; Li, Y.; Zhang, C.; Du, S.; Xie, W.; Wu, J.; Zheng, Q.; Deng, H.; Cheng, S. In-doping collaboratively controlling back interface and bulk defects to achieve efficient flexible CZTSSe solar cells. J. Energy Chem. 2024, 89, 10–17. [Google Scholar] [CrossRef]
- Wang, C.; Wang, T.; Liu, Y.; Li, M.; Ma, D.; Ding, Z.; Zhu, Y.; Sun, Y.; Sun, X.; Shi, L. Improvement of Performance of CZTSSe Solar Cells by the Synergistic Effect of Back Contact Modification and Ag Doping. ACS Appl. Mater. Interfaces 2024, 16, 26182–26194. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zeng, F.; Song, N.; Jiang, L.; Han, Z.; Su, Z.; Yan, C.; Wen, X.; Hao, X.; Liu, Y. Kesterite Cu2ZnSn(S, Se)4 solar cells with beyond 8% efficiency by a sol-gel and selenization process. ACS Appl. Mater. Interfaces 2015, 7, 14376–14383. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wang, Y.; Liu, R.; He, Y.; Zhang, Y.; Liu, C.; Luan, H.; Yang, Y.; Zhu, C. Enhancing the performance of CZTSSe solar cells via an alternate spin-coating process with DMSO and DMF. Sol. Energy Mater. Sol. Cells 2024, 274, 112976. [Google Scholar] [CrossRef]
- Tombak, A.; Ocak, Y.S.; Genişel, M.F.; Kilicoglu, T. Electrical and optical properties of Cu2ZnSnS4 grown by a thermal co-evaporation method and its diode application. Mater. Sci. Semicond. Process. 2014, 28, 98–102. [Google Scholar] [CrossRef]
- Hwang, S.K.; Yoon, J.H.; Kim, J.Y. Current status and future prospects of kesterite Cu2ZnSn(S, Se)4 (CZTSSe) thin film solar cells prepared via electrochemical deposition. ChemElectroChem 2024, 11, e202300729. [Google Scholar] [CrossRef]
- Atasoy, Y.; Bacaksız, E.; Çiriş, A.; Olğar, M.A.; Zan, R.; Ali, A.M.A.-d.; Küçükömeroğlu, T.; Başol, B.M. Improved CZTSe solar cell efficiency via silver and germanium alloying. Sol. Energy 2024, 267, 112247. [Google Scholar] [CrossRef]
- Zhou, T.; Huang, J.; Qian, S.; Wang, X.; Yang, G.; Yao, B.; Li, Y.; Jiang, Y.; Liu, Y. Further boosting solar cell performance via bandgap-graded Ag doping in Cu2ZnSn(S, Se)4 solar cells compared to uniform Ag doping. ACS Appl. Mater. Interfaces 2022, 15, 1073–1084. [Google Scholar] [CrossRef]
- Ava, C.A.; Ocak, Y.S.; Asubay, S.; Celik, O. The influence of Ge substitution and H2S annealing on Cu2ZnSnS4 thin films. Opt. Mater. 2021, 121, 111565. [Google Scholar] [CrossRef]
- Adiguzel, S.; Kaya, D.; Genisel, M.F.; Celik, O.; Tombak, A.; Ocak, Y.S.; Turan, R. Reactively sputtered Cu2ZnTiS4 Thin film as low-cost earth-abundant absorber. J. Electron. Mater. 2017, 46, 3976–3981. [Google Scholar] [CrossRef]
- Du, Y.; Wang, S.; Tian, Q.; Zhao, Y.; Chang, X.; Xiao, H.; Deng, Y.; Chen, S.; Wu, S.; Liu, S. Defect engineering in earth-abundant Cu2ZnSn(S, Se)4 photovoltaic materials via Ga3+-doping for over 12% efficient solar cells. Adv. Funct. Mater. 2021, 31, 2010325. [Google Scholar] [CrossRef]
- Shen, Z.; Wang, S.; Liu, Y.; Sun, Y.; Wu, J.; Guo, H.; Zhang, K.; Zhang, S.; Liu, F.; Zhang, Y. Li2S doping into CZTSe drives the large improvement of VOC of solar cell. J. Energy Chem. 2021, 62, 637–644. [Google Scholar] [CrossRef]
- Guo, Q.; Ford, G.M.; Yang, W.-C.; Hages, C.J.; Hillhouse, H.W.; Agrawal, R. Enhancing the performance of CZTSSe solar cells with Ge alloying. Sol. Energy Mater. Sol. Cells 2012, 105, 132–136. [Google Scholar] [CrossRef]
- Hironiwa, D.; Sakai, N.; Kato, T.; Sugimoto, H.; Tang, Z.; Chantana, J.; Minemoto, T. Impact of annealing treatment before buffer layer deposition on Cu2ZnSn(S, Se)4 solar cells. Thin Solid Film. 2015, 582, 151–153. [Google Scholar] [CrossRef]
- Zhang, W.-C.; Tang, J.-Y.; Niu, Y.-H.; Huang, R.; Chen, L.; Jiang, M.-Y. Study the best ratio of S and Se in CZTSSe solar cells with nontoxic buffer layer. J. Renew. Sustain. Energy 2021, 13, 033701. [Google Scholar] [CrossRef]
- Yadav, S.; Chauhan, R.; Mishra, R.; Kumar, S. Investigating Ag2S quantum dot buffer layer in CZTSSe photovoltaics for enhanced performance. Phys. Scr. 2023, 98, 125106. [Google Scholar] [CrossRef]
- Ren, G.; Zhuang, D.; Zhao, M.; Wei, Y.; Wu, Y.; Li, X.; Lyu, X.; Wang, C.; Li, Y. CZTSSe solar cell with an efficiency of 10.19% based on absorbers with homogeneous composition and structure using a novel two-step annealing process. Sol. Energy 2020, 207, 651–658. [Google Scholar] [CrossRef]
- Yao, L.; Ao, J.; Jeng, M.-J.; Bi, J.; Gao, S.; Sun, G.; He, Q.; Zhou, Z.; Sun, Y.; Chang, L.-B. A CZTSe solar cell with 8.2% power conversion efficiency fabricated using electrodeposited Cu/Sn/Zn precursor and a three-step selenization process at low Se pressure. Sol. Energy Mater. Sol. Cells 2017, 159, 318–324. [Google Scholar] [CrossRef]
- Haass, S.G.; Diethelm, M.; Werner, M.; Bissig, B.; Romanyuk, Y.E.; Tiwari, A.N. 11.2% Efficient Solution Processed Kesterite Solar Cell with a Low Voltage Deficit. Adv. Energy Mater. 2015, 5, 1301465. [Google Scholar] [CrossRef]
- Asuo, I.M.; Fourmont, P.; Ka, I.; Gedamu, D.; Bouzidi, S.; Pignolet, A.; Nechache, R.; Cloutier, S.G. Highly Efficient and Ultrasensitive Large-Area Flexible Photodetector Based on Perovskite Nanowires. Small 2019, 15, e1804150. [Google Scholar] [CrossRef]
- Zhao, R.-Y.; Liu, G.-N.; Liu, Q.-S.; Niu, P.-F.; Xu, R.-D.; Wang, Z.-H.; Wei, T.-H.; Zhang, J.; Sun, Y.-Q.; Li, C. (3-Phenylpyridin-1-ium) SbI4: Coulomb Interaction-Assembled Lead-free Hybrid Perovskite-like Semiconductor. Cryst. Growth Des. 2019, 20, 1009–1015. [Google Scholar] [CrossRef]
- Hajjiah, A.; Gorji, N.E. SEM and TEM image analysis for morphology and phase transition of CZTS, Sb2Se3 and perovskite thin films under thermal stress. J. Electron. Mater. 2025, 54, 523–530. [Google Scholar] [CrossRef]
- Cretì, A.; Prete, P.; Lovergine, N.; Lomascolo, M. Enhanced optical absorption of GaAs near-band-edge transitions in GaAs/AlGaAs core-shell nanowires: Implications for nanowire solar cells. ACS Appl. Nano Mater. 2022, 5, 18149–18158. [Google Scholar] [CrossRef]
- Chen, G.; Sun, G.; Ding, Y.J.; Prete, P.; Miccoli, I.; Lovergine, N.; Shtrikman, H.; Kung, P.; Livneh, T.; Spanier, J.E. Direct measurement of band edge discontinuity in individual core-shell nanowires by photocurrent spectroscopy. Nano Lett. 2013, 13, 4152–4157. [Google Scholar] [CrossRef]
- Cingolani, R.; Di Dio, M.; Lomascolo, M.; Rinaldi, R.; Prete, P.; Vasanelli, L.; Vanzetti, L.; Bassani, F.; Bonanni, A.; Sorba, L. Photocurrent spectroscopy of Zn1−xCdxSe/ZnSe quantum wells in p-i-n heterostructures. Phys. Rev. B 1994, 50, 12179. [Google Scholar] [CrossRef]
- Güzel, R.; Ocak, Y.S.; Karuk, Ş.N.; Ersöz, A.; Say, R. Light harvesting and photo-induced electrochemical devices based on bionanocage proteins. J. Power Sources 2019, 440, 227119. [Google Scholar] [CrossRef]
- Najm, A.S.; Alwash, S.A.; Sulaiman, N.H.; Chowdhury, M.; Techato, K. N719 dye as a sensitizer for dye-sensitized solar cells (DSSCs): A review of its functions and certain rudimentary principles. Environ. Prog. Sustain. Energy 2023, 42, e13955. [Google Scholar] [CrossRef]
- Wu, Q.; Xue, C.; Li, Y.; Zhou, P.; Liu, W.; Zhu, J.; Dai, S.; Zhu, C.; Yang, S. Kesterite Cu2ZnSnS4 as a Low-Cost Inorganic Hole-Transporting Material for High-Efficiency Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 28466–28473. [Google Scholar] [CrossRef]
- González-Castillo, J.; Vigil-Galán, O.; Rodríguez, E.; Jiménez-Olarte, D.; Leal, J. Cu6Sn5 binary phase as a precursor material of the CZTSe compound: Optimization of the synthesis process, physical properties and its performance as an absorbing material in a solar cell. Mater. Sci. Semicond. Process. 2021, 134, 106016. [Google Scholar] [CrossRef]
- Sun, L.; Shen, H.; Huang, H.; Raza, A.; Zhao, Q. Effect of evaporated Sb layer on performance of flexible CZTSSe thin film solar cell. Sol. Energy 2019, 193, 267–274. [Google Scholar] [CrossRef]
- Wang, Z.; Brodusch, N.; Gauvin, R.; Demopoulos, G.P. Lithium-doped Cu2ZnSnS4 superstrate solar cells with 5% efficiency-An alternative to thin film kesterite photovoltaics. Nano Energy 2018, 53, 130–134. [Google Scholar] [CrossRef]
- Moore, J.E.; Hages, C.J.; Agrawal, R.; Lundstrom, M.S.; Gray, J.L. The importance of band tail recombination on current collection and open-circuit voltage in CZTSSe solar cells. Appl. Phys. Lett. 2016, 109, 021102. [Google Scholar] [CrossRef]
- Kim, J.; Shin, B. Strategies to reduce the open-circuit voltage deficit in Cu2ZnSn(S, Se)4 thin film solar cells. Electron. Mater. Lett. 2017, 13, 373–392. [Google Scholar] [CrossRef]
- Gong, Y.; Qiu, R.; Niu, C.; Fu, J.; Jedlicka, E.; Giridharagopal, R.; Zhu, Q.; Zhou, Y.; Yan, W.; Yu, S. Ag incorporation with controlled grain growth enables 12.5% efficient kesterite solar cell with open circuit voltage reached 64.2% Shockley-Queisser limit. Adv. Funct. Mater. 2021, 31, 2101927. [Google Scholar] [CrossRef]
- Risch, L.; Vauche, L.; Redinger, A.; Dimitrievska, M.; Sánchez, Y.; Saucedo, E.; Unold, T.; Goislard, T.; Ruiz, C.; Escoubas, L. Overcoming the V oc limitation of CZTSe solar cells. In Proceedings of the 2016 IEEE 43rd Photovoltaic Specialists Conference (PVSC), Portland, OR, USA, 5–10 June 2016; pp. 0188–0192. [Google Scholar] [CrossRef]
- Ma, H.; Zhu, Q.; Zou, L.; Xu, B.; Wang, H.; Ge, R.; Yue, F.; Zhang, Y.; Sun, L.; Chen, Y. Bulk Carrier Recombination Mechanisms and Photovoltage Deficit in Kesterite Solar Cells. Adv. Energy Mater. 2025, 15, 2405033. [Google Scholar] [CrossRef]
- Gokmen, T.; Gunawan, O.; Todorov, T.K.; Mitzi, D.B. Band tailing and efficiency limitation in kesterite solar cells. Appl. Phys. Lett. 2013, 103, 103506. [Google Scholar] [CrossRef]
- Ren, G.; Zhuang, D.; Zhao, M.; Wei, Y.; Wu, Y.; Li, X.; Lyu, X.; Wang, C.; Li, Y. Cu2ZnSn(S, Se)4 solar cell with slight band tailing states achieves 11.83% efficiency by selenizing sputtered Cu-Zn-Sn-S precursor. J. Power Sources 2020, 479, 228747. [Google Scholar] [CrossRef]
- Chantana, J.; Kawano, Y.; Nishimura, T.; Mavlonov, A.; Minemoto, T. Impact of Urbach energy on open-circuit voltage deficit of thin-film solar cells. Sol. Energy Mater. Sol. Cells 2020, 210, 110502. [Google Scholar] [CrossRef]
- Li, J.; Wang, D.; Li, X.; Zeng, Y.; Zhang, Y. Cation substitution in earth-abundant kesterite photovoltaic materials. Adv. Sci. 2018, 5, 1700744. [Google Scholar] [CrossRef]
- Gong, Y.; Xin, H.; Ding, L. V oc deficit in kesterite solar cells. J. Semicond. 2021, 42, 100201. [Google Scholar] [CrossRef]
- Rey, G.; Larramona, G.; Bourdais, S.; Choné, C.; Delatouche, B.; Jacob, A.; Dennler, G.; Siebentritt, S. On the origin of band-tails in kesterite. Sol. Energy Mater. Sol. Cells 2018, 179, 142–151. [Google Scholar] [CrossRef]
- Gang, M.G.; Karade, V.C.; Suryawanshi, M.P.; Yoo, H.; He, M.; Hao, X.; Lee, I.J.; Lee, B.H.; Shin, S.W.; Kim, J.H. A facile process for partial Ag substitution in kesterite Cu2ZnSn(S, Se)4 solar cells enabling a device efficiency of over 12%. ACS Appl. Mater. Interfaces 2021, 13, 3959–3968. [Google Scholar] [CrossRef]
- Kumar, M.; Dubey, A.; Adhikari, N.; Venkatesan, S.; Qiao, Q. Strategic review of secondary phases, defects and defect-complexes in kesterite CZTS-Se solar cells. Energy Environ. Sci. 2015, 8, 3134–3159. [Google Scholar] [CrossRef]
- Ritzer, M.; Schönherr, S.; Schöppe, P.; Larramona, G.; Choné, C.; Gurieva, G.; Johannes, A.; Ritter, K.; Martínez-Criado, G.; Schorr, S. Interplay of Performance-Limiting Nanoscale Features in Cu2ZnSn(S, Se)4 Solar Cells. Phys. Status Solidi A 2020, 217, 2000456. [Google Scholar] [CrossRef]
- Schorr, S.; Gurieva, G.; Guc, M.; Dimitrievska, M.; Pérez-Rodríguez, A.; Izquierdo-Roca, V.; Schnohr, C.S.; Kim, J.; Jo, W.; Merino, J.M. Point defects, compositional fluctuations, and secondary phases in non-stoichiometric kesterites. J. Phys. Energy 2019, 2, 012002. [Google Scholar] [CrossRef]
- Chen, S.; Yang, J.-H.; Gong, X.-G.; Walsh, A.; Wei, S.-H. Intrinsic point defects and complexes in the quaternary kesterite semiconductor Cu2ZnSnS4. Phys. Rev. B-Condens. Matter Mater. Phys. 2010, 81, 245204. [Google Scholar] [CrossRef]
- Mutter, D.; Dunham, S.T. Calculation of defect concentrations and phase stability in Cu2ZnSnS4 and Cu2ZnSnSe4 from stoichiometry. IEEE J. Photovolt. 2015, 5, 1188–1196. [Google Scholar] [CrossRef]
- Li, C.; Shen, Y.; Song, H.; Wang, Y.; Chen, S.; Qi, R.; Cheng, Y.; Duan, C.-G.; Huang, R. Microstructure of Cu2S nanoprecipitates and its effect on electrical and thermal properties in thermoelectric Cu2Zn0.2Sn0.8S3 ceramics. AIP Adv. 2018, 8, 085105. [Google Scholar] [CrossRef]
- Xu, B.; Qin, X.; Lin, J.; Chen, J.; Tong, H.; Qi, R.; Yue, F.; Chen, Y.; Yang, P.; Chu, J. Positive role of inhibiting CZTSSe decomposition on intrinsic defects and interface recombination of 12.03% efficient kesterite solar cells. Sol. RRL 2022, 6, 2200256. [Google Scholar] [CrossRef]
- Minbashi, M.; Ghobadi, A.; Yazdani, E.; Ahmadkhan Kordbacheh, A.; Hajjiah, A. Efficiency enhancement of CZTSSe solar cells via screening the absorber layer by examining of different possible defects. Sci. Rep. 2020, 10, 21813. [Google Scholar] [CrossRef]
- Wei, H.; Li, Y.; Cui, C.; Wang, X.; Shao, Z.; Pang, S.; Cui, G. Defect suppression for high-efficiency kesterite CZTSSe solar cells: Advances and prospects. Chem. Eng. J. 2023, 462, 142121. [Google Scholar] [CrossRef]
- Qin, X.; Xu, B.; Lin, J.; Chen, J.; Tong, H.; Chen, Y.; Yang, P.; Chu, J.; Sun, L. Above 10% efficient electrodeposited Cu2ZnSn(S, Se)4 solar cell achieved by modifying precursor. Sol. Energy Mater. Sol. Cells 2022, 242, 111781. [Google Scholar] [CrossRef]
- Chen, S.; Wang, L.-W.; Walsh, A.; Gong, X.; Wei, S.-H. Abundance of CuZn+ SnZn and 2CuZn+ SnZn defect clusters in kesterite solar cells. Appl. Phys. Lett. 2012, 101, 223901. [Google Scholar] [CrossRef]
- dos Santos Araujo, M.; dos Santos, H.L.S.; Medina, M.; Salomao, A.C.; Mascaro, L.H.; Junior, M.A.S.A. Vanquishing CZTSSe deep defects to enhance photoelectrocatalytic water splitting. Electrochim. Acta 2023, 464, 142935. [Google Scholar] [CrossRef]
- Ma, S.; Li, H.; Hong, J.; Wang, H.; Lu, X.; Chen, Y.; Sun, L.; Yue, F.; Tomm, J.W.; Chu, J. Origin of band-tail and deep-donor states in Cu2ZnSnS4 solar cells and their suppression through Sn-poor composition. J. Phys. Chem. Lett. 2019, 10, 7929–7936. [Google Scholar] [CrossRef]
- Geng, H.; Wang, M.; Wang, S.; Kou, D.; Zhou, Z.; Zhou, W.; Qi, Y.; Yuan, S.; Han, L.; Meng, Y. Two-step cooling strategy for synergistic control of CuZn and SnZn defects enabling 12.87% efficiency (Ag, Cu)2ZnSn(S, Se)4 solar cells. Adv. Funct. Mater. 2023, 33, 2210551. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Z.; Cao, L.; Kou, D.; Yuan, S.; Zheng, Z.; Yang, G.; Tian, Q.; Wu, S.; Liu, S. Suppressed interface defects by GeSe2 post-deposition treatment enables high-efficiency kesterite solar cells. Adv. Funct. Mater. 2023, 33, 2211315. [Google Scholar] [CrossRef]
- Xie, T.; Han, L.; Chu, L.; Han, M.; Jian, Y.; Chi, J.; Zhong, X.; Liu, T.; Kou, D.; Zhou, W. Passivation of Deep-Level Defects through Chemical Environment Regulation for Efficient CZTSSe Solar Cells Based on Active Selenium Adsorption-Desorption Process. Adv. Funct. Mater. 2025, 35, 2414940. [Google Scholar] [CrossRef]
- Cui, X.-P.; Ma, Q.; Zhou, W.-H.; Kou, D.-X.; Zhou, Z.-J.; Meng, Y.-N.; Qi, Y.-F.; Yuan, S.-J.; Han, L.-T.; Wu, S.-X. Suppressing interface recombination in CZTSSe solar cells by simple selenization with synchronous interface gradient doping. Nanoscale 2023, 15, 185–194. [Google Scholar] [CrossRef]
- Wang, A.; Huang, J.; Yan, C.; He, G.; Cui, X.; Yuan, X.; Zhou, S.; He, M.; Qiu, T.; Zhao, C. Cd-Free High-Bandgap Cu2ZnSnS4 Solar Cell with 10.7% Certified Efficiency Enabled by Engineering Sn-Related Defects. Adv. Funct. Mater. 2024, 34, 2407063. [Google Scholar] [CrossRef]
- Kaur, K.; Sood, M.; Kumar, N.; Nazari, H.H.; Gudavalli, G.S.; Dhakal, T.P.; Kumar, M. Critical role of Zn/Sn ratio to enhance Cu-Zn-Sn-S solar cell efficiency by suppressing detrimental Cu2−xS secondary phase. Sol. Energy Mater. Sol. Cells 2018, 179, 22–30. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Jia, Z.-J.; Zhao, Z.-Y. Secondary phases in Cu2ZnSnS4 thin film solar cell: The role of interfaces. Phys. B Condens. Matter 2022, 626, 413539. [Google Scholar] [CrossRef]
- Temgoua, S.; Bodeux, R.; Naghavi, N.; Delbos, S. Effects of SnSe2 secondary phases on the efficiency of Cu2ZnSn(Sx, Se1−x)4 based solar cells. Thin Solid Film. 2015, 582, 215–219. [Google Scholar] [CrossRef]
- Vauche, L.; Risch, L.; Arasimowicz, M.; Sánchez, Y.; Saucedo, E.; Pasquinelli, M.; Goislard de Monsabert, T.; Grand, P.-P.; Jaime-Ferrer, S. Detrimental effect of Sn-rich secondary phases on Cu2ZnSnSe4 based solar cells. J. Renew. Sustain. Energy 2016, 8, 033502. [Google Scholar] [CrossRef]
- Xie, H.; Sánchez, Y.; López-Marino, S.; Espíndola-Rodríguez, M.; Neuschitzer, M.; Sylla, D.; Fairbrother, A.; Izquierdo-Roca, V.; Pérez-Rodríguez, A.; Saucedo, E. Impact of Sn(S, Se) secondary phases in Cu2ZnSn(S, Se) 4 solar cells: A chemical route for their selective removal and absorber surface passivation. ACS Appl. Mater. Interfaces 2014, 6, 12744–12751. [Google Scholar] [CrossRef]
- Oh, S.; Lim, S.Y.; Son, D.-H.; Kang, J.-K.; Yoo, H.; Kim, D.-H.; Cheong, H. Optical Detection of SnSe2 Formation on CZTSSe Thin-Film Solar Cells. ACS Appl. Energy Mater. 2022, 5, 11774–11779. [Google Scholar] [CrossRef]
- Zaki, M.Y.; Velea, A. Recent progress and challenges in controlling secondary phases in kesterite CZT (S/Se) thin films: A critical review. Energies 2024, 17, 1600. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Kim, S.-H.; Hong, S.; Son, D.-H.; Kim, Y.-I.; Kim, S.; Ahn, K.; Yang, K.-J.; Kim, D.-H.; Kang, J.-K. Secondary phase formation mechanism in the Mo-back contact region during sulfo-selenization using a metal precursor: Effect of wettability between a liquid metal and substrate on secondary phase formation. ACS Appl. Mater. Interfaces 2019, 11, 23160–23167. [Google Scholar] [CrossRef]
- Altamura, G.; Vidal, J. Impact of minor phases on the performances of CZTSSe thin-film solar cells. Chem. Mater. 2016, 28, 3540–3563. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, F.; Luo, Z.; Wu, Y.; Dong, X.; Chen, J.; Zhang, X.; Li, Y. Exploring the promoting effect of lanthanum passivation on the photovoltaic performance of CZTSSe solar cells. J. Chem. Phys. 2024, 161, 234201. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Kong, D.; Hu, G.; Li, J.; Xing, Y.; Li, W. Innovative surface passivation of CZTSSe thin films: Ammonium sulfide treatment with plasma etching. Mater. Sci. Semicond. Process. 2024, 181, 108651. [Google Scholar] [CrossRef]
- Wang, L.; Chu, L.; Zhou, Z.; Zhou, W.; Kou, D.; Meng, Y.; Qi, Y.; Yuan, S.; Han, L.; Yang, G. Synergistic crystallization modulation and defects passivation in kesterite via anion-coordinate precursor engineering for efficient solar cells. Adv. Sci. 2024, 11, 2405016. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Zhao, Y.; Luo, D.; Zhang, X.; Zhao, J.; Su, Z.; Chen, S.; Liang, G. Potassium doping for grain boundary passivation and defect suppression enables highly-efficient kesterite solar cells. Chin. Chem. Lett. 2024, 35, 109468. [Google Scholar] [CrossRef]
- Meng, R.; Liu, Y.; Xu, X.; Wu, L.; Li, J.; Feng, L.; Xu, H.; Shao, S.; Guo, H.; Zhang, Y. Grain Boundaries Passivation to Attain High Efficient Cu2ZnSn(Sx,Se1−x)4 Solar Cells. Adv. Funct. Mater. 2025, 2506959. [Google Scholar] [CrossRef]
- Siqin, L.; Xin, W.; Liu, R.; Luan, H.; Wang, L.; Wang, Y.; Li, S.; Guo, J.; He, Y.; Zhang, J. Cu2ZnSn(S, Se)4 solar cells with over 10% power conversion efficiency enabled by dual passivation strategy. Sol. Energy Mater. Sol. Cells 2024, 272, 112880. [Google Scholar] [CrossRef]
- Enkhbat, T.; Enkhbayar, E.; Otgontamir, N.; Sharif, M.H.; Mina, M.S.; Kim, S.Y.; Kim, J. High efficiency CZTSSe solar cells enabled by dual Ag-passivation approach via aqueous solution process. J. Energy Chem. 2023, 77, 239–246. [Google Scholar] [CrossRef]
- Charghandeh, R.; Abbasi, A. Improving the efficiency of CZTSSe thin-film solar cells using an optimized Al2O3 rear surface passivation layer. J. Mater. Sci. Mater. Electron. 2023, 34, 394. [Google Scholar] [CrossRef]
- Jian, Y.; Han, L.; Kong, X.; Xie, T.; Kou, D.; Zhou, W.; Zhou, Z.; Yuan, S.; Meng, Y.; Qi, Y. Segmented Control of Selenization Environment for High-Quality Cu2ZnSn(S, Se)4 Films Toward Efficient Kesterite Solar Cells. Small Methods 2024, 8, 2400041. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, C.; Chen, S.; Zheng, Z.; Fan, P.; Li, Y.; Tan, M.; Yan, C.; Zhang, X.; Su, Z. Unveiling the selenization reaction mechanisms in ambient air-processed highly efficient kesterite solar cells. Adv. Energy Mater. 2023, 13, 2300521. [Google Scholar] [CrossRef]
- Guo, J.; Mao, Y.; Ao, J.; Han, Y.; Cao, C.; Liu, F.; Bi, J.; Wang, S.; Zhang, Y. Microenvironment Created by SnSe2 Vapor and Pre-Selenization to Stabilize the Surface and Back Contact in Kesterite Solar Cells. Small 2022, 18, 2203354. [Google Scholar] [CrossRef] [PubMed]
- Zaki, M.Y.; Sava, F.; Simandan, I.D.; Stavarache, I.; Velea, A.; Pintilie, L. Optimization of CZTSe Thin Films Using Sequential Annealing in Selenium and Tin-Selenium Environments. Inorg. Chem. 2024, 64, 1–10. [Google Scholar] [CrossRef]
- Zhu, X.; Meng, R.; Shao, S.; Feng, L.; Shang, L.; Liu, H.; Wang, Y.; Guo, H.; Zhang, Y. Tailoring selenization dynamics: How heating rate manipulates nucleation and growth boosts efficiency in kesterite solar cells. J. Chem. Phys. 2025, 162, 034702. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Meng, R.; Dong, J.; Xu, X.; Zhang, J.; Zhang, Y. Tailoring Li assisted CZTSe film growth under controllable selenium partial pressure and solar cells. J. Chem. Phys. 2024, 161, 124709. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, J.; Xu, X.; Meng, F.; Xiang, C.; Lou, L.; Yin, K.; Duan, B.; Wu, H.; Shi, J. Ge bidirectional diffusion to simultaneously engineer back interface and bulk defects in the absorber for efficient CZTSSe solar cells. Adv. Mater. 2022, 34, 2202858. [Google Scholar] [CrossRef]
- Neuschitzer, M.; Rodriguez, M.E.; Guc, M.; Marquez, J.A.; Giraldo, S.; Forbes, I.; Perez-Rodriguez, A.; Saucedo, E. Revealing the beneficial effects of Ge doping on Cu2ZnSnSe4 thin film solar cells. J. Mater. Chem. A 2018, 6, 11759–11772. [Google Scholar] [CrossRef]
- Jimenez-Arguijo, A.; Navarro Güell, A.; Sanchez, Y.; Malerba, C.; Valentini, M.; Becker, P.; Choubrac, L.; Unold, T.; Jehl Li-Kao, Z.; Giraldo, S. Small atom doping: A synergistic strategy to reduce SnZn recombination center concentration in Cu2ZnSnSe4. Sol. RRL 2022, 6, 2200580. [Google Scholar] [CrossRef]
- Simya, O.; Anefnaf, I.; Punathil, P.; Artegiani, E.; Torabi, N.; Mukhtar, M.; Romeo, A. Impact of Lithium as Interfacial Treatment for CZTSSe Solar Cells. In Proceedings of the 2024 IEEE 52nd Photovoltaic Specialist Conference (PVSC), Seattle, WA, USA, 9–14 June 2024; pp. 0640–0644. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, J.; Siqin, L.; Yang, C.; Wang, L.; Li, S.; Liu, R.; Luan, H.; Zhu, C. Enhancing the photovoltaic efficiency of CZTSSe thin-film solar cells via Ag-doping induced defect modulation. Sol. Energy Mater. Sol. Cells 2024, 277, 113138. [Google Scholar] [CrossRef]
- Park, J.; Lee, M.; Karade, V.; Shin, S.J.; Yoo, H.; Shim, H.; Gour, K.S.; Kim, D.; Hwang, J.; Shin, D. Suppression of defects through cation substitution: A strategic approach to improve the performance of kesterite Cu2ZnSn(S, Se)4 solar cells under indoor light conditions. Sol. RRL 2021, 5, 2100020. [Google Scholar] [CrossRef]
- Karade, V.; Kim, K.; Yun, J.H.; Kim, J.H. Effect of Double Cation Substitution on Nonradiative Recombination Losses in Cu2ZnSn(S, Se)4 Solar Cells. In Proceedings of the 2023 IEEE 50th Photovoltaic Specialists Conference (PVSC), San Juan, PR, USA, 11–16 June 2023; p. 1. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Tseng, S.-C.; Chen, W.-C.; Yin, G.-C.; Chen, B.-Y.; Chen, K.-H.; Chen, L.-C.; Chen, C.-Y. Visualization of anion vacancy defect annihilation in CZTSe solar cells by hydrogen-assisted selenization with in operando x-ray nanoprobe studies. ACS Appl. Mater. Interfaces 2024, 16, 64656–64663. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-T.; Hu, F.; Huang, J.-H.; Chang, C.-h.; Lai, C.-c.; Yen, Y.-T.; Huang, H.-Y.; Hong, H.-F.; Wang, Z.M.; Shen, C.-H. Improved efficiency of a large-area Cu(In, Ga)Se2 solar cell by a nontoxic hydrogen-assisted solid Se vapor selenization process. ACS Appl. Mater. Interfaces 2014, 6, 4842–4849. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-C.; Chen, C.-Y.; Lin, Y.-R.; Chang, J.-K.; Chen, C.-H.; Chiu, Y.-P.; Wu, C.-I.; Chen, K.-H.; Chen, L.-C. Interface engineering of CdS/CZTSSe heterojunctions for enhancing the Cu2ZnSn(S, Se)4 solar cell efficiency. Mater. Today Energy 2019, 13, 256–266. [Google Scholar] [CrossRef]
- Hong, C.W.; Shin, S.W.; Suryawanshi, M.P.; Gang, M.G.; Heo, J.; Kim, J.H. Chemically deposited CdS buffer/kesterite Cu2ZnSnS4 solar cells: Relationship between CdS thickness and device performance. ACS Appl. Mater. Interfaces 2017, 9, 36733–36744. [Google Scholar] [CrossRef]
- Lee, J.; Enkhbat, T.; Han, G.; Sharif, M.H.; Enkhbayar, E.; Yoo, H.; Kim, J.H.; Kim, S.; Kim, J. Over 11% efficient eco-friendly kesterite solar cell: Effects of S-enriched surface of Cu2ZnSn(S, Se)4 absorber and band gap controlled (Zn, Sn) O buffer. Nano Energy 2020, 78, 105206. [Google Scholar] [CrossRef]
- Yan, C.; Liu, F.; Song, N.; Ng, B.K.; Stride, J.A.; Tadich, A.; Hao, X. Band alignments of different buffer layers (CdS, Zn (O, S), and In2S3) on Cu2ZnSnS4. Appl. Phys. Lett. 2014, 104, 173901. [Google Scholar] [CrossRef]
- Bencherif, H. Towards a high efficient Cd-free double CZTS layers kesterite solar cell using an optimized interface band alignment. Sol. Energy 2022, 238, 114–125. [Google Scholar] [CrossRef]
- Ghorbani, E. On efficiency of earth-abundant chalcogenide photovoltaic materials buffered with CdS: The limiting effect of band alignment. J. Phys. Energy 2020, 2, 025002. [Google Scholar] [CrossRef]
- Liu, F.; Yan, C.; Huang, J.; Sun, K.; Zhou, F.; Stride, J.A.; Green, M.A.; Hao, X. Nanoscale microstructure and chemistry of Cu2ZnSnS4/CdS interface in kesterite Cu2ZnSnS4 solar cells. Adv. Energy Mater. 2016, 6, 1600706. [Google Scholar] [CrossRef]
- Zhang, B.; Han, L.; Ying, S.; Li, Y.; Yao, B. Enhanced efficiency of Cu2 ZnSn(S, Se)4 solar cells via anti-reflectance properties and surface passivation by atomic layer deposited aluminum oxide. RSC Adv. 2018, 8, 19213–19219. [Google Scholar] [CrossRef]
- Xianfeng, Z.; Masakazu, K.; Akira, Y. Comparison of Ag (In, Ga) Se2/Mo and Cu(In, Ga)Se2/Mo Interfaces in Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 16215–16220. [Google Scholar] [CrossRef]
- Gour, K.S.; Karade, V.; Jang, J.S.; Jo, E.; Babar, P.; Korade, S.; Yoo, H.; Kim, S.; Kim, D.; Park, J. Nanoscale rear-interface passivation in Cu2ZnSn(S, Se)4 solar cells through the CuAlO2 intermediate layer. ACS Appl. Energy Mater. 2021, 4, 5222–5229. [Google Scholar] [CrossRef]
- Sawa, H.B.; Babucci, M.; Keller, J.; Platzer Björkman, C.; Mlyuka, N.R.; Samiji, M.E. Effects of Al2O3 Rear Interface Passivation on the Performance of Bifacial Kesterite-Based Solar Cells with Fluorine-Doped Tin Dioxide Back Contact. Phys. Status Solidi B 2024, 261, 2400080. [Google Scholar] [CrossRef]
- Gour, K.S.; Karade, V.C.; Lee, M.; Jang, J.S.; Jo, E.; Babar, P.; Shim, H.; Yun, J.S.; Park, J.; Kim, J.H. Engineering of interface and bulk properties in Cu2ZnSn(S, Se)4 thin-film solar cells with ultrathin CuAlO2 intermediate layer and Ge doping. ACS Appl. Energy Mater. 2022, 5, 2024–2035. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Hong, S.; Kim, S.-H.; Son, D.-H.; Kim, Y.-I.; Kim, S.; Heo, Y.-W.; Kang, J.-K.; Kim, D.-H. Effect of Al2O3 dot patterning on CZTSSe solar cell characteristics. Nanomaterials 2020, 10, 1874. [Google Scholar] [CrossRef]
- Zhang, A.; Zhou, Z.; Zhou, W.; Kou, D.; Meng, Y.; Qi, Y.; Yuan, S.; Wu, S. Chemical dynamics of back contact with MoO3 interfacial layer in kesterite solar cells: Microstructure evolution and photovoltaic performance. Sol. RRL 2019, 3, 1900131. [Google Scholar] [CrossRef]
- Xu, B.; Ma, C.; Lu, X.; Liu, Y.; Zhang, Q.; Chen, Y.; Yang, P.; Chu, J.; Sun, L. Beyond 10% efficient Cu2ZnSn(S, Se)4 solar cells: Effects of the introduction of SnS powder during selenization process. Sol. Energy Mater. Sol. Cells 2020, 210, 110522. [Google Scholar] [CrossRef]
- Kim, Y.-I.; Son, D.-H.; Lee, J.; Sung, S.-J.; Kang, J.-K.; Kim, D.-H.; Yang, K.-J. Secondary Phase and Defects in Cu2ZnSnSe4 Solar Cells with Decreasing Absorber Layer Thickness. Curr. Photovolt. Res. 2021, 9, 84–95. [Google Scholar] [CrossRef]
- Yan, C.; Huang, J.; Sun, K.; Johnston, S.; Zhang, Y.; Sun, H.; Pu, A.; He, M.; Liu, F.; Eder, K. Cu2ZnSnS4 solar cells with over 10% power conversion efficiency enabled by heterojunction heat treatment. Nat. Energy 2018, 3, 764–772. [Google Scholar] [CrossRef]
- Li, J.; Kim, S.; Nam, D.; Liu, X.; Kim, J.; Cheong, H.; Liu, W.; Li, H.; Sun, Y.; Zhang, Y. Tailoring the defects and carrier density for beyond 10% efficient CZTSe thin film solar cells. Sol. Energy Mater. Sol. Cells 2017, 159, 447–455. [Google Scholar] [CrossRef]
- Wu, Y.; Sui, Y.; He, W.; Zeng, F.; Wang, Z.; Wang, F.; Yao, B.; Yang, L. Substitution of Ag for Cu in Cu2ZnSn(S, Se)4: Toward wide band gap absorbers with low antisite defects for thin film solar cells. Nanomaterials 2020, 10, 96. [Google Scholar] [CrossRef]
- Kaur, K.; Arora, K.; Behzad, B.; Qiao, Q.; Kumar, M. Nanoscale charge transport and local surface potential distribution to probe defect passivation in Ag doped Cu2ZnSnS4 absorbing layer. Nanotechnology 2018, 30, 065706. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.K.; Chen, S.; Xiang, H.; Gong, X.G.; Walsh, A.; Park, J.S.; Repins, I.; Wei, S.H. Engineering solar cell absorbers by exploring the band alignment and defect disparity: The case of Cu-and Ag-based kesterite compounds. Adv. Funct. Mater. 2015, 25, 6733–6743. [Google Scholar] [CrossRef]
- Chen, G.; Wang, W.; Chen, S.; Whang, Z.; Huang, Z.; Zhang, B.; Kong, X. Bandgap engineering of Cu2ZnSn1−xGexS(e)4 by adjusting Sn-Ge ratios for almost full solar spectrum absorption. J. Alloys Compd. 2017, 718, 236–245. [Google Scholar] [CrossRef]
- He, M.; Zhang, X.; Huang, J.; Li, J.; Yan, C.; Kim, J.; Chen, Y.S.; Yang, L.; Cairney, J.M.; Zhang, Y. High efficiency Cu2ZnSn(S, Se)4 solar cells with shallow LiZn acceptor defects enabled by solution-based Li post-deposition treatment. Adv. Energy Mater. 2021, 11, 2003783. [Google Scholar] [CrossRef]
- Xin, H.; Vorpahl, S.; Collord, A.; Braly, I.; Uhl, A.; Krueger, B.; Ginger, D.; Hillhouse, H. Lithium-doping inverts the nanoscale electric field at the grain boundaries in Cu2ZnSn(S, Se)4 and increases photovoltaic efficiency. Phys. Chem. Chem. Phys. 2015, 17, 23859–23866. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Sun, Q.; Deng, H.; Xie, W.; Zhang, C.; Wu, J.; Zheng, Q.; Cheng, S. Enhancing carrier transport in flexible CZTSSe solar cells via doping Li strategy. J. Energy Chem. 2022, 75, 8–15. [Google Scholar] [CrossRef]
- Su, Z.; Liang, G.; Fan, P.; Luo, J.; Zheng, Z.; Xie, Z.; Wang, W.; Chen, S.; Hu, J.; Wei, Y. Device postannealing enabling over 12% efficient solution-processed Cu2ZnSnS4 solar cells with Cd2+ substitution. Adv. Mater. 2020, 32, 2000121. [Google Scholar] [CrossRef]
- He, M.; Huang, J.; Li, J.; Jang, J.S.; Suryawanshi, U.P.; Yan, C.; Sun, K.; Cong, J.; Zhang, Y.; Kampwerth, H. Systematic efficiency improvement for Cu2ZnSn(S, Se)4 solar cells by double cation incorporation with Cd and Ge. Adv. Funct. Mater. 2021, 31, 2104528. [Google Scholar] [CrossRef]
- Kim, S.Y.; Son, D.H.; Kim, S.H.; Kim, Y.I.; Kim, S.; Ahn, K.; Yang, K.J.; Kang, J.K.; Kim, D.H. Effect of Cu-Sn-Se liquid phase on grain growth and efficiency of CZTSSe solar cells. Adv. Energy Mater. 2020, 10, 1903173. [Google Scholar] [CrossRef]
- Yuan, X.; Li, J.; Sun, K.; Huang, J.; Cui, X.; Wang, A.; Xie, B.; Hoex, B.; Green, M.; Hao, X. Improved carrier collection efficiency in CZTS solar cells by Li-enhanced liquid-phase-assisted grain growth. EcoEnergy 2024, 2, 181–191. [Google Scholar] [CrossRef]
- Zeleke, M.A.; Kuo, D.-H. Synthesis and characterization of Ge doped Cu2ZnSn(S, Se)4 bulk in the presence of reactive liquid phase sintering aid. Ceram. Int. 2020, 46, 27226–27231. [Google Scholar] [CrossRef]
- Liu, X.; Chang, S.; Liu, J.; Qiao, Y.; Jia, E.; Shen, X.; Li, S.; Cheng, K.; Du, Z. Facile Sb2Se3 and Se co-selenization process improves the performance of Cu2ZnSnSe4 solar cells. J. Power Sources 2021, 491, 229581. [Google Scholar] [CrossRef]
- Zhao, B.; Deng, Y.; Cao, L.; Zhu, J.; Zhou, Z. Doping of Sb into Cu2ZnSn(S, Se)4 absorber layer via Se&Sb2Se3 co-selenization strategy for enhancing open-circuit voltage of kesterite solar cells. Front. Chem. 2022, 10, 974761. [Google Scholar] [CrossRef]
- Wang, S.; Shen, Z.; Liu, Y.; Zhang, Y. Growth mechanism and properties of nanostructure Cu2ZnSnSe4 thin films and solar cells. ACS Appl. Nano Mater. 2023, 6, 16515–16523. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Wang, H.; Wu, L.; Wang, J.; Liu, W.; Zhou, Z.; He, Q.; Sun, Y. On the growth process of Cu2ZnSn(S, Se)4 absorber layer formed by selenizing Cu-ZnS-SnS precursors and its photovoltaic performance. Sol. Energy Mater. Sol. Cells 2015, 132, 363–371. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Kim, S.-H.; Son, D.-H.; Yoo, H.; Kim, S.; Kim, S.; Kim, Y.-I.; Park, S.-N.; Jeon, D.-H.; Lee, J. Effect of Metal-Precursor Stacking Order on Volume-Defect Formation in CZTSSe Thin Film: Formation Mechanism of Blisters and Nanopores. ACS Appl. Mater. Interfaces 2022, 14, 30649–30657. [Google Scholar] [CrossRef] [PubMed]
- Caballero, R.; Haass, S.G.; Andres, C.; Arques, L.; Oliva, F.; Izquierdo-Roca, V.; Romanyuk, Y.E. Effect of magnesium incorporation on solution-processed kesterite solar cells. Front. Chem. 2018, 6, 5. [Google Scholar] [CrossRef]
- Haque, K.; Baten, M.Z. On the prospect of CZTSSe-based thin film solar cells for indoor photovoltaic applications: A simulation study. AIP Adv. 2019, 9, 055326. [Google Scholar] [CrossRef]
- Jo, E.; Gang, M.G.; Shim, H.; Suryawanshi, M.P.; Ghorpade, U.V.; Kim, J.H. 8% Efficiency Cu2ZnSn(S, Se)4(CZTSSe) thin film solar cells on flexible and lightweight molybdenum foil substrates. ACS Appl. Mater. Interfaces 2019, 11, 23118–23124. [Google Scholar] [CrossRef]
- López Mariño, S. New Processing Approaches for Cu2ZnSnSe4-Based Solar Cells. Ph.D. Thesis, Universitat Politècnica de Catalunya, Barcelona, Spain, 2016. [Google Scholar]
- Boutebakh, F.; Batibay, D.; Aida, M.; Ocak, Y.; Attaf, N. Thermal sulfurization effect on sprayed CZTS thin filmsproperties and CZTS/CdS solar cells performances. Mater. Res. Express 2018, 5, 015511. [Google Scholar] [CrossRef]
- Yang, K.-J.; Kim, S.; Kim, S.-Y.; Ahn, K.; Son, D.-H.; Kim, S.-H.; Lee, S.-J.; Kim, Y.-I.; Park, S.-N.; Sung, S.-J. Flexible Cu2ZnSn(S, Se)4 solar cells with over 10% efficiency and methods of enlarging the cell area. Nat. Commun. 2019, 10, 2959. [Google Scholar] [CrossRef]
- Xie, W.; Yan, Q.; Sun, Q.; Li, Y.; Zhang, C.; Deng, H.; Cheng, S. A progress review on challenges and strategies of flexible Cu2ZnSn(S, Se)4 solar cells. Sol. RRL 2023, 7, 2201036. [Google Scholar] [CrossRef]
- Becerril-Romero, I.; Acebo, L.; Oliva, F.; Izquierdo-Roca, V.; López-Marino, S.; Espíndola-Rodríguez, M.; Neuschitzer, M.; Sanchez, Y.; Placidi, M.; Pérez-Rodríguez, A. C ZTS e solar cells developed on polymer substrates: Effects of low-temperature processing. Prog. Photovolt. Res. Appl. 2018, 26, 55–68. [Google Scholar] [CrossRef]
- Park, J.; Nam, H.; Song, B.-G.; Burak, D.; Jang, H.S.; Lee, S.Y.; Cho, S.-H.; Park, J.-K. Performance Enhancement in Powder-Fabricated Cu2(ZnSn)Se4 Solar Cell by Roll Compression. Materials 2023, 16, 1076. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Meng, R.; Xu, X.; Wu, L.; Sun, Y.; Liu, Y.; Wang, Z.; Wang, N.; Li, M.; Zhang, Y. Facile Tailor on the Surface of Mo Foil Toward High-Efficient Flexible CZTSSe Solar Cells. Small Methods 2025, 9, 2401084. [Google Scholar] [CrossRef]
- Yu, X.; Cheng, S.; Yan, Q.; Fu, J.; Jia, H.; Sun, Q.; Yang, Z.; Wu, S. Efficient flexible Mo foil-based Cu2ZnSn(S, Se)4 solar cells from In-doping technique. Sol. Energy Mater. Sol. Cells 2020, 209, 110434. [Google Scholar] [CrossRef]
- Tai, K.F. Investigating the Open-Circuit Voltage Deficit in Cu2ZnSn(S, Se)4 Solar Cells. Ph.D. Thesis, Nanyang Technological University, Singapore, 2015. [Google Scholar]
- Sun, L.; Shen, H.; Huang, H.; Raza, A.; Shang, H. Performance enhancement of flexible CZTSSe solar cells on optimized roughness substrate. Opt. Eng. 2018, 57, 077101. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, B.; Ding, Z.; Li, Y. Performance Degradation in Solution-Processed Cu2ZnSn(S, Se)4 Solar Cells Based on Different Oxidation States of Copper Salts. ACS Appl. Energy Mater. 2022, 5, 11740–11747. [Google Scholar] [CrossRef]
- Park, H.K.; Cho, Y.; Kim, J.; Kim, S.; Kim, S.; Kim, J.; Yang, K.-J.; Kim, D.-H.; Kang, J.-K.; Jo, W. Flexible kesterite thin-film solar cells under stress. npj Flex. Electron. 2022, 6, 91. [Google Scholar] [CrossRef]
- Sun, K.; Liu, F.; Huang, J.; Yan, C.; Song, N.; Sun, H.; Xue, C.; Zhang, Y.; Pu, A.; Shen, Y. Flexible kesterite Cu2ZnSnS4 solar cells with sodium-doped molybdenum back contacts on stainless steel substrates. Sol. Energy Mater. Sol. Cells 2018, 182, 14–20. [Google Scholar] [CrossRef]
- Min, J.-H.; Jeong, W.-L.; Kim, K.; Lee, J.-S.; Kim, K.-P.; Kim, J.; Gang, M.G.; Hong, C.W.; Kim, J.H.; Lee, D.-S. Flexible high-efficiency CZTSSe solar cells on diverse flexible substrates via an adhesive-bonding transfer method. ACS Appl. Mater. Interfaces 2020, 12, 8189–8197. [Google Scholar] [CrossRef]
- Deng, H.; Sun, Q.; Yang, Z.; Li, W.; Yan, Q.; Zhang, C.; Zheng, Q.; Wang, X.; Lai, Y.; Cheng, S. Novel symmetrical bifacial flexible CZTSSe thin film solar cells for indoor photovoltaic applications. Nat. Commun. 2021, 12, 3107. [Google Scholar] [CrossRef]
- Li, J.; Hao, W.; Duan, J.; Xu, J. Stability enhancement of flexible CZTSSe solar cells by using nanopore array substrate. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Wuhan, China, 14–16 June 2019; p. 012037. [Google Scholar] [CrossRef]
- Kaur, K.; Kumar, M. Progress and prospects of CZTSSe/CdS interface engineering to combat high open-circuit voltage deficit of kesterite photovoltaics: A critical review. J. Mater. Chem. A 2020, 8, 21547–21584. [Google Scholar] [CrossRef]
- Awadallah, O.; Cheng, Z. In Situ Raman characterization of Cu2ZnSnS4 solar absorber material. In Proceedings of the 2015 IEEE 42nd Photovoltaic Specialist Conference (PVSC), New Orleans, LA, USA, 14–19 June 2015; pp. 1–6. [Google Scholar] [CrossRef]
- Lee, M.-H.; Lin, Y.-P.; Hsieh, T.-E. Preparation of CU2ZnSn(SxSe1−x)4(CZTSSe) Single-Phase Sputtering Target By Sintering Binary Alloy Powders and Its Applications to Cztsse Thin-Film Solar Cell. In Electrochemical Society Meeting Abstracts 231; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2017; p. 2070. [Google Scholar] [CrossRef]
- Fontané, X.; Izquierdo-Roca, V.; Fairbrother, A.; Espíndola-Rodríguez, M.; López-Marino, S.; Placidi, M.; Jawhari, T.; Saucedo, E.; Pérez-Rodríguez, A. Selective detection of secondary phases in Cu2ZnSn(S, Se)4 based absorbers by pre-resonant Raman spectroscopy. In Proceedings of the 2013 IEEE 39th Photovoltaic Specialists Conference (PVSC), Tampa, FL, USA, 16–21 June 2013; pp. 2581–2584. [Google Scholar] [CrossRef]
- López-Vergara, F.; Galdámez, A.; Barahona, P.; Manriquez, V. Effect of the selenium content in the optical properties of the kesterite Cu2ZnSnS4−xSex phases. J. Chil. Chem. Soc. 2016, 61, 3291–3294. [Google Scholar] [CrossRef]
- Schorr, S. The crystal structure of kesterite type compounds: A neutron and X-ray diffraction study. Sol. Energy Mater. Sol. Cells 2011, 95, 1482–1488. [Google Scholar] [CrossRef]
- Haass, S.G.; Andres, C.; Figi, R.; Schreiner, C.; Bürki, M.; Romanyuk, Y.E.; Tiwari, A.N. Complex interplay between absorber composition and alkali doping in high-efficiency kesterite solar cells. Adv. Energy Mater. 2018, 8, 1701760. [Google Scholar] [CrossRef]
- Guc, M.; Levcenko, S.; Bodnar, I.V.; Izquierdo-Roca, V.; Fontane, X.; Volkova, L.V.; Arushanov, E.; Pérez-Rodríguez, A. Polarized Raman scattering study of kesterite type Cu2ZnSnS4 single crystals. Sci. Rep. 2016, 6, 19414. [Google Scholar] [CrossRef]
- Fernandes, P.; Salomé, P.; Da Cunha, A. Study of polycrystalline Cu2ZnSnS4 films by Raman scattering. J. Alloys Compd. 2011, 509, 7600–7606. [Google Scholar] [CrossRef]
- Aytug Ava, C.; Ocak, Y.S.; Celik, O.; Asubay, S. Deposition and Characterization of Si Substituted Cu2ZnSnS4 Thin Films. Silicon 2023, 15, 451–458. [Google Scholar] [CrossRef]
- Siebentritt, S.; Schorr, S. Kesterites-a challenging material for solar cells. Prog. Photovolt. Res. Appl. 2012, 20, 512–519. [Google Scholar] [CrossRef]
- Drabavičius, A.; Naujokaitis, A.; Stalnionis, G.; Giraitis, R.; Mockus, Z.; Kanapeckaitė, S.; Kalinauskas, P.; Nedzinskas, R.; Niaura, G.; Juškėnas, R. Photoelectrochemical, Raman spectroscopy, XRD and photoluminescence study of disorder in electrochemically deposited kesterite thin film. J. Alloys Compd. 2020, 824, 153853. [Google Scholar] [CrossRef]
- Lee, T.; Hamim Sharif, M.; Enkhbayar, E.; Enkhbat, T.; Salahuddin Mina, M.; Kim, J. Defect passivation for kesterite CZTSSe solar cells via in situ Al2O3 incorporation into the bulk CZTSSe absorber. Sol. RRL 2022, 6, 2100862. [Google Scholar] [CrossRef]
- Choudhury, K.R.; Cao, Y.; Caspar, J.V.; Farneth, W.E.; Guo, Q.; Ionkin, A.S.; Johnson, L.K.; Lu, M.; Malajovich, I.; Radu, D. Characterization and understanding of performance losses in a highly efficient solution-processed CZTSSe thin-film solar cell. In Proceedings of the 2012 38th IEEE Photovoltaic Specialists Conference, Austin, TX, USA, 3–8 June 2012; pp. 001471–001474. [Google Scholar] [CrossRef]
- Gunawan, O.; Gokmen, T.; Warren, C.W.; Cohen, J.D.; Todorov, T.K.; Barkhouse, D.A.R.; Bag, S.; Tang, J.; Shin, B.; Mitzi, D.B. Electronic properties of the Cu2ZnSn(Se, S)4 absorber layer in solar cells as revealed by admittance spectroscopy and related methods. Appl. Phys. Lett. 2012, 100, 253905. [Google Scholar] [CrossRef]
- Naylor, M.C.; Tiwari, D.; Sheppard, A.; Laverock, J.; Campbell, S.; Ford, B.; Xu, X.; Jones, M.D.; Qu, Y.; Maiello, P. Ex situ Ge-doping of CZTS nanocrystals and CZTSSe solar absorber films. Faraday Discuss. 2022, 239, 70–84. [Google Scholar] [CrossRef]
- Grini, S.; Ross, N.; Sky, T.N.; Persson, C.; Platzer-Björkman, C.; Vines, L. Secondary ion mass spectrometry as a tool to study selenium gradient in Cu2ZnSn(S, Se)4. Phys. Status Solidi C 2017, 14, 1600187. [Google Scholar] [CrossRef]
- Li, J.; Huang, J.; Ma, F.; Sun, H.; Cong, J.; Privat, K.; Webster, R.F.; Cheong, S.; Yao, Y.; Chin, R.L. Unveiling microscopic carrier loss mechanisms in 12% efficient Cu2ZnSnSe4 solar cells. Nat. Energy 2022, 7, 754–764. [Google Scholar] [CrossRef]
- Saadaldin, A.; Slyamov, A.M.; Stuckelberger, M.E.; Jørgensen, P.S.; Rein, C.; Lucas, M.M.; Ramos, T.; Rodriguez-Fernandez, A.; Bernard, D.; Andreasen, J.W. Multi-modal characterization of kesterite thin-film solar cells: Experimental results and numerical interpretation. Faraday Discuss. 2022, 239, 160–179. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, W.; Feng, Y.; Li, Z.; Ma, X.; Liu, X.; Wu, X.; Zhang, Y.; Yang, C.; Lu, X. Band bending near grain boundaries of Cu2ZnSn(S, Se)4 thin films and its effect on photovoltaic performance. Nano Energy 2018, 51, 37–44. [Google Scholar] [CrossRef]
- Nisika, N.; Ghosh, A.; Kaur, K.; Bobba, R.S.; Qiao, Q.; Kumar, M. Engineering Cu2ZnSnS4 grain boundaries for enhanced photovoltage generation at the Cu2ZnSnS4/TiO2 heterojunction: A nanoscale investigation using Kelvin probe force microscopy. J. Appl. Phys. 2021, 130, 195301. [Google Scholar] [CrossRef]
- Thersleff, T.; Giraldo, S.; Neuschitzer, M.; Saucedo, E.; Leifer, K. Spatial correlation of structure/composition trends in Cu2ZnSnSe4-based solar cells with sub-nanometer resolution. In Proceedings of the 2015 Materials Research Society Fall Meeting, Boston, MA, USA, 29 November–4 December 2015. [Google Scholar]
- Hadermann, J.; Batuk, M. Solar cell structure at micro-and nanoscale through TEM. Acta Cryst 2017, 70, C1424. [Google Scholar] [CrossRef]
- Ali, S.S.; Mohamed, W.; Mohamed, H. Effect of series and shunt resistance on the performance of CZTSe thin film solar cell. Sohag J. Sci. 2025, 10, 75–79. [Google Scholar] [CrossRef]
- Nakane, A.; Tampo, H.; Tamakoshi, M.; Fujimoto, S.; Kim, K.M.; Kim, S.; Shibata, H.; Niki, S.; Fujiwara, H. Quantitative determination of optical and recombination losses in thin-film photovoltaic devices based on external quantum efficiency analysis. J. Appl. Phys. 2016, 120, 064505. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, X.; Wu, H.; Wang, J.; Lou, L.; Yin, K.; Gong, Y.; Shi, J.; Luo, Y.; Li, D. Control of the phase evolution of kesterite by tuning of the selenium partial pressure for solar cells with 13.8% certified efficiency. Nat. Energy 2023, 8, 526–535. [Google Scholar] [CrossRef]
- Pakštas, V.; Grincienė, G.; Selskis, A.; Balakauskas, S.; Talaikis, M.; Bruc, L.; Curmei, N.; Niaura, G.; Franckevičius, M. Improvement of CZTSSe film quality and superstrate solar cell performance through optimized post-deposition annealing. Sci. Rep. 2022, 12, 16170. [Google Scholar] [CrossRef]
- Hsu, W.-C.; Zhou, H.; Luo, S.; Song, T.-B.; Hsieh, Y.-T.; Duan, H.-S.; Ye, S.; Yang, W.; Hsu, C.-J.; Jiang, C. Spatial element distribution control in a fully solution-processed nanocrystals-based 8.6% Cu2ZnSn(S, Se)4 device. ACS Nano 2014, 8, 9164–9172. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, T.; Cojocaru-Mirédin, O.; Choi, P.; Mousel, M.; Redinger, A.; Siebentritt, S.; Raabe, D. Atom probe study of Cu2ZnSnSe4 thin-films prepared by co-evaporation and post-deposition annealing. Appl. Phys. Lett. 2013, 102, 042101. [Google Scholar] [CrossRef]
- Le Phuong, Q.; Okano, M.; Yamashita, G.; Nagai, M.; Ashida, M.; Nagaoka, A.; Yoshino, K.; Kanemitsu, Y. Photocarrier dynamics in undoped and Na-doped Cu2ZnSnS4 single crystals revealed by ultrafast time-resolved terahertz spectroscopy. Appl. Phys. Express 2015, 8, 062303. [Google Scholar] [CrossRef]
- Ghadiri, E.; Shin, D.; Shafiee, A.; Warren, W.S.; Mitzi, D.B. Grain-resolved ultrafast photophysics in Cu2BaSnS4−x se x semiconductors using pump-probe diffuse reflectance spectroscopy and microscopy. ACS Appl. Mater. Interfaces 2018, 10, 39615–39623. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Bernardi, S.; Zhang, Y. Microscopic structure differences in CZTSe quaternary alloys prepared by different techniques revealed by spatially-resolved laser-induced-modification Raman spectroscopy. arXiv 2017, arXiv:1706.08000 2017. [Google Scholar]
- Wei, Z.; Newman, M.J.; Tsoi, W.C.; Watson, T.M. Raman mapping analysis for removal of surface secondary phases of CZTS films using chemical etching. Appl. Phys. Lett. 2016, 109, 123902. [Google Scholar] [CrossRef]
- Salomé, P.M.; Fernandes, P.A.; Leitao, J.P.; Sousa, M.G.; Teixeira, J.P.; da Cunha, A.F. Secondary crystalline phases identification in Cu2ZnSnSe4 thin films: Contributions from Raman scattering and photoluminescence. J. Mater. Sci. 2014, 49, 7425–7436. [Google Scholar] [CrossRef]
- Li, B.; Tan, X.; Zhao, J.; Han, X. Experimental and modeling analysis of photocarrier dynamics in CZTSSe using temperature-dependent photoluminescence. Mater. Sci. Eng. B 2022, 284, 115897. [Google Scholar] [CrossRef]
- Halim, M.A.; Islam, M.M.; Luo, X.; Sakurai, T.; Sakai, N.; Kato, T.; Sugimoto, H.; Tampo, H.; Shibata, H.; Niki, S. Photocarrier recombination dynamics in Cu2ZnSn(S, Se)4 and Cu(In, Ga)Se2 studied by temperature-dependent time resolved Photoluminescence (TR-PL). In Proceedings of the 2015 IEEE 42nd Photovoltaic Specialist Conference (PVSC), New Orleans, LA, USA, 14–19 June 2015; pp. 1–4. [Google Scholar] [CrossRef]
- Ananthoju, B.; Mohapatra, J.; Jangid, M.K.; Bahadur, D.; Medhekar, N.; Aslam, M. Cation/anion substitution in Cu2ZnSnS4 for improved photovoltaic performance. Sci. Rep. 2016, 6, 35369. [Google Scholar] [CrossRef]
- Ocak, Y.S. Influence of substrate temperature on CuxSnSx+1 thin films prepared by Co-sputtering and H2S annealing. Mater. Res. Express 2020, 6, 126443. [Google Scholar] [CrossRef]
- Yu, S.M.; Lim, K.-S.; Khalkar, A.; Yoo, J.-B. Selenization of Cu2ZnSnS4 thin film using a Se metal-organic source for solar cell applications. Appl. Phys. A 2016, 122, 767. [Google Scholar] [CrossRef]
- He, J.; Lee, L.T.L.; Yang, S.; Li, Q.; Xiao, X.; Chen, T. Printable highly catalytic Pt-and TCO-Free counter electrode for dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2014, 6, 2224–2229. [Google Scholar] [CrossRef] [PubMed]
- Reyes, O.; Sanchez, M.F.; Pal, M.; Llorca, J.; Sebastian, P. Evolution pathway of CZTSe nanoparticles synthesized by microwave-assisted chemical synthesis. Adv. Nano Res. 2017, 5, 203. [Google Scholar]
- Kim, Y.S.; Yun, S.J. Studies on polycrystalline ZnS thin films grown by atomic layer deposition for electroluminescent applications. Appl. Surf. Sci. 2004, 229, 105–111. [Google Scholar] [CrossRef]
- Nanda, J.; Sapra, S.; Sarma, D.; Chandrasekharan, N.; Hodes, G. Size-selected zinc sulfide nanocrystallites: Synthesis, structure, and optical studies. Chem. Mater. 2000, 12, 1018–1024. [Google Scholar] [CrossRef]
- Jo, E.; Kim, S.G.; Gour, K.S.; Jang, S.; Jang, J.S.; Kim, J.H. Improved Jsc by Increasing the Absorber Layer Thickness of Monoclinic-Dominated Cu2SnS3 Thin Film Solar Cells Fabricated on Flexible Mo Foil. Sol. RRL 2022, 6, 2100743. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, I.-H. Characterization of a co-evaporated Cu2SnS3 thin-film solar cell. Thin Solid Film. 2019, 669, 351–354. [Google Scholar] [CrossRef]
- Anandh, B.; Ganesh, A.S.; Nandakumar, P.; Saranya, D. Exploring the structural and optical properties of MoSe2 for solar cell performance. J. Phys. Conf. Ser. 2024, 2886, 012006. [Google Scholar] [CrossRef]
- Indirajith, R.; Rajalakshmi, M.; Ramamurthi, K.; Ahamed, M.B.; Gopalakrishnan, R. Synthesis of ZnSe nano particles, deposition of ZnSe thin films by electron beam evaporation and their characterization. Ferroelectrics 2014, 467, 13–21. [Google Scholar] [CrossRef]
- Biacchi, A.J.; Vaughn, D.D.; Schaak, R.E. Synthesis and crystallographic analysis of shape-controlled SnS nanocrystal photocatalysts: Evidence for a pseudotetragonal structural modification. J. Am. Chem. Soc. 2013, 135, 11634–11644. [Google Scholar] [CrossRef] [PubMed]
- Mali, J.M.; Arbuj, S.S.; Ambekar, J.D.; Rane, S.B.; Mulik, U.P.; Amalnerkar, D.P. Hydrothermal synthesis of SnS2 faceted nano sheets and their visible light driven photocatalytic performance. Sci. Adv. Mater. 2013, 5, 1994–1998. [Google Scholar] [CrossRef]
- Olgar, M.A.; Atasoy, Y.; Başol, B.M.; Tomakin, M.; Aygun, G.; Ozyuzer, L.; Bacaksız, E. Influence of copper composition and reaction temperature on the properties of CZTSe thin films. J. Alloys Compd. 2016, 682, 610–617. [Google Scholar] [CrossRef]
- Márquez-Prieto, J.; Forbes, I. Evolution of phases in two-stage vacuum processed thin film Cu2ZnSnSe4 absorber layers. Mater. Res. Innov. 2014, 18, 515–518. [Google Scholar] [CrossRef]
- Fernandes, P.; Sousa, M.; Salome, P.M.; Leitão, J.; Da Cunha, A. Thermodynamic pathway for the formation of SnSe and SnSe2 polycrystalline thin films by selenization of metal precursors. CrystEngComm 2013, 15, 10278–10286. [Google Scholar] [CrossRef]
- Neuwirth, M.; Zhou, H.; Schnabel, T.; Ahlswede, E.; Kalt, H.; Hetterich, M. A multiple-selenization process for enhanced reproducibility of Cu2ZnSn(S, Se)4 solar cells. Appl. Phys. Lett. 2016, 109, 233903. [Google Scholar] [CrossRef]
- Himmrich, M.; Haeuseler, H. Far infrared studies on stannite and wurtzstannite type compounds. Spectrochim. Acta Part A Mol. Spectrosc. 1991, 47, 933–942. [Google Scholar] [CrossRef]
- Fontané, X.; Izquierdo-Roca, V.; Saucedo, E.; Schorr, S.; Yukhymchuk, V.; Valakh, M.Y.; Pérez-Rodríguez, A.; Morante, J. Vibrational properties of stannite and kesterite type compounds: Raman scattering analysis of Cu2(Fe, Zn)SnS4. J. Alloys Compd. 2012, 539, 190–194. [Google Scholar] [CrossRef]
- Dimitrievska, M.; Oliva, F.; Guc, M.; Giraldo, S.; Saucedo, E.; Pérez-Rodríguez, A.; Izquierdo-Roca, V. Defect characterisation in Cu2ZnSnSe4 kesterites via resonance Raman spectroscopy and the impact on optoelectronic solar cell properties. J. Mater. Chem. A 2019, 7, 13293–13304. [Google Scholar] [CrossRef]
- Hobson, T.D.; Hutter, O.S.; Fleck, N.; Daniels, L.M.; Major, J.D.; Ng, T.M.; Durose, K. Vegard Relation and Raman Band Reference Data Generated from Bulk Crystals of Kesterite-Phase Composition Series Cu2ZnSnS4xSe4−4x (CZTSSe, 0 ≤ x ≤ 1). Cryst. Growth Des. 2020, 20, 2164–2173. [Google Scholar] [CrossRef]
- Liu, T.; Yao, Y.-C.; Liu, F.-R.; Cheng, L.; Bao, Y.-F.; Kong, W.-J.; Qiao, M.; Wang, T.-J. Enhanced Raman intensity in ZnS planar and channel waveguide structures via carbon ion implantation. Opt. Mater. 2021, 112, 110733. [Google Scholar] [CrossRef]
- Kim, I.Y.; Lee, J.Y.; Ghorpade, U.V.; Suryawanshi, M.; Lee, D.S.; Kim, J.H. Influence of annealing temperature on the properties and solar cell performance of Cu2SnS3 (CTS) thin film prepared using sputtering method. J. Alloys Compd. 2016, 688, 12–17. [Google Scholar] [CrossRef]
- Choi, H.; Lee, J.; Shin, S.; Lee, J.; Lee, S.; Park, H.; Kwon, S.; Lee, N.; Bang, M.; Lee, S.-B. Fabrication of high crystalline SnS and SnS2 thin films, and their switching device characteristics. Nanotechnology 2018, 29, 215201. [Google Scholar] [CrossRef]
- Zhen, Z.-Q.; Wang, H.-Y. Density functional study of the electronic, elastic, and lattice dynamic properties of SnS2. Acta Phys. Pol. A 2020, 137, 1095–1100. [Google Scholar] [CrossRef]
- Ye, T.; Li, H.; Xiao, T.; Sun, Z. Bionic Design of Iron-Doped MoSe2 Catalyst for Efficient Nitrogen Fixation. ChemSusChem 2024, 17, e202400448. [Google Scholar] [CrossRef]
- Mosquera, E.; Carvajal, N.; Morel, M.; Marín, C. Fabrication of ZnSe nanoparticles: Structural, optical and Raman studies. J. Lumin. 2017, 192, 814–817. [Google Scholar] [CrossRef]
- Marcano, G.; Rincón, C.; López, S.; Pérez, G.S.; Herrera-Pérez, J.; Mendoza-Alvarez, J.; Rodríguez, P. Raman spectrum of monoclinic semiconductor Cu2SnSe3. Solid State Commun. 2011, 151, 84–86. [Google Scholar] [CrossRef]
- Tanaka, T.; Sueishi, T.; Saito, K.; Guo, Q.; Nishio, M.; Yu, K.M.; Walukiewicz, W. Existence and removal of Cu2Se second phase in coevaporated Cu2ZnSnSe4 thin films. J. Appl. Phys. 2012, 111, 053522. [Google Scholar] [CrossRef]
- Nelson, A.J.; Sinha, K.; Moreland, J. Growth and Characterization of the Binary Defect Alloy Cu2−xSe and the Relation to II-VI/I-III-VI Heterojunction Formation. MRS Online Proc. Libr. 1995, 378, 249–254. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ocak, Y.S.; Bayansal, F. Advancing Earth-Abundant CZTSSe Solar Cells: Recent Progress in Efficiency and Defect Engineering. Nanomaterials 2025, 15, 1617. https://doi.org/10.3390/nano15211617
Ocak YS, Bayansal F. Advancing Earth-Abundant CZTSSe Solar Cells: Recent Progress in Efficiency and Defect Engineering. Nanomaterials. 2025; 15(21):1617. https://doi.org/10.3390/nano15211617
Chicago/Turabian StyleOcak, Yusuf Selim, and Fatih Bayansal. 2025. "Advancing Earth-Abundant CZTSSe Solar Cells: Recent Progress in Efficiency and Defect Engineering" Nanomaterials 15, no. 21: 1617. https://doi.org/10.3390/nano15211617
APA StyleOcak, Y. S., & Bayansal, F. (2025). Advancing Earth-Abundant CZTSSe Solar Cells: Recent Progress in Efficiency and Defect Engineering. Nanomaterials, 15(21), 1617. https://doi.org/10.3390/nano15211617







