Recent Advances in Doping and Polymer Hybridization Strategies for Enhancing ZnO-Based Gas Sensors
Abstract
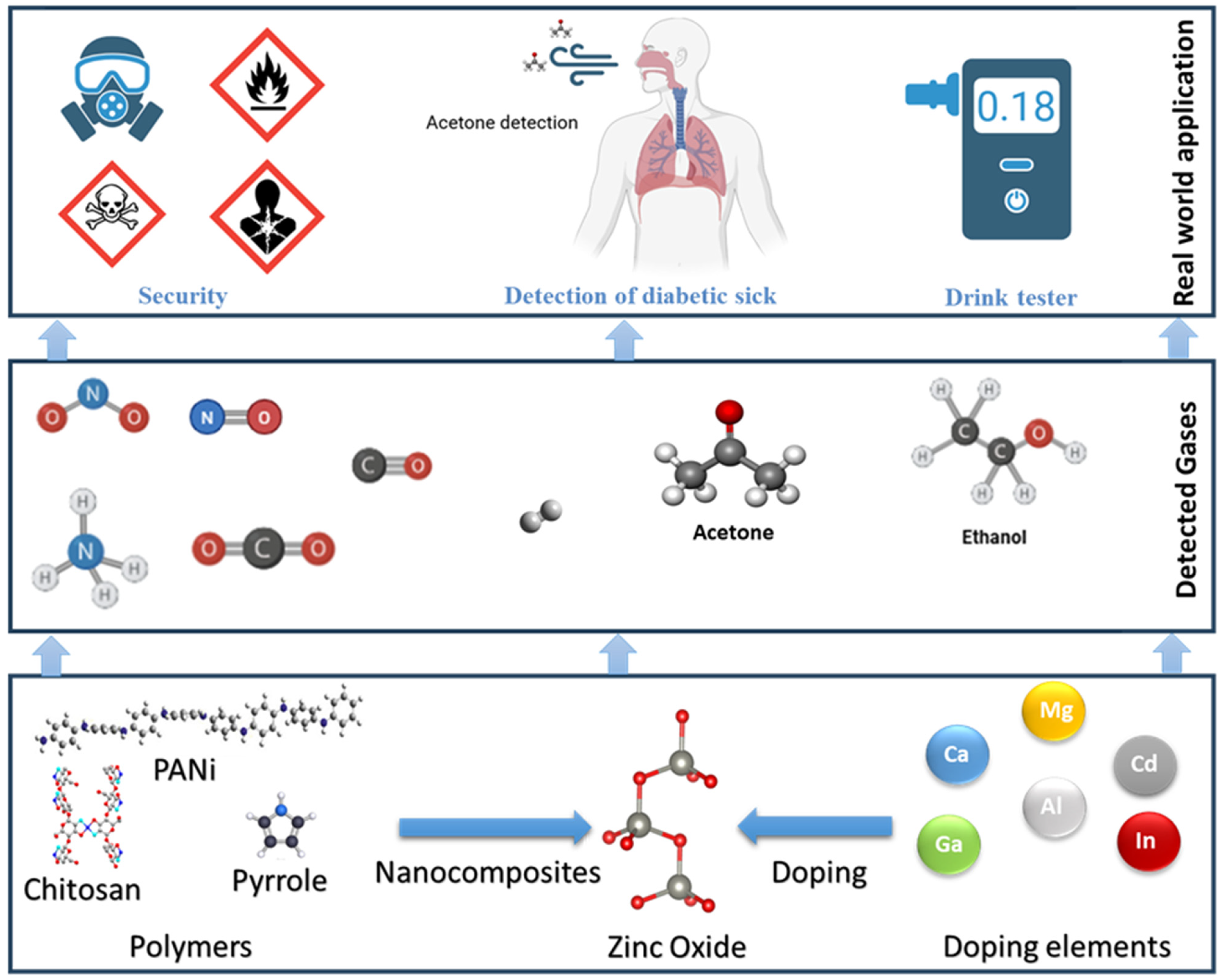
1. Introduction
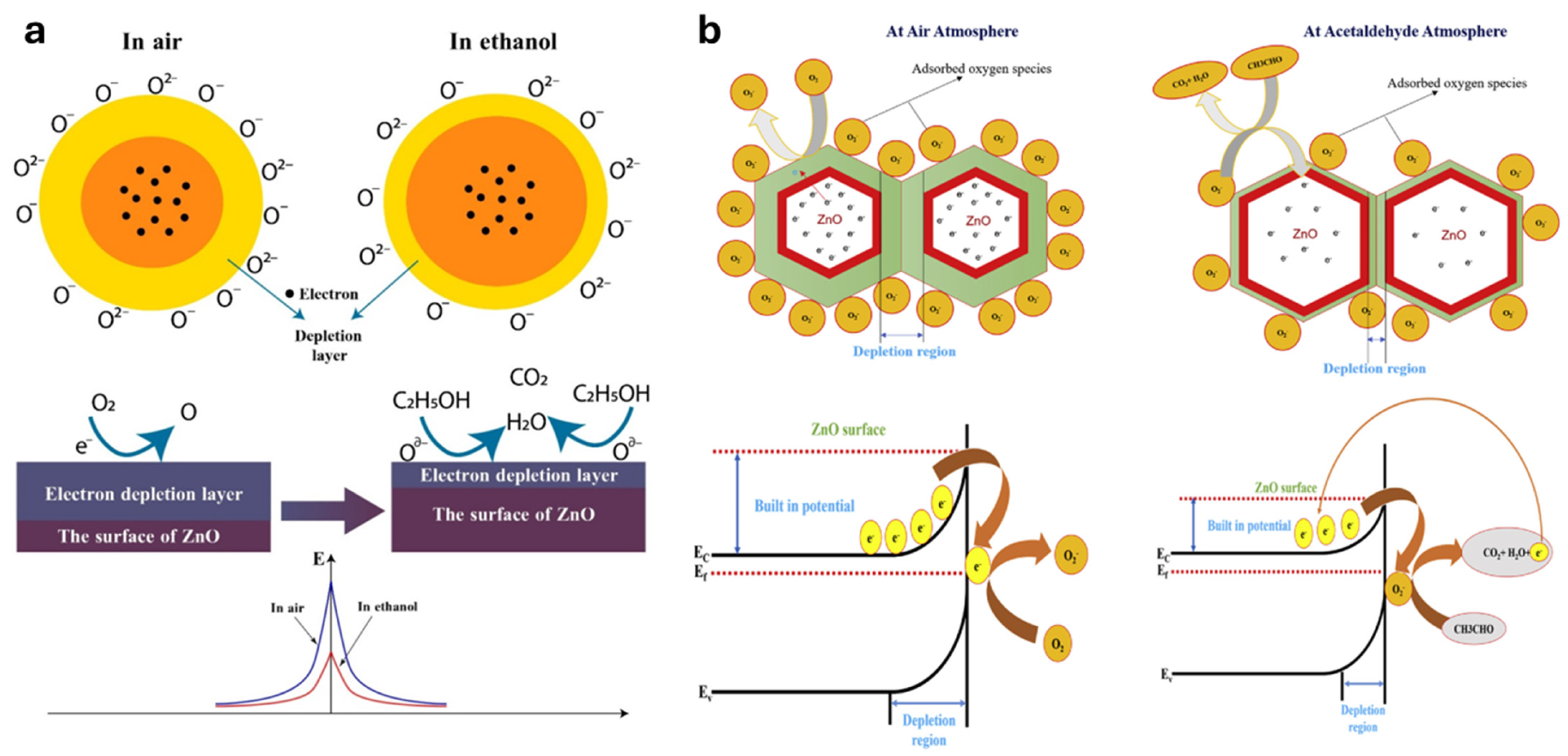
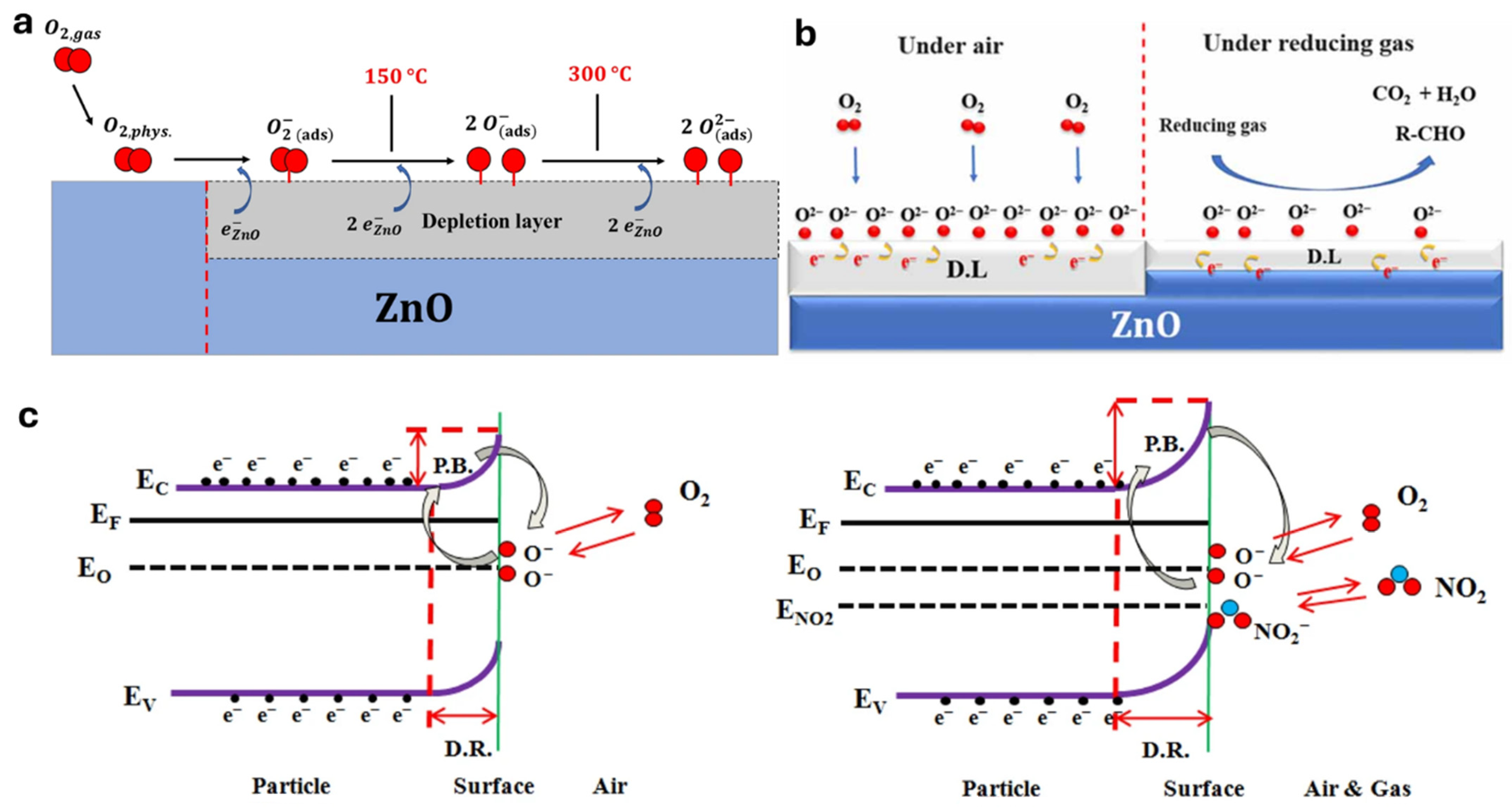
2. ZnO Gas Sensing Mechanism
2.1. Surface Reactions with Gases
2.2. Role of Defects and Polymer Synergy in ZnO Gas Sensing Mechanisms
3. Fabrication and Characterization of ZnO-Based Gas Sensors
3.1. Sol–Gel Synthesis of ZnO Nanostructures for Gas Sensing
3.2. Gas Sensor Instrumentation and Measurement Methodology
4. Dopant Strategies
4.1. Doping Effect on the Sensitivity
4.2. Doping Effects on the Response and Recovery Dynamics of ZnO-Based Gas Sensors
4.3. Effect of Doping Elements on the Reproducibility of ZnO-Based Gas Sensors
4.4. Effect of Doping Elements on the Selectivity Behavior of ZnO Gas Sensors
5. Integration of Polymer Matrices
5.1. Gas Sensing Behavior of PANi–ZnO Polymer Nanocomposites
5.2. Gas Sensing Performance of Polypyrrole/ZnO Nanocomposites
6. Challenges and Future Perspectives
| Sample Name | Gas Name | Gas Conc. | Response | Response/Recovery Times | Sensing Temp. | Ref. |
|---|---|---|---|---|---|---|
| Doping by transition metal | ||||||
| Fe-doped ZnO thick film | NH3 | 100 ppm | 85% (resistance) | ~50 s/~60 s | 150 °C | [215] |
| Al-doped ZnO nanoparticles | CO | 50 ppm | 2.5 (S value) | 30 s/45 s | 200 °C | [216] |
| Ga-doped ZnO nanoparticles | CO2 | 100 ppm | 1.8 (S value) | 40 s/60 s | 250 °C | [217] |
| CuO-ZnO composite | Acetaldehyde | 50 ppm | 18.2 | 23 s/36 s | 200 °C | [218] |
| Cu-doped ZnO thin film | Propane (C3H8) | 1000 ppm | ~6 × 104 | Not specified | 300 °C | [219] |
| Co-ZnO nanoflower (10% Co) | Isopropanol | 5 ppm | 12.2 | 330 s/475 s | 225 °C | [220] |
| Mn-doped ZnO thin film | Ammonia (NH3) | 200 ppm | 23% | 44 s/65 s | 250 °C | [221] |
| Doping by Rare earth | ||||||
| Gd-doped ZnO film | NH3 | 100 ppm | S = 18.2 | 39 s/11 s | Room Temp | [222] |
| La-doped ZnO film | H2S | 100 ppm | S = 14.5 | 42 s/13 s | 300 °C | [205] |
| La-doped ZnO film | CO2 | 500 ppm | S = 3.2 | 29 s/17 s | Room Temp | [205] |
| ZnO-150 (Ce-doped) | NO2 | Not specified | 132.44% | 231.7 s/732.5 s | Room temperature | [223] |
| Dy-doped ZnO film | NO2 | 1 ppm | S = 10.3 | 35 s/15 s | Room Temp | [224] |
| Nanocomposites with conductive polymer | ||||||
| ZnO-PANI nanocomposite | NH3 | 100–500 ppm | Sensitivity increases with ZnO wt% (max at 6 wt%) | 10–30 s/up to 1200 s (20 min) | Room Temp | [225] |
| PANI/nano-ZnO FET sensor | H2 | 100 ppm | Enhanced vs pure PANI | Not specified | Room Temp | [226] |
| PANi/ZnO NR composite | NH3 | 0.05/2.5 ppm | 130% (0.05 ppm)/20,920% (2.5 ppm) | Not specified | Room Temp | [213] |
| ZnO/PANI composite | Ethanol | 100 ppm | S = 20 | ~20 s | Room Temp | [227] |
| Chitosan-PEG/ZnO composite | Acetone | 0.5–5 ppm | LOD ≤ 0.96 ppb; linear and selective | ~5 min exposure/recovery | ~29 °C | [228] |
| ZnO/PANI nanocomposite | NH3 | 100 ppm | 4300% | Not specified | Room Temp | [229] |
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, X.; Dai, Y.; Zhang, C.; Zhang, Y.; Zong, W.; Zhang, W.; Chen, R.; Zhu, J.; Hu, X.; Wang, M.; et al. When it’s heavier: Interfacial and solvation chemistry of isotopes in aqueous electrolytes for Zn-ion batteries. Angew. Chem. 2023, 135, e202300608. [Google Scholar] [CrossRef]
- Fang, Q.; Sun, Q.; Ge, J.; Wang, H.; Qi, J. Multidimensional Engineering of Nanoconfined Catalysis: Frontiers in Carbon-Based Energy Conversion and Utilization. Catalysts 2025, 15, 477. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, X.; He, N.; Song, F.; Wang, X. Investigation on novel deep eutectic solvents with high carbon dioxide adsorption performance. J. Environ. Chem. Eng. 2025, 13, 117870. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, L.; Xiao, C.; Huang, Z.; Zhao, F.; Yin, J. Highly efficient upconversion photodynamic performance of rare-earth-coupled dual-photosensitizers: Ultrafast experiments and excited-state calculations. Nanophotonics 2024, 13, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, Y.; Zhang, G.; Liu, Y.; Han, Z.; Zhang, H.; Xu, Q.; Zhao, J.; Jin, M.; Song, D.; et al. Directly printing high-resolution, high-performance 3D curved electronics based on locally polarized electric-field-driven vertical jetting. Addit. Manuf. 2024, 96, 104579. [Google Scholar] [CrossRef]
- Zhang, H.; Chang, Y.; Xu, Y.; Liu, C.; Xiao, X.; Li, J.; Ma, X.; Wang, Y.; Guo, H. Design and fabrication of a chalcogenide hollow-core anti-resonant fiber for mid-infrared applications. Opt. Express 2023, 31, 7659–7670. [Google Scholar] [CrossRef]
- Zhang, H.H.; Chao, J.B.; Wang, Y.W.; Liu, Y.; Yao, H.M.; Zhao, Z.P.; Niu, K. 5G base station antenna array with heatsink radome. IEEE Trans. Antennas Propag. 2024, 72, 2270–2278. [Google Scholar] [CrossRef]
- Luo, Y.X.; Dong, Y.L. Strain measurement at up to 3000 °C based on ultraviolet-digital image correlation. NDT E Int. 2024, 146, 103155. [Google Scholar] [CrossRef]
- Ma, C.; Huang, S.; Li, M.; He, J.; Totis, G.; Hua, C.; Cui, G.; Wang, L.; Xue, R.; Tan, Z.; et al. Highly efficient heat dissipation method of grooved heat pipe for thermal behavior regulation for spindle system working in low rotational speed. Int. Commun. Heat Mass Transf. 2025, 169, 109575. [Google Scholar] [CrossRef]
- Ma, C.; Li, M.; Liu, J.; Li, M.; He, J.; Totis, G.; Hua, C.; Wang, L.; Cui, G.; Xue, R.; et al. High-efficiency topology optimization method for thermal-fluid problems in cooling jacket of high-speed motorized spindle. Int. Commun. Heat Mass Transf. 2025, 169, 109533. [Google Scholar] [CrossRef]
- Zhou, H.; Guo, J.; Zhu, G.; Xie, F.; Tang, X.; Luo, X. Highly efficient preparation of crystalline yttrium carbonate in sodium carbonate system: Formation and growth mechanism. J. Rare Earths 2025, 43, 1492–1501. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, L.; Wang, J.; Pan, H.; Dou, M.; Teng, Y.; Fu, X.; Liu, Z.; Huang, X.; Wang, M. Bagasse-based porous flower-like MoS2/carbon composites for efficient microwave absorption. Carbon Lett. 2025, 35, 145–160. [Google Scholar] [CrossRef]
- Ni, Z.L.; Ma, J.S.; Liu, Y.; Li, B.H.; Nazarov, A.A.; Li, H.; Yuan, Z.P.; Ling, Z.C.; Wang, X.X. Numerical analysis of ultrasonic spot welding of Cu/Cu joints. J. Mater. Eng. Perform. 2025, 34, 20624–20635. [Google Scholar] [CrossRef]
- Liu, K.; Feng, M.; Zhao, W.; Sun, J.; Dong, W.; Wang, Y.; Mian, A. Pixel-Level Noise Mining for Weakly Supervised Salient Object Detection. IEEE Trans. Neural Netw. Learn. Syst. 2025, 36, 18815–18829. [Google Scholar] [CrossRef]
- Sha, X.; Zhu, Y.; Sha, X.; Guan, Z.; Wang, S. ZHPO-LightXBoost an integrated prediction model based on small samples for pesticide residues in crops. Environ. Model. Softw. 2025, 188, 106440. [Google Scholar] [CrossRef]
- Sha, X.; Guan, Z.; Wang, Y.; Han, J.; Wang, Y.; Chen, Z. SSC-Net: A multi-task joint learning network for tongue image segmentation and multi-label classification. Digit. Health 2025, 11, 20552076251343696. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Qi, L.; Du, H.; Yang, J.; Chen, Z. AlignFusionNet: Efficient Cross-modal Alignment and Fusion for 3D Semantic Occupancy Prediction. IEEE Access 2025, 13, 125003–125015. [Google Scholar] [CrossRef]
- Feng, K.; Hong, H.; Tang, K.; Wang, J. Statistical tests for replacing human decision makers with algorithms. Manag. Sci. 2025. [Google Scholar] [CrossRef]
- Lei, M.; Hou, B.; Zhang, S.; Chang, R.; Ji, Y.; Chen, K.; Sun, J.; Liu, C.; Payne, G.F.; Qu, X. de novo Fabrication of Dense Collagen Matrices with Patterned Hierarchical Structures for Corneal Stromal Tissue Repair. Adv. Mater. 2025, 37, 2502279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhou, J.; Jiang, X.; Shi, T.; Jin, X.; Ren, Y.; Ji, K.; Xin, Z.; Zhang, Z.; Yin, C.; et al. MoS2 Nanozyme—Chlorella Hydrogels: Pioneering a Hepatocellular Carcinoma Integrative Therapy. Adv. Funct. Mater. 2025, 35, 2417125. [Google Scholar] [CrossRef]
- Gao, P.; Cao, M.; Wang, X.; Zhang, H.; Chen, X.; Song, Z.; Zhang, M.; Li, L.; Yin, C.; Yuan, J.; et al. G Protein—Coupled Receptor Kinase 3 Exacerbates Diabetic Heart Injuries Through Direct Phosphorylation of Cannabinoid Receptor 2 in Humans and Mice. Circulation 2025, 152, 882–898. [Google Scholar] [CrossRef]
- Nan, Y.; Zhu, M.; Wang, Q.; Du, X.; Xu, C.; Huang, Y.; Liu, Y.; Zhou, S.; Qiu, Y.; Chu, X.; et al. Nanobody-engineered bispecific IL-18 mimetics drive antitumor immunity by engaging CD8+ T cell and evading IL-18BP in preclinical models. Mol. Ther. 2025, 33, 4988–5002. [Google Scholar] [CrossRef]
- Yin, X.; Lai, Y.; Zhang, X.; Zhang, T.; Tian, J.; Du, Y.; Li, Z.; Gao, J. Targeted sonodynamic therapy platform for holistic integrative Helicobacter pylori therapy. Adv. Sci. 2025, 12, 2408583. [Google Scholar] [CrossRef]
- Zhang, T.; Zheng, Y.; Chen, T.; Gu, Y.; Gong, Y.; Wang, D.; Li, Z.; Du, Y.; Zhang, L.; Gao, J. Biomaterials mediated 3R (remove-remodel-repair) strategy: Holistic management of Helicobacter pylori infection. J. Nanobiotechnol. 2025, 23, 475. [Google Scholar] [CrossRef]
- Chen, Z.; Li, B.; Wang, B. Robust stability design for inverters using phase lag in proportional-resonant controllers. IEEE Trans. Ind. Electron. 2024, 72, 2655–2668. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, H.; Xia, Y.; Chen, Q.; Ye, Y. Equivalence relation analysis and design of repetitive controllers and multiple quasi-resonant controllers for single-phase inverters. IEEE J. Emerg. Sel. Top. Power Electron. 2025, 13, 3338–3349. [Google Scholar] [CrossRef]
- Liu, W.; Gao, Z.; Wei, Z.; Zhang, L.; Guo, G.; Mumtaz, S. Compensator-Based Fixed-Time Prescribed Performance Control of Vehicular Platoon With Input Nonlinearities: A Performance Boundary Self-Adjusting Approach. IEEE Trans. Intell. Transp. Syst. 2025. early access. [Google Scholar] [CrossRef]
- Gao, Z.; Wei, Z.; Liu, W.; Zhang, L.; Wen, S.; Guo, G. Global Prescribed Performance Control for 2024, 2-D Plane Vehicular Platoons With Small Overshoot: A Fixed-Time Composite Sliding Mode Control Approach. IEEE Trans. Intell. Transp. Syst. 2025. early access. [Google Scholar]
- Liu, Y.; Jiang, L.; Qi, Q.; Xie, K.; Xie, S. Online computation offloading for collaborative space/aerial-aided edge computing toward 6G system. IEEE Trans. Veh. Technol. 2023, 73, 2495–2505. [Google Scholar] [CrossRef]
- Tian, A.; Zhang, W.; Hei, J.; Hua, Y.; Liu, X.; Wang, J.; Gao, R. Resistance reduction method for building transmission and distribution systems based on an improved random forest model: A tee case study. Build. Environ. 2025, 282, 113256. [Google Scholar] [CrossRef]
- Li, B.; Wang, X.; Khurshid, A.; Saleem, S.F. Environmental governance, green finance, and mitigation technologies: Pathways to carbon neutrality in European industrial economies. Int. J. Environ. Sci. Technol. 2025, 22, 14899–14912. [Google Scholar] [CrossRef]
- Wang, C.; Yang, L.; Hu, M.; Wang, Y.; Zhao, Z. On-demand airport slot management: Tree-structured capacity profile and coadapted fire-break setting and slot allocation. Transp. A Transp. Sci. 2024, 1–35. [Google Scholar] [CrossRef]
- Ye, D.; Wang, B.; Wu, L.; Del Rio-Chanona, E.A.; Sun, Z. PO-SRPP: A decentralized pivoting path planning method for self-reconfigurable satellites. IEEE Trans. Ind. Electron. 2024, 71, 14318–14327. [Google Scholar] [CrossRef]
- Xu, C.; Huang, X.; Hu, Q.; Xue, W.; Zhou, K.; Li, X.; Nan, Y.; Ju, D.; Wang, Z.; Zhang, X. Modulating autophagy to boost the antitumor efficacy of TROP2-directed antibody-drug conjugate in pancreatic cancer. Biomed. Pharmacother. 2024, 180, 117550. [Google Scholar] [CrossRef]
- Ji, X.; Jiang, P.; Jiang, Y.; Chen, H.; Wang, W.; Zhong, W.; Zhang, X.; Zhao, W.; Zang, D. Toward enhanced aerosol particle adsorption in never-bursting bubble via acoustic levitation and controlled liquid compensation. Adv. Sci. 2023, 10, 2300049. [Google Scholar] [CrossRef]
- Li, J.; Han, D.; Weng, T.H.; Wu, H.; Li, K.C.; Castiglione, A. A secure data storage and sharing scheme for port supply chain based on blockchain and dynamic searchable encryption. Comput. Stand. Interfaces 2025, 91, 103887. [Google Scholar] [CrossRef]
- Han, D.; Shi, J.; Zhao, J.; Wu, H.; Zhou, Y.; Li, L.H.; Khan, M.K.; Li, K.C. LRCN: Layer-residual Co-Attention Networks for visual question answering. Expert Syst. Appl. 2025, 263, 125658. [Google Scholar] [CrossRef]
- Chen, X.; Dai, A. Quantifying contributions of external forcing and internal variability to Arctic warming during 1900–2021. Earth’s Future 2024, 12, e2023EF003734. [Google Scholar] [CrossRef]
- Zhou, H.; Guo, J.; Zhu, G.; Xu, H.; Tang, X.; Luo, X. Flotation behavior and mechanism of smithsonite under the system of bidentate ligand sulfide sodium thiocyanate. Sep. Purif. Technol. 2024, 334, 126086. [Google Scholar] [CrossRef]
- Feng, C.; Feng, Z.; Mao, R.; Li, G.; Zhong, Y.; Ling, K. Prediction of vitrinite reflectance of shale oil reservoirs using nuclear magnetic resonance and conventional log data. Fuel 2023, 339, 127422. [Google Scholar] [CrossRef]
- Yue, T.; Zhang, W.; Pei, H.; Danzeng, D.; He, J.; Yang, J.; Luo, Y.; Zhang, Z.; Xiong, S.; Yang, X.; et al. Monascus pigment-protected bone marrow-derived stem cells for heart failure treatment. Bioact. Mater. 2024, 42, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, W.; Li, X.; Zhang, Y. Guardians of the ledger: Protecting decentralized exchanges from state derailment defects. IEEE Trans. Reliab. 2024, 74, 3629–3641. [Google Scholar] [CrossRef]
- Yi, X.; Zhao, R.; Lin, Y. The impact of nighttime car body lighting on pedestrians’ distraction: A virtual reality simulation based on bottom-up attention mechanism. Saf. Sci. 2024, 180, 106633. [Google Scholar] [CrossRef]
- Ren, F.; Liu, X.; Charles, V.; Zhao, X.; Balsalobre-Lorente, D. Integrated efficiency and influencing factors analysis of ESG and market performance in thermal power enterprises in China: A hybrid perspective based on parallel DEA and a benchmark model. Energy Econ. 2025, 141, 108138. [Google Scholar] [CrossRef]
- Wei, J.; Fan, P.; Huang, Y.; Zeng, H.; Jiang, R.; Wu, Z.; Zhang, Y.; Hu, Z. (±)-Hypandrone A, a pair of polycyclic polyprenylated acylphloroglucinol enantiomers with a caged 7/6/5/6/6 pentacyclic skeleton from Hypericum androsaemum. Org. Chem. Front. 2024, 11, 3459–3464. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, M.; Li, D.; Wu, R.; Lin, R.; Yang, C. An AUV-enabled dockable platform for long-term dynamic and static monitoring of marine pastures. IEEE J. Ocean. Eng. 2024, 50, 276–293. [Google Scholar] [CrossRef]
- Feng, C.; Shi, Y.; Hao, J.; Wang, Z.; Mao, Z.; Li, G.; Jiang, Z. Nuclear magnetic resonance features of low-permeability reservoirs with complex wettability. Pet. Explor. Dev. 2024, 44, 274–279. [Google Scholar] [CrossRef]
- Jing, H.; Lin, Q.; Liu, M.; Liu, H. Electromechanical braking systems and control technology: A survey and practice. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2024, 239, 09544070241271826. [Google Scholar] [CrossRef]
- Yang, C.; Chen, Y.; Sun, W.; Zhang, Q.; Diao, M.; Sun, J. Extreme soil salinity reduces N and P metabolism and related microbial network complexity and community immigration rate. Environ. Res. 2025, 264, 120361. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shi, X.L.; Wang, J.; Shi, K.; Wang, Q. Effect of different hydrogen donors on the catalytic conversion of levulinic acid to γ-valerolactone over non-noble metal catalysts. J. Ind. Eng. Chem. 2024, 138, 17–33. [Google Scholar] [CrossRef]
- Xu, J.; Fan, L.; Chen, C.; Lu, G.; Li, B.; Tu, T. Study on fuel injection stability improvement in marine low-speed dual-fuel engines. Appl. Therm. Eng. 2024, 253, 123729. [Google Scholar] [CrossRef]
- Long, Q.; Yan, H.; Wu, H.; Qiu, S.; Zhou, X.; Qiu, T. Influence mechanism of leaching agent anions on the leaching of aluminium impurities in ionic-type rare earth ores: A DFT simulation combined with experimental verification. Sep. Purif. Technol. 2025, 354, 128768. [Google Scholar] [CrossRef]
- Li, Y.; Ma, H.; Zhang, Y.; He, T.; Li, B.; Ren, H.; Feng, J.; Sheng, J.; Li, K.; Qian, Y.; et al. PGLYRP2 drives hepatocyte-intrinsic innate immunity by trapping and clearing hepatitis B virus. J. Clin. Investig. 2025, 135, e188083. [Google Scholar] [CrossRef]
- Sha, X.; Si, X.; Zhu, Y.; Wang, S.; Zhao, Y. Automatic three-dimensional reconstruction of transparent objects with multiple optimization strategies under limited constraints. Image Vis. Comput. 2025, 160, 105580. [Google Scholar] [CrossRef]
- Liu, R.; Shen, W. Data Acquisition of Exercise and Fitness Pressure Measurement Based on Artificial Intelligence Technology. SLAS Technol. 2025, 33, 100328. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Liu, Z.; Qin, Y.; Su, K.; Yu, Z. Thermal Property of Reservoir Rocks at Thermal–Mechanical Coupled Conditions and Resultant Impact on Performance of Geothermal Systems. Rock Mech. Rock Eng. 2025, 58, 8773–8798. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, B.; Chen, L.; Tian, F.; Xu, J.; Liu, J.; Yang, Q.; Zhu, B. Effect of lateral stress and loading paths on direct shear strength and fracture of granite under true triaxial stress state by a self-developed device. Eng. Fract. Mech. 2025, 318, 110952. [Google Scholar] [CrossRef]
- Yue, T. Some results on the nonuniform polynomial dichotomy of discrete evolution families. Hiroshima Math. J. 2025, 55, 183–201. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, C.; Li, M.; Qi, Z.; Yang, X.; Lin, Y.; Cao, S. A review on curve edge based architectures under lateral loads. Thin-Walled Struct. 2025, 217, 113849. [Google Scholar] [CrossRef]
- Yu, Y.; Jia, T.; Lin, X.; Bao, Y.; Chang, S.; Sun, J.; Gao, T.; Shi, J.; Ai, S.; Yuan, K. Unveiling causal relationship between white matter tracts and psychiatric disorders. Commun. Biol. 2025, 8, 1221. [Google Scholar] [CrossRef]
- Lv, Z.; Zhao, Q.; Sun, X.M.; Wu, Y. Finite-time control design for a coaxial tilt-rotor UAV. IEEE Trans. Ind. Electron. 2024, 71, 16132–16142. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, A.; Zhang, L.; Cui, R.; Lv, B.; Xiao, Z.; Li, D.; Quan, Z.; Xu, X. Light modulated magnetism and spin–orbit torque in a heavy metal/ferromagnet heterostructure based on van der Waals-layered ferroelectric materials. Appl. Phys. Lett. 2023, 123, 092406. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Y.; Hu, G. Words that matter: A cross-disciplinary investigation of importance markers in 3MT presentations. Engl. Specif. Purp. 2025, 80, 91–108. [Google Scholar] [CrossRef]
- Lu, Y.; Lnong, X.; Mao, Y.; Qin, L.; Liao, X.; Zhao, L. Structural characterization and hypoglycemic activity of a polysaccharide from Litchi chinensis Sonn.(litchi) pericarp. Food Chem. 2025, 493, 145994. [Google Scholar] [CrossRef]
- Liu, J.; Ma, C.; Li, M.; He, J.; Totis, G.; Hua, C.; Cui, G.; Wang, L.; Xue, R.; Tan, Z.; et al. A compressed tensor-based edge-deployable framework for multi-source thermal error compensation in face gear machining. Adv. Eng. Inform. 2025, 68, 103802. [Google Scholar] [CrossRef]
- Chen, X.; Ma, L.; Bo, Y.; Xia, B.; Guo, Y.; Shang, Y.; Yan, F.; Huang, E.; Shi, W.; Ding, R.; et al. Single-nucleus RNA sequencing and machine learning identify CACNA1A as a myocyte-specific biomarker for sudden unexplained death in schizophrenia. Forensic Sci. Int. 2025, 377, 112646. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Chen, T.; Chen, J.; Huang, B.; Wang, G. Temperature effects on single cavitation bubble dynamics under the free field condition: Experimental and theoretical investigations on water. Ultrason. Sonochem. 2025, 120, 107520. [Google Scholar] [CrossRef]
- Sun, L.; Li, M.; Song, Z.; Fu, T.; Hao, X.; Li, Y.; Liu, J.; Matsveichuk, N.; Sotskov, Y. An Integrated MILP Model for Scheduling of Steelmaking-Continuous Casting with Cranes. IEEE Robot. Autom. Lett. 2025, 10, 10426–10433. [Google Scholar] [CrossRef]
- Weber, R.; Watson, A.; Forter, M.; Oliaei, F. Persistent organic pollutants and landfills-a review of past experiences and future challenges. Waste Manag. Res. 2025, 29, 107–121. [Google Scholar] [CrossRef]
- Moline, J.M.; Golden, A.L.; Bar-Chama, N.; Smith, E.; Rauch, M.E.; Chapin, R.E.; Perreault, S.D.; Schrader, S.M.; Suk, W.A.; Landrigan, P.J. Exposure to hazardous substances and male reproductive health: A research framework. Environ. Health Perspect. 2000, 108, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Vellingiri, A.; Mohanasundaram, K.; Tamilselvan, K.S.; Maheswar, R.; Ganesh, N. Multiple sensor based human detection robots: A review. Int. J. Smart Sens. Intell. Syst. 2023, 16, 1–17. [Google Scholar] [CrossRef]
- Kumari, S.; Choudhury, A.; Karki, P.; Simon, M.; Chowdhry, J.; Nandra, A.; Sharma, P.; Sengupta, A.; Yadav, A.; Raju, M.P.; et al. Next-Generation Air Quality Management: Unveiling Advanced Techniques for Monitoring and Controlling Pollution. Aerosol Sci. Eng. 2025, 1–22. [Google Scholar] [CrossRef]
- Banga, I.; Paul, A.; Poudyal, D.C.; Muthukumar, S.; Prasad, S. Recent advances in gas detection methodologies with a special focus on environmental sensing and health monitoring applications—A critical review. ACS Sens. 2023, 8, 3307–3319. [Google Scholar] [CrossRef]
- Ahmed, S.; Kumar, S. Carbon monoxide toxicity and its management: A review. Int. J. Adv. Res. Med. Chem. 2020, 2, 11–19. [Google Scholar]
- Sharma, S.; Ghoshal, S.K. Hydrogen the future transportation fuel: From production to applications. Renew. Sustain. Energy Rev. 2015, 43, 1151–1158. [Google Scholar] [CrossRef]
- Nazir, H.; Muthuswamy, N.; Louis, C.; Jose, S.; Prakash, J.; Buan, M.E.; Kannan, A.M. Is the H2 economy realizable in the foreseeable future? Part III: H2 usage technologies, applications, and challenges and opportunities. Int. J. Hydrogen Energy 2020, 45, 28217–28239. [Google Scholar] [CrossRef]
- Qazi, U.Y. Future of hydrogen as an alternative fuel for next-generation industrial applications; challenges and expected opportunities. Energies 2022, 15, 4741. [Google Scholar] [CrossRef]
- Duncan, I.J. Does methane pose significant health and public safety hazards? A review. Environ. Geosci. 2015, 22, 85–96. [Google Scholar] [CrossRef]
- Prasad, S.; Zhao, L.; Gomes, J. Methane and natural gas exposure limits. Epidemiology 2011, 22, S251. [Google Scholar] [CrossRef]
- Oyewunmi, T. Natural gas in a carbon-constrained world: Examining the role of institutions in curbing methane and other fugitive emissions. LSU J. Energy L. Resour. 2021, 9, 87. [Google Scholar]
- Randall, D.J.; Tsui, T.K.N. Ammonia toxicity in fish. Mar. Pollut. Bull. 2002, 45, 17–23. [Google Scholar] [CrossRef]
- Dasarathy, S.; Mookerjee, R.P.; Rackayova, V.; Rangroo Thrane, V.; Vairappan, B.; Ott, P.; Rose, C.F. Ammonia toxicity: From head to toe? Metab. Brain Dis. 2017, 32, 529–538. [Google Scholar] [CrossRef]
- Nemery, B. Metal toxicity and the respiratory tract. Eur. Respir. J. 1990, 3, 202–219. [Google Scholar] [CrossRef] [PubMed]
- Patocka, J.; Kuca, K. Irritant compounds: Respiratory irritant gases. Milit. Med. Sci. Lett. 2014, 83, 73–82. [Google Scholar] [CrossRef]
- Aydın, H.; İlkılıç, C. Air pollution, pollutant emissions and harmfull effects. J. Eng. Technol. 2017, 1, 8–15. [Google Scholar]
- Bhandarkar, S. Vehicular pollution, their effect on human health and mitigation measures. Veh. Eng. 2013, 1, 33–40. [Google Scholar]
- Hilliard, J.C.; Wheeler, R.W. Nitrogen dioxide in engine exhaust. SAE Trans. 1979, 88, 2343–2354. [Google Scholar]
- Epping, R.; Koch, M. On-site detection of volatile organic compounds (VOCs). Molecules 2023, 28, 1598. [Google Scholar] [CrossRef]
- Sun, X.; Shao, K.; Wang, T. Detection of volatile organic compounds (VOCs) from exhaled breath as noninvasive methods for cancer diagnosis. Anal. Bioanal. Chem. 2016, 408, 2759–2780. [Google Scholar] [CrossRef]
- Jones, A.W.; Musshoff, F.; Krämer, T.; Steuer, A.E.; Gerostamoulos, D.; Drummer, O.H.; Skopp, G. Toxicology of Specific Substances. In Handbook of Forensic Medicine, 2nd ed.; Willey: Hoboken, NJ, USA, 2022; pp. 1167–1312. [Google Scholar]
- Wu, A.; McKay, C. Recommendations for the use of laboratory tests to support poisoned patients who present to the emergency department. Clin. Chem. 2003, 49, 357–379. [Google Scholar] [CrossRef]
- Jones, A.W.; Musshoff, F.; Kraemer, T.; Schwaninger, A.E.; Gerostamoulos, D.; Drummer, O.H.; Schänzer, W. Toxicology of specific substances. In Handbook of Forensic Medicine; Willey: Hoboken, NJ, USA, 2014; pp. 900–993. [Google Scholar]
- Loomis, T.A. Formaldehyde toxicity. Arch. Pathol. Lab. Med. 1979, 103, 321–324. [Google Scholar] [PubMed]
- Songur, A.; Ozen, O.A.; Sarsilmaz, M. The toxic effects of formaldehyde on the nervous system. Rev. Environ. Contam. Toxicol. 2010, 203, 105–118. [Google Scholar]
- Gupta, K.C.; Ulsamer, A.G.; Preuss, P.W. Formaldehyde in indoor air: Sources and toxicity. Environ. Int. 1982, 8, 349–358. [Google Scholar] [CrossRef]
- Benedict, B.; Kristensen, S.M.; Duxin, J.P. What are the DNA lesions underlying formaldehyde toxicity? DNA Repair 2024, 138, 103667. [Google Scholar] [CrossRef]
- Herberger, S.; Herold, M.; Ulmer, H.; Burdack-Freitag, A.; Mayer, F. Detection of human effluents by a MOS gas sensor in correlation to VOC quantification by GC/MS. Build. Environ. 2010, 45, 2430–2439. [Google Scholar] [CrossRef]
- Bautista, Q.; Benamara, M.; Zhao, S.; Gómez, E.; Serrà, A. Efficient organic pollutant mineralization via PMS-sonophotocatalysis with doped-ZnO-CNT aerogels. Appl. Catal. O Open 2025, 199, 207027. [Google Scholar] [CrossRef]
- Bujaldón, R.; Benamara, M.; Dhahri, R.; Gómez, E.; Serrà, A. Attuning doped ZnO-based composites for an effective light-driven mineralization of pharmaceuticals via PMS activation. Chemosphere 2024, 357, 142127. [Google Scholar] [CrossRef]
- Benamara, M.; Iben Nassar, K.; Rivero-Antúnez, P.; Essid, M.; Soreto Teixeira, S.; Zhao, S.; Esquivias, L. Study of electrical and dielectric behaviors of copper-doped zinc oxide ceramic prepared by spark plasma sintering for electronic device applications. Nanomaterials 2024, 14, 402. [Google Scholar] [CrossRef] [PubMed]
- Benamara, M.; Nassar, K.I.; Soltani, S.; Kallekh, A.; Dhahri, R.; Dahman, H.; El Mir, L. Light-enhanced electrical behavior of a Au/Al-doped ZnO/p-Si/Al heterostructure: Insights from impedance and current–voltage analysis. RSC Adv. 2023, 13, 28632–28641. [Google Scholar] [CrossRef] [PubMed]
- Benamara, M.; Massoudi, J.; Dahman, H.; Ly, A.; Dhahri, E.; Debliquy, M.; Lahem, D. Study of room temperature NO2 sensing performances of ZnO1−x (x= 0, 0.05, 0.10). Appl. Phys. A 2022, 128, 31. [Google Scholar] [CrossRef]
- Benamara, M.; Teixeira, S.S.; Graça, M.P.F.; Valente, M.A.; Jakka, S.K.; Dahman, H.; Lahem, D. Study of ZnO room temperature NO2 sensor under illumination prepared by auto-combustion. Appl. Phys. A 2021, 127, 706. [Google Scholar] [CrossRef]
- Meng, F.; Li, G.; Ji, H.; Yuan, Z. Investigation on oxygen vacancy regulation mechanism of ZnO gas sensors under temperature modulation mode to distinguish alcohol homologue gases. Sens. Actuators B Chem. 2025, 423, 136747. [Google Scholar] [CrossRef]
- Hung, P.T.; Thao, D.T.H.; Hung, N.M.; Van Hoang, N.; Hoat, P.D.; Van Thin, P.; Heo, Y.W. H2S gas sensing properties of ZnO–SnO2 branch–stem nanowires grown on a copper foil. Scr. Mater. 2025, 255, 116372. [Google Scholar] [CrossRef]
- Nguyet, T.T.; Le, D.T.T.; Van Duy, N.; Xuan, C.T.; Ingebrandt, S.; Vu, X.T.; Hoa, N.D. A sigh-performance hydrogen gas sensor based on Ag/Pd nanoparticle-functionalized ZnO nanoplates. RSC Adv. 2023, 13, 13017–13029. [Google Scholar] [CrossRef]
- Nag, S.; Dey, S.; Das, D.; Guha, P.K. Adsorption-Mediated n-Type ZnO Surface Reconstruction for Optically Enhanced Volatile Organic Compound Sensing. ACS Appl. Electron. Mater. 2022, 4, 3825–3833. [Google Scholar] [CrossRef]
- Sun, K.; Zhan, G.; Zhang, L.; Wang, Z.; Lin, S. Highly sensitive NO2 gas sensor based on ZnO nanoarray modulated by oxygen vacancy with Ce doping. Sens. Actuators B Chem. 2023, 379, 133294. [Google Scholar] [CrossRef]
- Kumar, N.; Srivastava, A.K.; Patel, H.S.; Gupta, B.K.; Varma, G.D. Facile synthesis of ZnO–reduced graphene oxide nanocomposites for NO2 gas sensing applications. Eur. J. Inorg. Chem. 2015, 2015, 1912–1923. [Google Scholar] [CrossRef]
- Franco, M.A.; Conti, P.P.; Andre, R.S.; Correa, D.S. A review on chemiresistive ZnO gas sensors. Sens. Actuators Rep. 2022, 4, 100100. [Google Scholar] [CrossRef]
- Xuan, J.; Zhao, G.; Sun, M.; Jia, F.; Wang, X.; Zhou, T.; Liu, B. Low-temperature operating ZnO-based NO2 sensors: A review. RSC Adv. 2020, 10, 39786–39807. [Google Scholar] [CrossRef]
- Krishna, K.G.; Umadevi, G.; Parne, S.; Pothukanuri, N. Zinc oxide based gas sensors and their derivatives: A critical review. J. Mater. Chem. C 2023, 11, 3906–3925. [Google Scholar] [CrossRef]
- Bochenkov, V.E.; Sergeev, G.B. Sensitivity, selectivity, and stability of gas-sensitive metal-oxide nanostructures. Met. Oxide Nanostructures Their Appl. 2010, 3, 31–52. [Google Scholar]
- Hooshmand, S.; Kassanos, P.; Keshavarz, M.; Duru, P.; Kayalan, C.I.; Kale, İ.; Bayazit, M.K. Wearable nano-based gas sensors for environmental monitoring and encountered challenges in optimization. Sensors 2023, 23, 8648. [Google Scholar] [CrossRef]
- Mamun, M.A.A.; Yuce, M.R. Recent progress in nanomaterial enabled chemical sensors for wearable environmental monitoring applications. Adv. Funct. Mater. 2020, 30, 2005703. [Google Scholar] [CrossRef]
- Bulowski, W.; Knura, R.; Socha, R.P.; Basiura, M.; Skibińska, K.; Wojnicki, M. Thin Film Semiconductor Metal Oxide Oxygen Sensors: Limitations, Challenges, and Future Progress. Electronics 2024, 13, 3409. [Google Scholar] [CrossRef]
- Bessadok, M.N.; Bouri, A.; Ananias, D.; El Mir, L. Improvement of luminescence properties of Eu-doped ZnO nanoparticles followed by their incorporation into a silica aerogel matrix. Emergent Mater. 2025, 8, 775–791. [Google Scholar] [CrossRef]
- Mrabet, S.; Ihzaz, N.; Bessadok, M.N.; Vázquez-Vázquez, C.; Alshammari, M.; Lemine, O.M.; El Mir, L. Structural, optical, and magnetic behavior and the nucleation of a Griffiths-like phase in (Ca, V)-doped ZnO nanoparticles. Dalton Trans. 2025, 54, 7400–7414. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, R.; Singh, R.K. Progress on transition metal-doped ZnO nanoparticles and its application. Ind. Eng. Chem. Res. 2019, 58, 17130–17163. [Google Scholar] [CrossRef]
- Kanwal, F.; Javed, T.; Hussain, F.; Wasim, M.; Batool, M. Enhanced dye photodegradation through ZnO and ZnO-based photocatalysts doped with selective transition metals: A review. Environ. Technol. Rev. 2024, 13, 754–793. [Google Scholar] [CrossRef]
- Samadi, M.; Zirak, M.; Naseri, A.; Khorashadizade, E.; Moshfegh, A.Z. Recent progress on doped ZnO nanostructures for visible-light photocatalysis. Thin Solid Film. 2016, 605, 2–19. [Google Scholar] [CrossRef]
- Zegebreal, L.T.; Tegegne, N.A.; Hone, F.G. Recent progress in hybrid conducting polymers and metal oxide nanocomposite for room-temperature gas sensor applications: A review. Sens. Actuators A Phys. 2023, 359, 114472. [Google Scholar] [CrossRef]
- Husain, A.; Shariq, M.U. Polypyrrole nanocomposites as promising gas/vapour sensing materials: Past, present and future prospects. Sens. Actuators A Phys. 2023, 359, 114504. [Google Scholar] [CrossRef]
- Chowdhury, M.S.H.; Khan, M.M.R.; Shohag, M.R.H.; Rahman, S.; Paul, S.K.; Rahman, M.M.; Rahman, M.M. Easy synthesis of PPy/TiO2/ZnO composites with superior photocatalytic performance, efficient supercapacitors and nitrite sensor. Heliyon 2023, 9, e19564. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Han, L.; Hu, H.; Zhang, R. A review on polymers and their composites for flexible electronics. Mater. Adv. 2023, 4, 726–746. [Google Scholar] [CrossRef]
- Asture, A.; Rawat, V.; Srivastava, C.; Vaya, D. Investigation of properties and applications of ZnO polymer nanocomposites. Polym. Bull. 2023, 80, 3507–3545. [Google Scholar] [CrossRef]
- Chowdhury, N.K.; Bhowmik, B. Micro/nanostructured gas sensors: The physics behind the nanostructure growth, sensing and selectivity mechanisms. Nanoscale Adv. 2021, 3, 73–93. [Google Scholar] [CrossRef]
- Shakeel, A.; Rizwan, K.; Farooq, U.; Iqbal, S.; Altaf, A.A. Advanced polymeric/inorganic nanohybrids: An integrated platform for gas sensing applications. Chemosphere 2022, 294, 133772. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Sysoev, V.V.; Brzhezinskaya, M.; Solomatin, M.A.; Gabrelian, V.S.; Kirilenko, D.A.; Stolyarova, D.Y.; Saveliev, S.D.; Shvidchenko, A.V.; Cherviakova, P.D.; et al. Rationalizing Graphene–ZnO Composites for Gas Sensing via Functionalization with Amines. Nanomaterials 2024, 14, 735. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Glukhova, O.E.; Sysoev, V.V.; Barkov, P.V.; Ryzhkov, S.A.; Stolyarova, D.Y.; Saveliev, S.D.; Khalturin, B.G.; Varezhnikov, A.S.; Solomatin, M.A.; et al. Delving into the Effect of ZnO Nanoparticles on the Chemistry and Electronic Properties of Aminated Graphene: Ab Initio and Experimental Probing. Surf. Interfaces 2025, 65, 106501. [Google Scholar] [CrossRef]
- Almaev, A.V.; Karipbayev, Z.T.; Kakimov, A.B.; Yakovlev, N.N.; Kukenov, O.I.; Korchemagin, A.O.; Akmetova-Abdik, G.A.; Kumarbekov, K.K.; Zhunusbekov, A.M.; Mochalov, L.A.; et al. High-Temperature Methane Sensors Based on ZnGa2O4:Er Ceramics for Combustion Monitoring. Technologies 2025, 13, 286. [Google Scholar] [CrossRef]
- Grigorjeva, L.; Millers, D.; Smits, K.; Zolotarjovs, A. Gas sensitive luminescence of ZnO coatings obtained by plasma electrolytic oxidation. Sens. Actuators A Phys. 2015, 234, 290–293. [Google Scholar] [CrossRef]
- Berzina, B.; Trinkler, L.; Korsaks, N.; Ruska, R.; Krieke, G.; Sarakovskis, A. F-center luminescence and oxygen gas sensing properties of AlN nanoparticles. Sens. Transducers 2019, 238, 87–93. [Google Scholar]
- Zafar, Z.; Yi, S.; Li, J.; Li, C.; Zhu, Y.; Zada, A.; Yue, X. Recent development in defects engineered photocatalysts: An overview of the experimental and theoretical strategies. Energy Environ. Mater. 2022, 5, 68–114. [Google Scholar] [CrossRef]
- Sturaro, M.; Della Gaspera, E.; Michieli, N.; Cantalini, C.; Emamjomeh, S.M.; Guglielmi, M.; Martucci, A. Degenerately doped metal oxide nanocrystals as plasmonic and chemoresistive gas sensors. ACS Appl. Mater. Interfaces 2016, 8, 30440–30448. [Google Scholar] [CrossRef] [PubMed]
- Park, C.O.; Akbar, S.A. Ceramics for chemical sensing. J. Mater. Sci. 2003, 38, 4611–4637. [Google Scholar] [CrossRef]
- Mondal, B.; Das, J.; Roychaudhuri, C.; Mukharjee, N.; Saha, H. Enhanced sensing properties of ZnO-SnO2 based composite type gas sensor. Eur. Phys. J. Appl. Phys. 2016, 73, 10301. [Google Scholar] [CrossRef]
- Jagtap, S.; Priolkar, K. Study on effect of [OH−] linkages on physical, electrical, and gas sensing properties of ZnO nanoparticles. IEEE Sens. J. 2015, 15, 4700–4707. [Google Scholar] [CrossRef]
- Tit, N.; Othman, W.; Shaheen, A.; Ali, M. High selectivity of N-doped ZnO nano-ribbons in detecting H2, O2 and CO2 molecules: Effect of negative-differential resistance on gas-sensing. Sens. Actuators B Chem. 2018, 270, 167–178. [Google Scholar] [CrossRef]
- Yadav, M.; Kumar, M.; Chaudhary, S.; Yadav, K.; Sharma, A. A review on chemiresistive hybrid zinc oxide and nanocomposites for gas sensing. Ind. Eng. Chem. Res. 2023, 62, 11259–11278. [Google Scholar] [CrossRef]
- Heiland, G.; Kohl, D. Physical and chemical aspects of oxidic semiconductor gas sensors. Chem. Sens. Technol. 1988, 1, 15–38. [Google Scholar]
- Trincado, M.; Grützmacher, H.; Prechtl, M.H. CO2-based hydrogen storage–Hydrogen generation from formaldehyde/water. Phys. Sci. Rev. 2018, 3, 20170013. [Google Scholar]
- Yuan, C.; Ma, J.; Zou, Y.; Li, G.; Xu, H.; Sysoev, V.V.; Deng, Y. Modeling interfacial interaction between gas molecules and semiconductor metal oxides: A new view angle on gas sensing. Adv. Sci. 2022, 9, 2203594. [Google Scholar] [CrossRef] [PubMed]
- Aleksanyan, M.; Sayunts, A.; Shahkhatuni, G.; Simonyan, Z.; Shahnazaryan, G.; Aroutiounian, V. Gas sensor based on ZnO nanostructured film for the detection of ethanol vapor. Chemosensors 2022, 10, 245. [Google Scholar] [CrossRef]
- Subbiah, D.K.; Mani, G.K.; Babu, K.J.; Das, A.; Rayappan, J.B.B. Nanostructured ZnO on cotton fabrics–A novel flexible gas sensor & UV filter. J. Clean. Prod. 2018, 194, 372–382. [Google Scholar]
- Huang, Y.; Yu, Y.; Yu, Y.; Zhang, B. Oxygen vacancy engineering in photocatalysis. Sol. RRL 2020, 4, 2000037. [Google Scholar] [CrossRef]
- Ciftyurek, E.; Li, Z.; Schierbaum, K. Adsorbed oxygen ions and oxygen vacancies: Their concentration and distribution in metal oxide chemical sensors and influencing role in sensitivity and sensing mechanisms. Sensors 2022, 23, 29. [Google Scholar] [CrossRef]
- Rezaei, M.; Nezamzadeh-Ejhieh, A.; Massah, A.R. A comprehensive review on the boosted effects of anion vacancy in the heterogeneous photocatalytic degradation, Part II: Focus on oxygen vacancy. ACS Omega 2024, 9, 6093–6127. [Google Scholar] [CrossRef]
- Zhao, L.C.; He, Y.; Deng, X.; Xia, X.H.; Liang, J.; Yang, G.L.; Wang, H. Ultrasound-assisted extraction of syringin from the bark of Ilex rotunda thumb using response surface methodology. Int. J. Mol. Sci. 2012, 13, 7607–7616. [Google Scholar] [CrossRef]
- Ni, Z.; Ma, J.; Nazarov, A.A.; Yuan, Z.; Wang, X.; Ao, S.; Qin, J. Improving the weldability and mechanical property of ultrasonic spot welding of Cu sheets through a surface gradient structure. J. Mater. Res. Technol. 2025, 36, 2652–2668. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, T.; Gu, Q.; Shang, J. Adsorption Removal of NO2 Under Low-Temperature and Low-Concentration Conditions: A Review of Adsorbents and Adsorption Mechanisms. Adv. Mater. 2025, 37, 2401623. [Google Scholar] [CrossRef] [PubMed]
- Potyrailo, R.A.; Surman, C.; Nagraj, N.; Burns, A. Materials and transducers toward selective wireless gas sensing. Chem. Rev. 2011, 111, 7315–7354. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, M.T.; Donat, F.; Bork, A.H.; Grey, C.P.; Müller, C.R. CO2 capture at medium to high temperature using solid oxide-based sorbents: Fundamental aspects, mechanistic insights, and recent advances. Chem. Rev. 2021, 121, 12681–12745. [Google Scholar] [CrossRef]
- Kohl, D. Surface processes in the detection of reducing gases with SnO2-based devices. Sens. Actuators 1989, 18, 71–113. [Google Scholar] [CrossRef]
- Gadkari, A.B.; Shinde, T.J.; Vasambekar, P.N. Ferrite gas sensors. IEEE Sens. J. 2010, 11, 849–861. [Google Scholar] [CrossRef]
- Schöllhorn, B.; Germain, J.P.; Pauly, A.; Maleysson, C.; Blanc, J.P. Influence of peripheral electron-withdrawing substituents on the conductivity of zinc phthalocyanine in the presence of gases. Part 1: Reducing gases. Thin Solid Film. 1998, 326, 245–250. [Google Scholar] [CrossRef]
- Benamara, M.; Massoudi, J.; Dahman, H.; Dhahri, E.; El Mir, L.; Ly, A.; Debliquy, M.; Lahem, D. High response to sub-ppm level of NO2 with 50% RH of ZnO sensor obtained by an auto-combustion method. J. Mater. Sci. Mater. Electron. 2020, 31, 14249–14260. [Google Scholar] [CrossRef]
- Dhahri, R.; Benamara, M.; Nassar, K.I.; Elkenany, E.B.; Al-Syadi, A.M. Zinc oxide-based sensor prepared by modified sol–gel route for detection of low concentrations of ethanol, methanol, acetone, and formaldehyde. Semicond. Sci. Technol. 2024, 39, 115021. [Google Scholar] [CrossRef]
- Ji, Q.; Bi, L.; Zhang, J.; Cao, H.; Zhao, X.S. The role of oxygen vacancies of ABO3 perovskite oxides in the oxygen reduction reaction. Energy Environ. Sci. 2020, 13, 1408–1428. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.Q.; Fu, X.; Zhang, N.; Xu, Y.J. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef]
- Hjiri, M.; Soltani, S.; Jbeli, A.; Mustapha, N.; Ahmed Althumairi, N.; Benamara, M.; Valente, M.A. Tunable Electrical Properties of Cobalt-Doped Maghemite Nanoparticles for Advanced Resistive and Thermistor Applications. Nanomaterials 2025, 15, 534. [Google Scholar] [CrossRef]
- Bembibre, A.; Benamara, M.; Hjiri, M.; Gómez, E.; Alamri, H.R.; Dhahri, R.; Serra, A. Visible-light driven sonophotocatalytic removal of tetracycline using Ca-doped ZnO nanoparticles. Chem. Eng. J. 2022, 427, 132006. [Google Scholar] [CrossRef]
- Roy, S.; Pan, S.; De, P. Recent progress on polymeric probes for formaldehyde sensing: A comprehensive review. Sci. Technol. Adv. Mater. 2024, 25, 2423597. [Google Scholar] [CrossRef]
- Zhang, T.; Ou-Yang, J.; Yang, X.; Zhu, B. Transferred PMN-PT Thick Film on Conductive Silver Epoxy. Materials 2018, 11, 1621. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Day, C.M.; Garg, S.; Nayak, Y.; Shenoy, P.A.; Nayak, U.Y. Polymeric functionalization of mesoporous silica nanoparticles: Biomedical insights. Int. J. Pharm. 2024, 660, 124314. [Google Scholar] [CrossRef]
- Wawrzyniak, J. Advancements in improving selectivity of metal oxide semiconductor gas sensors opening new perspectives for their application in food industry. Sensors 2023, 23, 9548. [Google Scholar] [CrossRef]
- Sharma, A.; Eadi, S.B.; Noothalapati, H.; Otyepka, M.; Lee, H.D.; Jayaramulu, K. Porous materials as effective chemiresistive gas sensors. Chem. Soc. Rev. 2024, 53, 2530–2577. [Google Scholar] [CrossRef]
- Kamalasanan, M.N.; Chandra, S. Sol-gel synthesis of ZnO thin films. Thin Solid Film. 1996, 288, 112–115. [Google Scholar] [CrossRef]
- Chu, S.Y.; Yan, T.M.; Chen, S.L. Characteristics of sol-gel synthesis of ZnO-based powders. J. Mater. Sci. Lett. 2000, 19, 349–352. [Google Scholar] [CrossRef]
- Perveen, R.; Shujaat, S.; Qureshi, Z.; Nawaz, S.; Khan, M.I.; Iqbal, M. Green versus sol-gel synthesis of ZnO nanoparticles and antimicrobial activity evaluation against panel of pathogens. J. Mater. Res. Technol. 2020, 9, 7817–7827. [Google Scholar] [CrossRef]
- Ben Amor, I.; Hemmami, H.; Laouini, S.E.; Mahboub, M.S.; Barhoum, A. Sol-gel synthesis of ZnO nanoparticles using different chitosan sources: Effects on antibacterial activity and photocatalytic degradation of AZO Dye. Catalysts 2022, 12, 1611. [Google Scholar] [CrossRef]
- Luo, L.; Guo, Y.; Zhu, T.; Zheng, Y. Adsorption species distribution and multicomponent adsorption mechanism of SO2, NO, and CO2 on commercial adsorbents. Energy Fuels 2017, 31, 11026–11033. [Google Scholar] [CrossRef]
- Verma, G.; Gupta, A. Next-Generation Chemiresistive Wearable Breath Sensors for Non-Invasive Healthcare Monitoring: Advances in Composite and Hybrid Materials. Small 2025, 21, 2411495. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, M.V.; Milovanovic, V.; Vasiljevic, Z.Z.; Stamenkovic, Z. Semiconductor gas sensors: Materials, technology, design, and application. Sensors 2020, 20, 6694. [Google Scholar] [CrossRef]
- Strelcov, E.; Dmitriev, S.; Button, B.; Cothren, J.; Sysoev, V.; Kolmakov, A. Evidence of the self-heating effect on surface reactivity and gas sensing of metal oxidenanowire chemiresistors. Nanotechnology 2008, 19, 355502. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Baker, L.R.; Somorjai, G.A. Role of hot electrons and metal–oxide interfaces in surface chemistry and catalytic reactions. Chem. Rev. 2015, 115, 2781–2817. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Xiao, H.; Wang, Z.; Peng, Q.; Jin, K.; Sun, J. Hydrogen sensing properties of Pd/SnO2 nano-spherical composites under UV enhancement. Sens. Actuators B Chem. 2021, 346, 130557. [Google Scholar] [CrossRef]
- Zhao, W.J.; Ding, K.L.; Chen, Y.S.; Xie, F.Y.; Xu, D. Optimized low frequency temperature modulation for improving the selectivity and linearity of SnO2 gas sensor. IEEE Sens. J. 2020, 20, 10433–10443. [Google Scholar] [CrossRef]
- Jaballah, S.; Dahman, H.; Neri, G.; El Mir, L. Effect of Al and Mg Co-doping on the microstructural and gas-sensing characteristics of ZnO nanoparticles. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1653–1667. [Google Scholar] [CrossRef]
- Hjiri, M.; Dhahri, R.; Omri, K.; El Mir, L.; Leonardi, S.G.; Donato, N.; Neri, G. Effect of indium doping on ZnO based-gas sensor for CO. Mater. Sci. Semicond. Process. 2014, 27, 319–325. [Google Scholar] [CrossRef]
- Jaballah, S.; Alaskar, Y.; AlShunaifi, I.; Ghiloufi, I.; Neri, G.; Bouzidi, C.; El Mir, L. Effect of Al and Mg doping on reducing gases detection of ZnO nanoparticles. Chemosensors 2021, 9, 300. [Google Scholar] [CrossRef]
- Benamara, M.; Rivero-Antúnez, P.; Dahman, H.; Essid, M.; Bouzidi, S.; Debliquy, M.; El Mir, L. Selective and rapid detection of acetone using aluminum-doped ZnO-based sensors. J. Sol-Gel Sci. Technol. 2023, 108, 13–27. [Google Scholar] [CrossRef]
- Singh, P.; Kushwaha, C.S.; Singh, V.K.; Dubey, G.C.; Shukla, S.K. Chemiresistive sensing of volatile ammonia over zinc oxide encapsulated polypyrrole based nanocomposite. Sens. Actuators B Chem. 2021, 342, 130042. [Google Scholar] [CrossRef]
- Pinheiro, M.G.; de Souza, E.F.; Chagas, L.H.; Zonetti, P.C.; Gonzalez, G.G.; Huaman, N.R.; Appel, L.G. The role of oxygen vacancies and Zn in isobutene synthesis from ethanol employing Zn, Zr-based catalysts. Catal. Sci. Technol. 2024, 14, 2794–2805. [Google Scholar] [CrossRef]
- Mahdhi, H.; Haddad, N.; Ţălu, Ş.; Ghribi, F.; Djessas, K.; Ayadi, Z.B. Impact of calcium doping on the properties of ZnO thin films: A structural and optical analysis. J. Alloys Compd. 2025, 1020, 179291. [Google Scholar] [CrossRef]
- Lokhande, V.; Malavekar, D.; Kim, C.; Vinu, A.; Ji, T. Order within Disorder: Unveiling the Potential of High Entropy Materials in Energy Storage and Electrocatalysis. Energy Storage Mater. 2024, 72, 103718. [Google Scholar] [CrossRef]
- Yang, L.; He, R.; Chai, J.; Qi, X.; Xue, Q.; Bi, X.; Cabot, A. Synthesis Strategies for High Entropy Nanoparticles. Adv. Mater. 2025, 37, 2412337. [Google Scholar] [CrossRef]
- Çolak, H.; Karaköse, E. Gadolinium (III)-doped ZnO nanorods and gas sensing properties. Mater. Sci. Semicond. Process. 2022, 139, 106329. [Google Scholar] [CrossRef]
- Zheng, B.; Fan, J.; Chen, B.; Qin, X.; Wang, J.; Wang, F.; Liu, X. Rare-earth doping in nanostructured inorganic materials. Chem. Rev. 2022, 122, 5519–5603. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, W.; Li, Q.; Liu, H.; Wang, X. Preparations and applications of zinc oxide based photocatalytic materials. Adv. Sens. Energy Mater. 2023, 2, 100069. [Google Scholar] [CrossRef]
- Benamara, M.; Ly, A.; Soltani, S.; Essid, M.; Dahman, H.; Dhahri, R.; Lahem, D. Enhanced detection of low concentration volatile organic compounds using advanced doped zinc oxide sensors. RSC Adv. 2023, 13, 30230–30242. [Google Scholar] [CrossRef] [PubMed]
- Hjiri, M.; Algessair, S.; Dhahri, R.; Albargi, H.B.; Mansour, N.B.; Assadi, A.A.; Neri, G. Ammonia gas sensors based on undoped and Ca-doped ZnO nanoparticles. RSC Adv. 2024, 14, 5001–5011. [Google Scholar] [CrossRef]
- Dhahri, R.; Hjiri, M.; El Mir, L.; Alamri, H.; Bonavita, A.; Iannazzo, D.; Neri, G. CO sensing characteristics of In-doped ZnO semiconductor nanoparticles. J. Sci. Adv. Mater. Devices 2017, 2, 34–40. [Google Scholar] [CrossRef]
- Monroy, J.G.; González-Jiménez, J.; Blanco, J.L. Overcoming the slow recovery of MOX gas sensors through a system modeling approach. Sensors 2012, 12, 13664–13680. [Google Scholar] [CrossRef] [PubMed]
- Fonollosa, J.; Sheik, S.; Huerta, R.; Marco, S. Reservoir computing compensates slow response of chemosensor arrays exposed to fast varying gas concentrations in continuous monitoring. Sens. Actuators B Chem. 2015, 215, 618–629. [Google Scholar] [CrossRef]
- Subha, P.P.; Jayaraj, M.K. Enhanced room temperature gas sensing properties of low temperature solution processed ZnO/CuO heterojunction. BMC Chem. 2019, 13, 4. [Google Scholar] [CrossRef]
- Martinelli, E.; Santonico, M.; Pennazza, G.; Paolesse, R.; D’Amico, A.; Di Natale, C. Short time gas delivery pattern improves long-term sensor reproducibility. Sens. Actuators B Chem. 2011, 156, 753–759. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, F.C.; Peng, X.W.; Yin, X. A rapid discreteness correction scheme for reproducibility enhancement among a batch of MOS gas sensors. Sens. Actuators A Phys. 2014, 205, 170–176. [Google Scholar] [CrossRef]
- Casanova-Chafer, J. Advantages of Slow Sensing for Ambient Monitoring: A Practical Perspective. Sensors 2023, 23, 8784. [Google Scholar] [CrossRef]
- Zong, B.; Wu, S.; Yang, Y.; Li, Q.; Tao, T.; Mao, S. Smart gas sensors: Recent developments and future prospective. Nano-Micro Lett. 2025, 17, 54. [Google Scholar] [CrossRef]
- Soltabayev, B.; Yergaliuly, G.; Ajjaq, A.; Beldeubayev, A.; Acar, S.; Bakenov, Z.; Mentbayeva, A. Quick NO gas sensing by Ti-doped flower–rod-like ZnO structures synthesized by the SILAR method. ACS Appl. Mater. Interfaces 2022, 14, 41555–41570. [Google Scholar] [CrossRef]
- Wang, C.N.; Li, Y.L.; Gong, F.L.; Zhang, Y.H.; Fang, S.M.; Zhang, H.L. Advances in doped ZnO nanostructures for gas sensor. Chem. Rec. 2020, 20, 1553–1567. [Google Scholar] [CrossRef]
- Patial, P.; Deshwal, M. Selectivity and sensitivity property of metal oxide semiconductor based gas sensor with dopants variation: A review. Trans. Electr. Electron. Mater. 2022, 23, 6–18. [Google Scholar] [CrossRef]
- Moosavi, F.; Bahrololoom, M.E.; Kamjou, R.; Mirzaei, A.; Leonardi, S.G.; Neri, G. Hydrogen sensing properties of Co-doped ZnO nanoparticles. Chemosensors 2018, 6, 61. [Google Scholar] [CrossRef]
- Abdelkarem, K.; Saad, R.; El Sayed, A.M.; Fathy, M.I.; Shaban, M.; Hamdy, H. Design of high-sensitivity La-doped ZnO sensors for CO2 gas detection at room temperature. Sci. Rep. 2023, 13, 18398. [Google Scholar] [CrossRef]
- El Fidha, G.; Bitri, N.; Mahjoubi, S.; Chaabouni, F.; Llobet, E.; Casanova-Chafer, J. Dysprosium doped zinc oxide for NO2 Gas sensing. Sensors 2022, 22, 5173. [Google Scholar] [CrossRef]
- Wang, Z.; Bockstaller, M.R.; Matyjaszewski, K. Synthesis and applications of ZnO/polymer nanohybrids. ACS Mater. Lett. 2021, 3, 599–621. [Google Scholar] [CrossRef]
- Sheikh, M.; Pazirofteh, M.; Dehghani, M.; Asghari, M.; Rezakazemi, M.; Valderrama, C.; Cortina, J.L. Application of ZnO nanostructures in ceramic and polymeric membranes for water and wastewater technologies: A review. Chem. Eng. J. 2020, 391, 123475. [Google Scholar] [CrossRef]
- Fatima, S.; Ahamad, S.; Mishra, N.C.; Gaikwad, K.K. Polymer-based conductive ink: A comprehensive review. Polym. Bull. 2025, 82, 5275–5323. [Google Scholar] [CrossRef]
- Anisimov, Y.A.; Evitts, R.W.; Cree, D.E.; Wilson, L.D. Polyaniline/biopolymer composite systems for humidity sensor applications: A review. Polymers 2021, 13, 2722. [Google Scholar] [CrossRef]
- Sharma, S.; Sudhakara, P.; Omran, A.A.B.; Singh, J.; Ilyas, R.A. Recent trends and developments in conducting polymer nanocomposites for multifunctional applications. Polymers 2021, 13, 2898. [Google Scholar] [CrossRef]
- Foronda, J.R.F.; Aryaswara, L.G.; Santos, G.N.C.; Raghu, S.N.; Muflikhun, M.A. Broad-class volatile organic compounds (VOCs) detection via polyaniline/zinc oxide (PANI/ZnO) composite materials as gas sensor application. Heliyon 2023, 9, e13544. [Google Scholar] [CrossRef]
- Murugesan, T.; Kumar, R.R.; Anbalagan, A.K.; Lee, C.H.; Lin, H.N. Interlinked polyaniline/ZnO nanorod composite for selective NO2 gas sensing at room temperature. ACS Appl. Nano Mater. 2022, 5, 4921–4930. [Google Scholar] [CrossRef]
- Dong, V.T.; Hung, P.T.; Vuong, L.Q.; Khanh, D.D.; Huong, N.T. Zinc Oxide/Polypyrrole particle-decorated rod structure for NO2 detection at low temperature. Vietnam J. Sci. Technol. 2023, 63, 485–494. [Google Scholar]
- Waghchaure, R.H.; Koli, P.B.; Adole, V.A.; Jagdale, B.; Pawar, T.B. Transition metals Ni2+, Fe3+ incorporated modified ZnO thick film sensors to monitor the environmental and industrial pollutant gases. Orient. J. Chem. 2020, 36, 1049. [Google Scholar] [CrossRef]
- Pineda-Reyes, A.M.; Herrera-Rivera, M.R.; Rojas-Chávez, H.; Cruz-Martínez, H.; Medina, D.I. Recent advances in ZnO-based carbon monoxide sensors: Role of doping. Sensors 2021, 21, 4425. [Google Scholar] [CrossRef]
- Taha, I.; Abdulhamid, Z.M.; Straubinger, R.; Emwas, A.H.; Polychronopoulou, K.; Anjum, D.H. Ga-doped ZnO nanoparticles for enhanced CO2 gas sensing applications. Sci. Rep. 2024, 14, 29712. [Google Scholar] [CrossRef]
- Rodrigues, J.; Borge, V.; Jain, S.; Shimpi, N.G. Enhanced acetaldehyde sensing performance of spherical shaped copper doped ZnO nanostructures. ChemistrySelect 2023, 8, e202203967. [Google Scholar] [CrossRef]
- Gómez-Pozos, H.; Arredondo, E.J.L.; Maldonado Álvarez, A.; Biswal, R.; Kudriavtsev, Y.; Pérez, J.V.; Olvera Amador, M.D.L.L. Cu-doped ZnO thin films deposited by a sol-gel process using two copper precursors: Gas-sensing performance in a propane atmosphere. Materials 2016, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ly, A.; Lahem, D.; Martin, J.D.; Romain, A.C.; Zhang, C.; Debliquy, M. Role of cobalt in Co-ZnO nanoflower gas sensors for the detection of low concentration of VOCs. Sens. Actuators B Chem. 2022, 360, 131674. [Google Scholar] [CrossRef]
- Varshney, P.; Mahajan, S.; Sharma, R. Comparative studies of Pure and Mn doped ZnO thin film for gas sensor and Magnetic applications. J. Emerg. Technol. Innov. Res. 2019, 6, 321–324. [Google Scholar]
- Sharma, N.; Choudhury, S.P. Gas sensing using metal oxide semiconductor doped with rare earth elements: A review. Mater. Sci. Eng. B 2024, 307, 117505. [Google Scholar] [CrossRef]
- Jian, J.C.; Chang, Y.C.; Chang, S.P.; Chang, S.J. Biotemplate-assisted growth of ZnO in gas sensors for ppb-level NO2 detection. ACS Omega 2023, 9, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Sayago, I.; Santos, J.P.; Sánchez-Vicente, C. The effect of rare earths on the response of photo UV-activate ZnO gas sensors. Sensors 2022, 22, 8150. [Google Scholar] [CrossRef] [PubMed]
- Mehto, A.; Mehto, V.R.; Chauhan, J.; Singh, I.; Pandey, R. Preparation and characterization of polyaniline/ZnO composite sensor. J. Nanomed. Res. 2017, 5, 1–12. [Google Scholar]
- Karakuş, M.Ö.; Çetin, H. Fabrication and Characterization of Polyaniline/ZnO Nanocomposite Field Effect Transistor Based Hydrogen Gas Sensor. Erciyes Üniversitesi Fen Bilimleri Enstitüsü Fen Bilimleri Dergisi 2021, 37, 99–109. [Google Scholar]
- Masemola, C.M.; Moloto, N.; Tetana, Z.; Linganiso, L.Z.; Motaung, T.E.; Linganiso-Dziike, E.C. Advances in Polyaniline-Based Composites for Room-Temperature Chemiresistor Gas Sensors. Processes 2025, 13, 401. [Google Scholar] [CrossRef]
- Usman, F.; Dennis, J.O.; Mkawi, E.M.; Al-Hadeethi, Y.; Meriaudeau, F.; Fen, Y.W.; Sulieman, A. Acetone vapor-sensing properties of chitosan-polyethylene glycol using surface plasmon resonance technique. Polymers 2020, 12, 2586. [Google Scholar] [CrossRef]
- Guan, Y.; Wang, C.; Yu, H.; Zou, Z.; Zhou, Y.; Cao, G.; Yao, J. Flexible ZnO/PANI/nonwoven nanocomposite based high-sensitive NH3 gas sensor via vapor phase polymerization method. Mater. Sci. Energy Technol. 2020, 3, 862–867. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustapha, N.; Ben Abdelaziz, B.; Benamara, M.; Hjiri, M. Recent Advances in Doping and Polymer Hybridization Strategies for Enhancing ZnO-Based Gas Sensors. Nanomaterials 2025, 15, 1609. https://doi.org/10.3390/nano15211609
Mustapha N, Ben Abdelaziz B, Benamara M, Hjiri M. Recent Advances in Doping and Polymer Hybridization Strategies for Enhancing ZnO-Based Gas Sensors. Nanomaterials. 2025; 15(21):1609. https://doi.org/10.3390/nano15211609
Chicago/Turabian StyleMustapha, Nazir, Boutheina Ben Abdelaziz, Majdi Benamara, and Mokhtar Hjiri. 2025. "Recent Advances in Doping and Polymer Hybridization Strategies for Enhancing ZnO-Based Gas Sensors" Nanomaterials 15, no. 21: 1609. https://doi.org/10.3390/nano15211609
APA StyleMustapha, N., Ben Abdelaziz, B., Benamara, M., & Hjiri, M. (2025). Recent Advances in Doping and Polymer Hybridization Strategies for Enhancing ZnO-Based Gas Sensors. Nanomaterials, 15(21), 1609. https://doi.org/10.3390/nano15211609