Advanced Optoelectronic Applications of Nanopillar Arrays Fabricated by Glancing Angle Deposition
Abstract
1. Introduction
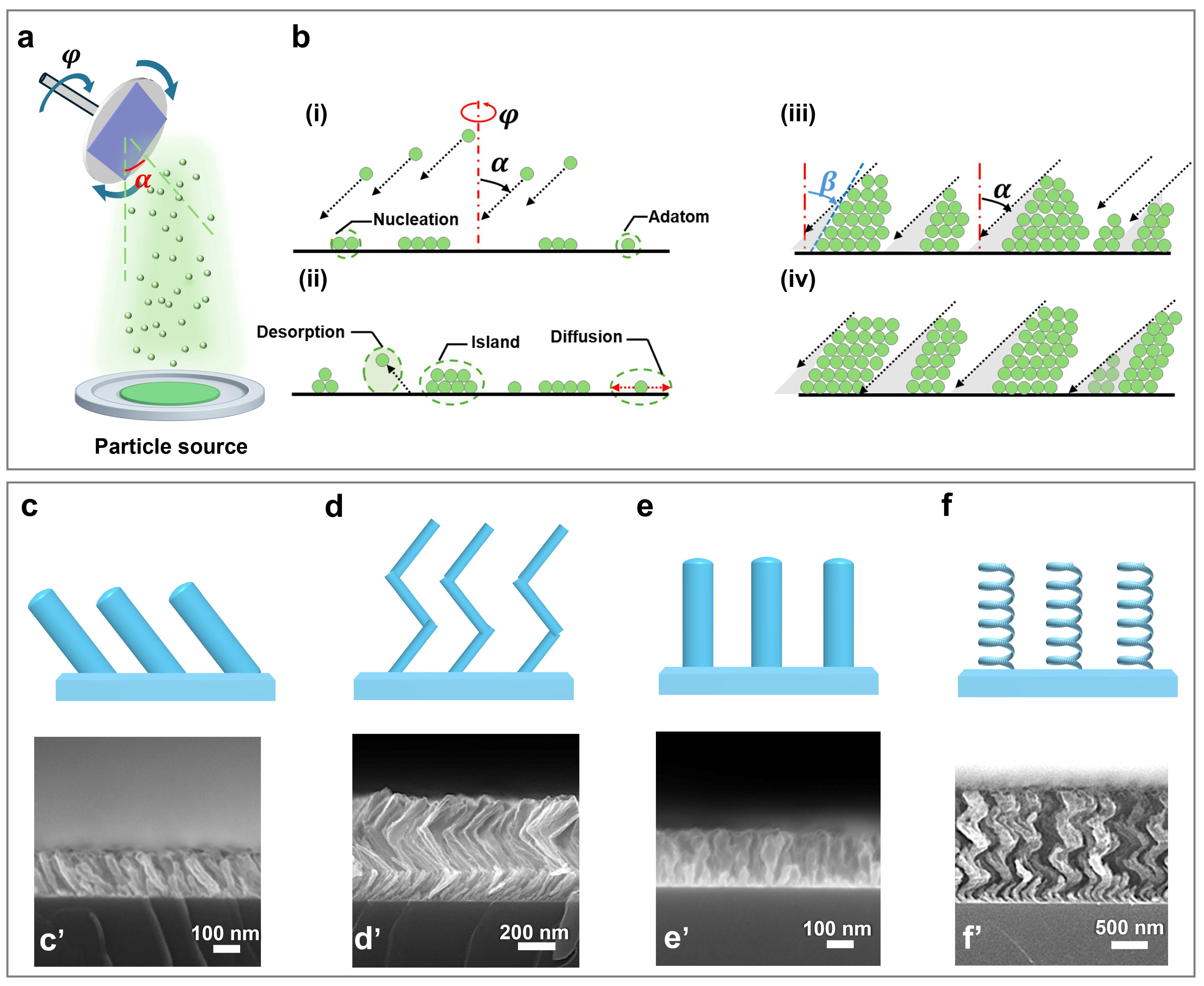
2. GLAD of Nanopillar Arrays
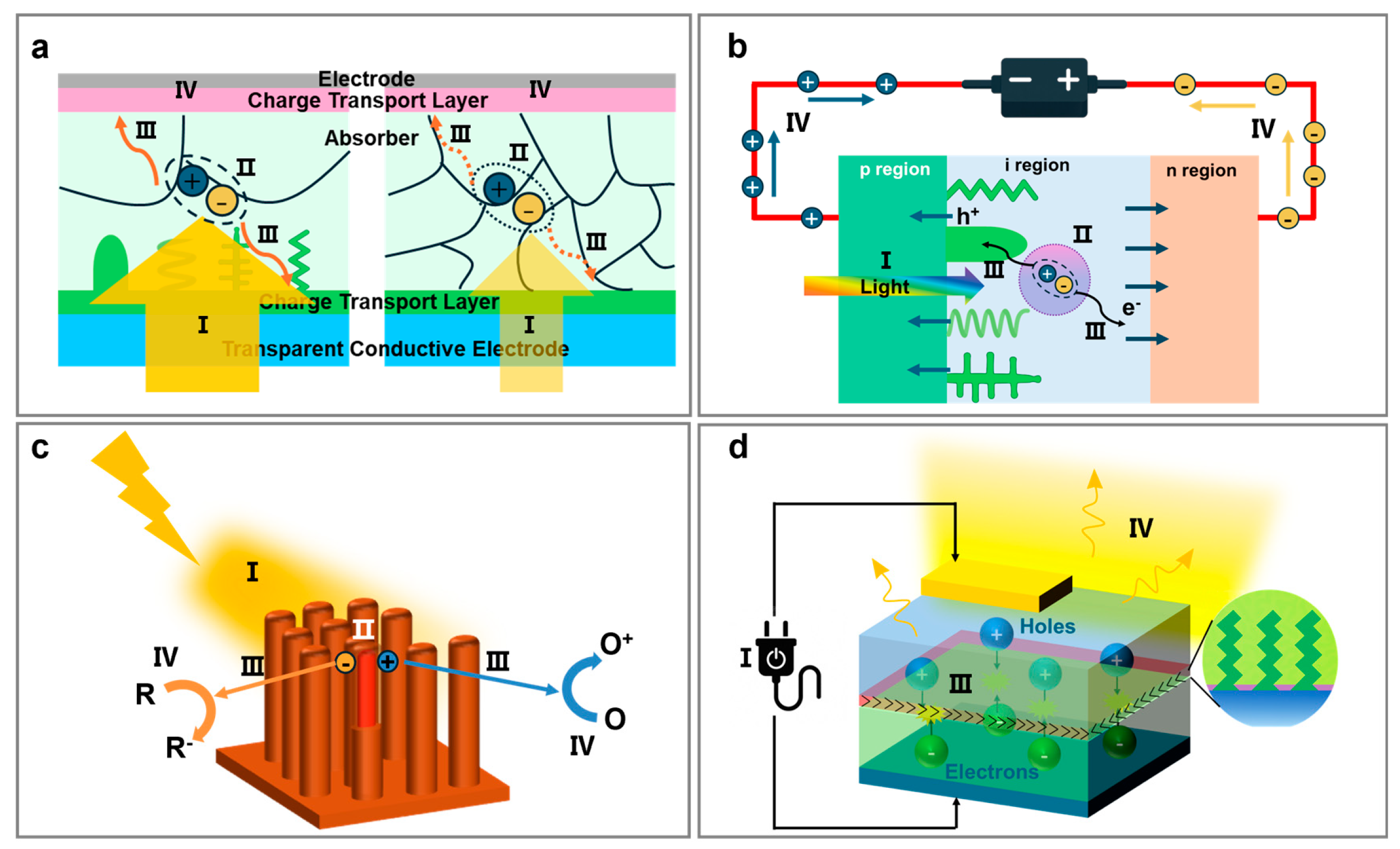
3. Fundamental Functions of Nanopillar Arrays in Diverse Optoelectronic Devices
4. Applications of Nanopillar Arrays in Photovoltaic Devices
4.1. Non-Perovskite Solar Cells

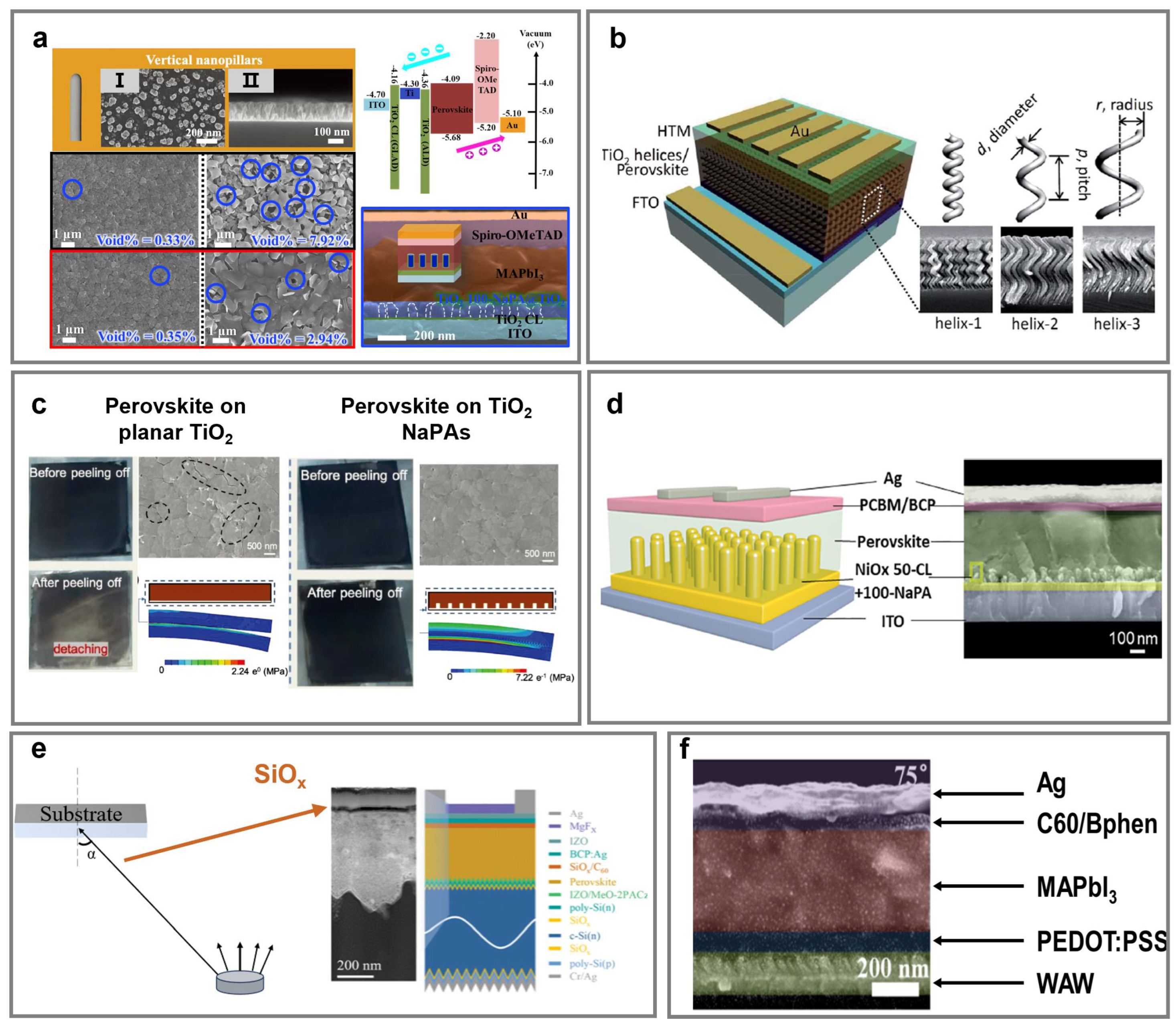
4.2. Perovskite Solar Cells
4.2.1. Electron Transporting Layers
4.2.2. Hole Transporting Layer and Interface Engineering
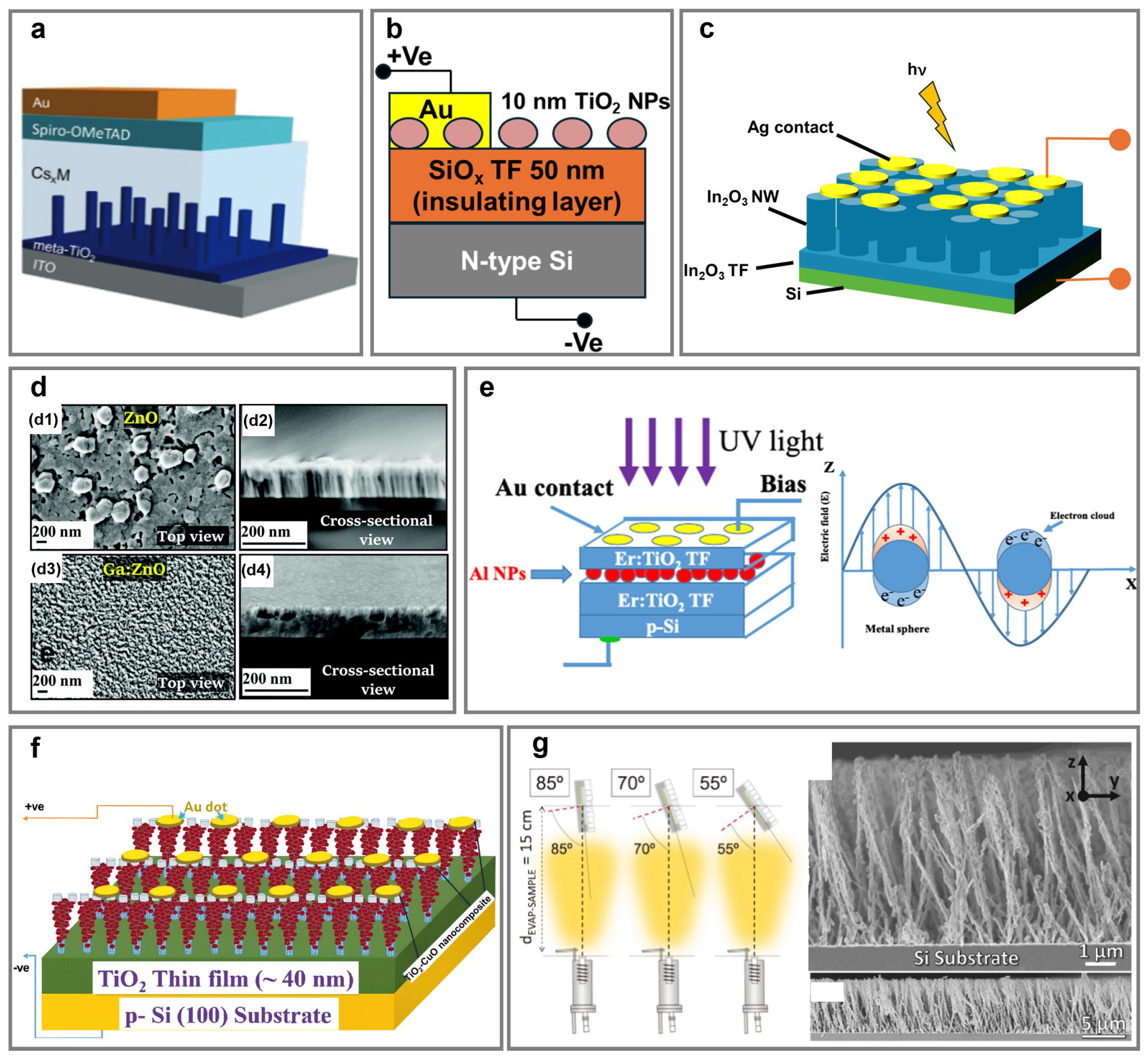
5. Photodetection
5.1. UV Photodetection Using NaPAs Made from Wide-Bandgap Oxide Semiconductors
5.2. Broadband (UV-Visible-Near-Infrared) Photodetection Using Heterostructure Photodetectors
5.3. Emerging Polarization-Sensitive Photodetection
6. Photocatalysis
6.1. Wide-Bandgap NaPAs for UV-Driven Photocatalysis
6.2. Narrow-Bandgap NaPAs for Visible Light-Driven Photocatalysis
6.3. Interface Engineering of NaPAs to Enhance Photocatalysis
6.3.1. Heterojunction Structures
6.3.2. Core@shell Structures
6.3.3. Doping and Defect-Engineering of NaPAs for Bandgap Modulation
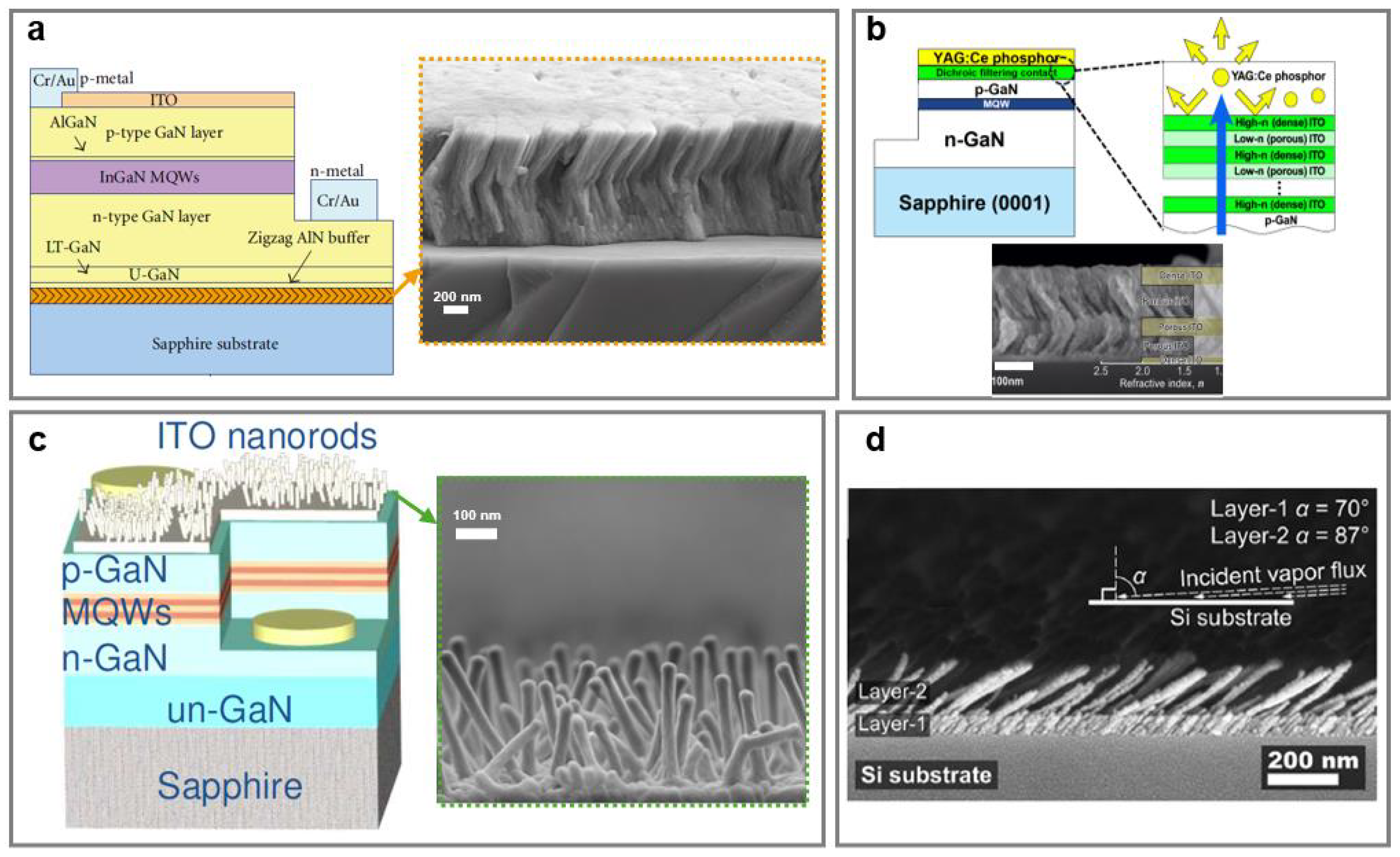
7. Light Emitting Diodes
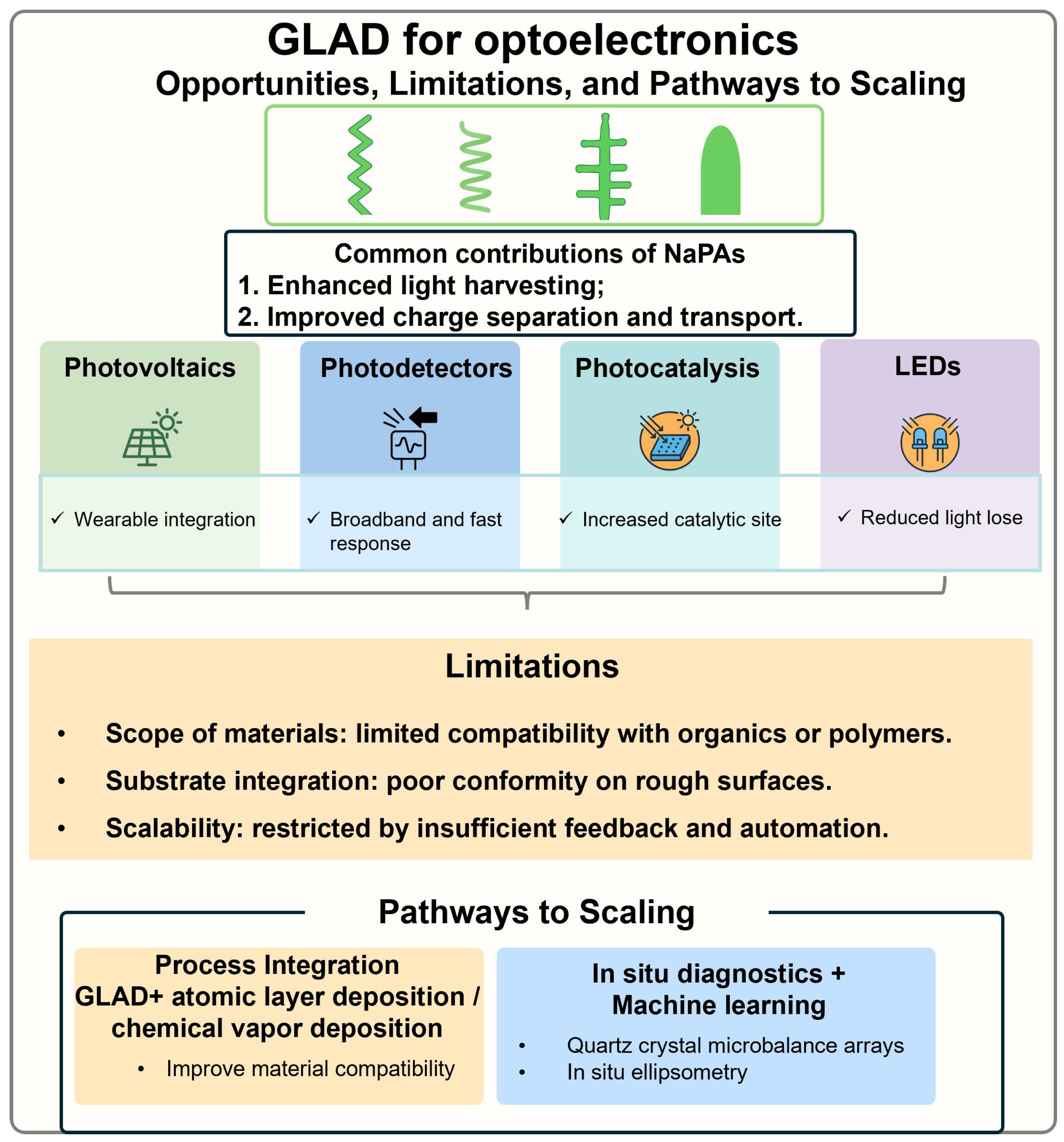
8. Summary and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jung, H.S.; Park, N.-G. Perovskite Solar Cells: From Materials to Devices. Small 2015, 11, 10–25. [Google Scholar] [CrossRef]
- Wöhrle, D.; Meissner, D. Organic Solar Cells. Adv. Mater. 1991, 3, 129–138. [Google Scholar] [CrossRef]
- Kaelin, M.; Rudmann, D.; Tiwari, A.N. Low Cost Processing of Cigs Thin Film Solar Cells. Sol. Energy 2004, 77, 749–756. [Google Scholar] [CrossRef]
- Ramakrishna Reddy, K.T.; Koteswara Reddy, N.; Miles, R.W. Photovoltaic Properties of Sns Based Solar Cells. Sol. Energy Mater. Sol. Cells 2006, 90, 3041–3046. [Google Scholar] [CrossRef]
- Saran, R.; Curry, R.J. Lead Sulphide Nanocrystal Photodetector Technologies. Nat. Photonics 2016, 10, 81–92. [Google Scholar] [CrossRef]
- Long, M.; Wang, P.; Fang, H.; Hu, W. Progress, Challenges, and Opportunities for 2d Material Based Photodetectors. Adv. Funct. Mater. 2019, 29, 1803807. [Google Scholar] [CrossRef]
- Cao, F.; Liu, L.; Li, L. Short-Wave Infrared Photodetector. Mater. Today 2023, 62, 327–349. [Google Scholar] [CrossRef]
- Huang, Y.; Hsiang, E.-L.; Deng, M.-Y.; Wu, S.-T. Mini-Led, Micro-Led and Oled Displays: Present Status and Future Perspectives. Light Sci. Appl. 2020, 9, 105. [Google Scholar] [CrossRef]
- Pimputkar, S.; Speck, J.S.; DenBaars, S.P.; Nakamura, S. Prospects for Led Lighting. Nat. Photonics 2009, 3, 180–182. [Google Scholar] [CrossRef]
- Rappaport, T.S.; Murdock, J.N.; Gutierrez, F. State of the Art in 60-Ghz Integrated Circuits and Systems for Wireless Communications. Proc. IEEE 2011, 99, 1390–1436. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Sun, Z.; Hasan, T.; Ferrari, A.C. Graphene Photonics and Optoelectronics. Nat. Photonics 2010, 4, 611–622. [Google Scholar]
- Soref, R.A. Silicon-Based Optoelectronics. Proc. IEEE 2002, 81, 1687–1706. [Google Scholar] [CrossRef]
- Ostroverkhova, O. Organic Optoelectronic Materials: Mechanisms and Applications. Chem. Rev. 2016, 116, 13279–13412. [Google Scholar] [CrossRef]
- Fröschl, T.; Hörmann, U.; Kubiak, P.; Kučerová, G.; Pfanzelt, M.; Weiss, C.K.; Behm, R.J.; Hüsing, N.; Kaiser, U.; Landfester, K. High Surface Area Crystalline Titanium Dioxide: Potential and Limits in Electrochemical Energy Storage and Catalysis. Chem. Soc. Rev. 2012, 41, 5313–5360. [Google Scholar] [CrossRef]
- Chen, G.; Seo, J.; Yang, C.; Prasad, P.N. Nanochemistry and Nanomaterials for Photovoltaics. Chem. Soc. Rev. 2013, 42, 8304–8338. [Google Scholar] [CrossRef] [PubMed]
- Roduner, E. Size Matters: Why Nanomaterials Are Different. Chem. Soc. Rev. 2006, 35, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pepin, A. Nanofabrication: Conventional and Nonconventional Methods. Electrophoresis 2001, 22, 187–207. [Google Scholar] [CrossRef]
- Nikam, A.; Prasad, B.; Kulkarni, A. Wet Chemical Synthesis of Metal Oxide Nanoparticles: A Review. CrystEngComm 2018, 20, 5091–5107. [Google Scholar] [CrossRef]
- Baldo, M.; Deutsch, M.; Burrows, P.; Gossenberger, H.; Gerstenberg, M.; Ban, V.; Forrest, S. Organic Vapor Phase Deposition. Adv. Mater. 1998, 10, 1505–1514. [Google Scholar] [CrossRef]
- Carlsson, J.-O. Selective Vapor-Phase Deposition on Patterned Substrates. Crit. Rev. Solid State Mater. Sci. 1990, 16, 161–212. [Google Scholar] [CrossRef]
- Lakeman, C.D.; Payne, D.A. Sol-Gel Processing of Electrical and Magnetic Ceramics. Mater. Chem. Phys. 1994, 38, 305–324. [Google Scholar] [CrossRef]
- Ju, D.; Xu, H.; Zhang, J.; Guo, J.; Cao, B. Direct Hydrothermal Growth of Zno Nanosheets on Electrode for Ethanol Sensing. Sens. Actuators B 2014, 201, 444–451. [Google Scholar] [CrossRef]
- Tonelli, D.; Scavetta, E.; Gualandi, I. Electrochemical Deposition of Nanomaterials for Electrochemical Sensing. Sensors 2019, 19, 1186. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Iqbal, M.; Lin, J.; Luo, X.; Jiang, B.; Malgras, V.; Wu, K.C.-W.; Kim, J.; Yamauchi, Y. Electrochemical Deposition: An Advanced Approach for Templated Synthesis of Nanoporous Metal Architectures. Acc. Chem. Res. 2018, 51, 1764–1773. [Google Scholar] [CrossRef]
- Birnie, D., III. Spin Coating Technique. In Sol-Gel Technologies for Glass Producers and Users; Springer: Boston, MA, USA, 2004; pp. 49–55. [Google Scholar]
- Berger, L.-M. Application of Hardmetals as Thermal Spray Coatings. Int. J. Refract. Met. Hard Mater 2015, 49, 350–364. [Google Scholar] [CrossRef]
- Tang, X.; Yan, X. Dip-Coating for Fibrous Materials: Mechanism, Methods and Applications. J. Sol-Gel Sci. Technol. 2017, 81, 378–404. [Google Scholar]
- Hess, D.; Jensen, K.F.; Anderson, T. Chemical Vapor Deposition: A Chemical Engineering Perspective. Rev. Chem. Eng. 1985, 3, 97–186. [Google Scholar] [CrossRef]
- Helmersson, U.; Lattemann, M.; Bohlmark, J.; Ehiasarian, A.P.; Gudmundsson, J.T. Ionized Physical Vapor Deposition (Ipvd): A Review of Technology and Applications. Thin Solid Films 2006, 513, 1–24. [Google Scholar] [CrossRef]
- Johnson, R.W.; Hultqvist, A.; Bent, S.F. A Brief Review of Atomic Layer Deposition: From Fundamentals to Applications. Mater. Today 2014, 17, 236–246. [Google Scholar] [CrossRef]
- Panjan, P.; Drnovšek, A.; Gselman, P.; Čekada, M.; Panjan, M. Review of Growth Defects in Thin Films Prepared by Pvd Techniques. Coatings 2020, 10, 447. [Google Scholar] [CrossRef]
- Huang, Z.F.; Harris, K.D.; Brett, M.J. Morphology Control of Nanotube Arrays. Adv. Mater. 2009, 21, 2983–2987. [Google Scholar] [CrossRef]
- Deng, J.H.; Huang, Z.F. Radiative Loss-Determined Circular Dichroism of Plasmonic Nanospirals with Bendable Stability of Chiroptical Activity. RSC Adv. 2016, 6, 84348–84353. [Google Scholar] [CrossRef]
- Lau, W.F.; Bai, F.; Huang, Z.F. Ballistic Glancing Angle Deposition of Inclined Ag Nanorods Limited by Adatom Diffusion. Nanotechnology 2013, 24, 465707. [Google Scholar] [CrossRef] [PubMed]
- Tait, R.N.; Smy, T.; Brett, M.J. Modelling and Characterization of Columnar Growth in Evaporated Films. Thin Solid Film. 1993, 226, 196–201. [Google Scholar] [CrossRef]
- Alvarez, R.; Lopez-Santos, C.; Parra-Barranco, J.; Rico, V.; Barranco, A.; Cotrino, J.; Gonzalez-Elipe, A.R.; Palmero, A. Nanocolumnar Growth of Thin Films Deposited at Oblique Angles: Beyond the Tangent Rule. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 2014, 32, 041802. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, P.; Lin, K.; Chan, F.H.L.; Gao, Q.; Lau, W.F.; Roy, V.A.L.; Zhang, H.; Lai, K.W.C.; Huang, Z.F.; et al. Extracellular Nanomatrix-Induced Self-Organization of Neural Stem Cells into Miniature Substantia Nigra-Like Structures with Therapeutic Effects on Parkinsonian Rats. Adv. Sci. 2019, 6, 1901822. [Google Scholar] [CrossRef]
- Tam, S.W.; Cheung, A.K.L.; Qin, P.; Zhang, S.; Huang, Z.F.; Yung, K.K.L. Extracellular Silica Nanomatrices Promote in Vitro Maturation of Anti-Tumor Dendritic Cells Via Activation of Focal Adhesion Kinase. Adv. Mater. 2025, 37, 2314358. [Google Scholar] [CrossRef]
- Huang, Z.F.; Hawkeye, M.M.; Brett, M.J. Enhancement in Broadband and Quasi-Omnidirectional Antireflection of Nanopillar Arrays by Ion Milling. Nanotechnology 2012, 23, 275703. [Google Scholar] [CrossRef]
- Huang, Z.F.; Bai, F. Wafer-Scale, Three-Dimensional Helical Porous Thin Films Deposited at a Glancing Angle. Nanoscale 2014, 6, 9401–9409. [Google Scholar] [CrossRef]
- Yang, L.; Ma, Y.; Lin, C.; Qu, G.; Bai, X.; Huang, Z. Nanohelix-Induced Optical Activity of Liquid Metal Nanoparticles. Small 2022, 18, 2200620. [Google Scholar] [CrossRef]
- Borrás, A.; Cotrino, J.; González-Elipe, A.R. Type of Plasmas and Microstructures of TiO2 Thin Films Prepared by Plasma Enhanced Chemical Vapor Deposition. J. Electrochem. Soc. 2007, 154, P152. [Google Scholar] [CrossRef]
- Huang, Z.F.; Liu, J.J. Chiroptically Active Metallic Nanohelices with Helical Anisotropy. Small 2017, 13, 1701883. [Google Scholar] [CrossRef]
- Oliva-Ramirez, M.; González-García, L.; Parra-Barranco, J.; Yubero, F.; Barranco, A.; González-Elipe, A.R. Liquids Analysis with Optofluidic Bragg Microcavities. ACS Appl. Mater. Interfaces 2013, 5, 6743–6750. [Google Scholar] [CrossRef]
- Schubert, M.F.; Mont, F.W.; Chhajed, S.; Poxson, D.J.; Kim, J.K.; Schubert, E.F. Design of Multilayer Antireflection Coatings Made from Co-Sputtered and Low-Refractive-Index Materials by Genetic Algorithm. Opt. Express 2008, 16, 5290–5298. [Google Scholar] [CrossRef]
- Liu, J.J.; Yang, L.; Zhang, H.; Wang, J.F.; Huang, Z.F. Ultraviolet-Visible Chiroptical Activity of Aluminum Nanostructures. Small 2017, 13, 1701112. [Google Scholar] [CrossRef]
- Yang, L.; Nandi, P.; Ma, Y.C.; Liu, J.J.; Mirsaidov, U.; Huang, Z.F. Binary Chiral Nanoparticles Exhibit Amplified Optical Activity and Enhanced Refractive Index Sensitivity. Small 2020, 16, 1906048. [Google Scholar] [CrossRef]
- Ma, Y.C.; Yang, L.; Hu, X.C.; Zhang, M.; Qu, G.P.; Bai, X.P.; Sun, H.F.; Zhu, F.; Zhong, X.Y.; Chen, X.; et al. Multielementary Alloy Chiral Nanoparticles with Strong Optical Activities. Adv. Opt. Mater. 2023, 11, 2300696. [Google Scholar] [CrossRef]
- Liu, J.J.; Ni, Z.Y.; Nandi, P.; Mirsaidov, U.; Huang, Z.F. Chirality Transfer in Galvanic Replacement Reactions. Nano Lett. 2019, 19, 7427–7433. [Google Scholar] [CrossRef]
- Ni, Z.Y.; Zhu, Y.M.; Liu, J.J.; Yang, L.; Sun, P.; Gu, M.; Huang, Z.F. Extension of Compositional Space to the Ternary in Alloy Chiral Nanoparticles through Galvanic Replacement Reactions. Adv. Sci. 2020, 7, 2001321. [Google Scholar] [CrossRef]
- Liu, Y.; Rzhevskii, A.; Rigos, S.; Xie, W.Y.; Zhang, S.B.; Lu, T.M.; Wang, G.C. A Study of Parylene Coated Pd/Mg Nanoblabes for Reversible Hydrogen Storage. Int. J. Hydrog. Energy 2013, 38, 5019–5029. [Google Scholar] [CrossRef]
- Shougaijam, B.; Singh, S.S. Enhanced Dye-Sensitized Solar Cell Performance Using Ag Nanoparticle-Decorated TiO2 Nanowire Photoanode Using the Glad Technique. Opt. Quantum Electron. 2024, 56, 1434. [Google Scholar] [CrossRef]
- Yadav, J.; Raturi, P.; Yadav, S.; Singh, J.P. Zig-Zag Ag2s Nanostructures for Superior Optical Absorption and Photoelectrochemical Water Splitting Performance. Renew. Energy 2021, 179, 2256–2266. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, P.; Wu, Z.; Li, J.; Wang, X.; Xiao, T.; Yang, L.; Zheng, Z.; Huang, Z. Titanium Nanopillar Arrays Functioning as Electron Transporting Layers for Efficient, Anti-Aging Perovskite Solar Cells. Small 2021, 17, 2004778. [Google Scholar] [CrossRef]
- Pihosh, Y.; Turkevych, I.; Ye, J.; Goto, M.; Kasahara, A.; Kondo, M.; Tosa, M. Photocatalytic Properties of TiO2 Nanostructures Fabricated by Means of Glancing Angle Deposition and Anodization. J. Electrochem. Soc. 2009, 156, K160. [Google Scholar] [CrossRef]
- Manas, S.H.; Choudhuri, B.; Chinnamuthu, P. Reduction of Interface State Density in Coaxial Tio2/Ge Nanowire Assembly-Based Heterostructure and Superior Photodetection. J. Alloys Compd. 2021, 853, 157344. [Google Scholar] [CrossRef]
- Pihosh, Y.; Nandal, V.; Minegishi, T.; Katayama, M.; Yamada, T.; Seki, K.; Sugiyama, M.; Domen, K. Development of a Core–Shell Heterojunction Ta3N5-Nanorods/BaTaO2N Photoanode for Solar Water Splitting. ACS Energy Lett. 2020, 5, 2492–2497. [Google Scholar] [CrossRef]
- Wu, S.; Ou, K.; Zhang, W.; Ni, Y.; Tang, Y.; Xia, Y.; Wang, H. Fe2O3/TiO2/WO3/Ti3C2Tx Heterojunction Composite Material for Efficient Photoelectrochemical Water Splitting. Appl. Phys. A 2024, 130, 155. [Google Scholar] [CrossRef]
- Wattanawikkam, C.; Bootchanont, A.; Porjai, P.; Jetjamnong, C.; Kowong, R.; Lertvanithphol, T.; Chananonnawathorn, C.; Chirawatkul, P.; Chanlek, N.; Nakajima, H.; et al. Phase Evolution in Annealed Ni-Doped WO3 Nanorod Films Prepared Via a Glancing Angle Deposition Technique for Enhanced Photoelectrochemical Performance. Appl. Surf. Sci. 2022, 584, 152581. [Google Scholar] [CrossRef]
- González-García, L.; González-Valls, I.; Lira-Cantu, M.; Barranco, A.; González-Elipe, A.R. Aligned TiO2 Nanocolumnar Layers Prepared by Pvd-Glad for Transparent Dye Sensitized Solar Cells. Energy Environ. Sci. 2011, 4, 3426–3435. [Google Scholar] [CrossRef]
- Chen, C.-W.; Tsai, H.-W.; Wang, Y.-C.; Su, T.-Y.; Yang, C.-H.; Lin, W.-S.; Lin, Z.-H.; Huang, J.-S.; Chueh, Y.-L. Design of Novel TiO2–SiO2 Core–Shell Helical Nanostructured Anti-Reflective Coatings on Cu(in,Ga)Se2 Solar Cells with Enhanced Power Conversion Efficiency. J. Mater. Chem. A 2019, 7, 11452–11459. [Google Scholar] [CrossRef]
- Lee, J.-W.; Lee, S.H.; Ko, H.-S.; Kwon, J.; Park, J.H.; Kang, S.M.; Ahn, N.; Choi, M.; Kim, J.K.; Park, N.-G. Opto-Electronic Properties of TiO2 Nanohelices with Embedded HC(NH2)2PbI3 Perovskite Solar Cells. J. Mater. Chem. A 2015, 3, 9179–9186. [Google Scholar] [CrossRef]
- Pan, B.; Gu, J.; Xu, X.; Xiao, L.; Zhao, J.; Zou, G. Interface Engineering of High Performance All-Inorganic Perovskite Solar Cells Via Low-Temperature Processed TiO2 Nanopillar Arrays. Nano Res. 2021, 14, 3431–3438. [Google Scholar] [CrossRef]
- Xiao, T.; Zhao, J.; Sun, P.; Li, P.; Zhang, Y.; Zhao, N.; Ren, Z.; Li, G.; Huang, Z.; Zheng, Z. Sensitive, High-Speed, and Broadband Perovskite Photodetectors with Built-in TiO2 Metalenses. Small 2021, 17, 2102694. [Google Scholar] [CrossRef]
- Chakrabartty, S.; Mondal, A.; Sarkar, M.B.; Choudhuri, B.; Saha, A.K.; Bhattacharyya, A. TiO2 Nanoparticles Arrays Ultraviolet-a Detector with Au Schottky Contact. IEEE Photonics Technol. Lett. 2014, 26, 1065–1068. [Google Scholar] [CrossRef]
- Mondal, S.; Dalal, A.; Mondal, A. Al Nanoparticles Decorated Er:TiO2 Thin Film Based Plasmonic Photodetector. Ceram. Int. 2023, 49, 6289–6298. [Google Scholar] [CrossRef]
- Choudhuri, B.; Mondal, A.; Dwivedi, S.M.M.D.; Henini, M. Fabrication of Novel Transparent Co3O4-TiO2 Nanowires P-N Heterojunction Diodes for Multiband Photodetection Applications. J. Alloys Compd. 2017, 712, 7–14. [Google Scholar] [CrossRef]
- Yasuda, Y.; Kobayashi, S.-I.; Hoshi, Y. Photocatalytic Properties of Annealed Tio2 Films with Controlled Structure Fabricated Using Oxygen-Ion-Assisted Reactive Evaporation with Glancing Angle Deposition Technique. AIP Adv. 2022, 12, 015016. [Google Scholar] [CrossRef]
- Taschuk, M.T.; Steele, J.J.; van Popta, A.C.; Brett, M.J. Photocatalytic Regeneration of Interdigitated Capacitor Relative Humidity Sensors Fabricated by Glancing Angle Deposition. Sens. Actuators, B 2008, 134, 666–671. [Google Scholar] [CrossRef]
- Yao, K.; Basnet, P.; Sessions, H.; Larsen, G.K.; Murph, S.E.H.; Zhao, Y. Fe2O3–TiO2 Core–Shell Nanorod Arrays for Visible Light Photocatalytic Applications. Catal. Today 2016, 270, 51–58. [Google Scholar] [CrossRef]
- Wang, B.; Qi, H.; Liu, Z.; Jin, Y.; Wang, H.; Yuan, J.; Zhao, J.; Shao, J. Structure, Chemical State and Photocatalytic Activity of TiO2−X Nanostructured Thin Films by Glancing Angle Deposition Technique. J. Alloys Compd. 2017, 716, 299–305. [Google Scholar] [CrossRef]
- Wei, X.; Ou, K.; Wang, J.; Li, K.; Wu, S.; Zhang, W.; Xia, Y.; Wang, H. Novel Visible Light-Induced ZnSe/TiO2 Nanorod Heterojunction for Efficient Photocatalysis and Degradation of Methyl Orange. J. Mater. Sci. Mater. Electron. 2023, 34, 1339. [Google Scholar]
- Kwon, H.; Ham, J.; Kim, D.Y.; Oh, S.J.; Lee, S.; Oh, S.H.; Schubert, E.F.; Lim, K.-G.; Lee, T.-W.; Kim, S.; et al. Three-Dimensional Nanostructured Indium-Tin-Oxide Electrodes for Enhanced Performance of Bulk Heterojunction Organic Solar Cells. Adv. Energy Mater. 2014, 4, 1301566. [Google Scholar]
- Cao, B.; He, X.; Sorge, J.B.; Lalany, A.; Ahadi, K.; Afshar, A.; Olsen, B.C.; Hauger, T.C.; Mobarok, M.H.; Li, P.; et al. Understanding the Effects of a High Surface Area Nanostructured Indium Tin Oxide Electrode on Organic Solar Cell Performance. ACS Appl. Mater. Interfaces 2017, 9, 38706–38715. [Google Scholar] [CrossRef]
- Oh, S.J.; Go, G.; Lee, J.-L.; Schubert, E.F.; Cho, J.; Kim, J.K. Enhanced Phosphor Conversion Efficiency of Gan-Based White Light-Emitting Diodes Having Dichroic-Filtering Contacts. J. Mater. Chem. C 2013, 1, 5733–5740. [Google Scholar][Green Version]
- Chiu, C.H.; Yu, P.; Chang, C.H.; Yang, C.S.; Hsu, M.H.; Kuo, H.C.; Tsai, M.A. Oblique Electron-Beam Evaporation of Distinctive Indium-Tin-Oxide Nanorods for Enhanced Light Extraction from Ingan/Gan Light Emitting Diodes. Opt. Express 2009, 17, 21250–21256. [Google Scholar][Green Version]
- Kim, J.K.; Chhajed, S.; Schubert, M.F.; Schubert, E.F.; Fischer, A.J.; Crawford, M.H.; Cho, J.; Kim, H.; Sone, C. Light-Extraction Enhancement of Gainn Light-Emitting Diodes by Graded-Refractive-Index Indium Tin Oxide Anti-Reflection Contact. Adv. Mater. 2008, 20, 801–804. [Google Scholar][Green Version]
- Ferhati, H.; Martin, N.; Djeffal, F. Boosting the Efficiency of Sns Solar Cells through Reactively Sputter-Deposited Ag-Nanostructured Layer/SnO2 Film at Glancing Angles. Plasmonics 2025, 20, 741–752. [Google Scholar] [CrossRef]
- Sun, P.; Qu, G.; Hu, Q.; Ma, Y.; Liu, H.; Xu, Z.-X.; Huang, Z. Highly Efficient Large-Area Flexible Perovskite Solar Cells Containing Tin Oxide Vertical Nanopillars without Oxygen Vacancies. ACS Appl. Energy Mater. 2022, 5, 3568–3577. [Google Scholar] [CrossRef]
- Ferhati, H.; Djeffal, F.; Martin, N.; Benhaya, A. Tunable Properties of Snox Sputter-Deposited by Rgpp and Glad Techniques: A Potential Candidate for Photosensing and All-Oxide Solar Cells. Sol. Energy 2024, 268, 112305. [Google Scholar] [CrossRef]
- Guo, X.; Ying, Z.; Li, X.; Zhang, M.; Su, S.; Zheng, J.; Du, H.; Sun, Y.; Wu, J.; Liu, L.; et al. Oblique-Angle Damage-Free Evaporation of Silicon Oxide Electron-Selective Passivation Contacts for Efficient and Stable Perovskite and Perovskite/Topcon Tandem Solar Cells. Adv. Energy Mater. 2025, 15, 2403021. [Google Scholar]
- Mondal, A.; Singh, N.K.; Chinnamuthu, P.; Dhar, J.C.; Bhattacharyya, A.; Choudhury, S. Enlarged Photodetection Using SiOx Nanowire Arrays. IEEE Photonics Technol. Lett. 2012, 24, 2020–2023. [Google Scholar] [CrossRef]
- Adhikari, D.; Junda, M.M.; Bastola, E.; Koirala, P.; Ellingson, R.J.; Collins, R.W.; Podraza, N.J. Glancing Angle Deposited Cdte: Nanostructured Films and Impact on Solar Cell Performance. Surf. Coat. Technol. 2020, 381, 125127. [Google Scholar] [CrossRef]
- Cong, S.; Zou, G.; Lou, Y.; Yang, H.; Su, Y.; Zhao, J.; Zhang, C.; Ma, P.; Lu, Z.; Fan, H.; et al. Fabrication of Nickel Oxide Nanopillar Arrays on Flexible Electrodes for Highly Efficient Perovskite Solar Cells. Nano Lett. 2019, 19, 3676–3683. [Google Scholar] [CrossRef]
- Pooja, P.; Chinnamuthu, P. Robust Ultraviolet Photodetection by Tailored Polycrystalline In2o3 Nanowire Synthesized Using Glancing Angle Deposition. IEEE Sens. J. 2021, 21, 13192–13199. [Google Scholar] [CrossRef]
- Liu, X.; Guo, X.; Lv, Y.; Hu, Y.; Lin, J.; Fan, Y.; Zhang, N.; Liu, X. Enhanced Performance and Flexibility of Perovskite Solar Cells Based on Microstructured Multilayer Transparent Electrodes. ACS Appl. Mater. Interfaces 2018, 10, 18141–18148. [Google Scholar] [CrossRef]
- Choudhury, A.; Biswas, I.; Gupta, R.; Dey, A.; Mondal, A. Glad Synthesized Ga2O3 Nanowire-Based Photodiode. Appl. Phys. A 2024, 130, 752. [Google Scholar] [CrossRef]
- Meitei, P.N.; Singh, N.K. Self-Powered Photodetector Based on a Ag Nanoparticle-Decorated Gd2O3 Nanorod. ACS Appl. Electron. Mater. 2023, 5, 1521–1525. [Google Scholar] [CrossRef]
- Raman, R.; Nath, A.; Sarkar, M.B. Influence of Annealing Temperature on ZrO2 Nanoparticles for Improved Photodetection. IEEE Electron Device Lett. 2022, 43, 918–921. [Google Scholar] [CrossRef]
- Devi, N.M.; BaQais, A.; Debnath, A.K.; Alam, M.W.; Singh, N.K. Improved Photodetection Capabilities of Ag@CeO2 Nanorod Composite Array Using Glad Technique. Ceram. Int. 2022, 48, 30107–30117. [Google Scholar] [CrossRef]
- Soni, A.; Mulchandani, K.; Mavani, K.R. Uv Activated Visible-Blind Ga:Zno Photodetectors Using the Glad Technique: A Comparative Study in Different Gas Atmospheres and Temperatures. J. Mater. Chem. C 2020, 8, 7837–7846. [Google Scholar] [CrossRef]
- Wolcott, A.; Smith, W.A.; Kuykendall, T.R.; Zhao, Y.; Zhang, J.Z. Photoelectrochemical Study of Nanostructured ZnO Thin Films for Hydrogen Generation from Water Splitting. Adv. Funct. Mater. 2009, 19, 1849–1856. [Google Scholar] [CrossRef]
- Swain, S.; Thakur, I.; Chatterjee, S.; Kulkarni, N.A.; Ayyub, P.; Chaudhary, Y.S. Array of Cu2O Nano-Columns Fabricated by Oblique Angle Sputter Deposition and Their Application in Photo-Assisted Proton Reduction. J. Appl. Phys. 2015, 117, 024303. [Google Scholar] [CrossRef]
- Singh, S.S.; Jamir, A.; Longkumer, B.; Devi, N.M.; Shougaijam, B.; Singh, N.K. Fabrication of Cu2O Nanorod Using Glancing Angle Deposition Technique for Photodetector Application. Appl. Phys. A 2024, 130, 837. [Google Scholar] [CrossRef]
- Brozak, M.; Cansizoglu, H.; Karabacak, T. Deposition of Quaternary Sputtered Cigs Nanorods Via Glancing Angle Deposition. Phys. Status Solidi (RRL)—Rapid Res. Lett. 2017, 11, 1600326. [Google Scholar] [CrossRef]
- Castillo-Seoane, J.; Contreras-Bernal, L.; Obrero-Perez, J.M.; García-Casas, X.; Lorenzo-Lázaro, F.; Aparicio, F.J.; Lopez-Santos, C.; Rojas, T.C.; Anta, J.A.; Borrás, A.; et al. Highly Anisotropic Organometal Halide Perovskite Nanowalls Grown by Glancing-Angle Deposition. Adv. Mater. 2022, 34, 2107739. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-C.; Tien, C.-H.; Liu, X.; Xu, B. Zigzag and Helical Aln Layer Prepared by Glancing Angle Deposition and Its Application as a Buffer Layer in a Gan-Based Light-Emitting Diode. J. Nanomater. 2012, 2012, 409123. [Google Scholar] [CrossRef]
- Yan, X.; Shatalov, M.; Saxena, T.; Shur, M.S. Deep-Ultraviolet Tailored- and Low-Refractive Index Antireflection Coatings for Light-Extraction Enhancement of Light Emitting Diodes. J. Appl. Phys. 2013, 113, 163105. [Google Scholar] [CrossRef]
- Lee, H.K.; Kim, M.S.; Yu, J.S. Improved Device Performance of Algainp-Based Vertical Light-Emitting Diodes with Low-N Ato Antireflective Coating Layer. Microelectron. Eng. 2013, 104, 29–32. [Google Scholar] [CrossRef]
- Van Dijken, J.G.; Fleischauer, M.D.; Brett, M.J. Controlled Nanostructuring of Cupc Thin Films Via Glancing Angle Deposition for Idealized Organic Photovoltaic Architectures. J. Mater. Chem. 2011, 21, 1013–1019. [Google Scholar] [CrossRef]
- Jiang, Q.; Xing, Y. Improved Performance of Small Molecule Organic Solar Cells by Incorporation of a Glancing Angle Deposited Donor Layer. Sci. Rep. 2020, 10, 5766. [Google Scholar] [CrossRef]
- Robbie, K.; Sit, J.; Brett, M. Advanced Techniques for Glancing Angle Deposition. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 1998, 16, 1115–1122. [Google Scholar]
- Rahchamani, S.Z.; Rezagholipour Dizaji, H.; Ehsani, M.H. Study of Structural and Optical Properties of Zns Zigzag Nanostructured Thin Films. Appl. Surf. Sci. 2015, 356, 1096–1104. [Google Scholar] [CrossRef]
- Liu, J.; Feng, X. Synthetic Tailoring of Graphene Nanostructures with Zigzag-Edged Topologies: Progress and Perspectives. Angew. Chem. Int. Ed. 2020, 59, 23386–23401. [Google Scholar] [CrossRef]
- Beaudry, A.L.; LaForge, J.M.; Tucker, R.T.; Sorge, J.B.; Adamski, N.L.; Li, P.; Taschuk, M.T.; Brett, M.J. Directed Branch Growth in Aligned Nanowire Arrays. Nano Lett. 2014, 14, 1797–1803. [Google Scholar] [CrossRef]
- Gish, D.A.; Nsiah, F.; McDermott, M.T.; Brett, M.J. Localized Surface Plasmon Resonance Biosensor Using Silver Nanostructures Fabricated by Glancing Angle Deposition. Anal. Chem. 2007, 79, 4228–4232. [Google Scholar] [CrossRef]
- He, Y.; Fu, J.; Zhao, Y. Oblique Angle Deposition and Its Applications in Plasmonics. Front. Phys. 2014, 9, 47–59. [Google Scholar] [CrossRef]
- Ren, H.; Chen, J.-D.; Li, Y.-Q.; Tang, J.-X. Recent Progress in Organic Photodetectors and Their Applications. Adv. Sci. 2021, 8, 2002418. [Google Scholar] [CrossRef]
- Huo, N.; Konstantatos, G. Recent Progress and Future Prospects of 2d-Based Photodetectors. Adv. Mater. 2018, 30, 1801164. [Google Scholar] [CrossRef]
- Konstantatos, G.; Sargent, E.H. Nanostructured Materials for Photon Detection. Nat. Nanotechnol. 2010, 5, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Ravi Anusuyadevi, P.; Aymonier, C.; Luque, R.; Marre, S. Nanostructured Materials for Photocatalysis. Chem. Soc. Rev. 2019, 48, 3868–3902. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.H. Leds for Solid-State Lighting: Performance Challenges and Recent Advances. IEEE J. Sel. Top. Quantum Electron. 2009, 15, 1028–1040. [Google Scholar] [CrossRef]
- Hamidnia, M.; Luo, Y.; Wang, X.D. Application of Micro/Nano Technology for Thermal Management of High Power Led Packaging—A Review. Appl. Therm. Eng. 2018, 145, 637–651. [Google Scholar] [CrossRef]
- Bergh, A.A.; Dean, P.J. Light-Emitting Diodes. Proc. IEEE 1972, 60, 156–223. [Google Scholar] [CrossRef]
- Xi, J.-Q.; Schubert, M.F.; Kim, J.K.; Schubert, E.F.; Chen, M.; Lin, S.-Y.; Liu, W.; Smart, J.A. Optical Thin-Film Materials with Low Refractive Index for Broadband Elimination of Fresnel Reflection. Nat. Photonics 2007, 1, 176–179. [Google Scholar] [CrossRef]
- Ho, P.K.; Kim, J.-S.; Burroughes, J.H.; Becker, H.; Li, S.F.; Brown, T.M.; Cacialli, F.; Friend, R.H. Molecular-Scale Interface Engineering for Polymer Light-Emitting Diodes. Nature 2000, 404, 481–484. [Google Scholar] [CrossRef]
- Lee, S.; Wang, Y.; Hou, X.; Tang, C. Interfacial Electronic Structures in an Organic Light-Emitting Diode. Appl. Phys. Lett. 1999, 74, 670–672. [Google Scholar] [CrossRef]
- Rhee, S.; Hahm, D.; Seok, H.-J.; Chang, J.H.; Jung, D.; Park, M.; Hwang, E.; Lee, D.C.; Park, Y.-S.; Kim, H.-K. Steering Interface Dipoles for Bright and Efficient All-Inorganic Quantum Dot Based Light-Emitting Diodes. ACS Nano 2021, 15, 20332–20340. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Yu, K.; Someya, T. The Future of Flexible Organic Solar Cells. Adv. Energy Mater. 2020, 10, 2000765. [Google Scholar] [CrossRef]
- Wang, R.; Mujahid, M.; Duan, Y.; Wang, Z.-K.; Xue, J.; Yang, Y. A Review of Perovskites Solar Cell Stability. Adv. Funct. Mater. 2019, 29, 1808843. [Google Scholar] [CrossRef]
- Lee, T.D.; Ebong, A.U. A Review of Thin Film Solar Cell Technologies and Challenges. Renew. Sustain. Energy Rev. 2017, 70, 1286–1297. [Google Scholar] [CrossRef]
- Walheim, S.; Schäffer, E.; Mlynek, J.; Steiner, U. Nanophase-Separated Polymer Films as High-Performance Antireflection Coatings. Science 1999, 283, 520–522. [Google Scholar] [CrossRef]
- Malinkiewicz, O.; Yella, A.; Lee, Y.H.; Espallargas, G.M.; Graetzel, M.; Nazeeruddin, M.K.; Bolink, H.J. Perovskite Solar Cells Employing Organic Charge-Transport Layers. Nat. Photonics 2014, 8, 128–132. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, S.; Xie, Z.; Walsh, A. Point Defect Engineering in Thin-Film Solar Cells. Nat. Rev. Mater. 2018, 3, 194–210. [Google Scholar] [CrossRef]
- Ball, J.M.; Petrozza, A. Defects in Perovskite-Halides and Their Effects in Solar Cells. Nat. Energy 2016, 1, 16149. [Google Scholar] [CrossRef]
- Tsakalakos, L. Nanostructures for Photovoltaics. Mater. Sci. Eng. R Rep. 2008, 62, 175–189. [Google Scholar] [CrossRef]
- Narasimhan, V.K.; Cui, Y. Nanostructures for Photon Management in Solar Cells. Nanophotonics 2013, 2, 187–210. [Google Scholar] [CrossRef]
- Nozik, A.J. Nanoscience and Nanostructures for Photovoltaics and Solar Fuels. Nano Lett. 2010, 10, 2735–2741. [Google Scholar] [CrossRef]
- Bierman, M.J.; Jin, S. Potential Applications of Hierarchical Branching Nanowires in Solar Energy Conversion. Energy Environ. Sci. 2009, 2, 1050–1059. [Google Scholar] [CrossRef]
- Dong, Q.; Chen, M.; Liu, Y.; Eickemeyer, F.T.; Zhao, W.; Dai, Z.; Yin, Y.; Jiang, C.; Feng, J.; Jin, S.; et al. Flexible Perovskite Solar Cells with Simultaneously Improved Efficiency, Operational Stability, and Mechanical Reliability. Joule 2021, 5, 1587–1601. [Google Scholar] [CrossRef]
- Savagatrup, S.; Printz, A.D.; O’Connor, T.F.; Zaretski, A.V.; Rodriquez, D.; Sawyer, E.J.; Rajan, K.M.; Acosta, R.I.; Root, S.E.; Lipomi, D.J. Mechanical Degradation and Stability of Organic Solar Cells: Molecular and Microstructural Determinants. Energy Environ. Sci. 2015, 8, 55–80. [Google Scholar] [CrossRef]
- Reza Reisi, A.; Hassan Moradi, M.; Jamasb, S. Classification and Comparison of Maximum Power Point Tracking Techniques for Photovoltaic System: A Review. Renew. Sustain. Energy Rev. 2013, 19, 433–443. [Google Scholar] [CrossRef]
- Yang, Z.; Jiang, Y.; Wang, Y.; Li, G.; You, Q.; Wang, Z.; Gao, X.; Lu, X.; Shi, X.; Zhou, G.; et al. Supramolecular Polyurethane “Ligaments” Enabling Room-Temperature Self-Healing Flexible Perovskite Solar Cells and Mini-Modules. Small 2024, 20, 2307186. [Google Scholar]
- Yang, Z.; Wei, J.; Liu, Y.; Jiang, Y.; Liu, L.; Li, Y.; Yu, S.; Xing, Z.; Wang, Z.; Chen, Y.; et al. Radical P-Doping Spiro-Ometad for Efficient, Stable and Self-Healing Flexible Perovskite Solar Cells. Adv. Mater. 2025, 37, 2417404. [Google Scholar] [CrossRef]
- Granqvist, C.G. Solar Energy Materials. Adv. Mater. 2003, 15, 1789–1803. [Google Scholar] [CrossRef]
- Gao, M.; Zhu, L.; Peh, C.K.; Ho, G.W. Solar Absorber Material and System Designs for Photothermal Water Vaporization Towards Clean Water and Energy Production. Energy Environ. Sci. 2019, 12, 841–864. [Google Scholar] [CrossRef]
- Wenham, S.R.; Green, M.A. Silicon Solar Cells. Prog. Photovoltaics Res. Appl. 1996, 4, 3–33. [Google Scholar]
- Radziemska, E. Thermal Performance of Si and Gaas Based Solar Cells and Modules: A Review. Prog. Energy Combust. Sci. 2003, 29, 407–424. [Google Scholar] [CrossRef]
- Wang, J.; Feng, Y.; He, Y. Advancements in Recycling Technologies for Waste Cigs Photovoltaic Modules. Nano Energy 2024, 128, 109847. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, Q.; Ding, Y.; Hou, G.; Zhao, Y. Passivating Contacts for High-Efficiency Silicon-Based Solar Cells: From Single-Junction to Tandem Architecture. Nano Energy 2022, 92, 106712. [Google Scholar] [CrossRef]
- Ramanujam, J.; Bishop, D.M.; Todorov, T.K.; Gunawan, O.; Rath, J.; Nekovei, R.; Artegiani, E.; Romeo, A. Flexible Cigs, Cdte and a-Si:H Based Thin Film Solar Cells: A Review. Prog. Mater Sci. 2020, 110, 100619. [Google Scholar]
- Wei, H.; Li, Y.; Cui, C.; Wang, X.; Shao, Z.; Pang, S.; Cui, G. Defect Suppression for High-Efficiency Kesterite Cztsse Solar Cells: Advances and Prospects. Chem. Eng. J. 2023, 462, 142121. [Google Scholar] [CrossRef]
- Ferekides, C.S.; Balasubramanian, U.; Mamazza, R.; Viswanathan, V.; Zhao, H.; Morel, D.L. CdTe Thin Film Solar Cells: Device and Technology Issues. Sol. Energy 2004, 77, 823–830. [Google Scholar] [CrossRef]
- Massiot, I.; Cattoni, A.; Collin, S. Progress and Prospects for Ultrathin Solar Cells. Nat. Energy 2020, 5, 959–972. [Google Scholar] [CrossRef]
- Zi, Y.; Zhu, J.; Hu, L.; Wang, M.; Huang, W. Nanoengineering of Tin Monosulfide (Sns)-Based Structures for Emerging Applications. Small Sci. 2022, 2, 2100098. [Google Scholar] [CrossRef]
- Sugathan, V.; John, E.; Sudhakar, K. Recent Improvements in Dye Sensitized Solar Cells: A Review. Renew. Sustain. Energy Rev. 2015, 52, 54–64. [Google Scholar] [CrossRef]
- Kippelen, B.; Brédas, J.-L. Organic Photovoltaics. Energy Environ. Sci. 2009, 2, 251–261. [Google Scholar] [CrossRef]
- Mazzio, K.A.; Luscombe, C.K. The Future of Organic Photovoltaics. Chem. Soc. Rev. 2015, 44, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, J.-W.; Jung, H.S.; Shin, H.; Park, N.-G. High-Efficiency Perovskite Solar Cells. Chem. Rev. 2020, 120, 7867–7918. [Google Scholar] [CrossRef]
- Miyasaka, T.; Kulkarni, A.; Kim, G.M.; Öz, S.; Jena, A.K. Perovskite Solar Cells: Can We Go Organic-Free, Lead-Free, and Dopant-Free? Adv. Energy Mater. 2020, 10, 1902500. [Google Scholar] [CrossRef]
- Li, Y. Molecular Design of Photovoltaic Materials for Polymer Solar Cells: Toward Suitable Electronic Energy Levels and Broad Absorption. Acc. Chem. Res. 2012, 45, 723–733. [Google Scholar]
- Zhou, Y.; Taima, T.; Miyadera, T.; Yamanari, T.; Kitamura, M.; Nakatsu, K.; Yoshida, Y. Glancing Angle Deposition of Copper Iodide Nanocrystals for Efficient Organic Photovoltaics. Nano Lett. 2012, 12, 4146–4152. [Google Scholar] [CrossRef] [PubMed]
- Gospodinova, N.; Tomšík, E. Hydrogen-Bonding Versus Π–Π Stacking in the Design of Organic Semiconductors: From Dyes to Oligomers. Prog. Polym. Sci. 2015, 43, 33–47. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, T.; Wang, Q.; Heinz Mayrhofer, P. Nanostructured Zig-Zag Γ-MO2N Thin Films Produced by Glancing Angle Deposition for Flexible Symmetrical Solid-State Supercapacitors. Mater. Des. 2023, 225, 111432. [Google Scholar] [CrossRef]
- Pedrosa, P.; Machado, D.; Fiedler, P.; Vasconcelos, B.; Alves, E.; Barradas, N.P.; Martin, N.; Haueisen, J.; Vaz, F.; Fonseca, C. Electrochemical Characterization of Nanostructured Ag:Tin Thin Films Produced by Glancing Angle Deposition on Polyurethane Substrates for Bio-Electrode Applications. J. Electroanal. Chem. 2016, 768, 110–120. [Google Scholar] [CrossRef]
- Karabacak, T. Thin-Film Growth Dynamics with Shadowing and Re-Emission Effects. J. Nanophotonics 2011, 5, 052501–052518. [Google Scholar] [CrossRef]
- Leem, J.W.; Yu, J.S. Glancing Angle Deposited Ito Films for Efficiency Enhancement of a-Si:H/Μc-Si:H Tandem Thin Film Solar Cells. Opt. Express 2011, 19, A258–A269. [Google Scholar] [CrossRef]
- Beaudry, A.L.; Tucker, R.T.; LaForge, J.M.; Taschuk, M.T.; Brett, M.J. Indium Tin Oxide Nanowhisker Morphology Control by Vapour–Liquid–Solid Glancing Angle Deposition. Nanotechnology 2012, 23, 105608. [Google Scholar] [CrossRef] [PubMed]
- Tucker, R.T.; Beaudry, A.L.; LaForge, J.M.; Taschuk, M.T.; Brett, M.J. A Little Ribbing: Flux Starvation Engineering for Rippled Indium Tin Oxide Nanotree Branches. Appl. Phys. Lett. 2012, 101, 193101. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Huang, Y.F.; Jen, Y.J.; Ganguly, A.; Chen, K.H.; Chen, L.C. Anti-Reflecting and Photonic Nanostructures. Mater. Sci. Eng. R Rep. 2010, 69, 1–35. [Google Scholar] [CrossRef]
- Yu, P.; Chang, C.-H.; Chiu, C.-H.; Yang, C.-S.; Yu, J.-C.; Kuo, H.-C.; Hsu, S.-H.; Chang, Y.-C. Efficiency Enhancement of Gaas Photovoltaics Employing Antireflective Indium Tin Oxide Nanocolumns. Adv. Mater. 2009, 21, 1618–1621. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, D.; Wang, K.; Yang, Q.; Qian, J.; Zhou, J.; Liu, S.; Yang, D. Lattice Mismatch at the Heterojunction of Perovskite Solar Cells. Angew. Chem. Int. Ed. 2024, 63, e202405878. [Google Scholar]
- Ran, C.; Xu, J.; Gao, W.; Huang, C.; Dou, S. Defects in Metal Triiodide Perovskite Materials Towards High-Performance Solar Cells: Origin, Impact, Characterization, and Engineering. Chem. Soc. Rev. 2018, 47, 4581–4610. [Google Scholar] [CrossRef]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The Emergence of Perovskite Solar Cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Park, N.-G. Perovskite Solar Cells: An Emerging Photovoltaic Technology. Mater. Today 2015, 18, 65–72. [Google Scholar] [CrossRef]
- Jena, A.K.; Kulkarni, A.; Miyasaka, T. Halide Perovskite Photovoltaics: Background, Status, and Future Prospects. Chem. Rev. 2019, 119, 3036–3103. [Google Scholar] [CrossRef]
- Kovalenko, M.V.; Protesescu, L.; Bodnarchuk, M.I. Properties and Potential Optoelectronic Applications of Lead Halide Perovskite Nanocrystals. Science 2017, 358, 745–750. [Google Scholar] [CrossRef]
- Fang, Y.; Jiang, Y.; Yang, Z.; Xu, Z.; Wang, Z.; Lu, X.; Gao, X.; Zhou, G.; Liu, J.-M.; Gao, J. A Nontoxic Nfm Solvent for High-Efficiency Perovskite Solar Cells with a Widened Processing Window. ACS Appl. Mater. Interfaces 2022, 14, 47758–47764. [Google Scholar] [CrossRef]
- Li, N.; Niu, X.; Chen, Q.; Zhou, H. Towards Commercialization: The Operational Stability of Perovskite Solar Cells. Chem. Soc. Rev. 2020, 49, 8235–8286. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Li, M.; Siffalovic, P.; Cao, G.; Tian, J. From Scalable Solution Fabrication of Perovskite Films Towards Commercialization of Solar Cells. Energy Environ. Sci. 2019, 12, 518–549. [Google Scholar]
- Rider, D.A.; Tucker, R.T.; Worfolk, B.J.; Krause, K.M.; Lalany, A.; Brett, M.J.; Buriak, J.M.; Harris, K.D. Indium Tin Oxide Nanopillar Electrodes in Polymer/Fullerene Solar Cells. Nanotechnology 2011, 22, 085706. [Google Scholar] [CrossRef] [PubMed]
- Robertson, I.M.; Schaffer, G.B. Review of Densification of Titanium Based Powder Systems in Press and Sinter Processing. Powder Metall. 2010, 53, 146–162. [Google Scholar] [CrossRef]
- Wu, Z.; Li, P.; Zhao, J.; Xiao, T.; Hu, H.; Sun, P.; Wu, Z.; Hao, J.; Sun, C.; Zhang, H.; et al. Low-Temperature-Deposited Tio2 Nanopillars for Efficient and Flexible Perovskite Solar Cells. Adv. Mater. Interfaces 2021, 8, 2001512. [Google Scholar] [CrossRef]
- Chen, W.; Li, X.; Li, Y.; Li, Y. A Review: Crystal Growth for High-Performance All-Inorganic Perovskite Solar Cells. Energy Environ. Sci. 2020, 13, 1971–1996. [Google Scholar] [CrossRef]
- Lin, X.; Cui, D.; Luo, X.; Zhang, C.; Han, Q.; Wang, Y.; Han, L. Efficiency Progress of Inverted Perovskite Solar Cells. Energy Environ. Sci. 2020, 13, 3823–3847. [Google Scholar] [CrossRef]
- Yao, Y.; Cheng, C.; Zhang, C.; Hu, H.; Wang, K.; De Wolf, S. Organic Hole-Transport Layers for Efficient, Stable, and Scalable Inverted Perovskite Solar Cells. Adv. Mater. 2022, 34, 2203794. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhu, K. Rapid Advances Enabling High-Performance Inverted Perovskite Solar Cells. Nat. Rev. Mater. 2024, 9, 399–419. [Google Scholar] [CrossRef]
- Ono, L.K.; Liu, S.; Qi, Y. Reducing Detrimental Defects for High-Performance Metal Halide Perovskite Solar Cells. Angew. Chem. Int. Ed. 2020, 59, 6676–6698. [Google Scholar] [CrossRef] [PubMed]
- Khenkin, M.V.; Katz, E.A.; Abate, A.; Bardizza, G.; Berry, J.J.; Brabec, C.; Brunetti, F.; Bulović, V.; Burlingame, Q.; Di Carlo, A.; et al. Consensus Statement for Stability Assessment and Reporting for Perovskite Photovoltaics Based on Isos Procedures. Nat. Energy 2020, 5, 35–49. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, T.; Xie, R.; Wang, Z.; Hu, W. How to Characterize Figures of Merit of Two-Dimensional Photodetectors. Nat. Commun. 2023, 14, 2224. [Google Scholar] [CrossRef]
- Cansizoglu, M.F.; Engelken, R.; Seo, H.-W.; Karabacak, T. High Optical Absorption of Indium Sulfide Nanorod Arrays Formed by Glancing Angle Deposition. ACS Nano 2010, 4, 733–740. [Google Scholar] [CrossRef]
- Leem, J.W.; Yu, J.S. Broadband and Wide-Angle Distributed Bragg Reflectors Based on Amorphous Germanium Films by Glancing Angle Deposition. Opt. Express 2012, 20, 20576–20581. [Google Scholar] [CrossRef]
- Chen, H.; Liu, H.; Zhang, Z.; Hu, K.; Fang, X. Nanostructured Photodetectors: From Ultraviolet to Terahertz. Adv. Mater. 2016, 28, 403–433. [Google Scholar] [CrossRef]
- Li, Z.; Li, Z.; Zuo, C.; Fang, X. Application of Nanostructured TiO2 in Uv Photodetectors: A Review. Adv. Mater. 2022, 34, 2109083. [Google Scholar] [CrossRef]
- Zhai, T.; Li, L.; Wang, X.; Fang, X.; Bando, Y.; Golberg, D. Recent Developments in One-Dimensional Inorganic Nanostructures for Photodetectors. Adv. Funct. Mater. 2010, 20, 4233–4248. [Google Scholar] [CrossRef]
- Lee, G.; Ko, R.; Kang, S.; Kim, Y.J.; Kim, Y.-J.; Yoo, H. Nanostructure Engineering by Oblique Angle Deposition for Photodetectors and Other Applications. Micromachines 2025, 16, 865. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubizadeh, P.; Eshraghi, M.J.; Hajati, S.; Naderi, N. Ultrahigh Performance of Β-Ga2O3-Based Msm Solar-Blind Photodetectors Fabricated Via the Glancing Angle Deposition Technique. ACS Appl. Electron. Mater. 2023, 5, 4220–4232. [Google Scholar] [CrossRef]
- Nath, A.; Mahajan, B.K.; Singh, L.R.; Vishwas, S.; Nanda, R.K.; Sarkar, M.B. Enhancing Detectivity of Indium-Oxide-Based Photodetectors Via Vertical Nanostructuring through Glancing Angle Deposition. J. Electron. Mater. 2021, 50, 3722–3730. [Google Scholar] [CrossRef]
- Li, Z.; Yan, T.; Fang, X. Low-Dimensional Wide-Bandgap Semiconductors for Uv Photodetectors. Nat. Rev. Mater. 2023, 8, 587–603. [Google Scholar] [CrossRef]
- Xie, C.; Lu, X.-T.; Tong, X.-W.; Zhang, Z.-X.; Liang, F.-X.; Liang, L.; Luo, L.-B.; Wu, Y.-C. Recent Progress in Solar-Blind Deep-Ultraviolet Photodetectors Based on Inorganic Ultrawide Bandgap Semiconductors. Adv. Funct. Mater. 2019, 29, 1806006. [Google Scholar] [CrossRef]
- Savage, N.O.; Akbar, S.A.; Dutta, P.K. Titanium Dioxide Based High Temperature Carbon Monoxide Selective Sensor. Sens. Actuators, B 2001, 72, 239–248. [Google Scholar] [CrossRef]
- Diebold, U. The Surface Science of Titanium Dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Wu, D.; Guo, J.; Wang, C.; Ren, X.; Chen, Y.; Lin, P.; Zeng, L.; Shi, Z.; Li, X.J.; Shan, C.-X.; et al. Ultrabroadband and High-Detectivity Photodetector Based on Ws2/Ge Heterojunction through Defect Engineering and Interface Passivation. ACS Nano 2021, 15, 10119–10129. [Google Scholar] [CrossRef]
- Devi, N.M.; Singh, N.K. Plasmon-Induced Ag Decorated Ceo2 Nanorod Array for Photodetector Application. Nanotechnology 2020, 31, 225203. [Google Scholar] [CrossRef] [PubMed]
- Soni, A.; Mulchandani, K.; Mavani, K.R. Effects of Substrates on the Crystalline Growth and Uv Photosensitivity of Glancing Angle Deposited Porous Zno Nanostructures. Sens. Actuators, A 2020, 313, 112140. [Google Scholar] [CrossRef]
- Lim, S.; Um, D.-S.; Ha, M.; Zhang, Q.; Lee, Y.; Lin, Y.; Fan, Z.; Ko, H. Broadband Omnidirectional Light Detection in Flexible and Hierarchical Zno/Si Heterojunction Photodiodes. Nano Res. 2017, 10, 22–36. [Google Scholar] [CrossRef]
- Zhao, Q.; Jie, W.; Wang, T.; Castellanos-Gomez, A.; Frisenda, R. Inse Schottky Diodes Based on Van Der Waals Contacts. Adv. Funct. Mater. 2020, 30, 2001307. [Google Scholar] [CrossRef]
- von Wenckstern, H.; Splith, D.; Schmidt, F.; Grundmann, M.; Bierwagen, O.; Speck, J.S. Schottky Contacts to In2O3. APL Mater. 2014, 2, 046104. [Google Scholar] [CrossRef]
- Ferhati, H.; Djeffal, F.; Martin, N. Highly Improved Responsivity of Self-Powered Uv–Visible Photodetector Based on TiO2/Ag/TiO2 Multilayer Deposited by Glad Technique: Effects of Oriented Columns and Nano-Sculptured Surface. Appl. Surf. Sci. 2020, 529, 147069. [Google Scholar]
- Sepúlveda, B.; Angelomé, P.C.; Lechuga, L.M.; Liz-Marzán, L.M. Lspr-Based Nanobiosensors. Nano Today 2009, 4, 244–251. [Google Scholar] [CrossRef]
- Li, W.; Ren, K.; Zhou, J. Aluminum-Based Localized Surface Plasmon Resonance for Biosensing. TrAC Trends Anal. Chem. 2016, 80, 486–494. [Google Scholar] [CrossRef]
- Li, Y.; DiStefano, J.G.; Murthy, A.A.; Cain, J.D.; Hanson, E.D.; Li, Q.; Castro, F.C.; Chen, X.; Dravid, V.P. Superior Plasmonic Photodetectors Based on Au@MoS2 Core–Shell Heterostructures. ACS Nano 2017, 11, 10321–10329. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wang, F.; Chen, H.; Wang, Y.; Jiang, M.; Fang, X.; Zhao, D. Solar-Blind Avalanche Photodetector Based on Single Zno–Ga2O3 Core–Shell Microwire. Nano Lett. 2015, 15, 3988–3993. [Google Scholar] [CrossRef]
- Ghosh, C.; Dey, A.; Biswas, I.; Gupta, R.K.; Yadav, V.S.; Yadav, A.; Yadav, N.; Zheng, H.; Henini, M.; Mondal, A. Cuo–TiO2 Based Self-Powered Broad Band Photodetector. Nano Mater. Sci. 2024, 6, 345–354. [Google Scholar] [CrossRef]
- Retamal, J.R.D.; Chen, C.-Y.; Lien, D.-H.; Huang, M.R.S.; Lin, C.-A.; Liu, C.-P.; He, J.-H. Concurrent Improvement in Photogain and Speed of a Metal Oxide Nanowire Photodetector through Enhancing Surface Band Bending Via Incorporating a Nanoscale Heterojunction. ACS Photonics 2014, 1, 354–359. [Google Scholar] [CrossRef]
- Dai, M.; Chen, H.; Wang, F.; Long, M.; Shang, H.; Hu, Y.; Li, W.; Ge, C.; Zhang, J.; Zhai, T.; et al. Ultrafast and Sensitive Self-Powered Photodetector Featuring Self-Limited Depletion Region and Fully Depleted Channel with Van Der Waals Contacts. ACS Nano 2020, 14, 9098–9106. [Google Scholar] [CrossRef]
- Delahoy, A.E.; Chen, L.; Akhtar, M.; Sang, B.; Guo, S. New Technologies for Cigs Photovoltaics. Sol. Energy 2004, 77, 785–793. [Google Scholar] [CrossRef]
- Lan, C.; Dong, R.; Zhou, Z.; Shu, L.; Li, D.; Yip, S.; Ho, J.C. Large-Scale Synthesis of Freestanding Layer-Structured Pbi2 and Mapbi3 Nanosheets for High-Performance Photodetection. Adv. Mater. 2017, 29, 1702759. [Google Scholar] [CrossRef]
- Lian, Z.; Yan, Q.; Lv, Q.; Wang, Y.; Liu, L.; Zhang, L.; Pan, S.; Li, Q.; Wang, L.; Sun, J.-L. High-Performance Planar-Type Photodetector on (100) Facet of Mapbi3 Single Crystal. Sci. Rep. 2015, 5, 16563. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Domen, K. Particulate Photocatalysts for Light-Driven Water Splitting: Mechanisms, Challenges, and Design Strategies. Chem. Rev. 2020, 120, 919–985. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 Photocatalysis and Related Surface Phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the Anatase to Rutile Phase Transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Su, Y.; Li, H.; Ma, H.; Robertson, J.; Nathan, A. Controlling Surface Termination and Facet Orientation in Cu2O Nanoparticles for High Photocatalytic Activity: A Combined Experimental and Density Functional Theory Study. ACS Appl. Mater. Interfaces 2017, 9, 8100–8106. [Google Scholar] [CrossRef]
- Bai, S.; Wang, L.; Li, Z.; Xiong, Y. Facet-Engineered Surface and Interface Design of Photocatalytic Materials. Adv. Sci. 2017, 4, 1600216. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor Heterojunction Photocatalysts: Design, Construction, and Photocatalytic Performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef]
- Wu, S.; Ou, K.; Zhang, W.; Ni, Y.; Xia, Y.; Wang, H. TiO2 Nanorod Arrays/Ti3c2tx Mxene Nanosheet Composites with Efficient Photocatalytic Activity. Nanotechnology 2024, 35, 155705. [Google Scholar] [CrossRef]
- Zhong, C.J.; Maye, M.M. Core–Shell Assembled Nanoparticles as Catalysts. Adv. Mater. 2001, 13, 1507–1511. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Asefa, T.; Guo, H.; Biradar, A.V.; Peng, D.-L.; Zboril, R.; Varma, R.S. Core–Shell Nanoparticles: Synthesis and Applications in Catalysis and Electrocatalysis. Chem. Soc. Rev. 2015, 44, 7540–7590. [Google Scholar] [CrossRef]
- Rawool, S.A.; Pai, M.R.; Banerjee, A.M.; Arya, A.; Ningthoujam, R.S.; Tewari, R.; Rao, R.; Chalke, B.; Ayyub, P.; Tripathi, A.K.; et al. Pn Heterojunctions in Nio:TiO2 Composites with Type-Ii Band Alignment Assisting Sunlight Driven Photocatalytic H2 Generation. Appl. Catal., B 2018, 221, 443–458. [Google Scholar] [CrossRef]
- Madhavi, V.; Kondaiah, P.; Shaik, H.; Kumar, K.N.; Kumar Naik, T.S.S.; Rao, G.M.; Ramamurthy, P.C. Fabrication of Porous 1d WO3 Nrs and WO3/BiVO4 Hetero Junction Photoanode for Efficient Photoelectrochemical Water Splitting. Mater. Chem. Phys. 2021, 274, 125095. [Google Scholar] [CrossRef]
- Shuang, S.; Xie, Z.; Zhang, Z. Enhanced Photocatalytic Properties of Cds Nanoparticles Decorated A-Fe2O3 Nanopillar Arrays under Visible Light. J. Colloid Interface Sci. 2017, 494, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, T.; Yawata, K.; Nosaka, Y. Photocatalytic Reactivity for O2− and Oh Radical Formation in Anatase and Rutile TiO2 Suspension as the Effect of H2O2 Addition. Appl. Catal., A 2007, 325, 105–111. [Google Scholar] [CrossRef]
- Yan, D.; Li, Y.; Huo, J.; Chen, R.; Dai, L.; Wang, S. Defect Chemistry of Nonprecious-Metal Electrocatalysts for Oxygen Reactions. Adv. Mater. 2017, 29, 1606459. [Google Scholar] [CrossRef]
- Xie, C.; Yan, D.; Li, H.; Du, S.; Chen, W.; Wang, Y.; Zou, Y.; Chen, R.; Wang, S. Defect Chemistry in Heterogeneous Catalysis: Recognition, Understanding, and Utilization. ACS Catal. 2020, 10, 11082–11098. [Google Scholar] [CrossRef]
- Antartis, D.A.; Mott, R.N.; Chasiotis, I. Silicon Nanosprings Fabricated by Glancing Angle Deposition for Ultra-Compliant Films and Interfaces. Mater. Des. 2018, 144, 182–191. [Google Scholar] [CrossRef]
- Jensen, M.O.; Brett, M.J. Periodically Structured Glancing Angle Deposition Thin Films. IEEE Trans. Nanotechnol. 2005, 4, 269–277. [Google Scholar] [CrossRef]
- Singh, J.; Wolfe, D.E. Review Nano and Macro-Structured Component Fabrication by Electron Beam-Physical Vapor Deposition (Eb-Pvd). J. Mater. Sci. 2005, 40, 1–26. [Google Scholar] [CrossRef]
- Reinhold, E.; Faber, J. Large Area Electron Beam Physical Vapor Deposition (Eb-Pvd) and Plasma Activated Electron Beam (Eb) Evaporation — Status and Prospects. Surf. Coat. Technol. 2011, 206, 1653–1659. [Google Scholar] [CrossRef]
- Hou, X.; Choy, K.L. Processing and Applications of Aerosol-Assisted chemical Vapor Deposition. Chem. Vap. Deposition 2006, 12, 583–596. [Google Scholar] [CrossRef]
- Marchand, P.; Hassan, I.A.; Parkin, I.P.; Carmalt, C.J. Aerosol-Assisted Delivery of Precursors for Chemical Vapour Deposition: Expanding the Scope of Cvd for Materials Fabrication. Dalton Trans. 2013, 42, 9406–9422. [Google Scholar] [CrossRef]
- Profijt, H.B.; Potts, S.E.; van de Sanden, M.C.M.; Kessels, W.M.M. Plasma-Assisted Atomic Layer Deposition: Basics, Opportunities, and Challenges. J. Vac. Sci. Technol. A 2011, 29, 050801. [Google Scholar] [CrossRef]
- Gardella, M.; Zambito, G.; Ferrando, G.; Barusso, L.F.; Chennuboina, R.; Repetto, L.; Barelli, M.; Giordano, M.C.; de Mongeot, F.B. Maskless Synthesis of Van Der Waals Heterostructure Arrays Engineered for Light Harvesting on Large Area Templates. Small 2025, 21, 2400943. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Dhar, J.C. Low Interface State Density and Large Capacitive Memory Window Using Rf Sputtered Nio Nanoparticles Decorated Mgzno Thin Film. Sci. Rep. 2025, 15, 2322. [Google Scholar] [CrossRef]
- Manzeli, S.; Ovchinnikov, D.; Pasquier, D.; Yazyev, O.V.; Kis, A. 2d Transition Metal Dichalcogenides. Nat. Rev. Mater. 2017, 2, 1–15. [Google Scholar] [CrossRef]
- Ling, X.; Wang, H.; Huang, S.; Xia, F.; Dresselhaus, M.S. The Renaissance of Black Phosphorus. Proc. Natl. Acad. Sci. USA 2015, 112, 4523–4530. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, B.; Zou, X.; Cheng, H.-M. Chemical Vapor Deposition Growth and Applications of Two-Dimensional Materials and Their Heterostructures. Chem. Rev. 2018, 118, 6091–6133. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Lee, H.-B.-R. Atomic Layer Deposition on 2d Materials. Chem. Mater. 2017, 29, 3809–3826. [Google Scholar] [CrossRef]
- Wang, F.K.; Yang, S.J.; Zhai, T.Y. 2d Bi2se3 Materials for Optoelectronics. iScience 2021, 24, 103291. [Google Scholar] [CrossRef]
- Mellnik, A.R.; Lee, J.; Richardella, A.; Grab, J.L.; Mintun, P.J.; Fischer, M.H.; Vaezi, A.; Manchon, A.; Kim, E.-A.; Samarth, N. Spin-Transfer Torque Generated by a Topological Insulator. Nature 2014, 511, 449–451. [Google Scholar] [CrossRef]
- Zhang, L.-W.; Yang, Y.-Q.; Chen, J.; Zhang, L. Photogalvanic Effect Induced Charge and Spin Photocurrent in Group-V Monolayer Systems. Front. Phys. 2023, 18, 62301. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, H.; Zhang, J.; Li, S.; Luo, D.; Wang, J.; Jin, K.; Sun, J. Circular Photogalvanic Effect in Oxide Two-Dimensional Electron Gases. Phys. Rev. Lett. 2022, 128, 187401. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.A.; Saif, M.T.A. Application of Mems Force Sensors for in Situ Mechanical Characterization of Nano-Scale Thin Films in Sem and Tem. Sens. Actuators A 2002, 97–98, 239–245. [Google Scholar] [CrossRef]
- Kondekar, N.P.; Boebinger, M.G.; Woods, E.V.; McDowell, M.T. In Situ Xps Investigation of Transformations at Crystallographically Oriented MoS2 Interfaces. ACS Appl. Mater. Interfaces 2017, 9, 32394–32404. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.; Wang, S. In Situ Rheed Monitor of the Growth of Epitaxial Anatase TiO2 Thin Films. Appl. Surf. Sci. 2001, 185, 47–51. [Google Scholar] [CrossRef]
- Brune, H. Epitaxial Growth of Thin Films. Surf. Interface Sci. 2014, 4, 421–477. [Google Scholar]
- Venables, J.A. Atomic Processes in Crystal Growth. Surf. Sci. 1994, 299–300, 798–817. [Google Scholar] [CrossRef]
- Hearst, M.A.; Dumais, S.T.; Osuna, E.; Platt, J.; Scholkopf, B. Support Vector Machines. IEEE Intell. Syst. Appl. 1998, 13, 18–28. [Google Scholar] [CrossRef]
- De Ville, B. Decision Trees. Wiley Interdiscip. Rev. Comput. Stat. 2013, 5, 448–455. [Google Scholar] [CrossRef]
- Rigatti, S.J. Random Forest. J. Insur. Med. 2017, 47, 31–39. [Google Scholar] [CrossRef]
- Dai, J.; Ji, X.; Niu, X.; Jiao, H.; Cheng, X.; Wang, Z.; Zhang, J. Advanced Deep Learning-Based Strategy for Optical Inversion Engineering of Optical Coatings. Opt. Express 2025, 33, 10057–10068. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Cho, H.; Kwon, C.; Ji, Y.; Ryu, S. Optimizing Chamber Systems for Deposition Processes in the Semiconductor Industry with Deep Learning Framework: Tackling Small Simulation Datasets. J. Intell. Manuf. 2025, 1–17. [Google Scholar] [CrossRef]
- Liu, Q.; Baghdasaryan, T.; Ottevaere, H. Data-Driven Investigation of Thickness Variations in Multilayer Thin Film Coatings. J. Phys. Photonics 2024, 6, 045020. [Google Scholar] [CrossRef]
- Lin, Z.-P.; Li, Y.; Haque, S.A.; Ganose, A.M.; Kafizas, A. Insights from Experiment and Machine Learning for Enhanced TiO2 Coated Glazing for Photocatalytic Nox Remediation. J. Mater. Chem. A 2024, 12, 13281–13298. [Google Scholar] [CrossRef]

| Material | Deposition Method | Refs. |
|---|---|---|
| Metals | ||
| Ag | Electron beam evaporation | [52,53] |
| Ti | Electron beam evaporation, Sputtering | [54,55] |
| Ge | Electron beam evaporation | [56] |
| Ta | Sputtering | [57] |
| Fe | Electron beam evaporation | [58] |
| Ni | Sputtering | [59] |
| Inorganic compounds | ||
| TiO2 | Electron beam evaporation | [52,58,60,61,62,63,64,65,66,67,68,69,70,71,72] |
| ITO | Electron beam evaporation | [73,74,75,76,77] |
| SnOx | Sputtering, electron beam evaporation | [78,79,80] |
| SiOx | Electron beam evaporation | [81,82] |
| SiO2 | Electron beam evaporation | [61] |
| CdTe | Thermal evaporation | [83] |
| NiOx | Electron beam evaporation | [84] |
| In2O3 | Electron beam evaporation | [85] |
| WO3 | Sputtering, thermal evaporation, electron beam evaporation | [58,59,86] |
| Ga2O3 | Thermal evaporation | [87] |
| Gd2O3 | Electron beam evaporation | [88] |
| ZrO2 | Electron beam evaporation | [89] |
| CeO2 | Electron beam evaporation | [90] |
| ZnO | Pulsed laser deposition, sputtering | [91,92] |
| Co3O4 | Electron beam evaporation | [67] |
| Cu2O | Electron beam evaporation, sputtering | [93,94] |
| CIGS | Sputtering | [95] |
| PbI2 | Thermal evaporation | [96] |
| Fe2O3 | Electron beam evaporation | [70] |
| AlN | Vapor–liquid–solid | [97] |
| Al2O3 | Electron beam evaporation | [98] |
| Antimony (Sb)-doped tin oxide | Sputtering | [99] |
| Organic molecules | ||
| Copper phthalocyanine | Thermal evaporation | [100] |
| Chloroaluminum phthalocyanine | Thermal evaporation | [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Y.; Yang, L.; Huang, Z. Advanced Optoelectronic Applications of Nanopillar Arrays Fabricated by Glancing Angle Deposition. Nanomaterials 2025, 15, 1555. https://doi.org/10.3390/nano15201555
Fang Y, Yang L, Huang Z. Advanced Optoelectronic Applications of Nanopillar Arrays Fabricated by Glancing Angle Deposition. Nanomaterials. 2025; 15(20):1555. https://doi.org/10.3390/nano15201555
Chicago/Turabian StyleFang, Yating, Lin Yang, and Zhifeng Huang. 2025. "Advanced Optoelectronic Applications of Nanopillar Arrays Fabricated by Glancing Angle Deposition" Nanomaterials 15, no. 20: 1555. https://doi.org/10.3390/nano15201555
APA StyleFang, Y., Yang, L., & Huang, Z. (2025). Advanced Optoelectronic Applications of Nanopillar Arrays Fabricated by Glancing Angle Deposition. Nanomaterials, 15(20), 1555. https://doi.org/10.3390/nano15201555







