Biomass-Derived Hard Carbon Anodes for Sodium-Ion Batteries: Recent Advances in Synthesis Strategies
Abstract
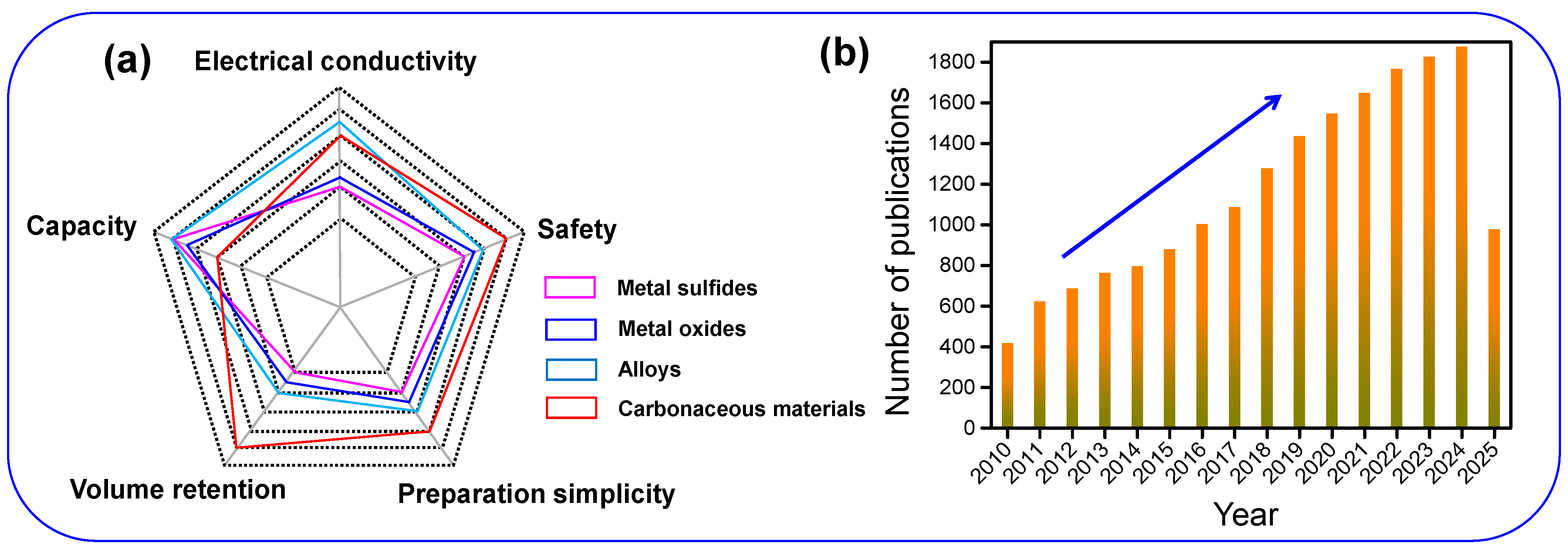
1. Introduction
2. Basic Characteristics of HC
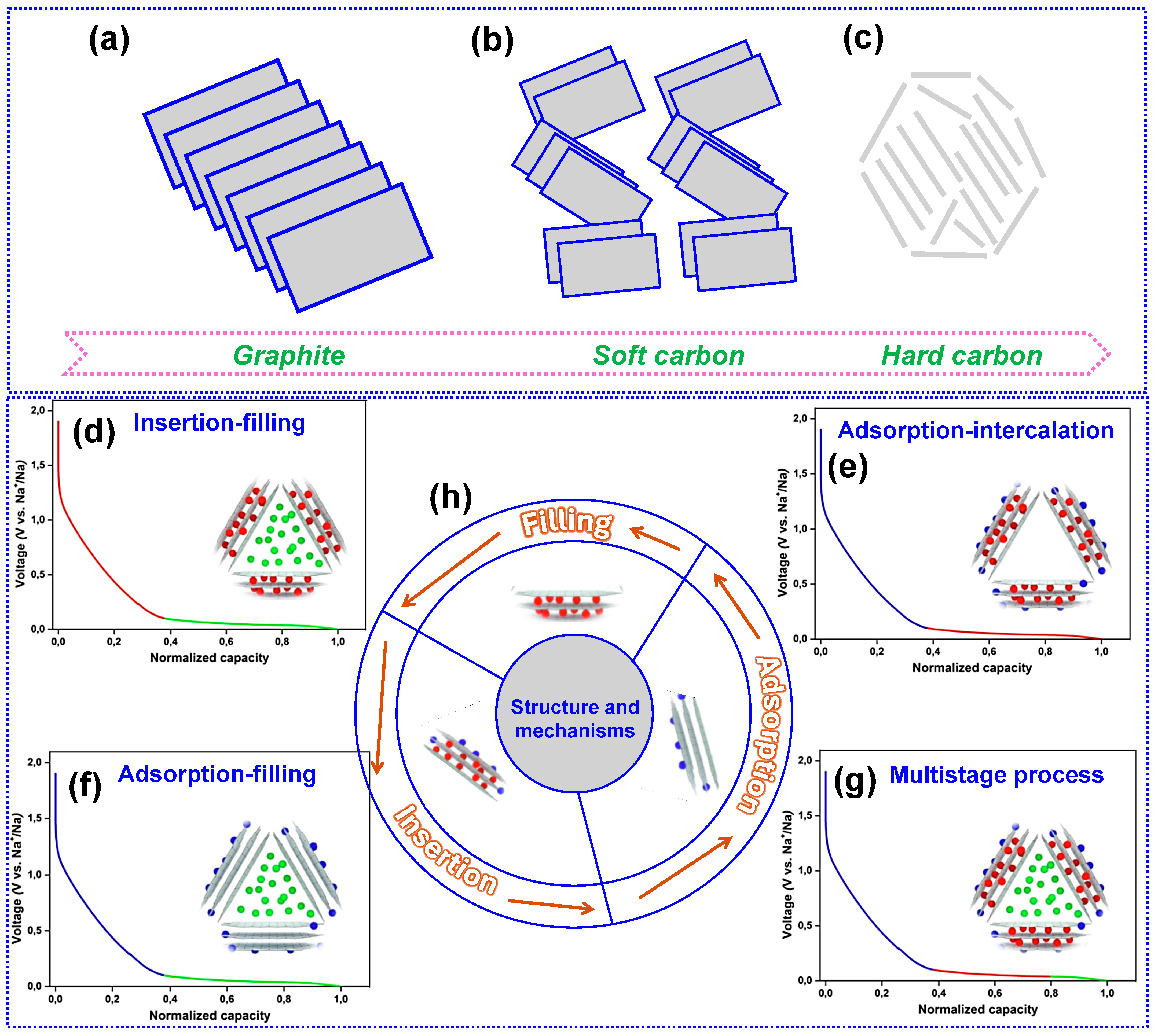
2.1. Structure of HC
2.2. Na Storage Mechanism of HC
2.2.1. Insertion-Filling Mechanism
2.2.2. Adsorption-Insertion Mechanism
2.2.3. Adsorption-Filling Mechanism
2.2.4. Multi-Step Storage Mechanism
2.2.5. Three Modes of Na+ Storage in HC
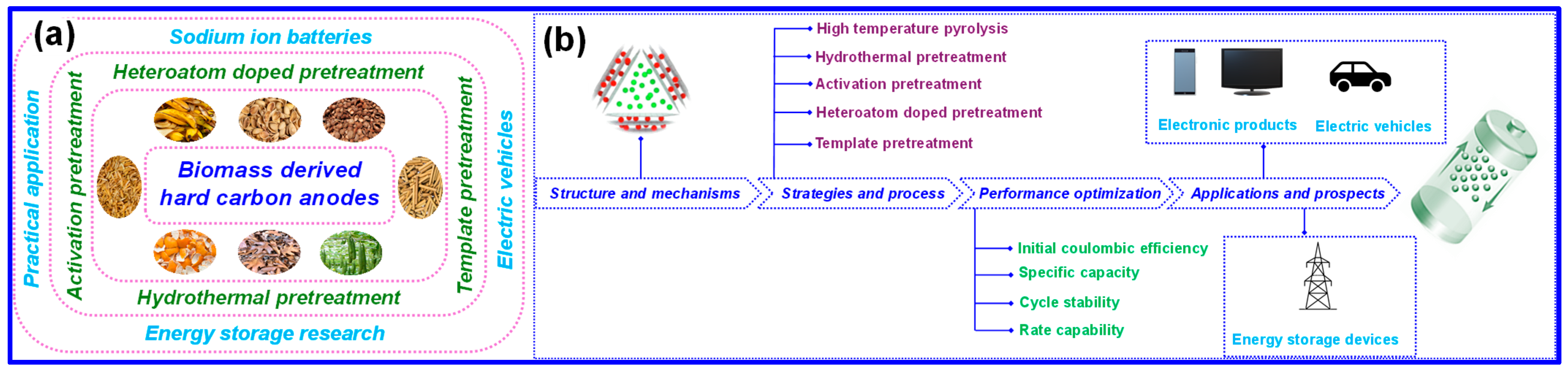
3. Numerous Preparation Approaches
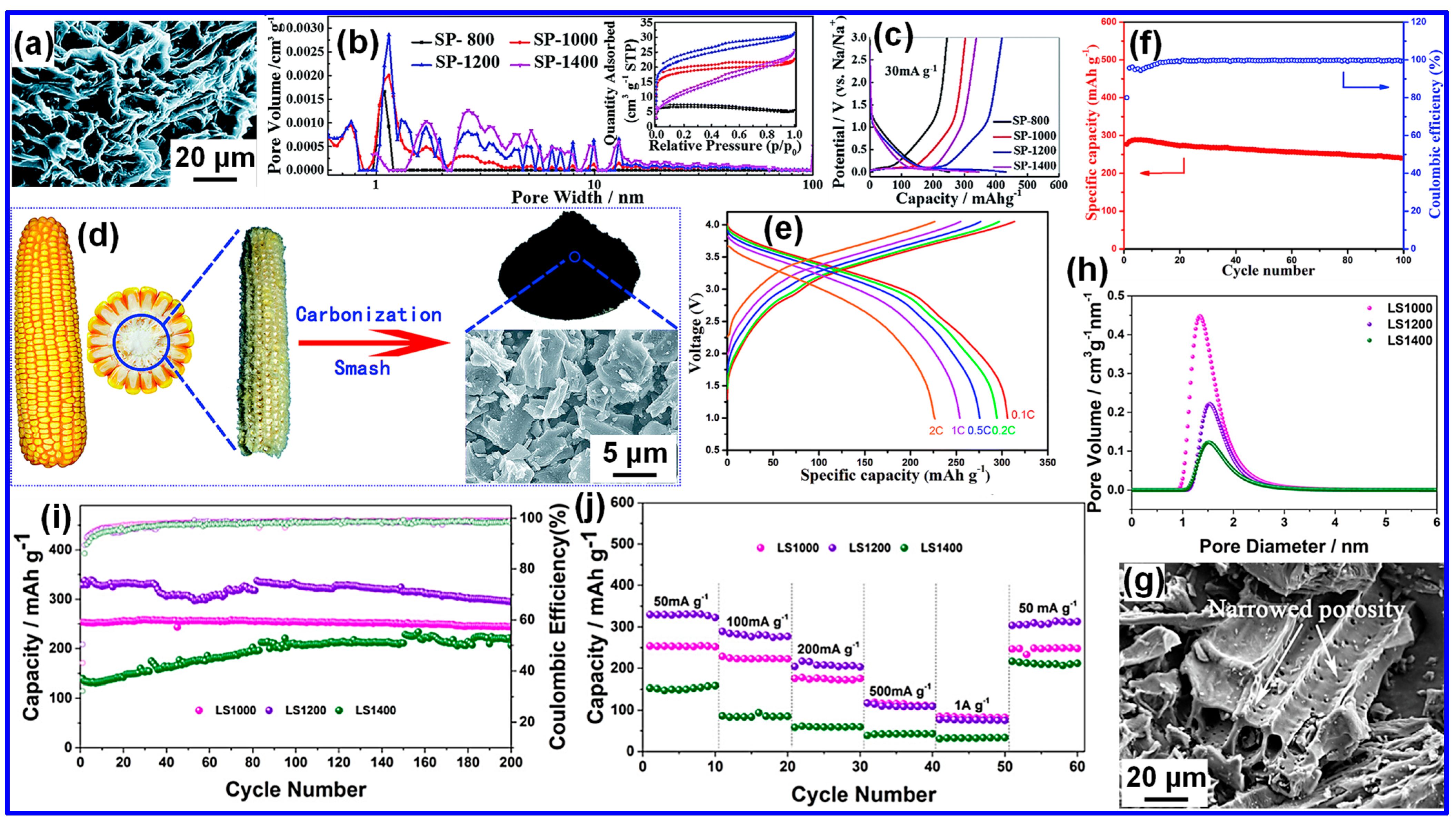
3.1. High-Temperature Pyrolysis
3.1.1. Single-Step High-Temperature Pyrolysis
3.1.2. Two-Step High-Temperature Pyrolysis
3.1.3. Summary of High-Temperature Pyrolysis
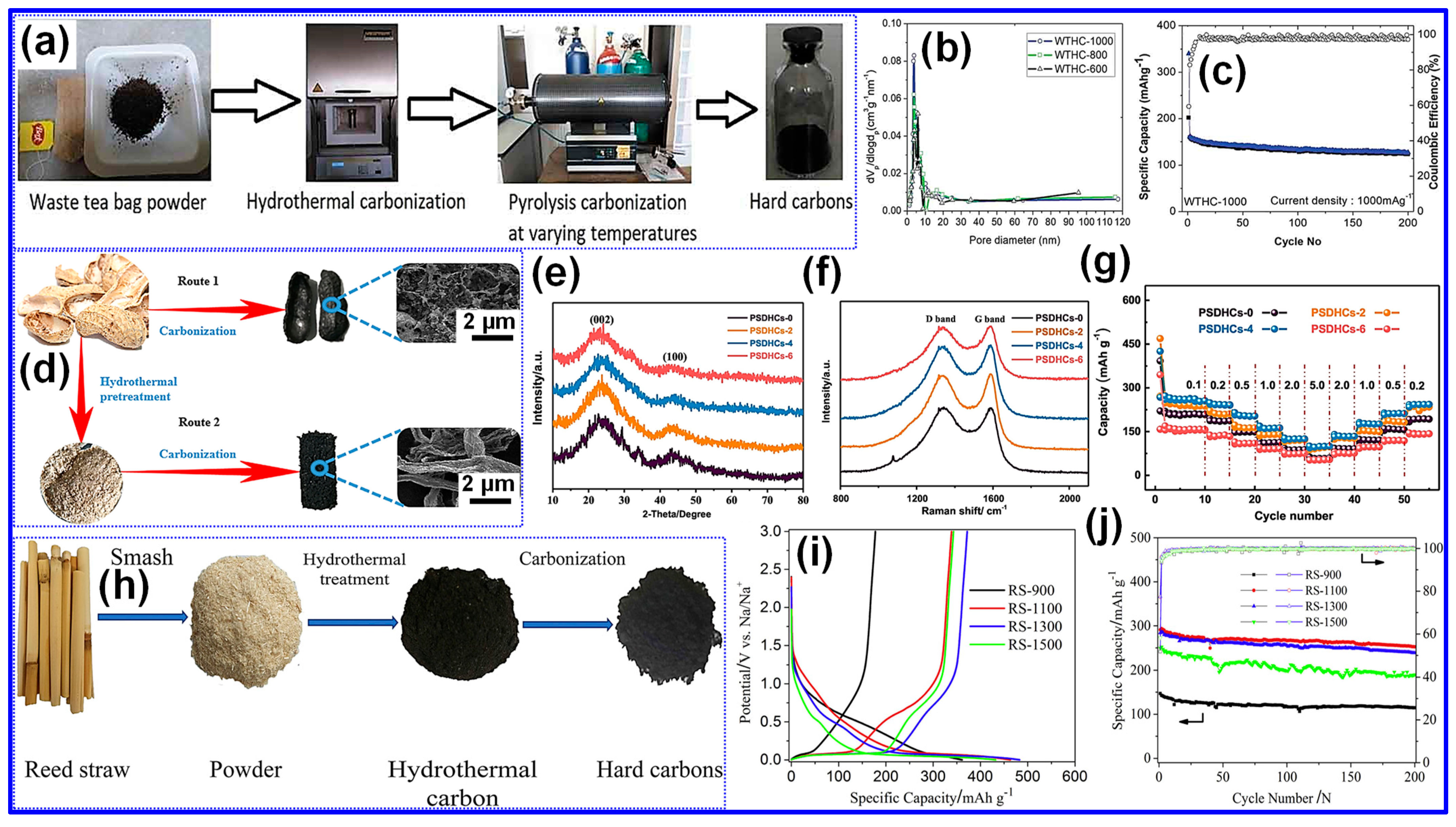
3.2. Hydrothermal Treatment
3.3. Activation Pretreatment
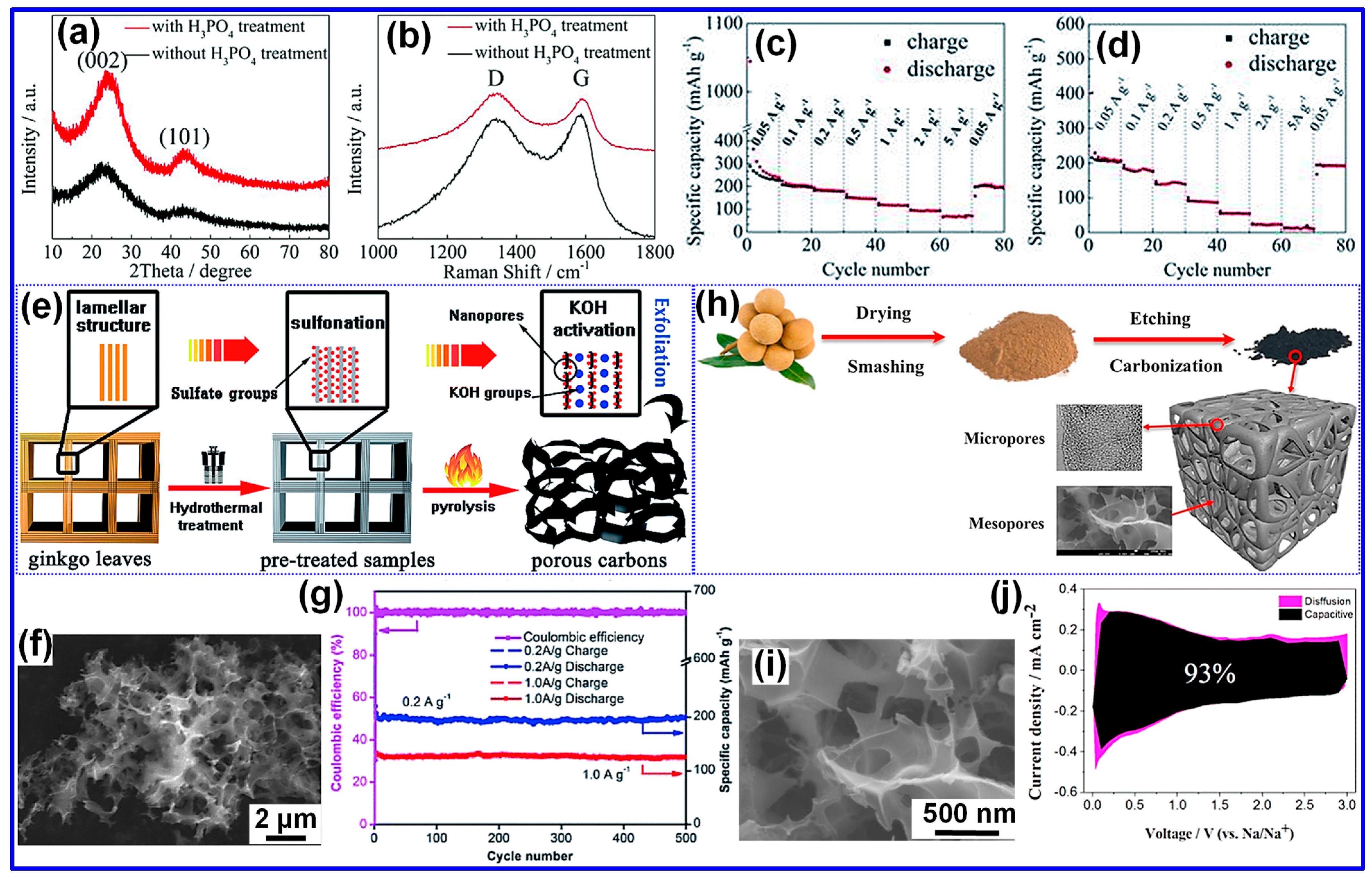
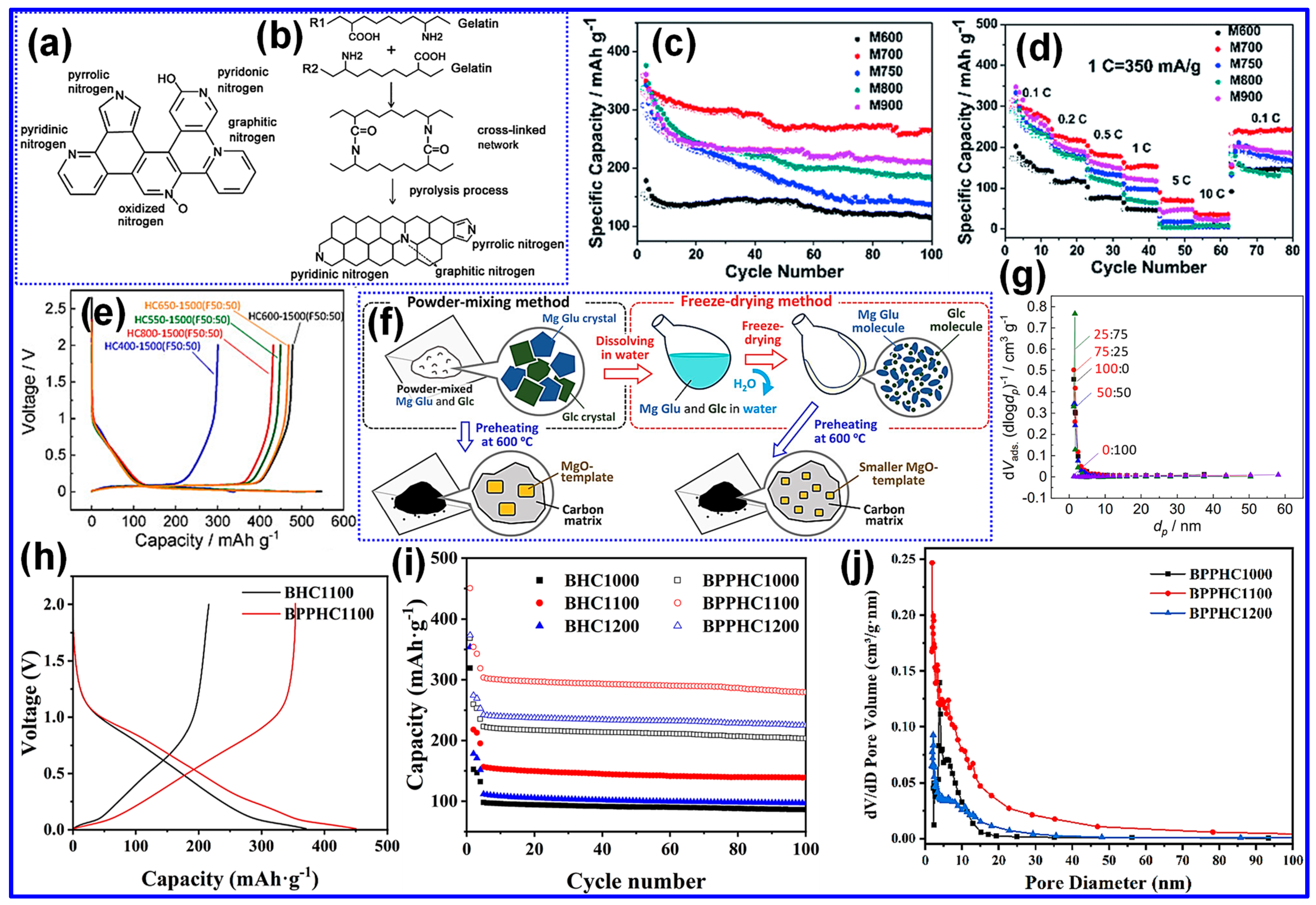
3.4. Heteroatom Doping
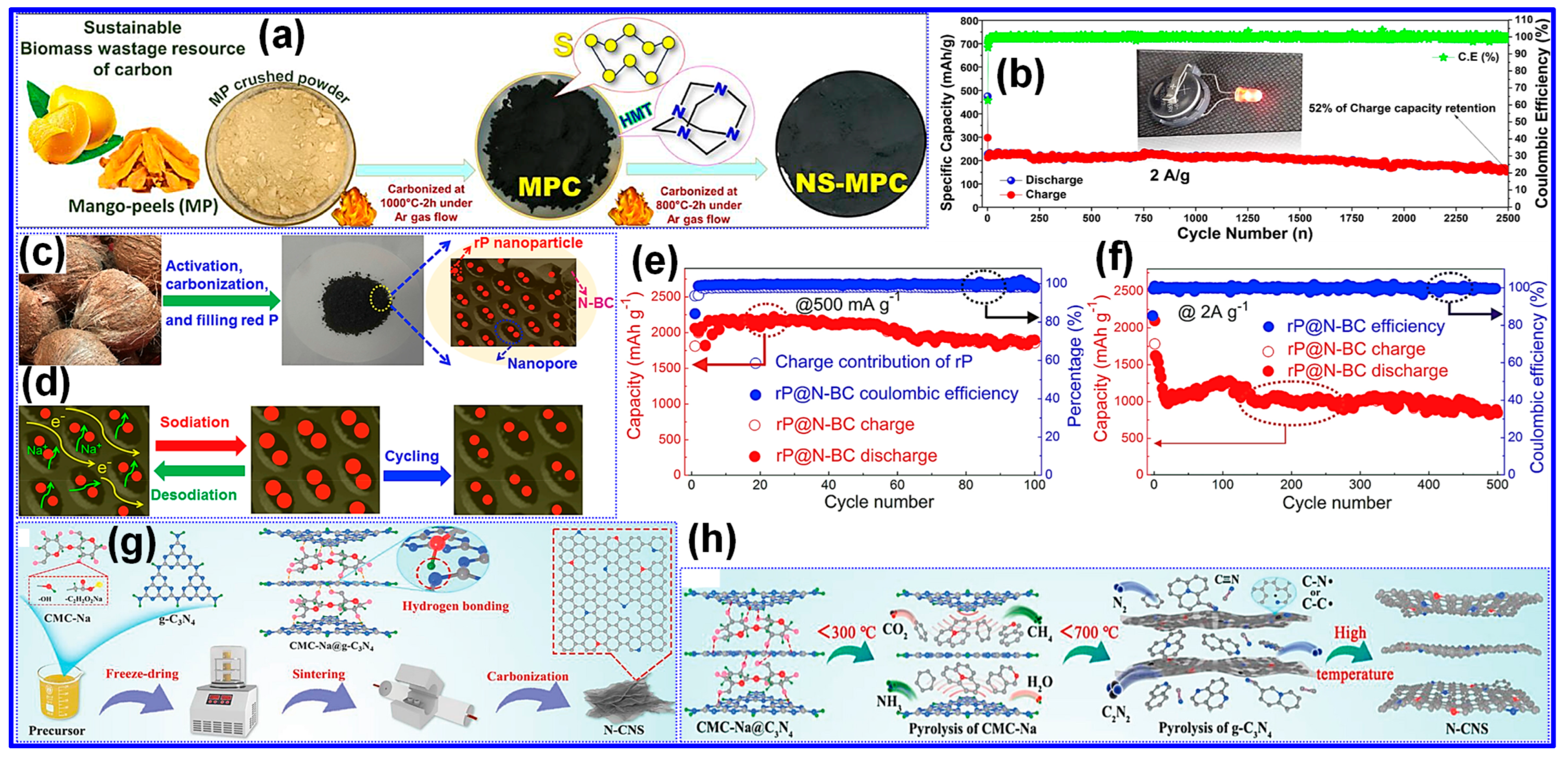
3.5. Template Pretreatment
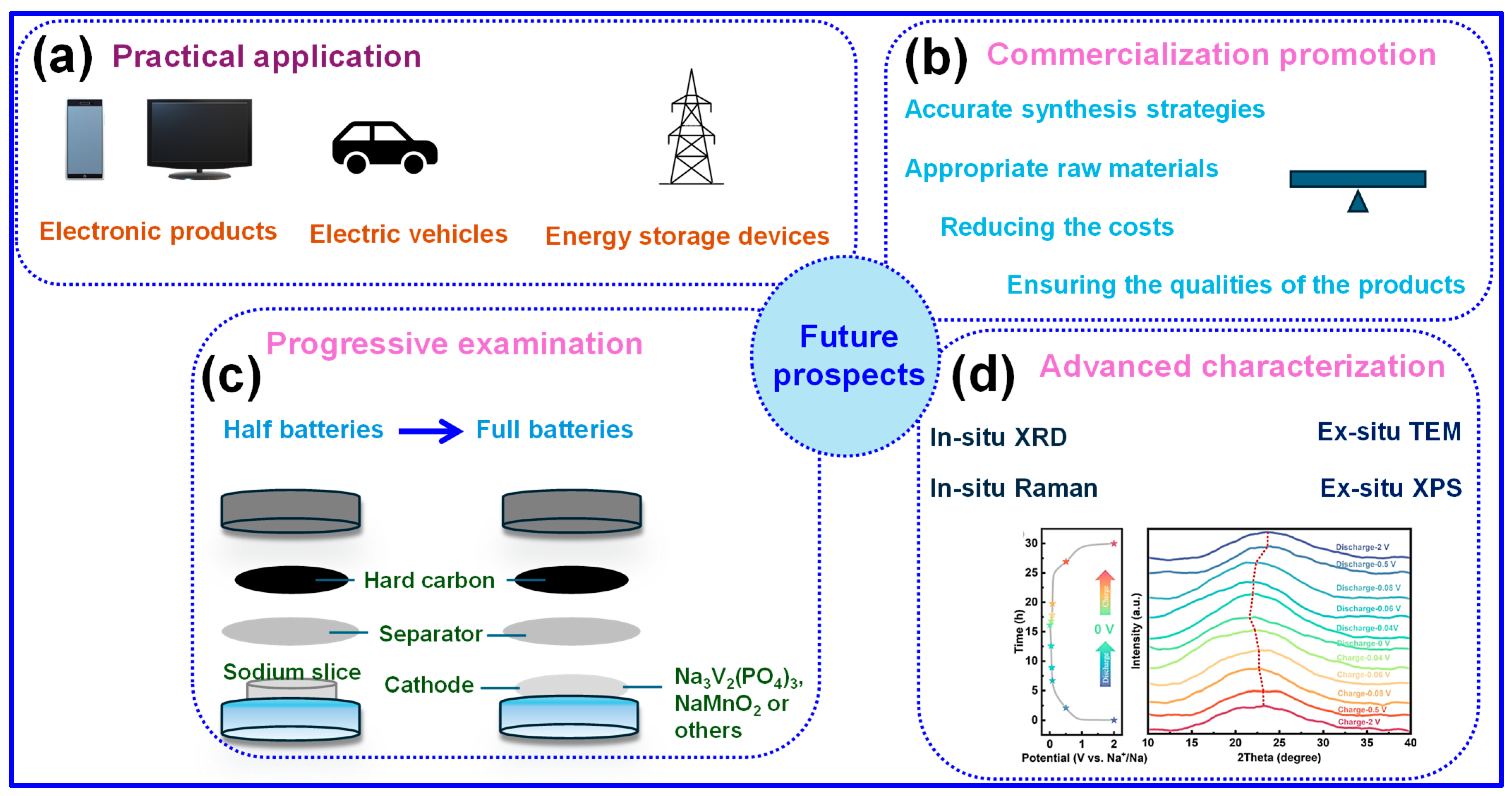
4. Conclusions and Future Perspectives
4.1. Conclusions
4.2. Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lokhande, P.E.; Misal, P.; Kalubarme, R.S.; Kulkarni, M.V.; Rednam, U.; Padlkar, S.; Asbahi, B.A.A. Scalable microwave-assisted production of Ti3C2Tx MXene for next-generation Li-ion and Na-ion batteries. Diam. Relat. Mater. 2025, 157, 112503. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, X.L.; Yang, Y.L.; Suo, G.Q.; Zhang, L.; Lu, S.; Chen, Z.G. Biomass-Derived Carbon for High-Performance Batteries: From Structure to Properties. Adv. Funct. Mater. 2022, 32, 2201584. [Google Scholar] [CrossRef]
- Dong, Y.T.; Chen, Y.M.; Zeng, Q.H.; Feng, J.; Fang, M.M.; Shi, Z.Q.; Liu, J.J.; Sheng, Y.L.; Yue, X.Y.; Liang, Z. Challenges and Strategies of Fast-Charging Li-Ion Batteries with a Focus on Li Plating. Energy Mater. Adv. 2024, 5, 0113. [Google Scholar] [CrossRef]
- Gong, C.; Pu, S.D.; Zhang, S.M.; Yuan, Y.; Ning, Z.; Yang, S.; Gao, X.W.; Chau, C.; Li, Z.X.; Liu, J.L.; et al. The role of an elastic interphase in suppressing gas evolution and promoting uniform electroplating in sodium metal anodes. Energy Environ. Sci. 2023, 16, 535–545. [Google Scholar] [CrossRef]
- Zhang, W.; Peng, J.; Hua, W.; Liu, Y.; Wang, J.; Liang, Y.; Lai, W.H.; Jiang, Y.; Huang, Y.; Zhang, W.; et al. Architecting Amorphous Vanadium Oxide/MXene Nanohybrid via Tunable Anodic Oxidation for High-Performance Sodium-Ion Batteries. Adv. Energy Mater. 2021, 11, 2100757. [Google Scholar] [CrossRef]
- Gao, W.; Lu, Y.; Tan, X.; Wang, T.; Yu, Y.; Lu, Y.; Zhang, X.H.; Wang, J.; Liu, Y.; Liu, X.; et al. Sodiophilic design for sodium-metal batteries: Progress and prospects. Energy Environ. Sci. 2025, 18, 1630–1657. [Google Scholar] [CrossRef]
- Wan, G.L.; Peng, B.; Zhao, L.; Wang, F.; Yu, L.; Liu, R.; Zhang, G.Q. Dual-strategy modification on P2-Na0.67Ni0.33Mn0.67O2 realizes stable high-voltage cathode and high energy density full cell for sodium-ion batteries. SusMat 2023, 3, 58–71. [Google Scholar] [CrossRef]
- Hasa, I.; Passerini, S.; Hassoun, J. Toward high energy density cathode materials for sodium-ion batteries: Investigating the beneficial effect of aluminum doping on the P2-type structure. J. Mater. Chem. A 2017, 5, 4467–4477. [Google Scholar] [CrossRef]
- BhaskaraRao, B.V.; Pabba, D.P.; Aepuru, R.; Fakhrabadi, A.A.; Lokhande, P.; Udayabhaskar, R.; Vera, M.R.; González, R.E. Fe3O4 nanoparticles intercalated reduced graphene oxide nanosheets for supercapacitor and lithium-ion battery anode performance. J. Mater. Sci. Mater. Electron. 2023, 34, 1910. [Google Scholar] [CrossRef]
- Thomas, P.; Billaud, D. Electrochemical insertion of sodium into hard carbons. Electrochim. Acta 2002, 47, 3303–3307. [Google Scholar] [CrossRef]
- Xiao, L.; Cao, Y.; Xiao, J.; Wang, W.; Kovarik, L.; Nie, Z.; Liu, J. High capacity, reversible alloying reactions in SnSb/C nanocomposites for Na-ion battery applications. Chem. Commun. 2012, 48, 3321–3323. [Google Scholar] [CrossRef]
- Zhao, L.F.; Hu, Z.; Lai, W.H.; Tao, Y.; Peng, J.; Miao, Z.C.; Wang, Y.X.; Chou, S.L.; Liu, H.K.; Dou, S.X. Hard Carbon Anodes: Fundamental Understanding and Commercial Perspectives for Na-Ion Batteries beyond Li-Ion and K-Ion Counterparts. Adv. Energy Mater. 2021, 11, 2002704. [Google Scholar] [CrossRef]
- Hasegawa, G.; Kanamori, K.; Kannari, N.; Ozaki, J.; Nakanishi, K.; Abe, T. Hard Carbon Anodes for Na-Ion Batteries: Toward a Practical Use. ChemElectroChem 2015, 2, 1917–1920. [Google Scholar] [CrossRef]
- Wan, H.; Hu, X. Sulfur-doped honeycomb-like carbon with outstanding electrochemical performance as an anode material for lithium and sodium ion batteries. J. Colloid Interface Sci. 2020, 558, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.Y.; Lv, W.M.; Mu, C.; Zhao, J.; Wang, B. Activated hard carbon from orange peel for lithium/sodium ion battery anode with long cycle life. J. Alloys Compd. 2017, 701, 870–874. [Google Scholar] [CrossRef]
- Yu, P.; Tang, W.; Wu, F.F.; Zhang, C.; Luo, H.Y.; Liu, H.; Wang, Z.G. Recent progress in plant-derived hard carbon anode materials for sodium-ion batteries: A review. Rare Met. 2020, 39, 1019–1033. [Google Scholar] [CrossRef]
- Bommier, C.; Luo, W.; Gao, W.Y.; Greaney, A.; Ma, S.Q.; Ji, X. Predicting capacity of hard carbon anodes in sodium-ion batteries using porosity measurements. Carbon 2014, 76, 165–174. [Google Scholar] [CrossRef]
- Dou, X.W.; Hasa, I.; Saurel, D.; Jauregui, M.; Buchholz, D.; Rojo, T.; Passerini, S. Impact of the Acid Treatment on Lignocellulosic Biomass Hard Carbon for Sodium-Ion Battery Anodes. ChemSusChem 2018, 11, 3276–3285. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Zhu, X.S.; Liu, Y.; Fang, Y.; Zhou, X.; Bao, J.C. Rice husk-derived hard carbons as high-performance anode materials for sodium-ion batteries. Carbon 2018, 127, 658–666. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Li, M. Porous Hard Carbon Derived from Walnut Shell as an Anode Material for Sodium-Ion Batteries. JOM 2018, 70, 1387–1391. [Google Scholar] [CrossRef]
- Xu, S.D.; Zhao, Y.; Liu, S.; Ren, X.X.; Chen, L.; Shi, W.J.; Wang, X.M.; Zhang, D. Curly hard carbon derived from pistachio shells as high-performance anode materials for sodium-ion batteries. J. Mater. Sci. 2018, 53, 12334–12351. [Google Scholar] [CrossRef]
- Dou, X.W.; Geng, C.; Buchholz, D.; Passerini, S. Research Update: Hard carbon with closed pores from pectin-free apple pomace waste for Na-ion batteries. APL Mater. 2018, 6, 047501. [Google Scholar] [CrossRef]
- Zhu, X.M.; Jiang, X.Y.; Liu, X.L.; Xiao, L.; Cao, Y.L. A green route to synthesize low-cost and high-performance hard carbon as promising sodium-ion battery anodes from sorghum stalk waste. Green Energy Environ. 2017, 2, 310–315. [Google Scholar] [CrossRef]
- Zhang, T.; Mao, J.; Liu, X.L.; Xuan, M.J.; Bi, K.; Zhang, X.L.; Hu, J.H.; Fan, J.J.; Chen, S.; Shao, G.S. Pinecone biomass-derived hard carbon anodes for high-performance sodium-ion batteries. RSC Adv. 2017, 7, 41504–41511. [Google Scholar] [CrossRef]
- Cao, L.; Hui, W.; Xu, Z.W.; Huang, J.F.; Zheng, P.; Li, J.; Sun, Q.Q. Rape seed shuck derived-lamellar hard carbon as anodes for sodium-ion batteries. J. Alloys Compd. 2017, 695, 632–637. [Google Scholar] [CrossRef]
- Lotfabad, E.M.; Ding, J.; Cui, K.; Kohandehghan, A.; Kalisvaart, W.P.; Hazelton, M.; Mitlin, D. High-Density Sodium and Lithium Ion Battery Anodes from Banana Peels. ACS Nano 2014, 8, 7115–7129. [Google Scholar] [CrossRef]
- Zhang, F.; Yao, Y.G.; Wan, J.; Henderson, D.; Zhang, X.G.; Hu, L.B. High Temperature Carbonized Grass as a High Performance Sodium Ion Battery Anode. ACS Appl. Mater. Interfaces 2017, 9, 391–397. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, W.; Peng, J.; Guo, Y.X.; Cheng, L.H.; Chen, N.; Du, R.; Huang, Y.H.; Xue, L.H.; Zhang, W.X. Pore Structure Modification of Pitch-Derived Hard Carbon for Enhanced Pore Filling Sodium Storage. Energy Technol. 2022, 10, 2200612. [Google Scholar] [CrossRef]
- Bottoni, L.; Darjazi, H.; Sbrascini, L.; Staffolani, A.; Gabrielli, S.; Pastore, G.; Tombesi, A.; Nobili, F. Electrochemical Characterization of Charge Storage at Anodes for Sodium-Ion Batteries Based on Corncob Waste-Derived Hard Carbon and Binder. ChemElectroChem 2023, 10, e202201117. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, Z.; Xie, L.; Mao, Y.; Ji, W.J.; Liu, Z.J.; Wei, X.X.; Su, F.Y.; Chen, C.M. Releasing Free Radicals in Precursor Triggers the Formation of Closed Pores in Hard Carbon for Sodium-Ion Batteries. Adv. Mater. 2024, 36, 2401249. [Google Scholar] [CrossRef]
- Yu, C.; Hou, H.Y.; Liu, X.X.; Yao, Y.; Liao, Q.; Dai, Z.P.; Li, D.D. Old-loofah-derived hard carbon for long cyclicity anode in sodium ion battery. Int. J. Hydrogen Energy 2018, 43, 3253–3260. [Google Scholar] [CrossRef]
- Kuai, J.; Xie, J.; Wang, J.D.; Chen, J.Y.; Liu, F.; Xu, X.W.; Tu, J.; Cheng, J.P. Comparison and optimization of biomass-derived hard carbon as anode materials for sodium-ion batteries. Chem. Phy. Lett. 2024, 842, 141214. [Google Scholar] [CrossRef]
- Li, C.J.; Li, J.; Zhang, Y.C.; Cui, X.; Lei, H.; Li, G. Heteroatom-doped hierarchically porous carbons derived from cucumber stem as high-performance anodes for sodium-ion batteries. J. Mater. Sci. 2019, 54, 5641–5657. [Google Scholar] [CrossRef]
- Ishaq, M.; Jabeen, M.; He, Y.S.; Che, H.Y.; Xu, W.; Zhao, S.Z.; Shen, Y.X.; Li, L.; Ma, Z.F. Unveiling the Critical Role of Pre-Hydrothermal Effect in Plant Biowaste-Derived Hard Carbon for Superior Rate Capability and Cycle Life in Sodium-Ion Batteries. Adv. Energy Mater. 2025, 15, 2403142. [Google Scholar] [CrossRef]
- Sun, N.; Liu, H.; Xu, B. Facile synthesis of high performance hard carbon anode materials for sodium ion batteries. J. Mater. Chem. A 2015, 3, 20560–20566. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Zhang, T.; Wang, F.J.; Ran, F. High-efficiently doping nitrogen in kapok fiber-derived hard carbon used as anode materials for boosting rate performance of sodium-ion batteries. J. Energy Chem. 2024, 96, 472–482. [Google Scholar] [CrossRef]
- Chen, D.Q.; Zhang, W.; Luo, K.Y.; Song, Y.; Zhong, Y.J.; Liu, Y.X.; Wang, G.K.; Zhong, B.; Wu, Z.G.; Guo, X.D. Hard carbon for sodium storage: Mechanism and optimization strategies toward commercialization. Energy Environ. Sci. 2021, 14, 2244–2262. [Google Scholar] [CrossRef]
- Nayak, P.K.; Yang, L.T.; Brehm, W.G.; Adelhelm, P. From Lithium-Ion to Sodium-Ion Batteries: Advantages, Challenges, and Surprises. Angew. Chem. Int. Ed. 2018, 57, 102–120. [Google Scholar] [CrossRef]
- Nyathi, M.S.; Clifford, C.B.; Schobert, H.H. Characterization of graphitic materials prepared from different rank Pennsylvania anthracites. Fuel 2013, 114, 244–250. [Google Scholar] [CrossRef]
- Zhong, B.; Liu, C.; Xiong, D.; Cai, J.M.; Li, J.; Li, D.X.; Cao, Z.W.; Song, B.; Deng, W.T.; Peng, H.J.; et al. Biomass-Derived Hard Carbon for Sodium-Ion Batteries: Basic Research and Industrial Application. ACS Nano 2024, 18, 16468–16488. [Google Scholar] [CrossRef]
- Qiu, S.; Xiao, L.; Sushko, M.L.; Han, K.S.; Shao, Y.; Yan, M.; Liang, X.M.; Mai, L.Q.; Feng, J.W.; Cao, Y.L.; et al. Manipulating Adsorption-Insertion Mechanisms in Nanostructured Carbon Materials for High-Efficiency Sodium Ion Storage. Adv. Energy Mater. 2017, 7, 1700403. [Google Scholar] [CrossRef]
- Alvira, D.; Antorán, D.; Manyà, J.J. Plant-derived hard carbon as anode for sodium-ion batteries: A comprehensive review to guide interdisciplinary research. Chem. Eng. J. 2022, 447, 137468. [Google Scholar] [CrossRef]
- Sun, N.; Qiu, J.; Xu, B. Understanding of Sodium Storage Mechanism in Hard Carbons: Ongoing Development under Debate. Adv. Energy Mater. 2022, 12, 2200715. [Google Scholar] [CrossRef]
- Stevens, D.; Dahn, J. High Capacity Anode Materials for Rechargeable Sodium-Ion Batteries. J. Electrochem. Soc. 2000, 147, 1271. [Google Scholar] [CrossRef]
- Stevens, D.; Dahn, J. The Mechanisms of Lithium and Sodium Insertion in Carbon Materials. J. Electrochem. Soc. 2001, 148, A803. [Google Scholar] [CrossRef]
- Aniskevich, Y.; Yu, J.H.; Kim, J.Y.; Komaba, S.; Myung, S.T. Tracking Sodium Cluster Dynamics in Hard Carbon with a Low Specific Surface Area for Sodium-Ion Batteries. Adv. Energy Mater. 2024, 14, 2304300. [Google Scholar] [CrossRef]
- Cao, Y.L.; Xiao, L.F.; Sushko, M.L.; Wang, W.; Schwenzer, B.; Xiao, J.; Nie, Z.; Saraf, L.V.; Yang, Z.G.; Liu, J. Sodium Ion Insertion in Hollow Carbon Nanowires for Battery Applications. Nano Lett. 2012, 12, 3783–3787. [Google Scholar] [CrossRef]
- Chen, X.Y.; Fang, Y.L.; Tian, J.; Lu, H.Y.; Ai, X.P.; Yang, H.; Cao, Y.L. Electrochemical Insight into the Sodium-Ion Storage Mechanism on a Hard Carbon Anode. ACS Appl. Mater. Interfaces 2021, 13, 18914–18922. [Google Scholar] [CrossRef]
- Zhang, B.; Ghimbeu, C.M.; Laberty, C.; Guterl, C.V.; Tarascon, J.M. Correlation Between Microstructure and Na Storage Behavior in Hard Carbon. Adv. Energy Mater. 2016, 6, 1501588. [Google Scholar] [CrossRef]
- Li, Y.M.; Hu, Y.S.; Titirici, M.M.; Chen, L.Q.; Huang, X. Hard Carbon Microtubes Made from Renewable Cotton as High-Performance Anode Material for Sodium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1600659. [Google Scholar] [CrossRef]
- Bai, P.X.; He, Y.W.; Zou, X.X.; Zhao, X.X.; Xiong, P.X.; Xu, Y.H. Elucidation of the Sodium-Storage Mechanism in Hard Carbons. Adv. Energy Mater. 2018, 8, 1703217. [Google Scholar] [CrossRef]
- Bommier, C.; Surta, T.W.; Dolgos, M.; Ji, X. New Mechanistic Insights on Na-Ion Storage in Nongraphitizable Carbon. Nano Lett. 2015, 15, 5888–5892. [Google Scholar] [CrossRef] [PubMed]
- Alvin, S.; Yoon, D.; Chandra, C.; Cahyadi, H.S.; Park, J.H.; Chang, W.Y.; Chung, K.Y.; Kim, J. Revealing sodium ion storage mechanism in hard carbon. Carbon 2019, 145, 67–81. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, S.J.; Chang, J.H.; Seo, H.K.; Lee, J.Y.; Yuk, J.M. Atomic visualization of a non-equilibrium sodiation pathway in copper sulfide. Nat. Commun. 2018, 9, 922. [Google Scholar] [CrossRef]
- Li, Y.; Qian, J.; Zhang, M.H.; Wang, S.; Wang, Z.H.; Li, M.S.; Bai, Y.; An, Q.; Xu, H.J.; Wu, F.; et al. Co-Construction of Sulfur Vacancies and Heterojunctions in Tungsten Disulfide to Induce Fast Electronic/Ionic Diffusion Kinetics for Sodium-Ion Batteries. Adv. Mater. 2020, 32, 2005802. [Google Scholar] [CrossRef]
- Thompson, M.; Xia, Q.B.; Hu, Z.; Zhao, X.S. A review on biomass-derived hard carbon materials for sodium-ion batteries. Mater. Adv. 2021, 2, 5881–5905. [Google Scholar] [CrossRef]
- Chen, C.; Huang, Y.; Meng, Z.Y.; Xu, Z.P.; Liu, P.; Li, T. Multi-heteroatom doped porous carbon derived from insect feces for capacitance-enhanced sodium-ion storage. J. Energy Chem. 2021, 54, 482–492. [Google Scholar] [CrossRef]
- Liu, P.; Li, Y.M.; Hu, Y.S.; Li, H.; Chen, L.Q.; Huang, X.J. A waste biomass derived hard carbon as a high-performance anode material for sodium-ion batteries. J. Mater. Chem. A 2016, 4, 13046–13052. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, M.H.; Bai, Y.; Wang, X.; Dong, R.; Wu, C. Lotus Seedpod-Derived Hard Carbon with Hierarchical Porous Structure as Stable Anode for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 12554–12561. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Li, X.; Dong, P.; Wu, G.; Xiao, J.; Zeng, X.Y.; Zhang, Y.J.; Sun, X.L. Honeycomb-like Hard Carbon Derived from Pine Pollen as High-Performance Anode Material for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 42796–42803. [Google Scholar] [CrossRef]
- Lu, P.R.; Xia, J.L.; Dong, X.L. Rapid Sodium-Ion Storage in Hard Carbon Anode Material Derived from Ganoderma lucidum Residue with Inherent Open Channels. ACS Sustain. Chem. Eng. 2019, 7, 14841–14847. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.X.; Meng, Q.S.; Jensen, A.C.S.; Zhang, Q.Q.; Zhang, Q.H.; Tong, Y.X.; Qi, Y.; Gu, L.; Titirici, M.M.; et al. Regulating Pore Structure of Hierarchical Porous Waste Cork-Derived Hard Carbon Anode for Enhanced Na Storage Performance. Adv. Energy Mater. 2019, 9, 1902852. [Google Scholar] [CrossRef]
- Tang, Y.; He, J.W.; Peng, J.; Yang, J.; Wu, Z.Y.; Liu, P.; Zhou, K.J.; Hu, S.H.; Hu, L.; Wang, X.Y. Electrochemical Behavior of the Biomass Hard Carbon Derived from Waste Corncob as a Sodium-Ion Battery Anode. Energy Fuels 2024, 38, 7389–7398. [Google Scholar] [CrossRef]
- Li, X.Y.; Zhang, S.; Tang, J.J.; Yang, J.; Wen, K.; Wang, J.; Wang, P.; Zhou, X.Y.; Zhang, Y.G. Structural design of biomass-derived hard carbon anode materials for superior sodium storage via increasing crystalline cellulose and closing the open pores. J. Mater. Chem. A 2024, 12, 21176–21189. [Google Scholar] [CrossRef]
- Gao, T.T.; Zhou, Y.H.; Jiang, Y.; Xue, Z.; Ding, Y.H. Bamboo waste derived hard carbon as high performance anode for sodium-ion batteries. Diam. Relat. Mater. 2024, 150, 111737. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefining 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Antero, R.V.P.; Alves, A.C.F.; de Oliveira, S.B.; Ojala, S.A.; Brum, S.S. Challenges and alternatives for the adequacy of hydrothermal carbonization of lignocellulosic biomass in cleaner production systems: A review. J. Clean. Prod. 2020, 252, 119899. [Google Scholar] [CrossRef]
- Arie, A.A.; Tekin, B.; Demir, E.; Cakan, R.D. Hard carbons derived from waste tea bag powder as anodes for sodium ion battery. Mater. Technol. 2019, 34, 515–524. [Google Scholar] [CrossRef]
- Ren, X.X.; Xu, S.D.; Liu, S.; Chen, L.; Zhang, D.; Qiu, L. Lath-shaped biomass derived hard carbon as anode materials with super rate capability for sodium-ion batteries. J. Electroanal. Chem. 2019, 841, 63–72. [Google Scholar] [CrossRef]
- Wang, J.; Yan, L.; Ren, Q.J.; Fan, L.L.; Zhang, F.M.; Shi, Z.Q. Facile hydrothermal treatment route of reed straw-derived hard carbon for high performance sodium ion battery. Electrochim. Acta 2018, 291, 188–196. [Google Scholar] [CrossRef]
- Gaddam, R.R.; Niaei, A.H.F.; Hankel, M.; Searles, D.J.; Kumar, N.A.; Zhao, X.S. Capacitance-enhanced sodium-ion storage in nitrogen-rich hard carbon. J. Mater. Chem. A 2017, 5, 22186–22192. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Kumaresan, T.K.; Masilamani, S.A.; Raman, K.; Karazhanov, S.Z.; Subashchandrabose, R. High performance sodium-ion battery anode using biomass derived hard carbon with engineered defective sites. Electrochim. Acta 2021, 368, 137574. [Google Scholar] [CrossRef]
- Pozio, A.; Aurora, A.; Prosini, P. Hard carbon for sodium batteries: Wood precursors and activation with first group hydroxide. J. Power Sources 2020, 449, 227555. [Google Scholar] [CrossRef]
- Hong, K.; Qie, L.; Zeng, R.; Yi, Z.; Zhang, W.; Wang, D.; Yin, W.; Wu, C.; Fan, Q.; Zhang, W.; et al. Biomass derived hard carbon used as a high performance anode material for sodium ion batteries. J. Mater. Chem. A 2014, 2, 12733–12738. [Google Scholar] [CrossRef]
- Wu, C.; Yang, Y.R.; Zhang, Y.H.; Xu, H.; Huang, W.; He, X.X.; Chen, Q.H.; Dong, H.H.; Li, L.; Wu, X.Q.; et al. Industrial-Scale Hard Carbon Designed to Regulate Electrochemical Polarization for Fast Sodium Storage. Angew. Chem. Int. Ed. 2024, 63, e202406889. [Google Scholar] [CrossRef]
- Zhou, S.; Tang, Z.; Pan, Z.; Huang, Y.C.; Zhao, L.; Zhang, X.; Sun, D.; Tang, Y.; Dhmees, A.S.; Wang, H. Regulating closed pore structure enables significantly improved sodium storage for hard carbon pyrolyzing at relatively low temperature. SusMat 2022, 2, 357–367. [Google Scholar] [CrossRef]
- Xu, H.; Song, H.; Sun, M.; Zhang, Y.H.; Feng, X.Y.; Qin, W.; Wu, C.; Chou, S.; Wu, X.Q. Molecular-level precursor regulation strategy aids fast-charging hard carbon anodes for sodium-ion batteries. Nano Energy 2025, 137, 110824. [Google Scholar] [CrossRef]
- Hao, E.; Liu, W.; Liu, S.; Zhang, Y.; Wang, H.L.; Chen, S.G.; Cheng, F.; Zhao, S.; Yang, H.Z. Rich sulfur doped porous carbon materials derived from ginkgo leaves for multiple electrochemical energy storage devices. J. Mater. Chem. A 2017, 5, 2204–2214. [Google Scholar] [CrossRef]
- Luo, D.H.; Han, P.; Shi, L.; Huang, J.; Yu, J.; Lin, Y.; Du, J.G.; Yang, B.; Li, C.H.; Zhu, C.Z.; et al. Biomass-derived nitrogen/oxygen co-doped hierarchical porous carbon with a large specific surface area for ultrafast and long-life sodium-ion batteries. Appl. Surf. Sci. 2018, 462, 713–719. [Google Scholar] [CrossRef]
- Lv, W.M.; Wen, F.S.; Xiang, J.Y.; Zhao, J.; Li, L.; Wang, L.; Liu, Z.Y.; Tian, Y.J. Peanut shell derived hard carbon as ultralong cycling anodes for lithium and sodium batteries. Electrochim. Acta 2015, 176, 533–541. [Google Scholar] [CrossRef]
- Li, Y.; Chen, M.H.; Liu, B.; Zhang, Y.; Liang, X.; Xia, X.H. Heteroatom Doping: An Effective Way to Boost Sodium Ion Storage. Adv. Energy Mater. 2020, 10, 2000927. [Google Scholar] [CrossRef]
- Senthil, C.; Park, J.W.; Shaji, N.; Sim, G.S.; Lee, C.W. Biomass seaweed-derived nitrogen self-doped porous carbon anodes for sodium-ion batteries: Insights into the structure and electrochemical activity. J. Energy Chem. 2022, 64, 286–295. [Google Scholar] [CrossRef]
- Yang, T.Z.; Qian, T.; Wang, M.F.; Shen, X.W.; Xu, N.; Sun, Z.Z.; Yan, C.L. A Sustainable Route from Biomass Byproduct Okara to High Content Nitrogen-Doped Carbon Sheets for Efficient Sodium Ion Batteries. Adv. Mater. 2016, 28, 539–545. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, Y.; Chen, C.; Wang, M.Y.; Liu, P. Phosphorus-doped porous biomass carbon with ultra-stable performance in sodium storage and lithium storage. Electrochim. Acta 2019, 321, 134698. [Google Scholar] [CrossRef]
- Muruganantham, R.; Wang, F.M.; Liu, W.R. A green route N, S-doped hard carbon derived from fruit-peel biomass waste as an anode material for rechargeable sodium-ion storage applications. Electrochim. Acta 2022, 424, 140573. [Google Scholar] [CrossRef]
- Tian, W.; Wang, L.; Huo, K.; He, X.M. Red phosphorus filled biomass carbon as high-capacity and long-life anode for sodium-ion batteries. J. Power Sources 2019, 430, 60–66. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.N.; Feng, S.; Liu, D.; Wang, G.X.; Tan, Q.L.; Jiang, S.T.; Yuan, J.J. N, S self-doped porous carbon with enlarged interlayer distance as anode for high performance sodium ion batteries. Int. J. Energy Res. 2021, 45, 7082–7092. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Hu, Z.; Fan, C.L.; Gao, P.; Zhang, R.S.; Liu, Z.X.; Liu, J.S.; Liu, J. Novel Structural Design and Adsorption/Insertion Coordinating Quasi-Metallic Na Storage Mechanism toward High-performance Hard Carbon Anode Derived from Carboxymethyl Cellulose. Small 2023, 19, 2303296. [Google Scholar] [CrossRef]
- Guan, Z.X.; Liu, H.; Xu, B.; Hao, X.; Wang, Z.X.; Chen, L. Gelatin-pyrolyzed mesoporous carbon as a high-performance sodium-storage material. J. Mater. Chem. A 2015, 3, 7849–7854. [Google Scholar] [CrossRef]
- Kamiyama, A.; Kubota, K.; Igarashi, D.; Youn, Y.; Tateyama, Y.; Ando, H.; Gotoh, K.; Komaba, S. MgO-Template Synthesis of Extremely High Capacity Hard Carbon for Na-Ion Battery. Angew. Chem. Int. Ed. 2021, 60, 5114–5120. [Google Scholar] [CrossRef]
- Yin, T.; Zhang, Z.; Xu, L.; Li, C.; Han, D.D. Preparation of green high-performance biomass-derived hard carbon materials from bamboo powder waste. ChemistryOpen 2024, 13, e202300178. [Google Scholar] [CrossRef]
- Tonnoir, H.; Huo, D.; Canevesi, R.L.S.; Fierro, V.; Celzard, A.; Janot, R. Tannin-based hard carbons as high-performance anode materials for sodium-ion batteries. Mater. Today Chem. 2022, 23, 100614. [Google Scholar] [CrossRef]
- Sun, N.; Guan, Z.X.; Liu, Y.; Cao, Y.L.; Zhu, Q.Z.; Liu, H.; Wang, Z.X.; Zhang, P.; Xu, B. Extended “Adsorption-Insertion” Model: A New Insight into the Sodium Storage Mechanism of Hard Carbons. Adv. Energy Mater. 2019, 9, 1901351. [Google Scholar] [CrossRef]
- Alvin, S.; Yoon, D.; Chandra, C.; Susanti, R.F.; Chang, W.Y.; Ryu, C.K.; Kim, J.H. Extended flat voltage profile of hard carbon synthesized using a two-step carbonization approach as an anode in sodium ion batteries. J. Power Sources 2019, 430, 157–168. [Google Scholar] [CrossRef]
- Zheng, P.; Liu, T.; Yuan, X.Y.; Zhang, L.F.; Liu, Y.; Huang, J.F.; Guo, S.W. Enhanced Performance by Enlarged Nano-pores of Holly Leaf-derived Lamellar Carbon for Sodium-ion Battery Anode. Sci. Rep. 2016, 6, 26246. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, G.; Deng, X.; Tian, Y.; Xiao, X.; Deng, W.; Hou, H.; Zou, G.; Ji, X. Strongly Coupled Interfacial Engineering Inspired by Robotic Arms Enable High-Performance Sodium-Ion Capacitors. Adv. Funct. Mater. 2022, 32, 2205453. [Google Scholar] [CrossRef]
- Li, D.; Zhu, Y.; Xu, E.; Wang, H.; Chen, T.; Quan, J.; Zhang, Y.; Wang, L.; Jiang, Y. Ion- and air-tailored micro-honeycomb structures for superior Na-ion storage in coir-derived hard carbon. New J. Chem. 2019, 43, 10449–10457. [Google Scholar] [CrossRef]
- Wang, H.; Yu, W.; Shi, J.; Mao, N.; Chen, S.G.; Liu, W. Biomass derived hierarchical porous carbons as high-performance anodes for sodium-ion batteries. Electrochim. Acta 2016, 188, 103–110. [Google Scholar] [CrossRef]
- Liu, C.; Song, Z.; Deng, X.; Xu, S.; Zheng, R.; Deng, W.; Hou, H.S.; Zou, G.Q.; Ji, X.B. Interfacial/bulk synergetic effects accelerating charge transferring for advanced lithium-ion capacitors. Chin. Chem. Lett. 2024, 35, 109081. [Google Scholar] [CrossRef]
- Qin, D.; Liu, Z.Y.; Zhao, Y.Z.; Xu, G.; Zhang, F.; Zhang, X.G. A sustainable route from corn stalks to N, P-dual doping carbon sheets toward high performance sodium-ion batteries anode. Carbon 2018, 130, 664–671. [Google Scholar] [CrossRef]
- Wu, C.; Huang, W.; Zhang, Y.H.; Chen, Q.H.; Li, L.; Zhang, Y.J.; Wu, X.Q.; Chou, S.L. Revisiting the critical role of metallic ash elements in the development of hard carbon for advancing sodium-ion battery applications. eScience 2025, 5, 100371. [Google Scholar] [CrossRef]
- LeGe, N.; Zhang, Y.H.; Lai, W.H.; He, X.X.; Wang, Y.X.; Zhao, L.; Liu, M.; Wu, X.Q.; Chou, S.L. Potassium escaping balances the degree of graphitization and pore channel structure in hard carbon to boost plateau sodium storage capacity. Chem. Sci. 2025, 16, 1179–1188. [Google Scholar] [CrossRef]
- Wang, Y.X.; Li, M.; Zhang, Y.; Zhang, N. Defect-rich hard carbon designed by heteroatom escape assists sodium storage performance for sodium-ion batteries. Chem. Eng. J. 2024, 499, 156115. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Kang, Y.; Du, H.; Jia, T.; Xu, J.; Huang, Y.; Zhao, Y.; Kang, F.; Li, B.H. Cathode Recycling of Spent Sodium Ion Batteries. Energy Mater. Adv. 2024, 5, 0128. [Google Scholar] [CrossRef]


| Preparation Approach | Anode Material | Specific Capacity (mAh g−1) [Current Density (A g−1/C-Rate)] | Cycle Stability (%) [CURRENT Density (A g−1/C-Rate)] | Number of Cycles | Ref. |
|---|---|---|---|---|---|
| High-temperature pyrolysis | HCC1300 | 298 [0.03 A g−1] | 97 [0.2 C] | 100 | [58] |
| CC-1600 | 358 [0.03 A g−1] | 71 [2 C] | 2000 | [62] | |
| SP-1200 | 430 [0.03 A g−1] | 97.5 [0.05 A g−1] | 200 | [35] | |
| A-2.25-6-T | 342 [0.03 A g−1] | 88.5 [0.3 A g−1] | 400 | [64] | |
| LS1200 | 329 [0.05 A g−1] | 89.7 [0.05 A g−1] | 200 | [59] | |
| CDHC-1300 | 311 [0.03 A g−1] | 83.5 [0.1 C] | 1000 | [63] | |
| Hydrothermal pretreatment | PSDHCs-4 | 256 [0.1 C] | 97 [0.1 C] | 100 | [69] |
| WTHC-1000 | 375 [0.01 A g−1] | 81 [0.1 A g−1] | 100 | [68] | |
| RS-1300 | 372 [0.1 C] | 84 [0.4 C] | 200 | [70] | |
| Activation pretreatment | LPC-800 | 351 [0.1 A g−1] | 85.4 [0.2 A g−1] | 50 | [80] |
| H3PO4-AC | 288 [0.05 A g−1] | 84.6 [0.2 A g−1] | 220 | [75] | |
| ACGL | 320 [5 A g−1] | 99 [0.2 A g−1] | 500 | [79] | |
| Heteroatom-doped pretreatment | rP@N-BC | 248 [0.05 A g−1] | 74.8 [0.5 A g−1] | 100 | [87] |
| NS-MPC | 400 [0.1 A g−1] | 52 [2 A g−1] | 2500 | [86] | |
| N-CNS-1050 | 305 [0.05 A g−1] | 76.5 [0.5 A g−1] | 2000 | [89] | |
| Template pretreatment | BPPHC1100 | 354 [0.05 A g−1] | 92 [1 C] | 100 | [92] |
| M750 | 360 [0.1 A g−1] | 51 [0.1 A g−1] | 100 | [90] | |
| HC600-1500(F50:50) | 478 [0.025 A g−1] | 96 [0.25 A g−1] | 25 | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitchamsetti, N.; Kim, K.-h.; Han, H.; Mhin, S. Biomass-Derived Hard Carbon Anodes for Sodium-Ion Batteries: Recent Advances in Synthesis Strategies. Nanomaterials 2025, 15, 1554. https://doi.org/10.3390/nano15201554
Kitchamsetti N, Kim K-h, Han H, Mhin S. Biomass-Derived Hard Carbon Anodes for Sodium-Ion Batteries: Recent Advances in Synthesis Strategies. Nanomaterials. 2025; 15(20):1554. https://doi.org/10.3390/nano15201554
Chicago/Turabian StyleKitchamsetti, Narasimharao, Kyoung-ho Kim, HyukSu Han, and Sungwook Mhin. 2025. "Biomass-Derived Hard Carbon Anodes for Sodium-Ion Batteries: Recent Advances in Synthesis Strategies" Nanomaterials 15, no. 20: 1554. https://doi.org/10.3390/nano15201554
APA StyleKitchamsetti, N., Kim, K.-h., Han, H., & Mhin, S. (2025). Biomass-Derived Hard Carbon Anodes for Sodium-Ion Batteries: Recent Advances in Synthesis Strategies. Nanomaterials, 15(20), 1554. https://doi.org/10.3390/nano15201554





