Facile Green Synthesis of N-Type InP Thin-Film Photoanodes with Enhanced Photoelectrochemical Performance for Solar Hydrogen Generation
Abstract
1. Introduction
2. Experiments
2.1. Chemicals
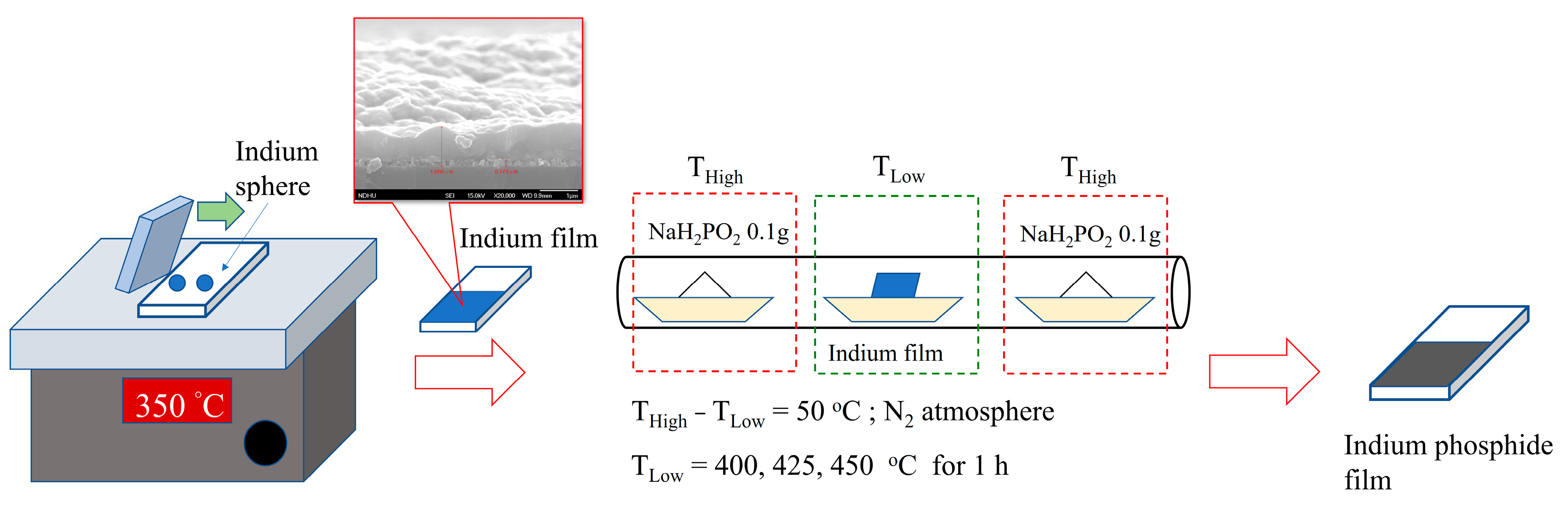
2.2. Preparation of InP Thin Films
2.3. Characterization
2.4. Photoelectrochemical Measurements
3. Results and Discussion
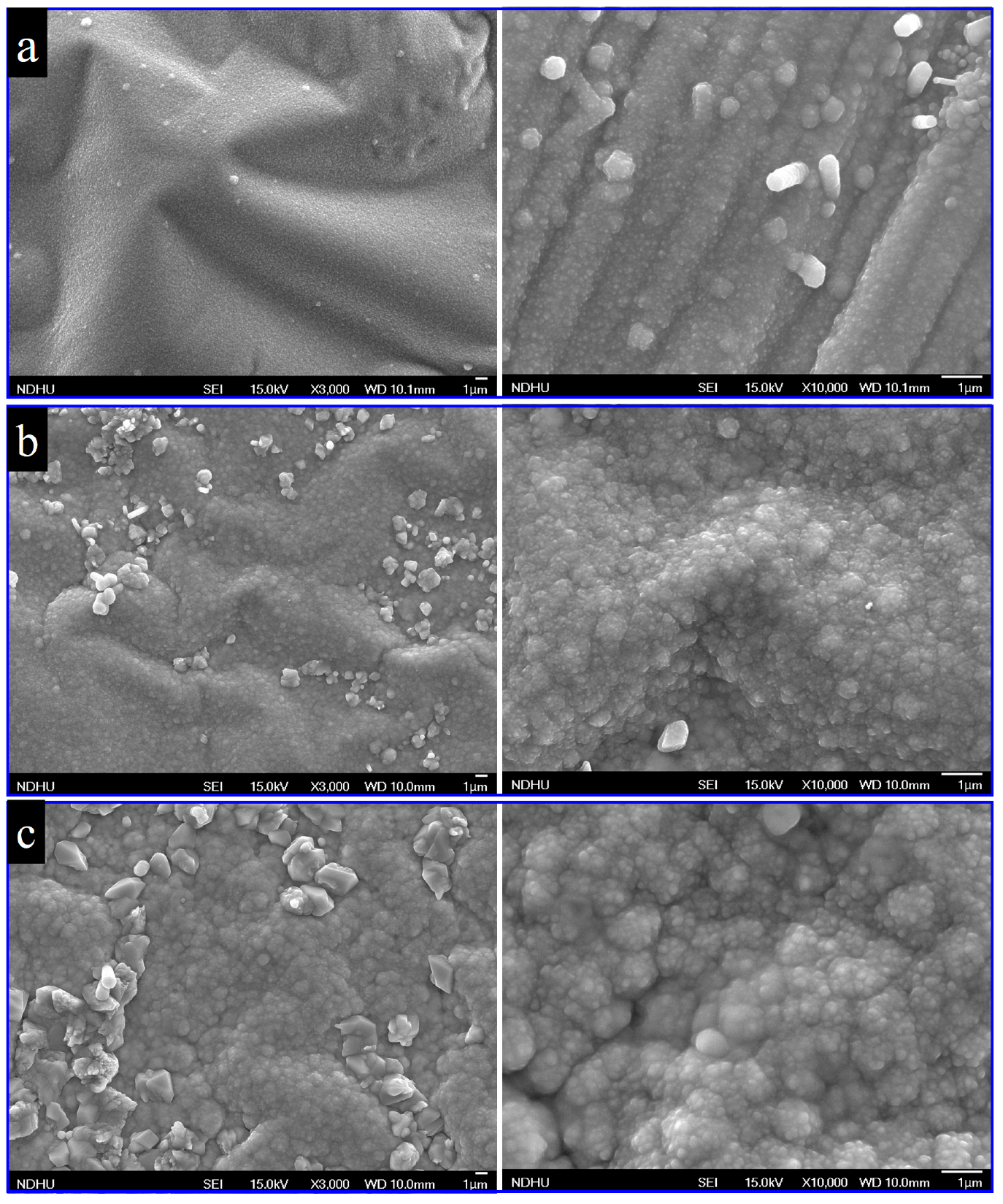
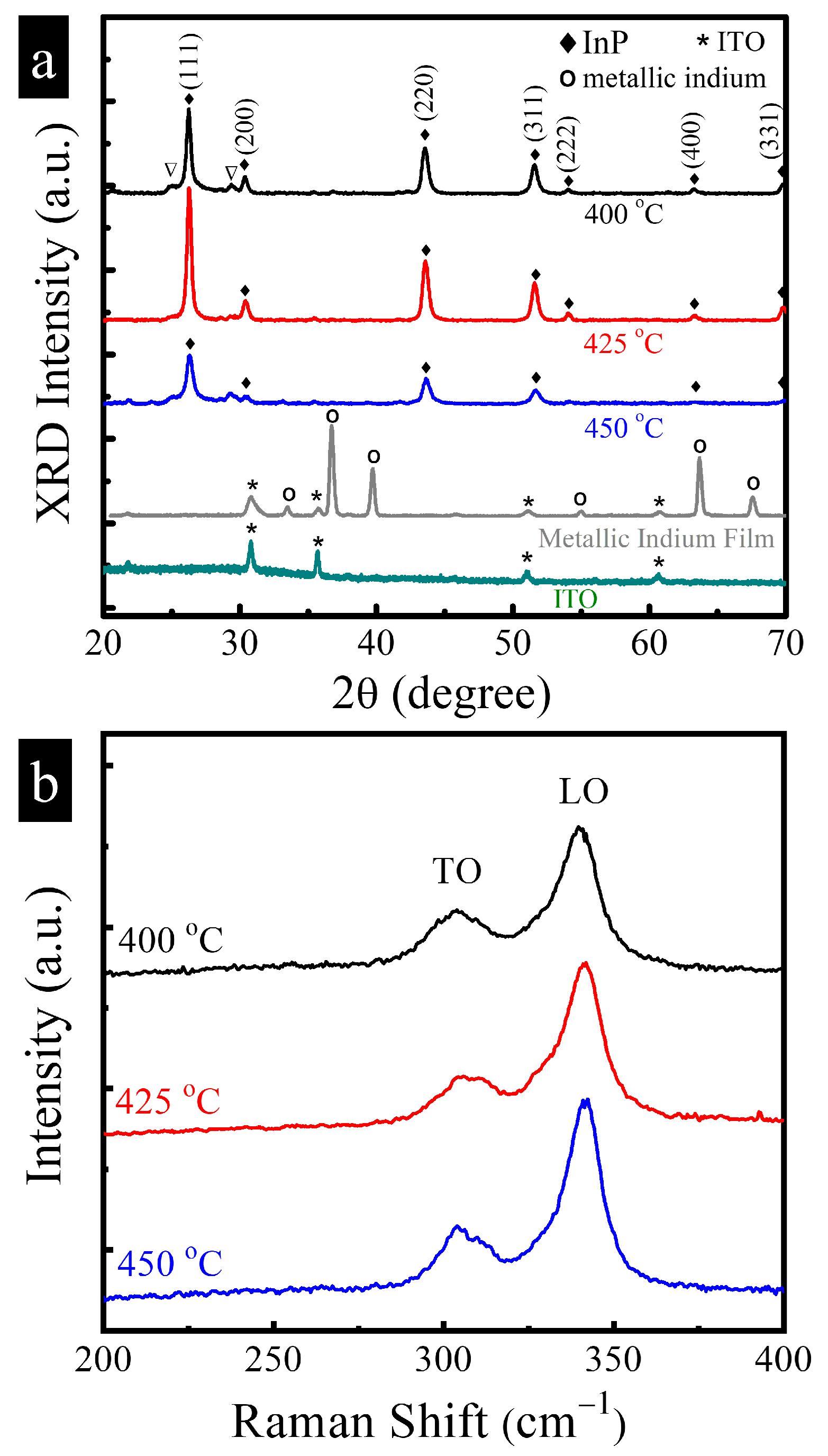
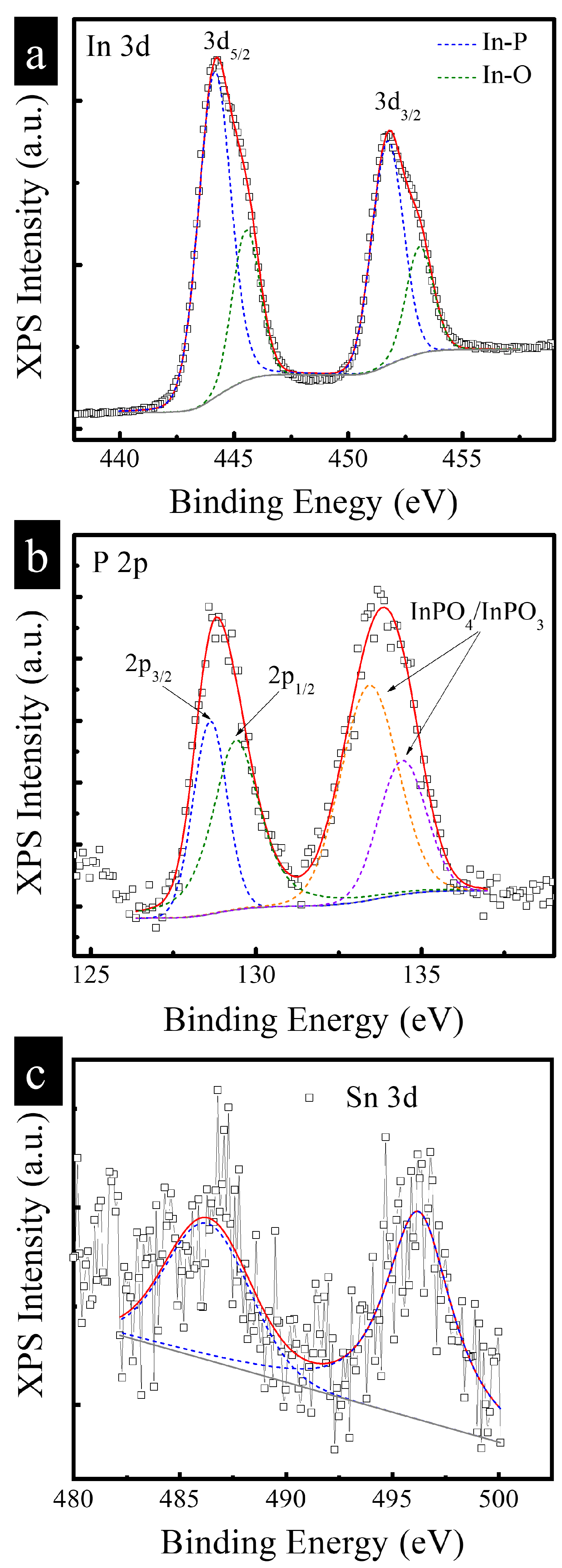
3.1. Characterization of Structure and Composition
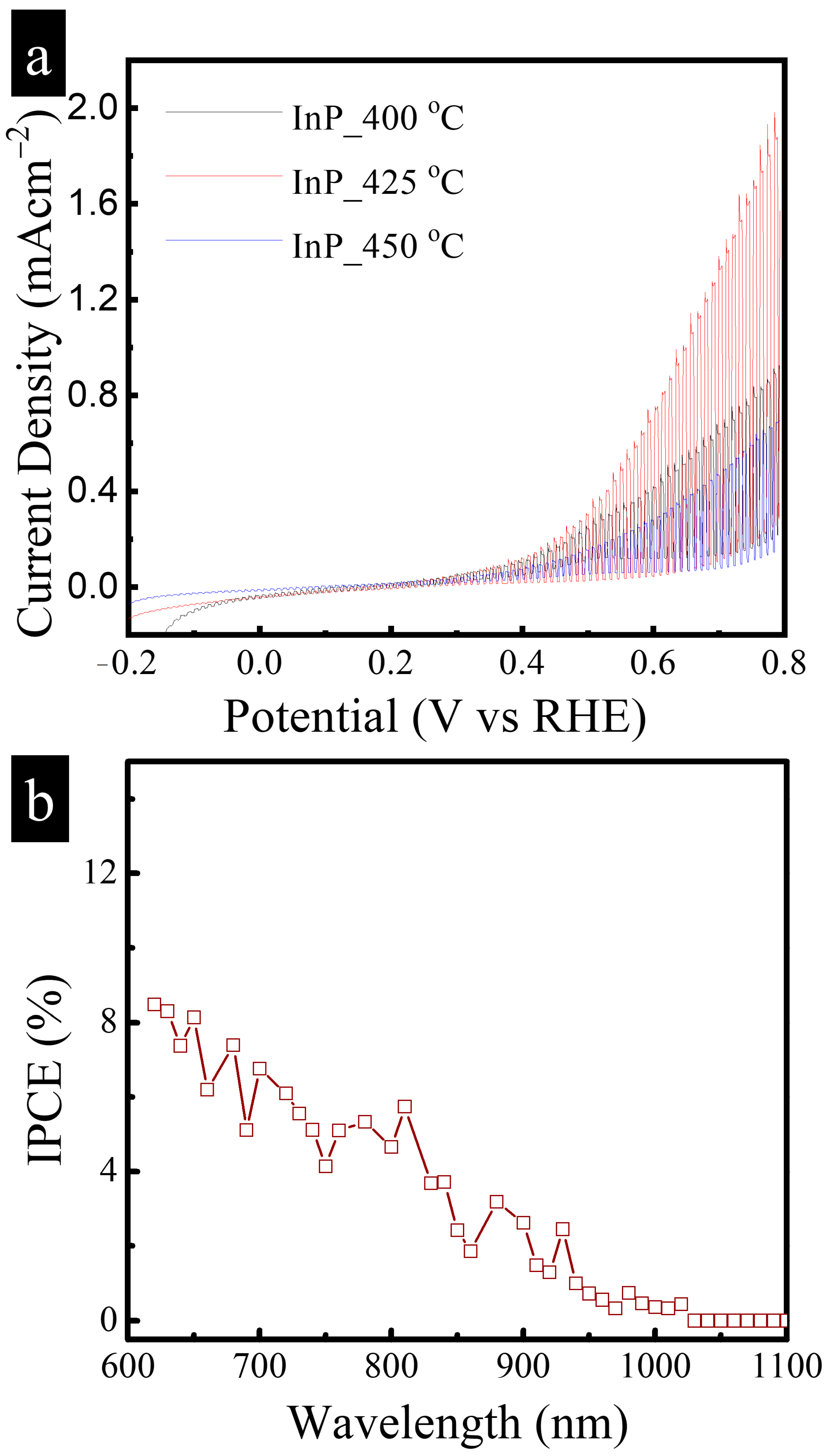
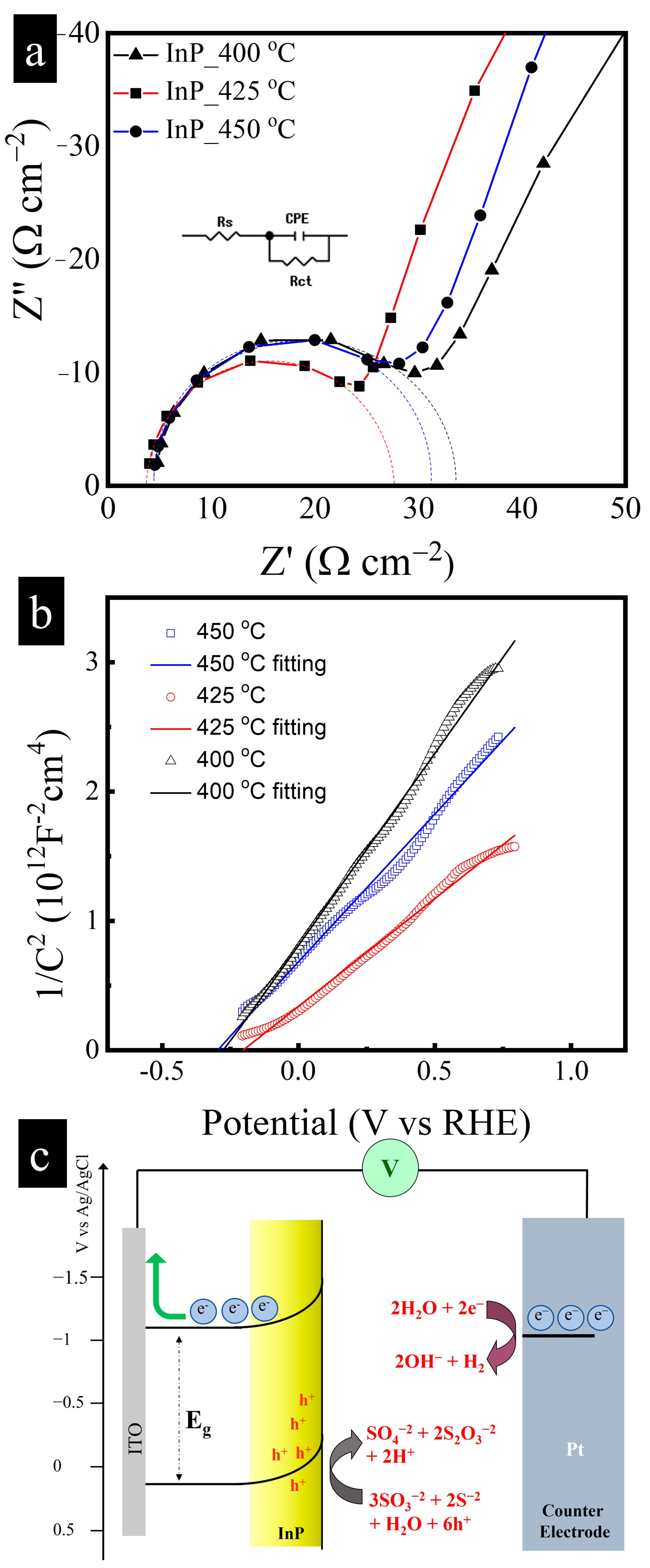
3.2. PEC Performance of InP Film Photoanodes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khaselev, O.; Turner, J.A. A monolithic photovoltaic-photoelectrochemical device for hydrogen production via water splitting. Science 1998, 280, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Lin, H.Y.; Hsu, Y.K. Boosting Photoelectrochemical Water Splitting via InPOx-Coated TiO2 Nanowire Photoanodes. Molecules 2025, 30, 3482. [Google Scholar] [CrossRef] [PubMed]
- Nisar, M.; Khan, N.; Qadir, M.I.; Shah, Z. Recent Advances in TiO2-Based Photocatalysts for Efficient Water Splitting to Hydrogen. Nanomaterials 2025, 15, 984. [Google Scholar] [CrossRef] [PubMed]
- Shabdan, Y.; Markhabayeva, A.; Bakranov, N.; Nuraje, N. Photoactive Tungsten-Oxide Nanomaterials for Water-Splitting. Nanomaterials 2020, 10, 1871. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Du, X.; Li, M.; Chen, H.; Hu, B.; Ding, H.; Wang, N.; Jin, L.; Liu, W. Enhanced photoelectrochemical water splitting performance of α-Fe2O3 photoanodes through Co-modification with Co single atoms and g-C3N4. Chem. Sci. 2024, 15, 12973–12982. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Jui, H.Y.; Chen, Y.W.; Hsu, Y.K. Record High Photocurrent Density of In:Ag2S/ZnO Heterojunction Enabled by Controllable Doping for Efficient Solar Hydrogen Production. J. Alloys Compd. 2025, 1010, 177325. [Google Scholar] [CrossRef]
- Zheng, Q.; Wang, J.; Huang, F.; Huang, Z.; Tian, S.; Chen, Q.; Pei, Y.; Zheng, K.; Tian, J. Constructing InP/ZnSe Quantum Dots with Shell Gradient In3+ Doping for Photoelectrochemical Cells. ACS Energy Lett. 2024, 9, 2358–2366. [Google Scholar] [CrossRef]
- Narangari, P.R.; Butson, J.D.; Tan, H.H.; Jagadish, C.; Karuturi, S. Surface-Tailored InP Nanowires via Self-Assembled Au Nanodots for Efficient and Stable Photoelectrochemical Hydrogen Evolution. Nano Lett. 2021, 21, 6967–6974. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Cui, Y.; Vervuurt, R.H.J.; Dam, D.V.; Veldhoven, R.P.J.V.; Hofmann, J.P.; Bol, A.A.; Haverkort, J.E.M.; Notten, P.H.L.; Bakkers, E.P.A.M.; et al. High-Effi ciency InP-Based Photocathode for Hydrogen Production by Interface Energetics Design and Photon Management. Adv. Funct. Mater. 2016, 26, 679–686. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, M.; Ma, L.; Zhong, M.; Zhu, C.; Zhang, B.; Wang, F.; Song, J.; Ma, L.; Shen, W. Unique Three-Dimensional InP Nanopore Arrays for Improved Photoelectrochemical Hydrogen Production. ACS Appl. Mater. Interfaces 2016, 8, 22493–22500. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, J.; Dai, K.; Li, H.; Zhang, M.; Zhu, G.; Zheng, M. In-situ synthesis of Au decorated InP nanopore arrays for enhanced photoelectrochemical hydrogen production. J. Alloys Compd. 2019, 774, 610–617. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Q.; Zhang, Z.; Lia, T.; Zhang, S. Synthesis of InP nanofibers from tri(m-tolyl)phosphine: An alternative route to metal phosphide nanostructures. Dalton Trans. 2010, 39, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, X.; Wei, L.; Zhang, T.; Wang, A.; Liu, C.; Prins, R. Disproportionation of hypophosphite and phosphite. Dalton Trans. 2017, 46, 6366–6378. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo-Fernandez, J.; Chavez-Angel, E.; Sanatinia, R.; Kataria, H.; Anand, S.; Lourdudoss, S.; Sotomayor-Torres, C.M. Thermal conductivity of epitaxially grown InP: Experiment and simulation. CrystEngComm 2017, 19, 1879–1887. [Google Scholar] [CrossRef]
- Virieux, H.; Troedec, M.L.; Cros-Gagneux, A.; Ojo, W.S.; Delpech, F.; Nayral, C.; Martinez, H.; Chaudret, B. InP/ZnS Nanocrystals: Coupling NMR and XPS for Fine Surface and Interface Description. J. Am. Chem. Soc. 2012, 134, 19701–19708. [Google Scholar] [CrossRef] [PubMed]
- Ezzedini, M.; Zeydi, I.; El Kazzi, S.; Jiang, S.; Guo, W.; Sfaxi, L.; M’ghaieth, R.; Maaref, H.; Merckling, C. Comprehensive study of Cp2Mg p-type doping of InP with MOVPE growth technique. J. Alloys Compd. 2015, 651, 344–349. [Google Scholar] [CrossRef]
- Jayarathna, R.A.; Heo, J.H.; Kim, E.T. Enhanced Photoelectrochemical Water Splitting of In2S3 Photoanodes by Surface Modulation with 2D MoS2 Nanosheets. Nanomaterials 2024, 14, 1628. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, S.; Wang, K.; Wang, X. Enhancing the PEC water splitting performance of In2O3 nanorods by a wet chemical reduction. Int. J. Hydrogen Energy 2022, 47, 38219–38228. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-C.; Lin, H.-Y.; Hsu, Y.-K. Facile Green Synthesis of N-Type InP Thin-Film Photoanodes with Enhanced Photoelectrochemical Performance for Solar Hydrogen Generation. Nanomaterials 2025, 15, 1544. https://doi.org/10.3390/nano15201544
Chen Y-C, Lin H-Y, Hsu Y-K. Facile Green Synthesis of N-Type InP Thin-Film Photoanodes with Enhanced Photoelectrochemical Performance for Solar Hydrogen Generation. Nanomaterials. 2025; 15(20):1544. https://doi.org/10.3390/nano15201544
Chicago/Turabian StyleChen, Ying-Chu, Heng-Yi Lin, and Yu-Kuei Hsu. 2025. "Facile Green Synthesis of N-Type InP Thin-Film Photoanodes with Enhanced Photoelectrochemical Performance for Solar Hydrogen Generation" Nanomaterials 15, no. 20: 1544. https://doi.org/10.3390/nano15201544
APA StyleChen, Y.-C., Lin, H.-Y., & Hsu, Y.-K. (2025). Facile Green Synthesis of N-Type InP Thin-Film Photoanodes with Enhanced Photoelectrochemical Performance for Solar Hydrogen Generation. Nanomaterials, 15(20), 1544. https://doi.org/10.3390/nano15201544




