Abstract
The development of high-performance supercapacitor electrodes is crucial to meet the increasing demand for efficient and sustainable energy storage systems. Cobalt oxide (Co3O4), with its high theoretical capacitance, is a promising electrode material, but its practical application is hindered by poor conductivity limitations and structural instability during cycling. In this work, lanthanum La3+-doped Co3O4 nanocubes were synthesized via a hydrothermal approach to tailor their structural and electrochemical properties. Different doping concentrations (1, 3, and 5%) were introduced to investigate their influence systematically. X-ray diffraction confirmed the retention of the spinel phase with clear evidence of La3+ incorporation into the Co3O4 lattice. Also, Raman spectroscopy validated the structural integrity through characteristic Co-O vibrational modes. Scanning electron microscopy analysis revealed uniform cubic morphologies across all samples. The formation of the cubic spinel structure of 1% La3+-doped Co3O4 are confirmed from XPS and TEM studies. Electrochemical evaluation in a 3 M KOH electrolyte demonstrated that 1% La3+-doped Co3O4 nanocubes delivered the highest performance, achieving a specific capacitance of 1312 F g−1 at 1 A g−1 and maintaining a 79.8% capacitance retention and a 97.12% Coulombic efficiency over 10,000 cycles at 5 Ag−1. It can be demonstrated that La3+ doping is an effective strategy to enhance the charge storage capability and cycling stability of Co3O4, offering valuable insights for the rational design of next-generation supercapacitor electrodes.
1. Introduction
The rising global demand for energy, coupled with growing environmental concerns, has placed immense pressure on the search for efficient, sustainable, and environmentally benign energy storage technologies. The depletion of fossil fuel reserves and the release of greenhouse gases are exacerbating these challenges, thereby intensifying research into alternative electrochemical systems for energy storage and conversion [1,2,3]. Among the wide range of candidates, including capacitors, batteries, and fuel cells, supercapacitors have emerged as a promising class of devices. They bridge the performance gap between traditional capacitors and batteries by offering both high power density and relatively high energy density. The performance of supercapacitors strongly depends on both material and operational factors, including the electrode–electrolyte interface, electrical contact with the current collector, operating temperature, and the applied current [4,5]. In recent decades, they have attracted considerable attention due to their advantageous features, including fast charge–discharge kinetics, high Coulombic efficiency, long cycle life, and cost-effective fabrication. Based on their charge storage mechanisms, supercapacitors are broadly categorized as: (i) electric double-layer capacitors (EDLCs), which rely on carbon-based nanostructures such as activated carbon, graphene, and carbon nanotubes, and (ii) pseudocapacitors, which utilize transition-metal oxides (TMOs) such as RuO2, NiO, Fe3O4, Mn3O4, V2O5, and Co3O4 materials to store charge through rapid and reversible Faradaic reactions [6,7,8,9,10,11,12].
Co3O4 nanoparticles have garnered considerable research interest among metal oxide nanoparticles due to their affordability and superior electroactivity. Co3O4 being a transition metal oxide, can display various oxidation states (Co2+, Co3+, and Co4+), enhancing its complex redox chemistry. Co3O4 is a multifunctional, antiferromagnitic p-type semiconductor characterized by a spinel crystal structure. The direct optical band gaps of Co3O4 nanoparticles are approximately 1.48 and 2.19 eV, facilitating their applications in several domains including catalysis [13], gas detection [14], water splitting [15], and biological [16] uses. Furthermore, Co3O4 has arisen as a viable electrode material for electrochemical energy storage systems, especially supercapacitors, due to its significant theoretical specific capacity of approximately 3560 F g−1 and the availability of numerous redox active sites that promote rapid and reversible faradaic reaction. However, its practical application is hindered by poor intrinsic conductivity, high internal resistance, and a relatively small electroactive surface area, resulting in capacitances that are far below the theoretical value [12,17,18]. To overcome these limitations, two primary strategies have been widely explored: (1) morphology engineering to expose more active sites, and (2) cation doping to improve conductivity and electrochemical activity [19,20]. An assortment of dopants such as Ni, Mn, Fe, Cu, Zn, and Cr has been integrated into the Co3O4 lattice to lower resistance and create more redox-active sites [21,22,23,24,25,26,27]. Additionally, Co3O4-based composites combined with carbon materials, conducting polymers and other metal compounds significantly improve its electrochemical performance for energy storage applications. For example, Annu et al. summarized that cobalt-based nanocomposite combined with conducting polymers such as polypyrrole (PPy) and polyaniline (PANI) exhibits superior electrochemical performance owing to the synergistic effect of cobalt oxide’s high capacitance and the polymers conductivity and pseudocapacitive behavior, making them promising for use in supercapacitors, batteries, and hybrid devices [28]. Deng et al. incorporated cadmium into porous Co3O4 nanosheets through a co-precipitation approach, and the optimized composition with 5% Cd delivered a specific capacitance of 737 F g−1at 1 A g−1, retaining 96% of its capacity after 1000 cycles [29]. Similarly, chromium-doped Co3O4 nanoflowers synthesized by Faisal et al. via a hydrothermal method exhibited a maximum capacitance of 1283 F g−1 at 6 at.% Cr and maintained 72.86% of their initial capacitance after 1000 charge–discharge cycles [2]. Aadil et al. reported the growth of mesoporous Ag-doped Co3O4 nanosheets on nickel foam, which offered a large surface area (176 m2 g−1) and excellent electrochemical properties, including 1425 F g−1at 1 A g−1, 92.84% retention at 10 A g−1, and stable cycling with 96.4% retention over 5000 cycles [30]. Additionally, Ce-doped Co3O4 nanoflakes were produced by Faisal et al. via the hydrothermal method and demonstrated an optimal performance at 5.0 at.% Ce, yielding 1309.6 F g−1 at 5 mV s−1, with 90.86% capacitance retention after 2000 cyclic voltammograms, and a rate capability of 82.87% at 10 A g−1 [31]. Furthermore, Aslam et al. incorporated Ni, Mn, and Zn dopants into Co3O4 via a co-precipitation process, where Mn0.05Co2.95O4 exhibited the most promising pseudocapacitive behavior with 80.8 F g−1 at 1 A g−1 along with excellent cycling stability [32].
More recently, rare earth metal doping (La, Nd, Gd, and Sm) has been investigated as a promising approach to further enhance the electrical conductivity and supercapacitor performance of Co3O4 [33]. In particular, Sm-doped Co3O4 electrodes exhibited excellent performance with a capacitance retention of 93.18%. The high ionic radii and strong complexation ability of rare earth ions can induce lattice distortion, resulting in smaller crystallite sizes, enhanced electrical conductivity, and reduced charge transfer resistance. Specifically, La3+ ions (with an ionic radius of 1.16 Å) exhibit a strong affinity for oxygen-containing functional groups, which stabilizes the surface structure and enhances electrical conductivity when incorporated into the Co3O4 [34]. In addition, various synthesis strategies have been explored for the fabrication of nanostructured Co3O4, including sol–gel, spray pyrolysis, chemical deposition, solvothermal, co-precipitation, and hydrothermal processes. Among these, the hydrothermal method stands out due to its simplicity, scalability, and precise control over morphology through reaction parameters (temperature, duration, precursor concentration) [34,35,36,37,38,39,40,41].
Therefore, in this study, La-doped Co3O4 nanocubes were synthesized via a facile hydrothermal method with varying La concentrations (1–5 mol%). The impact of La doping on the structural, morphological, and electrochemical properties was systematically evaluated. The results demonstrate that incorporating 1 mol% La significantly enhances the electrochemical performance, establishing La-doped Co3O4 as a highly promising electrode material for next-generation supercapacitor applications.
2. Materials and Methods
2.1. Chemical Materials
All chemicals and solvents used in this work were of reagent grade and employed without further purification. Cobalt (II) nitrate hexahydrate (Co(NO3)2·6H2O), lanthanum (III) nitrate hexahydrate (La(NO3)3·6H2O, 99.99%), and polyvinylidene fluoride (PVDF) were purchased from Sigma-Aldrich (Saint-Louis, MS, USA). Potassium hydroxide (KOH) pellets were obtained from Molychem. Additional materials included deionized (DI) water, ethanol (99.5%), activated carbon, and N-Methyl-2-pyrrolidone (NMP).
2.2. Preparation of Lanthanum-Doped Co3O4 Nanoparticles
Lanthanum-doped Co3O4 nanoparticles were synthesized via a hydrothermal method, as illustrated in Figure 1. Solution A was prepared by dissolving 1 mol L−1 Co(NO3)2·6H2O in 20 mL of DI water under constant stirring. Lanthanum nitrate was added in molar ratios of 1%, 3%, and 5% with respect to Co2+ ions, and the solution was until complete dissolution. Separately, solution B was prepared by dissolving 1 mol L−1 KOH in 20 mL of DI water. The solution was subsequently added drop wise to solution A while continuously stirring to achieve a homogenous mixture, with the pH of the combined solution maintained at 9 to ensure regulated nucleation and development of the nanoparticles. The resulting solution was transferred into a 100 mL Teflon-lined stainless-steel autoclave and heated at 200 °C for 24 h. After cooling to room temperature. The precipitated was collected, washed four times with ethanol and deionized water in order to remove impurities, and afterwards dried at 80 °C for 12 h. The dried powder was then calcinated at 400 °C for 4 h, utilizing a controlled heating rate of 3 °C min−1. The final products were labeled as 1%, 3%, and 5% La-Co3O4, reflecting the different lanthanum contents.
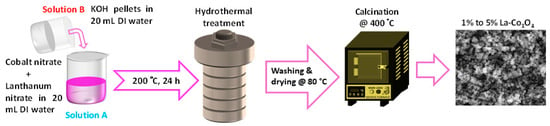
Figure 1.
Schematic representation of the synthesis procedure for the preparation of La (1–5%) doped cobalt oxide nanocubes.
2.3. Methods
The microstructural properties of the Co3O4 nanocubes were characterized by X-ray diffraction (XRD, Miniflex600, Rigaku, Tokyo, Japan) using CuKα radiation (λ = 1.5406 Å) as X-ray source. Surface morphology was examined using a field emission scanning electron microscope (FESEM) (Carl Ziess, Jena, Germany) and a high-resolution transmission electron microscope (HR-TEM JEM-2100, JEOL, Tokyo, Japan). Raman spectroscopy (Renishaw, Wottenunder-Edge, UK) equipped with a 532 nm excitation laser (power of 50 mW) was employed to analyze the vibrational modes and to confirm the phase purity of the samples. The valance state and the elemental composition were analyzed with X-ray photoelectron spectroscopy (XPS, Kratos AXIS-Nova, Shimadzu, Tokyo, Japan).
The electrochemical performance of the prepared cobalt oxide electrodes was evaluated in a 3 mol L−1 KOH aqueous electrolyte within a voltage range of 0.0 to 0.5 V at room temperature, using a CHI 608C electrochemical workstation (CH Instruments Inc., Austin, TX, USA) in a three-electrode glass cell. The working electrode was prepared by combining 80 wt.% active material, 10 wt.% PVDF, and 10 wt.% carbon black. After grinding these components for 3 h, a small amount of NMP solution was added to form a smooth slurry. Cleaned nickel foam pieces (1.5 cm2) were coated with the slurry through a drop-casting technique and dried overnight at 100 °C. For electrode preparation, commercial nickel foam sheets were cut into 1.5 cm × 1 cm pieces. Some foam pieces were immersed in 3 mol L−1 hydrochloric acid and then sonicated for 15 min to remove any residual hydrocarbons on the surface. The cleaned foams were then rinsed with deionized water and ethanol in an ultrasonic bath and dried in a vacuum oven for later use. The electrode mass loading was ~2 mg cm−2. The Ag/AgCl and a platinum strip were used as reference and counter electrodes, respectively.
3. Results and Discussion
3.1. Structure
Figure 2a presents the XRD patterns of pure and La-doped Co3O4 nanocubes with varying La contents (1, 3, and 5 mol%). The diffraction peaks of pristine Co3O4 sample are well indexed to the cubic spinel phase (JCPDS No. 00-042-1467), corresponding to the (111), (220), (311), (222), (400), (422), (511), (440), (531), (620), (533), and (622) planes. This confirms the formation of a single-phase spinel structure with a space group of Fd3m [42]. The absence of additional peaks further indicates the high phase purity of the samples. For the La-doped samples, no reflections corresponding to La-based secondary phases (e.g., La2O3 or LaCoO3) were observed, suggesting successful substitution of La3+ into the Co3O4 lattice or the presence of amorphous La species below the detection limit of XRD. However, slight peak shifts, variations in relative intensities, and changes in peak broadness reveal structural modifications induced by lanthanum incorporation (Figure 2b).
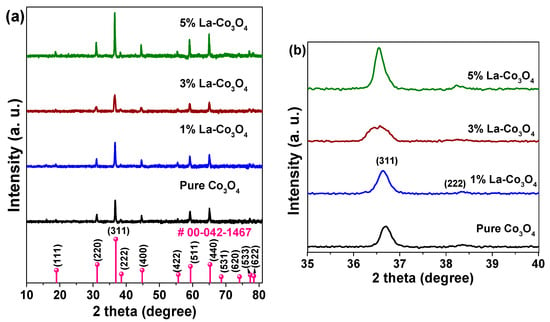
Figure 2.
(a) XRD spectra of pure and La (1–5%) doped Co3O4 nanocubes recorded using a CuKα X-ray source. (b) Expanded view of (311) and (222) diffraction planes.
At the 1 mol% La doping level, the (311) reflection exhibits slight broadening and enhanced intensity compared to the undoped sample. This is attributed to the lattice distortion and microstrain resulting from the substitution of Co3+ (ionic radius ~0.545 Å) with larger La3+ ions (ionic radius ~1.16 Å). The size mismatch introduces internal strain and crystallographic defects such as dislocations and vacancies. Increasing the La concentration to 3 mol% further broadens the peaks and decreases their intensity, implying a higher density of defects and microstrain, possibly due to excessive La incorporation that disrupts the spinel structure and limits crystallite growth. Interestingly, at 5 mol% La doping, the (311) peak becomes sharper and more intense than those of the 1% and 3% doped samples. This suggests partial recovery of crystallinity, likely due to saturation of La3+ solubility in the spinel lattice. At this level, La3+ ions may segregate toward grain boundaries or form amorphous La-rich domains undetectable by XRD, thereby relieving lattice strain and allowing coherent crystal growth, particularly along the (311) orientation.
The crystallite size (D) was estimated using Debye–Scherrer′s equation [43]
where λ is the X-ray wavelength (1.5406 Å for Cu Kα), β is the full width at half maximum (FWHM) in radians, and θ is the Bragg angle of the (311) planes. The dislocation density (δ) and microstrain (ε) were calculated using the relations [44,45]
The calculated values are summarized in Table 1. Pristine Co3O4exhibits a crystallite size of 30.6 nm. At the 1% La-doping content, the size slightly increases to 32.4 nm, which may result from slight lattice expansion due to the limited La3+ substitution into the Co3O4 lattice. At the 3% La-doping level, the crystallite size decreases sharply to 17.5 nm, accompanied by a notable increase in dislocation density and microstrain, which confirms a greater degree of structural disorder. In contrast, at the 5% La-doping content, the crystallite size increases to 38.0 nm with a decrease in microstrain, consistent with a La segregation and a partial strain relaxation at high doping levels.

Table 1.
Crystallite size, dislocation density, and microstrain of the pristine and La-doped (1–5%) Co3O4 samples.
The Raman spectra (Figure 3) of pristine and La-doped Co3O4 nanocubes exhibit five Raman-active modes at 194, 478.5, 520.1, 616.5, and 684.6 cm−1, corresponding to the F2g, Eg, F2g, F2g, and A1g phonon modes, respectively. These are in good agreement with the reported Raman modes of Co3O4. The band at 194 cm−1 arises from the translational vibration of Co2+–O2− at tetrahedral sites, while the 684.6 cm−1 band corresponds to the symmetric stretching vibration of Co3+–O2− in CoO6octahedra. The bands at 478.5, 520.1, and 616.5 cm−1 are associated with bending and stretching modes of Co–O bonds in octahedral sites. Slight shifts in peak positions with increasing La doping suggest lattice distortion and particle size effects. Notably, no Raman peaks attributed to La-based phases were detected, supporting the XRD results [46,47,48].
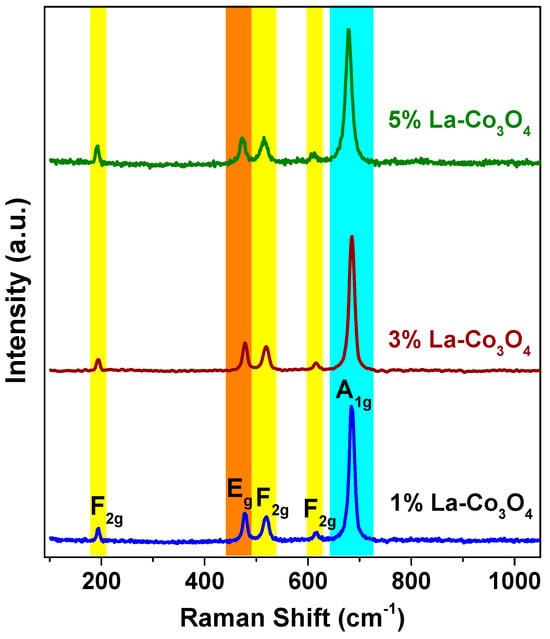
Figure 3.
Raman spectra of La-doped (1–5%) Co3O4 nanocubes recorded with a 532 nm excitation laser (power of 50 mW).
3.2. XPS Analysis
The surface chemical states of the 1 mol% La-doped Co3O4nanocubes were investigated using XPS experiments (Figure 4). The survey spectrum (Figure 4a) confirms the presence of Co 2p, O 1s, La 3d, and C 1s peaks, as well as minor Auger features, validating the successful incorporation of La into the cobalt oxide lattice. The high-resolution Co 2p spectrum (Figure 4b) displays two prominent peaks at 779.5 eV (Co 2p3/2) and 794.6 eV (Co 2p1/2), along with corresponding shake-up satellites. Deconvolution reveals contributions from both Co3+ (779.4 eV and 794.5 eV) and Co2+ (780.7 eV and 796.0 eV), confirming the mixed-valence state characteristic of the spinel Co3O4 phase. The presence of shake-up satellites further supports this coexistence [46,49]. The O 1s spectrum (Figure 4c) consists of three distinct components at 529.7 eV (lattice oxygen, Olatt), 531.2 eV (surface-adsorbed oxygen species, Oads), and 532.4 eV (hydroxyl groups or adsorbed water molecules, Ow) [50,51]. The dominance of the Olatt contribution indicates a well-ordered crystalline structure and effective oxygen incorporation within the spinel structure. The high-resolution La 3d spectrum (Figure 4d) reveals a broad spectral profile, deconvoluted into multiple peaks centered at 833.7, 837.7, 840.6, 844.2, and 847.7 eV. These values exhibit slight deviations from the standard binding energies typically associated with La3+ (La 3d5/2 at ~834 eV and La 3d3/2 at ~851 eV) [52]. The observed shifts and broadening may result from lattice distortions due to the low La doping level, leading to modified local coordination environments around the La ions. Quantitative XPS analysis reveals a surface La atomic percentage of 0.69%, which aligns reasonably well with the intended 1 mol% doping level, considering the surface-sensitive characteristics of XPS. The calculated La/(La + Co) atomic ratio is around 2.85, indicating a slight surface enrichment, a phenomenon commonly observed in metal oxide systems upon doping [53,54]. These results collectively confirm the successful substitution of La3+ into the Co3O4 lattice, likely occupying Co3+ sites and preserving the spinel structure.
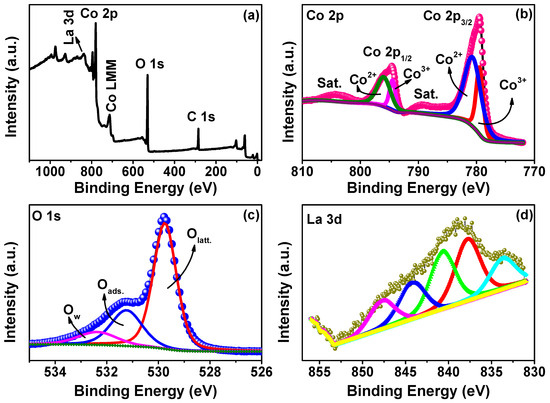
Figure 4.
(a) XPS survey spectrum and high-resolution (b) Co 2p XPS spectrum, (c) O 1s XPS spectrum, and (d) La 3d XPS spectrum of the 1 mol% La-doped Co3O4 sample.
3.3. Morphology
The surface morphology of La-doped Co3O4 samples was analyzed via field-emission scanning electron microscopy, as shown in Figure 5. All samples exhibit a well-defined cubic-like morphology, typical of spinel Co3O4 nanostructures. However, the La doping concentration significantly influences the uniformity and surface texture of the resulting particles. At a low doping level of 1 mol% La (Figure 5a), the particles appear as highly uniform nanocubes with smooth surfaces and sharply defined edges. This morphology suggests a high degree of crystallinity and minimal structural distortion, both of which are beneficial for enhanced electrochemical performance due to increased surface area and improved charge transport pathways. Increasing the La content to 3 mol% (Figure 5b) largely retains the cubic morphology, but noticeable surface roughness and particle aggregation begin to emerge.
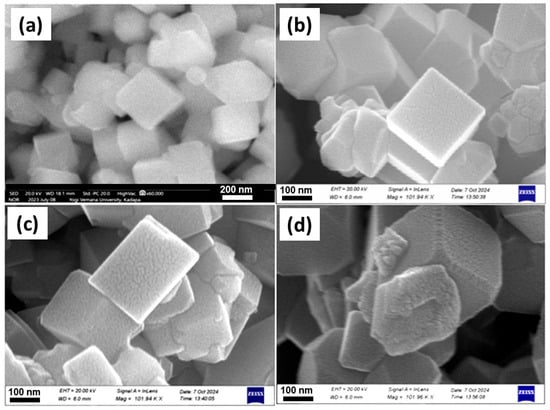
Figure 5.
FE-SEM images of La-doped Co3O4 nanocubes synthesized via hydrothermal method: (a) pristine, (b) 1 mol%, (c) 3 mol%, and (d) 5 mol% La-doped sample.
This result suggests a partial distortion of the crystal growth process, probably resulting from an excessive La incorporation, which may cause lattice strain or substitutional defects. The morphology of the 5 mol% La-Co3O4 sample (Figure 5c) exhibits a trend towards greater irregularity, characterized by less-defined cube structures and rough surfaces. The agglomeration of particles is more pronounced, and the uniformity of the nanostructures is significantly reduced. Such morphological deterioration may hinder electrochemical performance due to reduced active surface area and impaired ion/electron diffusion. These observations suggest that a low level of La doping (1 mol%) is optimal for maintaining a desirable nanocubic morphology and maximizing electrochemical performance. In contrast, higher La concentrations negatively affect particle uniformity and crystallinity, likely due to disruption of the spinel lattice during growth.
The elemental composition of the synthesized 1 mol% La-doped Co3O4 sample was analyzed with energy dispersive X-ray spectroscopy (EDS). The EDS spectrum is given as supplementary data in Figure S1 (Supplementary Materials). The presence of cobalt, oxygen, and lanthanum at their respective binding energies confirm a stoichiometric ratio consistent with the spinel Co3O4 phase with cobalt (26.59 at.%), oxygen (71.45 at.%) and lanthanum (1.96 at%) present in a stoichiometric ratio consistent with the spinel cobalt oxide (Co3O4) phase. The elemental mapping also confirms the uniform distribution of cobalt and oxygen throughout the scanning area of the nanocubes.
High-resolution transmission electron microscopy (HR-TEM) was utilized to examine the nanostructural properties of 1% La-doped Co3O4 nanocubes. Figure 6a illustrates a TEM picture that distinctly reveals a well-defined cubic morphology with sharp edges, thereby verifying the successful synthesis of homogeneous nanocubes. The particle size distribution obtained from many TEM images (Figure 6b) reveals an average particle size of approximately 131 nm. The lattice fringes in the HR-TEM image (Figure 6c) display an interplanar spacing of 0.249 nm, corresponding to the (311) plane of 1% La-Co3O4. The FFT pattern depicted in Figure 6c further substantiates the crystallinity of the nanocubes and the existence of distinctly resolved lattice planes. The minor increase in interplanar spacing relative to pure Co3O4 is due to the integration of bigger La3+ ions into the spinel lattice, resulting in localized lattice strain and minor distortions while preserving the overall cubic symmetry. The selected area electron diffraction (SAED) pattern (Figure 6d) exhibits bright spots accompanied by somewhat diffused rings, signifying the well-formed crystalline character of the nanocubes with a preferential orientation along the specific crystallographic axes. The integrated HR-TEM and SAED findings align remarkably with XRD investigations, validating that La doping marginally enlarges the lattice, maintains crystallinity, and does not alter the distinctive cubic shape of Co3O4. These structural alterations are anticipated to improve electrochemical characteristics by increasing active sites and enhancing ion transport channels within the nanocubes.
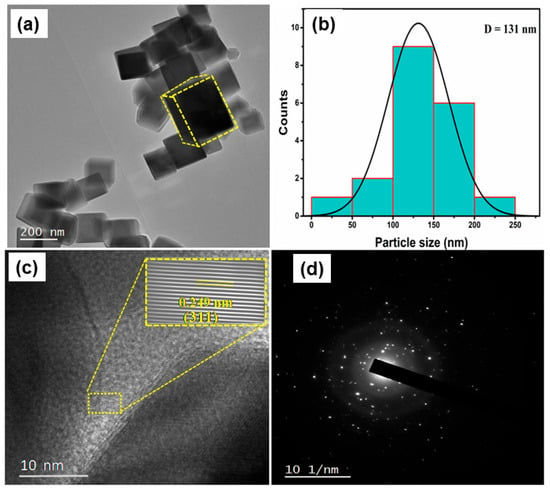
Figure 6.
HR-TEM analysis of 1 mol% La-doped Co3O4 nanocubes: (a) TEM image, (b) histogram of particle size distribution, (c) HR-TEM lattice fringe image, and (d) SAED pattern.
3.4. Electrochemical Properties
The electrochemical properties of La-doped Co3O4 electrodes were systematically studied using electrochemical impedance spectroscopy (EIS), cyclic voltammetry (CV), and galvanostatic charge–discharge (GCD) in a three-electrode configuration. The working electrodes consisted of La-doped Co3O4 composites with 1%, 3%, and 5% La content, while Ag/AgCl and Pt foil were used as the reference and counter electrodes, respectively. All measurements were carried out in a 3 mol L−1 KOH aqueous electrolyte.
Figure 7a shows the Nyquist plots of the La-doped Co3O4 electrodes. Each spectrum features a high-frequency intercept on the real axis, a depressed semicircle in the medium-frequency region, and an inclined line in the low-frequency region. The intercept corresponds to the solution resistance (Rs = 0.24 Ω), which reflects the combined effects of electrolyte, electrode, and contact resistances. The diameter of the semicircle represents the charge-transfer resistance (Rct) at the electrode/electrolyte interface. At the same time, the inclined line is associated with Warburg impedance, arising from ion diffusion processes within the porous electrode [55]. Among the samples, the 1% La-doped Co3O4 electrode shows the smallest semicircle diameter, indicating the lowest Rct (2.2 Ω) and the most efficient charge-transfer kinetics. This improvement arises from enhanced conductivity and better electronic interactions at low La doping. However, higher doping levels (3% and 5%) increase Rct, likely due to structural distortions or blockage of electroactive sites caused by excess La incorporation. The inset equivalent circuit model (Rs, Rct, a constant phase element Q for double-layer capacitance, and a Warburg element W) fits the experimental data well, supporting this interpretation. Overall, the EIS results confirm that 1% La doping achieves the best balance between conductivity and charge transfer, whereas higher doping concentrations degrade the electrochemical pathways. Figure 7b displays the CV profiles of the La-doped Co3O4 electrodes recorded at the scan rate of 10 mVs−1 within the potential window of 0 to 0.5 V (vs. Ag/AgCl). All electrodes show pronounced and nearly symmetric anodic and cathodic peaks, corresponding to the reversible Faradaic redox transitions of Co2+/Co3+ in an alkaline electrolyte according the following relationships [56,57]
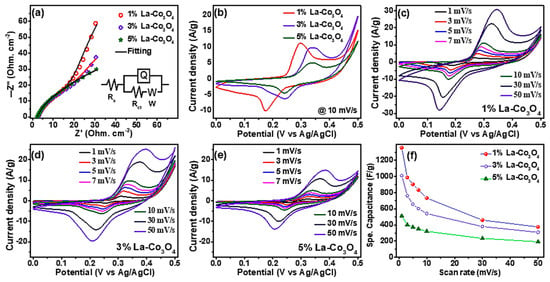
Figure 7.
(a) EIS spectra of La-Co3O4 electrodes with 1–5% La content; (b) CV curves at a scan rate of 10 mVs−1 for 1 to 5% La-Co3O4 electrodes; (c–e) CV curves of (c) 1%, (d) 3%, and (e) 5% La-Co3O4 electrodes at scan rates of 1 to 50 mVs−1; and (f) variation in specific capacitance with scan rate for 1–5% La-Co3O4 electrodes.
The 1% La-doped Co3O4 electrode delivers the highest peak currents and integrated CV area, indicating superior redox activity and faster kinetics compared with the 3% and 5% La-doped samples. At higher doping levels (3% La), partial blocking of electroactive sites and lattice distortions hinder the redox processes. Figure 7c–e presents the CV responses of the 1%, 3%, and 5% La-doped Co3O4 electrodes at scan rates in the range 1–50 mV s−1. As the scan rate increases, anodic peak potentials shift positively (from 0.26 to 0.35 V for 1% La; 0.30 to 0.39 V for 3% La; and 0.31 to 0.38 V for 5% La), while cathodic peaks shift negatively (from 0.19 to 0.13 V for 1% La, and 0.25 to 0.20 V for 3% and 5% La), consistent with polarization effects and internal resistance contributions [22,58]. Notably, the peak shapes remain well-preserved, indicating rapid electron/ion transport, as well as excellent reversibility. The specific capacitance (Cs) was calculated from the CV curves using the relation [59,60]:
where is the CV integral area, m is the active mass, v is the scan rate, and ΔV represents the potential window. Results are given in Table 2. As shown in Figure 7f and Table 2, the 1% La-doped Co3O4 electrode delivers the highest specific capacitance across all scan rates, followed by 3% and 5% La-doped Co3O4. A decrease in capacitance is observed with increasing scan rate for all electrodes, attributed to limited ion diffusion into the electrode’s deeper active sites at higher sweep rates [61]. These findings confirm that moderate La doping (approximately 1%) enhances electrochemical performance by promoting conductivity and redox kinetics, while excessive doping limits ion accessibility and compromises structural stability.

Table 2.
Specific capacitance of La doped Co3O4 samples at various scan rates.
The charge–storage mechanism was further analyzed using the power–law relationship [55,62,63]:
where ip is the peak current, v is the scan rate, and b reflects the dominant mechanism (0.5—diffusion-controlled; 1.0—capacitive-controlled). The calculated b-values were 0.65 (1% La-Co3O4), 0.61 (3% La-Co3O4), and 0.75 (5% La-Co3O4) (Figure 8a–c), suggesting mixed capacitive- and diffusion-controlled processes in all samples. The contribution percentages are listed in Table S1 (Supplementary Materials file).
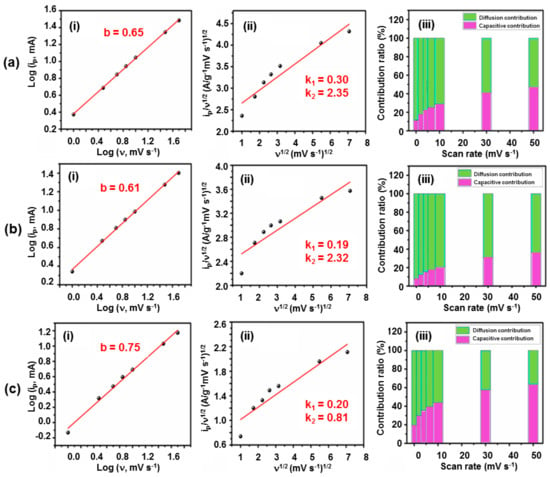
Figure 8.
Determination of the diffusive and capacitive contributions for (a) 1%, (b) 3%, and (c) 5% La-doped Co3O4 electrodes with nanocubic morphology. (i) Plot of Log (i) vs. Log (v), (ii) plot of vs., and (iii) diffusion/capacitive contribution ratio vs. scan rate.
The capacitive and diffusion contributions were quantified using:
where k1ν and k2ν1/2 represent the capacitive and diffusion-controlled contributions, respectively [59,63]. The capacitive and diffusion-controlled contributions in the electrodes at various scan rates were presented in Figure 8a(ii)–c(ii). This analysis reveals that the overall electrochemical performance of the La-doped Co3O4 electrodes is a result of the combined effects of both surface-controlled and diffusion-controlled processes. The relative dominance of these mechanisms varies with scan rate, as illustrated by the contribution plots in Figure 8a(iii)–c(iii). With increasing scan rate, a slight decrease in diffusion-controlled behavior is observed, indicating that surface-controlled redox reactions become more dominant under rapid charge–discharge conditions. The trend suggests that, at higher scan rates, ion intercalation into the bulk electrode is partially restricted due to the limited diffusion time, thereby favoring electrochemical reactions that occur at or near the electrode surface. At 50 mV s−1 scan rate, the capacitive contributions were calculated as 47.44% (1% La-Co3O4), 36.67% (3% La-Co3O4), and 63.58% (5% La-Co3O4). The 1% La-Co3O4 electrode demonstrates the most balanced contributions, enabling both rapid surface reflections and efficient ion intercalation.
The potential versus time plots at constant applied currents (GCD curves) were utilized to measure the specific capacitance, rate capability, and cycling stability of the electrodes. Figure 9a–c shows the GCD plots of the 1% La-Co3O4, 3% La-Co3O4, and 5% La-Co3O4 electrodes at current densities ranging from 1 to 5 A g−1 in the potential window of 0 to 0.4 V. The shape of the charge and discharge profiles confirms the high reversible electrochemical behavior. Based on the discharge time (from 0.4 to 0 V), the specific capacitances of the electrodes were calculated by using the following equation [57,60]:
where I represents the applied current, Δt denotes the discharge time, m is the active mass of the electrode, and ΔV refers to the potential window. The calculated specific capacitances of the electrode at corresponding current densities are presented in Figure 9d.
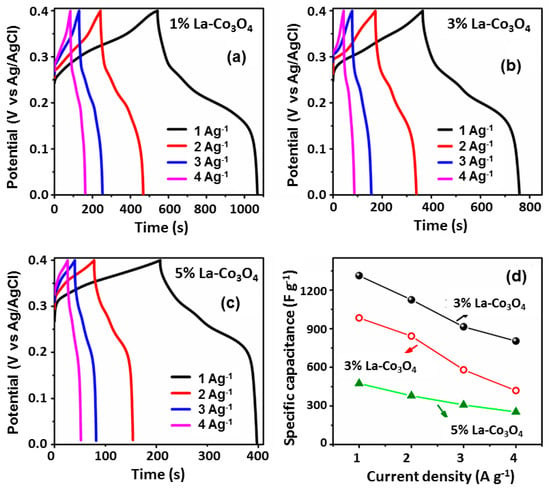
Figure 9.
GCD curves of La-doped Co3O4 at different doping levels: (a) 1% La-, (b) 3% La-, and (c) 5% La-doped. (d) Variation in the specific capacitance with applied current densities for La-doped Co3O4 electrodes.
Among all electrodes, 1% La-Co3O4 exhibited the highest specific capacitances at all current densities, i.e., 1312, 1124, 915, and 804 F g−1 at current densities of 1, 2, 3, and 4 A g−1, respectively. Likewise, 3% La-Co3O4 delivered 984, 842, 579, and 420 F g−1, while 5% La-Co3O4 shows 474, 379, 308, and 253 F g−1 at the same current densities, respectively. The specific capacitance of all electrodes decreases with increasing applied current densities due to the limitation on the interaction between electrolyte ions and the electrode material at higher current densities. In addition, the cycling stability behavior of the high specific capacitance delivered sample (1%La-doped Co3O4) was recorded at 5 A g−1 for 10,000 cycles, as shown in Figure 10. It demonstrates a Coulombic efficiency of 97.12% along with 79.8% capacitance retention even after 10,000 cycles.
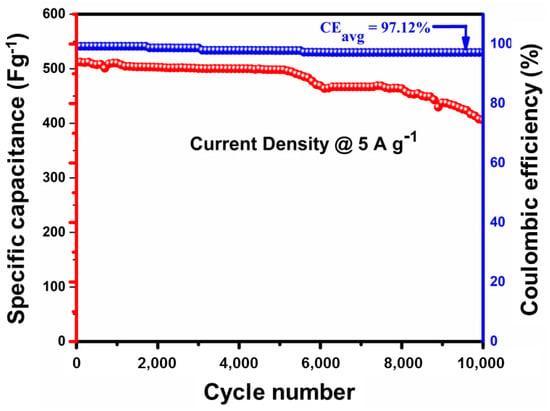
Figure 10.
Cycle stability of the specific capacitance and Coulombic efficiency carried out at 5 A g−1 current density for the 1% La-doped Co3O4 nanocubes.
The electrochemical properties of the La-doped Co3O4 electrodes compare well with those of pristine Co3O4 (see Figures S2 and S3 showing CV, GCD, long-term cycling and EIS results).
The structural study shows considerable differences in crystallite size, dislocation density, and microstrain between pristine and La-Co3O4 composites. The 3% La-Co3O4 sample has the smallest crystallite size (17.5 nm), excellent dislocation density (3.27 × 1015 lines m−2), and maximum microstrain (0.0066 rd). Such structural distortions and lattice defects have been shown to produce extra active sites and increase electrolyte ion accessibility by forming high-energy grain boundaries and defect-mediated diffusion channels [64]. Electrochemical experiments reveal that the 1% La-Co3O4 electrode exhibits the maximum capacitance (1355 F g−1 at 1 mV s−1), outperforming both the 3% and 5% La-Co3O4 electrodes. This better performance is due to the optimal balance of crystallite size and lattice imperfections. Excessive defects, such as those found in the 3% La-Co3O4 sample, can hinder long-range crystallinity and reduce electrical conductivity, lowering overall capacitance [65]. However, the moderate microstrain and dislocation density increase the number of electroactive sites and promote the number of electroactive sites and fast Faradaic reactions. The 5% La-Co3O4 sample has the highest crystallite size (38.0 nm) and lowest dislocation density (0.692 × 1015 lines/m2), but has a lower capacitance (511 F g−1 at 1 mV s−1). This trend indicates that crystallite overgrowth and low defect density limit the number of accessible electroactive sites, thereby reducing ion/electron transport efficiency [66]. Furthermore, the capacitance of all electrodes decreases as the scan rate increases owing to diffusion restrictions and reduced ion penetration into the deeper porous networks. The tendency is particularly prominent in the 3% La-Co3O4 sample, corresponding with its significant microstrain, which may generate localized lattice distortions but inhibit effective electron transport under rapid charge–discharge circumstances. The 1% La-Co3O4 electrode preserves greater capacitance at higher scan rates, indicating that an intermediate defect density and moderate crystallite size offer a better balance of structural integrity, conductivity, and electrochemical activity. In conclusion, the correlation between microstructural characteristics and electrochemical performance highlights that controlled La incorporation into Co3O4 enables defect engineering, where moderate strain and dislocation density enhance electrochemical kinetics. In contrast, excessive or insufficient defect concentrations impact capacitive behavior.
Furthermore, the specific capacitance and cycling stability of the as-prepared La-doped electrode were compared with those of previously reported earth-rare-doped cobalt oxide electrodes, as presented in Table 3. The results demonstrate its superior electrochemical performance in a three-electrode system compared to the earlier cobalt oxide electrodes.

Table 3.
Comparison of the electrochemical performance of the as-prepared La-doped Co3O4 electrode with other rare earth metal-doped (La, Nd, Gd, Sm, Ce, Pr, and Eu) cobalt oxides. Cycle numbers are marked in parenthesis.
4. Conclusions
In summary, La-doped Co3O4 nanocubes were synthesized via a facile hydrothermal method, and the effects of La incorporation on their structural, morphological, and electrochemical properties were systematically studied. Structural analyses (XRD, Raman, and XPS) confirmed the successful substitution of La3+ into the Co3O4 lattice, introducing lattice strain, dislocations, and oxygen vacancies that modify the crystallinity and defect density of the spinel framework. Morphological observations revealed that low-level doping (1 mol%) preserved the nanocubic architecture, characterized by smooth surfaces and uniform particle distribution. In contrast, higher La concentrations (3–5 mol%) disrupted the growth process, leading to aggregation, surface roughening, and reduced structural order. Electrochemical investigations demonstrated that these structural differences strongly influence redox kinetics, conductivity, and charge storage. The 1% La-Co3O4 electrode achieved the highest specific capacitance (1312 Fg−1 at 1 Ag−1), fast ion/electron transport, as evidenced by a low charge transfer resistance, and remarkable durability, with 79.8% capacitance retention and 97.12% Coulombic efficiency over 10,000 cycles at 5 Ag−1. These results concluded that low La doping effectively balances crystallinity, defect engineering, and morphology control to maximize electrochemical activity, whereas excessive doping compromises structural integrity and hinders charge transport. Therefore, this study not only identifies 1% La-Co3O4 as an optimal electrode material for high-performance supercapacitors but also provides valuable insights into the role of controlled doping strategies in tailoring spinel-type oxides for next-generation energy storage systems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano15191515/s1, Figure S1: EDS spectrum for 1% La-doped Co3O4 powder; Figure S2: Electrochemical properties of the pristine Co3O4 electrode. (a) Cyclic voltammograms of recorded at various scan rates (5–100 mV s−1), (b) log (ip) vs. log (scan rate) graph, (c) GCD curves recorded at various current densities (1–4 A g−1), and (d) Cycling stability performance at a current density of 3 A g−1; Figure S3: Nyquist plot of the pristine Co3O4 electrode; Table S1: Contribution of capacitive- and diffusion-controlled processes in all La-doped Co3O4 samples.
Author Contributions
Conceptualization, O.M.H.; methodology, B.H.; formal analysis, M.D. (Mudda Deepak); software: M.D. (Merum Dhananjaya); investigation, B.H. and M.D. (Mudda Deepak); resources, O.M.H.; data curation, M.D.; writing—original draft preparation, M.D. (Merum Dhananjaya); writing—review and editing, O.M.H. and C.M.J.; supervision, O.M.H. and C.M.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data are contained in this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CV | cyclic voltammetry |
| DI | deionized |
| EDLCs | electric double-layer capacitors |
| EIS | electrochemical impedance spectroscopy |
| FESEM | field emission scanning electron microscopy |
| GCD | galvanostatic charge discharge |
| NMP | N-Methyl-2-pyrrolidone |
| PVDF | polyvinylidene fluoride |
| TMO | transition-metal oxide |
| XRD | X-ray diffraction |
| XPS | X-ray photoelectron spectroscopy |
References
- Abbas, Q.; Mirzaeian, M.; Hunt, M.R.C.; Hall, P.; Raza, R. Current state and tuture prospects for electrochemical energy storage and conversion systems. Energies 2020, 13, 5847. [Google Scholar] [CrossRef]
- Ali, F.; Khalid, N.R. Facile synthesis and properties of chromium-doped cobalt oxide (Cr-Doped Co3O4) nanostructures for supercapacitor applications. Appl. Nanosci. 2020, 10, 1481–1488. [Google Scholar] [CrossRef]
- Qiu, Q.; Yang, Y.; Liang, F.; Wang, G.; Han, X.; Zang, C.; Ge, M. Recent advances in biomimetic porous materials for real-world applications. Biomimetics 2025, 10, 521. [Google Scholar] [CrossRef]
- Yoo, H.D.; Markevich, E.; Salitra, G.; Sharon, D.; Aurbach, D. On the challenge of developing advanced technologies for electrochemical energy storage and conversion. Mater. Today 2014, 17, 110–121. [Google Scholar] [CrossRef]
- Cheng, B.-H.; Zeng, R.J.; Jiang, H. Recent developments of post-modification of biochar for electrochemical energy storage. Bioresour. Technol. 2017, 246, 224–233. [Google Scholar] [CrossRef]
- Joseph, J.; Kunhi Kannan, S.; Surendran, K.K.; Navin, B.; Joseph, M.G. Safe and wide voltage electrolytes with n, p-doped porous carbon for supercapacitors. J. Power Sources 2025, 656, 238105. [Google Scholar] [CrossRef]
- Alfadhli, S.; Khasim, S.; Darwish, A.A.A.; Al-nahdi, K.; Abdelkader, M.; Gamal, R.; Hamdalla, T.A. Facile green synthesis of silver doped NiO nanoparticles using aloe vera latex for efficient energy storage and photocatalytic applications. Heliyon 2025, 11, e41322. [Google Scholar] [CrossRef]
- Joseph, N.; Shafi, P.M.; Sethulakshmi, J.S.; Karthik, R.; Bose, A.C.; Shim, J.-J. Three dimensional NiO nanonetwork electrode for efficient electrochemical energy storage application. Electrochim. Acta 2021, 399, 139392. [Google Scholar] [CrossRef]
- Tipsawat, P.; Wongpratat, U.; Phumying, S.; Chanlek, N.; Chokprasombat, K.; Maensiri, S. Magnetite (Fe3O4) nanoparticles: Synthesis, characterization and electrochemical properties. Appl. Surf. Sci. 2018, 446, 287–292. [Google Scholar] [CrossRef]
- Shaik, D.P.; Pitcheri, R.; Qiu, Y.; Hussain, O.M. Hydrothermally synthesized porous Mn3O4 nanoparticles with enhanced electrochemical performance for supercapacitors. Ceram. Int. 2019, 45, 2226–2233. [Google Scholar] [CrossRef]
- Dhananjaya, M.; Prakash, N.G.; Narayana, A.L.; Hussain, O.M. Microstructural and Supercapacitive Properties of One-Dimensional Vanadium Pentoxide Nanowires Synthesized by Hydrothermal Method. Appl. Phys. A 2018, 124, 185. [Google Scholar] [CrossRef]
- Haritha, B.; Deepak, M.; Hussain, O.M.; Julien, C.M. Morphological engineering of battery-type cobalt oxide electrodes for high-performance supercapacitors. Physchem 2025, 5, 11. [Google Scholar] [CrossRef]
- Drummer, S.; Mkhari, O.; Chowdhury, M. Green synthesis of Co3O4 nanoparticles using spent coffee: Application in catalytic and photocatalytic dye degradation. Next Nanotechnol. 2024, 6, 100069. [Google Scholar] [CrossRef]
- Xiao, S.; Xue, Y.; Li, Z.; Wu, C.; Zhang, Y.; Wu, P.; Tang, J.; Zhang, X.; Yuan, H.; Li, Y. High-performance Co3O4 nanoparticle-based gas sensor for leakage detection of eco-friendly insulating gas C4F7N. ACS Sens. 2025, 10, 2976–2985. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.N.; Hoang Nguyen, L.B.; Bui, T.K.; Nguyen, K.Q.; Pham, V.V. Review of Water Splitting Electrolysis over Cobalt Oxide Nanomaterials. ACS Appl. Nano Mater. 2025, 8, 3254–3271. [Google Scholar] [CrossRef]
- Sahu, M.; Singh, S.; Prajapati, S.; Verma, D.K.; Shin, D.K. From green chemistry to biomedicine: The sustainable symphony of cobalt oxide nanoparticles. RSC Adv. 2024, 14, 32733–32758. [Google Scholar] [CrossRef]
- Gopalakrishnan, P.P.; Thiyagaraj, S. A dual-function platform of green cobalt oxide nanoparticles for antimicrobial activity and high-performance symmetric supercapacitors. J. Inorg. Organomet. Polym. Mater. 2025. [Google Scholar] [CrossRef]
- Nguyen, D.T.A.; Alene, A.N.; Teshager, A.A.; Worku, A.K.; Abate, G.Y.; Li, H.-T. Enhanced electrochemical activity of boron doped cobalt oxide nanoparticles towards supercapacitor application. Electrochim. Acta 2025, 510, 145318. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, H.; Han, L.; Tao, K. Vacancy engineering of metal–organic framework derivatives for supercapacitors and electrochemical water splitting. Chem. Commun. 2025, 61, 8830–8842. [Google Scholar] [CrossRef]
- Ma, Y.; Bai, Y.; Liang, B.; Yang, R.; Jiang, X.; Zheng, S.; Zhang, C.; Hu, C. Doping-induced morphology modulation for boosting the capacity and stability of nanocrystals assembled Ni1-xCoxSe2. Appl. Surf. Sci. 2022, 579, 152156. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Mariappan, R.; Krishnamoorthy, E.; Bakkiyaraj, R. Effect of nickel doping on cobalt oxide nanoparticles for energy storage applications. Ionics 2024, 30, 2069–2082. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Mariappan, R. Characterization and electrochemical performance of Mn-doped Co3O4 nanoparticles for supercapacitor applications. J. Mater. Sci.: Mater. Electron. 2023, 34, 2092. [Google Scholar] [CrossRef]
- Lee, C.; Kim, S.K.; Choi, J.-H.; Chang, H.; Jang, H.D. Electrochemical performances of iron-cobalt oxides nanoparticles Loaded crumpled graphene for supercapacitor. J. Alloys Compd. 2018, 735, 2030–2037. [Google Scholar] [CrossRef]
- Lu, C.; Yang, Y.; Li, S.; Zhu, M. Nanosheet floral clusters of Fe-doped Co3O4 for high-performance supercapacitors. Mater. Chem. Front. 2024, 8, 2282–2292. [Google Scholar] [CrossRef]
- Kumar, S.; Reddy, L.; Hatshan, M.R.; Roy, N.; Kim, J.S.; Joo, S.W. Comprehensive characterization of octahedral Co3O4 doped with Cu induces oxygen vacancies as a battery-type redox active electrode material for supercapacitors. Ceram. Int. 2024, 50, 34726–34739. [Google Scholar]
- Guo, X.; Liu, Y.; Feng, L.; Xu, P.; Yang, Y.; Gao, X.; Sui, H.; Wang, X.; Yin, X.; Ma, X. Zn-substituted Co3O4 crystal anchored on porous carbon nanofibers for high performance supercapacitors. Surf. Interfaces 2024, 53, 105048. [Google Scholar] [CrossRef]
- Mallikarjuna, K.; Reddy, G.R.; Ghanem, M.A.; Dillip, G.R.; Joo, S.W. Insights of oxygen vacancies engineered structural, morphological, and electrochemical attributes of Cr-doped Co3O4 nanoparticles as redox active battery-type electrodes for hybrid supercapacitors. J. Energy Storage 2024, 100, 113751. [Google Scholar] [CrossRef]
- Annu; Park, S.S.; Alam, M.N.; Yewale, M.; Shin, D.K. Unraveling the electrochemical insights of cobalt oxide/conducting polymer hybrid materials for supercapacitor, battery, and supercapattery applications. Polymers 2024, 16, 2907. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Xiao, X.; Chen, G.; Wang, L.; Wang, Y. Cd-doped porous Co3O4 nanosheets as electrode material for high performance supercapacitor application. Electrochim. Acta 2016, 196, 316–327. [Google Scholar] [CrossRef]
- Aadil, M.; Zulfiqar, S.; Shahid, M.; Haider, S.; Shakir, I.; Warsi, M.F. Binder free mesoporous Ag-doped Co3O4 nanosheets with outstanding cyclic stability and rate capability for advanced supercapacitor applications. J. Alloys Compd. 2020, 844, 156062. [Google Scholar] [CrossRef]
- Ali, F.; Khalid, N.R.; Nabi, G.; Ul-Hamid, A.; Ikram, M. Hydrothermal synthesis of cerium-doped Co3O4 nanoflakes as electrode for supercapacitor application. Int. J. Energy Res. 2021, 45, 1999–2010. [Google Scholar] [CrossRef]
- Aslam, S.; Ramay, S.M.; Mahmood, A.; Mustafa, G.M.; Zawar, S.; Atiq, S. Electrochemical performance of transition metal doped Co3O4 as electrode material for supercapacitor applications. J. Sol-Gel Sci. Technol. 2023, 105, 360–369. [Google Scholar] [CrossRef]
- Garcia, M.F.L.; Arzuza, L.C.C.; Neves, G.A.; Loureiro, F.J.A.; Morales, M.A.; Macedo, D.A.; Lira, H.L.; Menezes, R.R. Structure and morphological properties of cobalt-oxide-based (Co3O4) materials as electrodes for supercapacitors: A brief review. Materials 2025, 18, 413. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Durai, G.; Tatarchuk, T.; Sumathi, M.; Kuppusami, P.; Qin, J.; Choi, M.Y. Synthesis of hierarchical structured rare earth metal–doped Co3O4 by polymer combustion method for high performance electrochemical supercapacitor electrode materials. Ionics 2020, 26, 2051–2061. [Google Scholar] [CrossRef]
- Priya, M.; Premkumar, V.K.; Vasantharani, P.; Sivakumar, G. Structural and electrochemical properties of ZnCo2O4 nanoparticles synthesized by hydrothermal method. Vacuum 2019, 167, 307–312. [Google Scholar] [CrossRef]
- Desai, R.S.; Jadhav, V.S.; Morankar, P.J.; Patil, S.B.; Sadale, S.B.; Pardeshi, S.R.; Lad, D.D.; Patil, P.S.; Jeon, C.-W.; Dalavi, D.S. Hydrothermal synthesis of self-supported hierarchical microflowers of Co3O4 nanowires for potential supercapacitor application. J. Electroanal. Chem. 2025, 976, 118800. [Google Scholar] [CrossRef]
- Yuanchun, Q.; Yanbao, Z.; Zhishen, W. Preparation of cobalt oxide nanoparticles and cobalt powders by solvothermal process and their characterization. Mater. Chem. Phys. 2008, 110, 457–462. [Google Scholar] [CrossRef]
- UmaSudharshini, A.; Bououdina, M.; Venkateshwarlu, M.; Manoharan, C.; Dhamodharan, P. Low temperature solvothermal synthesis of pristine Co3O4 nanoparticles as potential supercapacitor. Surf. Interfaces 2020, 19, 100535. [Google Scholar] [CrossRef]
- Turan, E.; Zeybekoğlu, E.; Kul, M. Effects of bath temperature and deposition time on Co3O4 thin films produced by chemical bath deposition. Thin Solid Film. 2019, 692, 137632. [Google Scholar] [CrossRef]
- Soltani, S.; Rozati, S.M.; Askari, M.B. Optical and electrochemical properties of spinel cubic nanostructured thin film Co3O4 prepared by spray pyrolysis. Phys. B: Condens. Matter 2022, 625, 413464. [Google Scholar] [CrossRef]
- Shaheen, I.; Ahmad, K.S.; Zequine, C.; Gupta, R.K.; Thomas, A.G.; Malik, M.A. Modified sol-gel synthesis of Co3O4 nanoparticles using organic template for electrochemical energy storage. Energy 2021, 218, 119502. [Google Scholar] [CrossRef]
- Jogdand, S.S.; Joshi, S.S. Effect of polymer combustion synthesis on thermal, spectroscopic, structural, and magnetic properties of Co3O4 nanoparticles. Polyhedron 2025, 269, 117419. [Google Scholar] [CrossRef]
- Guru Prakash, N.; Dhananjaya, M.; Lakshmi Narayana, A.; Hussain, O.M. One-dimensional MoO3/Pd nanocomposite electrodes for high performance supercapacitors. Mater. Res. Express 2019, 6, 085543. [Google Scholar] [CrossRef]
- Shanmugavalli, V.; Vishista, K. Low-cost synthesis of cubic spinel structured high efficient NiCo2O4/polyaniline nanocomposite for supercapacitor application. Mater. Res. Express 2019, 6, 045021. [Google Scholar] [CrossRef]
- Yewale, M.A.; Kadam, R.A.; Kaushik, N.K.; Koduru, J.R.; Velhal, N.B.; Nakate, U.T.; Jadhavar, A.A.; Sali, N.D.; Shin, D.K. Interconnected plate-like NiCo2O4 microstructures for supercapacitor application. Mater. Sci. Eng. B 2023, 287, 116072. [Google Scholar] [CrossRef]
- Efremova, A.; Rajkumar, T.; Szamosvölgyi, Á.; Sápi, A.; Baán, K.; Szenti, I.; Gómez-Pérez, J.; Varga, G.; Kiss, J.; Halasi, G.; et al. Complexity of a Co3O4 system under ambient-pressure CO2 methanation: Influence of bulk and surface properties on the catalytic performance. J. Phys. Chem. C 2021, 125, 7130–7141. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, X.; Hu, X.; Zhou, W.; Zhao, Y. Effect of formic acid treatment on the structure and catalytic activity of Co3O4 for N2O decomposition. Catal. Lett. 2019, 149, 1026–1036. [Google Scholar] [CrossRef]
- Lao, Y.; Zhu, N.; Jiang, X.; Zhao, J.; Dai, Q.; Wang, X. Effect of Ru on the activity of Co3O4 catalysts for chlorinated aromatics oxidation. Catal. Sci. Technol. 2018, 8, 4797–4811. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, Y.; Zhang, Y.; Zhou, J.; Xiao, W.; Gu, D. Highly ordered mesoporous cobalt oxide as heterogeneous catalyst for aerobic oxidative aromatization of N-heterocycles. ChemCatChem 2021, 13, 3679–3686. [Google Scholar] [CrossRef]
- Bae, J.; Shin, D.; Jeong, H.; Kim, B.-S.; Han, J.W.; Lee, H. Highly water-resistant La-doped Co3O4 catalyst for CO oxidation. ACS Catal. 2019, 9, 10093–10100. [Google Scholar] [CrossRef]
- Wei, X.; Barkaoui, S.; Chen, J.; Cao, G.; Wu, Z.; Wang, F.; Li, G. Investigation of Au/Co3O4 nanocomposites in glycol oxidation by tailoring Co3O4 morphology. Nanoscale Adv. 2021, 3, 1741–1746. [Google Scholar] [CrossRef]
- Gao, L.; Yao, Y.; Ma, Y.; Huang, J.; Chen, Y.; Chen, L.; Jia, L. La-doped LaFeCoOOH bifunctional materials for oxygen evolution reaction and supercapacitors. Adv. Funct. Mater. 2025, 36, 2507282. [Google Scholar] [CrossRef]
- Liu, M.; Bao, M.; Cao, H.; Yu, X.; Zhao, S.Q. Rare earth induced lattice distortion of Bi2WO6 to effectively improve photocatalytic performance: Experimental and DFT calculations. Opt. Mater. 2024, 155, 115798. [Google Scholar] [CrossRef]
- Das, D.; Prakash, J.; Bandyopadhyay, A.; Balhara, A.; Goutam, U.K.; Acharya, R.; Gupta, S.K.; Sudarshan, K. Modulating the effective ionic radii of trivalent dopants in ceria using a combination of dopants to improve catalytic efficiency for the oxygen evolution reaction. RSC Adv. 2024, 14, 17801–17813. [Google Scholar] [CrossRef] [PubMed]
- Rosaiah, P.; Zhu, J.; Hussain, O.M.; Qiu, Y. Facile and cost-effective synthesis of flower-like RGO/Fe3O4 nanocomposites with ultra-long cycling stability for supercapacitors. Ionics 2019, 25, 655–664. [Google Scholar] [CrossRef]
- Merum, D.; Pitcheri, R.; Gonuguntla, V.; Joo, S.W. Synthesis and properties of oxygen-deficient cobalt oxide nanocubes for supercapacitor application. Mater. Lett. 2023, 347, 134585. [Google Scholar] [CrossRef]
- Pitcheri, R.; Mooni, S.P.; Radhalayam, D.; Nora, M.; Roy, S.; Al-Zahrani, F.A.M.; Suneetha, M. Effect of Ce-doping on the structural, morphological, and electrochemical features of Co3O4 nanoparticles synthesized by solution combustion method for battery-type supercapacitors. Ceram. Int. 2024, 50, 50504–50515. [Google Scholar] [CrossRef]
- Kuang, L.; Ji, F.; Pan, X.; Wang, D.; Chen, X.; Jiang, D.; Zhang, Y.; Ding, B. Mesoporous MnCo2O4.5 nanoneedle arrays electrode for high-performance asymmetric supercapacitor application. Chem. Eng. J. 2017, 315, 491–499. [Google Scholar] [CrossRef]
- Merum, D.; Parvin, N.; Vattikuti, S.V.P.; Nallapureddy, R.R.; Pitcheri, R.; Shkir, M.; Manthrammel, M.A.; Banerjee, A.N.; Joo, S.W. Impact of Co-doping on the microstructural and electrochemical features of mesoporous 3D oval–shaped Ni3-xCoxV2O8 electrodes for high-performance hybrid supercapacitors. J. Energy Storage 2023, 61, 106674. [Google Scholar] [CrossRef]
- Shaik, D.P.M.D.; Kumar, M.V.S.; Reddy, P.N.K.; Hussain, O.M. High electrochemical performance of spinel Mn3O4 over Co3O4 nanocrystals. J. Mol. Struct. 2021, 1241, 130619. [Google Scholar] [CrossRef]
- Lakshmi Narayana, A.; Dhananjaya, M.; Guru Prakash, N.; Hussain, O.M.; Julien, C.M. Nanocrystalline Li2TiO3 electrodes for supercapattery application. Ionics 2017, 23, 3419–3428. [Google Scholar] [CrossRef]
- Deepak, M.; Hussain, O.M.; Julien, C.M. Microstructure and electrochemical properties of pure and vanadium-doped Li4Ti5O12 nanoflakes for high performance supercapacitors. Inorganics 2025, 13, 223. [Google Scholar] [CrossRef]
- Naresh, B.; Sreekanth, T.V.M.; Suma, C.N.; Sunil Kumar, K.; Yoo, K.; Kim, J. Urea-driven synthesis of MnCo2O4 nanocomposites for high-performance supercapacitors. J. Alloys Compd. 2025, 1037, 182232. [Google Scholar] [CrossRef]
- Aboelazm, E.A.; Ali, G.A.; Algarni, H.; Yin, H.; Zhong, Y.L.; Chong, K.F. Magnetic electrodeposition of the hierarchical cobalt oxide nanostructure from spent lithium-ion batteries: Its application as a supercapacitor electrode. J. Phys. Chem. C 2018, 122, 12200–12206. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Q.; Wang, T. Morphology-controllable synthesis of cobalt oxalates and their conversion to mesoporous Co3O4 nanostructures for application in supercapacitors. Inorg. Chem. 2011, 6482–6492. [Google Scholar] [CrossRef] [PubMed]
- Ali, G.A.; Fouad, O.A.; Makhlouf, S.A.; Yusoff, M.M.; Chong, K.F. Co3O4/SiO2 nanocomposites for supercapacitor application. J. Solid State Electrochem. 2014, 18, 2505–2512. [Google Scholar] [CrossRef]
- Alrowaily, A.W.; Abdullah, M.; Alotaibi, B.M.; Alyousef, H.A.; Alotiby, M.F.; Aman, S.; Al-Sehemi, A.G.; Henaish, A.M.A. Hydrothermally synthesized neodymium-doped Co3O4 nanostructures as electrode for supercapacitor applications. J. Mater. Sci. Mater. Electron. 2025, 36, 246. [Google Scholar] [CrossRef]
- Yang, D.; Xu, M.; Liang, X.; Wang, J.; Fang, W.; Zhu, C.; Wang, F. Facile synthesis of Pr-doped Co3O4 nanoflakes on the nickel-foam for high performance supercapacitors. Electrochim. Acta 2022, 406, 139815. [Google Scholar] [CrossRef]
- Wei, C.; Liu, K.; Tao, J.; Kang, X.; Hou, H.; Cheng, C.; Zhang, D. Self-template synthesis of hybrid porous Co3O4-CeO2 hollow polyhedrons for high-performance supercapacitors. Chem. Asian J. 2018, 13, 111–117. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhou, W.; Li, D.; Liang, Y.; Chao, S.; Zhao, X.; Zhang, M.; Xu, J. Rare earth doping engineering tailoring advanced oxygen-vacancy Co3O4 with tunable structures for high-efficiency energy storage. Small 2023, 19, 2206956. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).