Synthesis and Characterization of a Nanoscale Hyaluronic Acid-Specific Probe for Magnetic Particle Imaging and Magnetic Resonance Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. IONP
2.3. Purification of IONP Intermediates and HAIONPs by Magnetic Separation During Synthesis
2.4. Analytic Measurements
2.5. Nuclear Magnetic Resonance (NMR)-Relaxivities
2.6. Magnetic Particle Spectroscopy (MPS) and Direct Current Magnetization (DCM) Characterization
2.7. Dynamic Light Scattering (DLS) and ζ-Potential
2.8. Bicinchoninic Acid (BCA) Assay to Determine the Amount of HA Specific Peptide Incorporated Within HAIONPs
2.9. Quartz Crystal Microbalance (QCM) Device
2.10. QCM Measurement and Evaluation
2.11. Preparation of Samples for MPS Adsorption Measurements
2.11.1. Adsorption of HAIONPs on GAG-Coated QCM Sensors, Static Without Flow
2.11.2. Adsorption of HAIONPs on GAG-Coated QCM Sensors After QCM Affinity Measurements
2.12. MPS Measurement of the Adsorption of HAIONPs on GAG-Coated QCM Sensors Without Flow and QCM Sensors After Affinity Measurement
2.13. Preparation of IONP Control Sample (IONPC) by Treatment of Synomag®-D with Magnetic LS Columns
2.14. Synthesis
2.14.1. Chondroitin Sulfate L-Cysteine Conjugate (CS-cys)
2.14.2. Hyaluronic Acid L-Cysteine Conjugate (HA-cys)
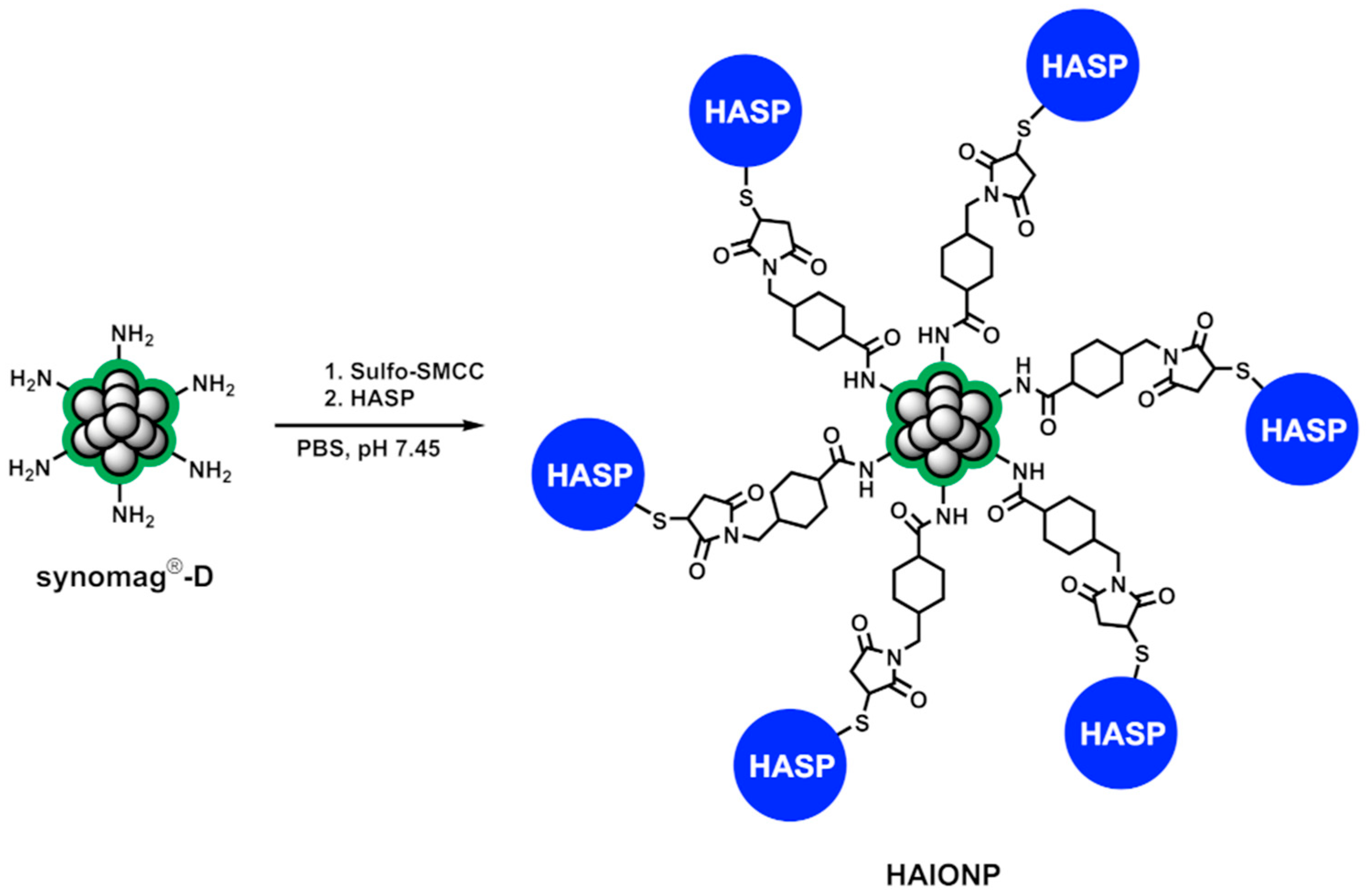
2.14.3. Synthesis of HA-Specific Iron Oxide Nanoparticles (HAIONPs)
3. Results and Discussion
3.1. Synthesis of HAIONPs
3.2. Characterization of HAIONPs
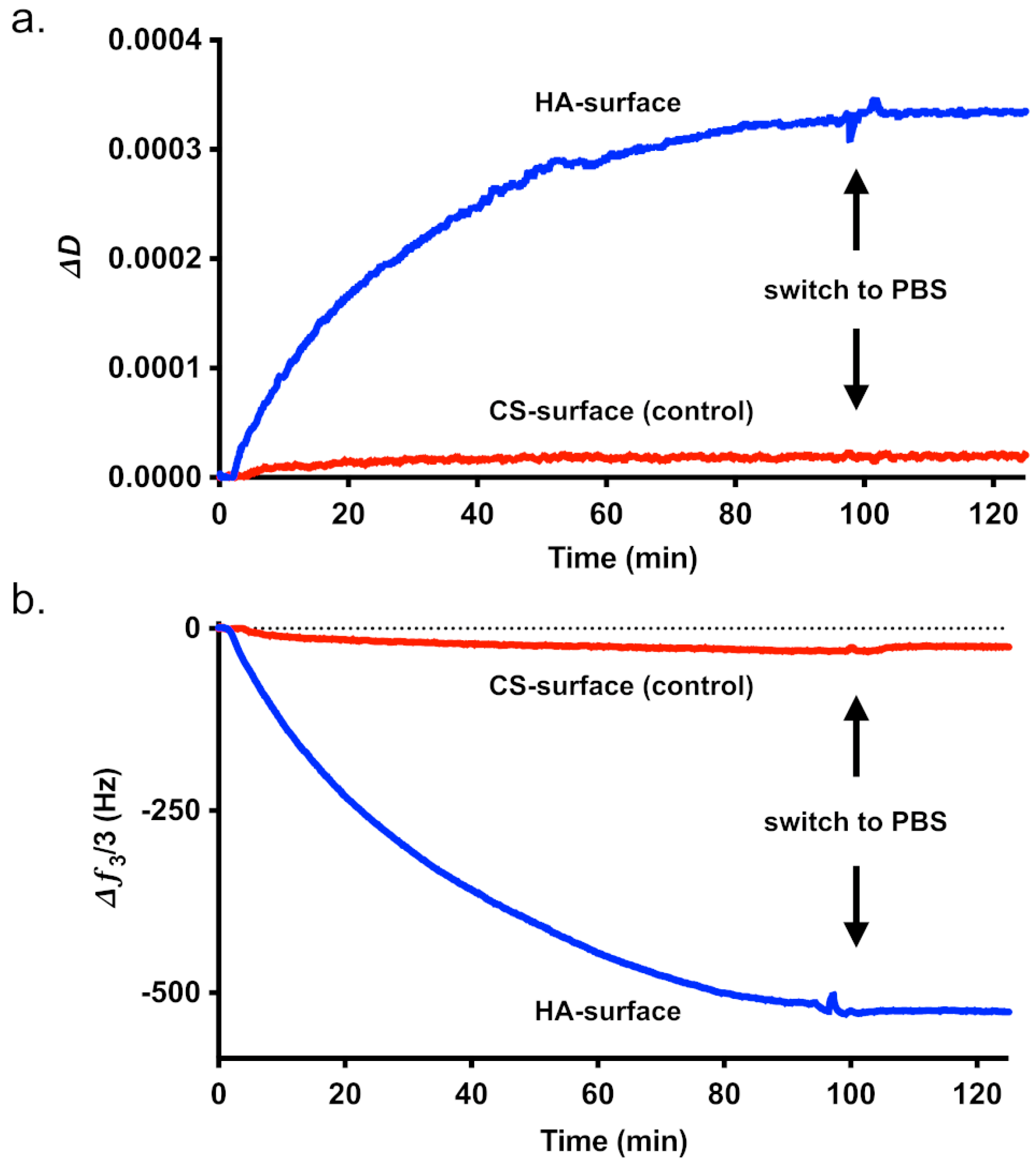
3.3. QCM Affinity Measurements
3.4. MPS Measurements of HAIONP Adsorption on GAG-Coated QCM Sensors
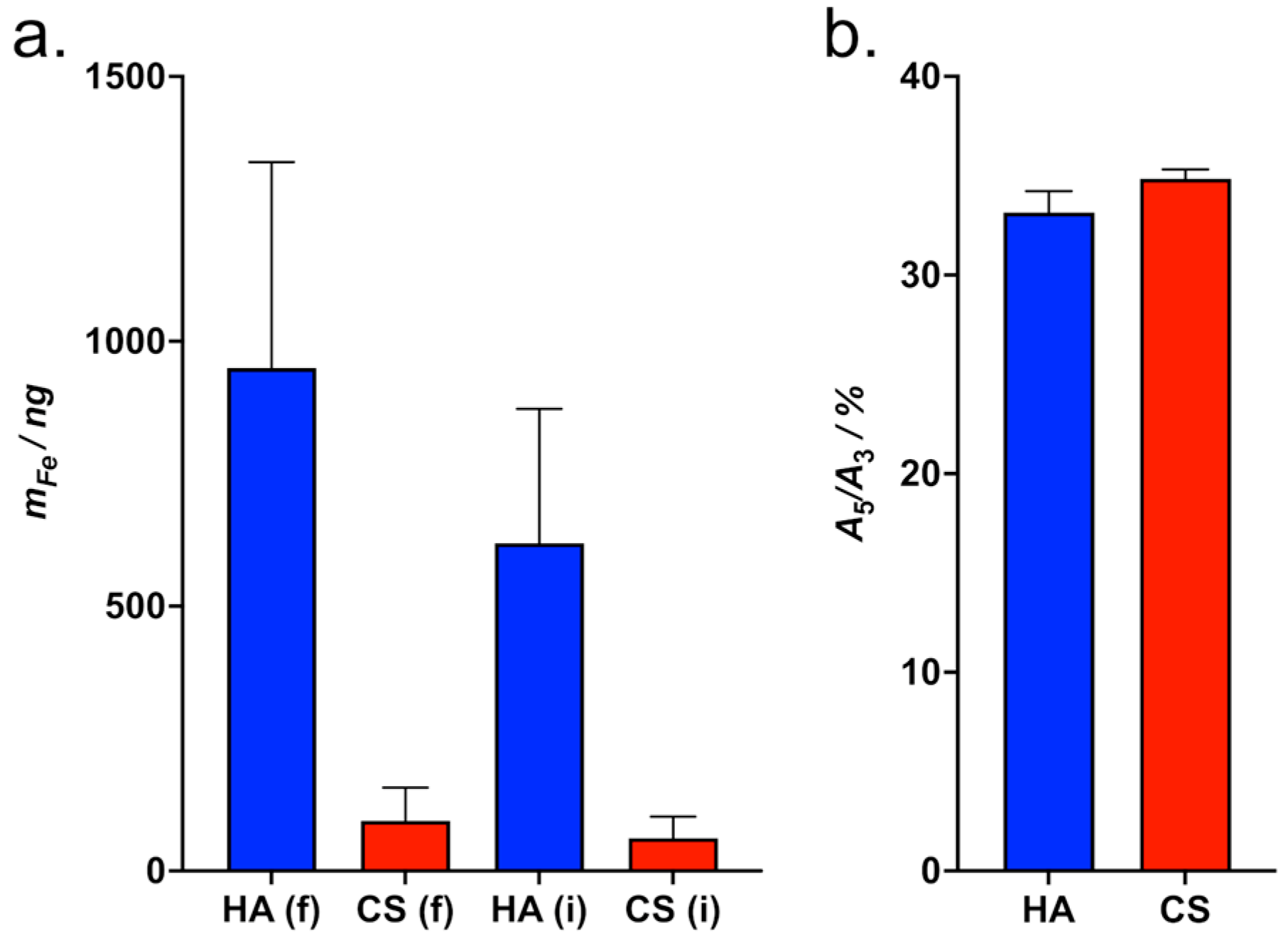
3.4.1. MPS Measurement of the Adsorption of HAIONPs on GAG-Coated QCM Sensors Without Flow
3.4.2. MPS Measurement of the Adsorption of HAIONPs on GAG-Coated QCM Sensors After QCM Affinity Measurements
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCA | bicinchoninic acid |
| CS | chondroitin sulfate |
| CS-cys | chondroitin sulfate L-cysteine conjugate |
| DCM | direct current magnetization |
| DLS | dynamic light scattering |
| DTT | 1,4-dithiothreitol |
| ECM | extracellular matrix |
| EDC | 1-ethyl-3-(3-dimetylaminopropyl)-carbodiimide |
| EDTA | ethylenediaminetetraacetic acid |
| GAGs | glycosaminoglycans |
| HA | hyaluronic acid |
| HA-cys | hyaluronic acid L-cysteine conjugate |
| HAIONP | HA-specific iron oxide nanoparticle |
| HAS | HA synthases |
| HASP | HA-specific peptide |
| IONP | iron oxide nanoparticle |
| IONPC | IONP control sample (treated with magnetic columns) |
| MPI | magnetic particle imaging |
| MPS | magnetic particle spectroscopy |
| MRI | magnetic resonance imaging |
| n.d. | not determined |
| NHS | N-hydroxysuccinimide |
| NMR | nuclear magnetic resonance |
| PBS | phosphate-buffered saline |
| QCM | quartz crystal microbalance |
| RT | room temperature |
| Sulfo-SMCC | sulfosuccinimidyl-trans-4-(N-maleimidomethyl)cyclohexane-1-carboxylate |
References
- Urbi, Z.; Azmi, N.S.; Hossain, M.S. Advances in the pharmacotherapeutic applications of hyaluronic acid: A comprehensive review. Mater. Today Proc. 2024, 107, 147–151. [Google Scholar] [CrossRef]
- Antonio, J.D.S.; Iozzo, R.V. Glycosaminoglycans: Structure and biological functions. Encycl. Life Sci. 2001. [Google Scholar] [CrossRef]
- Wang, Q.; Chi, L. The Alterations and Roles of Glycosaminoglycans in Human Diseases. Polymers 2022, 14, 5014. [Google Scholar] [CrossRef]
- Ricard-Blum, S.; Vives, R.R.; Schaefer, L.; Gotte, M.; Merline, R.; Passi, A.; Heldin, P.; Magalhaes, A.; Reis, C.A.; Skandalis, S.S.; et al. A biological guide to glycosaminoglycans: Current perspectives and pending questions. FEBS J. 2024, 291, 3331–3366. [Google Scholar] [CrossRef]
- Perez, S.; Makshakova, O.; Angulo, J.; Bedini, E.; Bisio, A.; de Paz, J.L.; Fadda, E.; Guerrini, M.; Hricovini, M.; Hricovini, M.; et al. Glycosaminoglycans: What Remains to Be Deciphered? JACS Au 2023, 3, 628–656. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.D.; Pournoori, N.; Saksala, E.; Oommen, O.P. Glycosaminoglycans’ for brain health: Harnessing glycosaminoglycan based biomaterials for treating central nervous system diseases and in-vitro modeling. Biomaterials 2024, 309, 122629. [Google Scholar] [CrossRef] [PubMed]
- Karangelis, D.E.; Kanakis, I.; Asimakopoulou, A.P.; Karousou, E.; Passi, A.; Theocharis, A.D.; Triposkiadis, F.; Tsilimingas, N.B.; Karamanos, N.K. Glycosaminoglycans as Key Molecules in Atherosclerosis: The Role of Versican and Hyaluronan. Curr. Med. Chem. 2010, 17, 4018–4026. [Google Scholar] [CrossRef] [PubMed]
- Stern, R. Hyaluronan catabolism: A new metabolic pathway. Eur. J. Cell Biol. 2004, 83, 317–325. [Google Scholar] [CrossRef]
- Parnigoni, A.; Viola, M.; Karousou, E.; Rovera, S.; Giaroni, C.; Passi, A.; Vigetti, D. Hyaluronan in pathophysiology of vascular diseases: Specific roles in smooth muscle cells, endothelial cells, and macrophages. Am. J. Physiol. Cell Physiol. 2022, 323, C505–C519. [Google Scholar] [CrossRef]
- Altman, R.; Bedi, A.; Manjoo, A.; Niazi, F.; Shaw, P.; Mease, P. Anti-Inflammatory Effects of Intra-Articular Hyaluronic Acid: A Systematic Review. Cartilage 2019, 10, 43–52. [Google Scholar] [CrossRef]
- Turley, E.A.; Noble, P.W.; Bourguignon, L.Y. Signaling properties of hyaluronan receptors. J. Biol. Chem. 2002, 277, 4589–4592. [Google Scholar] [CrossRef]
- Chen, C.G.; Gubbiotti, M.A.; Kapoor, A.; Han, X.; Yu, Y.; Linhardt, R.J.; Iozzo, R.V. Autophagic degradation of HAS2 in endothelial cells: A novel mechanism to regulate angiogenesis. Matrix Biol. 2020, 90, 1–19. [Google Scholar] [CrossRef]
- Sinova, R.; Zadnikova, P.; Safrankova, B.; Nesporova, K. Anti-HA antibody does not detect hyaluronan. Glycobiology 2021, 31, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Ikemoto, H.; Lingasamy, P.; Anton Willmore, A.-M.; Hunt, H.; Kurm, K.; Tammik, O.; Scodeller, P.; Simón-Gracia, L.; Kotamraju, V.R.; Lowy, A.M.; et al. Hyaluronan-binding peptide for targeting peritoneal carcinomatosis. Tumor Biol. 2017, 39, 1010428317701628. [Google Scholar] [CrossRef] [PubMed]
- Amemiya, K.; Nakatani, T.; Saito, A.; Suzuki, A.; Munakata, H. Hyaluronan-binding motif identified by panning a random peptide display library. Biochim. Biophys. Acta 2005, 1724, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; Prestwich, G.D. Hyaluronan-binding proteins: Tying up the giant. J. Biol. Chem. 2002, 277, 4585–4588. [Google Scholar] [CrossRef]
- Yang, B.; Yang, B.L.; Savani, R.C.; Turley, E.A. Identification of a common hyaluronan binding motif in the hyaluronan binding proteins RHAMM, CD44 and link protein. EMBO J. 1994, 13, 286–296. [Google Scholar] [CrossRef]
- Weizenecker, J.; Gleich, B.; Rahmer, J.; Dahnke, H.; Borgert, J. Three-dimensional real-time in vivo magnetic particle imaging. Phys. Med. Biol. 2009, 54, L1–L10. [Google Scholar] [CrossRef]
- Billings, C.; Langley, M.; Warrington, G.; Mashali, F.; Johnson, J.A. Magnetic Particle Imaging: Current and Future Applications, Magnetic Nanoparticle Synthesis Methods and Safety Measures. Int. J. Mol. Sci. 2021, 22, 7651. [Google Scholar] [CrossRef]
- Katti, G.; Ara, S.A.; Shireen, A. Magnetic resonance imaging (MRI)—A review. Int. J. Dent. Clin. 2011, 3, 65–70. [Google Scholar]
- Moser, E.; Stadlbauer, A.; Windischberger, C.; Quick, H.H.; Ladd, M.E. Magnetic resonance imaging methodology. Eur. J. Nucl. Med. Mol. Imaging 2009, 36 (Suppl. 1), S30–S41. [Google Scholar] [CrossRef]
- Szwargulski, P.; Wilmes, M.; Javidi, E.; Thieben, F.; Graeser, M.; Koch, M.; Gruettner, C.; Adam, G.; Gerloff, C.; Magnus, T.; et al. Monitoring Intracranial Cerebral Hemorrhage Using Multicontrast Real-Time Magnetic Particle Imaging. ACS Nano 2020, 14, 13913–13923. [Google Scholar] [CrossRef]
- Salimi, M.; Kuddannaya, S.; Bulte, J.W.M. Pharmacokinetic Profiling of Unlabeled Magnetic Nanoparticles Using Magnetic Particle Imaging as a Novel Cold Tracer Assay. Nano Lett. 2024, 24, 15557–15564. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chiu-Lam, A.; Rivera-Rodriguez, A.; DeGroff, R.; Savliwala, S.; Sarna, N.; Rinaldi-Ramos, C.M. Long circulating tracer tailored for magnetic particle imaging. Nanotheranostics 2021, 5, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, M.O.; Gerzenshtein, I.K.; Stoppel, W.L.; Rinaldi-Ramos, C.M. Advances in Vascular Diagnostics using Magnetic Particle Imaging (MPI) for Blood Circulation Assessment. Adv. Healthc. Mater. 2024, 13, e2400612. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. A colorimetric method for determining low concentrations of mercaptans. Arch. Biochem. Biophys. 1958, 74, 443–450. [Google Scholar] [CrossRef]
- Winther, J.R.; Thorpe, C. Quantification of thiols and disulfides. Biochim. Biophys. Acta 2014, 1840, 838–846. [Google Scholar] [CrossRef]
- Reynolds, F.; O’Loughlin, T.; Weissleder, R.; Josephson, L. Method of determining nanoparticle core weight. Anal. Chem. 2005, 77, 814–817. [Google Scholar] [CrossRef]
- Thomas, J.P.; Zhao, L.; Ding, K.; Heinig, N.F.; Leung, K.T. Near-surface oxidized sulfur modifications and self-assembly of thiol-modified aptamer on Au thin film substrates influenced by piranha treatment. ACS Appl. Mater. Interfaces 2012, 4, 5945–5948. [Google Scholar] [CrossRef]
- Berthou, M.; Pallotta, A.; Beurton, J.; Chaigneau, T.; Athanassiou, A.; Marcic, C.; Marchioni, E.; Boudier, A.; Clarot, I. Gold nanostructured membranes to concentrate low molecular weight thiols, a proof of concept study. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2022, 1198, 123244. [Google Scholar] [CrossRef]
- Easley, A.D.; Ma, T.; Eneh, C.I.; Yun, J.; Thakur, R.M.; Lutkenhaus, J.L. A practical guide to quartz crystal microbalance with dissipation monitoring of thin polymer films. J. Polym. Sci. 2021, 60, 1090–1107. [Google Scholar] [CrossRef]
- Dixon, M.C. Quartz crystal microbalance with dissipation monitoring: Enabling real-time characterization of biological materials and their interactions. J. Biomol. Tech. 2008, 19, 151–158. [Google Scholar]
- Komorek, P.; Martin, E.; Jachimska, B. Adsorption and Conformation Behavior of Lysozyme on a Gold Surface Determined by QCM-D, MP-SPR, and FTIR. Int. J. Mol. Sci. 2021, 22, 1322. [Google Scholar] [CrossRef]
- Wu, K.; Su, D.; Saha, R.; Liu, J.; Chugh, V.K.; Wang, J.-P. Magnetic Particle Spectroscopy: A Short Review of Applications Using Magnetic Nanoparticles. ACS Appl. Nano Mater. 2020, 3, 4972–4989. [Google Scholar] [CrossRef]
- Imhoff, E.D.; Melnyk, A.; Rinaldi-Ramos, C.M. Characterization and evaluation of commercial tracers for x-space magnetic particle imaging. J. Magn. Magn. Mater. 2025, 620, 172889. [Google Scholar] [CrossRef] [PubMed]
- Hickey, J.W.; Schneck, J.P. Enrich and expand rare antigen-specific T cells with magnetic nanoparticles. J. Vis. Exp. 2018, 141, e58640. [Google Scholar] [CrossRef] [PubMed]
- Noble, J.E.; Bailey, M.J. Quantitation of protein. Methods Enzymol. 2009, 463, 73–95. [Google Scholar] [PubMed]
- Olson, B.J. Assays for determination of protein concentration. Curr. Protoc. Pharmacol. 2016, 73, A.3A.1–A.3A.32. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential–What they are and what they are not? J. Control Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Kratz, H.; Eberbeck, D.; Wagner, S.; Taupitz, M.; Schnorr, J. Synthetic routes to magnetic nanoparticles for MPI. Biomed. Tech./Biomed. Eng. 2013, 58, 509–515. [Google Scholar] [CrossRef]
- Kratz, H.; Taupitz, M.; Ariza de Schellenberger, A.; Kosch, O.; Eberbeck, D.; Wagner, S.; Trahms, L.; Hamm, B.; Schnorr, J. Novel magnetic multicore nanoparticles designed for MPI and other biomedical applications: From synthesis to first in vivo studies. PLoS ONE 2018, 13, e0190214. [Google Scholar] [CrossRef] [PubMed]
- Ariza de Schellenberger, A.; Kratz, H.; Farr, T.D.; Lowa, N.; Hauptmann, R.; Wagner, S.; Taupitz, M.; Schnorr, J.; Schellenberger, E.A. Labeling of mesenchymal stem cells for MRI with single-cell sensitivity. Int. J. Nanomed. 2016, 11, 1517–1535. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Bai, R.; Yi, J.; Zhou, H.; Xian, J.; Chen, C. Designing Smart Iron Oxide Nanoparticles for MR Imaging of Tumors. Chem. Biomed. Imaging 2023, 1, 315–339. [Google Scholar] [CrossRef] [PubMed]
- Kratz, H.; Mohtashamdolatshahi, A.; Eberbeck, D.; Kosch, O.; Hauptmann, R.; Wiekhorst, F.; Taupitz, M.; Hamm, B.; Schnorr, J. MPI Phantom Study with A High-Performing Multicore Tracer Made by Coprecipitation. Nanomaterials 2019, 9, 1466. [Google Scholar] [CrossRef]
- Remmo, A.; Löwa, N.; Wiekhorst, F.; Peter, J. Enhanced characterization of a magnetic particle imaging tracer combining field-flow fractionation and magnetic particle spectroscopy. Int. J. Magn. Part. Imaging IJMPI 2022, 8 (Suppl. 1), 2203040. [Google Scholar] [CrossRef]
- Li, X.; Yu, G.; Jin, K.; Yin, Z. Hyaluronic acid L-cysteine conjugate exhibits controlled-release potential for mucoadhesive drug delivery. Die Pharm. Int. J. Pharm. Sci. 2012, 67, 224–228. [Google Scholar] [CrossRef]
- Suchaoin, W.; Bonengel, S.; Griessinger, J.A.; Pereira de Sousa, I.; Hussain, S.; Huck, C.W.; Bernkop-Schnurch, A. Novel bioadhesive polymers as intra-articular agents: Chondroitin sulfate-cysteine conjugates. Eur. J. Pharm. Biopharm. 2016, 101, 25–32. [Google Scholar] [CrossRef]
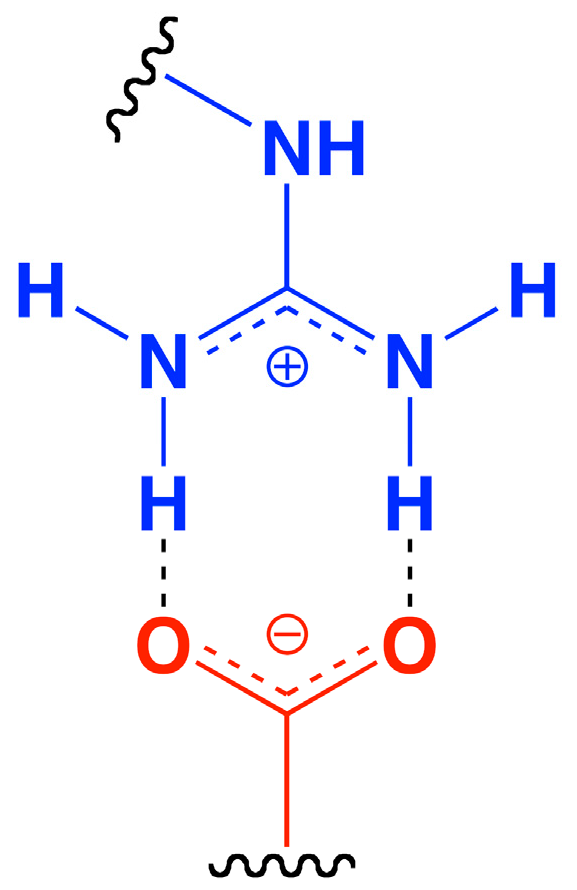
- Donald, J.E.; Kulp, D.W.; DeGrado, W.F. Salt bridges: Geometrically specific, designable interactions. Proteins 2011, 79, 898–915. [Google Scholar] [CrossRef]
- Vemulapalli, S.P.B.; Becker, S.; Griesinger, C.; Rezaei-Ghaleh, N. Combined High-Pressure and Multiquantum NMR and Molecular Simulation Propose a Role for N-Terminal Salt Bridges in Amyloid-Beta. J. Phys. Chem. Lett. 2021, 12, 9933–9939. [Google Scholar] [CrossRef]
- Behan, J.A.; Xie, Z.; Wang, Y.-F.; Yang, X.; Aastrup, T.; Yan, Y.; Adumeau, L.; Dawson, K.A. Quartz Crystal Microbalance Method to Measure Nanoparticle–Receptor Interactions and Evaluate Nanoparticle Design Efficiency. JACS Au 2023, 3, 1623–1633. [Google Scholar] [CrossRef]
- Alexander, T.E.; Lozeau, L.D.; Camesano, T.A. QCM-D characterization of time-dependence of bacterial adhesion. Cell Surf. 2019, 5, 100024. [Google Scholar] [CrossRef]
- Buchholz, O.; Sajjamark, K.; Franke, J.; Wei, H.; Behrends, A.; Munkel, C.; Gruttner, C.; Levan, P.; von Elverfeldt, D.; Graeser, M.; et al. In situ theranostic platform combining highly localized magnetic fluid hyperthermia, magnetic particle imaging, and thermometry in 3D. Theranostics 2024, 14, 324–340. [Google Scholar] [CrossRef]
- Carlton, H.; Salimi, M.; Arepally, N.; Bentolila, G.; Sharma, A.; Bibic, A.; Newgren, M.; Goodwill, P.; Attaluri, A.; Korangath, P.; et al. Ranking Magnetic Colloid Performance for Magnetic Particle Imaging and Magnetic Particle Hyperthermia. Adv. Funct. Mater. 2025, 35, 2412321. [Google Scholar] [CrossRef]
- Lei, S.; He, J.; Gao, P.; Wang, Y.; Hui, H.; An, Y.; Tian, J. Magnetic Particle Imaging-Guided Hyperthermia for Precise Treatment of Cancer: Review, Challenges, and Prospects. Mol. Imaging Biol. 2023, 25, 1020–1033. [Google Scholar] [CrossRef]
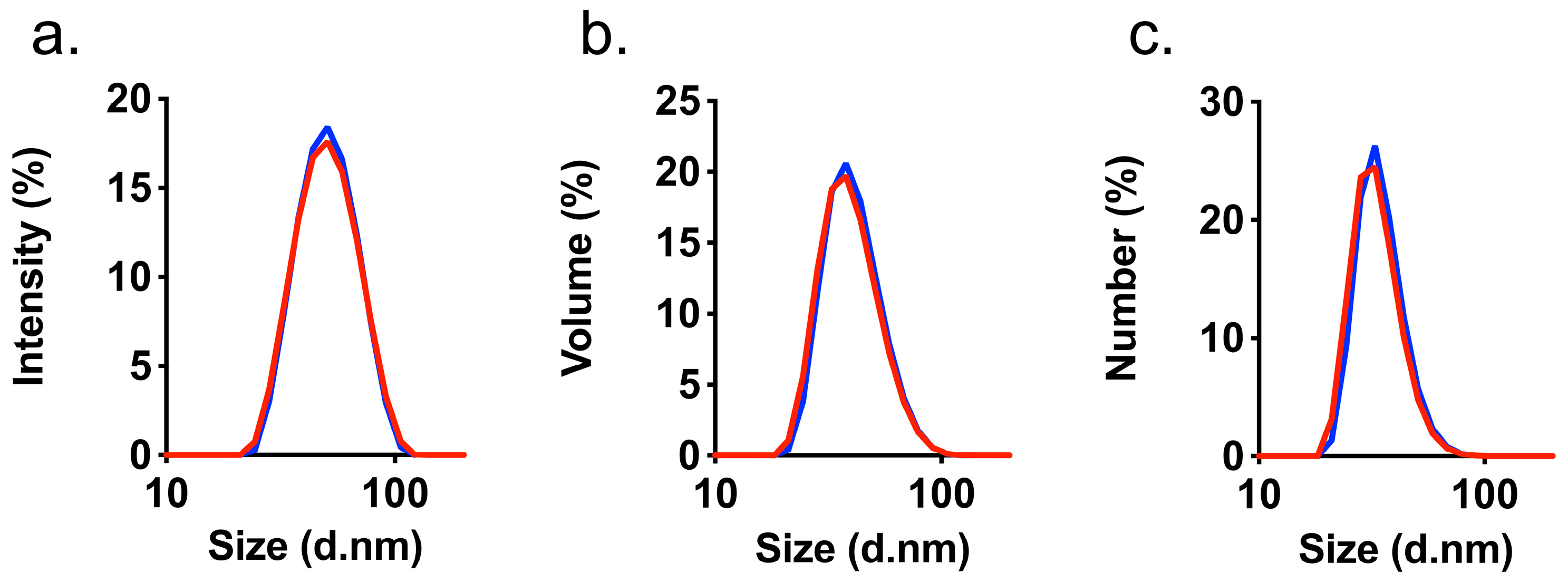

| IONPs | r1 L mmol−1s−1 | r2 L mmol−1s−1 | dV DLS nm | Z-Average nm | PDI | ζ-Potential mV |
|---|---|---|---|---|---|---|
| synomag®-D | 14 | 432 | 41.0 ± 12.83 | 48.0 | 0.082 | −1.77 ± 6.07 |
| HAIONPs | 13 | 421 | 41.9 ± 12.59 | 48.5 | 0.071 | −2.82 ± 9.02 |
| 1. HAIONP Addition | 2. PBS Addition | |||
|---|---|---|---|---|
| GAG | HA | CS | HA | CS |
| ∆ƒ3/3 (Hz) | −548 ± 33 | −21 ± 7 | 11 ± 13 | 7 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kratz, H.; Eberbeck, D.; Wiekhorst, F.; Taupitz, M.; Schnorr, J. Synthesis and Characterization of a Nanoscale Hyaluronic Acid-Specific Probe for Magnetic Particle Imaging and Magnetic Resonance Imaging. Nanomaterials 2025, 15, 1505. https://doi.org/10.3390/nano15191505
Kratz H, Eberbeck D, Wiekhorst F, Taupitz M, Schnorr J. Synthesis and Characterization of a Nanoscale Hyaluronic Acid-Specific Probe for Magnetic Particle Imaging and Magnetic Resonance Imaging. Nanomaterials. 2025; 15(19):1505. https://doi.org/10.3390/nano15191505
Chicago/Turabian StyleKratz, Harald, Dietmar Eberbeck, Frank Wiekhorst, Matthias Taupitz, and Jörg Schnorr. 2025. "Synthesis and Characterization of a Nanoscale Hyaluronic Acid-Specific Probe for Magnetic Particle Imaging and Magnetic Resonance Imaging" Nanomaterials 15, no. 19: 1505. https://doi.org/10.3390/nano15191505
APA StyleKratz, H., Eberbeck, D., Wiekhorst, F., Taupitz, M., & Schnorr, J. (2025). Synthesis and Characterization of a Nanoscale Hyaluronic Acid-Specific Probe for Magnetic Particle Imaging and Magnetic Resonance Imaging. Nanomaterials, 15(19), 1505. https://doi.org/10.3390/nano15191505






