Albumin-Coated Copper Oxide Nanoparticles for Radiosensitization of Human Glioblastoma Cells Under Clinically Relevant X-Ray Irradiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of BSA-Coated CuO Nanoparticles (BSA@CuO-NPs)
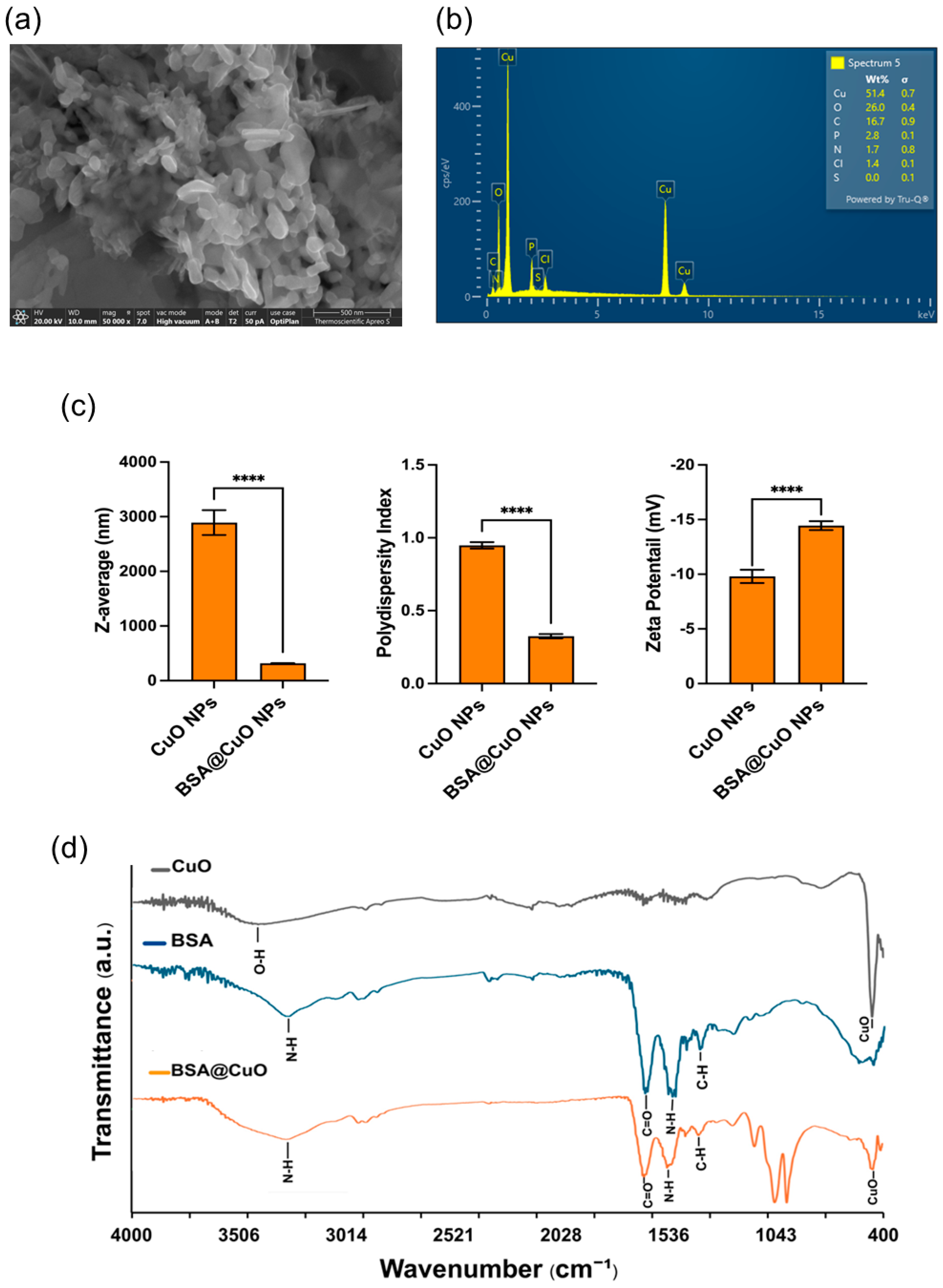
2.2. Characterization
2.2.1. Hydrodynamic Size and Zeta Potential
2.2.2. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-Ray (EDX) Spectroscopy
2.2.3. Fourier-Transform Infrared (FTIR) Spectroscopy
2.3. Cell Culture Conditions and Treatment Groups
2.4. MTT Assay and IC20 Determination
2.5. Irradiation Setup
2.6. Clonogenic Survival Assay
2.7. γ-H2AX Assay for DNA Damage Assessment
2.8. Apoptosis and Cell Cycle Analysis
2.9. Statistical Analysis
3. Results
3.1. Results of Synthesis and Characterization of BSA@CuO-NPs
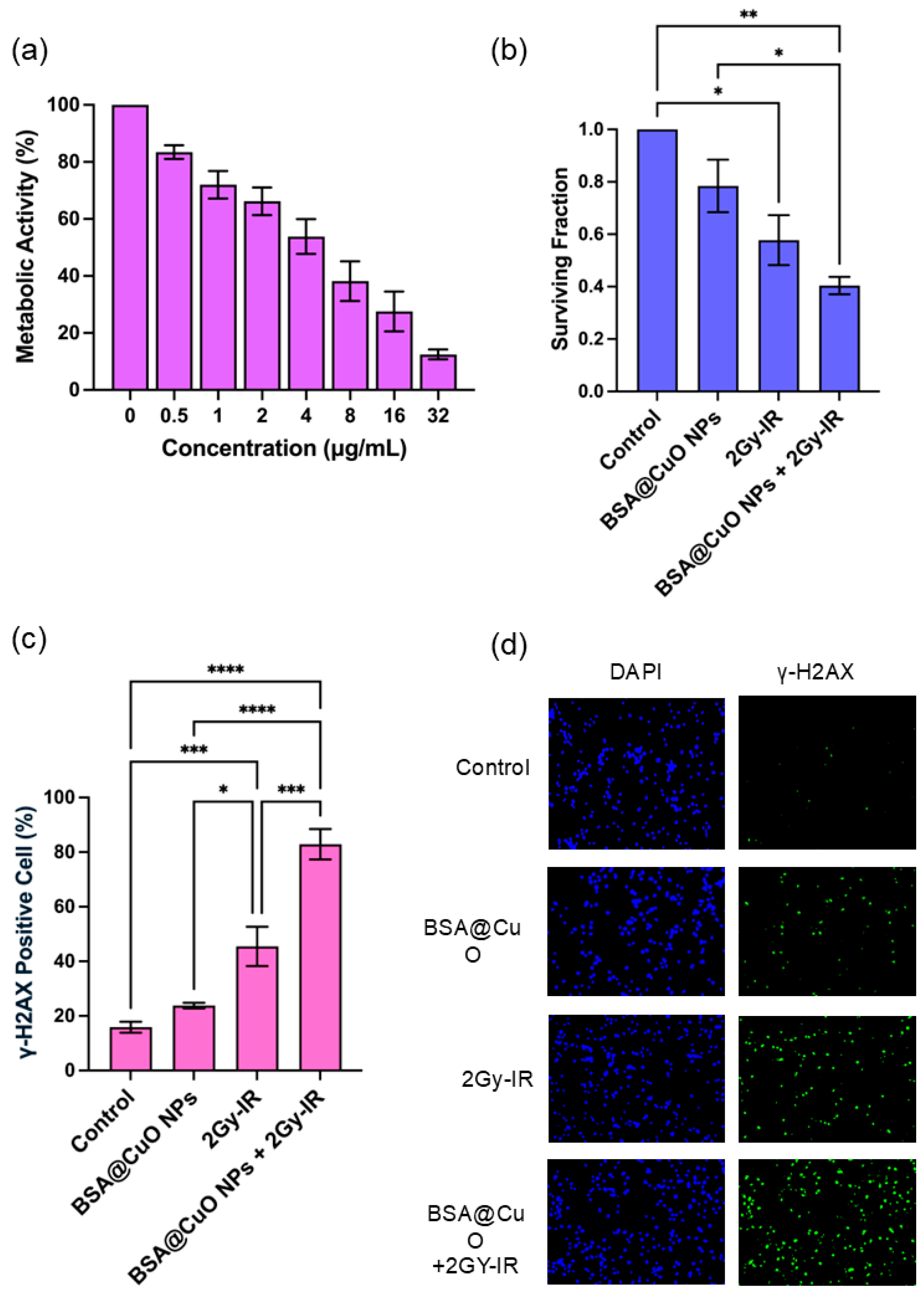
3.2. Metabolic Activity and IC20 Determination of BSA@CuO-NPs in U87-MG Cells
3.3. Radiosensitization Potential of BSA@CuO-NPs Assessed by Clonogenic Survival
3.4. BSA@CuO-NPs Enhance Radiation-Induced DNA Damage in U87-MG Cells
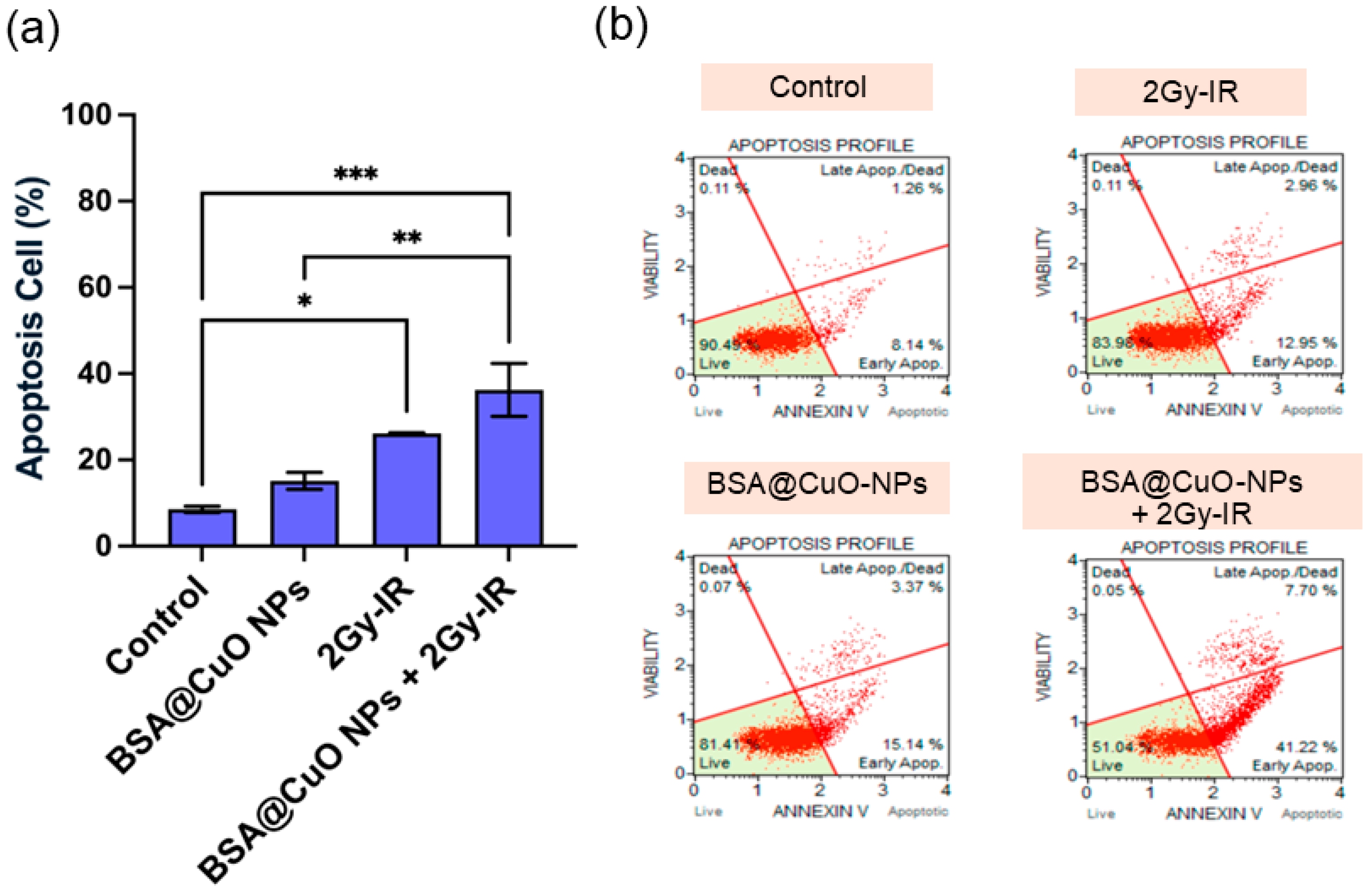
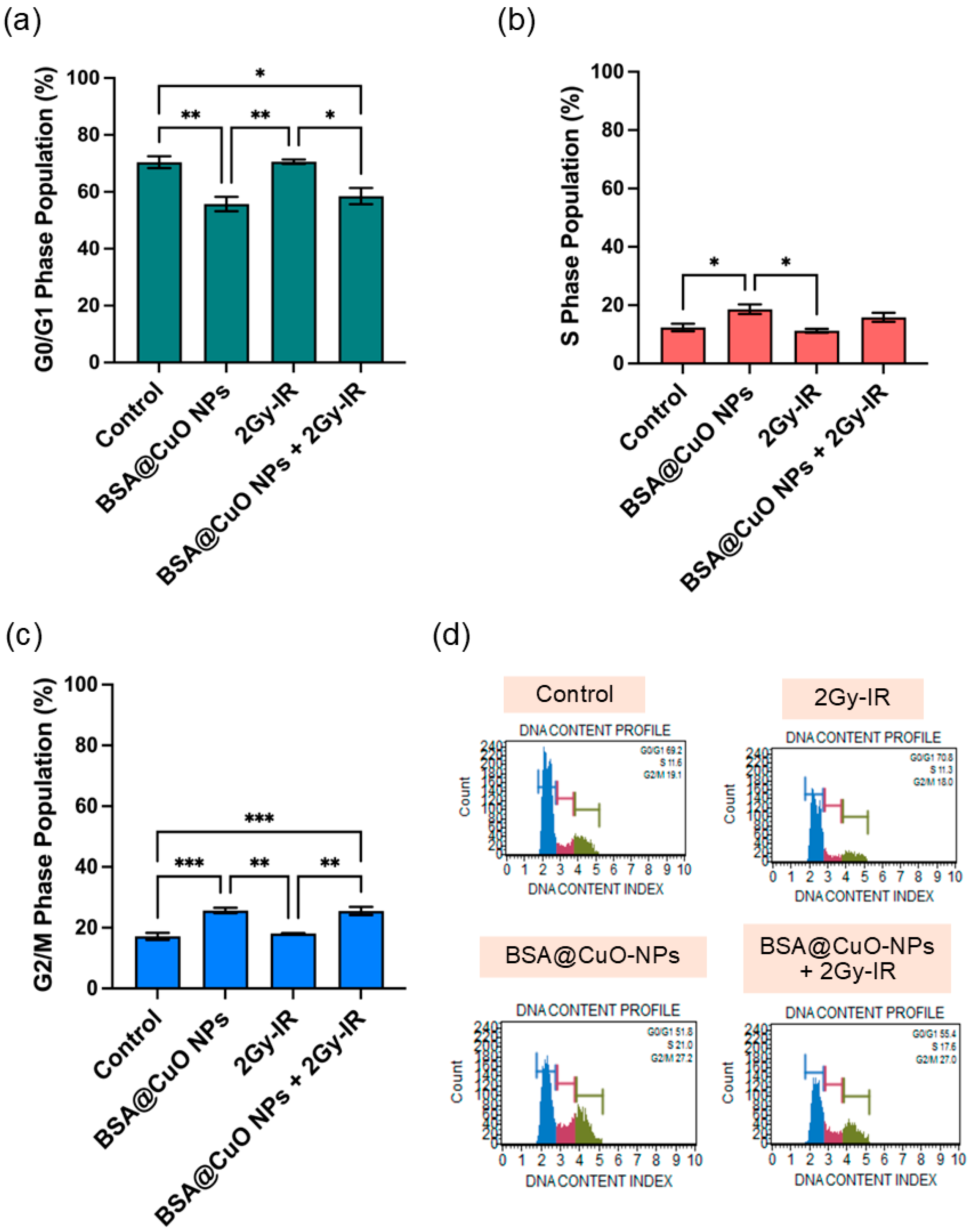
3.5. Induction of Apoptosis and Cell Cycle Disruption Following Combined Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cella, E.; Bosio, A.; Lombardi, G. New Insights into Glioblastoma. Int. J. Mol. Sci. 2024, 25, 4090. [Google Scholar] [CrossRef]
- Korbecki, J.; Kojder, K.; Grochans, S.; Cybulska, A.M.; Simi, D. Epidemiology of Glioblastoma Multiforme—Literature Review. Cancers 2022, 14, 2412. [Google Scholar]
- Abedi, A.A.; Grunnet, K.; Christensen, I.J.; Michaelsen, S.R.; Muhic, A.; Møller, S.; Hasselbalch, B.; Poulsen, H.S.; Urup, T. A Prognostic Model for Glioblastoma Patients Treated With Standard Therapy Based on a Prospective Cohort of Consecutive Non-Selected Patients From a Single Institution. Front. Oncol. 2021, 11, 597587. [Google Scholar] [CrossRef] [PubMed]
- Osuka, S. Targeting adaptive radioresistance in glioblastoma. Neuro-oncology 2022, 24, 1071–1073. [Google Scholar] [CrossRef] [PubMed]
- Vilar, J.B.; Christmann, M.; Tomicic, M.T. Alterations in Molecular Profiles Affecting Glioblastoma Resistance to Radiochemotherapy: Where Does the Good Go? Cancers 2022, 14, 2416. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.W.; Gao, G.; Jia, H.R.; Zhang, X.; Zhao, J.; Ma, N.; Liu, J.B.; Liu, P.; Wu, F.G. Copper Oxide Nanoparticles Induce Enhanced Radiosensitizing Effect via Destructive Autophagy. ACS Biomater. Sci. Eng. 2019, 5, 1569–1579. [Google Scholar] [CrossRef]
- Jackson, N.; Cecchi, D.; Beckham, W.; Chithrani, D.B. Application of High-Z Nanoparticles to Enhance Current Radiotherapy Treatment. Molecules 2024, 29, 2438. [Google Scholar] [CrossRef]
- He, M.; Chen, S.; Yu, H.; Fan, X.; Wu, H.; Wang, Y.; Wang, H.; Yin, X. Advances in Nanoparticle-Based Radiotherapy for Cancer Treatment. iScience 2025, 28, 111602. [Google Scholar] [CrossRef]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA Damage Response in Cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Xiao, S.; Wang, X.; Chen, B.; Mu, M.; Han, B.; Chen, N.; Guo, G. Enhancing Tumor Radiotherapy Sensitivity through Metal Nanomaterials: A Comprehensive Review. Malig. Spectr. 2024, 1, 243–262. [Google Scholar] [CrossRef]
- Choi, J.; Kim, G.; Cho, S.B.; Im, H.J. Radiosensitizing High-Z Metal Nanoparticles for Enhanced Radiotherapy of Glioblastoma Multiforme. J. Nanobiotechnol. 2020, 18, 122. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Huang, H.; Jiang, Z. Nanoparticle-Based Radiosensitization Strategies for Improving Radiation Therapy. Front. Pharmacol. 2023, 14, 1145551. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Guan, J. Nanoparticle-Based Drug Delivery Systems for Cancer Therapy. Smart Mater. Med. 2020, 1, 10–19. [Google Scholar] [CrossRef]
- Song, Y.; Tan, K.B.; Zhou, S.F.; Zhan, G. Biocompatible Copper-Based Nanocomposites for Combined Cancer Therapy. ACS Biomater. Sci. Eng. 2024, 10, 3673–3692. [Google Scholar] [CrossRef]
- Wei, Q.; Pan, Y.; Zhang, Z.; Yan, S.; Li, Z. Copper-Based Nanomaterials for Biomedical Applications. Chem. Eng. J. 2024, 483, 149040. [Google Scholar] [CrossRef]
- Tsymbal, S.; Li, G.; Agadzhanian, N.; Sun, Y.; Zhang, J.; Dukhinova, M.; Fedorov, V.; Shevtsov, M. Recent Advances in Copper-Based Organic Complexes and Nanoparticles for Tumor Theranostics. Molecules 2022, 27, 7066. [Google Scholar] [CrossRef]
- Sarfraz, S.; Javed, A.; Sharif Mughal, S.; Bashir, M.; Rehman, A.; Parveen, S.; Khushi, A.; Kamran Khan, M. Copper Oxide Nanoparticles: Reactive Oxygen Species Generation and Biomedical Applications. Int. J. Comput. Theor. Chem. 2020, 8, 40. [Google Scholar] [CrossRef]
- Chaudhry, M.A.; Omaruddin, R.A. Transcriptional Changes of Mitochondrial Genes in Irradiated Cells Proficient or Deficient in P53. J. Genet. 2012, 91, 105–110. [Google Scholar] [CrossRef]
- Sanità, G.; Carrese, B.; Lamberti, A. Nanoparticle Surface Functionalization: How to Improve Biocompatibility and Cellular Internalization. Front. Mol. Biosci. 2020, 7, 587012. [Google Scholar] [CrossRef]
- Lieberwirth, I.; Kokkinopoulou, M.; Han, S.; Simon, J.; Landfester, K.; Mailänder, V. The Role of the Protein Corona in the Uptake Process of Nanoparticles. Microsc. Microanal. 2018, 24 (Suppl. S1), 1404–1405. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, B. Bovine Serum Albumin as a Versatile Platform for Cancer Imaging and Therapy. Curr. Med. Chem. 2017, 25, 2938–2953. [Google Scholar] [CrossRef]
- Loureiro, A.; Abreu, A.S.; Sárria, M.P.; Figueiredo, M.C.O.; Saraiva, L.M.; Bernardes, G.J.L.; Gomes, A.C.; Cavaco-Paulo, A. Functionalized Protein Nanoemulsions by Incorporation of Chemically Modified BSA. RSC Adv. 2015, 5, 4976–4983. [Google Scholar] [CrossRef]
- Chaiwaree, S.; Prapan, A.; Suwannasom, N.; Laporte, T.; Neumann, T.; Pruß, A.; Georgieva, R.; Bäumler, H. Doxorubicin–Loaded Human Serum Albumin Submicron Particles: Preparation, Characterization and in Vitro Cellular Uptake. Pharmaceutics 2020, 12, 224. [Google Scholar] [CrossRef] [PubMed]
- Atloo, T.; Mohammadkhani, R.; Mohammadi, A.; Zaboli, K.A.; Kaboli, S.; Rahimi, H.; Nosrati, H.; Danafar, H. The Bovine Serum Albumin Coated Copper Oxide Nanoparticle for Curcumin Delivery in Biological Environment: In-Vitro Drug Release. J. Polym. Environ. 2022, 30, 3203–3208. [Google Scholar] [CrossRef]
- Lu, V.M.; Crawshay-Williams, F.; White, B.; Elliot, A.; Hill, M.A.; Townley, H.E. Cytotoxicity, Dose-Enhancement and Radiosensitization of Glioblastoma Cells with Rare Earth Nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 132–143. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Keleştemur, S.; Altunbek, M.; Culha, M. Influence of EDC/NHS Coupling Chemistry on Stability and Cytotoxicity of ZnO Nanoparticles Modified with Proteins. Appl. Surf. Sci. 2017, 403, 455–463. [Google Scholar] [CrossRef]
- Esfandfar, P.; Falahati, M.; Saboury, A.A. Spectroscopic Studies of Interaction between CuO Nanoparticles and Bovine Serum Albumin. J. Biomol. Struct. Dyn. 2016, 34, 1962–1968. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.M.; Bourassa, M.W.; Smith, R.J. FTIR Spectroscopic Imaging of Protein Aggregation in Living Cells. Biochim. Biophys. Acta. 2013, 1828, 2339–2346. [Google Scholar] [CrossRef]
- Ahmad, Z.; Shepherd, J.H.; Shepherd, D.V.; Ghose, S.; Kew, S.J.; Cameron, R.E.; Best, S.M.; Brooks, R.A.; Wardale, J.; Rushton, N. Effect of 1-Ethyl-3-(3-Dimethylaminopropyl) Carbodiimide and N-Hydroxysuccinimide Concentrations on the Mechanical and Biological Characteristics of Cross-Linked Collagen Fibres for Tendon Repair. Regen. Biomater. 2015, 2, 77–85. [Google Scholar] [CrossRef]
- Aires, A.; Ocampo, S.M.; Cabrera, D.; de la Cueva, L.; Salas, G.; Teran, F.J.; Cortajarena, A.L. BSA-Coated Magnetic Nanoparticles for Improved Therapeutic Properties. J. Mater. Chem. B 2015, 3, 6239–6247. [Google Scholar] [CrossRef]
- Xie, L.; Tong, W.; Yu, D.; Xu, J.; Li, J.; Gao, C. Bovine Serum Albumin Nanoparticles Modified with Multilayers and Aptamers for PH-Responsive and Targeted Anti-Cancer Drug Delivery. J. Mater. Chem. 2012, 22, 6053–6060. [Google Scholar] [CrossRef]
- Tirado-Miranda, M.; Schmitt, A.; Callejas-Fernández, J.; Fernández-Barbero, A. The Aggregation Behaviour of Protein-Coated Particles: A Light Scattering Study. Eur. Biophys. J. 2003, 32, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Lee, H. Hydrodynamics and Aggregation of Nanoparticles with Protein Corona: Effects of Protein Concentration and Ionic Strength. Small 2024, 20, 2403913. [Google Scholar] [CrossRef]
- Sharma, I.; Sharaf, M.G.; Pawar, A.; Milley, A.; Unsworth, L.D. Hemocompatibility of Albumin-Modified Magnetic Nanoparticles. Int. J. Mol. Sci. 2024, 25, 11975. [Google Scholar] [CrossRef] [PubMed]
- Mattix, B.; Moore, T.; Uvarov, O.; Pollard, S.; O’Donnell, L.; Park, K.; Horne, D.; Dhulekar, J.; Reese, L.; Nguyen, D.; et al. Effects of Polymeric Nanoparticle Surface Properties on Interaction with Brain Tumor Environment. Nano Life 2013, 3, 1343003. [Google Scholar] [CrossRef]
- Varzandeh, M.; Sabouri, L.; Mansouri, V.; Gharibshahian, M.; Beheshtizadeh, N.; Hamblin, M.R.; Rezaei, N. Application of Nano-Radiosensitizers in Combination Cancer Therapy. Bioeng. Transl. Med. 2023, 8, e10498. [Google Scholar] [CrossRef]
- Gupta, G.; Cappellini, F.; Farcal, L.; Gornati, R.; Bernardini, G.; Fadeel, B. Copper Oxide Nanoparticles Trigger Macrophage Cell Death with Misfolding of Cu/Zn Superoxide Dismutase 1 (SOD1). Part. Fibre Toxicol. 2022, 19, 33. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Nuryadi, E.; Komatsu, S.; Hirota, Y.; Shibata, A.; Oike, T.; Nakano, T. Robustness of Clonogenic Assays as a Biomarker for Cancer Cell Radiosensitivity. Int. J. Mol. Sci. 2019, 20, 4148. [Google Scholar] [CrossRef]
- Yi, X.; Chen, L.; Chen, J.; Maiti, D.; Chai, Z.; Liu, Z.; Yang, K. Biomimetic Copper Sulfide for Chemo-Radiotherapy: Enhanced Uptake and Reduced Efflux of Nanoparticles for Tumor Cells under Ionizing Radiation. Adv. Funct. Mater. 2018, 28, 1705161. [Google Scholar] [CrossRef]
- Al Zaki, A.; Cormode, D.; Tsourkas, A.; Dorsey, J.F. Increasing the Therapeutic Efficacy of Radiotherapy Using Nanoparticles. In Cancer Drug Discovery and Development; Humana Press: Cham, Switzerland, 2017; pp. 241–265. [Google Scholar] [CrossRef]
- Angelé-Martínez, C.; Nguyen, K.V.T.; Ameer, F.S.; Anker, J.N.; Brumaghim, J.L. Reactive Oxygen Species Generation by Copper(II) Oxide Nanoparticles Determined by DNA Damage Assays and EPR Spectroscopy. Nanotoxicology 2017, 11, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Möller, L.; Mo, L. Copper Oxide Nanoparticles Are Highly Toxic A Comparison between Metal Oxide Nanoparticles and Carbon Nanotubes—Chemical Research in Toxicology (ACS Publications). Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef]
- Mah, L.J.; Vasireddy, R.S.; Tang, M.M.; Georgiadis, G.T.; El-Osta, A.; Karagiannis, T.C. Quantification of ΓH2AX Foci in Response to Ionising Radiation. J. Vis. Exp. 2010, 6, e1957. [Google Scholar] [CrossRef]
- Szumiel, I. Analysis of DNA Double-Strand Breaks by Means of γ-H2AX Foci. In Chromosomal Alterations: Methods, Results and Importance in Human Health; Springer: Berlin/Heidelberg, Germany, 2007; pp. 145–160. [Google Scholar] [CrossRef]
- Böcker, W.; Iliakis, G. Computational Methods for Analysis of Foci: Validation for Radiation-Induced γ-H2AX Foci in Human Cells. Radiat. Res. 2006, 165, 113–124. [Google Scholar] [CrossRef]
- Pawlik, T.M.; Keyomarsi, K. Role of Cell Cycle in Mediating Sensitivity to Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 928–942. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.M.; Chi, C.W.; Lai, J.C.Y.; Chen, Y.J.; Kou, Y.R. TLC388 Induces DNA Damage and G2 Phase Cell Cycle Arrest in Human Non-Small Cell Lung Cancer Cells. Cancer Control 2020, 27, 1073274819897975. [Google Scholar] [CrossRef]
- Gazdova, M.; Michalkova, R.; Kello, M.; Vilkova, M.; Kudlickova, Z.; Baloghova, J.; Mirossay, L.; Mojzis, J. Chalcone-Acridine Hybrid Suppresses Melanoma Cell Progression via G2/M Cell Cycle Arrest, DNA Damage, Apoptosis, and Modulation of MAP Kinases Activity. Int. J. Mol. Sci. 2022, 23, 12266. [Google Scholar] [CrossRef]
- Shafagh, M.; Rahmani, F.; Delirezh, N. CuO Nanoparticles Induce Cytotoxicity and Apoptosis in Human K562 Cancer Cell Line via Mitochondrial Pathway, through Reactive Oxygen Species and P53. Iran. J. Basic Med. Sci. 2015, 18, 993–1000. [Google Scholar]
- Chakraborty, S.; Prakash, P.; Shah, J.; Mayya, C.; Singh, S.; Ranganathan, R.; Soppina, V.; Jones, E.V.; Misra, S.K. CuO Nanoparticles as Copper-Ion Reservoirs for Elesclomol-Mediated Intracellular Oxidative Stress: Implications for Anticancer Therapies. ACS Appl. Nano Mater. 2022, 5, 1607–1620. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suwannasing, C.; Suwannasom, N.; Iamcharoen, P.; Dokkham, R.; Maun, P.; Srisai, P.; Bäumler, H.; Prapan, A. Albumin-Coated Copper Oxide Nanoparticles for Radiosensitization of Human Glioblastoma Cells Under Clinically Relevant X-Ray Irradiation. Nanomaterials 2025, 15, 1376. https://doi.org/10.3390/nano15171376
Suwannasing C, Suwannasom N, Iamcharoen P, Dokkham R, Maun P, Srisai P, Bäumler H, Prapan A. Albumin-Coated Copper Oxide Nanoparticles for Radiosensitization of Human Glioblastoma Cells Under Clinically Relevant X-Ray Irradiation. Nanomaterials. 2025; 15(17):1376. https://doi.org/10.3390/nano15171376
Chicago/Turabian StyleSuwannasing, Chanyatip, Nittiya Suwannasom, Pattawat Iamcharoen, Rachan Dokkham, Panupong Maun, Pitchayuth Srisai, Hans Bäumler, and Ausanai Prapan. 2025. "Albumin-Coated Copper Oxide Nanoparticles for Radiosensitization of Human Glioblastoma Cells Under Clinically Relevant X-Ray Irradiation" Nanomaterials 15, no. 17: 1376. https://doi.org/10.3390/nano15171376
APA StyleSuwannasing, C., Suwannasom, N., Iamcharoen, P., Dokkham, R., Maun, P., Srisai, P., Bäumler, H., & Prapan, A. (2025). Albumin-Coated Copper Oxide Nanoparticles for Radiosensitization of Human Glioblastoma Cells Under Clinically Relevant X-Ray Irradiation. Nanomaterials, 15(17), 1376. https://doi.org/10.3390/nano15171376








