A First-Principles Investigation of the Structural, Electronic, Optical, and Mechanical Properties of Hydrogen Storage Ordered Vacancy Double Perovskite X2MH6 Materials
Abstract
1. Introduction
2. Computational Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M.I.; Al-Ghamdi, S.G. Hydrogen economy for sustainable development in GCC countries: A SWOT analysis considering current situation, challenges, and prospects. Int. J. Hydrogen Energy 2023, 48, 10315–10344. [Google Scholar] [CrossRef]
- Yang, L.; Cao, Y.; Xu, Z.; Qu, N. First-principles prediction of hydrogen storage capabilities in Pd-based alkali metal hydride X2PdH4 (X = Na, K, Rb, and Cs). Int. J. Hydrogen Energy 2025, 100, 444–455. [Google Scholar] [CrossRef]
- Ahmed, B.; Tahir, M.B.; Ali, A.; Sagir, M. DFT insights on structural, electronic, optical and mechanical properties of double perovskites X2FeH6 (X = Ca and Sr) for hydrogen-storage applications. Int. J. Hydrogen Energy 2024, 50, 316–323. [Google Scholar] [CrossRef]
- Ahmed, B.; Tahir, M.B.; Nazir, S.; Alzaid, M.; Ali, A.; Sagir, M.; Alrobei, H. An Ab-initio simulation of boron-based hydride perovskites XBH3 (X = Cs and Rb) for advance hydrogen storage system. Comput. Theor. Chem. 2023, 1225, 114173. [Google Scholar] [CrossRef]
- Al, S. Theoretical investigations of elastic and thermodynamic properties of LiXH4 compounds for hydrogen storage. Int. J. Hydrogen Energy 2019, 44, 1727–1734. [Google Scholar] [CrossRef]
- Jena, P. Materials for hydrogen storage: Past, present, and future. J. Phys. Chem. Lett. 2011, 2, 206–211. [Google Scholar] [CrossRef]
- Lai, Q.; Paskevicius, M.; Sheppard, D.A.; Buckley, C.E.; Thornton, A.W.; Hill, M.R.; Gu, Q.; Mao, J.; Huang, Z.; Liu, H.K.; et al. Hydrogen storage materials for mobile and stationary applications: Current state of the art. ChemSusChem. 2015, 8, 2789–2825. [Google Scholar] [CrossRef]
- Hirscher, M.; Yartys, V.A.; Baricco, M.; Von Colbe, J.B.; Blanchard, D.; Bowman, R.C., Jr.; Broom, D.P.; Buckley, C.E.; Chang, F.; Chen, P.; et al. Materials for hydrogen-based energy storage–past, recent progress and future outlook. J. Alloys Compd. 2020, 827, 153548. [Google Scholar] [CrossRef]
- Koufi, A.; Ziat, Y.; Belkhanchi, H. Study of the Gravimetric, Electronic and Thermoelectric Properties of XAlH3 (X = Be, Na, K) as hydrogen storage perovskite using DFT and the BoltzTrap Software Package. Sol. Energy Sustain. Dev. 2024, 14, 53–66. [Google Scholar] [CrossRef]
- Bouhadda, Y.; Rabehi, A.; Boudouma, Y.; Fenineche, N.; Drablia, S.; Meradji, H. Hydrogen solid storage: First-principles study of ZrNiH3. Int. J. Hydrogen Energy 2009, 34, 4997–5002. [Google Scholar] [CrossRef]
- Chattaraj, D.; Dash, S.; Majumder, C. Structural, electronic, elastic, vibrational and thermodynamic properties of ZrNi and ZrNiH3: A comprehensive study through first principles approach. Int. J. Hydrogen Energy 2016, 41, 20250–20260. [Google Scholar] [CrossRef]
- Simonovic, B.; Mentus, S.; Dimitrijevic, R.; Šusic, M. Multiple hydriding/dehydriding of Zr1.02Ni0.98 alloy. Int. J. Hydrogen Energy 1999, 24, 449–454. [Google Scholar] [CrossRef]
- Korst, W.L. The crystal structure of NiZrH3. J. Phys. Chem. 1962, 66, 370–372. [Google Scholar] [CrossRef]
- Westlake, D. Stoichiometries and interstitial site occupation in the hydrides of ZrNi and other isostructural intermetallic compounds. J. Less-Common. Met. 1980, 75, 177–185. [Google Scholar] [CrossRef]
- Westlake, D.; Shaked, H.; Mason, P.; McCart, B.; Mueller, M.; Matsumoto, T.; Amano, M. Interstitial site occupation in ZrNiH. J. Less-Common. Met. 1982, 88, 17–23. [Google Scholar] [CrossRef][Green Version]
- Zelai, T. Study of magnetic, thermoelectric, and mechanical properties of double perovskites Be2XH6 (X = Cr and Mn) for spintronic and hydrogen-storage applications. Inorg. Chem. Commun. 2024, 165, 112579. [Google Scholar] [CrossRef]
- Guan, S.; Zhou, J.; Sun, S.; Peng, Q.; Guo, X.; Liu, B.; Zhou, X.; Tang, Y. Nonmetallic Se/N Co-doped amorphous carbon anode collaborates to realize ultra-high capacity and fast potassium storage for potassium dual-ion batteries. Adv. Funct. Mater. 2024, 34, 2314890. [Google Scholar] [CrossRef]
- Zhao, X.H.; Wei, X.N.; Tang, T.Y.; Gao, L.K.; Xie, Q.; Lu, L.M.; Tang, Y.L. First-principles study on the structural, electronic and optical properties of vacancy-ordered double perovskites Cs2PtI6 and Rb2PtI6. Opt. Mater. 2021, 114, 110952. [Google Scholar] [CrossRef]
- Mubashir, M.; Ali, M.; Bibi, Z.; Younis, M.; Muzamil, M. Efficient hydrogen storage in LiMgF3: A first principle study. Int. J. Hydrogen Energy 2024, 50, 774–786. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Cui, Z.; Sun, Y.; Li, J.; Qu, J. Combination method for the calculation of elastic constants. Phys. Rev. B 2007, 75, 214101. [Google Scholar] [CrossRef]
- Togo, A.; Tanaka, I. First principles phonon calculations in materials science. Scr. Mater. 2015, 108, 1–5. [Google Scholar] [CrossRef]
- Wang, X.; Wei, Q.; Luo, J.; Jia, X.; Zhang, M.; Zhu, X.; Wei, B. Pressure-Induced Phase Transitions and Electronic Structure Evolution of Ba4Au. Materials 2025, 18, 3728. [Google Scholar] [CrossRef] [PubMed]
- Saal, J.E.; Kirklin, S.; Aykol, M.; Meredig, B.; Wolverton, C. Materials design and discovery with high-throughput density functional theory: The open quantum materials database (OQMD). JOM 2013, 65, 1501–1509. [Google Scholar] [CrossRef]
- Kirklin, S.; Saal, J.E.; Meredig, B.; Thompson, A.; Doak, J.W.; Aykol, M.; Rühl, S.; Wolverton, C. The Open Quantum Materials Database (OQMD): Assessing the accuracy of DFT formation energies. npj Comput. Mater. 2015, 1, 15010. [Google Scholar] [CrossRef]
- Mustafa, G.M.; Younas, B.; Alkhaldi, H.D.; Mera, A.; Alqorashi, A.K.; Hakami, J.; Mahmoud, S.A.; Boukhris, I.; Mahmood, Q. First principle study of physical aspects and hydrogen storage capacity of magnesium-based double perovskite hydrides Mg2XH6 (X = Cr, Mn). Int. J. Hydrogen Energy 2024, 95, 300–308. [Google Scholar] [CrossRef]
- Baysal, M.; Surucu, G.; Deligoz, E.; Ozısık, H. The effect of hydrogen on the electronic, mechanical and phonon properties of LaMgNi4 and its hydrides for hydrogen storage applications. Int. J. Hydrogen Energy 2018, 43, 23397–23408. [Google Scholar] [CrossRef]
- Gencer, A.; Surucu, G. Investigation of structural, electronic and lattice dynamical properties of XNiH3 (X = Li, Na and K) perovskite type hydrides and their hydrogen storage applications. Int. J. Hydrogen Energy 2019, 44, 15173–15182. [Google Scholar] [CrossRef]
- Gencer, A.; Surucu, G. Enhancement of hydrogen storage properties of Ca3CH antiperovskite compound with hydrogen doping. Int. J. Energy Res. 2020, 44, 567–573. [Google Scholar] [CrossRef]
- Baaddi, M.; Chami, R.; Baalla, O.; Quaoubi, S.E.; Saadi, A.; Omari, L.E.H.; Chafi, M. The effect of strain on hydrogen storage characteristics in K2NaAlH6 double perovskite hydride through first principle method. Environ. Sci. Pollut. Res. 2024, 31, 62056–62064. [Google Scholar] [CrossRef]
- Pugh, S.F. XCII. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1954, 45, 823–843. [Google Scholar] [CrossRef]
- Umer, M.; Murtaza, G.; Ahmad, N.; Ayyaz, A.; Raza, H.H.; Usman, A.; Liaqat, A.; Manoharadas, S. First principles investigation of structural, mechanical, thermodynamic, and electronic properties of Al-based perovskites XAlH3 (X=K, Rb, Cs) for hydrogen storage. Int. J. Hydrogen Energy 2024, 61, 820–830. [Google Scholar] [CrossRef]
- Xu, N.; Song, R.; Chen, S.; Chen, Y.; Li, S.; Jiang, Z. First-principles study on the structure, mechanical, electrical, optical, kinetic, thermodynamic and hydrogen storage properties of the hydride perovskites XScH3 (X = K, Rb, Cs) for hydrogen storage applications. J. Energy Storage 2025, 107, 114945. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, M.; Jia, X.; Wei, Q. Study of the stable structures and properties of Ru2Al5. Chin. Phys. B 2025, 34, 016301. [Google Scholar] [CrossRef]
- Wei, Q.; Yang, J.; Jia, X.; Luo, J.; Zhang, M.; Zhu, X. Crystal structures, mechanical properties, and electronic structure analysis of ternary FeCrAl alloys. Phys. Lett. A 2025, 533, 130228. [Google Scholar] [CrossRef]
- Chen, X.Q.; Niu, H.; Li, D.; Li, Y. Modeling hardness of polycrystalline materials and bulk metallic glasses. Intermetallics 2011, 19, 1275–1281. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, W.; Chen, L.; Zhang, Y.; Wang, H.; Zhang, M.; Wei, Q. Structural, strength and fracture mechanisms of superconducting transition metal nitrides TM3N5 (TM = W and Mo). Phys. Chem. Chem. Phys. 2025, 27, 6134–6145. [Google Scholar] [CrossRef]
- Fine, M.; Brown, L.; Marcus, H. Elastic constants versus melting temperature in metals. Scr. Metall. 1984, 18, 951–956. [Google Scholar] [CrossRef]
- Hussain, S.; Rehman, J.U.; Tahir, M.B.; Hussain, A. First-principles study of structural, mechanical, optical, and electronic properties of double perovskite RbBa2Ti3O10 material for photocatalytic applications. Int. J. Hydrogen Energy 2024, 78, 1123–1132. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef]
- Gupta, S.L.; Kumar, S.; Panwar, S. Ab initio studies of newly proposed zirconium based novel combinations of hydride perovskites ZrXH3 (X = Zn, Cd) as hydrogen storage applications. Int. J. Hydrogen Energy 2024, 55, 1465–1475. [Google Scholar] [CrossRef]
- Shockley, W.; Queisser, H.J. Detailed Balance Limit of Efficiency of p-n Junction Solar Cells. J. Appl. Phys. 1961, 32, 510–519. [Google Scholar] [CrossRef]
- Roldan-Carmona, C.; Malinkiewicz, O.; Betancur, R.; Longo, G.; Momblona, C.; Jaramillo, F.; Camacho, L.; Bolink, H.J. High efficiency single-junction semitransparent perovskite solar cells. Energy Environ. Sci. 2014, 7, 2968–2973. [Google Scholar] [CrossRef]
- Leguy, A.M.A.; Azarhoosh, P.; Alonso, M.I.; Campoy-Quiles, M.; Weber, O.J.; Yao, J.; Bryant, D.; Weller, M.T.; Nelson, J.; Walsh, A.; et al. Experimental and theoretical optical properties of methylammonium lead halide perovskites. Nanoscale 2016, 8, 6317–6327. [Google Scholar] [CrossRef]
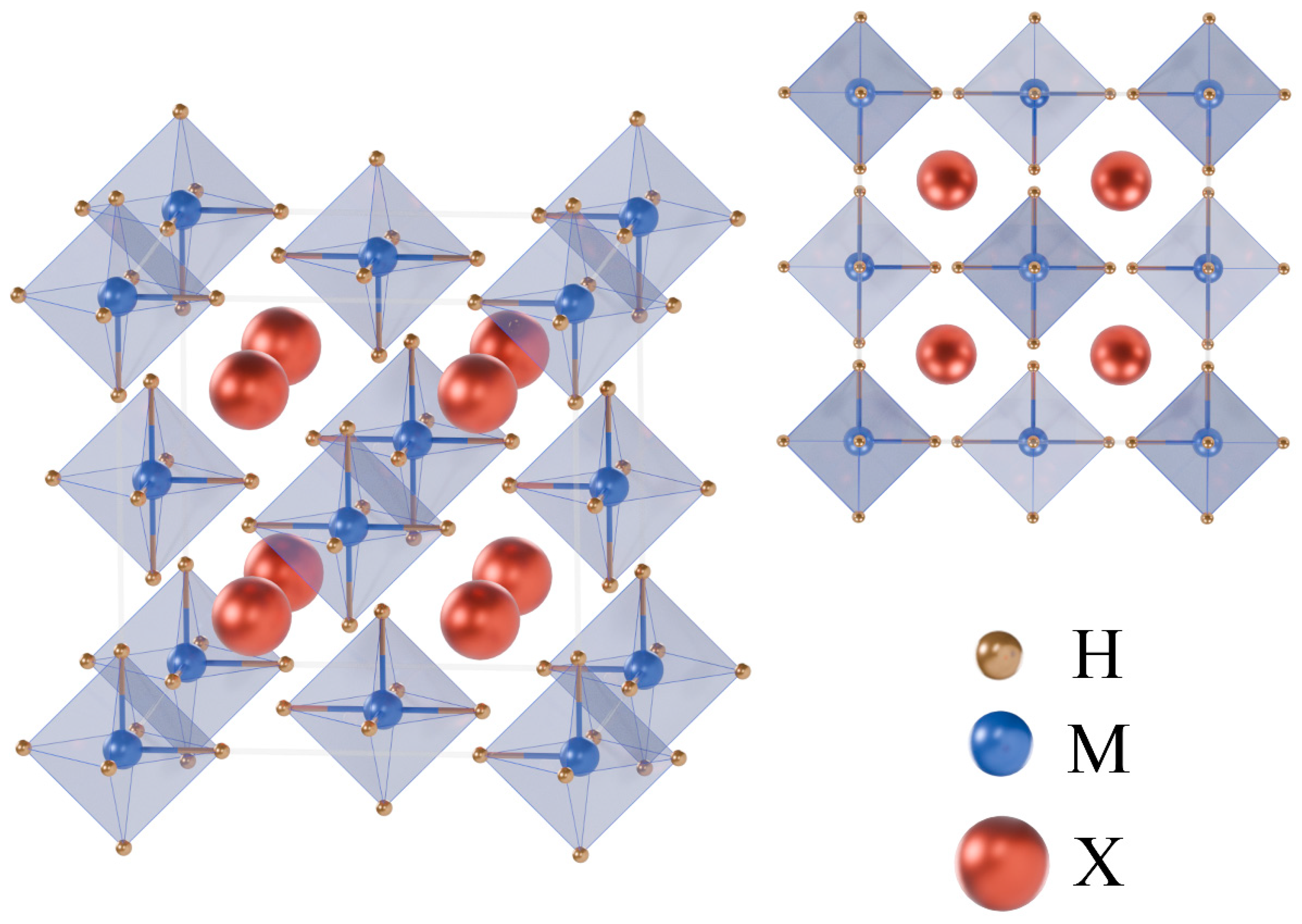
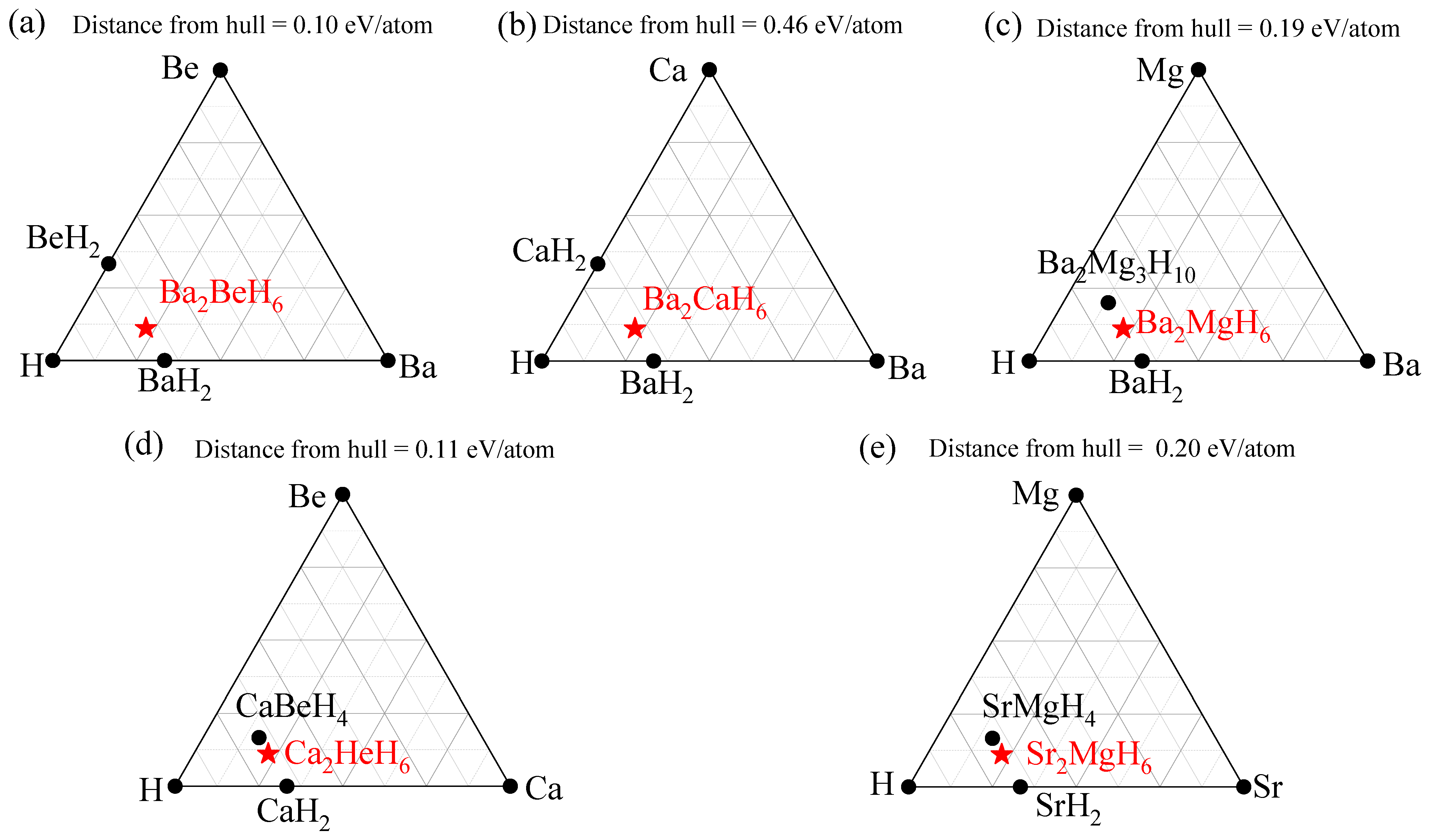




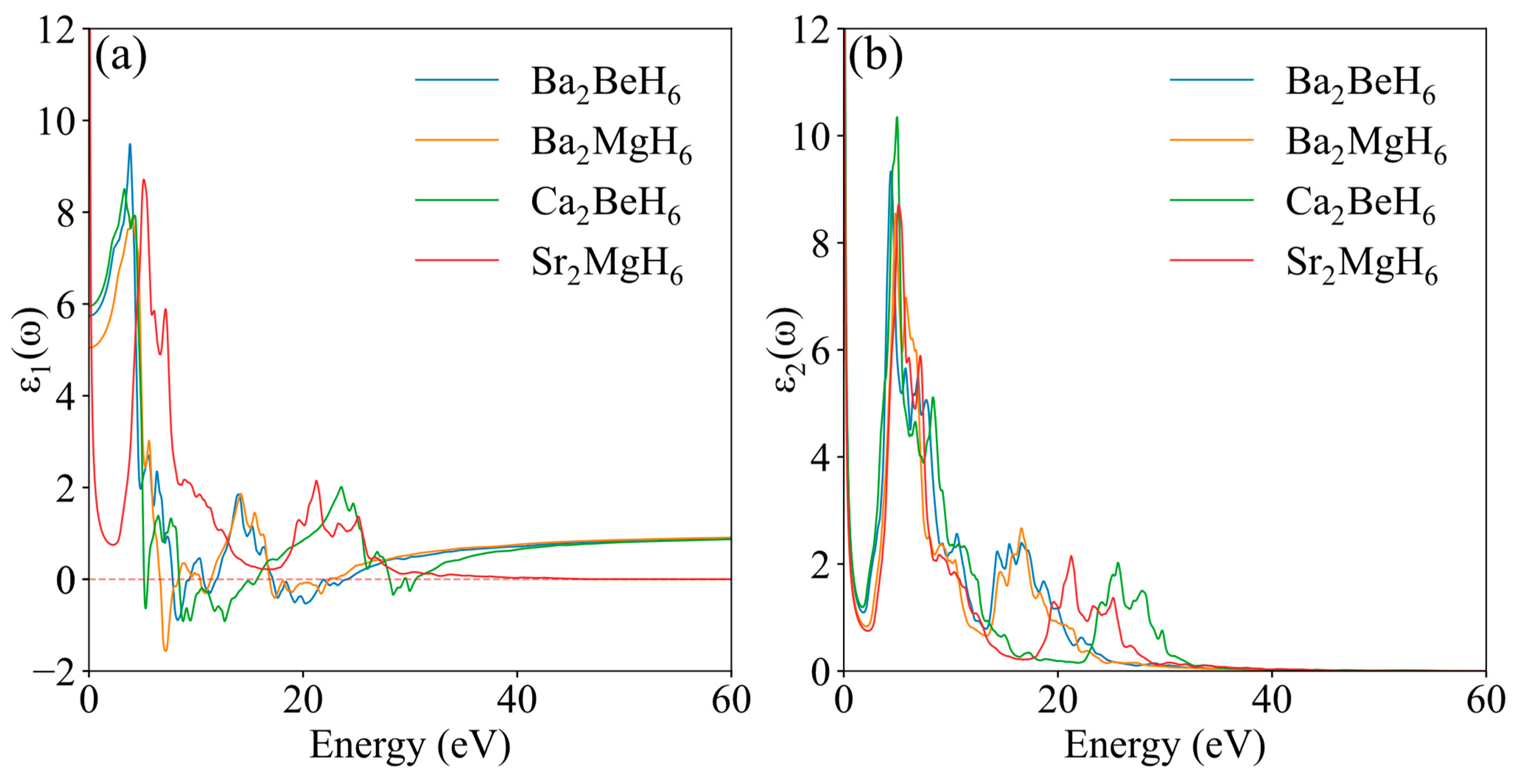
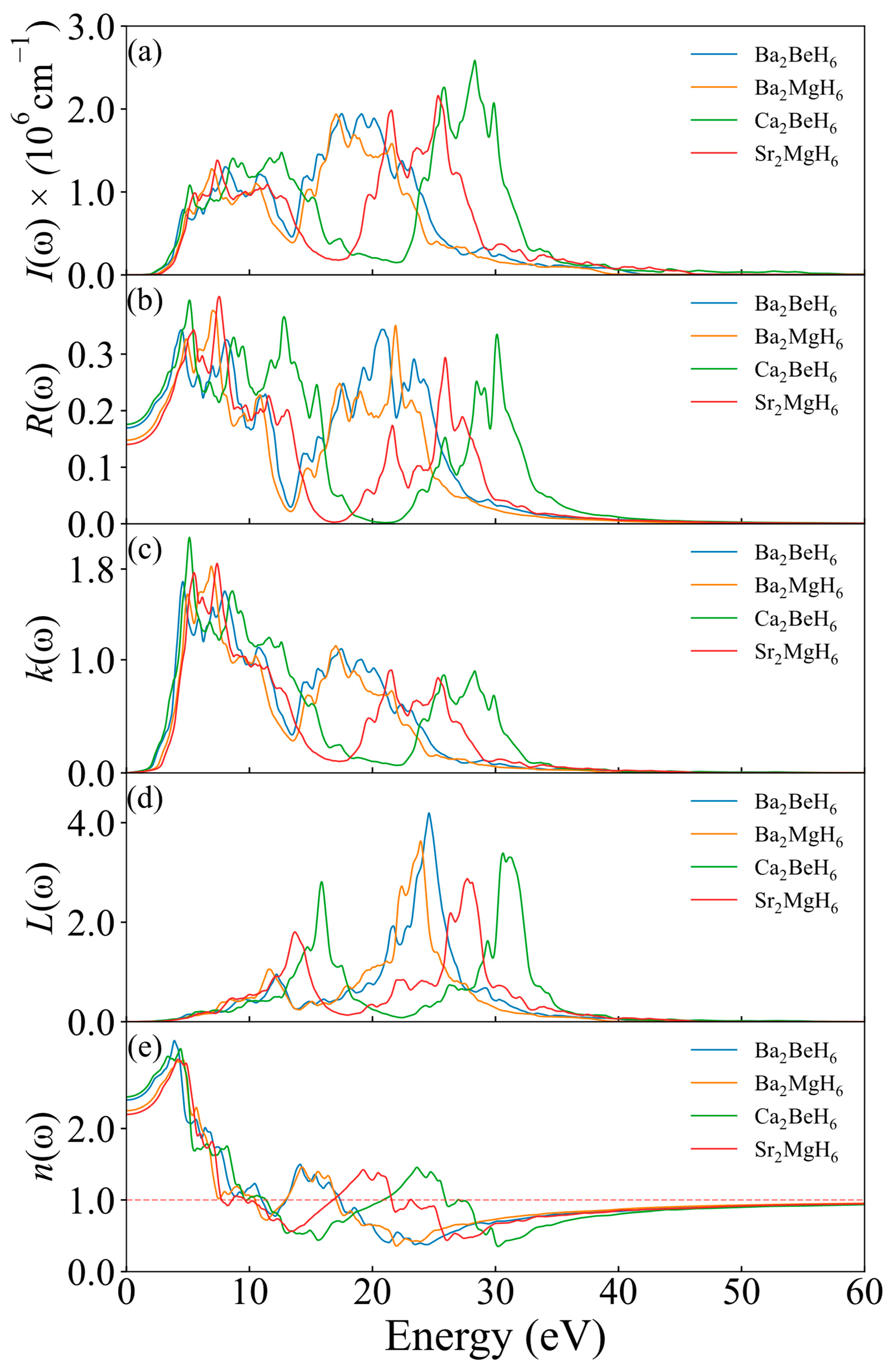
| Ba2BeH6 | Ba2CaH6 | Ba2MgH6 | Ca2BeH6 | Sr2MgH6 | |
|---|---|---|---|---|---|
| a () | 7.577 | 8.102 | 7.915 | 6.757 | 7.511 |
| V () | 435.023 | 531.921 | 495.765 | 308.527 | 423.737 |
| (eV/atom) | −0.75 | −0.57 | −0.74 | −0.84 | −0.75 |
| C11 (GPa) | 112 | 79 | 92 | 154 | 109 |
| C12 (GPa) | 26 | 17 | 20 | 25 | 20 |
| C44 (GPa) | 37 | 24 | 32 | 61 | 40 |
| Ba2BeH6 | Ba2MgH6 | Ca2BeH6 | Sr2MgH6 | |
|---|---|---|---|---|
| 1.47 | 1.98 | 6.32 | 2.91 | |
| 22.90 | 20.10 | 32.29 | 23.51 |
| Structure | A | B | G | E | B/G | HChen | Tm | |
|---|---|---|---|---|---|---|---|---|
| Ba2BeH6 | 0.86 | 54.3 | 39.4 | 95.3 | 1.4 | 0.208 | 8.7 | 706.67 |
| Ba2MgH6 | 0.89 | 44.0 | 33.9 | 80.8 | 1.3 | 0.194 | 8.5 | 671.22 |
| Ca2BeH6 | 0.95 | 67.9 | 62.3 | 143.1 | 1.1 | 0.149 | 17.1 | 700.78 |
| Sr2MgH6 | 0.90 | 49.5 | 41.5 | 97.3 | 1.2 | 0.173 | 11.3 | 671.22 |
| KAlH3 [35] | 0.14 | 1.19 | 11.61 | 8.79 | 0.10 | −0.26 | ||
| RbAlH3 [35] | 0.53 | 7.05 | 5.83 | 14.21 | 1.21 | 0.28 | ||
| CsAlH3 [35] | 0.18 | 1.69 | 0.61 | 2.00 | 2.79 | 0.39 | ||
| KScH3 [36] | 1.01 | 40.41 | 29.26 | 70.72 | 1.38 | 0.21 | ||
| RbScH3 [36] | 1.13 | 39.04 | 29.41 | 70.52 | 1.33 | 0.20 | ||
| CsScH3 [36] | 1.28 | 35.46 | 26.54 | 63.73 | 1.34 | 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.; Wei, Q.; Wang, X.; Zhang, M.; Wei, B. A First-Principles Investigation of the Structural, Electronic, Optical, and Mechanical Properties of Hydrogen Storage Ordered Vacancy Double Perovskite X2MH6 Materials. Nanomaterials 2025, 15, 1339. https://doi.org/10.3390/nano15171339
Luo J, Wei Q, Wang X, Zhang M, Wei B. A First-Principles Investigation of the Structural, Electronic, Optical, and Mechanical Properties of Hydrogen Storage Ordered Vacancy Double Perovskite X2MH6 Materials. Nanomaterials. 2025; 15(17):1339. https://doi.org/10.3390/nano15171339
Chicago/Turabian StyleLuo, Jing, Qun Wei, Xinyu Wang, Meiguang Zhang, and Bing Wei. 2025. "A First-Principles Investigation of the Structural, Electronic, Optical, and Mechanical Properties of Hydrogen Storage Ordered Vacancy Double Perovskite X2MH6 Materials" Nanomaterials 15, no. 17: 1339. https://doi.org/10.3390/nano15171339
APA StyleLuo, J., Wei, Q., Wang, X., Zhang, M., & Wei, B. (2025). A First-Principles Investigation of the Structural, Electronic, Optical, and Mechanical Properties of Hydrogen Storage Ordered Vacancy Double Perovskite X2MH6 Materials. Nanomaterials, 15(17), 1339. https://doi.org/10.3390/nano15171339








