Progress in Nanofluid Technology: From Conventional to Green Nanofluids for Biomedical, Heat Transfer, and Machining Applications
Abstract
1. Introduction
2. Overview of Conventional and Green Nanofluids Production
2.1. Conventional Synthesis of Nanoparticles
2.2. Green Synthesis of Nanoparticles
2.2.1. Principles of Green Synthesis
2.2.2. The Role of Plants and Microorganisms in Nanoparticle Synthesis
2.3. Conventional and Green Nanofluid Formulation Techniques
3. Factors Affecting the Physicochemical Properties of Nanofluids
3.1. Size of Nanoparticles
3.2. Shape of Nanoparticles
3.3. Concentration of Nanoparticles
3.4. Dispersion of Nanoparticles
3.5. Intrinsic Thermal Conductivity of Nanoparticles
3.6. Properties of the Base Fluid
4. Biomedical Applications of Nanofluids
4.1. Biomedical Applications of Conventional Nanofluids
4.1.1. Targeted Drug Delivery and Cancer Therapy
4.1.2. Theranostics and Imaging
4.1.3. Antimicrobial and Antioxidant Applications
4.2. Biomedical Applications of Green Nanofluids
4.2.1. Targeted Drug Delivery and Cancer Therapy
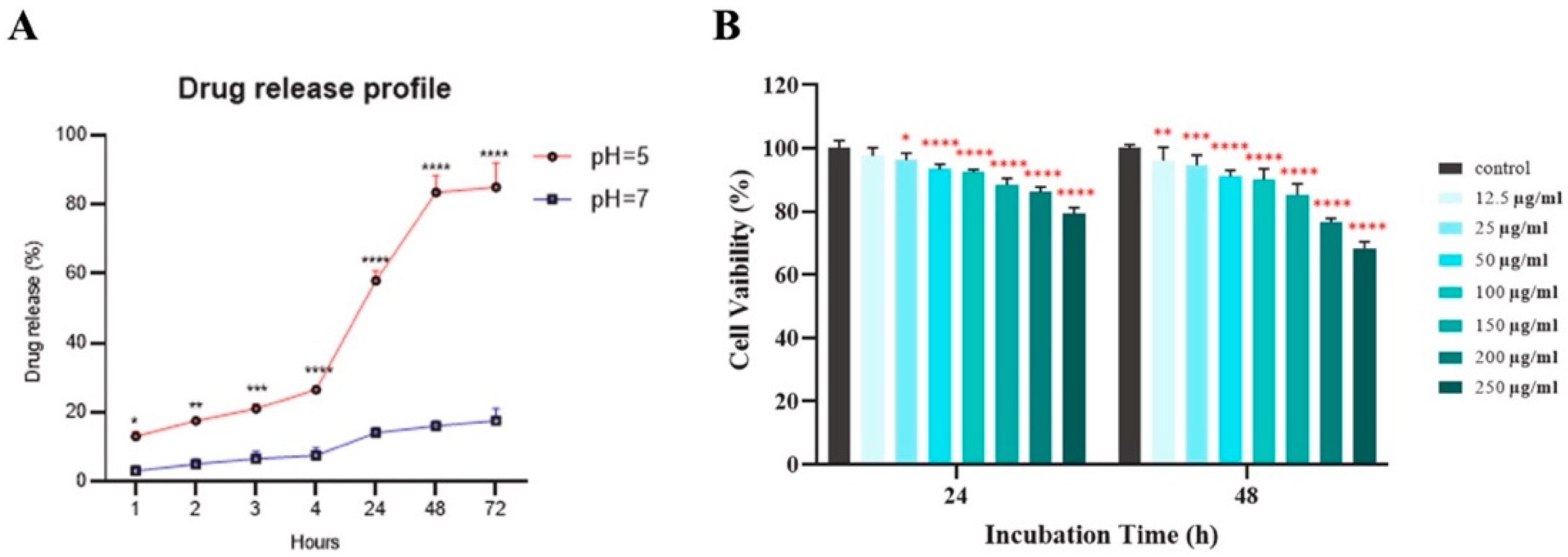
4.2.2. Theranostics and Imaging
4.2.3. Antimicrobial and Antioxidant Applications
4.3. Comparative Insights into Green and Conventional Derived Nanofluids for Biomedical Applications
5. Heat Transfer Applications of Nanofluids
5.1. Heat Transfer Applications of Conventional Nanofluids
5.1.1. Solar Panels and Collectors
5.1.2. Electronics Cooling
5.1.3. Engine Cooling
5.1.4. Nuclear Reactors
5.1.5. Space Technology
5.1.6. Transformers
5.1.7. Heat Pipes
5.2. Heat Transfer Applications of Green Nanofluids
5.2.1. Solar Panels and Collectors
5.2.2. Electronics and Engine Cooling
5.2.3. Transformers and Heat Pipes
5.2.4. Other Applications
5.2.5. Comparative Insights into Green and Conventional Derived Nanofluids for Heat Transfer Applications
6. Machining Applications of Nanofluids
6.1. Machining Applications in Conventional Nanofluids
6.2. Machining Applications in Green Nanofluids
6.3. Comparative Insights into Green and Conventional Derived Nanofluids for Machining Applications
7. Costs and Industrial Commercialization of Nanofluids
7.1. Economic Considerations in Nanofluid Production and Performance
7.2. Commercialization Attempts and Barriers to Industrial Implementation
7.3. Environmental Implications and Sustainability
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qiu, L.; Zhu, N.; Feng, Y.; Michaelides, E.E.; Żyła, G.; Jing, D.; Zhang, X.; Norris, P.M.; Markides, C.N.; Mahian, O. A review of recent advances in thermophysical properties at the nanoscale: From solid state to colloids. Phys. Rep. 2020, 843, 1–81. [Google Scholar] [CrossRef]
- Hwang, Y.; Lee, J.; Lee, C.; Jung, Y.; Cheong, S.; Lee, C.; Ku, B.; Jang, S. Stability and thermal conductivity characteristics of nanofluids. Thermochim. Acta 2007, 455, 70–74. [Google Scholar] [CrossRef]
- Sajid, M.U.; Ali, H.M. Recent advances in application of nanofluids in heat transfer devices: A critical review. Renew. Sustain. Energy Rev. 2019, 103, 556–592. [Google Scholar] [CrossRef]
- Choi, S.U.; Eastman, J.A. Enhancing Thermal Conductivity of Fluids with Nanoparticles; Argonne National Lab. (ANL): Argonne, IL, USA, 1995. [Google Scholar]
- Mamat, H.; Ramadan, M. Nanofluids: Thermal Conductivity and Applications. Encycl. Smart Mater. 2022, 3, 288–296. [Google Scholar]
- Eastman, J.A.; Choi, U.; Li, S.; Thompson, L.; Lee, S. Enhanced thermal conductivity through the development of nanofluids. MRS Online Proc. Libr. (OPL) 1996, 457, 3–11. [Google Scholar] [CrossRef]
- Moita, A.; Moreira, A.; Pereira, J. Nanofluids for the next generation thermal management of electronics: A review. Symmetry 2021, 13, 1362. [Google Scholar] [CrossRef]
- Pathak, S.K.; Kumar, R.; Goel, V.; Pandey, A.; Tyagi, V. Recent advancements in thermal performance of nano-fluids charged heat pipes used for thermal management applications: A comprehensive review. Appl. Therm. Eng. 2022, 216, 119023. [Google Scholar] [CrossRef]
- Razzaq, I.; Xinhua, W.; Rasool, G.; Sun, T.; Shflot, A.S.; Malik, M.Y.; Abbas, K.; Ali, S.; Ali, A. Nanofluids for advanced applications: A comprehensive review on Preparation methods, properties, and environmental impact. ACS Omega 2025, 10, 5251–5282. [Google Scholar] [CrossRef] [PubMed]
- Vatani, A. Heat Transfer from Electrically Heated Microwire in Magnetic and Optical Nanofluids. Ph.D. Thesis, Griffith University, Gold Coast, Australia, 2017. [Google Scholar]
- Das, S.K.; Choi, S.U.; Patel, H.E. Heat transfer in nanofluids—A review. Heat Transf. Eng. 2006, 27, 3–19. [Google Scholar] [CrossRef]
- Kavitha, T.; Rajendran, A.; Durairajan, A.; Shanmugam, A. Heat Transfer Enhancement Using Nanofluids and Innovative Methods. An Overview. Int. J. Mech. Eng. Technol. 2012, 3, 769–782. [Google Scholar]
- Enjavi, Y.; Sedghamiz, M.A.; Rahimpour, M.R. Application of Nanofluids in Drug Delivery and Disease Treatment. In Nanofluids and Mass Transfer; Elsevier: Amsterdam, The Netherlands, 2022; pp. 449–465. [Google Scholar]
- Mekheimer, K.S.; Hasona, W.; Abo-Elkhair, R.; Zaher, A. Peristaltic blood flow with gold nanoparticles as a third grade nanofluid in catheter: Application of cancer therapy. Phys. Lett. A 2018, 382, 85–93. [Google Scholar] [CrossRef]
- Sharifi, I.; Shokrollahi, H.; Amiri, S. Ferrite-based magnetic nanofluids used in hyperthermia applications. J. Magn. Magn. Mater. 2012, 324, 903–915. [Google Scholar] [CrossRef]
- Soufi, G.J.; Iravani, S. Eco-Friendly and sustainable synthesis of biocompatible nanomaterials for diagnostic imaging: Current challenges and future perspectives. Green Chem. 2020, 22, 2662–2687. [Google Scholar] [CrossRef]
- Amin, A.R.; Ali, A.; Ali, H.M. Application of nanofluids for machining processes: A comprehensive review. Nanomaterials 2022, 12, 4214. [Google Scholar] [CrossRef]
- Kadirgama, K. A comprehensive review on the application of nanofluids in the machining process. Int. J. Adv. Manuf. Technol. 2021, 115, 2669–2681. [Google Scholar] [CrossRef]
- Sah, N.K.; Singh, R.; Sharma, V. Experimental investigations into thermophysical, wettability and tribological characteristics of ionic liquid based metal cutting fluids. J. Manuf. Process. 2021, 65, 190–205. [Google Scholar] [CrossRef]
- Chu, A.; Li, C.; Zhou, Z.; Liu, B.; Zhang, Y.; Yang, M.; Gao, T.; Liu, M.; Zhang, N.; Dambatta, Y.S. Nanofluids minimal quantity lubrication machining: From mechanisms to application. Lubricants 2023, 11, 422. [Google Scholar] [CrossRef]
- Kumar, L.H.; Kazi, S.; Masjuki, H.; Zubir, M. A review of recent advances in green nanofluids and their application in thermal systems. Chem. Eng. J. 2022, 429, 132321. [Google Scholar] [CrossRef]
- Kaur, K.; Jeet, K. Electrical conductivity of water-based nanofluids prepared with graphene–carbon nanotube hybrid. Fuller. Nanotub. Carbon Nanostruct. 2017, 25, 726–734. [Google Scholar] [CrossRef]
- Areekara, S.; Sabu, A.S.; Mathew, A.; Saravanan, B. Statistical analysis on the stratification effects of bioconvective EMHD nanofluid flow past a stretching sheet: Application in theranostics. Heat Transf. 2021, 50, 6680–6702. [Google Scholar] [CrossRef]
- Lei, M.; Gao, M.; Yang, X.; Zou, Y.; Alghamdi, A.; Ren, Y.; Deng, Y. Size-controlled Au nanoparticles incorporating mesoporous ZnO for sensitive ethanol sensing. ACS Appl. Mater. Interfaces 2021, 13, 51933–51944. [Google Scholar] [CrossRef]
- Ashfield, L. Complex-shaped Metal Nanoparticles: Bottom-Up Syntheses and Applications. Platin. Met. Rev. 2013, 57, 123–126. [Google Scholar] [CrossRef]
- Grzelczak, M.; Pérez-Juste, J.; Mulvaney, P.; Liz-Marzán, L.M. Shape control in gold nanoparticle synthesis. In Colloidal Synthesis of Plasmonic Nanometals; Jenny Stanford Publishing: New York, NY, USA, 2020; pp. 197–220. [Google Scholar]
- De Carvalho, J.; De Medeiros, S.; Morales, M.; Dantas, A.; Carriço, A. Synthesis of magnetite nanoparticles by high energy ball milling. Appl. Surf. Sci. 2013, 275, 84–87. [Google Scholar] [CrossRef]
- Kim, M.; Osone, S.; Kim, T.; Higashi, H.; Seto, T. Synthesis of nanoparticles by laser ablation: A review. KONA Powder Part. J. 2017, 34, 80–90. [Google Scholar] [CrossRef]
- Li, W.; Shah, S.I.; Huang, C.-P.; Jung, O.; Ni, C. Metallorganic chemical vapor deposition and characterization of TiO2 nanoparticles. Mater. Sci. Eng. B 2002, 96, 247–253. [Google Scholar] [CrossRef]
- Wahab, R.; Ansari, S.G.; Dar, M.A.; Kim, Y.S.; Shin, H.S. Synthesis of Magnesium Oxide Nanoparticles by Sol-Gel Process. Mater. Sci. Forum 2007, 558–559, 983–986. [Google Scholar] [CrossRef]
- Tazikeh, S.; Akbari, A.; Talebi, A.; Talebi, E. Synthesis and characterization of tin oxide nanoparticles via the Co-precipitation method. Mater. Sci.-Pol. 2014, 32, 98–101. [Google Scholar] [CrossRef]
- Hyeon, T. Chemical synthesis of magnetic nanoparticles. Chem. Commun. 2003, 927–934. [Google Scholar] [CrossRef] [PubMed]
- De Souza, C.D.; Nogueira, B.R.; Rostelato, M.E.C. Review of the methodologies used in the synthesis gold nanoparticles by chemical reduction. J. Alloys Compd. 2019, 798, 714–740. [Google Scholar] [CrossRef]
- Gupta, D.; Boora, A.; Thakur, A.; Gupta, T.K. Green and sustainable synthesis of nanomaterials: Recent advancements and limitations. Environ. Res. 2023, 231, 116316. [Google Scholar] [CrossRef]
- Rudra, S. Harnessing Nature’s Power for Eco-Friendly Metal Nanoparticle Synthesis and Applications. Authorea Prepr. 2024. [Google Scholar] [CrossRef]
- Nyabadza, A.; McCarthy, É.; Makhesana, M.; Heidarinassab, S.; Plouze, A.; Vazquez, M.; Brabazon, D. A review of physical, chemical and biological synthesis methods of bimetallic nanoparticles and applications in sensing, water treatment, biomedicine, catalysis and hydrogen storage. Adv. Colloid Interface Sci. 2023, 321, 103010. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.; Bhaskar, R.; Vyavhare, V.; Dhadge, R.; Khaire, V.; Patil, Y. Overview on methods of synthesis of nanoparticles. Int. J. Curr. Pharm. Res. 2021, 13, 11–16. [Google Scholar] [CrossRef]
- Krishnia, L.; Thakur, P.; Thakur, A. Synthesis of nanoparticles by physical route. In Synthesis and Applications of Nanoparticles; Springer: Singapore, 2022; pp. 45–59. [Google Scholar]
- Silva, A.A.; Sousa, A.M.F.; Furtado, C.R.; Carvalho, N.M. Green magnesium oxide prepared by plant extracts: Synthesis, properties and applications. Mater. Today Sustain. 2022, 20, 100203. [Google Scholar] [CrossRef]
- Patel, M. Green Synthesis of Nanoparticles: A Solution to Environmental Pollution. In Handbook of Solid Waste Management: Sustainability Through Circular Economy; Springer: Singapore, 2022; pp. 1965–1993. [Google Scholar]
- Mishra, M.; Sharma, M.; Dubey, R.; Kumari, P.; Ranjan, V.; Pandey, J. Green synthesis interventions of pharmaceutical industries for sustainable development. Curr. Res. Green Sustain. Chem. 2021, 4, 100174. [Google Scholar] [CrossRef]
- Cardoso, B.; Nobrega, G.; Afonso, I.S.; Ribeiro, J.E.; Lima, R.A. Sustainable green synthesis of metallic nanoparticle using plants and microorganisms: A review of biosynthesis methods, mechanisms, toxicity, and applications. J. Environ. Chem. Eng. 2025, 13, 116921. [Google Scholar] [CrossRef]
- Omran, B.A.; Baek, K.-H. Valorization of agro-industrial biowaste to green nanomaterials for wastewater treatment: Approaching green chemistry and circular economy principles. J. Environ. Manag. 2022, 311, 114806. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Kharisov, B.I.; Oliva González, C.M.; Méndez, Y.P.; López, I. Greener synthesis of chemical compounds and materials. R. Soc. Open Sci. 2019, 6, 191378. [Google Scholar] [CrossRef]
- Aswathi, V.; Meera, S.; Maria, C.A.; Nidhin, M. Green synthesis of nanoparticles from biodegradable waste extracts and their applications: A critical review. Nanotechnol. Environ. Eng. 2023, 8, 377–397. [Google Scholar] [CrossRef]
- Rodríguez-Félix, F.; Graciano-Verdugo, A.Z.; Moreno-Vásquez, M.J.; Lagarda-Díaz, I.; Barreras-Urbina, C.G.; Armenta-Villegas, L.; Olguín-Moreno, A.; Tapia-Hernández, J.A. Trends in Sustainable Green Synthesis of Silver Nanoparticles Using Agri-Food Waste Extracts and Their Applications in Health. J. Nanomater. 2022, 2022, 8874003. [Google Scholar] [CrossRef]
- Hano, C.; Abbasi, B.H. Plant-Based Green Synthesis of Nanoparticles: Production, Characterization and Applications. Biomolecules 2022, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Huston, M.; DeBella, M.; DiBella, M.; Gupta, A. Green synthesis of nanomaterials. Nanomaterials 2021, 11, 2130. [Google Scholar] [CrossRef]
- Mustapha, T.; Misni, N.; Ithnin, N.R.; Daskum, A.M.; Unyah, N.Z. A review on plants and microorganisms mediated synthesis of silver nanoparticles, role of plants metabolites and applications. Int. J. Environ. Res. Public Health 2022, 19, 674. [Google Scholar] [CrossRef]
- Hosseingholian, A.; Gohari, S.; Feirahi, F.; Moammeri, F.; Mesbahian, G.; Moghaddam, Z.; Ren, Q. Recent advances in green synthesized nanoparticles: From production to application. Mater. Today Sustain. 2023, 24, 100500. [Google Scholar] [CrossRef]
- Nath, P.C.; Ojha, A.; Debnath, S.; Sharma, M.; Sridhar, K.; Nayak, P.K.; Inbaraj, B.S. Biogeneration of valuable nanomaterials from agro-wastes: A comprehensive review. Agronomy 2023, 13, 561. [Google Scholar] [CrossRef]
- Pandit, C.; Roy, A.; Ghotekar, S.; Khusro, A.; Islam, M.N.; Emran, T.B.; Lam, S.E.; Khandaker, M.U.; Bradley, D.A. Biological agents for synthesis of nanoparticles and their applications. J. King Saud Univ. Sci. 2022, 34, 101869. [Google Scholar] [CrossRef]
- Trzcińska-Wencel, J.; Wypij, M.; Rai, M.; Golińska, P. Biogenic nanosilver bearing antimicrobial and antibiofilm activities and its potential for application in agriculture and industry. Front. Microbiol. 2023, 14, 1125685. [Google Scholar] [CrossRef]
- Tan, Y.N.; Lee, J.Y.; Wang, D.I. Uncovering the design rules for peptide synthesis of metal nanoparticles. J. Am. Chem. Soc. 2010, 132, 5677–5686. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wu, H.; Pu, S.; Zhang, W.; Liao, X.; Shi, B. One-step room-temperature synthesis of Au@ Pd core–shell nanoparticles with tunable structure using plant tannin as reductant and stabilizer. Green Chem. 2011, 13, 950–957. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Yassin, M.T.; Mostafa, A.A.-F.; Al-Askar, A.A.; Al-Otibi, F.O. Facile green synthesis of silver nanoparticles using aqueous leaf extract of Origanum majorana with potential bioactivity against multidrug resistant bacterial strains. Crystals 2022, 12, 603. [Google Scholar] [CrossRef]
- Álvarez-Chimal, R.; García-Pérez, V.I.; Álvarez-Pérez, M.A.; Tavera-Hernández, R.; Reyes-Carmona, L.; Martínez-Hernández, M.; Arenas-Alatorre, J.Á. Influence of the particle size on the antibacterial activity of green synthesized zinc oxide nanoparticles using Dysphania ambrosioides extract, supported by molecular docking analysis. Arab. J. Chem. 2022, 15, 103804. [Google Scholar] [CrossRef]
- Matos, Â.; Novelli, E.; Tribuzi, G. Use of algae as food ingredient: Sensory acceptance and commercial products. Front. Food Sci. Technol. 2022, 2, 989801. [Google Scholar] [CrossRef]
- Patel, A.K.; Albarico, F.P.J.B.; Perumal, P.K.; Vadrale, A.P.; Nian, C.T.; Chau, H.T.B.; Anwar, C.; ud din Wani, H.M.; Pal, A.; Saini, R. Algae as an emerging source of bioactive pigments. Bioresour. Technol. 2022, 351, 126910. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, D.; Verduin, J.J.; Shah, M.; Sharma, S.B.; Poinern, G.E.J. A review of current research into the biogenic synthesis of metal and metal oxide nanoparticles via marine algae and seagrasses. J. Nanosci. 2017, 2017, 8013850. [Google Scholar] [CrossRef]
- Khanna, P.; Kaur, A.; Goyal, D. Algae-based metallic nanoparticles: Synthesis, characterization and applications. J. Microbiol. Methods 2019, 163, 105656. [Google Scholar] [CrossRef]
- Dogmaz, S.; Cavas, L. Biohydrogen production via green silver nanoparticles synthesized through biomass of Ulva lactuca bloom. Bioresour. Technol. 2023, 379, 129028. [Google Scholar] [CrossRef]
- Alarif, W.M.; Shaban, Y.A.; Orif, M.I.; Ghandourah, M.A.; Turki, A.J.; Alorfi, H.S.; Tadros, H.R.Z. Green Synthesis of TiO2 Nanoparticles Using Natural Marine Extracts for Antifouling Activity. Mar. Drugs 2023, 21, 62. [Google Scholar] [CrossRef] [PubMed]
- Raj, C.D.; Muthukumar, K.; Dahms, H.U.; James, R.A.; Kandaswamy, S. Structural characterization, antioxidant and anti-uropathogenic potential of biogenic silver nanoparticles using brown seaweed Turbinaria ornata. Front. Microbiol. 2023, 14, 1072043. [Google Scholar] [CrossRef]
- Żymańczyk-Duda, E.; Brzezińska-Rodak, M.; Klimek-Ochab, M.; Duda, M.; Zerka, A. Yeast as a versatile tool in biotechnology. In Yeast-Industrial Applications; IntechOpen: London, UK, 2017. [Google Scholar]
- Zhang, X.; Yan, S.; Tyagi, R.; Surampalli, R. Synthesis of nanoparticles by microorganisms and their application in enhancing microbiological reaction rates. Chemosphere 2011, 82, 489–494. [Google Scholar] [CrossRef]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef]
- Liu, D.; Ding, L.; Sun, J.; Boussetta, N.; Vorobiev, E. Yeast cell disruption strategies for recovery of intracellular bio-active compounds—A review. Innov. Food Sci. Emerg. Technol. 2016, 36, 181–192. [Google Scholar] [CrossRef]
- Perego, P.; Howell, S.B. Molecular mechanisms controlling sensitivity to toxic metal ions in yeast. Toxicol. Appl. Pharmacol. 1997, 147, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Hulkoti, N.I.; Taranath, T. Biosynthesis of nanoparticles using microbes—A review. Colloids Surf. B Biointerfaces 2014, 121, 474–483. [Google Scholar] [CrossRef]
- Iravani, S. Bacteria in nanoparticle synthesis: Current status and future prospects. Int. Sch. Res. Not. 2014, 2014, 359316. [Google Scholar] [CrossRef] [PubMed]
- Tsekhmistrenko, S.; Bityutskyy, V.; Tsekhmistrenko, O.; Horalskyi, L.; Tymoshok, N.; Spivak, M. Bacterial synthesis of nanoparticles: A green approach. Biosyst. Divers. 2020, 28, 9–17. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Chen, Z.-S.; Chen, G. Biosynthesis of nanoparticles by microorganisms and their applications. J. Nanomater. 2011, 2011, 270974. [Google Scholar] [CrossRef]
- Ingold, C.T.; Hudson, H.J. The Biology of Fungi; Springer: Dordrecht, The Netherlands, 1993. [Google Scholar]
- Sakurai, T.; Kataoka, K. Basic and applied features of multicopper oxidases, CueO, bilirubin oxidase, and laccase. Chem. Rec. 2007, 7, 220–229. [Google Scholar] [CrossRef]
- Šebesta, M.; Vojtková, H.; Cyprichová, V.; Ingle, A.P.; Urík, M.; Kolenčík, M. Mycosynthesis of metal-containing nanoparticles—Fungal metal resistance and mechanisms of synthesis. Int. J. Mol. Sci. 2022, 23, 14084. [Google Scholar] [CrossRef] [PubMed]
- Consolo, V.F.; Torres-Nicolini, A.; Alvarez, V.A. Mycosinthetized Ag, CuO and ZnO nanoparticles from a promising Trichoderma harzianum strain and their antifungal potential against important phytopathogens. Sci. Rep. 2020, 10, 20499. [Google Scholar] [CrossRef]
- Hashem, A.H.; Abdelaziz, A.M.; Askar, A.A.; Fouda, H.M.; Khalil, A.M.; Abd-Elsalam, K.A.; Khaleil, M.M. Bacillus megaterium-mediated synthesis of selenium nanoparticles and their antifungal activity against Rhizoctonia solani in faba bean plants. J. Fungi 2021, 7, 195. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.e.; Tung, S.; Schneider, E.; Xi, S. A review on development of nanofluid preparation and characterization. Powder Technol. 2009, 196, 89–101. [Google Scholar] [CrossRef]
- Kumar, S.A.; Meenakshi, K.S.; Narashimhan, B.; Srikanth, S.; Arthanareeswaran, G. Synthesis and characterization of copper nanofluid by a novel one-step method. Mater. Chem. Phys. 2009, 113, 57–62. [Google Scholar] [CrossRef]
- Soleimani, H.; Baig, M.K.; Yahya, N.; Khodapanah, L.; Sabet, M.; Demiral, B.M.; Burda, M. Impact of carbon nanotubes based nanofluid on oil recovery efficiency using core flooding. Results Phys. 2018, 9, 39–48. [Google Scholar] [CrossRef]
- Hemben, A.; Chianella, I.; Leighton, G.J.T. Surface engineered iron oxide nanoparticles generated by inert gas condensation for biomedical applications. Bioengineering 2021, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-J.; Hu, Y.-X.; Liu, M.-C.; Kong, L.-B.; Hu, Y.-M.; Han, W.; Luo, Y.-C.; Kang, L. Mechanical alloying synthesis of Ni3S2 nanoparticles as electrode material for pseudocapacitor with excellent performances. J. Alloys Compd. 2016, 656, 138–145. [Google Scholar] [CrossRef]
- Bhuyan, R.K.; Pamu, D.; Sahoo, B.K.; Sarangi, A.K. Structural and thermal study of Mg2TiO4 nanoparticles synthesized by mechanical alloying method. Micro Nanosyst. 2020, 12, 87–91. [Google Scholar] [CrossRef]
- Waje, S.B.; Hashim, M.; Yusoff, W.D.W.; Abbas, Z. X-ray diffraction studies on crystallite size evolution of CoFe2O4 nanoparticles prepared using mechanical alloying and sintering. Appl. Surf. Sci. 2010, 256, 3122–3127. [Google Scholar] [CrossRef]
- Ahmad, P.; Khandaker, M.U.; Khan, Z.R.; Amin, Y.M. Synthesis of boron nitride nanotubes via chemical vapour deposition: A comprehensive review. RSC Adv. 2015, 5, 35116–35137. [Google Scholar] [CrossRef]
- Gulino, G.; Vieira, R.; Amadou, J.; Nguyen, P.; Ledoux, M.J.; Galvagno, S.; Centi, G.; Pham-Huu, C. C2H6 as an active carbon source for a large scale synthesis of carbon nanotubes by chemical vapour deposition. Appl. Catal. A Gen. 2005, 279, 89–97. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; RanjithKumar, D.; Lee, Y.R. Direct growth of iron oxide nanoparticles filled multi-walled carbon nanotube via chemical vapour deposition method as high-performance supercapacitors. Int. J. Hydrogen Energy 2019, 44, 2349–2360. [Google Scholar] [CrossRef]
- Kumar, D.; Agarwal, G.; Tripathi, B.; Vyas, D.; Kulshrestha, V. Characterization of PbS nanoparticles synthesized by chemical bath deposition. J. Alloys Compd. 2009, 484, 463–466. [Google Scholar] [CrossRef]
- Yew, Y.P.; Shameli, K.; Miyake, M.; Kuwano, N.; Bt Ahmad Khairudin, N.B.; Bt Mohamad, S.E.; Lee, K.X. Green synthesis of magnetite (Fe3O4) nanoparticles using seaweed (Kappaphycus alvarezii) extract. Nanoscale Res. Lett. 2016, 11, 276. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.M.; Babar, H.; Shah, T.R.; Sajid, M.U.; Qasim, M.A.; Javed, S. Preparation techniques of TiO2 nanofluids and challenges: A review. Appl. Sci. 2018, 8, 587. [Google Scholar] [CrossRef]
- Yang, L.; Hu, Y. Toward TiO2 nanofluids—Part 2: Applications and challenges. Nanoscale Res. Lett. 2017, 12, 446. [Google Scholar] [CrossRef] [PubMed]
- Kumarage, G.W.; Hakkoum, H.; Comini, E. Recent advancements in TiO2 nanostructures: Sustainable synthesis and gas sensing. Nanomaterials 2023, 13, 1424. [Google Scholar] [CrossRef]
- Kalsi, S.; Kumar, S.; Kumar, A.; Alam, T.; Dobrotă, D. Thermophysical properties of nanofluids and their potential applications in heat transfer enhancement: A review. Arab. J. Chem. 2023, 16, 105272. [Google Scholar] [CrossRef]
- Evanoff, D.D.; Chumanov, G. Size-controlled synthesis of nanoparticles. 2. Measurement of extinction, scattering, and absorption cross sections. J. Phys. Chem. B 2004, 108, 13957–13962. [Google Scholar] [CrossRef]
- Oh, E.; Delehanty, J.B.; Sapsford, K.E.; Susumu, K.; Goswami, R.; Blanco-Canosa, J.B.; Dawson, P.E.; Granek, J.; Shoff, M.; Zhang, Q. Cellular uptake and fate of PEGylated gold nanoparticles is dependent on both cell-penetration peptides and particle size. ACS Nano 2011, 5, 6434–6448. [Google Scholar] [CrossRef] [PubMed]
- Perrault, S.D.; Walkey, C.; Jennings, T.; Fischer, H.C.; Chan, W.C. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 2009, 9, 1909–1915. [Google Scholar] [CrossRef] [PubMed]
- Panja, P.; Jana, N.R. Arginine-terminated nanoparticles of< 10 nm size for direct membrane penetration and protein delivery for straight access to cytosol and nucleus. J. Phys. Chem. Lett. 2020, 11, 2363–2368. [Google Scholar]
- Osaki, F.; Kanamori, T.; Sando, S.; Sera, T.; Aoyama, Y. A quantum dot conjugated sugar ball and its cellular uptake. On the size effects of endocytosis in the subviral region. J. Am. Chem. Soc. 2004, 126, 6520–6521. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Kim, B.Y.; Rutka, J.T.; Chan, W.C. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 2008, 3, 145–150. [Google Scholar] [CrossRef]
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat. Mater. 2005, 4, 435–446. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble metals on the nanoscale: Optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef]
- Murphy, C.J.; Vartanian, A.M.; Geiger, F.M.; Hamers, R.J.; Pedersen, J.; Cui, Q.; Haynes, C.L.; Carlson, E.E.; Hernandez, R.; Klaper, R.D. Biological responses to engineered nanomaterials: Needs for the next decade. ACS Cent. Sci. 2015, 1, 117–123. [Google Scholar] [CrossRef]
- Lee, N.; Yoo, D.; Ling, D.; Cho, M.H.; Hyeon, T.; Cheon, J. Iron oxide based nanoparticles for multimodal imaging and magnetoresponsive therapy. Chem. Rev. 2015, 115, 10637–10689. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef]
- Vivero-Escoto, J.L.; Huxford-Phillips, R.C.; Lin, W. Silica-based nanoprobes for biomedical imaging and theranostic applications. Chem. Soc. Rev. 2012, 41, 2673–2685. [Google Scholar] [CrossRef]
- Jang, S.P.; Choi, S.U.S. Effects of Various Parameters on Nanofluid Thermal Conductivity. J. Heat Transf. 2007, 129, 617–623. [Google Scholar] [CrossRef]
- Timofeeva, E.V.; Smith, D.S.; Yu, W.; France, D.M.; Singh, D.; Routbort, J.L. Particle size and interfacial effects on thermo-physical and heat transfer characteristics of water-basedα-SiC nanofluids. Nanotechnology 2010, 21, 215703. [Google Scholar] [CrossRef]
- Ambreen, T.; Kim, M.-H. Influence of particle size on the effective thermal conductivity of nanofluids: A critical review. Appl. Energy 2020, 264, 114684. [Google Scholar] [CrossRef]
- Farooq, S.; Habib, M.; Cardozo, O.; Ullah, K.; Pandey, A.; Said, Z. Exploring the impact of particle stability, size, and morphology on nanofluid thermal conductivity: A comprehensive review for energy applications. Adv. Colloid Interface Sci. 2025, 341, 103495. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yu, B.; Zou, M.; Xu, P. A new model for heat conduction of nanofluids based on fractal distributions of nanoparticles. J. Phys. D Appl. Phys. 2006, 39, 4486. [Google Scholar] [CrossRef]
- Anoop, K.; Sundararajan, T.; Das, S.K. Effect of particle size on the convective heat transfer in nanofluid in the developing region. Int. J. Heat Mass Transf. 2009, 52, 2189–2195. [Google Scholar] [CrossRef]
- Ambreen, T.; Kim, M.-H. Effects of variable particle sizes on hydrothermal characteristics of nanofluids in a microchannel. Int. J. Heat Mass Transf. 2018, 120, 490–498. [Google Scholar] [CrossRef]
- Qin, J.; Tao, Y.; Liu, Q.; Li, Z.; Zhu, Z.; He, N. Experimental and theoretical studies of different parameters on the thermal conductivity of nanofluids. Micromachines 2023, 14, 964. [Google Scholar] [CrossRef] [PubMed]
- Hemmat, E.; Saedodin, S.; Wongwises, S.; Toghraie, D. An experimental study on the effect of diameter on thermal conductivity and dynamic viscosity of Fe/Water nanofluids. J. Therm. Anal. Calorim. 2015, 119, 1817–1824. [Google Scholar] [CrossRef]
- Reynwar, B.J.; Illya, G.; Harmandaris, V.A.; Müller, M.M.; Kremer, K.; Deserno, M. Aggregation and vesiculation of membrane proteins by curvature-mediated interactions. Nature 2007, 447, 461–464. [Google Scholar] [CrossRef]
- Vácha, R.; Martinez-Veracoechea, F.J.; Frenkel, D. Receptor-mediated endocytosis of nanoparticles of various shapes. Nano Lett. 2011, 11, 5391–5395. [Google Scholar] [CrossRef]
- Jahn, M.; Patze, S.; Hidi, I.J.; Knipper, R.; Radu, A.I.; Mühlig, A.; Yüksel, S.; Peksa, V.; Weber, K.; Mayerhöfer, T. Plasmonic nanostructures for surface enhanced spectroscopic methods. Analyst 2016, 141, 756–793. [Google Scholar] [CrossRef]
- Ma, X.; Sim, S.J. Single plasmonic nanostructures for biomedical diagnosis. J. Mater. Chem. B 2020, 8, 6197–6216. [Google Scholar] [CrossRef]
- Ankamwar, B. Size and shape effect on biomedical applications of nanomaterials. In Biomedical Engineering—Technical Applications in Medicine; InTech: London, UK, 2012. [Google Scholar]
- Murshed, S.; Leong, K.; Yang, C. Enhanced thermal conductivity of TiO2—Water based nanofluids. Int. J. Therm. Sci. 2005, 44, 367–373. [Google Scholar] [CrossRef]
- Glory, J.; Bonetti, M.; Helezen, M.; Mayne-L’Hermite, M.; Reynaud, C. Thermal and electrical conductivities of water-based nanofluids prepared with long multiwalled carbon nanotubes. J. Appl. Phys. 2008, 103, 094309. [Google Scholar] [CrossRef]
- Jeong, J.; Li, C.; Kwon, Y.; Lee, J.; Kim, S.H.; Yun, R. Particle shape effect on the viscosity and thermal conductivity of ZnO nanofluids. Int. J. Refrig. 2013, 36, 2233–2241. [Google Scholar] [CrossRef]
- Timofeeva, E.V.; Routbort, J.L.; Singh, D. Particle shape effects on thermophysical properties of alumina nanofluids. J. Appl. Phys. 2009, 106, 014304. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, S.-H.; Lee, J.-H.; Jang, S.P. Effect of particle shape on suspension stability and thermal conductivities of water-based bohemite alumina nanofluids. Energy 2015, 90, 1290–1297. [Google Scholar] [CrossRef]
- Maheshwary, P.; Handa, C.; Nemade, K. A comprehensive study of effect of concentration, particle size and particle shape on thermal conductivity of titania/water based nanofluid. Appl. Therm. Eng. 2017, 119, 79–88. [Google Scholar] [CrossRef]
- Cui, W.; Cao, Z.; Li, X.; Lu, L.; Ma, T.; Wang, Q. Experimental investigation and artificial intelligent estimation of thermal conductivity of nanofluids with different nanoparticles shapes. Powder Technol. 2022, 398, 117078. [Google Scholar] [CrossRef]
- Pashirova, T.; Shaihutdinova, Z.; Souto, E.; Masson, P.; Mironov, V. Nanoparticle Concentration as an Important Parameter for Characterization of Dispersion and Its Applications in Biomedicine. Colloid J. 2023, 85, 770–781. [Google Scholar] [CrossRef]
- Jun, Y.-w.; Seo, J.-w.; Cheon, J. Nanoscaling laws of magnetic nanoparticles and their applicabilities in biomedical sciences. Acc. Chem. Res. 2008, 41, 179–189. [Google Scholar] [CrossRef]
- Issa, B.; Obaidat, I.M.; Albiss, B.A.; Haik, Y. Magnetic nanoparticles: Surface effects and properties related to biomedicine applications. Int. J. Mol. Sci. 2013, 14, 21266–21305. [Google Scholar] [CrossRef]
- Guo, J.; Qin, S.; Wei, Y.; Liu, S.; Peng, H.; Li, Q.; Luo, L.; Lv, M. Silver nanoparticles exert concentration-dependent influences on biofilm development and architecture. Cell Prolif. 2019, 52, e12616. [Google Scholar] [CrossRef]
- Kwon, Y.-M.; Xia, Z.; Glyn-Jones, S.; Beard, D.; Gill, H.S.; Murray, D.W. Dose-dependent cytotoxicity of clinically relevant cobalt nanoparticles and ions on macrophages in vitro. Biomed. Mater. 2009, 4, 025018. [Google Scholar] [CrossRef]
- Åberg, C. Kinetics of nanoparticle uptake into and distribution in human cells. Nanoscale Adv. 2021, 3, 2196–2212. [Google Scholar] [CrossRef]
- Kato, H. Tracking nanoparticles inside cells. Nat. Nanotechnol. 2011, 6, 139–140. [Google Scholar] [CrossRef]
- Esfe, M.H.; Esfandeh, S.; Afrand, M.; Rejvani, M.; Rostamian, S.H. Experimental evaluation, new correlation proposing and ANN modeling of thermal properties of EG based hybrid nanofluid containing ZnO-DWCNT nanoparticles for internal combustion engines applications. Appl. Therm. Eng. 2018, 133, 452–463. [Google Scholar] [CrossRef]
- Esfe, M.H.; Yan, W.-M.; Akbari, M.; Karimipour, A.; Hassani, M. Experimental study on thermal conductivity of DWCNT-ZnO/water-EG nanofluids. Int. Commun. Heat Mass Transf. 2015, 68, 248–251. [Google Scholar] [CrossRef]
- Rostamian, S.H.; Biglari, M.; Saedodin, S.; Esfe, M.H. An inspection of thermal conductivity of CuO-SWCNTs hybrid nanofluid versus temperature and concentration using experimental data, ANN modeling and new correlation. J. Mol. Liq. 2017, 231, 364–369. [Google Scholar] [CrossRef]
- Jana, S.; Salehi-Khojin, A.; Zhong, W.-H. Enhancement of fluid thermal conductivity by the addition of single and hybrid nano-additives. Thermochim. Acta 2007, 462, 45–55. [Google Scholar] [CrossRef]
- Hong, J.; Kim, D. Effects of aggregation on the thermal conductivity of alumina/water nanofluids. Thermochim. Acta 2012, 542, 28–32. [Google Scholar] [CrossRef]
- Philip, J.; Shima, P.D. Thermal properties of nanofluids. Adv. Colloid Interface Sci. 2012, 183, 30–45. [Google Scholar] [CrossRef]
- Keblinski, P.; Eastman, J.A.; Cahill, D.G. Nanofluids for thermal transport. Mater. Today 2005, 8, 36–44. [Google Scholar] [CrossRef]
- Li, Q.; Xuan, Y.; Wang, J. Experimental investigations on transport properties of magnetic fluids. Exp. Therm. Fluid Sci. 2005, 30, 109–116. [Google Scholar] [CrossRef]
- Wang, X.-j.; Zhu, D.-s. Investigation of pH and SDBS on enhancement of thermal conductivity in nanofluids. Chem. Phys. Lett. 2009, 470, 107–111. [Google Scholar] [CrossRef]
- Yoo, D.-H.; Hong, K.; Yang, H.-S. Study of thermal conductivity of nanofluids for the application of heat transfer fluids. Thermochim. Acta 2007, 455, 66–69. [Google Scholar] [CrossRef]
- Gowda, R.; Sun, H.; Wang, P.; Charmchi, M.; Gao, F.; Gu, Z.; Budhlall, B. Effects of particle surface charge, species, concentration, and dispersion method on the thermal conductivity of nanofluids. Adv. Mech. Eng. 2010, 2, 807610. [Google Scholar] [CrossRef]
- Chen, L.; Xie, H.; Li, Y.; Yu, W. Nanofluids containing carbon nanotubes treated by mechanochemical reaction. Thermochim. Acta 2008, 477, 21–24. [Google Scholar] [CrossRef]
- Hwang, Y.; Ahn, Y.; Shin, H.; Lee, C.; Kim, G.; Park, H.; Lee, J. Investigation on characteristics of thermal conductivity enhancement of nanofluids. Curr. Appl. Phys. 2006, 6, 1068–1071. [Google Scholar] [CrossRef]
- Agarwal, R.; Verma, K.; Agrawal, N.K.; Singh, R. Sensitivity of thermal conductivity for Al2O3 nanofluids. Exp. Therm. Fluid Sci. 2017, 80, 19–26. [Google Scholar] [CrossRef]
- Agarwal, R.; Verma, K.; Agrawal, N.K.; Duchaniya, R.K.; Singh, R. Synthesis, characterization, thermal conductivity and sensitivity of CuO nanofluids. Appl. Therm. Eng. 2016, 102, 1024–1036. [Google Scholar] [CrossRef]
- Sundar, L.S.; Singh, M.K.; Sousa, A.C. Enhanced thermal properties of nanodiamond nanofluids. Chem. Phys. Lett. 2016, 644, 99–110. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46. [Google Scholar] [CrossRef]
- Hervault, A.; Thanh, N.T.K. Magnetic nanoparticle-based therapeutic agents for thermo-chemotherapy treatment of cancer. Nanoscale 2014, 6, 11553–11573. [Google Scholar] [CrossRef]
- Rivas, J.; Bañobre-López, M.; Piñeiro-Redondo, Y.; Rivas, B.; López-Quintela, M. Magnetic nanoparticles for application in cancer therapy. J. Magn. Magn. Mater. 2012, 324, 3499–3502. [Google Scholar] [CrossRef]
- Kolen’ko, Y.V.; Bañobre-López, M.; Rodríguez-Abreu, C.; Carbó-Argibay, E.; Sailsman, A.; Piñeiro-Redondo, Y.; Cerqueira, M.F.; Petrovykh, D.Y.; Kovnir, K.; Lebedev, O.I. Large-scale synthesis of colloidal Fe3O4 nanoparticles exhibiting high heating efficiency in magnetic hyperthermia. J. Phys. Chem. C 2014, 118, 8691–8701. [Google Scholar] [CrossRef]
- Lee, K.J.; Yoon, S.H.; Jang, J. Carbon nanofibers: A novel nanofiller for nanofluid applications. Small 2007, 3, 1209–1213. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, B.D.; Fernandes, D.E.; Amorim, C.O.; Amaral, V.S.; Coutinho, P.J.; Rodrigues, A.R.O.; Castanheira, E.M. Magnetoliposomes with calcium-doped magnesium ferrites anchored in the lipid surface for enhanced DOX release. Nanomaterials 2023, 13, 2597. [Google Scholar] [CrossRef]
- Alyautdin, R.; Khalin, I.; Nafeeza, M.I.; Haron, M.H.; Kuznetsov, D. Nanoscale drug delivery systems and the blood–brain barrier. Int. J. Nanomed. 2014, 9, 795–811. [Google Scholar] [CrossRef]
- Luo, Z.; Chen, C.Y.; Li, S. Improving Tumor Targeting and Penetration for Nanoparticle-Mediated Cancer Therapy. Small Methods 2025, 2401860. [Google Scholar] [CrossRef]
- Shinde, V.R.; Revi, N.; Murugappan, S.; Singh, S.P.; Rengan, A.K. Enhanced permeability and retention effect: A key facilitator for solid tumor targeting by nanoparticles. Photodiagnosis Photodyn. Ther. 2022, 39, 102915. [Google Scholar] [CrossRef]
- Han, H.S.; Choi, K.Y. Advances in nanomaterial-mediated photothermal cancer therapies: Toward clinical applications. Biomedicines 2021, 9, 305. [Google Scholar] [CrossRef]
- Périgo, E.A.; Hemery, G.; Sandre, O.; Ortega, D.; Garaio, E.; Plazaola, F.; Teran, F.J. Fundamentals and Advances in Magnetic Hyperthermia. Appl. Phys. Rev. 2015, 2, 041302. [Google Scholar] [CrossRef]
- Chicheł, A.; Skowronek, J.; Kubaszewska, M.; Kanikowski, M. Hyperthermia–description of a method and a review of clinical applications. Rep. Pract. Oncol. Radiother. 2007, 12, 267–275. [Google Scholar] [CrossRef]
- Jordan, A.; Scholz, R.; Wust, P.; Fähling, H.; Felix, R. Magnetic fluid hyperthermia (MFH): Cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J. Magn. Magn. Mater. 1999, 201, 413–419. [Google Scholar] [CrossRef]
- Alnasraui, A.H.F.; Joe, I.H.; Al-Musawi, S. Investigation of Folate-Functionalized Magnetic-Gold Nanoparticles Based Targeted Drug Delivery for Liver: In Vitro, In Vivo and Docking Studies. ACS Biomater. Sci. Eng. 2024, 10, 6299–6313. [Google Scholar] [CrossRef] [PubMed]
- Parsa, F.; Setoodehkhah, M.; Atyabi, S.M. Loading and release study of ciprofloxacin from silica-coated magnetite modified by iron-based metal-organic framework (MOF) as a nonocarrier in targeted drug delivery system. Inorg. Chem. Commun. 2023, 155, 111056. [Google Scholar] [CrossRef]
- Ding, Q.; Liu, D.; Guo, D.; Yang, F.; Pang, X.; Che, R.; Zhou, N.; Xie, J.; Sun, J.; Huang, Z. Shape-controlled fabrication of magnetite silver hybrid nanoparticles with high performance magnetic hyperthermia. Biomaterials 2017, 124, 35–46. [Google Scholar] [CrossRef]
- Mendes, R.; Pedrosa, P.; Lima, J.C.; Fernandes, A.R.; Baptista, P.V. Photothermal enhancement of chemotherapy in breast cancer by visible irradiation of Gold Nanoparticles. Sci. Rep. 2017, 7, 10872. [Google Scholar] [CrossRef]
- Rodriguez, A.F.; Dos Santos, C.C.; Lüdtke-Buzug, K.; Bakenecker, A.C.; Chaves, Y.O.; Mariúba, L.A.; Brandt, J.V.; Amantea, B.E.; de Santana, R.C.; Marques, R.F. Evaluation of antiplasmodial activity and cytotoxicity assays of amino acids functionalized magnetite nanoparticles: Hyperthermia and flow cytometry applications. Mater. Sci. Eng. C 2021, 125, 112097. [Google Scholar] [CrossRef]
- Ito, A.; Shinkai, M.; Honda, H.; Kobayashi, T. Medical application of functionalized magnetic nanoparticles. J. Biosci. Bioeng. 2005, 100, 1–11. [Google Scholar] [CrossRef]
- Hou, Y.-K.; Zhang, Z.-J.; Li, R.-T.; Peng, J.; Chen, S.-Y.; Yue, Y.-R.; Zhang, W.-H.; Sun, B.; Chen, J.-X.; Zhou, Q. Remodeling the tumor microenvironment with core–shell nanosensitizer featuring dual-modal imaging and multimodal therapy for breast cancer. ACS Appl. Mater. Interfaces 2023, 15, 2602–2616. [Google Scholar] [CrossRef]
- Yoon, Y.I.; Pang, X.; Jung, S.; Zhang, G.; Kong, M.; Liu, G.; Chen, X. Smart gold nanoparticle-stabilized ultrasound microbubbles as cancer theranostics. J. Mater. Chem. B 2018, 6, 3235–3239. [Google Scholar] [CrossRef]
- Park, I.-K.; Ng, C.-P.; Wang, J.; Chu, B.; Yuan, C.; Zhang, S.; Pun, S.H. Determination of nanoparticle vehicle unpackaging by MR imaging of a T2 magnetic relaxation switch. Biomaterials 2008, 29, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, A.; Zambelli, V.; Ferretti, A.M.; Salerno, D.; Bellani, G.; Polito, L. Design of functionalized gold nanoparticle probes for computed tomography imaging. Contrast Media Mol. Imaging 2016, 11, 405–414. [Google Scholar] [CrossRef]
- Ivask, A.; ElBadawy, A.; Kaweeteerawat, C.; Boren, D.; Fischer, H.; Ji, Z.; Chang, C.H.; Liu, R.; Tolaymat, T.; Telesca, D. Toxicity mechanisms in Escherichia coli vary for silver nanoparticles and differ from ionic silver. ACS Nano 2014, 8, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Bedlovičová, Z.; Strapáč, I.; Baláž, M.; Salayová, A. A brief overview on antioxidant activity determination of silver nanoparticles. Molecules 2020, 25, 3191. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Muñoz, R.; Meza-Villezcas, A.; Fournier, P.; Soria-Castro, E.; Juarez-Moreno, K.; Gallego-Hernández, A.; Bogdanchikova, N.; Vazquez-Duhalt, R.; Huerta-Saquero, A. Enhancement of antibiotics antimicrobial activity due to the silver nanoparticles impact on the cell membrane. PLoS ONE 2019, 14, e0224904. [Google Scholar] [CrossRef]
- Ipe, D.S.; Kumar, P.S.; Love, R.M.; Hamlet, S.M. Silver nanoparticles at biocompatible dosage synergistically increases bacterial susceptibility to antibiotics. Front. Microbiol. 2020, 11, 1074. [Google Scholar] [CrossRef]
- Khatoon, N.; Alam, H.; Khan, A.; Raza, K.; Sardar, M. Ampicillin silver nanoformulations against multidrug resistant bacteria. Sci. Rep. 2019, 9, 6848. [Google Scholar] [CrossRef]
- Saeb, A.T.; Alshammari, A.S.; Al-Brahim, H.; Al-Rubeaan, K.A. Production of silver nanoparticles with strong and stable antimicrobial activity against highly pathogenic and multidrug resistant bacteria. Sci. World J. 2014, 2014, 704708. [Google Scholar] [CrossRef]
- Liao, S.; Zhang, Y.; Pan, X.; Zhu, F.; Jiang, C.; Liu, Q.; Cheng, Z.; Dai, G.; Wu, G.; Wang, L. Antibacterial activity and mechanism of silver nanoparticles against multidrug-resistant Pseudomonas aeruginosa. Int. J. Nanomed. 2019, 14, 1469–1487. [Google Scholar] [CrossRef]
- Azadpour, A.; Hajrasouliha, S.; Khaleghi, S. Green synthesized-silver nanoparticles coated with targeted chitosan nanoparticles for smart drug delivery. J. Drug Deliv. Sci. Technol. 2022, 74, 103554. [Google Scholar] [CrossRef]
- Chinnathambi, A.; Alahmadi, T.A. Zinc nanoparticles green-synthesized by Alhagi maurorum leaf aqueous extract: Chemical characterization and cytotoxicity, antioxidant, and anti-osteosarcoma effects. Arab. J. Chem. 2021, 14, 103083. [Google Scholar] [CrossRef]
- Hussain, A.; Oves, M.; Alajmi, M.F.; Hussain, I.; Amir, S.; Ahmed, J.; Rehman, M.T.; El-Seedi, H.R.; Ali, I. Biogenesis of ZnO nanoparticles using Pandanus odorifer leaf extract: Anticancer and antimicrobial activities. RSC Adv. 2019, 9, 15357–15369. [Google Scholar] [CrossRef] [PubMed]
- Sanaeimehr, Z.; Javadi, I.; Namvar, F. Antiangiogenic and antiapoptotic effects of green-synthesized zinc oxide nanoparticles using Sargassum muticum algae extraction. Cancer Nanotechnol. 2018, 9, 3. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Shah, M.; Hashmi, S.S.; Nazir, M.; Naz, S.; Ahmad, W.; Khan, I.U.; Hano, C. Green bio-assisted synthesis, characterization and biological evaluation of biocompatible ZnO NPs synthesized from different tissues of milk thistle (Silybum marianum). Nanomaterials 2019, 9, 1171. [Google Scholar] [CrossRef] [PubMed]
- Ahlam, A.A.; Shaniba, V.; Jayasree, P.; Manish Kumar, P. Spondias pinnata (lf) Kurz leaf extract derived zinc oxide nanoparticles induce dual modes of apoptotic-necrotic death in HCT 116 and K562 cells. Biol. Trace Elem. Res. 2021, 199, 1778–1801. [Google Scholar] [CrossRef]
- Prasad, K.S.; Prasad, S.K.; Veerapur, R.; Lamraoui, G.; Prasad, A.; Prasad, M.N.; Singh, S.K.; Marraiki, N.; Syed, A.; Shivamallu, C. Antitumor potential of green synthesized ZnONPs using root extract of Withania somnifera against human breast cancer cell line. Separations 2021, 8, 8. [Google Scholar] [CrossRef]
- Ali, S.; Sudha, K.G.; Karunakaran, G.; Kowsalya, M.; Kolesnikov, E.; Rajeshkumar, M.P. Green synthesis of stable antioxidant, anticancer and photocatalytic activity of zinc oxide nanorods from Leea asiatica leaf. J. Biotechnol. 2021, 329, 65–79. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, J.N.; Prabhu, A.; Nagaraja, G.; Navada, M.; Kouser, S.; Manasa, D. Unravelling the human triple negative breast cancer suppressive activity of biocompatible zinc oxide nanostructures influenced by Vateria indica (L.) fruit phytochemicals. Mater. Sci. Eng. C 2021, 122, 111887. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S.; Kumar, S.V.; Ramaiah, A.; Agarwal, H.; Lakshmi, T.; Roopan, S.M. Biosynthesis of zinc oxide nanoparticles usingMangifera indica leaves and evaluation of their antioxidant and cytotoxic properties in lung cancer (A549) cells. Enzym. Microb. Technol. 2018, 117, 91–95. [Google Scholar] [CrossRef]
- Umamaheswari, A.; Prabu, S.L.; John, S.A.; Puratchikody, A. Green synthesis of zinc oxide nanoparticles using leaf extracts of Raphanus sativus var. Longipinnatus and evaluation of their anticancer property in A549 cell lines. Biotechnol. Rep. 2021, 29, e00595. [Google Scholar] [CrossRef]
- Palai, P.K.; Mondal, A.; Chakraborti, C.K.; Banerjee, I.; Pal, K. Green synthesized amino-PEGylated silver decorated graphene nanoplatform as a tumor-targeted controlled drug delivery system. SN Appl. Sci. 2019, 1, 269. [Google Scholar] [CrossRef]
- Braim, F.S.; Ab Razak, N.N.A.N.; Aziz, A.A.; Dheyab, M.A.; Ismael, L.Q. Rapid green-assisted synthesis and functionalization of superparamagnetic magnetite nanoparticles using Sumac extract and assessment of their cellular toxicity, uptake, and anti-metastasis property. Ceram. Int. 2023, 49, 7359–7369. [Google Scholar] [CrossRef]
- Yusefi, M.; Shameli, K.; Su Yee, O.; Teow, S.-Y.; Hedayatnasab, Z.; Jahangirian, H.; Webster, T.J.; Kuča, K. Green synthesis of Fe3O4 nanoparticles stabilized by a Garcinia mangostana fruit peel extract for hyperthermia and anticancer activities. Int. J. Nanomed. 2021, 16, 2515–2532. [Google Scholar] [CrossRef]
- Jiananda, A.; Sari, E.K.; Larasati, D.A.; Tumbelaka, R.M.; Ardiyanti, H.; Darmawan, M.Y.; Istiqomah, N.I.; Wicaksono, S.T.; Suharyadi, E. Optical, microstructural, and magnetic hyperthermia properties of green-synthesized Fe3O4/carbon dots nanocomposites utilizing Moringa oleifera extract and watermelon rinds. Carbon Trends 2023, 13, 100305. [Google Scholar] [CrossRef]
- Mosleh-Shirazi, S.; Kasaee, S.R.; Dehghani, F.; Kamyab, H.; Kirpichnikova, I.; Chelliapan, S.; Firuzyar, T.; Akhtari, M.; Amani, A.M. Investigation through the anticancer properties of green synthesized spinel ferrite nanoparticles in present and absent of laser photothermal effect. Ceram. Int. 2023, 49, 11293–11301. [Google Scholar] [CrossRef]
- Faid, A.H.; Shouman, S.A.; Thabet, N.A.; Badr, Y.A.; Sliem, M.A. Laser enhanced combinatorial chemo-photothermal therapy of green synthesis gold nanoparticles loaded with 6mercaptopurine on breast cancer model. J. Pharm. Innov. 2023, 18, 144–148. [Google Scholar] [CrossRef]
- Larasati, D.A.; Puspitarum, D.L.; Darmawan, M.Y.; Istiqomah, N.I.; Partini, J.; Aliah, H.; Suharyadi, E. Green synthesis of CoFe2O4/ZnS composite nanoparticles utilizing Moringa Oleifera for magnetic hyperthermia applications. Results Mater. 2023, 19, 100431. [Google Scholar] [CrossRef]
- Kharey, P.; Goel, M.; Husain, Z.; Gupta, R.; Sharma, D.; Palani, I.; Gupta, S. Green synthesis of biocompatible superparamagnetic iron oxide-gold composite nanoparticles for magnetic resonance imaging, hyperthermia and photothermal therapeutic applications. Mater. Chem. Phys. 2023, 293, 126859. [Google Scholar] [CrossRef]
- Alomar, T.S.; AlMasoud, N.; Awad, M.A.; AlOmar, R.S.; Merghani, N.M.; El-Zaidy, M.; Bhattarai, A. Designing green synthesis-based silver nanoparticles for antimicrobial theranostics and cancer invasion prevention. Int. J. Nanomed. 2024, 19, 4451–4464. [Google Scholar] [CrossRef] [PubMed]
- Parvathalu, K.; Chinmayee, S.; Preethi, B.; Swetha, A.; Maruthi, G.; Pritam, M.; Sreenivas, B.; Naidu, S.R.; Merlinsheeba, G.; Murali, B. Green synthesis of silver nanoparticles using Argyreia nervosa leaf extract and their antimicrobial activity. Plasmonics 2023, 18, 1075–1081. [Google Scholar] [CrossRef]
- Aldorkee, S.Y.; Al-Janabi, A.A.H.S. Antimicrobial Activity of the Nanoparticle Form of Greens (Lemon, Black Seeds or Flax) with Silver on Drug-resistant Human Pathogens. Pharm. Nanotechnol. 2023, 11, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Meti, R.S.; Neelagund, S.; Urs, D.; Dharmappa, K.; Kotresh, K. Green synthesis of silver nanoparticles from Acacia sinuata seed extract and evaluation of their mosquitocidal and anticancer (Caco-2 and MG-63 cell) activity. Biomass Convers. Biorefinery 2025, 15, 175–184. [Google Scholar] [CrossRef]
- Naveed, M.; Mahmood, S.; Aziz, T.; Azeem, A.; Rajpoot, Z.; Rehman, S.u.; Al-Asmari, F.; Alahmari, A.S.; Saleh, O.; Sameeh, M.Y. Green-synthesis of silver nanoparticles AgNPs from Podocarpus macrophyllus for targeting GBM and LGG brain cancers via NOTCH2 gene interactions. Sci. Rep. 2024, 14, 25489. [Google Scholar] [CrossRef]
- Ma, W.; Zhu, G.; Zhang, Y.; Guo, J. Green synthesis of ZnO NPs with long-lasting and ultra-high antimicrobial activity. Surf. Interfaces 2024, 50, 104506. [Google Scholar] [CrossRef]
- Mazhar, M.W.; Ishtiaq, M.; Maqbool, M.; Arshad, A.; Alshehri, M.A.; Alhelaify, S.S.; Alharthy, O.M.; Shukry, M.; Sayed, S.M. Green synthesis of anethole-loaded zinc oxide nanoparticles enhances antibacterial strategies against pathogenic bacteria. Sci. Rep. 2024, 14, 24671. [Google Scholar] [CrossRef]
- Shanmugam, R.; Anandan, J.; Abalkhail, T.; Alqahtani, A.M.; Roy, A. Green synthesis of iron oxide nanoparticles using Cissus rotundifolia and its antibacterial activity against wound pathogens. J. Indian Chem. Soc. 2025, 102, 101599. [Google Scholar] [CrossRef]
- Kummara, S.; Patil, M.B.; Uriah, T. Synthesis, characterization, biocompatible and anticancer activity of green and chemically synthesized silver nanoparticles–a comparative study. Biomed. Pharmacother. 2016, 84, 10–21. [Google Scholar] [CrossRef]
- Sreelekha, E.; George, B.; Shyam, A.; Sajina, N.; Mathew, B. A comparative study on the synthesis, characterization, and antioxidant activity of green and chemically synthesized silver nanoparticles. BioNanoScience 2021, 11, 489–496. [Google Scholar] [CrossRef]
- Akshaykranth, A.; Jayarambabu, N.; Tumu, V.R.; Rajaboina, R.K. Comparative study on antibacterial activity of MgO nanoparticles synthesized from Lawsonia inermis leaves extract and chemical methods. J. Inorg. Organomet. Polym. Mater. 2021, 31, 2393–2400. [Google Scholar] [CrossRef]
- Aravind, M.; Amalanathan, M.; Mary, M.S.M. Synthesis of TiO2 nanoparticles by chemical and green synthesis methods and their multifaceted properties. SN Appl. Sci. 2021, 3, 409. [Google Scholar] [CrossRef]
- Barabadi, H.; Mojab, F.; Vahidi, H.; Marashi, B.; Talank, N.; Hosseini, O.; Saravanan, M. Green synthesis, characterization, antibacterial and biofilm inhibitory activity of silver nanoparticles compared to commercial silver nanoparticles. Inorg. Chem. Commun. 2021, 129, 108647. [Google Scholar] [CrossRef]
- Wong, K.V.; De Leon, O. Applications of nanofluids: Current and future. Advances in mechanical engineering 2010, 2, 519659. [Google Scholar] [CrossRef]
- Ebaid, M.S.; Al-busoul, M.; Ghrair, A.M. Performance enhancement of photovoltaic panels using two types of nanofluids. Heat Transf. 2020, 49, 2789–2812. [Google Scholar] [CrossRef]
- Ramadass, G.; Vijayalakshmi, M.; Natarajan, E. Energy investigation in serpentine heat exchanger using aluminum oxide nanofluid on solar photovoltaic/thermal system. J. Test. Eval. 2020, 48, 1031–1054. [Google Scholar] [CrossRef]
- Shahsavar, A.; Eisapour, M.; Talebizadehsardari, P. Experimental evaluation of novel photovoltaic/thermal systems using serpentine cooling tubes with different cross-sections of circular, triangular and rectangular. Energy 2020, 208, 118409. [Google Scholar] [CrossRef]
- Hussien, H.A.; Noman, A.H.; Abdulmunem, A.R. Indoor investigation for improving the hybrid photovoltaic/thermal system performance using nanofluid (Al2O3-water). Eng. Tech. J. 2015, 33, 889–901. [Google Scholar] [CrossRef]
- Rajput, N.S.; Shukla, D.D.; Rajput, D.; Sharm, S.K. Performance analysis of flat plate solar collector using Al2O3/distilled water nanofluid: An experimental investigation. Mater. Today Proc. 2019, 10, 52–59. [Google Scholar] [CrossRef]
- Michael, J.J.; Iniyan, S. Performance of copper oxide/water nanofluid in a flat plate solar water heater under natural and forced circulations. Energy Convers. Manag. 2015, 95, 160–169. [Google Scholar] [CrossRef]
- Colangelo, G.; Favale, E.; De Risi, A.; Laforgia, D. A new solution for reduced sedimentation flat panel solar thermal collector using nanofluids. Appl. Energy 2013, 111, 80–93. [Google Scholar] [CrossRef]
- Lee, Y.; Jeong, H.; Sung, Y. Thermal Absorption Performance Evaluation of Water-Based Nanofluids (CNTs, Cu, and Al2O3) for Solar Thermal Harvesting. Energies 2021, 14, 4875. [Google Scholar] [CrossRef]
- Ni, G.; Miljkovic, N.; Ghasemi, H.; Huang, X.; Boriskina, S.V.; Lin, C.-T.; Wang, J.; Xu, Y.; Rahman, M.M.; Zhang, T. Volumetric solar heating of nanofluids for direct vapor generation. Nano Energy 2015, 17, 290–301. [Google Scholar] [CrossRef]
- Sidik, N.A.C.; Adamu, I.M.; Jamil, M.M.; Kefayati, G.; Mamat, R.; Najafi, G. Recent progress on hybrid nanofluids in heat transfer applications: A comprehensive review. Int. Commun. Heat Mass Transf. 2016, 78, 68–79. [Google Scholar] [CrossRef]
- Hasan, A.; Alazzam, A.; Abu-Nada, E. Direct absorption solar collectors: Fundamentals, modeling approaches, design and operating parameters, advances, knowledge gaps, and future prospects. Prog. Energy Combust. Sci. 2024, 103, 101160. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Fang, X.; Zhang, Z. Reduced graphene oxide dispersed nanofluids with improved photo-thermal conversion performance for direct absorption solar collectors. Sol. Energy Mater. Sol. Cells 2017, 163, 125–133. [Google Scholar] [CrossRef]
- Ahmad, S.; Saidur, R.; Mahbubul, I.; Al-Sulaiman, F. Optical properties of various nanofluids used in solar collector: A review. Renew. Sustain. Energy Rev. 2017, 73, 1014–1030. [Google Scholar] [CrossRef]
- Khanafer, K.; Vafai, K. A review on the applications of nanofluids in solar energy field. Renew. Energy 2018, 123, 398–406. [Google Scholar] [CrossRef]
- Yu, W.; France, D.M.; Choi, S.U.; Routbort, J.L. Review and Assessment of Nanofluid Technology for Transportation and Other Applications; Argonne National Lab. (ANL): Argonne, IL, USA, 2007. [Google Scholar]
- Colangelo, G.; Favale, E.; Milanese, M.; de Risi, A.; Laforgia, D. Cooling of electronic devices: Nanofluids contribution. Appl. Therm. Eng. 2017, 127, 421–435. [Google Scholar] [CrossRef]
- Korpyś, M.; Al-Rashed, M.; Dzido, G.; Wójcik, J. CPU Heat Sink Cooled by Nanofluids and Water: Experimental and Numerical Study. Comput. Aided Chem. Eng. 2013, 32, 409–414. [Google Scholar]
- Turgut, A.; Elbasan, E. Nanofluids for electronics cooling. In Proceedings of the 2014 IEEE 20th International Symposium for Design and Technology in Electronic Packaging (SIITME), Bucharest, Romania, 23–26 October 2014; pp. 35–37. [Google Scholar]
- Roberts, N.A.; Walker, D. Convective performance of nanofluids in commercial electronics cooling systems. Appl. Therm. Eng. 2010, 30, 2499–2504. [Google Scholar] [CrossRef]
- Rafati, M.; Hamidi, A.; Niaser, M.S. Application of nanofluids in computer cooling systems (heat transfer performance of nanofluids). Appl. Therm. Eng. 2012, 45, 9–14. [Google Scholar] [CrossRef]
- Yousefi, T.; Mousavi, S.A.; Farahbakhsh, B.; Saghir, M. Experimental investigation on the performance of CPU coolers: Effect of heat pipe inclination angle and the use of nanofluids. Microelectron. Reliab. 2013, 53, 1954–1961. [Google Scholar] [CrossRef]
- Dash, R.K.; Borca-Tasciuc, T.; Purkayastha, A.; Ramanath, G. Electrowetting on dielectric-actuation of microdroplets of aqueous bismuth telluridenanoparticle suspensions. Nanotechnology 2007, 18, 475711. [Google Scholar] [CrossRef]
- Adham, A.M.; Mohd-Ghazali, N.; Ahmad, R. Thermal and hydrodynamic analysis of microchannel heat sinks: A review. Renew. Sustain. Energy Rev. 2013, 21, 614–622. [Google Scholar] [CrossRef]
- Wang, Z.; Sundén, B.; Li, Y. A novel optimization framework for designing multi-stream compact heat exchangers and associated network. Appl. Therm. Eng. 2017, 116, 110–125. [Google Scholar] [CrossRef]
- Bai, M.; Xu, Z.; Lv, J. Application of Nanofluids in Engine Cooling System; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2008. [Google Scholar]
- Paul, G.; Hirani, H.; Kuila, T.; Murmu, N. Nanolubricants dispersed with graphene and its derivatives: An assessment and review of the tribological performance. Nanoscale 2019, 11, 3458–3483. [Google Scholar] [CrossRef]
- Wambsganss, M.W. Thermal Management Concepts for Higher-Efficiency Heavy Vehicles; SAE Technical Paper; SAE International: Warrendale, PA, USA, 1999; pp. 41–47. [Google Scholar]
- Saidur, R.; Leong, K.; Mohammed, H.A. A review on applications and challenges of nanofluids. Renew. Sustain. Energy Rev. 2011, 15, 1646–1668. [Google Scholar] [CrossRef]
- Leong, K.Y.; Saidur, R.; Kazi, S.; Mamun, A. Performance investigation of an automotive car radiator operated with nanofluid-based coolants (nanofluid as a coolant in a radiator). Appl. Therm. Eng. 2010, 30, 2685–2692. [Google Scholar] [CrossRef]
- Abdolbaqi, M.K.; Mamat, R.; Sidik, N.A.C.; Azmi, W.; Selvakumar, P. Experimental investigation and development of new correlations for heat transfer enhancement and friction factor of BioGlycol/water based TiO2 nanofluids in flat tubes. Int. J. Heat Mass Transf. 2017, 108, 1026–1035. [Google Scholar] [CrossRef]
- Kulkarni, D.P.; Vajjha, R.S.; Das, D.K.; Oliva, D. Application of aluminum oxide nanofluids in diesel electric generator as jacket water coolant. Appl. Therm. Eng. 2008, 28, 1774–1781. [Google Scholar] [CrossRef]
- Gu, Z. History review of nuclear reactor safety. Ann. Nucl. Energy 2018, 120, 682–690. [Google Scholar] [CrossRef]
- Rahnama, Z.; Ansarifar, G. Predicting and optimizing the thermal-hydraulic, natural circulation, and neutronics parameters in the NuScale nuclear reactor using nanofluid as a coolant via machine learning methods through GA, PSO and HPSOGA algorithms. Ann. Nucl. Energy 2021, 161, 108375. [Google Scholar] [CrossRef]
- Buongiorno, J.; Hu, L. 8. Innovative Technologies: Two-Phase Heat Transfer in Water-Based Nanofluids for Nuclear Applications Final Report; Massachusetts Institute of Technology: Cambridge, MA, USA, 2009. [Google Scholar]
- Yu, W.; Xie, H. A review on nanofluids: Preparation, stability mechanisms, and applications. J. Nanomater. 2012, 2012, 435873. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, S.; Gangacharyulu, D. Nanofluids Preparation and Stability for HeatTransfer Applications—A Review. Int. J. Comp. Appl. 2016, 975, 8887. [Google Scholar]
- Das, S.K.; Choi, S.U.; Yu, W.; Pradeep, T. Nanofluids: Science and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Chen, C.; Feng, S.; Peng, H.; Peng, X.; Chaoyue, L.; Zhang, R. Thermocapillary convection flow and heat transfer characteristics of graphene nanoplatelet based nanofluid under microgravity. Microgravity Sci. Technol. 2021, 33, 40. [Google Scholar] [CrossRef]
- Yanaoka, H.; Inafune, R. Frequency response of three-dimensional natural convection of nanofluids under microgravity environments with gravity modulation. Numer. Heat Transf. Part A Appl. 2023, 83, 745–769. [Google Scholar] [CrossRef]
- Kamal, M.H.A.; Ali, A.; Lim, Y.J.; Rawi, N.A.; Shafie, S. Shape Effect of Cu, Al2O3 and TiO2 Nanoparticles on Stagnation Point Nanofluid Flow in a Microgravity Environment. Data Anal. Appl. Math. 2021, 2, 1–13. [Google Scholar] [CrossRef]
- Das, D.; Vajjha, R.; Strandberg, R. Enhancement of the Performance of Thermal Control Systems Using Nanofluids. In Proceedings of the Thermal and Fluids Analysis Workshop 2010, League City, TX, USA, 16–20 August 2010. [Google Scholar]
- Ungar, E.K.; Erickson, L.R. Assessment of the Use of Nanofluids in Spacecraft Active Thermal Control Systems. In Proceedings of the AIAA SPACE 2011 Conference & Exposition, Long Beach, CA, USA, 27–29 September 2011. [Google Scholar]
- Kuo, K.K.; Risha, G.A.; Evans, B.J.; Boyer, E. Potential usage of energetic nano-sized powders for combustion and rocket propulsion. MRS Online Proc. Libr. 2003, 800, 39–50. [Google Scholar] [CrossRef]
- Choi, C.; Yoo, H.; Oh, J. Preparation and heat transfer properties of nanoparticle-in-transformer oil dispersions as advanced energy-efficient coolants. Curr. Appl. Phys. 2008, 8, 710–712. [Google Scholar] [CrossRef]
- Makmud, M.; Illias, H.; Chee, C. Partial discharge behaviour within palm oil-based Fe2O3 nanofluids under AC voltage. IOP Conf. Ser. Mater. Sci. Eng. 2017, 210, 012034. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, F.; Li, J.; Ran, H.; Huang, D. Influence of copper particles on breakdown voltage and frequency-dependent dielectric property of vegetable insulating oil. Energies 2017, 10, 938. [Google Scholar] [CrossRef]
- Cavallini, A.; Karthik, R.; Negri, F. The effect of magnetite, graphene oxide and silicone oxide nanoparticles on dielectric withstand characteristics of mineral oil. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 2592–2600. [Google Scholar] [CrossRef]
- Du, Y.; Lv, Y.; Li, C.; Chen, M.; Zhong, Y.; Zhou, J.; Li, X.; Zhou, Y. Effect of semiconductive nanoparticles on insulating performances of transformer oil. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 770–776. [Google Scholar] [CrossRef]
- Segal, V.; Nattrass, D.; Raj, K.; Leonard, D. Accelerated thermal aging of petroleum-based ferrofluids. J. Magn. Magn. Mater. 1999, 201, 70–72. [Google Scholar] [CrossRef]
- Mergos, J.A.; Athanassopoulou, M.D.; Argyropoulos, T.G.; Dervos, C.T. Dielectric properties of nanopowder dispersions in paraffin oil. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 1502–1507. [Google Scholar] [CrossRef]
- Miao, J.; Dong, M.; Ren, M.; Wu, X.; Shen, L.; Wang, H. Effect of nanoparticle polarization on relative permittivity of transformer oil-based nanofluids. J. Appl. Phys. 2013, 113, 204103. [Google Scholar] [CrossRef]
- Jeong, G.-Y.; Jang, S.P.; Lee, H.-Y.; Lee, J.-C.; Choi, S.; Lee, S.-H. Magnetic-thermal-fluidic analysis for cooling performance of magnetic nanofluids comparing with transformer oil and air by using fully coupled finite element method. IEEE Trans. Magn. 2013, 49, 1865–1868. [Google Scholar] [CrossRef]
- Pîslaru–Dănescu, L.; Morega, A.M.; Morega, M.; Stoica, V.; Marinică, O.M.; Nouraş, F.; Păduraru, N.; Borbáth, I.; Borbáth, T. Prototyping a ferrofluid-cooled transformer. IEEE Trans. Ind. Appl. 2013, 49, 1289–1298. [Google Scholar] [CrossRef]
- Du, Y.; Lv, Y.; Li, C.; Chen, M.; Zhou, J.; Li, X.; Zhou, Y.; Tu, Y. Effect of electron shallow trap on breakdown performance of transformer oil-based nanofluids. J. Appl. Phys. 2011, 110, 104104. [Google Scholar] [CrossRef]
- Lv, Y.; Du, Y.; Li, C.; Qi, B.; Zhong, Y.; Chen, M. TiO2 nanoparticle induced space charge decay in thermal aged transformer oil. Appl. Phys. Lett. 2013, 102, 132902. [Google Scholar] [CrossRef]
- Herchl, F.; Marton, K.; Tomčo, L.; Kopčanský, P.; Timko, M.; Koneracká, M.; Kolcunová, I. Breakdown and partial discharges in magnetic liquids. J. Phys. Condens. Matter 2008, 20, 204110. [Google Scholar] [CrossRef]
- Elnaggar, M.; Edwan, E. Heat pipes for computer cooling applications. In Electronics Cooling; InTech.: London, UK, 2016; Volume 51. [Google Scholar]
- Li, J.; Qiao, L.; Lv, W.; Zeng, X.; Chen, M. Performance response analysis of battery module with nanofluids pulsating heat pipes under normal and high-temperature charging scenarios. Appl. Therm. Eng. 2024, 257, 124339. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, H.; Liu, L.; Zhang, M.; Wang, Y.; Zhang, Y.; Zhou, B. Enhancement of start-up and thermal performance in pulsating heat pipe with GO/water nanofluid. Powder Technol. 2021, 384, 414–422. [Google Scholar] [CrossRef]
- Afsari, K.; Emami, M.R.S.; Zahmatkesh, S.; Klemeš, J.J.; Bokhari, A. Optimizing the thermal performance of the thermosyphon heat pipe for energy saving with graphene oxide nanofluid. Energy 2023, 274, 127422. [Google Scholar] [CrossRef]
- Hinge, H.; Dhokane, N.; Barhatte, S. Study of Effect of Nanofluid on Performance of Heat Pipe. Int. Conf. Ideas Impact Innov. Mech. Eng. ICIIIME 2017, 5, 336–339. [Google Scholar]
- Nobrega, G.; Souza, R.; Cardoso, B.; Afonso, I.; Pereira, J.; Cardoso, E.; Moita, A.; Ribeiro, J.; Lima, R. Experimental evaluation of green nanofluids in heat exchanger made oF PDMS. Therm. Sci. Eng. Prog. 2024, 55, 102978. [Google Scholar] [CrossRef]
- Cardoso, B.; Nobrega, G.; Machado, M.; Lima, R.A. Green synthesis of copper ferrite-based nanofluids using Chlorella vulgaris for heat transfer enhancement. J. Mol. Liq. 2025, 428, 127498. [Google Scholar] [CrossRef]
- Anand, A.; Srivastava, V.; Singh, S.; Shukla, A.; Choubey, A.K.; Sharma, A. Development of nano-enhanced phase change materials using manganese dioxide nanoparticles obtained through green synthesis. Energy Storage 2022, 4, e344. [Google Scholar] [CrossRef]
- Al Aboushi, A.; Abdelhafez, E.; Hamdan, M. Finned PV Natural Cooling Using Water-Based TiO2 Nanofluid. Sustainability 2022, 14, 12987. [Google Scholar] [CrossRef]
- Mustafa, J.; Alqaed, S.; Sajadi, S.M.; Aybar, H.Ş. Enhancing solar panel cooling efficiency: A study on the influence of nanofluid inclusion and pin fin shape during melting and freezing of phase change materials. Front. Energy Res. 2024, 12, 1344061. [Google Scholar] [CrossRef]
- Ranjbarzadeh, R.; Moradikazerouni, A.; Bakhtiari, R.; Asadi, A.; Afrand, M. An experimental study on stability and thermal conductivity of water/silica nanofluid: Eco-friendly production of nanoparticles. J. Clean. Prod. 2019, 206, 1089–1100. [Google Scholar] [CrossRef]
- Kumar, L.H.; Kazi, S.; Masjuki, H.; Zubir, M.; Jahan, A.; Sean, O.C. Experimental study on the effect of bio-functionalized graphene nanoplatelets on the thermal performance of liquid flat plate solar collector. J. Therm. Anal. Calorim. 2022, 147, 1657–1674. [Google Scholar] [CrossRef]
- Kumar, L.H.; Kazi, S.; Masjuki, H.; Zubir, M.; Jahan, A.; Bhinitha, C. Energy, exergy and economic analysis of liquid flat-plate solar collector using green covalent functionalized graphene nanoplatelets. Appl. Therm. Eng. 2021, 192, 116916. [Google Scholar] [CrossRef]
- Ramadhan, A.I.; Sari, A.M.; Saptaji, K.; Rahardja, I.B.; Umar, E.; Perdana, S.Y.; Azmi, W.H. Characterization and Stability of ZrO2-SiO2 Nanofluids from Local Minerals Indonesia as Green Nanofluids to Application Radiator Cooling System. J. Adv. Res. Fluid Mech. Therm. Sci. 2023, 111, 126–140. [Google Scholar] [CrossRef]
- Jebali, M.; Colangelo, G.; Gómez-Merino, A.I. Green synthesis, characterization, and empirical thermal conductivity assessment of ZnO nanofluids for high-efficiency heat-transfer applications. Materials 2023, 16, 1542. [Google Scholar] [CrossRef]
- Hao, N.V.; Tung, D.H.; Thao, T.T.; Hoa, V.X.; Thoan, N.H.; Tan, P.T.; Minh, P.N.; Fal, J.; Żyła, G.; Trinh, P.V. High thermal conductivity of green nanofluid containing Ag nanoparticles prepared by using solution plasma process with Paramignya trimera extract. J. Therm. Anal. Calorim. 2023, 148, 7579–7590. [Google Scholar] [CrossRef]
- Sarafraz, M.; Hormozi, F.; Peyghambarzadeh, S. Thermal performance and efficiency of a thermosyphon heat pipe working with a biologically ecofriendly nanofluid. Int. Commun. Heat Mass Transf. 2014, 57, 297–303. [Google Scholar] [CrossRef]
- Sarafraz, M.; Hormozi, F. Intensification of forced convection heat transfer using biological nanofluid in a double-pipe heat exchanger. Exp. Therm. Fluid Sci. 2015, 66, 279–289. [Google Scholar] [CrossRef]
- Sadri, R.; Hosseini, M.; Kazi, S.; Bagheri, S.; Zubir, N.; Solangi, K.; Zaharinie, T.; Badarudin, A. A bio-based, facile approach for the preparation of covalently functionalized carbon nanotubes aqueous suspensions and their potential as heat transfer fluids. J. Colloid Interface Sci. 2017, 504, 115–123. [Google Scholar] [CrossRef]
- Sadri, R.; Hosseini, M.; Kazi, S.; Bagheri, S.; Abdelrazek, A.H.; Ahmadi, G.; Zubir, N.; Ahmad, R.; Abidin, N. A facile, bio-based, novel approach for synthesis of covalently functionalized graphene nanoplatelet nano-coolants toward improved thermo-physical and heat transfer properties. J. Colloid Interface Sci. 2018, 509, 140–152. [Google Scholar] [CrossRef]
- Sone, B.; Diallo, A.; Fuku, X.; Gurib-Fakim, A.; Maaza, M. Biosynthesized CuO nano-platelets: Physical properties & enhanced thermal conductivity nanofluidics. Arab. J. Chem. 2020, 13, 160–170. [Google Scholar]
- Dewanjee, D.; Kundu, B. A review of applications of green nanofluids for performance improvement of solar collectors. Renew. Energy 2024, 240, 122182. [Google Scholar] [CrossRef]
- Maddah, H.; Rezazadeh, M.; Maghsoudi, M.; NasiriKokhdan, S. The effect of silver and aluminum oxide nanoparticles on thermophysical properties of nanofluids. J. Nanostructure Chem. 2013, 3, 28. [Google Scholar] [CrossRef]
- Thakur, M.; Gangacharyulu, D.; Singh, G. Effect of temperature and multiwalled carbon nanotubes concentration on thermophysical properties of water base nanofluid. Int. J. Mech. Prod. Eng. Res. Dev. (IJMPERD) 2017, 7, 4151–4160. [Google Scholar]
- Iranmanesh, S.; Mehrali, M.; Sadeghinezhad, E.; Ang, B.C.; Ong, H.C.; Esmaeilzadeh, A. Evaluation of viscosity and thermal conductivity of graphene nanoplatelets nanofluids through a combined experimental–statistical approach using respond surface methodology method. Int. Commun. Heat Mass Transf. 2016, 79, 74–80. [Google Scholar] [CrossRef]
- Darvanjooghi, M.H.K.; Esfahany, M.N. Experimental investigation of the effect of nanoparticle size on thermal conductivity of in-situ prepared silica–ethanol nanofluid. Int. Commun. Heat Mass Transf. 2016, 77, 148–154. [Google Scholar] [CrossRef]
- Esfe, M.H.; Saedodin, S.; Akbari, M.; Karimipour, A.; Afrand, M.; Wongwises, S.; Safaei, M.R.; Dahari, M. Experimental investigation and development of new correlations for thermal conductivity of CuO/EG–water nanofluid. Int. Commun. Heat Mass Transf. 2015, 65, 47–51. [Google Scholar] [CrossRef]
- Moradnazhad, M.; Unver, H.O. Energy efficiency of machining operations: A review. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2017, 231, 1871–1889. [Google Scholar] [CrossRef]
- Davim, J.P. Machining: Fundamentals and Recent Advances; Springer: London, UK, 2008. [Google Scholar]
- Dhar, N.R.; Islam, M.W.; Islam, S.; Mithu, M.A.H. The influence of minimum quantity of lubrication (MQL) on cutting temperature, chip and dimensional accuracy in turning AISI-1040 steel. J. Mater. Process. Technol. 2006, 171, 93–99. [Google Scholar] [CrossRef]
- Shokoohi, Y.; Khosrojerdi, E.; Shiadhi, B.R. Machining and ecological effects of a new developed cutting fluid in combination with different cooling techniques on turning operation. J. Clean. Prod. 2015, 94, 330–339. [Google Scholar] [CrossRef]
- Goindi, G.S.; Sarkar, P. Dry machining: A step towards sustainable machining–challenges and future directions. J. Clean. Prod. 2017, 165, 1557–1571. [Google Scholar] [CrossRef]
- Braga, D.U.; Diniz, A.E.; Miranda, G.W.; Coppini, N.L. Using a minimum quantity of lubricant (MQL) and a diamond coated tool in the drilling of aluminum–silicon alloys. J. Mater. Process. Technol. 2002, 122, 127–138. [Google Scholar] [CrossRef]
- Hiran Gabriel, D.; Parthiban, M.; Kantharaj, I.; Beemkumar, N. A review on sustainable alternatives for conventional cutting fluid applications for improved machinability. Mach. Sci. Technol. 2023, 27, 157–207. [Google Scholar] [CrossRef]
- Esfe, M.H.; Bahiraei, M.; Mir, A. Application of conventional and hybrid nanofluids in different machining processes: A critical review. Adv. Colloid Interface Sci. 2020, 282, 102199. [Google Scholar] [CrossRef]
- Sharma, A.K.; Tiwari, A.K.; Dixit, A.R. Progress of nanofluid application in machining: A review. Mater. Manuf. Process. 2015, 30, 813–828. [Google Scholar] [CrossRef]
- Daungthongsuk, W.; Wongwises, S. A critical review of convective heat transfer of nanofluids. Renew. Sustain. Energy Rev. 2007, 11, 797–817. [Google Scholar] [CrossRef]
- Kakaç, S.; Pramuanjaroenkij, A. Review of convective heat transfer enhancement with nanofluids. Int. J. Heat Mass Transf. 2009, 52, 3187–3196. [Google Scholar] [CrossRef]
- He, Y.; Jin, Y.; Chen, H.; Ding, Y.; Cang, D.; Lu, H. Heat transfer and flow behaviour of aqueous suspensions of TiO2 nanoparticles (nanofluids) flowing upward through a vertical pipe. Int. J. Heat Mass Transf. 2007, 50, 2272–2281. [Google Scholar] [CrossRef]
- Lee, C.-G.; Hwang, Y.-J.; Choi, Y.-M.; Lee, J.-K.; Choi, C.; Oh, J.-M. A study on the tribological characteristics of graphite nano lubricants. Int. J. Precis. Eng. Manuf. 2009, 10, 85–90. [Google Scholar] [CrossRef]
- Reddy, N.S.K.; Rao, P.V. Experimental investigation to study the effect of solid lubricants on cutting forces and surface quality in end milling. Int. J. Mach. Tools Manuf. 2006, 46, 189–198. [Google Scholar] [CrossRef]
- Gujar, J.G.; Patil, S.S.; Sonawane, S.S. A review on nanofluids: Synthesis, stability, and uses in the manufacturing industry. Curr. Nanomater. 2023, 8, 303–318. [Google Scholar] [CrossRef]
- Roy, S.; Ghosh, A. High speed turning of AISI 4140 steel using nanofluid through twin jet SQL system. In Proceedings of the ASME 2013 International Manufacturing Science and Engineering Conference Collocated with the 41st North American Manufacturing Research Conference, Madison, WI, USA, 10–14 June 2013; p. V002T004A002. [Google Scholar]
- Sharma, A.K.; Tiwari, A.K.; Dixit, A.R.; Singh, R.K.; Singh, M. Novel uses of alumina/graphene hybrid nanoparticle additives for improved tribological properties of lubricant in turning operation. Tribol. Int. 2018, 119, 99–111. [Google Scholar] [CrossRef]
- Rahmati, B.; Sarhan, A.A.; Sayuti, M. Morphology of surface generated by end milling AL6061-T6 using molybdenum disulfide (MoS2) nanolubrication in end milling machining. J. Clean. Prod. 2014, 66, 685–691. [Google Scholar] [CrossRef]
- Jamil, M.; Khan, A.M.; Hegab, H.; Gupta, M.K.; Mia, M.; He, N.; Zhao, G.; Song, Q.; Liu, Z. Milling of Ti–6Al–4V under hybrid Al2O3-MWCNT nanofluids considering energy consumption, surface quality, and tool wear: A sustainable machining. Int. J. Adv. Manuf. Technol. 2020, 107, 4141–4157. [Google Scholar] [CrossRef]
- Vryzas, Z.; Kelessidis, V.C. Nano-based drilling fluids: A review. Energies 2017, 10, 540. [Google Scholar] [CrossRef]
- Huang, W.-T.; Wu, D.-H.; Chen, J.-T. Robust design of using nanofluid/MQL in micro-drilling. Int. J. Adv. Manuf. Technol. 2016, 85, 2155–2161. [Google Scholar] [CrossRef]
- Lee, P.H.; Nam, T.S.; Li, C.; Lee, S.W. Environmentally-Friendly Nano-Fluid Minimum Quantity Lubrication (MQL) Meso-Scale Grinding Process Using Nano-Diamond Particles. In Proceedings of the 2010 International Conference on Manufacturing Automation (ICMA), Hong Kong, China, 13–15 December 2010; pp. 44–49. [Google Scholar]
- Khandekar, S.; Sankar, M.R.; Agnihotri, V.; Ramkumar, J. Nano-cutting fluid for enhancement of metal cutting performance. Mater. Manuf. Process. 2012, 27, 963–967. [Google Scholar] [CrossRef]
- Zhou, C.; Guo, X.; Zhang, K.; Cheng, L.; Wu, Y. The coupling effect of micro-groove textures and nanofluids on cutting performance of uncoated cemented carbide tools in milling Ti-6Al-4V. J. Mater. Process. Technol. 2019, 271, 36–45. [Google Scholar] [CrossRef]
- Vázquez, K.; Cantú, D.; Segura, A.; Araiz, F.; Peña-Parás, L.; Maldonado, D. Application of Nanofluids to improve tool life in machining processes. In Proceedings of the 4th Conference and Exhibition on Lubrication, Maintenance and Tribotechnology (LUBMAT) 2014, Manchester, UK, 25–27 June 2014; pp. 1–8. [Google Scholar]
- Khan, M.A.A.; Hussain, M.; Lodhi, S.K.; Zazoum, B.; Asad, M.; Afzal, A. Green metalworking fluids for sustainable machining operations and other sustainable systems: A review. Metals 2022, 12, 1466. [Google Scholar] [CrossRef]
- Afonso, I.S.; Nobrega, G.; Lima, R.; Gomes, J.R.; Ribeiro, J.E. Conventional and recent advances of vegetable oils as metalworking fluids (MWFs): A review. Lubricants 2023, 11, 160. [Google Scholar] [CrossRef]
- Dennison, M.S.; Jebabalan, S.K.; Barik, D. Applicability of nano-cutting fluids for enhanced cooling, low tool wear, and high tribological performance during machining—A review. Discov. Appl. Sci. 2024, 6, 663. [Google Scholar] [CrossRef]
- Salem, A.; Hopkins, C.; Imad, M.; Hegab, H.; Darras, B.; Kishawy, H.A. Environmental analysis of sustainable and traditional cooling and lubrication strategies during machining processes. Sustainability 2020, 12, 8462. [Google Scholar] [CrossRef]
- Abukhshim, N.; Mativenga, P.; Sheikh, M.A. Heat generation and temperature prediction in metal cutting: A review and implications for high speed machining. Int. J. Mach. Tools Manuf. 2006, 46, 782–800. [Google Scholar] [CrossRef]
- Duc, T.M.; Long, T.T.; Chien, T.Q. Performance Evaluation of MQL Parameters Using Al2O3 and MoS2 Nanofluids in Hard Turning 90CrSi Steel. Lubricants 2019, 7, 40. [Google Scholar] [CrossRef]
- Hu, S.; Li, C.; Zhou, Z.; Liu, B.; Zhang, Y.; Yang, M.; Li, B.; Gao, T.; Liu, M.; Cui, X. Nanoparticle-enhanced coolants in machining: Mechanism, application, and prospects. Front. Mech. Eng. 2023, 18, 53. [Google Scholar] [CrossRef]
- Rahman, S.S.; Ashraf, M.Z.I.; Amin, A.N.; Bashar, M.; Ashik, M.F.K.; Kamruzzaman, M. Tuning nanofluids for improved lubrication performance in turning biomedical grade titanium alloy. J. Clean. Prod. 2019, 206, 180–196. [Google Scholar] [CrossRef]
- Rao, G.K.M.; Padmini, R.; Krishna, S.V. Performance evaluation of eco-friendly nano fluids in machining. In Proceedings of the International Conference on Recent Advances in Robotics, Aeronautical and Mechanical Engineering, Athens, Greece, 14–16 May 2013. [Google Scholar]
- Sharma, A.K.; Singh, R.K.; Dixit, A.R.; Tiwari, A.K. Characterization and experimental investigation of Al2O3 nanoparticle based cutting fluid in turning of AISI 1040 steel under minimum quantity lubrication (MQL). Mater. Today Proc. 2016, 3, 1899–1906. [Google Scholar] [CrossRef]
- Padmini, R.; Krishna, P.V.; Rao, G.K.M. Effectiveness of vegetable oil based nanofluids as potential cutting fluids in turning AISI 1040 steel. Tribol. Int. 2016, 94, 490–501. [Google Scholar] [CrossRef]
- Minh, D.T.; The, L.T.; Bao, N.T. Performance of Al2O3 nanofluids in minimum quantity lubrication in hard milling of 60Si2Mn steel using cemented carbide tools. Adv. Mech. Eng. 2017, 9, 1687814017710618. [Google Scholar] [CrossRef]
- Afonso, I.S.; Pereira, J.; Ribeiro, A.E.; Amaral, J.S.; Rodrigues, N.; Gomes, J.R.; Lima, R.; Ribeiro, J. Analysis of a vegetable oil performance in a milling process by MQL lubrication. Micromachines 2022, 13, 1254. [Google Scholar] [CrossRef]
- Lawal, S.A.; Choudhury, I.A.; Nukman, Y. Application of vegetable oil-based metalworking fluids in machining ferrous metals—A review. Int. J. Mach. Tools Manuf. 2012, 52, 1–12. [Google Scholar] [CrossRef]
- Sofiah, A.; Samykano, M.; Pandey, A.; Kadirgama, K.; Sharma, K.; Saidur, R. Immense impact from small particles: Review on stability and thermophysical properties of nanofluids. Sustain. Energy Technol. Assess. 2021, 48, 101635. [Google Scholar] [CrossRef]
- Charalampous, P. Performance Investigation of Coated Carbide Tools in Milling Procedures. Appl. Sci. 2025, 15, 3765. [Google Scholar] [CrossRef]
- Elsheikh, A.; Ali, A.B.; Saba, A.; Faqeha, H.; Alsaati, A.A.; Maghfuri, A.M.; Abd-Elaziem, W.; El Ashmawy, A.A.; Ma, N. A review on sustainable machining: Technological advancements, health and safety considerations, and related environmental impacts. Results Eng. 2024, 24, 103042. [Google Scholar] [CrossRef]
- Deshpande, S.; Deshpande, Y. A review on cooling systems used in machining processes. Mater. Today Proc. 2019, 18, 5019–5031. [Google Scholar] [CrossRef]
- Sen, B.; Mia, M.; Gupta, M.K.; Rahman, M.A.; Mandal, U.K.; Mondal, S.P. Influence of Al2O3 and Palm Oil–Mixed Nano-Fluid on Machining Performances of Inconel-690: IF-THEN Rules–Based FIS Model in Eco-Benign Milling. Int. J. Adv. Manuf. Technol. 2019, 103, 3389–3403. [Google Scholar] [CrossRef]
- Makhesana, M.A.; Patel, K.M.; Krolczyk, G.M.; Danish, M.; Singla, A.K.; Khanna, N. Influence of MoS2 and graphite-reinforced nanofluid-MQL on surface roughness, tool wear, cutting temperature and microhardness in machining of Inconel 625. CIRP J. Manuf. Sci. Technol. 2023, 41, 225–238. [Google Scholar] [CrossRef]
- Sharmin, I.; Gafur, M.A.; Dhar, N.R. Preparation and evaluation of a stable CNT-water based nano cutting fluid for machining hard-to-cut material. SN Appl. Sci. 2020, 2, 626. [Google Scholar] [CrossRef]
- Barhoum, A.; Jeevanandam, J.; Rastogi, A.; Samyn, P.; Boluk, Y.; Dufresne, A.; Danquah, M.K.; Bechelany, M. Plant celluloses, hemicelluloses, lignins, and volatile oils for the synthesis of nanoparticles and nanostructured materials. Nanoscale 2020, 12, 22845–22890. [Google Scholar] [CrossRef]
- Gokapai, V.; Pothana, P.; Ling, K. Nanoparticles in Drilling Fluids: A Review of Types, Mechanisms, Applications, and Future Prospects. Eng 2024, 5, 2462–2495. [Google Scholar] [CrossRef]
- Nam, J.S.; Kim, D.H.; Chung, H.; Lee, S.W. Optimization of environmentally benign micro-drilling process with nanofluid minimum quantity lubrication using response surface methodology and genetic algorithm. J. Clean. Prod. 2015, 102, 428–436. [Google Scholar] [CrossRef]
- Eltaggaz, A.; Hegab, H.; Deiab, I.; Kishawy, H.A. Hybrid nano-fluid-minimum quantity lubrication strategy for machining austempered ductile iron (ADI). Int. J. Interact. Des. Manuf. (IJIDeM) 2018, 12, 1273–1281. [Google Scholar] [CrossRef]
- Pal, A.; Chatha, S.S.; Sidhu, H.S. Assessing the lubrication performance of various vegetable oil-based nano-cutting fluids via eco-friendly MQL technique in drilling of AISI 321 stainless steel. J. Braz. Soc. Mech. Sci. Eng. 2022, 44, 148. [Google Scholar] [CrossRef]
- Mosleh, M.; Shirvani, K.A.; Smith, S.T.; Belk, J.H.; Lipczynski, G. A study of minimum quantity lubrication (MQL) by nanofluids in orbital drilling and tribological testing. J. Manuf. Mater. Process. 2019, 3, 5. [Google Scholar] [CrossRef]
- Nam, J.S.; Lee, P.-H.; Lee, S.W. Experimental characterization of micro-drilling process using nanofluid minimum quantity lubrication. Int. J. Mach. Tools Manuf. 2011, 51, 649–652. [Google Scholar] [CrossRef]
- Ibrahim, A.M.M.; Wei, L.; Mourad, A.-h.I.; Mohamed, A.; Abd El-Naby, A.M.; Al Soufi, M.S.; Ezzat, M.F.; Elsheikh, A. Cooling and lubrication techniques in grinding: A state-of-the-art review, applications, and sustainability assessment. Chin. J. Aeronaut. 2023, 36, 76–113. [Google Scholar] [CrossRef]
- Kishore, K.; Sinha, M.K.; Singh, A.; Archana; Gupta, M.K.; Korkmaz, M.E. A comprehensive review on the grinding process: Advancements, applications and challenges. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2022, 236, 10923–10952. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.; Zhang, Y.; Yang, M.; Jia, D.; Liu, G.; Hou, Y.; Li, R.; Zhang, N.; Wu, Q. Experimental assessment of an environmentally friendly grinding process using nanofluid minimum quantity lubrication with cryogenic air. J. Clean. Prod. 2018, 193, 236–248. [Google Scholar] [CrossRef]
- Li, B.; Li, C.; Zhang, Y.; Wang, Y.; Jia, D.; Yang, M.; Zhang, N.; Wu, Q.; Han, Z.; Sun, K. Heat transfer performance of MQL grinding with different nanofluids for Ni-based alloys using vegetable oil. J. Clean. Prod. 2017, 154, 1–11. [Google Scholar] [CrossRef]
- Bai, X.; Jiang, J.; Li, C.; Dong, L.; Ali, H.M.; Sharma, S. Tribological performance of different concentrations of Al2O3 nanofluids on minimum quantity lubrication milling. Chin. J. Mech. Eng. 2023, 36, 11. [Google Scholar] [CrossRef]
- Sinha, M.K.; Madarkar, R.; Ghosh, S.; Rao, P.V. Application of eco-friendly nanofluids during grinding of Inconel 718 through small quantity lubrication. J. Clean. Prod. 2017, 141, 1359–1375. [Google Scholar] [CrossRef]
- Saravanan, R.; Sathish, T.; Vijayan, V.; Rajkumar, S.; Sharma, S.; Li, C.; Zhang, Y.; Sharma, K.; Eldin, S.M. Eco-friendly MoS2/waste coconut oil nanofluid for machining of magnesium implants. Rev. Adv. Mater. Sci. 2023, 62, 20220296. [Google Scholar] [CrossRef]
- Krishna, P.V.; Srikant, R.R.; Rao, D.N. Experimental investigation on the performance of nanoboric acid suspensions in SAE-40 and coconut oil during turning of AISI 1040 steel. Int. J. Mach. Tools Manuf. 2010, 50, 911–916. [Google Scholar] [CrossRef]
- Tong, Y.; Ding, Y.; Guo, W.; Wang, S.; Cho, H. Heat transfer and lubrication performance of palm oil-Al2O3 nanofluid compared to traditional cutting fluid. ScienceAsia 2022, 48, 69–74. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Jia, D.; Zhang, D.; Zhang, X. Experimental evaluation of MoS2 nanoparticles in jet MQL grinding with different types of vegetable oil as base oil. J. Clean. Prod. 2015, 87, 930–940. [Google Scholar] [CrossRef]
- Shen, B.; Malshe, A.P.; Kalita, P.; Shih, A.J. Performance of novel MoS2 nanoparticles based grinding fluids in minimum quantity lubrication grinding. Trans. Namri/SME 2008, 36, 357–364. [Google Scholar]
- Mukherjee, S.; Wciślik, S.; Mishra, P.C.; Chaudhuri, P. Nanofluids: Critical issues, economics and sustainability perspectives. Particuology 2024, 87, 147–172. [Google Scholar] [CrossRef]
- Alagumalai, A.; Qin, C.; Solomin, E.; Yang, L.; Zhang, P.; Otanicar, T.; Kasaeian, A.; Chamkha, A.J.; Rashidi, M.M.; Wongwises, S. Conceptual analysis framework development to understand barriers of nanofluid commercialization. Nano Energy 2022, 92, 106736. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Raphael, W.; Tavares, J.R. Nanofluids as heat transfer fluids: Hurdles to industrial application and economic considerations. Can. J. Chem. Eng. 2022, 100, 3311–3320. [Google Scholar] [CrossRef]
- Wciślik, S. A simple economic and heat transfer analysis of the nanoparticles use. Chem. Pap. 2017, 71, 2395–2401. [Google Scholar] [CrossRef] [PubMed]
- Charitidis, C.A.; Georgiou, P.; Koklioti, M.A.; Trompeta, A.-F.; Markakis, V. Manufacturing nanomaterials: From research to industry. Manuf. Rev. 2014, 1, 11. [Google Scholar] [CrossRef]
- Alirezaie, A.; Hajmohammad, M.H.; Ahangar, M.R.H.; Esfe, M.H. Price-performance evaluation of thermal conductivity enhancement of nanofluids with different particle sizes. Appl. Therm. Eng. 2018, 128, 373–380. [Google Scholar] [CrossRef]
- Alirezaie, A.; Hajmohammad, M.H.; Alipour, A. Do nanofluids affect the future of heat transfer? “A benchmark study on the efficiency of nanofluids”. Energy 2018, 157, 979–989. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mishra, P.C.; Chaudhuri, P. Thermo-economic performance analysis of Al2O3-water nanofluids—An experimental investigation. J. Mol. Liq. 2020, 299, 112200. [Google Scholar] [CrossRef]
- Scalbi, S.; Masoni, P. Comparative environmental assessment of nanofluid application in refrigeration of power electronic traction systems. P-ESEM 2015, 2, 93. [Google Scholar]
- Induranga, A.; Galpaya, C.; Vithanage, V.; Indupama, A.; Maduwantha, K.; Gunawardana, N.; Wijesekara, D.; Amarasinghe, P.; Nilmalgoda, H.; Gunasena, K. Nanofluids for Heat Transfer: Advances in Thermo-Physical Properties, Theoretical Insights, and Engineering Applications. Energies 2025, 18, 1935. [Google Scholar] [CrossRef]
- Gonçalves, I.; Souza, R.; Coutinho, G.; Miranda, J.; Moita, A.; Pereira, J.E.; Moreira, A.; Lima, R. Thermal conductivity of nanofluids: A review on prediction models, controversies and challenges. Appl. Sci. 2021, 11, 2525. [Google Scholar] [CrossRef]
- Buongiorno, J.; Venerus, D.C.; Prabhat, N.; McKrell, T.; Townsend, J.; Christianson, R.; Tolmachev, Y.V.; Keblinski, P.; Hu, L.-w.; Alvarado, J.L. A benchmark study on the thermal conductivity of nanofluids. J. Appl. Phys. 2009, 106, 094312. [Google Scholar] [CrossRef]
- Souza, R.R.; Faustino, V.; Gonçalves, I.M.; Moita, A.S.; Bañobre-López, M.; Lima, R. A review of the advances and challenges in measuring the thermal conductivity of nanofluids. Nanomaterials 2022, 12, 2526. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, A.; Packnezhad, M.; Moshrefi-Torbati, M.; Walsh, F. Erosion–corrosion synergism in an alumina/sea water nanofluid. Microfluid. Nanofluidics 2014, 17, 225–232. [Google Scholar] [CrossRef]
- Anitha, S.; Safaei, M.R.; Rajeswari, S.; Pichumani, M. Thermal and energy management prospects of γ-AlOOH hybrid nanofluids for the application of sustainable heat exchanger systems. J. Therm. Anal. Calorim. 2021, 147, 6941–6957. [Google Scholar] [CrossRef]
- Thakur, P.; Kumar, N.; Sonawane, S.S. Enhancement of pool boiling performance using MWCNT based nanofluids: A sustainable method for the wastewater and incinerator heat recovery. Sustain. Energy Technol. Assess. 2021, 45, 101115. [Google Scholar] [CrossRef]
- Khan, A.M.; Gupta, M.K.; Hegab, H.; Jamil, M.; Mia, M.; He, N.; Song, Q.; Liu, Z.; Pruncu, C.I. Energy-based cost integrated modelling and sustainability assessment of Al-GnP hybrid nanofluid assisted turning of AISI52100 steel. J. Clean. Prod. 2020, 257, 120502. [Google Scholar] [CrossRef]
- Kishawy, H.A.; Hegab, H.; Deiab, I.; Eltaggaz, A. Sustainability assessment during machining Ti-6Al-4V with nano-additives-based minimum quantity lubrication. J. Manuf. Mater. Process. 2019, 3, 61. [Google Scholar] [CrossRef]
- Ilyas, S.U.; Shamsuddin, R.; Xiang, T.K.; Estellé, P.; Pendyala, R. Rheological profile of graphene-based nanofluids in thermal oil with hybrid additives of carbon nanotubes and nanofibers. J. Mol. Liq. 2023, 376, 121443. [Google Scholar] [CrossRef]
- Li, F.; Li, L.; Zhong, G.; Zhai, Y.; Li, Z. Effects of ultrasonic time, size of aggregates and temperature on the stability and viscosity of Cu-ethylene glycol (EG) nanofluids. Int. J. Heat Mass Transf. 2019, 129, 278–286. [Google Scholar] [CrossRef]
- Lee, S.H.S.; Hatton, T.A.; Khan, S.A. Microfluidic continuous magnetophoretic protein separation using nanoparticle aggregates. Microfluid. Nanofluidics 2011, 11, 429–438. [Google Scholar] [CrossRef]
- Elsaid, K.; Olabi, A.; Wilberforce, T.; Abdelkareem, M.A.; Sayed, E.T. Environmental impacts of nanofluids: A review. Sci. Total Environ. 2021, 763, 144202. [Google Scholar] [CrossRef] [PubMed]
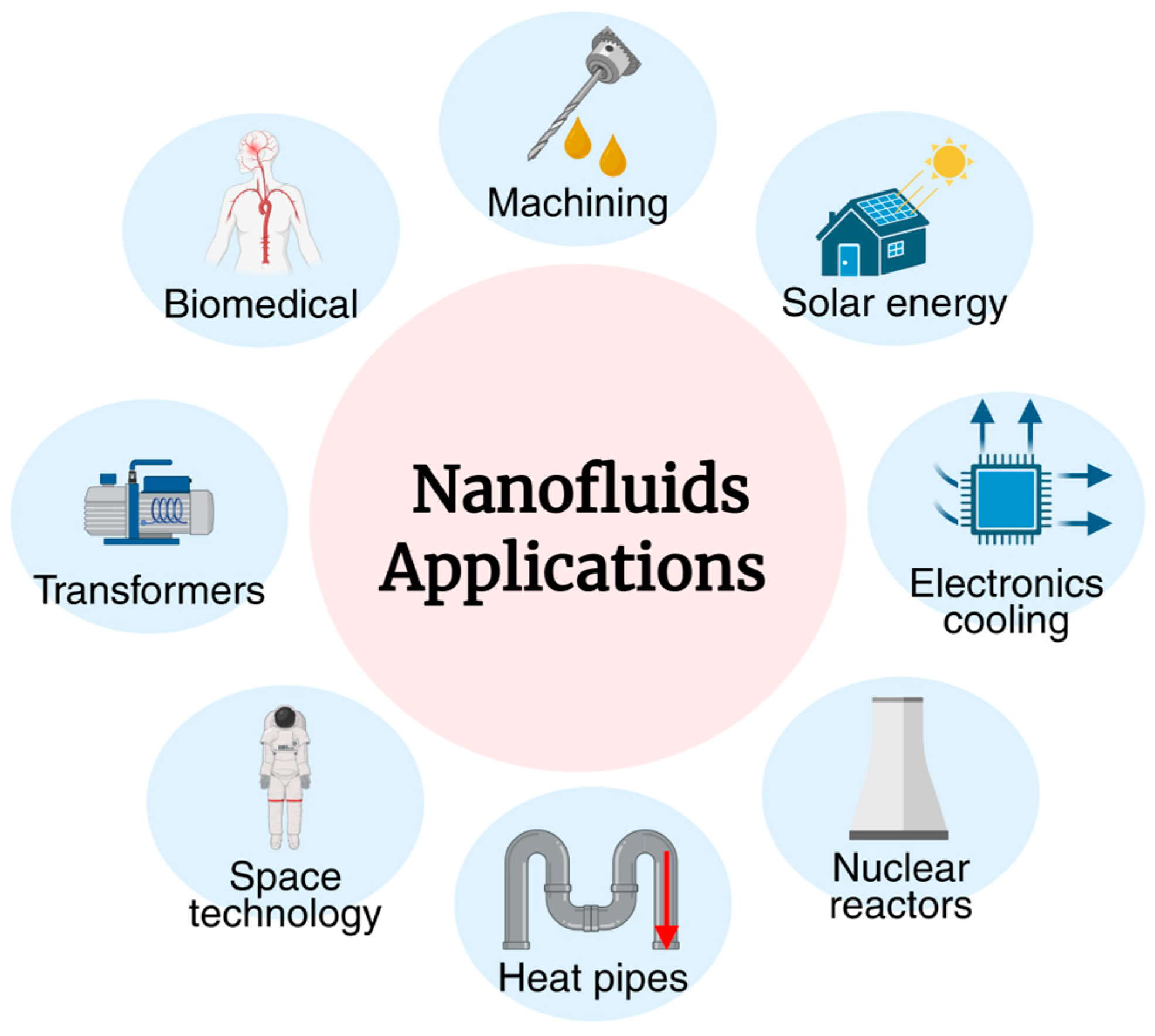
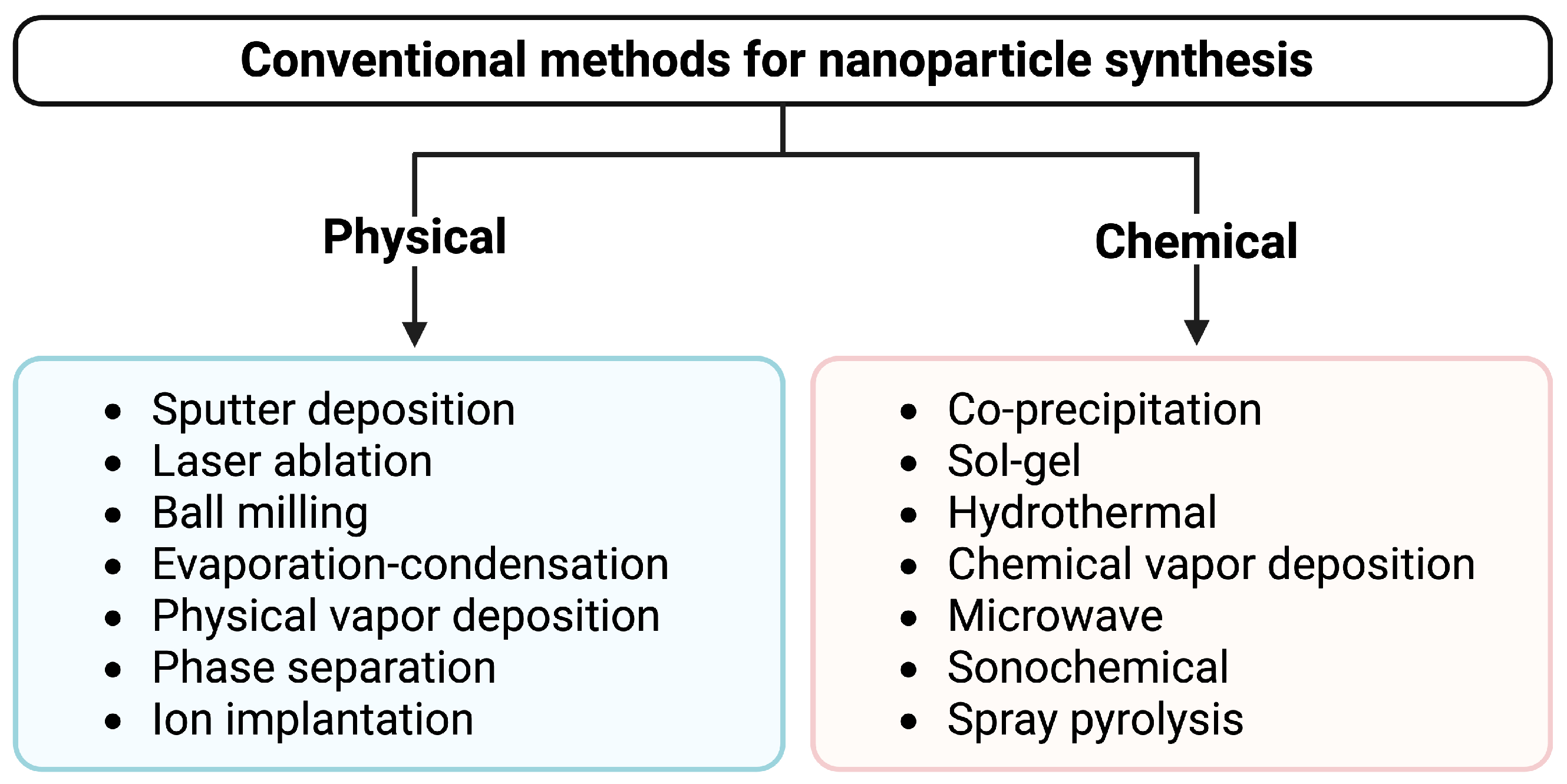
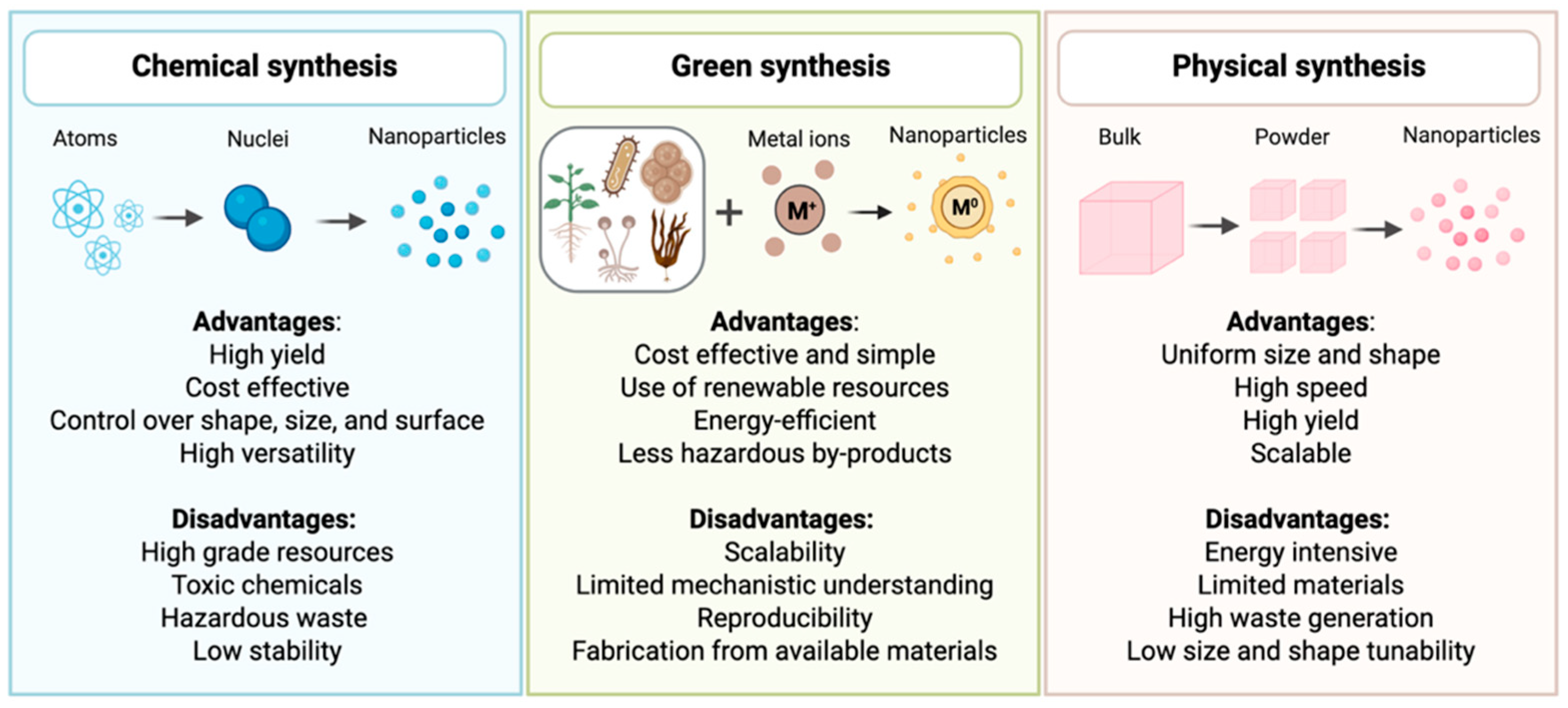
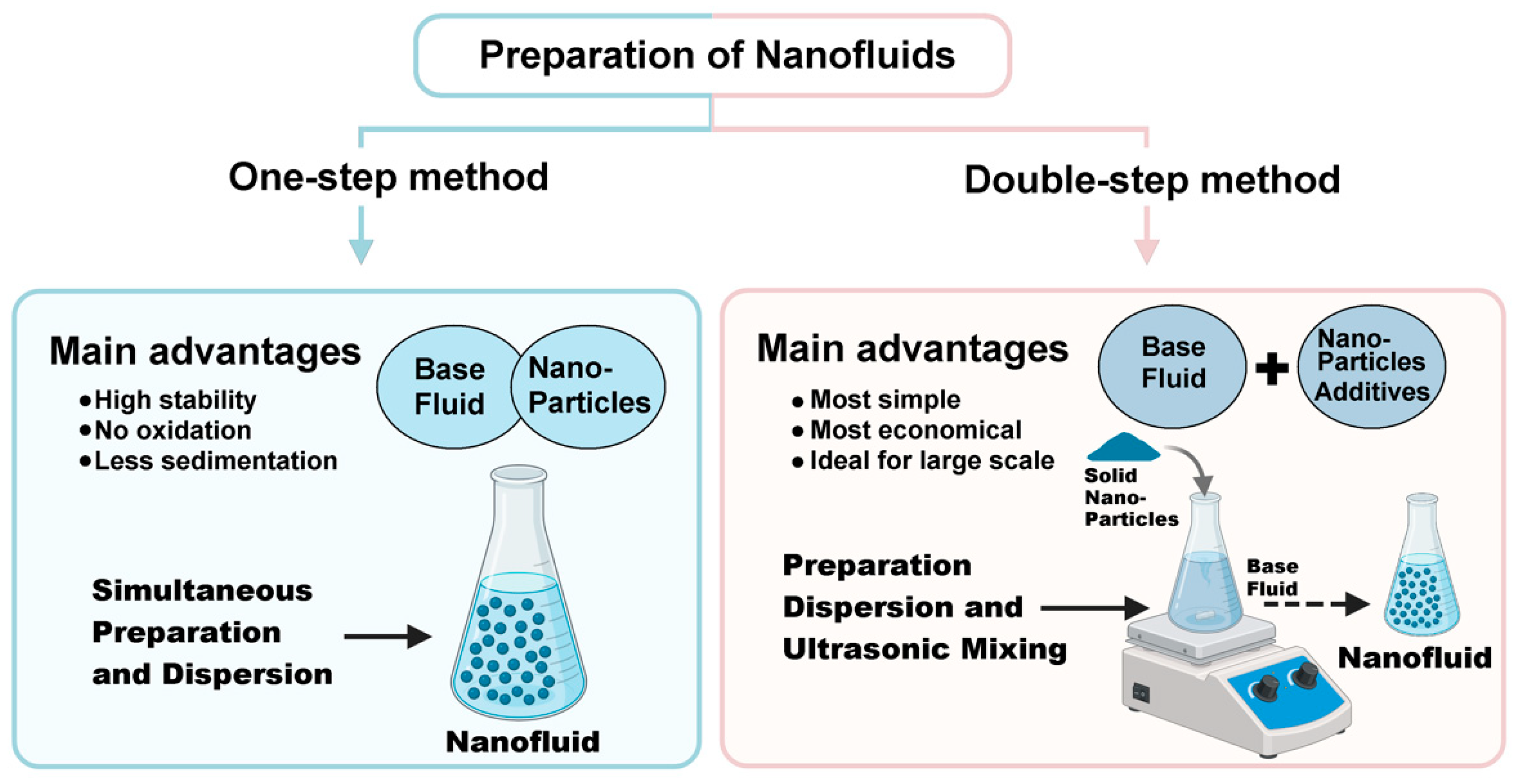
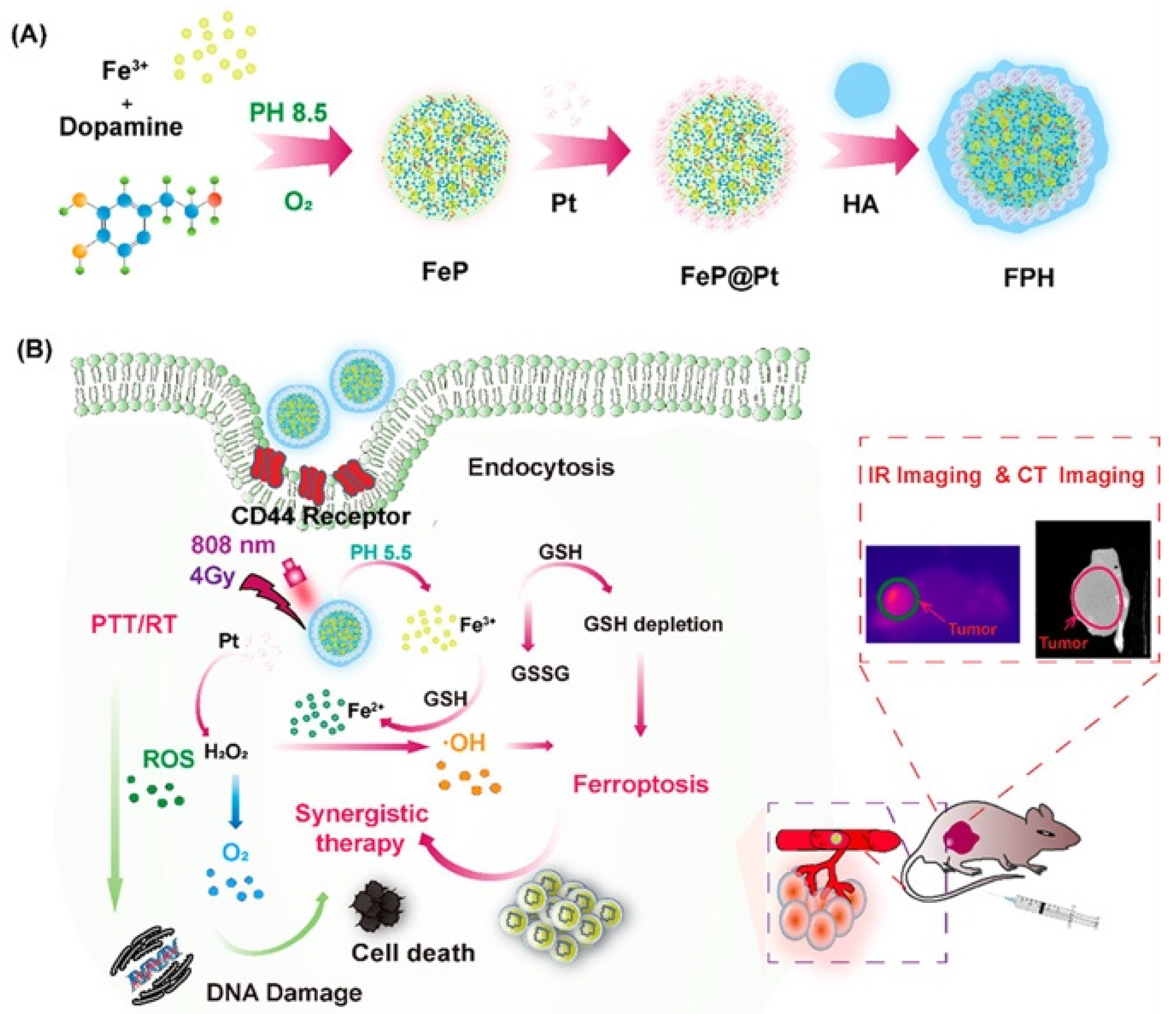
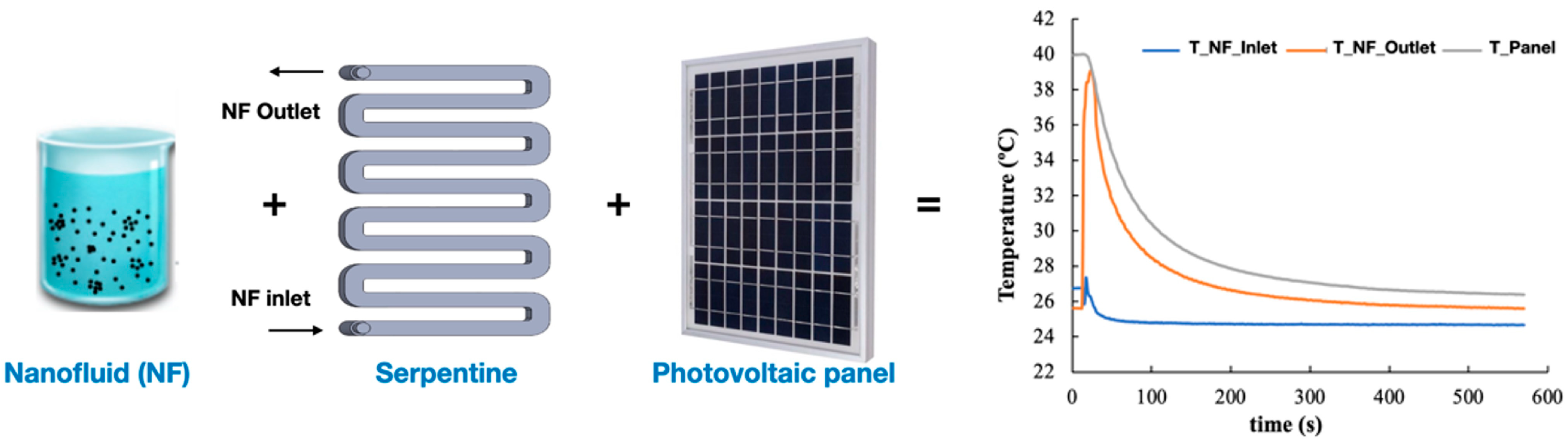

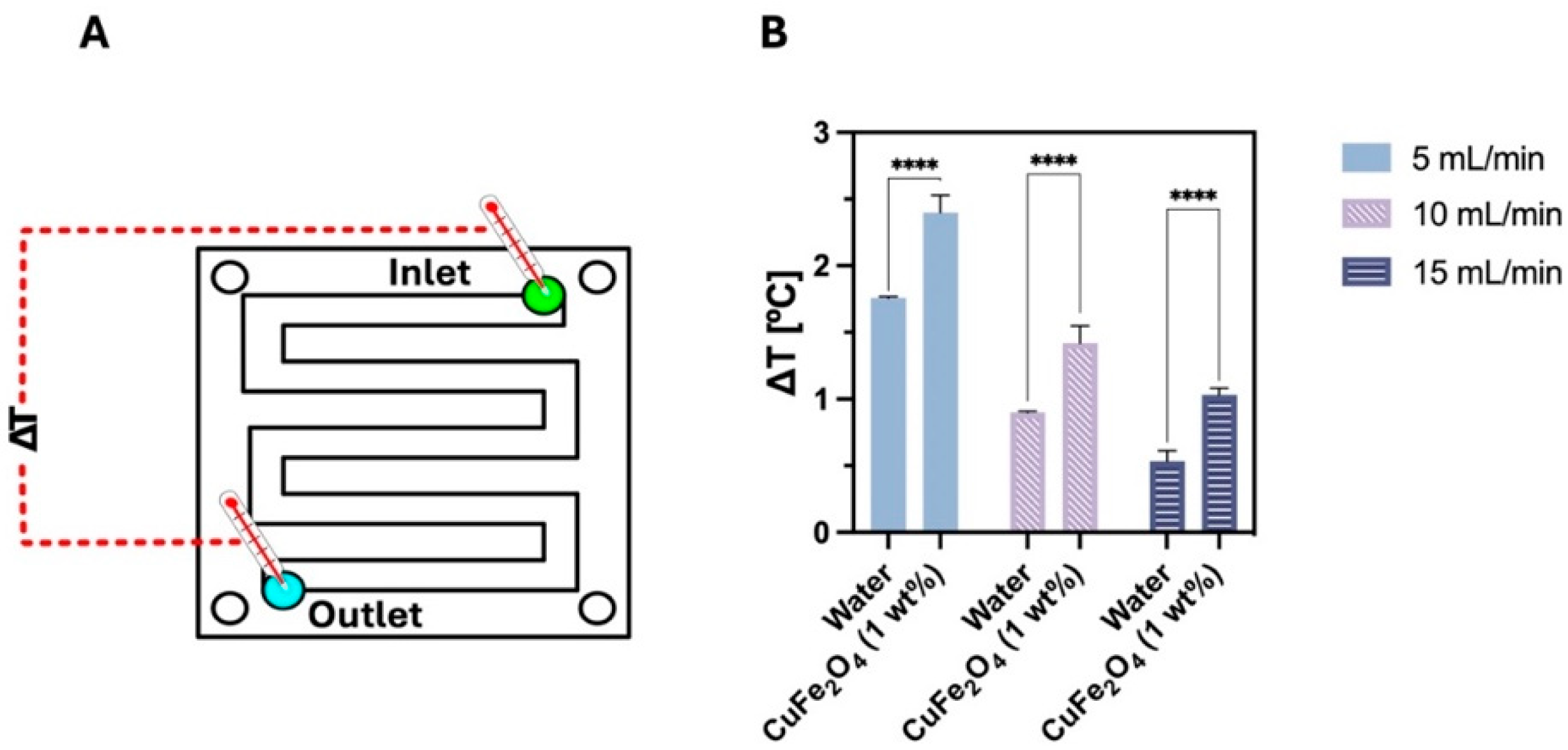
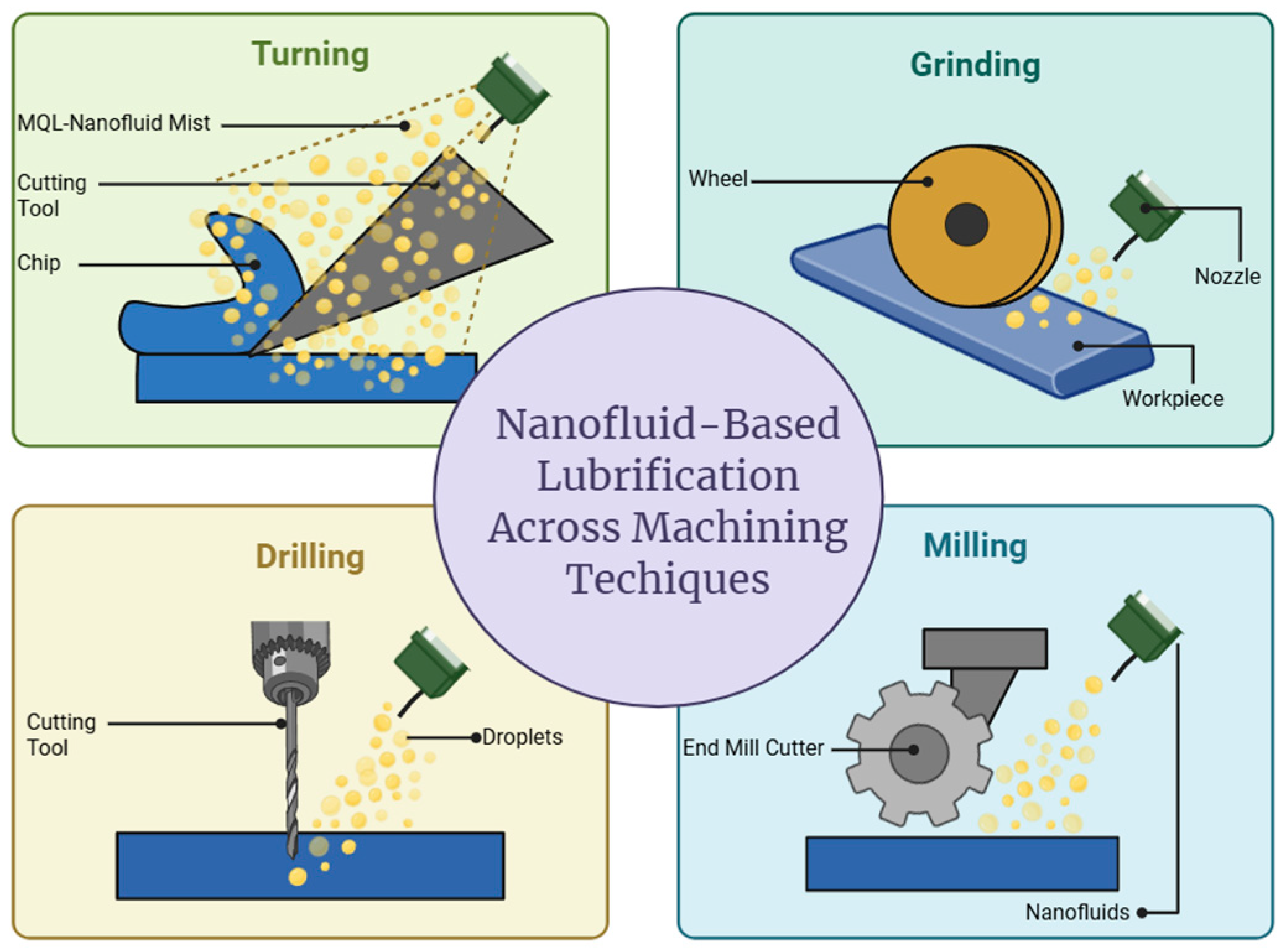

| NP | Synthesis Approach | Toxicity and Biocompatibility | Environmental Impact | Main Differences | Applications | Ref. |
|---|---|---|---|---|---|---|
| Ag | Chemical: Sodium citrate reduction | High toxicity to human dermal fibroblasts (HDFa); ROS ↑; non-selective cytotoxicity | Chemical waste, higher energy input | Higher cytotoxicity, chemical residues, less biocompatibility | Antibacterial, basic biomedical | [210] |
| Green: Azadirachta indica (Neem) leaf extract | Non-toxic to HDFa and RBCs; selective cancer cell (NCI-H460) apoptosis; ROS ↑ only in cancer cells | Eco-friendly, minimal waste | Safer profile, selective cancer cytotoxicity, natural capping/stabilizing agents | Anticancer, biomedical, drug delivery | ||
| Ag | Chemical: Sodium citrate method | Reduced antioxidant activity; lower DPPH scavenging % | Lower stability, generates waste | Weaker antioxidant capacity, poor stability over time | General lab use, short-term antimicrobial | [211] |
| Green: Mussaenda frondosa leaf extract | Higher antioxidant activity (↑ DPPH scavenging); non-toxic profile | Sustainable, minimal chemical load | Better stability, phytochemical surface functionalization | Biomedical, antioxidant applications | ||
| Ag | Chemical: Commercial (Sigma-Aldrich) | MIC = 8 µg/mL (vs. S. aureus); lower biofilm inhibition | Synthetic chemicals; not eco-friendly | Less effective at lower doses, weaker biofilm suppression, stable colloid | Antibacterial, anti-biofilm | [214] |
| Green: Zataria multiflora extract | MIC = 4 µg/mL (vs. S. aureus); better biofilm inhibition at 0.5–2× MIC | Eco-friendly; plant-based | Better inhibition at low conc., phytochemical capping, stable at pH 9 | Antibacterial, anti-biofilm | ||
| MgO | Chemical: NaOH + Magnesium acetate | Smaller inhibition zones against B. subtilis, E. coli, etc. | Requires strong base; more energy input | Larger particles, lower activity | General antimicrobial | [212] |
| Green: Lawsonia inermis extract | Larger inhibition zones at all tested doses (20–80 µL) | Green route, plant-derived | Better porosity, biocompatibility | Biomedical, antimicrobial | ||
| TiO2 | Chemical: Hydrothermal | Lower antibacterial effect | Ethanol use; higher temp calcination | Lower photocatalytic and antimicrobial efficiency | Photocatalysis, bactericide | [213] |
| Green: Jasmine flower extract | Higher inhibition zones | Minimal by-products, bio-safe | Higher biological activity, cleaner synthesis | Photocatalysis, antibacterial |
| NP | Synthesis Approach | Base Fluid | Thermal Conductivity Increase | Colloidal Stability | Types of Testing | Ref. |
|---|---|---|---|---|---|---|
| Ag | Microwave- assisted chemical precipitation | Distilled water | Up to ~29% at 1 vol.% | ---- | Thermal conductivity and viscosity measurements in static fluid (KD2 Pro, Brookfield viscometer) | [294] |
| Green tea (Camellia sinensis) | Water–Ethylene Glycol 50% | Up to 37% at 1 vol.% | Up to 12 days | Forced convection test in a double-pipe heat exchanger (flowing fluid); thermal conductivity measured with KD2 Decagon | [289] | |
| MWCNT | Conventional two-step with gum Arabic (0.25 wt.%) and 3 h ultrasonication | Deionized water | Up to 23% at 0.8 vol.% | Stable (confirmed by UV spectroscopy) | Thermal conductivity (KD2 Pro), specific heat (DSC), in static fluid | [295] |
| MWCNT | Green: Clove (Syzygium aromaticum) | Deionized wate | Up to 20% at 0.08 wt.% | Up to 60 days | Thermal conductivity and viscosity measurements in static fluid | [290] |
| GNP | Direct dispersion in distilled water via ultrasonication | Distilled water | Up to ~31% at 0.1 wt.% | ------ | KD2 Pro thermal analyzer | [296] |
| CGNP | Green: Clove (Syzygium aromaticum) | Deionized water | Up to 22.92% at 0.1 wt.% | Up to 63 days (UV–Vis analysis) | Thermal conductivity (KD2 Pro) in static fluid | [291] |
| GGNP | Green: Gallic acid | Distilled water | Up to 17.76% at 0.1 wt.% | >60 days without agglomeration (confirmed by visual inspection and stability curve) | Thermal conductivity via KD2 Pro analyzer; application in flat-plate solar collector (forced flow system, 0.5–1.5 L/min) | [283] |
| SiO2 | Conventional Stöber method | Ethanol | Up to ~60% at 1.17 vol.%, | ------ | KD2 Pro—Transient Hot-Wire | [297] |
| SiO2 | Green: Rice husk | Deionized water | Up to 38.2% at 3.0 vol.% | >180 days | Thermal conductivity: transient hot-wire (KD2 Pro) | [282] |
| CuO | Two-step method | Etilenoglicol –Water (40:60) | 36.97% at 2.0 (wt.%) | 75 days | KD2 Pro Transient hot-wire | [298] |
| CuO | Green: Callistemon viminalis | Deionized Water | Up to 34% at 9% (vol.%) | Stable with PVP | KD2 Pro Transient Hot-Wire | [292] |
| Types of NPs | Size of NPs (nm) | Approach | Base Fluid | Process | Performance | Ref. |
|---|---|---|---|---|---|---|
| MoS2 | <5 | Conv. | Castrol Syntilo 9930 | Turning | Lowered cutting and feed forces, cutting zone temperature, tool wear, and improved surface finish compared to the conventional coolant. | [358] |
| Green | Waste coconut oil | |||||
| Nano boric acid | 50 | Conv. | SAE-40 oil | Suspensions at 0.5% concentration significantly reduced cutting temperature, flank wear, and surface roughness, with coconut oil showing superior performance over SAE-40 due to better lubricating properties. | [359] | |
| Green | Coconut oil | |||||
| Al2O3 | 20 | Conv. | Traditional cutting fluid | Milling | At 1.0 vol.% and 20 nm, the palm oil-based Al2O3 NF reduced workpiece surface temperature by more than 10 °C and decreased milling force deviations by 8–13% compared to conventional cutting fluid. | [360] |
| Green | Palm oil | |||||
| MoS2 | 50 | Conv. | Liquid paraffin 2 wt.% | Grinding | In MQL grinding of Grade 45 steel, the MoS2–soybean oil NF significantly lowered grinding forces and friction while improving G-ratio and surface quality compared to a comparable MoS2–paraffin oil NF under identical conditions. | [361] |
| Green | Palm oil 2 wt.% | |||||
| Rapeseed oil 2 wt.% | ||||||
| Soybean oil 2 wt.% | ||||||
| MoS2 | ~250 | Conv. | Cimtech500 | Reduction in grinding forces (up to 27%) and enhanced G-ratio (up to 46%), with soybean oil achieving a 9% force reduction and 15% G-ratio improvement compared to its base oil alone, with minimal fluid usage (5 mL/min). | [362] | |
| Paraffin oil | ||||||
| Green | Soybean oil | |||||
| CANMIST oil | ||||||
| ND | 30 | Conv. | Paraffin oil | Drilling | 1 vol.% achieved the greatest reduction in torque (31.3%) and thrust force (32.2%), while 2 vol.% led to a slight performance decline due to possible particle agglomeration. | [351] |
| Green | Vegetable oil | At 2 vol.%, the NF matched paraffin oil in reducing torque (31.3%) and thrust force (30.9%), while significantly improving chip evacuation and burr removal. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoso, B.D.; Souza, A.; Nobrega, G.; Afonso, I.S.; Neves, L.B.; Faria, C.; Ribeiro, J.; Lima, R.A. Progress in Nanofluid Technology: From Conventional to Green Nanofluids for Biomedical, Heat Transfer, and Machining Applications. Nanomaterials 2025, 15, 1242. https://doi.org/10.3390/nano15161242
Cardoso BD, Souza A, Nobrega G, Afonso IS, Neves LB, Faria C, Ribeiro J, Lima RA. Progress in Nanofluid Technology: From Conventional to Green Nanofluids for Biomedical, Heat Transfer, and Machining Applications. Nanomaterials. 2025; 15(16):1242. https://doi.org/10.3390/nano15161242
Chicago/Turabian StyleCardoso, Beatriz D., Andrews Souza, Glauco Nobrega, Inês S. Afonso, Lucas B. Neves, Carlos Faria, João Ribeiro, and Rui A. Lima. 2025. "Progress in Nanofluid Technology: From Conventional to Green Nanofluids for Biomedical, Heat Transfer, and Machining Applications" Nanomaterials 15, no. 16: 1242. https://doi.org/10.3390/nano15161242
APA StyleCardoso, B. D., Souza, A., Nobrega, G., Afonso, I. S., Neves, L. B., Faria, C., Ribeiro, J., & Lima, R. A. (2025). Progress in Nanofluid Technology: From Conventional to Green Nanofluids for Biomedical, Heat Transfer, and Machining Applications. Nanomaterials, 15(16), 1242. https://doi.org/10.3390/nano15161242










