Carbon Restrains the Precipitation of Cu-Rich Nanoparticles in CuFeMnNi HEAs
Abstract
1. Introduction
2. Experiment and Simulation
2.1. Materials
2.2. Characterization
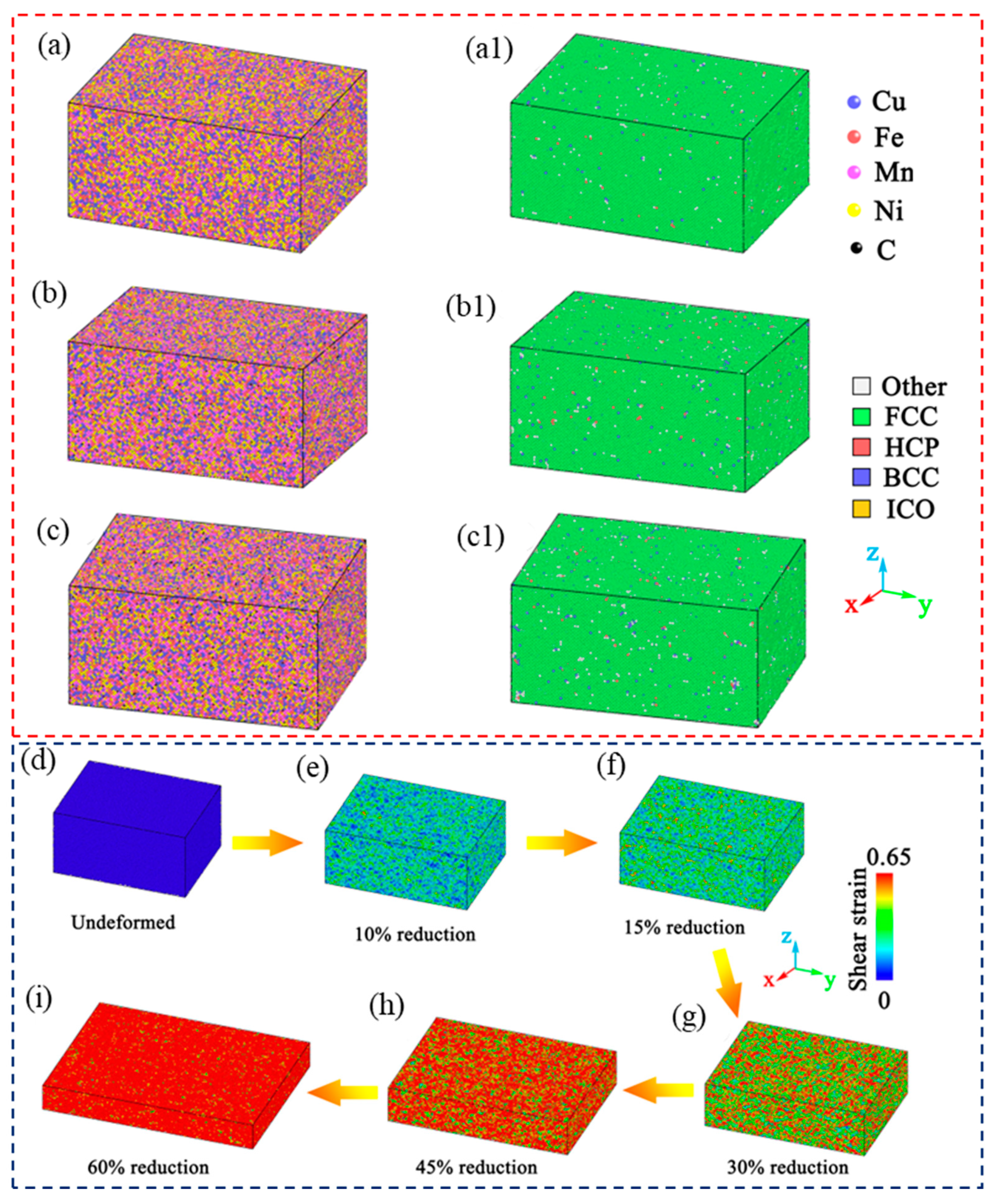
2.3. Simulation
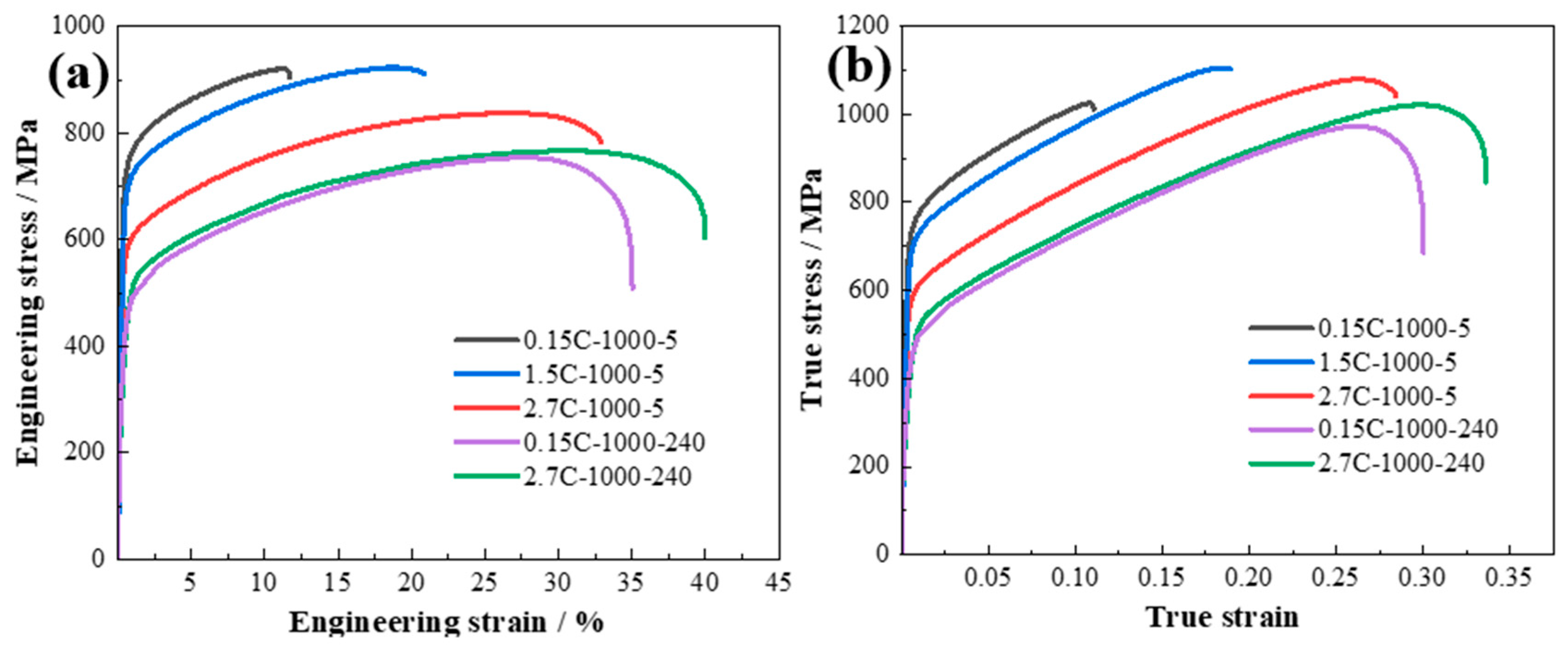
3. Results
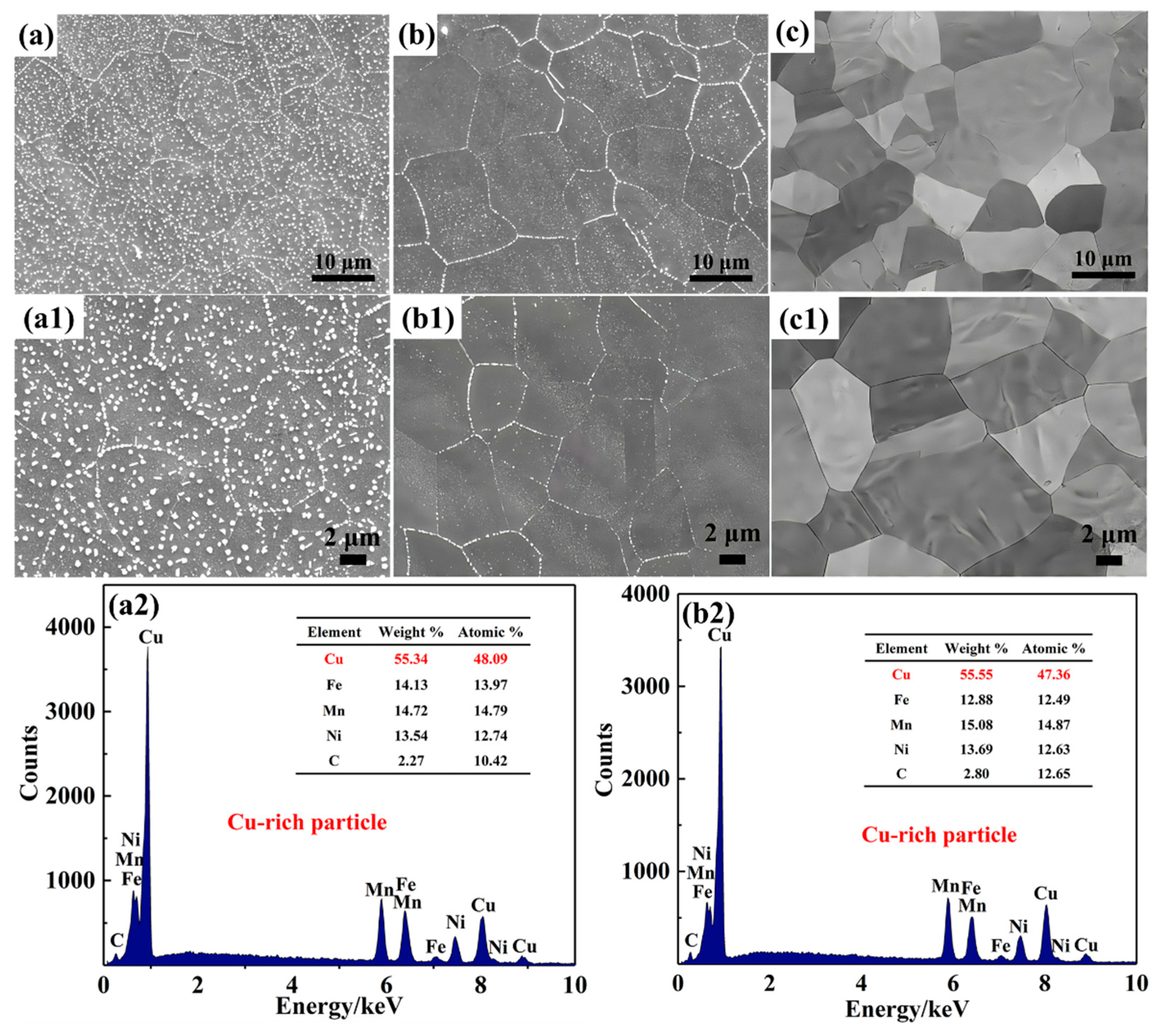
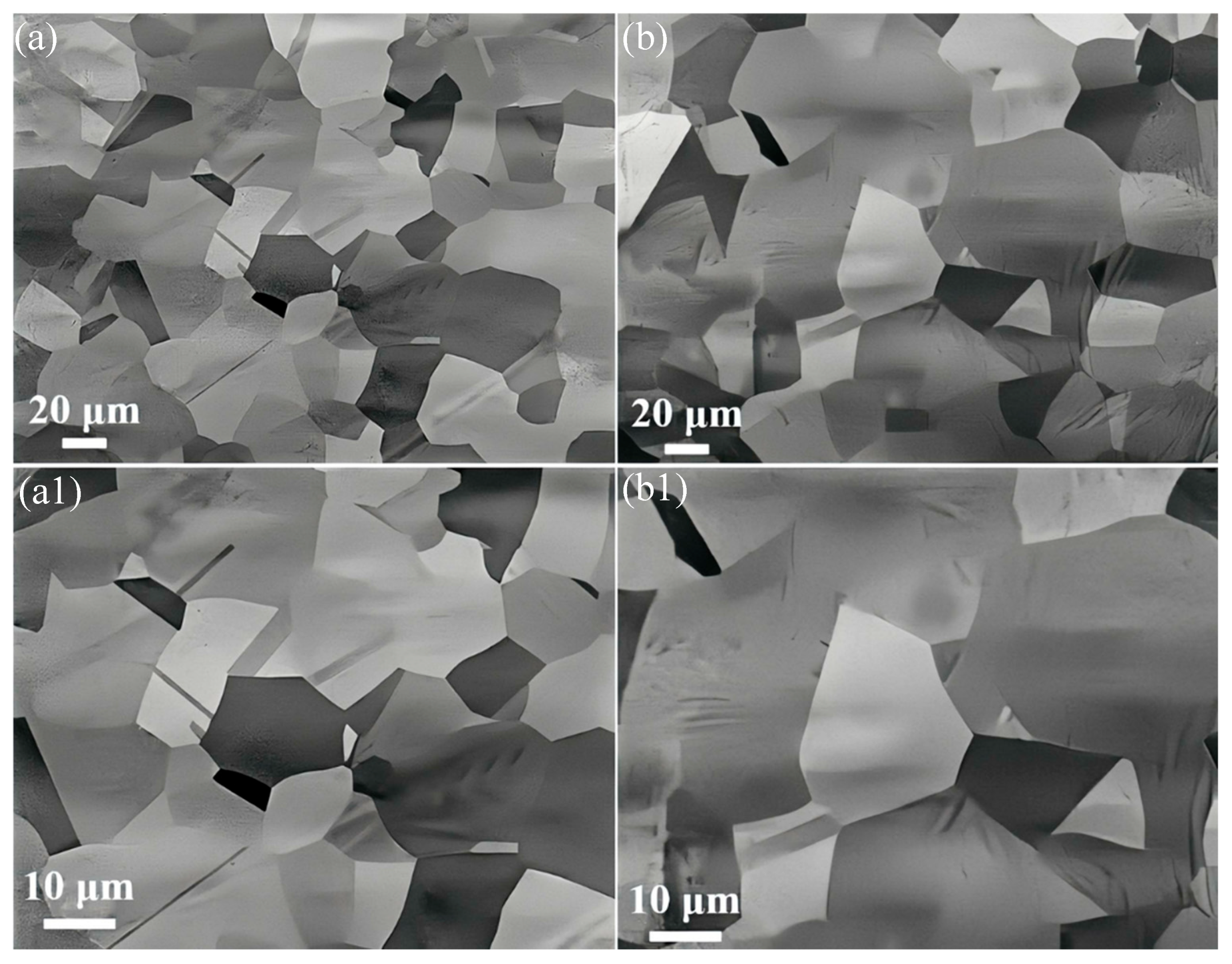
3.1. Effect of C on the Precipitation of Submicron Cu-Rich Particles
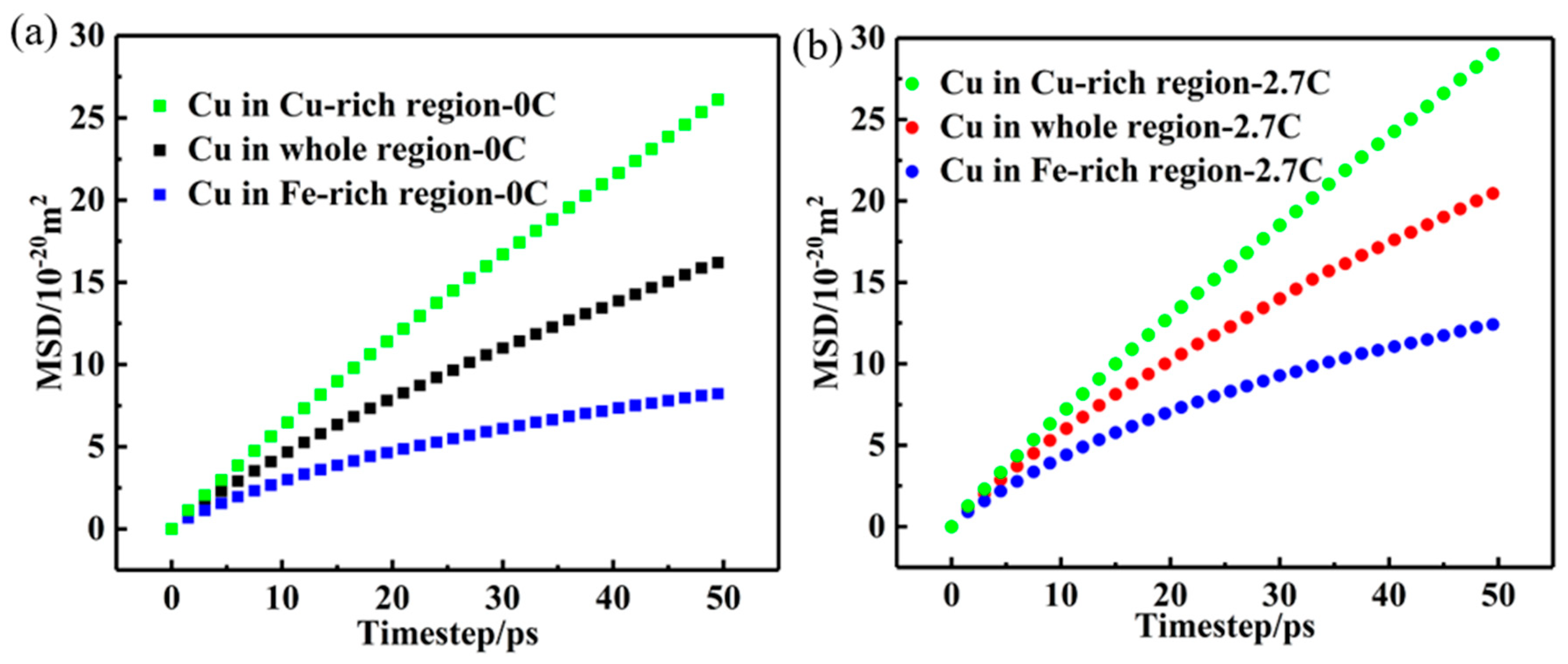
3.2. Effect of Carbon on Diffusion of Substitutional Atoms
4. Discussion
4.1. Diffusion Mechanism of Different C Concentrations
4.2. Strengthening Mechanisms
5. Conclusions
- (1)
- With the increase in C content in the CuFeMnNi HEAs, the size and area fraction of the precipitated Cu-rich nanoparticles decrease significantly, finally disappearing completely.
- (2)
- After holding the alloy at 1000 °C for 4 h, no large Cu-rich particles were found in the C-doped CuFeMnNi HEAs, while the grain growth of the 0.15-1000-5 alloy is extremely sluggish (increased by ~4 μm) because of the slow dissolution of Cu-rich nanoparticles and their continuous pinning effect on grain boundaries.
- (3)
- During the high-temperature annealing at 1000 °C, the excessive C greatly increases the diffusion speed and diffusion distance of Cu atoms in the C-doped CuFeMnNi HEAs. Thus, Cu atoms have more opportunities to fully diffuse to the Fe-rich region (and even to the adjacent Cu-rich region) to achieve homogenization, thereby inhibiting the precipitation of Cu-rich nanoparticles.
- (4)
- The increasing carbon content enables an extraordinary ductility of 33%. The 1.5C-1000-5 alloy displays a superior strength–ductility combination, with the yield strength of 695 ± 10 MPa, ultimate tensile strength of 925 ± 20 MPa, and ductility of 21.5%. These results are attributed to the fact that the Cu-rich nanoparticles can significantly elevate the work hardening ability of the 1.5C-1000-5 alloy. In addition, the high diffusion channels induced by short-time annealing significantly inhibit the grain growth, leading to an increase in the ductility.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mat. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Sun, L.; He, Z.; Jia, N. Local chemical order enables an ultrastrong and ductile high-entropy alloy in a cryogenic environment. Sci. Adv. 2024, 10, eadq6398. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Zhao, P.L. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- He, M.Y.; Jia, N.; Liu, X.C.; Shen, Y.F.; Zuo, L. Abnormal chemical composition fluctuations in multi-principal-element alloys induced by simple cyclic deformation. J. Mater. Sci. Technol. 2021, 113, 287–295. [Google Scholar] [CrossRef]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Yu, Y.; Ren, B.; Liu, Z.X.; Li, T.Y.; Wang, L.J.; Qiao, Z.H. Design of a novel CoCrFeNiCu0.3 high entropy alloy with desirable mechanical, corrosion and anti-bacterial properties via adjusting Cu distribution. Mater. Today Commun. 2023, 35, 105946. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, N.N.; Zhu, S.Y.; Qiao, Z.H.; Zhang, J.B.; Yang, J.; Liu, W.M. A novel Cu-doped high entropy alloy with excellent comprehensive performances for marine application. J. Mater. Sci. Technol. 2021, 69, 48–59. [Google Scholar] [CrossRef]
- Wang, J.M.; Jiang, H.; Chang, X.X.; Zhang, L.J.; Wang, H.X.; Zhu, L.; Qin, S.X. Effect of Cu content on the microstructure and corrosion resistance of AlCrFeNi3Cux high entropy alloys. Corros. Sci. 2023, 221, 111313. [Google Scholar] [CrossRef]
- Li, Z.; Qiao, D.X.; Xu, Y.; Zhou, E.Z.; Yang, C.T.; Yuan, X.Y.; Lu, Y.P.; Gu, J.-D.; Wolfgang, S.; Xu, D.; et al. Cu-bearing high-entropy alloys with excellent antiviral properties. J. Mater. Sci. Technol. 2021, 84, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Burla, A.; Khandelwal, M.; Vaidya, M. Antibacterial properties of Cu containing complex concentrated alloys. Mater. Today Commun. 2022, 33, 104915. [Google Scholar] [CrossRef]
- Wang, M.; Shen, Y.; Jia, N.; Xue, W.; Wang, Z. Ultrahigh strength triggered by BCC and B2 eutectic-phase interfaces in a novel FeCrVNiAl high-entropy alloy. Mater. Res. Lett. 2024, 12, 745–755. [Google Scholar] [CrossRef]
- Cai, Y.C.; Chen, Y.; Luo, Z.; Gao, F.; Li, L. Manufacturing of FeCoCrNiCux medium-entropy alloy coating using laser cladding technology. Mater. Des. 2017, 133, 91–108. [Google Scholar] [CrossRef]
- Zhu, J.M.; Meng, J.L.; Liang, J.L. Microstructure and mechanical properties of multi-principal component AlCoCrFeNiCux alloy. Rare Met. 2016, 35, 385–389. [Google Scholar] [CrossRef]
- Gao, N.; Hu, Q.; Wang, Y.S.; Liu, P.; Wang, Q.; Fan, Z.T.; Zhang, Y.L.; Liu, X.W. Controlled precipitation and strengthening in a CuFeNiMn medium-entropy alloy. Mat. Sci. Eng. A 2022, 852, 143694. [Google Scholar] [CrossRef]
- He, M.Y.; Shen, Y.F.; Jia, N.; Liaw, P.K.; Zuo, L. Achieving sustainable strain hardening in a carbon-doped CuFeMnNi high-entropy alloy via dual-level heterogeneous microstructures. J. Alloy. Compd. 2023, 939, 168831. [Google Scholar] [CrossRef]
- Thompson, A.P.; Aktulga, H.M.; Berger, R.; Bolintineanu, D.S.; Brown, W.M.; Crozier, P.S.; in ’t Veld, P.J.; Kohlmeyer, A.; Moore, S.G.; Nguyen, T.D.; et al. LAMMPS—a flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comput. Phys. Commun. 2022, 271, 108171. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO–the Open Visualization Tool. Model. Simulat. Mater. Sci. Eng. 2010, 18, 2154–2162. [Google Scholar] [CrossRef]
- Farkas, D.; Caro, A. Model interatomic potentials and lattice strain in a high-entropy alloy. J. Mater. Res. 2018, 33, 3218–3225. [Google Scholar] [CrossRef]
- Stuart, S.J.; Tutein, A.B.; Harrison, J.A. A reactive potential for hydrocarbons with intermolecular interactions. J. Chem. Phys. 2000, 112, 6472–6486. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Riyahi, K.A.; Kundin, J.; Lukianova, O.; Esakkiraja, N.; Paul, A.; Divinski, S.; Steinbach, I. Carbon effect on thermo-kinetics of Co-Cr-Fe-Mn-Ni high entropy alloys: A computational study validated by interdiffusion experiments. Acta Mater. 2023, 261, 119358. [Google Scholar] [CrossRef]
- Allen, M.P.; Tildesley, D.J. Computer Simulation of Liquids; Oxford University: Oxford, UK, 1987. [Google Scholar]
- Liu, Y.Y.; Jia, G.B.; Bin, Y. Molecular dynamics simulation on diffusion properties of Pb-Mg alloy. Sci. China Technol. Sci. 2010, 53, 2328–2332. [Google Scholar] [CrossRef]
- Lukianova, O.A.; Kulitckii, V.; Rao, Z.; Li, Z.; Wilde, G.; Divinski, S.V. Self-diffusion in carbon-alloyed CoCrFeMnNi high entropy alloys. Acta Mater. 2022, 237, 118136. [Google Scholar] [CrossRef]
- Shen, Y.F.; Dong, X.X.; Song, X.T.; Jia, N. Carbon content-tuned martensite transformation in low-alloy TRIP steels. Sci. Rep. 2019, 9, 7559. [Google Scholar] [CrossRef]
- Gaertner, D.; Abrahams, K.; Kottke, J.; Esin, V.A.; Steinbach, I.; Wilde, G.; Divinski, S.V. Concentration-dependent atomic mobilities in FCC CoCrFeMnNi high-entropy alloys. Acta Mater. 2019, 166, 357–370. [Google Scholar] [CrossRef]
- Lu, E.; Zhao, J.; Makkonen, I.; Mizohata, K.; Li, Z.; Hua, M.; Djurabekova, F.; Tuomisto, F. Enhancement of vacancy diffusion by C and N interstitials in the equiatomic FeMnNiCoCr high entropy alloy. Acta Mater. 2021, 215, 117093. [Google Scholar] [CrossRef]
- Kresse, T.; Borchers, C.; Kirchheim, R. Vacancy–carbon complexes in bcc iron: Correlation between carbon content, vacancy concentration and diffusion coefficient. Scr. Mater. 2013, 69, 690–693. [Google Scholar] [CrossRef]
- Wang, M.; Shen, Y.; Jia, N.; Xue, W.; Wang, X. Multistage precipitation triggering 3 GPa compressive strength and superior corrosion resistance in a FeCrVNiAl alloy. Mater. Futures 2025, 4, 035004. [Google Scholar] [CrossRef]
- Li, W.; Xie, D.; Li, D.; Zhang, Y.; Gao, Y.; Liaw, P.K. Mechanical behavior of high entropy alloys. Prog. Mater. Sci. 2021, 118, 100777. [Google Scholar] [CrossRef]
- He, J.Y.; Wang, H.; Huang, H.L.; Xu, X.D.; Chen, M.W.; Wu, Y.; Liu, X.J.; Nieh, T.G.; An, K.; Lu, Z.P. A precipitation-hardened high-entropy alloy with outstanding tensile properties. Acta Mater. 2016, 102, 187–196. [Google Scholar] [CrossRef]
- Williamson, G.K.; Smallman, R.E. III. Dislocation densities in some annealed and cold-worked metals from measurements on the X-ray debye-scherrer spectrum. Philos. Mag. 1956, 1, 34–46. [Google Scholar] [CrossRef]
- He, C.; Shen, Y.; Xue, W.; Fan, Z.; Zhou, Y. Nanosized κ-Carbide and B2 Boosting Strength Without Sacrificing Ductility in a Low-Density Fe-32Mn-11Al Steel. Nanomaterials 2024, 15, 48. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.W.; Shen, Y.F.; Jia, N.; Li, Y.J.; Xue, W.Y. Controllable selection of martensitic variant enables concurrent enhancement of strength and ductility in a low-carbon steel. Inter. J. Plast. 2023, 168, 103704. [Google Scholar] [CrossRef]



| Alloy | Diffusion Coefficient/10−10m2·s−1 | |||
|---|---|---|---|---|
| Cu | Fe | Mn | Ni | |
| CuFeMnNi | 5.12 | 3.62 | 3.23 | 3.04 |
| 1.5C-1000-240 | 5.93 | 3.93 | 3.44 | 3.13 |
| 2.7C-1000-240 | 6.61 | 4.24 | 3.64 | 3.35 |
| C Content/at. % | Region | Chemical Compositions/at. % | ||||
|---|---|---|---|---|---|---|
| Cu | Fe | Mn | Ni | C | ||
| 0C | Fe-rich region | 12 | 41 | 25 | 22 | - |
| Cu-rich region | 41 | 12 | 25 | 22 | - | |
| 2.7C | Fe-rich region | 11.3 | 40.5 | 24 | 21.5 | 2.7 |
| Cu-rich region | 40.5 | 11.3 | 24 | 21.5 | 2.7 | |
| C Content/at. % | Diffusion Coefficient/10−10m2·s−1 | ||
|---|---|---|---|
| Cu-Rich Region | Entire Region | Fe-Rich Region | |
| 0 | 8.57 | 5.12 | 2.13 |
| 2.7 | 9.57 | 6.61 | 3.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; He, M.; Shen, Y.; Xue, W.; Fan, Z. Carbon Restrains the Precipitation of Cu-Rich Nanoparticles in CuFeMnNi HEAs. Nanomaterials 2025, 15, 1223. https://doi.org/10.3390/nano15161223
Wang M, He M, Shen Y, Xue W, Fan Z. Carbon Restrains the Precipitation of Cu-Rich Nanoparticles in CuFeMnNi HEAs. Nanomaterials. 2025; 15(16):1223. https://doi.org/10.3390/nano15161223
Chicago/Turabian StyleWang, Mingze, Mengyuan He, Yongfeng Shen, Wenying Xue, and Zhijian Fan. 2025. "Carbon Restrains the Precipitation of Cu-Rich Nanoparticles in CuFeMnNi HEAs" Nanomaterials 15, no. 16: 1223. https://doi.org/10.3390/nano15161223
APA StyleWang, M., He, M., Shen, Y., Xue, W., & Fan, Z. (2025). Carbon Restrains the Precipitation of Cu-Rich Nanoparticles in CuFeMnNi HEAs. Nanomaterials, 15(16), 1223. https://doi.org/10.3390/nano15161223







