Abstract
This work presents a method for preparing an Fe2O3/MWCNT/Al composite electrode without the use of a binder. Synthesizing the composite material directly on conductive substrates allows one to obtain ready-made supercapacitor electrodes characterized by high values of specific capacity, as well as resistance to numerous charge/discharge cycles. Using an array of multi-walled carbon nanotubes (MWCNTs) as a conductive base for the synthesis of iron oxide allows for the production of a composite material that combines the positive properties of both materials. The Fe2O3/MWCNT/Al composite was formed using electrochemical oxidation of the MWCNT/Al material in a mixture of 0.1 M aqueous solution of Fe(NH4)2(SO4)2 (iron ammonium sulfate) and 0.08 M CH3COONa (sodium acetate) in a 1:1 ratio. The proposed approaches to fabricating composite electrodes provide excellent performance characteristics, namely high cyclic stability and fast response time. For the first time, an Fe2O3/MWCNT/Al composite was obtained using electrochemical oxidation of Fe2+ on the surface of MWCNTs grown directly on aluminum foil. The specific capacitance of the obtained composite material reaches 175 F/g at a scanning rate of 100 mV/s. The capacity loss during cyclic measurements does not exceed 25% after 10,000 charge/discharge cycles.
1. Introduction
Creating reliable electrochemical energy storage devices is a primary task for the storage, conversion, and further use of renewable (clean) energy [1,2,3]. There are two main types of energy storage and accumulation devices: supercapacitors and lithium-ion batteries [4,5,6]. Supercapacitors (SCs) are electrochemical energy sources characterized by high power, long life, and high stability over multiple charge/discharge cycles, as well as safety and environmental friendliness [7,8,9]. The performance of supercapacitors directly depends on the active material of the electrode [10,11]. Much work has been performed using transition metal oxides as active materials of electrodes [12,13,14]. The redox behavior of these oxides is related to the multivalent nature of transition metals. Promising materials include iron-based materials such as hematite (Fe2O3) [15,16,17], magnetite (Fe3O4) [18], FeOOH [19], FeOx [20], and CoFe2O4 [21]. They have attracted attention as promising materials for SC electrodes due to their high theoretical capacitance, wide negative potential range (−1.2–0 V), abundance in nature, low cost, and non-toxicity [22,23,24]. Despite their high values of specific capacitance, most transition metal oxides have low conductivity, poor stability, a low charging rate, and a short life during the charge/discharge process [25,26]. One way to improve the conductivity of iron-based materials is to combine them with conductive carbon materials [27,28,29]. Due to the synergistic effect of the high capacitance of iron oxides and the high conductivity of carbon materials, composite materials demonstrate better electrochemical characteristics than the original pseudocapacitive materials [30,31,32]. Carbon nanotubes are characterized by high conductivity, a large specific surface area, mesoporosity, environmental friendliness, and chemical stability. These properties make them a promising material for supercapacitor electrodes [33,34].
Conventional electrode manufacturing processes use binders, which usually have high resistivity and low electrochemical activity. Their use degrades the electrochemical performance of the electrode by increasing the internal resistance [35]. In addition, conventional binders are often based on organic solvents, which can affect the environment. Therefore, the study of binder-free electrode preparation methods has become an important area of research to improve the performance of supercapacitors. The preparation of binder-free electrodes increases the amount of active material in the electrodes and also significantly increases the conductivity of the electrodes. One way to prepare binder-free electrodes is to grow electrochemically active materials on conductive substrates [36].
Synthesis of MWCNTs on metal substrates can improve electrochemical properties due to a decrease in the contact resistance between the active electrode material and the current collector [37,38]. Aluminum is a promising material for supercapacitor electrodes due to its high electrical conductivity and plasticity [39]. Electrodes made of aluminum foil coated with an MWCNT layer are suitable for use in flexible supercapacitors [40]. The aluminum foil acts as a conductive substrate for the synthesis of the MWCNT array and as a current collector for the finished electrode. In turn, the MWCNT array acts as a conductive base for the formation of an iron oxide layer and as the active material in the supercapacitor electrode. Composite electrodes based on carbon nanotubes with transition metal oxides combine physical and chemical mechanisms of charge accumulation on a single electrode. Nanotube-based materials promote charge generation through the formation of an electric double-layer capacitor (EDLC) and also provide a large specific surface area [41], which in turn increases the contact area between the electrolyte and the pseudocapacitive materials applied to the MWCNTs. In this case, pseudocapacitive materials increase the specific capacitance values due to fast redox reactions [42,43]. There are various technologies for the production of composite materials. Methods such as hydrothermal deposition [44], electrochemical deposition [45,46,47,48,49], and electrospinning [50] are used to produce an electrode without a binder.
In this work, the possibility of producing a composite electrode based on multi-walled carbon nanotubes and iron oxide without the use of binders was investigated. Thin films of Fe2O3 were deposited on the surface of carbon nanotube arrays grown on aluminum foil with electrochemical deposition. The optimal conditions for preliminary electrochemical oxidation of the carbon nanotubes were selected: 10 min in 0.005 M aqueous Na2SO4 solution at an oxidation voltage of 4 V. This allowed us to obtain a composite electrode with high resistance to numerous charge/discharge cycles (10,000). The dependence of the specific capacity and stability of the electrode material on the annealing temperature was studied. The electrochemical properties of the composite were studied as a function of the parameters of iron hydroxide film formation on the surface of the carbon nanotube array. The optimal conditions for composite formation were selected, which led to a fivefold increase in specific capacitance: electrochemical oxidation of MWCNT/Al samples in an aqueous solution (Fe(NH4)2(SO4)2 0.1 M and CH3COONa 0.08 M) at a voltage sweep rate of 2 mV/s, followed by annealing at 300 °C. All samples were characterized by scanning electron microscopy (SEM), Raman spectroscopy (RS), X-ray photoelectron spectroscopy (XPS), cyclic voltammetry (CV), galvanostatic charge/discharge (GCD), and electrochemical impedance spectroscopy (EIS) methods.
2. Materials and Methods
2.1. Synthesis of MWCNTs on Aluminum Foil
The synthesis of carbon nanotubes was carried out using a previously developed method [51]. A CVD synthesis setup was used for deposition. MWCNTs were deposited at atmospheric pressure onto aluminum foil substrates (99.0%, Russia) coated with a catalyst layer. The deposition temperature was 600 °C, and the synthesis duration was 1 h. Ethanol consumption was 6–7 mL/h (96 wt.%, Russia).
2.2. Synthesis of Fe2O3/MWCNT/Al Composite Material
Pre-oxidized MWCNT/Al samples were used to prepare the Fe2O3/MWCNT/Al composite material. Electrochemical oxidation of the MWCNT/Al material was carried out in a two-electrode cell. A platinum wire served as a counter electrode. A strip of aluminum foil coated with an MWCNT layer acted as an anode during the oxidation process. A 0.005 M Na2SO4 (high purity grade, Russia) solution was used as an electrolyte. The oxidation time was 10 min at a voltage of 4 V [52]. A three-electrode electrochemical cell was used to form the Fe2O3/MWCNT/Al composite material. The MWCNT/Al sample served as the working electrode. A saturated calomel electrode was used as a reference electrode, and a pure platinum wire was used as a counter electrode.
The process was carried out using a P-40X potentiostat (Electrochemical Instruments, Chernogolovka, Russia). The electrolyte was a mixture of 0.1 M aqueous solution of Fe(NH4)2(SO4)2 (iron ammonium sulfate (special purity grade, Russia)) and 0.08 M CH3COONa (sodium acetate (special purity grade, Russia)) in a 1:1 ratio. The voltage range was from −10 to 700 mV, and the voltage sweep rate was from 2 to 100 mV/s. After the formation of the iron hydroxide layer, the samples were washed in distilled water and dried in air for 24 h. The resulting material was annealed in air at temperatures of 200, 300, and 400 °C. The heating rate for the formation of the composite material was 15 °C/min. For further studies, samples prepared as follows were selected:
- (a)
- Synthesis of MWCNTs on the surface of aluminum foil, electrochemical oxidation of MWCNTs;
- (b)
- Synthesis of MWCNTs on the surface of aluminum foil, electrochemical oxidation of MWCNTs, formation of an iron hydroxide layer on the surface of MWCNTs at a voltage sweep rate of 2 mV/s, calcinations at a temperature of 200 °C;
- (c)
- Synthesis of MWCNTs on the surface of aluminum foil, electrochemical oxidation of MWCNTs, formation of an iron hydroxide layer on the surface of MWCNTs at a voltage sweep rate of 2 mV/s, calcinations at a temperature of 300 °C;
- (d)
- Synthesis of MWCNTs on the surface of aluminum foil, electrochemical oxidation of MWCNTs, formation of an iron hydroxide layer on the surface of MWCNTs at a voltage sweep rate of 2 mV/s, calcinations at a temperature of 400 °C.
2.3. Structural Characterization
The Raman spectra of the obtained samples were studied in the range of 150–2000 cm−1 with a spectral resolution of 9–15 cm−1 using a Bruker Senterra micro-Raman system coupled to an OLYMPUS BX51 optical microscope. The spectra were recorded under excitation by a solid-state laser at a wavelength of 532 nm. The laser operating power was 5 mW. X-ray photoelectron spectra of the sample were obtained on a SPECS electron spectrometer with a FOIBOS-150 energy analyzer (SPECS GmbH, Berlin, Germany) using non-monochromatic Al Kα radiation (1486.6 eV). The spectra were measured at an anode voltage of 12.5 kV and an emission current of 19.0 mA on the XR50 X-ray source. The spectra obtained were processed using CasaXPS software (version 2.3.25PR1.0). To compare and study the morphology of the samples obtained, an AURIGA CrossBeam microscope (Carl Zeiss NTS, Germany) was used in scanning mode with an acceleration voltage of 5 kV. An attachment to the JEOL 6490 INCA Oxford Instruments electron microscope was used to study the elemental composition of the samples. The electron accelerating voltage was 15 kV, and the current was ~1 nA.
2.4. Electrochemical Measurements
Electrochemical tests on the original and modified samples (cyclic voltammetry, impedance spectroscopy) were carried out in a three-electrode cell. A saturated calomel electrode was used as the reference electrode. A platinum wire was used as the counter electrode. The working electrode was a strip of aluminum foil (0.75 × 2 cm2) coated on both sides with a composite material. A 0.5 M aqueous solution of Na2SO4 was used as the electrolyte. Electrochemical impedance measurements were carried out in the presence of a 0.5 M aqueous solution of Na2SO4 at a constant potential of 0 V, superimposed on an alternating potential with an amplitude of 20 mV in the frequency range from 50 kHz to 100 mHz. Electrochemical measurements were performed using a P-40X potentiostat equipped with an FRA-24M frequency analyzer module (Electrochemical Instruments, Chernogolovka, Russia).
3. Results and Discussion
3.1. Comparison of Particle Size and Morphology Based on the SEM Images and Discussion of the Influence of Temperature
An array of carbon nanotubes grown directly on aluminum foil can serve as a base for obtaining Fe2O3/MWCNT/Al composites with high specific capacitance values due to the pseudocapacitive properties of iron oxide. An analysis of the literature shows that the functionalization of the carbon material plays an important role in the formation of an iron oxide layer on the surface of a carbon material [53]. The technique of electrochemical oxidation in a low Na2SO4 solution allows for the optimal conditions for processing the MWCNT/Al material to be selected. It is important to select conditions for pre-oxidation of the sample that not only ensure further formation of the composite but also do not lead to the destruction of the MWCNT layer. During the experiments, it was found that the optimal processing conditions are achieved with electrochemical oxidation of MWCNT/Al samples for 10 min in 0.005 M aqueous Na2SO4 solution at an oxidation voltage of 4 V. Fe(NH4)2(SO4)2 was used as the source of iron in the formation of the composite. CH3COONa was used as a reducing agent to prevent destruction of the MWCNT layer. An iron hydroxide layer was formed on the MWCNT surface [54]. The resulting material was annealed in air at 200, 300, and 400 °C to form the Fe2O3/MWCNT/Al composite material.
To select the optimal conditions for the preparation of the composite, preliminary tests were carried out at different voltage sweep rates. Energy-dispersive X-ray spectroscopy (EDX) of the surface layer of all processed samples showed the presence of carbon, oxygen, and iron as the main components of the active layer. Data on their concentrations are given in Table 1.

Table 1.
Concentrations of elements in the active layer of Fe2O3/MWCNT/Al samples depending on the voltage sweep rate.
The results obtained show that, at a voltage sweep rate of 2 mV/s, the composite material contained the greatest amount of not only iron but also oxygen. The samples obtained at a voltage sweep rate of 2 mV/s were selected for further investigation. To convert the iron hydroxide layer to oxide, the material was annealed at 200, 300, and 400 °C. The morphology of the composite material was studied using scanning electron microscopy. Figure 1 shows the scanning electron microscopy images of carbon nanomaterials (Figure 1a) and Fe2O3/MWCNT/Al composites (Figure 1b–d). The studies were carried out on samples obtained at annealing temperatures of 200 (Figure 1b), 300 (Figure 1c), and 400 °C (Figure 1d). In all cases, we observed intertwined MWCNTs, and in the case of the composite material, the surface of the carbon nanotubes was covered with a layer of iron oxide.
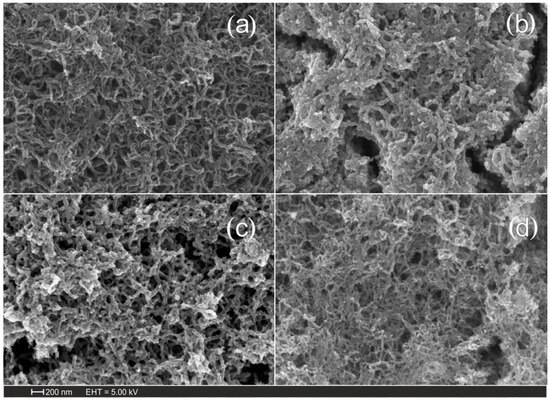
Figure 1.
SEM images of (a) MWCNT/Al, (b) 200 °C—Fe2O3/MWCNT/Al, (c) 300 °C—Fe2O3/MWCNT/Al, and (d) 400 °C—Fe2O3/MWCNT/Al.
The chemical structure of the composite material was studied using Raman spectroscopy and X-ray photoelectron spectroscopy. Figure 2 shows the Raman spectra for the MWCNT/Al (1) and Fe2O3/MWCNT/Al (2–4) composite material samples.
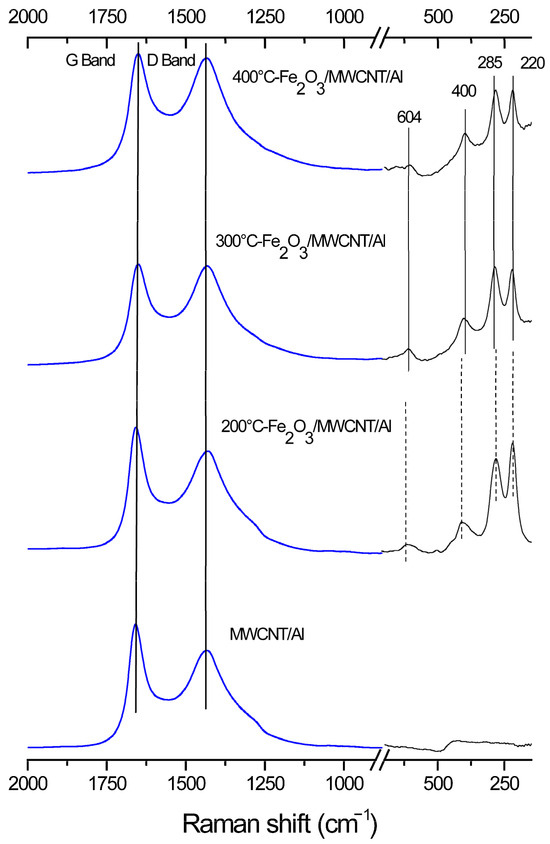
Figure 2.
Raman shifts of MWCNT/Al and Fe2O3/MWCNT/Al (200, 300, and 400 °C).
All spectra show two broad peaks at about 1335 and 1600 cm−1 [55], which are attributed to MWCNTs (D and G, respectively). Band G corresponds to the vibrations of the hexagonal crystal lattice of carbon, and band D characterizes the disorder of the MWCNT structure [56]. Since hematite belongs to the crystal space group D6 3d, the phonon modes A1g (220 cm−1) and Eg (285, 400 and 604 cm−1) can be distinguished in the spectra obtained [57]. The results show that three samples are covered with a layer of hematite. However, for the composite obtained at 200 °C, the decrease in the intensity of peak Eg1 (400 cm−1) to the intensity of peak Eg (285 cm−1), as well as a slight shift in peaks A1g and Eg (285 cm−1) towards the low-frequency region, may indicate the less perfect crystalline structure of the material [58]. This may also be indicated by a change in the ratio of the intensities of peaks A1g and Eg (285 cm−1) [59].
Typical XPS spectra are shown in Figure 3. The main intense lines in the survey spectrum with AlKα excitation are the series of iron, carbon, and oxygen spectra.
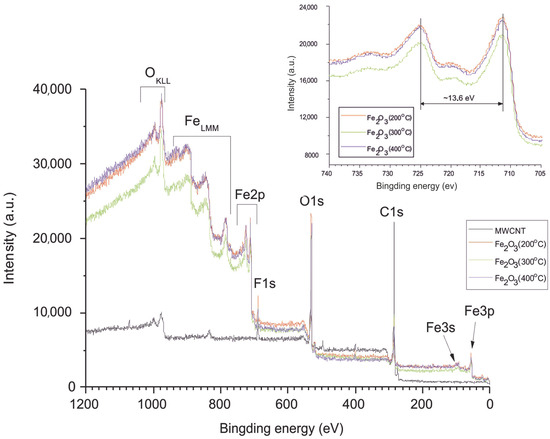
Figure 3.
Long scan of the XPS spectrum with the inset showing the high-resolution Fe2p region of MWCNT/Al and Fe2O3/MWCNT/Al (200, 300, and 400 °C).
The high-resolution spectrum of Fe2p (Figure 3, inset) shows, for all iron-containing samples, two distinct peaks (Fe 2p 3/2 and 2p 1/2) separated by a broad satellite peak at 711.4 and 725 eV, respectively. The separation of the spin energies of Fe 2p 3/2 and 2p 1/2 is 13.6 eV, indicating that Fe in the composite is in the Fe3+ state [60]. Figure 4 and Figure 5 show the high-resolution XPS spectra of carbon (C1s) and oxygen (O1s). As can be seen from Figure 4, the spectra of carbon (C1s) are multi-component [61]. For sample 1, the spectrum corresponds to lightly oxidized carbon. The carbon in samples 2-4 is more oxidized. The C1s spectrum can be resolved into three peaks centered at 284.6 eV (C-C), 286.2 eV (C-O), and 288.6 eV (C=O) [62].
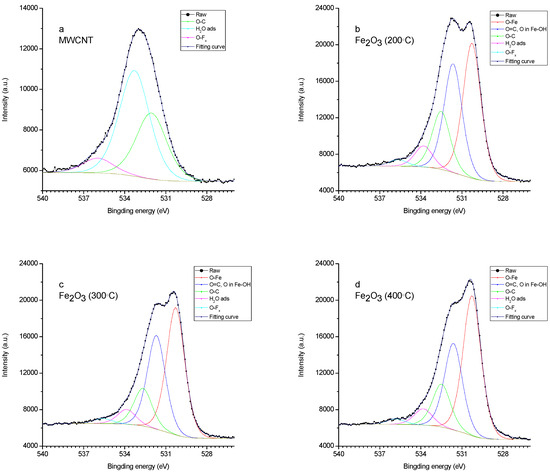
Figure 4.
XPS spectrum (C1s) of (a) MWCNT/Al, (b) 200 °C—Fe2O3/MWCNT/Al, (c) 300 °C—Fe2O3/MWCNT/Al, and (d) 400 °C—Fe2O3/MWCNT/Al.
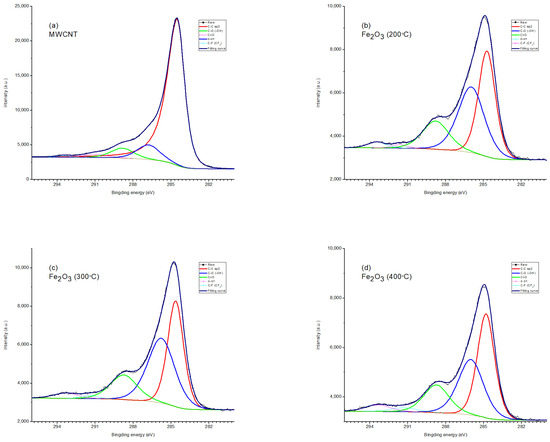
Figure 5.
XPS spectrum (O1s) of (a) MWCNT/Al, (b) 200 °C—Fe2O3/MWCNT/Al, (c) 300 °C—Fe2O3/MWCNT/Al, and (d) 400 °C—Fe2O3/MWCNT/Al.
The oxygen spectrum is also multi-component (Figure 5). In sample 1, the main contribution comes from the bond lines with carbon and hydrogen in the water adsorbed by the sample. In samples 2-4, there are two lines (530.2 eV and 531.7 eV) associated with iron in the oxide and hydroxide [63]. In the sample obtained at an annealing temperature of 200 °C, the line corresponding to the hydroxide is more pronounced.
Based on the obtained data, it can be concluded that, with all methods of forming a composite material, a layer of Fe2O3 is formed on the surface of the carbon nanotube array. The optimal calcination stage is 300 °C.
3.2. Study of the Electrochemical Properties of Fe2O3/MWCNT/Al Composites
A series of electrochemical measurements were carried out to investigate the possibility of using the obtained Fe2O3/MWCNT/Al material as supercapacitor electrodes. The ability of a system to accumulate charge is usually determined by the specific capacitance (Cspm) of the electrode materials and is calculated from data obtained with cyclic voltammetry methods [64]. A common unit of measurement for specific capacitance is F/g. The specific capacitance for the CV method is calculated using Equation (1).
Cspm = ∫ I d V: (ΔV × v × mel)
In expression (1), ∫IdV is the total area under the CV curve, i.e., the accumulated charge; ΔV is the voltage range (V); ν is the scan rate (V/s); and mel is the mass of the active material on the working surface of the electrode.
The cyclic voltammetry method showed a significant increase in the capacitance of the MWCNT/Al samples after the formation of the Fe2O3/MWCNT/Al composite material. Figure 6 shows examples of cyclic voltammograms for the initial MWCNT/Al samples, the electrochemically oxidized samples, and the composite material obtained at different voltage sweep rates.
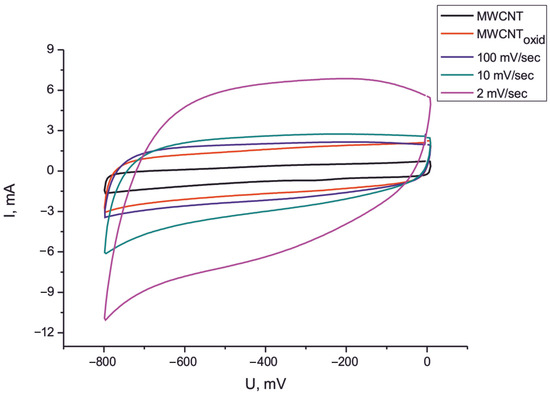
Figure 6.
CVs of initial electrode (1), oxidized sample (2), Fe2O3/MWCNT/Al composite (100 mV/s) (3), Fe2O3/MWCNT/Al composite (10 mV/s), and (4) Fe2O3/MWCNT/Al composite (2 mV/s).
From the plots shown in Figure 6, it is clear that the specific capacitance of the composite material Fe2O3/MWCNT/Al obtained at different voltage sweep rates (100, 10, and 2 mV/s) is significantly higher than that of the original MWCNT/Al sample. From the CV results, it can be concluded that the maximum increase in specific capacitance was achieved for the sample obtained at a voltage sweep rate of 2 mV/s (175 F/g at a scan rate of 100 mV/s and 230 F/g at a scan rate of 2 mV/s). No significant increase in specific capacitance was observed as the voltage sweep rate was increased. At 10 mV/s, the specific capacitance increased by a factor of about 3, and at 100 mV/s, it increased by a factor of about 2. The increase in the specific capacitance of the composite material is due to the pseudocapacitive properties of the iron oxide. Redox reactions take place at the electrode surface. Since an aqueous solution of sodium sulfate is used as the electrolyte, the charge storage mechanism can be described with the following equations [65,66,67,68]:
(Fe2O3)surf + Na+ + e− ‹–› (Fe2O3−, Na+)surf
(Fe2O3−, Na+)surf + Na+ + e− ‹–› (Fe2O32−, 2Na+)surf
Fe2O3 + 2OH− = Fe2O3 (OH)2 + 2e−
Fe2O3 + 2e− + 3H2O = 2Fe (OH)2 + 2OH−
The electrochemical properties of the Fe2O3/MWCNT/Al electrodes were further investigated using electrochemical impedance spectroscopy. Figure 7a,b show Nyquist plots of the original MWCNT/Al sample, the pre-oxidized MWCNT/Al sample, and the Fe2O3/MWCNT/Al sample formed at a voltage sweep rate of 2 mV/s. The use of a Randles-like equivalent circuit is optimal for the obtained material [69,70,71].
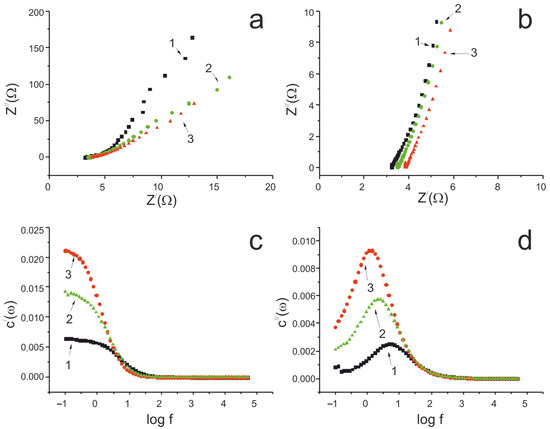
Figure 7.
(a) Nyquist plots for a three-electrode cell (full range); (b) Nyquist plots for a three-electrode cell (middle range); (c) frequency dependence of real capacitance; and (d) frequency dependence of imaginary capacitance. As-prepared MWCNT/Al sample—1; oxidized MWCNT/Al sample—2; and Fe2O3/MWCNT/Al sample—3.
In the low-frequency range, the Nyquist plots for the initial, oxidized, and composite Fe2O3/MWCNT/Al samples were almost linear, indicating good capacitive characteristics (Figure 7a). The ohmic resistance of the initial MWCNT/Al sample was 3.2 ohms, which increased to 3.5 ohms after electrochemical oxidation in a Na2SO4 solution. Further deposition of Fe2O3 on the MWCNT surface did not significantly increase the ohmic resistance (3.85 ohms). As a result, the Fe2O3 in the Fe2O3/MWCNT/Al composite electrode has good electrical contact with the conductive substrate. At the same time, the slope of the Nyquist plot for the Fe2O3/MWCNT/Al sample decreased in the low-frequency region compared to the original MWCNT/Al sample (Figure 7a), which can be explained by the pseudocapacitive properties of Fe2O3.
The frequency dependence of the actual capacitance confirms an increase in the electrode capacitance of about 1.5 times after electrochemical oxidation and about 5 times after Fe2O3 deposition (Figure 7c). This study showed that, during the anodic oxidation of MWCNTs, the increase in the specific capacity of the material is due to the increase in oxygen-containing groups covalently bonded to the surface of the nanotubes. Another reason is the increase in the porosity and specific surface area of the MWCNTs resulting from the electrochemical etching of the nanotube surface [52]. The specific capacitance of the Fe2O3/MWCNT/Al composite increases due to the oxidation reduction reactions taking place on the surface of electrodes (2) and (3). The imaginary capacitance dependence curves have maxima, the position of which characterizes the charge/discharge rate. The τr values for the initial MWCNT/Al electrode, the oxidized sample, and the final Fe2O3/MWCNT/Al sample were 0.22 s, 0.48 s, and 0.88 s, respectively. Pseudocapacitance limits rapid charge/ion transfer and results in a long response time. Therefore, the Fe2O3/MWCNT/Al electrode has a higher τr value of 0.88 s. However, when applied to pseudocapacitors, this value indicates the very good charge/discharge characteristics of the resulting binder-free composite electrodes [72].
The galvanostatic charge/discharge (GCD) test is one of the most reliable methods for assessing specific capacity. Figure 8 shows the results of measuring the specific capacity for composite materials obtained at the calcination stages of 200, 300, and 400 °C. The specific capacity for this method is calculated using the following formula [64]:
Cspm = I/((dV/dt) mel)
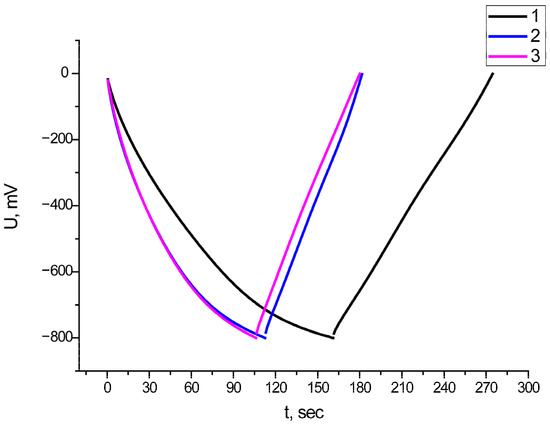
Figure 8.
GCD of initial electrode: 1—composite obtained at a temperature of 200 °C; 2—composite obtained at a temperature of 300 °C; and 3—composite obtained at a temperature of 400 °C.
In expression (6), I is the applied current, dV/dt is the slope of the discharge curve, and mel is the mass of the active material on the working surface of the electrode.
The average specific capacitance value for all composite materials was about 125 F/g at a current density of 2 A/g. The Coulomb efficiency was 70% for the composite material Fe2O3/MWCNT/Al obtained at the calcination stages of 200 and 400 °C, and it was 60% for the composite material Fe2O3/MWCNT/Al obtained at the calcination stage of 300 °C.
An important feature of SC electrodes is their resistance to multiple charge/discharge cycles. A series of measurements were taken at different calcination steps. Figure 8 shows the resistance of the Fe2O3/MWCNT/Al composite to multiple charge/discharge cycles as a function of annealing temperature.
Figure 9 shows that the Fe2O3/MWCNT/Al composite exhibited the highest cycle resistance at an annealing temperature of 300 °C. These results suggest that annealing temperatures below 300 °C may not be sufficient to convert iron hydroxide to oxide. On the other hand, the MWCNT layer can be destroyed at a temperature of 400 °C.
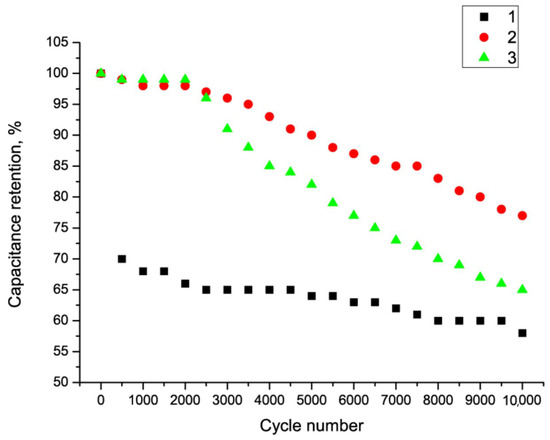
Figure 9.
Capacitance evolution of Fe2O3/MWCNT/Al composite during multiple charge–discharge cycles as a function of annealing temperature: 1—composite obtained at a temperature of 200 °C; 2—composite obtained at a temperature of 300 °C; and 3—composite obtained at a temperature of 400 °C.
As noted in the Introduction, many papers devoted to the study of supercapacitors made on the basis of carbon materials and iron oxide using various methods have been published. Therefore, it is interesting to compare the results of this work with previously published ones. Table 2 shows the literature data for various composite materials based on iron oxide.

Table 2.
Literary data. Parameter formation for composite material and corresponding electrochemical capacity values.
It is evident that the values of the specific capacity of the composite materials we obtained are comparable to published values. At the same time, the composite material is highly resistant to numerous charge/discharge cycles. Undoubtedly, the main advantage of the method of direct formation of a composite material on a metal substrate is the possibility of obtaining a ready-to-use material for flexible electrodes of a supercapacitor.
4. Conclusions
In summary, a method for preparing a binder-free Fe2O3/MWCNT/Al composite electrode has been developed. For the first time, conditions for the electrochemical oxidation of MWCNTs grown directly on the surface of aluminum foil were selected to allow for the formation of an Fe2O3/MWCNT/Al composite. Fe2O3/MWCNT/Al electrodes without a binder were obtained via electrochemical oxidation of MWCNT/Al samples in a mixture of aqueous solutions (Fe(NH4)2(SO4)2 0.1 M and CH3COONa 0.08 M). Electrochemical oxidation of MWCNT/Al samples in an aqueous solution (Fe(NH4)2(SO4)2 0.1 M and CH3COONa 0.08 M) at a voltage sweep rate of 2 mV/s, followed by annealing at 300 °C, increases the specific capacitance of the active material of the samples by a factor of 5 to 175 F/g. At the same time, the studied material maintains excellent adhesion and electrical contact with the aluminum substrate. The Fe2O3/MWCNT/Al electrodes obtained have a high resistance to multiple charge/discharge cycles. After 10,000 cycles, the capacitance loss does not exceed 25%. The resulting material can be used as a ready-made flexible electrode (cathode) for a supercapacitor.
Author Contributions
Conceptualization, A.N.R.; methodology, A.A.M.; investigation, A.A.M., E.E.Y., M.A.K., and V.I.K.; writing—original draft preparation, A.A.M.; writing—review and editing, A.A.M., E.E.Y., M.A.K., and V.I.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, via grant number 24-22-00240.
Data Availability Statement
Dataset available on request from the authors: The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to the Data Availability Statement. This change does not affect the scientific content of the article.
References
- Dissanayake, K.; Kularatna-Abeywardana, D. A review of supercapacitors: Materials, technology, challenges, and renewable energy applications. J. Energy Storage 2024, 96, 112563. [Google Scholar] [CrossRef]
- Blaabjerg, F.; Ionel, D.M. Renewable energy devices and systems–state-of-the-art technology, research and development, challenges and future trends. Electr. Power Compon. Syst. 2015, 43, 1319–1328. [Google Scholar] [CrossRef]
- Zeng, T.; Meng, L.; Cheng, L.; Wang, R.; Ran, Z.; Liu, D.; Fu, J.; He, J.; Zhou, Q.; Li, Q.; et al. Scalable Hybrid Films of Polyimidesdle.; Ch Quantum Dots for Hightume.; Cheng Capacitive Energy Storage Utilizing Quantum Confinement Effect. Adv. Funct. Mater. 2024, 35, 2419278. [Google Scholar] [CrossRef]
- Zhan, Y.; Ren, X.; Zhao, S.; Guo, Z. Enhancing prediction of electron affinity and ionization energy in liquid organic electrolytes for lithium-ion batteries using machine learning. J. Power Sources 2025, 629, 235992. [Google Scholar] [CrossRef]
- Chen, X.; Wei, S.; Wang, J.; Tong, F.; Söhnel, T.; Waterhouse, G.I.; Zhang, W.; Kennedy, J.; Taylor, M.P. Lithium insertion/extraction mechanism in Mg2Sn anode for lithium-ion batteries. Intermetallics 2024, 169, 108306. [Google Scholar] [CrossRef]
- Cao, M.; Chen, W.; Ma, Y.; Huang, H.; Luo, S.; Zhang, C. Cross-linked K2Ti4O9 nanoribbon arrays with superior rate capability and cyclability for lithium-ion batteries. Mater. Lett. 2020, 279, 128495. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, M.; Chen, X. Supercapacitors for renewable energy applications: A review. Micro Nano Eng. 2023, 21, 100229. [Google Scholar] [CrossRef]
- Ariyarathna, T.; Kularatna, N.; Gunawardane, K.; Jayananda, D.; Steyn-Ross, D.A. Development of supercapacitor technology and its potential impact on new power converter techniques for renewable energy. IEEE J. Emerg. Sel. Top. Ind. Electron. 2021, 2, 267–276. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abbas, Q.; Al Makky, A.; Abdelkareem, M.A. Supercapacitors as next generation energy storage devices: Properties and applications. Energy 2022, 248, 123617. [Google Scholar] [CrossRef]
- Attia, S.Y.; Mohamed, S.G.; Barakat, Y.F.; Hassan, H.H.; Zoubi, W.A. Supercapacitor electrode materials: Addressing challenges in mechanism and charge storage. Rev. Inorg. Chem. 2022, 42, 53–88. [Google Scholar] [CrossRef]
- Gao, L.; Cao, M.; Zhang, C.; Li, J.; Zhu, X.; Guo, X.; Toktarbay, Z. Zinc selenide/cobalt selenide in nitrogen-doped carbon frameworks as anode materials for high-performance sodium-ion hybrid capacitors. Adv. Compos. Hybrid Mater. 2024, 7, 144. [Google Scholar] [CrossRef]
- Yadav, M.S. Metal oxides nanostructure-based electrode materials for supercapacitor application. J. Nanoparticle Res. 2020, 22, 367. [Google Scholar] [CrossRef]
- Ansari, M.Z.; Seo, K.M.; Kim, S.H.; Ansari, S.A. Critical aspects of various techniques for synthesizing metal oxides and fabricating their composite-based supercapacitor electrodes: A review. Nanomaterials 2022, 12, 1873. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhu, M.; Liu, J.; Li, X.; Liu, J. Flexible asymmetric supercapacitor with high energy density based on optimized MnO2 cathode and Fe2O3 anode. Chin. Chem. Lett. 2018, 30, 750–756. [Google Scholar] [CrossRef]
- Phakkhawan, A.; Suksangrat, P.; Srepusharawoot, P.; Ruangchai, S.; Klangtakai, P.; Pimanpang, S.; Amornkitbamrung, V. Reagent-and solvent-mediated Fe2O3 morphologies and electrochemical mechanism of Fe2O3 supercapacitors. J. Alloys Compd. 2022, 919, 165702. [Google Scholar] [CrossRef]
- Shivakumara, S.; Penki, T.R.; Munichandraiah, N. High specific surface area α-Fe2O3 nanostructures as high performance electrode material for supercapacitors. Mater. Lett. 2014, 131, 100–103. [Google Scholar] [CrossRef]
- Azimov, F.; Kim, J.; Choi, S.M.; Jung, H.M. Synergistic Effects of Fe2O3 Nanotube/Polyaniline Composites for an Electrochemical Supercapacitor with Enhanced Capacitance. Nanomaterials 2021, 11, 1557. [Google Scholar] [CrossRef]
- Nithya, V.D.; Arul, N.S. Progress and development of Fe3O4 electrodes for supercapacitors. J. Mater. Chem. A 2016, 4, 10767–10778. [Google Scholar] [CrossRef]
- Barik, R.; Jena, B.K.; Mohapatra, M. Metal doped mesoporous FeOOH nanorods for high performance supercapacitors. RSC Adv. 2017, 7, 49083–49090. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, D.; Ran, G.; Song, Q.; Masson, J.F. Controllable design of polycrystalline synergies: Hybrid FeOx nanoparticles applicable to electrochemical sensing antineoplastic drug in mammalian cells. Sens. Actuators B Chem. 2018, 275, 1–9. [Google Scholar] [CrossRef]
- Kumbhar, V.S.; Jagadale, A.D.; Shinde, N.M.; Lokhande, C.D. Chemical synthesis of spinel cobalt ferrite (CoFe2O4) nano-flakes for supercapacitor application. Appl. Surf. Sci. 2012, 259, 39–43. [Google Scholar] [CrossRef]
- Owusu, K.A.; Qu, L.; Li, J.; Wang, Z.; Zhao, K.; Yang, C.; Hercule, K.M.; Lin, C.; Shi, C.; Wei, Q.; et al. Low-crystalline iron oxide hydroxide nanoparticle anode for high-performance supercapacitors. Nat. Commun. 2017, 8, 14264. [Google Scholar] [CrossRef] [PubMed]
- Lorkit, P.; Panapoy, M.; Ksapabutr, B. Iron oxide-based supercapacitor from ferratrane precursor via sol–gel-hydrothermal process. Energy Procedia 2014, 56, 466–473. [Google Scholar] [CrossRef]
- Yadav, A.A.; Hunge, Y.M.; Ko, S.; Kang, S.W. Chemically synthesized iron-oxide-based pure negative electrode for solid-state asymmetric supercapacitor devices. Materials 2022, 15, 6133. [Google Scholar] [CrossRef]
- Zeng, Y.; Yu, M.; Meng, Y.; Fang, P.; Lu, X.; Tong, Y. Iron-based supercapacitor electrodes: Advances and challenges. Adv. Energy Mater. 2016, 6, 1601053. [Google Scholar] [CrossRef]
- Xu, B.; Zheng, M.; Tang, H.; Chen, Z.; Chi, Y.; Wang, L.; Zhang, L.; Chen, Y.; Pang, H. Iron oxide-based nanomaterials for supercapacitors. Nanotechnology 2019, 30, 204002. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Zhang, B.; Han, D.; Gong, Z.; Li, X. Fe2O3/Porous Carbon Composite Derived from Oily Sludge Waste as an Advanced Anode Material for Supercapacitor Application. Nanomaterial 2022, 12, 3819. [Google Scholar] [CrossRef]
- Jiang, S.H.; Ding, J.; Wang, R.H.; Chen, F.Y.; Sun, J.; Deng, Y.X.; Li, X.L. Solvothermal-induced construction of ultra-tiny Fe2O3 nanoparticles/graphene hydrogels as binder-free high-capacitance anode for supercapacitors. Rare Met. 2021, 40, 3520–3530. [Google Scholar] [CrossRef]
- Xia, Q.; Xia, T.; Wu, X. PPy decorated α-Fe2O3 nanosheets as flexible supercapacitor electrodes. Rare Met. 2022, 41, 1195–1201. [Google Scholar] [CrossRef]
- Samuel, E.; Aldalbahi, A.; El-Newehy, M.; El-Hamshary, H.; Yoon, S.S. Flexible and freestanding manganese/iron oxide carbon nanofibers for supercapacitor electrodes. Ceram. Int. 2022, 48, 18374–18383. [Google Scholar] [CrossRef]
- Ishaq, S.; Moussa, M.; Kanwal, F.; Ayub, R.; Van, T.N.; Azhar, U.; Losic, D. One step strategy for reduced graphene oxide/cobalt-iron oxide/polypyrrole nanocomposite preparation for high performance supercapacitor electrodes. Electrochim. Acta 2022, 427, 140883. [Google Scholar] [CrossRef]
- Hsiao, C.; Lee, C.; Tai, N. Reduced graphene oxide/oyster shell powers/iron oxide composite electrode for high performance supercapacitors. Electrochim. Acta 2021, 391, 138868. [Google Scholar] [CrossRef]
- Tundwal, A.; Kumar, H.; Binoj, B.J.; Sharma, R.; Kumar, G.; Kumari, R.; Dhayal, A.; Yadav, A.; Singh, D.; Kumar, P. Developments in conducting polymer-, metal oxide-, and carbon nanotube-based composite electrode materials for supercapacitors: A review. RSC Adv. 2024, 14, 9406–9439. [Google Scholar] [CrossRef]
- Baby, A.; Vigneswaran, J.; Jose, S.P.; Davis, D.; PB, S. Hybrid architecture of Multiwalled carbon nanotubes/nickel sulphide/polypyrrole electrodes for supercapacitor. Mater. Today Sustain. 2024, 26, 100727. [Google Scholar] [CrossRef]
- Gerard, O.; Ramesh, S.; Ramesh, K.; Numan, A.; Khalid, M.; Tiong, S.K. Fast and green synthesis of battery-type nickel-cobalt phosphate (NxCyP) binder-free electrode for supercapattery. Chem. Eng. J. 2024, 497, 154842. [Google Scholar] [CrossRef]
- Kumbhar, M.B.; Patil, V.V.; Chandak, V.S.; Gunjakar, J.L.; Kulal, P.M. Enhancing energy storage with binder-free nickel oxide cathodes in flexible hybrid asymmetric solid-state supercapacitors. J. Alloys Compd. 2025, 1010, 177311. [Google Scholar] [CrossRef]
- Avasthi, P.; Kumar, A.; Balakrishnan, V. Aligned CNT forests on stainless steel mesh for flexible supercapacitor electrode with high capacitance and power density. ACS Appl. Nano Mater. 2019, 2, 1484–1495. [Google Scholar] [CrossRef]
- Hussain, S.; Amade, R.; Moreno, H.; Bertran, E. RF-PECVD growth and nitrogen plasma functionalization of CNTs on copper foil for electrochemical applications. Diam. Relat. Mater 2014, 49, 55–61. [Google Scholar] [CrossRef]
- Vicentini, R.; Costa, L.H.; Nunes, W.; Vilas Boas, O.; Soares, D.M.; Alves, T.A.; Real, C.; Bueno, C.; Peterlevitz, A.C.; Zanin, H. Direct growth of mesoporous Carbon on aluminum foil for supercapacitors devices. J. Mater. Sci. Mater. Electron. 2018, 29, 10573–10582. [Google Scholar] [CrossRef]
- Ghai, V.; Chatterjee, K.; Agnihotri, P.K. Vertically aligned carbon nanotubes-coated aluminium foil as flexible supercapacitor electrode for high power applications. Carbon Lett. 2021, 31, 473–481. [Google Scholar] [CrossRef]
- Das, H.T.; Dutta, S.; Balaji, T.E.; Das, N.; Das, P.; Dheer, N.; Kanojia, R.; Ahuja, P.; Ujjain, S.K. Recent trends in carbon nanotube electrodes for flexible supercapacitors: A review of smart energy storage device assembly and performance. Chemosensors 2022, 10, 223. [Google Scholar] [CrossRef]
- Kanwade, A.; Shirage, P.M. A review on synergy of transition metal oxide nanostructured materials: Effective and coherent choice for supercapacitor electrodes. J. Energy Storage 2022, 55, 105692. [Google Scholar] [CrossRef]
- Liu, R.; Zhou, A.; Zhang, X.; Mu, J.; Che, H.; Wang, Y.; Wang, T.T.; Zhang, Z.; Kou, Z. Fundamentals, advances and challenges of transition metal compounds-based supercapacitors. Chem. Eng. J. 2021, 412, 128611. [Google Scholar] [CrossRef]
- Greenwood, D.; Lim, K.; Patsios, C.; Lyons, P.; Lim, Y.; Taylor, P. Frequency response services designed for energy storage. Appl. Energy 2017, 203, 115–127. [Google Scholar] [CrossRef]
- Tian, Y.; Cai, Y.; Chen, Y.; Jia, M.; Hu, H.; Xie, W.; Li, D.; Song, H.; Guo, S.; Zhang, X. Accessing the O Vacancy with Anionic Redox Chemistry Toward Superior Electrochemical Performance in O3 type Na-Ion Oxide Cathode. Adv. Funct. Mater. 2024, 34, 2316342. [Google Scholar] [CrossRef]
- Li, X.; Xiong, S.; Li, G.; Xiao, S.; Zhang, C.; Ma, Y. Effect of microstructure on electrochemical performance of electrode materials for microsupercapacitor. Mater. Lett. 2023, 346, 134481. [Google Scholar] [CrossRef]
- Minakshi, M.; Aughterson, R.; Sharma, P.; Sunda, A.P.; Ariga, K.; Shrestha, L.K. Micelle-Assisted Electrodeposition of γ-MnO2 on Lead Anodes: Structural and Electrochemical Insights. ChemNanoMat, 2025; in press. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Xiao, J.; Tian, X.; Yuan, S. Enhancing electrochemical performance of ultrasmall Fe2O3-embedded carbon nanotubes via combusting-induced high-valence dopants. J. Mater. Sci. Technol. 2023, 134, 142–150. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, W.; Wang, R.; Sun, J.; Gao, L. Controllable synthesis of 3D binary nickel-cobalt hydroxide/graphene/nickel foam as a binder-free electrode for high-performance supercapacitors. J. Mater. Chem. A 2015, 3, 12530–12538. [Google Scholar] [CrossRef]
- Wang, J.; Li, C.; Yang, Z.; Chen, D. Chemical vapor deposition-assisted fabrication of a graphene-wrapped MnO/carbon nanofibers membrane as a high-rate and long-life anode for lithium ion batteries. RSC Adv. 2017, 7, 50973–50980. [Google Scholar] [CrossRef]
- Redkin, A.N.; Mitina, A.A.; Yakimov, E.E. Simple technique of multiwalled carbon nanotubes growth on aluminum foil for supercapacitors. Mater. Sci. Eng. B 2021, 272, 115342. [Google Scholar] [CrossRef]
- Redkin, A.N.; Mitina, A.A.; Yakimov, E.E.; Kabachkov, E.N. Electrochemical Improvement of the MWCNT/Al Electrodes for Supercapacitors. Materials 2021, 14, 7612. [Google Scholar] [CrossRef]
- Meng, X.; Huang, J.; Zhu, G.; Xu, Y.; Zhu, S.; Li, Q.; Chen, M.; Lin, M.C. Fe2O3 nanoparticles anchored on thermally oxidized MWCNTs as anode material for lithium-ion battery. Nanotechnology 2022, 34, 015602. [Google Scholar] [CrossRef]
- Rokade, A.; Jadhav, Y.; Jathar, S.; Rahane, S.; Barma, S.; Rahane, G.; Thawarkar, S.; Vairale, P.; Punde, A.; Shah, S.; et al. Realization of electrochemically grown a-Fe2O3 thin films for photoelectrochemical water splitting application. Eng. Sci. 2021, 17, 242–255. [Google Scholar] [CrossRef]
- Roy, D.; Kanojia, S.; Mukhopadhyay, K.; Eswara Prasad, N. Analysis of carbon-based nanomaterials using Raman spectroscopy: Principles and case studies. Bull. Mater. Sci. 2021, 44, 31. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Wang, C.; Zhang, Y.; Xu, L.; Zhu, K.; Xie, S. Raman Characterization of Aligned Carbon Nanotubes Produced by Thermal Decomposition of Hydrocarbon Vapor. Appl. Phys. Lett 1997, 70, 2684. [Google Scholar] [CrossRef]
- De Faria, D.L.A.; Venâncio Silva, S.; de Oliveira, M.T. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Lohaus, C.; Steinert, C.; Brötz, J.; Klein, A.; Jaegermann, W. Systematic investigation of the electronic structure of hematite thin films. Adv. Mater. Interfaces 2017, 4, 1700542. [Google Scholar] [CrossRef]
- Marshall, C.P.; Stockdale, G.; Carr, C.A. Raman Spectroscopy of Geological Varieties of Hematite of Varying Crystallinity and Morphology. J. Raman Spectrosc. 2025, 56, 590–597. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Fang, D. A review on C1s XPS-spectra for some kinds of carbon materials. Fuller. Nanotub. Carbon Nanostructures 2020, 28, 1048–1058. [Google Scholar] [CrossRef]
- Orisekeh, K.; Singh, B.; Olanrewaju, Y.; Kigozi, M.; Ihekweme, G.; Umar, S.; Anye, V.; Bello, A.; Parida, S.; Soboyejo, W.O. Processing of α-Fe2O3 nanoparticles on activated carbon cloth as binder-free electrode material for supercapacitor energy storage. J. Energy Storage 2021, 33, 102042. [Google Scholar] [CrossRef]
- Li, M.; He, H. Study on electrochemical performance of multi-wall carbon nanotubes coated by iron oxide nanoparticles as advanced electrode materials for supercapacitors. Vacuum 2017, 143, 371–379. [Google Scholar] [CrossRef]
- Stoller, M.D.; Ruoff, R.S. Best practice methods for determining an electrode material’s performance for ultracapacitors. Energy Environ. Sci. 2010, 3, 1294–1301. [Google Scholar] [CrossRef]
- Abdi, A.; Trari, M. Investigation on photoelectrochemical and pseudo-capacitance properties of the non-stoichiometric hematite α-Fe2O3 elaborated by sol–gel. Electrochim. Acta 2013, 111, 869–875. [Google Scholar] [CrossRef]
- Toupin, M.; Brousse, T.; Bélanger, D. Charge storage mechanism of MnO2 electrode used in aqueous electrochemical capacitor. Chem. Mater. 2004, 16, 3184–3190. [Google Scholar] [CrossRef]
- Aldalbahi, A.; Samuel, E.; Alotaibi, B.S.; El-Hamshary, H.; Yoon, S.S. Reduced graphene oxide supersonically sprayed on wearable fabric and decorated with iron oxide for supercapacitor applications. J. Mater. Sci. Technol. 2021, 82, 47–56. [Google Scholar] [CrossRef]
- Anjana, R.; Hanamantrao, D.P.; Banu, G.N.; Raja, V.; Isaac, R.R.; John, J.S.; Vediappan, K.; Jose, S.P.; Neppolian, B.; Sajan, D. Hydrothermal synthesis of graphitic carbon nitride/Ce doped Fe2O3 heterostructures for supercapattery device and hydrogen evolution reaction. J. Energy Storage 2025, 116, 116021. [Google Scholar] [CrossRef]
- Moya, A.A. Identification of characteristic time constants in the initial dynamic response of electric double layer capacitors from high-frequency electrochemical impedance. J. Power Sources 2018, 397, 124–133. [Google Scholar] [CrossRef]
- Devillers, N.; Jemei, S.; Péra, M.C.; Bienaimé, D.; Gustin, F. Review of characterization methods for supercapacitor modeling. J. Power Sources 2014, 246, 596–608. [Google Scholar] [CrossRef]
- Mainka, J.; Gao, W.; He, N.; Dillet, J.; Lottin, O. A General Equivalent Electrical Circuit Model for the characterization of MXene/graphene oxide hybrid-fiber supercapacitors by electrochemical impedance spectroscopy–Impact of fiber length. Electrochim. Acta 2022, 404, 139740. [Google Scholar] [CrossRef]
- Perdana, M.Y.; Johan, B.A.; Abdallah, M.; Hossain, M.E.; Aziz, M.A.; Baroud, T.N.; Drmosh, Q.A. Understanding the Behavior of Supercapacitor Materials via Electrochemical Impedance Spectroscopy: A Review. Chem. Rec. 2024, 24, e202400007. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).