Quantum Diamond Microscopy of Individual Vaterite Microspheres Containing Magnetite Nanoparticles
Abstract
1. Introduction
2. Experimental Methods
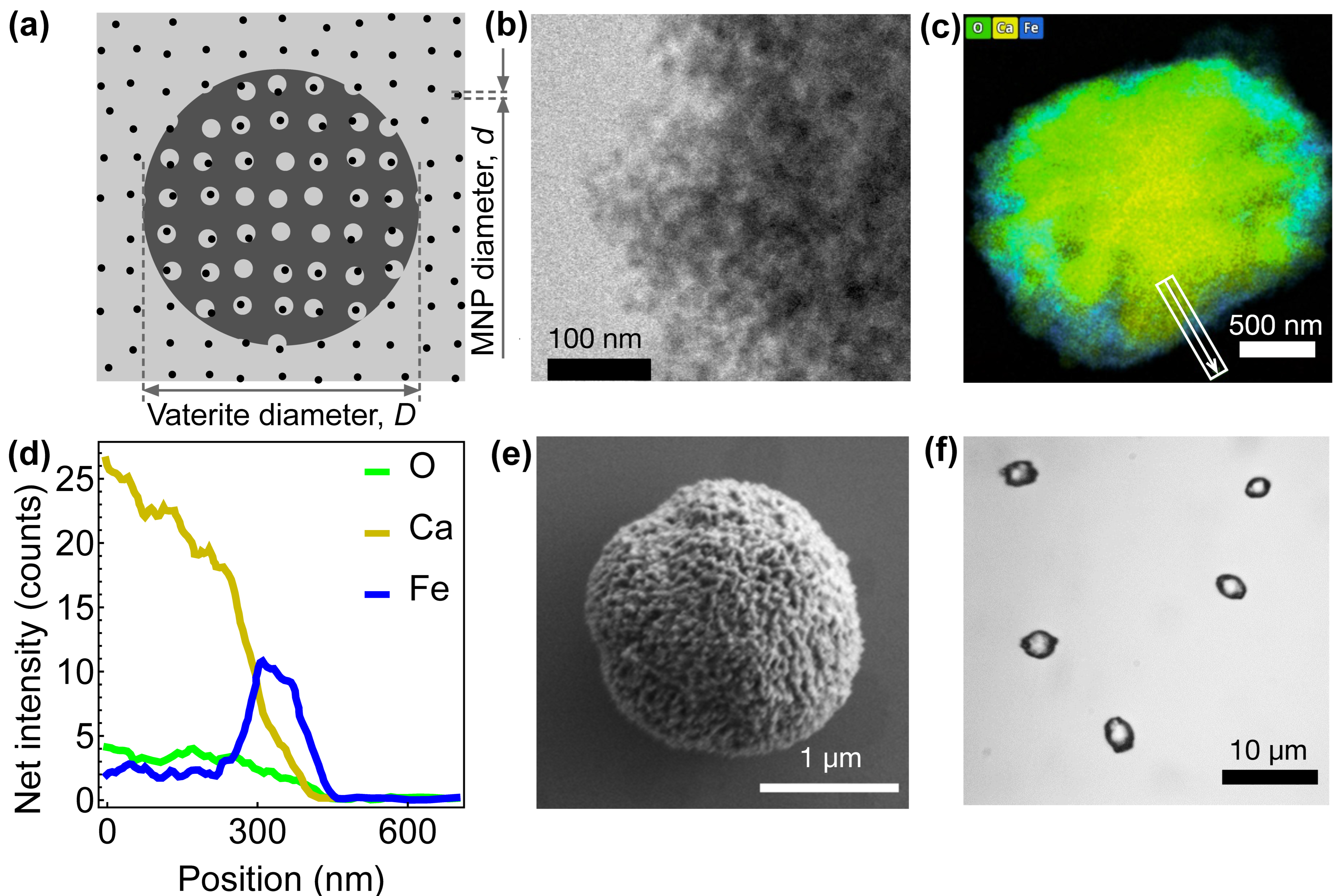
2.1. Synthesis of Fe3O4-Loaded Vaterite Microspheres
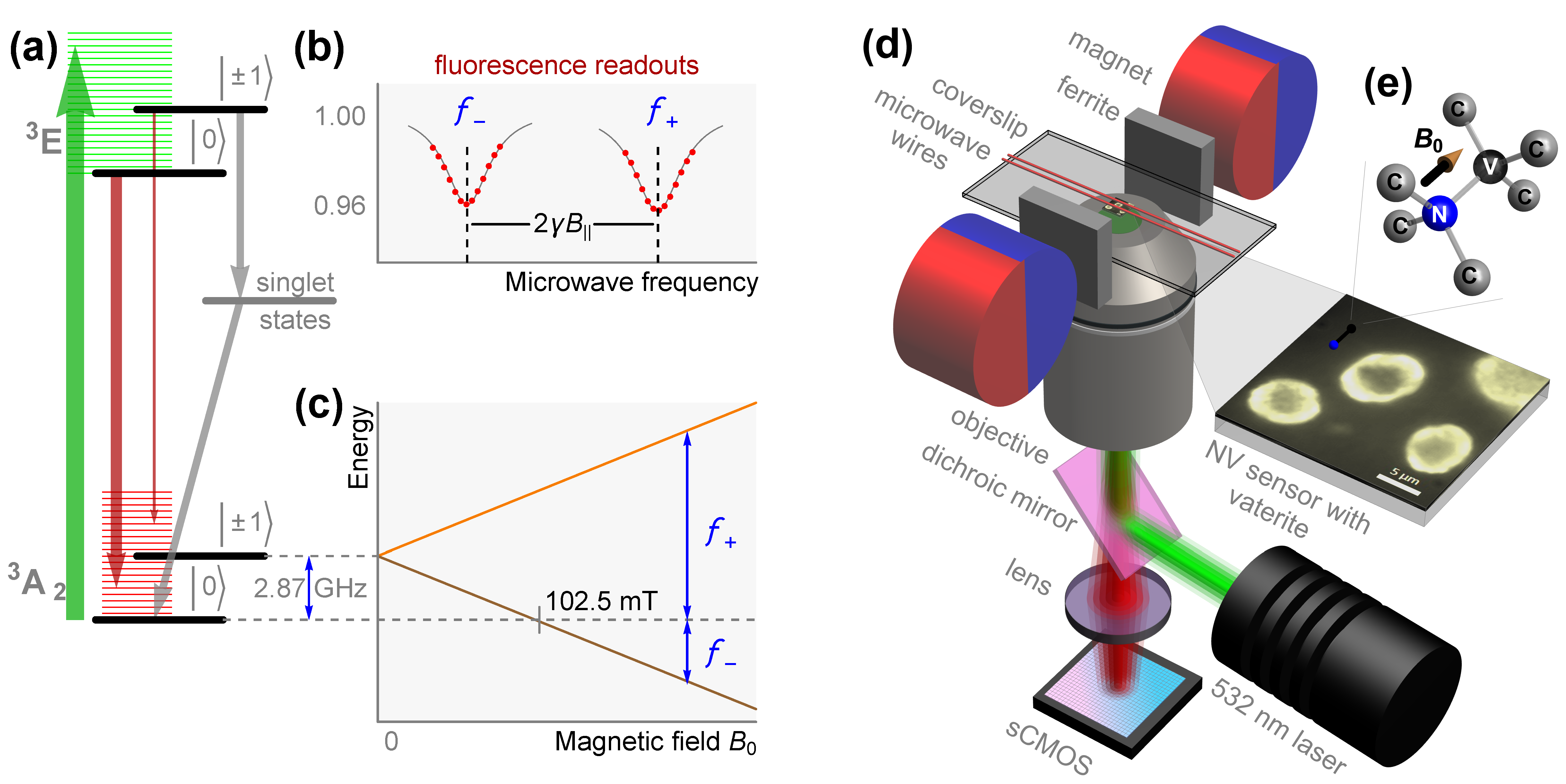
2.2. Quantum Diamond Microscopy
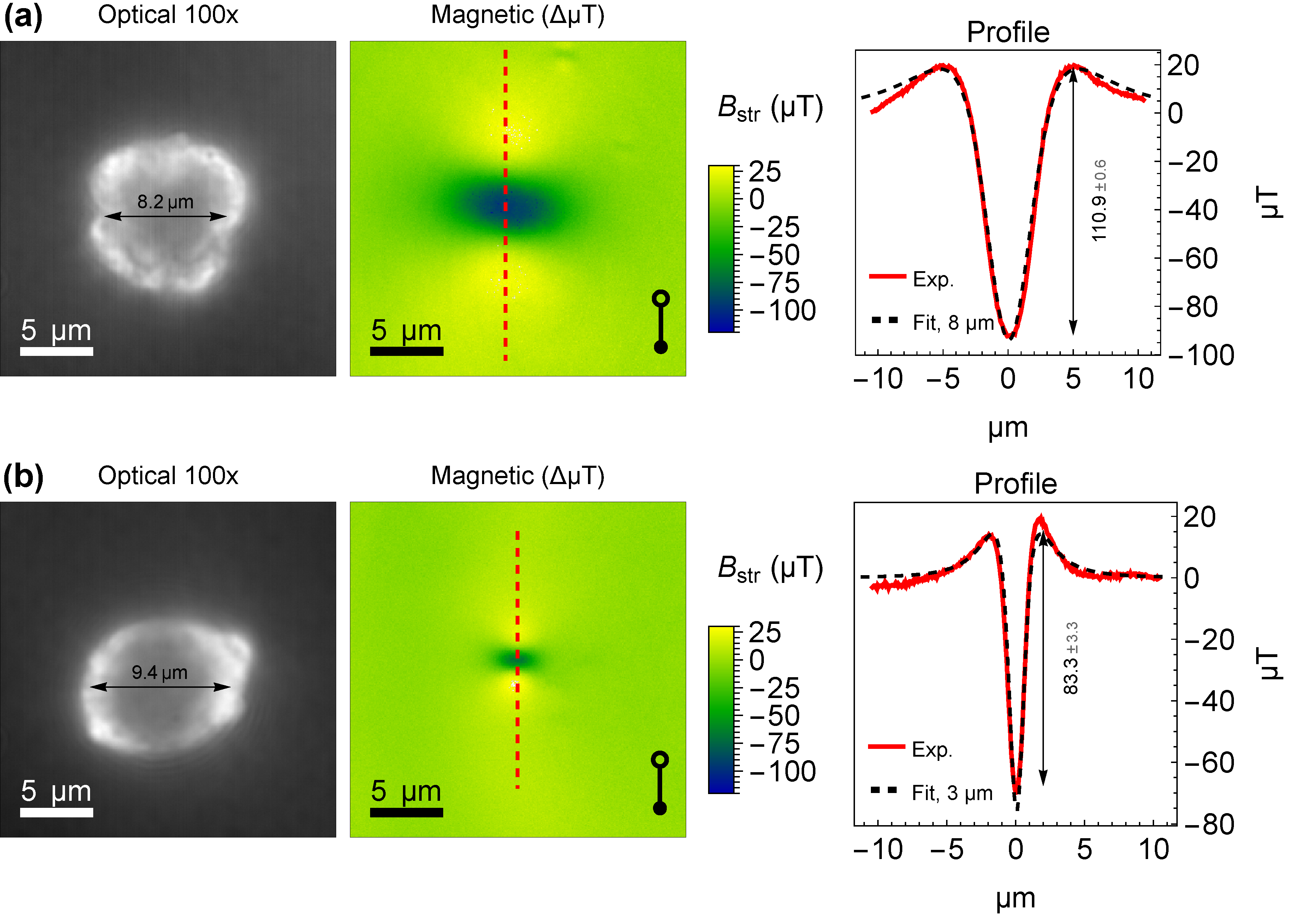
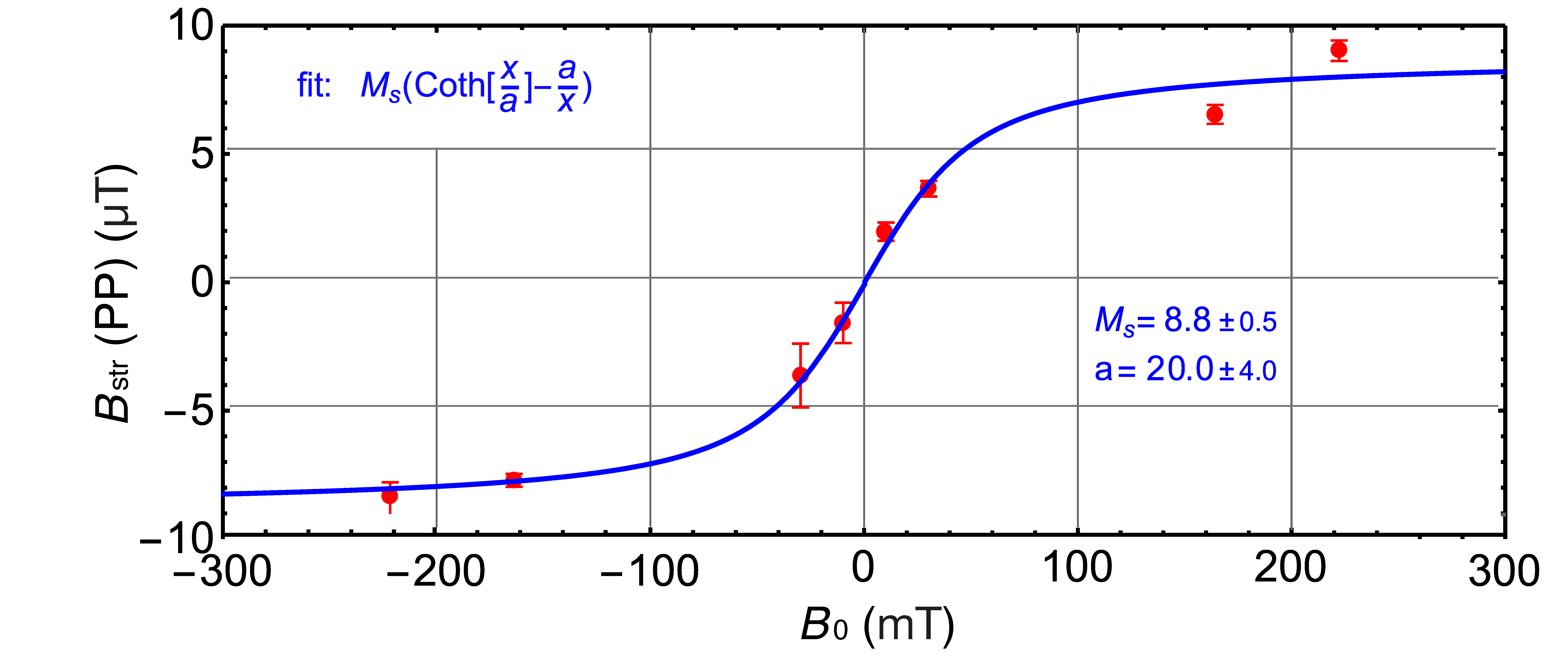
3. Results and Discussion
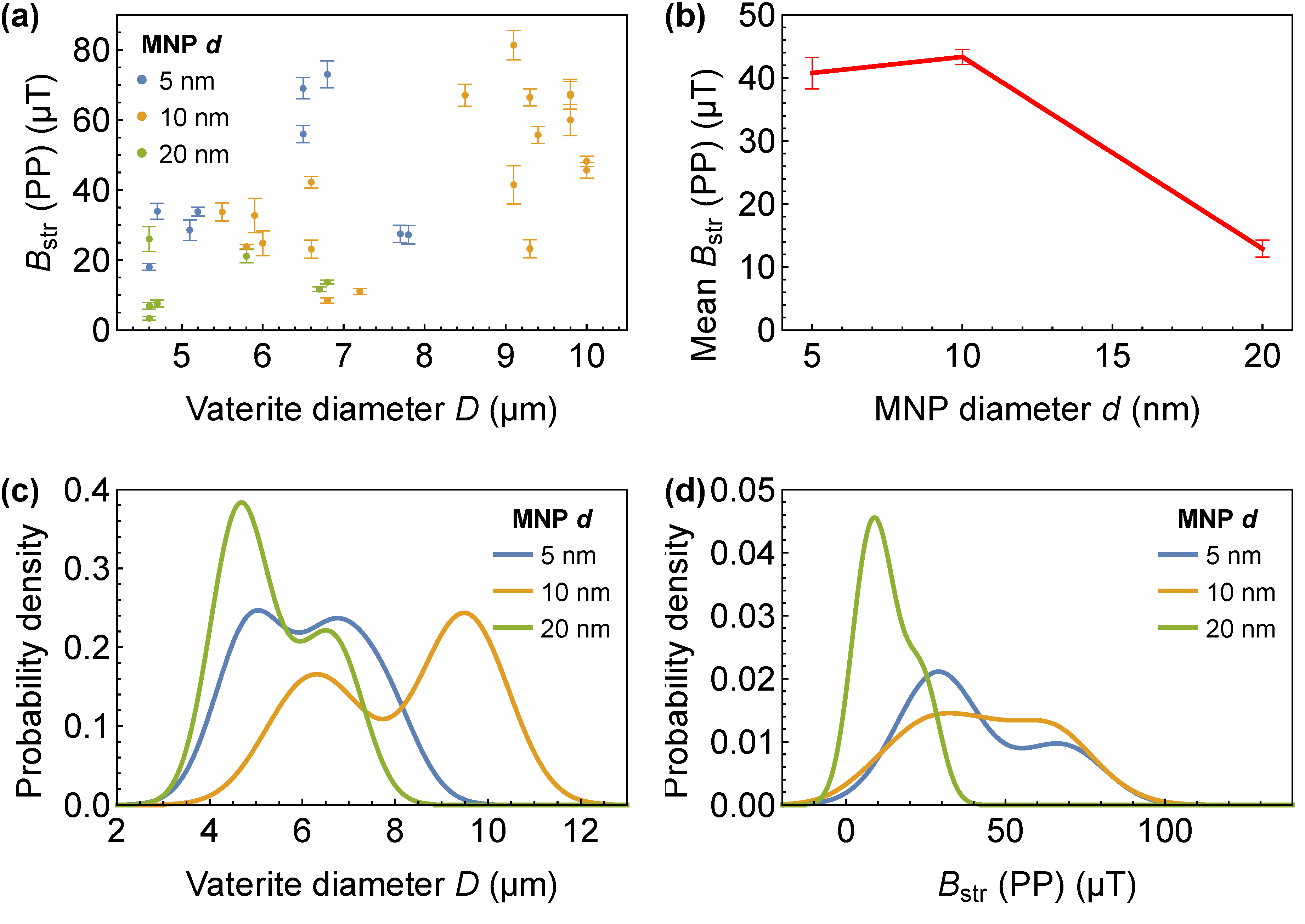
3.1. Magnetic Imaging of Individual Particles
3.2. Batch Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| NV | nitrogen-vacancy center |
| MNP | magnetic nanoparticles |
| QDM | quantum diamond microscope |
| ODMR | optically detected magnetic resonance |
| SQUID | superconducting quantum interference device |
| TEM | transmission electron microscopy |
| EDS | energy-dispersive spectroscopy |
| PP | peak-to-peak |
References
- Vikulina, A.S.; Feoktistova, N.A.; Balabushevich, N.G.; Skirtach, A.G.; Volodkin, D. The mechanism of catalase loading into porous vaterite CaCO3 crystals by co-synthesis. Phys. Chem. Chem. Phys. 2018, 20, 8822–8831. [Google Scholar] [CrossRef]
- Demina, P.A.; Saveleva, M.S.; Verkhovskii, R.A.; Anisimov, R.A.; Pidenko, P.S.; Svenskaya, Y.I. Investigation and release profile optimization for vaterite-based carriers loaded with betamethasone dipropionate. BioNanoScience 2024, 15, 61. [Google Scholar] [CrossRef]
- Huang, Y.; Skirtach, A.G.; Parakhonskiy, B.V. Systematic study of stability, loading efficiency and release mechanisms, and cellular interaction of vaterite with various sizes. Ceram. Int. 2024, 50, 7469–7479. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Vikulina, A.; Cave, G.W.; Loughlin, M.; Puddu, V.; Volodkin, D. Vaterite vectors for the protection, storage and release of silver nanoparticles. J. Colloid Interface Sci. 2023, 631, 165–180. [Google Scholar] [CrossRef]
- Bahrom, H.; Goncharenko, A.A.; Fatkhutdinova, L.I.; Peltek, O.O.; Muslimov, A.R.; Koval, O.Y.; Eliseev, I.E.; Manchev, A.; Gorin, D.; Shishkin, I.I.; et al. Controllable synthesis of calcium carbonate with different geometry: Comprehensive analysis of particle formation, cellular uptake, and biocompatibility. ACS Sustain. Chem. Eng. 2019, 7, 19142–19156. [Google Scholar] [CrossRef]
- Parakhonskiy, B.V.; Yashchenok, A.M.; Donatan, S.; Volodkin, D.V.; Tessarolo, F.; Antolini, R.; Möhwald, H.; Skirtach, A.G. Macromolecule Loading into Spherical, Elliptical, Star-Like and Cubic Calcium Carbonate Carriers. ChemPhysChem 2014, 15, 2817–2822. [Google Scholar] [CrossRef] [PubMed]
- Azarian, M.H.; Sutapun, W. Vaterite CaCO3-Ag hybrid microspheres: An overview of synthesis techniques and applications. Nanocomposites 2024, 11, 1–19. [Google Scholar] [CrossRef]
- Xia, H.; Zhou, M.; Wei, X.; Zhang, X.; Wu, Z. Slow and Sustained Release of Carbonate Ions from Amino Acids for Controlled Hydrothermal Growth of Alkaline-Earth Carbonate Single Crystals. ACS Omega 2020, 5, 14123–14132. [Google Scholar] [CrossRef]
- Gilad, H.; Ushkov, A.; Kolchanov, D.; Machnev, A.; Salgals, T.; Bobrovs, V.; Barhum, H.; Ginzburg, P. Optomechanically and Thermo-Optically Driven Interactions Between Gilded Vaterite Particles in Bubbles. Laser Photonics Rev. 2025, 19, 2500081. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. Appl. Phys. 2003, 36, R167. [Google Scholar] [CrossRef]
- Choukrani, G.; Maharjan, B.; Park, C.H.; Kim, C.S.; Kurup Sasikala, A.R. Biocompatible superparamagnetic sub-micron vaterite particles for thermo-chemotherapy: From controlled design to in vitro anticancer synergism. Mater. Sci. Eng. C 2020, 106, 110226. [Google Scholar] [CrossRef]
- Markina, N.E.; Markin, A.V.; Zakharevich, A.M.; Goryacheva, I.Y. Calcium carbonate microparticles with embedded silver and magnetite nanoparticles as new SERS-active sorbent for solid phase extraction. Microchim. Acta 2017, 184, 3937–3944. [Google Scholar] [CrossRef]
- Parakhonskiy, B.V.; Abalymov, A.; Ivanova, A.; Khalenkow, D.; Skirtach, A.G. Magnetic and silver nanoparticle functionalized calcium carbonate particles—Dual functionality of versatile, movable delivery carriers which can surface-enhance Raman signals. J. Appl. Phys. 2019, 126, 203102. [Google Scholar] [CrossRef]
- Frandsen, B.A.; Read, C.; Stevens, J.; Walker, C.; Christiansen, M.; Harrison, R.G.; Chesnel, K. Superparamagnetic dynamics and blocking transition in Fe3O4 nanoparticles probed by vibrating sample magnetometry and muon spin relaxation. Phys. Rev. Mater. 2021, 5, 054411. [Google Scholar] [CrossRef]
- Vékony, V.; Matta, C.; Pál, P.; Szabó, I.A. Structural and magnetic characterisation of a biocompatible magnetic nanoparticle assembly. J. Magn. Magn. Mater. 2022, 545, 168772. [Google Scholar] [CrossRef]
- Schwarz, T.; Wölbing, R.; Reiche, C.F.; Müller, B.; Martínez-Pérez, M.J.; Mühl, T.; Büchner, B.; Kleiner, R.; Koelle, D. Low-Noise YBa2Cu3O7 Nano-SQUIDs for Performing Magnetization-Reversal Measurements on Magnetic Nanoparticles. Phys. Rev. Appl. 2015, 3, 044011. [Google Scholar] [CrossRef]
- Hajalilou, A.; Tavakoli, M.; Parvini, E. The Principle of SQUID Magnetometry and Its Contribution in MNPs Evaluation. In Magnetic Nanoparticles: Synthesis, Characterization, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2023; pp. 135–155. [Google Scholar] [CrossRef]
- Johnson, C.E.; Johnson, J.A.; Hah, H.Y.; Cole, M.; Gray, S.; Kolesnichenko, V.; Kucheryavy, P.; Goloverda, G. Mössbauer studies of stoichiometry of Fe3O4: Characterization of nanoparticles for biomedical applications. Hyperfine Interact. 2016, 237, 27. [Google Scholar] [CrossRef]
- Iacob, N.; Palade, P.; Comanescu, C.; Crisan, O.; Toderascu, L.I.; Socol, G.; Schinteie, G.; Kuncser, V. Comprehensive Methodology for Evaluating the Drug Loading of Iron Oxide Nanoparticles Using Combined Magnetometry and Mössbauer Spectroscopy. Molecules 2025, 30, 676. [Google Scholar] [CrossRef]
- Adolphi, N.L.; Huber, D.L.; Bryant, H.C.; Monson, T.C.; Fegan, D.L.; Lim, J.; Trujillo, J.E.; Tessier, T.E.; Lovato, D.M.; Butler, K.S.; et al. Characterization of single-core magnetite nanoparticles for magnetic imaging by SQUID relaxometry. Phys. Med. Biol. 2010, 55, 5985–6003. [Google Scholar] [CrossRef]
- Araujo, J.F.; Tahir; Arsalani, S.; Freire, F.L.; Mariotto, G.; Cremona, M.; Mendoza, L.A.; Luz-Lima, C.; Zaman, Q.; Del Rosso, T.; et al. Novel scanning magnetic microscopy method for the characterization of magnetic nanoparticles. J. Magn. Magn. Mater. 2020, 499, 166300. [Google Scholar] [CrossRef]
- Sebastian, T.; Schultheiss, K.; Obry, B.; Hillebrands, B.; Schultheiss, H. Micro-focused Brillouin light scattering: Imaging spin waves at the nanoscale. Front. Phys. 2015, 3, 35. [Google Scholar] [CrossRef]
- Kirk, K.J.; Chapman, J.N.; Wilkinson, C.D.W. Lorentz microscopy of small magnetic structures (invited). J. Appl. Phys. 1999, 85, 5237–5242. [Google Scholar] [CrossRef]
- Sievers, S.; Braun, K.; Eberbeck, D.; Gustafsson, S.; Olsson, E.; Schumacher, H.W.; Siegner, U. Quantitative Measurement of the Magnetic Moment of Individual Magnetic Nanoparticles by Magnetic Force Microscopy. Small 2012, 8, 2675–2679. [Google Scholar] [CrossRef] [PubMed]
- Levine, E.V.; Turner, M.J.; Kehayias, P.; Hart, C.A.; Langellier, N.; Trubko, R.; Glenn, D.R.; Fu, R.R.; Walsworth, R.L. Principles and techniques of the quantum diamond microscope. Nanophotonics 2019, 8, 1945–1973. [Google Scholar] [CrossRef]
- Fescenko, I.; Laraoui, A.; Smits, J.; Mosavian, N.; Kehayias, P.; Seto, J.; Bougas, L.; Jarmola, A.; Acosta, V.M. Diamond magnetic microscopy of malarial hemozoin nanocrystals. Phys. Rev. Appl. 2019, 3, 034029. [Google Scholar] [CrossRef]
- Berzins, A.; Smits, J.; Petruhins, A.; Rimsa, R.; Mozolevskis, G.; Zubkins, M.; Fescenko, I. NV microscopy of thermally controlled stresses caused by thin Cr2O3 films. Opt. Express 2023, 31, 17950. [Google Scholar] [CrossRef]
- Le Sage, D.; Arai, K.; Glenn, D.R.; DeVience, S.J.; Pham, L.M.; Rahn-Lee, L.; Lukin, M.D.; Yacoby, A.; Komeili, A.; Walsworth, R.L. Optical magnetic imaging of living cells. Nature 2013, 496, 486–489. [Google Scholar] [CrossRef]
- Glenn, D.R.; Fu, R.R.; Kehayias, P.; Le Sage, D.; Lima, E.A.; Weiss, B.P.; Walsworth, R.L. Micrometer-scale magnetic imaging of geological samples using a quantum diamond microscope. Geochem. Geophys. Geosystems 2017, 18, 3254–3267. [Google Scholar] [CrossRef]
- Glenn, D.R.; Lee, K.; Park, H.; Weissleder, R.; Yacoby, A.; Lukin, M.D.; Lee, H.; Walsworth, R.L.; Connolly, C.B. Single-cell magnetic imaging using a quantum diamond microscope. Nat. Methods 2015, 12, 736–738. [Google Scholar] [CrossRef]
- Mosavian, N.; Hubert, F.; Smits, J.; Kehayias, P.; Silani, Y.; Richards, B.A.; Acosta, V.M. Super-Resolution Diamond Magnetic Microscopy of Superparamagnetic Nanoparticles. ACS Nano 2024, 18, 6523–6532. [Google Scholar] [CrossRef] [PubMed]
- Radu, V.; Price, J.C.; Levett, S.J.; Narayanasamy, K.K.; Bateman-Price, T.D.; Wilson, P.B.; Mather, M.L. Dynamic Quantum Sensing of Paramagnetic Species Using Nitrogen-Vacancy Centers in Diamond. ACS Sens. 2019, 5, 703–710. [Google Scholar] [CrossRef]
- Martínez-Mera, I.; Espinosa-Pesqueira, M.; Pérez-Hernández, R.; Arenas-Alatorre, J. Synthesis of magnetite (Fe3O4) nanoparticles without surfactants at room temperature. Mater. Lett. 2007, 61, 4447–4451. [Google Scholar] [CrossRef]
- Hadadian, Y.; Masoomi, H.; Dinari, A.; Ryu, C.; Hwang, S.; Kim, S.; Cho, B.k.; Lee, J.Y.; Yoon, J. From Low to High Saturation Magnetization in Magnetite Nanoparticles: The Crucial Role of the Molar Ratios Between the Chemicals. ACS Omega 2022, 7, 15996–16012. [Google Scholar] [CrossRef] [PubMed]
- Nkurikiyimfura, I.; Wang, Y.; Safari, B.; Nshingabigwi, E. Temperature-dependent magnetic properties of magnetite nanoparticles synthesized via coprecipitation method. J. Alloys Compd. 2020, 846, 156344. [Google Scholar] [CrossRef]
- Sukhorukov, G.B.; Volodkin, D.V.; Günther, A.M.; Petrov, A.I.; Shenoy, D.B.; Möhwald, H. Porous calcium carbonate microparticles as templates for encapsulation of bioactive compounds. J. Mater. Chem. 2004, 14, 2073–2081. [Google Scholar] [CrossRef]
- Fescenko, I. Ultra-fast detection of the center frequency of a spectral line from amplitude-weighted average. arXiv 2023, arXiv:2303.12544. [Google Scholar] [CrossRef]
- Berzins, A.; Grube, H.; Lazda, R.; Hannig, M.A.; Smits, J.; Fescenko, I. Tunable magnetic field source for magnetic field imaging microscopy. Ultramicroscopy 2022, 242, 113624. [Google Scholar] [CrossRef]
- Auzinsh, M.; Berzins, A.; Budker, D.; Busaite, L.; Ferber, R.; Gahbauer, F.; Lazda, R.; Wickenbrock, A.; Zheng, H. Hyperfine level structure in nitrogen-vacancy centers near the ground-state level anticrossing. Phys. Rev. B 2019, 100, 075204. [Google Scholar] [CrossRef]
- Noskov, R.E.; Machnev, A.; Shishkin, I.I.; Novoselova, M.V.; Gayer, A.V.; Ezhov, A.A.; Shirshin, E.A.; German, S.V.; Rukhlenko, I.D.; Fleming, S.; et al. Golden Vaterite as a Mesoscopic Metamaterial for Biophotonic Applications. Adv. Mater. 2021, 33, 2008484. [Google Scholar] [CrossRef]
- German, S.V.; Novoselova, M.V.; Bratashov, D.N.; Demina, P.A.; Atkin, V.S.; Voronin, D.V.; Khlebtsov, B.N.; Parakhonskiy, B.V.; Sukhorukov, G.B.; Gorin, D.A. High-efficiency freezing-induced loading of inorganic nanoparticles and proteins into micron- and submicron-sized porous particles. Sci. Rep. 2018, 8, 17763. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jani, M.; Barhum, H.; Alnis, J.; Attrash, M.; Amro, T.; Bar-Gill, N.; Salgals, T.; Ginzburg, P.; Fescenko, I. Quantum Diamond Microscopy of Individual Vaterite Microspheres Containing Magnetite Nanoparticles. Nanomaterials 2025, 15, 1141. https://doi.org/10.3390/nano15151141
Jani M, Barhum H, Alnis J, Attrash M, Amro T, Bar-Gill N, Salgals T, Ginzburg P, Fescenko I. Quantum Diamond Microscopy of Individual Vaterite Microspheres Containing Magnetite Nanoparticles. Nanomaterials. 2025; 15(15):1141. https://doi.org/10.3390/nano15151141
Chicago/Turabian StyleJani, Mona, Hani Barhum, Janis Alnis, Mohammad Attrash, Tamara Amro, Nir Bar-Gill, Toms Salgals, Pavel Ginzburg, and Ilja Fescenko. 2025. "Quantum Diamond Microscopy of Individual Vaterite Microspheres Containing Magnetite Nanoparticles" Nanomaterials 15, no. 15: 1141. https://doi.org/10.3390/nano15151141
APA StyleJani, M., Barhum, H., Alnis, J., Attrash, M., Amro, T., Bar-Gill, N., Salgals, T., Ginzburg, P., & Fescenko, I. (2025). Quantum Diamond Microscopy of Individual Vaterite Microspheres Containing Magnetite Nanoparticles. Nanomaterials, 15(15), 1141. https://doi.org/10.3390/nano15151141






