Steady Shear Rheology of Suspensions of Mixtures of Starch Nanoparticles and Cellulose Nanocrystals
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of NCC and SNP Suspensions
2.3. Preparation of Suspensions of SNP and NCC Mixtures
2.4. Measurements
3. Results and Discussion
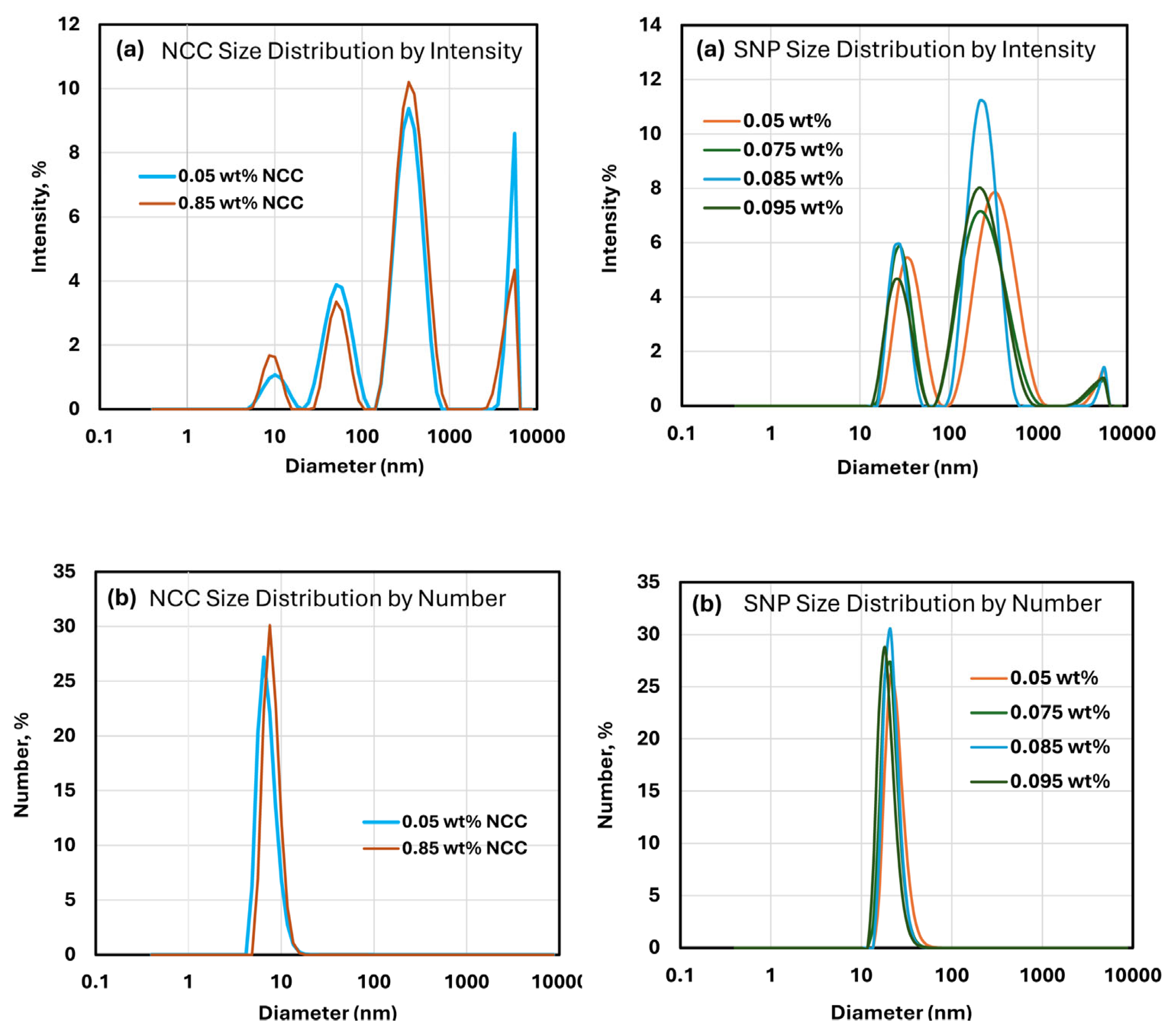
3.1. Particle Size Analysis of NCC and SNPs
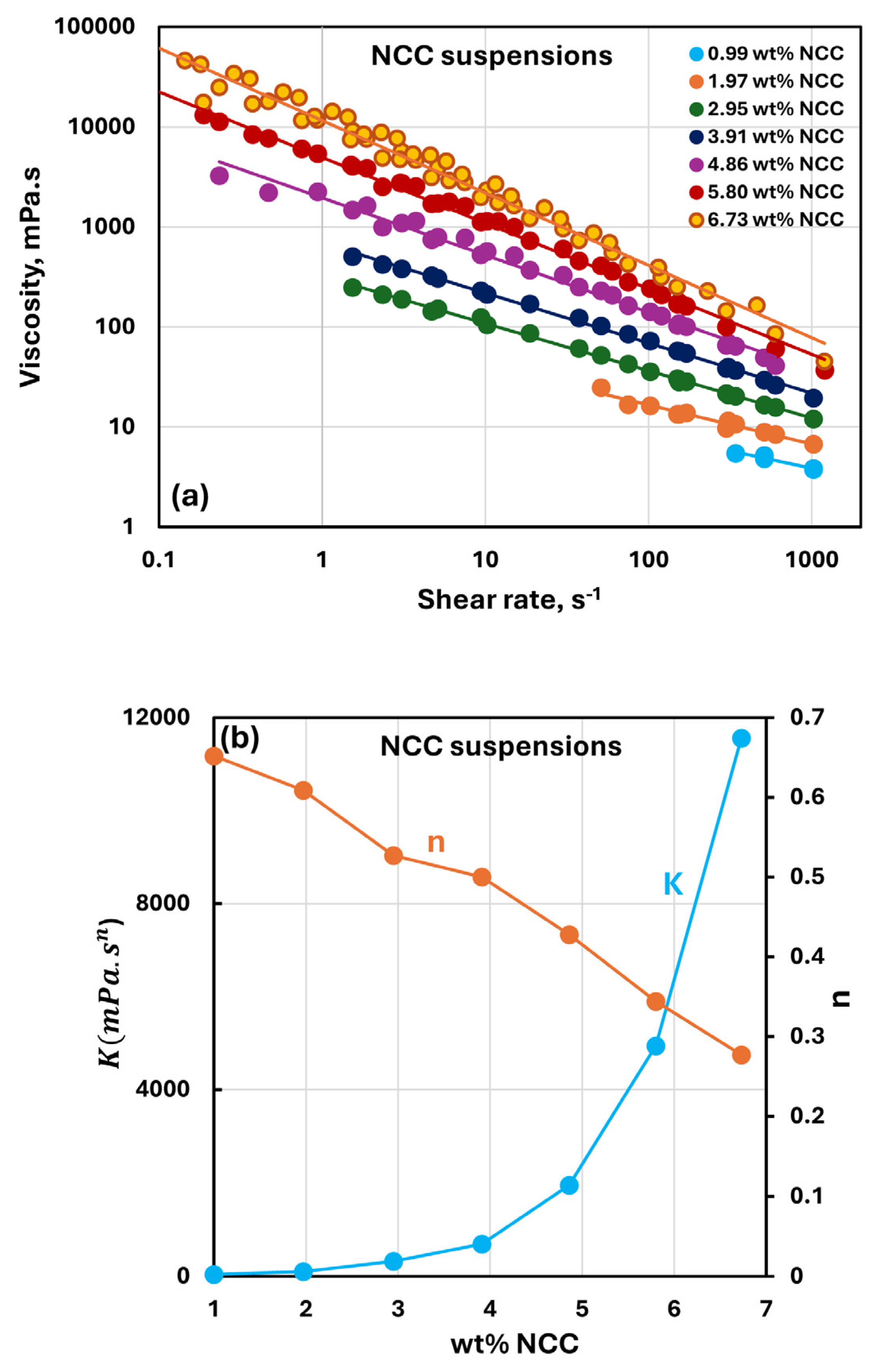
3.2. Rheology of Suspensions of NCC
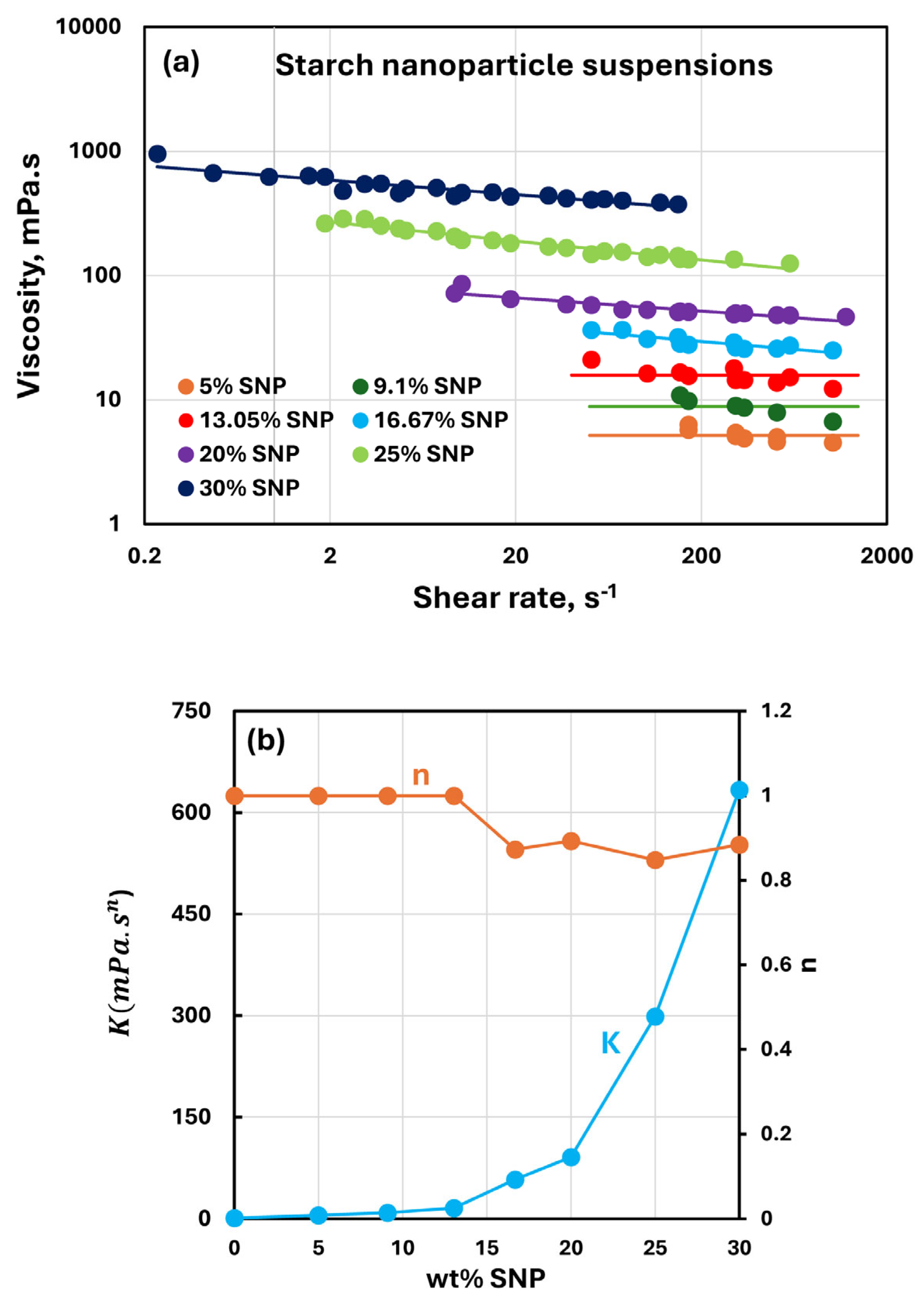
3.3. Rheology of Suspensions of SNPs
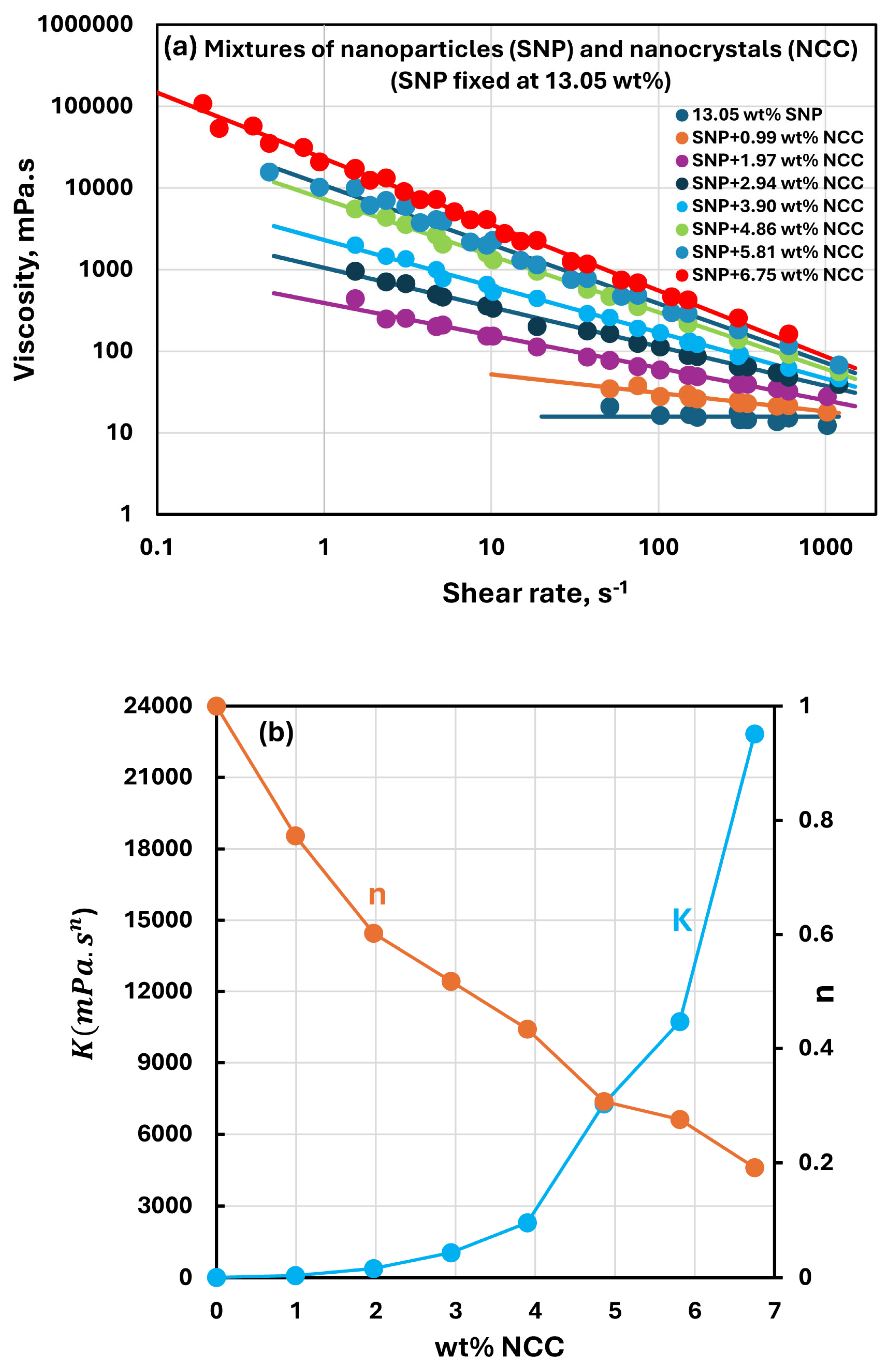
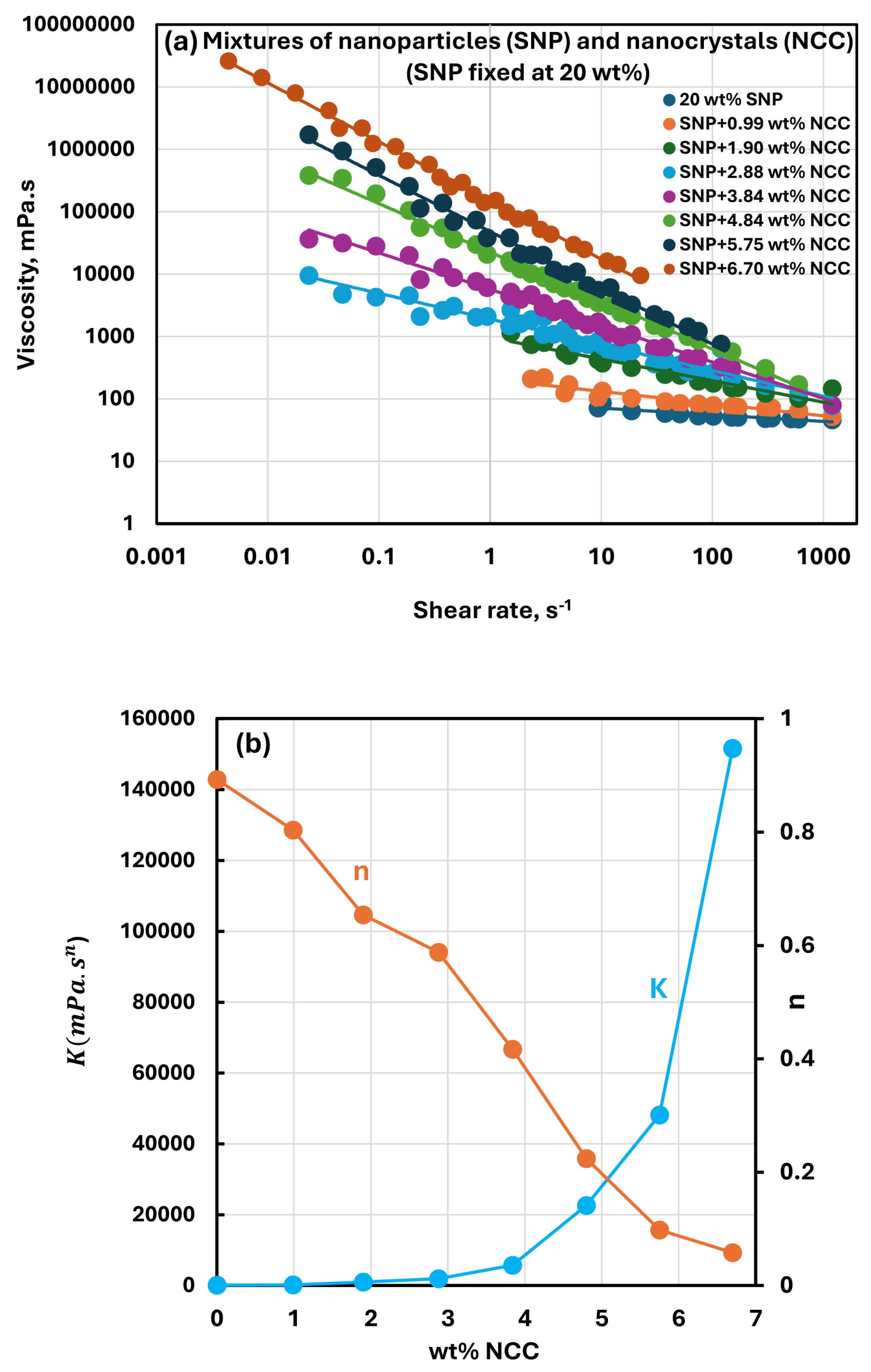
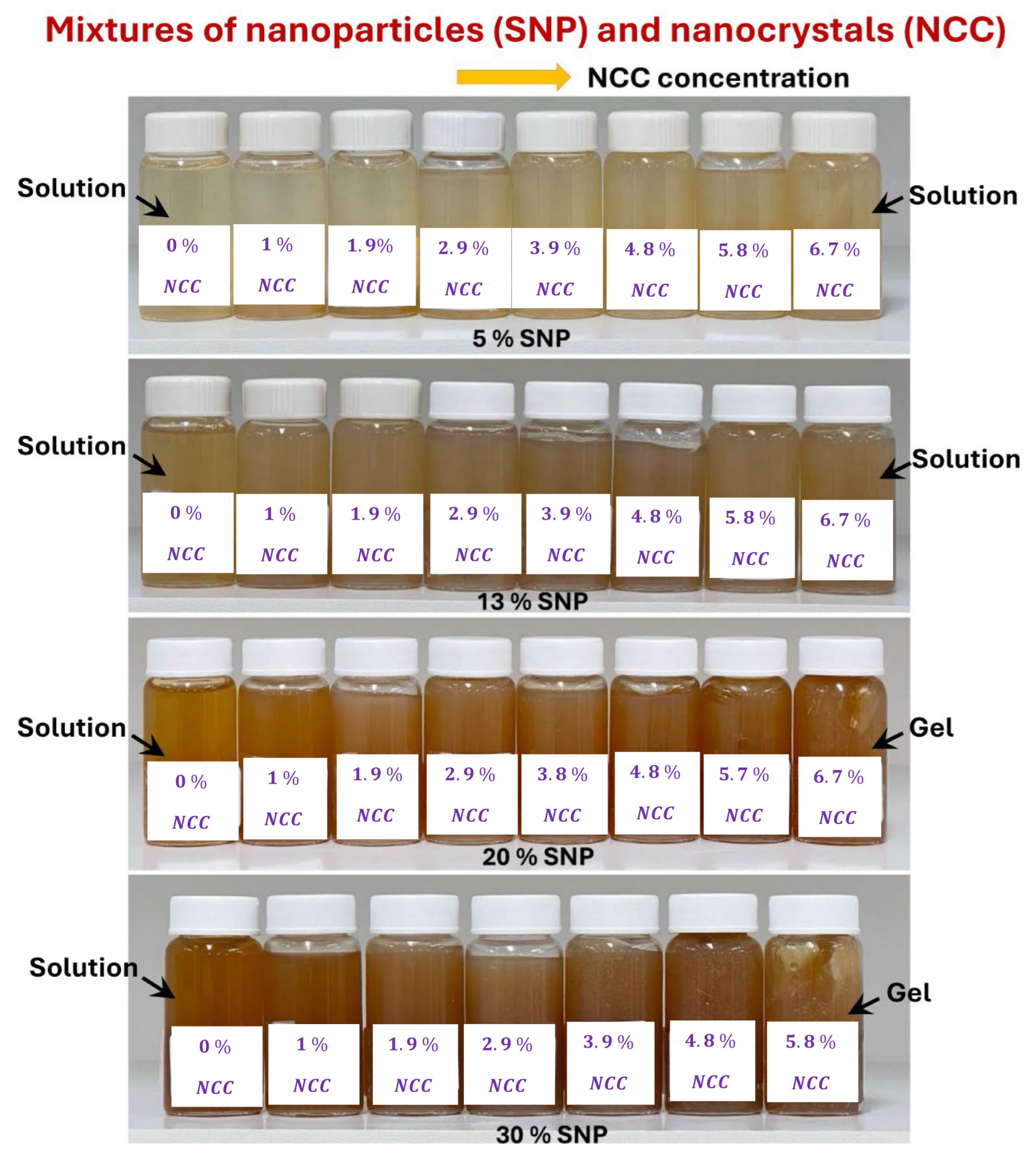
3.4. Rheology of Suspensions of Mixtures of NCC and SNPs
- The suspensions of mixtures of NCC and SNPs are Newtonian at low concentrations of NCC and SNPs in the mixture suspension. The mixture suspensions become shear-thinning pseudoplastic, that is, the viscosity decreases with an increase in shear rate, at high concentrations of NCC and SNPs in the mixture suspension.
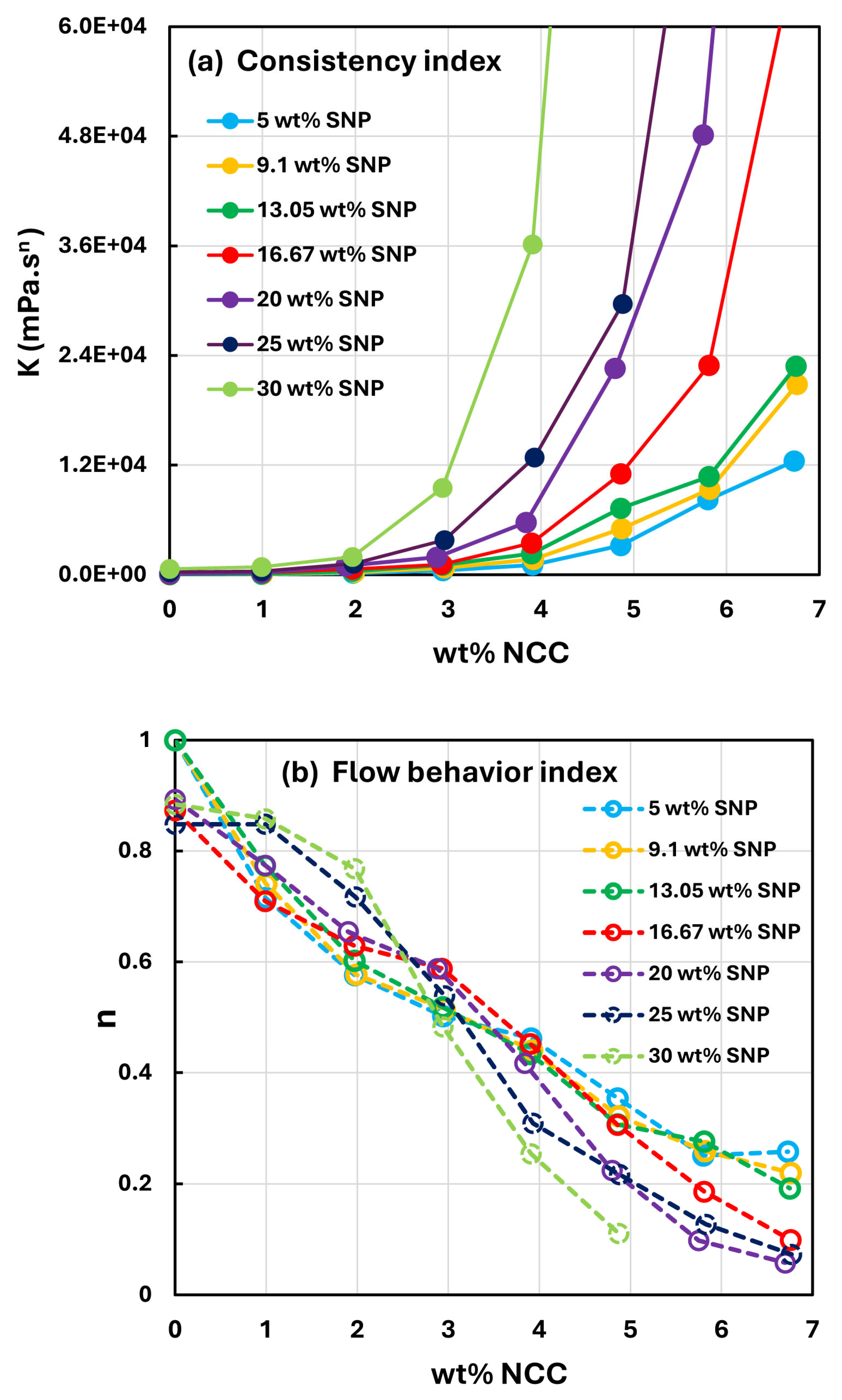
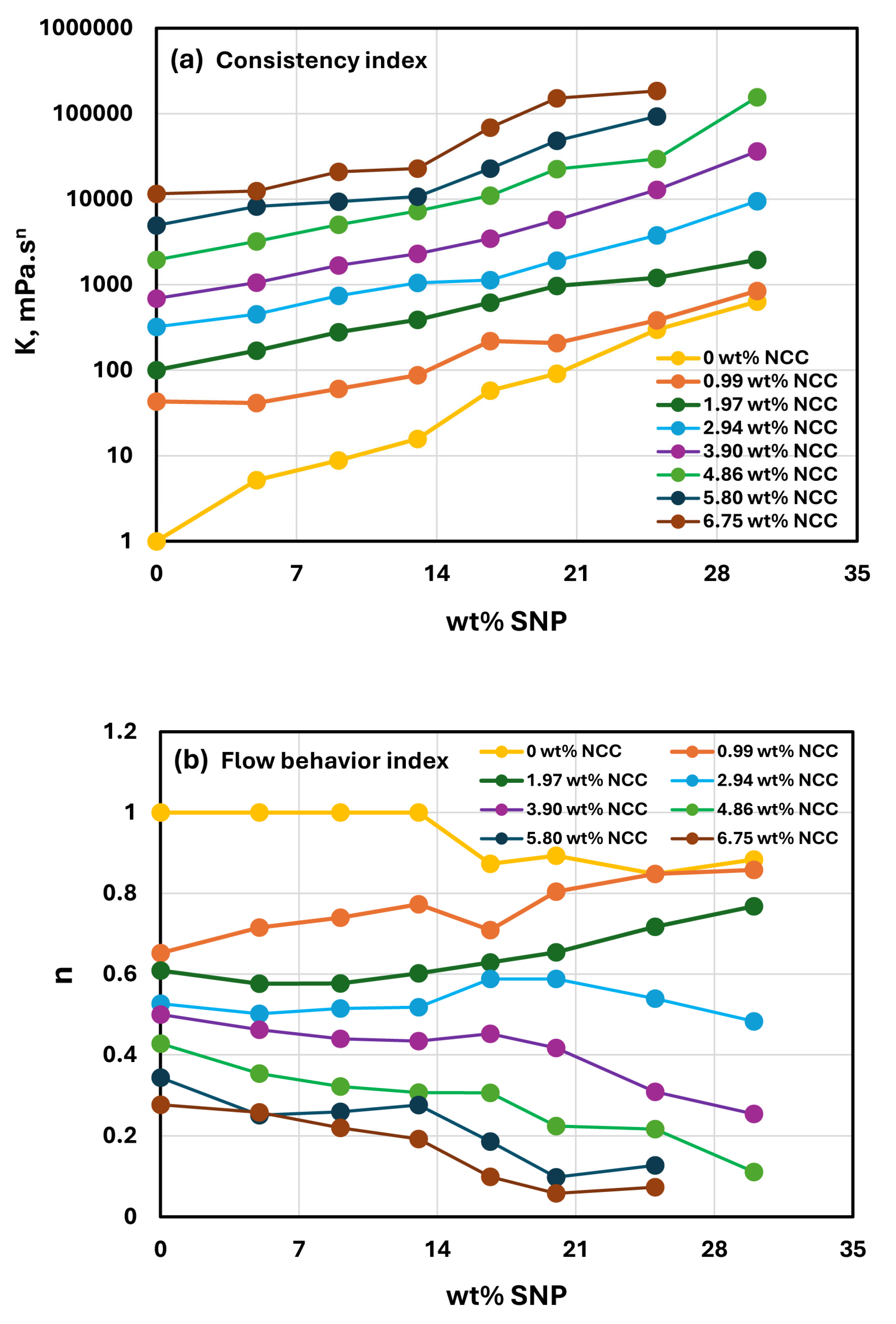
- At a fixed concentration of SNPs, the mixture suspensions become more viscous and shear-thinning with an increase in NCC concentration. In other words, the viscosity versus shear rate plot shifts upwards and becomes steeper with an increase in NCC concentration. The consistency index increases whereas the flow behavior index decreases with an increase in the NCC concentration of the mixture suspension.
- The consistency index of suspensions of mixtures of NCC and SNPs is strongly dependent on both NCC and SNP concentrations of the mixtures. For example, the consistency index increases substantially with an increase in SNP concentration at any fixed concentration of NCC.


4. Conclusions
- The NCC suspensions are shear-thinning and follow a power-law model over the NCC concentration range of 0.99 to 6.73 wt%. The consistency and the degree of shear-thinning of suspensions both increase with an increase in NCC concentration.
- The SNP suspensions are Newtonian up to an SNP concentration of about 13 wt%. At higher SNP concentrations, suspensions become modestly shear-thinning. However, the consistency index continues to increase substantially with an increase in SNP concentration.
- The suspensions of mixtures of NCC and SNPs are non-Newtonian shear-thinning over the entire range of concentrations investigated. The degree of shear-thinning in suspensions of mixtures of NCC and SNPs is strongly dependent on the NCC concentration and less severely dependent on the SNP concentration. The consistency of the suspension mixture increases substantially with increases in NCC and SNP concentrations. However, NCC concentration has a stronger effect on consistency as compared with SNP concentration.
- Our future work in this area will explore dynamic (oscillatory) shear properties of suspensions of NCC, SNPs, and their blends.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pal, A.; Pal, R. Rheology of emulsions thickened by starch nanoparticles. Nanomaterials 2022, 12, 2391. [Google Scholar] [CrossRef] [PubMed]
- Himashree, P.; Sengar, A.S.; Sunil, C.K. Food thickening agents: Sources, chemistry, properties and applications—A review. Int. J. Gastron. Food Sci. 2022, 27, 100468. [Google Scholar] [CrossRef]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef]
- Hydrocolloids Market Size, Share & Growth Report, 2030. Available online: https://www.grandviewresearch.com/industry-analysis/hydrocolloids-market (accessed on 7 June 2025).
- How to Thicken Liquids for a Medical Diet. Available online: https://www.verywellhealth.com/what-are-thickened-liquids-1192165 (accessed on 7 June 2025).
- Pal, R. Modeling of sedimentation and creaming in suspensions and Pickering emulsions. Fluids 2019, 4, 186. [Google Scholar] [CrossRef]
- Qin, Q.; Zeng, S.; Duan, G.; Liu, Y.; Han, X.; Yu, R.; Huang, Y.; Zhang, C.; Han, J.; Jiang, S. “Bottom-up” and “top-down” strategies toward strong cellulose-based materials. Chem. Soc. Rev. 2024, 53, 9306–9343. [Google Scholar] [CrossRef]
- Marla, H.; Rizki, D.I.; Mardawati, E.; Djali, M.; Mohammad, M.; Cahyana, Y. Starch nanoparticles: Preparation, properties, and applications. Polymers 2023, 15, 1167. [Google Scholar] [CrossRef] [PubMed]
- Marcuello, C.; Foulon, L.; Chabbert, B.; Molinari, M.; Aguie-Beghin, V. Langmuir—Blodgett Procedure to Precisely Control the Coverage of Functionalized AFM Cantilevers for SMFS Measurements: Application with Cellulose Nanocrystals. Langmuir 2018, 34, 9376–9386. [Google Scholar] [CrossRef]
- Saraswat, P.; Singh, S.; Prasad, M.; Misra, R.; Rajput, V.D.; Ranjan, R. Applications of bio-based nanomaterials in environment and agriculture: A review on recent progresses. Hybrid Adv. 2023, 4, 100097. [Google Scholar] [CrossRef]
- Bagal-Kestwal, D.R.; Kestwal, R.M.; Chiang, B.H. Bio-based Nanomaterials and Their Bionanocomposites. In Nanomaterials and Nanocomposites: Zero-to Three-Dimensional Materials and Their Composites, 1st ed.; Visakh, P.M., Morlanes, M.J.M., Eds.; Wiley-VCH: Weinheim, Germany, 2016; pp. 255–330. [Google Scholar]
- Li, H.; Yoshida, S.; Mitani, N.; Egusa, M.; Takagi, M.; Izawa, H.; Matsumoto, T.; Kaminaka, H.; Ifuku, S. Disease resistance and growth promotion activities of chitin/cellulose nanofiber from spent mushroom substrate to plant. Carbohydr. Polym. 2022, 284, 119233. [Google Scholar] [CrossRef]
- Bolade, O.P.; Williams, A.B.; Benson, N.U. Green synthesis of iron-based nanomaterials for environmental remediation: A review. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100279. [Google Scholar] [CrossRef]
- Rao, V.; Sundararajan, P.; Ramakrishnan, C.; Ramachandran, G.N. Conformational studies of amylose. In Conformation of Biopolymers; Ramachandran, G.N., Ed.; Academic Press: London, UK, 1967; pp. 721–737. [Google Scholar]
- Krassig, H.A. Cellulose: Structure, Accessibility and Reactivity; Gordon and Breach Science: Philadelphia, PA, USA, 1993. [Google Scholar]
- Pérez, S.; Mazeau, K. Conformations, structures, and morphologies of celluloses. In Polysaccharides: Structural Diversity and Functional Versatility, 2nd ed.; Dumitriu, S., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 41–68. [Google Scholar]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Zhang, Y.; Nypelö, T.; Salas, C.; Arboleda, J.; Hoeger, I.C.; Rojas, O.J. Cellulose Nanofibrils: From Strong Materials to Bioactive Surfaces. J. Renew. Mater. 2013, 1, 195–211. [Google Scholar] [CrossRef]
- Heinze, T. Cellulose: Structure and properties. In Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials; Rojas, O., Ed.; Springer: Cham, Germany, 2016; pp. 1–52. [Google Scholar]
- Yu, H.; Qin, Z.; Liang, B.; Liu, N.; Zhou, Z.; Chen, L. Facile extraction of thermally stable cellulose nanocrystals with a high yield of 93% through hydrochloric acid hydrolysis under hydrothermal conditions. J. Mater. Chem. A. 2013, 1, 3938–3944. [Google Scholar] [CrossRef]
- Islam, M.; Chen, L.; Sisler, J.; Tam, K. Cellulose nanocrystal (CNC)–inorganic hybrid systems: Synthesis, properties and applications. J. Mater. Chem. B. 2018, 6, 864–883. [Google Scholar] [CrossRef] [PubMed]
- Abitbol, T.; Palermo, A.; Moran-Mirabal, J.M.; Cranston, E.D. Fluorescent labeling and characterization of cellulose nanocrystals with varying charge contents. Biomacromolecules 2013, 14, 3278–3284. [Google Scholar] [CrossRef] [PubMed]
- Sadeghifar, H.; Filpponen, I.; Clarke, S.P.; Brougham, D.F.; Argyropoulos, D.S. Production of cellulose nanocrystals using hydrobromic acid and click reactions on their surface. J. Mater. Sci. 2011, 46, 7344–7355. [Google Scholar] [CrossRef]
- Kaushik, M.; Moores, A. Nanocelluloses as versatile supports for metal nanoparticles and their applications in catalysis. Green Chem. 2016, 18, 622–637. [Google Scholar] [CrossRef]
- Hu, S.; Fang, Y.; Liang, C.; Turunen, M.; Ikkala, O.; Zhang, H. Thermally trainable dual network hydrogels. Nat. Commun. 2023, 14, 3717. [Google Scholar] [CrossRef]
- Kondo, T. The relationship between intramolecular hydrogen bonds and certain physical properties of regioselectively substituted cellulose derivatives. J. Polymer Sci. Part B Polym. Phys. 1997, 35, 717–723. [Google Scholar] [CrossRef]
- Heinze, T.; Liebert, T. Unconventional methods in cellulose functionalization. Prog. Polym. Sci. 2001, 26, 1689–1762. [Google Scholar] [CrossRef]
- Kumar, A.; Jyske, T.; Petrič, M. Delignified wood from understanding the hierarchically aligned cellulosic structures to creating novel functional materials: A review. Adv. Sustain. Syst. 2021, 5, 2000251. [Google Scholar] [CrossRef]
- Torres, F.G.; De-la-Torre, G.E. Synthesis, characteristics, and applications of modified starch nanoparticles: A review. Int. J. Biol. Macromol. 2022, 194, 289–305. [Google Scholar] [CrossRef]
- Le Corre, D.; Bras, J.; Dufresne, A. Starch nanoparticles: A review. Biomacromolecules 2010, 11, 1139–1153. [Google Scholar] [CrossRef]
- Sreejit, V.; Preetha, R.; Mubeena, S.A.; Dhananjay, S. Green synthesized nano starch: Preparation and emerging avenues in food industry. J. Nanoparticle Res. 2025, 27, 1–20. [Google Scholar] [CrossRef]
- Lin, X.; Sun, S.; Wang, B.; Zheng, B.; Guo, Z. Structural and physicochemical properties of lotus seed starch nanoparticles prepared using ultrasonic-assisted enzymatic hydrolysis. Ultrason. Sonochemistry 2020, 68, 105199. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Xue, L.; Hu, Y.; Qiu, C.; Jin, Z.; Xu, X.; Wang, J. Green fabrication and characterization of debranched starch nanoparticles via ultrasonication combined with recrystallization. Ultrason. Sonochemistry 2020, 66, 105074. [Google Scholar] [CrossRef]
- Ghanaatpishehsanaei, G.; Pal, R. Rheology of suspensions of solid particles in liquids thickened by starch nanoparticles. Colloids Interfaces 2023, 7, 52. [Google Scholar] [CrossRef]
- Chopra, H.; Mohanta, Y.K.; Rauta, P.R.; Ahmed, R.; Mahanta, S.; Mishra, P.K.; Panda, P.; Rabaan, A.A.; Alshehri, A.A.; Othman, B.; et al. An insight into advances in developing nanotechnology based therapeutics, drug delivery, diagnostics and vaccines: Multidimensional applications in tuberculosis disease management. Pharmaceuticals 2023, 16, 581. [Google Scholar] [CrossRef]
- Masina, N.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Govender, M.; Indermun, S.; Pillay, V. A review of the chemical modification techniques of starch. Carbohydr. Polym. 2017, 157, 1226–1236. [Google Scholar] [CrossRef]
- Lu, Q.; Pal, R. Steady shear rheology and surface activity of polymer-surfactant mixtures. Polymers 2025, 17, 364. [Google Scholar] [CrossRef]

| Starch Nanoparticle (SNP) Concentration in SNP–Water Suspension (wt%) | Nanocrystalline Cellulose (NCC) Concentration in NCC–SNP–Water Suspension (wt%) |
|---|---|
| 5.0 | Seven concentrations: 0.99, 1.98, 2.95, 3.91, 4.86, 5.80, 6.73 |
| 9.1 | Seven concentrations: 1.0, 1.99, 2.96, 3.92, 4.87, 5.82, 6.76 |
| 13.05 | Seven concentrations: 0.99, 1.97, 2.94, 3.90, 4.86, 5.81, 6.75 |
| 16.67 | Seven concentrations: 0.99, 1.97, 2.93, 3.90, 4.86, 5.81, 6.76 |
| 20.0 | Seven concentrations: 0.99, 1.90, 2.88, 3.84, 4.84, 5.75, 6.70 |
| 25.0 | Seven concentrations: 0.99, 1.98, 2.96, 3.93, 4.88, 5.83, 6.77 |
| 30.0 | Five concentrations: 0.99, 1.97, 2.94, 3.91, 4.87 |
| Device | (cm) | Length of Inner Cylinder (cm) | Gap-Width (cm) | |
|---|---|---|---|---|
| Fann 35A/SR-12 | 1.72 | 1.84 | 3.8 | 0.12 |
| Haake Roto-visco RV 12 with MV I | 2.00 | 2.1 | 6.0 | 0.10 |
| Haake Roto-visco RV 12 with MV II | 1.84 | 2.1 | 6.0 | 0.26 |
| Haake Roto-visco RV 12 with MV III | 1.52 | 2.1 | 6.0 | 0.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alizadeh, H.; Pal, R. Steady Shear Rheology of Suspensions of Mixtures of Starch Nanoparticles and Cellulose Nanocrystals. Nanomaterials 2025, 15, 966. https://doi.org/10.3390/nano15130966
Alizadeh H, Pal R. Steady Shear Rheology of Suspensions of Mixtures of Starch Nanoparticles and Cellulose Nanocrystals. Nanomaterials. 2025; 15(13):966. https://doi.org/10.3390/nano15130966
Chicago/Turabian StyleAlizadeh, Hanie, and Rajinder Pal. 2025. "Steady Shear Rheology of Suspensions of Mixtures of Starch Nanoparticles and Cellulose Nanocrystals" Nanomaterials 15, no. 13: 966. https://doi.org/10.3390/nano15130966
APA StyleAlizadeh, H., & Pal, R. (2025). Steady Shear Rheology of Suspensions of Mixtures of Starch Nanoparticles and Cellulose Nanocrystals. Nanomaterials, 15(13), 966. https://doi.org/10.3390/nano15130966







