A Comprehensive Overview of Co3O4 Nanoparticles: Solution Combustion Synthesis and Potential Applications
Abstract
1. Introduction
2. Materials and Methods
3. Cobalt (II) (III) Oxide (Co3O4) Nanoparticles
Characteristics
4. Results and Discussion
| No. | Used Precursors and Fuel Solution | Electrolyte | Specific Capacitance, Fg−1 | Surface Area/m2g−1 | Pure Volume/cm3g−1 | TA, °C | Reaction T, °C | Particle Size/Diameter, nm | Proposed Applications | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | (Co(NO3)2·6H2O) and (CO(NH2)2) as fuel | Alkaline | 1060 | 3 | 0.02 | 600 | 36 | As the anode material for Li-ion batteries | [23] | |
| 2 | (Co(NO3)2·6H2O) and glycine, NH2CH2COOH | 1 M KOH | 10.45 | 300 | 500 | 13.1 | Best-performing electrode obtaining | [24] | ||
| 3 | (Co(CH3-CO2)2 4H2O) and urea (CH4N2O) as fuel | 500 | 70 | Catalysis and energy storage applications | [19] | |||||
| 4 | Cobalt nitrate hexahydrate and 2-imidazolidinone hemihydrate (ethylenurea) | 500 | 26.0 | Sensitive sensors for the safety of environmental and healthcare | [1] | |||||
| 5 | (Co(NO3)2·6H2O) and methanol as feul | 1 M KOH | 3560 | 500 | Electrode for electrochemical applications | [26] | ||||
| 6 | 5 g (Co(CH3- CO2)2·6H2O) and 1.72 g urea (CH4N2O) as fuel and 15 mL deionized water | KOH | 400 | 900 | 50 | Active for oxygen evolution reaction (OER) | [67] | |||
| 7 | (Co(CH3-CO2)2∙6H2O) and citric acid monohydrate (C6H8O7·H2O) and ammonium nitrate (NH4NO3) were used as fuel | 362.8 | 17.9 | 0.095 | 350 | 550 | 26.1 | Supercapaci tors electrode materials | [25] | |
| 8 | 3M(Co(NO3)2∙6H2O), 6M glycine (C2H5NO2), 10% by weight of cobalt nitrate (nitric acid) and 50 mL deionized water | 700 | 90 | 292.66 | 260 | 260 | 20–65 | Gas sensors | [18] | |
| 9 | Co(NO3)2⋅6H2O and urea, NH2CONH2 with 100 mL deionized water | 3 M KOH | 212 | 69.34 | 0.0431 | 600 | 13.64 | High-performance electrodes for supercapaci tors | [47] | |
| 10 | (CoCl2∙6H2O), D-glucose, fructose, maltose, sucrose | 1 M KOH | 600 | Non-enzyme glucose detection | [68] | |||||
| 11 | Cobalt nitrate, urea as fuel and deionized water | 1.4 | 0.016 | 400 | 30–50 | In catalysts as coatings | [69] | |||
| 12 | (CoCl2∙6H2O), (AgNO3) and (NH3), in deionized water | 0.1 M KOH | 992.7 | 407.33 | 0.1155 | 12.98 | Supercapaci tors application | [11] | ||
| 13 | (CoCl2∙6H2O), (AgNO3) and (NH3), in deionized water | 0.1 M KOH | 53.06 | 0.07425 | 19.37 | Supercapaci tors application | [11] |
4.1. Characterization
4.2. Application
4.2.1. Supercapacitors and Pseudocapacitors
| No. | Material | Preparation | Electrolyte | Specific Capacitance, Fg−1 | Current Density, A g−1 | Retention | Cycles | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Cobalt oxide | SCS | 2 M KOH | 54 | 10 | 82% | 10,000 | [66] |
| 2 | Cobalt oxide thin film | Heating of an alkaline bath of cobalt salt | KOH 0.25 to 2.0 M | 118 | [67] | |||
| 3 | Spinel-nanostructured Co3O4 powder | SCS | 100 | 0.05−5 | 75% | 100 | [23] | |
| 4 | Co3O4 NPs | Solid-state calcination | 100 | 1.1 | 50 | [4] | ||
| 5 | Co3O4 NPs | SCS | 1 M KOH | 182 | 0.5 | 71% | 2000 | [88] |
| 6 | Hexagonal Co3O4 | SCS | 6 M KOH | 227 | 1 | 95% | 1000 | [103] |
| 7 | Co3O4 thin films | Electrodeposition | KOH | 235 | [94] | |||
| 8 | Co3O4 nanoflake | Hydrothermal | 2 M KOH | 351 | good | 4000 | [104] | |
| 9 | Co3O4 nanospheres | One pot hydrothermal | 182 | 1 | 93.75% | 8000 | [90] | |
| 10 | Cobalt oxide | SCS | 2 M KOH | 351 | 0.85 | 98.6% | 1000 | [95] |
| 11 | Cobalt oxide flakes | Potentiodynamic approach | 0.1 M Na2SO4 | 396.67 | better cyclic retention | 1600 | [105] | |
| 12 | Marigold 3D flower like Co3O4 NPs | SCS | 3 M KOH | 603 | 97.6% | 5000 | [47] | |
| 13 | Cobalt oxide | Electrode Position | PH 12 | 504 | 600 | [106] | ||
| 14 | Co3O4@C NPs | Simple Thermolysis | 2 M KOH | 642 | 1 | [38] | ||
| 15 | Co3O4 nanoflakes | Cathodic potential step | 598.9 | 6.25 | [107] | |||
| 16 | Pure Co3O4 NPs and Co3O4 /graphite nanocomposite | Co-precipitation | 6 M KOH | 239.5 for pure 395.04 for Co3O4/ graphite | 0.5 | 2.68% | 1000 | [108] |
4.2.2. Batteries
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abu-Zied, B.M.; Alam, M.M.; Asiri, A.M.; Ahmed, J.; Rahman, M.M. Efficient hydroquinone sensor development based on Co3O4 nanoparticle. Microchem. J. 2020, 157, 104972. [Google Scholar] [CrossRef]
- Zhao, J.; Zheng, C.; Gao, J.; Gui, J.; Deng, L.; Wang, Y.; Xu, R. Co3O4 nanoparticles embedded in laser-induced graphene for a flexible and highly sensitive enzyme-free glucose biosensor. Sens. Actuators B Chem. 2021, 347, 130653. [Google Scholar] [CrossRef]
- Sharifi, S.L.; Shakur, H.R.; Mirzaei, A.; Hosseini, M.H. Characterisation of cobalt oxide Co3O4 nanoparticles prepared by various methods: Effect of calcination temperatures on size, dimension and catalytic decomposition of hydrogen peroxide. Int. J. Nanosci. Nanotechnol. 2013, 9, 51–58. [Google Scholar]
- Mahmood, Z.H.; Jarosova, M.; Kzar, H.H.; Machek, P.; Zaidi, M.; Dehno Khalaji, A.; Kadhim, M.M. Synthesis and characterization of Co3O4 nanoparticles: Application as performing anode in Li-ion batteries. J. Chin. Chem. Soc. 2022, 69, 657–662. [Google Scholar] [CrossRef]
- Liu, D.; Wang, X.; Wang, X.; Tian, W.; Bando, Y.; Golberg, D. Co3O4 nanocages with highly exposed {110} facets for high-performance lithium storage. Sci. Rep. 2013, 3, 2543. [Google Scholar] [CrossRef]
- Han, S.; Wang, C.; Huang, Y.; Zhang, X.; Liu, J.; Li, Y. Graphene frameworks supported cobalt oxide with tunable morphologies for enhanced lithium storage behaviors. J. Mater. Sci. 2016, 51, 4856–4863. [Google Scholar] [CrossRef]
- Meghdadi, S.; Amirnasr, M.; Zhiani, M.; Zand, Z.; Heydari, M. Facile synthesis of cobalt oxide nanoparticles by thermal decomposition of cobalt(II) carboxamide complexes: Application as oxygen evolution reaction electrocatalyst in alkaline water electrolysis. Electrocatalysis 2017, 8, 122–131. [Google Scholar] [CrossRef]
- Al Kiey, S.A.; Sery, A.A.; Farag, H.K. Sol-gel synthesis of nanostructured cobalt oxide in four different ionic liquids. J. Sol-Gel Sci. Technol. 2023, 106, 37–43. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, Y.; Liu, W.; Zhang, S.; Liu, G.; Chen, H.; Qiu, J. Hydrothermal synthesis and electrochemical performance of Co3O4/reduced graphene oxide nanosheet composites for supercapacitors. Electrochim. Acta 2013, 112, 120–126. [Google Scholar] [CrossRef]
- Farhadi, S.; Javanmard, M.; Nadri, G. Characterization of cobalt oxide nanoparticles prepared by the thermal decomposition. Acta Chim. Slov. 2016, 63, 335–343. [Google Scholar] [CrossRef]
- Alem, A.F.; Worku, A.K.; Ayele, D.W.; Wubieneh, T.A.; abebaw Teshager, A.; Admasu, B.T.; Yemata, T.A. Ag doped Co3O4 nanoparticles for high-performance supercapacitor application. Heliyon 2023, 9, e13286. [Google Scholar] [CrossRef] [PubMed]
- Al-Senani, G.M.; Al-Saeedi, S.I. The use of synthesized CoO/Co3O4 nanoparticles as a corrosion inhibitor of low-carbon steel in 1 M HCl. Materials 2022, 15, 3129. [Google Scholar] [CrossRef] [PubMed]
- Hashami, M. Synthesized Co3O4 nanoparticles by solution combustion method and their environmental remediation applications. Vestn. Toraighyrov Univ. 2023, 4, 47–57. [Google Scholar] [CrossRef]
- Hafeez, M.; Shaheen, R.; Akram, B.; Haq, S.; Mahsud, S.; Ali, S.; Khan, R.T. Green synthesis of cobalt oxide nanoparticles for potential biological applications. Mater. Res. Express 2020, 7, 025019. [Google Scholar] [CrossRef]
- Govindasamy, R.; Raja, V.; Singh, S.; Govindarasu, M.; Sabura, S.; Rekha, K.; Rajeswari, V.D.; Alharthi, S.S.; Vaiyapuri, M.; Sudarmani, R. Green synthesis and characterization of cobalt oxide nanoparticles using Psidium guajava leaves extracts, and their photocatalytic and biological activities. Molecules 2022, 27, 5646. [Google Scholar] [CrossRef]
- Abouali, S.; Akbari Garakani, M.; Zhang, B.; Xu, Z.L.; Kamali Heidari, E.; Huang, J.Q.; Kim, J.K. Electrospun carbon nanofibers with in situ encapsulated Co3O4 nanoparticles as electrodes for high-performance supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 13503–13511. [Google Scholar] [CrossRef]
- Varma, A.; Mukasyan, A.S.; Rogachev, A.S.; Manukyan, K.V. Solution combustion synthesis of nanoscale materials. Chem. Rev. 2016, 116, 14493–14586. [Google Scholar] [CrossRef]
- Keneshbekova, A.; Imash, A.; Kaidar, B.; Yensep, E.; Ilyanov, A.; Artykbaeva, M.; Smagulova, G. Morphological features of Co3O4 nanoparticles obtained by solution combustion method. Combust. Plasma Chem. 2023, 21, 159–171. [Google Scholar]
- Kumar, G.P.; Jawahar, I.N.; Biju, V. Synthesis of nanocrystalline Co3O4 through solution combustion method: Effect of fuel to oxidizer ratio on structural and physical properties. J. Mater. Sci. Mater. Electron. 2021, 32, 14919–14931. [Google Scholar] [CrossRef]
- Hashami, M.; Mansurov, Z.A. Synthesis of Co3O4 nanoparticles by solution combustion synthesis (SCS) and their structure morphology: A mini-review. Combust. Plasma Chem. 2025, 23, 53–62. [Google Scholar]
- Dudnik, O.V.; Lakiza, S.M.; Marek, I.O.; Red’ko, V.P.; Makudera, A.O.; Ruban, O.K. Advanced Approaches for Producing Nanocrystalline and Fine-Grained ZrO2-Based Powders (Review) I. Mechanical, Physical and Chemical Methods (Excluding ‘Wet’Chemistry). Powder Metall. Met. Ceram. 2025, 63, 426–443. [Google Scholar] [CrossRef]
- Kingsley, J.J.; Patil, K.C. A novel combustion process for the synthesis of fine particle α-alumina and related oxide materials. Mater. Lett. 1988, 6, 427–432. [Google Scholar] [CrossRef]
- Michalska, M.; Xu, H.; Shan, Q.; Zhang, S.; Dall’Agnese, Y.; Gao, Y.; Krajewski, M. Solution combustion synthesis of a nanometer-scale Co3O4 anode material for Li-ion batteries. Beilstein J. Nanotechnol. 2021, 12, 424–431. [Google Scholar] [CrossRef]
- Acedera, R.A.E.; Gupta, G.; Mamlouk, M.; Balela, M.D.L. Solution combustion synthesis of porous Co3O4 nanoparticles as oxygen evolution reaction (OER) electrocatalysts in alkaline medium. J. Alloys Compd. 2020, 836, 154919. [Google Scholar] [CrossRef]
- Deng, J.; Kang, L.; Bai, G.; Li, Y.; Li, P.; Liu, X.; Liang, W. Solution combustion synthesis of cobalt oxides (Co3O4 and Co3O4/CoO) nanoparticles as supercapacitor electrode materials. Electrochim. Acta 2014, 132, 127–135. [Google Scholar] [CrossRef]
- Murayama, M.; Kobayashi, N.; Matsushima, Y.; Unuma, H. Low-temperature synthesis of porous Co3O4 thin films through two approaches: Metal organic framework-decomposition and solution combustion methods. J. Ceram. Soc. Jpn. 2019, 127, 741–746. [Google Scholar] [CrossRef]
- El-Shafie, A.S.; Ahsan, I.; Radhwani, M.; Al-Khangi, M.A.; El-Azazy, M. Synthesis and application of cobalt oxide (Co3O4)-impregnated olive stones biochar for the removal of rifampicin and tigecycline: Multivariate controlled performance. Nanomaterials 2022, 12, 379. [Google Scholar] [CrossRef] [PubMed]
- Pagar, T.; Ghotekar, S.; Pagar, K.; Pansambal, S.; Oza, R. A review on bio-synthesized Co3O4 nanoparticles using plant extracts and their diverse applications. J. Chem. Rev. 2019, 1, 260–270. [Google Scholar]
- Jahani, M.; Khavari-Nejad, R.A.; Mahmoodzadeh, H.; Saadatmand, S. Effects of cobalt oxide nanoparticles (Co3O4 NPs) on ion leakage, total phenol, antioxidant enzymes activities and cobalt accumulation in Brassica napus L. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 48, 1260–1275. [Google Scholar] [CrossRef]
- Kaur, A.; Kaur, H.; Kumar, S.; Singh, P.P.; Bala, K.; Kumar, S.; Singh, G. Crafting Superior Photocatalytic Potential: Synergistic Precipitation-Hydrothermal Customization of CTAB-Engineered Co3O4 Nanoparticles. J. Clust. Sci. 2025, 36, 11. [Google Scholar] [CrossRef]
- Yan, Z.; Gong, W.; Liu, X.; Gao, A.; Li, Y.; Wang, Y.; Lin, J. A novel hierarchical Co3O4/ZnIn2S4 0D/3D pn heterojunction nanocomposite for efficient visible-light-driven hydrogen production. Fuel 2025, 393, 134959. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Ahmad, S.; Ullah, H.; Alqarni, S.A.; Yao, S.; Khan, K.A.; Zaki, M.E. Thermal and electrical properties of PVDF modified Co3O4 functionalized MWCNTs. RSC Adv. 2025, 15, 8740–8749. [Google Scholar] [CrossRef]
- Ifijen, I.H.; Faderin, E.; Okafor, C.E.; Onyinyechi, O.L.; Aworinde, O.R.; Iorkula, T.H.; Udokpoh, N.U. Dual Functionality of Cobalt Oxide Nanoparticles: Exploring Their Potential as Antimicrobial and Anticancer Agents. Biomed. Mater. Devices 2025, 1–46. [Google Scholar] [CrossRef]
- Fan, X.; Xu, Y.; Ma, C.; He, W. In-situ growth of Co3O4 nanoparticles based on electrospray for an acetone gas sensor. J. Alloys Compd. 2021, 854, 157234. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Rashad, M.; Moharram, A.H.; Abdel-Rahim, M.A. Promising methane gas sensor synthesized by microwave-assisted Co3O4 nanoparticles. Mater. Sci. Semicond. Process. 2016, 46, 1–5. [Google Scholar] [CrossRef]
- Sisakyan, N.; Chilingaryan, G.; Manukyan, A.; Mukasyan, A.S. Combustion synthesis of materials for application in supercapacitors: A review. Nanomaterials 2023, 13, 3030. [Google Scholar] [CrossRef]
- Afrooze, A.; Shaik, D.P. Porous Co3O4 nanospheres synthesized via solution combustion method for supercapacitors. Chem. Pap. 2023, 77, 1201–1211. [Google Scholar] [CrossRef]
- Edison, T.N.J.I.; Atchudan, R.; Sethuraman, M.G.; Lee, Y.R. Supercapacitor performance of carbon supported Co3O4 nanoparticles synthesized using Terminalia chebula fruit. J. Taiwan Inst. Chem. Eng. 2016, 68, 489–495. [Google Scholar] [CrossRef]
- Lakra, R.; Kumar, R.; Thatoi, D.N.; Sahoo, P.K.; Soam, A. Synthesis and characterization of cobalt oxide (Co3O4) nanoparticles. Mater. Today Proc. 2021, 41, 269–271. [Google Scholar] [CrossRef]
- Do, J.S.; Weng, C.H. Preparation and characterization of CoO used as anodic material of lithium battery. J. Power Sources 2005, 146, 482–486. [Google Scholar] [CrossRef]
- Souvi, S.O.; Danset, D.; Alikhani, M.E.; Manceron, L. Formation and structure of Co2O4: A combined ir matrix isolation and theoretical study. J. Phys. Chem. A 2010, 114, 11399–11407. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Saikia, B.J. Synthesis, characterization and biological applications of cobalt oxide (Co3O4) nanoparticles. Chem. Phys. Impact 2023, 6, 100137. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, X.; Han, Z.; Wang, Y.; Jiang, H.; Zhang, F.; Huang, C. Dual modification of cobalt silicate nanobelts by Co3O4 nanoparticles and phosphorization boosting oxygen evolution reaction properties. J. Colloid Interface Sci. 2025, 679, 1036–1045. [Google Scholar] [CrossRef]
- Mumtaz, M.; Mumtaz, A. Approaching capacity limit: A comprehensive structural and electrochemical study of capacity enhancement in Co3O4 nanoparticles. Mater. Sci. Eng. B 2025, 314, 117988. [Google Scholar] [CrossRef]
- Akhter, N.; Liaquat, A.; Murtaza, F.; Yaqoob, A.; Sharif, S.; Jummah, N.; Luque, R. One-step phytosynthesis of Co3O4 nanoparticles and study of their catalytic, antioxidant, antibiofilm and antibacterial activities. Results Chem. 2025, 14, 102151. [Google Scholar] [CrossRef]
- Devi, V.S.; Athika, M.; Duraisamy, E.; Prasath, A.; Sharma, A.S.; Elumalai, P. Facile sol-gel derived nanostructured spinel Co3O4 as electrode material for high-performance supercapattery and lithium-ion storage. J. Energy Storage 2019, 25, 100815. [Google Scholar] [CrossRef]
- Afrooze, A.; Shaik, D.P. Marigold flower like Co3O4 nanoparticles as high performance electrodes for supercapacitors. Inorg. Chem. Commun. 2024, 159, 111663. [Google Scholar] [CrossRef]
- Hou, C.; Wang, B.; Murugadoss, V.; Vupputuri, S.; Chao, Y.; Guo, Z.; Du, W. Recent advances in Co3O4 as anode materials for high-performance lithium-ion batteries. Eng. Sci. 2020, 11, 19–30. [Google Scholar] [CrossRef]
- Aldashukurova, G.B.; Mironenko, A.V.; Mansurov, Z.A.; Shikina, N.V.; Yashnik, S.A.; Kuznetsov, V.V.; Ismagilov, Z.R. Synthesis gas production on glass cloth catalysts modified by Ni and Co oxides. J. Energy Chem. 2013, 22, 811–818. [Google Scholar] [CrossRef]
- Maurya, M.P.; Raitani, K.; Gyanprakash, M.; Rastogi, C.K.; Krishna, R.H.; Manjunatha, C. The copper interlayer tunes the reactivity of cobalt oxide towards the oxygen evolution reaction. Mater. Today Proc. 2023, 79, 193–197. [Google Scholar] [CrossRef]
- Vennela, A.B.; Mangalaraj, D.; Muthukumarasamy, N.; Agilan, S.; Hemalatha, K.V. Structural and optical properties of Co3O4 nanoparticles prepared by sol-gel technique for photocatalytic application. Int. J. Electrochem. Sci. 2019, 14, 3535–3552. [Google Scholar] [CrossRef]
- Meher, S.K.; Rao, G.R. Ultralayered Co3O4 for high-performance supercapacitor applications. J. Phys. Chem. C 2011, 115, 15646–15654. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X.; Wang, X.; Yang, X.; Lu, L. Preparation and electrochemical properties of mesoporous Co3O4 crater-like microspheres as supercapacitor electrode materials. Curr. Appl. Phys. 2010, 10, 1422–1426. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Z.; Li, Z.; Wang, B.; Yan, Y.; Liu, Q.; Jiang, Z. Green synthesis of graphene nanosheets/ZnO composites and electrochemical properties. J. Solid State Chem. 2011, 184, 1421–1427. [Google Scholar] [CrossRef]
- Xu, J.; Gao, L.; Cao, J.; Wang, W.; Chen, Z. Preparation and electrochemical capacitance of cobalt oxide (Co3O4) nanotubes as supercapacitor material. Electrochim. Acta 2010, 56, 732–736. [Google Scholar] [CrossRef]
- Zhu, Y.; Ji, X.; Pan, C.; Sun, Q.; Song, W.; Fang, L.; Banks, C.E. A carbon quantum dot decorated RuO2 network: Outstanding supercapacitances under ultrafast charge and discharge. Energy Environ. Sci. 2013, 6, 3665–3675. [Google Scholar] [CrossRef]
- Liu, Z.; Mi, J.; Yang, Y.; Tan, X.; Lv, C. Easy synthesis of phosphorus-incorporated three-dimensionally ordered macroporous carbons with hierarchical pores and their use as electrodes for supercapacitors. Electrochim. Acta 2014, 115, 206–215. [Google Scholar] [CrossRef]
- Yin, X.; Wang, Z.; Wang, J.; Yan, G.; Xiong, X.; Li, X.; Guo, H. One-step facile synthesis of porous Co3O4 microspheres as anode materials for lithium-ion batteries. Mater. Lett. 2014, 120, 73–75. [Google Scholar] [CrossRef]
- Han, D.; Xu, P.; Jing, X.; Wang, J.; Yang, P.; Shen, Q.; Zhang, M. Trisodium citrate assisted synthesis of hierarchical NiO nanospheres with improved supercapacitor performance. J. Power Sources 2013, 235, 45–53. [Google Scholar] [CrossRef]
- Qing, X.; Liu, S.; Huang, K.; Lv, K.; Yang, Y.; Lu, Z.; Liang, X. Facile synthesis of Co3O4 nanoflowers grown on Ni foam with superior electrochemical performance. Electrochim. Acta 2011, 56, 4985–4991. [Google Scholar] [CrossRef]
- Li, Y.; Tan, B.; Wu, Y. Freestanding mesoporous quasi-single-crystalline Co3O4 nanowire arrays. J. Am. Chem. Soc. 2006, 128, 14258–14259. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Miao, Z.; Wu, T.; Zhang, Z.; Yang, X. Micro/nano-structure Co3O4 as high capacity anode materials for lithium-ion batteries and the effect of the void volume on electrochemical performance. J. Power Sources 2014, 248, 28. [Google Scholar] [CrossRef]
- Sahoo, P.; Djieutedjeu, H.; Poudeu, P.F. Co3O4 nanostructures: The effect of synthesis conditions on particles size, magnetism and transport properties. J. Mater. Chem. A 2013, 1, 15022–15030. [Google Scholar] [CrossRef]
- Gyulasaryan, H.; Kuzanyan, A.; Manukyan, A.; Mukasyan, A.S. Combustion synthesis of magnetic nanomaterials for biomedical applications. Nanomaterials 2023, 13, 1902. [Google Scholar] [CrossRef]
- Ortiz-Quiñonez, J.L.; Pal, U.; Villanueva, M.S. Structural, magnetic, and catalytic evaluation of spinel Co, Ni, and Co–Ni ferrite nanoparticles fabricated by low-temperature solution combustion process. ACS Omega 2018, 3, 14986–15001. [Google Scholar] [CrossRef]
- Zallouz, S.; Réty, B.; Vidal, L.; Le Meins, J.M.; Matei Ghimbeu, C. Co3O4 nanoparticles embedded in mesoporous carbon for supercapacitor applications. ACS Appl. Nano Mater. 2021, 4, 5022–5037. [Google Scholar] [CrossRef]
- Singhal, A.; Bisht, A.; Kumar, A.; Sharma, S. One pot, rapid synthesis of Co3O4 by solution combustion method and its electrochemical properties in different electrolytes. J. Electroanal. Chem. 2016, 776, 152–161. [Google Scholar] [CrossRef]
- Yu, J.; Ni, Y.; Zhai, M. Highly selective non-enzyme glucose detection based on Co-CoO-Co3O4 nanocomposites prepared via a solution-combustion and subsequent heat-treating route. J. Alloys Compd. 2017, 723, 904–911. [Google Scholar] [CrossRef]
- Wójcik, S.; Ercolino, G.; Gajewska, M.; Quintero, C.W.M.; Specchia, S.; Kotarba, A. Robust Co3O4 α-Al2O3|cordierite structured catalyst for N2O abatement–Validation of the SCS method for active phase synthesis and deposition. Chem. Eng. J. 2019, 377, 120088. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, X.; Ma, W.; Wu, M.; Qian, N.; Lu, W. Novel three-dimensional Co3O4 dendritic superstructures: Hydrothermal synthesis, formation mechanism and magnetic properties. CrystEngComm 2013, 15, 1389–1396. [Google Scholar] [CrossRef]
- Rashad, M.; Rüsing, M.; Berth, G.; Lischka, K.; Pawlis, A. CuO and Co3O4 nanoparticles: Synthesis, characterizations, and Raman spectroscopy. J. Nanomater. 2013, 2013, 714853. [Google Scholar] [CrossRef]
- Zou, D.; Yi, Y.; Song, Y.; Guan, D.; Xu, M.; Ran, R.; Shao, Z. The BaCe0.16Y0.04Fe0.8O3−δ nanocomposite: A new high-performance cobalt-free triple-conducting cathode for protonic ceramic fuel cells operating at reduced temperatures. J. Mater. Chem. A 2022, 10, 5381–5390. [Google Scholar] [CrossRef]
- Tong, X.; Hai, Z.; Cui, D.; Gao, L.; Zhang, Q.; Xu, H.; Liu, J. Investigation of lattice distortion of Co3O4 nanoparticles prepared by a carbon-assisted method. Microelectron. Eng. 2016, 159, 17–20. [Google Scholar] [CrossRef]
- Ravina Srivastava, G.; Dalela, S.; Kumar, S.; Nasit, M.; Singh, J.; Alvi, P.A. Study of structural, optical, surface and electrochemical properties of Co3O4 nanoparticles for energy storage applications. Interactions 2024, 245, 85. [Google Scholar] [CrossRef]
- Srijith, S.; Asitha, L.R.; Amritha, R.S.; Ridhun, M. Structural and Electrical Characterization of Cobalt Oxide Nanoparticles. Mater. Chem. Phys 2023, 1, 07–15. [Google Scholar]
- Jogdand, S.S.; Joshi, S.S. Effect of polymer combustion synthesis on thermal, spectroscopic, structural, and magnetic properties of Co3O4 nanoparticles. Polyhedron 2025, 269, 117419. [Google Scholar] [CrossRef]
- Zhang, L.; Deng, Z.; Miao, B.; He, H.; Poh, C.K.; Zhang, L.; Chan, S.H. Aqueous Gel-Casting Synthesis and the Characterization of Cobalt Oxide as a Catalyst Precursor for Sodium Borohydride Hydrolysis. Catalysts 2025, 15, 380. [Google Scholar] [CrossRef]
- Groven, L.J.; Pfeil, T.L.; Pourpoint, T.L. Solution combustion synthesized cobalt oxide catalyst precursor for NaBH4 hydrolysis. Int. J. Hydrogen Energy 2013, 38, 6377–6380. [Google Scholar] [CrossRef]
- Khort, A.; Podbolotov, K.; Serrano-García, R.; Gun’ko, Y. One-step solution combustion synthesis of cobalt nanopowder in air atmosphere: The fuel effect. Inorg. Chem. 2018, 57, 1464–1473. [Google Scholar] [CrossRef]
- Huang, M.; Lv, S.; Zhou, C. Thermal decomposition kinetics of glycine in nitrogen atmosphere. Thermochim. Acta 2013, 552, 60–64. [Google Scholar] [CrossRef]
- Yablokov, V.Y.; Smel’tsova, I.L.; Zelyaev, I.A.; Mitrofanova, S.V. Studies of the rates of thermal decomposition of glycine, alanine, and serine. Russ. J. Gen. Chem. 2009, 79, 1704–1706. [Google Scholar] [CrossRef]
- Sato, N. Decomposition of glycine in high temperature and high pressure water. Kagaku Kogaku Ronbunshu 2002, 28, 113–117. [Google Scholar] [CrossRef]
- Wei, X.; Kang, J.; Gan, L.; Wang, W.; Yang, L.; Wang, D.; Qi, J. Recent advances in Co3O4-based composites: Synthesis and application in combustion of methane. Nanomaterials 2023, 13, 1917. [Google Scholar] [CrossRef]
- Wang, Z.; Hou, X.; Shen, J.; Li, T. Supported cobalt oxide nanocrystals: Morphology control and catalytic performance for styrene oxidation. RSC Adv. 2016, 6, 89503–89509. [Google Scholar] [CrossRef]
- Teng, F.; Chen, M.; Li, G.; Teng, Y.; Xu, T.; Hang, Y.; Zhu, Y. High combustion activity of CH4 and catalluminescence properties of CO oxidation over porous Co3O4 nanorods. Appl. Catal. B Environ. 2011, 110, 133–140. [Google Scholar] [CrossRef]
- Jadhav, H.S.; Rai, A.K.; Lee, J.Y.; Kim, J.; Park, C.J. Enhanced electrochemical performance of flower-like Co3O4 as an anode material for high performance lithium-ion batteries. Electrochim. Acta 2014, 146, 270–277. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, C.; Li, S.; Sun, Y. First principles prediction of CH 4 reactivities with Co3O4 nanocatalysts of different morphologies. Phys. Chem. Chem. Phys. 2017, 19, 30874–30882. [Google Scholar] [CrossRef]
- Trivedi, S.; Prasad, R. Reactive calcination route for synthesis of active Mn–Co3O4 spinel catalysts for abatement of CO–CH4 emissions from CNG vehicles. J. Environ. Chem. Eng. 2016, 4, 1017–1028. [Google Scholar] [CrossRef]
- Yao, X.; Xin, X.; Zhang, Y.; Wang, J.; Liu, Z.; Xu, X. Co3O4 nanowires as high capacity anode materials for lithium ion batteries. J. Alloys Compd. 2012, 521, 95–100. [Google Scholar] [CrossRef]
- Al Jahdaly, B.A.; Abu-Rayyan, A.; Taher, M.M.; Shoueir, K. Phytosynthesis of Co3O4 nanoparticles as the high energy storage material of an activated carbon/Co3O4 symmetric supercapacitor device with excellent cyclic stability based on a Na2SO4 aqueous electrolyte. ACS Omega 2022, 7, 23673–23684. [Google Scholar] [CrossRef] [PubMed]
- Waris, A.; Din, M.; Ali, A.; Afridi, S.; Baset, A.; Khan, A.U.; Ali, M. Green fabrication of Co and Co3O4 nanoparticles and their biomedical applications: A review. Open Life Sci. 2021, 16, 14–30. [Google Scholar] [CrossRef]
- Anele, A.; Obare, S.; Wei, J. Recent trends and advances of Co3O4 nanoparticles in environmental remediation of bacteria in wastewater. Nanomaterials 2022, 12, 1129. [Google Scholar] [CrossRef]
- Wang, X.; Mou, H.; Zhao, Q.; Sun, Y.; Zeng, Z.; Zhao, H.; Xu, J. Carbon Monoxide Combustion on Metal Oxide Supported Au@CuxO Catalysts at Low Temperature. Combust. Sci. Technol. 2021, 193, 2505–2513. [Google Scholar] [CrossRef]
- Liu, F.; Su, H.; Jin, L.; Zhang, H.; Chu, X.; Yang, W. Facile synthesis of ultrafine cobalt oxide nanoparticles for high-performance supercapacitors. J. Colloid Interface Sci. 2017, 505, 796–804. [Google Scholar] [CrossRef]
- Manteghi, F.; Kazemi, S.H.; Peyvandipour, M.; Asghari, A. Preparation and application of cobalt oxide nanostructures as electrode materials for electrochemical supercapacitors. RSC Adv. 2015, 5, 76458–76463. [Google Scholar] [CrossRef]
- Xu, G.L.; Li, J.T.; Huang, L.; Lin, W.; Sun, S.G. Synthesis of Co3O4 nano-octahedra enclosed by {111} facets and their excellent lithium storage properties as anode material of lithium ion batteries. Nano Energy 2013, 2, 394–402. [Google Scholar] [CrossRef]
- Halder, S.; Roy, S.; Roy, S.; Chakraborty, C. Enriching oxygen vacancy in Co3O4 by solution combustion synthesis for enhanced supercapacitive property. J. Phys. Chem. C 2023, 127, 18279–18290. [Google Scholar] [CrossRef]
- Alex, J.; Silambarasan, S.; Jose, A.K.; AswathyW, F.; Maiyalagan, T.; Aravind, A.; Sajan, D. Cerium-doped Co3O4 spinel structures synthesized by modified combustion route as an excellent material for electrochemical applications. Ceram. Int. 2025, 51, 3185–3197. [Google Scholar] [CrossRef]
- Nguyen, D.T.A.; Alene, A.N.; Teshager, A.A.; Worku, A.K.; Abate, G.Y.; Li, H.T. Enhanced electrochemical activity of boron doped cobalt oxide nanoparticles towards supercapacitor application. Electrochim. Acta 2025, 510, 145318. [Google Scholar] [CrossRef]
- Indumathi, N.; Sridevi, C.; Madona, J.; Gokulavani, G.; Prabhu, S. Fabrication of novel chitosan decorated reduced graphene oxide/cobalt oxide hybrid nanocomposite electrode material for supercapacitor applications. Inorg. Chem. Commun. 2025, 171, 113544. [Google Scholar] [CrossRef]
- Balachandran, S.; Jothi, K.J.; Albeshr, M.F.; Anbalagan, G.; Kumar, R.D.; Prakash, N.; Kumar, A.S.K. Evaluation of nitrogen-doped cobalt oxide with a flower-like architecture for high-performance supercapacitors. J. Energy Storage 2025, 120, 116479. [Google Scholar] [CrossRef]
- Darko, S.A.; Puplampu, S.T.; Yeboah, D.; Mensah-Darkwa, K. Enhancing the supercapacitive performance of soot material induced with manganese cobaltite (MnCo2O4) for supercapacitor application. Next Mater. 2025, 7, 100382. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, X.; Shioyama, H.; Mukai, T.; Sakai, T.; Xu, Q. Converting cobalt oxide subunits in cobalt metal-organic framework into agglomerated Co3O4 nanoparticles as an electrode material for lithium ion battery. J. Power Sources 2010, 195, 857–861. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Li, L.; Shi, S.J.; Xiong, Q.Q.; Zhao, X.Y.; Wang, X.L.; Tu, J.P. Synthesis of porous Co3O4 nanoflake array and its temperature behavior as pseudo-capacitor electrode. J. Power Sources 2014, 256, 200–205. [Google Scholar] [CrossRef]
- Niveditha, C.V.; Aswini, R.; Fatima, M.J.; Ramanarayan, R.; Pullanjiyot, N.; Swaminathan, S. Feather like highly active Co3O4 electrode for supercapacitor application: A potentiodynamic approach. Mater. Res. Express 2018, 5, 065501. [Google Scholar] [CrossRef]
- Kalyani, M.; Emerson, R.N. High-performance supercapacitor cobalt oxide thin films by the influence of copper substrate. Int. J. Pure Appl. Phys 2018, 14, 115–124. [Google Scholar]
- Kazemi, S.H.; Asghari, A. High performance supercapacitors based on the electrodeposited Co3O4 nanoflakes on electro-etched carbon fibers. Electrochim. Acta 2014, 138, 9–14. [Google Scholar] [CrossRef]
- Gopalakrishnan, M.; Srikesh, G.; Mohan, A.; Arivazhagan, V. In-situ synthesis of Co3O4/graphite nanocomposite for high-performance supercapacitor electrode applications. Appl. Surf. Sci. 2017, 403, 578–583. [Google Scholar]
- Goudar, J.A.; SN, T.; Chapi, S.; MV, M.; Saeb, M.R.; Salami-Kalajahi, M. Cobalt-Based Materials in Supercapacitors and Batteries: A Review. Adv. Energy Sustain. Res. 2025, 6, 2400271. [Google Scholar] [CrossRef]
- Garcia, M.F.; Arzuza, L.C.; Neves, G.A.; Loureiro, F.J.; Morales, M.A.; Macedo, D.A.; Menezes, R.R. Structure and Morphological Properties of Cobalt-Oxide-Based (Co3O4) Materials as Electrodes for Supercapacitors: A Brief Review. Materials 2025, 18, 413. [Google Scholar] [CrossRef]
- Kumar, P.S.; Nadar, N.R.; Sharma, S.C.; Das, B.K. Development of solution combustion-synthesized RGO/ZnO:1Eu3+ nanocomposite for supercapacitor and fingerprint visualization applications. Diam. Relat. Mater. 2025, 153, 112103. [Google Scholar] [CrossRef]
- Haritha, B.; Deepak, M.; Hussain, O.M.; Julien, C.M. Morphological Engineering of Battery-Type Cobalt Oxide Electrodes for High-Performance Supercapacitors. Physchem 2025, 5, 11. [Google Scholar] [CrossRef]
- Pitcheri, R.; Mooni, S.P.; Radhalayam, D.; Nora, M.; Roy, S.; Al-Zahrani, F.A.M.; Suneetha, M. Effect of Ce-doping on the structural, morphological, and electrochemical features of Co3O4 nanoparticles synthesized by solution combustion method for battery-type supercapacitors. Ceram. Int. 2024, 50, 50504–50515. [Google Scholar] [CrossRef]
- Mallikarjuna, K.; Reddy, G.R.; Ghanem, M.A.; Dillip, G.R.; Joo, S.W. Insights of oxygen vacancies engineered structural, morphological, and electrochemical attributes of Cr-doped Co3O4 nanoparticles as redox active battery-type electrodes for hybrid supercapacitors. J. Energy Storage 2024, 100, 113751. [Google Scholar] [CrossRef]
- Wen, W.; Wu, J.M.; Tu, J.P. A novel solution combustion synthesis of cobalt oxide nanoparticles as negative-electrode materials for lithium ion batteries. J. Alloys Compd. 2012, 513, 592–596. [Google Scholar] [CrossRef]
- Drummer, S.; Mkhari, O.; Chowdhury, M. Green synthesis of Co3O4 nanoparticles using spent coffee: Application in catalytic and photocatalytic dye degradation. Next Nanotechnol. 2024, 6, 100069. [Google Scholar] [CrossRef]
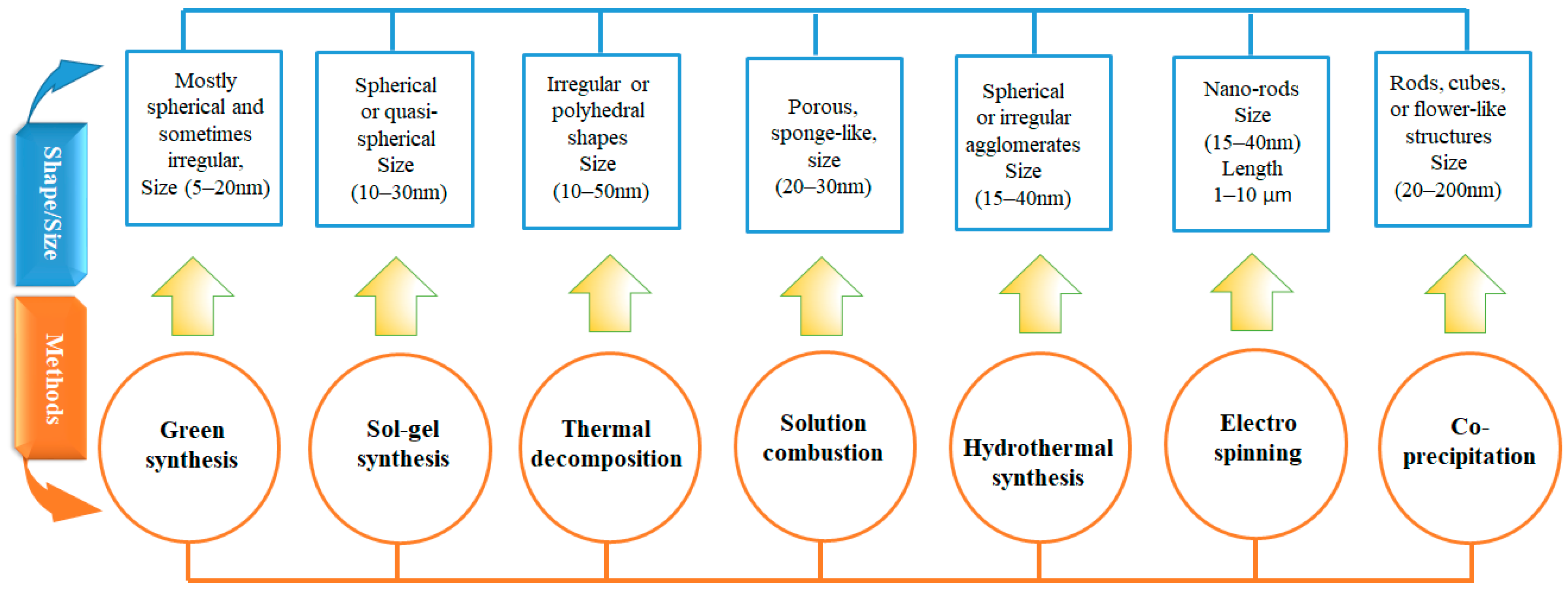
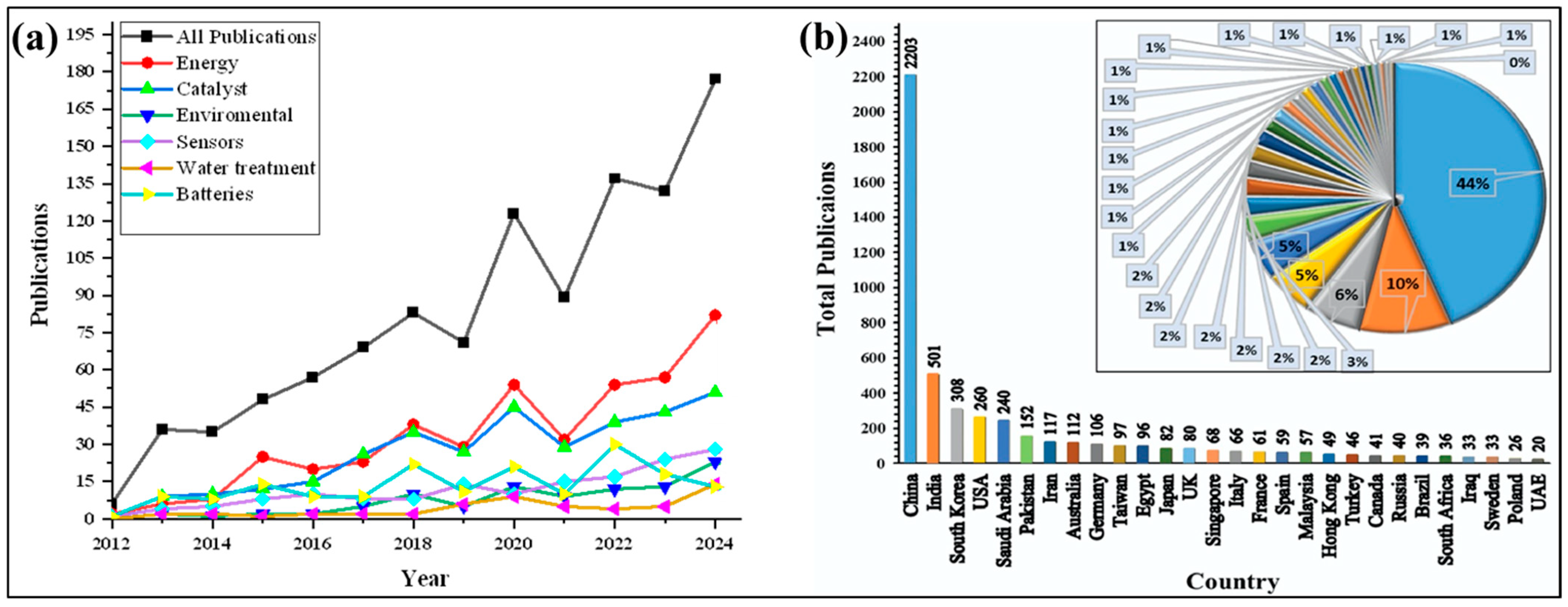
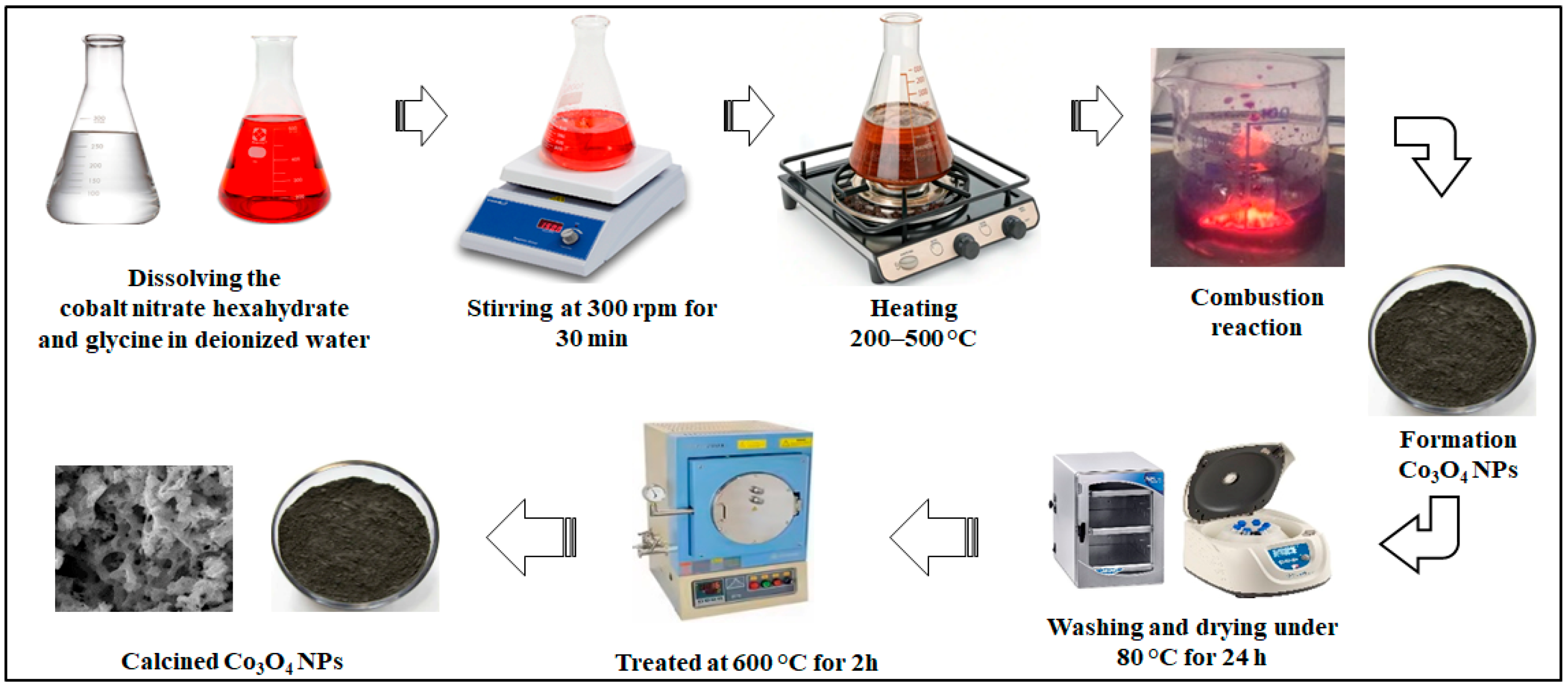
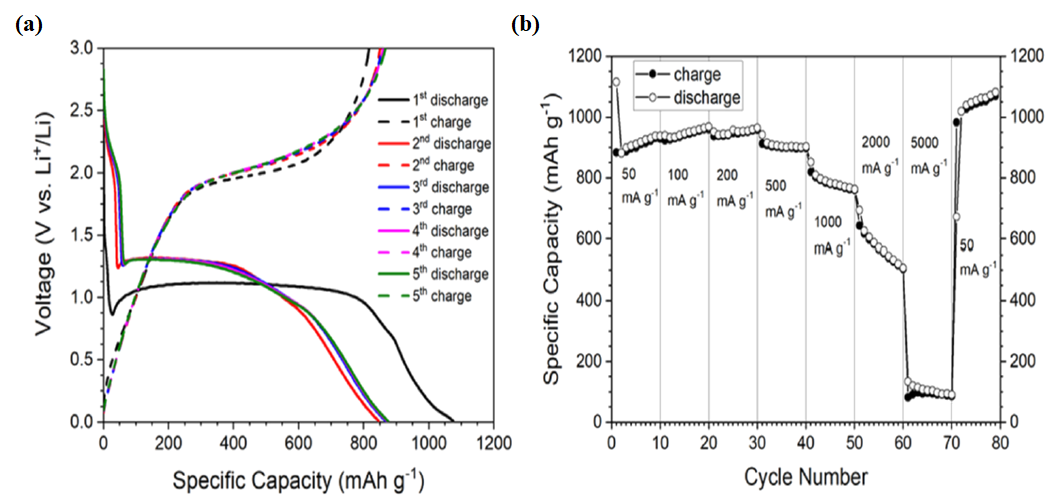
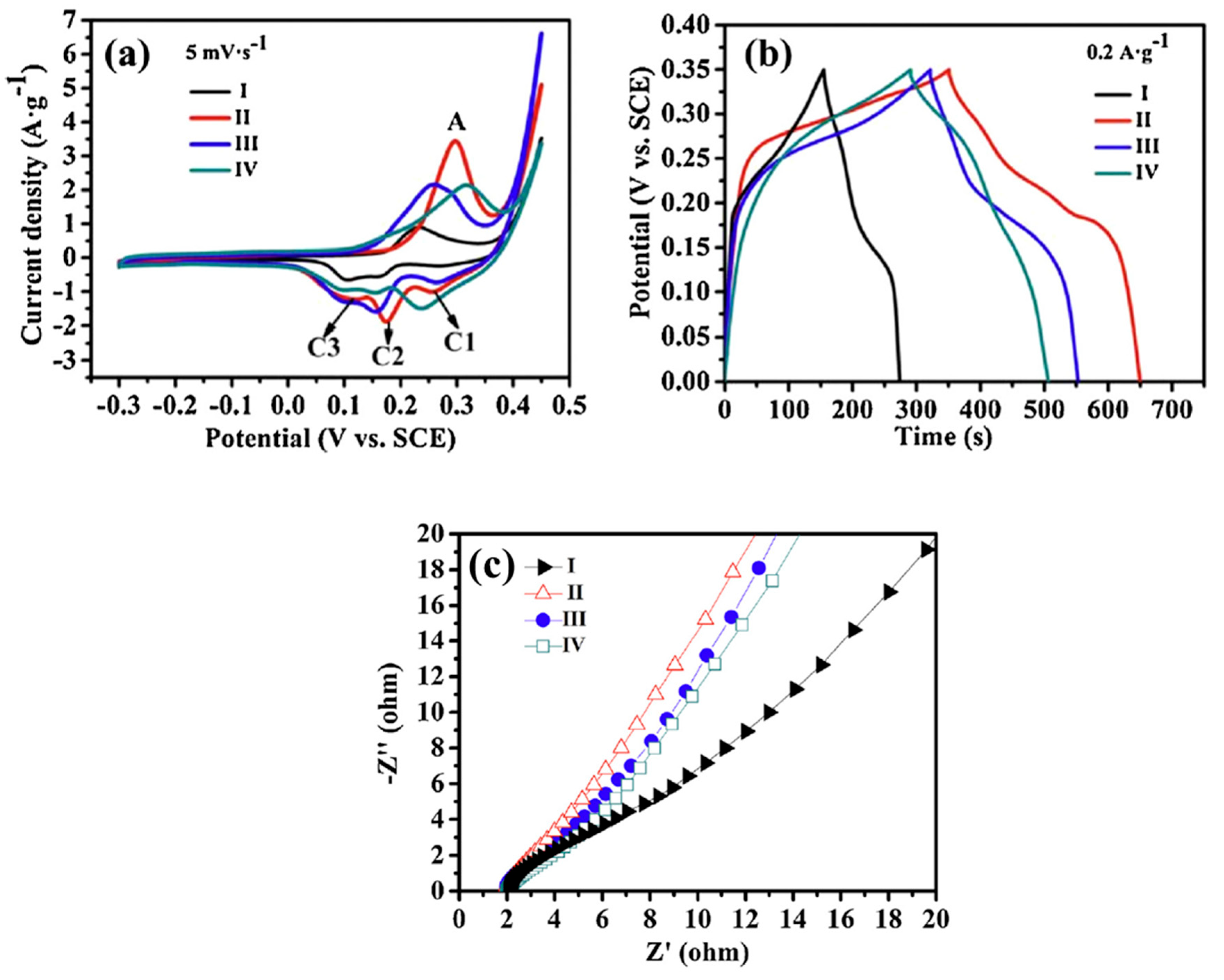
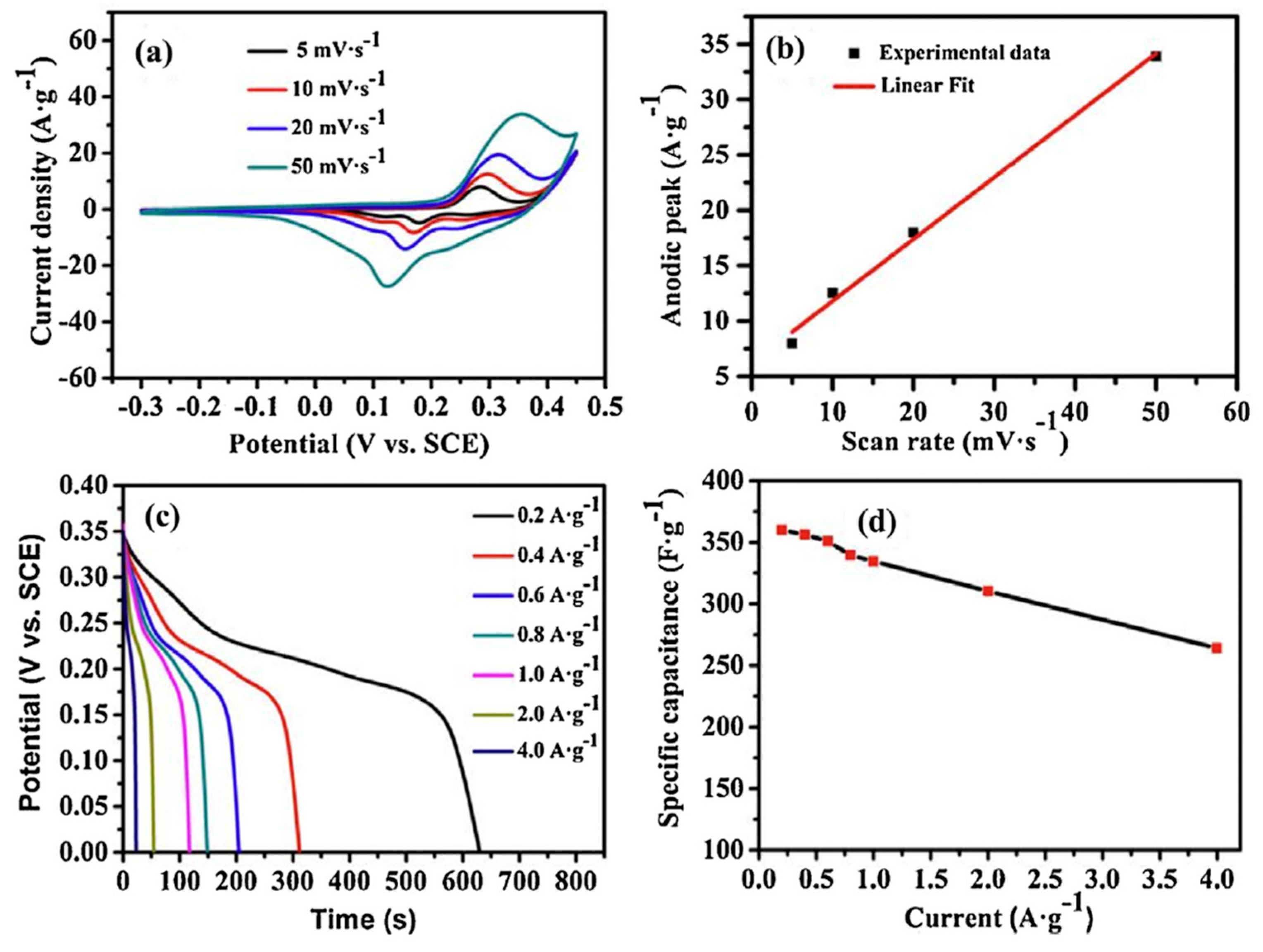
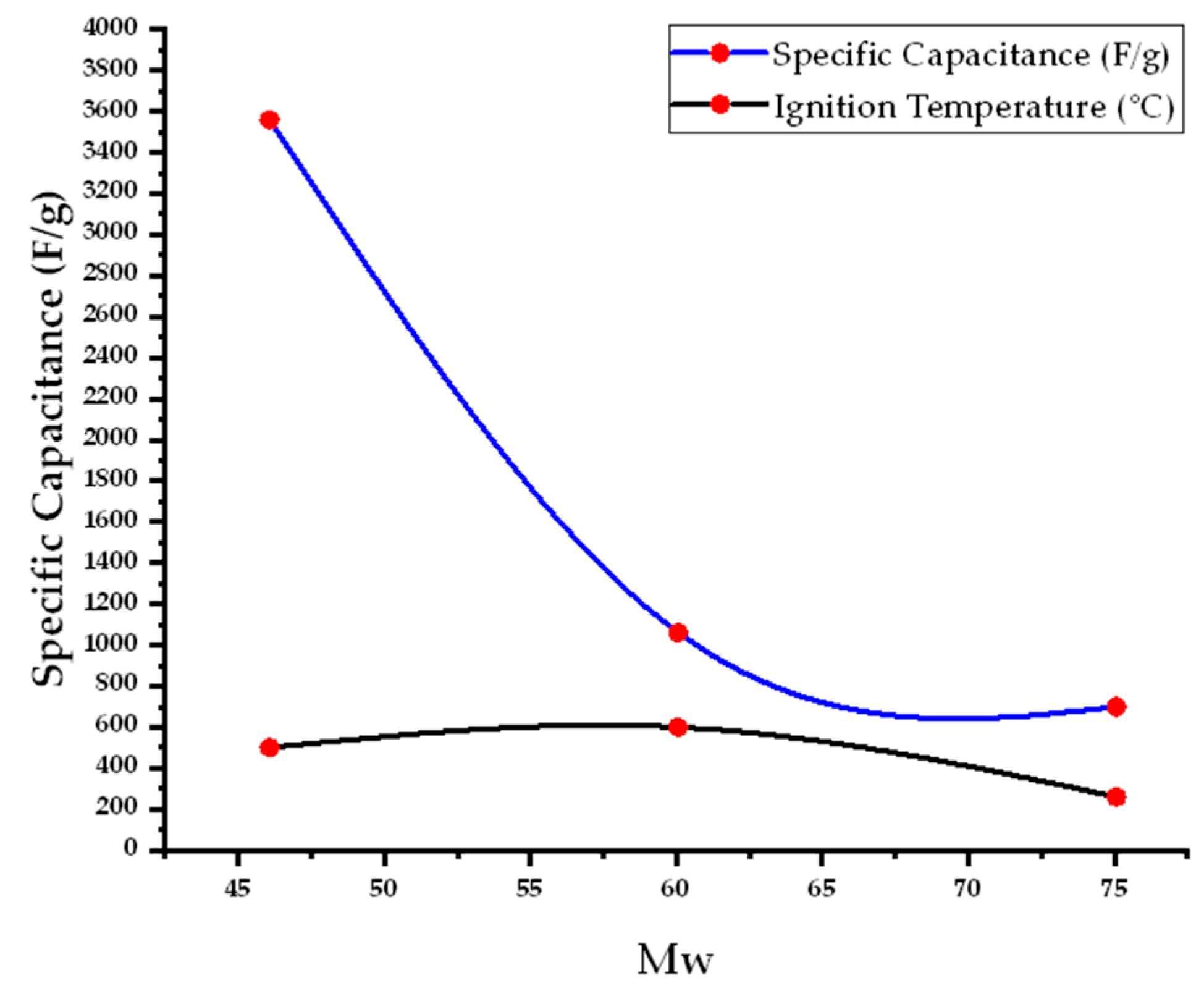
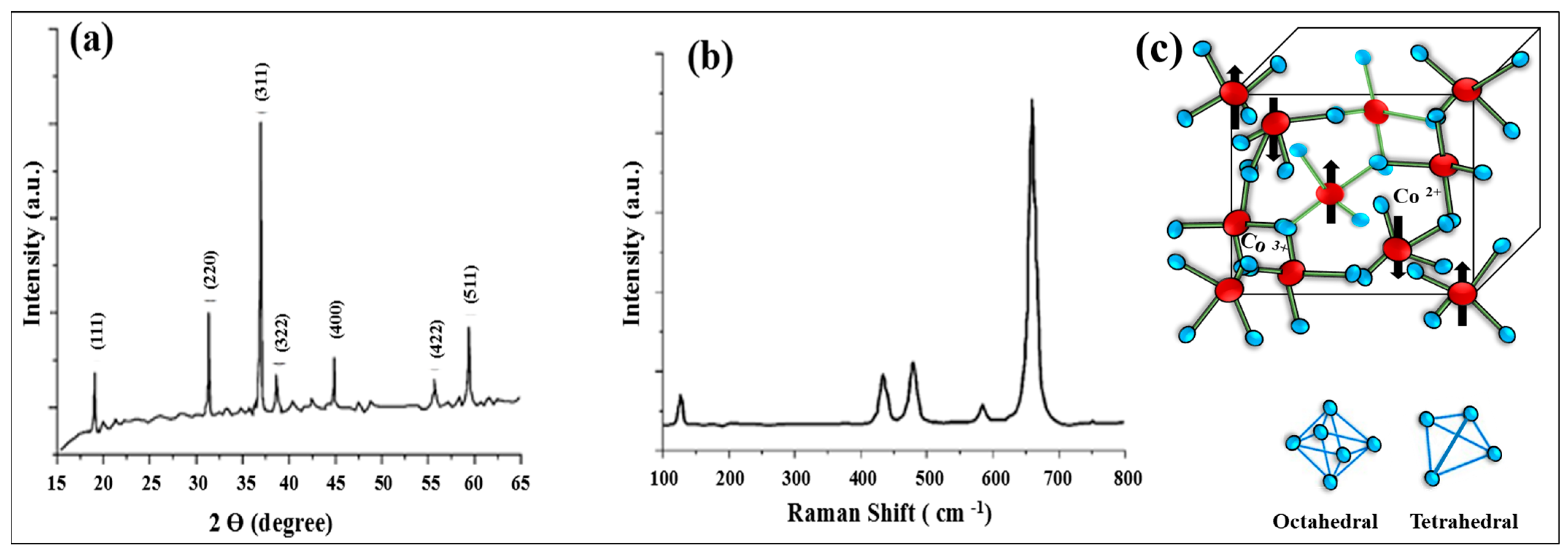
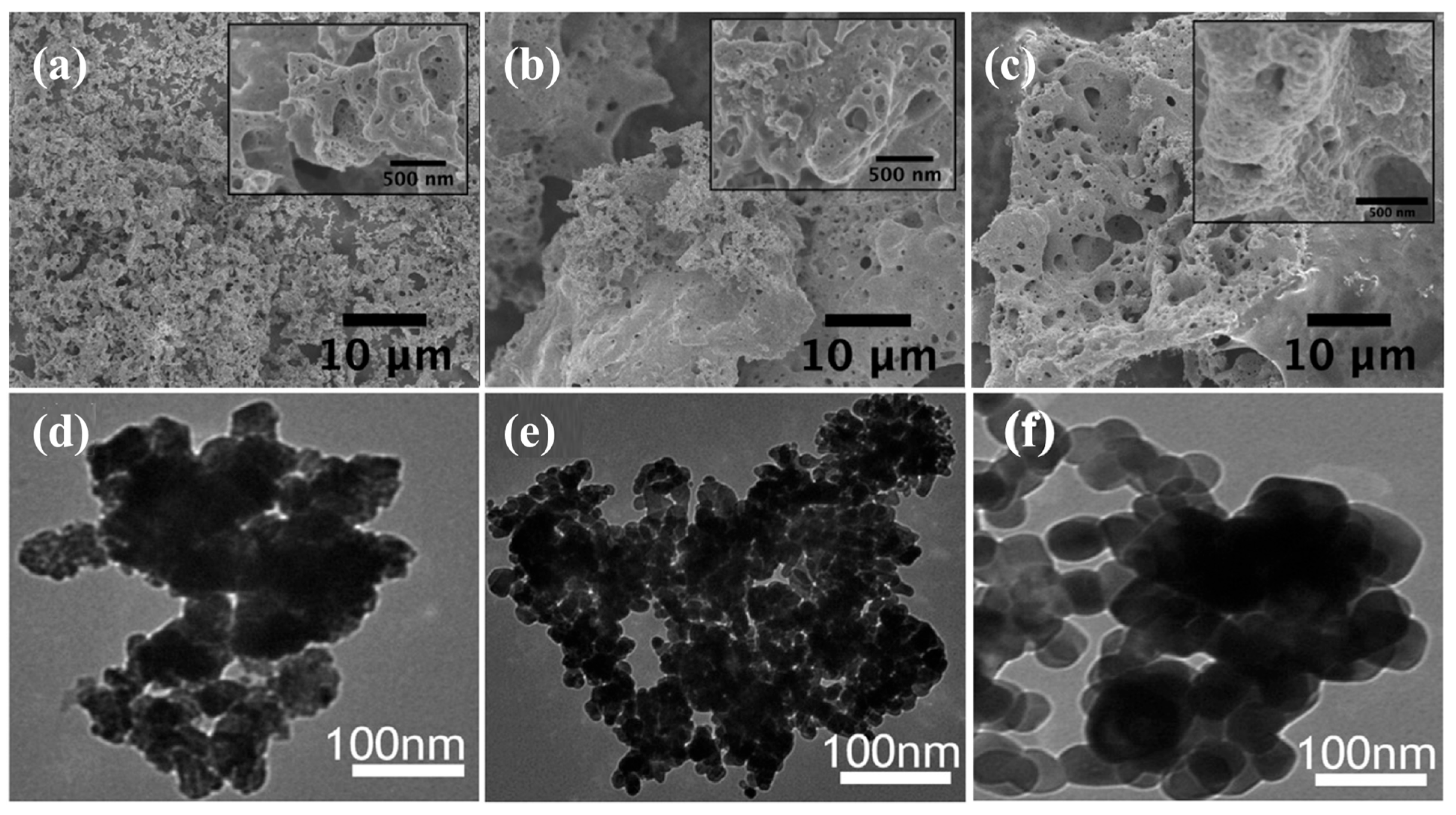
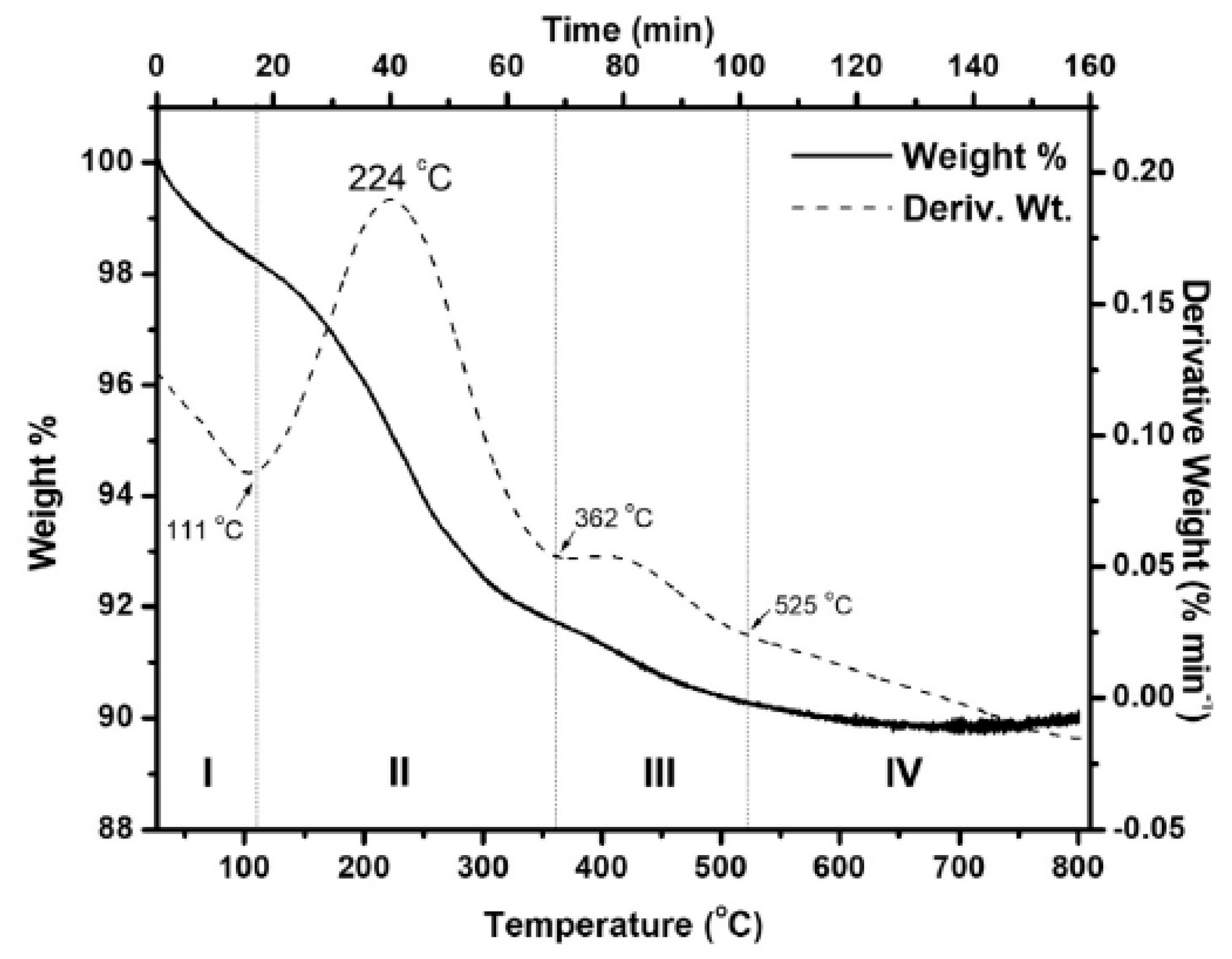
| No. | Fuel | F/O Ratio(φ) | Temperature (°C) | Particle Size nm | Morphology | Ref. |
|---|---|---|---|---|---|---|
| 1 | Glycine | 1 | 500 | 20 | Agglomerated NPs | [23] |
| 2 | Urea | 0.75–1.25 | 400–500 | 30–70 | Porous clusters | [19] |
| 3 | Citric acid | 1.0–2.0 | 300–600 | 25–50 | Crystalline porous | [19] |
| 4 | Glycine | 1.2 | 500 | 45 | Hollow spheres | [37] |
| 5 | Plant extract | 1.5 | 450 | <50 | Spherical porous | [38] |
| No. | Synthesis Method | Raw Materials | Catalyst | Temperature, °C | SSA, m2g−1 | References |
|---|---|---|---|---|---|---|
| 1 | SCS | (C4H6O4Co·4H2O) and d-(+)(C6H12O6) | Spinel-structured Co3O4 powder | 700 | 3 | [23] |
| 2 | SCS | (Co(NO3)2·6H2O and urea ((NH2)2CO) | Co3O4 NPs | 300–800 | 39–2 | [63] |
| 3 | Hydrothermal | (Co(NO3)2·6H2O) and urea (CO(NH2)2) in deionized water | Co3O4 nanoplate, nanorod, NPs | 325 | 45.5–111.4, 112.6 | [87] |
| 4 | Sol–gel | (Co(NO3)2·6H2O) and (C2H5-OH) | Co3O4 NPs | 150–550–650 | 15–46–42 | [84] |
| 5 | Sol–gel | (Co(NO3)2·6H2O) and PEG in deionized water | Co3O4 nanorod | 90–350–700 | 170.2–48–20.9 | [85] |
| 6 | Reactive calcination route | (Co(NO3)2·6H2O) and (Mn(NO3)2·4H2O) in deionized water | Mn promoted Co3O4 spinel (Cat-R) | 340–380–420 | 127.94–94.5–57.43 | [88] |
| 7 | SCS | (Co(NO3)2·6H2O) and urea (CO(NH2)2) in deionized water | Nano-crystalline Co3O4 | 600 | 10 | [67] |
| 8 | Co-precipitation | (CoCl2. 6H2O), (AgNO3) and (NH3), in deionized water | Single-cubic Co3O4 nanostructure Ag doped | 407.33 | [11] |
| No. | Synthesis Method | Dopant | Specific Capacity (mAh g−1) | Cycling Stability (Retention% @ Cycles) | Ref. |
|---|---|---|---|---|---|
| 1 | SCS (morphology-controlled) | None | 580–710 | 90 @ 100 | [112] |
| 2 | SCS | Ce | 812 | 85 @ 200 | [113] |
| 3 | SCS | Cr | >720 | 95 @ 200 | [114] |
| 4 | SCS | None | 630 | 80 @ 80 | [115] |
| 5 | SCS (refined) | None | >720 | 90 @ 150 | [23] |
| 6 | Advanced SCS + conductive composite | Multi-doping (e.g., Ce + Cr) | >700 | Needs investigation (≥300 cycles desired) | Future direction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mashan, T.T.; Hashami, M.; Bergeneva, N.S.; Nurmukhanbetova, N.N.; Beisebayeva, A.S.; Nazhipkyzy, M.; Mamatova, G.U.; Zhaxybayeva, A.G. A Comprehensive Overview of Co3O4 Nanoparticles: Solution Combustion Synthesis and Potential Applications. Nanomaterials 2025, 15, 932. https://doi.org/10.3390/nano15120932
Mashan TT, Hashami M, Bergeneva NS, Nurmukhanbetova NN, Beisebayeva AS, Nazhipkyzy M, Mamatova GU, Zhaxybayeva AG. A Comprehensive Overview of Co3O4 Nanoparticles: Solution Combustion Synthesis and Potential Applications. Nanomaterials. 2025; 15(12):932. https://doi.org/10.3390/nano15120932
Chicago/Turabian StyleMashan, Togzhan T., Muhammad Hashami, Nurgul S. Bergeneva, Nurgul N. Nurmukhanbetova, Aigul S. Beisebayeva, Meruyert Nazhipkyzy, Gulnar U. Mamatova, and Aigerim G. Zhaxybayeva. 2025. "A Comprehensive Overview of Co3O4 Nanoparticles: Solution Combustion Synthesis and Potential Applications" Nanomaterials 15, no. 12: 932. https://doi.org/10.3390/nano15120932
APA StyleMashan, T. T., Hashami, M., Bergeneva, N. S., Nurmukhanbetova, N. N., Beisebayeva, A. S., Nazhipkyzy, M., Mamatova, G. U., & Zhaxybayeva, A. G. (2025). A Comprehensive Overview of Co3O4 Nanoparticles: Solution Combustion Synthesis and Potential Applications. Nanomaterials, 15(12), 932. https://doi.org/10.3390/nano15120932









