Surface Modification of Fe-Based Perovskite Oxide via Sr0.95Ce0.05CoO3−δ Infiltration: A Strategy for Thermochemical Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Ba0.5Sr0.5Fe0.8Cu0.2O3−δ (BSFC) and Sr0.95Ce0.05CoO3−δ (SCC) Perovskite Powder
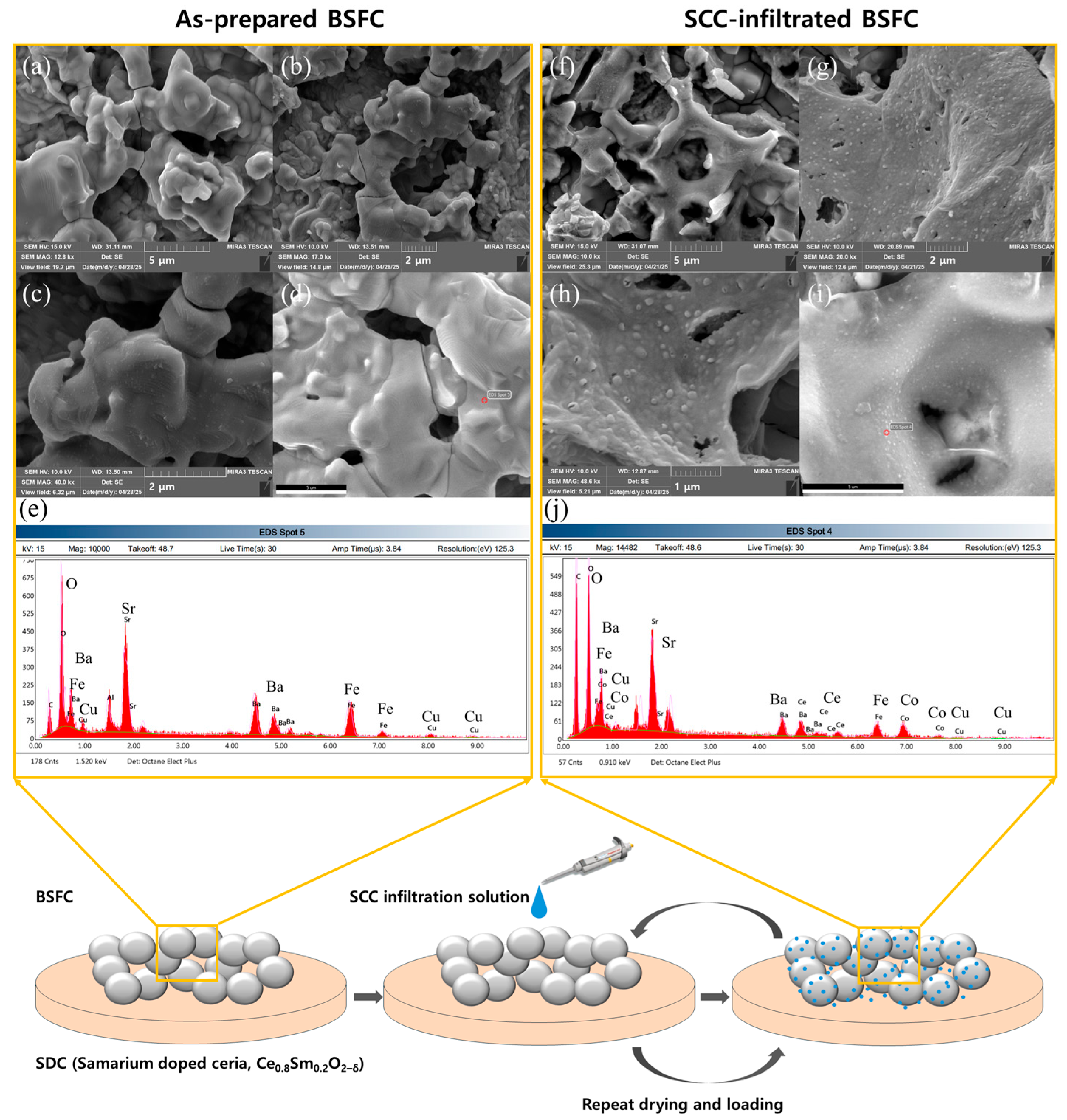
2.2. Surface Modification of BSFC with SCC Infiltration
2.3. Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mitchell, R.H.; Welch, M.D.; Chakhmouradian, A.R. Nomenclature of the perovskite supergroup: A hierarchical system of classification based on crystal structure and composition. Mineral. Mag. 2017, 81, 411–461. [Google Scholar] [CrossRef]
- Mishra, S.; Choudhary, R.N.P.; Parida, S.K. Structural, dielectric, electrical and optical properties of Li/Fe modified barium tungstate double perovskite for electronic devices. Ceram. Int. 2022, 48, 17020–17033. [Google Scholar] [CrossRef]
- Sun, X.; Yuan, Y.; Liu, S.; Zhao, H.; Yao, S.; Sun, Y.; Zhang, M.; Liu, Y.; Lin, Z. Recent advances in perovskite oxides for oxygen evolution reaction: Structures, mechanisms, and strategies for performance enhancement. Adv. Funct. Mater. 2025, 35, 2416705. [Google Scholar] [CrossRef]
- He, J.; Xu, X.; Li, M.; Zhou, S.; Zhou, W. Recent advances in perovskite oxides for non-enzymatic electrochemical sensors: A review. Anal. Chim. Acta 2023, 1251, 341007. [Google Scholar] [CrossRef]
- Gao, W.; Zhu, Y.; Wang, Y.; Yuan, G.; Liu, J.M. A review of flexible perovskite oxide ferroelectric films and their application. J. Mater. 2020, 6, 1–16. [Google Scholar] [CrossRef]
- Shen, F.; Lu, K. Comparison of different perovskite cathodes in solid oxide fuel cells. Fuel Cells 2018, 18, 457–465. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, T.; Qi, H.; Tu, B. The investigation of co-assembled high-performance (Ba, Sm/Sr)(Co, Zr) O3−δ composite perovskite cathodes for intermediate-temperature solid oxide fuel cell. Mater. Lett. 2024, 355, 135513. [Google Scholar] [CrossRef]
- Zhao, L.; He, B.; Zhang, X.; Peng, R.; Meng, G.; Liu, X. Electrochemical performance of novel cobalt-free oxide Ba0.5Sr0.5Fe0.8Cu0.2O3−δ for solid oxide fuel cell cathode. J. Power Sources 2010, 195, 1859–1861. [Google Scholar] [CrossRef]
- Mushtaq, N.; Lu, Y.; Xia, C.; Dong, W.; Wang, B.; Wang, X.; Shah, M.A.K.Y.; Rauf, S.; Jingjing, N.; Hu, E.; et al. Design principle and assessing the correlations in Sb-doped Ba0.5Sr0.5FeO3−δ perovskite oxide for enhanced oxygen reduction catalytic performance. J. Catal. 2021, 395, 168–177. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, X.; Li, M.; O’Hayre, R.P.; Yang, W. Nanoparticles at grain boundaries inhibit the phase transformation of perovskite membrane. Nano Lett. 2015, 15, 7678–7683. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, K.; Liu, Y.; Li, W.; Cai, L.; Zhu, X.; Cheng, M.; Yang, W. Structure and electrochemical properties of cobalt-free perovskite cathode materials for intermediate-temperature solid oxide fuel cells. Electrochim. Acta 2018, 279, 224–230. [Google Scholar] [CrossRef]
- Yu, X.; Long, W.; Jin, F.; He, T. Cobalt-free perovskite cathode materials SrFe1−xTixO3−δ and performance optimization for intermediate-temperature solid oxide fuel cells. Electrochim. Acta 2014, 123, 426–434. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, S.; Han, H.; Tang, K.; Xia, C. Cobalt-free double perovskite oxide as a promising cathode for solid oxide fuel cells. ACS Appl. Mater. Interfaces 2023, 15, 8253–8262. [Google Scholar] [CrossRef]
- Kim, S.; Kim, G.; Manthiram, A. A review on infiltration techniques for energy conversion and storage devices: From fundamentals to applications. Sustain. Energy Fuels 2021, 5, 5024–5037. [Google Scholar] [CrossRef]
- Zeng, R.; Huang, Y. Enhancing surface activity of La0.6Sr0.4CoO3−δ cathode by a simple infiltration process. Int. J. Hydrogen Energy 2017, 42, 7220–7225. [Google Scholar] [CrossRef]
- Wu, T.; Yu, B.; Zhang, W.; Chen, J.; Zhao, S. Fabrication of a high-performance nano-structured Ln1−xSrxMO3−δ (Ln = La, Sm; M = Mn, Co, Fe) SOC electrode through infiltration. RSC Adv. 2016, 6, 68379–68387. [Google Scholar] [CrossRef]
- Niu, Y.; Huo, W.; Yu, Y.; Li, W.; Chen, Y.; Lv, W. Cathode infiltration with enhanced catalytic activity and durability for intermediate-temperature solid oxide fuel cells. Chin. Chem. Lett. 2022, 33, 674–682. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, H.; Yao, C.; Lou, H.; Chen, M.; Lang, X.; Cai, K. Review of SOFC cathode performance enhancement by surface modifications: Recent advances and future directions. Energy Fuels 2023, 37, 3470–3487. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Cai, C.; Xue, K.; Bian, L.; An, S. Ce-doped promotes the phase stability and electrochemical performance of SrCoO3−δ cathode for intermediate-temperature solid oxide fuel cells. J. Power Sources 2024, 592, 233932. [Google Scholar] [CrossRef]
- Wei, Q.T.; Guo, R.S.; Wang, F.H.; Li, H.L. Structure and electrical properties of SrCoO3−δ doped by CeO2. J. Mater. Sci. 2005, 40, 1317–1319. [Google Scholar] [CrossRef]
- Yang, W.; Hong, T.; Li, S.; Ma, Z.; Sun, C.; Xia, C.; Chen, L. Perovskite Sr1−xCexCoO3−δ (0.05 ≤ x ≤ 0.15) as superior cathodes for intermediate temperature solid oxide fuel cells. ACS Appl. Mater. Interfaces 2013, 5, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wu, H.; Song, K.; Beshiwork, B.A.; Luo, X.; Tian, D.; Zhu, S.; Lu, X.; Ding, Y.; Chen, Y.; et al. Tuning Ba0.5Sr0.5Co0.8Fe0.2O3−δ cathode to high stability and activity via Ce-doping for ceramic fuel cells. Ceram. Int. 2022, 48, 31418–31427. [Google Scholar] [CrossRef]
- Lim, T.; Jo, K.; Kim, Y.N.; Lee, H. Valence electronic structure on oxygen reduction reaction kinetics of Cu-doped Ba0.5Sr0.5FeO3−δ for IT-SOFCs. Mater. Lett. 2024, 372, 136959. [Google Scholar] [CrossRef]
- Acharya, S.R.; Popczun, E.J.; Paudel, H.P.; Natesakhawat, S.; Lekse, J.W.; Duan, Y. Ba1-x SrxFeO3−δ as an improved oxygen storage material for chemical looping air separation: A computational and experimental study. Sustain. Energy Fuels 2025, 9, 2340–2354. [Google Scholar] [CrossRef]
- Fitriana, F.; Muniroh, M.; Zainuri, M.; Kidkhunthod, P.; Kato, M.; Suasmoro, S. XRD, XANES, and Electrical Conductivity Analysis of La-and Zr-Doped Ba0.5Sr0.5Fe0.9Cu0.1O3−δ Suitable for IT-SOFC Cathodes. J. Electron. Mater. 2021, 50, 5838–5845. [Google Scholar] [CrossRef]
- Fitriana, F.; Baity, P.S.N.; Zainuri, M.; Kidkhunthod, P.; Suasmoro, S. Crystal structure and Cu/Fe K-edge analysis of Ba0.5Sr0.5Fe1x2212xCuxO3−δ (x = 0–0.2) and the influence on conductivity. J. Phys. Chem. Solids 2021, 154, 110065. [Google Scholar] [CrossRef]
- Essehaity, A.S.M.; Aman, D.; Abd El-Hafiz, D.R.; Khedr, G.E.; Mikhail, S.; Abdel-Monem, Y.K. Exploration of Hierarchically Porous Perovskite Ba0.5Sr0.5FeO3 Catalyst Through Experimental and Computational Investigations for Sustainable Synthesis of Acrolein. J. Inorg. Organomet. Polym. Mater. 2024, 34, 4214–4228. [Google Scholar] [CrossRef]
- Wu, Y.C.; Ye, T.Y.; Zhou, Z. Study on microstructure and physical properties of Ba0.5Sr0.5Fe1-xCuxO3−δ–Ce 0.8Sm0.15Ca0.05O1.875 cathode materials used in solid oxide fuel cell. Int. J. Hydrogen Energy 2024, 52, 928–937. [Google Scholar] [CrossRef]
- Yu, Z.; Xu, Y.; Guo, R.; Zeng, J.; Xie, Z.; Zhang, M.; Fang, Y.; Qi, X.; Lü, Z.; Li, H. The effect of A-site deficiency on the performance of Fe-based perovskite La0.5Ba0.5-xFeO3−δ cathodes for intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2025, 100, 355–364. [Google Scholar] [CrossRef]
- Zheng, X.; Li, B.; Shen, L.; Cao, Y.; Zhan, Y.; Zheng, S.; Wang, S.; Jiang, L. Oxygen vacancies engineering of Fe doped LaCoO3 perovskite catalysts for efficient H2S selective oxidation. Appl. Catal. B Environ. 2023, 329, 122526. [Google Scholar] [CrossRef]
- Zheng, X.; Li, B.; Huang, R.; Jiang, W.; Shen, L.; Lei, G.; Wang, S.; Zhan, Y.; Jiang, L. Asymmetric Oxygen Vacancy-Promoted Synthesis of Aminoarenes from Nitroarenes Using Waste H2S as a “Hydrogen Donor”. ACS Catal. 2024, 14, 10245–10259. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, H.; Li, J.; Guo, X.; Hu, Q.; Yang, Z.; Yu, F.; Li, G. Modified La0.6Sr0.4Co0.2Fe0.8O3−δ cathodes with the infiltration of Er0.4Bi1.6O3 for intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2021, 46, 22932–22941. [Google Scholar] [CrossRef]
- Yao, C.; Meng, J.; Liu, X.; Zhang, X.; Meng, F.; Wu, X.; Meng, J. Effects of Bi doping on the microstructure, electrical and electrochemical properties of La2-xBixCu0.5Mn1.5O6 (x = 0, 0.1 and 0.2) perovskites as novel cathodes for solid oxide fuel cells. Electrochim. Acta 2017, 229, 429–437. [Google Scholar] [CrossRef]


| Parameters | Composition | |
|---|---|---|
| BSFC | SCC | |
| a = b = c [Å] | 3.9492(5) | 3.8490(2) |
| Volume [Å3] | 61.593(5) | 57.024(3) |
| Space group | Pm-3m | Pm-3m |
| Rp [%] | 3.05 | 3.32 |
| Rwp [%] | 5.87 | 4.53 |
| Rexp [%] | 2.24 | 2.07 |
| χ2 | 6.87 | 4.79 |
| Sample | Fe3+ (%) | Fe4+ (%) | Average Oxidation State |
|---|---|---|---|
| BSFC | 47.77 | 52.23 | 3.52 (±0.0015) |
| BSFC+SCC | 50.82 | 49.18 | 3.49 (±0.0017) |
| Sample | Olat | Oads | Omoi | Area Ratio of | |||
|---|---|---|---|---|---|---|---|
| Peak (eV) | Area | Peak | Area | Peak | Area | Oads + Omoi/Olat + Oads + Omoi | |
| BSFC | 527.98 | 25,270.25 | 530.81 | 54,085.33 | 532.90 | 10,093.92 | 0.72 |
| BSFC+SCC | 528.04 | 22,827.22 | 530.88 | 57,041.56 | 532.96 | 11,667.61 | 0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, T.; Lee, H. Surface Modification of Fe-Based Perovskite Oxide via Sr0.95Ce0.05CoO3−δ Infiltration: A Strategy for Thermochemical Stability. Nanomaterials 2025, 15, 934. https://doi.org/10.3390/nano15120934
Lim T, Lee H. Surface Modification of Fe-Based Perovskite Oxide via Sr0.95Ce0.05CoO3−δ Infiltration: A Strategy for Thermochemical Stability. Nanomaterials. 2025; 15(12):934. https://doi.org/10.3390/nano15120934
Chicago/Turabian StyleLim, Taeheun, and Heesoo Lee. 2025. "Surface Modification of Fe-Based Perovskite Oxide via Sr0.95Ce0.05CoO3−δ Infiltration: A Strategy for Thermochemical Stability" Nanomaterials 15, no. 12: 934. https://doi.org/10.3390/nano15120934
APA StyleLim, T., & Lee, H. (2025). Surface Modification of Fe-Based Perovskite Oxide via Sr0.95Ce0.05CoO3−δ Infiltration: A Strategy for Thermochemical Stability. Nanomaterials, 15(12), 934. https://doi.org/10.3390/nano15120934






