Bio-Based Nanomaterials for Groundwater Arsenic Remediation: Mechanisms, Challenges, and Future Perspectives
Abstract
1. Introduction
1.1. Sources and Environmental Presence
1.2. Role of Bio-Based Nanomaterials in Groundwater Arsenic Remediation
2. Mechanisms of Arsenic Remediation
2.1. Adsorption
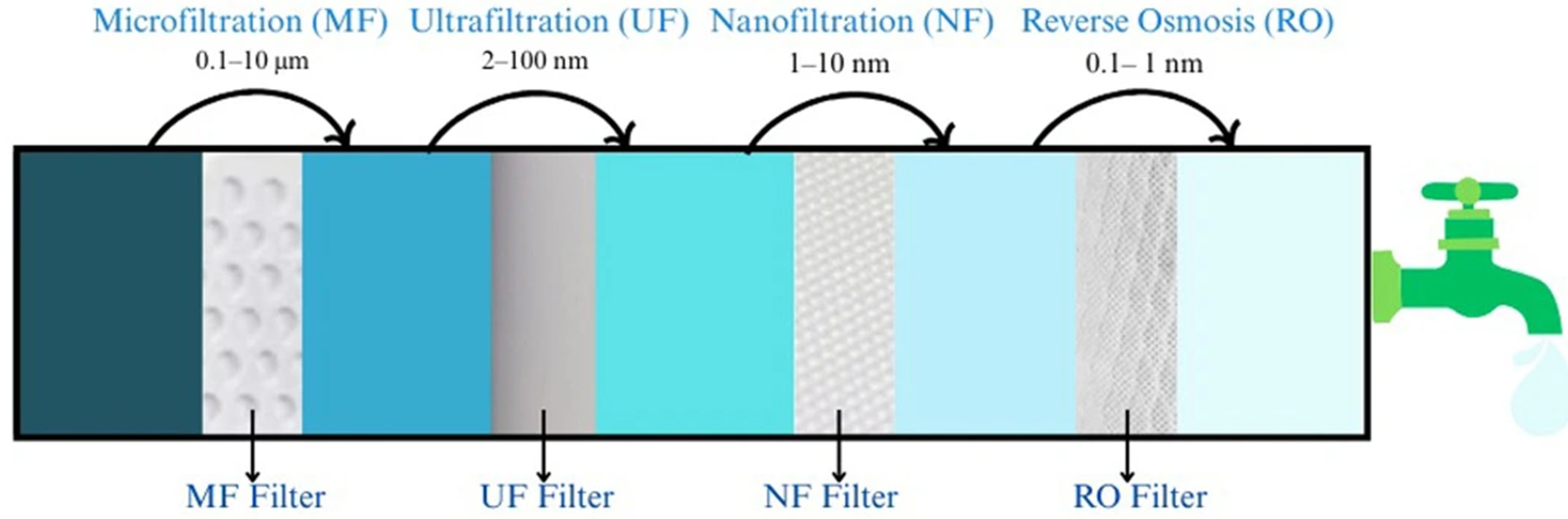
2.2. Filtration/Membrane Technology
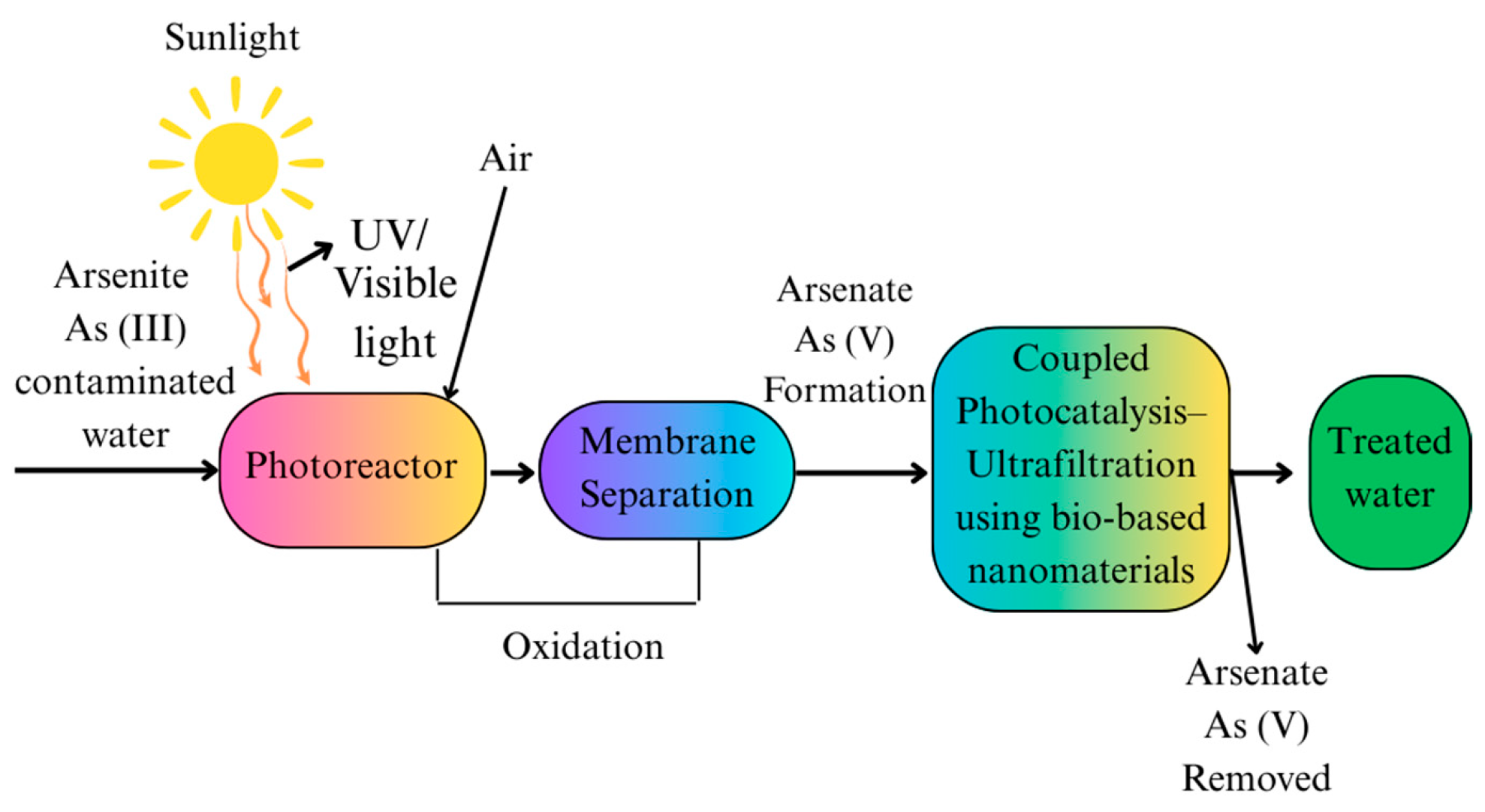
2.3. Photocatalysis
2.4. Redox Reactions
2.5. Complexation
2.6. Ion Exchange
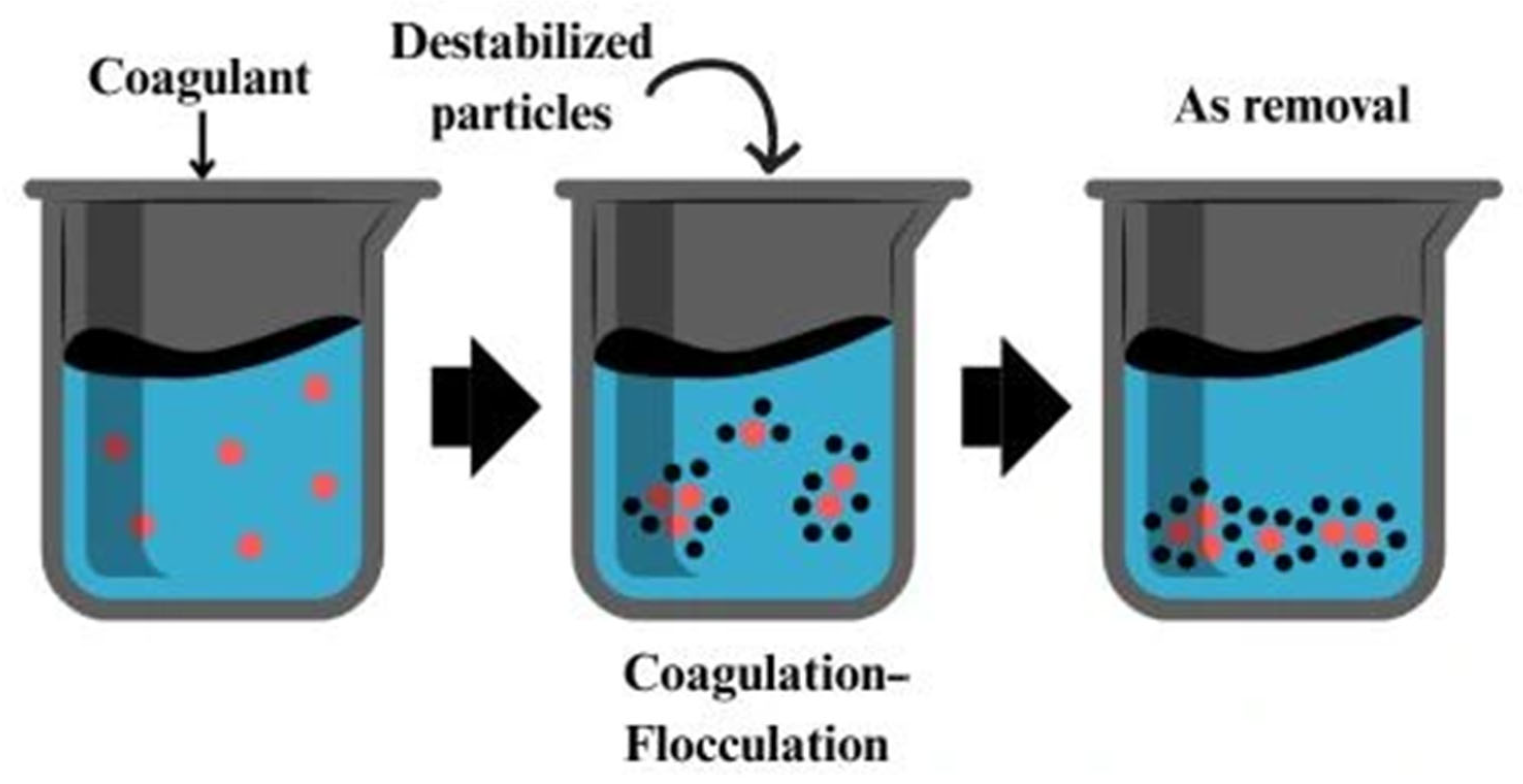
2.7. Coagulation–Flocculation
3. Challenges of and Advances in Arsenic Remediation
3.1. Challenges of Arsenic Remediation
3.1.1. Stability and Selectivity
3.1.2. Affordable Scalability
3.1.3. Ability to Regenerate and Recycle
3.2. Recent Advances in Arsenic Remediation
3.2.1. Bio- Nano- Materials Design
3.2.2. Surface Modifications
3.2.3. Hybrid Systems
4. Future Perspectives and Research Directions
4.1. Integration of Bio-Based Nanomaterials with Smart Sensing Technologies
4.2. Development of Sustainable Arsenic-Contaminated Water-Treatment Frameworks
4.3. Smart Design and Life-Cycle Integration for Bio-Based Arsenic Adsorbents
4.4. Green Synthesis and Life-Cycle Assessment (LCA)
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shaji, E.; Santosh, M.; Sarath, K.V.; Prakash, P.; Deepchand, V.; Divya, B.V. Arsenic contamination of groundwater: A global synopsis with focus on the Indian Peninsula. Geosci. Front. 2021, 12, 101079. [Google Scholar] [CrossRef]
- Bundschuh, J.; Schneider, J.; Alam, M.A.; Niazi, N.K.; Herath, I.; Parvez, F.; Tomaszewska, B.; Guilherme, L.R.G.; Maity, J.P.; López, D.L.; et al. Seven potential sources of arsenic contamination in Latin America and their environmental and health impacts. Sci. Total Environ. 2021, 780, 146274. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bi, Y.; Mi, W.; Xie, S.; Ji, L. Land-use change caused by anthropogenic activities increase fluoride and arsenic contamination in groundwater and human health risk. J. Hazard. Mater. 2021, 406, 124337. [Google Scholar] [CrossRef]
- Fatoki, J.O.; Badmus, J.A. Arsenic as an environmental and human health antagonist: A review of its toxicity and disease initiation. J. Hazard. Mater. Adv. 2022, 5, 100052. [Google Scholar] [CrossRef]
- World Health Organization. Arsenic. Available online: https://www.who.int/news-room/fact-sheets/detail/arsenic (accessed on 20 March 2025).
- Argos, M.; Kalra, T.; Rathouz, P.J.; Chen, Y.; Pierce, B.; Parvez, F.; Islam, T.; Ahmed, A.; Rakibuz-Zaman, M.; Hasan, R.; et al. Arsenic Exposure from Drinking Water, and All-Cause and Chronic-Disease Mortalities in Bangladesh (HEALS): A Prospective Cohort Study. Lancet 2010, 376, 252–258. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Mise, N.; Ichihara, S. Arsenic Contamination in Food Chain in Bangladesh: A Review on Health Hazards, Socioeconomic Impacts and Implications. Hyg. Environ. Health Adv. 2022, 2, 100004. [Google Scholar] [CrossRef]
- Flanagan, S.V.; Johnston, R.B.; Zheng, Y. Arsenic in Tube Well Water in Bangladesh: Health and Economic Impacts and Implications for Arsenic Mitigation. Bull. World Health Organ. 2012, 90, 839–846. [Google Scholar] [CrossRef]
- Thakur, B.K.; Gupta, V.; Bhattacharya, P.; Chakraborty, T. Impact of socioeconomic factors on households’ willingness to pay for arsenic-free safe drinking water—A case study of Bihar, India. Groundw. Sustain. Dev. 2022, 19, 100837. [Google Scholar] [CrossRef]
- Cervantes, G.I.V.; Esquivel, D.F.G.; Ortega, D.R.; Ayala, T.B.; Chávez, L.A.R.; López-López, H.E.; Salazar, A.; Flores, I.; Pineda, B.; Gómez-Manzo, S.; et al. Mechanisms associated with cognitive and behavioral impairment induced by arsenic exposure. Cells 2023, 12, 2537. [Google Scholar] [CrossRef]
- Kuo, C.-C.; Moon, K.A.; Wang, S.-L.; Silbergeld, E.; Navas-Acien, A. The association of arsenic metabolism with cancer, cardiovascular disease, and diabetes: A systematic review of the epidemiological evidence. Environ. Health Perspect. 2017, 125, 087001. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Branscum, A.; Welch, B.M.; Megowan, M.; Bethel, J.W.; Odden, M.C.; Joya, S.A.; Hasan, M.O.S.I.; Lin, P.I.; Mostofa, G.; et al. A prospective cohort study of in utero and early childhood arsenic exposure and infectious disease in 4-to 5-year-old Bangladeshi children. Environ. Epidemiol. 2020, 4, e086. [Google Scholar] [CrossRef]
- Mohapatra, B.; Saha, A.; Chowdhury, A.N.; Kar, A.; Kazy, S.K.; Sar, P. Geochemical, metagenomic, and physiological characterization of the multifaceted interaction between microbiome of an arsenic contaminated groundwater and aquifer sediment. J. Hazard. Mater. 2021, 412, 125099. [Google Scholar] [CrossRef]
- Clark, A.J.; Labaj, A.L.; Smol, J.P.; Campbell, L.M.; Kurek, J. Arsenic and mercury contamination and complex aquatic bioindicator responses to historical gold mining and modern watershed stressors in urban Nova Scotia, Canada. Sci. Total Environ. 2021, 787, 147374. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, B.; Pan, F.; Fu, Y.; Guo, W.; Guo, Z.; Liu, H. Effects of manganese, iron and sulfur geochemistry on arsenic migration in the estuarine sediment of a small river in Xiamen, Southeast China. Environ. Pollut. 2022, 293, 118570. [Google Scholar] [CrossRef]
- Tang, Z.; Zhao, F.-J. The roles of membrane transporters in arsenic uptake, translocation and detoxification in plants. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2449–2484. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, P.; Wei, X.; Peng, H.; Hu, L.; Zhu, X. Migration, transformation of arsenic, and pollution controlling strategies in paddy soil-rice system: A comprehensive review. Sci. Total Environ. 2024, 951, 175500. [Google Scholar] [CrossRef]
- Hussain, M.M.; Bibi, I.; Niazi, N.K.; Shahid, M.; Iqbal, J.; Shakoor, M.B.; Ahmad, A.; Shah, N.S.; Bhattacharya, P.; Mao, K.; et al. Arsenic biogeochemical cycling in paddy soil–rice system: Interaction with various factors, amendments and mineral nutrients. Sci. Total Environ. 2021, 773, 145040. [Google Scholar] [CrossRef]
- Gelaye, Y. Public health and economic burden of heavy metals in Ethiopia: Review. Heliyon 2024, 10, e39022. [Google Scholar] [CrossRef]
- Yunus, F.M.; Khan, S.; Chowdhury, P.; Milton, A.H.; Hussain, S.; Rahman, M. A Review of Groundwater Arsenic Contamination in Bangladesh: The Millennium Development Goal Era and Beyond. Int. J. Environ. Res. Public Health 2016, 13, 215. [Google Scholar] [CrossRef]
- Khan, N.; Hoque, S.F.; Mahmud, Z.H.; Islam, M.R.; Alam, M.A.U.; Islam, M.S.; Charles, K.J. Water Quality and Unseen Health Outcomes: A Cross-Sectional Study on Arsenic Contamination, Subclinical Disease and Psychosocial Distress in Bangladesh. SSM Ment. Health 2024, 6, 100344. [Google Scholar] [CrossRef]
- Raju, N.J. Arsenic in the geo-environment: A review of sources, geochemical processes, toxicity and removal technologies. Environ. Res. 2022, 203, 111782. [Google Scholar] [CrossRef]
- Muzaffar, S.; Khan, J.; Srivastava, R.; Gorbatyuk, M.S.; Athar, M. Mechanistic understanding of the toxic effects of arsenic and warfare arsenicals on human health and environment. Cell Biol. Toxicol. 2023, 39, 85–110. [Google Scholar] [CrossRef]
- University College London. Arsenic. Available online: https://blogs.ucl.ac.uk/irdr/tag/arsenic/ (accessed on 20 March 2025).
- Khan, W.S.; Asmatulu, E.; Uddin, M.N.; Asmatulu, R. Recycling and Reusing of Engineering Materials, Recycling for Sustainable Developments; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 9780128224618. [Google Scholar]
- Uddin, M.N.; Rab, M.F.; Islam, A.K.M.N.; Asmatulu, E.; Rahman, M.M.; Asmatulu, R. Nanostructured Hybrid Hydrogels for Solar-Driven Clean Water Harvesting from the Atmosphere. Materials 2022, 15, 7538. [Google Scholar] [CrossRef]
- Uddin, M.N.; Desai, F.; Asmatulu, E. Engineered Nanomaterials in the Environment: Bioaccumulation, Biomagnification, and Biotransformation. Environ. Chem. Lett. 2020, 18, 1073–1083. [Google Scholar] [CrossRef]
- Preetha, J.S.Y.; Arun, M.; Vidya, N.; Kowsalya, K.; Halka, J.; Ondrasek, G. Biotechnology Advances in Bioremediation of Arsenic: A Review. Molecules 2023, 28, 1474. [Google Scholar] [CrossRef]
- Saud, A.; Gupta, S.; Allal, A.; Preud’homme, H.; Shomar, B.; Zaidi, S.J. Progress in the Sustainable Development of Biobased (Nano)materials for Application in Water Treatment Technologies. ACS Omega 2024, 9, 29088–29113. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Sreekumari, S.S. Adsorption characteristics of heavy metal ions and arsenate on chitosan-based composite: Equilibrium and kinetics studies. J. Hazard. Mater. 2011, 190, 449–460. [Google Scholar] [CrossRef]
- Hesami, F.; Bina, B.; Ebrahimi, A.; Amin, M.M. Arsenic removal by coagulation using ferric chloride and chitosan from water. Int. J. Environ. Health Eng. 2013, 2, 17. [Google Scholar] [CrossRef]
- Chauhan, K.; Singh, P.; Sen, K.; Singhal, R.K.; Thakur, V.K. Recent Advancements in the Field of Chitosan/Cellulose-Based Nanocomposites for Maximizing Arsenic Removal from Aqueous Environment. ACS Omega 2024, 9, 27766–27788. [Google Scholar] [CrossRef]
- Sinha, S.; Nigam, S.; Solanki, S.; Batra, L.; Chug, P.; Singh, R. Prospects on Arsenic Remediation Using Organic Cellulose-Based Adsorbents. Ind. Crops Prod. 2023, 201, 116928. [Google Scholar] [CrossRef]
- Motloung, M.T.; Magagula, S.I.; Kaleni, A.; Sikhosana, T.S.; Lebelo, K.; Mochane, M.J. Recent Advances on Chemically Functionalized Cellulose-Based Materials for Arsenic Removal in Wastewater: A Review. Water 2023, 15, 793. [Google Scholar] [CrossRef]
- Sharma, G.; Verma, Y.; Lai, C.W.; Naushad, M.; Iqbal, J.; Kumar, A.; Dhiman, P. Biochar and Biosorbents Derived from Biomass for Arsenic Remediation. Heliyon 2024, 10, e36288. [Google Scholar] [CrossRef]
- Chatzimichailidou, S.; Xanthopoulou, M.; Tolkou, A.K.; Katsoyiannis, I.A. Biochar Derived from Rice by-Products for Arsenic and Chromium Removal by Adsorption: A Review. J. Compos. Sci. 2023, 7, 59. [Google Scholar] [CrossRef]
- Chang Chien, S.W.; Weng, C.M.; Chou, J.S.; Liu, C.C. Application of δ-MnO2 and Biochar Materials in an Arsenic-Contaminated Groundwater. Water Environ. Res. 2022, 94, e10811. [Google Scholar] [CrossRef]
- Shaikh, W.A.; Alam, M.A.; Alam, M.O.; Chakraborty, S.; Owens, G.; Bhattacharya, T.; Mondal, N.K. Enhanced Aqueous Phase Arsenic Removal by a Biochar-Based Iron Nanocomposite. Environ. Technol. Innov. 2020, 19, 100936. [Google Scholar] [CrossRef]
- Tabassum, R.A.; Shahid, M.; Niazi, N.K.; Dumat, C.; Zhang, Y.; Imran, M.; Bakhat, H.F.; Hussain, I.; Khalid, S. Arsenic Removal from Aqueous Solutions and Groundwater Using Agricultural Biowastes-Derived Biosorbents and Biochar: A Column-Scale Investigation. Int. J. Phytoremediat. 2019, 21, 509–518. [Google Scholar] [CrossRef]
- Chowdhury, S.; Chowdhury, I.R.; Kabir, F.; Mazumder, M.A.J.; Zahir, M.H.; Alhooshani, K. Alginate-based biotechnology: A review on the arsenic removal technologies and future possibilities. J. Water Supply Res. Technol. AQUA 2019, 68, 369–389. [Google Scholar] [CrossRef]
- Saleh, S.M.; Younis, A.M.; Ali, R.; Elkady, E.M. A Novel and Eco-Friendly Algae Amino-Modified Nanoparticles with Significant Environmental Effect for the Removal of As(III) and As(V) from Water. Environ. Adv. 2024, 16, 100550. [Google Scholar] [CrossRef]
- Sandu, T.; Chiriac, A.L.; Zaharia, A.; Iordache, T.V.; Sarbu, A. New Trends in Preparation and Use of Hydrogels for Water Treatment. Gels 2025, 11, 238. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, J.H.; Lee, S.L.; Hwang, S.W.; Seo, D.C. Adsorption Behavior of Arsenic onto Lignin-Based Biochar Decorated with Zinc. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127095. [Google Scholar] [CrossRef]
- Maia, L.C.; Soares, L.C.; Gurgel, L.V.A. A Review on the Use of Lignocellulosic Materials for Arsenic Adsorption. J. Environ. Manag. 2021, 288, 112397. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Sharma, M.; Kumari, A.; Shrestha, S.; Shrestha, B. Arsenic Removal from Water by Adsorption onto Iron Oxide/Nano-Porous Carbon Magnetic Composite. Appl. Sci. 2019, 9, 3732. [Google Scholar] [CrossRef]
- Ainiwaer, M.; Zhang, T.; Zhang, N.; Yin, X.; Su, S.; Wang, Y.; Zeng, X. Synergistic Removal of As(III) and Cd(II) by Sepiolite-Modified Nanoscale Zero-Valent Iron and a Related Mechanistic Study. J. Environ. Manag. 2022, 319, 115658. [Google Scholar] [CrossRef]
- Angai, J.U.; Ptacek, C.J.; Pakostova, E.; Bain, J.G.; Verbuyst, B.R.; Blowes, D.W. Removal of Arsenic and Metals from Groundwater Impacted by Mine Waste Using Zero-Valent Iron and Organic Carbon: Laboratory Column Experiments. J. Hazard. Mater. 2022, 424, 127295. [Google Scholar] [CrossRef] [PubMed]
- Alijani, H.; Shariatinia, Z. Effective Aqueous Arsenic Removal Using Zero Valent Iron Doped MWCNT Synthesized by In Situ CVD Method Using Natural α-Fe2O3 as a Precursor. Chemosphere 2017, 171, 502–511. [Google Scholar] [CrossRef]
- Dang, N.T.T.; Nguyen, T.T.A.; Phan, T.D.; Tran, H.; Dang, P.V.; Nguyen, H.Q. Synthesis of Silica Nanoparticles from Rice Husk Ash. Sci. Technol. Dev. J. 2017, 20, 50–54. [Google Scholar] [CrossRef]
- Mukherjee, A.; Sarkar, D.; Sasmal, S. A Review of Green Synthesis of Metal Nanoparticles Using Algae. Front. Microbiol. 2021, 12, 693899. [Google Scholar] [CrossRef]
- Liu, P.; Liang, Q.; Luo, H.; Fang, W.; Geng, J. Synthesis of Nano-Scale Zero-Valent Iron-Reduced Graphene Oxide-Silica Nano-Composites for the Efficient Removal of Arsenic from Aqueous Solutions. Environ. Sci. Pollut. Res. 2019, 26, 33507–33516. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhong, H.; He, Z.; Hu, L. Efficient Removal of Arsenite by a Composite of Amino Modified Silica Supported MnO2/Fe–Al Hydroxide (SNMFA) Prepared from Biotite. J. Environ. Manag. 2021, 291, 112678. [Google Scholar] [CrossRef]
- Vievard, J.; Alem, A.; Pantet, A.; Ahfir, N.-D.; Arellano-Sánchez, M.G.; Devouge-Boyer, C.; Mignot, M. Bio-Based Adsorption as Ecofriendly Method for Wastewater Decontamination: A Review. Toxics 2023, 11, 404. [Google Scholar] [CrossRef]
- Gupta, A.D.; Rene, E.R.; Giri, B.S.; Pandey, A.; Singh, H. Adsorptive and Photocatalytic Properties of Metal Oxides towards Arsenic Remediation from Water: A Review. J. Environ. Chem. Eng. 2021, 9, 106376. [Google Scholar] [CrossRef]
- Ahmad, H.; Bibi, H.; Murugesan, C.; Ahmad, S.; Kyriakopoulos, G. Sustainable Wastewater Treatment Strategies in Effective Abatement of Emerging Pollutants. Water 2024, 16, 2893. [Google Scholar] [CrossRef]
- Ungureanu, G.; Santos, S.; Boaventura, R.; Botelho, C. Arsenic and Antimony in Water and Wastewater: Overview of Removal Techniques with Special Reference to Latest Advances in Adsorption. J. Environ. Manag. 2015, 151, 326–342. [Google Scholar] [CrossRef]
- Nisticò, R.; Celi, L.R.; Prevot, A.B.; Carlos, L.; Magnacca, G.; Zanzo, E.; Martin, M. Sustainable Magnet-Responsive Nanomaterials for the Removal of Arsenic from Contaminated Water. J. Hazard. Mater. 2018, 342, 260–269. [Google Scholar] [CrossRef]
- Wen, Z.; Xi, J.; Lu, J.; Zhang, Y.; Cheng, G.; Zhang, Y.; Chen, R. Porous Biochar-Supported MnFe2O4 Magnetic Nanocomposite as an Excellent Adsorbent for Simultaneous and Effective Removal of Organic/Inorganic Arsenic from Water. J. Hazard. Mater. 2021, 411, 124909. [Google Scholar] [CrossRef]
- Chakraborty, R.; Asthana, A.; Singh, A.K.; Jain, B.; Susan, A.B.H. Adsorption of heavy metal ions by various low-cost adsorbents: A review. Int. J. Environ. Anal. Chem. 2022, 102, 342–379. [Google Scholar] [CrossRef]
- Musah, M.; Azeh, Y.; Mathew, J.T.; Umar, M.T.; Abdulhamid, Z.; Muhammad, A.I. Adsorption Kinetics and Isotherm Models: A Review. Caliphate J. Sci. Technol. 2022, 4, 20–26. [Google Scholar] [CrossRef]
- Qiu, H.; Lv, L.; Pan, B.-C.; Zhang, Q.-J.; Zhang, W.-M.; Zhang, Q.-X. Critical Review in Adsorption Kinetic Models. J. Zhejiang Univ. Sci. A 2009, 10, 716–724. [Google Scholar] [CrossRef]
- Azizian, S.; Eris, S. Adsorption Isotherms and Kinetics. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 33, pp. 445–509. [Google Scholar] [CrossRef]
- López-Luna, J.; Ramírez-Montes, L.E.; Martinez-Vargas, S.; Martínez, A.I.; Mijangos-Ricardez, O.F.; González-Chávez, M.D.C.A.; Vázquez-Hipólito, V. Linear and Nonlinear Kinetic and Isotherm Adsorption Models for Arsenic Removal by Manganese Ferrite Nanoparticles. SN Appl. Sci. 2019, 1, 977. [Google Scholar] [CrossRef]
- Abiodun, O.-A.O.; Oluwaseun, O.; Oladayo, O.K.; Abayomi, O.; George, A.A.; Opatola, E.; Orah, R.F.; Isukuru, E.J.; Ede, I.C.; Oluwayomi, O.T.; et al. Remediation of Heavy Metals Using Biomass-Based Adsorbents: Adsorption Kinetics and Isotherm Models. Clean Technol. 2023, 5, 934–960. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, R.; Chen, S.; Zhu, J.; Wu, P.; Huang, J.; Qi, S. Arsenic (III) Removal from Aqueous Solution Using TiO2-Loaded Biochar Prepared by Waste Chinese Traditional Medicine Dregs. RSC Adv. 2022, 12, 7720–7734. [Google Scholar] [CrossRef] [PubMed]
- Sherlala, A.I.A.; Raman, A.A.A.; Bello, M.M.; Buthiyappan, A. Adsorption of Arsenic Using Chitosan Magnetic Graphene Oxide Nanocomposite. J. Environ. Manag. 2019, 246, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, H.H.; Demarchi, C.A.; Rodrigues, C.A.; Greneche, J.M.; Nedelko, N.; Ślawska-Waniewska, A. Adsorption of As(III) on Chitosan-Fe-Crosslinked Complex (Ch-Fe). Chemosphere 2011, 82, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Yee, J.-J.; Arida, C.V.J.; Futalan, C.M.; de Luna, M.D.G.; Wan, M.-W. Treatment of Contaminated Groundwater via Arsenate Removal Using Chitosan-Coated Bentonite. Molecules 2019, 24, 2464. [Google Scholar] [CrossRef]
- Ayub, A.; Raza, Z.A.; Majeed, M.I.; Tariq, M.R.; Irfan, A. Development of Sustainable Magnetic Chitosan Biosorbent Beads for Kinetic Remediation of Arsenic Contaminated Water. Int. J. Biol. Macromol. 2020, 163, 603–617. [Google Scholar] [CrossRef]
- Abdelwahab, H.E.; Elhag, M.; El Sadek, M.M. Removal of As(V) and Cr(VI) Using Quinoxaline Chitosan Schiff Base: Synthesis, Characterization and Adsorption Mechanism. BMC Chem. 2024, 18, 225. [Google Scholar] [CrossRef]
- Hashimi, S.Q.; Hong, S.-H.; Lee, C.-G.; Park, S.-J. Adsorption of Arsenic from Water Using Aluminum-Modified Food Waste Biochar: Optimization Using Response Surface Methodology. Water 2022, 14, 2712. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, S.; Feng, L.; Zhou, J.; Li, T.; Zhao, Z.; Wang, W. Fresh Biomass Derived Biochar with High-Load Zero-Valent Iron Prepared in One Step for Efficient Arsenic Removal. J. Clean. Prod. 2022, 352, 131616. [Google Scholar] [CrossRef]
- Villena-Martínez, E.M.; Alvizuri-Tintaya, P.A.; Lora-García, J.; Torregrosa-López, J.I.; Lo-Iacono-Ferreira, V.G. Reverse Osmosis Modeling Study of Lead and Arsenic Removal from Drinking Water in Tarija and La Paz, Bolivia. Processes 2022, 10, 1889. [Google Scholar] [CrossRef]
- Algieri, C.; Pugliese, V.; Coppola, G.; Curcio, S.; Calabro, V.; Chakraborty, S. Arsenic Removal from Groundwater by Membrane Technology: Advantages, Disadvantages, and Effect on Human Health. Groundw. Sustain. Dev. 2022, 19, 100815. [Google Scholar] [CrossRef]
- Hamid, N.H.A.; Rushdan, A.I.; Nordin, A.H.; Norrrahim, M.N.F.; Muhamad, S.N.H.; Tahir, M.I.H.M.; Rosli, N.S.B.; Pakrudin, N.H.M.; Roslee, A.S.; Asyraf, M.R.M.; et al. A Review: The State-of-the-Art of Arsenic Removal in Wastewater. Water Reuse 2024, 14, 279–311. [Google Scholar] [CrossRef]
- Siddique, T.A.; Dutta, N.K.; Choudhury, N.R. Nanofiltration for Arsenic Removal: Challenges, Recent Developments, and Perspectives. Nanomaterials 2020, 10, 1323. [Google Scholar] [CrossRef]
- Gokcek, O.B.; Uzal, N. Arsenic Removal by the Micellar-Enhanced Ultrafiltration Using Response Surface Methodology. Water Supply 2020, 20, 574–585. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Hakami, M.; Zacharof, M.-P.; Abu Homod, H.; Alsadun, A. Mercury, Arsenic and Lead Removal by Air Gap Membrane Distillation: Experimental Study. Water 2020, 12, 1574. [Google Scholar] [CrossRef]
- Xiao, T.; Zhu, Z.; Li, L.; Shi, J.; Li, Z.; Zuo, X. Membrane fouling and cleaning strategies in microfiltration/ultrafiltration and dynamic membrane. Sep. Purif. Technol. 2023, 318, 123977. [Google Scholar] [CrossRef]
- Wang, X.; Liu, W.; Li, D.; Ma, W. Arsenic (V) Removal from Groundwater by GE-HL Nanofiltration Membrane: Effects of Arsenic Concentration, pH, and Co-Existing Ions. Front. Environ. Sci. Eng. 2009, 3, 428–433. [Google Scholar] [CrossRef]
- Boussouga, Y.-A.; Mohankumar, M.B.; Gopalakrishnan, A.; Welle, A.; Schäfer, A.I. Removal of Arsenic (III) via Nanofiltration: Contribution of Organic Matter Interactions. Water Res. 2021, 201, 117315. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Talukdar, A.; Sengupta, S.; Das, T.; Dey, A.; Gupta, K.; Dutta, N. Arsenic contaminated water remediation: A state-of-the-art review in synchrony with sustainable development goals. Groundw. Sustain. Dev. 2023, 23, 101000. [Google Scholar] [CrossRef]
- Morales-Jiménez, M.; Palacio, D.A.; Palencia, M.; Meléndrez, M.F.; Rivas, B.L. Bio-Based Polymeric Membranes: Development and Environmental Applications. Membranes 2023, 13, 625. [Google Scholar] [CrossRef]
- Li, K.; Lu, Y.; Zhou, C.; Liu, Z.; Luo, L.; Zhou, A.; Shao, S. Toward one-step As(III) removal in ultrafiltration with in situ BioMnOx cake layer: Mechanism and feasibility insights. Water 2025, 273, 123087. [Google Scholar] [CrossRef]
- Harisha, R.S.; Hosamani, K.M.; Keri, R.S.; Nataraj, S.K.; Aminabhavi, T.M. Arsenic removal from drinking water using thin film composite nanofiltration membrane. Desalination 2010, 252, 75–80. [Google Scholar] [CrossRef]
- Pookrod, Y.P.; Haller, K.J.; Scamehorn, J.F. Removal of arsenic anions from water using polyelectrolyte enhanced ultrafiltration. Sep. Sci. Technol. 2004, 39, 811–831. [Google Scholar] [CrossRef]
- Pezeshki, H.; Hashemi, M.; Rajabi, S. Removal of arsenic as a potentially toxic element from drinking water by filtration: A mini review of nanofiltration and reverse osmosis techniques. Heliyon 2023, 9, e14246. [Google Scholar] [CrossRef] [PubMed]
- Molinari, R.; Argurio, P. Arsenic Removal from Water by Coupling Photocatalysis and Complexation-Ultrafiltration Processes: A Preliminary Study. Water Res. 2017, 109, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Fontana, K.B.; Lenzi, G.G.; Seára, E.C.R.; Chaves, E.S. Comparison of Photocatalysis and Photolysis Processes for Arsenic Oxidation in Water. Ecotoxicol. Environ. Saf. 2018, 151, 127–131. [Google Scholar] [CrossRef]
- Mahamallik, P.; Swain, R. A Mini-Review on Arsenic Remediation Techniques from Water and Future Trends. Water Sci. Technol. 2023, 87, 3108–3123. [Google Scholar] [CrossRef]
- Chen, N.; Wang, X.; Wan, Y.; Luo, Y.; Huang, Y.; Zhang, L. Simulated Solar Light Driven Fe(III)/Fe(II) Redox Cycle for Roxarsone Degradation and Simultaneous Arsenate Immobilization. J. Hazard. Mater. 2020, 394, 121635. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, G.; Naushad, M.; Al-Muhtaseb, A.A.H.; García-Peñas, A.; Mola, G.T.; Si, C.; Stadler, F.J. Bio-inspired and biomaterials-based hybrid photocatalysts for environmental detoxification: A review. Chem. Eng. J. 2020, 382, 122937. [Google Scholar] [CrossRef]
- Wang, H.; Murugananthan, M.; Zhang, Y. Graphitic Carbon Nitride Based Photocatalysis for Redox Conversion of Arsenic(III) and Chromium(VI) in Acid Aqueous Solution. Appl. Catal. B Environ. 2019, 248, 349–356. [Google Scholar] [CrossRef]
- Lei, D.; Zhang, W.; Chen, L.; Liu, X.; Li, Y.; Wang, Y.; Liu, J. Simultaneous Removal of Arsenic(III) and Chromium(VI) over ZnFe2O4 {100}/{111} Z-Scheme Photocatalyst: Facet-Dependent Active Site and Overlooked As(III)/Cr(VI) Complex. J. Clean. Prod. 2023, 383, 135493. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.; Lai, C.; Zeng, G.; Huang, D.; Cheng, M.; Wang, J.; Chen, F.; Zhou, C.; Xiong, W. BiOX (X = Cl, Br, I) photocatalytic nanomaterials: Applications for fuels and environmental management. Adv. Colloid Interface Sci. 2018, 254, 76–93. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshni, N.; Nath, P.; Nagahanumaiah; Chanda, N. Sustainable removal of arsenate, arsenite and bacterial contamination from water using biochar stabilized iron and copper oxide nanoparticles and associated mechanism of the remediation process. J. Water Process Eng. 2020, 37, 101495. [Google Scholar] [CrossRef]
- Moreira, V.R.; Lebron, Y.A.R.; Santos, L.V.S.; de Paula, E.C.; Amaral, M.C.S. Arsenic Contamination, Effects and Remediation Techniques: A Special Look onto Membrane Separation Processes. Process Saf. Environ. Prot. 2021, 148, 604–623. [Google Scholar] [CrossRef]
- Cuong, D.V.; Wu, P.C.; Chen, L.I.; Hou, C.H. Active MnO2/biochar composite for efficient As(III) removal: Insight into the mechanisms of redox transformation and adsorption. Water Res. 2021, 188, 116495. [Google Scholar] [CrossRef]
- Yamen, R.; Bashir, H.; Bibi, I.; Shaheen, S.M.; Niazi, N.K.; Shahid, M.; Hussain, M.M.; Antoniadis, V.; Shakoor, M.B.; Al-Solaimani, S.G.; et al. A critical review on arsenic removal from water using biochar-based sorbents: The significance of modification and redox reactions. Chem. Eng. J. 2020, 396, 125195. [Google Scholar] [CrossRef]
- Shi, W.; Ma, J.; Gao, F.; Dai, R.; Su, X.; Wang, Z. Metal-organic framework with a redox-active bridge enables electrochemically highly selective removal of arsenic from water. Environ. Sci. Technol. 2023, 57, 6342–6352. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Maiti, A. Optimized Synthesis and Characterization of Laterite–Biochar Composite for Arsenic Removal: Examining Colloidal Stability and As(III) Oxidation. Biochar 2024, 6, 17. [Google Scholar] [CrossRef]
- Alka, F.; Shahir, S.; Ibrahim, N.; Ndejiko, M.J.; Vo, D.-V.N.; Manan, F.A. Arsenic Removal Technologies and Future Trends: A Mini Review. J. Clean. Prod. 2021, 278, 123805. [Google Scholar] [CrossRef]
- Zhang, S.; Yi, X.; He, D.; Tang, X.; Chen, Y.; Zheng, H. Recent progress and perspectives of typical renewable bio-based flocculants: Characteristics and application in wastewater treatment. Environ. Sci. Pollut. Res. 2024, 31, 46877–46897. [Google Scholar] [CrossRef]
- Siddiqui, S.I.; Naushad, M.; Chaudhry, S.A. Promising prospects of nanomaterials for arsenic water remediation: A comprehensive review. Process Saf. Environ. Prot. 2019, 126, 60–97. [Google Scholar] [CrossRef]
- Sánchez, J.; Dax, D.; Tapiero, Y.; Xu, C.; Willför, S. Bio-based hydrogels with ion exchange properties applied to remove Cu(II), Cr(VI), and As(V) ions from water. Front. Bioeng. Biotechnol. 2021, 9, 656472. [Google Scholar] [CrossRef] [PubMed]
- De Vargas Brião, G.; de Andrade, J.R.; da Silva, M.G.C.; Vieira, M.G.A. Removal of toxic metals from water using chitosan-based magnetic adsorbents: A review. Environ. Chem. Lett. 2020, 18, 1145–1168. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Mohamed, A.A. The use of chitosan-based composites for environmental remediation: A review. Int. J. Biol. Macromol. 2023, 242, 124787. [Google Scholar] [CrossRef] [PubMed]
- Zia, Q.; Tabassum, M.; Gong, H.; Li, J. A review on chitosan for the removal of heavy metals ions. J. Fiber Bioeng. Inform. 2019, 3, 103–128. [Google Scholar] [CrossRef]
- Kolya, H.; Kang, C.-W. Bio-based polymeric flocculants and adsorbents for wastewater treatment. Sustainability 2023, 15, 9844. [Google Scholar] [CrossRef]
- Yang, R.; Li, H.; Huang, M.; Yang, H.; Li, A. A review on chitosan-based flocculants and their applications in water treatment. Water Res. 2016, 95, 59–89. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Ajaj, Y.; Mahmoud, Z.H.; Ghadir, G.K.; Alani, Z.K.; Hussein, M.M.; Hussein, S.A.; Karim, M.M.; Al-Khalidi, A.; Abbas, J.K.; et al. Adsorption of Heavy Metal Ions Using Chitosan/Graphene Nanocomposites: A Review Study. Results Chem. 2024, 7, 101332. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Abdullah, S.R.S.; Imron, M.F.; Said, N.S.M.; Ismail, N.I.; Hasan, H.A.; Othman, A.R.; Purwanti, I.F. Challenges and Opportunities of Biocoagulant/Bioflocculant Application for Drinking Water and Wastewater Treatment and Its Potential for Sludge Recovery. Int. J. Environ. Res. Public Health 2020, 17, 9312. [Google Scholar] [CrossRef]
- Shih, M.C. An Overview of Arsenic Removal by Pressure-Driven Membrane Processes. Desalination 2005, 172, 85–97. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, F.; Han, C.; Houda, C.; Hao, M.; Wang, Q. Research Progress on Adsorption of Arsenic from Water by Modified Biochar and Its Mechanism: A Review. Water 2022, 14, 1691. [Google Scholar] [CrossRef]
- Rahman, M.S.; Reza, A.S.; Sattar, G.S.; Siddique, M.A.B.; Akbor, M.A.; Moniruzzaman, M.; Shafiuzzaman, S.M. Mobilization Mechanisms and Spatial Distribution of Arsenic in Groundwater of Western Bangladesh: Evaluating Water Quality and Health Risk Using EWQI and Monte Carlo Simulation. Chemosphere 2024, 366, 143453. [Google Scholar] [CrossRef] [PubMed]
- Burbano, A.A.; Lassalle, V.L.; Horst, M.F.; Méndez, A. The Effect of Carbon Coating on the Arsenite Sorption by Magnetic Carbon Nanocomposites. Int. J. Environ. Sci. Technol. 2025, 22, 4749–4760. [Google Scholar] [CrossRef]
- Feng, W.Y.; Wei, S.D.; Liu, C.B.; Chen, T.; Tang, Y.H.; Ma, J.H.; Yin, K.; Luo, S.L. Efficient removal of arsenic from groundwater using iron oxide nanoneedle array-decorated biochar fibers with high Fe utilization and fast adsorption kinetics. J. Hazard. Mater. 2019, 373, 141–151. [Google Scholar] [CrossRef]
- Nikić, J.; Jazić, J.M.; Watson, M.; Agbaba, J. Application of Nanomaterials in Water Treatment: Arsenic and Natural Organic Matter Removal. Recent Pat. Nanotechnol. 2021, 15, 197–224. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, J.; Ren, Z.; Xiang, W.; Sifat, I.; Zhang, W.; Zhu, J.; Li, B. Next Generation Decentralized Water Systems: A Water-Energy-Infrastructure-Human Nexus (WEIHN) Approach. Environ. Sci. Water Res. Technol. 2023, 9, 2446–2471. [Google Scholar] [CrossRef]
- Lata, S.; Samadder, S.R. Removal of Arsenic from Water Using Nano Adsorbents and Challenges: A Review. J. Environ. Manag. 2016, 166, 387–406. [Google Scholar] [CrossRef]
- Shaumbwa, V.R.; Liu, D.; Archer, B.; Li, J.; Su, F. Preparation and Application of Magnetic Chitosan in Environmental Remediation and Other Fields: A Review. J. Appl. Polym. Sci. 2021, 138, 51241. [Google Scholar] [CrossRef]
- Alsaiari, N.S.; Alzahrani, F.M.; Katubi, K.M.; Amari, A.; Rebah, F.B.; Tahoon, M.A. Polyethylenimine-Modified Magnetic Chitosan for the Uptake of Arsenic from Water. Appl. Sci. 2021, 11, 5630. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Dave, P.N. Water Purification Using Nanotechnology: An Emerging Opportunity. Chem. Methodol. 2019, 3, 115–144. [Google Scholar] [CrossRef]
- Rahman, M.M.; Khan, K.H.; Parvez, M.M.H.; Irizarry, N.; Uddin, M.N. Polymer Nanocomposites with Optimized Nanoparticle Dispersion and Enhanced Functionalities for Industrial Applications. Processes 2025, 13, 994. [Google Scholar] [CrossRef]
- Lin, Y.; Jin, X.; Khan, N.I.; Owens, G.; Chen, Z. Bimetallic Fe/Ni Nanoparticles Derived from Green Synthesis for the Removal of Arsenic (V) in Mine Wastewater. J. Environ. Manag. 2022, 301, 113838. [Google Scholar] [CrossRef] [PubMed]
- Chutia, G.P.; Phukan, K. Facile Synthesis of Fe3O4@biochar@SO3H as Magnetically Separable Bronsted Acid Nanocatalyst for Biodiesel Production from Different Oil Feedstocks. Ind. Crops Prod. 2024, 215, 118578. [Google Scholar] [CrossRef]
- Uddin, M.N.; Dhanasekaran, P.S.; Asmatulu, R. Synthesis, Characterization, and Applications of Polymer-Based Biomaterials. In Advances in Nanotechnology; Bartul, Z., Trenor, J., Eds.; Nova Science Publishers: New York, NY, USA, 2019; Volume 22, pp. 129–168. [Google Scholar]
- Uddin, M.N.; Desai, F.; Asmatulu, E. Review of Bioaccumulation, Biomagnification, and Biotransformation of Engineered Nanomaterials. In Nanotoxicology and Nanoecotoxicology; Kumar, V., Guleria, P., Ranjan, S., Dasgupta, N., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2021; Volume 67, pp. 129–168. [Google Scholar] [CrossRef]
- Choi, J.; Kim, B.H. Ligands of Nanoparticles and Their Influence on the Morphologies of Nanoparticle-Based Films. Nanomaterials 2024, 14, 1685. [Google Scholar] [CrossRef] [PubMed]
- Pakizeh, M.; Moradi, A.; Ghassemi, T. Chemical Extraction and Modification of Chitin and Chitosan from Shrimp Shells. Eur. Polym. J. 2021, 159, 110709. [Google Scholar] [CrossRef]
- Zahedi, A.; Liyanapathirana, R.; Thiyagarajan, K. Biodegradable and Renewable Antennas for Green IoT Sensors: A Review. IEEE Access 2024, 12, 189749–189775. [Google Scholar] [CrossRef]
- Yuan, M.; Li, C.; Wang, M.; Cao, H.; Ye, T.; Hao, L.; Wu, X.; Yin, F.; Yu, J.; Xu, F. Low-Cost, Portable, On-Site Fluorescent Detection of As(III) by a Paper-Based Microfluidic Device Based on Aptamer and Smartphone Imaging. Microchim. Acta 2023, 190, 109. [Google Scholar] [CrossRef]
- Paranjape, P.; Sadgir, P. Heavy Metal Removal Using Plant Origin Biomass and Agricultural Waste-Derived Biomass from Aqueous Media: A Review. Water Conserv. Sci. Eng. 2023, 8, 9. [Google Scholar] [CrossRef]
- Tang, Z.; Ma, D.; Chen, Q.; Wang, Y.; Sun, M.; Lian, Q.; Shang, J.; Wong, P.K.; He, C.; Xia, D.; et al. Nanomaterial-Enabled Photothermal-Based Solar Water Disinfection Processes: Fundamentals, Recent Advances, and Mechanisms. J. Hazard. Mater. 2022, 437, 129373. [Google Scholar] [CrossRef]
- Varamesh, A.; Abraham, B.D.; Wang, H.; Berton, P.; Zhao, H.; Gourlay, K.; Minhas, G.; Lu, Q.; Bryant, S.L.; Hu, J. Multifunctional Fully Biobased Aerogels for Water Remediation: Applications for Dye and Heavy Metal Adsorption and Oil/Water Separation. J. Hazard. Mater. 2023, 457, 131824. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Removal of Various Pollutants from Water and Wastewater by Modified Chitosan Adsorbents. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2331–2386. [Google Scholar] [CrossRef]
- Wang, B.; Xuan, J.; Yang, X.; Bai, Z. Synergistic DFT-Guided Design and Microfluidic Synthesis of High-Performance Ion-Imprinted Biosorbents for Selective Heavy Metal Removal. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127030. [Google Scholar] [CrossRef]
- Uddin, M.N.; Subeshan, B.; Rahman, M.M.; Asmatulu, R. Bioinspired Electrospun Nanocomposites: An Emerging Technology of Atmospheric Fog Water Generator. In Proceedings of the International Conference on Mechanical, Industrial and Energy Engineering, Khulna, Bangladesh, 19–21 December 2020. [Google Scholar]
- Del Pilar Rodríguez-Rojas, M.; Bustos-Terrones, V.; Díaz-Cárdenas, M.Y.; Vázquez-Vélez, E.; Martínez, H. Life Cycle Assessment of Green Synthesis of TiO2 Nanoparticles vs. Chemical Synthesis. Sustainability 2024, 16, 7751. [Google Scholar] [CrossRef]
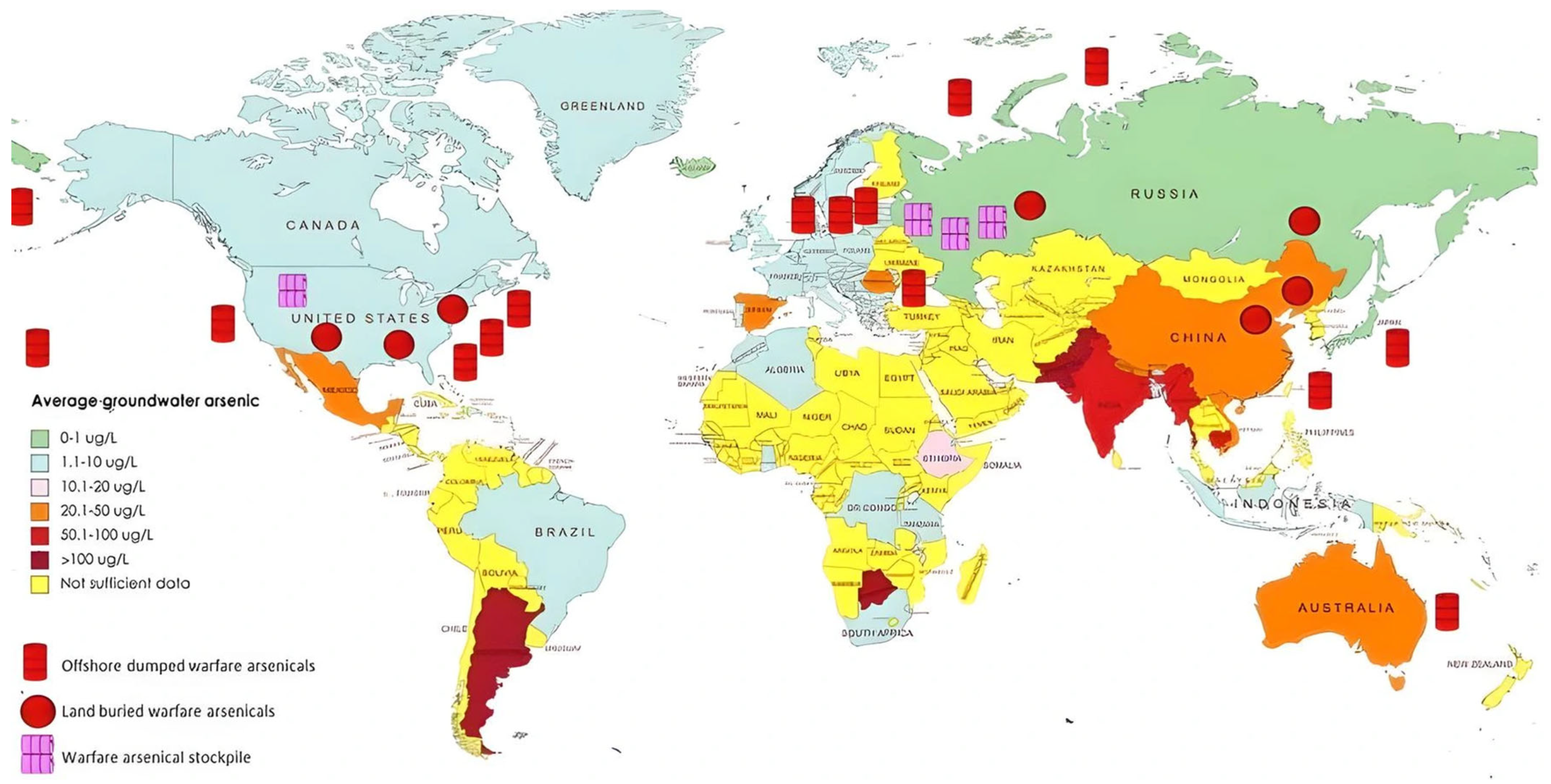
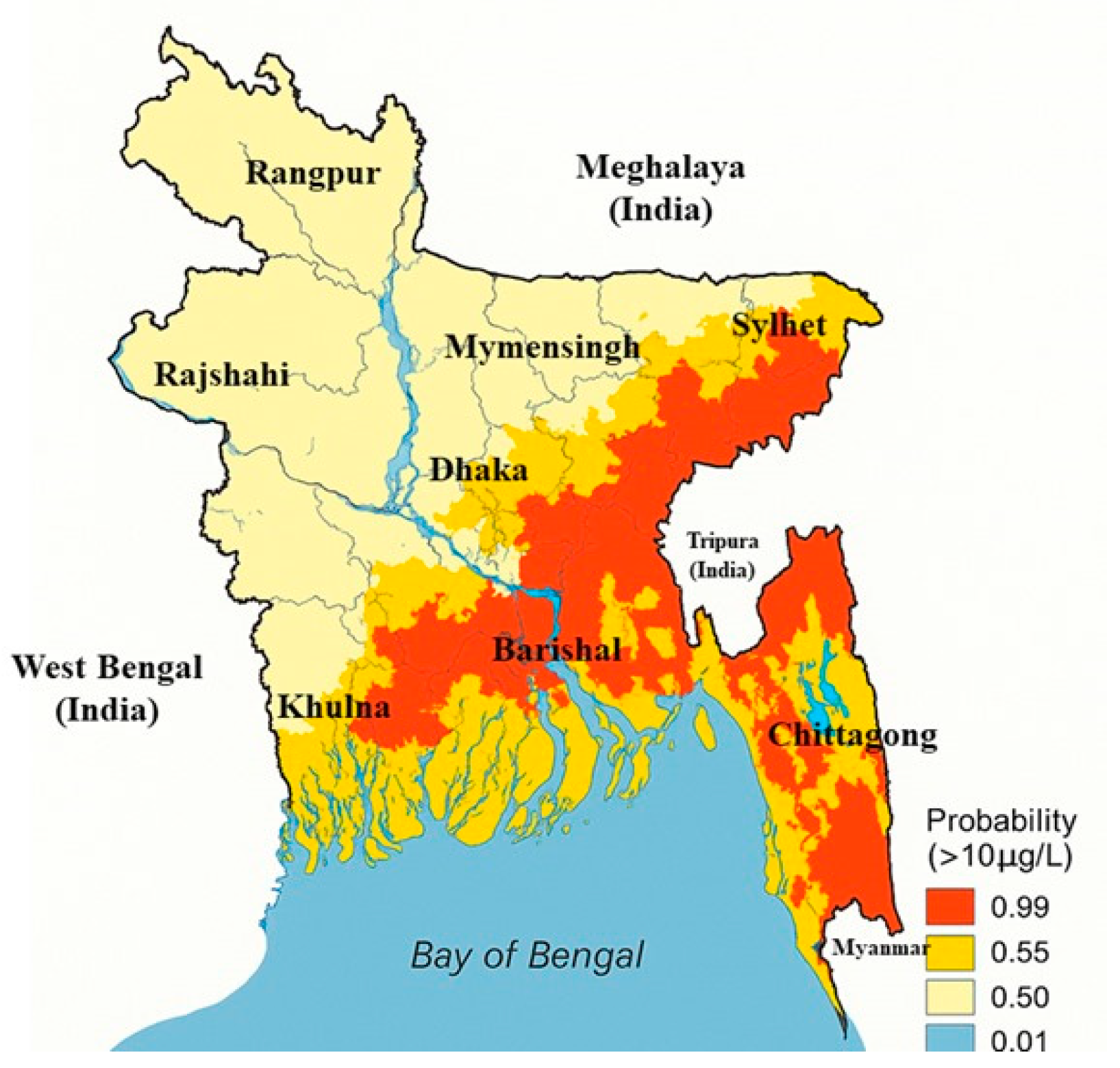

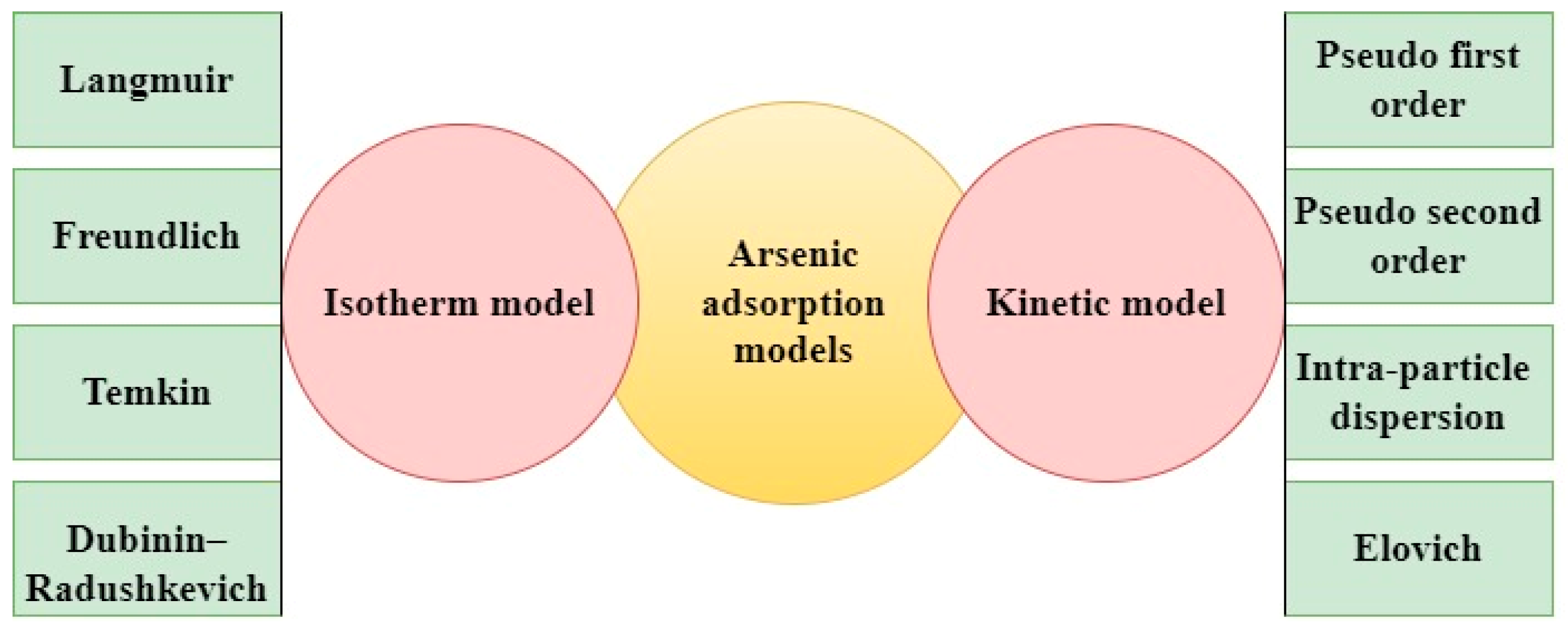
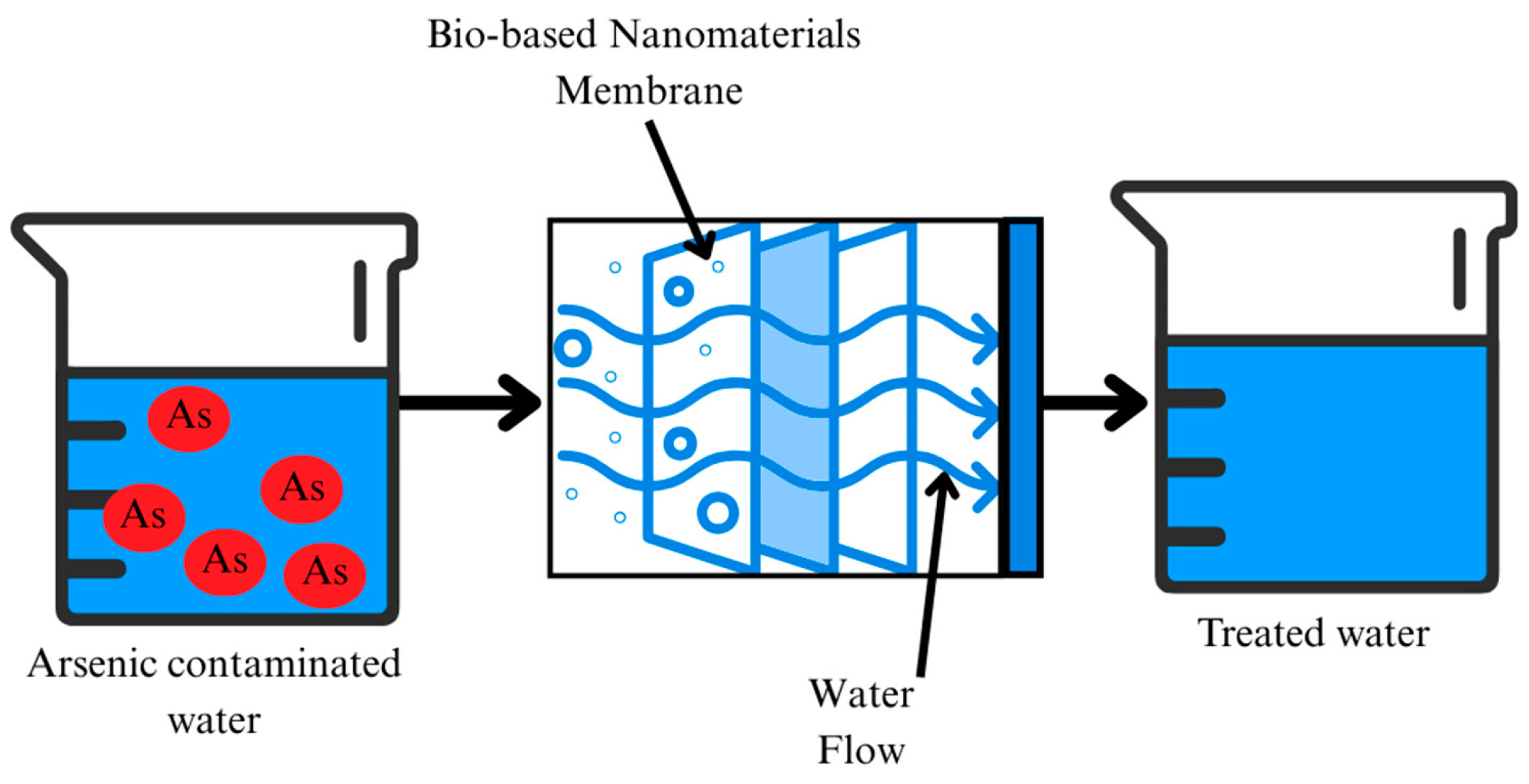


| Category | Effect | Reference |
|---|---|---|
| Human Health | Skin lesions, cancers (skin, lung, bladder), cardiovascular disease | [9] |
| Neurological effects and cognitive impairment in children | [10] | |
| Diabetes, hypertension, and metabolic disorders | [11] | |
| Adverse birth outcomes due to prenatal arsenic exposure | [12] | |
| Environment | Disruption of microbial communities in arsenic-contaminated sites | [13] |
| Decreased aquatic biodiversity | [14] | |
| Sediment arsenic accumulation and benthic toxicity | [15] | |
| Agriculture | Phytotoxic effects: reduced plant growth and yield loss | [16] |
| Arsenic accumulation in rice and vegetables | [17] | |
| Microbial community and enzyme activity decline in agricultural soil | [18] | |
| Socio-economic | Health care costs and productivity loss in arsenic-affected regions | [19] |
| Loss of income and agricultural productivity | [20] | |
| Mental health challenges among chronically exposed populations | [21] |
| Category | Source | Arsenic (kt/a) |
|---|---|---|
| Natural | Windblown dusts | 2.6 |
| Sea salt spray | 1.7 | |
| Volcanoes | 3.8 | |
| Forest fires | 0.19 | |
| Anthropogenic | Fossil-fuel combustion | 0.81 |
| Non-ferrous metal production | 3.46 | |
| Iron and steel production | 0.35 | |
| Cement production | 0.27 | |
| Waste disposal | 0.12 | |
| Biogenic | Continental particulates | 0.26 |
| Continental volatiles | 1.3 | |
| Marine | 2.3 |
| Bio-Based Nanomaterial | Derived Sources | Possible Nanostructure/Form | As Species Targeted | Remediation Mechanism(s) | Reference |
|---|---|---|---|---|---|
| Chitosan | Shrimp shells, fungi | Nanoparticles, beads, films | As (V), As (III) | Adsorption, coagulation/flocculation ion exchange | [30,31,32] |
| Cellulose | Plant biomass | Aerogels, membranes | As (V) | Adsorption | [33,34] |
| Modified Biochar | Rice husk, sawdust | Porous nanosheets, powder | As (III), As (V) | Adsorption, redox reaction | [35,36,37,38,39] |
| Algae-Based | Green/brown algae | Hydrogel, nanopowder | As (III), As (V) | Complexation, ion exchange | [40,41,42] |
| Lignin-Based | Forestry/agricultural waste | Nanoparticles | As (III), As (V) | Adsorption, complexation, ion exchange | [43,44] |
| Biogenic nZVI | Green tea, eucalyptus | Zero-valent iron nanoparticles | As (V) | Reduction, adsorption | [45,46,47,48] |
| Nano-silica | Algae, agricultural residue | Nanoparticles | As (V) | Adsorption | [50,51,52] |
| Bio-Based Adsorbent | Adsorbate | Optimum pH | Kinetic Constants k1: Pseudo 1st Order (/min); k2: Pseudo 2nd Order (g/mg·min); kp: Intra-Particle Diffusion (mg/g·(min)1/2); β: Desorption Constant (g/mg) (Elovich Model); qe: Equilibrium Adsorption Capacity (mg/g) | Surface Area (m2/g) | Maximum Adsorption Capacity (Qm) (mg/g) | Reference |
|---|---|---|---|---|---|---|
| TiO2-loaded biochar | As (III) | – | k1 = 0.0020 k2 = 0.0284 kp = 1.6106 β = 0.516 (For initial concentration 80 mg/L) | 128.22 | 58.456 | [65] |
| Chitosan–magnetic graphene oxide nanocomposite | As (III) | 7.3 | k1 = 0.0767 k2 = 0.0317 kp = 0.5755 | 152.38 | 45 | [66] |
| Chitosan–Fe-crosslinked complex | As (III) | 9.0 | k1 = 0.0024 (qe = 2.51) k2 = 0.0042 (qe = 1.69) kp = 42.05 | – | 13.4 | [67] |
| Chitosan-coated bentonite | As (V) | – | k1 = 0.0117 (qe = 0.002201) k2 = 4.502 (qe = 0.00834) kp = 1.519 × 109 | – | – | [68] |
| Control chitosan biosorbent beads (CCBB) and Magnetic chitosan biosorbent beads (MCBB) | As (III), As (V) | 6.7 | For CCBB, k1 = 0.000667, k2 = 0.27 [As (III)] k1 = 0.000975, k2 = 0.17 [As (V)] For MCBB, k1 = 0.00078, k2 = 0.40 [As (III)] k1 = 0.00076, k2 = 0.32 [As (V)] | 38.27 (CCBB) 52.48 (MCBB) | For CCBB 18.87 As (III) 26.13 As (V) For MCBB 73.69 As (III) 79.49 As (V) | [69] |
| Chitosan quinoxaline Schiff base (CsQ) and cross-linked chitosan quinoxaline Schiff base (CsQG) | As (V) | 7 (CsQ) 6 (CsQG) | For CsQ, k1 = 0.0027, K2 = 00.064 For CsQG, k1 = 0.0196, K2 = 0.0195 | – | 8.811 (CsQ) 31.95 (CsQG) | [70] |
| Aluminum-modified food-waste biochar | As (III) | – | k1 = 0.00496 (qe = 19.5) k2 = 0.000317 (qe = 20.5) | – | 52.2 | [71] |
| Zero-valent iron/biochar composite | As (III), As (V) | – | – | – | 129.24 As (III) 127.15 As (V) | [72] |
| Membrane Type | Pore Size | As Species | Initial As Concentration | pH Range | Removal Efficiency (%) | Reference |
|---|---|---|---|---|---|---|
| Microfiltration (MF) | 0.1–10 μm | As (III) | 20–50 mg/L | 7.6–7.9 | 96% | [79,80] |
| Ultrafiltration (UF) | 2–100 nm | As (V) | 100 ng/L | 8 | >99% | [75,84,86] |
| Nanofiltration (NF) | 1–10 nm | As (V) | 0–200 μg/L | 6.75 | >99% | [80,81] |
| Reverse Osmosis (RO) | 0.1–1 nm | As (III), As (V) | 10–1000 μg/L | 5.5–8.5 | >99% | [75,87] |
| Method | Mechanism | Nanomaterials Used | Mode of Action | Advantages | Disadvantages | Reference |
|---|---|---|---|---|---|---|
| Adsorption | Binding of arsenic ions to adsorbent surfaces | Chitosan-coated NPs, MnFe2O4-biochar, iron oxide nanoneedles | Physical/chemical binding, surface complexation, electrostatic interactions | -Cost-effective and simple -High efficiency with materials like chitosan and biochar -Easy regeneration | -Limited selectivity in the presence of competing ions -Potential fouling after multiple uses | [58,59,60] |
| Filtration/Membranes | Physical separation and selective transport using nanoporous structures | Nanofiltration membranes, bio-nanocomposites, TiO2-coated, iron oxide nanofiber filters | Physical blocking, size exclusion coupled with adsorption | -High selectivity -Can achieve >99% arsenic removal -Good for continuous-flow systems | -Expensive fabrication and operation -Membrane fouling -Not always feasible for rural areas | [73,76,77] |
| Photocatalysis | Light-driven oxidation combined with adsorption | BiOI, TiO2, biochar-based composites, carbon nanotube hybrids | Oxidation of As (III) to As (V), followed by adsorption | -Uses solar energy -Effective for As (III) to As (V) conversion | -Depends on light source -Some materials have low visible light efficiency | [88,89] |
| Redox Reactions | Oxidation-reduction conversion to less toxic and more adsorbable arsenic forms | Biochar@Fe/Cu, MnO2-biochar, redox-active MOFs (e.g., ferrocene-based) | Redox transformation followed by adsorption | -Converts toxic As (III) to less toxic As (V) -Synergistic adsorption–redox activity | -May require specific pH conditions -Material degradation possible over time | [98,99,100] |
| Complexation | Formation of stable complexes between functional groups and arsenic ions | Biochar@Fe/Cu, chitosan-stabilized magnetic NPs, MnFe2O4 nanocomposites | Surface complexation via hydroxyl, carboxyl, and amino groups, sometimes aided by photocatalysis | -High binding affinity with functional groups -Can be selective and efficient | -Functional group leaching may reduce long-term efficiency -Surface modification can be costly | [104] |
| Ion Exchange | Replacement of arsenic ions (As5+/As3+) with functional groups (e.g., –NH3⁺, –OH) present on the nanomaterial surface | Chitosan nanoparticles, chitin nanofibers | Amino and hydroxyl groups bind arsenic ions through electrostatic attraction and ligand exchange | -Good for low-concentration arsenic -Reversible process -Eco-friendly materials like chitosan | -Limited capacity -Sensitive to competing anions -Regeneration of chemicals may reduce sustainability | [107,108] |
| Coagulation–flocculation | Neutralization of surface charges and formation of aggregates that trap arsenic species | Modified chitosan (e.g., with Fe3+, Al3+), chitin-based nanoflocculants | Positively charged biopolymers interact with negatively charged arsenate/arsenite or suspended solids to form flocs | -Fast and scalable -Uses biodegradable coagulants like chitosan -Low-cost option | -Produces sludge -Less effective for dissolved arsenic -Needs post-treatment (filtration/sedimentation) | [109,110,112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.M.; Uddin, M.N.; Parvez, M.M.H.; Mohotadi, M.A.A.; Ferdush, J. Bio-Based Nanomaterials for Groundwater Arsenic Remediation: Mechanisms, Challenges, and Future Perspectives. Nanomaterials 2025, 15, 933. https://doi.org/10.3390/nano15120933
Rahman MM, Uddin MN, Parvez MMH, Mohotadi MAA, Ferdush J. Bio-Based Nanomaterials for Groundwater Arsenic Remediation: Mechanisms, Challenges, and Future Perspectives. Nanomaterials. 2025; 15(12):933. https://doi.org/10.3390/nano15120933
Chicago/Turabian StyleRahman, Md. Mahbubur, Md. Nizam Uddin, Md Mahadi Hassan Parvez, Md. Abdullah Al Mohotadi, and Jannatul Ferdush. 2025. "Bio-Based Nanomaterials for Groundwater Arsenic Remediation: Mechanisms, Challenges, and Future Perspectives" Nanomaterials 15, no. 12: 933. https://doi.org/10.3390/nano15120933
APA StyleRahman, M. M., Uddin, M. N., Parvez, M. M. H., Mohotadi, M. A. A., & Ferdush, J. (2025). Bio-Based Nanomaterials for Groundwater Arsenic Remediation: Mechanisms, Challenges, and Future Perspectives. Nanomaterials, 15(12), 933. https://doi.org/10.3390/nano15120933









