Removal of PET Microfibers from Simulated Wastewater Using Magnetic Nano-Ferric-Loaded Biochar: High Adsorption and Regeneration Performance
Abstract
1. Introduction
2. Methods and Materials
2.1. Chemicals and Reagents
2.2. Preparation of FBC
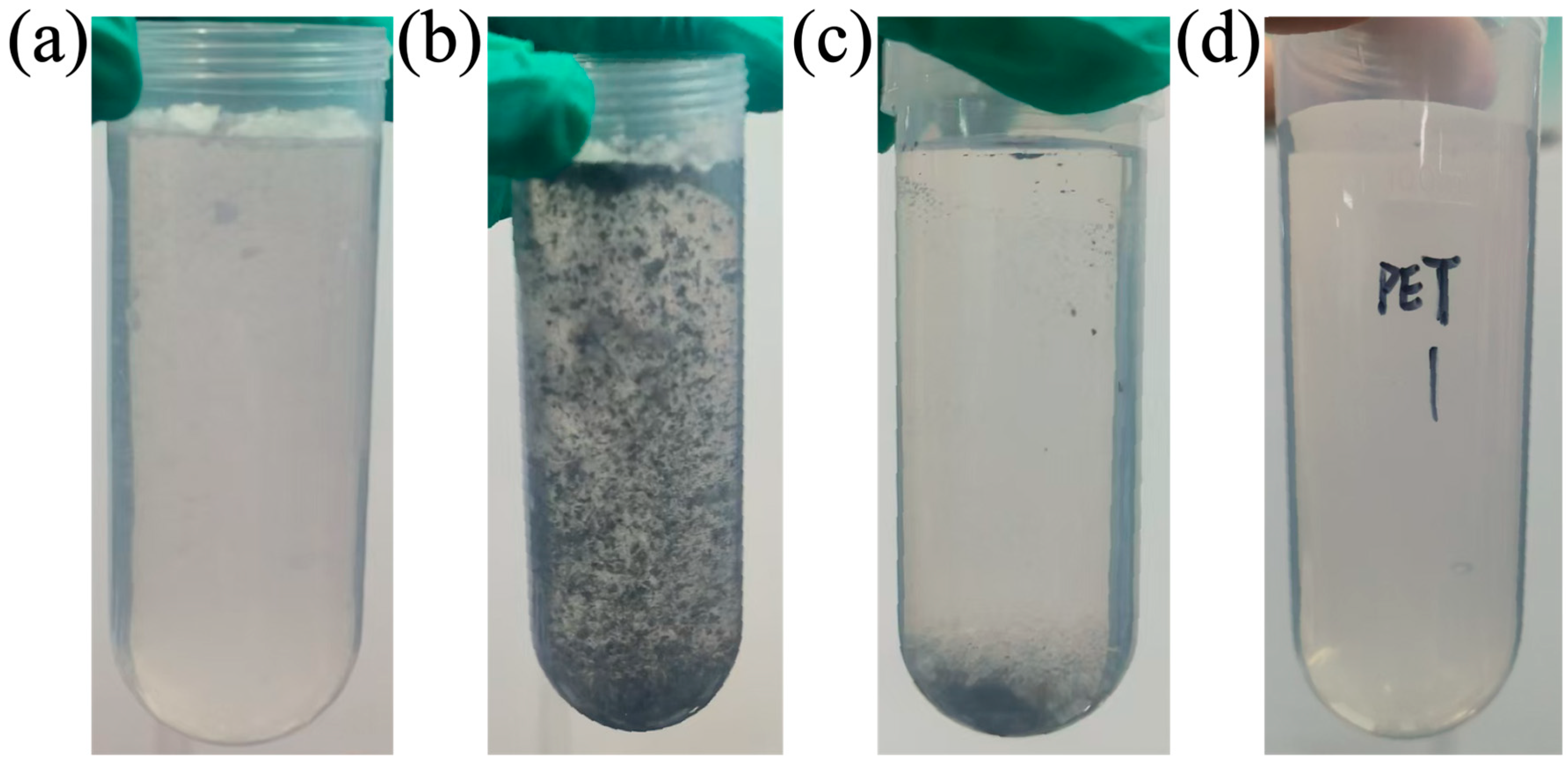
2.3. Quantitation of PET
2.4. Batch Adsorption Experiments
2.4.1. Effect of FBC Dosage on PET Adsorption
2.4.2. Effect of pH on PET Adsorption
2.4.3. Adsorption Isotherms
2.4.4. Adsorption Kinetics
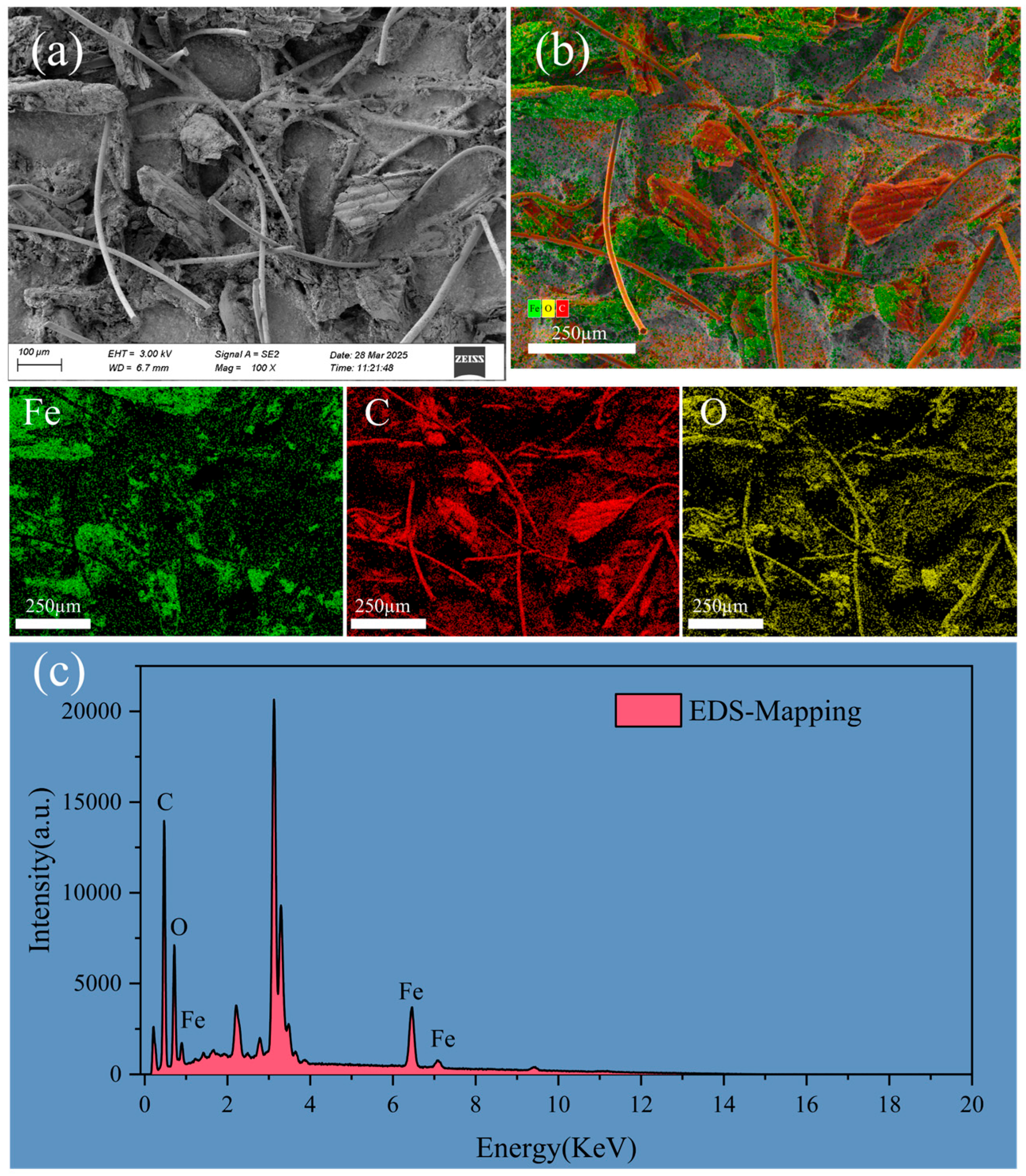
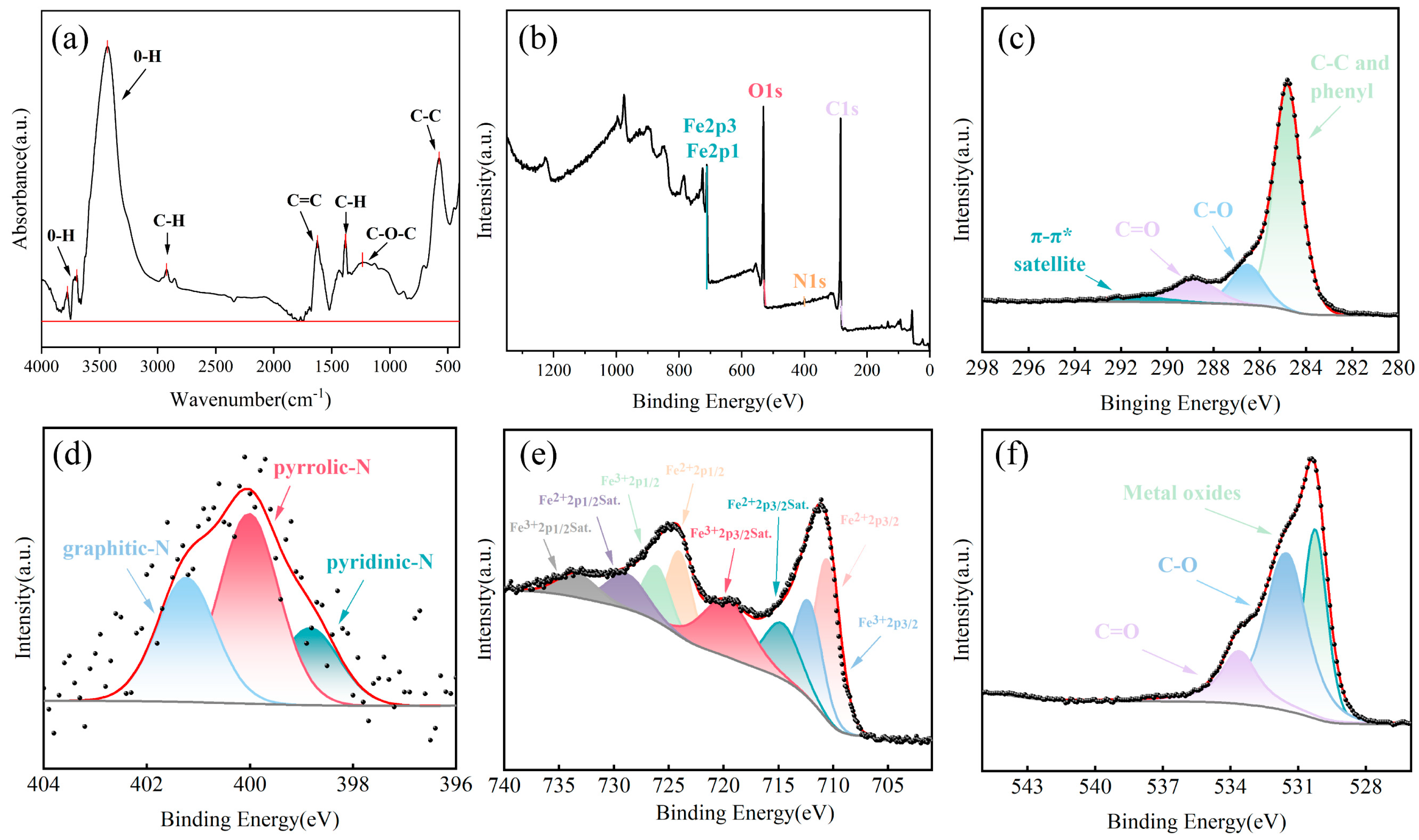
2.5. Characterization Methods
2.6. Regeneration Performance of FBC
3. Results and Discussion
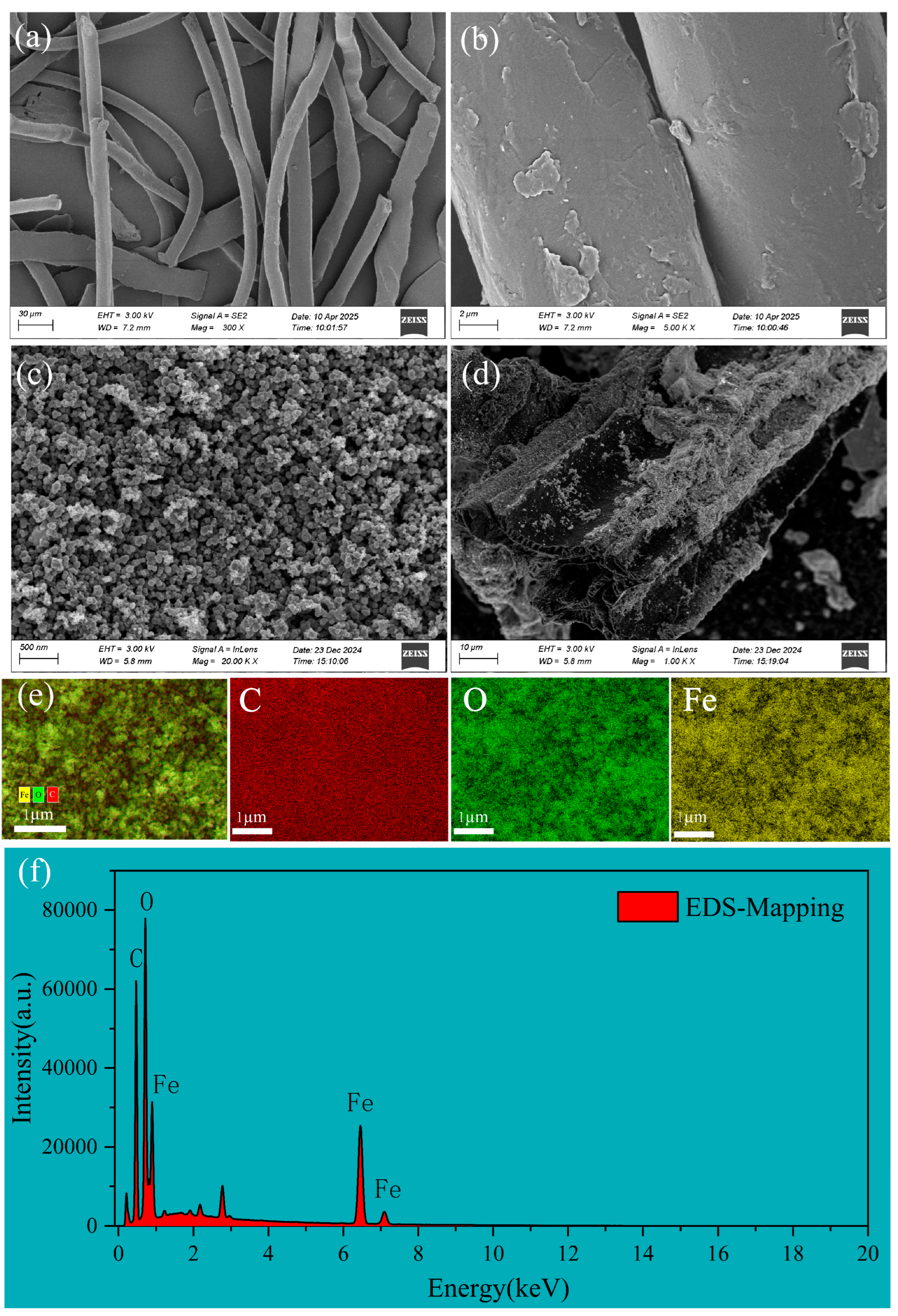
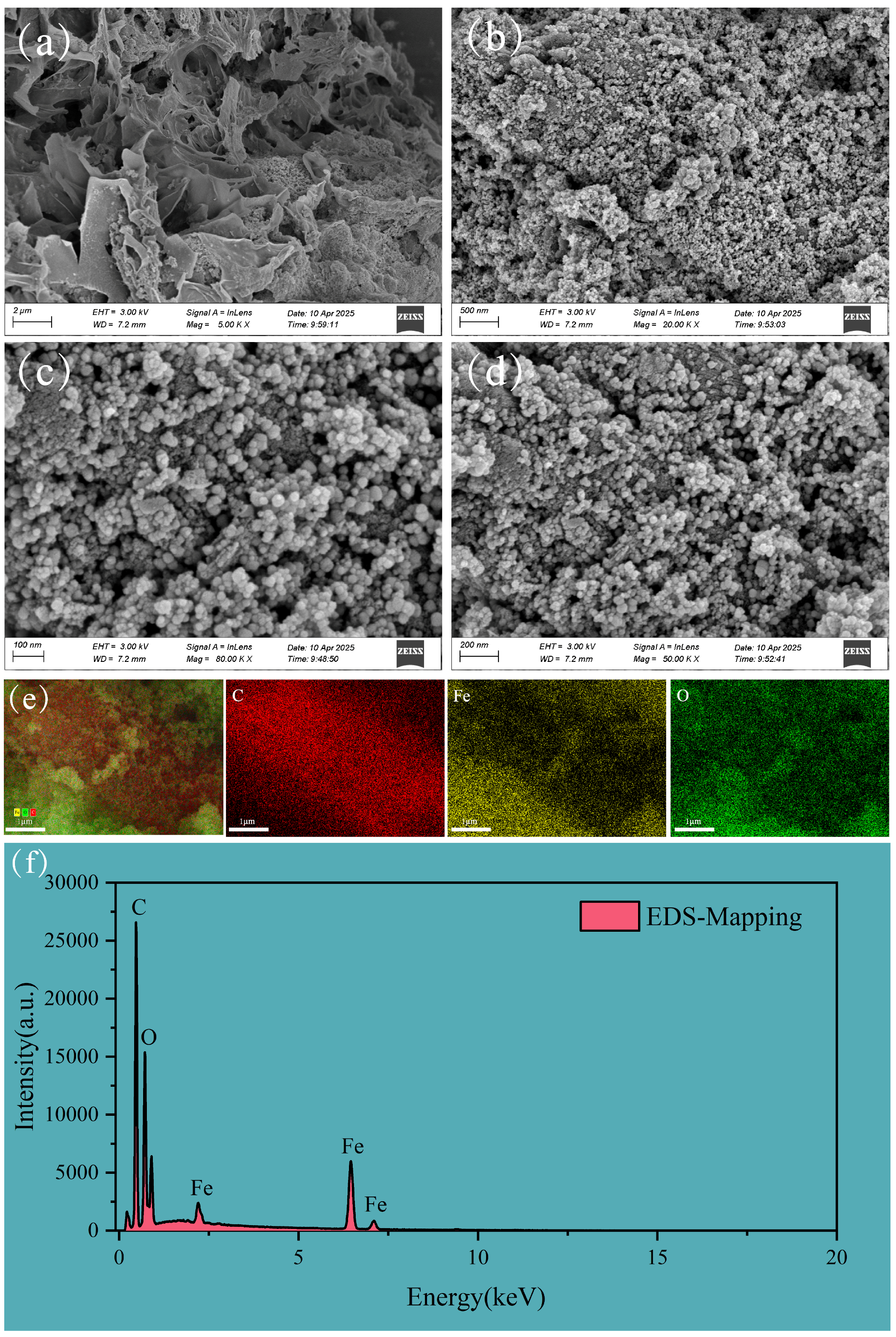
3.1. Characterization of FBC and PET
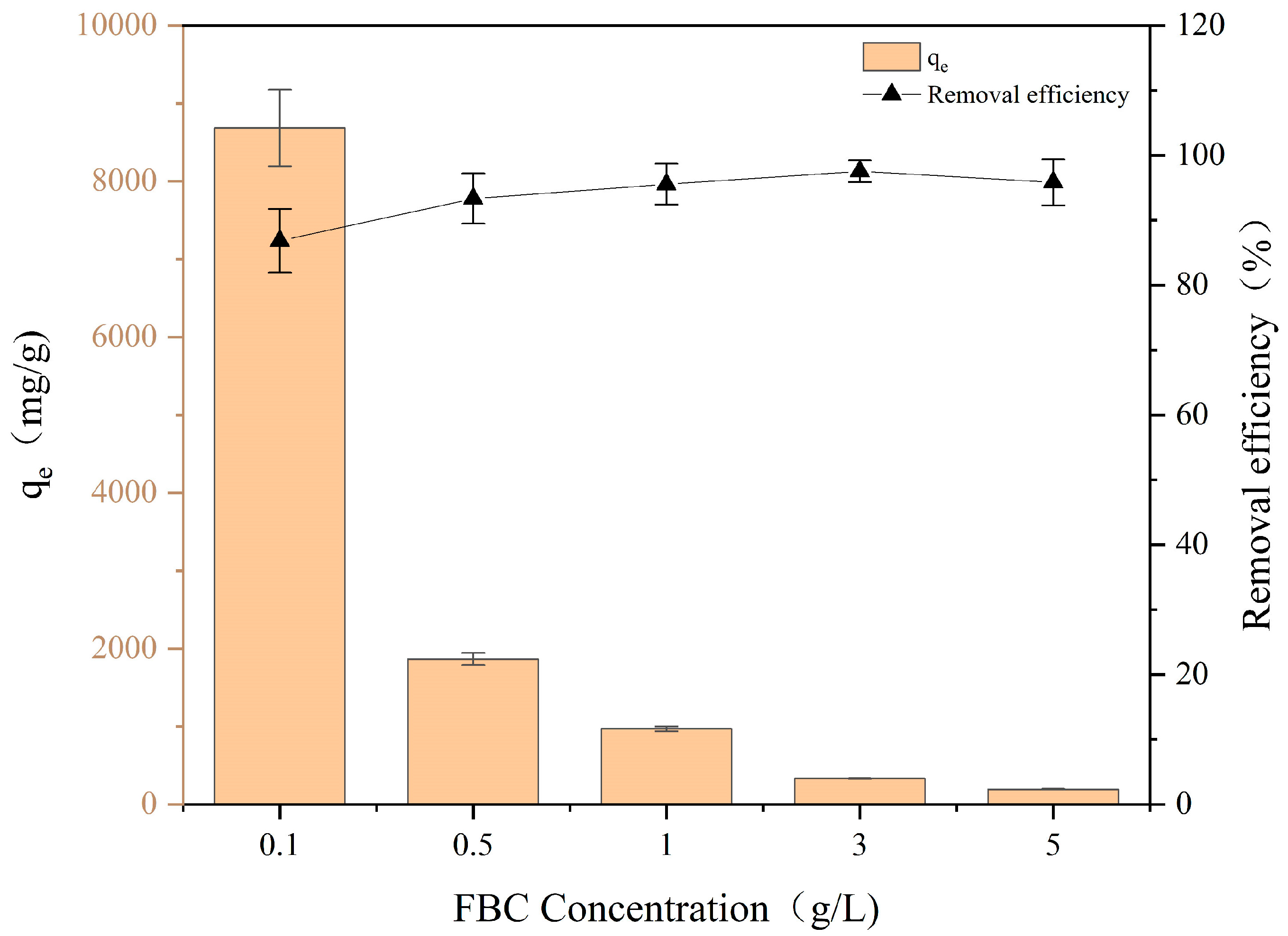
3.2. Effect of FBC Dosage on PET Adsorption
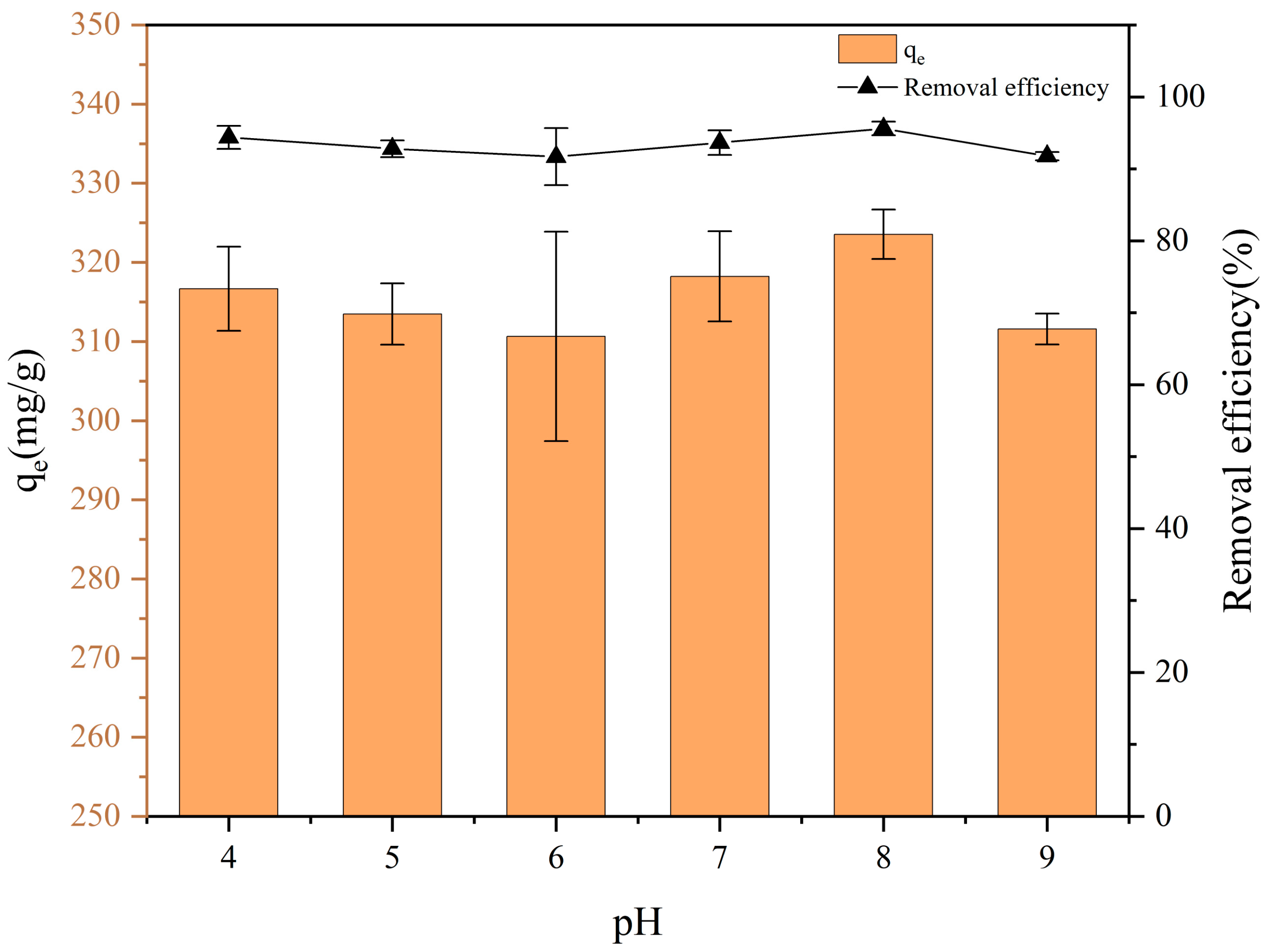
3.3. Effect of Solution PH on PET Adsorption
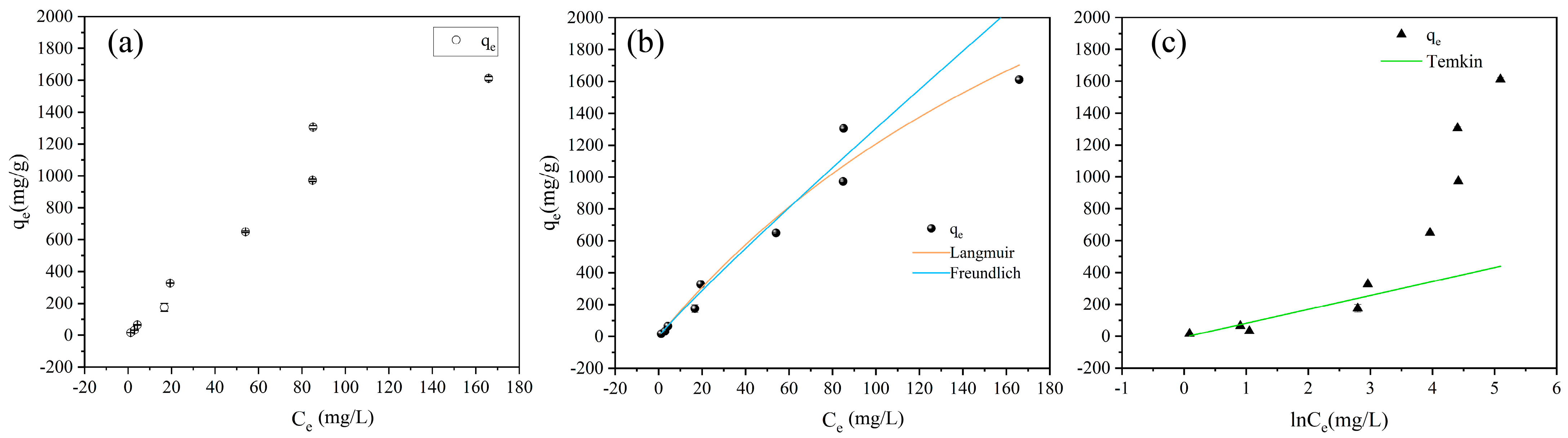
3.4. Adsorption Isotherms
3.5. Adsorption Kinetics
3.6. Proposed Adsorption Mechanisms
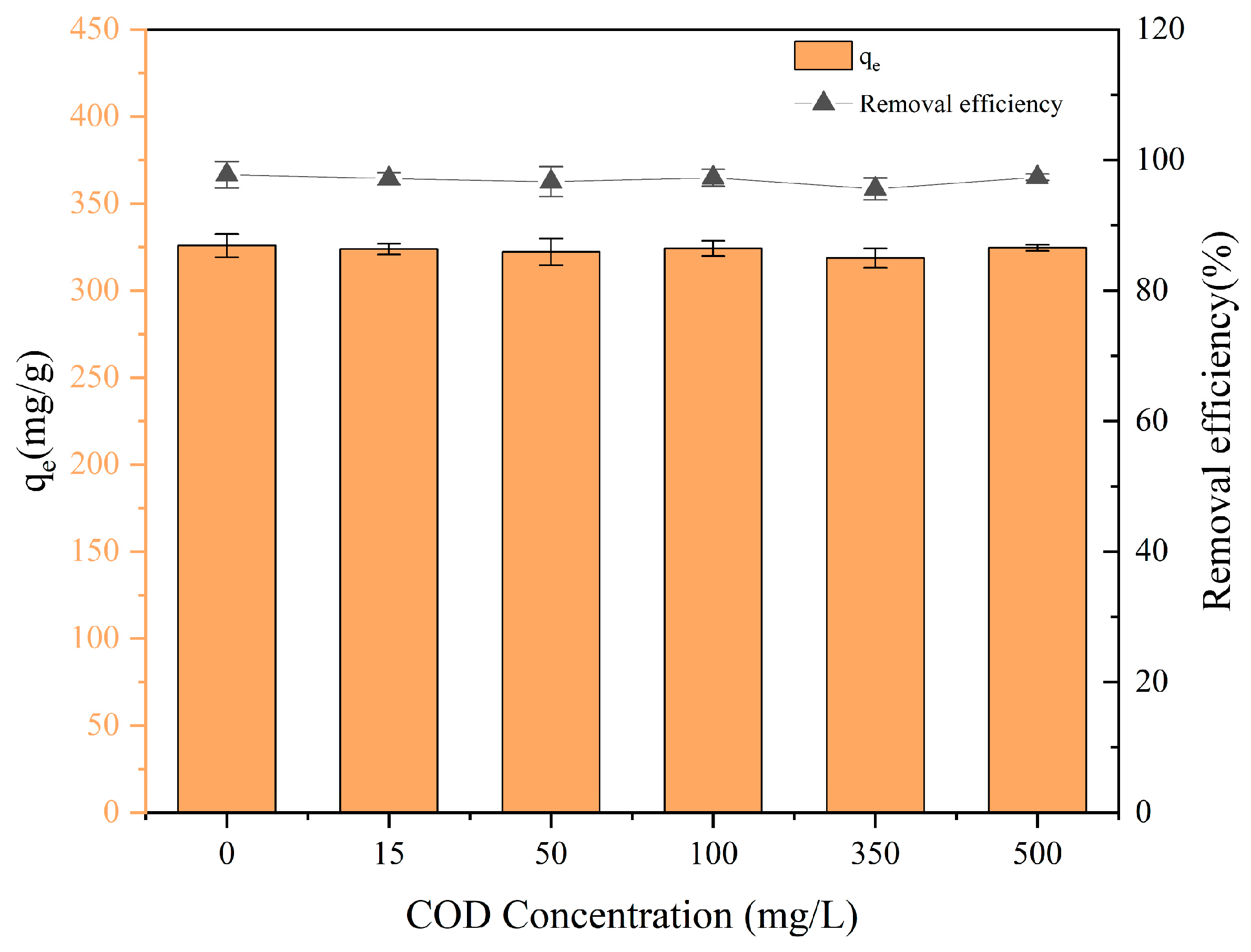
3.7. Effect of Co-Existed COD on PET Adsorption
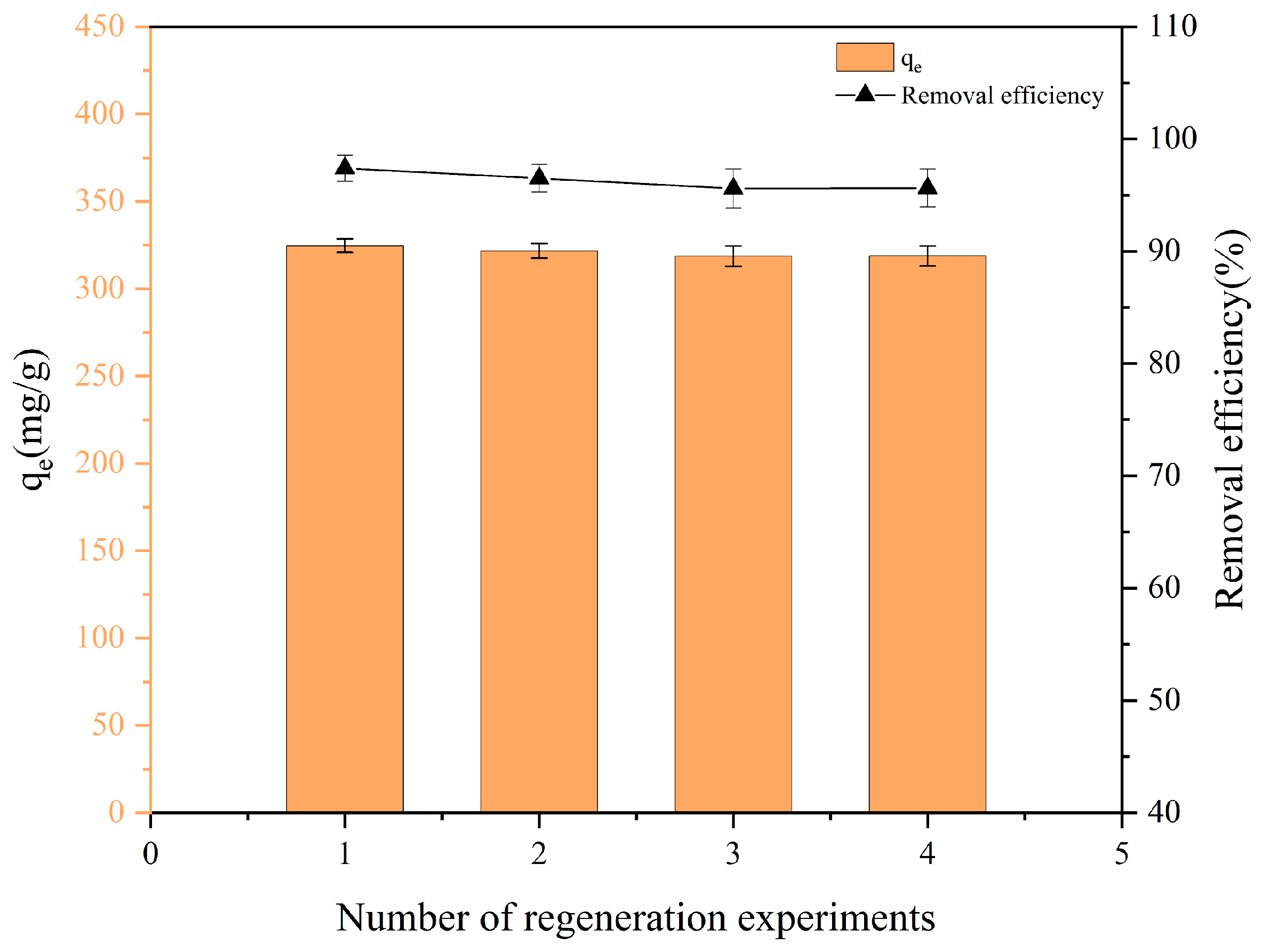
3.8. Regeneration Performance of FBC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Borrelle, S.B.; Ringma, J.; Law, K.L.; Monnahan, C.C.; Lebreton, L.; McGivern, A.; Murphy, E.; Jambeck, J.; Leonard, G.H.; Hilleary, M.A.; et al. Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science 2020, 369, 1515–1518. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Fang, Y.; Wang, Y.; Wang, F.; Shang, L.; Zhong, R. Microplastic pollution in soils, plants, and animals: A review of distributions, effects and potential mechanisms. Sci. Total Environ. 2022, 850, 157857. [Google Scholar] [CrossRef]
- Bostan, N.; Ilyas, N.; Akhtar, N.; Mehmood, S.; Saman, R.U.; Sayyed, R.Z.; Shatid, A.A.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Pandiaraj, S. Toxicity assessment of microplastic (MPs); a threat to the ecosystem. Environ. Res. 2023, 234, 116523. [Google Scholar] [CrossRef]
- Jing, M.; Li, C.; Wang, L.; Wang, T.a.; Xue, X.; Zhang, X.; Ma, Y. Toxicological Research Progress of Microplastics:Toxicological Effects of Microplastics on Microorganism, Algae, Fish and Mammal. Asian J. Ecotoxicol. 2022, 17, 265–280. [Google Scholar]
- Sun, M.-Y.; Guo, J.-Y.; Wang, X.-Y.; Chang, X. Sources and distributions of microplastics and the hazards to plants, animals and human health:A review. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2024, 35, 2301–2312. [Google Scholar]
- Lehner, R.; Weder, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Emergence of Nanoplastic in the Environment and Possible Impact on Human Health. Environ. Sci. Technol. 2019, 53, 1748–1765. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Shi, Q.; Tang, J. Research Progress on Human Exposure and Health Risks of Microplastics. Asian J. Ecotoxicol. 2022, 17, 354–365. [Google Scholar]
- Phuong Linh, N.; Pramanik, B.K.; Shah, K.; Roychand, R. Pathway, classification and removal efficiency of microplastics in wastewater treatment plants. Environ. Pollut. 2019, 255, 113326. [Google Scholar] [CrossRef]
- Iyare, P.U.; Ouki, S.K.; Bond, T. Microplastics removal in wastewater treatment plants: A critical review. Environ. Sci. Water Res. Technol. 2020, 6, 2664–2675. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Liu, H.; Guo, X.; Zhang, X.; Yao, X.; Cao, Z.; Zhang, T. A review of the removal of microplastics in global wastewater treatment plants: Characteristics and mechanisms. Environ. Int. 2021, 146, 106277. [Google Scholar] [CrossRef] [PubMed]
- Bodzek, M.; Pohl, A.; Rosik-Dulewska, C. Microplastics in Wastewater Treatment Plants: Characteristics, Occurrence and Removal Technologies. Water 2024, 16, 3574. [Google Scholar] [CrossRef]
- Jiang, F.; Wang, M.; Ding, J.; Cao, W.; Sun, C. Occurrence and Seasonal Variation of Microplastics in the Effluent from Wastewater Treatment Plants in Qingdao, China. J. Mar. Sci. Eng. 2022, 10, 58. [Google Scholar] [CrossRef]
- Sol, D.; Laca, A.; Laca, A.; Diaz, M. Approaching the environmental problem of microplastics: Importance of WWTP treatments. Sci. Total Environ. 2020, 740, 140016. [Google Scholar] [CrossRef] [PubMed]
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F.; Huerta-Lwanga, E.; Geissen, V. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total Environ. 2019, 671, 411–420. [Google Scholar] [CrossRef]
- Koshti, R.; Mehta, L.; Samarth, N. Biological Recycling of Polyethylene Terephthalate: A Mini-Review. J. Polym. Environ. 2018, 26, 3520–3529. [Google Scholar] [CrossRef]
- Ben Zair, M.M.; Jakarni, F.M.; Muniandy, R.; Hassim, S. A Brief Review: Application of Recycled Polyethylene Terephthalate in Asphalt Pavement Reinforcement. Sustainability 2021, 13, 1303. [Google Scholar] [CrossRef]
- De Falco, F.; Gullo, M.P.; Gentile, G.; Di Pace, E.; Cocca, M.; Gelabert, L.; Brouta-Agnesa, M.; Rovira, A.; Escudero, R.; Villalba, R.; et al. Evaluation of microplastic release caused by textile washing processes of synthetic fabrics. Environ. Pollut. 2018, 236, 916–925. [Google Scholar] [CrossRef]
- Soong, Y.H.V.; Sobkowicz, M.J.; Xie, D.M. Recent Advances in Biological Recycling of Polyethylene Terephthalate (PET) Plastic Wastes. Bioeng. -Basel 2022, 9, 98. [Google Scholar] [CrossRef]
- Yao, J.; Liu, Y.; Gu, Z.; Zhang, L.; Guo, Z. Deconstructing PET: Advances in enzyme engineering for sustainable plastic degradation. Chem. Eng. J. 2024, 497, 154183. [Google Scholar] [CrossRef]
- Jia, Q.-L.; Chen, H.; Zhao, X.; Li, L.; Nie, Y.-H.; Ye, J.-F. Removal of Microplastics by Different Treatment Processes in Shanghai Large Municipal Wastewater Treatment Plants. Huan Jing Ke Xue Huanjing Kexue 2019, 40, 4105–4112. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhang, Y.; Mo, A.; Jiang, J.; Liang, Y.; Cao, X.; He, D. Removal of microplastics in water: Technology progress and green strategies. Green Anal. Chem. 2022, 3, 100042. [Google Scholar] [CrossRef]
- Dalmau-Soler, J.; Ballesteros-Cano, R.; Boleda, M.R.; Paraira, M.; Ferrer, N.; Lacorte, S. Microplastics from headwaters to tap water: Occurrence and removal in a drinking water treatment plant in Barcelona Metropolitan area (Catalonia, NE Spain). Environ. Sci. Pollut. Res. 2021, 28, 59462–59472. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yi, L.; Wang, N.; Liu, Z.; Jiang, W.; Chen, Z. Research Progress on the Removal of Microplastics in Drinking Water. Environ. Sci. Technol. 2021, 44, 211–218. [Google Scholar]
- Li, R.; Li, N.; Liang, L.; Yan, B.; Chen, G.; Hou, L.a. Research Progress on Removal Methods of Microplastics from Aquatic Environment. Technol. Water Treat. 2022, 48, 12. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z.; Wei, J.; Qian, F.; Song, Y. Research progress on sources, ecotoxicological effect and treatment technology of microplastics in water. J. Environ. Eng. Technol. 2023, 13, 1883–1892. [Google Scholar]
- Wang, Y.; Wang, H.; Yan, Z.; Cheng, J.; Wang, Q.; Zhao, C. Research progress on pollution status and removal technology of microplastics in freshwater. Mod. Chem. Ind. 2023, 43, 1. [Google Scholar]
- Taniguchi, I.; Yoshida, S.; Hiraga, K.; Miyamoto, K.; Kimura, Y.; Oda, K. Biodegradation of PET: Current Status and Application Aspects. Acs Catal. 2019, 9, 4089–4105. [Google Scholar] [CrossRef]
- Li, L.; Gao, X.; Qi, H.; Li, C.; Lu, F.; Mao, S.; Qin, H. Research progress of modern biotechnology-promoted green degradation of polyethylene terephthalate in plastics. Synth. Biol. J. 2022, 3, 763–780. [Google Scholar]
- Ali, I.; Tan, X.; Li, J.; Peng, C.; Wan, P.; Naz, I.; Duan, Z.; Ruan, Y. Innovations in the Development of Promising Adsorbents for the Remediation of Microplastics and Nanoplastics-A Critical Review. Water Res. 2023, 230, 119526. [Google Scholar] [CrossRef]
- Kumar, R.; Verma, A.; Rakib, M.R.J.; Gupta, P.K.; Sharma, P.; Garg, A.; Girard, P.; Aminabhavi, T.M. Adsorptive behavior of micro(nano)plastics through biochar: Co-existence, consequences, and challenges in contaminated ecosystems. Sci. Total Environ. 2023, 856, 159097. [Google Scholar] [CrossRef]
- Imreova, Z.; Stanova, A.V.; Zazimal, F.; Debnarova, S.; Vrana, L.; Petrovicova, N.; Tulipanova, A.; Lukac, T.; Vegh, D.; Styskalik, A.; et al. Low-cost carbon-based sorbents for the removal of pharmaceuticals from wastewaters. J. Water Process Eng. 2024, 61, 105181. [Google Scholar] [CrossRef]
- Tan, X.-f.; Liu, Y.-g.; Gu, Y.-l.; Xu, Y.; Zeng, G.-m.; Hu, X.-j.; Liu, S.-b.; Wang, X.; Liu, S.-m.; Li, J. Biochar-based nano-composites for the decontamination of wastewater: A review. Bioresour. Technol. 2016, 212, 318–333. [Google Scholar] [CrossRef]
- Xiao, Z.; Xiao, Y.; Xiao, M.; Zheng, C.; Hao, J.; Fan, M.; Wang, W. Preparation of magnetic hydrotalcite/biochar composite and its adsorption performance for phosphorus in aqueous solution. Environ. Pollut. Control 2020, 42, 1090–1095,1101. [Google Scholar]
- Yi, Y.; Huang, Z.; Lu, B.; Xian, J.; Tsang, E.P.; Cheng, W.; Fang, J.; Fang, Z. Magnetic biochar for environmental remediation: A review. Bioresour. Technol. 2020, 298, 122468. [Google Scholar] [CrossRef] [PubMed]
- Diao, Y.; Shan, R.; Li, M.; Gu, J.; Yuan, H.; Chen, Y. Efficient Adsorption of a Sulfonamide Antibiotic in Aqueous Solutions with N-doped Magnetic Biochar: Performance, Mechanism, and Reusability. Acs Omega 2023, 8, 879–892. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Tu, G.; Zhao, D.; Tsang, P.E.; Fang, Z. Biomass waste components significantly influence the removal of Cr(VI) using magnetic biochar derived from four types of feedstocks and steel pickling waste liquor. Chem. Eng. J. 2019, 360, 212–220. [Google Scholar] [CrossRef]
- Sukoviene, A.; Ali, S.; Jagminas, A.; Ramanavicius, S. Magnetic Cobalt and Other Types of Ferrite Nanoparticles: Synthesis Aspects and Novel Strategies for Application in Wastewater Treatment (Review). Appl. Sci. Basel 2025, 15, 857. [Google Scholar] [CrossRef]
- Keshta, B.E.; Gemeay, A.H.; Sinha, D.K.; Elsharkawy, S.; Hassan, F.; Rai, N.; Arora, C. State of the art on the magnetic iron oxide Nanoparticles: Synthesis, Functionalization, and applications in wastewater treatment. Results Chem. 2024, 7, 101388. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; Yu, S.; Cui, M. Removal of pristine and aged microplastics from water by magnetic biochar: Adsorption and magnetization. Sci. Total Environ. 2023, 875, 162647. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Mei, Q.; Dong, B.; Dai, X.; Ding, G.; Zeng, E.Y. Microplastics in sewage sludge from the wastewater treatment plants in China. Water Res. 2018, 142, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yu, Y.; Ge, S. Characteristics and Adsorption/Dissolution Behaviors of Micro-plastics Affected by Sludge Treatment Process:a Review. Environ. Sci. Technol. 2022, 45, 76–85. [Google Scholar]
- Sun, W.; Ni, J. Applicability of Langmuir equation to copper sorption by loess. Environ. Chem. 2002, 21, 37–44. [Google Scholar]
- Sorensen, L.; Rogers, E.; Altin, D.; Salaberria, I.; Booth, A.M. Sorption of PAHs to microplastic and their bioavailability and toxicity to marine copepods under co-exposure conditions. Environ. Pollut. 2020, 258, 113844. [Google Scholar] [CrossRef]
- Deng, H.; Mao, Z.; Xu, H.; Zhang, L.; Zhong, Y.; Sui, X. Synthesis of fibrous LaFeO3 perovskite oxide for adsorption of Rhodamine B. Ecotoxicol. Environ. Saf. 2019, 168, 35–44. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Kumar, P.; Kapur, M.; Mondal, M.K. Phosphate removal from aqueous solutions by nano-alumina for the effective remediation of eutrophication. Environ. Prog. Sustain. Energy 2019, 38, S77–S85. [Google Scholar] [CrossRef]
- Hernandez, L.M.; Xu, E.G.; Larsson, H.C.; Tahara, R.; Maisuria, V.B.; Tufenkji, N. Plastic teabags release billions of microparticles and nanoparticles into tea. Environ. Sci. Technol. 2019, 53, 12300–12310. [Google Scholar] [CrossRef]
- Yu, J.; Wang, P.; Ni, F.; Cizdziel, J.; Wu, D.; Zhao, Q.; Zhou, Y. Characterization of microplastics in environment by thermal gravimetric analysis coupled with Fourier transform infrared spectroscopy. Mar. Pollut. Bull. 2019, 145, 153–160. [Google Scholar] [CrossRef]
- Xiong, Y.D.; Lin, K.; Yan, M.; Jiang, Y.Z. Fabrication and Magnetic Properties of FINEMET Alloy Powder Cores. Rare Met. Mater. Eng. 2014, 43, 1951–1954. [Google Scholar]
- Suzuki, A.; Miyake, Y.; Shibata, R.; Takai, K. Spin and charge interactions between nanographene host and ferrocene. Beilstein J. Org. Chem. 2024, 20, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, T.; Shibutani, S.; Yamauchi, S. An investigation into carbon structures in iron-loaded charcoal based on the Raman shift and line-shape of the G’-band. J. Wood Sci. 2023, 69, 8. [Google Scholar] [CrossRef]
- Yamagishi, T.; Yamauchi, S.; Suzuki, K.; Suzuki, T.; Kurimoto, Y.; Takayama, T.; Sakai, Y. Mossbauer and Raman spectroscopic characterization of iron and carbon in iron-loaded Japanese cypress charcoal. J. Wood Sci. 2020, 66, 82. [Google Scholar] [CrossRef]
- Karimzadeh, I.; Aghazadeh, M.; Ganjali, M.R.; Doroudi, T.; Kolivand, P.H. Preparation and characterization of iron oxide (Fe3O4) nanoparticles coated with polyvinylpyrrolidone/polyethylenimine through a facile one-pot deposition route. J. Magn. Magn. Mater. 2017, 433, 148–154. [Google Scholar] [CrossRef]
- Banik, C.; Lawrinenko, M.; Bakshi, S.; Laird, D.A. Impact of Pyrolysis Temperature and Feedstock on Surface Charge and Functional Group Chemistry of Biochars. J. Environ. Qual. 2018, 47, 452–461. [Google Scholar] [CrossRef]
- Fang, D.; He, F.; Xie, J.L.; Xue, L.H. Calibration of Binding Energy Positions with C1s for XPS Results. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2020, 35, 711–718. [Google Scholar] [CrossRef]
- Mamtani, K.; Jain, D.; Zemlyanov, D.; Celik, G.; Luthman, J.; Renkes, G.; Co, A.C.; Ozkan, U.S. Probing the Oxygen Reduction Reaction Active Sites over Nitrogen-Doped Carbon Nanostructures (CNx) in Acidic Media Using Phosphate Anion. Acs Catal. 2016, 6, 7249–7259. [Google Scholar] [CrossRef]
- Cao, S.Y.; Chen, B.J.; Yu, F.F.; Xu, X.W.; Yao, Y.Y. Preparation of MoO2@nitrogen doped carbon composites for degradation of organic pollutants. Chin. J. Inorg. Chem. 2023, 39, 80–90. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Frankcombe, T.J.; Liu, Y. Interpretation of Oxygen 1s X-ray Photoelectron Spectroscopy of ZnO. Chem. Mater. 2023, 35, 5468–5474. [Google Scholar] [CrossRef]
- Sajid, M.; Ihsanullah, I. Magnetic layered double hydroxide-based composites as sustainable adsorbent materials for water treatment applications: Progress, challenges, and outlook. Sci. Total Environ. 2023, 880, 163299. [Google Scholar] [CrossRef]
- Su, J.; Zhan, J. Research progress of microplastic removal from water environment by biochar. Chem. Ind. Eng. Prog. 2023, 42, 5445–5458. [Google Scholar]
- Zhuang, J.K.; Ma, L.; Qiu, Y.H. Characterization of the surface charge property and porosity of track-etched polymer membranes. Electrophoresis 2022, 43, 2428–2435. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, C.; Huang, Q.-X.; Chi, Y.; Yan, J.-H. Adsorption and thermal degradation of microplastics from aqueous solutions by Mg/Zn modified magnetic biochars. J. Hazard. Mater. 2021, 419, 126486. [Google Scholar] [CrossRef]
- Singh, N.; Khandelwal, N.; Ganie, Z.A.; Tiwari, E.; Darbha, G.K. Eco-friendly magnetic biochar: An effective trap for nanoplastics of varying surface functionality and size in the aqueous environment. Chem. Eng. J. 2021, 418, 129405. [Google Scholar] [CrossRef]
- Weber, T.W.; Chakravorti, R.K. Pore and solid diffusion models for fixed-bed adsorbers. AIChE J. 1974, 20, 228–238. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Wang, Q.; Feng, C.; Zhang, Z.; Lei, Z.; Shimizu, K. Insights into mathematical characteristics of adsorption models and physical meaning of corresponding parameters. J. Mol. Liq. 2018, 254, 20–25. [Google Scholar] [CrossRef]
- Shi, Q.; Guo, S.; Tang, J.; Lyu, H.; Ri, C.; Sun, H. Enhanced removal of aged and differently functionalized polystyrene nanoplastics using ball-milled magnetic pinewood biochars. Environ. Pollut. 2023, 316, 120696. [Google Scholar] [CrossRef]
- Ganie, Z.A.; Khandelwal, N.; Tiwari, E.; Singh, N.; Darbha, G.K. Biochar-facilitated remediation of nanoplastic contaminated water: Effect of pyrolysis temperature induced surface modifications. J. Hazard. Mater. 2021, 417, 126096. [Google Scholar] [CrossRef]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Cao, Y.; Zhao, M.; Ma, X.; Song, Y.; Zuo, S.; Li, H.; Deng, W. A critical review on the interactions of microplastics with heavy metals: Mechanism and their combined effect on organisms and humans. Sci. Total Environ. 2021, 788, 147620. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.Z.; Bahar, M.M.; Sarkar, B.; Donne, S.W.; Ok, Y.S.; Palansooriya, K.N.; Kirkham, M.B.; Chowdhury, S.; Bolan, N. Biochar and its importance on nutrient dynamics in soil and plant. Biochar 2020, 2, 379–420. [Google Scholar] [CrossRef]
- Zeghioud, H.; Mouhamadou, S. Dye Removal Characteristics of Magnetic Biochar Derived from Sewage Sludge: Isotherm, Thermodynamics, Kinetics, and Mechanism. Water Air Soil Pollut. 2023, 234, 233. [Google Scholar] [CrossRef]
- Xiu, L.; Gu, W.; Sun, Y.; Wu, D.; Wang, Y.; Zhang, H.; Zhang, W.; Chen, W. The fate and supply capacity of potassium in biochar used in agriculture. Sci. Total Environ. 2023, 902, 165969. [Google Scholar] [CrossRef]
- Kasera, N.; Kolar, P.; Hall, S.G. Nitrogen-doped biochars as adsorbents for mitigation of heavy metals and organics from water: A review. Biochar 2022, 4, 17. [Google Scholar] [CrossRef]
- Ma, Y.; Lu, T.; Tang, J.; Li, P.; Mašek, O.; Yang, L.; Wu, L.; He, L.; Ding, Y.; Gao, F. One-pot hydrothermal synthesis of magnetic N-doped sludge biochar for efficient removal of tetracycline from various environmental waters. Sep. Purif. Technol. 2022, 297, 121426. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Bąk, J.; Kozioł, M.; Pylychuk, L. Investigations of heavy metal ion sorption using nanocomposites of iron-modified biochar. Nanoscale Res. Lett. 2017, 12, 433. [Google Scholar] [CrossRef]
- Papadopoulou, K.; Tarani, E.; Ainali, N.M.; Chrissafis, K.; Wurzer, C.; Masek, O.; Bikiaris, D.N. The Effect of Biochar Addition on Thermal Stability and Decomposition Mechanism of Poly(butylene succinate) Bionanocomposites. Molecules 2023, 28, 5330. [Google Scholar] [CrossRef]
- Qi, C.; Yuan, Z.; Hu, J.; Wang, H.; Zhu, Y.; Palocz-Andresen, M.; Zhu, N.; Lou, Z. The synergistic pyrolysis effects of polyethylene terephthalate with the additive of sewage sludge. J. Clean. Prod. 2024, 450, 141889. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, N.; Xing, C.; Cui, Q.; Sun, Q. The adsorption, regeneration and engineering applications of biochar for removal organic pollutants: A review. Chemosphere 2019, 223, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Vergnes, H.; Pruksathorn, K.; Klamklang, S.; Damlonglerd, S. Electrochemical Incineration of Organic Pollutants for Wastewater Treatment: Past, Present and Prospect. In Organic Pollutants Ten Years After the Stockholm Convention—Environmental and Analytical Update; Puzyn, T., Mostrag, A., Eds.; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar]
- Ghosh, P.; Ghime, D. Advanced Oxidation Processes: A Powerful Treatment Option for the Removal of Recalcitrant Organic Compounds. In Advanced Oxidation Processes—Applications, Trends, and Prospects; Bustillo-Lecompte, C.F., Ed.; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Domingos, R.d.A.; da Fonseca, F.V. Evaluation of adsorbent and ion exchange resins for removal of organic matter from petroleum refinery wastewaters aiming to increase water reuse. J. Environ. Manag. 2018, 214, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Devaisy, S.; Kandasamy, J.; Aryal, R.; Johir, M.A.H.; Ratnaweera, H.; Vigneswaran, S. Removal of Organics with Ion-Exchange Resins (IEX) from Reverse Osmosis Concentrate. Membranes 2023, 13, 136. [Google Scholar] [CrossRef]
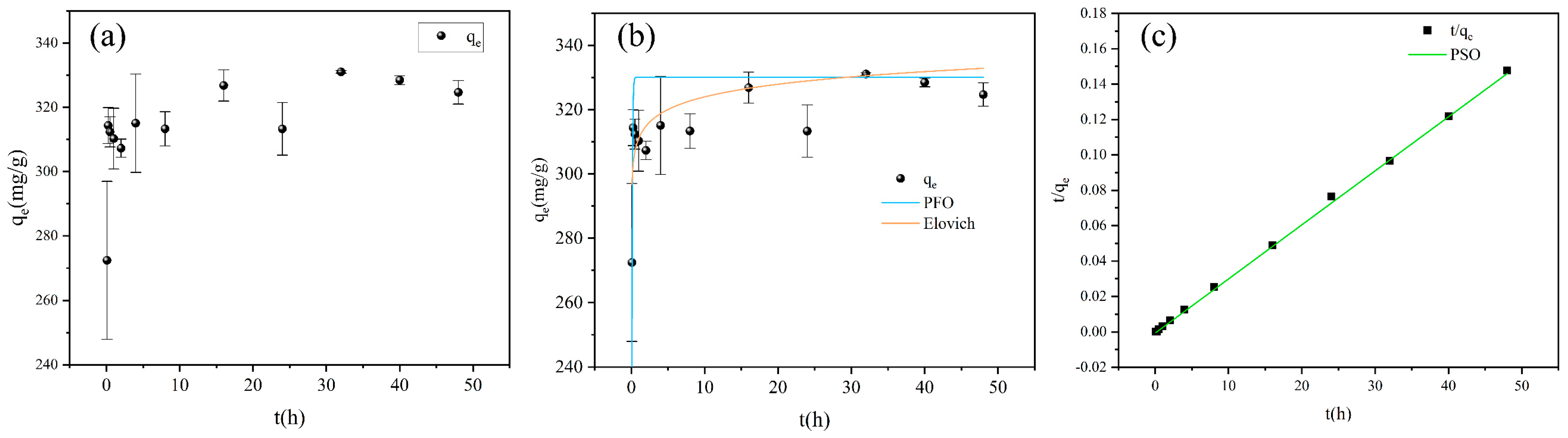
| Langmuir | Freundlich | Temkin | ||||||
|---|---|---|---|---|---|---|---|---|
| qe (mg/g) | KL | R2 | n | KF | R2 | lnf | KT | R2 |
| 4500 | 0.0037 | 0.9743 | 1.06 | 17.23 | 0.9560 | −0.064 | 87.22 | 0.6560 |
| PFO | PSO | Elovich | ||||||
|---|---|---|---|---|---|---|---|---|
| qe (mg/g) | K1 | R2 | qe (mg/g) | K2 | R2 | α | β | R2 |
| 330 | 14.39 | 0.07711 | 328 | −0.014 | 0.9974 | 3.93 | 0.18 | 0.7320 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, B.; Duan, N.; Xia, H.; Li, Y.; Xu, H.; Geng, Y.; Wang, X. Removal of PET Microfibers from Simulated Wastewater Using Magnetic Nano-Ferric-Loaded Biochar: High Adsorption and Regeneration Performance. Nanomaterials 2025, 15, 905. https://doi.org/10.3390/nano15120905
Song B, Duan N, Xia H, Li Y, Xu H, Geng Y, Wang X. Removal of PET Microfibers from Simulated Wastewater Using Magnetic Nano-Ferric-Loaded Biochar: High Adsorption and Regeneration Performance. Nanomaterials. 2025; 15(12):905. https://doi.org/10.3390/nano15120905
Chicago/Turabian StyleSong, Beisi, Nini Duan, Huaguo Xia, Yuan Li, Hongbin Xu, Ying Geng, and Xin Wang. 2025. "Removal of PET Microfibers from Simulated Wastewater Using Magnetic Nano-Ferric-Loaded Biochar: High Adsorption and Regeneration Performance" Nanomaterials 15, no. 12: 905. https://doi.org/10.3390/nano15120905
APA StyleSong, B., Duan, N., Xia, H., Li, Y., Xu, H., Geng, Y., & Wang, X. (2025). Removal of PET Microfibers from Simulated Wastewater Using Magnetic Nano-Ferric-Loaded Biochar: High Adsorption and Regeneration Performance. Nanomaterials, 15(12), 905. https://doi.org/10.3390/nano15120905






