Efficient Removal of Micro-Sized Degradable PHBV Microplastics from Wastewater by a Functionalized Magnetic Nano Iron Oxides-Biochar Composite: Performance, Mechanisms, and Material Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Quantification of PHBV MPs
2.2. Preparation of MFe@BC
2.3. Batch Adsorption Experiments
2.3.1. Effects of MFe@BC Dosage
2.3.2. Effect of Solution pH on Adsorption
2.3.3. Adsorption Isotherms
2.3.4. Adsorption Kinetics
2.3.5. Effect of Co-Existed COD on PHBV Adsorption
2.3.6. Characterization of PHBV and MFe@BC
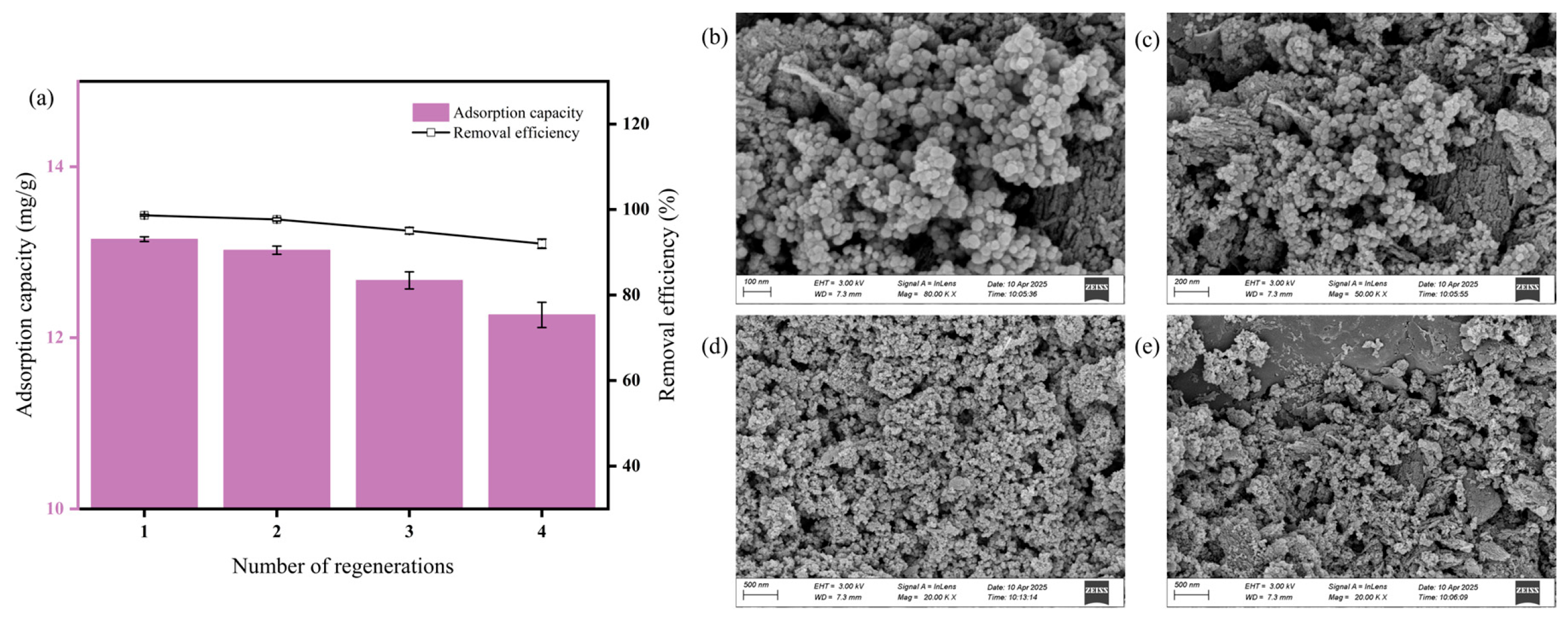
2.3.7. Regeneration of MFe@BC
3. Results and Discussion
3.1. Characterization of MFe@BC
3.2. Effect of MFe@BC Dosage on PHBV Adsorption
3.3. Effect of Solution pH on PLA Adsorption by MFe@BC
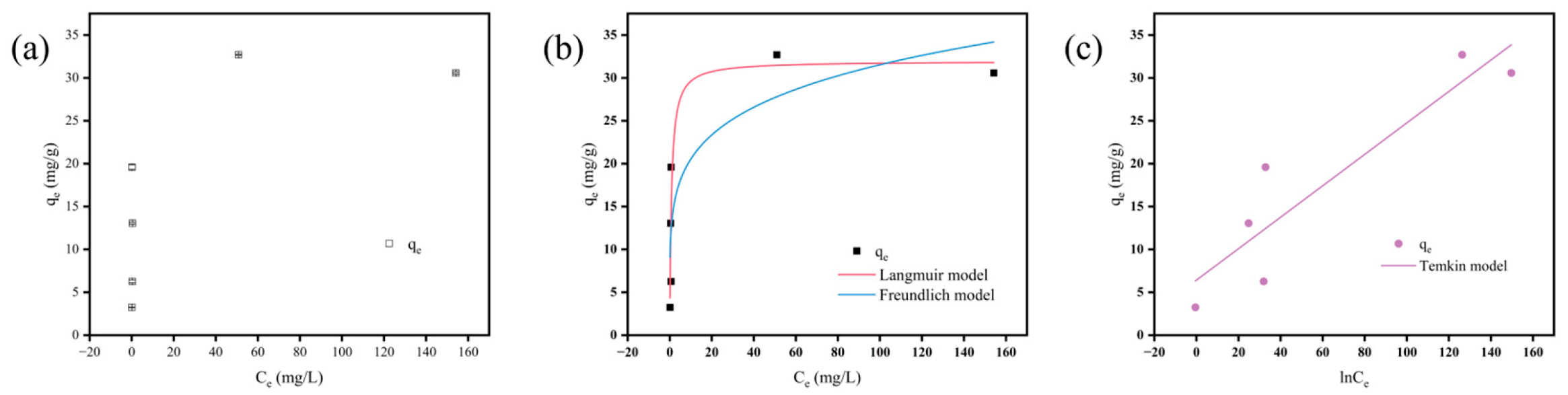
3.4. Adsorption Isotherms
3.5. Adsorption Kinetics
3.6. Effect of Co-Existing COD on PHBV Adsorption
3.7. Potential Mechanism of PHBV Adsorption by MFe@BC
3.8. Regeneration of MFe@BC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alimi, O.S.; Budarz, J.F.; Hernandez, L.M.; Tufenkji, N. Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef]
- Liao, Q.; Qu, J.; Wang, J.; Gao, F. Pollution Analysis of Plastic and Improving Proposal in Marine Environment. World Sci.-Tech. R&D 2015, 37, 206–211, 217. [Google Scholar]
- Qiu, Y.P.; Zheng, Z.Z.; Zhou, Z.L.; Sheng, G.D. Effectiveness and mechanisms of dye adsorption on a straw-based biochar. Bioresour. Technol. 2009, 100, 5348–5351. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.; Turner, A. Human exposure to microplastics: A study in Iran. J. Hazard. Mater. 2021, 403, 123799. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shi, G.; Revell, L.E.; Zhang, J.; Zuo, C.; Wang, D.; Le Ru, E.C.; Wu, G.; Mitrano, D.M. Long-range atmospheric transport of microplastics across the southern hemisphere. Nat. Commun. 2023, 14, 7898. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Huang, J.; Zheng, Y.; Yang, Y.; Zhang, Y.; He, F.; Chen, H.; Quan, G.; Yan, J.; Li, T.; et al. Environmental occurrences, fate, and impacts of microplastics. Ecotoxicol. Environ. Saf. 2019, 184, 109612. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Qiao, R.X.; Zhang, S.Y.; Wang, G.X. Metabolomic profiling reveals the intestinal toxicity of different length of microplastic fibers on zebrafish (Danio rerio). J. Hazard. Mater. 2021, 403, 123663. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Yuan, G.D. Phosphate affects adsorption and desorption of oxytetracycline in the seawater-sediment systems. Environ. Sci. Pollut. Res. 2018, 25, 28160–28168. [Google Scholar] [CrossRef]
- Hüffer, T.; Metzelder, F.; Sigmund, G.; Slawek, S.; Schmidt, T.C.; Hofmann, T. Polyethylene microplastics influence the transport of organic contaminants in soil. Sci. Total Environ. 2019, 657, 242–247. [Google Scholar] [CrossRef]
- Zhang, S.W.; Han, B.; Sun, Y.H.; Wang, F.Y. Microplastics influence the adsorption and desorption characteristics of Cd in an agricultural soil. J. Hazard. Mater. 2020, 388, 121775. [Google Scholar] [CrossRef]
- Hink, L.; Rohrbach, S.; Rehkopf, J.; Sehl, E.; Agarwal, S.; Feldhaar, H.; Horn, M.A. Poly(L-lactide) mineralisation under environmental conditions is enhanced in earthworm guts. Soil Biol. Biochem. 2024, 196, 109485. [Google Scholar] [CrossRef]
- Tong, H.; Zhong, X.; Duan, Z.; Yi, X.; Cheng, F.; Xu, W.; Yang, X. Micro- and nanoplastics released from biodegradable and conventional plastics during degradation: Formation, aging factors, and toxicity. Sci. Total Environ. 2022, 833, 155275. [Google Scholar] [CrossRef] [PubMed]
- Ju, D.; Han, L.; Li, F.; Chen, S.; Dong, L. Poly(ε-caprolactone) composites reinforced by biodegradable poly(3-hydroxybutyrate-co-3-hydroxyvalerate) fiber. Int. J. Biol. Macromol. 2014, 67, 343–350. [Google Scholar] [CrossRef]
- Xiang, H.; Chen, Z.; Zheng, N.; Zhang, X.; Zhu, L.; Zhou, Z.; Zhu, M. Melt-spun microbial poly(3-hydroxybutyrate-co-3-hydroxyvalerate) fibers with enhanced toughness: Synergistic effect of heterogeneous nucleation, long-chain branching and drawing process. Int. J. Biol. Macromol. 2019, 122, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yu, H.-Y.; Li, Y.; Abdalkarim, S.Y.H.; Zhu, J.; Zhou, Y. “Soft-rigid” synergistic reinforcement of PHBV composites with functionalized cellulose nanocrystals and amorphous recycled polycarbonate. Compos. Part B-Eng. 2021, 206, 108542. [Google Scholar] [CrossRef]
- Philip, S.; Keshavarz, T.; Roy, I. Polyhydroxyalkanoates: Biodegradable polymers with a range of applications. J. Chem. Technol. Biotechnol. 2007, 82, 233–247. [Google Scholar] [CrossRef]
- Lyshtva, P.; Voronova, V.; Barbir, J.; Leal Filho, W.; Kroeger, S.D.; Witt, G.; Miksch, L.; Sabowski, R.; Gutow, L.; Frank, C.; et al. Degradation of a poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) compound in different environments. Heliyon 2024, 10, e24770. [Google Scholar] [CrossRef]
- Reay, M.K.; Graf, M.; Greenfield, L.M.; Bargiela, R.; Onyije, C.; Lloyd, C.E.M.; Bull, I.D.; Evershed, R.P.; Golyshin, P.N.; Chadwick, D.R.; et al. Microbial degradation of bioplastic (PHBV) is limited by nutrient availability at high microplastic loadings. Environ. Sci.-Adv. 2025, 4, 133–146. [Google Scholar] [CrossRef]
- Brown, R.W.; Chadwick, D.R.; Zang, H.; Graf, M.; Liu, X.; Wang, K.; Greenfield, L.M.; Jones, D.L. Bioplastic (PHBV) addition to soil alters microbial community structure and negatively affects plant-microbial metabolic functioning in maize. J. Hazard. Mater. 2023, 441, 129959. [Google Scholar] [CrossRef]
- Laranjeiro, F.; Rotander, A.; Lopez-Ibanez, S.; Vilas, A.; Seilitz, F.S.; Clerandeau, C.; Sampalo, M.; Rial, D.; Bellas, J.; Cachot, J.; et al. Comparative assessment of the acute toxicity of commercial bio-based polymer leachates on marine plankton. Sci. Total Environ. 2024, 946, 174403. [Google Scholar] [CrossRef]
- Cao, Z.; Kim, C.; Li, Z.; Jung, J. Comparing environmental fate and ecotoxicity of conventional and biodegradable plastics: A critical review. Sci. Total Environ. 2024, 951, 175735. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska, K.; Ormaniec, P. Microbial Succession on Microplastics in Wastewater Treatment Plants: Exploring the Complexities of Microplastic-Microbiome Interactions. Microb. Ecol. 2024, 87, 105. [Google Scholar] [CrossRef] [PubMed]
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef]
- Lu, Y.; Li, M.-C.; Lee, J.; Liu, C.; Mei, C. Microplastic remediation technologies in water and wastewater treatment processes: Current status and future perspectives. Sci. Total Environ. 2023, 868, 161618. [Google Scholar] [CrossRef]
- Agboola, O.D.; Benson, N.U. Physisorption and Chemisorption Mechanisms Influencing Micro (Nano) Plastics-Organic Chemical Contaminants Interactions: A Review. Front. Environ. Sci. 2021, 9, 678574. [Google Scholar] [CrossRef]
- Verma, A.; Sharma, G.; Kumar, A.; Dhiman, P.; Mola, G.T.; Shan, A.; Si, C. Microplastic pollutants in water: A comprehensive review on their remediation by adsorption using various adsorbents. Chemosphere 2024, 352, 141365. [Google Scholar] [CrossRef]
- Ji, G.; Xing, Y.; You, T. Biochar as adsorbents for environmental microplastics and nanoplastics removal. J. Environ. Chem. Eng. 2024, 12, 113377. [Google Scholar] [CrossRef]
- Sadeghi, M.H.; Tofighy, M.A.; Mohammadi, T. One-dimensional graphene for efficient aqueous heavy metal adsorption: Rapid removal of arsenic and mercury ions by graphene oxide nanoribbons (GONRs). Chemosphere 2020, 253, 126647. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Shi, J.; Wang, Y.; Tong, H.; Zhu, Y.; Xu, L.; Wang, Y.; Zhang, B.; Tao, Y.; Dai, X.; et al. Applications of functionalized magnetic biochar in environmental remediation: A review. J. Hazard. Mater. 2022, 434, 128841. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Q.; Wang, Y.; Cui, X.; Liu, Y.; Ruan, R.; Wu, X.; Cao, L.; Zhao, L.; Zheng, H. Preparation and application of metal-modified biochar in the purification of micro-polystyrene polluted aqueous environment. J. Environ. Manag. 2023, 347, 119158. [Google Scholar] [CrossRef]
- Rhein, F.; Nirschl, H.; Kaegi, R. Separation of Microplastic Particles from Sewage Sludge Extracts Using Magnetic Seeded Filtration. Water Res. X 2022, 17, 100155. [Google Scholar] [CrossRef]
- Li, N.; Xia, Y.; He, X.W.; Li, W.J.; Yuan, L.H.; Wu, X.; Qin, Y.H.; Yuan, R.; Gong, X. Glucose Addition Enhanced the Advanced Treatment of Coking Wastewater. Water 2021, 13, 3365. [Google Scholar] [CrossRef]
- Saleem, M.H.; Mfarrej, M.F.B.; Khan, K.A.; Alharthy, S.A. Emerging trends in wastewater treatment: Addressing microorganic pollutants and environmental impacts. Sci. Total Environ. 2024, 913, 169755. [Google Scholar] [CrossRef]
- Paul, A.; Reese, M.; Goldhammer, T.; Schmalsch, C.; Weber, J.; Bannick, C.G. Spectroscopic evidence for adsorption of natural organic matter on microplastics. Appl. Res. 2024, 3, e202200126. [Google Scholar] [CrossRef]
- Choma, J.; Jagiello, J.; Jaroniec, M. Assessing the contribution of micropores and mesopores from nitrogen adsorption on nanoporous carbons: Application to pore size analysis. Carbon 2021, 183, 150–157. [Google Scholar] [CrossRef]
- Xu, Y.H.; Qu, Y.P.; Yang, Y.J.; Qu, B.; Shan, R.; Yuan, H.R.; Sun, Y. Study on Efficient Adsorption Mechanism of Pb2+ by Magnetic Coconut Biochar. Int. J. Mol. Sci. 2022, 23, 14053. [Google Scholar] [CrossRef] [PubMed]
- Guel-Nájar, N.A.; Rios-Hurtado, J.C.; Muzquiz-Ramos, E.M.; Dávila-Pulido, G.I.; González-Ibarra, A.A.; Pat-Espadas, A.M. Magnetic Biochar Obtained by Chemical Coprecipitation and Pyrolysis of Corn Cob Residues: Characterization and Methylene Blue Adsorption. Materials 2023, 16, 3127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zheng, H.H.; Wu, J.; Chen, W.; Chen, Y.Q.; Xuezhi, G.; Yang, H.P.; Chen, H.P. Physicochemical and adsorption properties of biochar from biomass-based pyrolytic polygeneration: Effects of biomass species and temperature. Biochar 2021, 3, 657–670. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; Yu, S.; Cui, M. Removal of pristine and aged microplastics from water by magnetic biochar: Adsorption and magnetization. Sci. Total Environ. 2023, 875, 162647. [Google Scholar] [CrossRef]
- Das, K.; Sukul, U.; Chen, J.-S.; Sharma, R.K.; Banerjee, P.; Dey, G.; Taharia, M.; Wijaya, C.J.; Lee, C.-I.; Wang, S.-L.; et al. Transformative and sustainable insights of agricultural waste-based adsorbents for water defluoridation: Biosorption dynamics, economic viability, and spent adsorbent management. Heliyon 2024, 10, e29747. [Google Scholar] [CrossRef]
- Xiang, Y.; Xu, Z.; Zhou, Y.; Wei, Y.; Long, X.; He, Y.; Zhi, D.; Yang, J.; Luo, L. A sustainable ferromanganese biochar adsorbent for effective levofloxacin removal from aqueous medium. Chemosphere 2019, 237, 124464. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S. Review of Second-Order Models for Adsorption Systems. ChemInform 2006, 136, 681–689. [Google Scholar]
- Wu, S. Carbon-Based Adsorbents for Microplastic Removal from Wastewater. Materials 2024, 17, 5428. [Google Scholar] [CrossRef]
- Babalar, M.; Siddiqua, S.; Sakr, M.A. A novel polymer coated magnetic activated biochar-zeolite composite for adsorption of polystyrene microplastics: Synthesis, characterization, adsorption and regeneration performance. Sep. Purif. Technol. 2024, 331, 125582. [Google Scholar] [CrossRef]
- Bashir, M.; Ahanger, M.A.; Gani, K.M. Investigations on adsorptive removal of PVC microplastics from aqueous solutions usingPinus roxburghii–derived biochar. Environ. Sci. Pollut. Res. 2024, 31, 59416–59429. [Google Scholar] [CrossRef]
- Tian, J.; Li, Y.; Sun, N.; Mi, S.; Xie, Y.; Chen, Z. Enhanced dyes adsorption from wastewater via Fe3O4 nanoparticles functionalized activated carbon. J. Hazard. Mater. 2019, 373, 397–407. [Google Scholar]
- Li, J.; Zhao, H.; Ma, C.; Han, Q.; Li, M.; Liu, H. Preparation of Fe3O4@polyoxometalates Nanocomposites and Their Efficient Adsorption of Cationic Dyes from Aqueous Solution. Nanomaterials 2019, 9, 649. [Google Scholar] [CrossRef]
- Khayyun, T.S.; Mseer, A.H. Comparison of the experimental results with the Langmuir and Freundlich models for copper removal on limestone adsorbent. Appl. Water Sci. 2019, 9, 170. [Google Scholar] [CrossRef]
- Guo, S.; Zou, Z.; Chen, Y.; Long, X.; Liu, M.; Li, X.; Tan, J.; Chen, R. Synergistic effect of hydrogen bonding and π-π interaction for enhanced adsorption of rhodamine B from water using corn straw biochar? Environ. Pollut. 2023, 320, 121060. [Google Scholar] [CrossRef]
- Zaidi, R.; Khan, S.U.; Farooqi, I.H.; Azam, A. Investigation of kinetics and adsorption isotherm for fluoride removal from aqueous solutions using mesoporous cerium-aluminum binary oxide nanomaterials. Rsc Adv. 2021, 11, 28744–28760. [Google Scholar] [CrossRef]
- Li, S.X.; Wang, T.Y.; Guo, J.H.; Dong, Y.F.; Wang, Z.S.; Gong, L.; Li, X.N. Polystyrene microplastics disturb the redox homeostasis, carbohydrate metabolism and phytohormone regulatory network in barley. J. Hazard. Mater. 2021, 415, 125614. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Nie, D.Y.; Sang, M.; Wang, W.W.; Nie, G.Z. Adsorption of Rhodamine B and Pb(II) from aqueous solution by MoS2 nanosheet modified biochar: Fabrication, performance, and mechanisms. Bioresour. Technol. 2023, 386, 129548. [Google Scholar] [CrossRef]
- Abdurahman, A.; Cui, K.; Wu, J.; Li, S.; Gao, R.; Dai, J.; Liang, W.; Zeng, F. Adsorption of dissolved organic matter (DOM) on polystyrene microplastics in aquatic environments: Kinetic, isotherm and site energy distribution analysis. Ecotoxicol. Environ. Saf. 2020, 198, 110658. [Google Scholar] [CrossRef] [PubMed]
- Lesiak, B.; Rangam, N.; Jiricek, P.; Gordeev, I.; Toth, J.; Kover, L.; Mohai, M.; Borowicz, P. Surface Study of Fe3O4 Nanoparticles Functionalized With Biocompatible Adsorbed Molecules. Front. Chem. 2019, 7, 642. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zaloga, J.; Ding, Y.; Liu, Y.; Janko, C.; Pischetsrieder, M.; Alexiou, C.; Boccaccini, A.R. Facile preparation of multifunctional superparamagnetic PHBV microspheres containing SPIONs for biomedical applications. Sci. Rep. 2016, 6, 23140. [Google Scholar] [CrossRef]
- Alp, E.; Cirak, T.; Demirbilek, M.; Turk, M.; Guven, E. Targeted delivery of etoposide to osteosarcoma cells using poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) nanoparticles. Turk. J. Biol. 2017, 41, 719–733. [Google Scholar] [CrossRef]








| Specific Surface Area | Porosity | Average Pore Size |
|---|---|---|
| 525.1104 m2/g | 0.567678 cm3/g | 4.3243 nm |
| Model | Parameters | ||
|---|---|---|---|
| Langmuir | qe | mg/g | 1.26 |
| KL | L/mg | 31.96 | |
| R2 | 0.8440 | ||
| Freundlich | n | 0.19 | |
| KF | (mg/g)(L/mg)1/n | 13.35 | |
| R2 | 0.7309 | ||
| Temkin | lnf | 3.71 | |
| KT | mg/g | 3.87 | |
| R2 | 0.7986 |
| Model | Parameters | ||
|---|---|---|---|
| PFO | qe | mg/g | 0.44 |
| K1 | min−1 | −1.64 × 10−4 | |
| R2 | 0.1177 | ||
| PSO | qe | mg/g | 12.99 |
| K2 | g/(mg·min) | −0.06 | |
| R2 | 0.9999 | ||
| Elovich | α | mg/(g·min) | 1.80 × 10203 |
| β | g/mg | 36.87 | |
| R2 | 0.1404 |
| Before Adsorption | After Adsorption | ||||
|---|---|---|---|---|---|
| Peak Position (eV) | Proportion (%) | Peak Position (eV) | Proportion (%) | ||
| C | C-C | 283.86 | 72.99 | 283.06 | 84.03 |
| C-O-C | 285.14 | 21.17 | 284.77 | 5.88 | |
| O-C=O | 287.84 | 5.84 | 286.96 | 10.08 | |
| Fe | Fe2p1/2 | 726.46 | 12.09 | 725.40 | 8.23 |
| 712.92 | 27.48 | 712.60 | 18.93 | ||
| Fe2p3/2 | 724.28 | 8.79 | 722.28 | 18.11 | |
| 710.85 | 19.78 | 709.48 | 41.15 | ||
| sattlite | 731.73 | 4.67 | 730.30 | 4.12 | |
| 718.93 | 22.53 | 717.50 | 9.47 | ||
| N | Ta4p3/2 | 400.10 | 23.76 | 399.55 | 31.42 |
| 399.04 | 41.58 | 398.78 | 30.27 | ||
| N1s | 398.77 | 34.65 | 396.90 | 38.31 | |
| O | Organic C-O | 532.05 | 51.02 | 532.00 | 38.76 |
| Metal oxide | 530.31 | 25.51 | 530.24 | 38.76 | |
| Metal carbonate | 529.16 | 23.47 | 528.67 | 22.48 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, H.; Duan, N.; Song, B.; Li, Y.; Xu, H.; Geng, Y.; Wang, X. Efficient Removal of Micro-Sized Degradable PHBV Microplastics from Wastewater by a Functionalized Magnetic Nano Iron Oxides-Biochar Composite: Performance, Mechanisms, and Material Regeneration. Nanomaterials 2025, 15, 915. https://doi.org/10.3390/nano15120915
Xia H, Duan N, Song B, Li Y, Xu H, Geng Y, Wang X. Efficient Removal of Micro-Sized Degradable PHBV Microplastics from Wastewater by a Functionalized Magnetic Nano Iron Oxides-Biochar Composite: Performance, Mechanisms, and Material Regeneration. Nanomaterials. 2025; 15(12):915. https://doi.org/10.3390/nano15120915
Chicago/Turabian StyleXia, Huaguo, Nini Duan, Beisi Song, Yuan Li, Hongbin Xu, Ying Geng, and Xin Wang. 2025. "Efficient Removal of Micro-Sized Degradable PHBV Microplastics from Wastewater by a Functionalized Magnetic Nano Iron Oxides-Biochar Composite: Performance, Mechanisms, and Material Regeneration" Nanomaterials 15, no. 12: 915. https://doi.org/10.3390/nano15120915
APA StyleXia, H., Duan, N., Song, B., Li, Y., Xu, H., Geng, Y., & Wang, X. (2025). Efficient Removal of Micro-Sized Degradable PHBV Microplastics from Wastewater by a Functionalized Magnetic Nano Iron Oxides-Biochar Composite: Performance, Mechanisms, and Material Regeneration. Nanomaterials, 15(12), 915. https://doi.org/10.3390/nano15120915






