Experimental and Artificial Neuron Network Insights into the Removal of Organic Dyes from Wastewater Using a Clay/Gum Arabic Nanocomposite †
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Clay/Gum Arabic Nanocomposite
2.2. Batch Adsorption Experiments
2.3. Kinetic Studies
2.4. Adsorption Isotherm Models
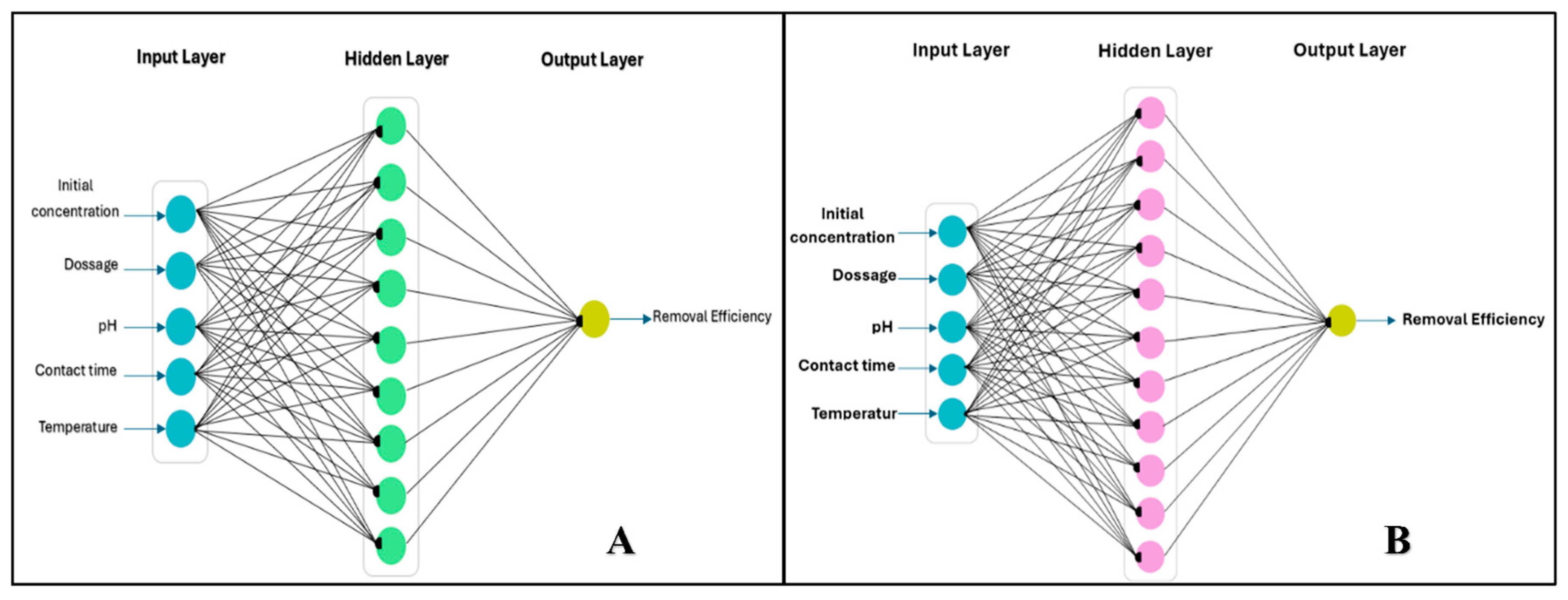
2.5. Artificial Neural Networks (ANN)
2.6. Reusability of CG/NC
2.7. Zeta Potential (ZP)
2.8. Instrumentation
3. Results and Discussion
3.1. Characterization
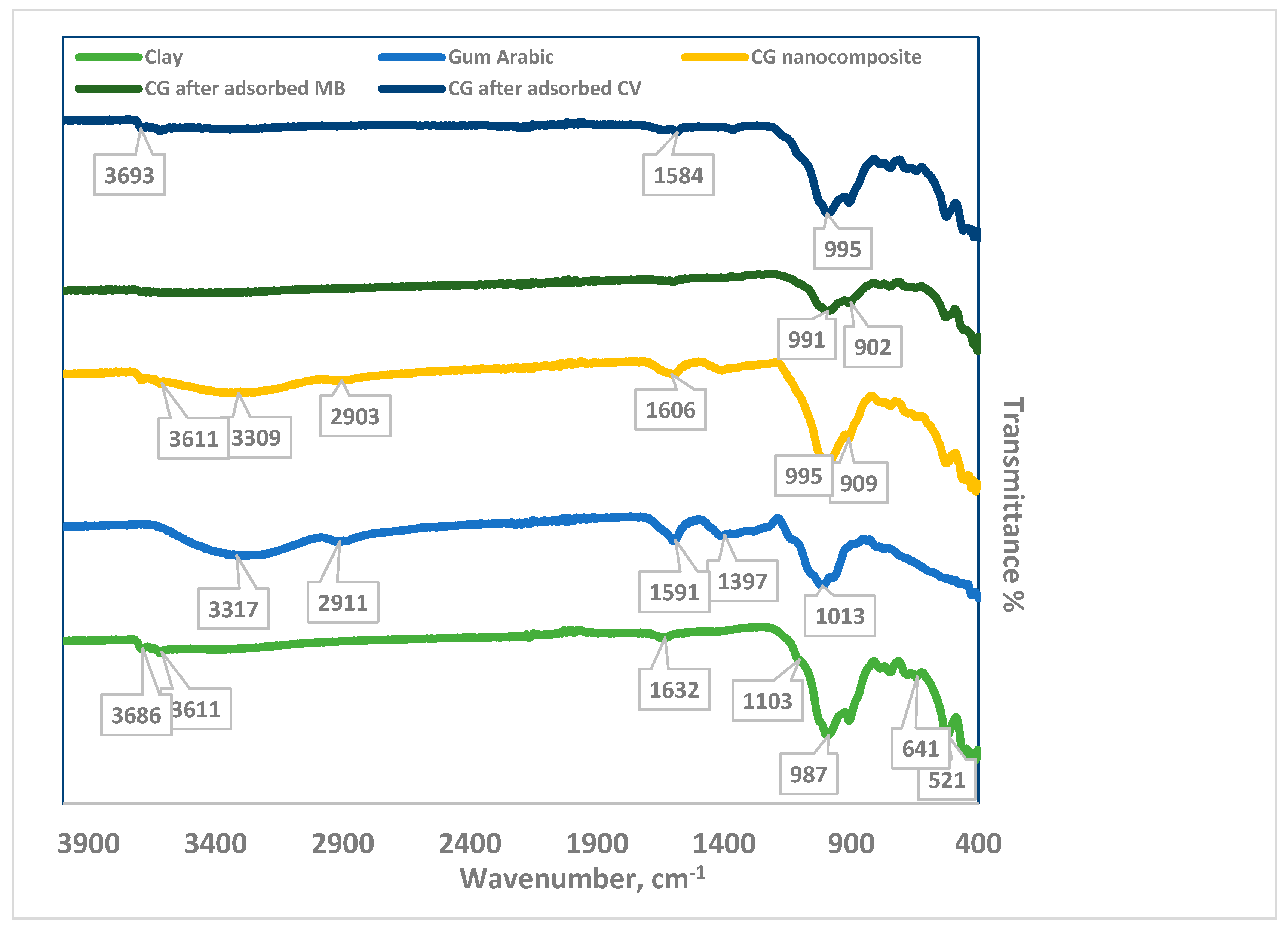
3.1.1. Fourier Transform Infrared Spectroscopy (FTIR)
3.1.2. Scanning Electron Microscopy (SEM-EDX)
3.1.3. Brunauer–Emmett–Teller (BET) Analysis
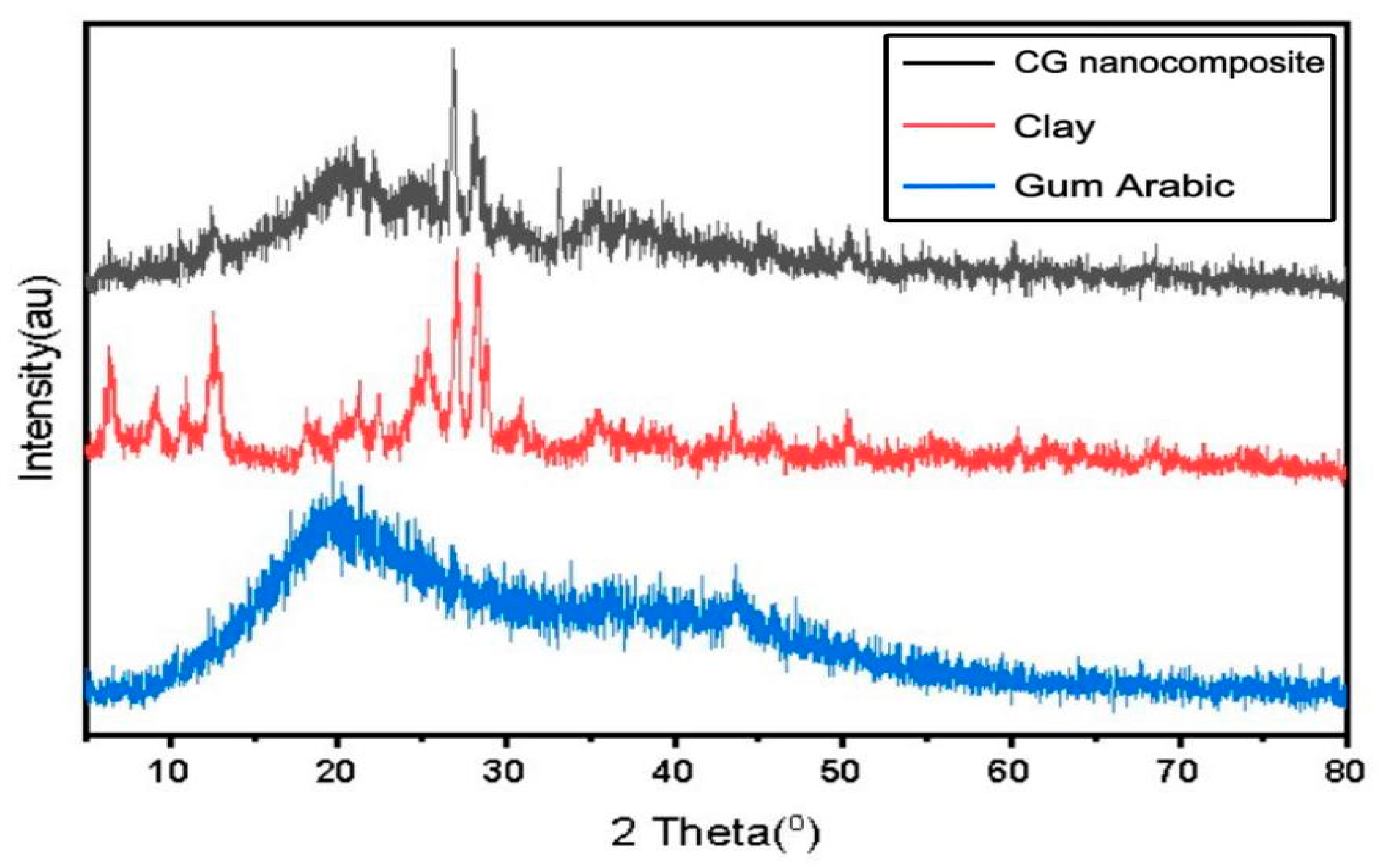
3.1.4. X-Ray Powder Diffraction (XRD)
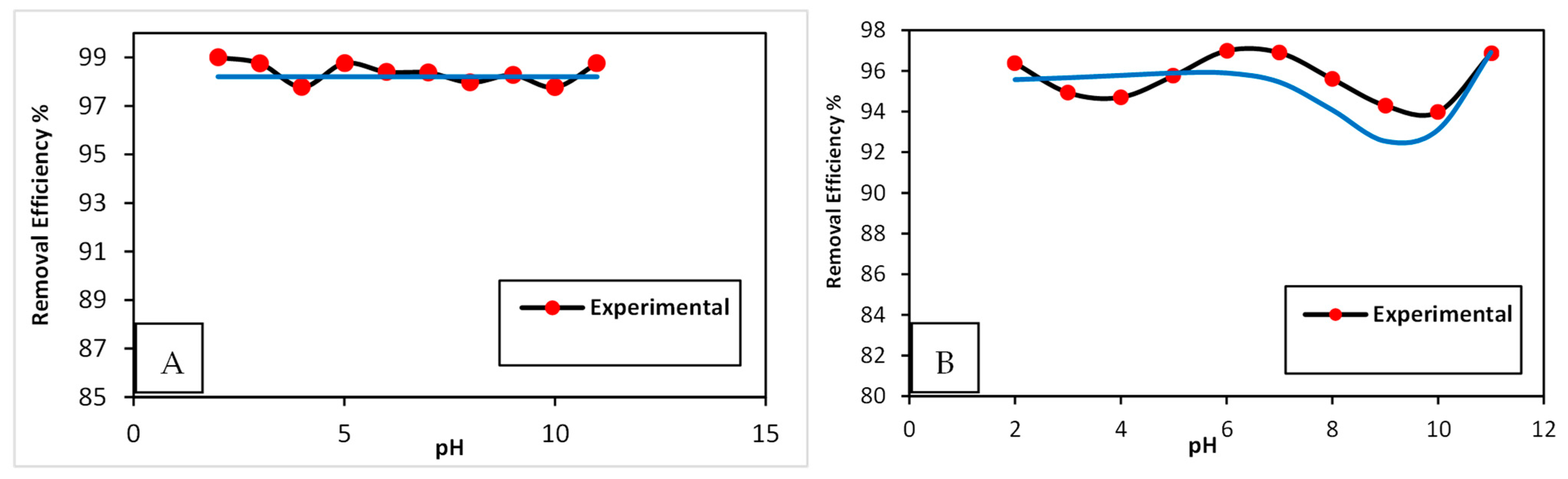
3.2. Artificial Neural Network (ANN)
3.3. Adsorption Studies
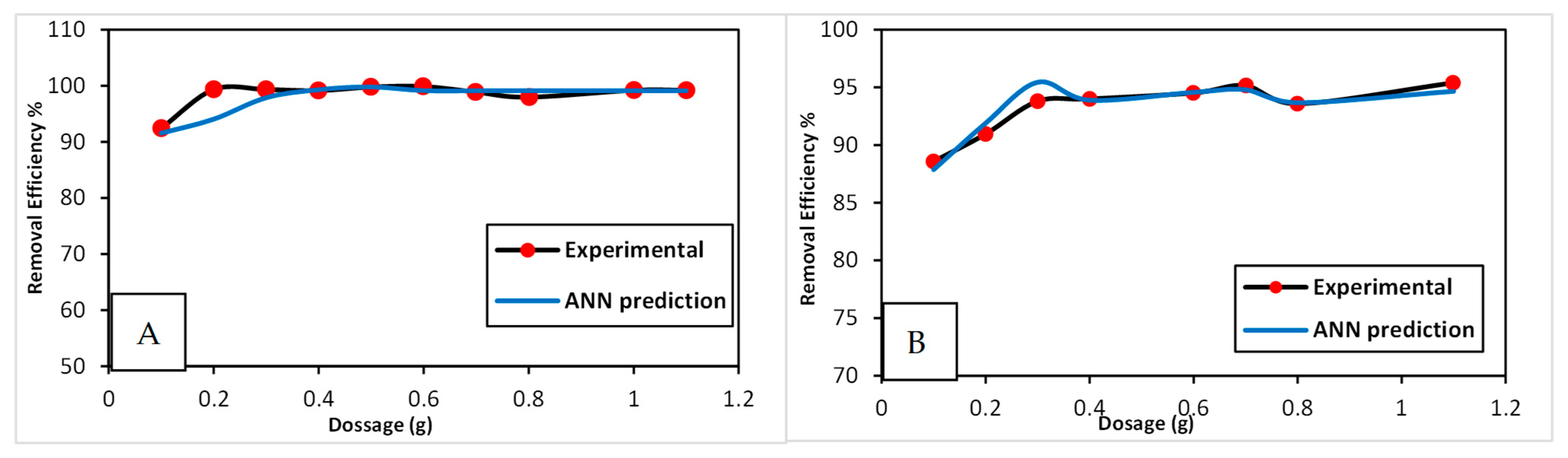
3.3.1. Effect of Dosage of CG/MC
3.3.2. Effect of pH
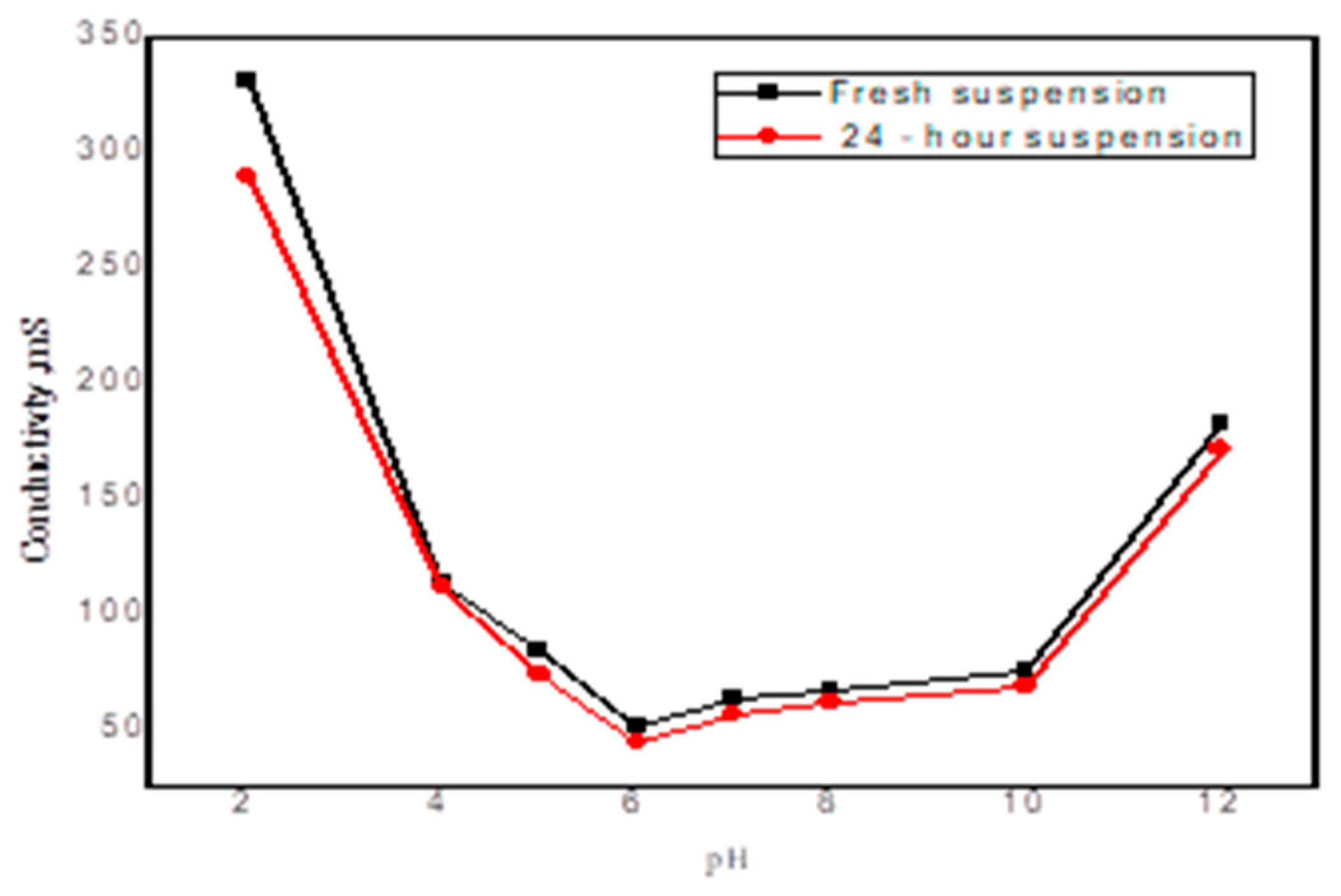
3.3.3. Zeta Potential
3.3.4. Effect of Contact Time
3.3.5. Effect of Initial Concentration
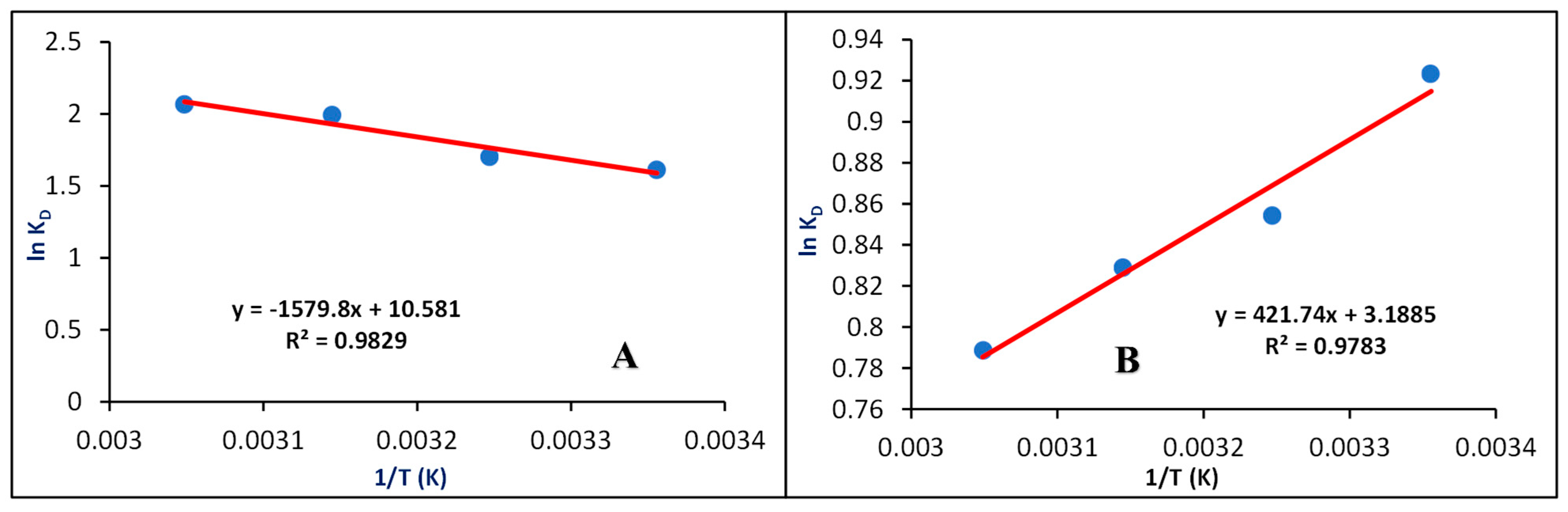
3.3.6. Effect of Temperature
3.4. Optimizing the ANN Model
3.5. Thermodynamic Factors
3.6. Adsorption Isotherm Models
3.6.1. Langmuir Isotherm Model
3.6.2. Freundlich Isotherm Model
3.6.3. Temkin Isotherm Model
| Langmuir Isotherm | |||
| qmax (mg/g) | KL (L/g) | R2 | |
| MB | 66.7 | 0.159 | 0.991 |
| CV | 52.9 | 0.298 | 0.981 |
| Freundlich Isotherm | |||
| N | Kf (mg/g)/(mg/L) | R2 | |
| MB | 1.89 | 10.1 | 0.975 |
| CV | 2.76 | 16.5 | 0.880 |
| Temkin Isotherm | |||
| A(L/g) | B | R2 | |
| MB | 2.5 | 12.08 | 0.929 |
| CV | 2.6 | 28.55 | 0.975 |
3.7. Results of the Kinetic Studies
3.7.1. The Pseudo-First-Order
3.7.2. The Pseudo-Second-Order
3.7.3. The Intra-Particle Diffusion Model
| Pseudo-First-Order | |||
| qe (mg/g) | k1 (min−1) | R2 | |
| MB | 5.694 | 0.000137 | 0.701 |
| CV | 4.028 | 0.00035 | 0.323 |
| Pseudo-second-order | |||
| qe (mg/g) | k2 (mg/g.min) | R2 | |
| MB | 5.694 | 1.1876 | 0.997 |
| CV | 4.028 | 1.9537 | 0.999 |
| Intra-particle diffusion | |||
| kid (mg/g.min) | I | R2 | |
| MB | 6.228 | 39.725 | 0.671 |
| CV | 4.111 | 12.827 | 0.511 |
| Elovich | |||
| α | β | R2 | |
| MB | 3.40 × 1029 | 10.98 | 0.704 |
| CV | 3.16 × 106 | 5.89 | 0.849 |
3.7.4. The Elovich Model
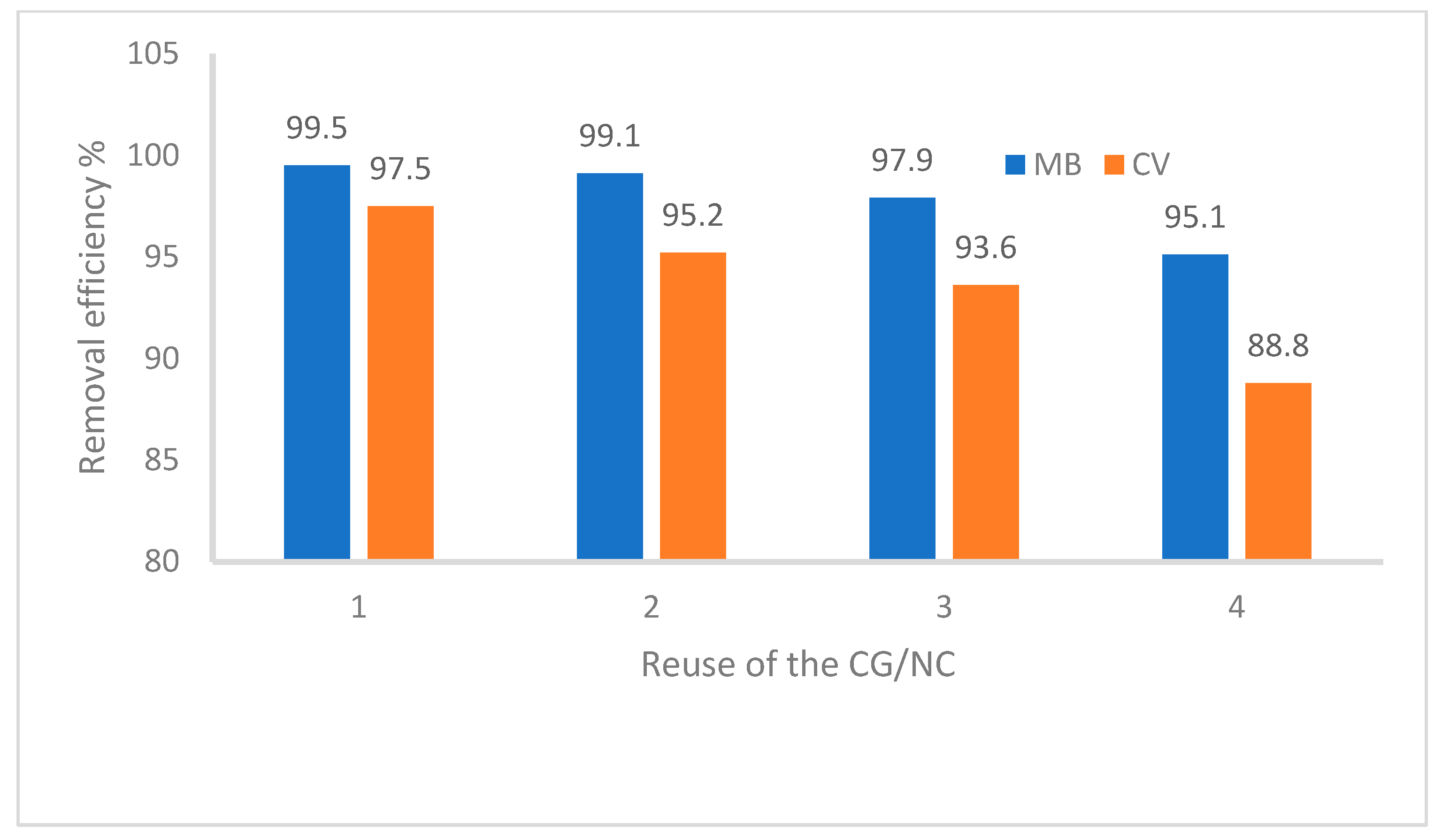
3.8. Results of the CG/NC Reusability
3.9. Comparison with Other Adsorbents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jaramillo-Fierro, X.; González, S.; Jaramillo, H.A.; Medina, F. Synthesis of the ZnTiO3/TiO2 Nanocomposite Supported in Ecuadorian Clays for the Adsorption and Photocatalytic Removal of Methylene Blue Dye. Nanomaterials 2020, 10, 1891. [Google Scholar] [CrossRef]
- Ibupoto, A.S.; Qureshi, U.A.; Ahmed, F.; Khatri, Z.; Khatri, M.; Maqsood, M.; Brohi, R.Z. Reusable carbon nanofibers for efficient removal of methylene blue from aqueous solution. Chem. Eng. Res. Des. 2018, 136, 744–752. [Google Scholar] [CrossRef]
- Ouaddari, H.; Abbou, B.; Lebkiri, I.; Habsaoui, A.; Ouzzine, M.; Fath Allah, R. Removal of Methylene Blue by adsorption onto natural and purified clays: Kinetic and thermodynamic study. Chem. Phys. Impact 2024, 8, 100405. [Google Scholar] [CrossRef]
- Sandikci, M.N.; Isik, B. Fabrication of calcium alginate/gum arabic/egg shell composite microbeads for adsorptive removal of methylene blue dye from aqueous solutions. Macromol. Res. 2025. [Google Scholar] [CrossRef]
- Singh, V.; Sapehia, R.; Dhiman, V. Removal of methylene blue dye by green synthesized NiO/ZnO nanocomposites. Inorg. Chem. Commun. 2025, 162, 112267. [Google Scholar] [CrossRef]
- Bulut, Y.; Aydın, H. A kinetics and thermodynamics study of methylene blue adsorption on wheat shells. Desalination 2006, 194, 259–267. [Google Scholar] [CrossRef]
- Hoslett, J.; Ghazal, H.; Mohamad, N.; Jouhara, H. Removal of methylene blue from aqueous solutions by biochar prepared from the pyrolysis of mixed municipal discarded material. Sci. Total Environ. 2020, 714, 136832. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Liu, X.; Wu, J.; Zhu, X.; Bai, Z.; Yu, Z. Preparation of β-cyclodextrin/graphene oxide and its adsorption properties for methylene blue. Colloids Surf. B Biointerfaces 2021, 200, 111605. [Google Scholar] [CrossRef]
- Rehman, M.U. Physicochemical characterization of Pakistani clay for adsorption of methylene blue: Kinetic, isotherm and thermodynamic study. Mater. Chem. Phys. 2021, 269, 124722. [Google Scholar] [CrossRef]
- Patil, M.R.; Shrivastava, V. Adsorptive removal of methylene blue from aqueous solution by polyaniline-nickel ferrite nanocomposite: A kinetic approach. Desalination Water Treat. 2016, 57, 5879–5887. [Google Scholar] [CrossRef]
- Chang, J. Adsorption of methylene blue onto Fe3O4/activated montmorillonite nanocomposite. Appl. Clay Sci. 2016, 119, 132–140. [Google Scholar] [CrossRef]
- Alzahrani, E. Gum Arabic-coated magnetic nanoparticles for methylene blue removal. Int. J. Innov. Res. Sci. Eng. Technol. 2014, 3, 15118–15129. [Google Scholar] [CrossRef]
- Azhar-ul-Haq, M.; Javed, T.; Abid, M.A.; Masood, H.T.; Muslim, N. Adsorptive removal of hazardous crystal violet dye onto banana peel powder: Equilibrium, kinetic and thermodynamic studies. J. Dispers. Sci. Technol. 2024, 45, 475–490. [Google Scholar] [CrossRef]
- Chowdhury, S.; Chakraborty, S.; Das, P. Adsorption of crystal violet from aqueous solution by citric acid modified rice straw: Equilibrium, kinetics, and thermodynamics. Sep. Sci. Technol. 2013, 48, 1339–1348. [Google Scholar] [CrossRef]
- Sulthana, R.; Taqui, S.N.; Syed, U.T. Adsorption of crystal violet dye from aqueous solution using industrial pepper seed spent: Equilibrium, thermodynamic, and kinetic studies. Adsorpt. Sci. Technol. 2022, 2022, 9009214. [Google Scholar] [CrossRef]
- Khattri, S.; Singh, M. Use of Sagaun sawdust as an adsorbent for the removal of crystal violet dye from simulated wastewater. Environ. Prog. Sustain. Energy 2012, 31, 435–442. [Google Scholar] [CrossRef]
- Loulidi, I.; Boukhlifi, F.; Ouchabi, M.; Amar, A. Adsorption of crystal violet onto an agricultural waste residue: Kinetics, isotherm, thermodynamics, and mechanism of adsorption. Sci. World J. 2020, 2020, 5873521. [Google Scholar] [CrossRef]
- AL-Shehri, H.S.; Almudaifer, E.; Alorabi, A.Q.; Alanazi, H.S.; Alkorbi, A.S.; Alharthi, F.A. Effective adsorption of crystal violet from aqueous solutions with effective adsorbent: Equilibrium, mechanism studies and modeling analysis. Environ. Pollut. Bioavailab. 2021, 33, 214–226. [Google Scholar] [CrossRef]
- Senthilkumaar, S.; Kalaamani, P.; Subburaam, C. Liquid phase adsorption of crystal violet onto activated carbons derived from male flowers of coconut tree. J. Hazard. Mater. 2006, 136, 800–808. [Google Scholar] [CrossRef]
- Ahmad, R. Studies on adsorption of crystal violet dye from aqueous solution onto Coniferous pinus bark powder (CPBP). J. Hazard. Mater. 2009, 171, 767–773. [Google Scholar] [CrossRef]
- Laskar, N.; Kumar, U. Adsorption of crystal violet from wastewater by modified Bambusa tulda. KSCE J. Civ. Eng. 2018, 22, 2755–2763. [Google Scholar] [CrossRef]
- Mulla, B.; Ioannou, K.; Kotanidis, G.; Ioannidis, I.; Constantinides, G.; Baker, M.; Hinder, S.; Mitterer, C.; Pashalidis, I.; Kostoglou, N.; et al. Removal of Crystal Violet Dye from Aqueous Solutions through Adsorption onto Activated Carbon Fabrics. C 2024, 10, 19. [Google Scholar] [CrossRef]
- Ben Tahar, L.; Mogharbel, R.; Hameed, Y.; Noubigh, A.; Abualreish, M.J.A.; Alanazi, A.H.; Hatshan, M.R. Enhanced removal of the crystal violet dye from aqueous medium using tripolyphosphate–functionalized Zn–substituted magnetite nanoparticles. Results Chem. 2025, 14, 102152. [Google Scholar] [CrossRef]
- Khan, M.I.; Almesfer, M.K.; Elkhaleefa, A.M.; Aamary, A.; Ali, I.H.; Shamim, M.Z.; Shoukry, H. Efficient adsorption of hexavalent chromium ions onto novel ferrochrome slag/polyaniline nanocomposite: ANN modeling, isotherms, kinetics, and thermodynamic studies. Environ. Sci. Pollut. Res. Int. 2022, 29, 86665–86679. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, E.; Moghaddam, M.R.A.; Kowsari, E. A systematic and critical review on development of machine learning based-ensemble models for prediction of adsorption process efficiency. J. Clean. Prod. 2022, 379, 134588. [Google Scholar] [CrossRef]
- Çelekli, A.; Geyik, F. Artificial neural networks (ANN) approach for modeling of removal of Lanaset Red G on Chara contraria. Bioresour. Technol. 2011, 102, 5634–5638. [Google Scholar] [CrossRef]
- Khonde, R.; Pandharipande, S. Artificial Neural Network modeling for adsorption of dyes from aqueous solution using rice husk carbon. Int. J. Comput. Appl. 2012, 41, 1–5. [Google Scholar]
- Sharma, K.; Sharma, S.; Sharma, V.; Mishra, P.K.; Ekielski, A.; Sharma, V.; Kumar, V. Methylene Blue Dye Adsorption from Wastewater Using Hydroxyapatite/Gold Nanocomposite: Kinetic and Thermodynamics Studies. Nanomaterials 2021, 11, 1403. [Google Scholar] [CrossRef]
- Khan, M.I.; Yahya, S.A.; ElKhaleefa, A.; Shigidi, I.; Ali, I.H.; Rehan, M.; Pirzada, A.M. Toxic Anionic Azo Dye Removal from Artificial Wastewater by Using Polyaniline/Clay Nanocomposite Adsorbent: Isotherm, Kinetics and Thermodynamic Study. Processes 2025, 13, 827. [Google Scholar] [CrossRef]
- Ali, I.H.; Bani-Fwaz, M.Z.; El-Zahhar, A.A.; Marzouki, R.; Jemmali, M.; Ebraheem, S.M. Gum Arabic-Magnetite Nanocomposite as an Eco-Friendly Adsorbent for Removal of Lead(II) Ions from Aqueous Solutions: Equilibrium, Kinetic and Thermodynamic Studies. Separations 2021, 8, 224. [Google Scholar] [CrossRef]
- Elkhaleefa, A.; Ali, I.H.; Brima, E.I.; Shigidi, I.; Elhag, A.B.; Karama, B. Evaluation of the Adsorption Efficiency on the Removal of Lead(II) Ions from Aqueous Solutions Using Azadirachta indica Leaves as an Adsorbent. Processes 2021, 9, 559. [Google Scholar] [CrossRef]
- Hussain, S.A.; Demirci, Ş.; Özbayoğlu, G. Zeta potential measurements on three clays from Turkey and effects of clays on coal flotation. J. Colloid Interface Sci. 1996, 184, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Shehu, Z. Synthesis, Characterization and Antibacterial Activity of Kaolin/Gum Arabic Nanocomposite on Escherichia Coli and Pseudomonas Aeruginosa. Res. J. Nanosci. Eng. 2018, 2, 23–29. [Google Scholar] [CrossRef]
- Idrissi, M. Degradation of crystal violet by heterogeneous Fenton-like reaction using Fe/Clay catalyst with H2O2. J. Mater. Environ. Sci. 2016, 7, 50–58. [Google Scholar]
- Adikary, S.; Wanasinghe, D. Characterization of locally available Montmorillonite clay using FTIR technique. Annu. Trans. Inst. Eng. Sri Lanka 2012, 1, 140–145. [Google Scholar]
- Upadhyay, A. Ethylene scavenging film based on corn starch-gum acacia impregnated with sepiolite clay and its effect on quality of fresh broccoli florets. Food Biosci. 2022, 46, 101556. [Google Scholar] [CrossRef]
- Nayak, A.K.; Das, B.; Maji, R. Calcium alginate/gum Arabic beads containing glibenclamide: Development and in vitro characterization. Int. J. Biol. Macromol. 2012, 51, 1070–1078. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S. Mesoporous Iraqi red kaolin clay as an efficient adsorbent for methylene blue dye: Adsorption kinetic, isotherm and mechanism study. Surf. Interfaces 2020, 18, 100422. [Google Scholar] [CrossRef]
- Dai, F.; Zhuang, Q.; Huang, G.; Deng, H.; Zhang, X. Infrared Spectrum Characteristics and Quantification of OH Groups in Coal. ACS Omega 2023, 8, 17064–17076. [Google Scholar] [CrossRef]
- Alorabi, A.Q.; Hassan, M.S.; Alam, M.M.; Zabin, S.A.; Alsenani, N.I.; Baghdadi, N.E. Natural Clay as a Low-Cost Adsorbent for Crystal Violet Dye Removal and Antimicrobial Activity. Nanomaterials 2021, 11, 2789. [Google Scholar] [CrossRef]
- Amirabadi, S.; Milani, J.M.; Sohbatzadeh, F. Application of dielectric barrier discharge plasma to hydrophobically modification of gum arabic with enhanced surface properties. Food Hydrocoll. 2020, 104, 105724. [Google Scholar] [CrossRef]
- Mecheri, R.; Zobeidi, A.; Atia, S.; Neghmouche Nacer, S.; Salih, A.A.M.; Benaissa, M.; Ghernaout, D.; Arni, S.A.; Ghareba, S.; Elboughdiri, N. Modeling and Optimizing the Crystal Violet Dye Adsorption on Kaolinite Mixed with Cellulose Waste Red Bean Peels: Insights into the Kinetic, Isothermal, Thermodynamic, and Mechanistic Study. Materials 2023, 16, 4082. [Google Scholar] [CrossRef]
- Marsh, A. Phase formation behaviour in alkali activation of clay mixtures. Appl. Clay Sci. 2019, 175, 10–21. [Google Scholar] [CrossRef]
- Bashir, M.; Haripriya, S. Assessment of physical and structural characteristics of almond gum. Int. J. Biol. Macromol. 2016, 93, 476–482. [Google Scholar] [CrossRef]
- Emam, H.E. Arabic gum as bio-synthesizer for Ag–Au bimetallic nanocomposite using seed-mediated growth technique and its biological efficacy. J. Polym. Environ. 2019, 27, 210–223. [Google Scholar] [CrossRef]
- Yadav, V.B.; Gadi, R.; Kalra, S. Synthesis and characterization of novel nanocomposite by using kaolinite and carbon nanotubes. Appl. Clay Sci. 2018, 155, 30–36. [Google Scholar] [CrossRef]
- Nasuha, N.; Hameed, B.H.; Din, A.T. Rejected tea as a potential low-cost adsorbent for the removal of methylene blue. J. Hazard. Mater. 2010, 175, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Elsagan, Z.; AbdElhafez, S. Lignin from agro-industrial waste to an efficient magnetic adsorbent for hazardous crystal violet removal. Molecules 2022, 27, 1831. [Google Scholar] [CrossRef] [PubMed]
- Al-Shahrani, S. Phenomena of removal of crystal violet from wastewater using Khulays natural bentonite. J. Chem. 2020, 2020, 4607657. [Google Scholar] [CrossRef]
- Elsherif, K.M.; El-Dali, A.; Alkarewi, A.A.; Ewlad-Ahmed, A.M.; Treban, A. Adsorption of crystal violet dye onto olive leaves powder: Equilibrium and kinetic studies. Chem. Int. 2021, 7, 79–89. [Google Scholar]

| Sample | Surface Area (m2/g) |
|---|---|
| Clay | 14.34 |
| Gum arabic | 0.3835 |
| CG/NC | 46.91 |
| Initial Concentration (mg/L) | Dosage CG/NC (g) | pH | Contact Time (min) | Temperature (°C) | R (%) Predicted | R (%) Experimentally | |
|---|---|---|---|---|---|---|---|
| MB | 50.00 | 0.30 | 7.00 | 7.00 | 24.00 | 99.15 | 97.31 |
| CV | 47.90 | 0.29 | 7.00 | 3.80 | 50.00 | 99.00 | 96.40 |
| MB | |||
| T, K | ΔG° (kJ/mol) | ΔS° (J/mol) | ΔH° (kJ/mol) |
| 298 | −3.991 | ||
| 308 | −4.356 | 88.01 | 13.13 |
| 318 | −5.266 | ||
| 328 | −5.631 | ||
| CV | |||
| T, K | ΔG° (kJ/mol) | ΔS° (J/mol) | ΔH° (kJ/mol) |
| 298 | −2.288 | ||
| 308 | −2.187 | 26.51 | −3.50 |
| 318 | −2.191 | ||
| 328 | −2.150 | ||
| Adsorbent | Adsorbate | Isotherm Model | Optimum pH | Kinetic Model | Enthalpy | qmax (mg/g) | Adsorbent Mass (g L−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| (RT) | MB | Langmuir | 6–7 | Second order | - | 147 | 12.5 | [47] |
| (Fe3O4/Mt) | MB | Langmuir | 7.37 | Second order | - | 106.38 | 12.5 | [11] |
| (IRKC) | MB | Langmuir-Freundlich | 8 | First order | - | 240.4 | 10.0 | [38] |
| PANI-NiFe2O4 | MB | Langmuir | 9 | Second order | - | 6.65 | 80.0 | [10] |
| (WHS) | MB | Langmuir | 7 | Second order | endothermic | 21.50 | 20.0 | [6] |
| (LCF) | CV | Langmuir-Freundlich | 7 | First order | exothermic | 34.12 | 5.0 | [48] |
| Khulays natural bentonite | CV | Langmuir-Freundlich | 5.3 | Second order | endothermic | 263 | 5.0 | [49] |
| (NAJL) | CV | Langmuir | 9 | First order | exothermic | 315.2 | 1.0 | [18] |
| (OLP) | CV | Langmuir | 7.5 | Second order | - | 181.1 | 4.0 | [50] |
| (AS) | CV | Langmuir | 6 | Second order | endothermic | 12.2 | 10.0 | [17] |
| CG/NC | MB | Langmuir | 7 | Second order | endothermic | 66.7 | 4.0 | this study |
| CG/NC | CV | Langmuir | 7 | Second order | exothermic | 52.9 | 6.0 | this study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqahtani, M.F.; Ali, I.H.; Siddeeg, S.M.; Maiz, F.; Eltahir, S.B.; Alarfaji, S.S. Experimental and Artificial Neuron Network Insights into the Removal of Organic Dyes from Wastewater Using a Clay/Gum Arabic Nanocomposite. Nanomaterials 2025, 15, 857. https://doi.org/10.3390/nano15110857
Alqahtani MF, Ali IH, Siddeeg SM, Maiz F, Eltahir SB, Alarfaji SS. Experimental and Artificial Neuron Network Insights into the Removal of Organic Dyes from Wastewater Using a Clay/Gum Arabic Nanocomposite. Nanomaterials. 2025; 15(11):857. https://doi.org/10.3390/nano15110857
Chicago/Turabian StyleAlqahtani, Malak F., Ismat H. Ali, Saifeldin M. Siddeeg, Fethi Maiz, Sawsan B. Eltahir, and Saleh S. Alarfaji. 2025. "Experimental and Artificial Neuron Network Insights into the Removal of Organic Dyes from Wastewater Using a Clay/Gum Arabic Nanocomposite" Nanomaterials 15, no. 11: 857. https://doi.org/10.3390/nano15110857
APA StyleAlqahtani, M. F., Ali, I. H., Siddeeg, S. M., Maiz, F., Eltahir, S. B., & Alarfaji, S. S. (2025). Experimental and Artificial Neuron Network Insights into the Removal of Organic Dyes from Wastewater Using a Clay/Gum Arabic Nanocomposite. Nanomaterials, 15(11), 857. https://doi.org/10.3390/nano15110857







