Powering the Future: Unveiling the Potential of Na, K, and Mg Solid-State Batteries
Abstract
1. Introduction
2. Na-Ion Solid-State Battery Systems: Progress and Challenges
2.1. Cathode Considerations in the Context of Na-Ion Batteries
2.1.1. Layered Structures of P2 and O3-Type NaTMO2 Cathode Materials
P2-Type Metal Oxide Cathode Materials
O3-Type Metal Oxide Cathode Materials
Polyanion-Type Cathode Materials
Phosphate-Based Cathode Materials
Pyrophosphates for Cathode Materials
2.1.2. Transition Metal Sulfates NaxMy(SO4)z (M = Fe, Mn, Co, Ni)
2.1.3. Transition Metal Silicates Na2MSiO4 (M = Fe, Mn, Co)
2.1.4. Prussian Blue (PB) and Its Analogues (PBAs)
2.1.5. Other Types of Cathode Materials
NASICON-Based Compounds
Organic Compounds
Hexacyanometalates
2.2. Anode Materials for SSSBs
2.2.1. Sodium Metal Anodes
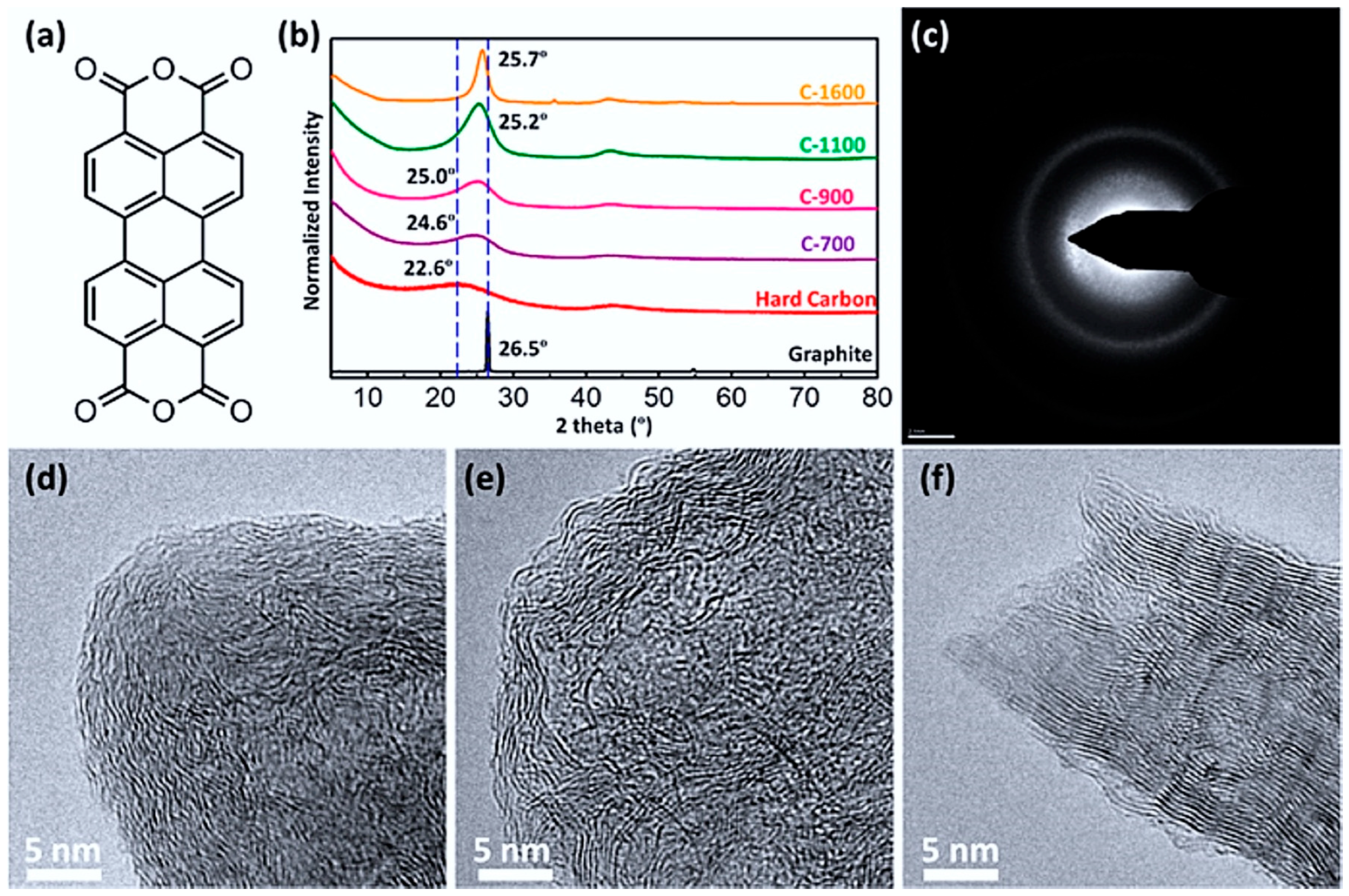
2.2.2. Carbon-Based Anode Materials
2.3. Electrolytes for Sodium-Ion Solid-State Batteries
2.3.1. Inorganic Solid Electrolytes
2.3.2. Composite Polymer Electrolytes
| Systems | Fillers | Electrochemical Stability Window/V (vs. Na+/Na) | Ionic Conductivity | Ionic Transference Number | References |
|---|---|---|---|---|---|
| PMA/PEG-NaClO4 | Al2O3 | 4.5 | 1.46 × 10−4 at 70 °C | - | [75] |
| PEO-NaClO4 | NaAlO2 | 4.5 | 7.4 × 10−5 at 30 °C | 0.6 | [89] |
| PEO-NaClO4 | TiO2 | - | 2.62 × 10−4 at 60 °C | - | [20] |
| PVDF-HFP-NaTf | NZSP | 5.0 | 1.2 × 10−4 at 0 °C | 0.92 | [75] |
| PEO-SN/PAN-NaClO4 | NZSP | 4.8 | 1.36 × 10−4 at 25 °C | 0.42 | [90] |
2.3.3. Solid Polymer Electrolytes
3. Other SSB Systems
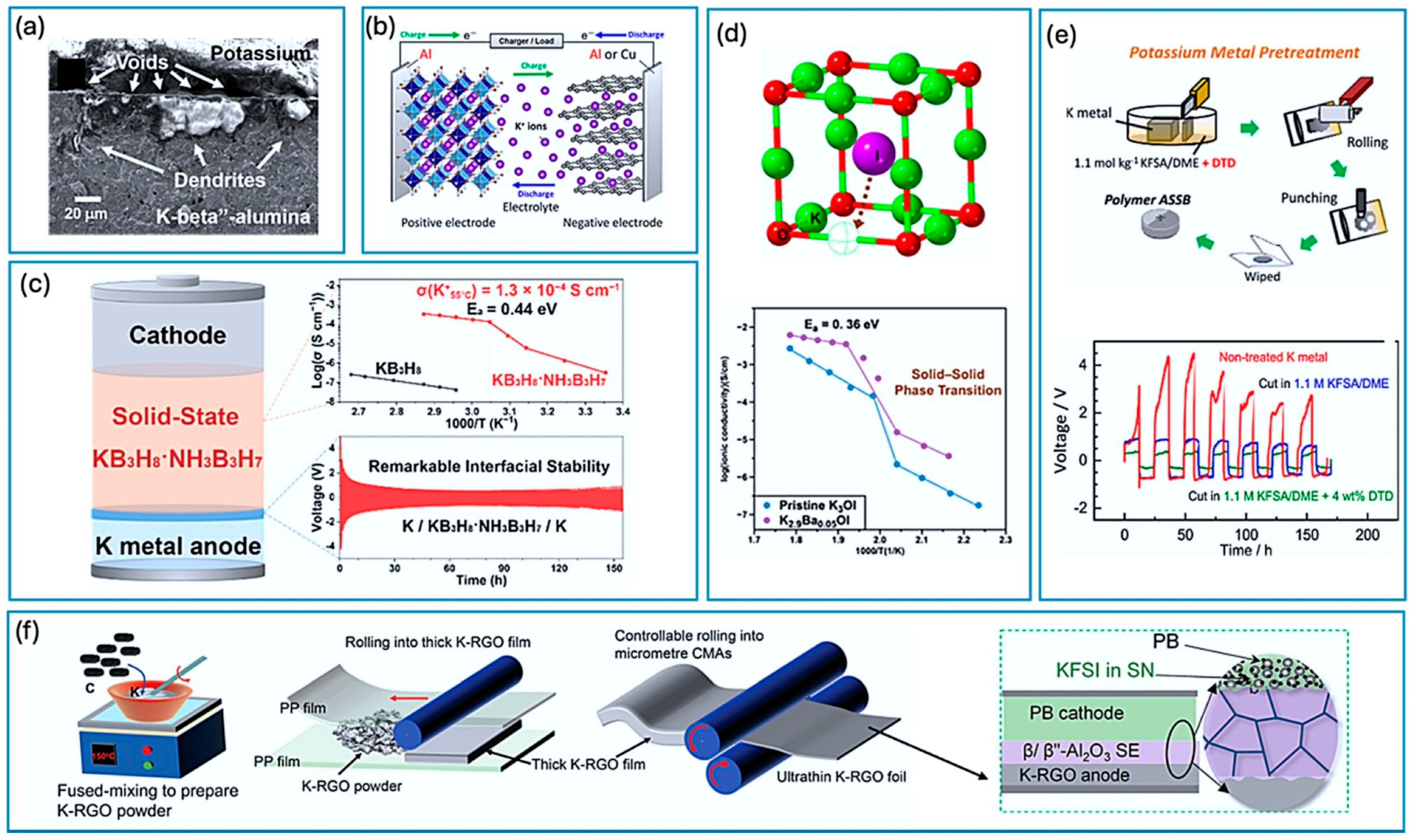
3.1. Solid-State K-Ion Batteries (SSKBs)
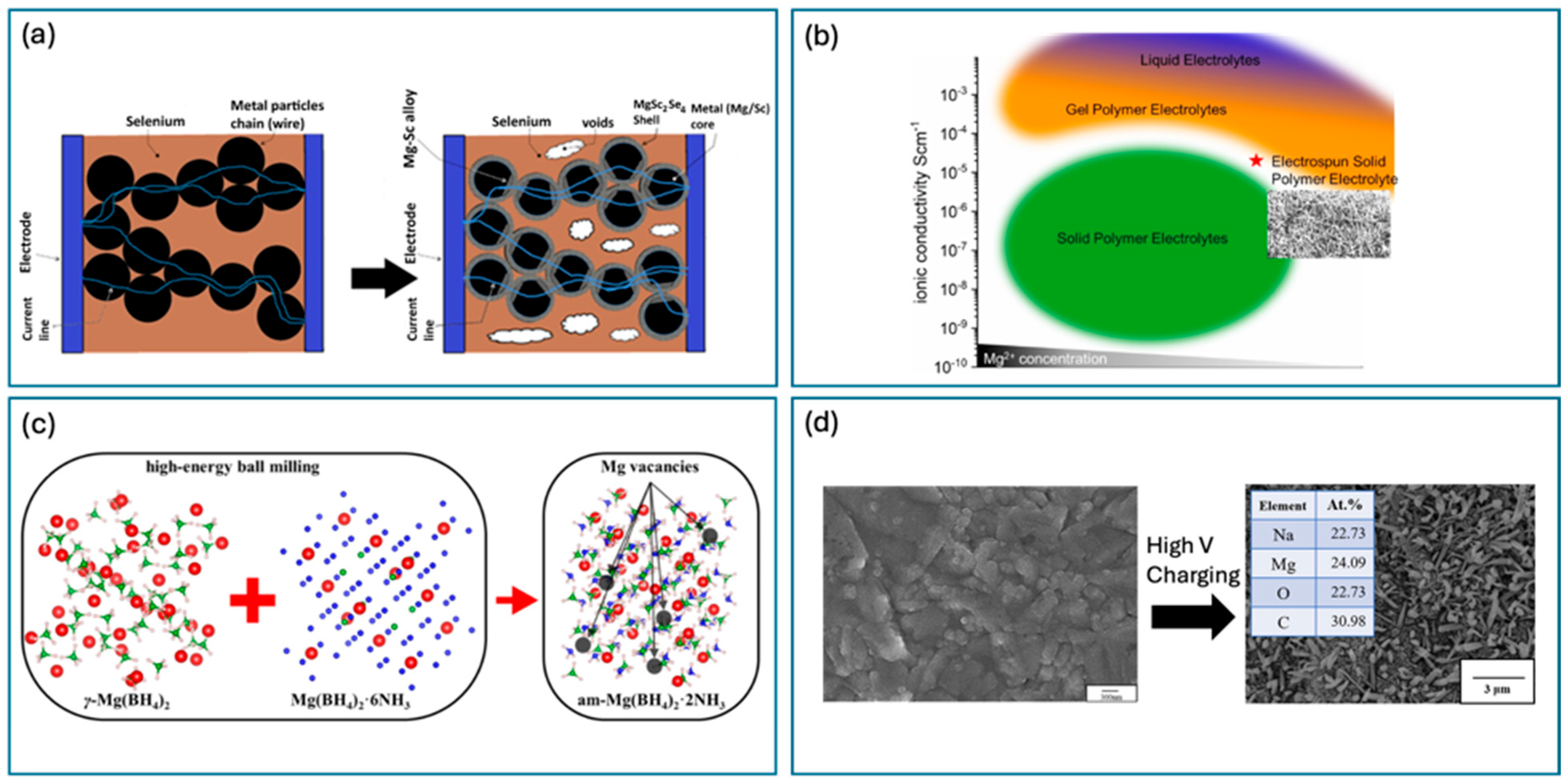
3.2. Solid-State Mg-Ion Batteries (SSMBs)
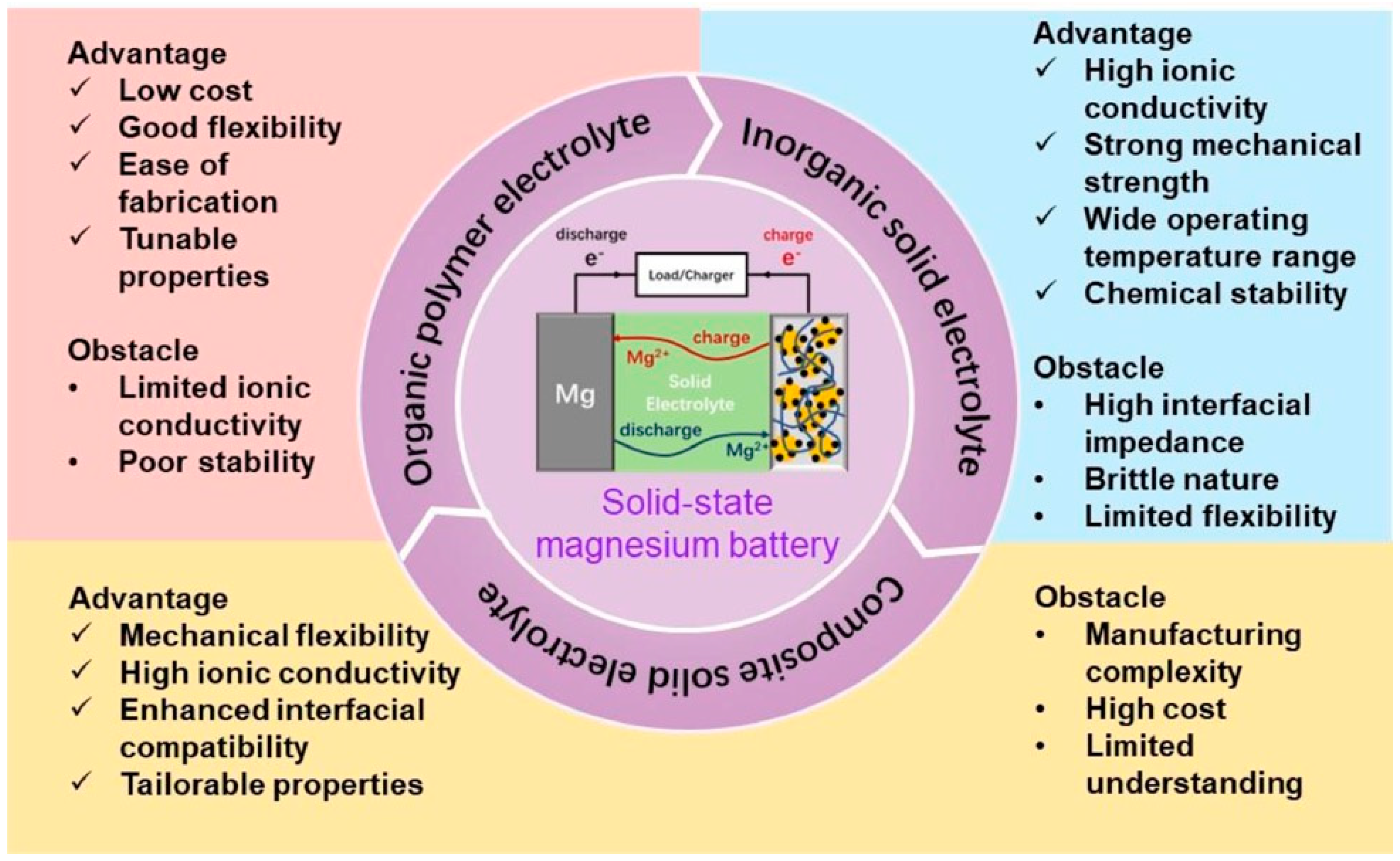
4. Present Challenges and Future Perspectives
4.1. Present Challenges
4.2. Future Perspectives in Materials Innovation
4.2.1. Polyanion-Type Cathode Materials for Na-Ion Solid-State Batteries
4.2.2. Phosphate-Based Cathode Materials for Na-Ion Solid-State Batteries
4.2.3. Pyrophosphates as Cathode Materials for Na-Ion Solid-State Batteries
4.2.4. Transition Metal Sulfates of NaxMy (SO4)z (M = Fe, Mn, Co, Ni) for Na-Ion Solid-State Batteries
4.2.5. Transition Metal Silicates of Na2MSiO4 (M = Fe, Mn, Co) for Na-Ion Solid-State Batteries
4.2.6. Further Classes of Cathode Materials for Na-Ion Solid-State Batteries
4.2.7. Future Direction with Inorganic Solid Electrolytes for Na-Ion Solid-State Batteries
4.2.8. Future Directions for K-Ion Solid-State Batteries
4.2.9. Future Directions for Mg-Solid State Batteries
5. Summary and Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALD | Atomic layer deposition |
| ASSLB | All-solid-state lithium-ion battery |
| CBM | Conduction band minimum |
| CCD | Critical current density |
| CEI | Cathode electrolyte interphase |
| CNT | Carbon nanotube |
| CV | Cyclic voltammetry |
| DFT | Density functional theory |
| DMF | N,N-dimethylformamide |
| EBSD | Electron backscatter diffraction |
| EIS | Electrochemical impedance spectroscopy |
| EELS | Electron energy loss spectroscopy |
| EV | Electric vehicle |
| GB | Grain boundary |
| HAADF-STEM | High-angle annular dark-field scanning transmission electron microscope |
| HOMO | Highest occupied molecular orbital |
| ISE | Inorganic solid electrolyte |
| LATP | Li1.3Al0.3Ti1.7(PO4)3 |
| LAGP | Li1.5Al0.5Ge1.5(PO4)3 |
| LGPS | Li10GeP2S12 |
| LIBs | Lithium-ion batteries |
| LiPON | Lithium phosphorus oxynitride |
| LISICON | Lithium superionic conductor |
| LPS | Li3PS4: Li7P3S11 |
| LPSCl | Li6PS5Cl |
| LLZO | Li7La3Zr2O12 |
| LTMO | Layered transition metal oxide |
| LTO | Li4Ti5O12 |
| LUMO | Lowest unoccupied molecular orbital |
| LYBC | Li3Y(Br3Cl3) |
| LZO | Li6Zr2O7 |
| NASICON | Sodium superionic conductor |
| NaClO4 | Sodium perchlorate |
| NaFNFSI | (Sodium (fluorosulfonyl)(n-nonafluorobutanesulfonyl)imide) |
| NaPTAB | Sodium–Poly(tartaric acid) Borate |
| NMR | Nuclear magnetic resonance |
| PB | Prussian blue |
| PAN | Polyacrylonitrile |
| PBA | Prussian blue analog |
| PE | Polyethylene |
| PEO | Polyethylene glycol |
| PP | Polypropylene |
| P(VDF-HFP) | Poly(vinylidene fluoride-co-hexafluoropropylene) |
| RCC | Current constriction resistance |
| RCT | Charge transfer resistance |
| rGO | Reduced graphene oxide |
| Rint | Interfacial resistance |
| SEM | Scanning electron microscope |
| SEI | Solid electrolyte interphase |
| SGLE | Single-Ion Gel Polymer Electrolyte |
| SHE | Standard hydrogen electrode |
| SPE | Solid polymer electrolyte |
| SSB | Solid-state battery |
| SSE | Solid-state electrolyte |
| SSKB | Solid-state K-ion battery |
| SSMB | Solid-state Mg-ion battery |
| SSSB | Sodium-ion solid-state battery |
| TiO2 | Titanium dioxide |
| VBM | Valence band maximum |
| XAS | X-ray absorption spectroscopy |
| XPS | X-ray photoelectron spectroscopy |
References
- Shang, R.; Kurban, M.; Ma, Y.; Ozkan, M.; Ozkan, C.S. Rational design of SnO2 thin film coated cathode with function of entrapping polysulfides for performance enhanced Li–S batteries. J. Power Sources 2024, 597, 234144. [Google Scholar] [CrossRef]
- Zerrin, T.; Shang, R.; Dong, B.; Aguilar, E.C.; Malvin, J.; Ozkan, M.; Ozkan, C.S. An overlooked parameter in Li–S batteries: The impact of electrolyte-to-sulfur ratio on capacity fading. Nano Energy 2022, 104, 107913. [Google Scholar] [CrossRef]
- Shang, R.; Zerrin, T.; Dong, B.; Ozkan, C.S.; Ozkan, M. Sustainable and Low-Cost Lithium-Ion Batteries: Nonconventional Electrode Chemistries and State of Health Characterization. Technol. Innov. 2020, 21, 1–23. [Google Scholar] [CrossRef]
- Li, Y.; Dong, B.; Zerrin, T.; Jauregui, E.; Wang, X.; Hua, X.; Ravichandran, D.; Shang, R.; Xie, J.; Ozkan, M.; et al. State-of-health prediction for lithium-ion batteries via electrochemical impedance spectroscopy and artificial neural networks. Energy Storage 2020, 2, e186. [Google Scholar] [CrossRef]
- Zerrin, T.; Kurban, M.; Dickson, M.M.; Ozkan, M.; Ozkan, C.S. Suppression of the Shuttle Effect in Li–S Batteries via Magnetron Sputtered TiO2 Thin Film at the Electrode–Electrolyte Interface. ACS Appl. Energy Mater. 2020, 3, 1515–1529. [Google Scholar] [CrossRef]
- Li, C.; Liu, C.; Wang, W.; Bell, J.; Mutlu, Z.; Ahmed, K.; Ye, R.; Ozkan, M.; Ozkan, C.S. Towards flexible binderless anodes: Silicon/carbon fabrics via double-nozzle electrospinning. Chem. Commun. 2016, 52, 11398–11401. [Google Scholar] [CrossRef]
- Ma, Y.; Shang, R.; Liu, Y.; Lake, R.; Ozkan, M.; Ozkan, C.S. Enabling fast-charging capability for all-solid-state lithium-ion batteries. J. Power Sources 2023, 559, 232647. [Google Scholar] [CrossRef]
- Wang, H.; Ozkan, C.S.; Zhu, H.; Li, X. Advances in solid-state batteries: Materials, interfaces, characterizations, and devices. MRS Bull. 2023, 48, 1221–1229. [Google Scholar] [CrossRef]
- Zhou, W.; Li, Y.; Xin, S.; Goodenough, J.B. Rechargeable Sodium All-Solid-State Battery. ACS Cent. Sci. 2017, 3, 52–57. [Google Scholar] [CrossRef]
- Dong, Y.; Wen, P.; Shi, H.; Yu, Y.; Wu, Z.-S. Solid-State Electrolytes for Sodium Metal Batteries: Recent Status and Future Opportunities. Adv. Funct. Mater. 2024, 34, 2213584. [Google Scholar] [CrossRef]
- Man, Y.; Jaumaux, P.; Xu, Y.; Fei, Y.; Mo, X.; Wang, G.; Zhou, X. Research development on electrolytes for magnesium-ion batteries. Sci. Bull. 2023, 68, 1819–1842. [Google Scholar] [CrossRef] [PubMed]
- Jung, P.; Kim, M. Recent Research Progress on All-Solid-State Mg Batteries. Batteries 2024, 9, 570. [Google Scholar]
- Yang, Y.; Zhang, Y.; Li, Y.; Chen, X.; Hao, Y.; Chang, G.; Huang, C.; Qian, Y.; Liu, Z.; Tang, Q.; et al. High-performance lithium–sulfur batteries enabled by a dual-functional separator with a built-in polysulfide trap and fast ion transport channel. Nano Energy 2024, 122, 109303. [Google Scholar] [CrossRef]
- Du, G.; Tao, M.; Liu, D.; Aslam, M.K.; Qi, Y.; Jiang, J.; Li, Y.; Bao, S.-J.; Xu, M. Facile synthesis of hierarchical NiCo2S4 nanosheets on carbon cloth as a binder-free electrode for high-performance supercapacitors. J. Colloid Interface Sci. 2021, 582, 932–939. [Google Scholar] [CrossRef]
- Lin, X.; Zhao, Y.; Wang, C.; Luo, J.; Fu, J.; Xiao, B.; Gao, Y.; Li, W.; Zhang, S.; Xu, J. A Dual Anion Chemistry-Based Superionic Glass Enabling Long-Cycling All-Solid-State Sodium-Ion Batteries. Angew. Chem. 2024, 136, e202314181. [Google Scholar] [CrossRef]
- Ma, L.; Li, X.; Zhang, G.; Huang, Z.; Han, C.; Li, H.; Tang, Z.; Zhi, C. A Record-High Ion Storage Capacity of T-Graphene as Two-Dimensional Anode Material for Li-ion and Na-ion Batteries. Energy Storage Mater. 2020, 31, 451–458. [Google Scholar] [CrossRef]
- Chu, I.-H.; Kompella, C.S.; Nguyen, H.; Zhu, Z.; Hy, S.; Deng, Z.; Meng, Y.S.; Ong, S.P. Room-Temperature All-solid-state Rechargeable Sodium-ion Batteries with a Cl-doped Na3PS4 Superionic Conductor. Sci. Rep. 2016, 6, 33733. [Google Scholar] [CrossRef]
- Hayashi, A.; Noi, K.; Tanibata, N.; Nagao, M.; Tatsumisago, M. High sodium ion conductivity of glass–ceramic electrolytes with cubic Na3PS4. J. Power Sources 2014, 258, 420–423. [Google Scholar] [CrossRef]
- Guo, J.-Z.; Yang, A.-B.; Gu, Z.-Y.; Wu, X.-L.; Pang, W.-L.; Ning, Q.-L.; Li, W.-H.; Zhang, J.-P.; Su, Z.-M. Quasi-Solid-State Sodium-Ion Full Battery with High-Power/Energy Densities. ACS Appl. Mater. Interfaces 2018, 10, 17903–17910. [Google Scholar] [CrossRef]
- Ni, Y.L.; Cheng, M.-Y.; Cheng, J.H.; Rick, J.; Hwang, B.-J. Solid-state polymer nanocomposite electrolyte of TiO2/PEO/NaClO4 for sodium ion batteries. J. Power Sources 2015, 278, 375–381. [Google Scholar]
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef] [PubMed]
- U.S. Geological Survey 2024. Lithium. In Mineral Commodity Summaries; U.S. Geological Survey: Reston, VA, USA, 2024; pp. 100–101. [Google Scholar]
- Huang, S.; Jiang, L.; Chen, Y.; Chen, L. Preparation and Application of Thin-Sodium Metal. Innov. Mater. 2024, 2, 100077. [Google Scholar] [CrossRef]
- Pan, H.; Hu, Y.-S.; Chen, L. Room-temperature stationary sodium-ion batteries for large-scale electric energy storage. Energy Environ. Sci. 2013, 6, 2338–2360. [Google Scholar] [CrossRef]
- Gupta, P.; Pushpakanth, S.; Haider, M.A.; Basu, S. Understanding the Design of Cathode Materials for Na-Ion Batteries. ACS Omega 2022, 7, 5605–5614. [Google Scholar] [CrossRef]
- Hong, P.; Xu, C.; Yan, C.; Dong, Y.; Zhao, H.; Guo, Y. Prussian Blue and Its Analogues for Commercializing Fast-Charging Sodium-Ion Batteries. ACS Energy Lett. 2025, 10, 750–778. [Google Scholar] [CrossRef]
- CATL’s Naxtra Sodium-Ion Battery Achieved a Gravimetric Energy Density of 175 Wh/kg. 2025. Available online: https://www.reuters.com/technology/chinese-battery-maker-catl-launches-second-generation-fast-charging-battery-2025-04-21/ (accessed on 21 May 2025).
- Northvolt Developed a Sodium-Ion Battery with a Validated Energy Density of Over 160 Wh/kg. 2023. Available online: https://northvolt.com/articles/northvolt-sodium-ion/ (accessed on 21 May 2025).
- Gabriel, E.; Hou, D.; Lee, E.; Xiong, H. Multiphase layered transition metal oxide positive electrodes for sodium-ion batteries. Energy Sci. Eng. 2022, 10, 1672–1705. [Google Scholar] [CrossRef]
- Xian, L.; Li, M.; Qiu, D.; Qiu, C.; Zhang, Y.; Wang, Y.; Zhang, L. P3-Type Layered Na0.26Co1−xMnxO2 Cathode Induced by Mn Doping for High-Performance Sodium-Ion Batteries. J. Alloys Compd. 2022, 905, 163965. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, Z.; Chen, M.; Zou, C.; Jin, H.; Wang, S.; Gu, Q.; Chou, S. P2-Type Na2/3Ni1/3Mn2/3O2 as a Cathode Material with High-Rate and Long-Life for Sodium Ion Storage. J. Mater. Chem. A 2019, 7, 9215–9221. [Google Scholar] [CrossRef]
- Chen, T.; Wang, J.; Tan, B.; Zhang, K.J.; Banda, H.; Zhang, Y.; Kim, D.-H.; Dincă, M. High-Energy, High-Power Sodium-Ion Batteries from a Layered Organic Cathode. J. Am. Chem. Soc. 2025, 147, 6181–6192. [Google Scholar] [CrossRef]
- Xu, C.; Zhao, J.; Yang, C.; Hu, Y.-S. Polyanionic cathode materials for practical Na-ion batteries toward high energy density and long cycle life. ACS Cent. Sci. 2023, 9, 1721–1736. [Google Scholar] [CrossRef]
- Wazeer, W.; Nabil, M.M.; Feteha, M.; Soliman, M.B.; Kashyout, A.E. Ultra-fast green microwave assisted synthesis of NaFePO4-C nanocomposites for sodium ion batteries and supercapacitors. Sci. Rep. 2022, 12, 16307. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, X.; Li, Y.; Zhu, C.; Wang, C.; Zhang, Y.; Wang, Y.; Zhang, L. Easy Approach of Highly Electrochemical-Active Maricite NaFePO4 Cathode for Low-Cost and High-Rate Sodium-Ion Batteries. Appl. Phys. Lett. 2023, 123, 043903. [Google Scholar] [CrossRef]
- Chen, Y.; Woo, H.J.; Fadzil, S.A.F.S.M.; Tan, W.; Wang, F.; Arof, A.K.M. A Review on the Development of Solid-State Polymer Electrolytes for Sodium-Ion Batteries. ACS Appl. Nano Mater. 2022, 5, 4833–4840. [Google Scholar] [CrossRef]
- Kim, H. Sodium-Ion Battery: Can It Compete with Li-Ion? ACS Mater. Au 2023, 6, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, K.; Li, X.; Yu, R.; Zhang, X.; Huang, Y.; Chen, G.; Jamil, S.; Cao, S.; Xie, X. Improved cycle and air stability of P3-Na0.65Mn0.75Ni0.25O2 electrode for sodium-ion batteries coated with metal phosphates. Chem. Eng. J. 2019, 372, 1066–1076. [Google Scholar] [CrossRef]
- Kanwade, A.; Gupta, S.; Kankane, A.; Tiwari, M.K.; Srivastava, A.; Satrughna, J.A.K.; Yadav, S.C.; Shirage, P.M. Transition metal oxides as a cathode for indispensable Na-ion batteries. RSC Adv. 2022, 12, 23284–23310. [Google Scholar] [CrossRef]
- Mhaske, V.P.; Jilkar, S.; Yadav, M.D. Minireview on layered transition metal oxides synthesis using coprecipitation for sodium ion batteries cathode material: Advances and perspectives. Energy Fuels 2023, 37, 16221–16244. [Google Scholar] [CrossRef]
- Mathiyalagan, K.; Shin, D.; Lee, Y.-C. Recent progress in cathode materials for sodium-ion batteries: A review. J. Energy Chem. 2024, 90, 40–57. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kajiyama, M.; Iwatate, J.; Nishikawa, H.; Hitomi, S.; Okuyama, R.; Usui, R.; Yamada, Y.; Komaba, S. P2-type Nax[Fe1/2Mn1/2]O2 made from earth-abundant elements for rechargeable Na batteries. Nat. Mater. 2012, 11, 512–517. [Google Scholar] [CrossRef]
- Ni, Q.; Bai, Y.; Wu, F.; Wu, C. Polyanion-Type Electrode Materials for Sodium-Ion Batteries. Adv. Sci. 2017, 4, 16002.75. [Google Scholar] [CrossRef]
- Yoshida, H.; Yabuuchi, N.; Komaba, S. NaFe0.5Co0.5O2 as high energy and power positive electrode for Na-ion batteries. Electrochem. Commun. 2013, 34, 60–63. [Google Scholar] [CrossRef]
- Zhao, C.; Yao, Z.; Wang, Q.; Li, H.; Wang, J.; Liu, M.; Ganapathy, S.; Lu, Y.; Cabana, J.; Li, B. Revealing High Na-Content P2-Type Layered Oxides as Advanced Sodium-Ion Cathodes. J. Am. Chem. Soc. 2020, 142, 5742–5750. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Alvarado, J.; Xu, J.; Cle, R.L.J.; Kodur, M.; Tong, W.; Grey, C.P.; Meng, Y.S. Exploring Oxygen Activity in the High Energy P2-Type Na0.78Ni0.23Mn0.69O2 Cathode Material for Na-Ion Batteries. J. Am. Chem. Soc. 2017, 139, 4835–4845. [Google Scholar] [CrossRef]
- Lee, D.H.; Xu, J.; Meng, Y.S. An advanced cathode for Na-ion batteries with high rate and excellent structural stability. Phys. Chem. Chem. Phys. 2013, 15, 3304–3312. [Google Scholar] [CrossRef]
- De Ilarduya, J.M.; Otaegui, L.; del Amo, J.M.L.; Armand, M.; Singh, G. NaN3 addition, a strategy to overcome the problem of sodium deficiency in P2-Na0.67[Fe0.5Mn0.5]O2 cathode for sodium-ion battery. J. Power Sources 2017, 337, 197–203. [Google Scholar] [CrossRef]
- Palaniyandy, N. Recent progress of nanotechnology in the research framework of all-solid-state sodium-ion batteries. Curr. Opin. Electrochem. 2020, 21, 319–326. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, J.; Lee, D.H.; Dimov, N.; Meng, Y.S.; Okada, S. Electrochemical and thermal properties of P2-type Na2/3Fe1/3Mn2/3O2 for Na-ion batteries. J. Power Sources 2014, 264, 235–239. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Cation–Anion Co-Redox Induced High-Capacity Cathode for High-Energy and Safe Sodium-Ion Batteries. J. Phys. Chem. C 2018, 122, 13500–13507. [Google Scholar] [CrossRef]
- Yue, J.-L.; Zhou, Y.-N.; Yu, X.; Bak, S.-M.; Yang, X.-Q.; Fu, Z.-W. O3-type layered transition metal oxide Na(NiCoFeTi)1/4O2 as a high rate and long cycle life cathode material for sodium ion batteries. J. Mater. Chem. A 2015, 3, 23261–23267. [Google Scholar] [CrossRef]
- Vassilaras, P.; Kwon, D.-H.; Dacek, S.T.; Shi, T.; Seo, D.-H.; Ceder, G.; Kim, J.C. The role of cation ordering in the electrochemical performance of layered Na(Ni1/3Mn1/3Fe1/3)O2 cathodes for sodium-ion batteries. J. Mater. Chem. A 2017, 5, 4596–4606. [Google Scholar] [CrossRef]
- Kumar, B.S.; Pradeep, A.; Dutta, A.; Mukhopadhyay, A. Water-stable O3-type layered Na transition metal oxides enabling environment friendly ‘aqueous processing’ of electrodes with long-term electrochemical stability. J. Mater. Chem. A 2020, 8, 18064–18078. [Google Scholar] [CrossRef]
- Jin, T.; Li, H.; Zhu, K.; Wang, P.-F.; Liu, P.; Jiao, L. Polyanion-type cathode materials for sodium-ion batteries. Chem. Soc. Rev. 2020, 49, 2342–2377. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Yang, Y. Recent advances in the research of polyanion-type cathode materials for Li-ion batteries. Energy Environ. Sci. 2011, 4, 3223–3242. [Google Scholar] [CrossRef]
- Li, H.; Xu, M.; Zhang, Z.; Lai, Y.; Ma, J. Engineering of polyanion type cathode materials for sodium-ion batteries: Toward higher energy/power density. Adv. Funct. Mater. 2020, 30, 2000473. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Matsumoto, K.; Nohira, T.; Ding, C.; Yamamoto, T.; Hagiwara, R. Charge–discharge behavior of a Na2FeP2O7 positive electrode in an ionic liquid electrolyte between 253 and 363 K. Electrochim. Acta 2014, 133, 583–588. [Google Scholar] [CrossRef]
- Kee, Y.; Dimov, N.; Staikov, A.; Barpanda, P.; Lu, Y.-C.; Minami, K.; Okada, S. Insight into the limited electrochemical activity of NaVP2O7. RSC Adv. 2015, 5, 64991–64996. [Google Scholar] [CrossRef]
- Park, C.S.; Kim, H.; Shakoor, R.A.; Yang, E.; Lim, S.Y.; Kahraman, R.; Jung, Y.; Choi, J.W. Anomalous manganese activation of a pyrophosphate cathode in sodium ion batteries: A combined experimental and theoretical study. J. Am. Chem. Soc. 2013, 135, 2787–2792. [Google Scholar] [CrossRef]
- Shakoor, R.A.; Park, C.S.; Raja, A.A.; Shin, J.; Kahraman, R. A mixed iron–manganese based pyrophosphate cathode, Na2Fe0.5Mn0.5P2O7, for rechargeable sodium ion batteries. Phys. Chem. Chem. Phys. 2016, 18, 3929–3935. [Google Scholar] [CrossRef]
- Erragh, F.; Boukhari, A.; Elouadi, B.; Holt, E.M. Crystal Structures of Two Allotropic Forms of Na2CoP2O7. J. Crystallogr. Spectrosc. Res. 1991, 21, 321–326. [Google Scholar] [CrossRef]
- Barpanda, P.; Lu, J.; Ye, T.; Kajiyama, M.; Chung, S.-C.; Yabuuchi, N.; Komaba, S.; Yamada, A. A layer-structured Na2CoP2O7 pyrophosphate cathode for sodium-ion batteries. RSC Adv. 2013, 3, 3857–3860. [Google Scholar] [CrossRef]
- Li, S.; Guo, J.; Ye, Z.; Zhao, X.; Wu, S.; Mi, J.-X.; Wang, C.-Z.; Gong, Z.; McDonald, M.J.; Zhu, Z.; et al. Zero-Strain Na2FeSiO4 as Novel Cathode Material for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 17233–17238. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hautier, G.; Jain, A.; Moore, C.; Kang, B.; Doe, R.; Wu, L.; Zhu, Y.; Tang, Y.; Ceder, G. Carbonophosphates: A New Family of Cathode Materials for Li-Ion Batteries Identified Computationally. Chem. Mater. 2012, 24, 2009–2016. [Google Scholar] [CrossRef]
- Zhou, S.; Barim, G.; Morgan, B.J.; Melot, B.C.; Brutchey, R.L. Influence of rotational distortions on Li+- and Na+-intercalation in anti-NASICON Fe2(MoO4)3. Chem. Mater. 2016, 28, 4492–4500. [Google Scholar] [CrossRef]
- Wu, X.; Wu, C.; Wei, C.; Hu, L.; Qian, J.; Cao, Y.; Ai, X.; Wang, J.; Yang, H. Highly Crystallized Na2CoFe(CN)6 with Suppressed Lattice Defects as Superior Cathode Material for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 5393–5399. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; You, Y.; Vinu, A.; Mai, L. NASICONs-type solid-state electrolytes: The history, physicochemical properties, and challenges. Interdiscip. Mater. 2023, 2, 91–110. [Google Scholar] [CrossRef]
- Hu, J.; Hong, Y.; Guo, M.; Hu, Y.; Tang, W.; Xu, S.; Jia, S.; Wei, B.; Liu, S.; Fan, C.; et al. Recent Advances in Carbon-Based Electrodes for Energy Storage and Conversion Systems. Energy Storage Mater. 2023, 56, 267–299. [Google Scholar] [CrossRef]
- Niitani, K.; Ushiroda, S.; Kuwata, H.; Ohata, H.N.; Shimo, Y.; Hozumi, M.; Matsunaga, T.; Nakanishi, S. Hard Carbon Anode with a Sodium Carborane Electrolyte for Fast-Charging All-Solid-State Sodium-Ion Batteries. ACS Energy Lett. 2022, 7, 145–149. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, F.; Ming, F.; Alshareef, H.N. Sodium-ion battery anodes: Status and future trends. EnergyChem 2019, 1, 100012. [Google Scholar] [CrossRef]
- Luo, W.; Jian, Z.; Xing, Z.; Wang, W.; Bommier, C.; Lerner, M.M.; Ji, X. Electrochemically Expandable Soft Carbon as Anodes for Na-Ion Batteries. ACS Cent. Sci. 2015, 1, 516–522. [Google Scholar] [CrossRef]
- Zheng, Y.; Pan, Q.; Clites, M.; Byles, B.W.; Pomerantseva, E.; Li, C.Y. High-Capacity All-Solid-State Sodium Metal Battery with Hybrid Polymer Electrolytes. Adv. Energy Mater. 2018, 8, 1801885. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Seo, D.-H.; Shi, T.; Chen, S.; Tian, Y.; Kim, H.; Ceder, G. A High-Energy NASICON-Type Cathode Material for Na-Ion Batteries. Adv. Energy Mater. 2020, 10, 1903968. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Liu, S.; Tao, Z.; Chen, J. A Novel PMA/PEG-Based Composite Polymer Electrolyte for All-Solid-State Sodium-Ion Batteries. Nano Res. 2018, 11, 6244–6251. [Google Scholar] [CrossRef]
- Sångeland, C.; Mogensen, R.; Brandell, D.; Mindemark, J. Stable Cycling of Sodium Metal All-Solid-State Batteries with Polycarbonate-Based Polymer Electrolytes. ACS Appl. Polym. Mater. 2019, 1, 825–832. [Google Scholar] [CrossRef]
- Yi, S.; Yang, Z.; He, S.; Wang, Z.; Chen, Y.; Chen, L. Bond order control in sulfur-based electrolyte additive for low temperatures lithium metal batteries. J. Alloys Compd. 2025, 1022, 179846. [Google Scholar] [CrossRef]
- Chen, S.; Che, H.; Feng, F.; Liao, J.; Wang, H.; Yin, Y.; Ma, Z.-F. Poly(vinylene carbonate)-Based Composite Polymer Electrolyte with Enhanced Interfacial Stability to Realize High-Performance Room-Temperature Solid-State Sodium Batteries. ACS Appl. Mater. Interfaces 2019, 11, 43056–43065. [Google Scholar] [CrossRef]
- Sångeland, C.; Younesi, R.; Mindemark, J.; Brandell, D. Towards room temperature operation of all-solid-state Na-ion batteries through polyester–polycarbonate-based polymer electrolytes. Energy Storage Mater. 2019, 19, 31–38. [Google Scholar] [CrossRef]
- Law, H.M.; Yu, J.; Kwok, S.C.; Zhou, G.; Robson, M.J.; Wu, J.; Ciucci, F. A hybrid dual-salt polymer electrolyte for sodium metal batteries with stable room temperature cycling performance. Energy Storage Mater. 2022, 46, 182–191. [Google Scholar] [CrossRef]
- Tian, Z.; Zou, Y.; Liu, G.; Wang, Y.; Yin, J.; Ming, J.; Alshareef, H.N. Electrolyte Solvation Structure Design for Sodium Ion Batteries. Adv. Sci. 2022, 9, 2201207. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, W.; Ni, X.; Yu, H.; Zhang, C.; Wang, B.; Jiang, L.; He, H.; Chen, Y.; Chen, L. Steering Interfacial Renovation with Highly Electronegative Cl Modulated Trinity Effect for Exceptional Durable Zinc Anode. Adv. Funct. Mater. 2024, 34, 2404219. [Google Scholar] [CrossRef]
- Xiao, S.; Ren, L.; Liu, W.; Zhang, L.; Wang, Q. High-voltage polymer electrolytes: Challenges and progress. Energy Storage Mater. 2023, 63, 102970. [Google Scholar] [CrossRef]
- Komaba, S.; Hasegawa, T.; Dahbi, M.; Kubota, K. Potassium intercalation into graphite to realize high-voltage/high-power potassium-ion batteries and potassium-ion capacitors. Electrochem. Commun. 2015, 60, 172–175. [Google Scholar] [CrossRef]
- Hosaka, T.; Kubota, K.; Hameed, A.S.; Komaba, S. Research Development on K-Ion Batteries. Chem. Rev. 2020, 120, 6358–6466. [Google Scholar] [CrossRef] [PubMed]
- Madhani, V.; Kumar, D.; Kanchan, D.K.; Rathore, M.S. Recent advances and prospects of K-ion conducting polymer electrolytes. J. Electroanal. Chem. 2023, 935, 117334. [Google Scholar] [CrossRef]
- Tatara, R.; Ishihara, K.; Kosugi, M.; Aoki, K.; Takei, Y.; Matsui, T.; Takayama, T.; Komaba, S. Application of Potassium Ion Conducting KTiOPO4 as Effective Inner Solid-Contact Layer in All-Solid-State Potassium Ion-Selective Electrode. J. Electrochem. Soc. 2023, 170, 027507. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, P.; Zheng, J.; Chen, X.; Chen, X.-M.; Li, S.; Ji, C.; Wu, Y.; Chen, X. KB3H8·NH3B3H7 Complex as a Potential Solid-State Electrolyte with Excellent Stability against K Metal. ACS Appl. Mater. Interfaces 2022, 14, 17378–17387. [Google Scholar] [CrossRef]
- Chauhan, A.K.; Mishra, K.; Kumar, D.; Singh, A. Enhancing Sodium Ion Transport in a PEO-Based Solid Polymer Electrolyte System with NaAlO2 Active Fillers. J. Electron. Mater. 2021, 50, 5122–5133. [Google Scholar] [CrossRef]
- Niu, W.; Chen, L.; Liu, Y.; Fan, L.-Z. All-solid-state sodium batteries enabled by flexible composite electrolytes and plastic-crystal interphase. Chem. Eng. J. 2020, 384, 123233. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, Y.; Liang, X.; Lei, Y.; Yuan, T.; Lu, H.; Liu, Z.; Cao, Y.; Feng, J. Novel Sodium–poly(tartaric acid) borate-based single-ion conducting polymer electrolyte for sodium–metal batteries. ACS Appl. Energy Mater. 2020, 3, 10053–10060. [Google Scholar] [CrossRef]
- Aziam, H.; Larhrib, B.; Hakim, C.; Sabi, N.; Youcef, H.B.; Saadoune, I. Solid-state electrolytes for beyond lithium-ion batteries: A review. Renew. Sustain. Energy Rev. 2022, 167, 112694. [Google Scholar] [CrossRef]
- Hamada, M.; Tatara, R.; Kubota, K.; Kumakura, S.; Komaba, S. All-Solid-State Potassium Polymer Batteries Enabled by the Effective Pretreatment of Potassium Metal. ACS Energy Lett. 2022, 7, 2244–2246. [Google Scholar] [CrossRef]
- Li, C.; Jiang, X.; Qi, H.; Chen, D.; You, T.; Huang, S.; Yu, H.; Huang, Y.; Rao, M.; Li, G. Interfacial dual-modulation through deoxygenation effect and tuning hydrogen-bonding environment toward highly reversible Zn metal anodes. Energy Storage Mater. 2025, 75, 104012. [Google Scholar] [CrossRef]
- Yang, H.; Wu, N. Ionic conductivity and ion transport mechanisms of solid-state lithium-ion battery electrolytes: A review. Energy Sci. Eng. 2022, 10, 1643–1671. [Google Scholar] [CrossRef]
- Kim, J.G.; Son, B.; Mukherjee, S.; Schuppert, N.; Bates, A.; Kwon, O.; Choi, M.J.; Chung, H.Y.; Park, S. A review of lithium and non-lithium based solid state batteries. J. Power Sources 2015, 282, 299–322. [Google Scholar] [CrossRef]
- Janek, J.; Zeier, W.G. Challenges in speeding up solid-state battery development. Nat. Energy 2023, 8, 230–240. [Google Scholar] [CrossRef]
- Xia, W.; Zhao, Y.; Zhao, F.; Adair, K.; Zhao, R.; Li, S.; Zou, R.; Zhao, Y.; Sun, X. Antiperovskite Electrolytes for Solid-State Batteries. Chem. Rev. 2022, 122, 3763–3819. [Google Scholar] [CrossRef]
- Zheng, J.; Fang, H.; Fan, L.; Ren, Y.; Jena, P.; Wu, Y. Antiperovskite K3OI for K-Ion Solid State Electrolyte. J. Phys. Chem. Lett. 2021, 12, 7120–7126. [Google Scholar] [CrossRef]
- Jolly, D.S.; Perera, J.; Pu, S.D.; Melvin, D.L.R.; Adamson, P.; Bruce, P.G. High critical currents for dendrite penetration and voiding in potassium metal anode solid-state batteries. J. Solid State Electrochem. 2022, 26, 1961–1968. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, C.-Z.; Yuan, H.; Cheng, X.-B.; Huang, J.-Q.; Zhang, Q. Critical Current Density in Solid-State Lithium Metal Batteries: Mechanism, Influences, and Strategies. Adv. Funct. Mater. 2021, 31, 2009925. [Google Scholar] [CrossRef]
- Sarkar, S.; Thangadurai, V. Critical Current Densities for High-Performance All-Solid-State Li-Metal Batteries: Fundamentals, Mechanisms, Interfaces, Materials, and Applications. ACS Energy Lett. 2022, 7, 1492–1527. [Google Scholar] [CrossRef]
- Yu, H.-M.; Chen, D.-P.; Zhang, L.-J.; Huang, S.-Z.; Zhou, L.-J.; Kuang, G.-C.; Wei, W.-F.; Chen, L.-B.; Chen, Y.-J. Electrolyte engineering for optimizing anode/electrolyte interface towards superior aqueous zinc-ion batteries: A review. Trans. Nonferrous Met. Soc. China 2024, 34, 3118–3150. [Google Scholar] [CrossRef]
- Wu, J.-F.; Zhou, W.; Wang, Z.; Wang, W.-W.; Lan, X.; Yan, H.; Shi, T.; Hu, R.; Cui, X.; Xu, C.; et al. Building K–C Anode with Ultrahigh Self-Diffusion Coefficient for Solid State Potassium Metal Batteries Operating at −20 to 120 °C. Adv. Mater. 2023, 35, 2209833. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-J.; Hung, F.-Y.; He, Y.-T. Charge-Discharge Properties of Sputtered Mg Anode in Flexible All-Solid-State Mg-Ion Batteries. ACS Omega 2022, 7, 43161–43168. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Yuan, C.; Zhang, T.; Yu, X. Solid-State Electrolytes for Rechargeable Magnesium-Ion Batteries: From Structure to Mechanism. Small 2022, 18, 2106981. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, Q.; Jiang, R.; Ni, X.; You, T.; Li, C.; Deng, Y.; Xu, B.; Chen, Y.; Chen, L. Stereoisomeric engineering mediated zinc metal electrodeposition: Critical balance of solvation and adsorption capability. Adv. Powder Mater. 2025, 100276. [Google Scholar] [CrossRef]
- Pang, Y.; Zhu, Y.; Fang, F.; Sun, D.; Zheng, S. Advances in solid Mg-ion electrolytes for solid-state Mg batteries. J. Mater. Sci. Technol. 2023, 161, 136–149. [Google Scholar] [CrossRef]
- Kundu, S.; Solomatin, N.; Kraytsberg, A.; Ein-Eli, Y. MgSc2Se4 Solid Electrolyte for Rechargeable Mg Batteries: An Electric Field-Assisted All-Solid-State Synthesis. Energy Technol. 2022, 10, 2200896. [Google Scholar] [CrossRef]
- Yang, K.; Liu, D.; Qian, Z.; Jiang, D.; Wang, R. Computational Auxiliary for the Progress of Sodium-Ion Solid-State Electrolytes. ACS Nano 2021, 15, 17232–17246. [Google Scholar] [CrossRef]
- Dao, A.H.; López-Aranguren, P.; Černý, R.; Guiader, O.; Zhang, J.; Cuevas, F.; Latroche, M.; Jordy, C. Improvement of the ionic conductivity on new substituted borohydride argyrodites. Solid State Ion. 2019, 339, 114987. [Google Scholar] [CrossRef]
- Wang, M.; Wu, Y.; Qiu, M.; Li, X.; Li, C.; Li, R.; He, J.; Lin, G.; Qian, Q.; Wen, Z.; et al. Research progress in electrospinning engineering for all-solid-state electrolytes of lithium metal batteries. J. Energy Chem. 2021, 61, 253–268. [Google Scholar] [CrossRef]
- Walke, P.; Venturini, J.; Spranger, R.J.; van Wüllen, L.; Nilges, T. Fast Magnesium Conducting Electrospun Solid Polymer Electrolyte. Batter. Supercaps 2022, 5, e202200285. [Google Scholar] [CrossRef]
- Liu, F.; Cao, G.; Ban, J.; Lei, H.; Zhang, Y.; Shao, G.; Zhou, A.; Fan, L.Z.; Hu, J. Recent advances based on Mg anodes and their interfacial modulation in Mg batteries. J. Magnes. Alloys 2022, 10, 2699–2716. [Google Scholar] [CrossRef]
- Sun, Q.; Luo, S.; Huang, R.; Liu, Q.; Yan, S.; Lin, X. Insights on solid electrolytes for solid-state magnesium batteries: Progress and prospects. Energy Storage Mater. 2024, 103508. [Google Scholar] [CrossRef]
- Kondori, A.; Esmaeilirad, M.; Harzandi, A.M.; Amine, R.; Saray, M.T.; Yu, L.; Liu, T.; Wen, J.; Shan, N.; Wang, H.-H.; et al. A room temperature rechargeable Li2O-based lithium-air battery enabled by a solid electrolyte. Science 2023, 379, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Xing, Y.; Chen, N.; Li, L.; Wu, F.; Chen, R. Electrolytes for Rechargeable Lithium–Air Batteries. Angew. Chem. Int. Ed. 2020, 59, 2974–2997. [Google Scholar] [CrossRef]
- Banerjee, A.; Wang, X.; Fang, C.; Wu, E.A.; Meng, Y.S. Interfaces and Interphases in All-Solid-State Batteries with Inorganic Solid Electrolytes. Chem. Rev. 2020, 120, 6878–6933. [Google Scholar] [CrossRef]
- Choudhury, S.; Stalin, S.; Vu, D.; Warren, A.; Deng, Y.; Biswal, P.; Archer, L.A. Solid-state polymer electrolytes for high-performance lithium metal batteries. Nat. Commun. 2019, 10, 4398. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H. Composite Solid Electrolytes with NASICON-Type LATP and PVdF–HFP for Solid-State Lithium Batteries. Ind. Eng. Chem. Res. 2021, 60, 1494–1500. [Google Scholar] [CrossRef]
- Ding, Y.; Li, Y.; Wu, Z.-S. Recent advances and challenges in the design of Li–air batteries oriented solid-state electrolytes. Battery Energy 2023, 2, 20220014. [Google Scholar] [CrossRef]
- Chi, X.; Li, M.; Di, J.; Bai, P.; Song, L.; Wang, X.; Li, F.; Liang, S.; Xu, J.; Yu, J. A highly stable and flexible zeolite electrolyte solid-state Li–air battery. Nature 2021, 592, 551–557. [Google Scholar] [CrossRef]
- Wang, Y.; Sui, Y.; Xiao, Z.; Fei, W.; Deng, M.; Zhang, X.; Guo, H.; Wang, R.; Wu, L. Inner/Interface Engineered Iron/Manganese-Based Mixed Phosphate Cathode with High Energy Density and Ultra-Long Cycle Life for Sodium-Ion Batteries. Adv. Funct. Mater. 2025, 2500290. [Google Scholar] [CrossRef]
- Yan, B.; Sun, H.; Liu, X.; Fu, X.; Xu, C.; Zhang, T.; Tao, H.; Zhang, L.; Li, X.; Yang, X. Confining hollow ZnSe/NiSe microspheres in freestanding carbon nanofibers for flexible potassium-ion batteries. Energy Environ. Sci. 2024, 17, 3419–3432. [Google Scholar] [CrossRef]
- Wang, J.; Liu, F.; Qin, G.; He, X. Conformational gearing of black phosphorus anode via biomimetic adaptive mechanism for fast charging and low-temperature adaptability in potassium batteries. Energy Storage Mater. 2025, 104101. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Wang, X.; Xia, X.; Xie, D.; Gu, C.; Tu, J. Original growth mechanism for ultra-stable dendrite-free potassium metal electrode. Nano Energy 2019, 62, 367–375. [Google Scholar] [CrossRef]
- Piernas-Muñoz, M.J.; Zarrabeitia, M. Revisiting Intercalation Anode Materials for Potassium-Ion Batteries. Materials 2025, 18, 190. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Alahakoon, S.H.; Adair, K.; Zhang, S.; Xia, W.; Li, W.; Yu, C.; Feng, R.; Hu, Y.; Liang, J. An Air-Stable and Li-Metal-Compatible Glass-Ceramic Electrolyte enabling High-Performance All-Solid-State Li Metal Batteries. Adv. Mater. 2021, 33, 2006577. [Google Scholar] [CrossRef]
- Tan, H.; Wang, Y.; Liu, Y.; Zhang, C.; Xu, Y. Interface Stability and Dendrite Suppression in Potassium Metal Batteries with Solid Electrolytes. Adv. Func. Mater. 2025, 35, 2402163. [Google Scholar]
- Cao, K.; Ma, J.; Yue, Z.; Li, H.; Fan, Y.; Liu, H. Status and Challenges of Solid-State Electrolytes for Potassium Batteries. Small 2025, 21, 2300762. [Google Scholar] [CrossRef]
- Zhao-Karger, Z.; Fichtner, M. Beyond Intercalation Chemistry for Rechargeable Mg Batteries: A Short Review and Perspective. Front. Chem. 2019, 6, 656. [Google Scholar] [CrossRef]
- Jaschin, P.W.; Gao, Y.; Li, Y.; Bo, S. A Materials Perspective on Magnesium-Ion-Based Solid-State Electrolytes. J. Mater. Chem. A 2020, 8, 2875–2897. [Google Scholar] [CrossRef]
- Wang, Q.; Li, H.; Xu, T.; Chen, Y.; Yan, Y. Understanding Mg-ion Deposition Behavior on MgBi Alloy in Solid-State Form. Energy Mater. 2025, 5, 500022. [Google Scholar] [CrossRef]
- Abed, A.M.; Kumar, A.; Jain, V.; Yajid, M.S.A.; Chahar, M.; Raju, G.S.; Rusho, M.A.; Alkahtani, H.M. A C2B Two-Dimensional Monolayer with Superior Electrochemical Performance of Anode for Mg-Ion Batteries. Solid State Sci. 2025, 107857. [Google Scholar] [CrossRef]
- Jaafreh, R.; Kim, J.-G.; Hamad, K. Phonon DOS-Based Machine Learning Model for Designing High-Performance Solid Electrolytes in Li-Ion Batteries. J. Power Sources 2024, 606, 234575. [Google Scholar] [CrossRef]
- Helen, P.A.; Selvin, P.C.; Sakthivel, P. Quasi-solid-state plasticized chitosan biopolymer electrolyte with enhanced Mg2+ ion mobility for next-generation Mg ion battery. J. Solid State Electrochem. 2024, 28, 3147–3161. [Google Scholar] [CrossRef]
- Pang, Y.; Nie, Z.; Xu, F.; Sun, L.; Yang, J.; Sun, D.; Fang, F.; Zheng, S. Borohydride Ammoniate Solid Electrolyte Design for All-Solid-State Mg Batteries. Energy Environ. Mater. 2024, 7, e12527. [Google Scholar] [CrossRef]
- Marlton, F.P.; Yang, F.Z.; Everett, S.M.; Neuefeind, J.; Schmid, S. Understanding the influence of local structure distortions on Na-ion migration in perovskite solid electrolytes. J. Power Sources 2024, 617, 235154. [Google Scholar] [CrossRef]
- Luo, X.; Aguey-Zinsou, K.-F. NaBH4-Poly(Ethylene Oxide) Composite Electrolyte for All-Solid-State Na-Ion Batteries. Batteries 2024, 10, 316. [Google Scholar] [CrossRef]
- Wang, L.; Hao, J.; Su, G.; Yao, D.; Zhang, Y.; Liang, L.; Hou, L.; Yuan, C. Multi-Level Engineering from Surface to Bulk Enabling Highly Stable Ni-Rich O3-Type Cathode toward Temperature-Tolerant Quasi-Solid-State Na-Ion Batteries. Small 2025, 21, 2412537. [Google Scholar] [CrossRef]
- Cai, T.; Cai, M.; Mu, J.; Zhao, S.; Bi, H.; Zhao, W.; Dong, W.; Huang, F. High-Entropy Layered Oxide Cathode Enabling High-Rate for Solid-State Sodium-Ion Batteries. Nano-Micro Lett. 2024, 16, 10. [Google Scholar] [CrossRef]
- Thirupathi, R.; Sharma, S.; Bhattacharyya, S.; Omar, S. Tuning the cathode/solid electrolyte interface for high-performance solid-state Na-ion batteries. J. Am. Ceram. Soc. 2024, 107, 8328–8341. [Google Scholar] [CrossRef]
- Panigrahi, R.; Mallik, B.S. Mg-doped cathodic properties and solid-state ionic conduction in P2-type layered material for Na-ion batteries and supercapacitors. New J. Chem. 2024, 48, 2063–2072. [Google Scholar] [CrossRef]
- Wang, W.; Ding, M.; Chen, S.; Weng, J.; Zhang, P.; Yuan, W.; Bi, A.; Zhou, P. A novel composite solid electrolyte with ultrahigh ion transference number and stability for solid-state sodium metal batteries. Chem. Eng. J. 2024, 491, 151989. [Google Scholar] [CrossRef]
- Jin, M.; Xu, D.; Su, Z.; He, Z.; Chen, X.; Wu, R.; Guo, Y. A Practical Nonflammable Na4B36H34-Based Hydroborate Electrolyte for High-Voltage All-Solid-State Sodium Batteries. ACS Energy Lett. 2024, 9, 1176–1183. [Google Scholar] [CrossRef]
- Fu, C.; Li, Y.; Xu, W.; Feng, X.; Gu, W.; Liu, J.; Deng, W.; Wang, W.; Abeykoon, A.M.; Su, L. LaCl3-based sodium halide solid electrolytes with high ionic conductivity for all-solid-state batteries. Nat. Commun. 2024, 15, 4315. [Google Scholar] [CrossRef]
- Sung, J.; Heo, J.; Kim, D.-H.; Jo, S.; Ha, Y.-C.; Kim, D.; Ahn, S.; Park, J.-W. Recent advances in all-solid-state batteries for commercialization. Mater. Chem. Front. 2024, 8, 1861–1887. [Google Scholar] [CrossRef]
- Wu, M.; Liu, X.; Liu, H.; Li, D.; Qi, X.; Zeng, J.; Gao, L.; Nan, C.-W.; Fan, L.-Z. Fluorinated amorphous halides with improved ionic conduction and interfacial stability for all-solid-state sodium-ion batteries. Nat. Commun. 2025, 16, 2808. [Google Scholar] [CrossRef]
- Hu, L.; Li, H.; Chen, F.; Liu, Y.; Wang, J.; Ma, C. Surface-to-bulk engineering with high-valence W6+ enabling stabilized single-crystal LiNi0.9Co0.05Mn0.05O2 cathode. J. Energy Chem. 2024, 95, 1–8. [Google Scholar] [CrossRef]
- Dong, Z.L.; Gan, Y.; Martins, V.; Wang, X.; Fu, B.; Jin, E.; Gao, Y.; Hu, Y.; Lin, X.; Yuan, Y. Novel Sulfide–Chloride Solid-State Electrolytes with Tunable Anion Chemistry for High-Performance Sodium-Ion Batteries. Adv. Mater. 2025, 2503107. [Google Scholar] [CrossRef]
- Rosen, M.; Mahioui, S.; Schwab, C.; Dück, G.; Finsterbusch, M. Tape Casting of NASICON-Based Separators with High Conductivity for Na All-Solid-State Batteries. Electrochem 2025, 6, 5. [Google Scholar] [CrossRef]
- He, M.; Liu, S.; Wu, J.; Zhu, J. Review of cathode materials for sodium-ion batteries. Prog. Solid State Chem. 2024, 100452. [Google Scholar] [CrossRef]
- Rizvi, S.; Aladhyani, I.; Ding, Y.; Zhang, Q. Recent advances in doping Na3Zr2Si2PO12 (NASICON) solid-state electrolyte for sodium-ion batteries. Nano Energy 2024, 110009. [Google Scholar] [CrossRef]
- Zhu, R.-J.; Li, Z.-C.; Zhang, W.; Nasu, A.; Kobayashi, H.; Matsui, M. All-Solid-State Sodium-Ion Batteries: A Leading Contender in the Next-Generation Battery Race. J. Electrochem. 2024, 30, 2. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, W.; Liu, Y.; Zhou, M.; Pan, Z.; Yang, X. Contact Ion-Pair-Dominated Electrolyte Enabling Inorganic-Rich Solid–Electrolyte Interphase for Long-Cycling Magnesium Metal Anodes. ACS Energy Lett. 2025, 10, 552–561. [Google Scholar] [CrossRef]



| Type | Material | Advantage | Disadvantage | Specific Energy Density |
|---|---|---|---|---|
| LTMO | O2- and P3-type layered structures composed of transition metals (e.g., Mn, Fe, Co) |
|
| ~100–170 Wh/kg (gravimetric) ~250–375 Wh/L (volumetric) [29,30,31,32] |
| Polyanionic compounds | Polythiophene-modified NaFePO4 and maricite-phase NaFePO4 structures |
|
| ~100–150 Wh/kg (gravimetric) ~200–300 Wh/L (volumetric) [33,34,35] |
| PBAs | Open-framework cubic structures, typically Na1.92Fe2(CN)6 with Fe(CN)6 vacancies |
|
| ~160–175 Wh/kg (gravimetric) ~50 Wh/L (volumetric) [26,27,28,32,36,37] |
| Structure | a/Å | b/Å | c/Å | β/Å | Vol/Å | Ref. | |
|---|---|---|---|---|---|---|---|
| Na2FeP2O7 | Triclinic | 6.4299 | 9.4145 | 11.0110 | 85.465 | 573.39 | [58] |
| Na2CoP2O7 | Triclinic | 9.735 | 10.940 | 12.289 | 121.76 | 566.8 | [62] |
| Orthorhombic | 15.4061 | 10.2885 | 7.7031 | - | 1221.0 | [63] | |
| Tetragonal | 7.706 | 10.301 | - | - | - | [63] | |
| Na2MnP2O7 | Triclinic | 5.316 | 6.580 | 9.409 | 95.25 | 290.96 | [58] |
| β-Na2MnP2O7 | Triclinic | 9.922 | 11.086 | 12.473 | 121.94 | 599.4 | [58] |
| α-Na2CuP2O7 | Monoclinic | 8.823 | 13.494 | 5.108 | 92.77 | 607.5 | [63] |
| β-Na2CuP2O7 | Monoclinic | 14.728 | 5.698 | 8.067 | 115.15 | 612.8 | [63] |
| Solid Polymer Electrolyte | Electrochemical Stability Window/V (vs. Na+/NA) | Ionic Conductivity | Ionic Transference Number | References |
|---|---|---|---|---|
| PEO-NaFNFSI | 4.87 | 3.36 × 10−4 at 80 °C | 0.24 | [77] |
| PTMC-NaFSI | 4.8 | 5 × 10−5 at 25 °C | 0.48 | [76] |
| PVCA-NaTf | 5.3 | 1.2 × 10−4 at 25 °C | 0.60 | [78] |
| PCL-NaFSI | - | 1.28 × 10−4 at 25 °C | 0.50 | [79] |
| NaPTAB-SGPE | 4.8 | 1.43 × 10−4 at 60 °C | 0.91 | [91] |
| Type | Materials | Advantage | Disadvantage |
|---|---|---|---|
| Na-Ion SSB |
|
|
|
| K-Ion SSB |
|
|
|
| Mg-Ion SSB |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, R.; Ma, Y.; Anduaga-Quiros, K.; Briseno, G.; Ning, Y.; Chang, H.-J.; Ozkan, M.; Ozkan, C.S. Powering the Future: Unveiling the Potential of Na, K, and Mg Solid-State Batteries. Nanomaterials 2025, 15, 859. https://doi.org/10.3390/nano15110859
Shang R, Ma Y, Anduaga-Quiros K, Briseno G, Ning Y, Chang H-J, Ozkan M, Ozkan CS. Powering the Future: Unveiling the Potential of Na, K, and Mg Solid-State Batteries. Nanomaterials. 2025; 15(11):859. https://doi.org/10.3390/nano15110859
Chicago/Turabian StyleShang, Ruoxu, Yi Ma, Kathrine Anduaga-Quiros, Gustavo Briseno, Yuying Ning, Hung-Ju Chang, Mihrimah Ozkan, and Cengiz S. Ozkan. 2025. "Powering the Future: Unveiling the Potential of Na, K, and Mg Solid-State Batteries" Nanomaterials 15, no. 11: 859. https://doi.org/10.3390/nano15110859
APA StyleShang, R., Ma, Y., Anduaga-Quiros, K., Briseno, G., Ning, Y., Chang, H.-J., Ozkan, M., & Ozkan, C. S. (2025). Powering the Future: Unveiling the Potential of Na, K, and Mg Solid-State Batteries. Nanomaterials, 15(11), 859. https://doi.org/10.3390/nano15110859








