Treatment of Dairy Industry Wastewater and Crop Irrigation Water Using AgBr-Coupled Photocatalysts
Abstract
1. Introduction
2. Materials and Methods
2.1. Photocatalytic Material Preparation
2.2. Physicochemical Analysis of AgBr-Coupled Materials
2.3. Water Sampling and Analysis
2.4. Photocatalytic Treatment of Water Sources
3. Results and Discussion
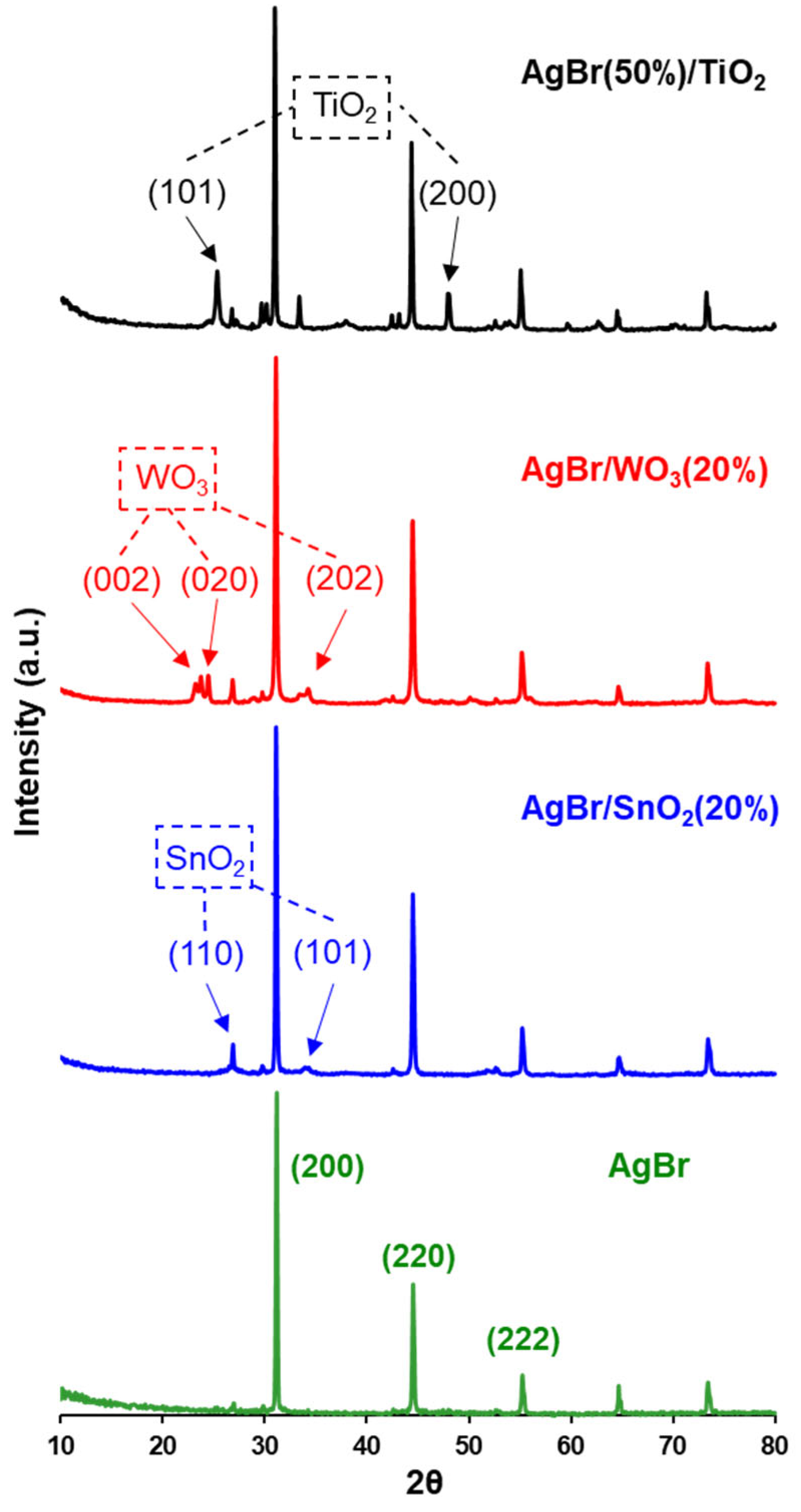
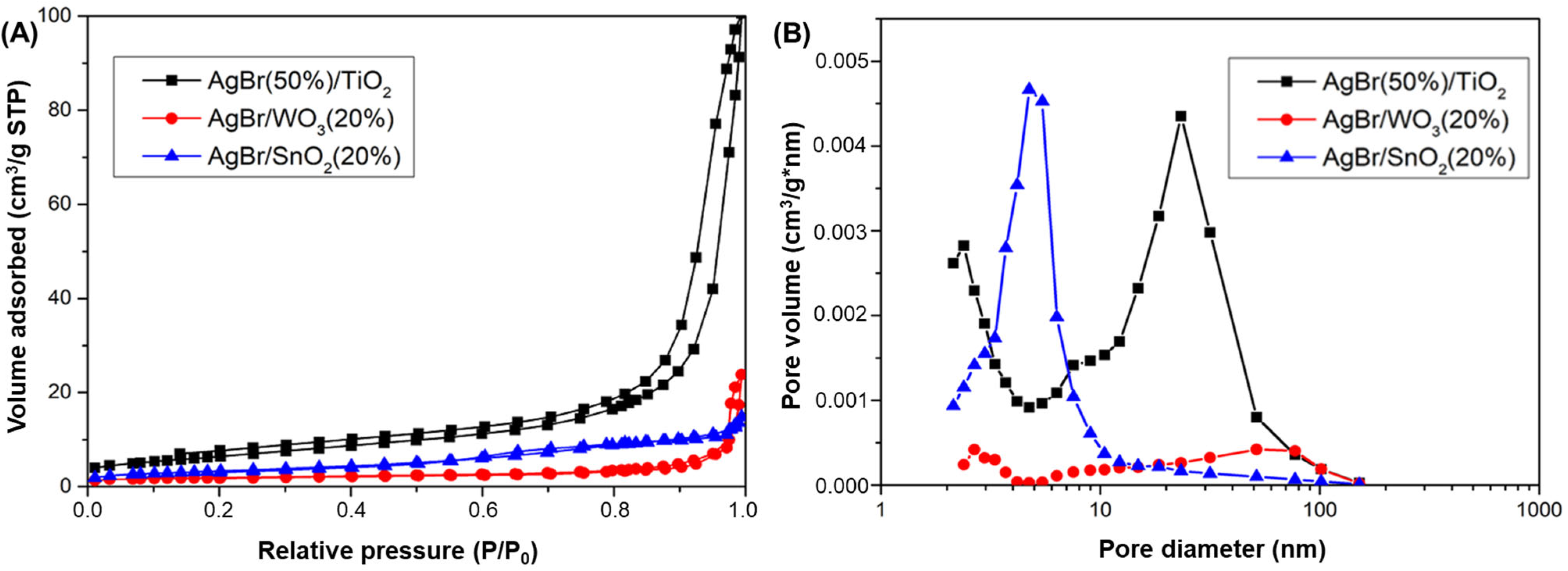
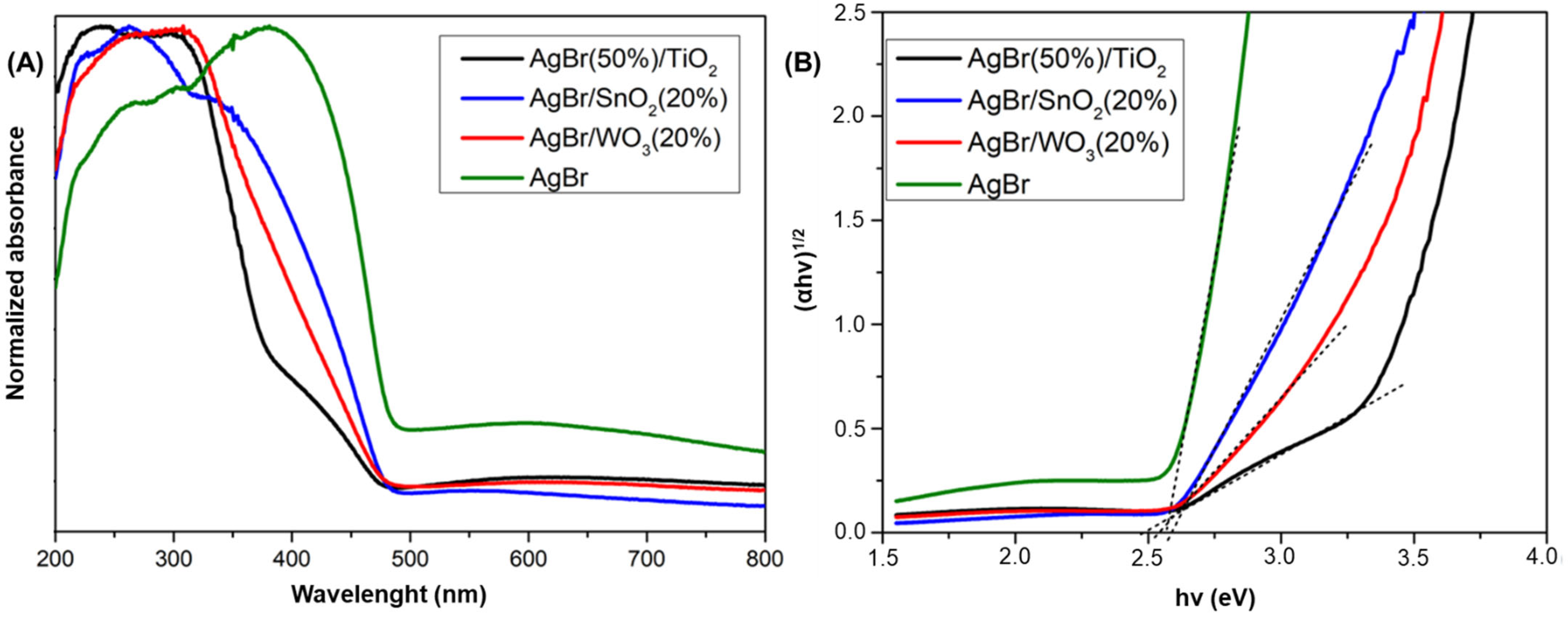
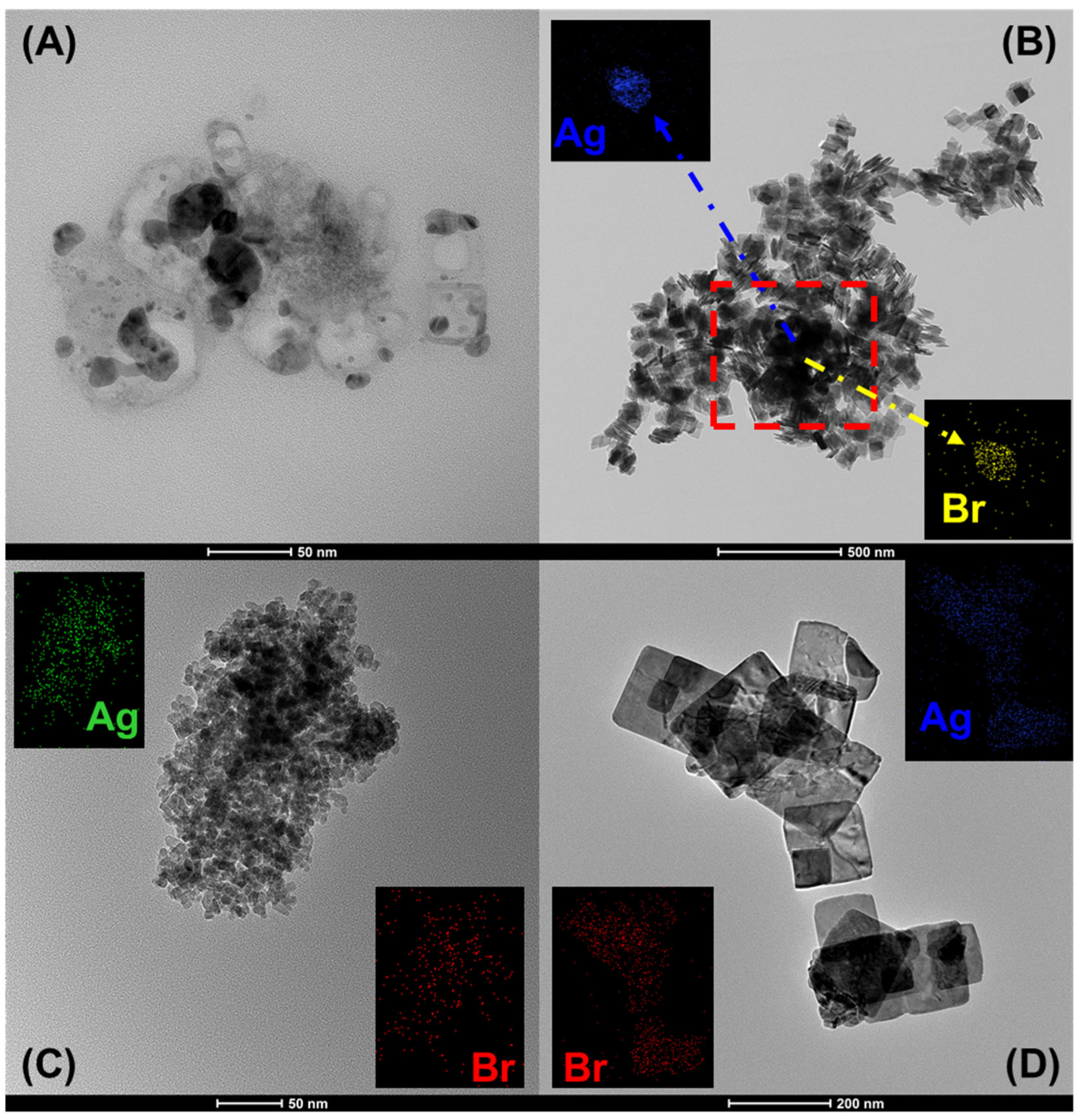
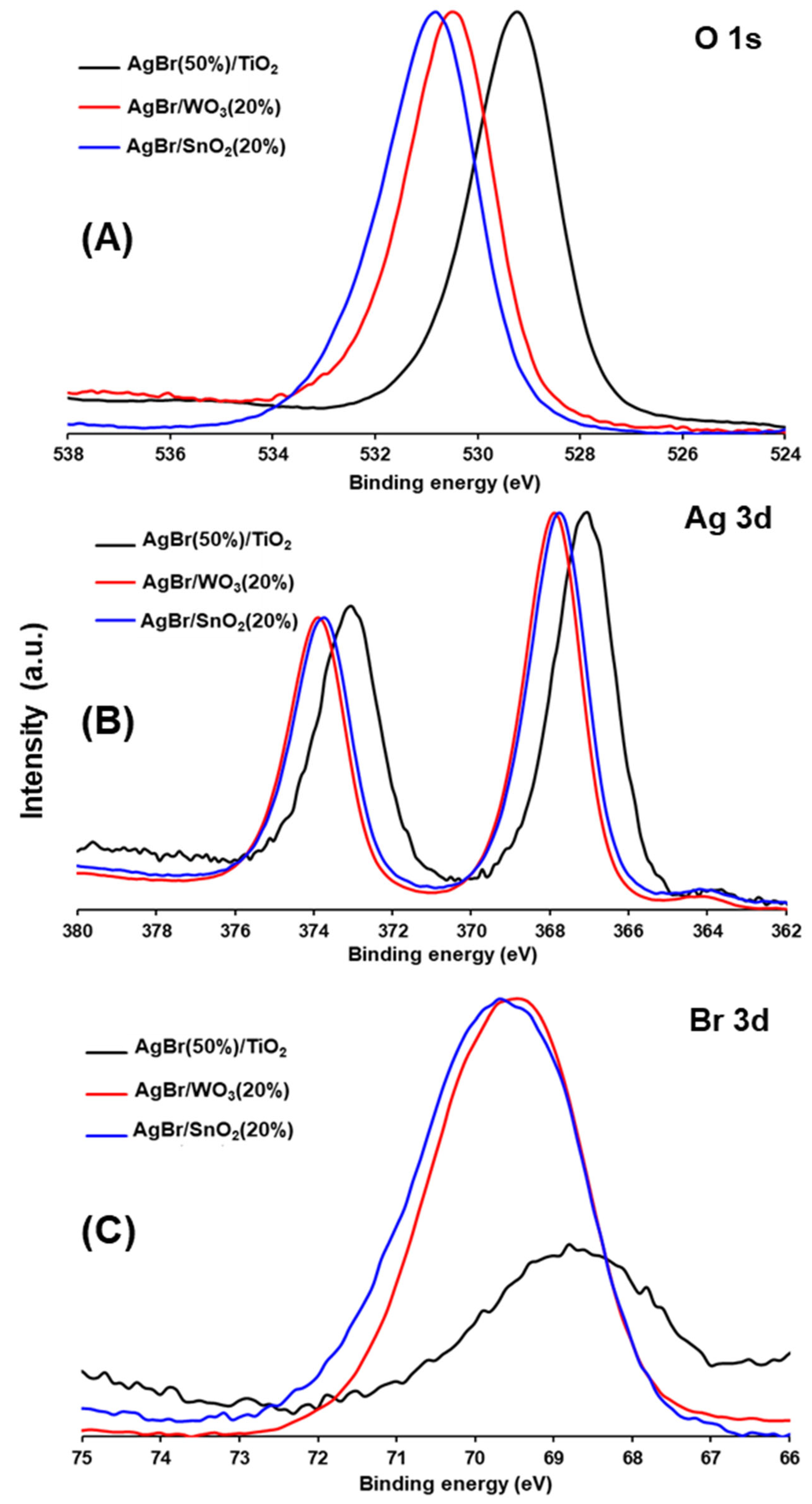
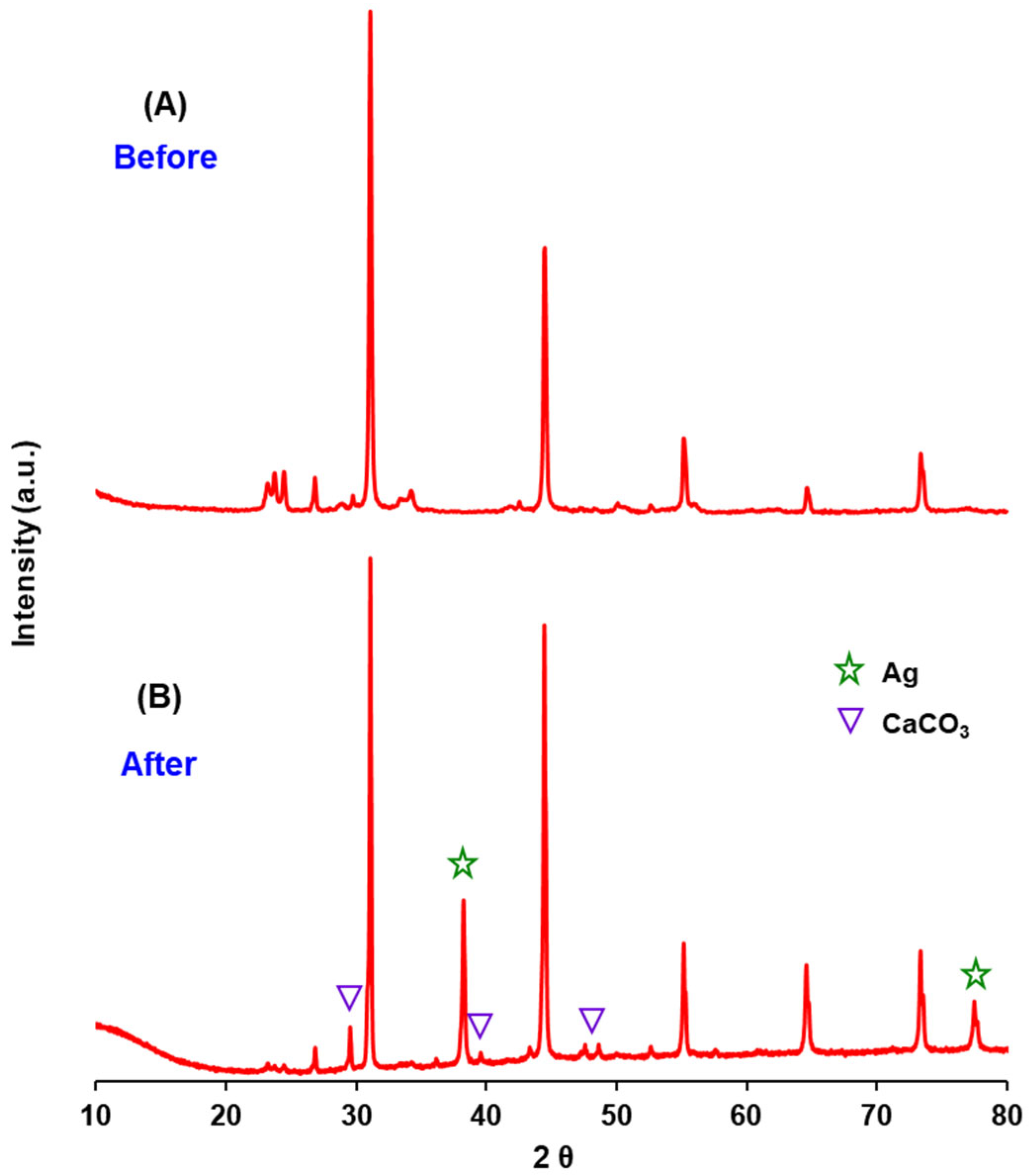
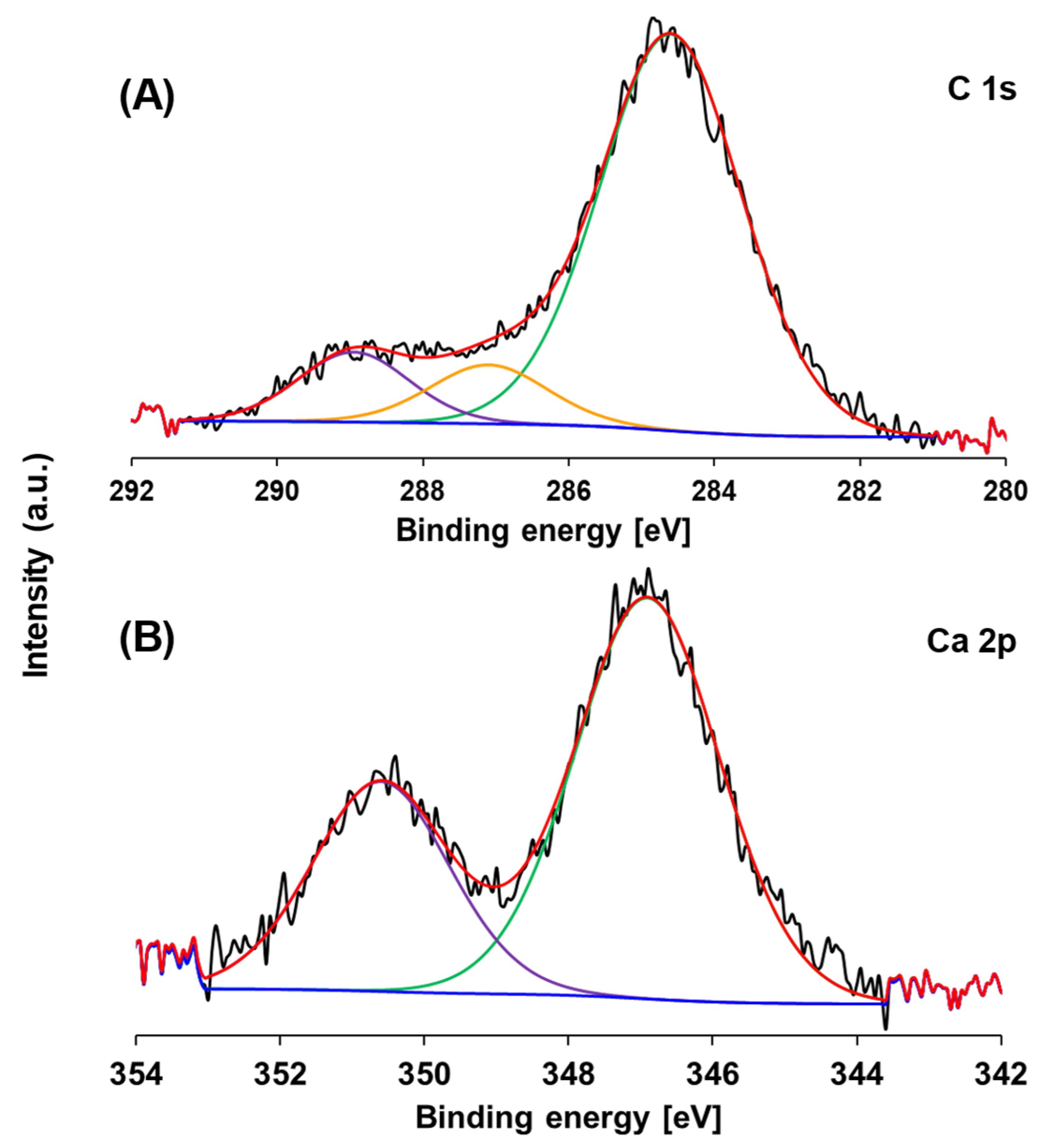
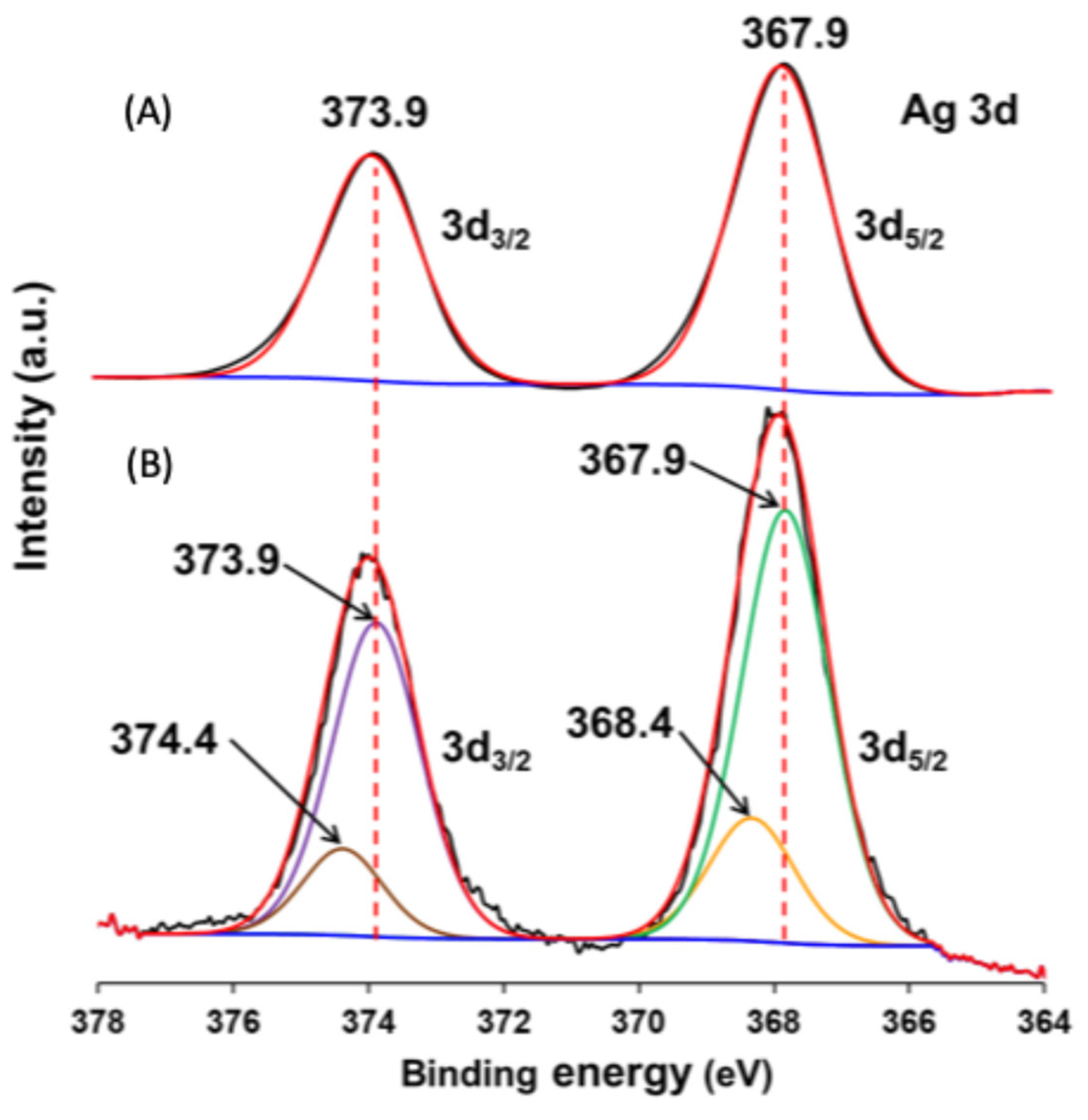
3.1. Physicochemical Properties of AgBr-Coupled Materials
3.2. Photocatalytic Treatment of Wastewater by AgBr-Coupled Photocatalysts
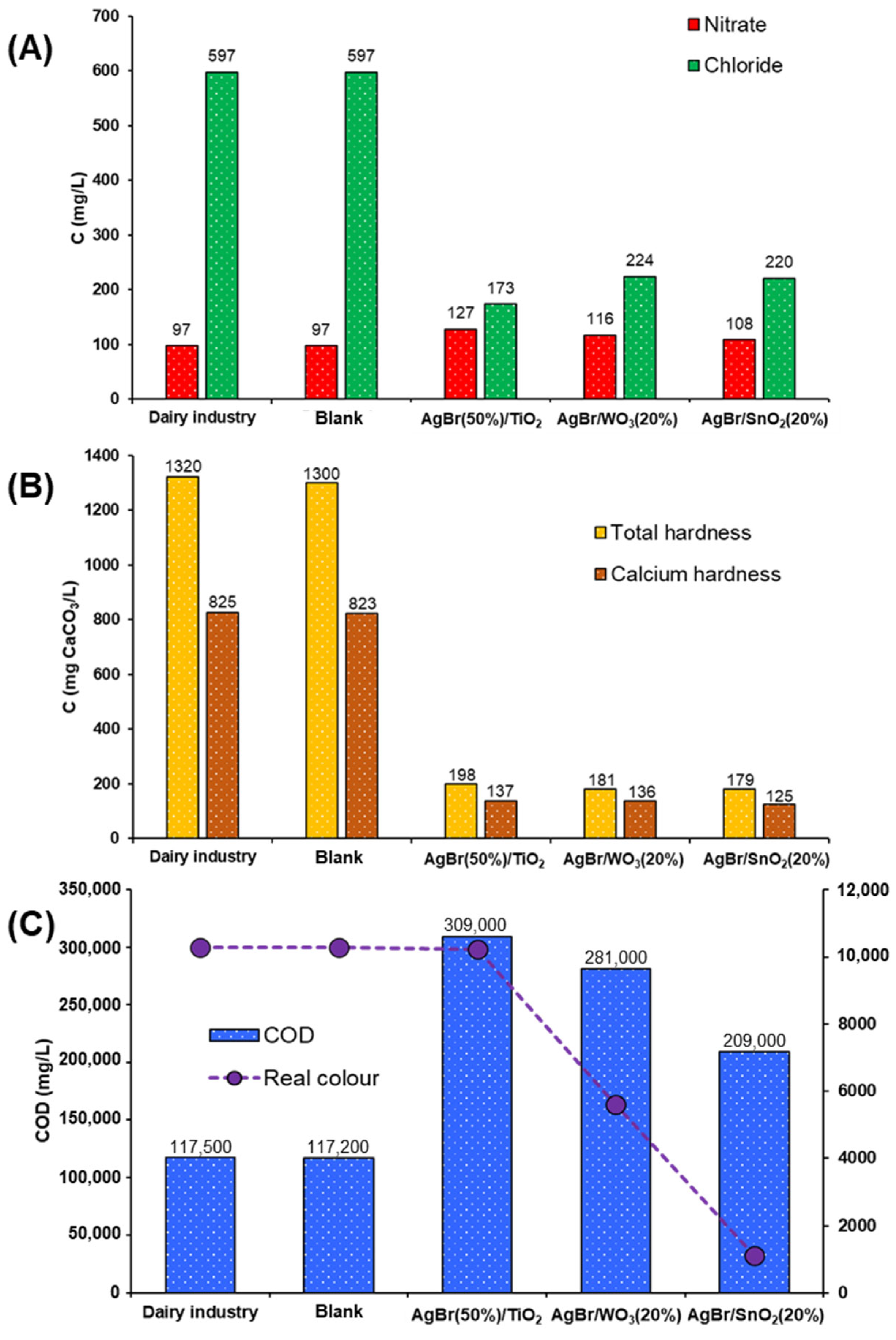
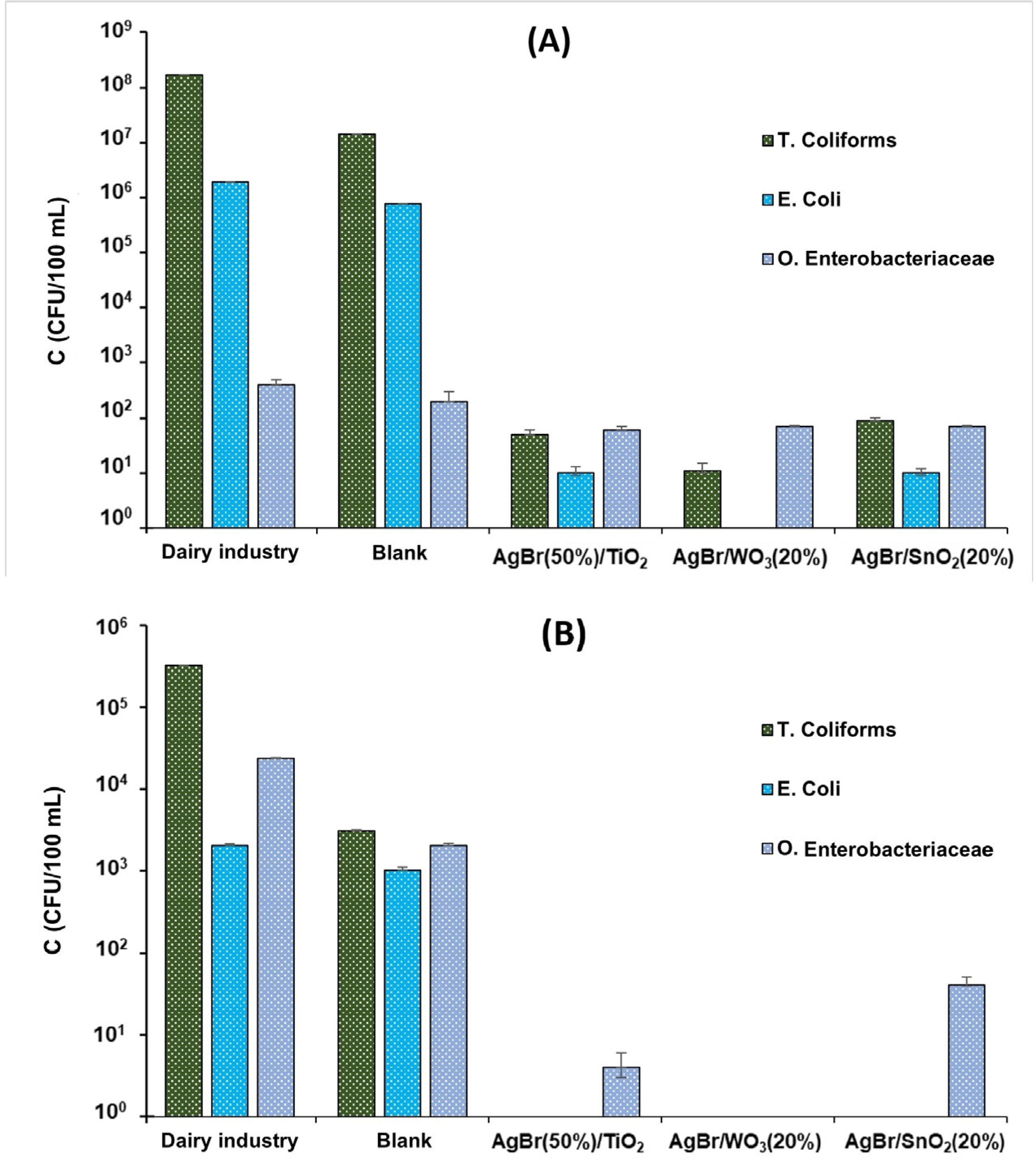
3.2.1. Dairy Industry Effluent
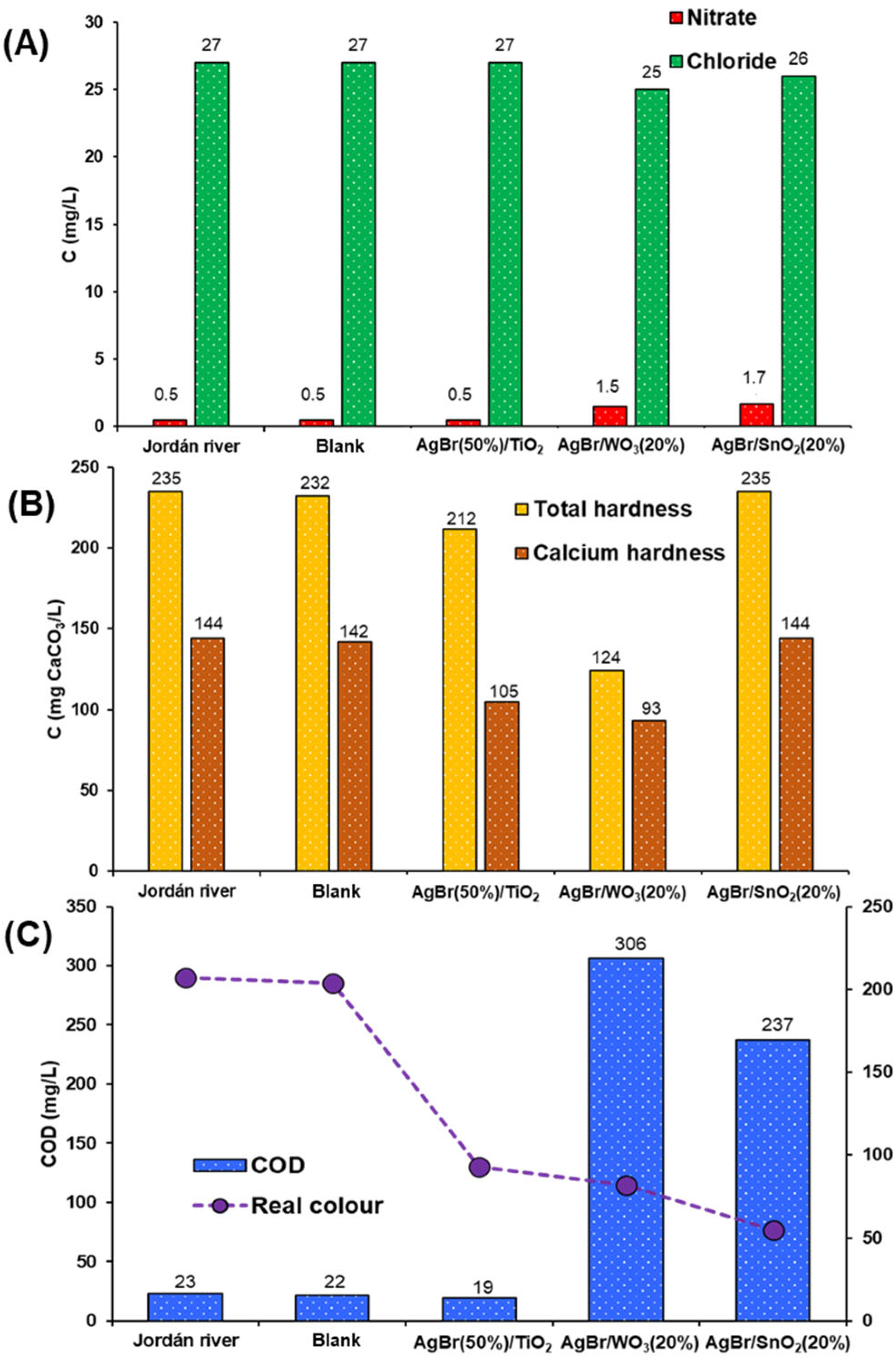
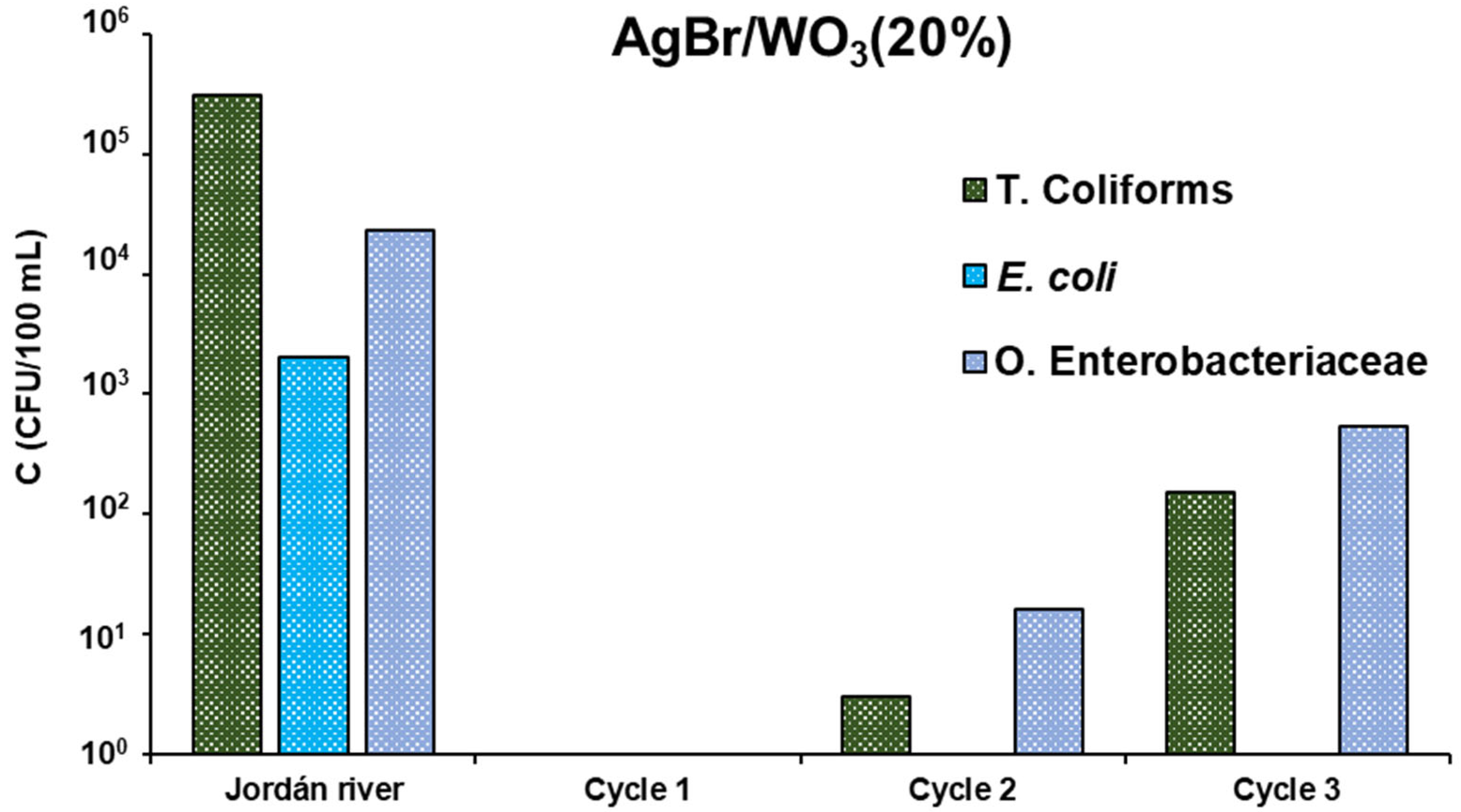
3.2.2. Jordán River
- (i)
- The water source samples are not comparable; it is because the complexity of the composition of each one is very different. On the one hand, the wastewater came from the outflow on an industrial facility, and on the other hand, the natural water sample was taken from a river highly polluted by different anthropogenic activities, with different industrial and domestic effluents, coming from towns located along the course of the river.
- (ii)
- The starting contents of chlorides and hardness are much higher in the dairy industry sample compared with the river sample.
- (iii)
- The pollutant diversity in the river water could be higher than in that from the dairy industry facility, thus leading to lower effectiveness of the treatment of the first sample.
- (iv)
- In the case of the Cl− content, the final value may be close to the maximum effectiveness of the treatment in the case of the river sample.
- (v)
- The dairy industry sample presented a COD content significantly higher than the river sample, thus indicating high organic load, which can affect behaviour under photocatalytic treatment.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, S.N.; Haider, W. Heterogeneous Photocatalysis and Its Potential Applications in Water and Wastewater Treatment: A Review. Nanotechnology 2018, 29, 342001. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Guo, Y.; Guo, R.; Liu, X. A Review of Visible Light-Active Photocatalysts for Water Disinfection: Features and Prospects. Chem. Eng. J. 2019, 373, 624–641. [Google Scholar] [CrossRef]
- Yan, H.; Deng, Y.; Shen, M.; Ye, Y.-X.; Zhu, F.; Yang, X.; Ouyang, G. Regulation the Reactive Oxygen Species on Conjugated Polymers for Highly Efficient Photocatalysis. Appl. Catal. B Environ. 2022, 314, 121488. [Google Scholar] [CrossRef]
- Lan, Y.; Hu, C.; Hu, X.; Qu, J. Efficient Destruction of Pathogenic Bacteria with AgBr/TiO2 under Visible Light Irradiation. Appl. Catal. B Environ. 2007, 73, 354–360. [Google Scholar] [CrossRef]
- Ganguly, P.; Byrne, C.; Breen, A.; Pillai, S.C. Antimicrobial Activity of Photocatalysts: Fundamentals, Mechanisms, Kinetics and Recent Advances. Appl. Catal. B Environ. 2018, 225, 51–75. [Google Scholar] [CrossRef]
- Yang, G.; Yin, H.; Liu, W.; Yang, Y.; Zou, Q.; Luo, L.; Li, H.; Huo, Y.; Li, H. Synergistic Ag/TiO2-N Photocatalytic System and Its Enhanced Antibacterial Activity towards Acinetobacter Baumannii. Appl. Catal. B Environ. 2018, 224, 175–182. [Google Scholar] [CrossRef]
- Akhavan, O. Lasting Antibacterial Activities of Ag–TiO2/Ag/a-TiO2 Nanocomposite Thin Film Photocatalysts under Solar Light Irradiation. J. Colloid Interface Sci. 2009, 336, 117–124. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Photocatalytic Reduction of Graphene Oxide Nanosheets on TiO2 Thin Film for Photoinactivation of Bacteria in Solar Light Irradiation. J. Phys. Chem. C 2009, 113, 20214–20220. [Google Scholar] [CrossRef]
- Zhang, S.; Gu, P.; Ma, R.; Luo, C.; Wen, T.; Zhao, G.; Cheng, W.; Wang, X. Recent Developments in Fabrication and Structure Regulation of Visible-Light-Driven g-C3N4-Based Photocatalysts towards Water Purification: A Critical Review. Catal. Today 2019, 335, 65–77. [Google Scholar] [CrossRef]
- Hernández-Alonso, M.D.; Fresno, F.; Suárez, S.; Coronado, J.M. Development of Alternative Photocatalysts to TiO2: Challenges and Opportunities. Energy Environ. Sci. 2009, 2, 1231–1257. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, S.-J. TiO2 Photocatalyst for Water Treatment Applications. J. Ind. Eng. Chem. 2013, 19, 1761–1769. [Google Scholar] [CrossRef]
- Iervolino, G.; Zammit, I.; Vaiano, V.; Rizzo, L. Limitations and Prospects for Wastewater Treatment by UV and Visible-Light-Active Heterogeneous Photocatalysis: A Critical Review. Top. Curr. Chem. 2019, 378, 7. [Google Scholar] [CrossRef]
- Parul; Kaur, K.; Badru, R.; Singh, P.P.; Kaushal, S. Photodegradation of Organic Pollutants Using Heterojunctions: A Review. J. Environ. Chem. Eng. 2020, 8, 103666. [Google Scholar] [CrossRef]
- Shen, J.; Shi, A.; Lu, J.; Lu, X.; Zhang, H.; Jiang, Z. Optimized fabrication of Cu-doped ZnO/calcined CoFe-LDH composite for efficient degradation of bisphenol a through synergistic visible-light photocatalysis and persulfate activation: Performance and mechanisms. Environ. Pollut. 2023, 323, 121186. [Google Scholar] [CrossRef] [PubMed]
- Van, K.N.; Huu, H.T.; Nguyen Thi, V.N.; Le Thi, T.L.; Truong, D.H.; Truong, T.T.; Dao, N.N.; Vo, V.; Tran, D.L.; Vasseghian, Y. Facile Construction of S-Scheme SnO2/g-C3N4 Photocatalyst for Improved Photoactivity. Chemosphere 2022, 289, 133120. [Google Scholar] [CrossRef] [PubMed]
- Abrishami-Rad, A.; Sadeghzadeh-Attar, A. Fe-Doped BiVO4 Photocatalyst Assisting SnO2 Nanorod Arrays for Efficient Visible-Light-Driven Degradation of Basic Red 46. J. Taiwan Inst. Chem. Eng. 2023, 151, 105110. [Google Scholar] [CrossRef]
- Tahir, M.B.; Ali, S.; Rizwan, M. A Review on Remediation of Harmful Dyes through Visible Light-Driven WO3 Photocatalytic Nanomaterials. Int. J. Environ. Sci. Technol. 2019, 16, 4975–4988. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, J.; Cao, H.; Guo, Z.; Rabeah, J.; Brückner, A.; Xie, Y. The Role of Ozone and Influence of Band Structure in WO3 Photocatalysis and Ozone Integrated Process for Pharmaceutical Wastewater Treatment. J. Hazard. Mater. 2018, 360, 481–489. [Google Scholar] [CrossRef]
- Piriyanon, J.; Takhai, P.; Patta, S.; Chankhanittha, T.; Senasu, T.; Nijpanich, S.; Juabrum, S.; Chanlek, N.; Nanan, S. Performance of Sunlight Responsive WO3/AgBr Heterojunction Photocatalyst toward Degradation of Rhodamine B Dye and Ofloxacin Antibiotic. Opt. Mater. 2021, 121, 111573. [Google Scholar] [CrossRef]
- Ma, M.; Yan, X.; Mao, Y.; Kang, H.; Yan, Q.; Chen, X.; Zhou, J.; Pan, L.; Li, Y.; Cui, L.; et al. CeVO4 Nanoparticle Coupled with Ag/AgBr as an Efficient Plasmonic Photocatalyst for Degradation of Rhodamine B: Construction, Characterization, and Mechanism Insight. J. Phys. Chem. Solids 2023, 181, 111535. [Google Scholar] [CrossRef]
- Deng, J.; Xue, R.; Huang, C.; Yang, J.; Li, L.; Yang, L.; Fan, X. Preparation of Z-Scheme Ag/AgBr/BiOBr Composite Photocatalyst for Effective Removal of Organic Pollutants. Chem. Phys. 2021, 548, 111228. [Google Scholar] [CrossRef]
- Al-Hamdi, A.M.; Rinner, U.; Sillanpää, M. Tin Dioxide as a Photocatalyst for Water Treatment: A Review. Process Saf. Environ. Prot. 2017, 107, 190–205. [Google Scholar] [CrossRef]
- Hosseinzadeh, G.; Sajjadi, S.M.; Mostafa, L.; Yousefi, A.; Vafaie, R.H.; Zinatloo-Ajabshir, S. Synthesis of Novel Direct Z-Scheme Heterojunction Photocatalyst from WO3 Nanoplates and SrTiO3 Nanoparticles with Abundant Oxygen Vacancies. Surf. Interfaces 2023, 42, 103349. [Google Scholar] [CrossRef]
- Sharma, S.; Dutta, V.; Raizada, P.; Hosseini-Bandegharaei, A.; Thakur, V.K.; Kalia, S.; Nguyen, V.-H.; Singh, P. Recent Advances in Silver Bromide-Based Z-Scheme Photocatalytic Systems for Environmental and Energy Applications: A Review. J. Environ. Chem. Eng. 2021, 9, 105157. [Google Scholar] [CrossRef]
- Zheng, H.; Li, P.; Gao, L.; Li, G. Hexagonal AgBr Crystal Plates for Efficient Photocatalysis through Two Methods of Degradation: Methyl Orange Oxidation and CrVI Reduction. RSC Adv. 2017, 7, 25725–25731. [Google Scholar] [CrossRef]
- Karim, A.V.; Krishnan, S.; Shriwastav, A. An Overview of Heterogeneous Photocatalysis for the Degradation of Organic Compounds: A Special Emphasis on Photocorrosion and Reusability. J. Indian Chem. Soc. 2022, 99, 100480. [Google Scholar] [CrossRef]
- Vaiano, V.; Matarangolo, M.; Murcia, J.J.; Rojas, H.; Navío, J.A.; Hidalgo, M.C. Enhanced Photocatalytic Removal of Phenol from Aqueous Solutions Using ZnO Modified with Ag. Appl. Catal. B Environ. 2018, 225, 197–206. [Google Scholar] [CrossRef]
- Thambiliyagodage, C. Efficient Photocatalysis of Carbon Coupled TiO2 to Degrade Pollutants in Wastewater—A Review. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100737. [Google Scholar] [CrossRef]
- Murcia, J.J.; Hernández-Laverde, M.; Rojas, H.; Muñoz, E.; Navío, J.A.; Hidalgo, M.C. Study of the Effectiveness of the Flocculation-Photocatalysis in the Treatment of Wastewater Coming from Dairy Industries. J. PhotoChem. Photobiol. A Chem. 2018, 358, 256–264. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Wu, B.; Zhou, T.; Wang, J.; Zhou, J.; Li, S.; Cao, F.; Qin, G. Preparation and Visible-Light-Driven Photocatalytic Property of AgX (X = Cl, Br, I) Nanomaterials. J. Alloys Compd. 2019, 776, 948–953. [Google Scholar] [CrossRef]
- Feng, T.; Liang, J.; Ma, Z.; Li, M.; Tong, M. Bactericidal Activity and Mechanisms of BiOBr-AgBr under Both Dark and Visible Light Irradiation Conditions. Colloids Surf. B Biointerfaces 2018, 167, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Ma, S.; Zhan, S. Superior Photocatalytic Disinfection Effect of Ag-3D Ordered Mesoporous CeO2 under Visible Light. Appl. Catal. B Environ. 2018, 224, 27–37. [Google Scholar] [CrossRef]
- Ekkachai Kanchanatip, P.K.; Neramittagapong, A. Photocatalytic Treatment of Real Liquid Effluent from Hydrothermal Carbonization of Agricultural Waste Using Metal Doped TiO2/UV System. J. Environ. Sci. Health Part A 2023, 58, 246–255. [Google Scholar] [CrossRef]
- Ahuja, T.; Brighu, U.; Saxena, K. Recent Advances in Photocatalytic Materials and Their Applications for Treatment of Wastewater: A Review. J. Water Process Eng. 2023, 53, 103759. [Google Scholar] [CrossRef]
- Motamedi, M.; Yerushalmi, L.; Haghighat, F.; Chen, Z. Recent Developments in Photocatalysis of Industrial Effluents։ A Review and Example of Phenolic Compounds Degradation. Chemosphere 2022, 296, 133688. [Google Scholar] [CrossRef]
- Puga, F.; Navío, J.A.; Hidalgo, M.C. Features of Coupled AgBr/WO3 Materials as Potential Photocatalysts. J. Alloys Compd. 2021, 867, 159191. [Google Scholar] [CrossRef]
- Puga, F.; Navío, J.A.; Hidalgo, M.C. Enhanced UV and Visible Light Photocatalytic Properties of Synthesized AgBr/SnO2 Composites. Sep. Purif. Technol. 2021, 257, 117948. [Google Scholar] [CrossRef]
- Puga, F.; Navío, J.A.; Paulete-Romero, M.A.; Córdoba, J.M.; Hidalgo, M.C. Exploring the Photocatalytic Activities of a Highly {001} Faceted TiO2 Sensitized by Coupling with AgBr or Ag3PO4. Mater. Sci. Eng. B 2022, 276, 115555. [Google Scholar] [CrossRef]
- Fariña, S.R.; Baudracco, J.; Bargo, F. Dairy Production in Diverse Regions: Latin America. In Encyclopedia of Dairy Sciences, 3rd ed.; McSweeney, P.L.H., McNamara, J.P., Eds.; Academic Press: Oxford, UK, 2022; pp. 244–252. ISBN 978-0-12-818767-8. [Google Scholar]
- Raghunath, B.V.; Punnagaiarasi, A.; Rajarajan, G.; Irshad, A.; Elango, A.; Mahesh Kumar, G. Impact of Dairy Effluent on Environment—A Review. In Integrated Waste Management in India; Environmental Science and Engineering; Springer: Cham, Switzerland, 2016; pp. 239–249. [Google Scholar]
- Rosa, D.R.; Duarte, I.C.S.; Katia Saavedra, N.; Varesche, M.B.; Zaiat, M.; Cammarota, M.C.; Freire, D.M.G. Performance and Molecular Evaluation of an Anaerobic System with Suspended Biomass for Treating Wastewater with High Fat Content after Enzymatic Hydrolysis. Bioresour. Technol. 2009, 100, 6170–6176. [Google Scholar] [CrossRef]
- Tikariha, A.; Sahu, O. Study of Characteristics and Treatments of Dairy Industry Waste Water. J. Appl. Environ. Microbiol. 2014, 2, 16–22. [Google Scholar]
- Truchado, P.; Hernandez, N.; Gil, M.I.; Ivanek, R.; Allende, A. Correlation between E. Coli Levels and the Presence of Foodborne Pathogens in Surface Irrigation Water: Establishment of a Sampling Program. Water Res. 2018, 128, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Decol, L.T.; Casarin, L.S.; Hessel, C.T.; Batista, A.C.F.; Allende, A.; Tondo, E.C. Microbial Quality of Irrigation Water Used in Leafy Green Production in Southern Brazil and Its Relationship with Produce Safety. Food Microbiol. 2017, 65, 105–113. [Google Scholar] [CrossRef]
- Venegas, C.; Diez, H.; Blanch, A.R.; Jofre, J.; Campos, C. Microbial Source Markers Assessment in the Bogotá River Basin (Colombia). J. Water Health 2015, 13, 801–810. [Google Scholar] [CrossRef]
- Onyeaka, H.N.; Nwabor, O.F. Chapter 3—Microbial Food Contamination and Foodborne Diseases. In Food Preservation and Safety of Natural Products; Onyeaka, H.N., Nwabor, O.F., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 19–37. ISBN 978-0-323-85700-0. [Google Scholar]
- da Silva Lannes, S.C. Prevalence of Foodborne Diseases in Latin America and the Caribbean. In Encyclopedia of Food Safety, 2nd ed.; Smithers, G.W., Ed.; Academic Press: Oxford, UK, 2024; pp. 104–112. ISBN 978-0-12-822520-2. [Google Scholar]
- Mohaghegh, N.; Endo-Kimura, M.; Wang, K.; Wei, Z.; Hassani Najafabadi, A.; Zehtabi, F.; Hosseinzadeh Kouchehbaghi, N.; Sharma, S.; Markowska-Szczupak, A.; Kowalska, E. Apatite-Coated Ag/AgBr/TiO2 Nanocomposites: Insights into the Antimicrobial Mechanism in the Dark and under Visible-Light Irradiation. Appl. Surf. Sci. 2023, 617, 156574. [Google Scholar] [CrossRef]
- Elahifard, M.R.; Rahimnejad, S.; Haghighi, S.; Gholami, M.R. Apatite-Coated Ag/AgBr/TiO2 Visible-Light Photocatalyst for Destruction of Bacteria. J. Am. Chem. Soc. 2007, 129, 9552–9553. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yu, Y.; Liu, Z.; Zuo, S.; Li, B. AgBr-Coupled TiO2: A Visible Heterostructured Photocatalyst for Degrading Dye Pollutants. Int. J. Photoenergy 2012, 2012, 254201. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, X.; Li, H.; Wang, K.; Gao, S.; Yin, Z.; Yu, H.; Zhu, X.; Xiong, Z.; Xie, Y. TiO2/SnO2 Double-Shelled Hollow Spheres-Highly Efficient Photocatalyst for the Degradation of Rhodamine B. Catal. Commun. 2015, 60, 129–133. [Google Scholar] [CrossRef]
- Shabanian-Broujeni, E.; Nezamzadeh-Ejhieh, A. The Coupled WO3-AgBr Nanocatalyst, Part II: Synthesis, Characterization, and the Boosted Photocatalytic Activity towards Metronidazole in an Aqueous Solution. Heliyon 2024, 10, e31353. [Google Scholar] [CrossRef]
- Fan, G.; Ning, R.; Yan, Z.; Luo, J.; Du, B.; Zhan, J.; Liu, L.; Zhang, J. Double photoelectron-transfer mechanism in Ag−AgCl/WO3/g-C3N4 photocatalyst with enhanced visible-light photocatalytic activity for trimethoprim degradation. J. Hazard. Mater. 2021, 403, 123964. [Google Scholar] [CrossRef]
- Fan, G.; Zheng, X.; Luo, J.; Peng, H.; Lin, H.; Bao, M.; Hong, L.; Zhou, J. Rapid synthesis of Ag/AgCl@ZIF-8 as a highly efficient photocatalyst for degradation of acetaminophen under visible light. Chem. Eng. J. 2018, 351, 782–790. [Google Scholar] [CrossRef]
- Liang, S.; Zhang, D.; Yao, X.; Han, R.; Zhang, Q.; Jin, C.; Pu, X.; Geng, Y. Deposition-Precipitation Synthesis of Yb3+/Er3+ Co-Doped BiOBr/AgBr Heterojunction Photocatalysts with Enhanced Photocatalytic Activity under Vis/NIR Light Irradiation. Sep. Purif. Technol. 2020, 238, 116450. [Google Scholar] [CrossRef]
- Hernández-Laverde, M.; Murcia, J.J.; Navío, J.A.; Hidalgo, M.C. Novel Heterostructured NaTaO3/WO3 Systems with Improved Photocatalytic Properties for Water Decontamination under UV and Visible Illumination. J. Mater. Sci. 2024, 59, 8669–8681. [Google Scholar] [CrossRef]
- Sun, X.; He, W.; Hao, X.; Ji, H.; Liu, W.; Cai, Z. Surface Modification of BiOBr/TiO2 by Reduced AgBr for Solar-Driven PAHs Degradation: Mechanism Insight and Application Assessment. J. Hazard. Mater. 2021, 412, 125221. [Google Scholar] [CrossRef]
- Katsumata, H.; Hayashi, T.; Taniguchi, M.; Suzuki, T.; Kaneco, S. Highly Efficient Visible-Light Driven AgBr/Ag3PO4 Hybrid Photocatalysts with Enhanced Photocatalytic Activity. Mater. Sci. Semicond. Process. 2014, 25, 68–75. [Google Scholar] [CrossRef]
- Wang, R.; Xu, M.; Xie, J.; Ye, S.; Song, X. A Spherical TiO2-Bi2WO6 Composite Photocatalyst for Visible-Light Photocatalytic Degradation of Ethylene. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125048. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Y.; Liu, F.; Zhang, L.; Chai, S. Fabrication of a Direct Z-Scheme Type WO3/Ag3PO4 Composite Photocatalyst with Enhanced Visible-Light Photocatalytic Performances. Appl. Surf. Sci. 2017, 393, 180–190. [Google Scholar] [CrossRef]
- Chen, H.; Hu, Y.; Ying, Z.; Xia, Y.; Ye, J.; Zhao, J.; Zhang, S. BiOI-SnO2 Heterojunction Design to Boost Visible-Light-Driven Photocatalytic NO Purification. Int. J. Environ. Res. Public Health 2023, 20, 4009. [Google Scholar] [CrossRef] [PubMed]
- Rice, E.W.; Bridgewater, L. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Ministerio de Ambiente y Desarrollo Sostenible. Resolución 631 de 2015; Ministerio de Ambiente y Desarrollo Sostenible: Bogotá, Colombia, 2015.
- Ministerio de Ambiente y Desarrollo Sostenible. Resolución 1207 de 2014; Ministerio de Ambiente y Desarrollo Sostenible: Bogotá, Colombia, 2014.
- Mirsalari, S.A.; Nezamzadeh-Ejhieh, A. A Ternary CdS/AgBr/Ag3PO4 Nanocomposite: Characterization and the Kinetics of Its Photocatalytic Activity. Environ. Sci. Pollut. Res. 2021, 28, 41651–41662. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yin, L.; Chen, Z.; Zhou, G.; Shi, H. Photodegradation of Methyl Orange Using Magnetically Recoverable AgBr@Ag3PO4/Fe3O4 Photocatalyst under Visible Light. J. Nanomater. 2014, 2014, 150150. [Google Scholar] [CrossRef]
- Liu, H.; Li, D.; Yang, X.; Li, H. Fabrication and Characterization of Ag3PO4/TiO2 Heterostructure with Improved Visible-Light Photocatalytic Activity for the Degradation of Methyl Orange and Sterilization of E.coli. Mater. Technol. 2019, 34, 192–203. [Google Scholar] [CrossRef]
- Fu, F.; Zhang, Y.; Yan, L.; Wang, Y.; Gao, X.; Wang, D. Preparation of Efficient Ag/AgBr/TiO2 Visible Light Photocatalyst for Destruction of MB. J. Mater. Sci. Mater. Electron. 2017, 28, 691–696. [Google Scholar] [CrossRef]
- Zuo, X.; Hu, J.; Chen, M. The Role and Fate of Inorganic Nitrogen Species during UVA/TiO2 Disinfection. Water Res. 2015, 80, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Grebel, J.E.; Pignatello, J.J.; Mitch, W.A. Effect of halide ions and carbonates on organic contaminant degradation by hydroxyl radical-based advanced oxidation processes in saline Waters. Environ. Sci. Technol. 2010, 44, 6822–6828. [Google Scholar] [CrossRef]
- Katz, A.; McDonagh, A.; Tijing, L.; Shon, H.K. Fouling and inactivation of titanium dioxide-based photocatalytic systems. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1880–1915. [Google Scholar] [CrossRef]
- Hirakawa, T.; Nosaka, Y. Properties of O2●− and OH. Formed in TiO2 aqueous suspensions by photocatalytic reaction and the influence of H2O2 and some ions. Langmuir 2002, 18, 3247–3254. [Google Scholar] [CrossRef]
- Munuera, G.; Navío, A.; Soria, J.; Gonzalez-Elipe, A.R. Photo-adsorption of oxygen on chlorinated TiO2 surfaces; a possible way to photo-oxy chlorinations. Stud. Surf. Sci. Catal. 1981, 7, 1185–1197. [Google Scholar] [CrossRef]
- Munuera, G.; Navío, A.; Rives-Arnau, V. Photogeneration of singlet oxygen from TiO2 surfaces. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1981, 77, 2747–2749. [Google Scholar] [CrossRef]
- Colina-Márquez, J.; Machuca-Martínez, F.; Salas, W. Enhancement of the Potential Biodegradability and the Mineralization of a Pesticides Mixture after Being Treated by a Coupled Process of TiO2-Based Solar Photocatalysis with Constructed Wetlands. Ing. Compet. 2013, 15, 181–190. [Google Scholar]
- Lei, S.; Guo, G.; Xiong, B.; Gong, W.; Mei, G. Disruption of Bacterial Cells by Photocatalysis of Montmorillonite Supported Titanium Dioxide. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2009, 24, 557–561. [Google Scholar] [CrossRef]
- Carré, G.; Hamon, E.; Ennahar, S.; Estner, M.; Lett, M.C.; Horvatovich, P.; Gies, J.P.; Keller, V.; Keller, N.; Andre, P. TiO2 Photocatalysis Damages Lipids and Proteins in Escherichia Coli. Appl. Environ. Microbiol. 2014, 80, 2573–2581. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, Y.; Huang, W.; Liu, Z.; Guo, R.; Chen, J. Synchronous Complete COD Reduction for Persistent Chemical-Industrial Organic Wastewater Using the Integrated Treatment System. Chem. Eng. J. 2022, 430, 133136. [Google Scholar] [CrossRef]
- Zhang, C.; Guisasola, A.; Baeza, J.A. Achieving Simultaneous Biological COD and Phosphorus Removal in a Continuous Anaerobic/Aerobic A-Stage System. Water Res. 2021, 190, 116703. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E. Self-Accumulated Ag Nanoparticles on Mesoporous TiO2 Thin Film with High Bactericidal Activities. Surf. Coat. Technol. 2010, 204, 3676–3683. [Google Scholar] [CrossRef]
- Mezzanotte, V.; Antonelli, M.; Citterio, S.; Nurizzo, C. Wastewater Disinfection Alternatives: Chlorine, Ozone, Peracetic Acid, and UV Light. Water Environ. Res. 2007, 79, 2373–2379. [Google Scholar] [CrossRef]
- Sontakke, S.; Modak, J.; Madras, G. Effect of Inorganic Ions, H2O2 and pH on the Photocatalytic Inactivation of Escherichia Coli with Silver Impregnated Combustion Synthesized TiO2 Catalyst. Appl. Catal. B Environ. 2011, 106, 453–459. [Google Scholar] [CrossRef]
- Li, X.Z.; Fan, C.M.; Sun, Y.P. Enhancement of Photocatalytic Oxidation of Humic Acid in TiO2 Suspensions by Increasing Cation Strength. Chemosphere 2002, 48, 453–460. [Google Scholar] [CrossRef]
- Chen, Q.; Gao, M.; Yu, M.; Zhang, T.; Wang, J.; Bi, J.; Dong, F. Efficient Photo-Degradation of Antibiotics by Waste Eggshells Derived AgBr-CaCO3 Heterostructure under Visible Light. Sep. Purif. Technol. 2023, 314, 123573. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, P.; Chen, H.; Yu, Q. Photocatalytic Performance and Dispersion Stability of Nanodispersed TiO2 Hydrosol in Electrolyte Solutions with Different Cations. J. Environ. Sci. 2020, 88, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Chong, R.; Cheng, X.; Chang, Z.; Li, D.; Zhang, L. Effects of Common Metal Cations on Ag3PO4-Photocatalytic Water Decontamination. J. Environ. Chem. Eng. 2015, 3, 1215–1222. [Google Scholar] [CrossRef]
- Nam, H.M.; Lim, S.K.; Kang, H.M.; Kim, J.M.; Moon, J.S.; Jang, K.C.; Kim, J.M.; Joo, Y.S.; Jung, S.C. Prevalence and Antimicrobial Susceptibility of Gram-Negative Bacteria Isolated from Bovine Mastitis between 2003 and 2008 in Korea. J. Dairy Sci. 2009, 92, 2020–2026. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Huang, B.; Qin, X.; Zhang, X.; Dai, Y.; Whangbo, M.-H. Ag/AgBr/WO3·H2O: Visible-Light Photocatalyst for Bacteria Destruction. Inorg. Chem. 2009, 48, 10697–10702. [Google Scholar] [CrossRef]
- Zhan, S.; Hou, Q.; Li, Y.; Ma, S.; Wang, P.; Li, Y.; Wang, H. AgBr/g-C3N4 Nanocomposites for Enhanced Visible-Light-Driven Photocatalytic Inactivation of Escherichia Coli. RSC Adv. 2018, 8, 34428–34436. [Google Scholar] [CrossRef]
- Amano, F.; Nogami, K.; Tanaka, M.; Ohtani, B. Correlation between Surface Area and Photocatalytic Activity for Acetaldehyde Decomposition over Bismuth Tungstate Particles with a Hierarchical Structure. Langmuir 2010, 26, 7174–7180. [Google Scholar] [CrossRef]
- Kominami, H.; Murakami, S.; Kato, J.; Kera, Y.; Ohtani, B. Correlation between Some Physical Properties of Titanium Dioxide Particles and Their Photocatalytic Activity for Some Probe Reactions in Aqueous Systems. J. Phys. Chem. B 2002, 106, 10501–10507. [Google Scholar] [CrossRef]
- Singh, K.; Nancy; Bhattu, M.; Singh, G.; Mubarak, N.M.; Singh, J. Light-Absorption-Driven Photocatalysis and Antimicrobial Potential of PVP-Capped Zinc Oxide Nanoparticles. Sci. Rep. 2023, 13, 13886. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, X.; Quan, Y.; Tian, Y.; Chen, J.; Li, L. Visible Light-Induced Photocatalytic and Antibacterial Adhesion Properties of Superhydrophilic TiO2 Nanoparticles. Sci. Rep. 2024, 14, 7940. [Google Scholar] [CrossRef]
- Ren, L.; Huo, W.; Li, G.; Choi, W.; An, T. Photocatalytic Mechanisms and Photocatalyst Deactivation during the Degradation of 5-Fluorouracil in Water. Catal. Today 2023, 410, 45–55. [Google Scholar] [CrossRef]
- Yan, X.J.; Tang, Y.; Ma, C.; Liu, Y.; Xu, J. Deactivation and Regeneration of Photocatalysts: A Review. Desalination Water Treat. 2018, 124, 160–176. [Google Scholar] [CrossRef]
- Yao, S.; Xue, S.; Zhang, J.; Shen, X. Characterization and Mechanism Analysis of AgBr Mixed Cuboid WO3 Rods with Enhanced Photocatalytic Activity. RSC Adv. 2016, 6, 93436–93444. [Google Scholar] [CrossRef]
- Wang, F.; Wang, T.; Lang, J.; Su, Y.; Wang, X. Improved Photocatalytic Activity and Durability of AgTaO3/AgBr Heterojunction: The Relevance of Phase and Electronic Structure. J. Mol. Catal. A Chem. 2017, 426, 52–59. [Google Scholar] [CrossRef]

| Water Quality Parameter | Resolution 631/2015 | Resolution 1207/2014 |
|---|---|---|
| COD (mg/L O2) | 450.0 | 450.0 |
| pH | 6.0–9.0 | 6.0–9.0 |
| Nitrates (mg/L) | Analysis * | 5.0 |
| Chlorides (mg Cl−/L) | 500.0 | 300.0 |
| Total hardness (mg CaCO3/L) | Analysis * | NR ** |
| Calcic hardness (mg CaCO3/L) | Analysis * | NR ** |
| Real colour (absorbance measured at wavelength of 436 nm) | Analysis * | NR ** |
| E. coli (CFU/100 mL) | NR ** | NR ** |
| Total coliforms (CFU/100 mL) | NR ** | NR ** |
| Other Enterobacteriaceae (CFU/100 mL) | NR ** | NR ** |
| Photocatalyst | Crystallite Size D (nm) AgBr(200) | SBET (m2/g) | Band Gap (eV) |
|---|---|---|---|
| AgBr | 175.5 | <1 | 2.60 |
| TiO2 | - | 91.0 | 3.10 |
| SnO2 | - | 111 | 3.92 |
| WO3 | - | 30.2 | 2.80 |
| AgBr(50%)/TiO2 | 242.4 | 23.4 | 2.48 (*) |
| AgBr/SnO2(20%) | 149.9 | 12.0 | 2.60 (*) |
| AgBr/WO3(20%) | 107.1 | 6.60 | 2.54 (*) |
| Semiconductor | Electronegativity | Eg | VB Position | CB Position |
|---|---|---|---|---|
| (X) | (eV) | (eV) vs. NHE | (eV) vs. NHE | |
| AgBr | 4.42 | 2.60 | 1.22 | −1.38 |
| TiO2 | 5.81 | 3.10 | 2.86 | −0.24 |
| WO3 | 6.33 | 2.80 | 3.23 | 0.43 |
| SnO2 | 6.25 | 3.92 | 3.71 | −0.21 |
| Quality Control Parameter | Dairy Industry | Jordán River |
|---|---|---|
| COD (mg/L) | 117,500 | 23 |
| pH | 6.75 | 6.68 |
| Nitrates (mg/L) | 97 | 0.5 |
| Chlorides (mg/L) | 597 | 27 |
| Total hardness (mgCaCO3/L) | 1320 | 235 |
| Calcium hardness (mgCaCO3/L) | 825 | 144 |
| Real colour (absorbance measured at wavelength of 436 nm) | 10,290 | 207 |
| E. coli (CFU/100 mL) | 2 × 106 | 2.0 × 103 |
| Total coliforms (CFU/100 mL) | 1.8 × 108 | 3.1 × 105 |
| Other Enterobacteriaceae (CFU/100 mL) | 4.0 × 102 | 2.3 × 104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Laverde, M.; Murcia, J.J.; Navío, J.A.; Hidalgo, M.C.; Puga, F. Treatment of Dairy Industry Wastewater and Crop Irrigation Water Using AgBr-Coupled Photocatalysts. Nanomaterials 2025, 15, 848. https://doi.org/10.3390/nano15110848
Hernández-Laverde M, Murcia JJ, Navío JA, Hidalgo MC, Puga F. Treatment of Dairy Industry Wastewater and Crop Irrigation Water Using AgBr-Coupled Photocatalysts. Nanomaterials. 2025; 15(11):848. https://doi.org/10.3390/nano15110848
Chicago/Turabian StyleHernández-Laverde, M., J. J. Murcia, J. A. Navío, M. C. Hidalgo, and F. Puga. 2025. "Treatment of Dairy Industry Wastewater and Crop Irrigation Water Using AgBr-Coupled Photocatalysts" Nanomaterials 15, no. 11: 848. https://doi.org/10.3390/nano15110848
APA StyleHernández-Laverde, M., Murcia, J. J., Navío, J. A., Hidalgo, M. C., & Puga, F. (2025). Treatment of Dairy Industry Wastewater and Crop Irrigation Water Using AgBr-Coupled Photocatalysts. Nanomaterials, 15(11), 848. https://doi.org/10.3390/nano15110848








