Trimetallic Fe-Zn-Mn (Oxy)Hydroxide-Enhanced Coffee Biochar for Simultaneous Phosphate and Ammonium Recovery and Recycling
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Spent Coffee Ground Waste
2.2. Biochar Synthesis from Spent Coffee Grounds
2.3. Biochar Activation
2.4. Physicochemical Characterization Methods
2.5. Evaluation of Phosphate and Ammonium Adsorption
2.5.1. Effect of pH on Phosphate and Ammonium Adsorption
2.5.2. Phosphate and Ammonium Adsorption Kinetics
2.5.3. Adsorption Isotherms
2.5.4. Thermodynamic Analysis
2.5.5. Biochar Regeneration
2.5.6. Phosphate Fractionation
2.6. Application to Real Wastewater
3. Results and Discussion
3.1. Physicochemical Properties of Biochar
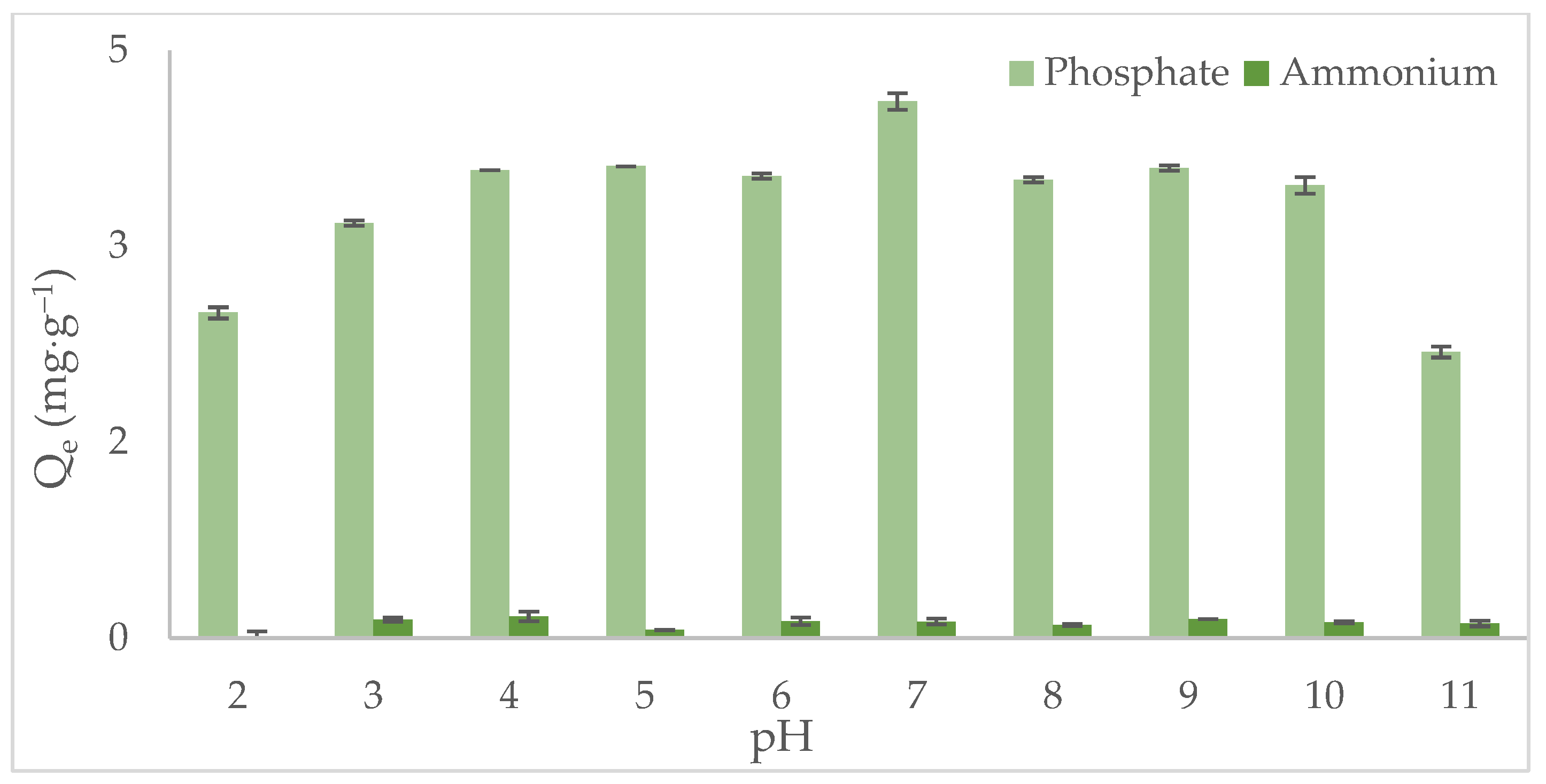
3.2. Influence of pH on Phosphate and Ammonium Adsorption
3.3. Kinetic of Phosphate and Ammonium Adsorption
3.4. Phosphate and Ammonium Adsorption Isotherm and Thermodynamic Analysis
3.5. Extraction of Phosphate Fractions from Phosphate-Loaded Biochar
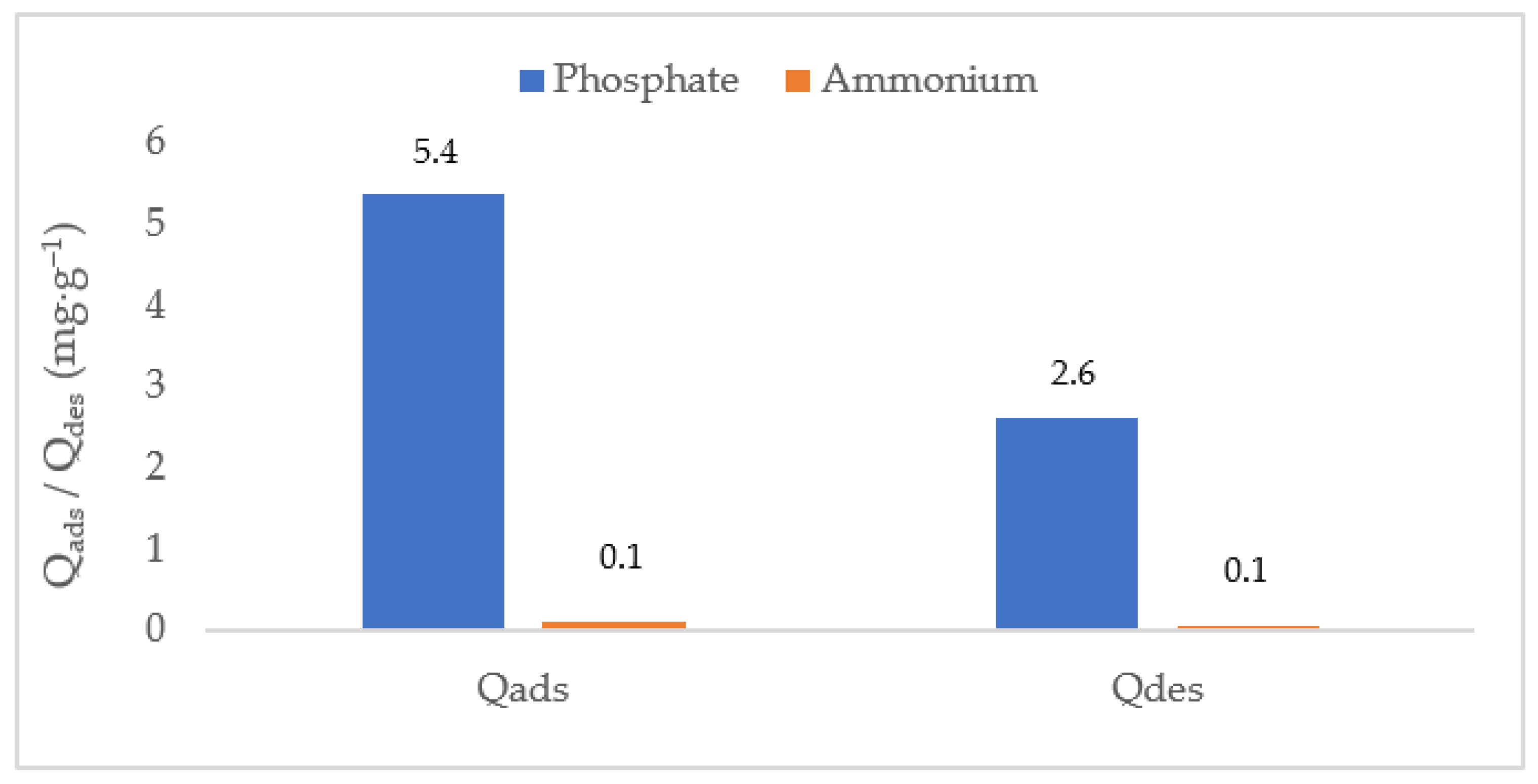
3.6. Evaluation of Biochar Regeneration Capacity
3.7. Phosphate and Ammonium Removal from Real Wastewater Using CB–M
3.8. Comparative Efficiency of CB–M for Phosphate and Ammonium Removal
| Biochar Source | Modification Strategy | Phosphate Adsorption Capacity (mg·g−1) | Ammonium Removal Capacity (mg·g−1) | Reference |
|---|---|---|---|---|
| Spent coffee grounds | CB–M biochar modified with Mn2+/Zn2+/Fe3+ (oxy)hydroxide nanoparticles | 42.6 | 2.79 | This study |
| 30.16 * | 1.84 * | |||
| Soybean straw | Impregnated with the prepared solutions MgCl2 and AlCl3 | 74.47 | 0.70 | [76] |
| Rape straw (RS) | Red mud and rape Straw mixing | 11.78 | 2.97 | [76] |
| Rice husk biochar | Biochar supported Mg(OH)2/bentonite composite (PMRB) | 125.36 | 58.20 | [77] |
| Sugarcane crop harvest residue | MgO particle-impregnated | 398 | 22 | [78] |
| Peanut shell biochar | Mg-doped biochar/bentonite composite bead (SA-Mg@BC/BT) | 132.2 | 39.5 | [54] |
| Rice straw biochar | Calcium alginate-biochar composite at 300 °C (CA-MRB300) | 31.38 | 1137.7 | [55] |
| Biochar from the pyrolysis of sludge fermented | Biochar from the pyrolysis of sludge fermented with rusty scrap iron and reduced iron powder (RSI-RIP) at 600 °C-ES600 | 12.70 | 10.72 | [79] |
| Crushed rice straws biochar | Immersion in MgCl2 solutions (HM-HP-HT) 2 M at 800 °C | 5.52 | 4.40 | [68] |
| Rice straw biochar | Modified by ferric chloride (Fe-HBC2) | 22.98 | 28.10 | [80] |
| Corn stalk biochar (BC) | Mg-modified biochar (MBC) | 64.48 | 36.27 | [81] |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhagowati, B.; Ahamad, K.U. A review on lake eutrophication dynamics and recent developments in lake modeling. Ecohydrol. Hydrobiol. 2019, 19, 155–166. [Google Scholar] [CrossRef]
- Yao, J.; Wang, G.; Xue, B.; Wang, P.; Hao, F.; Xie, G.; Peng, Y. Assessment of lake eutrophication using a novel multidimensional similarity cloud model. J. Environ. Manag. 2019, 248, 109259. [Google Scholar] [CrossRef] [PubMed]
- Li-Kun, Y.; Sen, P.; Xin-Hua, Z.; Xia, L. Development of a two-dimensional eutrophication model in an urban lake (China) and the application of uncertainty analysis. Ecol. Model. 2017, 345, 63–74. [Google Scholar] [CrossRef]
- Reis, C.R.G.; Pacheco, F.S.; Reed, S.C.; Tejada, G.; Nardoto, G.B.; Forti, M.C.; Ometto, J.P. Biological nitrogen fixation across major biomes in Latin America: Patterns and global change effects. Sci. Total Environ. 2020, 746, 140998. [Google Scholar] [CrossRef]
- Cruz, H.; Luckman, P.; Seviour, T.; Verstraete, W.; Laycock, B.; Pikaar, I. Rapid removal of ammonium from domestic wastewater using polymer hydrogels. Sci. Rep. 2018, 8, 2912. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Q. Microalgae-based nitrogen bioremediation. Algal Res. 2020, 46, 101775. [Google Scholar] [CrossRef]
- Cakmak, E.K.; Hartl, M.; Kisser, J.; Cetecioglu, Z. Phosphorus mining from eutrophic marine environment towards a blue economy: The role of bio-based applications. Water Res. 2022, 219, 118505. [Google Scholar] [CrossRef]
- Yu, H.; Lu, X.; Miki, T.; Matsubae, K.; Sasaki, Y.; Nagasaka, T. Sustainable phosphorus supply by phosphorus recovery from steelmaking slag: A critical review. Resour. Conserv. Recycl. 2022, 180, 106203. [Google Scholar] [CrossRef]
- Sun, H.; Mohammed, A.N.; Liu, Y. Phosphorus recovery from source-diverted blackwater through struvite precipitation. Sci. Total Environ. 2020, 743, 140747. [Google Scholar] [CrossRef]
- Perera, M.K.; Englehardt, J.D.; Cohn, J.L.; Dauer, E.A.; Shukla, D. Electrohydromodulation for phosphate recovery from wastewater. Sep. Purif. Technol. 2020, 247, 116909. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Guaya, D.; Hermassi, M.; Valderrama, C.; Farran, A.; Cortina, J.L. Recovery of ammonium and phosphate from treated urban wastewater by using potassium clinoptilolite impregnated hydrated metal oxides as N-P-K fertilizer. J. Environ. Chem. Eng. 2016, 4, 3519–3526. [Google Scholar] [CrossRef]
- Lin, X.; Xie, Y.; Lu, H.; Xin, Y.; Altaf, R.; Zhu, S.; Liu, D. Facile preparation of dual La-Zr modified magnetite adsorbents for efficient and selective phosphorus recovery. Chem. Eng. J. 2021, 413, 127530. [Google Scholar] [CrossRef]
- Brassard, P.; Godbout, S.; Raghavan, V. Soil biochar amendment as a climate change mitigation tool: Key parameters and mechanisms involved. J. Environ. Manag. 2016, 181, 484–497. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, F.; Li, J.; Cheng, K. Porous biochar-nanoscale zero-valent iron composites: Synthesis, characterization and application for lead ion removal. Sci. Total Environ. 2020, 746, 141037. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Varjani, S.; Saravanan, A. A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar] [CrossRef] [PubMed]
- Battista, F.; Zuliani, L.; Rizzioli, F.; Fusco, S.; Bolzonella, D. Biodiesel, biogas and fermentable sugars production from Spent coffee Grounds: A cascade biorefinery approach. Bioresour. Technol. 2021, 342, 125952. [Google Scholar] [CrossRef]
- Debevc, S.; Weldekidan, H.; Snowdon, M.R.; Vivekanandhan, S.; Wood, D.F.; Misra, M.; Mohanty, A.K. Valorization of almond shell biomass to biocarbon materials: Influence of pyrolysis temperature on their physicochemical properties and electrical conductivity. Carbon Trends 2022, 9, 100214. [Google Scholar] [CrossRef]
- da Silva Correia, I.K.; Santos, P.F.; Santana, C.S.; Neris, J.B.; Luzardo, F.H.M.; Velasco, F.G. Application of coconut shell, banana peel, spent coffee grounds, eucalyptus bark, piassava (Attalea funifera) and water hyacinth (Eichornia crassipes) in the adsorption of Pb2+ and Ni2+ ions in water. J. Environ. Chem. Eng. 2018, 6, 2319–2334. [Google Scholar] [CrossRef]
- Lee, K.T.; Du, J.T.; Chen, W.H.; Ubando, A.T.; Lee, K.T. Green additive to upgrade biochar from spent coffee grounds by torrefaction for pollution mitigation. Environ. Pollut. 2021, 285, 117244. [Google Scholar] [CrossRef]
- Mukherjee, A.; Borugadda, V.B.; Dynes, J.J.; Niu, C.; Dalai, A.K. Carbon dioxide capture from flue gas in biochar produced from spent coffee grounds: Effect of surface chemistry and porous structure. J. Environ. Chem. Eng. 2021, 9, 106049. [Google Scholar] [CrossRef]
- Gęca, M.; Khalil, A.M.; Tang, M.; Bhakta, A.K.; Snoussi, Y.; Nowicki, P.; Wiśniewska, M.; Chehimi, M.M. Surface Treatment of Biochar—Methods, Surface Analysis and Potential Applications: A Comprehensive Review. Surfaces 2023, 6, 179–213. [Google Scholar] [CrossRef]
- Guaya, D.; Cobos, H.; Valderrama, C.; Cortina, J.L. Effect of Mn2+/Zn2+/Fe3+ Oxy(Hydroxide) Nanoparticles Doping onto Mg-Al-LDH on the Phosphate Removal Capacity from Simulated Wastewater. Nanomaterials 2022, 12, 3680. [Google Scholar] [CrossRef] [PubMed]
- Guaya, D.; Maza, L.; Angamarca, A.; Mendoza, E.; García, L.; Valderrama, C.; Cortina, J.L. Fe3+/Mn2+ (Oxy)Hydroxide Nanoparticles Loaded onto Muscovite/Zeolite Composites (Powder, Pellets and Monoliths): Phosphate Carriers from Urban Wastewater to Soil. Nanomaterials 2022, 12, 3848. [Google Scholar] [CrossRef]
- Mei, Y.; Xu, J.; Zhang, Y.; Li, B.; Fan, S.; Xu, H. Effect of Fe–N modification on the properties of biochars and their adsorption behavior on tetracycline removal from aqueous solution. Bioresour. Technol. 2021, 325, 124732. [Google Scholar] [CrossRef]
- Huang, Q.; Tong, F.; Gao, Y.; Chen, J.; Zhou, D.; Qu, Z.; Fan, G.; Chen, W.; Shi, G. Enhanced simultaneous arsenite oxidation and sorption by Mn-modified biochar: Insight into the mechanisms under optimal modification condition. J. Environ. Chem. Eng. 2023, 11, 109612. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, Y.; Huang, Y.; Song, L.; Chen, H.; Zhu, S.; Tang, C. Enhanced adsorption of phosphate on orange peel-based biochar activated by Ca/Zn composite: Adsorption efficiency and mechanisms. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129728. [Google Scholar] [CrossRef]
- Shen, M.; Huang, Z.; Kuo, Y.; Hsu, J. Improvement of gaseous bioenergy production from spent coffee grounds Co-digestion with pulp wastewater by physical/chemical pretreatments. Int. J. Hydrogen Energy 2022, 47, 40664–40671. [Google Scholar] [CrossRef]
- Zhou, H.; Tan, Y.; Yang, Y.; Zhang, Y.; Lei, X.; Yuan, D. Application of FeMgMn layered double hydroxides for phosphate anions adsorptive removal from water. Appl. Clay Sci. 2021, 200, 105903. [Google Scholar] [CrossRef]
- Nguyen, N.-T.; Kang, S.-Y.; Chang, C.-T.; Hong, G.-B. Iron-Doped Biochar from Hospital Sludge for Efficient Arsenic Removal from Groundwater. J. Compos. Sci. 2024, 8, 509. [Google Scholar] [CrossRef]
- Ji, L.; Li, J.; Zhai, R.; Wang, J.; Wang, X.; Yan, S.; Hua, M. Metal Oxyhydroxide Catalysts Promoted CO2 Absorption and Desorption in Amine-Based Carbon Capture: A Feasibility Study. ACS Omega 2022, 7, 44620–44630. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, T.R.; Prelot, B. Adsorption processes for the removal of contaminants from wastewater: The perspective role of nano-materials and nanotechnology. In Nanomaterials for the Detection and Removal of Wastewater Pollutants; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liang, X.; Niyungeko, C.; Sun, T.; Liu, F.; Arai, Y. Effects of biochar amendments on soil phosphorus transformation in agricultural soils. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2019; Volume 158. [Google Scholar] [CrossRef]
- Mu, T.-H.; Sun, H.-N. Sweet Potato Leaf Polyphenols: Preparation, Individual Phenolic Compound Composition and Antioxidant Activity. In Polyphenols in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 365–380. [Google Scholar]
- Lonappan, L.; Rouissi, T.; Brar, S.K.; Verma, M.; Surampalli, R.Y. An insight into the adsorption of diclofenac on different biochars: Mechanisms, surface chemistry, and thermodynamics. Bioresour. Technol. 2018, 249, 386–394. [Google Scholar] [CrossRef]
- Campoverde, J.; Guaya, D. From Waste to Added-Value Product: Synthesis of Highly Crystalline LTA Zeolite from Ore Mining Tailings. Nanomaterials 2023, 13, 1295. [Google Scholar] [CrossRef]
- Kepp, K.P. Free Energies of Hydration for Metal Ions from Heats of Vaporization. J. Phys. Chem. A 2019, 123, 6536–6546. [Google Scholar] [CrossRef]
- Zhang, P.; O’Connor, D.; Wang, Y.; Jiang, L.; Xia, T.; Wang, L.; Tsang, D.C.; Ok, Y.S.; Hou, D. A green biochar/iron oxide composite for methylene blue removal. J. Hazard. Mater. 2020, 384, 121286. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Cao, X.; Mašek, O.; Zimmerman, A. Heterogeneity of biochar properties as a function of feedstock sources and production temperatures. J. Hazard. Mater. 2013, 256–257, 1–9. [Google Scholar] [CrossRef]
- Li, S.; Skelly, S. Physicochemical properties and applications of biochars derived from municipal solid waste: A review. Environ. Adv. 2023, 13, 100395. [Google Scholar] [CrossRef]
- Han, S.; Xiao, P. Catalytic degradation of tetracycline using peroxymonosulfate activated by cobalt and iron co-loaded pomelo peel biochar nanocomposite: Characterization, performance and reaction mechanism. Sep. Purif. Technol. 2022, 287, 120533. [Google Scholar] [CrossRef]
- Li, X.; Li, R.; Feng, X. Efficient Adsorption and Photocatalytic Degradation of Organic Pollutant by Ag3PO4/ZnO/Chitosan–Biochar Composites. Russ. J. Inorg. Chem. 2023, 68, 1386–1398. [Google Scholar] [CrossRef]
- Tan, W.-T.; Zhou, H.; Tang, S.-F.; Zeng, P.; Gu, J.-F.; Liao, B.-H. Enhancing Cd(II) adsorption on rice straw biochar by modification of iron and manganese oxides. Environ. Pollut. 2022, 300, 118899. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.-T.; Vo, T.-D.-H.; Tran, T.; Nguyen, T.-N.; Le, T.-N.C.; Bui, X.-T.; Bach, L.-G. Biochar derived from the spent coffee ground for ammonium adsorption from aqueous solution. Case Stud. Chem. Environ. Eng. 2021, 4, 100141. [Google Scholar] [CrossRef]
- Chen, W.-H.; Du, J.-T.; Lee, K.-T.; Ong, H.C.; Park, Y.-K.; Huang, C.-C. Pore volume upgrade of biochar from spent coffee grounds by sodium bicarbonate during torrefaction. Chemosphere 2021, 275, 129999. [Google Scholar] [CrossRef]
- Nguyen, V.-T.; Nguyen, T.-B.; Huang, C.; Chen, C.-W.; Bui, X.-T.; Dong, C.-D. Alkaline modified biochar derived from spent coffee ground for removal of tetracycline from aqueous solutions. J. Water Process Eng. 2021, 40, 101908. [Google Scholar] [CrossRef]
- Ma, W.; Fan, J.; Cui, X.; Wang, Y.; Yan, Y.; Meng, Z.; Gao, H.; Lu, R.; Zhou, W. Pyrolyzing spent coffee ground to biochar treated with H3PO4 for the efficient removal of 2,4-dichlorophenoxyacetic acid herbicide: Adsorptive behaviors and mechanism. J. Environ. Chem. Eng. 2022, 11, 109165. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, Q.; Yang, G.; Li, X.; Du, W.; Leong, Y.K.; Chang, J.-S. Enhanced chlortetracycline removal by iron oxide modified spent coffee grounds biochar and persulfate system. Chemosphere 2022, 301, 134654. [Google Scholar] [CrossRef]
- Silvaraja, J.; Yahya, N.Y.; Zainol, M.M.; Lee, Y.S. Preliminary investigations of sustainable magnetic catalyst-based biochar derived spent coffee ground for biodiesel production from waste cooking oil. Clean. Chem. Eng. 2025, 11, 100148. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, Z.; Hu, Y.; Abbey, L.; Cesarino, I.; Goonetilleke, A.; He, Q. Exploring the Properties and Potential Uses of Biocarbon from Spent Coffee Grounds: A Comparative Look at Dry and Wet Processing Methods. Processes 2023, 11, 2099. [Google Scholar] [CrossRef]
- Guaya, D.; Valderrama, C.; Farran, A.; Cortina, J.L. Modification of a natural zeolite with Fe(III) for simultaneous phosphate and ammonium removal from aqueous solutions. J. Chem. Technol. Biotechnol. 2015, 91, 1737–1746. [Google Scholar] [CrossRef]
- Guaya, D.; Valderrama, C.; Farran, A.; Cortina, J.L. Simultaneous nutrients (N,P) removal by using a hybrid inorganic sorbent impregnated with hydrated manganese oxide. J. Environ. Chem. Eng. 2017, 5, 1516–1525. [Google Scholar] [CrossRef]
- Xi, H.; Zhang, X.; Zhang, A.H.; Guo, F.; Yang, Y.; Lu, Z.; Ying, G.; Zhang, J. Concurrent removal of phosphate and ammonium from wastewater for utilization using Mg-doped biochar/bentonite composite beads. Sep. Purif. Technol. 2022, 285, 120399. [Google Scholar] [CrossRef]
- Feng, Q.; Chen, M.; Wu, P.; Zhang, X.; Wang, S.; Yu, Z.; Wang, B. Simultaneous reclaiming phosphate and ammonium from aqueous solutions by calcium alginate-biochar composite: Sorption performance and governing mechanisms. Chem. Eng. J. 2022, 429, 132166. [Google Scholar] [CrossRef]
- Valderrama, C.; Barios, J.I.; Caetano, M.; Farran, A.; Cortina, J.L. Kinetic evaluation of phenol/aniline mixtures adsorption from aqueous solutions onto activated carbon and hypercrosslinked polymeric resin (MN200). React. Funct. Polym. 2010, 70, 142–150. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.J.; Zhou, B.; Awasthi, M.K.; Ali, A.; Zhang, Z.; Gaston, L.A.; Lahori, A.H.; Mahar, A. Enhancing phosphate adsorption by Mg/Al layered double hydroxide functionalized biochar with different Mg/Al ratios. Sci. Total Environ. 2016, 559, 121–129. [Google Scholar] [CrossRef]
- Zhang, M.; Song, G.; Gelardi, D.L.; Huang, L.; Khan, E.; Mašek, O.; Parikh, S.J.; Ok, Y.S. Evaluating biochar and its modifications for the removal of ammonium, nitrate, and phosphate in water. Water Res. 2020, 186, 116303. [Google Scholar] [CrossRef] [PubMed]
- Biswas, B.; Adhikari, S.; Jahromi, H.; Ammar, M.; Baltrusaitis, J.; Torbert, A.; Linhoss, J.; Lamba, J. Magnesium doped biochar for simultaneous adsorption of phosphate and nitrogen ions from aqueous solution. Chemosphere 2024, 358, 142130. [Google Scholar] [CrossRef]
- Takaya, C.A.; Fletcher, L.A.; Singh, S.; Anyikude, K.U.; Ross, A.B. Phosphate and ammonium sorption capacity of biochar and hydrochar from different wastes. Chemosphere 2016, 145, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, X. The unit problem in the thermodynamic calculation of adsorption using the langmuir equation. Chem. Eng. Commun. 2014, 201, 1459–1467. [Google Scholar] [CrossRef]
- Deng, L.; Shi, Z. Synthesis and characterization of a novel Mg–Al hydrotalcite-loaded kaolin clay and its adsorption properties for phosphate in aqueous solution. J. Alloys Compd. 2015, 637, 188–196. [Google Scholar] [CrossRef]
- Trazzi, P.A.; Leahy, J.J.; Hayes, M.H.B.; Kwapinski, W. Adsorption and desorption of phosphate on biochars. J. Environ. Chem. Eng. 2016, 4, 37–46. [Google Scholar] [CrossRef]
- Fan, R.; Chen, C.; Lin, J.; Tzeng, J.; Huang, C.; Dong, C.; Huang, C.P. Adsorption characteristics of ammonium ion onto hydrous biochars in dilute aqueous solutions. Bioresour. Technol. 2019, 272, 465–472. [Google Scholar] [CrossRef]
- Xu, H.; Wang, B.; Zhao, R.; Wang, X.; Pan, C.; Jiang, Y.; Zhang, X.; Ge, B. Adsorption behavior and performance of ammonium onto sorghum straw biochar from water. Sci. Rep. 2022, 12, 5358. [Google Scholar] [CrossRef]
- Xia, S.; Liang, S.; Qin, Y.; Chen, W.; Xue, B.; Zhang, B.; Xu, G. Significant Improvement of Adsorption for Phosphate Removal by Lanthanum-Loaded Biochar. ACS Omega 2023, 8, 24853–24864. [Google Scholar] [CrossRef]
- Cai, G.; Ye, Z.-L. Concentration-dependent adsorption behaviors and mechanisms for ammonium and phosphate removal by optimized Mg-impregnated biochar. J. Clean. Prod. 2022, 349, 131453. [Google Scholar] [CrossRef]
- Scholz, F.; Kahlert, H. The calculation of the solubility of metal hydroxides, oxide-hydroxides, and oxides, and their visualisation in logarithmic diagrams. ChemTexts 2015, 1, 7. [Google Scholar] [CrossRef]
- Hermassi, M.; Guaya, D.; Gibert, O.; Valderrama, C.; Cortina, J.L. Valorisation of Nutrients in Wastewaters Using Reactive Inorganic Sorbents BT. In Phosphorus Recovery and Recycling; Ohtake, H., Tsuneda, S., Eds.; Springer: Singapore, 2019; pp. 457–482. [Google Scholar] [CrossRef]
- Guaya, D.; Valderrama, C.; Farran, A.; Armijos, C.; Cortina, J.L. Simultaneous phosphate and ammonium removal from aqueous solution by a hydrated aluminum oxide modified natural zeolite. Chem. Eng. J. 2015, 271, 204–213. [Google Scholar] [CrossRef]
- Pantoja, F.; Sukmana, H.; Beszédes, S.; László, Z. Removal of ammonium and phosphates from aqueous solutions by biochar produced from agricultural waste. J. Mater. Cycles Waste Manag. 2023, 25, 1921–1934. [Google Scholar] [CrossRef]
- Sisay, G.B.; Atisme, T.B.; Workie, Y.A.; Negie, Z.W.; Mekonnen, M.L. Mg/Zr modified nanobiochar from spent coffee grounds for phosphate recovery and its application as a phosphorous release fertilizer. Environ. Nanotechnol. Monit. Manag. 2023, 19, 100766. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Huo, J.; Zhang, X.; Wen, H.; Zhang, D.; Zhao, Y.; Kang, D.; Guo, W.; Ngo, H.H. Adsorption recovery of phosphorus in contaminated water by calcium modified biochar derived from spent coffee grounds. Sci. Total Environ. 2023, 909, 168426. [Google Scholar] [CrossRef]
- Shin, H.; Tiwari, D.; Kim, D.-J. Phosphate adsorption/desorption kinetics and P bioavailability of Mg-biochar from ground coffee waste. J. Water Process Eng. 2020, 37, 101484. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, B.; Feng, Q.; Chen, M.; Zhang, X.; Zhao, R. Recovery of nitrogen and phosphorus in wastewater by red mud-modified biochar and its potential application. Sci. Total Environ. 2023, 860, 160289. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.-P.; Li, Y.; Wang, X.; Zhao, J.; Xia, S. Simultaneous recovery of phosphate, ammonium and humic acid from wastewater using a biochar supported Mg(OH)2/bentonite composite. Environ. Sci. Water Res. Technol. 2019, 5, 931–943. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.J.; Zhou, B.; Zhang, Z.; Liu, S.; Lei, S.; Xiao, R. Simultaneous capture removal of phosphate, ammonium and organic substances by MgO impregnated biochar and its potential use in swine wastewater treatment. J. Clean. Prod. 2017, 147, 96–107. [Google Scholar] [CrossRef]
- Zhang, L.; Deng, F.; Liu, Z.; Ai, L. Removal of ammonia nitrogen and phosphorus by biochar prepared from sludge residue after rusty scrap iron and reduced iron powder enhanced fermentation. J. Environ. Manag. 2021, 282, 111970. [Google Scholar] [CrossRef]
- Huang, Z.; Chang, B.; Tang, Y.; Li, Q.; Zhang, Z.; Wei, S.; Chang, X.; Yang, Y.; Xu, C.; Hu, F.; et al. Co-adsorption performance and mechanism of ammonium and phosphate by iron-modified biochar in water. J. Water Process Eng. 2024, 67, 106209. [Google Scholar] [CrossRef]
- Tan, M.; Zhao, Y.; Quan, B.; Wu, Q.; Chi, D.; Zhang, W. Synergistic removal of ammonium and phosphate using ultrasonic Mg-based biochar composite: Mechanism, behavior and green recycling. J. Environ. Chem. Eng. 2024, 12, 111995. [Google Scholar] [CrossRef]





| Components | CB | CB–M |
|---|---|---|
| (%) | (%) | |
| SiO2 | 2.4 ± 0.3 | 0.7 ± 0.4 |
| P2O5 | 1.3 ± 0.1 | 2.5 ± 0.1 |
| K2O | 4.4 ± 0.0 | 1.3 ± 0.0 |
| CaO | 0.7 ± 0.0 | 2.2 ± 0.0 |
| MnO | − | 1.6 ± 0.0 |
| Fe2O3 | 0.1 ± 0.0 | 25.3 ± 0.0 |
| ZnO | − | 63.9 ± 0.1 |
| Model | Kinetic Parameters | ||
|---|---|---|---|
| Phosphate | Ammonium | ||
| Pseudo-first order | qe (mg∙g−1) | 4.98 | 0.07 |
| k1 (h−1) | 3.03 | 0.27 | |
| R2 | 0.98 | 0.90 | |
| Pseudo-second order | qe (mg∙g−1) | 6.38 | 0.12 |
| k2 (h−1) | 1.95 | 43.00 | |
| R2 | 1.00 | 1.00 | |
| Intraparticle diffusion | k1 (h−1) | 10.57 | 5 × 10−1 |
| R2 | 0.99 | 0.99 | |
| k2 (h−1) | 2.66 | 3.01 × 10−2 | |
| R2 | 0.96 | 0.97 | |
| Film diffusion | kF (h−1) | 0.05 | 4.54 × 10−3 |
| R2 | 0.98 | 0.90 | |
| Particle diffusion | kP (h−1) | 0.02 | 1.99 × 10−3 |
| R2 | 1.00 | 0.94 | |
| Model | T = 293.15 K | T = 299.15 K | T = 306.15 K | ||||
|---|---|---|---|---|---|---|---|
| PO43− | NH4+ | PO43− | NH4+ | PO43− | NH4+ | ||
| Langmuir | qm (mg∙g−1) | 42.6 | 2.79 | 57.8 | 2.84 | 72.0 | 2.95 |
| kL (L∙g−1) | 1.2 × 10–2 | 2.4 × 10–2 | 7.5 × 10–3 | 2.7 × 10–2 | 6.7 × 10–3 | 2.8 × 10–2 | |
| R2 | 0.98 | 0.60 | 0.95 | 0.56 | 0.92 | 0.80 | |
| Freundlich | kF (mg∙g−1) | 5.4 | 0.13 | 5.3 | 0.09 | 5.6 | 0.16 |
| 1/n | 0.3 | 0.62 | 0.3 | 0.74 | 0.3 | 0.59 | |
| R2 | 0.83 | 0.90 | 0.85 | 0.87 | 0.86 | 0.95 | |
| Temperature | ln kc | R2 | ΔG° | ΔS° | ΔH° | ||||
|---|---|---|---|---|---|---|---|---|---|
| (K) | PO43− | NH4+ | PO43− | NH4+ | PO43− | NH4+ | PO43− | NH4+ | |
| (kJ·mol−1) | (kJ·mol−1·K−1) | (kJ·mol−1) | |||||||
| 293.15 | 61.0 | 23.6 | 0.84 | −148.8 | −57.5 | −4 | 1.02 | 1450 | −241 |
| 299.15 | 39.2 | 27.4 | −97.6 | −68.1 | |||||
| 306.15 | 35.4 | 27.9 | −90.1 | −70.9 | |||||
| Fractions Type | Parameters | |
|---|---|---|
| Total adsorbed PO43− | qe (mg∙g−1) | 5.6 |
| PO43− labile fraction | % | 17.1 |
| qe (mg∙g−1) | 0.37 | |
| PO43− Fe-Mn-Zn bound | % | 72.1 |
| qe (mg∙g−1) | 1.6 | |
| PO43− Na-Mg-Ca bound | % | 6.3 |
| qe (mg∙g−1) | 0.1 | |
| PO43− Residual | % | 2.7 |
| qe (mg∙g−1) | 0.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guaya, D.; Campoverde, J.; Piedra, C.; Debut, A. Trimetallic Fe-Zn-Mn (Oxy)Hydroxide-Enhanced Coffee Biochar for Simultaneous Phosphate and Ammonium Recovery and Recycling. Nanomaterials 2025, 15, 849. https://doi.org/10.3390/nano15110849
Guaya D, Campoverde J, Piedra C, Debut A. Trimetallic Fe-Zn-Mn (Oxy)Hydroxide-Enhanced Coffee Biochar for Simultaneous Phosphate and Ammonium Recovery and Recycling. Nanomaterials. 2025; 15(11):849. https://doi.org/10.3390/nano15110849
Chicago/Turabian StyleGuaya, Diana, Jhuliana Campoverde, Camilo Piedra, and Alexis Debut. 2025. "Trimetallic Fe-Zn-Mn (Oxy)Hydroxide-Enhanced Coffee Biochar for Simultaneous Phosphate and Ammonium Recovery and Recycling" Nanomaterials 15, no. 11: 849. https://doi.org/10.3390/nano15110849
APA StyleGuaya, D., Campoverde, J., Piedra, C., & Debut, A. (2025). Trimetallic Fe-Zn-Mn (Oxy)Hydroxide-Enhanced Coffee Biochar for Simultaneous Phosphate and Ammonium Recovery and Recycling. Nanomaterials, 15(11), 849. https://doi.org/10.3390/nano15110849








