Abstract
Forest ecosystems represent a natural repository of biodiversity, bioenergy, food, timber, water, medicine, wildlife shelter, and pollution control. In many countries, forests offer great potential to provide biogenic resources that could be utilized for large-scale biotechnological synthesis and products. The evolving nanotechnology could be an excellent platform for the transformation of forest products into value-added nanoparticles (NPs). It also serves as a tool for commercial production, placing the forest at the heart of conservation and sustainable management strategies. NPs are groups of atoms with a size ranging from 1 to 100 nm. This review analyzes the scholarly articles published over the last 25 years on the forest and woody plant-based green synthesis of NPs, highlighting the plant parts and applications discussed. The biosynthesis of nanomaterials from plant extracts provides inexpensiveness, biocompatibility, biodegradability, and environmental nontoxicity to the resultant NPs. The leaf is the most critical organ in woody plants, and it is widely used in NP biosynthesis, perhaps due to its central functions of bioactive metabolite production and storage. Most biosynthesized NPs from tree species have been used and tested for medical applications. For sustainable advancements in forest-based nanotechnology, broader species coverage, expanded applications, and interdisciplinary collaboration are essential.
1. Introduction
Forests are fundamental natural resources that provide essential socioeconomic, environmental, educational, medical, and climate change mitigation services [1,2,3,4,5,6]. The ecosystems of forests represent a natural repository of food, fuelwood, timber, fiber, fodder, oxygen, water, biodiversity, fertile soil, medicine, wildlife shelter, and pollution control (Figure 1).

Figure 1.
The forest ecosystem as a vital source of resources and environmental services.
In many countries, forests offer great potential and could be utilized as an indispensable source for large-scale biotechnological synthesis and economic benefits [7,8,9,10]. Forests contain about 80% of the terrestrial biodiversity of the Earth’s biosphere [11]. With the renowned advancements in nanotechnology in different fields of life, it is possible to broadly exploit forest resources and diversity to produce various nanostructured materials and composites. Nanotechnology is an evolving and growing field in science, with many applications, especially in the biosciences and technology, resulting in new products from nanoparticles [12,13,14,15]. According to Javad et al. (2017) [16], nanoparticles (NPs) are defined as groups of atoms with a size ranging from one to one hundred nanometers (nm). Nanotechnology is a multidisciplinary science that deals with matter at the nanoscale size. Thus, nanomaterials (NMs) can be defined as materials with internal or external structures within the nanoscale dimension [17].
In recent decades, the biosynthetic approach has been widely adopted, using biological organisms such as plant extracts, fungi, and bacteria as viable and simple tools relative to routinely used toxic chemicals [16,18,19,20,21]. The chemical preparation of nanoparticles is the most well-known method, but the biological synthesis of nanomaterials has proven to be much simpler, more inexpensive, and more ecofriendly [16,22,23,24]. The effective use of renewable natural resources for innovative green synthesis is key to sustainable development in green chemistry [25,26]. The use of bio-based nanoparticles has gained particular attention because of their potential in plant production, medical uses, and industrial development, among other applications [27,28,29,30].
2. Forest Tree and Woody Plant-Based Synthesis of Nanoparticles
The green synthesis of nanoparticles from plant extracts is receiving notable interest due to their natural abundance, broad spectrum of bioactive metabolites, and vast applications in industrial, medical, agricultural, and environmental aspects [31,32,33,34]. Various plant parts are used for the synthesis of nanomaterials as they contain numerous compounds, such as steroids, gums, resins, polyphenols, saponins, terpenoids, alkaloids, fibers, rubber, latex, antioxidant enzymes, and others, which act as reducing and stabilizing agents in nanoparticle synthesis [17,19,35,36]. It has been reported that the use of plant materials as bio-reducing agents for the synthesis of nanoparticles is a cost-effective and environmentally benign option compared to the conventional chemical approach [26,37,38]. The green synthesis of nanoparticles using plant-biobased materials is shown in Figure 2. Forest trees and woody plants represent fundamental biogenic resources for the synthesis of NPs, which can also be used for further improvements in forestry, agricultural crops, medicine, and industrial sectors [39,40,41].
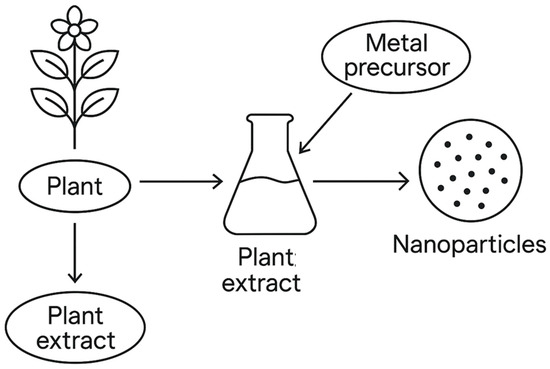
Figure 2.
Biosynthesis of nanoparticles.
The biosynthesis of nanomaterials from woody plant extracts provides biocompatibility, biodegradability, and environmental nontoxicity to the resultant nanoparticles [16,42,43,44,45,46,47]. Furthermore, biogenic material synthesis tends to be a more economical, simpler, and robust route for the synthesis of nanoparticles [48,49]. Green synthesis is a component of biotechnology that entails using the expertise and techniques of biology to manipulate molecular, genetic, and cellular processes, serving to increase the resulting services and products for utilization in numerous fields, including medicine, agriculture, forestry, and environmental safety [50,51]. In the forestry sector, biotechnology is considered to be an excellent platform for the transformation of forest materials into value-added products and serves as a tool for commercial production, placing the forest at the heart of conservation and sustainable management strategies [9,17]. Although forests tend to be fundamental biogenic sources for nanomaterial synthesis and an essential application hub, there are currently limited reviews in the forestry domain [51,52].
This review paper evaluates and analyzes the information published over the past 25 years regarding the green synthesis of nanoparticles (NPs) from forest and woody plant species and their components. Before delving into the analysis, we summarize the key parameters influencing the nanoparticles synthesized from plant extracts.
The green synthesis of nanoparticles using forest plant extracts relies heavily on several critical parameters that impact the efficiency, morphology, and stability of the resulting nanomaterials. One of the most significant factors is the pH; alkaline conditions accelerate the reduction of metal ions, typically leading to smaller and more stable nanoparticles, while acidic conditions slow the reduction process, often resulting in larger particles or aggregates [20,23]. The temperature is another vital variable—higher synthesis temperatures can speed up the reduction and nucleation rates, often yielding smaller nanoparticles. However, excessively high temperatures may degrade the plant-derived biomolecules that act as capping and stabilizing agents, thereby diminishing the nanoparticles’ stability [31]. The concentration of the plant extract is also crucial, as the bioactive compounds within the extract reduce metal ions and stabilize the nanoparticles, necessitating an optimal concentration to balance nucleation and growth. Excessive concentrations may lead to particle agglomeration due to an overload of organic compounds [23,31]. Likewise, optimizing the concentration of the metal salt is essential; lower concentrations promote the formation of nanoparticles within the nanoscale range, while higher concentrations can cause uncontrolled nucleation and aggregation [31]. Furthermore, the reaction time and stirring significantly affect the synthesis process. Extended reaction times can improve the stability and uniformity of nanoparticles but may also lead to larger particle sizes. Stirring facilitates even interaction between metal ions and phytochemicals, enhancing the consistency of the particle morphology [32]. Finally, the method employed to extract phytochemicals from plant materials—such as boiling, cold maceration, or sonication—can affect both the type and amount of bioactive compounds, ultimately influencing the nanoparticles’ characteristics, such as their size, shape, and functional properties [33].
2.1. Biosynthesis of NPs Using Leaf Extracts
Leaf extracts from many tree species are used to prepare a broad range of metal and metal oxide NPs, as shown in Table 1. The leaves of Eucalyptus, Mimusops, Azadirachta, Ekebergia, Abies, Debregeasia, Dalbergia, and various other tree species are used in the synthesis of silver NPs [40,53,54,55,56,57]. Silver chloride NPs are also produced using the leaves of kaffir lime (Citrus hystrix) [58], and green zero-valent iron NPs are produced using oak (Quercus spp.), mulberry (Morus alba), and cherry (Prunus cerasus) tree leaves [59]. Gold and iron NPs are prepared using leaf extracts from Acer pentapomicum, Ficus benghalensis, and Albizia lebbeck [60,61,62].
Various oxide NPs, including CuO NPs, MgO NPs, ZnO NPs, Fe2O3 NPs, and others, are fabricated from the leaf extracts of woody tree species [47,63,64,65,66]. It is noted that the leaf is regarded as the most important part of forest and woody plants used in NP biosynthesis, perhaps due to its central functions of metabolic activity and metabolite storage. Biologically active metabolites, such as terpenes, phenols, glycosides, alkaloids, and others, with antimicrobial activity, detergent properties, and pharmacological applications, are mainly produced in plant leaves and will be relocated to other parts when required [67,68,69,70].

Table 1.
Biosynthesis, size characterization, and applications of nanoparticles (NPs) using leaf extracts from various tree species.
Table 1.
Biosynthesis, size characterization, and applications of nanoparticles (NPs) using leaf extracts from various tree species.
| Tree Species/Part Used | Tree Family | Chemical Substrate Used for NP Synthesis | Nanoparticles (NPs)/ Size of NPs | Applications of NPs | Reference |
|---|---|---|---|---|---|
| Eucalyptus camaldulensis/leaves | Myrtaceae | Silver nitrate (AgNO3) solution | Silver nanoparticles (Ag NPs) 8.7–26.5 nm |
| [41] |
| Eucalyptus camaldulensis/leaves | Myrtaceae | AgNO3 | Ag NPs ˂100 nm |
| [71] |
| Eucalyptus chapmaniana/leaves | Myrtaceae | AgNO3 | Ag NPs 60 nm |
| [72] |
| Ecalyptus camaldulensis/leaves | Myrtaceae | AgNO3 | Ag NPs 28–68 nm |
| [56] |
| Eucalyptus grandis/leaves | Myrtaceae | AgNO3 | Ag NPs 1.7–52.9 nm |
| [73] |
| Eucalyptus oleosa/leaves | Myrtaceae | AgNO3 | Ag NPs 21 nm |
| [74] |
| Mimusops elengi/leaves | Sapotaceae | AgNO3 | Ag NPs 4–28 nm |
| [54] |
| Azadirachta indica/leaves | Meliaceae | AgNO3 | Ag NPs 10–100 nm |
| [53] |
| Ekebergia capensis/leaves | Meliaceae | AgNO3 | Ag NPs 20–120 nm |
| [55] |
| Abies webbiana/leaf | Pinaceae | AgNO3 | AW-Ag NPs Not included |
| [57] |
| Debregeasia salicifolia/leaves | Urticaceae | AgNO3 | Ag NPs 38.15 nm |
| [75] |
| Carissa carandas/leaves | Apocynaceae | AgNO3 | Ag NPs 28–06 nm |
| [76] |
| Thevetia peruviana/leaves | Apocynaceae | AgNO3 | Ag NPs 6.4–39.4 nm |
| [77] |
| Dalbergia sissoo/leaf | Fabaceae | AgNO3 | Ag NPs 10–25 nm |
| [40] |
| Mimusops elengi/leaf | Sapotaceae | AgNO3 | Ag NPs 55–83 |
| [78] |
| Prosopis juliflora/leaf | Mimosaceae | AgNO3 | Ag NPs 10–20 nm |
| [79] |
| Ziziphus Jujuba/leaf | Rhamnaceae | AgNO3 | Ag NPs 20–30 nm |
| [80] |
| Acer pentapomicum/leaf | Sapindaceae | Gold chloride | Au NPs 19–24 nm |
| [62] |
| Albizia lebbeck/leaves | Mimosaceae | Fecl3·6H2O | Iron nanoparticles ,size not included |
| [61] |
| Diospyros montana/leaf extract | Ebenaceae | AgNO3 | Ag NPs 61.69 nm |
| [81] |
| Ficus benghalensis/leaf extract | Moraceae | AgNO3 | Ag NPs 16 nm |
| [82] |
| Ficus benghalensis/leaf extract | Moraceae | Gold chloride | Au NPs 2–100 nm |
| [60] |
| Juglans regia/leaf extract | Juglandaceae | AgNO3 | Ag NPs 10–50 nm |
| [83] |
| Citrus hystrix (kaffir lime)/leaves | Rutaceae | Silver nitrate and gelatin | Ag and AgCl nanoparticles 20–50 nm |
| [58] |
| Prosopis juliflora/leaf extract | Mimosaceae | AgNO3 | Ag NPs 35–60 nm |
| [84] |
| Oak (Quercus brantii)/leaves | Fagaceae | AgNO3 | Ag NPs 6 nm |
| [85] |
| Teak (Tectona grandis)/leaves | Verbenaceae | AgNO3 | Ag NPs 26–28 nm |
| [86] |
| Acacia nilotica/leaves | Mimosaceae | AgNO3 | Ag NPs 20 nm |
| [87] |
| Cestrum nocturnum/leaf extract | Solanaceae | AgNO3 | Ag NPs 20 nm |
| [36] |
| Dalbergia sissoo/leaf extract | Fabaceae | Mg(NO3)2·6H2O | MgO NPs ˂50 nm |
| [47] |
| Melia azedarach/leaf extract | Meliaceae | Zn(NO3)26H2O | MaZnO NPs 30–40 nm |
| [66] |
| Agrewia optiva and Prunus persica/leaf extracts | Malvaceae and Rosaceae | Iron(II) chloride tetrahydrate (FeCl2·4H2O) | Iron oxide nanoparticles 14–17 nm |
| [64] |
| Albizia lebbeck/leaf extract | Mimosaceae | Copper sulfate | Copper oxide nanoparticles (CuO NPs) ˂100 nm |
| [63] |
| Tectona grandis/leaf extract | Verbenaceae | Zinc nitrate | Zinc oxide nanoparticles (ZnO NPs) 54 nm |
| [88] |
| Terminalia catappa/leaf | Combretaceae | Nd(NO3)3 | Neodimium oxide nanoparticles (Nd2O3 NPs) 40–60 nm |
| [65] |
| Rhus punjabensis/leaf extract | Anacardiaceae | Ferric chloride | Iron oxide NPs 41.5 nm |
| [89] |
| Rosa brunonii/leaves | Rosaceae | AgNO3 | Ag NPs ˂100 nm |
| [89] |
| Oak (Quercus peatrea), mulberry (Morus alba), and cherry (Prunus cerasus)/leaves | Fagaceae, Moraceae, and Rosaceae | Fe(III) solution | Green zero-valent iron nanoparticles (nZVIs) 10–30 nm |
| [59] |
2.2. Biosynthesis of NPs Using Fruit and Seed Extracts
A wide range of extracts from the fruits and seeds of woody plant species are used to manufacture metal, metal oxide, and quantum dot NPs (Table 2). The pods of Acaia nilotica and Parkia speciosa tree species were used to biosynthesize silver, iron, and tin oxide quantum dot nanoparticles [45,90,91,92]. Silver NPs were prepared using the fruit of Ficus benghalensis [93], the fruit pericarp of Sapindus emarginatus [94], the fruit peel of Citrus species [95], the fruit hulls of Quercus species [96], the cones of pine tree species [97], and seed extracts from teak [98] and other tree species. Fruit bark extracts from the oak tree are used in the synthesis of palladium NPs [99], and the walnut (Juglans regia) tree fruit husk has been used to fabricate silver chloride NPs [100]. The fruit hull extract of the oak tree was also used in producing ZnO and CuO NPs [101], as well as bimetallic silver/zinc oxide NPs [44].

Table 2.
Biosynthesis, size characterization, and applications of nanoparticles (NPs) using fruit and seed extracts from various tree species.
2.3. Biosynthesis of NPs Using Stem Bark Extracts
The biosynthesis of nanomaterials using stem bark extracts from woody plant species is an increasing trend (Table 3). Bark extracts from numerous tree species from different tree families are used to fabricate metal NPs, particularly silver NPs [109,110]. Silver NPs have been produced using the bark extracts of tree species from the families Mimosaceae [7,46,111,112], Moraceae [110,113], and Combretaceae [42,114], as well as tree species such as Boswellia ovalifoliolata and Pinus eldarica [115,116]. As populations in many regions, such as Africa, South America, and East Asia, are still using raw stem bark in the treatment of various ailments, scientific investigations should continue to elucidate the types and amounts of bioactive metabolites in the bark of various tree species [117,118,119,120].

Table 3.
Biosynthesis, size characterization, and applications of nanoparticles (NPs) using stem bark extracts from various tree species.
2.4. Biosynthesis of NPs Using Extracts of Different Plant Parts and Constituents
Various review articles have described the green synthesis of NPs using different parts and exudates from woody plants, such as gums, latex, rubber, oils, pollens, roots, cellulose, and mixed extracts from the roots and stems (Table 4). The rubber of Hevea brasiliensis, gum of Anogeissus latifolia, and latex of Ficus sycomorus and Calotropis procera were used to produce silver NPs [122,123,124,125]. Silver NPs were also fabricated using pollens from pine [126], roots from palm [127], and extracts from an Acacia rigidula root stem mixture [128]. Furthermore, oil of bitter orange (Citrus aurantium), leaf cellulose from screw pine (Pandanus tectorius), stem fiber from rubberwood (Hevea brasiliensis), and the tree and fruit pulp from oil palm (Elaeis guineensis) were used in the biosynthesis of chitosan NPs, cellulose nanocrystals, and cellulose nanofibers, respectively [129,130,131]. This broad range of extracts and constituents gained from various parts of woody plants, with potential suitability for NP fabrication, offers a promising and sustainable basis for biogenic resource-based nanotechnology if it is managed well.

Table 4.
Biosynthesis, size characterization, and applications of nanoparticles (NPs) using extracts and constituents of various parts of different tree species.
3. Applications of Woody Plant-Based Biosynthesized NPs
As shown in Table 1, Table 2, Table 3 and Table 4, applications of woody plant-based biosynthesized NPs as nanofertilizers and stimulators for plant growth have been documented. It has been reported that Ag NPs can be used to promote Acacia senegal and Acacia mellifera growth [41], and Fe2O3 NPs in Photinia fraserii and Cotinus coggygria promoted tree seedling growth [132]. Zinc oxide nanoparticles extracted from Citrus limon and Melia azedarach tree leaves were applied as pesticides for the protection of sweet potato and soybean, respectively, against pathogens [66,105], and chitosan NPs derived from Citrus aurantium were used to preserve the postharvest quality of button mushrooms [131]. According to the available literature, most biosynthesized nanoparticles from tree species have been used and tested for medical and antimicrobial applications. Silver NPs extracted from many tree species and families show biomedical effects [78,79] and antibacterial [40,55,95,121], antifungal [77,126], antioxidant [62,76], anticancer [107,111], and toxic degradation properties [79,80]. Iron and copper oxide nanoparticles obtained from Acacia nilotica exhibited an ability to eliminate several pathogens from human samples [91,104]. Gold NPs from Ficus benghalensis [60], iron oxide NPs from Agrewia optiva and Prunus persica [64], and zinc oxide NPs extracted from teak trees [88] have demonstrated potent antimicrobial activity against some Gram-positive and -negative bacteria.
It is worth mentioning that green synthesis may have negative consequences for the environment and plant habitats as the processes imply modifications in cellular, genetic, and molecular aspects. The nanoparticles’ quality, stability, size, and shape vary from one tree species to another, and the phytochemical richness significantly differs across plant species [17,133]. Therefore, the assessment of green synthesis and the applications of nanoparticles, their benefits, and their drawbacks must be performed concurrently. For sustainable research on nanotechnology in forestry, the coverage of a large range of tree species, alongside adopting an interdisciplinary collaboration approach that involves different institutions worldwide, would be of prime interest.
4. Comparative Overview
The analysis of the studies indicates that the leaves are the most frequently utilized plant parts for nanoparticle synthesis, comprising roughly 47% of all cases identified in our review. This preference likely stems from the high concentrations of bioactive phytochemicals in the leaves, which promote effective nanoparticle formation. Following the leaves, the fruits, seeds, and related structures (such as the fruit pericarp, testa, husk, hull, and cones) account for approximately 21% of the total, while the bark contributes 17%. Other plant parts, like the stems, roots, latex/gum/oil, and pollen, are used less often, together representing the remaining proportion.
This strong preference for leaves arises from several factors. Leaves are typically more accessible and renewable than other plant parts, which enables sustainable harvesting without causing significant harm to the plant. More importantly, they are abundant in a variety of bioactive phytochemicals, such as flavonoids, phenolics, and tannins, which act as effective reducing and stabilizing agents in the green synthesis of nanoparticles. Additionally, the large surface area and high metabolic activity of the leaves further improve the extraction of these compounds, leading to more efficient and reproducible nanoparticle formation.
Ag NPs represent the most widely synthesized nanoparticles, comprising 75% of the syntheses exhibited in this review. This dominance is primarily due to their well-established broad-spectrum antimicrobial properties, which make them highly sought after for a range of biomedical, environmental, and industrial uses. Silver ions are notably effective against numerous pathogenic microorganisms, with their antimicrobial effectiveness significantly heightened when reduced to the nanoscale, thanks to their increased surface areas and reactivity. Additionally, the synthesis of Ag NPs using plant extracts is an environmentally friendly process that is both straightforward and efficient. Plant phytochemicals like flavonoids, phenolics, tannins, and terpenoids function as reducing and stabilizing agents, facilitating the rapid and eco-conscious conversion of silver ions into stable nanoparticles. The interplay between silver chemistry and plant biochemistry allows a wide variety of plant species and parts to be used for Ag NP production, usually under mild reaction conditions and without requiring toxic chemicals or high-energy inputs. Moreover, the observable color change during Ag NP formation provides a simple, immediate sign of successful synthesis, enhancing their appeal in research.
Other nanoparticles, such as zinc oxide, gold, iron, copper oxide, and others, make up the remaining 25%. While these alternative nanoparticles showcase valuable properties, like photocatalytic activity, antioxidant effects, and targeted therapeutic potential, their synthesis using plant extracts is often more complex or less efficient. Additionally, despite their growing ranges of applications, they do not enjoy the same widespread recognition as Ag NPs. Consequently, Ag NPs continue to be the primary focus of research in plant-mediated nanoparticles, thanks to their proven effectiveness, straightforward synthesis, and versatility across diverse scientific and practical domains.
In terms of application, plant-derived nanoparticles are predominantly used for their antimicrobial properties, making up about 69% of the reported applications. This significant emphasis highlights the large global demand for innovative strategies to address microbial pathogens, particularly in light of the alarming increase in antibiotic-resistant bacteria and the drawbacks of traditional antimicrobial agents. Silver nanoparticles (Ag NPs), in particular, demonstrate broad-spectrum effectiveness against various bacteria, including both Gram-positive and Gram-negative types, as well as some fungi and viruses. Their potent antimicrobial activity stems from their capacity to disrupt microbial cell membranes, produce reactive oxygen species, and affect critical cellular functions, rendering them highly effective even against drug-resistant strains. The preference for antimicrobial research is also influenced by the straightforward and clear testing methods available, along with the direct and quantifiable results that they yield. Additionally, employing plant extracts for nanoparticle synthesis creates distinctive phytochemical coatings on the nanoparticles’ surfaces, enhancing their biocompatibility and further elevating their antimicrobial efficacy. This interaction between plant-derived substances and metallic nanoparticles significantly contributes to the prevalence of antimicrobial applications in the field.
Other significant applications include antioxidants (9%), anticancer/antiproliferative agents (13%), and environmental remediation or catalytic functions, like dye degradation (14%). Antioxidant uses harness the radical-scavenging properties of both nanoparticles and their phytochemical capping agents, offering potential advantages in food preservation, cosmetics, and medicine. The use of plant-based nanoparticles in anticancer and antiproliferative contexts is on the rise, with multiple studies showing that these nanoparticles can trigger apoptosis or slow down the growth of various cancer cell lines, typically with lower toxicity to healthy cells compared to traditional chemotherapeutics. Environmental remediation and catalytic functions, including the degradation of harmful dyes and pollutants, highlight the promise of these nanomaterials for sustainable water treatment and pollution management. Although antifungal and other specialized applications are reported less frequently, they are becoming increasingly noteworthy as the adaptability of plant-based nanoparticles gains recognition.
These trends collectively underscore the crucial role of leaf-derived phytochemicals in the eco-friendly synthesis of silver nanoparticles, which are primarily studied for their potent antimicrobial properties. At the same time, the data show a growing, albeit still secondary, interest in expanding the use of plant-based nanomaterials to address broader challenges in medicine, environmental remediation, and industry. This trend reflects both the proven efficacy and adaptability of Ag NPs and ongoing research into new plant sources, types of nanoparticles, and novel applications to meet future technological and societal demands.
5. Current Challenges and Research Opportunities in Forest Tree-Based Nanoparticle Synthesis
The synthesis of forest tree-based nanoparticles provides an eco-friendly alternative to traditional methods. Nevertheless, various challenges limit its broader use.
- The biochemical composition of tree extracts can vary due to the species, age, and environmental conditions, significantly impacting the nanoparticle synthesis outcomes. This variability influences the nanoparticle size, shape, and stability. For example, a study comparing silver nanoparticle synthesis with the use of extracts from collard greens, hazelnut, and green tea revealed that green tea extract, abundant in phenolic compounds, accelerated nanoparticle formation and produced smaller, more stable particles. In contrast, collard greens, having lower phenolic content, resulted in less effective nanoparticle synthesis with larger, more polydisperse particles [134]. Additionally, seasonal changes can modify plants’ phytochemical profiles [135].
- The lack of standardized protocols for plant-mediated nanoparticle synthesis creates considerable challenges regarding reproducibility and scalability. Differences in the plant species, cultivation conditions, and extraction methods may lead to inconsistencies in properties such as the size, shape, and stability of nanoparticles. For instance, variability in the phytochemical content of extracts, influenced by environmental and species factors, can cause batch-to-batch discrepancies in the synthesis results. This highlights the urgent need to standardize the extraction and synthesis methods to achieve consistent and reproducible outcomes across various studies and applications.
- The mass production and disposal of nanoparticles present serious environmental hazards, mainly because their release into air, water, and soil can result in ecosystem contamination and toxicity. Manufactured nanomaterials can enter the environment through intentional and accidental means, including emissions from production sites, waste discharge, spills during transport, and the degradation of consumer items [136].
- This necessitates detailed impact assessments. Research indicates that NPs can trigger oxidative stress, DNA damage, and inflammation in various organisms, threatening human health and ecosystems [137]. Furthermore, releasing nanoparticles into the environment might result in their accumulation in soil and water, potentially disrupting microbial communities and nutrient cycles [138]. Thus, a thorough evaluation of the environmental implications of nanoparticle synthesis and their applications is crucial, ensuring the adoption of sustainable practices to mitigate the adverse effects.
- Despite these obstacles, there are encouraging research prospects.
- The phytochemical profiling of tree extracts is vital in pinpointing the key compounds involved in NP synthesis, facilitating more controlled and efficient processes. Research has shown that various bioactive compounds in plant extracts, including polyphenols, flavonoids, terpenoids, and alkaloids, can serve as reducing and stabilizing agents during NP formation [139]. This highlights the significance of comprehensive phytochemical analysis in uncovering the nanoparticle synthesis mechanisms and optimizing the processes for targeted results.
- Utilizing forest waste materials such as sawdust, bark, and leaves for nanoparticle synthesis provides a sustainable approach that diminishes the environmental impact while also enhancing the value of by-products usually seen as waste. For example, lignin nanoparticles (LNPs) have been effectively extracted from the sawdust of Iroko (Milicia excelsa) and Norway spruce (Picea abies) trees. These LNPs were subsequently used to coat beech (Fagus sylvatica) wood, increasing its resistance to artificial weathering, showcasing wood waste’s potential in generating functional nanomaterials [140]. This emphasizes the practicality of using forest waste materials in nanoparticle synthesis, encouraging sustainability and reducing waste in the forest product industry.
- Environmental impact studies play a critical role in shaping the development of eco-friendly strategies for nanoparticle synthesis. Life cycle assessments (LCAs) have been utilized to evaluate the environmental effects of green synthesis methods versus traditional ones, showcasing advantages like decreased emissions and energy use. For example, an LCA focused on iron oxide nanoparticle synthesis using natural extracts highlighted a more sustainable approach with lower environmental burdens [141]. These evaluations reinforce the necessity of implementing green synthesis techniques to lessen ecological footprints and advocate for sustainability in the production of nanomaterials.
6. Conclusions
A review of the existing literature on the green synthesis of nanoparticles indicates that various forest and woody plants from diverse species and families serve as biogenic sources. The significant advancements in nanotechnology can be applied to harness the extensive resources and biodiversity found in forests to create a variety of nanostructured materials and composites. Green biosynthesis provides an environmentally friendly, cost-effective, and biocompatible method of creating nanoparticles, especially when compared to traditional chemical methods. The application of bio-based nanoparticles has garnered particular interest due to their potential roles in plant enhancement, medical applications, and industrial growth, among others. In the forestry sector, biotechnology is recognized as a valuable avenue for the transformation of forest products into nanoparticles with added value, serving as a means for commercial production and conservation initiatives. A review of the current academic literature shows that the most commonly biosynthesized nanoparticles from tree species have been explored for their medical and antimicrobial properties. However, adverse effects, particularly those of high concentrations of nanoparticles on environmental safety and plant health, have also been documented. Further research into nanotechnology’s applications is necessary to thoroughly evaluate the potential benefits and drawbacks related to various forestry aspects. Fostering sustainable nanotechnology research in forestry, including a broad range of tree species and families, is crucial, as is establishing interdisciplinary collaborations among scientific and development organizations. The variability in the phytochemical compositions among tree species and the lack of standardized protocols for the preparation of extracts and the synthesis of nanoparticles can impede the reproducibility of results. Additionally, the environmental implications of nanoparticle production and disposal raise significant concern, highlighting the need for thorough assessments to encourage eco-friendly practices. Opportunities exist to improve phytochemical profiling to pinpoint key compounds for nanoparticle synthesis, use forest waste, and employ advanced characterization methods to better understand nanoparticles’ properties and interactions. These efforts could enhance sustainable practices in nanotechnology within the forestry sector while mitigating the environmental impact risks.
Author Contributions
A.M.J.S. conceptualized and wrote the original draft. R.S. reviewed the draft. R.A.-Z. revised the aspects on nanotechnology and reshaped the figures. N.S. and R.M.A. reviewed the aspects on chemical substrates and tree taxonomy, respectively. All authors edited and provided a substantial review of the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The first author thanks the International Institute for Education’s Scholar Rescue Fund (IIE-SRF) for offering a Visiting Fellowship, and the University of Jordan for hosting and providing the research facilities.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- von Maydell, H.-J. Trees and Shrubs of the Sahel: Their Characteristics and Uses, 2nd ed.; Verlag Josef Margraft: Weikersheim, Germany, 1990; pp. 1–407. [Google Scholar]
- Dharan, N. Trees and Shrubs of East Africa, 2nd ed.; Struik Publishers (Pty) Ltd.: Cape Town, South Africa, 2005; p. 4. [Google Scholar]
- Freer-Smith, P.H.; Broadmeadow, M.S.; Lynch, J.M. Forests and Climate Change: The Knowledge-base for Action. In Forestry & Climate Change; Freer-Smith, P.H., Broadmeadow, M.S., Lynch, J.M., Eds.; CAB International: Oxfordshire, UK, 2007; pp. 7–14. [Google Scholar]
- Marshall, E. Health and Wealth from Medicinal Aromatic Plants: Diversification Booklet 17; FAO: Rome, Italy, 2011; pp. 1–23. [Google Scholar]
- Allona, I.; Kirst, M.; Boerjan, W.; Strauss, S.; Sederoff, R. Editorial: Forest Genomics and Biotechnology. Front. Plant Sci. 2019, 10, 1187. [Google Scholar] [CrossRef] [PubMed]
- Pelai, R.; Hagerman, S.; Kozak, R. Biotechnologies in agriculture and forestry: Governance insights from a comparative systematic review of barriers and recommendations. For. Policy Econ. 2020, 117, 102191. [Google Scholar] [CrossRef]
- Murugan, K.; Senthilkumar, B.; Senbagam, D.; Al-Sohaibani, S. Biosynthesis of silver nanoparticles using Acacia leucophloea extract and their antibacterial activity. Int. J. Nanomed. 2014, 9, 2431–2438. [Google Scholar]
- Myburg, A.A.; Hussey, S.; Wang, J.; Street, N.R.; Mizrachi, E. Systems and Synthetic Biology of Forest Trees: A Bioengineering Paradigm for Woody Biomass Feedstocks. Front. Plant Sci. 2019, 10, 775. [Google Scholar] [CrossRef]
- Bett, L.A.; Auer, C.; Karp, S.; Maranho, L. Forest biotechnology: Economic aspects and conservation implications. J. Biotechnol. Biodivers. 2021, 9, 107–117. [Google Scholar] [CrossRef]
- Chimene, A.F.; Julien, H.; Sidoine, M. Effect of Saline Stress on the Growth and Physiological Behavior of Young Planting Acacia nilotica in Nursery and After Transplantation. Plant 2024, 12, 131–141. [Google Scholar] [CrossRef]
- Niu, S.; Ding, J.; Xu, C.; Wang, J. Modern and future forestry based on biotechnology. Mod. Agric. 2023, 1, 27–33. [Google Scholar] [CrossRef]
- Balantrapu, K.; Goia, D.V. Silver nanoparticles for printable electronics and biological applications. J. Mater. Res. 2009, 24, 2828–2836. [Google Scholar] [CrossRef]
- Shibli, R.; Mohusaien, R.; Abu-Zurayk, R.; Qudah, T.; Tahtamouni, R. Silver Nanoparticles (Ag NPs) Boost Mitigation Powers of Chenopodium quinoa (Q6 Line) Grown under In Vitro Salt-Stressing Conditions. Water 2022, 14, 3099. [Google Scholar] [CrossRef]
- Kathiravan, V.; Ravi, S.; Velmurugan, A.; Elumalai, V.; Khatiwada, C. Green synthesis of silver nanoparticles using Croton sparsiflorus morong leaf extract and their antibacterial and antifungal activities. Spectrochim. Acta PartA Mol. Biomol. Spectrosc. 2015, 139, 200–205. [Google Scholar] [CrossRef]
- Malik, S.; Muhammad, K.; Waheed, Y. Nanotechnology: A Revolution in Modern Industry. Molecules 2023, 28, 661. [Google Scholar] [CrossRef] [PubMed]
- Javad, S.; Ghaffar, N.; Naseer, I.; Jabeen, K.; Aftab, A.; Shaheen, S. Antibacterial activity of phyto-mediated silver nanoparticles developed from Melia azedarach. Period. Biol. 2017, 119, 107–111. [Google Scholar] [CrossRef]
- Husen, A.; Jawaid, M. Nanomaterials for Agriculture and Forestry Applications; Husen, A., Jawaid, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 15–100. [Google Scholar]
- Zaheer, Z.; Rafiuddin. Silver nanoparticles to self-assembled films: Green synthesis and characterization. Colloids Surf. B Biointerfaces 2012, 90, 48–52. [Google Scholar] [CrossRef]
- Lokina, S.; Stephen, A.; Kaviyarasan, V.; Arulvasu, C.; Narayanan, V. Cytotoxicity and antimicrobial activities of green synthesized silver nanoparticles. Eur. J. Med. Chem. 2014, 76, 256–263. [Google Scholar] [CrossRef]
- Husen, A.; Siddiqi, K.S. Phytosynthesis of nanoparticles: Concept, controversy and application. Nano Res. Lett. 2014, 9, 229. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A.; Rao, R.A. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol 2018, 16, 14. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Babu, R.S.; Venkataraman, D.; Bilal, D.; Gurunathan, S.S. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf. Biointerfaces 2008, 65, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Das, R.K.; Pachapur, V.; Lonappan, L.; Naghdi, M.; Pulicharla, R.; Maiti, S.; Cledon, M.; Dalila, L.M.A.; Sarma, S.J.; Brar, S.K. Biological synthesis of metallic nanoparticles: Plants, animals and microbial aspects. Nanotechnol. Environ. Eng. 2017, 2, 18. [Google Scholar] [CrossRef]
- Sreekanth, T.V.; Nagajyothi, P.; Muthuraman, P.; Enkhtaivan, G.; Vattikuti, S.; Tettey, C.; Kim, D.H.; Shim, J.; Yoo, K. Ultra-sonication-assisted silver nanoparticles using Panax ginseng root extract and their anti-cancer and antiviral activities. J. Photochem. Photobiol. B Biol. 2018, 188, 6–11. [Google Scholar] [CrossRef]
- McKenzie, L.C.; Hutchison, J.E. Green nanoscience. Chim. Oggi 2004, 22, 30–33. [Google Scholar]
- Krishnaswamy, K.; Orsat, V. Insight into the nanodielectric properties of gold nanoparticles synthesized from maple leaf and pine needle extracts. Ind. Crops Prod. 2015, 66, 131–136. [Google Scholar] [CrossRef]
- dos Santos, R.M.; Neto, W.; Silverio, H.; Martins, D.; Dantas, N.; Pasquini, D. Cellulose nanocrystals from pineapple leaf: A new approach for the reuse of this agro-waste. Ind. Crops Prod. 2013, 50, 707–714. [Google Scholar] [CrossRef]
- Venkatesham, M.; Ayodhya, D.; Madhusudhan, A.; Veerabhadram, G. Synthesis of Stable Silver Nanoparticles Using Gum Acacia as Reducing and Stabilizing Agent and Study of Its Microbial Properties: A Novel Green Approach. Int. J. Green Nanotechnol. 2013, 4, 199–206. [Google Scholar] [CrossRef]
- Zhang, J.; Song, H.; Lin, L.; Zhuang, J.; Pang, C.; Liu, S. Microfibrillated cellulose from bamboo pulp and its properties. Biomass Bioenergy 2012, 39, 78–83. [Google Scholar] [CrossRef]
- Verma, S.K.; Kumar, P.; Mishra, A.; Khare, R.; Singh, D. Green nanotechnology: Illuminating the effects of bio-based nanoparticles on plant physiology. Biotechnol. Sustain. Mater. 2024, 1, 1. [Google Scholar] [CrossRef]
- Song, J.Y.; Kim, B.S. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess. Biosyst. Eng. 2009, 32, 79–84. [Google Scholar] [CrossRef]
- Rasheed, T.; Bilal, M.; Iqbal, H.; Li, C. Green biosynthesis of silver nanoparticles using leaves extract of Artemisia vulgaris and their potential biomedical applications. Colloids Surf. B Biointerfaces 2017, 158, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Alara, O.R.; Abdurahman, N.; Ukaegbu, C. Soxhlet extraction of phenolic compounds from Vernonia cinerea leaves and its antioxidant activity. J. Appl. Res. Med. Aromat. Plants 2018, 11, 12–17. [Google Scholar] [CrossRef]
- Sabela, M.; Talent Makhanya, T.; Kanchi, S.; Shahbaaz, M.; Idress, D.; Bisetty, K. One-pot biosynthesis of silver nanoparticles using Iboza Riparia and Ilex Mitis for cytotoxicity on human embryonic kidney cells. J. Photochem. Photobiol. B Biol. 2018, 178, 560–567. [Google Scholar] [CrossRef]
- Anwar, A.; Masri, A.; Rao, K.; Rajendran, K.; Khan, N.; Shah, M.; Siddiqui, R. Antimicrobial activities of green synthesized gums-stabilized nanoparticles loaded with flavonoids. Sci. Rep. 2019, 9, 3122. [Google Scholar] [CrossRef]
- Keshari, A.K.; Srivastava, R.; Singh, P.; Yadav, V.; Nath, G. Antioxidant and antibacterial activity of silver nanoparticles synthesized by Cestrum nocturnum. J. Ayurveda Integr. Med. 2020, 11, 37–44. [Google Scholar] [CrossRef]
- Wu, C.; Chen, D. Facile green synthesis of gold nanoparticles with gum arabic as a stabilizing agent and reducing agent. Gold Bull. 2010, 43, 234–240. [Google Scholar] [CrossRef]
- Tonoli, G.H.; Teixeira, E.; Correa, A.; Marconcini, J.; Caixeta, L.; Silvac, M.; Mattoso, L. Cellulose micro/nanofibres from Eucalyptus kraft pulp: Preparation and properties. Carbohydr. Polym. 2012, 89, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Vasyukova, I.; Gusev, A.; Zakharova, O.; Baranchikov, P.; Yevtushenko, N. Silver nanoparticles for enhancing the efficiency of micropropagation of gray poplar (Populus × canescens Aiton. Sm.). For. IOP Conf. Ser. Earth Environ. Sci. 2021, 875, 012053. [Google Scholar] [CrossRef]
- Khatun, H.; Alam, S.; Abdul Aziz, M.; Karim, M.R.; Rahman, M.H.; Rabbi, M.A.; Habib, M.R. Plant-assisted green preparation of silver nanoparticles using leaf extract of Dalbergia sissoo and their antioxidant, antibacterial and catalytic applications. Bioprocess Biosyst. Eng. 2024, 47, 1347–1362. [Google Scholar] [CrossRef] [PubMed]
- Abdalkreem, I.H.; Zayed, M.Z.; Shetta, N.D.; Yacout, M.M. Green synthesis of AgNPs and their effects on seed germination and seedling vigor of Acacia senegal and Acacia mellifera. J. Trop. For. Sci. 2024, 36, 391–401. [Google Scholar] [CrossRef]
- Edison, T.N.J.; Lee, Y.R.; Sethuraman, M.G. Green synthesis of silver nanoparticles using Terminalia cuneata and its catalytic action in reduction of direct yellow-12 dye. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 161, 122–129. [Google Scholar] [CrossRef]
- Mohanty, A.S.; Jena, B.S. Innate catalytic and free radical scavenging activities of silver nanoparticles synthesized using Dillenia indica bark extract. J. Colloid Interface Sci. 2017, 496, 513–521. [Google Scholar] [CrossRef]
- Sorbiun, M.; Mehr, E.; Ramazani, A.; Fardood, S. Biosynthesis of Ag, ZnO and bimetallic Ag/ZnO alloy nanoparticles by aqueous extract of oak fruit hull (Jaft) and investigation of photocatalytic activity of ZnO and bimetallic Ag/ZnO for degradation of basic violet 3 dye. J. Mater. Sci. Mater. Electron. 2018, 29, 2806–2814. [Google Scholar] [CrossRef]
- Begum, S.; Ahmaruzzaman, M. Green synthesis of SnO2 quantum dots using Parkia speciosa Hassk pods extract for the evaluation of anti-oxidant and photocatalytic properties. J. Photochem. Photobiol. B Biol. 2018, 184, 44–53. [Google Scholar] [CrossRef]
- Arya, G.; Kumari, R.M.; Pundir, R.; Chatterjee, S.; Gupta, N.; Kumar, A.; Chandra, R.; Nimesh, S. Versatile biomedical potential of biosynthesized silver nanoparticles from Acacia nilotica bark. J. Appl. Biomed. 2019, 17, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Akhtar, M.; Naveed Ashraf, N.; Najeeb, J.; Munir, H.; Awan, T.; Tahir, M.; Kabli, M. Green synthesis of magnesium oxide nanoparticles using Dalbergia sissoo extract for photocatalytic activity and antibacterial efficacy. Appl. Nanosci. 2020, 10, 2351–2364. [Google Scholar] [CrossRef]
- Yadaf, V.; Gupta, V. Comparative Analysis of Green Synthesis and Chemical Synthesis of Nanoparticles and its Applications. IJEP 2023, 43, 1022–1035. [Google Scholar]
- Irewale, A.T.; Dimkpa, C.; Agunbiade, F.; Oyetunde, O.; Elemike, E.; Oguzie, E. Unlocking sustainable agricultural development in Africa via bio-nanofertilizer application—Challenges, opportunities and prospects. Sci. Afr. 2024, 25, e02276. [Google Scholar] [CrossRef]
- Prasad, R.; Kumar, V.; Prasad, K.S. Nanotechnology in sustainable agriculture: Present concerns and future aspects. Afr. J. Biotechnol. 2014, 13, 705–713. [Google Scholar]
- Wagay, O.; Khan, S.; Rafeeq, J.; Pala, N.; Bhat, G.; Dutt, V.; Peerzada, I.A.; Malik, A.; Sofi, P.; Mushtaq, T.; et al. Nanotechnology and its potential application in forest and forest-based industries: A review. SKUAST J. Res. 2023, 25, 527–537. [Google Scholar] [CrossRef]
- Singh, D.; Sillu, D.; Kumar, A.; Agnihotri, S. Dual nanozyme characteristics of iron oxide nanoparticles alleviate salinity stress and promote the growth of an agroforestry tree, Eucalyptus tereticornis Sm. Environ. Sci. Nano 2021, 8, 1308–1325. [Google Scholar] [CrossRef]
- Tripathy, A.; Raichur, A.M.; Chandrasekaran, N.; Prathna, T.C.; Mukherjee, A. Process variables in biomimetic synthesis of silver nanoparticles by aqueous extract of Azadirachta indica (Neem) leaves. J. Nanopart Resour. 2010, 12, 237–246. [Google Scholar] [CrossRef]
- Ahmad, R.; Parrey, S.H.; Faisal, Q. Role of Cetyltrimethylammonium Bromide in the Green Synthesis of Silver Nanoparticles Using Mimusops elengi, Linn. (Maulsari) Leaf Extract. Adv. Nanoparticles 2016, 5, 44–52. [Google Scholar] [CrossRef]
- Anand, K.; Kaviyarasu, K.; Muniyasamy, S.; Roopan, S.; Gengan, R.; Chuturgoon, A. Bio-Synthesis of Silver Nanoparticles Using Agroforestry Residue and Their Catalytic Degradation for Sustainable Waste Management. J. Clust. Sci. 2017, 28, 2279–2291. [Google Scholar] [CrossRef]
- Alghoraibi, I.; Soukkarieh, C.; Zein, R.; Alahmad, A.; Walter, J.; Daghestani, M. Aqueous extract of Eucalyptus camaldulensis leaves as reducing and capping agent in biosynthesis of silver nanoparticles. Inorg. Nano-Met. Chem. 2020, 50, 895–902. [Google Scholar] [CrossRef]
- Sowmiya, K.; Prakash, J.T. Green-synthesis of silver nanoparticles using Abies webbiana leaves and evaluation of its antibacterial activity. J. Pharmacogn. Phytochem. 2018, 7, 2033–2036. [Google Scholar]
- Chankaew, C.; Somsri, S.; Tapala, W.; Mahatheeranont, S.; Saenjum, C.; Rujiwatra, A. Kaffir lime leaf extract mediated synthesis, anticancer activities and antibacterial kinetics of Ag and Ag/AgCl nanoparticles. Particuology 2018, 40, 160–168. [Google Scholar] [CrossRef]
- Poguberovic, S.; Krcmar, D.; Maletic, S.; Konya, Z.; Pilipovic, D.; Kerkez, D.; Roncevic, S. Removal of As(III) and Cr(VI) from aqueous solutions using “green” zero-valent iron nanoparticles produced by oak, mulberry and cherry leaf extracts. Ecol. Eng. 2016, 90, 42–49. [Google Scholar] [CrossRef]
- Francis, G.; Thombre, R.; Parekh, F.; Leksminarayan, P. Bioinspired Synthesis of Gold Nanoparticles Using Ficus benghalensis (Indian Banyan) Leaf Extract. Chem. Sci. Trans. 2014, 3, 470–474. [Google Scholar]
- Raju, C.A.I.; Bharadwaj, M.S.; Prem, K.; Satyanandam, K. Green Synthesis of Iron Nanoparticles using Albizia lebbeck leaves for Synthetic Dyes decolorization. Int. J. Sci. Eng. Technol. Res. 2016, 5, 3429–3434. [Google Scholar]
- Khan, S.; Bakht, J.; Syed, F. Green synthesis of gold nanoparticles using Acer pentapomicum leaves extract its characterization, antibacterial, antifungal and antioxidant bioassay. Dig. J. Nanomater. Biostructures 2018, 13, 579–589. [Google Scholar]
- Jayakumarai, G.; Gokulpriya, C.; Sudhapriya, R.; Sharmila, G.; Muthukumaran, C. Phytofabrication and characterization of monodisperse copper oxide nanoparticles using Albizia lebbeck leaf extract. Appl. Nanosci. 2015, 5, 1017–1021. [Google Scholar] [CrossRef]
- Mirza, A.U.; Kareem, A.; Nami, S.A.; Khan, M.S.; Rehman, S.; Bhat, S.A.; Mohammad, A.; Nishat, N. Biogenic synthesis of iron oxide nanoparticles using Agrewia optiva and Prunus persica phyto species: Characterization, antibacterial and antioxidant activity. J. Photochem. Photobiol. B Biol. 2018, 185, 262–274. [Google Scholar] [CrossRef]
- Lembang, M.S.; Yulizar, Y.Y.; Sudirman, S.; Apriandanu, D. A Facile Method for Green Synthesis of Nd2O3 Nanoparticles Using Aqueous Extract of Terminalia Catappa Leaf. AIP Conf. Proc. 2017, 2023, 020093-1–020093-6. [Google Scholar] [CrossRef]
- Lakshmeesha, T.R.; Murali, M.M.; Ansari, M.A.; Udayashankar, A.C.; Alzohairy, M.A.; Almatroudi, A.; Asiri, S.M.M.; Ashwini, B.; Kalagatur, N.K.; Nayak, C.S.; et al. Biofabrication of zinc oxide nanoparticles from Melia azedarach and its potential in controlling soybean seed-borne phytopathogenic fungi. Saudi J. Biol. Sci. 2020, 27, 1923–1930. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Huner, N.P.A. Introduction to Plant Physiology, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 459–479. [Google Scholar]
- Faleva, A.V.; Ulyanovskii, N.; Onuchina, A.; Falev, D.; Kosyakov, D. Comprehensive Characterization of Secondary Metabolites in Fruits and Leaves of Cloudberry (Rubus chamaemorus L.). Metabolites 2023, 13, 598. [Google Scholar] [CrossRef]
- Puri, J.B.; Shaikh, A.; Dhuldhaj, U.P. Moringa Leaves with Beneficial Secondary Metabolites. Int. J. Adv. Biol. Biomed. Res. 2023, 11, 164–171. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, X.; Yang, H.; Zhang, S.; Lyu, L.; Li, W.; Wu, W. Analysis of flavonoid-related metabolites in different tissues and fruit developmental stages of blackberry based on metabolome analysis. Food Res. Int. 2023, 163, 112313. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.E. Green synthesis, antimicrobial and cytotoxic effects of silver nanoparticles mediated by Eucalyptus camaldulensis leaf extract. Asian Pac. J. Trop. Biomed. 2015, 5, 382–386. [Google Scholar] [CrossRef]
- Sulaiman, G.M.; Mohammed, W.H.; Marzoog, T.R.; AlAmiery, A.A.; Kadhum, A.H.; Mohamad, A. Green synthesis, antimicrobial and cytotoxic effects of silver nanoparticles using Eucalyptus chapmaniana leaves extract. Asian Pac. J. Trop. Biomed. 2013, 3, 58–63. [Google Scholar] [CrossRef]
- Oliveira, L.M.F.; da Silva, U.P.; Braga, J.P.; Teixeira, A.V.; Ribon, A.; Varejão, E.; Coelho, E.; de Freitas, C.; Teixeira, R.; Moreira, R.; et al. Green Synthesis, Characterization and Antibacterial and Leishmanicidal Activities of Silver Nanoparticles Obtained from Aqueous Extract of Eucalyptus grandis. J. Braz. Chem. Soc. 2023, 34, 527–536. [Google Scholar] [CrossRef]
- Pourmortazavi, S.M.; Taghdiri, M.; Makari, V.; Rahimi-Nasrabadi, M. Procedure optimization for green synthesis of silver nanoparticles by aqueous extract of Eucalyptus oleosa. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 1249–1254. [Google Scholar] [CrossRef]
- Khan, J.; Irsa Naseem, I.; Bibi, S.; Ahmad, S.; Altaf, F.; Hafeez, M.; Almoneef, M.M.; Ahmad, K. Green Synthesis of Silver Nanoparticles (Ag-NPs) Using Debregeasia Salicifolia for Biological Applications. Materials 2023, 16, 129. [Google Scholar] [CrossRef]
- Singh, D.; Kumar, V.; Yadav, E.; Falls, N.; Singh, M.; Komal, U.; Verma, A. One-pot green synthesis and structural characterization of silver nanoparticles using aqueous leaves extract of Carissa carandas: Antioxidant, anticancer and antibacterial activities. IET Nanobiotechnology 2018, 12, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Oluwaniyi, O.O.; Adegoke, H.I.; Adesuji, E.T.; Alabi, A.B.; Bodede, S.O.; Labulo, A.H.; Oseghale, C.O. Biosynthesis of silver nanoparticles using aqueous leaf extract of Thevetia peruviana Juss and its antimicrobial activities. Appl. Nanosci. 2016, 6, 903–912. [Google Scholar] [CrossRef]
- Prakash, P.; Gnanaprakasama, P.; Emmanuel, R.; Arokiyaraj, S.; Saravanan, M. Green synthesis of silver nanoparticles from leaf extract of Mimusops elengi, Linn. for enhanced antibacterial activity against multi drug resistant clinical isolates. Colloids Surf. B Biointerfaces 2013, 108, 255–259. [Google Scholar] [CrossRef]
- Arya, G.; Kumari, R.M.; Sharma, N.; Gupta, N.; Kumar, A.; Chatterjee, S.; Nimesh, S. Catalytic, antibacterial and antibiofilm efficacy of biosynthesized silver nanoparticles using Prosopis juliflora leaf extract along with their wound healing potential. J. Photochem. Photobiol. B Biol. 2019, 190, 50–58. [Google Scholar] [CrossRef]
- Gavade, N.L.; Kadam, A.N.; Suwarnkar, M.B.; Ghodake, V.P.; Garadkar, K.M. Biogenic synthesis of multi-applicative silver nanoparticles by using Ziziphus Jujuba leaf extract. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, K.S.; Rashid, M.; Tajuddin Husen, A.; Rehman, S. Biofabrication of Silver Nanoparticles from Diospyros montana, Their Characterization and Activity Against Some Clinical Isolates. BioNanoScience 2019, 9, 302–312. [Google Scholar] [CrossRef]
- Saxena, A.; Tripathi, R.M.; Zafar, F.; Singh, P. Green synthesis of silver nanoparticles using aqueous solution of Ficus benghalensis leaf extract and characterization of their antibacterial activity. Mater. Lett. 2012, 67, 91–94. [Google Scholar] [CrossRef]
- Korbekandi, H.; Asghari, G.; Jalayer, S.; Jalayer, M.; Bandegani, M. Nanosilver Particle Production Using Juglans Regia L.(Walnut) Leaf Extract. Jundishapur J. Nat. Pharm. Prod. 2012, 8, 20–26. [Google Scholar] [CrossRef]
- Raja, K.; Saravanakumar, A.; Vijayakumar, R. Efficient synthesis of silver nanoparticles from Prosopis juliflora leaf extract and its antimicrobial activity using sewage. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 97, 490–494. [Google Scholar] [CrossRef]
- Korbekandi, H.; Chitsazi, M.; Asghari, G.; Najafi, R.; Badii, A.; Iravani, S. Green biosynthesis of silver nanoparticles using Quercus brantii (oak) leaves hydroalcoholic extract. Pharm. Biol. 2015, 53, 807–881. [Google Scholar] [CrossRef]
- Devadiga, A.; Shetty, K.V.; Saidutta, M.B. Timber industry waste-teak (Tectona grandis Linn.) leaf extract mediated synthesis of antibacterial silver nanoparticles. Int. Nano Lett. 2015, 5, 205–214. [Google Scholar] [CrossRef]
- Saratale, R.G.; Dattatray, G.; Saratale, G.; Cho, S.; Ghodake, G.; Kadam, A.; Kumar, S.; Mulla, S.I.; Kim, D.-S.; Jeon, B.-H.; et al. Phyto-fabrication of silver nanoparticles by Acacia nilotica leaves: Investigating their antineoplastic, free radical scavenging potential and application in H2O2 sensing. J. Taiwan Inst. Chem. Eng. 2019, 99, 239–249. [Google Scholar] [CrossRef]
- Senthilkumar, N.; Nandhakumar, E.; Priya, P.; Soni, D.; Vimalan, M.; Potheher, I.V. Synthesis of ZnO nanoparticles using leaf extract of Tectona grandis (L.) and their anti-bacterial, anti-arthritic, anti-oxidant and in vitro cytotoxicity activities. New J. Chem. 2017, 41, 10347–10356. [Google Scholar] [CrossRef]
- Naz, S.; Islam, M.; Tabassum, S.; Fernandes, N.; de Blanco, E.; Zia, M. Green synthesis of hematite (a-Fe2O3) nanoparticles using Rhus punjabensis extract and their biomedical prospect in pathogenic diseases and cancer. J. Mol. Struct. 2019, 1185, 1–7. [Google Scholar] [CrossRef]
- Bhagat, M.; Anand, R.; Datt, R.; Gupta, V.; Arya, S. Green Synthesis of Silver Nanoparticles Using Aqueous Extract of Rosa brunonii Lindl and Their Morphological, Biological and Photocatalytic Characterizations. J. Inorg. Organomet. Polym. Mater. 2019, 29, 1039–1047. [Google Scholar] [CrossRef]
- Da’na, E.; Taha, A.; Afkar, E. Green Synthesis of Iron Nanoparticles by Acacia nilotica Pods Extract and Its Catalytic, Adsorption, and Antibacterial Activities. Appl. Sci. 2018, 8, 1922. [Google Scholar] [CrossRef]
- Saheed, Y. Antifungal Potential of silver nanoparticles from Acacia nilotica pod against Dermatophytes. J. Drug Deliv. Ther. 2021, 11, 85–95. [Google Scholar] [CrossRef]
- Kotakadi, V.S.; Gaddam, S.A.; Venkata, S.K.; Prasad, T.N.; Sai Gopal, D.V. Ficus fruit-mediated biosynthesis of silver nanoparticles and their antibacterial activity against antibiotic resistant E. coli strains. Curr. Nanosci. 2015, 11, 527–538. [Google Scholar] [CrossRef]
- Swarnavalli, G.C.; Dinakaran, S.; Raman, N.; Jegadeesh, R.; Pereira, C. Bio inspired synthesis of monodispersed silver nano particles using Sapindus emarginatus pericarp extract: Study of antibacterial efficacy. J. Saudi Chem. Soc. 2015, 21, 172–179. [Google Scholar] [CrossRef]
- Niluxsshun, M.C.D.; Masilamani, K.; Mathiventhan, U. Green Synthesis of Silver Nanoparticles from the Extracts of Fruit Peel of Citrus tangerina, Citrus sinensis, and Citrus limon for Antibacterial Activities. Bioinorg. Chem. Appl. 2021, 6695734. [Google Scholar] [CrossRef]
- Heydari, R.; Rashidipour, M. Green Synthesis of Silver Nanoparticles Using Extract of Oak Fruit Hull (Jaft): Synthesis and In Vitro Cytotoxic Effect on MCF-7 Cells. Int. J. Breast Cancer 2015, 2015, 846743. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, P.; Lee, S.; Iydroose, M.; Lee, K.; Oh, B. Pine cone-mediated green synthesis of silver nanoparticles and their antibacterial activity against agricultural pathogens. Appl. Microbiol. Biotechnol. 2013, 97, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Rautela, A.; Rani, J.; Debnath, M. Green synthesis of silver nanoparticles from Tectona grandis seeds extract: Characterization and mechanism of antimicrobial action on different microorganisms. J. Anal. Sci. Technol. 2019, 10, 5. [Google Scholar] [CrossRef]
- Veisi, H.; Hemmati, S.; Qomi, M. Aerobic oxidation of benzyl alcohols through biosynthesized palladium nanoparticles mediated by Oak fruit bark extract as an efficient heterogeneous nanocatalyst. Tetrahedron Lett. 2017, 58, 4191–4196. [Google Scholar] [CrossRef]
- Baghkheirati, E.K.; Bagherieh-Najjar, M.; Fadafan, H.; Abdolzadeh, A. Synthesis and antibacterial activity of stable bio-conjugated nanoparticles mediated by walnut (Juglans regia) green husk extract. J. Exp. Nanosci. 2016, 11, 512–517. [Google Scholar] [CrossRef]
- Sorbiun, M.; Mehr, E.; Ramazani, A.; Fardood, S. Green Synthesis of Zinc Oxide and Copper Oxide Nanoparticles Using Aqueous Extract of Oak Fruit Hull (Jaft) and Comparing their Photocatalytic Degradation of Basic Violet 3. Int. J. Environ. Res. 2018, 12, 29–37. [Google Scholar] [CrossRef]
- Edison, T.N.J.; Sethuraman, M.J. Electrocatalytic Reduction of Benzyl Chloride by Green Synthesized Silver Nanoparticles Using Pod Extract of Acacia nilotica. ACS Sustain. Chem. Eng. 2013, 1, 1326–1332. [Google Scholar] [CrossRef]
- Veisi, H.; Hemmati, S.; Shirvani, H.; Veisi, H. Green synthesis and characterization of monodispersed silver nanoparticles obtained using oak fruit bark extract and their antibacterial activity. Appl. Organometalic Chem. 2016, 30, 387–391. [Google Scholar] [CrossRef]
- Ramesh, S.; Vinitha, U.G.; Anthony, S.P.; Muthuraman, M.S. Pods of Acacia nilotica mediated synthesis of copper oxide nanoparticles and it’s in vitro biological applications. Mater. Today Proc. 2021, 47, 751–756. [Google Scholar] [CrossRef]
- Hossain, A.; Abdallah, Y.; Ali, M.; Masum, M.; Bin Li, B.; Sun, G.; Meng, Y.; Wang, Y.; An, Q. Lemon-Fruit-Based Green Synthesis of Zinc Oxide Nanoparticles and Titanium Dioxide Nanoparticles against Soft Rot Bacterial Pathogen Dickeya dadantii. Biomolecules 2019, 9, 863. [Google Scholar] [CrossRef]
- Muralidharan, V.A.; Ramesh, S.; Muthukrishnan, L. Facile fabrication of Annona squamosa L. seed extract mediated silver nanoparticles challenged against biofilm forming oral pathogens. Plant Nano Biol. 2023, 3, 100023. [Google Scholar]
- Edison, T.N.J.; Atchudan, R.; Sethuraman, M.; Lee, Y. Reductive-degradation of carcinogenic azo dyes using Anacardium occidentale testa derived silver nanoparticles. J. Photochem. Photobiol. B Biol. 2016, 162, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Mehnath, S.; Sathishkumar, G.; Arivoli, A.; Rajan, M.; Praphakar, R.; Jeyaraj, M. Green synthesis of AgNPs by Walnut seed extract and its role in photocatalytic degradation of a textile dye effluent. Trans. Eng. Sci. 2017, 5, 31–40. [Google Scholar]
- Venkateswarlu, P.; Ankanna, S.; Prasad, T.N.; Elumalai, E.K.; Nagajyothi, P.C.; Savithramma, N. Green synthesis of silver nanoparticles using shorea tumbuggaia stem bark. Int. J. Drug Dev. Res. 2010, 2, 720–723. [Google Scholar]
- Saheed, Y.; Umar, A.F.; Iliyasu, M.Y. Potential of Silver Nano Particles Synthesized from Ficus sycomorus Linn Against Multidrug Resistant Shigella species Isolated from Clinical Specimens. Am. J. Life Sci. 2020, 8, 82–90. [Google Scholar]
- Arya, G.; Kumari, R.M.; Gupta, N.; Kumar, A.; Chandra, R.; Nimesh, S. Green synthesis of silver nanoparticles using Prosopis juliflora bark extract: Reaction optimization, antimicrobial and catalytic activities. Artif. Cells Nanomed. Biotechnol. 2018, 46, 985–993. [Google Scholar] [CrossRef]
- Shah, Z.; Hassan, S.; Shaheen, K.; Khan, S.A.; Gul, T.; Anwar, Y.; Al-shaeri, M.A.; Khan, M.; Khan, R.; Haleem, M.A.; et al. Synthesis of AgNPs coated with secondary metabolites of Acacia nilotica: An efficient antimicrobial and detoxification agent for environmental toxic organic pollutants. Mater. Sci. Eng. C 2020, 111, 110829. [Google Scholar] [CrossRef]
- Nayak, D.; Ashe, S.; Rauta, P.R.; Kumari, M.; Nayak, B. Bark extract mediated green synthesis of silver nanoparticles: Evaluation of antimicrobial activity and antiproliferative response against osteosarcoma. Mater. Sci. Eng. C 2016, 58, 44–52. [Google Scholar] [CrossRef]
- Ahmed, Q.; Gupta, N.; Kumar, A.; Nimesh, S. Antibacterial efficacy of silver nanoparticles synthesized employing Terminalia arjuna bark extract. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1192–1200. [Google Scholar] [CrossRef]
- Savithramma, N.; Ankanna, S.; Bhumi, G. Effect of nanoparticles on seed germination and seedling growth of Boswellia ovalifoliolata—An endemic and endangered medicinal tree taxon. Nano Vis. 2012, 2, 61–68. [Google Scholar]
- Iravani, S.; Zolfaghari, B. Green Synthesis of Silver Nanoparticles Using Pinus eldarica Bark Extract. BioMed Res. Int. 2013, 2013, 639725. [Google Scholar] [CrossRef] [PubMed]
- Travasarou, A.; Angelopoulou, M.; Vougogiannopoulou, K.; Papadopoulou, A.; Aligiannis, N.; Cantrell, C.L.; Kletsas, D.; Fokialakis, N.; Pratsinis, H. Bioactive Metabolites of the Stem Bark of Strychnos aff. darienensis and Evaluation of their Antioxidant and UV Protection Activity in Human Skin Cell Cultures. Cosmetics 2019, 6, 7. [Google Scholar] [CrossRef]
- Yin, M.L.; Li, C.; Wang, Y.; Fu, J.; Sun, Y.; Zhang, Q. Comparison analysis of metabolite profiling in seeds and bark of Ulmus parvifolia, a Chinese medicine species. Plant Signal. Behav. 2022, 17, e2138041. [Google Scholar] [CrossRef]
- Leutcha, P.; Jouda, J.; Tankeu, V.; Magnibou, L.; Wahab, A.; Choudhry, M.; Lannang, A. Secondary metabolites from the stem bark of Stereospermum acuminatissimum and their antimicrobial activity. Biochem. Syst. Ecol. 2023, 109, 104648. [Google Scholar] [CrossRef]
- Sadgrove, N.J.; Mitaine-Offer, A.; Khumalo, G.; Van Wyk, B. An overview of the phytochemistry of medicinal bark (trunk, stem or root) from the most popular southern African species. Phytochem. Rev. 2025. [Google Scholar] [CrossRef]
- Bharali, P.; Das, S.; Bhandari, N.; Das, A.K.; Kalta, M.C. Sunlight induced biosynthesis of silver nanoparticle from the bark extract of Amentotaxus assamica D.K. Ferguson and its antibacterial activity against Escherichia coli and Staphylococcus aureus. IET Nanobiotechnology 2019, 13, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Guidelli, E.J.; Ramos, A.P.; Zaniquelli, M.E.; Baffa, O. Green synthesis of colloidal silver nanoparticles using natural rubber latex extracted from Hevea brasiliensis. Spectrochim. Acta Part A 2011, 82, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Kora, A.J.; Beedu, S.R.; Jayaraman, A. Size-controlled green synthesis of silver nanoparticles mediated by gum ghatti (Anogeissus latifolia) and its biological activity. Org. Med. Chem. Lett. 2012, 2, 17. [Google Scholar] [CrossRef]
- Mohamed, N.H.; Ismail, M.A.; Abdel-Mageed, W.M.; Shoreit, A.A. Antimicrobial activity of latex silver nanoparticles using Calotropis procera. Asian Pac. J. Trop. Biomed. 2014, 4, 876–883. [Google Scholar] [CrossRef]
- Salem, W.M.; Haridy, M.; Sayed, W.F.; Hassan, N.H. Antibacterial activity of silver nanoparticles synthesized from latex and leaf extract of Ficus sycomorus. Ind. Crops Prod. 2014, 62, 228–234. [Google Scholar] [CrossRef]
- Khatami, M.; Mortazavi, S.; Kishani-Farahani, Z.; Abbas Amini, A.; Amini, E.; Heli, H. Biosynthesis of Silver Nanoparticles Using Pine Pollen and Evaluation of the Antifungal Efficiency. Iran. J. Biotech. 2017, 15, e1436. [Google Scholar] [CrossRef] [PubMed]
- Oves, M.; Aslam, M.; Rauf, M.; Qayyum, S.; Qari, H.; Khan, M.S.; Alam, M.Z.; Tabrez, S.; Pugazhendhi, A.; Ismail, I.M. Antimicrobial and anticancer activities of silver nanoparticles synthesized from the root hair extract of Phoenix dactylifera. Mater. Sci. Eng. C 2018, 89, 429–443. [Google Scholar] [CrossRef]
- Escarcega-Gonzalez, C.E.; Garza-Cervantes, J.A.; Vazques-Rodriques, A.; Montelongo-Peralta, L.Z.; Treviño-Gonzalez, M.T.; Castro, E.D.B.; Saucedo-Salazar, E.M.; Morales, R.M.C.; Regalado-Soto, D.I.; Treviño-González, F.M.; et al. In vivo antimicrobial activity of silver nanoparticles produced via a green chemistry synthesis using Acacia rigidula as a reducing and capping agent. Int. J. Nanomed. 2018, 13, 2349–2363. [Google Scholar] [CrossRef]
- Jonoobi, M.; Khazaeian, A.; Tahir, P.; Azry, S.; Oksman, K. Characteristics of cellulose nanofibers isolated from rubberwood and empty fruit bunches of oil palm using chemo-mechanical process. Cellulose 2011, 18, 1085–1095. [Google Scholar] [CrossRef]
- Sheltami, R.M.; Abdullah, I.; Ahmad, I.; Dufresne, A.; Kargarzadeh, H. Extraction of cellulose nanocrystals from mengkuang leaves (Pandanus tectorius). Carbohydr. Polym. 2012, 88, 772–779. [Google Scholar] [CrossRef]
- Karimirad, R.; Behnamian, M.; Dezhsetan, S.; Sonnenberg, A. Chitosan nanoparticles-loaded Citrus aurantium essential oil: A novel delivery system for preserving the postharvest quality of Agaricus bisporus. J. Sci. Food Agric. 2018, 98, 5112–5119. [Google Scholar] [CrossRef]
- Babali, N.; Nadaroglu, H.; Taki Demir, T.; Alayli, A. Use of Nano-iron Fertilizer Additive Produced by Green Synthesis in Flame Tree (Photinia frasserii) and Smoke Tree (Cotinus coggyria) Cultivation. Indones. J. Soc. Environ. Issues 2024, 5, 182–191. [Google Scholar]
- Schneider, G.F.; Diego Salazar, D.; Hildreth, S.; Helm, R.; Whitehead, S. Comparative Metabolomics of Fruits and Leaves in a Hyperdiverse Lineage Suggests Fruits Are a Key Incubator of Phytochemical Diversification. Front. Plant Sci. 2021, 12, 693739. [Google Scholar] [CrossRef] [PubMed]
- Demirel Bayik, G.; Baykal, B. Impact of Plant Species on the Synthesis and Characterization of Biogenic Silver Nanoparticles: A Comparative Study of Brassica oleracea, Corylus avellana, and Camellia sinensis. Nanomaterials 2024, 14, 1954. [Google Scholar] [CrossRef]
- Ogwu, M.C.; Izah, S.C.; Joshua, M.T. Ecological and environmental determinants of phytochemical variability in forest trees. Phytochem. Rev. 2025. [Google Scholar] [CrossRef]
- Ray, P.C.; Yu, H.; Fu, P.P. Toxicity and environmental risks of nanomaterials: Challenges and future needs. J. Environ. Sci. Health Part C 2009, 27, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Rim, K.T.; Song, S.W.; Kim, H.Y. Oxidative DNA damage from nanoparticle exposure and its application to workers’ health: A literature review. Saf. Health Work 2013, 4, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Kumah, E.A.; Fopa, R.D.; Harati, S.; Boadu, P.; Zohoori, F.V.; Pak, T. Human and environmental impacts of nanoparticles: A scoping review of the current literature. BMC Public Health 2023, 23, 1059. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Desimone, M.F.; Pandya, S.; Jasani, S.; George, N.; Adnan, M.; Aldarhami, A.; Bazaid, A.; Alderhami, S. Revisiting the Green Synthesis of Nanoparticles: Uncovering Influences of Plant Extracts as Reducing Agents for Enhanced Synthesis Efficiency and Its Biomedical Applications. Int. J. Nanomed. 2023, 18, 4727–4750. [Google Scholar] [CrossRef]
- Zikeli, F.; Vinciguerra, V.; D’Annibale, A.; Capitani, D.; Romagnoli, M.; Scarascia Mugnozza, G. Preparation of Lignin Nanoparticles from Wood Waste for Wood Surface Treatment. Nanomaterials 2019, 9, 281. [Google Scholar] [CrossRef]
- Patino-Ruiz, D.A.; Meramo-Hurtado, S.I.; Gonzalez-Delgado, A.D.; Herrera, A. Environmental sustainability evaluation of iron oxide nanoparticles synthesized via green synthesis and the coprecipitation method: A comparative life cycle assessment study. ACS Omega 2021, 6, 12410–12423. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).